Abstract
This review about the genus Laminaria sensu lato summarizes the extensive literature that has been published since the overview of the genus given by Kain in 1979. The recent proposal to divide the genus into the two genera Laminaria and Saccharina is acknowledged, but the published data are discussed under a ‘sensu lato’ concept, introduced here. This includes all species which have been considered to be ‘Laminaria’ before the division of the genus. In detail, after an introduction the review covers recent insights into phylogeny and taxonomy, and discusses morphotypes, ecotypes, population genetics and demography. It describes growth and photosynthetic performance of sporophytes with special paragraphs on the regulation of sporogenesis, regulation by endogenous rhythms, nutrient metabolism, storage products, and salinity tolerance. The biology of microstages is discussed separately. The ecology of these kelps is described with a focus on stress defence against abiotic and biotic factors and the role of Laminaria as habitat, its trophic interactions and its competition is discussed. Finally, recent developments in aquaculture are summarized. In conclusion to each section, as a perspective and guide to future research, we draw attention to the remaining gaps in the knowledge about the genus and kelps in general.
Introduction
The genus Laminaria is one of the most important macroalgal genera of the order Laminariales (=‘kelp’) in temperate to polar rocky coastal ecosystems, especially in the northern Hemisphere. This is reflected, amongst other things, by its high species numbers, its considerable overall biomass, its dominance and economic significance, and is expressed in the steadily increasing rate of publications on this topic since the 1970s. As Kain (Citation1979) stated, at sites where Laminaria “species can exist only members of the Lessoniaceae completely out compete them”. This overall importance is, however, only partially reflected in the knowledge we have about the species of the genus Laminaria sensu lato and, at present, we can only guess what causes the recent dramatic changes in biomass and occurrence within the genus reported throughout Europe (see Section 3: Demography of Laminaria communities).
The first attempt to structure the diverse information available was undertaken by Kain (Citation1971) for Laminaria hyperborea and by Kain (Citation1979) for the whole genus. She reviewed taxonomy and distribution, life history and thallus structure, physiological aspects, ecology and population ecology. The ‘state of the art’ outlined in Kain (Citation1979) represents the backbone for our current synopsis and we have tried to avoid repetition by including literature prior to her work only if needed in the context. We also faced the problem of the literature being exhaustive and proliferating and it has proved impossible to cover all single aspects and always to be objective. This especially concerns literature published in the Far East (i.e. particularly Japan, China, Russia and Korea), which was only partly accessible to us, and only if published in English. We hope that this bias has not led to major omissions or mistakes.
Since Kain's review in 1979, the application of new methods in molecular biology, biochemistry, ecophysiology and also ecology have drastically changed our perception of kelps. The major discovery was that kelps are very distant from higher land plants and have to be considered as protists in the broad sense: Classically brown algae, along with green and red algae were regarded as plants (e.g. Sitte et al., Citation2002). Among many other features they share the presence of plastids and of complex vegetative bodies, at least in their advanced groups. However, brown algae on the one hand and green and red algae on the other are fundamentally different with respect to the nature and origin of their plastids. Plastids of green and red algae originate from a primary endosymbiosis of a cyanobacterium in a eukaryotic host cell whereas brown algae descend from a secondary endosymbiosis of a unicellular red alga in a eukaryotic host cell (Valentin & Zetsche, Citation1990; Valentin et al., Citation1992). As a consequence the former plastid type is surrounded by two membranes and the latter by four membranes. The eukaryotic host cells of red algae, green algae and glaucocystophytes are closely related and form a monophyletic group (Baldauf, Citation2003). The eukaryotic host cell of brown algae, however, lies in a totally different branch of the tree of life within the Chromista and not the Plantae (e.g. Cavalier-Smith, Citation1998) and is closely related to diatoms or oomycetes (Baldauf, Citation2003). Brown algae in general and, therefore, also kelps may thus be regarded as photosynthetic protists, similar to haptophytes, dinoflagellates and cryptophytes. They form a fifth independent lineage of multicellular organisms, next to animals, fungi, green algae and land plants, and red algae. The evolution, physiology, and ecology of kelps must be seen in this broader context.
The new molecular methods have also drastically changed our concept of the phylogeny and taxonomy of kelps, which is still ongoing and it has become clear that many morphological criteria used in classical taxonomy evolved several times (see Section 1: Recent developments in phylogeny and taxonomy). As a consequence, the genus Laminaria was recently shown to be polyphyletic (Yoon et al., Citation2001) and a separation into the two genera Laminaria Lamouroux and a resurrected Saccharina Stackhouse was proposed (Lane et al., Citation2006; see Section 1: Recent developments in phylogeny and taxonomy) and is acknowledged here. As our review mostly covers the time-frame between the late 1970s and 2006, we have introduced a ‘sensu lato’ concept, which includes all species considered to belong to ‘Laminaria’ before the proposal of Lane et al. (Citation2006). As more work is needed until all Laminaria sensu lato species are assigned to the right genus and almost all cited references use old names, we stick to the old nomenclature except in , where a summarized species concept of the genera Laminaria and Saccharina is presented including the current names (see Section 1).
Table 1. Current concept of species within the genera Laminaria Lamouroux and Saccharina Stackhouse
Another important area of research, which has developed rapidly since Kain's (1979) review, is the investigation of the ecophysiology of Laminaria species under controlled laboratory conditions. This line of research provided new insights into the complex external and internal control mechanisms affecting growth and reproduction throughout the whole life cycle (see Sections 4–7). Most importantly, the influence of daylength as external trigger and endogenous rhythms as internal Zeitgeber became evident. The discovery that pheromones were released from mature oogonia to attract male spermatozoids was the first indication of chemical communication in kelps. Additionally, information became available on the role of temperature and irradiance (including ultraviolet radiation) for the regulation of growth, survival and physiological parameters. These data for the first time explained the present biogeography and partially also zonation patterns.
The current synopsis summarizes the information available for Laminaria sensu lato with a focus on recent insights into phylogeny and taxonomy, the regulation and control of all life-cycle stages, their physiology and ecology and also provides an overview of recent advances in aquaculture. Many data are presented in form of overview tables. A figure depicting life cycle regulation by abiotic and endogenous factors complements the review. Finally, each section finishes with a conclusion and draws attention to gaps in knowledge regarding Laminaria sensu lato and kelps in general.
1. Recent developments in phylogeny and taxonomy
The genus Laminaria is classified within the Laminariaceae, one of the ten families within the Laminariales (van den Hoek et al., Citation1995; Kawai & Sasaki, Citation2000; Lane et al., Citation2006), which is one of the presently recognized 13–17 orders in the Phaeophyceae (Bold & Wynne, Citation1985; van den Hoek et al., Citation1995; de Reviers & Rousseau, Citation1999; Graham & Wilcox, Citation1999). More than 200 species, subspecies and forms have been described in the genus Laminaria since its establishment by Lamouroux in 1813 (Lamouroux, Citation1813). At present, the taxonomic database ‘Algaebase’ lists 240 species names of which 34 are regarded as current (Guiry & Guiry, Citation2007). During the 20th century, it was recognized that the genus exhibits great morphological plasticity and that reliable characters for delineating species are scarce. Kain (Citation1979) reviewed and discussed some of the characters in use, such as presence/absence of mucilage ducts, mucilage duct anatomy or stipe anatomy and she and others (e.g. Burrows, Citation1964; Wilce, Citation1965; Chapman, Citation1975) came to the conclusion that they were too variable to serve as diagnostic characters. As for many other taxa, classification of the Laminariales has mainly been based on morphology, anatomy, chemical constituents and life-cycle characteristics and classification based on those criteria is now being challenged by molecular systematics (Fain et al., Citation1988; Saunders & Druehl, Citation1993; Boo et al., Citation1999; Yoon & Boo, Citation1999; Yotsukura et al., Citation1999; Kawai & Sasaki, Citation2000; Kraan & Guiry, Citation2000; Erting et al., Citation2004; Lane et al., Citation2006). Genes from all three genetic compartments, the nucleus, the plastid and the mitochondrion, in addition to total DNA, have been recently used for phylogenetic reconstruction and have also added useful information for taxonomic delineation.
Kain (Citation1979) comprehensively showed both the confusion that had arisen to delineate taxa and the progress that had taken place in the classification of the genus Laminaria. As a consequence of her analysis, some of the doubtful species have not been mentioned again in the literature after 1979, thereby indicating that Kain's ideas were accepted within the scientific community. Some other taxa, however, still await a proper taxonomic assessment. In order to summarize the current species concept, an annotated species list is presented in . Its background is further explained in the following. This concept expresses the views of the authors, is deduced from recent insights and includes all species names that, to our knowledge, have been in use since Kain (Citation1979).
The first attempts to unravel phylogenetic relationships within the genus Laminaria utilized single-copy DNA–DNA hybridization (Stam et al., Citation1988). Laminaria digitata, L. saccharina, L. hyperborea, L. rodriguezii, and L. ochroleuca were compared with Chorda filum, but only a distant relationship was found, and this was later confirmed by phylogenetic analyses using 18S rDNA sequences (e.g. Boo et al., Citation1999). It was assumed that the five Laminaria species investigated had all evolved simultaneously from their most common ancestor some 15–19 Ma ago.
Later studies have tried to reveal phylogenetic relationships within the genus Laminaria and the order Laminariales with ribosomal RNA markers, i.e. the nuclear small subunit 18S rDNA (SSU) and the nuclear large subunit 28S rDNA (LSU). Bhattacharya et al. (Citation1991) used restriction fragment length polymorphism of the 18S gene and found that restriction maps of L. agardhii, L. saccharina and L. longicruris were identical, indicating that little variation is present in this gene among the Laminariales, and that the investigated species might be conspecific. This had already been suggested by evaluation of the hollow stipe character, interfertility and transplant experiments (Mann, Citation1971; Chapman, Citation1973a, 1974a) so that little molecular variation was expected in this group of species. Later, sequence analyses confirmed that the ribosomal RNA is too conserved to distinguish between species (Saunders & Druehl, Citation1992; Boo et al., Citation1999; Erting et al., Citation2004).
A more variable marker, the nuclear encoded internal transcribed spacer (ITS) of the rDNA operon, was used to compare 10 out of a total of 12 non-digitate Laminaria species from Japan (Yotsukura et al., Citation1999). There was little divergence in the ITS region but, nevertheless, two groups became separated, a ‘L. saccharina group’ and a ‘L. japonica group’. The authors thus concluded that only two biological species were present in the non-digitate Laminaria complex from Japan. Using a similar marker in a comparison of four Laminaria species from the North Atlantic, Erting et al. (Citation2004) were able to distinguish L. digitata from L. hyperborea. These two species formed a monophyletic group, which was clearly separated from L. saccharina. Laminaria faeroensis, a species with restricted occurrence, seemed to be conspecific with L. saccharina indicating a subspecies status. The same may be true for L. longicruris. Cho et al. (Citation2000) and Lane et al. (Citation2006) reported identical RuBisCo (ribulose 1,5 bisphosphate carboxylase/oxygenase) spacer and ITS sequences in L. saccharina and L. longicruris from the NW Atlantic.
In cases where single markers failed to resolve the phylogeny of a given group, the use of concatenated alignment, i.e. a combination of two or more markers, may be helpful. Draisma et al. (Citation2001) used this approach and established a phylogeny of the brown algae based on nuclear encoded 18S rDNA and plastid encoded rbcL (=large subunit of RuBisCo) gene sequences. Their study revealed that the Laminariales are phylogenetically the most developed brown algal group, astonishingly together with the Ectocarpales. The complex thallus structure and obligate haplo-diplont heteromorphic life-cycle of the Laminariales have always been regarded as at the opposite extreme to the filamentous thallus structure and isomorphic haplo-diplont life cycle of Ectocarpales. This exemplifies that phylogenetical relationships do not compulsorily correspond to morphological and life cycle complexity and that the term ‘primitiveness’ has to be used with caution. The close relationship of the two orders was also indicated by a comparison of the mitochondrial genome of Laminaria digitata with that of Pylaiella littoralis, a member of the Ectocarpales. The two mitochondrial genomes shared unusual features which might be unique to the heterokont lineage in general (Oudot-Le Secq et al., Citation2002). Draisma et al. (Citation2001) showed that rbcL sequences had more phylogenetic resolving power than the SSU sequences. However, their approach was applied only to the ordinal level and would probably fail at the family or genus level as both genes are highly conserved.
At approximately the same time, Yoon et al. (Citation2001) used a similar approach to tackle the phylogeny and familial boundaries of the three ‘advanced’ kelp families, the Alariaceae, the Laminariaceae and the Lessoniaceae. These authors chose much more variable but shorter markers, namely the nuclear-encoded ITS within the SSU and LSU genes, and the plastid-encoded spacer region between the large and small subunits of RuBisCo (rbcL and rbcS). They found that both markers produced similar trees but that the bootstrap support increased in combined trees, thereby creating an ‘improved phylogenetic signal’. In their tree, the three families fell into eight distinct generic clades: Agarum, Alaria, Ecklonia, Egregia, Hedophyllum, Laminaria, Lessonia and Macrocystis. For the first time it became evident that the genus Laminaria might not be a monophyletic group because species of the genus fell in clearly separated clades: L. digitata, L. hyperborea, L. setchellii and L. sinclairii were in the ‘Laminaria clade’, whereas L. japonica, L. religiosa, L. diabolica, L. longipdalis, L. longissima and L. saccharina belonged to the ‘Hedophyllum clade’.
Further evidence for the polyphyly of the genus Laminaria was recently provided by Lane et al. (Citation2006). They performed a thorough phylogenetic analysis of the Laminariales including many Laminaria species again using multi-gene phylogenies from nuclear, plastid and mitochondrial genome sequences. The length of their alignment exceeded 6,000 bp and was significantly longer, and thus more informative, than those from earlier approaches. The result was a comprehensive and well-supported phylogeny of the Alariaceae, Laminariaceae and Lessoniaceae. The resulting trees were also supported by the previously published short and variable ITS1 data sets of Saunders & Druehl (Citation1993) and Druehl et al. (Citation1997). The three kelp families have been retained but with a major reorganization of some species and genera and the creation of a new family, Costariaceae. It became evident that several conspicuous morphological features, like sporophylls and splitting of the blade, have arisen several times within the evolution of the Laminariales, a finding further reinforced by Cho et al. (Citation2006) working with Lessoniopsis. The impact of Lane's study for the species-rich genus Laminaria is considerable. The authors showed that the genus Laminaria splits into two sub-groups (clades) as already suggested by Yoon et al. (Citation2001), although the two investigations showed different affiliations. The genus Laminaria is actually not monophyletic, and the different clades include species with diverse morphologies. Digitate and simple bladed species were found in both clades, so that the classical morphological separation of the genus Laminaria into the section ‘Digitatae’ and ‘Simplices’ (Agardh, Citation1868; Setchell, Citation1893, 1900), based on the ontogenetic blade splitting, becomes obsolete. To follow the rules of nomenclatural precedence, Lane et al. (Citation2006) suggested retaining the genus Laminaria, containing the type of the genus L. digitata, and resurrecting Stackhouse's genus Saccharina, as the type for this genus was S. plana (=L. saccharina). The new legitimate name for L. saccharina was proposed to be Saccharina latissima (Linnaeus) Lane, Mayes, Druehl & Saunders. Other Laminaria species included into the new genus Saccharina are: L. angustata, L. cichorioides, L. coriacea, L. dentigera, L. diabolica, L. groenlandica, L. japonica, L. longicruris, L. longipedalis, L. longissima, L. ochotensis, L. religiosa and L. yendoana. In addition to these species of Laminaria, Cymathaere japonica and the genera Hedophyllum and Kjellmaniella were included in the genus Saccharina and new name combinations were proposed (). The close relationship between Kjellmaniella and Hedophyllum and the Japanese ‘L. saccharina group’ had already been shown by Yotsukura et al. (Citation1999) and Yoon et al. (Citation2001) and supports the taxonomic decisions taken by Lane et al. (Citation2006).
In summary, the consequences of these molecular and other recent studies for the taxonomic relationships within the genus Laminaria sensu lato since Kain (Citation1979) are as follows (see also and footnotes): The perception of most species with either a digitate blade or a discoid or rhizomatous holdfast has not changed very much since Kain (Citation1979). There are 13 distinct species (L. abyssalis, L. digitata, L. ephemera, L. farlowii, L. hyperborea, L. longipes, L. ochroleuca, L. pallida, L. rodriguezii, L. setchellii, L. sinclairii, L. solidungula and L. yezoensis) that have partially been further substantiated by hybridization and molecular investigations (e.g. Stam et al., Citation1988; tom Dieck, Citation1992; tom Dieck & de Oliveira, 1993; Erting et al., Citation2004) and that are not challenged at the moment. It seems that L. digitata is the most plastic species within this group as 40 forms have been assigned to it (Guiry & Guiry, Citation2007) and the relationship to taxa such as Arctic L. nigripes or the very restricted L. complanata in the NE Pacific is still unclear.
Many of the varieties mentioned by Kain (Citation1979) have still not been worked upon in a taxonomic context. Two examples are N Pacific L. bongardiana and L. groenlandica. Kain (Citation1979) and Lüning & tom Dieck (Citation1990) further discussed the confusion related to these species: Wilce (Citation1960) synonymized simple-bladed L. cuneifolia from the N Atlantic with simple-bladed L. groenlandica from the same region; synonymy of these species with L. saccharina was then suggested by Kain (Citation1979). N Pacific L. groenlandica, however, is considered to be clearly different from N Atlantic L. saccharina with longitudinal splits starting from tears at the end of the blade (Druehl, Citation1968). Petrov (Citation1972) included L. groenlandica sensu Druehl (Citation1968) as a synonym of L. bongardiana, a concept adopted by Lüning & tom Dieck (Citation1990) but not further used thereafter. A genetic relationship between N Atlantic L. digitata and N Pacific L. groenlandica could not, however, be proven by hybridization experiments (L. bongardiana in tom Dieck, Citation1992). Recently, Gabrielson et al. (Citation2006) subsumed both L. groenlandica and L. bongardiana under L. subsimplex, but final evidence for their conspecificity is still missing ().
Furthermore, there are several local species that are recorded only in eastern Russia (Sea of Ochotsk; Laminaria appresirhiza, L. gurjanovae, L. inclinatorhiza, L. multiplicata) and are “unknown to the overwhelming majority of phycologists outside Russia” (Selivanova, pers. comm.). Similarly, the deep-water species off Brazil, in the Mediterranean Sea, and the Philippines (L. abyssalis, L. brasiliensis, L. philippinensis, L. rodriguezii) are poorly investigated. All these entities need molecular and morphological re-examination to clarify their relationships to the genera Laminaria and Saccharina.
Most recent progress has been achieved within the simple-bladed taxa of the NW-Pacific region (Yotsukura et al., Citation1999, 2001, 2002, 2006; Yoon et al., Citation2001; Lane et al., Citation2006). Originally, 15 species of Laminaria were described by Miyabe and co-workers for Japan (Tokida et al., Citation1980 and references therein), but recently only 13 species have been recognized (L. angustata, L. cichorioides, L. coriacea, L. diabolica, L. longipedalis, L. longissima, L. japonica, L. religiosa, L. ochotensis, L. sachalinensis, L. saccharina f. linearis, L. yendoana, L. yezoensis; Yoshida et al., Citation2000). Attempts to apply morphological characters for species distinction led to five distinct morphological groupings of the 13 species investigated along the coast of Hokkaido, Japan (Druehl et al., Citation1988a), but could not resolve the taxonomic problems. The same was true for crossing experiments and transplantation studies: some results supported the conservative taxonomy, but others supported those scientists who considered Japanese species to be varieties of a few species (citations in Funano, Citation1980; Tokida et al., Citation1980). Lewis (Citation1996) pointed out that chromosome numbers are of some use to delineate taxa and mentions the case of L. longissima (n = 30) and L. angustata (n = 22; Funano, Citation1980), two species with close morphological affinities but different chromosome numbers. Later studies, however, reported 32 chromosomes in L. angustata (Yabu & Yasui, Citation1991) and thereby again questioned the usefulness of chromosome numbers in species distinction. Nevertheless, Lewis (Citation1996) suggests that a “comparison of chromosome number together with an assessment of the ability to hybridize can be helpful in determining the taxonomic and genetic affinities of different entities”, but a proper correlation was never achieved within the Laminariales. As morphological and cytological characters in general only provided a few useful characters for species delimitation, we are left with the recent molecular evidence (Druehl, pers. comm.).
Two major species complexes were identified on molecular grounds, and were corroborated by morphology and crossing experiments, namely a Laminaria japonica- and a L. saccharina-complex, the first sub-divided into two sub-groups. The L. japonica-complex comprises L. diabolica, L. japonica, L. religiosa, and L. ochotensis and probably also L. longipedalis (Yoon et al., Citation2001; Yotsukura et al., Citation1999, 2006; Yotsukura, pers. comm.). ITS sequences of L. angustata and L. longissima show a close affinity to L. japonica, but these species were considered to be a sub-group of this complex. These two species also form a separate morphological group according to Kawashima (1989; cited in Yotsukura et al., Citation2006) sharing morphological characters and their main distribution range. The complete interfertility between L. diabolica, L. japonica, L. religiosa and L. ochotensis up to the F2 generation and the partial interfertility between these species and L. angustata (all crosses with male L. angustata did not become fertile; Funano, Citation1980; Druehl et al., Citation2005) further support the two sub-groups of the L. japonica-complex. Investigations to resolve their position better are continuing (Yotsukura, pers. comm.).
There is even more complexity in the Laminaria saccharina-complex because this complex is distributed in the North Atlantic and the North Pacific. Unpublished results show one nucleotide difference in the ITS regions between plants from Nova Scotia and British Columbia (Mayes, Citation1984) and suggest Atlantic and Pacific L. saccharina to be the same (Lane, pers. comm.), but the more variable 5S rDNA spacer of Atlantic L. saccharina was “not unambiguously alignable with the sequences of Japanese samples” (Yotsukura et al., Citation2006). Individuals from the Atlantic and the Pacific are morphologically similar and partially interfertile (see Section 2: Morphotypes, ecotypes and population dynamics). Most individuals of the Pacific population behave as annuals, but some persist into a second year and become fertile in spring (Druehl, pers. comm.). This is in contrast to most Atlantic populations, which are perennial except at their southern boundary (see Section 2: Morphotypes, ecotypes and population dynamics). Thus, final evidence for conspecifity is still missing. The Japanese ‘L. saccharina complex’ of Yotsukura et al. (Citation1999; L. cichorioides, L. coriacea, L. saccharina, L. yendoana) inferred from ITS sequences was changed into a ‘L. coriacea-complex’ according to more variable 5S rDNA spacer sequences comprising L. cichorioides, L. coriacea, L. sachalinensis and L. yendoana together with Kjellmaniella crassifolia, K. gyrata and Cymathaere japonica and perhaps L. saccharina (Lane et al., Citation2006; Yotsukura et al., Citation2006). The relationship between L. cichorioides and L. sachalinenis in this group is still not finally resolved (Yotsukura, pers. comm.). These developments reflect the current uncertainty about a final classification within this group of Japanese species.
In the Atlantic, Laminaria saccharina also forms a species-complex. There is considerable evidence from investigations of clinal morphological characters, transplantation and interfertility experiments, and a comparison of molecular markers (Mann, Citation1971; Chapman Citation1973a, 1974a, 1975; Kain, Citation1976; Lüning et al., Citation1978; Cho et al., Citation2000; Erting et al., Citation2004; Lane et al., Citation2006) that all non-digitate species with a branched holdfast in the N Atlantic (L. saccharina, L. longicruris, L. faroensis, L. agardhii, Atlantic L. groenlandica) are part of a single very plastic L. saccharina species complex. In addition to the morphological plasticity of this species complex, a physiological plasticity with a strong genetic component was demonstrated for Atlantic populations (see Section 2: Morphotypes, ecotypes and population dynamics). This suggests that L. saccharina is a huge Pacific-Atlantic species complex with a broad plasticity, as had already been assumed after the successful hybridization experiments in this species complex in the 1970s and 1980s (see Section 2: Morphotypes, ecotypes and population dynamics). Unpublished molecular data suggest that variation between European and North American populations of L. saccharina, L. longicruris and L. faroensis needs further evaluation (Lane, pers. comm.). Erting et al. (Citation2004), for example, suggested subspecies status for L. faroensis. Final taxonomic decisions are urgently needed, but can be made only when the concept of what constitutes a species in the Laminariales has been further explored. Interestingly, one morphological character, namely the ornamentation of the surface of blades, seemed to reflect phylogeny in Japanese simple-bladed species (Yotsukura et al., Citation2006), giving hope of finding at least some correlations between molecular phylogeny and visible characters in the future.
Conclusion
Molecular genetics brought with it the uncomfortable knowledge that many morphological characters used to subdivide the genus Laminaria did not reflect genetic relationships (Druehl, pers. comm.). As a consequence, not only the genus Laminaria, but the whole order Laminariales is in a state of taxonomic re-organization that has not yet come to an end. Whether morphological and cytological criteria will just be a tool to describe species on the shore, as suggested by the recent evidence, or will also have any evolutionary significance remains to be clarified. One major task will be to elucidate the molecular relationships within the N and S Atlantic Laminaria species, and between the N Atlantic and N Pacific species of the L. saccharina complex. Furthermore, the resolution of the position of local entities and deep-water populations has to be fitted into a new species concept.
2. Morphotypes, ecotypes and population dynamics
The genus Laminaria exhibits a large morphological and physiological variability. The question of whether the distinct morphologies observed in the field represent species or ecotypes has been discussed for a long time. As reviewed by Kain (Citation1979), a large number of investigations of interfertility among different morphological types and geographical varieties have been published since the first investigations by Schreiber (Citation1930).
Hybridization experiments
An overview of the results of the numerous hybridization experiments among Laminaria species or morphological and geographical varieties is given in . Broad interfertility was observed among different morphological forms of related taxa or within species in the N Atlantic. The bullate forms of L. saccharina and L. longicruris from the Irish Sea, Brittany and Canada and the non-bullate form of L. saccharina from Helgoland (North Sea) are all interfertile and showed that bullae were inherited as a dominant trait (Lüning, Citation1975; Lüning et al., Citation1978; Bolton et al., Citation1983). Similarly, the presence of hollow or solid stipes or of mucilage ducts did not hinder interfertility in NW Atlantic L. longicruris, L. saccharina and L. faroensis (Chapman, Citation1974a, 1975). These results stimulated further research and hybridization experiments were conducted between N Atlantic and N Pacific simple-bladed Laminaria species. Bolton et al. (Citation1983) were able to generate fertile offspring from crossings of L. saccharina from the NE Pacific with smooth and bullate L. saccharina from the Atlantic. Moreover, L. saccharina strains from these sites were interfertile with NE Atlantic L. longicruris, and crossings of L. ochotensis from Japan with several varieties of L. saccharina from the N Atlantic and N Pacific also resulted in fertile offspring. A few years before Funano (Citation1980) had already shown that, in simple bladed taxa of the N Pacific (L. angustata, L. diabolica, L. japonica, L. religiosa and L. ochotensis), broad interfertily occurred and F2 sporophytes were formed. This does not mean, however, that all species with a simple blade, the so-called Simplices-group (Agardh, Citation1868; Setchell, Citation1893, 1900), belong to one common macrospecies pool (see also Section 1: Recent developments in phylogeny and taxonomy). For example, only female L. longicruris were able to interbreed with male L. ochotensis (Bolton et al., Citation1983); the reciprocal cross resulted in “twisted and deformed sporophytes, which did not become more than 1 mm in length” (cf. Bolton et al., Citation1983). Male L. longicruris from W Atlantic, however, successfully interbred with female L. saccharina from Helgoland (North Sea) and Brittany (France; Lüning et al., Citation1978; Bolton et al., Citation1983).
Table 2. Hybridization tests within the genus Laminaria: overview of successful and unsuccessful crossing experiments. Hybrids with normal F1 morphology (different to parthenosporophytes and similar to parental generation) were considered to indicate successful crosses. Formation of sori and further generations further substantiate successful crossings. Irregular or deformed F1 sporophyte morphology indicates unsuccessful hybridization. For further discussion see text (Section 2: Morphotypes, ecotypes and population dynamics)
A comparable set of crossing experiments was conducted for digitate Laminaria species by tom Dieck (Citation1992). Strains of L. digitata from the NW Atlantic and NE Atlantic were found to be interfertile and F3 generations were formed, confirming the amphi-Atlantic distribution of this species. Attempts to cross diverse other digitate Laminaria species, however, failed to produce normal sporophytes corresponding to the parental generation: NE Pacific L. setchellii or L. bongardiana (=L. groenlandica sensu Druehl Citation1968) did not successfully interbreed with NE Atlantic L. digitata, L. hyperborea and L. ochroleuca. The same was true for the partially sympatric NE Atlantic L. digitata, L. hyperborea and L. ochroleuca (tom Dieck, Citation1992). These results are in agreement with those of Schreiber (Citation1930), but conflict with those of Cosson & Olivari (Citation1982), Cosson & Gayral (Citation1983) and Cosson (Citation1987), who hybridized L. digitata with L. saccharina, L. hyperborea, L. ochroleuca and Saccorhiza polyschides and produced small F1 sporophytes, several cm in length. Unfortunately, the authors did not explicitly describe how delimitation between hybrid sporophytes and parthenosporophytes was achieved. In an earlier study, parthenosporophytes developed at a rate of 23–28% similar to some of the hybrids reported later (Olivari, 1981 cited in Cosson & Gayral, Citation1983; see ). As tom Dieck (Citation1992) showed that some of the hybrid sporophytes had a normal morphology at an early microscopic stage but later became stunted or deformed and stopped growth, the apparently successful hybridizations between sympatric N Atlantic species need re-investigation. In this context, Cosson et al. (Citation1984) also assumed that a form of L. digitata with bifurcated stipes found along the Normandy coast might represent a hybrid between L. saccharina and L. digitata, but these forms are rarely found and could also be the result of mechanical damage to the transition zone.
In order to test N Atlantic/S Atlantic relationships in later experiments, the range of species involved in the crossing experiments was expanded (tom Dieck & de Oliveira, 1993): S Atlantic species Laminaria schinzii, L. pallida and L. abyssalis were all able to interbreed with each other, forming normal F1 sporophytes. Similarly, crossing N Atlantic L. digitata with L. pallida or L. abyssalis from the S Atlantic was successful, but crossing with L. schinzii from the same ocean was not. The other N Atlantic species, L. saccharina, L. ochroleuca and L. hyperborea, however, did not hybridize with Laminaria species from the S Atlantic. Here again, many of these unsuccessful breeding experiments initially produced normal microscopic sporophytes, which became stunted during further development and only formed small sporophytes differing from the parental or parthenogenetic generation (tom Dieck & de Oliveira, 1993). This suggests that fertilization may have taken place on many occasions due to the universal pheromone bouquet present in the Laminariaceae (Maier & Müller, Citation1986; Maier, Citation1995) but that further development was distorted. Tom Dieck (Citation1992) postulated that reproductive isolation develops gradually in Laminariales–a phenomenon that has been generally observed for the evolution of characters controlled by multiple genes (Barton, Citation1988). The observation that hybrid formation was expressed differently in reciprocal crosses (Saito, Citation1972; Lüning et al., Citation1978; Sanbonsuga & Neushul, Citation1978; Cosson, Citation1987) supports this theory.
It is believed, therefore, that interfertility is common in the whole genus. Irrespective of the fact that interfertility even occurs between at least some Laminaria species and species from sister genera and closely related families (Cosson & Gayral, Citation1983; Cosson, Citation1987; Druehl et al., Citation2005), the observed restrictions of interfertility in the genus Laminaria itself clearly show that there are borders and that, therefore, a macrospecies concept cannot be applied. Furthermore all crossing studies within the Laminariales so far do not conform to the classical proof of total interfertility, which demands assessment of offspring fertility, breeding of a F2 generation and back crosses. Thus, the reported results of interfertility and their impact for species delimitation have to be judged with care. However, if partial interfertility is possible, questions arise about how adaptations to local conditions may have evolved. One possible explanation might be the ability of several Laminaria species to form adult parthenogenetic sporophytes. Parthenogenesis in kelps was first described by Schreiber (Citation1930), but he only reported abnormal small morphologies of parthenosporophytes. Later studies revealed that in some cases adult, fertile parthenosporophytes with normal morphology may develop (e.g.: L. japonica: Fang et al., Citation1978; Lewis et al., Citation1993; Bai & Qin, Citation1998; L. saccharina, from aposporous gametophyte-like filaments: Ar Gall et al., Citation1996).
Exposure morphotypes
Gerard (Citation1987) showed that bullations of the blade were an adaptation to mechanical stress of the environment and this character did not hamper interfertility (Lüning, Citation1975). Another effect of mechanical stress on morphology was shown by Klinger & de Wreede (Citation1988) in studies with Laminaria setchellii. Plants of similar age from exposed sites had longer and thicker stipes than plants from less exposed sites. This is in contrast to earlier reports from Eastern Canada where stipe lengths were longer at calm-water sites (Chapman, Citation1973a; Gerard & Mann, Citation1979). Earlier reports had similarly shown that absence of wave action leads to broad and non-digitate blades in L. hyperborea, a morphotype described as L. cucullata in the past (Kain, Citation1979 and citations therein).
Mucilage ducts varieties
Mucilage duct types have been used to discriminate between Laminaria saccharina and L. longicruris in the past. Wilce (Citation1965) was the first to discredit this as a taxonomic character, suggesting that presence or absence of them is related to temperature conditions. Later, crossing experiments by Chapman (Citation1975; ) revealed complete interfertility among these species and showed that this character is of limited value for species discrimination. These findings were later strengthened by the observation of Calvin & Ellis (Citation1981) that, in N Atlantic L. groenlandica, considerable variability of mucilage duct types was induced by environmental conditions.
Iodine strains
Several members of the Phaeophyceae have been shown to accumulate trace elements to a large degree (Indergaard & Minsaas, 1991). This holds true for Laminaria, which has been used in the past in Europe as raw material for iodine extraction, especially along the coast of Brittany in France (Lüning, Citation1985). Later, Brinkhuis et al. (Citation1987) reported the selection of strains with exceptionally high accumulation rates for iodine in order to achieve a food supplement able to overcome iodine deficiency, resulting in goitre, a common disease, especially in central China.
Hollow stipe vs solid stipe variants
The problems of using the hollowness of the stipe as a taxonomic character and its ability to differentiate among species in the genus Laminaria have been discussed in a number of publications (e.g. Wilce, Citation1965; Mann, Citation1971; Chapman, Citation1973a, 1974a; Kain, Citation1979). It was shown that there is a significant genetic component in the expression of hollowness in the section Simplices. Crossing experiments and molecular work (Chapman, Citation1974a; Lüning et al., Citation1978; Bolton et al., Citation1983; Bhattacharya et al., Citation1991) have provided evidence that L. saccharina and L. longicruris are conspecific, further reducing the value of stipe hollowness as a character.
Temperature and light ecotypes
The influence of growth temperature on morphotypic and ecotypic differentiation was shown for six different Laminaria species (Okada et al., Citation1985; Gerard & DuBois, Citation1988). In general, sporophytes of L. angustata var. longissima, L. diabolica, L. japonica, L. ochotensis and L. religiosa incubated at high temperatures were rounder and more slender than those incubated at low temperatures. A clear ecotypic differentiation of two L. saccharina populations from the Atlantic coast of the USA was described by Gerard & DuBois (Citation1988). One population near the southern edge of the distribution (New York State) is exposed to ambient temperatures above 20°C in summer, whereas a second population from Maine is seldom exposed to temperatures exceeding 17°C. In the laboratory, adult sporophytes from New York survived and grew for 6 weeks at 20°C, but all plants from Maine died after 3 weeks. After temperature acclimation in the laboratory, plants from the two populations retained their distinctive growth and photosynthesis parameters, confirming the ecotypic differentiation of the strains. A similar observation was made by Lüning et al. (Citation1978), who mentioned in the discussion that the smooth form of L. saccharina from Helgoland tolerated summer temperatures of 18°C, whereas the bullate form from Nova Scotia died at temperatures above 16°C. Gerard (Citation1988) reported on the irradiance acclimation capabilities of ecotypes of L. saccharina. She showed that the acclimation range was related to the degree of variability in irradiance at the natural habitat of origin, decreasing with increasing depth of the population sampled. Specimens of L. saccharina harvested from shallow, deep or turbid water habitats along the coast of Maine exhibited large differences in photosynthetic parameters even after acclimation to ‘common garden conditions’ for six weeks after collection. These variations further resulted in marked differences in carbon assimilation and growth rates. The differences persisted even after cultivation in identical conditions, suggesting that a physiologically based ecotypic differentiation occurred on small spatial scales (Gerard, Citation1988).
Nutrient ecotypes
Gagné et al. (Citation1982) concluded that three genetically fixed nutrient strains were present in Laminaria longicruris, based on differences in growth pattern and the patterns of nitrogen and carbon storage, which in turn depended on inorganic nitrogen availability. At sites where nitrogen was available throughout the whole year, growth followed the seasonal availability of light and storage of nitrogen and carbon was small. At the site where nitrogen was abundant only in the winter months, growth mainly occurred during winter and carbon was stored during summer. At sites with intermediate nitrogen conditions, growth rates were maximal during summer and minimal during winter, when plants accumulated large nitrogen reserves. Espinoza & Chapman (Citation1983) investigated the influence of nitrate availability on physiological parameters of L. longicruris. Organisms from a nitrogen-rich and a nitrogen-poor habitat clearly differed in their acclimation potential to nitrogen depletion. As these differences were stable also during laboratory experiments, during which plants from both sites were incubated under similar conditions, a genetically fixed adaptation was postulated. However, this adaptation has not resulted in speciation in the L. saccharina/longicruris complex (see above).
Population genetics
New molecular tools like microsatellite markers (Billot et al., Citation1998), random amplified polymorphic DNA (RAPD) markers (Billot et al., Citation1999; Hu & Zhou, Citation2001; Wang et al., Citation2004; Xia & Wang, Citation2005) and the amplified fragment-length polymorphism (AFLP) technique (Erting et al., Citation2004) have provided new insights in population genetics and phylogeny of the genus Laminaria in recent years. Using microsatellite markers, a detailed study was performed in L. digitata in order to get information about the influence of habitat discontinuities on population genetic structure (Billot et al., Citation2003). Populations around Brittany (France) and the English Channel were analysed. Continuous, non-fragmented forests of L. digitata were genetically different at distances greater than 10 km, despite the absence of clear population boundaries. Habitat discontinuities accentuated the genetic differences and resulted in a reduced genetic variation of isolated stands.
A low degree of polymorphism was also found by Yotsukura et al. (Citation1999), analysing ribosomal DNA ITS1 and ITS2 sequences of 12 non-digitate Laminaria species from Hokkaido, Japan. A similar result was obtained by Neefus et al. (Citation1993) using isoenzyme gel electrophoresis. In his study, an extremely low degree of polymorphism both within and between populations of L. saccharina, L. longicruris, L. digitata and N Atlantic L. groenlandica was found. The fact that kelp and kelp-like species from other genera (Agarum cribosum, Alaria esculenta, Chorda tomentosa (=Halosiphon tomentosus), Macrocystis pyrifera) also exhibited low polymorphism raised the question whether the results of such studies on brown algae can be compared with analyses of higher plants or if the degree of polymorphism might be lower in the heterokont lineage in general, a field where more knowledge urgently is required.
Conclusion
At present, irrespective of the ongoing discussion about which species concept would be the most appropriate for the genus Laminaria or Heterokontae in general, a broad range of morphological plasticity and a large adaptive capacity of Laminaria species have been described. Sexual isolation of Laminaria species, as well as the degree of genetic polymorphism, seems to be unusually low but is combined with a large adaptation capacity as outlined above–a situation that is not yet understood and needs to be further investigated.
Summarizing, the statement made by Chapman (Citation1974b), that “most taxonomic treatments of algae may be criticized in that they lack the philosophy required for dealing with variation in population” seems still to be valid. As already shown for Alaria (Nakahara & Nakamura, Citation1973) and for Laminaria saccharina and Macrocystis integrifolia (Druehl et al., Citation2005), male kelp gametophytes are able to develop directly into apogamous sporophytes with normal sporophyte morphologies, a fact which would require male negative controls for accurate interpretation of the results of the hybridization experiments listed in , but have not been published. Moreover, as shown by Druehl et al. (Citation2005) molecular evidence is essential for establishing hybridizations in brown algae. In any case, it seems that a simple application of concepts derived from higher plants, including ‘alternative species concepts’ (e.g. Cracraft, Citation1989), will not solve the problems in brown algal species delimitation.
3. Demography of Laminaria communities
During the last decade, a growing number of reports have addressed the changes of demographic parameters in Laminaria stands (e.g. Breuer & Schramm, Citation1988; Givernaud et al., Citation1991; Lambert et al., Citation1992; Schaffelke et al., Citation1996; Sivertsen, Citation1997; Cosson, Citation1999; Klotchkova & Berezovskaya, Citation2000; Morizur, Citation2001; Moy et al., Citation2003; Britton-Simmons, Citation2004; Gehling & Bartsch, unpublished data). Proposed causes for the changes included an altered physical environment (e.g. Lyngby & Mortensen, Citation1996; Dayton et al., Citation1999), pollution (including eutrophication, e.g. Brown et al., Citation1990) and changes in biotic interactions (e.g. Sivertsen & Bjoerge, Citation1980; see also Section 14: Trophic interactions), but unequivocal evidence is mostly missing.
Influence of exposure
Kain (Citation1971, 1976) compared the age structure of Laminaria hyperborea stands on sheltered and exposed coasts and found a high percentage of young plants at exposed sites, indicating high mortality of old sporophytes. At shallow locations with less mechanical stress, the percentage of older sporophytes increased, and the low number of juveniles indicated competition for space in a saturated community. In contrast to most other algae, the age structure of Laminaria populations can be studied by counting the concentric annual growth rings in the stipe. Using this technique, Klinger & de Wreede (Citation1988) confirmed the results of Kain (Citation1971) and showed that the mean age of a L. setchellii population was inversely related to exposure.
Matrix models
In order to test whether the demographic parameters ‘mortality’ and ‘fecundity’ were related more to size or to age, Chapman (Citation1986) investigated 255 individuals of Laminaria longicruris. The rate of mortality was constant, producing a Type II (Deevey, Citation1947) survivorship curve. If the population was divided into age classes, size variation had no significant influence on the survivorship of the plants. However, for a long period these observations were of limited value because, as a result of the biphasic life-cycle of Laminaria, interpretation by means of common demographic models (see review by Caswell, Citation1986) was not possible. The first matrix models for algal species with a multiphasic life cycle were presented by Ang (Citation1987, 1991) and Ang & de Wreede (Citation1990). The applicability of these models was tested by means of data obtained from L. longicruris stands. These discrete matrix models realistically described the life-history processes of Laminaria (Chapman, Citation1993). He reported an analysis of a dataset consisting of age and fertility proxies for 68 individuals over a 9-year interval. Using size-based square matrix models, prediction of population dynamics was possible whereas the application of age-based fertility life tables failed (Chapman, Citation1993).
Age vs reproduction
With respect to demography, a number of interesting conclusions about a population of Laminaria digitata from Abbott's Harbour (Canada) were drawn, summarized by Chapman (Citation1993): (i) The species was found to be iteroparous, i.e. perennial, as was shown for L. longicruris (Chapman, Citation1986). (ii) Only 13% of the members of the cohort reproduced in 8.75 years. (iii) The minimum size for reproduction was found to be a total thallus length of 74 cm. (iv) Age of first reproduction was approximately 15 months. (v) Reproduction occurred among all individuals that survived for 60 months or more. (vi) Not all individuals reaching the minimum size for reproduction produced sporangia.
The life span and the reproductive effort have been shown to be temperature or latitude dependent in Laminaria saccharina and L. hyperborea (Lee & Brinkhuis, Citation1986; Sjøtun, Citation1993). Lee & Brinkhuis (Citation1986) described stands of L. saccharina at the southern distribution limit in which plants were annuals. At the other extreme, Sjøtun et al. (Citation1993) showed clear differences in life expectancy between L. hyperborea individuals from different geographical locations: a population from northern Norway (Finnmark) contained 13–18-year-old plants while oldest individuals in a population at the southern Norwegian Atlantic coast were 8–9 years old. Interestingly, the mean standing crop was similar at both sites indicating a ‘pure’ longevity effect with more juvenile plants per m2 in the south. This result was confirmed by Rinde & Sjøtun (Citation2005), showing a direct relation between longevity and increasing latitude for L. hyperborea along the Norwegian coast (58–71°N).
Growth vs age
With respect to size classes, Sjøtun (Citation1993) found an age-dependent elongation of the blade of Laminaria saccharina. In western Norway, 3-year-old sporophytes exhibited lower blade elongation rates than 2-year-old plants, whereas width growth was not affected by age. In a later study, Sjøtun et al. (Citation1995) showed that the highest allocation of growth to the stipes was found in 3- and 4-year-old sporophytes. Maximum stipe weight increase was observed, however, in 4- and 5-year-old plants while blade growth increased continuously with age (Sjøtun et al., Citation1995). Similar age-dependent growth was observed by Druehl et al. (Citation1987) for L. groenlandica. In first-year plants, maximum growth per season was delayed by 3–4 months compared with older-year classes. Interestingly, all year classes reached their highest wet weight during July/August (Druehl et al., Citation1987). Lüning (Citation1979) reported a similar age-dependent prolongation of the growth season in L. hyperborea. He showed that blade growth was reduced later in the year in first-year plants than in older individuals.
Density of stands and biomass
With respect to biomass and density of stands, a number of studies have been published and are summarized in . Many data are available for the northern Scandinavian coast up to the White Sea (Sjøtun et al., Citation1993; Schoschina, Citation1997; Sivertsen, Citation1997), but Sivertsen (Citation1997) presented the most detailed study from the Norwegian coast. Here kelp beds with large thalli of Laminaria hyperborea reached 20.7 individuals m− 2 decreasing to 9.7 individuals m− 2 in transition areas at the edge of the stands. Interestingly, in dense kelp forests, juvenile Laminaria sporophytes had a similar density to the adults (23.9 individuals m−2) whereas, in transition areas, the proportion of juveniles was clearly lower (approx. 30%, 3.6 individuals m−2). In harvesting areas, the density of juveniles was highest (59.1 individuals m−2) and resembled the total population density in unharvested kelp beds. The only data available on latitudinal variations are those of Rinde & Sjøtun (Citation2005). Here a significant decrease of density in canopy-forming L. hyperborea was observed with increasing latitude (12.6 individuals m−2 in the south to 6.0 individuals m−2 in the north). With respect to biomass of Laminaria stands, a number of reports from the northern Hemisphere have been published (e.g. Calvin & Ellis, Citation1978; Edwards, Citation1980; Chapman & Lindley, Citation1980a; Chapman, Citation1981, 1984; Dunton et al., Citation1982; Brady-Campbell et al., Citation1984; Kornfeldt, Citation1984; Smith, Citation1985; Egan & Yarish, Citation1990; Sjøtun et al., Citation1993; Schoschina, Citation1997; Sivertsen, Citation1997; ). Comparing biomass ranges from Arctic and cold boreal regions (Dunton & Dayton, Citation1995; and references therein), the same trend as for density of stands (see above) is apparent: 10-fold higher biomass values were reported from more southerly, cold temperate regions (10–40 kg m−2) than in the Arctic (0.4–1.8 kg m−2; ). In other publications most of the numbers given are from disturbed stands or a stand growing at suboptimal conditions or it is not clear whether the number is related to maximum values. The sparse and in most cases fragmented information about demography of stands does not yet allow any large-scale analysis of factors influencing the population structure. Systematic analyses, as done by Sivertsen (Citation1997) for Norwegian populations, are required from other regions as well to identify the abiotic and biotic factors structuring Laminaria kelp beds.
Table 3. Maximum biomass and density values achieved in diverse Laminaria bedsa
Conclusion
Irrespective of the growing number of demographic investigations on Laminaria stands the present knowledge about the main population parameters is still restricted to a few places. Moreover, the pronounced seasonal growth characteristics of Laminaria species (see Section 4: Growth and photosynthetic performance of sporophytes) would require seasonal sampling. Even in the few cases where the sampling was done in autumn, it was not proven whether the maximum biomass was reached for this specific stand, because growth characteristics have been shown to be influenced by site-specific conditions and age (see Section 2: Morphotypes, ecotypes and population dynamics). Therefore, the overview given in does not provide a complete picture and underlines the scattered knowledge rather than giving a comprehensive overview. Detailed investigations of biomass, stand densities and age structure, fertility etc., reached by individual species at optimum conditions along latitudinal gradients (temperature/light) and over the year are urgently needed.
4. Growth and photosynthetic performance of sporophytes
The growth and reproductive characteristics of Laminaria sporophytes have received substantial attention, especially with respect to the role of environmental factors. Within the Phaeophyceae, the ecophysiological traits and acclimation potential to environmental conditions have probably been best explored in the orders Fucales and Laminariales. While earlier studies mainly addressed performance of sporophytes by weight, length or area increase and thus growth, more recent studies have often used photosynthetic activity as an indicator of physiological performance. Different approaches will result in a different estimation of performance, since, for example, saturating irradiances are largely different for photosynthetic activity and growth (Lüning, Citation1979). In order to estimate the success of a species in a given habitat, growth may be ecologically more significant as this parameter integrates many physiological processes. In contrast, photosynthetic activity describes the major physiological process impacting growth and thereby is highly relevant per se. Growth performance depends on carbon allocation and is generally governed by abiotic forces such as light (irradiance, spectral composition, photoperiod), temperature, nutrient availability and the seasonal interaction of all factors. Information about the contribution of these abiotic factors to growth and photosynthetic performance of Laminaria sporophytes is diverse. As multifactorial experiments have rarely been conducted, it is still difficult to determine how environmental factors interact and contribute to the successful establishment of Laminaria species.
Seasonality
The seasonality of growth in N Atlantic species of Laminaria was extensively reviewed by Kain (Citation1979). Generally there is “a period of rapid growth from January to June and one of slow growth from July to September” (Kain, Citation1979). On Helgoland (North Sea), L. saccharina and L. hyperborea predominantly grow in winter and early spring (Lüning, Citation1979), as they do in Norway (Sjøtun, Citation1993; Sjøtun et al., Citation1996). In contrast, the growth period of L. digitata on Helgoland extends from spring to summer. While growth of L. hyperborea stops completely in July, growth of L. saccharina decreases substantially but does not cease. In contrast, L. digitata grows continuously through the summer and the growth rate in September is still 50% of the optimum (Lüning, Citation1979). In all species, a new blade is formed during each growth period while the old blade erodes. The seasonal variations in abiotic conditions affect growth performance, particularly in algae from high latitudes with a more pronounced seasonality of temperature, irradiance and photoperiod than those from temperate waters. The endemic, Arctic L. solidungula grows predominantly in winter under a thick cover of sea-ice (Chapman & Lindley, Citation1980b; Dunton et al., Citation1982). Growth in this species is fuelled by consumption of stored carbohydrates from the previous season's blade (Dunton & Schell, Citation1986), as shown earlier for L. hyperborea from Helgoland (Lüning, Citation1969; Lüning et al., Citation1973). In contrast, the growth of L. saccharina in the Arctic is closely tied to active photosynthesis in the new growing blade (Dunton, Citation1985; Henley & Dunton, Citation1995).
Most Laminaria species are so-called season anticipators, which grow and reproduce in a strategic annual rhythm in response to a trigger, e.g. daylength (Kain, Citation1989). Seasonal optima of photosynthetic capacities are mostly recorded in late winter to early summer at moderate light availability. Drew (Citation1983) provided a physiological baseline study for L. digitata, L. hyperborea and L. saccharina from Scotland. He measured seasonal photosynthetic performance in the laboratory at 10°C and found a spring peak of photosynthetic capacity in all three species. After adjustment of the metabolic rates to habitat temperatures, the seasonal maximum was shifted to summer. As the spring peak coincided with nutrient regeneration in the coastal system, and was followed by nutrient depletion in late spring, it was thought to depend on the presence of nutrients. Similarly, the C/N ratio showed a clear seasonal pattern with an increase from 7 in early spring to about 12 in summer corresponding to strong photosynthetic and growth activity, but at the expense of the internally stored N (Gévaert et al., Citation2001).
Photoperiod
Photoperiod is of major importance for growth performance of Laminaria sporophytes. The longer the day the higher the growth rates as exemplified in L. saccharina (Fortes & Lüning, Citation1980). When specimens are exposed to light–dark cycles, sporophytes grow faster during the illumination period so that Lüning (Citation1992) assumed an underlying diurnal rhythm (see Section 7: Endogenous rhythms controlling metabolism and development). Besides the significance of daylength for providing light energy, another very important function, photoperiod is its trigger for the regulation of seasonal growth. In L. saccharina and L. setchellii, short daylength treatments applied after a period of long days led to complete halt in growth within 1–5 weeks, but new blade growth is initiated after another few weeks in these conditions (Lüning, Citation1988; tom Dieck, Citation1991; see also Section 7: Endogenous rhythms controlling metabolism and development). Initiation of new blades in L. hyperborea is also triggered by short days (Lüning, Citation1986; ).
Fig. 1. Schematic representation of life-cycle control in Laminaria sensu lato by abiotic and endogenous factors assuming that regulation processes are similar within the genus. aLüning (Citation1986, 1988, 1992, 1994), tom Dieck (Citation1991), Schaffelke & Lüning (Citation1994), Makarov et al. (Citation1995), for species and further details see section 7: Endogenous rhythms controlling metabolism and development; b L. hyperborea, L. saccharina: Lüning (Citation1979), Fortes & Lüning (Citation1980), Lüning (Citation1988), L. setchellii: tom Dieck (Citation1991); c L. hyperborea: Lüning (Citation1986), L. setchellii: tom Dieck (Citation1991); d L. saccharina: Lüning (Citation1988), L. setchellii: tom Dieck (Citation1991); e L. digitata, L. saccharina: Buchholz & Lünig (Citation1999), Lüning et al. (Citation2000), L. japonica: Mizuta et al. (Citation1999b), for further detail see section 5: Sporogenesis and meiospore release; f L. japonica: Fukuhara et al. (Citation2002); g L. farlowii: Amsler & Neushul (Citation1990), L. japonica: Fukuhara et al. (Citation2002); h L. digitata, L. hyperborea, L. saccharina: Lüning (Citation1980); i iron: L. farlowii: Amsler & Neushul (Citation1989a), L. japonica: Motomura & Sakai (Citation1981, 1984), blue light: L. digitata, L. hyperborea, L. saccharina: Lüning & Dring (Citation1972, 1975), Lüning (Citation1980); j L. saccharina: Lüning (Citation1981), L. japonica: Tseng et al. (Citation1959); k(e.g.: L. digitata): Müller et al. (Citation1979), Maier et al. (Citation1988); lLüning (Citation1979), Wiencke & Fischer (Citation1990), Han & Kain (Citation1996); m L. hyperborea: Lüning (Citation1970), L. solidungula: Chapman & Lindley (Citation1980a), L. saccharina: Borum et al. (Citation2002); n L. abyssalis, L. bongardiana, L. digitata, L. hyperborea, L. longicruris, L. ochroleuca, L. pallida, L. schinzii, L. setchellii, L. solidungula: Bolton & Lüning (Citation1982), Lüning & Freshwater (Citation1988), tom Dieck (Citation1992), tom Dieck & de Oliveira (1993); oLüning & Dring (Citation1985), Lüning (Citation1993); p L. abyssalis, L. bongardiana, L. digitata, L. hyperborea, L. ochroleuca, L. pallida, L. schinzii, L. setchellii: tom Dieck (Citation1992), tom Dieck & de Oliveira (1993), Izquierdo et al. (Citation2002), L. longicruris: Yarish et al. (Citation1990); q L. digitata: Bartsch (unpublished data).
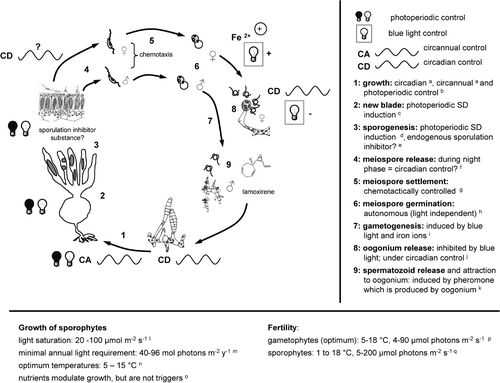
Irradiation
As the genus Laminaria inhabits almost the entire sublittoral from the surface at low tide to a depth where at least 1% of the surface light penetrates, it has to cope with both very intense irradiation and light limitation. Light acclimation of most species employs the full set of classical mechanisms, such as adjustment of the ratio of light harvesting complex to chlorophyll and the total pigment content (Dring, Citation1986), reaction centre stoichiometry (Machalek et al., Citation1996), and relative content of xanthophyll cycle pigments (Bruhn & Gerard, Citation1996; Hanelt et al., Citation1997a), but also morphological changes (Grzymski et al., Citation1997; Hanelt et al., Citation1997a). Consequently, the growth rates and strategies of Laminaria species are variable in high and low light environments. Depending on species, temperature and photoperiod, growth in Laminaria sporophytes is light saturated at irradiances of 20–100 µmol m−2 s−1 (), which, since this corresponds to about 1–5% of the maximal solar irradiance, is indicative of shade adaptation. Short daylengths lead to a decrease of growth rates, and elevated temperatures result in a shift in the saturation towards higher irradiances (Han & Kain, Citation1996; Wiencke & Fischer, Citation1990). In L. digitata from Helgoland, light saturation of growth was achieved at irradiances of 70 µmol m−2 s−1, which is about 50% of the irradiance necessary to saturate photosynthesis (Lüning, Citation1979). Irradiances of about 250 µmol m−2 s−1 inhibited growth by 50% compared with sporophytes kept at optimal irradiances of about 110 µmol m−2 s−1. At the same time, chl a and chl c contents decreased after one week of exposure. In a similar study on L. hyperborea, growth was inhibited at slightly lower irradiances of about 180 µmol m−2 s−1 (Han, Citation1993). Exposure for 1 h to sunlight at noon in October (∼500 µmol m−2 s−1) led to 100% die-off in L. hyperborea and to 50% die-off in L. digitata (Han & Kain, Citation1996).
The presence of several Laminaria species at great depths (down to about 100 m below sea level; ), or as understorey species, illustrates their acclimation potential to extremely low-light conditions. The maximum depth is dependent on the water type present (Jerlov, Citation1968). Deep-water populations in the Mediterranean Sea are surrounded by clear water of Jerlov Type Oceanic III, while deepest specimens of L. hyperborea at Helgoland (North Sea) are restricted to 8 m depth as this site is characterized by the silt-laden waters of the German Bight (Jerlov Type Coastal 7; Lüning & Dring, Citation1979; Lüning, Citation1990). Other records from the N Atlantic restrict L. hyperborea, for example, to a maximum depth of about 32 m (Lüning, Citation1990). In very turbid waters, such as the Bristol Channel (England), there is insufficient light in subtidal habitats to permit any kelp populations to develop (Dring, Citation1987). The dark tolerance of adult sporophytes is generally high, as indicated by sporophytes surviving the long Arctic winter or several months of turbid waters during winter in temperate regions (Dunton et al., Citation1982; Lüning, Citation1990). In contrast, juvenile cultured sporophytes are less tolerant of darkness. After 20 days of darkness, 4 and 23% of the sporophytes of L. hyperborea and L. digitata, respectively, were dead (Han & Kain, Citation1996).
Table 4. Examples of deepest Laminaria populations
The minimum light requirement for growth in young sporophytes of Laminaria hyperborea is about 1 µmol m−2 s−1 (Han & Kain, Citation1996). The minimum annual light requirement to support the growth of mature Laminaria sporophytes is about 70 mol photons m−2 for L. hyperborea from Helgoland (Lüning, Citation1990) and 50 mol photons m−2 for L. solidungula from the Arctic (Chapman & Lindley, Citation1980b). Arctic L. saccharina receives between 40 and 96 mol photons m−2 year−1, corresponding to 0.7–1.6% of surface irradiance at the lower depth limit of 15–20 m (Borum et al., Citation2002; ). Similarly, Lüning & Dring (Citation1979) calculated that 0.7 to 1.4% of the surface irradiance reached the deepest individuals of various Laminaria species in other sites.
In Laminaria solidungula from the Arctic, the photosynthetic light saturation point Ek is located at 20–30 µmol m−2 s−1 in meristematic and 38 µmol m−2 s−1 in non-meristematic tissue of adult plants (Dunton & Jodwalis, Citation1988). In other brown macroalgae of the Arctic, including L. saccharina, the Ek values are even lower (11.5–16.1 µmol m−2 s−1; Kühl et al., Citation2001). This corresponds to the light available in a deep (40–70 m) bed of L. abyssalis off the Brazilian coast, which receives only 4–15 µmol m−2 s−1 at midday (Yoneshigue-Valentin, Citation1990; Rodrigues et al., Citation2002). However, photosynthetic and dark respiration rates of L. ochroleuca growing at 50 m in the Strait of Messina do not satisfactorily explain its occurrence at that depth (Drew et al., Citation1982).
The photosynthetic parameters (light compensation point E c , light saturation point E k , irradiance at which P max is reached E sat, photosynthetic efficiency α, photosynthetic capacity P max) for selected Laminaria species are compared in . As the data presented are derived from small pieces of thallus only, they cannot be extrapolated to the whole thallus. The best thallus part for this kind of investigation is the blade because the ratio of photosynthetic to non-photosynthetic tissue is very low in stipes or holdfasts. Moreover, P max values estimated from gas exchange rates change in different ways when expressed per unit area or per unit dry weight because of variations in thickness, dry weight and activity associated with tissue age (Kain, Citation1979). Also, the respiratory quotient may change under different environmental conditions. Often just one side of the thallus was irradiated. In addition, the material itself (cultured vs field material), season of collection and temperature strongly influence the results of gas exchange measurements (Kain, Citation1979; Sakanishi et al., Citation1990; Davison et al., Citation1991). This makes it difficult to compare values from different studies, even when they are expressed in the same units. Consequently, the data in are presented in their original units. In future, fluorescence yield measurements will facilitate comparisons, because photosynthetic parameters are independent of chlorophyll content or the thickness and shape of the sample (Hanelt et al., Citation2003). Moreover, fluorescence yield can be measured directly in the field under natural conditions. This has already revealed that photosynthetic parameters even change with tide level (; Gévaert et al., Citation2003) or depth distribution (Hanelt et al., Citation2003).
Table 5. Photosynthetic parameters of Laminaria sporophytes
The acclimation potential of some species, such as Laminaria saccharina, to different irradiances is very high. Photoprotection enables photosynthesis to acclimate both to high (e.g. 1100 µmol m−2 s−1) and very low irradiances (e.g. 5 µmol m−2 s−1) in an extremely wide depth range between shallow water (1 m) and about 20 m depth as observed in the Arctic Kongsfjorden (Hanelt et al., Citation1997b). Other species, like the deep water L. abyssalis are extremely sensitive and the acclimation potential seems to be low since this species becomes irreversibly photoinhibited even after a short exposure to daylight at the water surface (Rodrigues et al., Citation2000, 2002).
Carbon fixation
Photosynthetic CO2-fixation and light-independent carbon fixation (LICF) were investigated in various life-history stages of Laminaria saccharina by Kremer & Markham (Citation1979). The photosynthetic rate was similar in all stages. Photosynthetic CO2-fixation was accompanied by substantial LICF, as indicated by the strong activity of phosphoenolpyruvate carboxykinase (PEPCK) in addition to RuBisCo activity. The LICF could not fully compensate for respiratory carbon losses, which were usually greater than 10% of P max.
Highest P max and LICF rates occur under high irradiances in Laminaria setchellii and decrease during periods of low irradiance (Cabello-Pasini & Alberte, Citation1997, 2001). In this species, the photosynthetic capacity is regulated by the abundance of RuBisCo while the LICF is controlled through the abundance of PEPCK (Cabello-Pasini & Alberte, Citation2001). In several brown algal species, LICF was less than 10% of the carboxylation capacity (Kremer & Küppers, Citation1977). Old tissue of L. saccharina exhibits a high photosynthetic capacity and contributes strongly to the carbon balance (Borum et al., Citation2002). In L. digitata, L. hyperborea and L. saccharina, carboxylation via RuBisCo and PEPCK exhibits maximum activity in the meristoderm, the main photosynthetic tissue, and follows a gradient from the outer to the inner tissues (Kremer, Citation1980).
When growth rates are lowest during summer in Arctic Laminaria solidungula, assimilatory surplus is stored as reserve material (Chapman & Lindley, Citation1980b) which may move towards the base of the blade, so that significant accumulation of the translocates occurs within the growing region of the blade and in growing haptera as shown for L. saccharina and L. hyperborea (Schmitz et al., Citation1972).
In Laminaria saccharina from the Arctic, respiration rates decrease under low light conditions, lowering the light compensation point to about 2 µmol m−2 s−1 so that photosynthesis is mostly balanced even during periods of ice cover (Borum et al., Citation2002). The rates of respiration ranged from 3 to 20 nmol O2 mg−1 dry wt h−1 (Borum et al., Citation2002) and were at the lower end of the typical range reported for macroalgae (15–125 nmol O2 mg−1 dry wt h−1; Markager & Sand-Jensen, Citation1994). In young sporophytes of L. hyperborea, the mannitol/laminaran reserve of the developing blade is sufficient to meet the requirements of dark respiration for only 7–10 days at 10°C under continuous darkness (Kremer, Citation1984). The β-carboxylation potential of PEPCK (i.e. LICF) decreases with the depletion of the stored carbohydrate. In darkness, the substrate for β-carboxylation is probably derived mainly from mannitol along with the glycolytic degradation of laminaran. The young blade of L. hyperborea cannot maintain a positive carbon balance under the irradiation conditions of midwinter and early spring, but relies on a supply of carbon from the old blade (Kremer, Citation1984). In spite of the constantly low temperatures, the photosynthetic performance of L. saccharina in the Arctic is fully comparable to that of macroalgae in the temperate regions, reflecting the ability to adapt to low temperatures through changes in Calvin-cycle enzyme activities (Davison, Citation1987).
Carbon uptake
A capacity to utilize HCO3 −–in addition to CO2–for carbon fixation is of great advantage for aquatic photosynthetic organisms. Numerous macroalgae are able to use HCO3 − as a carbon source (Maberly, Citation1990; Larsson & Axelsson, Citation1999). Laminaria spp. can utilize both sources (as indicated by carbon stable isotope ratios; see Raven et al., Citation2002). To acquire bicarbonate, some seaweeds take it up actively, and convert it to CO2 within the cell. Others have an external carbonic anhydrase (CA), converting HCO3 − to CO2, which then enters the cells by diffusion.
In Laminaria digitata and L. saccharina, carbon uptake generally depends on the presence of an external CA, while direct bicarbonate uptake takes place at the higher pH that may occur during calm or stagnant water conditions. Under these high pH conditions, the effectiveness of bicarbonate acquisition via CA activity is reduced (Axelsson et al., Citation2000; Klenell et al., Citation2004). In saturating irradiances of red light, photosynthesis of L. saccharina is stimulated by additional low irradiances of continuous blue light during conditions of limited dissolved inorganic carbon (Dring et al., Citation1994; Schmid et al., Citation1996). These authors proposed that photosynthesis is supported by a blue-light-activated release of CO2 from an internal store probably located in the vacuoles of the cortical tissue of the blades. The main photosynthetic tissue, however, is in the overlying meristoderm, and blue-light-activated mobilization of the CO2 store could stimulate O2 evolution only if an internal periplasmic CA is available to facilitate CO2 uptake from the cortex. Klenell et al. (Citation2002, 2004) suggested that photosynthetic carbon uptake in L. digitata depends on an external CA under both red and red plus blue light conditions, whereby blue light induces an increased activity of a P-type H+-ATPase in the plasma membrane. This CO2 uptake mechanism operates by a pH gradient across the cell membrane and involves proton excretion by H+-ATPase as a proton pump.
Photoinhibition and photodamage of photosynthesis
The ability of photosynthesis to acclimate to short-term increases in irradiance increases with the age of a Laminaria thallus (Hanelt et al., Citation1997a). Correspondingly, sensitivity to UV-radiation decreases with increasing age as shown for L. digitata, L. hyperborea and L. saccharina (Dring et al., Citation1996). Older sporophytes acclimate faster to high irradiation conditions than juvenile sporophytes, because inhibition and recovery of photosynthesis is faster in older individuals. Generally, a higher content of protective pigments (e.g. xanthophylls) is associated with the decrease in sensitivity to high light. The resistance of mature sporophytes, however, is not exclusively due to a greater content of such pigments. Changes in thallus structure during the development of the sporophytes are probably also responsible for elevated high irradiation resistance (Hanelt et al., Citation1997a) and for the differential sensitivity of Laminaria species to UV radiation (Roleda et al., Citation2006a). Laminaria individuals occurring naturally over a wide depth range are able to acclimate to the prevailing in situ irradiances (Hanelt, Citation1998; Franklin et al., Citation2003; Hanelt et al., Citation2003).
One important strategy to cope with higher irradiance levels in shallow waters is the ability to recover more rapidly from high-light stress than isolates from deeper waters (Bischof et al., Citation1998). Recovery has a two-phase kinetics with a slow component more dominant in the deep-water specimens and the fast component prevailing in shallow water isolates (Hanelt, Citation1998). This is related to two co-occurring mechanisms present in Laminariales: photoprotection and photoinactivation (i.e. chronic photoinhibition). Rapid conversion of xanthophylls causes the rapid decrease of photosynthetic efficiency representing the fast exponential component of the acclimation kinetics model (Hanelt, Citation1998). This state is rapidly reversible and is also responsible for fast acclimation during the recovery phase. The slow kinetics is attributed to photoinactivation, i.e. loss of functional photosystem (PS) II reaction centres, which are repaired only slowly in dim light. The capacity for photoprotection enables most species to grow close to the water surface, whereas a high photosynthetic efficiency allows growth in the dim light of deeper regions. In field sporophytes of L. saccharina, the effective quantum yield of PS II decreases strongly during a falling tide and high irradiance as a result of harmless heat dissipation (photoprotection; Gévaert et al., Citation2003). Photosynthesis recovers totally during the subsequent rising tide, indicating that no significant photosynthetic damage has occurred ().
In Laminaria saccharina, the sensitivity to excessive light is dependent on thallus age, its life history stage (Hanelt et al., Citation1997a), and temperature (Bruhn & Gerard, Citation1996). Even at optimal growth temperatures, L. saccharina is sensitive to excessive light, and this sensitivity increases at elevated temperatures because of disturbed repair processes (Bruhn & Gerard, Citation1996). As a consequence, growth is reduced at temperatures of 10–15°C and high irradiances of 250 µmol m−2 s−1, but not if the thalli are exposed to relatively low irradiances of 30 µmol m−2 s−1 (Fortes & Lüning, Citation1980). In cold Arctic waters (–1.8°C), Borum et al. (Citation2002) did not observe photoinactivation at irradiances of ≤250 µmol m−2 s−1, illustrating the strong temperature dependency of this process. In addition, light quality (i.e. its spectral composition) is also of great importance for growth and photosynthesis. Simulated underwater radiation and blue light resulted in higher growth rates than green, red or white fluorescent light at corresponding irradiances (Lüning & Dring, Citation1985; ).
Effects of UV radiation
Research on the ecophysiology of Laminariales sporophytes during the last 10 years has been dominated by studies of the impact of increased UV radiation on photosynthesis and growth. Most of these studies were conducted on Spitsbergen (Norway) in the Kongsfjorden and on the island of Helgoland in the North Sea (Dring et al., Citation2001). UV radiation was found to affect performance of kelps substantially, with the degree of UV-susceptibility depending on the species and on developmental stage (Dring et al., Citation1996; Hanelt et al., Citation1997a,b; Bischof et al., Citation2002). A comparative study of the stage-dependent sensitivity to UV radiation of the three Helgoland species Laminaria digitata, L. hyperborea and L. saccharina showed that germination of meiospores and growth of gametophytes were reduced, whereas growth of young and mature sporophytes was much less affected. Similarly, the photosynthetic efficiency of gametophytes was strongly affected by UV exposure, whereas young sporophytes and especially mature sporophytes exhibited a much greater UV tolerance (Dring et al., Citation1996; see also Section 6: Biology of microstages). Similar results were obtained for Arctic L. saccharina exposed to high photosynthetically active radiation (PAR; Hanelt et al., Citation1997a).
The ecological consequences of UV-exposure for growth have been demonstrated in several Laminaria species (Michler et al., Citation2002, Roleda et al., Citation2004, 2006b,c). In all species tested, growth rates were significantly higher in sporophytes exposed to PAR alone than in sporophytes exposed to a combination of PAR and UV radiation. In sporophytes exposed to UV-radiation, the energy demands for repair of DNA damage and synthesis of UV-absorbing compounds effectively diverted photosynthates at the expense of growth. Photosynthetic pigment content was not significantly different between treatments suggesting a capacity for acclimation to moderate UV irradiances. In another study by Roleda et al. (Citation2006a), the sensitivity of growth to UV radiation was correlated with the observed upper depth distribution limit of the three species of Laminaria on Helgoland (North Sea). This finding suggests that UV-radiation may play a role in determining the vertical zonation patterns of macroalgae on the shore (Bischof et al., Citation2006), a hypothesis that was further substantianted by experiments with meiospores (see Section 6: Biology of microstages).
Large seasonal differences were found in the UV-susceptibility of Laminaria saccharina in the Arctic. Specimens collected under sea-ice cover in spring, from clear water conditions in early summer and from turbid waters in high summer showed similar UV-induced inhibition of photosynthesis, but differences in UV sensitivity became apparent during the recovery phase (Bischof et al., Citation2002). Recovery from inhibition was incomplete in specimens collected early in the year, while individuals harvested in later seasons recovered completely from UV stress. In response to seasonal changes in underwater radiation conditions, marked changes in photosynthetic capacity and a substantial loss of chl a were observed (Aguilera et al., Citation2002; Bischof et al., Citation2002). In addition, the UV-susceptibility of L. saccharina, Alaria esculenta and Saccorhiza dermatodea was higher in specimens from deeper waters than in specimens from the upper sublittoral (Bischof et al., Citation1998), indicating an ability to acclimate to UV-B exposure.
Temperature
In addition to the radiation regime, temperature affects sporophyte performance in the field (Davison, Citation1991). Sporophytes of the endemic Arctic Laminaria solidungula grow at temperatures up to 15°C with an optimum at 5–10°C and exhibit an upper survival temperature of 16°C. Cold-temperate NE Pacific species grow between 0 and 18°C with optima between 5 and 15°C. The growth range of cold-temperate N Atlantic species extends from 0 to 20°C with optima between 5 and 15°C (Bolton & Lüning, Citation1982; Lüning, Citation1984; Lüning & Freshwater, Citation1988; tom Dieck, Citation1992; Wiencke et al., Citation1994) while warm-temperate Atlantic species grow at up to 23–24°C and have slightly elevated optima (tom Dieck & de Oliveira, 1993; ). Other warm-temperate species of Laminariales, such as Undaria pinnatifida, also exhibit higher temperature requirements with growth optima at 20°C (Akiyama, Citation1965). Generally, it has become apparent that growth optima and upper and lower limits for growth and survival follow the latitudinal gradient. A comparative and extensive overview of these parameters including Laminariales and other seaweed species is given by Wiencke et al. (Citation1994) and is not repeated here.
Growth temperature is clearly important to acquire heat tolerance. Photosynthesis in sporophytes of Laminaria saccharina grown at 0–5°C is strongly inhibited by temperatures between 15 and 20°C, whereas specimens grown at 10–20°C exhibit an increased temperature tolerance due to physiological changes in RuBisCo activity and the kinetics and efficiency of light harvesting electron transport systems (Davison, Citation1987; Davison & Davison, Citation1987). Complex metabolic interactions were suggested to operate in L. saccharina in order to optimize photosynthesis and growth over the wide range of temperatures and light levels occurring in the field (Machalek et al., Citation1996). Sporophytes grown at either 5 or 17°C and exposed to either 15 or 150 µmol m−2 s−1 showed distinct differences in photosynthetic performance. Higher concentrations of RuBisCo and thus higher maximal photosynthetic rates under standard temperatures were found in algae raised at 5°C. In specimens grown at 17°C, pigment contents, PS II reaction centre densities and the size of the fucoxanthin–Chl a/c protein complex increased, irrespective of cultivation irradiance. Curiously, similar physiological changes were also found in specimens raised at 5°C under low light conditions, but not in specimens raised at 150 µmol m−2 s−1 (Machalek et al., Citation1996). The obvious general similarity between acclimation of photosynthesis to high temperatures and that to low light (Gerard, Citation1988; Greene & Gerard, Citation1990) was also found in several microalgae (Maxwell et al., Citation1994).
Habitat-specific differences in the heat tolerance of Laminaria saccharina seemed to be partly attributable to the nutrient status. In heat-tolerant individuals, high N supply resulted in a higher density of PS II reaction centres, higher activities of Calvin-Cycle enzymes (e.g. RuBisCo) and increased photosynthetic capacity and optimum quantum yield (Gerard, Citation1997). The ability of heat tolerant ecotypes to accumulate and maintain high N reserves appeared to be decisive for the increased heat tolerance. Under a combination of N limitation and heat stress, heat tolerant specimens were able to fuel metabolic processes from their large N reserves. Thus, under these combined stress conditions, high rates of carbon fixation and the integrity of the photosynthetic apparatus were maintained (Gerard, Citation1997).
Conclusion
Overall, a wealth of data on growth and photosynthesis of Laminaria sporophytes is available today especially with respect to seasonality, irradiation and temperature conditions. However, one has to bear in mind that growth is an integrative parameter and that, even though photosynthesis is an important component for growth performance, it is only one component among others. In future, it would be desirable to measure a range of different physiological processes and to combine the data so that the growth performance under the various environmental conditions can be explained in a more holistic way. Ideally, such studies should include the investigation of gene expression, which would permit us to document the performance of the species from the molecular to the growth level.
5. Sporogenesis and meiospore release
The successful accomplishment of reproduction is crucial for recruitment of stands. Basic knowledge about development and phenology of meiosporangia and sori was already available by the middle of the last century (see Kain, Citation1979). Recent research has added information about the ultrastructural development of meiosporangia and meiospores and about internal and external regulation of sporogenesis and meiospore release.
Ultrastructural development of meio-sporangia
The general development of meiosporangia in Laminaria from meristodermal cells has been investigated in detail, for example by Nishibayashi & Inoh (Citation1956) and Ohmori (Citation1967). An ultrastructural study of L. angustata by Motomura (Citation1993) shed light on the cytological details of meiosporangium formation: When meristodermal cells start to elongate longitudinally, fibrous layers are deposited on their distal side. In the cytoplasm of the paraphyses, a large number of Golgi bodies and physodes become visible. Furthermore, electron-dense material, probably polyphenolic substances, accumulates in the cytoplasm of the paraphyses of L. angustata (Motomura, Citation1993). Similarly, the physodes of paraphyses and meiospores of L. digitata become enriched with the polyphenol phlorotannin (Wiencke et al., Citation2004; Gruber, pers. comm.). The higher phlorotannin level of reproductive rather than vegetative tissue found in several kelp species points to a protective role of phlorotannins in meiosporangia (van Alstyne et al., Citation1999).
The parent cell of the unilocular sporangium is the site of meiosis, as shown by earlier researchers (e.g. Evans, Citation1965). A characteristic feature is the accumulation of many lipid granules in the parent cell and in the developing sporangium. Similar structures have also been reported by Chi & Neushul (Citation1972) for Macrocystis pyrifera. Later Brzezinski et al. (Citation1993) and Reed et al. (Citation1999) confirmed that neutral lipids were the major storage product in the meiospores of kelps, including Laminaria. Motomura (Citation1993) further showed that, just before meiosis, the meiosporangial mother cell contains eight chloroplasts. They divide almost synchronously before the eight nuclei are formed through meiosis. The further divisions of both nuclei and chloroplasts are synchronized resulting in 32 meiospores. The coordination between chloroplast and nuclear divisions is presumably mediated by centrosomes (Motomura et al., Citation1997). After completing the nuclear divisions, the cap structure of the sporangium is formed. Many mitochondria gather at the tip of the sporangium, a phenomenon that has not been reported before in brown algae. There is evidence that the thick mucilage cap of the paraphysis consists of sulphated polysaccharides (fucoidan). Parallel to the cap formation, flagella are formed. The elongation of the anterior and posterior flagella occurs synchronously in each sporangium. Subsequently, the cytoplasm of the unilocular sporangium becomes highly vesiculated, lipid globules divide into smaller ones and chloroplasts migrate from the periphery to the centre to surround a nucleus. Individual meiospores develop when the plasmamembrane invaginates and ER fragments fuse to form large vesicles. After cleavage, each newly derived meiospore contains a nucleus, a Golgi body, a chloroplast, a pair of flagella, several mitochondria and small lipid granules (Motomura, Citation1993).
Fruiting periods
As Kain (Citation1979) pointed out, the appearance of sori in the field seems to be confined mostly to periods of low or no growth, although sori may be present throughout the year. As a consequence, many Laminaria species have their main fruiting period in autumn to winter coinciding with decreasing daylengths and temperatures. gives an overview of the fruiting periods of selected Laminaria species worldwide. Only a few species, such as L. digitata, L. ochroleuca and the annual rhizomatous species L. rodriguezii and L. ephemera, fruit mainly in summer. The variance of fruiting periods among different species seems to indicate a wide plasticity in this trait.
Table 6. Reproductive period of selected Laminaria species worldwide
Regulation of sporogenesis
Experiments under controlled conditions of light and temperature starting in the late 1980s provided evidence that internal and external triggers control growth and sporogenesis (). In two Laminaria species, L. saccharina from Helgoland (North Sea) and L. setchellii from Bamfield, USA, reproduction is controlled by photoperiod (Lüning, Citation1988; tom Dieck, Citation1991). Short photoperiods of 8 h per day given after long photoperiods of 16 h per day induced an immediate cessation of growth, followed by the formation of sori 6–14 weeks later. The sori of L. setchellii developed either on new blades after growth terminated, or on old, non-growing second-year blades (tom Dieck, Citation1991). In both species, sori were induced during the period of arrested growth, but eventually growth was resumed in unchanged short-day (SD) conditions. Similarly, in L. japonica, sori are formed only on blade parts that have stopped elongation (Mizuta et al., Citation1999a). Minimum induction time for sporogenesis in SD was about 3–4 weeks in L. saccharina (Preisler & Bartsch, unpublished data). Unusually short periods of 10 days until visible sorus formation in L. saccharina were achieved only if inductive short daylengths followed long day conditions (Pang & Lüning, Citation2004). Indirect evidence also points to a short day induction of sporogenesis in L. japonica (Mizuta et al., Citation1999a,b). Material taken from the field in summer during long day conditions and transferred to a 12:12 h light–dark cycle (which might still act as long-day signal; Buchholz & Lüning, Citation1999) did not become fertile, whereas late winter material from natural SD readily became fertile in experimental 12:12 h light–dark conditions (Mizuta et al., Citation1999a). Other species with a clear autumn to winter fruiting period, like L. hyperborea, are likely candidates for a short-day-dependent induction of sporogenesis, but further experimental evidence is missing.
A major breakthrough in the understanding of sporogenesis was achieved when Buchholz & Lüning (Citation1999) detected that isolated discs cut from the distal blades of Laminaria digitata and cultured separately from the parental sporophytes formed fertile tissue 5 months earlier than corresponding field material in a wide range of temperatures (6 and 12°C) and daylengths (8, 12 and 16 h light per day). Similar discs of meristematic tissue and the original whole plants remained sterile. The separation of vegetative tissue from growing sporophytes proved an effective method to investigate Laminaria sporogenesis under controlled laboratory conditions. A similar method was published in the same year for L. japonica (Mizuta et al., Citation1999b) but the isolation of discs from growing sporophytes was not mentioned as a sorus inducing factor. Subsequently, sporogenesis was successfully induced in L. saccharina (Buchholz & Lüning, Citation1999; Pang & Lüning, Citation2004; Preisler & Bartsch, unpublished data), but only if exposed to short daylengths. The method also worked with L. angustata, L. religiosa and L. ochotensis (Nimura et al., Citation2002) and L. cichorioides (Skriptsova & Titlyanov, Citation2003) indicating a general mechanism behind this artificial induction of sporogenesis.
The hypothesis that the interplay of growth and fertility is regulated via daylength and through internal hormonal substances (Lüning & tom Dieck, Citation1989) was further substantiated by Lüning et al. (Citation2000; ). Laminaria digitata produced sori on the distal side of horizontal cuts or holes in the blade of complete, growing sporophytes, while intact growing sporophytes remained sterile. This led to the idea of a ‘sporulation inhibitor substance’, which is produced by growing meristems and translocated distally thereby suppressing sporogenesis (Buchholz & Lüning, Citation1999; Lüning et al., Citation2000). In the field, sporogenesis is often initiated in distal blade portions as they expand distally and basally during maturation (e.g. Lee & Brinkhuis, Citation1986; Mizuta et al., Citation1999a; Bartsch, pers. obs.). In discs of L. digitata, sporangia develop more rapidly in older tissue taken far from the meristem than in younger tissue near the meristem (Buchholz & Lüning, Citation1999) and a gradient for optimum sorus development was observed along the whole longitudinal blade axis of this species (Bartsch, unpublished). Application of exogenous abscisic acid to excised sporophyte tissue suppresses surface expansion and promotes sorus formation in L. japonica (maximum: 10−5 M abscisic acid; Nimura & Mizuta, Citation2002). The hormonal antagonist, indole acetic acid (IAA), induces an opposite reaction. Application of 10−5 M IAA delays sorus formation by 4 to 7 weeks in L. japonica. Lower concentrations are not convincingly active, and higher concentrations are toxic (Kai et al., Citation2006). All observations support the idea of an inhibitor substance produced in the meristem. The auxin IAA or related substances might be candidates for this substance, since bioassay tests indicated that IAA activity was greater in vegetative parts than in sori of both L. japonica and Undaria pinnatifida (Kai et al., Citation2006). However, the existence and nature of this postulated inhibitor substance requires confirmation, and this will be one of the challenges in future Laminaria research.
Further studies have shown that tissue location, temperature, irradiance and nutrient conditions, as well as competition and life strategy, modify the reproductive output, but no single parameter is the sole decisive trigger (Buchholz & Lüning, Citation1999; Mizuta et al., Citation1999a,b; Bartsch, pers. observ.). Nutrient poor medium (nitrate and nitrite <0.25–0.45 µM, phosphate-P < 0.11–0.24 µM in contrast to Provasoli enriched seawater) delays sorus production and sorus size considerably in Laminaria japonica (Mizuta et al., Citation1999b). High phosphorus and nitrogen supply enhances sorus formation in L. angustata, L. japonica, L. ochotensis and L. religiosa. The sporogeneous tissue of these four species always had nitrogen values above 1.78 mg N cm−3 and phosphorus levels above 0.19 mg P cm−3, regardless of ambient nutrient conditions, and these concentrations were significantly higher than in non-sorus tissue (Nimura et al., Citation2002). The importance of a critical internal N and P accumulation for meiospore formation was also recently shown for Undaria pinnatifida and Alaria crassifolia (Kumura et al., Citation2006), indicating a strong influence of nutrients on reproduction of Laminariales in general. Canopy cover and water depth, combined with unusual temperature conditions during El Nino events, modulated the strictly seasonal reproductive period and output in L. farlowii in a Californian Macrocystis kelp forest (Dayton et al., Citation1999). In L. japonica, low water temperatures, long photoperiod and low nutrients delayed the onset of sorus formation (Mizuta et al., Citation1999b). Epibenthos such as the bryozoan crust Membranipora membranacea affected the size of sori in L. hyperborea and an influence of allelopathic substances was assumed by Kain (Citation1975).
Age and reproduction
The age at which sporophytes first reproduce is variable and seems to be dependent on life-cycle pattern, abiotic factors, size and weight. A comparison between two N Pacific species, the annual Laminaria ephemera and the perennial L. setchellii, revealed that L. ephemera becomes fertile for the first time after 54 days and L. setchellii only after 2.5 years (Klinger, Citation1984). There is, however, no correlation between age of sporophytes and magnitude of sorus production in these two species, in contrast to the kelp Pterygophora californica (De Wreede, Citation1984). In other long-lived kelps such as L. hyperborea, the time of first reproduction was reported to be 15 months to 5 years (Kain, Citation1975). Here, the reproductive peak was in 6-year-old plants. Size rather than age may determine the initiation of reproduction, as was shown for L. longicruris (Chapman, Citation1986) and for Cymathere triplicata (Roland, Citation1984). In the annual rhizomatous L. ephemera, reproduction was initiated earlier in large individuals but these also died earlier than comparable smaller individuals (Klinger, Citation1984).
Meiospore release
As already pointed out by Kain (Citation1979), artificial release of meiospores from sporangia is possible if fertile tissue is subjected to osmotic shock conditions, preferably also combined with a temperature change. Release of meiospores under natural conditions, however, has rarely been studied. An early indication that meiospore release is under environmental control came from the N Pacific kelp Nereocystis luetkeana (Amsler & Neushul, Citation1989a). In this species 80% of sori are released with a diel periodicity in the time period between 2 h before sunrise and 4 h after sunrise, indicating a possible underlying circadian control (see Section 7: Endogenous rhythms controlling metabolism and development). A recent publication on Laminaria japonica indicated that natural meiospore release is favoured by night conditions. The release during the dark period reached 76–88% of the total released meiospores (Fukuhara et al., Citation2002; ).
Information about the duration of meiospore release from individual sori is scattered, but all publications indicate that meiospore release occurs over a period of several months. The duration of meiospore release from each blade of Laminaria hyperborea is variable, ranging from about 20 to 65 days, but with a mean of 6 weeks (Kain, Citation1975). This is different in L. saccharina from Long Island Sound, USA, where release from individual sori was observed over a period of 5 months with a mean life span of sori of 2.5 months (Lee & Brinkhuis, Citation1986). In the case of L. japonica, meiospores were released from individual 1-cm discs bearing ripe sori over 17–24 days (Fukuhara et al., Citation2002). In laboratory grown sporophytes of L. setchellii, the period between the appearance of sori and release was 11–14 weeks (tom Dieck, Citation1991). Extended release is confined to previously sterile tissue in L. hyperborea (Kain, Citation1975). Sometimes, even though ripe sori are present, no meiospores are released. This is probably due to unfavourable temperature conditions in the field as was reported for L. saccharina at its southern distribution boundary in August, the warmest month (Lee & Brinkhuis, Citation1986). Similarly, in Helgoland, no meiospores were released by L. digitata during the exceptionally warm summers of 2003 and 2006, although sori were present (Gruber, Bartsch, pers. obs.). Epibenthos may also drastically influence meiospore release: complete Membranipora incrustation on L. longicruris resulted in 100-fold reduction of meiospore release and 50% one-sided incrustation caused 64% reduction (Saier & Chapman, Citation2004).
Reproductive effort
The reproductive effort in Laminaria is defined here, following the concept of DeWreede & Klinger (Citation1988), as the proportion of the surface area of the vegetative blade that is transformed to sorus. There are few data available on reproductive effort in Laminaria and kelps in general, and they do not allow the identification of general patterns (DeWreede & Klinger, Citation1988). The scattered information shows that reproductive effort in Laminaria is often quite low. In L. longicruris from Long Island Sound, USA, the mean percentage of sorus to blade area over the year ranged between 1 and 37%, with highest allocation of blade surface to reproduction in autumn (October, November) and lowest in spring to summer (van Patten & Yarish, Citation1993). In L. saccharina from the same site, only 2.4% (January) to 6.1% (August) of blades were covered with sori (Lee & Brinkhuis, Citation1986). Sorus allocation of other species is similarly low with 1–37% in perennial L. setchellii and 13–32% in annual L. ephemera (Klinger, Citation1984). In L. japonica, 1 to >20% of the blade surface may be covered by sori (Mizuta et al., Citation1999a). In contrast, fertile tissue of L. hyperborea may cover 80–90% of the blade with a mean of 70% in canopy sporophytes (Kain, Citation1975). There was a significant correlation between frond weight and sorus size; fertile plants always had blades above 80 g fresh weight. This may explain why artificial sorus induction was not possible in tank experiments with 1–2-year-old, small plants of L. hyperborea (20 cm long blades; Schaffelke, Citation1993). The reproductive effort reported for L. hyperborea indicates a different life strategy and allocation of resources to reproduction from the other species mentioned. As growth rate is negatively correlated with the allocation of blade surface to reproduction in many Laminaria ssp., van Patten & Yarish (Citation1993) assumed a cost of reproduction in terms of reduced growth in L. longicruris from Long Island Sound. Chapman (Citation1986) pointed out that the concept of ‘costs’ of reproduction vs growth “depends on the idea that different activities are alternatives and that a gain in one must be offset by a loss in another”. Whether this holds true has not yet been convincingly proven for Laminaria. Lee & Brinkhuis (Citation1986) analysed the carbon content of vegetative tissue in reproductive and non-reproductive plants of L. saccharina 10 cm above the meristem, and found higher values in reproductive sporophytes in some months. Additionally, fertile sporophytes were generally longer than non-reproductive individuals. This contrasts with the findings of Buchholz & Lüning (Citation1999), who showed that shorter L. digitata thalli had a greater capacity to form sori. In L. longicruris from Nova Scotia, fecundity is also related to thallus size but not age (Chapman, Citation1986), again an indirect sign of the importance of the nutritional status of the sporophytes for sporogenesis.
Few investigations allow the total output of meiospores in Laminaria spp. to be calculated. Billions of spores per m2 of substrate were produced in L. longicruris. Van Patten & Yarish (Citation1993) estimated 18.2 and 19.1 sporangia per 250 µm of linear transverse or longitudinal sections of sorus tissue in spring and autumn, respectively. This was equivalent to 5.3 and 5.84 × 105 sporangia per cm2 sorus giving 1.7–1.87 × 107 meiospores per cm2 sorus tissue, assuming 32 meiospores per sporangium. Sporophytes in spring had 130 cm2 of sorus, thereby producing 2.2 × 109 meiospores; in autumn, the sorus area per plant increased to 400 cm2 producing 7.5 × 109 meiospores. Chapman (Citation1984) estimated 8 × 109 meiospores m−2 substrate y−1 for L. longicruris (mean density over the year: 1.24 individuals m−2) from Nova Scotia and 20.02 × 109 meiospores m−2 substrate y−1 for L. digitata (mean density over the year: 3.2 individuals m−2). The same order of magnitude was calculated for L. setchellii by Klinger (Citation1984) with 3.6–3.8 × 108 meiospores per individual y−1. In contrast to this, Kain (Citation1975) counted about 4.7 × 105 meiospores mm−2 sorus during times of highest fertility for L. hyperborea and, hence, estimated a possible 3.3 × 106 meiospores mm−2 rock surface, (equivalent to 3.3 × 1012 meiospores m−2), which is three orders of magnitude more than calculated by Chapman (Citation1984). Calculations of spore density per m2 substrate are dependent, however, on the mean size of sori per individual kelp and the density of fertile sporophytes, as was emphasized by van Patten & Yarish (Citation1993). Following the recruitment success in the field of average stands of L. longicruris, Chapman (Citation1984) counted 9 × 106 benthic microscopic sporophytes m−2 y−1. From the known mortality rate of visible plants, he then calculated that only 1 visible sporophyte m−2 substrate y−1 will develop from this pool of microscopic sporophytes. For L. digitata, he calculated that 2 sporophytes m−2 y−1 are recruited from 1 × 106 benthic microscopic sporophytes. If this holds true generally, Laminaria meiospores make a considerable contribution to the phytoplankton food web in coastal systems (van Patten & Yarish, Citation1993).
Conclusion
In the last 25 years, there has been a considerable increase in our knowledge of the development of sporangia, the regulation of sporogenesis and the reproductive effort of Laminaria spp. but, nevertheless, the reproductive biology of these kelps is far from being well understood. We do not know whether and how increasing global temperatures and irradiation will affect reproductive periods, reproductive effort or meiospore release and, thereby, the recruitment of these ecosystem-building species. Quantitative background data are virtually missing for most species. These are urgently needed before future developments can be judged. The identification of the substance that is postulated to suppress the onset of sporogenesis during periods of rapid growth will advance our understanding of the interplay of growth and fecundity and will trigger new developments in aquaculture. Generally, the physiological regulation of fertility is poorly understood and needs new approaches. The contribution of the meiospores of Laminariales, or of seaweed propagules in general, to the food web of coastal systems has rarely been investigated and this represents a major missing link in food-web studies.
6. Biology of microstages: meiospores, gametophytes and gametes
The formation and release of motile meiospores and spermatozoids is a fundamental step in the life history of kelps, since it is important for enlarging the geographic and depth distribution and for mixing the genetic material between populations. The dispersal range of brown algal propagules is at least 200 m and is driven mainly by currents and water motion (Norton, Citation1992; Fredriksen et al., Citation1995). In the kelp Macrocystis, a range of 4 km was reported (Reed et al., Citation1988). As free-living kelp gametophytes are difficult to find in nature, most studies of microstages have been conducted in the laboratory. Recently, however, kelp gametophytes have been observed in the field, living endophytically within the cell walls of 17 species of red algae in the NE Pacific. The gametophytes may complete their whole reproductive cycle in the host and the endophytic nature of gametophytes is assumed to play an important role in the reproductive biology of kelps (Garbary et al., Citation1999).
Ultrastructure
The ultrastructure of meiospores, spermatozoids and eggs of Laminaria and related species has been reported in detail (Henry & Cole, Citation1982a,b; Motomura & Sakai, Citation1988; Motomura, Citation1989). The meiospores contain only one chloroplast with a comparatively low photosynthetic activity (Amsler & Neushul, Citation1991; Roleda et al., Citation2006d) while the spermatozoids contain two or three chloroplasts. Both types of propagules are small, wall-less cells, 4–8 µm in diameter and lack an eyespot (Clayton, Citation1992). The anterior flagellum of meiospores and spermatozoids consists of two parts, a mastigoneme-bearing basal part and a distal ‘whiplash’ portion, an extension of the two central microtubules of the axoneme. The posterior flagellum is short in the meiospores and long in the spermatozoids. In the latter, it tapers distally as the doublet microtubules become singlets and decrease in number. The cytoskeleton in the meiospores is well developed and consists of 1–2 bands of microtubules looping around the periphery of the spore. Spermatozoids possess one band of microtubules in the most anterior portion of the cell (Henry & Cole, Citation1982b). Eggs may also have two flagella as shown for L. angustata, which are eventually shed during liberation (Motomura & Sakai, Citation1988). Other unique characters of Laminaria eggs are absence of mastigonemes, widely spaced basal bodies and no flagellar rootlets. Conspicuous features of meiospores are lipid bodies, adhesion vesicles and phlorotannin-containing physodes. Storage lipids are the main energy source of spores, supporting swimming and, potentially, germination processes (Brzezinski et al., Citation1993; Reed et al., Citation1999). The adhesion vesicles contain adhesive material composed of glycoproteins, which are extruded when the spores settle (Olivera et al., Citation1980). Ultrastuctural studies on normal fertilization, zygote development and parthenogenesis of unfertilized eggs suggest that centrioles play an important role for normal morphogenesis (Motomura, Citation1990, 1991). Female meiospores contain an unusually large X-chromosome, as shown originally for Alaria esculenta, Chorda filum and four Laminaria species by Evans (Citation1963, 1965) and later confirmed by Yasui (Citation1992) for L. yendoana, which results in less DNA in male than in female meiospores. These features were used in a flow cytometry study to separate male and female DAPI-stained meiospores (Druehl et al., Citation1989a).
Nutrients and minerals
Chemotaxis as a nutrient-finding mechanism in Laminariales meiospores has been reported in Laminaria japonica, Pterygophora californica and Macrocystis pyrifera (Amsler & Neushul, Citation1990; Fukuhara et al., Citation2002). Nitrate stimulates positive chemotactic swimming and meiospore settlement in all three species, as does phosphate in the first two species. High concentrations of ammonia and Fe2+ are, however, inhibitory for growth and reproduction of M. pyrifera gametophytes; whereas lower concentrations of ammonia stimulate growth of the gametophytes, and low concentrations of Fe2+ stimulate gametogenesis (Amsler & Neushul, Citation1989b). Gametogenesis, and especially oogenesis, is induced by the presence of chelated iron (Motomura & Sakai, Citation1981, 1984; ) while allelochemicals from coralline red algae suppress the maturation of female gametophytes of Laminaria (Denboh et al., Citation1997). A short-term low-level exposure to zinc promotes the germination of meiospores in M. pyrifera (Anderson & Hunt, Citation1988).
Light and temperature
Gametogenesis in Laminaria is induced by blue light (400–512 nm; ). Quantum doses of 50 to 870 µmol cm−2 are necessary to induce 50% of the female gametophytes of L. saccharina, L. digitata and L. hyperborea to produce eggs (Lüning & Dring, Citation1975; Lüning, Citation1980). The optimal temperature for vegetative growth of gametophytes is species-specific and ranges between 10 and 19°C. At a given irradiance of PAR, the percentage of fertile gametophytes increases towards the lower limit of the optimal temperature ranges (Lüning, Citation1980). In several Laminaria species, an interaction of temperature and irradiance became visible. Low irradiances of 2 µmol m−2 s−1 were not sufficient to induce gametogenesis in most cases and 4.5 µmol m−2 s−1 produced optimal oogenesis only in optimal temperatures. The same was true for relatively high irradiances of 93 µmol m−2 s−1, which were detrimental at suboptimal temperatures (tom Dieck, Citation1992; tom Dieck & de Oliveira, 1993; ). Generally, freshly released meiospores show a higher fecundity than pre-cultivated red-light grown gametophytes (tom Dieck, Citation1992; Izquierdo et al., Citation2002), although ability of gametophytes to become fertile is still good after 30 years of cultivation under red-light conditions in many kelp species (Druehl et al., Citation2005; Bartsch, pers. obs.). The extremely low-light demand of gametophytes is also apparent in their capacity to withstand prolonged periods of darkness. The cultured gametophytes of nine Laminaria species survived 12–18 months of darkness and even became reproductive afterwards (Druehl & Boal, Citation1981; tom Dieck, Citation1993).
The photosynthetic capacity of meiospores is very low (). For example, meiospores of Laminaria farlowii exhibit a photosynthetic capacity (P max) of only 0.039 µmol O2 mg−1 chl a min−1, whereas sporophytes of L. saccharina achieved 1.5 µmol O2 mg−1 chl a min−1 (Amsler & Neushul, Citation1991; Benet et al., Citation1994). A characteristic feature of N Atlantic populations of L. digitata, L. saccharina and other kelps is the much higher photosynthetic capacity (measured as ETRmax) compared with populations of the same or other species in the Arctic (Roleda et al., Citation2005, 2006d), a difference, which may be explained by the different temperature regimes.
Table 7. Photosynthetic parameters of freshly released meiospores exposed to photosynthetic active radiation (PAR) and response of meiospores to UV radiation of Laminariales species and Saccorhiza from various regions
Temperature-dependent gametangia production in male and female gametophytes shows some correlation with geographical distribution. The N Pacific species Laminaria bongardiana (=L. groenlandica sensu Druehl Citation1968) and L. setchellii show optimal egg production between 5 and 10°C and no fertility at 17°C (tom Dieck, Citation1992) and the N Atlantic cold-temperate species L. longicruris, L. hyperborea and L. digitata have their optima at (5–)10 to 17°C (Yarish et al., Citation1990; tom Dieck, Citation1992), whereas the most southerly distributed species, L. ochroleuca, L. abyssalis, L. pallida and L. schinzii, show maximum fecundity at higher temperatures (15–18°C) and have reduced or no fertility at low temperatures of 5°C (tom Dieck & de Oliveira, 1993; Izquierdo et al., Citation2002; ). Oogenesis and sporophyte formation at 0°C was compared in L. saccharina, L. digitata and L. hyperborea. L. saccharina produces oogonia and sporophytes within 20 to 26 days of sporulation, indicating a good adaptation to Arctic conditions, while oogonium and sporophyte production is considerably delayed in the other two species (Sjøtun & Schoschina, Citation2002). In some species (e.g. Arctic L. solidungula), gametogenesis occurs only under short days (8 h light and less) and low temperature conditions (tom Dieck, Citation1989).
A seasonal acclimation to temperature was observed in Laminaria saccharina from Long Island Sound, USA. Meiospores released between February and April did not survive at 20°C, or showed reduced germination, in contrast to meiospores released in November, May and June (Lee & Brinkhuis, Citation1988). High temperatures may also affect the 1:1 sex ratio that has been assumed (since the work of Schreiber, Citation1930) to be present after release of sporangia. Higher temperatures resulted in a greater number of male gametophytes in L. saccharina from Long Island Sound, USA (Lee & Brinkhuis, Citation1988). Funano (Citation1983), however, observed the opposite, i.e. fewer males at both higher and lower temperatures in L. religiosa. Earlier reports had already shown the promotion of male gametophytes by adverse saline conditions (Schreiber, Citation1930; Hsiao & Druehl, 1973 in Kain, Citation1979) indicating that abiotic stress may generally alter the sex ratio. Whether this has any impact in natural field situations is unknown.
Survival temperature ranges of 47 species of Laminariales gametophytes were found to be related to their geographical distribution, in a similar way to gametangia production. Upper 2-week survival temperatures (UST) range between 19 and 30°C and are generally 1–2°C higher than the survival temperature of sporophytes. The lowest USTs (19–20°C) were encountered in Arctic to cold-temperate species (e.g. Laminaria solidungula) while warm temperate Japanese species exhibited the highest USTs (28–30°C). The lower 2-week survival temperatures of Laminariales gametophytes range between–1.5 and 8°C (tom Dieck, Citation1993; Wiencke et al., Citation1994 and citations therein).
Pheromones
Sexual reproduction in several brown seaweeds, including the orders Laminariales, Desmarestiales and Sporochnales, involves signalling chemicals or ‘pheromones’ (Müller et al., Citation1979, 1982, 1988). Lamoxirene (cis-2-cyclohepta-2′, 5′-dienyl-3-vinyloxirane) has been identified as the sperm-releasing pheromone in the Laminariaceae, Alariaceae and Lessoniaceae (Müller et al., Citation1979, Müller, Citation1989). The biochemical aspects of pheromone production and reception were described by Maier (Citation1995) and Pohnert & Boland (Citation2002). After release of the pheromone lamoxirene from eggs of Laminaria digitata, spermatozoids are rapidly released from the antheridia (threshold 10−11 mM; Maier et al., Citation1988) and swim directly towards the pheromone source (). Besides this positive chemotactic response, there is also a phobic response of the spermatozoids, when the concentration of the pheromone decreases, resulting in a reversal of the swimming direction. With reference to the effects of metal concentrations on reproductive processes, Maier (Citation1995) suggested that metal ions lower the ability of spermatozoids to find eggs due to interference with the pheromone attractant. Most species produce several pheromones, although spermatozoids from the same species do not react to all of them. This has led to the speculation that pheromones might have allelopathic functions. For example, they might be used to attract spermatozoids from a competitor to prevent fertilization of a competitor species (Müller, Citation1981).
Pollution
The early developmental stages (meiospores, gametes, gametophytes, microscopic sporophytes) are widely recognized to be most susceptible to a variety of environmental perturbations (Coelho et al., Citation2000). Limited information is available on their sensitivity to pollution (e.g. eutrophication, herbicides, trace metals and oil). In Laminaria hyperborea, the survival of germinating gametophytes in culture is reduced by pollutants including metals, herbicides and detergents. Development of sporophytes from the remaining viable gametophytes is delayed and sporophyte growth is inhibited by several pollutants (Hopkin & Kain, Citation1978). Detergents may also affect motility and settlement of meiospores of L. saccharina (Pybus, Citation1973). In both studies, juvenile sporophytes were found to be more sensitive to pollutants than meiospores or gametophytes. In Macrocystis pyrifera, nuclear migration during gametophyte development is inhibited by exposure to environmentally relevant levels of copper and arsenic (Garman et al., Citation1994). In L. saccharina, meiospore settlement and germination is not affected at copper concentrations below 500 µg l−1. Gametophyte development, growth and sporophyte production is, however, affected at concentrations higher than 50 µg l−1 (Chung & Brinkhuis, Citation1986).
Sewage discharge not only increases nutrient input but also increases suspended solid concentrations and lowers salinity, which is potentially stressful for brown algal propagules (Doblin & Clayton, Citation1995). In turbid coastal water, fine sediments reduce irradiance and inhibit photosynthesis. Spores attached to sediment grains can also be easily washed away by waves and water motion (Devinny & Volse, Citation1978), while the silt covering of rocks can prevent settlement of juvenile sporophytes (Norton, Citation1978) affecting the density and distribution of young Saccorhiza polyschides recruits. Sediment particles can also cover hard substrate and so bury early recruitment stages of macroalgae. Toxic chemicals in sewage have strong negative effects on the early settlement stages of Macrocystis pyrifera (Anderson & Hunt, Citation1988), but there is no information on this topic for Laminaria species.
Global climate change
Increasing information is available on the sensitivity of early life stages of seaweeds to enhanced UV radiation. After the early observations by Lüning and Neushul (Lüning & Neushul, Citation1978; Lüning, Citation1980), who found that meiospores and gametophytes of Laminaria and other kelp species do not survive exposure to full sunlight, research on the effects of UV radiation (UVR), especially on kelp meiospores under the scenario of stratospheric ozone depletion was strongly stimulated. Today we know that the motile meiospores are highly affected by UVR. Various cellular processes of meiospores are negatively affected by UVR, in particular photosynthesis (Roleda et al., Citation2005, 2006d), nuclear division (Huovinen et al., Citation2000) and motility (Makarov & Voskoboinikov, Citation2001). The inhibition of photosynthesis is the result of damage to the D1 reaction centre protein and part of the D1/D2 heterodimer of PS II (Richter et al., Citation1990). Nuclear division is thought to be impaired due to DNA damage by the formation of cyclobutane-pyrimidine dimers (Roleda et al., Citation2005) and possibly by negative effects on the cytoskeleton (cf. Schoenwaelder et al., Citation2003), which may also explain UV effects on motility. On the other hand, there are repair mechanisms operating, which mitigate damage. This is shown by the effective recovery of photosynthesis and the repair of DNA damage (Roleda et al., Citation2005, 2006d,e). DNA repair may be temperature dependent as the repair rates are higher in species/populations from the temperate zone than from the Arctic (). Damage may also be prevented by UV-absorbing phlorotannins, which are often exuded into the surrounding medium (Swanson & Druehl, Citation2002; Roleda et al., Citation2005, 2006b).
The balance between the damaging effects of UVR and the repair and protective mechanisms can be measured by the integrative parameter ‘germination’. If meiospores germinate after UVR exposure, the repair and protective mechanisms are strong enough to outweigh the damaging effects of UVR. Upon exposure to UVR, photosynthesis is inhibited strongly in all tested species. However, the recovery of photosynthesis in dim white light is much better in upper sublittoral than in deep water species (). DNA damage is higher and DNA repair is less efficient in species from deep waters compared with upper sublittoral species. As a result of these processes, germination of meiospores is least affected by UVR in upper sublittoral than in lower sublittoral species. This has been shown in a number of kelps from the Arctic, the N Atlantic and the S Pacific (; Roleda et al., Citation2005, 2006d,e; Véliz et al., Citation2006; Wiencke et al., Citation2000, 2007). In field experiments on Spitsbergen, the tolerance of meiospores to UVR was relatively high only in kelps from shallow waters and lower in Laminaria digitata from somewhat greater depths (Wiencke et al., Citation2006). These results support the suggestion of Wiencke et al. (Citation2000, 2004) that the UV susceptibility of meiospores influences the upper depth distribution limit of kelps. Moreover, size of meiospores of different Laminariales species in the N Pacific was correlated with the depth distribution of the adult sporophyte. Large meiospores were found among shallow-dwelling adult kelp exposed to high UVR. The prevalence of larger and more UV-tolerant meiospores in species and populations exposed to high UV environments suggests that kelp meiospores are pre-adapted to the UV environment of the parent plant (Swanson & Druehl, Citation2000).
As with the meiospores, there has been intensive research on the effects of different radiation conditions on the physiology of kelp gametophytes and juvenile sporophytes. After exposure to high PAR, photosynthesis is rapidly inhibited in gametophytes of Laminaria saccharina and in juvenile sporophytes, but recovers quickly (Hanelt et al., Citation1997a). In two Lessonia species, free meiospores show a higher UV sensitivity than settled spores, gametophytes and young sporophytes (Véliz et al., Citation2006). Among L. digitata, L. hyperborea and L. saccharina from Helgoland, the sensitivity of meiospores and gametophytes to UVR was higher than that of young and mature sporophytes (Dring et al., Citation1996).
Conclusion
The data available today provide insights into the importance of abiotic factors for the geographical and depth distribution and the ecology of Laminaria species. In the field, however, the meiospores are exposed to various environmental factors, which can change on tidal, daily or seasonal scales. The responses to such multifactorial complexes determine the fitness, recruitment and competitive strength of the species. Preliminary data on the combined effect of different radiation conditions and temperatures on meiospores of L. digitata from Spitsbergen indicate a reduced germination capacity and photosynthetic efficiency at low temperatures (2°C) combined with moderate UVB exposure (0.8 W m−2) compared with favourable temperature and radiation conditions (Müller, Roleda, Bischof & Wiencke, unpublished data). So, if UVB radiation increases further, the upper depth distribution limit will be shifted to deeper waters. If this is accompanied by a temperature increase of a few degrees, the zonation pattern will not change. This example shows that multifactorial investigations will provide important new insights into the ecological consequences of changing environmental conditions and may help in further elucidating ecotypic differentiation. They are, thus, urgently required in future kelp research.
7. Endogenous rhythms controlling metabolism and development
Like other organisms, macroalgae have to cope with periodic changes in their natural environment. Endogenous rhythms are internally generated oscillations in the organisms, which control physiological processes as well as behavioural activities. They enable organisms to anticipate the periodic changes and to prepare for them in advance. The hallmark of an endogenous rhythm is its persistence for more than one cycle without triggering clues from the environment. The period of the ‘free-running’ endogenous rhythm typically deviates from the exact period of the cycling environmental factor. Under natural conditions, the endogenous rhythm is entrained to the period of the environmental cycle by one or several Zeitgeber. Often light (day–night cycles, photoperiod) is the entraining factor, but temperature, nutrient availability and other factors can also act as a Zeitgeber (e.g. Lüning, Citation1991; Rensing & Ruoff, Citation2002; Stephan, Citation2002).
In the Laminariales, endogenous rhythms of the circadian (period about 24 h) and the circannual (period about 1 year) type have been described (see below). Circatidal rhythms (period about 12.4 h) or circalunar/semilunar rhythms (period approximately 2/4 weeks) are known in a number of marine animals (Neumann, Citation1981; Palmer, Citation1995). Although there are several reports of semilunar swarmer release in diverse seaweeds in the field (e.g.: Ulva: Smith, Citation1947; Monostroma: Ohno, Citation1972; Sargassum muticum: Fletcher, Citation1980), an endogenous free-running semilunar rhythm has only been confirmed in the brown alga Dictyota dichotoma (Müller, Citation1962) and there is partial evidence for Baltic Fucus vesiculosus (Andersson et al., Citation1994). Neither circatidal nor circalunar/-semilunar rhythms have been observed so far in members of the Laminariales.
Circadian rhythms
Circadian rhythms are the best-studied type of endogenous rhythms. They have been described in all groups of organisms (e.g. Hastings et al., Citation1991; Johnson & Kondo, Citation2001; McClung, Citation2001). Within algae, most studies have been on microalgae (Mittag, Citation2001; Suzuki & Johnson, Citation2001), but examples of circadian rhythms, mostly of photosynthetic activity and growth and/or cell division, have also been reported in macroalgae (e.g. Waaland & Cleland, Citation1972; Titlyanov et al., Citation1996; Lüning, Citation2001). Circadian growth rhythms were shown to be present in several members of the Laminariales (Laminaria abyssalis, L. digitata, L. japonica, L. longissima, L. pallida, L. schinzii, L. sinclairii, Pterygophora californica), and they may be a general feature in this group (Lüning, Citation1994; ). Maximal rates of growth under free-running conditions of continuous light peaked during the ‘subjective nights’, i.e. the phases of the 24-h light cycle, which correspond to the dark phases under day–night conditions. Interestingly, under day–night conditions, growth rate in P. californica increased at the end of the day and stopped during darkness. The reason for this apparent cessation of growth during darkness remains unclear; water loss due to osmotic effects or respiratory losses were discussed (Lüning, Citation1994).
Since DNA replication is a UV-sensitive process, it has been speculated that there was a strong evolutionary pressure on organisms to shift DNA replication and cell division into the dark periods to escape harmful UV radiation (Pittendrigh, Citation1993). Makarov et al. (Citation1995) demonstrated that nuclear division occurs predominantly during the dark phase under day–night conditions in Pterygophora californica, Laminaria schinzii and L. sinclairii. They also established that this was controlled by a circadian rhythm in P. californica. Maximum frequency of nuclear division preceded the peak of maximum growth by about 6 h. Because most cells of a thallus are not capable of division at any given time, it was concluded that the rhythm of growth, which consists of cell division and cell expansion, is not solely governed by the rhythm of cell division.
Another example of a process under circadian control in the Laminariales is egg release from female gametophytes of Laminaria saccharina (Lüning, Citation1981; ). Under light–dark conditions, release occurs over several days, but mainly during the first 30 min of darkness. This rhythmic pattern was maintained in continuous darkness and in continuous red or green light, but not in continuous white or blue light. Circadian regulation is also suspected to be involved in the timing of meiospore release (see Section 5: Sporogenesis and Meiospore release). It is likely that more processes, such as photosynthesis, are under circadian control in Laminariales because circadian oscillations of photosynthetic activity have been shown in several species of green, red, and brown algae (e.g. Britz & Briggs, Citation1976; Okada et al., Citation1978; Schmid & Dring, Citation1992; Granbom et al., Citation2001; Goulard et al., Citation2004).
Circannual rhythms
Annual growth cycles in several species of Laminariales provide rare examples of circannual rhythms in photosynthetic eukaryotic organisms. In nature, most Laminaria species exhibit a strong seasonality with maximum growth activity in winter and spring (see Section 4: Growth and photosynthetic performance of Sporophytes). For sporophytes of Pterygophora californica, Laminaria setchellii, and L. hyperborea, it was demonstrated that this behaviour is controlled by an endogenous annual rhythm of growth activity by following growth activity over up to 2 years in constant conditions (Lüning, Citation1991; tom Dieck, Citation1991; Schaffelke & Lüning, Citation1994; ). Similar experiments were conducted with L. digitata, but the results were ambiguous (Schaffelke & Lüning, Citation1994; Lüning, Citation2005). The growth rhythms in P. californica and L. setchellii were expressed in continuous long-day and night-break conditions, but not in short-day conditions. In night-break conditions, the long dark interval of a short-day regime is interrupted by a short light phase to exclude effects that are due only to the increased light dose under long-day conditions. The response of L. hyperborea was slightly different, since continuous long-days resulted in a cessation of growth in this species. Only continuous day–night cycles of 12:12 h light–darkness permitted the expression of a growth rhythm in this species. Short-days led to arrhythmic continuous growth in all three species. Species-specific daylength limits for the expression of circannual rhythmicity may be connected to the ecology and geographical distribution of a species, as was shown in birds (Gwinner, Citation1989, 2003).
Daylength was found to be the decisive Zeitgeber for the circannual rhythms since the free-running periods that varied within and between individual algae could be entrained to exactly 12 months by a simulated cycle of annual daylength (Lüning, Citation1991; tom Dieck, Citation1991; Lüning & Kadel, Citation1993; Schaffelke & Lüning, Citation1994). By modifying the day-length cycles, the algae can even be manipulated to follow ‘annual’ cycles as short as 3 months (Lüning & Kadel, Citation1993; Schaffelke & Lüning, Citation1994). Furthermore, the natural sinusoidal curve of the daylength cycle can be reduced to a rectangular skeleton cycle consisting of only two different photoperiods in P. californica (Lüning & Kadel, Citation1993). Circannual rhythms will probably also control growth in other species of Laminariales. For L. saccharina, L. bongardiana (=L. groenlandica sensu Druehl, Citation1968), Agarum cribrosum and Pleurophycus gardneri, it was possible to synchronize growth rhythms to artificial daylength cycles, but experimental problems prevented the proof of free-running circannual rhythmicity (Lüning & Kadel, Citation1993).
Photoperiodic effects and circadian rhythms
Endogenously generated circannual oscillations have to be distinguished from reactions that are exogenously triggered by environmental changes during the annual cycle. Here, a prominent example is the photoperiodic response, i.e. a reaction triggered by annual changes of daylength. The phenomenon is known to arise from the interaction of photoperiod with the circadian clock in higher plants and vertebrates, although via different mechanisms (e.g. Hastings & Follett, Citation2001; Suárez-López et al., Citation2001; Schultz & Kay, Citation2003). It is likely that algae also use the interaction of photoperiod with the circadian clock for daylength measurement. Several photoperiodic effects have been described in macroalgae; mostly short-day-dependent induction of reproduction or upright thallus formation, while only few long-day effects are known (Dring, Citation1984, 1988). In addition to the formation of new blades in Laminaria hyperborea (Lüning, Citation1986), the induction of sorus formation in L. saccharina and L. setchelli was shown to be a genuine short-day photoperiodic reaction (Lüning, Citation1988; tom Dieck, Citation1991; see also Section 5: Sporogenesis and meiospore release; ).
Temperature has a modulating effect on the time needed for sorus induction in Laminaria saccharina and L. setchelli (Lüning, Citation1988; tom Dieck, Citation1991). It was speculated that the influence of temperature allows the kelps to discriminate between warmer short days in autumn, when reproduction takes place in the field, and cooler short days in spring (Lüning, Citation1988). The endogenous circannual rhythm of growth will probably reinforce such a mechanism.
Molecular mechanisms
Apart from speculation, nothing is known about the underlying mechanisms of endogenous rhythms other than the circadian ones. The ‘circadian clock’ is driven by a network of delayed feedback loops of specialized genes, so-called ‘clock genes’. Components of the molecular oscillator have been characterized in animals, fungi, cyanobacteria and higher plants (see reviews by Roenneberg & Merrow, Citation2003; Dunlap & Loros, Citation2004; Gardner et al., Citation2006; Woelfle & Johnson, Citation2006), but so far not in eukaryotic algae. The ‘clock genes’ in turn coordinate the expression of a variety of ‘output genes’. Many ‘output genes’ were shown to exhibit circadian rhythmicity in higher plants and in some species of microalgae (e.g. Harmer et al., Citation2000; Mittag, Citation2001; Schaffer et al., Citation2001; Suzuki & Johnson, Citation2001; Kucho et al., Citation2005). Circadian gene expression has also been documented in three species of red macroalgae (Lopes et al., Citation2002; Jacobsen et al., Citation2003; Goulard et al., Citation2004), but not so far in brown algae.
Conclusion
Several examples of circadian and circannual rhythms as well as photoperiodic responses have been documented in the Laminariales. However, considering the widespread influence of endogenous rhythms on animal and higher plant physiology and development, it is likely that many more processes in the Laminariales and in other macroalgae as well are under control of endogenous ‘clocks’. Almost nothing is known about the molecular base and regulation of input and output pathways of these rhythms in macroalgae. The acculumation of genomic data on macroalgae should facilitate progress in this field. However, in the sequenced genome of the green microalga Chlamydomonas reinhardtii, no homologs to known central parts of circadian ‘clocks’ were found (Mittag et al., Citation2005). Thus, identification of core ‘clock genes’ in algae will probably not be as easy as one might have thought. Nonetheless, consideration of possible endogenous oscillations is an important prerequisite for the cultivation of algae and for experimental design.
8. Macro- and micronutrient metabolism
Growth and macronutrient availability
Many field observations on Laminaria species have confirmed that growth is significantly reduced or ceases altogether from late spring onwards (Kain, Citation1979; see Section 4: Growth and photosynthetic performance of Sporophytes). The first indication that this growth reduction was related to changing seasonal macronutrient conditions (nitrogen, phosphorus) was for L. longicruris (Chapman & Craigie, Citation1977). These authors showed for the first time that growth in the sea was nitrogen-limited and that the growth period could be extended by artificial fertilization, although ultimately the growth rate decreased in autumn. Later studies confirmed that the nitrogen and phosphorus contents of Laminaria blades decrease considerably in late spring (e.g. Davison et al., Citation1984; Conolly & Drew, Citation1985). In L. saccharina from the English Channel, tissue nitrogen was monitored over a complete seasonal cycle. Average nitrogen content ranged from 2.2 to 3.4% of dry weight (DW). There was a highly significant and positive relationship between carbon and nitrogen content and sporophyte length (Gévaert et al., Citation2001; for C:N ratios see ). For L. japonica, a nitrogen content of 1.3% in winter appeared to be critical in different tissue regions (Mizuta et al., Citation1997) and lower values were accompanied by a strong depression in photosynthetic activity. Elongation rates in L. japonica were high with approx. 1 m per month from winter to spring, but elongation ceased in June, when the phosphorus content of blades dropped below 1.3 mg P g−1 DW (Mizuta et al., Citation2003; ). Similar threshold values were described for nitrogen metabolism. Growth reduction occurred below 21 mg N g−1 DW and photosynthesis decreased below 13 mg N g−1 DW (Mizuta et al., Citation1997). Consequently, summer growth rates in L. longicruris, L. digitata and L. saccharina increased in more eutrophic sites along a nutrient gradient (Gagné et al., Citation1982; Conolly & Drew, Citation1985). Limiting nitrate concentrations caused a reduction in pigment content, maximum photosynthetic rate and quantum yield of PS II, as well as an increased light compensation point in L. solidungula (Henley & Dunton, Citation1997) while higher nitrate concentrations favoured growth and photosynthetic capacity in cultured sporophytes of L. saccharina (Chapman et al., Citation1978).
Table 8. Tissue nitrogen and carbon contents (as % of dry weight), carbon: nitrogen ratios (C:N), and rates of uptake of nitrate, ammonium and phosphate (µmol g−1 dry weight h−1) in different Laminaria species
Although these examples indicate that macronutrient availability is crucial for growth, the triggering mechanism for seasonal growth, at least in some Laminaria species, is an underlying circannual rhythm (Lüning, Citation1991; tom Dieck, Citation1991; Schaffelke & Lüning, Citation1994; see Section 7: Endogenous rhythms controlling metabolism and development). Lüning & tom Dieck (Citation1989) considered macronutrients to modulate but not to trigger growth (). This idea was confirmed when tank-cultivated L. digitata sporophytes that were kept in continuous SD maintained a higher growth rate throughout the summer than in in situ (long day) plants (Gómez & Lüning, Citation2001). The continuous growth of small first-year L. hyperborea thalli and the low C:N ratio of these individuals over the summer may indicate that first-year thalli are not as nitrogen-limited in summer as older plants (Sjøtun et al., Citation1996). Such first-year thalli may have higher nitrogen uptake rates because of their higher proportion of younger cells and tissues, as was shown for juvenile L. groenlandica by Harrison et al. (Citation1986). Preliminary experiments indicated, however, that the endogenous growth rhythm of juvenile Laminaria sporophytes only developed after a few weeks of the sporophyte ontogeny (Bartsch, unpublished data), which may also explain the longer growth season of juveniles. Since a multifactorial analysis of macronutrient limitation, temperature effects, underwater light climate and photoperiod has not been attempted, the weighted contribution of each factor to the summer growth depression in Laminaria species is still unknown.
Macronutrient uptake
The meristem and distal parts of Laminaria blades are capable of taking up nitrate. Although meristems of L. digitata exhibit very high nitrate uptake rates, their generally large nutrient demand has to be supplied, up to at least 70%, by import from more apical parts (e.g. Davison & Stewart, Citation1983). Direct translocation of nitrate is considered to be slow, but the basipetal transport of amino acids and its relation to growth rates was shown in the early 1970s (Lüning et al., Citation1972; Schmitz et al., Citation1972; Lüning et al., Citation1973). Maximum uptake rates amount to approx. 8 nmol nitrate cm−2 blade h−1 in L. japonica (Ozaki et al., Citation2001). Other authors have reported uptake rates of approx. 40 µmol nitrate g−1 DW d−1 in L. digitata (Gordillo et al., Citation2002; ). As nitrate reductase activity is very high in mature blades of L. digitata (i.e. in tissue regions where photosynthesis and carbon fixation are also enhanced), the hypothesis that nitrogen is translocated to the meristem in the form of amino acids is supported (Davison & Stewart, Citation1984). The nitrate reductase activity strictly follows the nitrate supply–seasonally with the ambient nitrate concentration (e.g. Gordillo et al., Citation2006; ) and locally with a higher activity in the blade as the region where nitrate uptake is highest (Davison et al., Citation1984). However, in two red algae, a diurnally changing activity of nitrate reductase was demonstrated, a phenomenon not yet taken into account for Laminaria (Lopes et al., Citation1997; Granbom et al., Citation2004) although there is evidence that polar brown algae have reduced nitrate uptake in darkness (Korb & Gerard, Citation2000a).
Nitrate uptake in Laminaria groenlandica was positively correlated with ambient macronutrient concentrations. First-year sporophytes of L. groenlandica showed a faster uptake than second-year algae (Harrison et al., Citation1986), suggesting ontogenetic and phenological modulations of nitrate uptake (Druehl et al., Citation1989b). However, it is difficult to assess whether this affects the resulting blade size, because blade length and width are influenced by the growth rate as well as by erosion and exposure to current (discussed in Druehl et al., Citation1989b). Ammonium is taken up simultaneously with nitrate, and nitrate uptake rates with and without ammonium were similar in L. groenlandica and L. abyssalis (Harrison et al., Citation1986; dā Costa Braga & Yoneshigue-Valentin, Citation1996). In contrast to these species, nitrate uptake in L. angustata var. longissima and L. solidungula decreased in the presence of ammonium (Korb & Gerard, Citation2000a; Machiguchi et al., Citation2006). Moreover, the uptake of ammonium seems to be much more efficient than that of nitrate, especially at ambient (low) concentrations (Rees, Citation2003). This can be explained by the fact that nitrate uptake is thought to be active and that nitrate can be stored in vacuoles, but its assimilation is energetically more expensive than that of ammonium, which is taken up passively, and not stored but rapidly assimilated into amino acids (Raven et al., Citation1992). Despite the presence of ammonium in the ambient seawater, its uptake has not been considered in most field investigations (). Although many authors provide nutrient uptake kinetic parameters such as V max and K s these values should be considered with great care since most are based on crude protein extracts or even intact thallus pieces, and, hence, do not reflect the real biochemical properties of the underlying enzymes. To characterize an enzyme biochemically, it must be isolated and purified to homogeneity, which is still a difficult task for Laminaria species because of strong cell walls and the presence of alginates, slime and phenolic compounds, which may interfere with enzymological approaches. In addition, epibiota such as bacteria can competitively influence algal nutrient uptake kinetics.
Maximum phosphate uptake rates of approx. 7 nmol cm−2 blade h−1 in Laminaria japonica or 1 µmol g−1 FW d−1 in L. digitata were measured at ambient concentrations of <10 µmol P l−1 with a rather low Km of 0.2 µM, indicating an efficient uptake system (Ozaki et al., Citation2001; Gordillo et al., Citation2002). Uptake rates decreased at lower PAR and temperatures (Ozaki et al., Citation2001) and when intracellular pools were high in L. japonica (Niemeyer, Citation1976; Mizuta et al., Citation2003). Inorganic phosphate was always preferred to organic phosphorus. Phosphatase activity in L. japonica increased from 0.07 to 0.2 µg P cm−2 h−1 when the cellular P concentrations decreased from 3.3 to 1.0 µg P mg−1 DW, which indicates the high phosphorus demand of the kelp and its ability to up-regulate uptake rates under external P-deficient conditions. Interestingly, the activity of phosphatase provided approx. 10-times the amount of phosphate that was actually taken up (Mizuta et al., Citation2003).
All measurements of enzyme activities and uptake kinetics may be hampered by epiphytes, especially bacteria, and may not, therefore, reflect the responses of the macroalga alone. Tissue washing and cleaning prior to experimental assays may reduce surface biofilms. Nevertheless, the contribution of bacteria to enzyme activities has to be checked carefully throughout the incubations, although it was found to be negligible in case of nitrate reductase (e.g. Davison & Stewart, Citation1984). The contribution of biofilms to the nutrient supply for Laminaria in general needs further attention in future studies.
Macronutrient storage and remobilization
There are several options for nitrogen storage in seaweeds. Pueschel & Korb (Citation2001) reported on the storage of nitrate in proteinaceous bodies in cells of Laminaria solidungula. Different types of storage molecules are used or remobilized on different time scales. Intracellular nitrate was exhausted after 1 month of external nitrogen depletion in L. solidungula. Labile organic nitrogen compounds, such as amino acids, soluble protein and chl a (Korb & Gerard, Citation2000b) were not utilized for another 2 months and proteinaceous bodies were absent after 7 months of nitrogen depletion (Pueschel & Korb, Citation2001). Thus, naturally nitrogen-deficient conditions of summer (<3 months in the Canadian Arctic, Chapman & Lindley, Citation1980b; approx. 7 months in cold-temperate Canada, Gagné et al., Citation1982) may well be compensated for by storage of various nitrogen-containing compounds. The concentrations of stored N in blades can exceed external concentrations by >1,000-times as shown in L. saccharina from Helgoland (North Sea; Chapman et al., Citation1978). In Japanese species, nitrogen and phosphorus concentrations were higher in fertile than in vegetative tissue (Nimura et al., Citation2002).
The other important macronutrient, phosphorus, is only rarely considered independently from nitrogen. An increase in phosphatase activity accompanied phosphorus depletion in the thallus of Laminaria japonica (see above; Mizuta et al., Citation2003). Sporophytes sampled in winter had a phosphate content of 5.3 µg P g−1 DW that continuously declined to 1.3 µg P g−1 DW until summer and their nucleic acid content followed these concentrations. Thus, L. japonica may sustain higher cell division rates through mobilization of phosphorus reserves, but a direct experimental proof is lacking. Although many red and brown algae store phosphorus immediately after replenishment (e.g. as polyphosphate granules, Niemeyer, Citation1976), there is no detailed information on the storage of phosphorus by Laminaria in relation to environmental factors and growth rates.
Iodine and other micronutrients
Iodine is accumulated approx. 30,000-fold by Laminaria species, which are the strongest iodine accumulators among all living systems and a major source of this element. Iodine contributes 0.25–5.0% to the dry matter of L. digitata sporophytes (Ar Gall et al., Citation2004). An iodine concentration of 5% of the dry weight equals about 80–85 mmol l−1 of cell water (recalculated on an approx. cell water content of 70% in Laminaria), which is very similar to the nitrate contents accumulated by L. digitata for osmotic acclimation (Davison & Reed, Citation1985a). Although not studied in detail, it is clear that such high iodine values will contribute significantly to the internal osmotic potential, and may also explain the anion deficits reported (Davison & Reed, Citation1985a). In L. japonica, iodine is stored as inorganic iodine, iodinated tyrosine and other iodinated compounds (Han et al., Citation2001). In L. digitata, iodine increases from the meristematic zone to the distal regions of the blade and from the stipe towards the holdfast (Küpper et al., Citation1998). Similar findings were reported for L. japonica by Wang et al. (Citation1996). In contrast, Yoshimura et al. (Citation1992) found particularly high iodine concentrations in the basal parts of L. japonica and L. angustata. In L. saccharina, iodine is translocated in the direction of the meristematic tissue (Amat & Srivastava, Citation1985).
Apart from iodine, several trace metals are taken up and accumulated by Laminaria species as essential micronutrients required for enzyme activation and photosynthetic electron transport (Stengel et al., Citation2005 and references therein). These authors measured copper, manganese and iron in L. digitata, and showed the highest concentrations of all metals were in the holdfast, ranging from 2.5 to 94 µg g−1 DW, as well as different distribution patterns in stipes and blades. The data were related to growth pattern and functional differences between thallus parts were analysed (Stengel et al., Citation2005). Iron, for example, was lower in the meristem and young tissue than in the holdfast and stipe, which was interpreted as small-scale metal limitation in actively growing tissue. The approach of Stengel et al. (Citation2005) is interesting since many publications on trace metals in Laminaria and other brown algae mention application of these algae in marine biomonitoring (Bryan & Hummerstone, Citation1973; Phillips, Citation1977; Amado et al., 1999) without examining taxa- and metal-specificities, as well as potential relationships between metabolism and metal accumulation.
Conclusion
The nutrient uptake mechanisms in Laminaria are understood only at a basic level. New biochemical approaches are urgently needed to characterize the properties of isolated and purified enzymes involved in nutrient uptake, as well as in all subsequent metabolic processes. In addition, molecular approaches could help us to understand better all aspects of nutrient-related gene regulation per se, or the effects of changing environmental conditions such as seasonally fluctuating nitrogen concentrations and their influence on molecular processes.
9. Storage compounds and growth substances
The statement of Kain (Citation1979) that “biochemical pathways of brown algae have not been of interest” still holds true in many ways and may account for the restricted information available about the genus Laminaria.
Storage carbohydrates
The phycocolloids alginate and fucoidan are present in the cell walls of most brown seaweeds and are economically important, but will not be considered here since they were reviewed recently by McHugh (Citation2003). The ecological significance and concentrations of the storage carbohydrates laminaran and mannitol in Laminaria were first investigated by Black (Citation1950) and Haug & Jensen (Citation1954). Apart from its multiple ecophysiological functions in Laminaria species, the sugar alcohol mannitol represents one of the main primary photosynthetic products, and serves as a storage compound, together with the polysaccharide laminaran (Kremer, Citation1980 and references therein). Due to the size of Laminaria sporophytes and their differentiation into holdfast, stipe and blade, distinct regions of carbon-sources and carbon-sinks exist along the thalli. They are biochemically connected by the translocation of various compounds through the highly specialized elongated sieve elements (=‘trumpet hyphae’; Schmitz & Lobban, Citation1976; Buggeln, Citation1983). There is much evidence that the pattern of translocation in Laminaria involves unidirectional transport from source to sink, i.e. from mature blade areas, which produce a surplus of photoassimilates, to the intercalary carbon- and nitrogen-requiring meristems and, to a lesser extent, stipes and haptera. In the sink tissues, the imported organic compounds are rapidly metabolized and incorporated into polysaccharides and proteins (Schmitz & Lobban, Citation1976). The translocated organic substances move at velocities of <10 cm h−1 and with rates of several gram DW h−1 cm−2 cross-sectional area of transporting sieve elements (Schmitz, Citation1981). These rates can be considered as high since they are similar to those of higher plants. However, the underlying biochemical and molecular biological mechanisms of translocation in Laminaria species have still not been studied.
There is clear evidence of biochemical differences in carbon fixation pathways among different Laminaria species, which can be related to the ontogenetic and physiological state of the tissue, but also to the ratio between carbon fixation via the Calvin cycle and LICF (Kremer, Citation1980; see also Section 4: Growth and photosynthetic performance of Sporophytes). The latter pathway, which takes place in the light as well as in darkness, seems to be mainly active in meristems to supply sufficient carbon skeletons and ATP for growth. The significance of ß-carboxylation in Laminaria species is seen during remobilization of stored mannitol as anaplerotic reactions, and as conservation of some carbon lost from respiration. Arctic L. solidungula is able to withstand long periods of light limitation, mainly by respiring carbon, and high LICF rates may account for growth observed during winter under the ice (Dunton & Schell, Citation1986). Cabello-Pasini & Alberte (Citation1997) did a comprehensive study on the significance of LICF in a broad taxonomic range of macroalgae. Except in L. setchellii, LICF rates were generally less than 5% of the maximum photosynthetic rates in most taxa, and thus can only partially compensate for the respiratory carbon losses, which accounted for about 10% of the maximum photosynthesis. In contrast, L. setchellii showed much higher tissue-specific LICF values between 4 and 27% of the maximum photosynthesis with the highest rates observed in the meristem (Cabello-Pasini & Alberte, Citation1997), which clearly indicates a taxa-specific significant role of this pathway in carbon acquisition. Since LICF rates, however, were only high under elevated incident irradiances, this pathway probably plays only a minor role under light-limiting conditions. From the data published so far on LICF it seems that, compared with many algal groups, brown algae and diatoms benefit from the occurrence of this pathway (Cabello-Pasini & Alberte, Citation1997 and references therein).
Mannitol metabolism is not well understood in Laminaria species, or in other brown algal taxa. Experimental evidence from Eisenia bicyclis, Dictyota dichotoma and Spatoglossum pacificum, however, indicate high activity levels of the anabolic enzymes mannitol-1-phosphate dehydrogenase (M1PDH) and mannitol-1-phosphatase (M1Pase; Yamaguchi et al., Citation1966, 1969; Ikawa et al., Citation1972). In addition, M1PDH has been reported in several Laminaria species (Kremer, Citation1980). However, the catabolic pathway for mannitol, which includes mannitol dehydrogenase (MDH) and a non-specific hexokinase (HK) and has been demonstrated in many other organisms, has not been reported in brown algae (Karsten et al., Citation1997). Therefore, it is not clear whether Laminaria spp. exhibit the complete mannitol cycle as described for the red alga Caloglossa leprieurii (Karsten et al., Citation1997).
Although Laminaria species can store carbon in monomeric compounds such as mannitol, they usually utilize polysaccharides, such as the ß-glucan laminaran (Schaffelke, Citation1995), because this polymer has only minor effects on the intracellular osmotic potential. Some laminaran molecules have mannitol instead of glucose at the reducing end (for chemical details see Percival, Citation1979 and references therein). While mannitol is stored in the cytoplasm, laminaran is mainly located in the chloroplasts, like starch in green algae. The soluble fraction of laminaran may also be stored in vacuoles (Rusanowski & Vadas, Citation1974). Although the chemistry of storage products in Laminariales is well understood, neither their biochemical formation and degradation nor their gene expression and regulation have been studied.
Strong seasonal changes in the content of mannitol and laminaran have been reported for various Laminaria species and have been related to the ecological strategy of these perennial kelps, which synthesize and store reserve products in summer and remobilize them for growth in winter and spring (e.g. Lüning et al., Citation1973; Chapman & Craigie, Citation1977, 1978; Küppers & Weidner, Citation1980; Honya et al., Citation1993; Sjøtun & Fredriksen, Citation1995; Sjøtun et al., Citation1996; see also Section 4: Growth and photosynthetic performance of Sporophytes). An annual carbon budget for L. longicruris indicated that 45% and 8% of fixed carbon was used for growth of the blade and stipe, respectively, and a further 12% as storage products (Hatcher et al., Citation1977). The remaining 35% was assumed to be lost as dissolved organic carbon.
Amino acids and lipids
Using 14C-labelling techniques, Hellebust & Haug (Citation1972) reported that the amino acid alanine played a quantitatively more important role than mannitol in the blade of Laminaria digitata. In addition, the amino acids glutamic acid and aspartic acid are translocated through the transporting sieve elements (Schmitz, Citation1981), indicating an important role for the transport of carbon and nitrogen between source and sink regions. The total amino acid concentration in the blade of L. japonica exhibited pronounced seasonal variations from 0.5 to 9.9% of the DW, and could be related to the inorganic nitrogen availability in the water column and physiological changes such as the development of sori (Honya et al., Citation1994). In L. solidungula, nitrogen may be stored in proteinaceous cytoplasmic inclusions, which can be remobilized during the summer months when water column nitrogen is low (Pueschel & Korb, Citation2001; see also Section 8: Macro- and micronutrient metabolism). In addition to the amino acids, lipid composition and concentrations showed strong seasonality in L. japonica (Honya et al., Citation1989, 1994), which was interpreted as biochemical adaptation to extreme temperature changes (Kostetsky et al., Citation2004). A comparison of young, mid-aged and old sporophytic tissue of the Arctic L. solidungula indicated a strong decrease of particular fatty acids (18:4(n–3)) with age, and a compensating increase in 20:5(n–3) (Graeve et al., Citation2002). In addition to environmental factors such as irradiance, salinity and temperature, the developmental stage has a strong influence on the fatty acid composition.
Plant hormones
Various higher plant growth substances are known from macroalgae. Like hormones in animals, they seem to regulate growth and reproduction of many algae (Bradley, Citation1991). In the Laminariales, abscisic acid (ABA; Schaffelke, Citation1995), auxin (Kai et al., Citation2006: indirect proof), gibberellin (Wildgoose et al., Citation1978) and cytokinin (Duan et al., Citation1995) have been reported. While increasing ABA concentrations in Laminaria japonica stimulated sorus formation and decreased vegetative growth (Nimura & Mizuta, Citation2002), the role of the other growth substances is not well understood in Laminaria. This is also true of the origin of these compounds, i.e. whether Laminaria species are biochemically capable of synthesizing growth substances by themselves, as are higher plants, or whether associated microorganisms are responsible.
Conclusion
The biosynthesis and regulation of mannitol and laminaran is not fully understood in biochemical terms. In addition, studies on molecular aspects of the underlying mechanisms such as gene expression and regulation are almost completely missing. Compared with the methodological approaches used in higher plant biology (metabolomics, proteomics, etc.), kelp biology is at least 5–10 years behind. Therefore, modern techniques should be applied to obtain a deeper insight into the functional genomics of Laminaria. The genome sequence of the brown alga Ectocarpus siliculosus will be available soon and will help to answer some of the open questions.
10. Salinity tolerance and osmotic acclimation
The typical sublittoral habitat of Laminaria represents quite a stable environment. Nevertheless some species, such as L. digitata, L. saccharina and L. hyperborea, can be exposed to major salinity changes at spring low tides (Lüning, Citation1990). At lowest water level, hyposaline conditions may be present due to the mixing of seawater with rain, snow or melt water, while hypersaline stress may occur due to evaporation during high insolation in summer or freezing-out of freshwater in winter. In addition, in estuaries and fjords which often exhibit extensive Laminaria stands (Schramm & Nienhuis, Citation1996), rivers or freshwater run-off mix with seawater and lead to diurnally and seasonally fluctuating salinity gradients. In Arctic waters, Laminaria species can be affected by melt water influx and calving glaciers (Hanelt et al., Citation2001). In addition to active processes which compensate for osmotic stress, Laminaria blades form multiple-layered, mat-like canopies at neap tides, protecting individual thalli against desiccation and other harmful abiotic factors (Lüning, Citation1990).
A high and constant water content of the cells seems to be an essential feature for vitality, and the optimal functioning of all metabolic activities. Morphological features, such as thick cell walls and mucilage layers, decrease or delay water loss, and thereby contribute to salinity tolerance. Osmotic acclimation in response to salinity changes, however, is the fundamental mechanism of salinity tolerance that conserves the stability of the intracellular milieu (homoeostasis), which is essential for maintaining an efficient functional state (Kirst, Citation1990). The acclimation process in Laminaria digitata involves the metabolic control of cellular concentrations of osmolytes. The major inorganic osmolytes are potassium, sodium, chloride and nitrate (Davison & Reed, Citation1985a), the cellular concentrations of which can be rapidly adjusted with little metabolic energy cost, especially compared with the cost of the biosynthesis or degradation of organic osmolytes (Kirst, Citation1990). However, protein and organelle function, enzyme activity and membrane integrity in macroalgae are adversely affected by increased inorganic ion concentrations and, hence, the biosynthesis and accumulation of organic osmolytes in the cytoplasm permit the generation of low water potentials without incurring metabolic damage (Yancey, Citation2005). For organic compounds that are tolerated at high intracellular concentrations, the term 'compatible solute' is used (Brown & Simpson, Citation1972).
The main organic osmolyte in Laminaria and most other brown algae is the sugar alcohol mannitol (Schmitz et al., Citation1972; Davison & Reed, Citation1985a,b). The concentration of this polyol is actively regulated in response to the external salinity. Because of its physicochemical properties, mannitol is one of the most potent organic osmolytes, not only balancing salinity stress, but also acting as an antioxidant, heat protectant (stabilization of proteins) and rapidly available respiratory substrate. It is important as an energy supply for maintenance metabolism under stress and for repair processes (Jennings et al., Citation1998; Iwamoto & Shiraiwa, Citation2005; Yancey, Citation2005). Although detailed studies on Laminaria species are lacking (Kremer, Citation1985), organic osmolytes in eukaryotic cells are typically localized in the cytoplasm (Yancey, Citation2005).
So far, few ecophysiological studies have been conducted on the salinity tolerance of Laminaria (Druehl, Citation1967; Pybus, Citation1973; Hopkin & Kain, Citation1978; Davison & Reed, Citation1985a,b and references therein; Gerard et al., Citation1987). The available data indicate that marine populations of L. saccharina exhibit optimal growth between salinities of 23 and 31‰, with a strong reduction of growth at 16‰ and high mortality below 8‰. Similar results were reported for L. hyperborea by Hopkin & Kain (Citation1978), and for Arctic L. digitata, L. saccharina and L. solidungula (Karsten, 2007). The photosynthesis of L. japonica was shown to be optimal between salinities of 28 and 36‰ (Niihara, Citation1975), whereas L. saccharina from the White Sea exposed to salinities between 24 and 26 was still able to photosynthesize at 6 and 8‰, but at highly reduced rates (Drobyshev, Citation1971). However, L. digitata, L. saccharina and L. solidungula from Spitsbergen exposed to hypo- and hypersaline salinities for 5 days died at salinities of 5 or 10‰, and photosynthetic performance (measured as F v ′/F m ′) was maximal between 25 and 34‰. Photosynthesis was reduced to about 50% of the control at salinities of 55–60‰ in these species (Karsten, 2007). In addition, nitrate uptake rates in L. digitata declined after exposure to seawater salinities of 17‰ (Gordillo et al., Citation2002). Ecotypic differentiation in terms of growth under different salinities has been reported in N Atlantic populations of L. saccharina originating from Long Island Sound, New York and Cape Neddick, Maine (Gerard et al., Citation1987), and might be considered as a mechanism to adapt to environmentally unfavourable conditions. Nevertheless, the genus Laminaria is stenohaline with respect to growth and photosynthesis. This is supported by a broad study of the benthic algal vegetation along the strong salinity gradient of the Hardangerfjord in Norway (Jorde & Klavestad, Citation1963). At 10 m depth, salinity inside the fjord may fluctuate seasonally between 18 and 30‰, and the variation is even higher (between 2–8 and 30‰) at 0–5 m depth. L. hyperborea occurred only in the outermost and, hence, fully marine areas of the fjord, probably because of the very low salinity tolerance of this species. In contrast, L. digitata and L. saccharina grew under both fully marine and brackish water conditions inside the Hardangerfjord with a similar horizontal distribution along the salinity gradient (Jorde & Klavestad, Citation1963), indicating a higher tolerance to hyposaline conditions. It seems that both species tolerate salinities down to about 15‰, at least temporarily.
Besides this more local role of salinity conditions on Laminaria distributions, the interactive effects of salinity and other abiotic factors such as temperature have to be considered. Low salinity may be compromised by temperature as shown for N Pacific L. groenlandica which cannot tolerate the low salinity, high temperature conditions encountered in areas subjected to snow-melt run-off, whereas L. saccharina can (Druehl, Citation1967). Both species, however, do well in areas subjected to winter rain run-off where cold conditions prevail (Druehl, Citation1967).
There are strong seasonal changes in the cytoplasmic composition of major inorganic and organic osmolytes in cells of Laminaria digitata (Davison & Reed, Citation1985a). Although this species accumulates high nitrate concentrations in spring, the mannitol content is low. During summer, nitrate is completely metabolized, and the gap in the osmotic potential is filled through the biosynthesis and accumulation of mannitol. This seasonal increase in mannitol concentration compensates for the intracellular decrease in nitrate rather than for changes in external salinity (Davison & Reed, Citation1985a).
Conclusion
In summary, although some physiological data are available on the salinity tolerance of Laminaria species, there have been almost no studies on the underlying biochemical and molecular mechanisms, such as ion transport across membranes, biosynthesis of mannitol, gene expression and regulation. The application of modern techniques such as metabolomics or proteomics is urgently needed to get a fundamental understanding of salinity stress responses.
11. Physiological defences against abiotic stress
In kelps, as in other seaweeds, abiotic stress may be either mechanical or physiological. Mechanical stress can induce wounding and may result from wave action, but kelps appear to be relatively well adapted to hydrodynamic forces (Laminaria saccharina: Kawamata, Citation2001a; L. hyperborea: Sjøtun et al., Citation1998) and, therefore, wounding as a result of animal action has received most attention (see Section 12: Defence against biotic stress factors). Physiological stress results from diverse situations, such as unfavourable light, temperature, salinity or nutrient conditions, as well as from chemical toxicity. In most cases, physiological stress will ultimately result in a malfunction of photosynthesis or growth (see also Section 4: Growth and photosynthetic performance of Sporophytes). As a consequence, photosynthesis slows down or becomes oversaturated, which results in increased pseudocyclic electron flow and in electron transfer to oxygen, yielding superoxide anions (•O2 −) and other reactive oxygen species (ROS), such as hydrogen peroxide (H2O2) or hydroxyl radicals (•OH). Physiological stress may also impair respiratory electron transport and mechanical stress may activate oxidases. Physiological stress (Fucus spp.; Collén & Davison, Citation1999) and wounding (Laminariales; Benet et al., Citation1994) are therefore generally detectable as an increase in ROS production (reviewed by Dring, Citation2006). If accumulation of ROS exceeds the capacity of cellular antioxidant systems, this process inhibits photosynthesis and becomes autodestructive due to oxidation of lipids, proteins and nucleic acids.
Oxidative stress in subtidal seaweeds, such as kelps, is largely unexplored. A study of seaweeds from Spitsbergen has demonstrated that the photosynthetic apparatus of Laminaria solidungula shows a similar sensitivity towards externally supplied H2O2 to other seaweeds from the lower subtidal, but is more sensitive than that of L. digitata and other species from the upper subtidal (Dummermuth et al., Citation2003). Therefore, the capacity for oxidative stress management appears to be correlated with general environmental stress resistance in kelps, as in other seaweeds.
Like most other living organisms, kelps are equipped with intracellular enzymatic detoxification systems and antioxidants of different chemical groups that diminish oxidative stress by elimination and reduction of ROS to less toxic and less reactive products. The presence of superoxide dismutase was reported in the sporophyte of Laminaria japonica (Liu et al., Citation2002a) and a gene homologue of superoxide dismutase is present in the gametophyte of L. digitata (Crepineau et al., Citation2000). On the other hand, catalase and ascorbate peroxidase have been reported so far only for L. japonica sporophytes (Huang et al., Citation2002) and glutathione reductase, another oxidative stress reducing enzyme that is present in Fucus spp. and other photosynthetic organisms, has not yet been demonstrated in kelps. Even an analysis of 1985 gene transcripts from protoplasts derived from L. digitata sporophytes did not reveal the expression of these three enzymes (Roeder et al., Citation2005), although such protoplasts were subject to severe oxidative stress (Benet et al., Citation1994) due to massive oxidative burst during tissue maceration (see Section 12: Defence against biotic stress factors). Increased expression of a stress-specific bromoperoxidase was observed, however (Roeder et al., Citation2005). Haloperoxidases in kelps have been and still are the subject of intensive research, mainly due to their importance as a major global source of volatile halocarbons, which play an important role in atmospheric ozone depletion and influence the lifetime of other greenhouse gases (Manley et al., Citation1992; Laturnus, Citation1996; Cota & Sturges, Citation1997; Goodwin et al., Citation1997; Carpenter & Liss, Citation2000; Carpenter et al., Citation2000; Laturnus, Citation2001; Malin et al., Citation2001; Ballschmiter, Citation2003; Palmer et al., Citation2005). Haloperoxidases catalyse the oxidation of halide ions (iodide only in the case of iodoperoxidase, iodide or bromide in the case of bromoperoxidase) to the more reactive hypohalous acid, and require H2O2. Polyhalogenated compounds may subsequently be formed from hypohalous acid via the haloform reaction, which results in sequential substitution of hydrogen atoms on a nucleophilic acceptor with halogen atoms (Ballschmiter, Citation2003).
Several haloperoxidases have been purified from Laminariales sporophytes and have repeatedly been shown to be dependent upon vanadium (De Boer et al., Citation1986; Jordan et al., Citation1991; Almeida et al., Citation2001; Colin et al., Citation2003). Two distinct isoforms of bromoperoxidase were isolated in these studies from Laminaria saccharina as well as from L. digitata, and one isoform from L. hyperborea. One isoform of iodoperoxidase has been isolated from each of L. hyperborea, L. ochroleuca and L. digitata. Jordan et al. (Citation1991) separated bromoperoxidases from cell-wall preparations, protoplasts and intact tissues of L. digitata and L. saccharina and found specific isoforms that were only present in the cell wall. The authors suggested that this was due to post-translational modification during or after excretion of the enzyme into the extracellular space. Correspondingly, histochemical tests revealed that haloperoxidases are mainly located near the outer cell wall in the external cortex region of the thallus (Almeida et al., Citation2001). Strong haloperoxidase activity was also detected around the mucilaginous channels in L. hyperborea. Studies on L. saccharina and L. digitata demonstrated that bromoperoxidase was active only in the blade, while iodoperoxidase was also detected in the stipe (Jordan et al., Citation1991; Mehrtens & Laturnus, Citation1997).
Experiments with kelps have consistently demonstrated increased production of volatile halogenated compounds under conditions of oxidative stress (Palmer et al., Citation2005), as well as in physiological or mechanical stress (Nightingale et al., Citation1995; Goodwin et al., Citation1997; Mehrtens & Laturnus, Citation1997). The inhibition of photosynthetic electron transport processes, and thus of H2O2 production, reduced halogenation as well (Goodwin et al., Citation1997). An important body of evidence thus indicates that haloperoxidase is a key enzyme for oxidative stress management in kelps and some authors consider that volatile halocarbons are only a byproduct of the removal of toxic active oxygen species (Manley, Citation2002). However, an involvement of haloperoxidases in biological defence has also been reported (see Section 12: Defence against biotic stress factors) and it is probable that the different isoforms of haloperoxidase play distinct roles (Colin et al., Citation2003). Iodoperoxidase, for example, has been suggested to be responsible for the accumulation of iodine from seawater (Ar Gall et al., Citation2004).
It is thought that iodine is taken up by sporophytes and gametophytes of Laminariales (see Section 8: Macro- and micronutrient metabolism) after haloperoxidase-mediated oxidation of iodide to hypoiodous acid (Küpper et al., Citation1998). The involvement of iodoperoxidase is indicated by the requirement of hydrogen peroxide for uptake (Küpper et al., Citation1998). The same authors also demonstrated that L. saccharina protoplasts do not accumulate iodine unless haloperoxidase is added to their medium, which indicates that the iodoperoxidase must be apoplastic. Interestingly, severe oxidative stress results in a net efflux of iodine (Küpper et al., Citation1998; Palmer et al., Citation2005). Correspondingly, lower concentrations of iodine were generally reported for plants exposed to higher irradiances and thus more photosynthetic stress: more iodine was detected in winter than in summer (L. digitata, Ar Gall et al., Citation2004), in high than in low latitudes (L. cichorioides, L. inclinatorhiza, L. japonica and other kelps, Saenko et al., Citation1978; L. digitata, Ar Gall et al., Citation2004) and in deeper than in shallow waters (L. cichorioides, L. inclinatorhiza, L. japonica and other kelps, Saenko et al., Citation1978).
High temperatures have been shown to accelerate the production of non-methane hydrocarbons such as isoprene in Laminaria digitata (Broadgate et al., Citation2004), suggesting that this compound–which is also produced by L. saccharina (Broadgate et al., Citation2004)–possibly plays a similar role as a thermoprotectant and antioxidant in kelps as it does in vascular plants.
Like most organisms, kelps respond to oxidative stress with an increased expression of specific cell-repair-related proteins. Protoplasts of Laminaria digitata sporophytes have been shown massively to upregulate heat-shock proteins HSP-70 and HSP-90, which play a crucial role in the recovery of cells from stress, in the prevention of protein aggregation and in the refolding of denaturated proteins (Roeder et al., Citation2005). The same study also revealed increased expression of the thioredoxin system (typically expressed during cellular oxidative stress) and of glutathione-S-transferase, which is known to detoxify lipid peroxidation products and endogenous toxic products.
In relation to the acclimation potential or defence mechanisms of Laminaria species against harmful UV-wavelengths (see also Section 4: Growth and photosynthetic performance of sporophytes), research has focussed on the synthesis and accumulation of UV-screening compounds, for example phlorotannins. Phlorotannins are similar in basic chemical properties to the condensed tannins of vascular plants and were reported to act as efficient sunscreens (Pavia et al., Citation1997; Schoenwaelder, Citation2002a; Henry & Van Alstyne, Citation2004; Wiencke et al., Citation2004; Roleda et al., Citation2006e). They are polymers of phloroglucinol (1,3,5-trihydroxybenzene) and are classified into six groups on the basis of the chemical structure of the polymer (Ragan & Glombitza, Citation1986). Phlorotannins are thought to be synthesized via a polyketide synthase type pathway (Arnold & Targett, Citation2002), although such an enzyme has not yet been detected in brown algae. The cytology of phlorotannin production has mainly been investigated in members of the Fucales and is probably similar in the Laminariales (reviewed by Ragan & Glombitza, Citation1986; Schoenwaelder, Citation2002b). Phlorotannins are localized in physodes, which are membrane-bound cytoplasmic vesicles. Fusion of physodes with cell membranes results in a secretion of phlorotannins (Schoenwaelder & Clayton, Citation1998a,b). They may then form complexes with alginic acid (Vreeland & Laetsch, Citation1990) or fuse after activation by haloperoxidases (Berglin et al., Citation2004) and thereby become insoluble components of the cell wall; they may even be excreted into the surrounding medium (Roleda et al., Citation2006b,e). Exposure of Laminariales species to UV-radiation usually results in an increase in the size of physodes (Wiencke et al., Citation2004) and/or increased exudation of phlorotannins (Swanson & Druehl, Citation2002). The shielding effect of external phlorotannin concentration has been demonstrated by Roleda et al. (Citation2006e) and Wiencke et al. (Citation2004). In several kelp species, lethal effects of exposing meiospores to UV-B were prevented by increased phlorotannin concentrations. In addition to their role in UV-screening, phlorotannins are likely to exhibit antioxidant activities since they have the potential to scavenge ROS. Furthermore, a function of phlorotannins as defence agents against predators is also suggested (see Section 12: Defence against biotic stress factors).
Conclusion
Most questions about anti-stress defence in kelps are still waiting for answers. For example, a comprehensive analysis of oxidative stress management has not yet been conducted with any subtidal macroalga. Laminariales would obviously be suitable subjects for such a study, given that the available information on anti-stress defence in kelps is somewhat broader than in many other subtidal species. As in most other algal groups, the management of defences against combined stresses in the Laminariales is nearly completely unknown and is still waiting for elucidation. New techniques such as differential displays of gene expression in Laminariales that are stressed in different ways may help in the near future to identify proteins that are involved in anti-stress defence.
12. Defence against biotic stress factors
In addition to abiotic stresses, which often result in oxidative stress (Section 11, above), seaweeds are subjected to a variety of biotic stress factors, such as intra- and interspecific competition, colonization or grazing (see Section 13: Laminaria as a habitat for epi- and endobionts). Most research on kelps has focused on the use of chemical anti-herbivory defences, but it has become obvious that multiple defensive strategies have evolved in kelps to counteract the detrimental effects of biotic stressors. Generally, defensive responses of Laminaria against biotic factors can be divided into physical, chemical, and associative traits. To date, only some of the strategies have been studied in detail in Laminaria species.
Physical defence
Physical responses will prevent or hamper the initial contact between the grazer and kelps. This is achieved in at least three ways. Firstly, the sweeping action of Laminaria fronds creates a visible protection zone along the margin of kelp beds (Velimirov & Griffiths, Citation1979). Inside this zone, the density of herbivores is significantly lower than further away from the kelp bed. The effectiveness of this defence depends on the strength of wave-induced water movements and on the flexibility of the stipe. For instance, the sweeping action of L. dentigera was of little effect due to its relatively stiff stipe (Konar & Estes, Citation2003). Secondly, the viscous mucous polysaccharide layer produced on blade surfaces of L. hyperborea is a possible mechanism to reduce attachment of grazers, as described for the herbivorous snail Ansates pellucida (Toth & Pavia, Citation2002b). Thirdly, the toughness of the epidermis may hamper attacks of grazers, as shown for L. longicruris, which successfully impeded attacks by the snail Lacuna vincta (Johnson & Mann, Citation1986). Similarly, the abalone Haliotis discus is unable to consume L. japonica prior to ontogenetic changes in radula morphology, which then allow the snail to penetrate the Laminaria-epidermis (Takami & Kawamura, Citation2003). On the other hand, Winter & Estes (Citation1992) showed that the morphology of L. sinclarii was not sufficient to deter Haliotis rufescens. This suggests that a defensive algal trait like toughness does not deter herbivores per se. Rather at least some Laminaria species seem to use different structural and chemical defences that interact or act in concert to deter grazers.
Chemical defence
The different aspects of chemical defences in macroalgae have been recently reviewed (Targett & Arnold, Citation2001; Potin et al., Citation2002; La Barre et al., Citation2004; Pohnert, Citation2004; Amsler & Fairhead, Citation2006; Dring, Citation2006). Numerous publications demonstrate the presence of pharmacologically active compounds (mostly antibiotics) in kelps (reviewed by Bhadury & Wright, Citation2004). These results are usually interpreted as an indication that kelps are well defended chemically. Despite their possible importance in medicine, such in vitro studies will not be reviewed here because they usually deal with tests of compounds at physiologically unrealistic concentrations and against human pathogens rather than ecologically relevant target organisms. Chemical defences of algae can be grouped into (i) inducible, (ii) constitutive (i.e. permanently available), and (iii) activated defences, with the latter being a special form of constitutive defence (Cetrulo & Hay, Citation2000). The regulation of many algal defences has not been investigated yet. Most defences are typically regarded as constitutive because evidence of activation or induction is missing. The following paragraphs will discuss chemical defences in Laminariales against fouling organisms, competitors, grazers and pathogens, starting with examples of constitutive defence forms.
Anti-fouling
Only very few studies have examined chemical anti-fouling defences in Laminaria species. The settlement of blue mussel (Mytilus edulis) was significantly reduced on thalli of L. saccharina, and Dobretsov (Citation1999) suggested that toxic compounds were responsible for this effect. Furthermore, it was shown that the exudates of L. saccharina deterred mussel spat from settlement on algal thalli (Dobretsov & Wahl, Citation2001). Good anti-fouling activity was demonstrated in L. saccharina (Wahl & Mark, Citation1999): the Laminaria thalli were more repellent than Delesseria and sea-grass surfaces resulting in reduced specific abundances of epibionts. Chemically mediated changes in competition were shown in two further studies: Laminaria meiospores were destroyed when grown together with coralline algae (Suzuki et al., Citation1998) and Denboh et al. (Citation1997) isolated a substance that was responsible for this allelophatic effect.
Phlorotannins
Phlorotannins have received the greatest interest as defence compounds. They are thought to function as cell wall constituents, UV sunscreens (see Sections 6: Biology of microstages, 11: Physiological defences against abiotic stress), antibacterial agents, fouling inhibitors, herbivore deterrents and digestion inhibitors. The potential antifouling and antibacterial roles of phlorotannins have been investigated in numerous studies (reviewed in Ragan & Glombitza, Citation1986; Amsler & Fairhead, Citation2006). Phlorotannins apparently play a role in wound healing. Wounding or ‘artificial grazing’ induced phlorotannins in Laminaria hyperborea (Toth & Pavia, Citation2002b), L. complanata and L. groenlandica (Hammerström et al., Citation1998), and three out of four other Laminariales (Steinberg, Citation1994; Hammerström et al., Citation1998). A cytological study revealed that, one day after wounding of Ecklonia radiata, phlorotannins accumulated around the wound sites and that dense accumulations were found throughout the medulla of the entire algal section after 9 days (Lüder & Clayton, Citation2004). The authors suggested that the increased presence of physodes at wound surfaces serves to reduce microbial infection. However, phlorotannins are not able to stop colonization around wounds or on the kelp thallus completely. For instance, Vairappan et al. (Citation2001) isolated three species of bacteria exclusively associated with thallus lesions of L. religiosa. Furthermore, Jennings & Steinberg (Citation1997) found no correlation between epiphyte load and tissue phlorotannin content, and concluded that natural concentrations of phlorotannins at the thallus surface of Ecklonia radiata were too low to reduce epiphytism by Ulva lactuca.
The anti-herbivory function of phlorotannins is controversial. Several studies support their anti-herbivory role, as a strong inverse correlation was reported between phenolic tissue content and tissue palatability of Laminaria longicruris (Johnson & Mann, Citation1986), L. pallida (Tugwell & Branch, Citation1989), L. sinclairii (Winter & Estes, Citation1992), L. dentigera (Steinberg, Citation1985), and Lessonia nigrescens (Martinez, Citation1996). However, phlorotannin concentrations did not explain herbivore food preferences for kelps in other studies (Ireland & Horn, Citation1991; Wakefield & Murray, Citation1998; Van Alstyne et al., Citation2001b). Some studies that support a defensive role of phlorotannins detected the highest phlorotannin concentrations in superficial tissues (L. pallida, Tugwell & Branch, Citation1989; L. hyperborea, Pedersen, Citation1980; Ecklonia and Eisenia, Shibata et al., Citation2004), suggesting that attacking grazers will immediately encounter the defensive potential of the alga. This may especially deter snails, as their radula penetrates the uppermost phlorotannin-enriched algal layers during initial grazing. Additional indirect support for the defensive properties of phlorotannins comes from the distribution of phlorotannins among tissues. Tissues of a high fitness-value were rich in phlorotannins, which matches with the predictions of defence theory (Tugwell & Branch, Citation1989; Martinez, Citation1996; but see Shibata et al., Citation2004). Tugwell & Branch (Citation1992) showed that phlorotannins can reduce digestibility through increased protein precipitation. Further supportive results for the grazing deterrent function of phlorotannins come from correlative studies, in which grazing simulations increased phlorotannin levels, but no feeding assays were conducted to confirm changes in the palatability of kelp (Hammerström et al., Citation1998). On the other hand, bio-assays with phlorotannin-containing fractions of Fucus vesiculosus extracts demonstrated that phlorotannins did not adversely affect grazing by the sea urchin Abracia punctulata (Deal et al., Citation2003). Similarly, phlorotannin-enriched feed did not adversely affect amphipod fitness (Kubanek et al., Citation2004). Another argument against the herbivory deterrent function of phlorotannin is its concentration in Laminaria. The phlorotannin concentration of seven Fucales species (average 2.17 to 5.8% of DW) was more than one order of magnitude higher than that in L. digitata (average 0.13% of DW; Connan et al., Citation2004), challenging the view that ambient phlorotannin levels in Laminaria-species can deter herbivores. This, however, needs further testing, as the sensitivity to phlorotannins may vary between intertidal (Fucales-associated) and subtidal grazers (Laminaria-associated).
The ambiguities of the role of phlorotannins as anti-herbivory substances may be due to incomplete extraction in earlier studies, which did not include cell-bound phlorotannins (Koivikko et al., Citation2005). On the other hand, phlorotannins represent a heterogeneous group of chemicals that presumably have different functions, as outlined above. Moreover, phlorotannin levels can vary considerably among seasons (Van Alstyne et al., Citation1999), among individuals of different age (Van Alstyne et al., Citation2001b), and at different spatial scales (Martinez, Citation1996; Toth & Pavia, Citation2002b), among individuals (Toth & Pavia, Citation2002b), among species along an intertidal gradient (Connan et al., Citation2004), and among populations (Van Alstyne et al., Citation1999, 2001a; Toth & Pavia, Citation2002b). This variation and the required sensitivity of the herbivore against the deterrent chemical(s) may explain the equivocal results obtained.
Induction of anti-herbivory defence in Laminaria has been shown only for L. japonica (Molis et al., 2008) and L. saccharina (Molis, unpubl. data), but data from other kelp genera are available. For instance, grazing by the amphipod Parhyalella rufoi lowered the palatability of Macrocystis angustifolia and of Lessonia nigrescens relative to ungrazed conspecifics (Macaya et al., Citation2005; Rothäusler et al., Citation2005). As in rockweeds (reviewed in Amsler, Citation2001), the species of herbivore and season both influence whether or not defences will be induced in Ecklonia cava (Molis et al., Citation2006). Phlorotannin concentration increases in response to artificial wounding in L. complanata and L. groenlandica, as well as in two other kelp species (Hammerström et al., Citation1998), but is reduced in L. hyperborea after exposure to the herbivorous snails Lacuna vincta and Ansates pellucida, indicating that phlorotannins do not function as an inducible chemical defence against these snail species (Toth & Pavia, Citation2002b). A good example of activated defence and currently the best-described example of a chemical defence mechanism in kelps is the oxidative burst in response to oligoalginates (see also Section 11: Physiological defences against abiotic stress). In sporophytes of L. digitata, the presence of oligoguluronate–a degradation product of alginate–resulted in a massive release of reactive oxygen species (, H2O2) by epidermal cells (Küpper et al., Citation2001). The same response was also observed in sporophytes, but not gametophytes, of L. hyperborea, L. ochroleuca, L. pallida, L. saccharina, Macrocystis pyrifera, Saccorhiza polyschides, Chorda filum and Lessonia nigrescens, so that the response appears to be universal in kelp and kelp-like sporophytes, but not in their gametophytes (Küpper et al., Citation2002). Alginate-degrading microorganisms also triggered an oxidative burst in L. japonica sporophytes (Liu et al., Citation2002b), which demonstrates that the oligoguluronate concentrations required for oxidative burst elicitation can be reached in vivo. H2O2 concentrations in the range released by L. digitata were toxic to alginate-degrading bacteria (Küpper et al., Citation2002) and axenic M. pyrifera was rapidly infected by pathogenic bacteria when the oxidative burst response was blocked with an NAD(P)H-oxidase inhibitor (Küpper et al., Citation2002). Treatment of nonaxenic M. pyrifera or L. digitata with the inhibitor also resulted in rapid degradation by their natural bacterial flora, which indicates that the oxidative burst must play an important role in the algal defence against bacteria and the maintenance of biofilms. The oxidative burst response also induced the resistance of M. pyrifera and L. digitata to the pathogenic brown algal endophytes Laminariocolax tomentosoides and Laminariocolax macrocystis. This response took 7 days to occur and probably involved induction or up-regulation of other structural or chemical defences (Küpper et al., Citation2002). Activity of alginate degrading microorganisms also induced programmed cell death in young L. japonica sporelings (Wang et al., Citation2006). This response was not due to external cell damage, but resulted from caspase and nuclease activation in L. japonica cells. It therefore appears similar to the hypersensitive responses that are typically observed in vascular plants after defence activation. Interestingly, over-expression of hypersensitive lesions in response to alginate-degrading bacteria may lead to massive losses of L. japonica sporelings in commercial kelp aquaculture and has been described as the so-called ‘rot disease’ (Ding, Citation1992). Recently, it was reported that L. digitata can recognize not only oligoalginates (i.e. endogenous elicitors), but also lipopolysaccharides from the outer cell envelope of a range of gram-negative bacterial taxa as exogenous elicitors of an oxidative burst and other early defence responses (Küpper et al., Citation2006).
Reactive oxygen species generated during the oxidative burst may play a role in biotic defence not only through direct cytotoxicity, but also through their peroxidase-catalysed reactions (see Section 11: Physiological defences against abiotic stress). In particular, the release of hypohalous acid and, subsequently, halogenated organic compounds by Laminaria digitata increases after oxidative burst elicitation (Malin et al., Citation2001; Palmer et al., Citation2005). Borchardt et al. (Citation2001) reported that hypohalous acid generated by L. digitata inactivated bacterial quorum-sensing signals and thereby caused dispersal of biofilms. In contrast, a role of halogenated organic compounds in the defence of kelps has not been demonstrated so far. However, bromoform, which is the main volatile halocarbon produced by L. digitata (Carpenter et al., Citation2000) and most other seaweeds (Carpenter & Liss, Citation2000), contributes to the defence against bacterial and algal epiphytes in red seaweeds (Ohsawa et al., Citation2001; Paul et al., Citation2006) and a similar effect in kelps may be possible.
Associative defence
Associative species interactions represent a spatial escape response, in which a vulnerable species gains protection from a natural enemy by associating with a protective host. There is only one known example in which this defence mechanism is attributed to kelps. Kelps are thought to be associatively defended against sea urchins by Desmarestia species. Grazing by sea urchins destroyed much of the kelp forests in several areas, for example off the coast of North America (Bernstein & Mann, Citation1982; see also Section 14: Tropic interactions), but discrete patches of kelp survived within urchin barrens (Konar & Estes, Citation2003). These refuges were surrounded by stands of the brown alga D. viridis (Gagnon et al., Citation2003). According to Gagnon et al. (Citation2006), D. viridis deterred sea urchins by its sweeping action, rather than its chemical content, i.e. sulphuric acid.
Conclusion
Relatively little information exists on anti-fouling defences in species of Laminaria, although more research has been conducted on anti-herbivory defences within the genus. Chemical defence mechanisms are best studied, but there are many uncertainties with respect to the identity of deterrent secondary metabolites, such as phlorotannins, and the way in which defences are deployed (inducible vs constitutive and activated defences). The possible role of multiple functions among the secondary metabolites of Laminaria species has been neglected, but its understanding would greatly improve our ability to predict interactions among consumers, epibionts, and Laminaria specimens. The interaction between abiotic and biotic stress factors is also nearly completely unexplored.
13. Laminaria as habitat for epi- and endobionts
The longevity and size of kelps and their worldwide geographical and sublittoral distribution contribute to one of their most important ecosystem functions: creating a habitat for a multitude of organisms with a high biodiversity. Recent insights into the role of microorganisms, endophytes, epiphytes and epizoobenthos within the Laminaria forests are outlined below.
Microorganisms
The diversity of microorganisms associated with algae, the type of association (specific or unspecific) and the interaction between algae and bacteria or fungi have been addressed in only a few studies, but there is growing evidence that macroalgae are influenced by their microbial epiphytes. The microbial colonization of Laminaria may be influenced by biotic and abiotic factors, such as tissue location and its age or its life history stage, the density and composition of the microbial communities, and the temperature, salinity and nutrient content of the seawater as well as secondary metabolites produced by the alga (see Section 12: Defence against biotic stress factors).
The abundance of epiphytic bacteria on the meristem and blade of Laminaria digitata from Brittany (France) varied between 106 bacteria cm−2 in winter and 6 × 107 bacteria cm−2 in summer (Corre & Prieur, Citation1990). A stable bacterial density of 6 × 107 bacteria cm−2 was observed at the distal end of the blade throughout the year. Seasonal variation was shown by cultivation experiments for several other Laminaria species (L. longicruris: 90 colony forming units (CFU) cm−2 in winter and 4 × 103 CFU cm−2 in summer, Laycock, Citation1974; L. pallida: 103 CFU cm−2 in winter and 107 CFU cm−2 in summer, Mazure & Field, Citation1980; L. digitata meristems: 2 × 103 CFU cm−2 in May, Davison & Stewart, Citation1984).
Bacteria and fungi associated with Laminaria might have a deleterious effect and/or might cause diseases. The composition of the bacterial population was studied for L. longicruris (Laycock, Citation1974). A psychrophilic population was dominant during winter, while mesophilic bacteria were associated with the decaying alga in the summer months. Isolates hydrolysing different substrates, like mannitol, alginate and laminaran, belonged to the genera Vibrio, Flavobacterium and Pseudomonas. Representatives of these three genera were also observed in L. japonica samples (Jiaozhou Bay, Qingdao, China; Duan et al., Citation1995). Dimitrieva & Dimitriev (Citation1996) compared natural and cultivated individuals of L. japonica in Kit Bay (Russia). They observed a decrease in macroalgal productivity during mariculture coinciding with fouling processes and occurrence of different associated bacteria. Strains belonging to Erwinia, Escherichia, Pseudomonas and coryneform bacteria were isolated from field plants. Pseudomonas and Alteromonas isolates were obtained from cultivated algae. Isolates of Alteromonas, especially, produced a variety of hydrolytic enzymes (e.g. lipase, DNase; Dimitrieva & Dimitriev, Citation1996).
Alteromonas and Pseudoalteromonas strains are discussed as infectious agents of Laminaria diseases. Alteromonas sp. has been suggested as the organism responsible for lesions and thallus bleaching symptoms in L. religiosa from Japan (Vairappan et al., Citation2001). Changes of abiotic factors, such as a decrease in salinity and an increase in the seawater temperature may also contribute to these regularly occurring symptoms. Since Alteromonas sp. has also been isolated from healthy kelp samples, in addition to Azomonas agilis, Azotobacter beijerinckii, Escherichia coli, Halobacterium sp. and Halococcus sp., it is probable that further factors, such as the cell density of Alteromonas sp., strain-specific differences in their biochemical profile, the interaction with other microorganisms and the physiological state of the alga, play an important role in the outbreak of these unspecified disease symptoms. Pseudoalteromonas elyakovii, another bacterial isolate from spot-wounded blades of L. japonica, was able to produce alginate-degrading enzymes. These were extracellular alginate lyase with a broad substrate spectrum (preference for poly-mannuronate and poly-guluronate) as well as intracellular enzymes degrading oligosaccharides generated from the digestion of the high molecular weight alginate (Sawabe et al., Citation1997, 1998a, 2000). The authors proposed that P. elyakovii might induce the spot disease. A second species of Pseudoalteromonas, P. bacteriolytica, might be the infectious agent of the red-spot disease of L. japonica (Sawabe et al., Citation1998b). Alginate degrading bacteria also cause the so-called ‘rot disease’ symptom in L. japonica blades (Wang et al., Citation2006) as well as in young L. japonica sporophytes, which results from an autodestructive hypersensitive response of the alga to alginate oligosaccharides (see Section 12: Defence against biotic stress factors).
Representatives of the fungal subdivision Ascomycotina are also known to colonize macroalgae (Kohlmeyer, Citation1979). A comparison between natural forests of Laminaria japonica and kelp farms in Russia revealed differences in the fungal community (Zvereva, Citation1998). The number of different fungal species was up to 1.8-times higher for farmed algae than for wild algae. Furthermore the diversity of fungal species decreased with the water depth and was twice as high on 2-year-old as on 1-year-old thalli of L. japonica. In summary, 37 different fungal species have been observed. The dominant genera were Penicillium, Aspergillus, Alternaria, Cladosporium, Dendrophyiella, Stemphylium, Fusarium (facultatively pathogenic) and Mucor.
Host specificity for the genus Laminaria is assumed for the fungal parasite Phycomelaina laminariae, which invades the meristoderm and outer cortical tissue of the stipes (Schatz, Citation1980). The parasite induces a disease, the formation of blackened spots, which only becomes evident on sporophytes after at least 1 year. The infection rate of L. saccharina by this parasite increased with increasing water temperatures, lowered nutrient availability and decreased growth rate between May and August. In addition, the infection by P. laminariae enhanced the colonization rate by other saprophytic fungi (Schatz, Citation1984a,b).
Laminaria-associated bacteria might also be beneficial in protecting the alga against microbial pathogens, predators, settlement of spores of common fouling algae and colonization by further fouling organisms. Attempts to isolate antibiotically active bacteria associated with L. saccharina from the Baltic Sea (Germany) revealed about 20 different species, mainly belonging to Actinobacteria, Gammaproteobacteria and Firmicutes (Wiese et al., unpublished data). These preliminary results led to the suggestion that associated bacteria might protect L. saccharina against microbial infections. A further favourable effect of bacteria on Laminaria has been described recently. Pseudoalteromonas porphyrae isolated from L. japonica was cultivated in a mariculture environment in the Sea of Japan (Dimitrieva et al., Citation2006). This bacterium displayed a growth-promoting effect on L. japonica: meiospore germination and blade extension was improved. This effect was linked to the bacterial production of a catalase, capable of protecting the alga against the toxic effects of hydrogen peroxide. Interestingly, the highest production rates of this catalase were reached during stress conditions, i.e. low salinity and low temperatures. Thus, the stress tolerance of Laminaria species might be enhanced by associated bacteria (see also Section 11: Physiological defences against abiotic stress).
Endophytes
Field observations show massive prevalence of infection by endophytic microalgae in kelp species in different parts of the world. In the 1990s, infection rates of Laminaria hyperborea and of L. saccharina in the NE Atlantic and the western Baltic Sea were as high as 25–100% and 70–100%, respectively (Lein et al., Citation1991; Peters & Schaffelke, Citation1996; Ellertsdottír & Peters, Citation1997). Frequent infections have also been reported in other geographical regions, such as in Laminaria species in the NW Pacific (Yoshida, Citation1980) and in several members of the Laminariales in the NE and SE Pacific (Apt, Citation1988; Peters, Citation1991). Even though infections by endophytic algae have been described as a common disease of kelp species for many decades, little is known about their ecological significance. However, it is reasonable to assume that they negatively influence the fitness and productivity of Laminaria sporophytes.
Endophytes of Laminaria are generally microscopic, morphologically simple, filamentous brown algae (e.g. Laminariocolax aecidioides, L. tomentosoides, Laminarionema elsbetiae), recently classified in the family Chordariaceae within the order Ectocarpales (Peters, Citation2003). European Laminaria saccharina is infested by Laminariocolax aecidioides and Laminarionema elsbetiae (Peters & Ellersdottír, Citation1996; Burkhardt & Peters, Citation1998; Peters & Burkhardt, Citation1998). The endophytes are distributed among host plants via zoospores from plurilocular sporangia. Zoospores of Laminariocolax aecidioides and Laminarionema elsbetiae attach to and penetrate the healthy host surface; no wounds or other openings are required for successful invasion of the host and no epiphytic stage precedes infection (Heesch & Peters, Citation1999). Thus, these endophytes are immediately invasive. This is noteworthy as a great number of epiphytic algae occur on kelps but most of these are unable to penetrate into the host. Thus, endophytes must have developed special attributes to achieve infection. Endophyte spores settle with their anterior end on the host surface and fibrillar adhesive material is formed around the attaching end. As no inward deflection of the host surface was observed, Heesch & Peters (Citation1999) proposed that the surface is locally dissolved by enzymes. A similar mechanism has been described for the green endophyte Acrochaete operculata, which infects the red alga Chondrus crispus (Correa & McLachlan, Citation1994).
Endophytic infection of Laminaria saccharina by Laminariocolax aecidioides has been divided into three disease categories according to Peters & Schaffelke (Citation1996): (i) Thalli are infected microscopically and disease symptoms are absent. (ii) Moderate symptoms (i.e. dark spots, ridges and small wart-like structures) are visible. Consequently, this endophytic infestation is called ‘dark spot disease’. (iii) Severe morphological changes, such as distorted stipes or crinkled blades may occur. Even though the presence of endophytes is not necessarily harmful to the host, plants with severe morphological changes are less flexible and hence more susceptible to wave action. Furthermore, negative effects of both endophytes and their polar and non-polar extracts on the growth rates of Laminaria sporophytes have been shown, indicating direct chemical interactions with the host tissue (Peters, pers. comm.).
In addition, endophytic infestation probably interferes with the fertility of kelp sporophytes. Sporangia can cover about 70% of the blade surface (Kain, Citation1975), so that 20% coverage of the blade surface by the ‘dark spot disease’ will diminish the potential reproductive area significantly (Lein et al., Citation1991). Furthermore, brittle thalli are more likely to be detached during storms, again reducing the potential reproductive area and/or period. On the other hand, Lein et al. (Citation1991) suggested that endophyte infection per se might inhibit the formation of sori, and this was also observed in L. digitata (Lüning et al., Citation2000) and in L. saccharina (Peters & Schaffelke, Citation1996). However, no underlying mechanism has so far been demonstrated.
Laminaria sporophytes can recognize attacks by endophytes and can initiate effective defence responses within minutes (see Section 12: Defence against biotic stress factors). However, endophytes seem also to have developed mechanisms that either eliminate the defence response of L. digitata or neutralize ROS (Küpper et al., Citation2002).
Epiphytic algae
Epiphytes are a common phenomenon on marine macroalgae. They may form obligate relationships (e.g. Notheia anomala and Hormosira banksii; Hallam et al., Citation1980) or merely occupy available space on the surface of larger species. The majority of species are facultative epiphytes, which are not host-specific and generally occur on non-living substrata as well. Wahl & Mark (Citation1999) even showed that macro- and microepiphytes usually preferred artificial substrata to Laminaria saccharina surfaces. The diversity of epiphytes on kelp species is highly variable. Furthermore, epiphytes show considerable spatial heterogeneity on kelps; holdfasts, stipes and blades are colonized by different species and to different degrees. Nearly 80 species of epiphytic green, brown and red algae were identified on Laminaria spp. from the Sea of Japan (Sukhoveeva, Citation1975). In contrast, Schultze et al. (Citation1990) only identified seven and eight algal species on L. hyperborea and L. digitata, respectively, at Helgoland (North Sea). Red algae dominated there throughout the year. Also Berdar et al. (Citation1978) found only seven algal species on L. ochroleuca in the Mediterranean Sea and >95% of the epiphyte biomass on L. hyperborea in Scotland was made up of only four species (Whittick, Citation1983). Especially the rough stipes of L. hyperborea support the development of a stable epiphytic flora. Epiphytes of L. digitata from the Isle of Man (UK) were dominated by ectocarpoid algae (Russell, Citation1983a).
Percentage cover, abundance and number of species of epiphytes on kelps increase with the age of the hosts (e.g. Laminaria saccharina: Russell, Citation1983b; L. hyperborea: Christie et al., Citation1994). The distal and oldest part of the kelp frond is most strongly covered by epiphytes. On the kelp stipes, epiphytes are confined to the lower, rugose and oldest parts, and are absent from the smooth and young area immediately below the blade. (Whittick, Citation1983).
Epiphyte composition also shows a differentiation along abiotic gradients of their habitat. For example, Palmaria palmata on Laminaria hyperborea is restricted to shallow sublittoral areas, while Phycodrys rubens is most abundant at 6 m depth and below (Whittick, Citation1983). Furthermore, due to light attenuation in the water column, epiphyte biomass decreases with water depth (L. hyperborea: Marshall, Citation1960; L. pallida: Allen & Griffiths, Citation1981). Whittick (Citation1983) reported up to 100 g DW of epiphytic biomass per L. hyperborea stipe of 5–7-year-old algae in 1–2 m depth and <10 g DW per stipe of the same age class in 12 m depth. Experimental removal of kelp canopies (L. hyperborea and L. digitata) at the Isle of Man (UK) resulted in pioneer settlement of typical epiphytic species, which suggests that competition for light with canopy algae restricts facultative epiphytes of kelps to the epiphytic habitat (Hawkins & Harkin, Citation1985). The authors concluded that epiphytes may constitute a reservoir from which recruitment of ephemeral species to other, often transient, habitats can occur.
Enhancement of nutrients in coastal waters is likely to cause large changes to benthic assemblages, such as a shift from slow-growing macroalgae to fast-growing turf-forming algae (Gorgula & Connell, Citation2004), and may also favour growth of epiphytic filamentous algae. Epiphyte abundance and biomass on Laminaria longicruris increased at an eutrophicated coast (Scheibling et al., Citation1999).
Epiphytic animals
Depending on their level of mobility, animals may live attached to or associated with macroalgae. The most abundant taxa include bryozoans, tube-building amphipods and gastropods (Schultze et al., Citation1990; Berman et al., Citation1992; Lambert et al., Citation1992; Okamura & Partridge Citation1999; Norderhaug et al., Citation2002; Christie et al., Citation2003), which are often attracted to settle on epiphytic turf algae that grow on kelps and provide suitable microhabitats for a variety of animals (Allen & Griffiths, Citation1981). The kelp-associated fauna represents a large food source for adjacent food webs, making kelp beds ecologically important as export centres. For instance 1–2% of the biomass of the mobile fauna emigrates daily from Laminaria hyperborea forests to pelagic and benthic food webs (Jørgensen & Christie, Citation2003). The total number of animal species reported to be associated with Laminaria species ranges from 32–107 for L. digitata (n = 4 to 8; Lippert et al., Citation2001) to 238 for L. hyperborea (n = 56; Christie et al., Citation2003). The number of mobile specimens living on a single kelp can exceed 7,000 individuals (Jørgensen & Christie, Citation2003). Besides abiotic factors (e.g. wave exposure) controlling the development and distribution of animals associated with kelps, the following biotic factors have been studied: (i) type of tissue, (ii) algal size, (iii) anti-fouling mechanisms, and (iv) level of competitiveness in larvae.
Type of tissue
The effect of tissue type (blade, stipe or holdfast) on faunal colonization has attracted most research. Large animals (macrofauna) have not been reported to be exclusively associated with blades of Laminaria hyperborea (Christie et al., Citation2003), but blades of L. ochroleuca host a specific meiofauna (Arroyo et al., Citation2004). As with epiphytic algae, blades appear to harbour the least number of animal species (Seed & Harris, Citation1980) and blade assemblages differ from holdfast and stipe assemblages (Schultze et al., Citation1990; Christie et al., Citation2003; Jørgensen & Christie, Citation2003). However, because of competition for space, inferior competitors may settle on less preferable tissue types, such as on the blades of L. saccharina (Seed & Harris, Citation1980). Low species diversity on blades is partly due to the flexibility of the substratum. The bryozoan Membranipora membranacea is one of the few, and sometimes the only species covering Laminaria blades to any great extent (Seed & Harris, Citation1980). Its zooids develop non-calcified bands, which prevent cracking of colonies on the flexible fronds (Ryland & Hayward, Citation1977). In addition, the ephemeral nature of Laminaria blades prevents the accumulation of large numbers of species (Norton et al., Citation1977; Christie et al., Citation2003). Temporal recruitment patterns of epiphytic animals must broadly correlate with the growth cycle of the Laminaria species on which they occur (Seed & Harris, Citation1980). Larvae settle preferentially on basal, i.e. younger parts of Laminaria blades (Seed, Citation1976; Brumbaugh et al., Citation1994), thereby prolonging the duration of the habitable substrate. Macrofaunal species composition in the holdfasts of L. ochroleuca is distinct from that on adjacent rock (Sheppard et al., Citation1977), while the meiofaunal species composition is similar (Arroyo et al., Citation2004). Compared with blades and stipes, the holdfast community of L. hyperborea is richer in species and of different composition, although the number of individuals was highest on the stipe (Christie et al., Citation2003; Jørgensen & Christie, Citation2003).
Algal size
The size of kelps strongly affects species composition of the associated fauna as shown for Laminaria hyperborea (Christie et al., Citation1998). Larger plants host more species and individuals than smaller conspecifics (Christie et al., Citation2003), indicating that kelp harvesting may prevent full re-establishment of the epifauna if times between trawling episodes are short (Christie et al., Citation1998). However, the species richness of animals inhabiting holdfasts was maximal on 6-year-old plants, which suggests that larger holdfasts may be accessible to predators, which eliminate part of the holdfast community (Christie et al., Citation1998).
Anti-fouling mechanisms
As anti-fouling protection, kelps are known to produce chemicals that affect recruitment patterns of larvae on the kelp surface (see also Section 12: Defence against biotic stress factors). For instance, gradients of antibiotics result in a preferential settlement of spirorbid larvae in the inner parts of the holdfast (Al-Ogily & Knight-Jones, Citation1977). Furthermore, Dobretsov & Wahl (Citation2001) suggest that exuded chemical cues of Laminaria saccharina inhibited settlement of mussel spat, and the copious production of mucus on the surface of kelp blades reduces survival of larvae (Norton et al., Citation1977).
Wave exposure
Water velocity is the most frequently listed abiotic factor affecting the abundance of sedentary animals living on Laminaria species. Schultze et al. (Citation1990) reported that the species richness of holdfast assemblages of L. digitata and L. hyperborea was higher in exposed than in sheltered sites on the island of Helgoland (Germany). The same study revealed a species-specific difference in wave-mediated colonization of the blade and stipe. The species richness of colonizers on L. hyperborea was similar in sheltered and exposed sites, while wave exposure reduced the species richness of colonizers on blades and stipes of L. digitata relative to sheltered sites. Increasing water movement reduced the abundance of bryozoans (Seed & Harris, Citation1980), while higher turbidity at sheltered sites was positively correlated with the dominance of suspension feeders and low diversity (Edwards, Citation1980). A species that seems to cope well with the high water flow regimes around Laminaria blades is the bryozoan Membranipora membranacea, sometimes covering blades completely. This bryozoan species has adapted to high water velocity environments by a reduction in zooid size (Okamura & Partridge, Citation1999). The miniaturized zooids enable M. membranacea to position the feeding structures into the low flow regimes of the boundary layer around the blade, from where sufficient food can be collected (Okamura & Partridge, Citation1999).
Effects on the host
Epiphytic animals on kelps are of major ecological relevance, as they exert direct and indirect effects on the fitness and performance of their host. In contrast to epiphytic algae, colonial animals, such as bryozoans, constitute a substantial mechanical barrier that has been shown to affect (i) nutrient uptake, (ii) defoliation, (iii) photosynthesis, (iv) consumption pressure, and (v) reproductive output. Whilst relatively little is known from species of the genus Laminaria, the ecological consequences of epiphytic animals on the giant kelp Macrocystis pyrifera have been studied in more detail. (i) Nutrient uptake: The bryozoan Membranipora membranacea and the hydroid Obelia geniculata stimulated kelp growth rates because excreted ammonium was taken up by the host M. pyrifera (Hepburn & Hurd, Citation2005; Hepburn et al., Citation2006). This suggests that epiphytic colonial animals may have positive effects on kelp fitness at oligotrophic sites (but see Hurd et al., Citation2000 for temporal variation). (ii) Defoliation: Kelp blades become more susceptible to breakage with increasing bryozoan cover (Dixon et al., Citation1981). Brittleness of blades was positively correlated with the abundance of encrusting bryozoans, which resulted in higher loss of fouled than of clean Laminaria blades during storms (Lambert et al., Citation1992). (iii) Photosynthesis: The pigment concentration of Macrocystis pyrifera was lowered in thallus parts under epiphytically growing bryozoans, but not where colonies of the hydroid Obelia geniculata were present (Hepburn et al., Citation2006). This species-specific effect of epiphytic animals on their kelp host may result from lowered nitrogen provision under bryozoan crusts or a damaging effect of the crust to the underlying algal tissue (Hepburn et al., Citation2006). (iv) Consumption pressure: Kelps experience a detrimental effect due to the attractiveness of their epibionts to consumers, which is described as ‘shared doom scenarios’ (sensu Wahl & Hay, Citation1995). For instance, kelp blades suffered greater loss than clean blades because predators incidentally consumed kelp when preying on the bryozoans (Dixon et al., Citation1981). Furthermore, urchin growth rates increased when bryozoan-covered kelps were eaten, which in turn increased overall consumption pressure on kelps (Scheibling et al., Citation1999). (v) Reproductive output: Epiphytic animals can affect Laminaria fitness by reducing spore output in species lacking the potential of vegetative propagation. For instance, crusts of the bryozoan Membranipora membranacea considerably suppressed meiospore liberation in L. longicruris (Saier & Chapman, Citation2004, see also Section 5: Sporogenesis and meiospore release).
Conclusion
Laminaria species act as hosts to species-rich assemblages of algae, animals and micro-organisms and this underpins one important ecosystem function of kelps: providing a suitable habitat for a great variety of species. Species associated with Laminaria either serve as food for higher trophic levels or are consumers of their host or the associated assemblage. The associated microorganisms may be ecologically important in spreading infectious algal diseases, protecting against fouling organisms and pathogens or producing substances that promote algal growth. Their role is still virtually unknown. The same is true for the ecological significance of endophytes. Their impact on kelp productivity and survival needs a closer look. The trophic connections are largely unexplored but suggest a complex and finely triggered interaction web among kelps and their associated fauna, flora and microorganisms. Future research on epiphytic and endophytic algae, animals and micoorganims of Laminaria species should examine the environmental factors that influence their colonization and the defensive mechanisms of the host.
14. Trophic interactions
Based on our current knowledge, little of the considerable biomass produced in kelp forests is consumed directly, but most enters a detritus-based food web. Consequently, assimilation of kelp carbon into higher trophic levels is not only related to the relative activity and abundance of herbivores that graze directly on Laminaria populations, but also to the supply of particulate and dissolved organic carbon (POC and DOC) derived from kelp production.
Although Laminaria is characterized by a heteromorphic life history, only grazing on sporophytes has been surveyed, probably because it is so difficult to find microscopic stages in the field. The different kinds of grazers that consume kelp sporophytes are usually divided into meso- and macrograzers. Since the effects of sea urchins on Laminaria populations are often more dramatic, research efforts have emphasized more macro- than mesograzer effects.
Macrograzers
In contrast to meso-herbivores, macrograzers such as sea urchins or fish are able to consume individual algae entirely. The strongest impact of consumers on Laminaria fronds is exerted by sea urchins, among which the genus Strongylocentrotus is most effective in destroying Laminaria beds. Worldwide, different species of Laminaria encounter different Strongylocentrotus species as their most important consumers (). Irrespective of the region, destructive consumption by sea urchins of kelp beds in general, or of Laminaria beds, has been reported (see ), which ultimately leads to ‘barren grounds’, where kelp beds have been replaced by crustose coralline algae alone (Lawrence, Citation1975; Mann, Citation1977). Consequently, kelp beds and barren grounds may be viewed as two distinct organizational stages of the ecosystem (Konar & Estes, Citation2003). There is some evidence that, at least in the NW Atlantic, the ‘kelp forests–barren ground’ stages have been part of a cyclic phenomenon since the early 1900s (Mann, Citation1977; Miller, Citation1985b analysed fishermen interviews), which has persisted until today at a larger spatial scale.
Table 9. Selected examples of sea-urchin grazing leading to barren grounds in Laminaria beds and their possible top-down controla
The establishment of high urchin densities seems to be of utmost importance in forming urchin fronts and, thus, the commencement of destructive herbivory events in intact kelp forests. At least two mechanisms have been proposed to promote urchin aggregation. First, changes in urchin behaviour in response to certain predators have been reported (Bernstein et al., Citation1981, 1983). While urchins hide in crevices to escape their predators, such as fishes or crabs, aggregations were thought to be an effective anti-predator defence at higher predator densities (Bernstein et al., Citation1981). Second, the absence of top-down controls was shown to increase urchin densities; for example the decrease of sea otter populations in Alaska led to enhanced sea urchin densities and, thus, to barren grounds (Estes et al., Citation1978; for more examples see ).
In the NW Atlantic between Maine and Newfoundland, where kelp beds mostly consist of Laminaria species, sea urchin grazing was investigated from the late 1960s onwards. At this time, the sea urchin Strongylocentrotus droebachiensis became locally abundant, grazed kelp beds of L. longicruris and L. digitata heavily, and converted large areas from Massachusetts to Labrador into ‘urchin-dominated barren grounds’ (e.g. Mann, Citation1977; Wharton & Mann, Citation1981; Keats et al., Citation1982, 1990; Pringle et al., Citation1982). Over-fishing of the top-predator, the American lobster (Homarus americanus) and predatory fish was suggested as a possible reason for the change from low to high sea urchin densities (Wharton & Mann, Citation1981; Pringle et al., Citation1982; Miller Citation1985a; Chapman & Johnson, Citation1990; Elner & Vadas, Citation1990; Vadas & Steneck, Citation1995). At high densities, sea urchins climb onto and bend down Laminaria blades, so that they can be completely consumed. Urchin fronts are possibly formed when threshold sea urchin densities are exceeded and animals develop a defensive response against certain sea urchin predators (Bernstein et al., Citation1981, 1983). Other studies have proposed that (e.g. after storms) the kelp:sea urchin ratio falls below a critical threshold and drifting kelp biomass is insufficient to satisfy the needs of the sea urchins (Miller, Citation1985a; Harrold & Reed, Citation1985; Vadas et al., Citation1986). This whole cascade was reviewed by Chapman & Johnson (Citation1990). Depending on the size and density of urchins, grazing fronts may advance at rates of up to 4.2 m per month (Miller & Mann, Citation1973; Scheibling et al., Citation1999). Laminaria populations could only persist in refuges at wave-exposed sites close to low tide level (Mann, Citation1977; Chapman, Citation1981).
A similar pattern was observed in Norway, where grazing is most severe in the sheltered areas of fjords, and the outer, more wave-exposed sites served as Laminaria refuges (Christie & Rinde, Citation1995; Sivertsen, Citation1997; Sjøtun et al., Citation2000). In Japan, L. religiosa was restricted to refuge populations in shallow areas (Akaike et al., Citation1999; Kuwahara, Citation2003). Therefore, as on North American coasts, Laminaria refuges exist only in shallow areas with high wave movement or sites with high water velocities (Kawamata, Citation1998, Akaike et al., Citation1999; Dotsu et al., Citation1999). Here, the grazers are susceptible to the whiplash effect of kelp blades (Kawamata, Citation2001b; see Section 12: Defence against biotic stress factors). Miller (Citation1985b) also suggested that very sheltered sites, or isolated boulder patches function as refuge habitats inaccessible to destructive sea urchin grazing in Nova Scotia. From these refuge sites, Laminaria can potentially re-colonize areas over distances of at least 600 m as calculated for L. longicruris (Chapman, Citation1981), depending on local hydrodynamics (Johnson & Mann, Citation1988). After the formation of barren grounds, sea urchins in Nova Scotia switched their diet to ephemeral algae and persisted with low growth rates, consuming any new Laminaria recruits from refuge stands before these could reproduce (Chapman, Citation1981). In the NW Atlantic, Laminaria is a highly preferred food source of best nutritional value for Strongylocentrotus droebachiensis (Larson et al., Citation1980). Since lobsters, which prey on sea urchins, need kelp beds as a habitat (Wharton & Mann, Citation1981; Bologna & Steneck, Citation1993), there is a positive feedback that prevents the increase of predator pressure and thereby stabilizes the barren grounds (Mann, Citation1977; Bernstein et al., Citation1981). Only mass mortalities from diseases (e.g. Paramoeba sp. in NW Atlantic, Scheibling Citation1986; the endoparasitic nematode Echinomermella matsi in Norway, Hagen, Citation1987), or experimental removals were able to establish new kelp beds, at least temporarily (e.g. Novaczek & McLachlan, Citation1986; Scheibling, Citation1986; Johnson & Mann, Citation1988; Keats et al., Citation1990; Scheibling & Raymond, Citation1990; Scheibling et al., Citation1999).
The interaction of abiotic factors and grazing was shown in southern California. Although kelp forests are mainly dominated by other kelp genera (Macrocystis, Pterygophora) in the NE Pacific, Laminaria spp. have also been affected by sea urchin grazing. At Naples Reef (west of Santa Barbara), severe storms were able to shift the community state between barren grounds and kelp forests. Ebeling et al. (Citation1985) showed that a single winter storm destroyed giant kelp forests and thereby deprived Strongylocentrotus spp. of a supply of kelp litter. Consequently, hungry sea urchins, without predator control due to over-fishing, consumed all remaining algae including L. farlowii and created barren grounds (see also Dayton et al., Citation1992). Two years later, another storm removed most sea urchins because of the lack of shelter, and kelp forests, including L. farlowii, became established again within 1 year.
Several examples of trophic cascades of different lengths have been documented, which explain the parallel development of high sea urchin densities and barren grounds (e.g. Sivertsen & Bjoerge, Citation1980; Wharton & Mann, Citation1981; Tegner & Dayton, Citation1991). The studies of Estes et al. (Citation1998, Citation2004) from the NE Pacific revealed possibly the most comprehensive example in this context, linking oceanic and coastal ecosystems. Since the early 1970s, it has become clear that low densities or absence of the over-harvested sea otter (Enhydra lutris) was the main reason for high abundances of sea urchins in Alaska (Estes & Palmisano, Citation1974; Estes et al., Citation1978; Simenstad et al., Citation1978; Duggins, Citation1980; Estes & Duggins, Citation1995). Estes et al. (Citation1998, 2004) provided evidence that, because of overexploitation of fish stocks or predation by killer whales, pinniped numbers were reduced in oceanic ecosystems. The killer whales then attacked sea otters from coastal environments as a substitute for the decreasing pinniped and whale stocks. Consequently, predation pressure on urchins was decreased via a four-level trophic cascade, in which killer whales decreased sea otters, which increased sea urchin density, destroying kelp beds, including several Laminaria species (L. dentigera, L. groenlandica, L. longipes, L. yezoensis, see Estes et al., Citation1978).
In SE Alaska, unusual events, such as a very high supply of diatoms together with masses of washed up pelagic salps, may occasionally redirect sea urchin grazing from macroalgae for enough time to allow the re-establishment of macroalgal communities, including Laminaria (Duggins, Citation1981). Locally, starfish predation may also control some Strongylocentrotus species and thus release macroalgae from grazing pressure (Duggins, Citation1983).
The natural recolonization of barren grounds by Laminaria hyperborea was followed on Norwegian coasts. After removal of sea urchins, a succession of ephemeral algae and dense stands of rapidly growing L. saccharina took place, and the latter was ultimately replaced by the slower growing L. hyperborea. The density of sea urchins determined whether succession remained in the early phase of ephemeral macroalgae or if it continued towards Laminaria growth (Leinaas & Christie, Citation1996). Laboratory incubations of stones from the field revealed that microscopic Laminaria recruits were generally present in barren areas (Sjøtun et al., Citation1995).
Besides natural recolonization, a multitude of management actions have been proposed to overcome the barren ground state. In Japan, the commercial interest is considerable since the fishing and aquaculture industries are dependent on harvests of Laminaria and its consumers (sea urchins, abalone). As management actions here, (i) fencing, (ii) removal of sea urchins and (iii) change of hydrography to enhance water velocity have been proposed (Kawai et al., Citation2003; Kuwahara, Citation2003), and numerical models of interactions between Laminaria and Strongylocentrotus have been developed to enhance the yield of both species (Kawamata, Citation1997; Yoshimori et al., Citation1998). Diets containing Laminaria enhance the gonad quality of sea urchins and thus their marketability (Agatsuma, Citation1998). Sakai (Citation2001) claimed that “intensive grazing of sea urchin on the seaweeds is a good means to remove competitive algae” and, as a result, proposed that the release of sea urchin juveniles as restocking management would enhance both the sea urchin (S. intermedius) and Laminaria fishery. Management action in southern California included release of quicklime to suppress high sea urchin densities. This, together with diseases of sea urchins, led to expansions of kelp forests, in which L. dentigera was dominant at an intermediate successional phase and was finally replaced by Macrocystis pyrifera except in shallow locations (Pearse & Hines, Citation1979). Unfortunately, many studies in Japan have been published only in Japanese and are thus not directly available for large parts of the scientific community.
In summary, decreases in the predators of sea urchins (especially Strongylocentrotus spp.), enhancement of populations of top predators or changes in their feeding behaviour, can result in trophic cascades, leading ultimately to destruction of kelp forests and to the establishment of barren grounds dominated by coralline red algae. These are stable states and need management or catastrophic die-backs of the herbivores to allow re-establishment of kelp forests. Re-establishment of Laminaria beds is often possible from refuges, in which high water velocities preclude effective sea urchin grazing. The loss of kelp biomass has dramatic consequences for total primary production. In St. Margaret's Bay in Nova Scotia, a loss of 60% of the biomass was reported (Chapman, Citation1981). Furthermore a huge array of algae and animals associated with the kelp forest habitat is affected (see Section 13: Laminaria as a habitat for epi- and endobionts).
Mesograzers
Knowledge of trophic interactions between meso-herbivores and Laminaria-species is scarce. This is surprising, given the reported seasonally high abundance of mesograzers such as snails, amphipods, and isopods which use Laminaria as habitat (Toth & Pavia, Citation2002b; see also Section 13: Laminaria as a habitat or epi- and endobionts). The effect of mesograzers on Laminaria populations is usually of only limited temporal and spatial relevance, and it seems to need unusual conditions to be substantial. One example is the study of Chess (Citation1993), who reported that grazing by the amphipod Peramphithoe stypotrupetes in northern California led to total destruction of an entire L. setchellii bed. Mating pairs of adult amphipods and their offspring formed hollow chambers in the stipes, fed on them and grew by boring more holes until the kelp eventually died. The destruction, however, occurred only after calm winters and was promoted by the trochid gastropod Tegula pulligo, which was able to enter the Laminaria blade and by its grazing pre-conditioned the kelp for infestation by the amphipod.
Some mesograzer species, such as the small gastropod Lacuna vincta, may develop in mass on Laminaria. Thus, despite its small size, it may have a significant impact on sporophytes of several Laminaria species (e.g. L. digitata: Brady-Campbell et al., Citation1984; L. hyperborea: Toth & Pavia, Citation2002b; Fredriksen, Citation2003; L. longicruris: Johnson & Mann, Citation1986; L. saccharina: Brady-Campbell et al., Citation1984; R. Karez, pers. obs.). Johnson & Mann (Citation1986) found that Lacuna consumed only 0.05% of total blade biomass of L. longicruris available per season, although grazing was obvious and led to loss of marginal blade parts after storms. Grazing had no negative effect overall on L. longicruris abundance. The authors even proposed that Lacuna enhances survival of Laminaria by reducing blade area and thus the risk of being torn away by storms. This contrasts with earlier findings of Fralick et al. (Citation1974) that Lacuna grazed L. saccharina and L. digitata stipes heavily, leading to extensive destruction of kelp forests. This was accompanied, however, by exceptionally low salinity conditions.
Several laboratory studies indicate tissue-specific consumption patterns. In general, older blade parts are preferred to meristematic tissues, probably due to the relatively high polyphenol content of the latter (Johnson & Mann, Citation1986; Toth & Pavia, Citation2002b). The isopod Idotea granulosa and the gastropods Gibbula cineraria and Lacuna vincta prefer sori of Laminaria digitata to non-reproductive blade and meristematic tissue, reflecting a potential impact of mesograzers on the recruitment success of L. digitata (Enge & Molis, unpublished data).
The type of damage that mesograzers inflict on kelp species can vary from superficial (e.g. Ansates pellucida (formerly Patina pellucida) on Laminaria hyperborea (Toth & Pavia, Citation2002a,b) to complete penetration (e.g. Lacuna vincta on L. digitata sori; Enge, pers. comm.). Superficial attacks may target epiphytic diatoms (Paul et al., Citation1977). Takami & Kawamura (Citation2003) showed that ontogenetic changes in the development of the radula morphology coincide with the dietary changes. Prior to these morphological changes, the diet of juvenile Haliotis discus mainly consists of epiphytic diatoms, while the well-developed radula of adults allows deeper penetration of algal thalli and the consumption of kelp tissue. Diet analysis using stable isotopes revealed that A. pellucida consumes L. hyperborea. In contrast, other small gastropods, such as Gibbula sp. and Calliostoma zizyphinum, which are abundant on Laminaria, belong to the higher trophic levels of detritivores or microphagous carnivores (Fredriksen, Citation2003). For some mesograzers, Laminaria tissue may be of low nutritive value, as suggested by the relatively long retention period of Laminaria pieces in the guts of Haliotis midae (Day & Cook, Citation1995). Superficial grazing may increase light and nutrient availability for kelp tissues but, although such a beneficial role of mesograzers is known from studies with Fucus vesiculosus (Jormalainen et al., Citation2003), experimental evidence is missing for Laminaria species. In other cases, lethal herbivory on freshly recruited whole sporophytes has been reported, e.g. when ‘small herbivorous sea-snails’ fed on small L. japonica sporophytes and thus prevented the development of dense populations (Asano et al., Citation1990). The opposite was reported from Nova Scotia, where dense limpet and chiton populations did not prevent re-establishement of macroalgae, including the dominant kelp L. longicruris (Johnson & Mann, Citation1993). In California, the opisthobranch gastropod Aplysia vaccaria may graze occasionally on blades of L. farlowii and exert a negative effect on its population (Dayton et al., Citation1992). In South Africa, two Patella species depend on kelp including L. pallida: P. granatina feeds on drifting kelp and debris, while P. aregenvillei actively feeds on attached Laminaria (Bustamante et al., Citation1995).
The detrital pathway
Finally, most of the Laminaria biomass degrades and, in this form, becomes an important food source for a multitude of animals. Enormous quantities of blade material may be broken off seasonally by storms and are either washed ashore (e.g. Thornton, Citation2004) or down slopes, where they may form thick layers. The upper parts of these layers are still inhabited by the associated fauna, but bacteria and cyanobacteria dominate the lower parts (e.g. Bedford & Moore, Citation1984; Tzetlin et al., Citation1997). Furthermore, small fragments are broken off from healthy thalli through water motion, or organic material is lost as mucilage and other dissolved organic matter (DOM). It seems likely that L. digitata is partly responsible for formation of transparent exopolymer particles (TEP) from macroalgal detritus (Thornton, Citation2004).
These small fractions are further processed by bacteria (Robinson et al., Citation1982, Rieper-Kirchner, Citation1990; Uchida, Citation1996) and may then be used by suspension feeders (Duggins & Eckmann, Citation1997) or they may be grazed by protozoans (Linley et al., Citation1981; Newell & Lucas, Citation1981; Rogerson, Citation1991) and meiofauna (Rieper-Kirchner, Citation1990), before they enter higher trophic levels such as crustaceans and gastropods (Norderhaug et al., Citation2003) and finally fish and birds (Duggins et al., Citation1989; Fredriksen, Citation2003). However, Duggins & Eckman (Citation1997) suggest also that fresh, unprocessed particulate organic matter (POM) of Laminaria may be used directly due to its relatively low polyphenolic content. However, some ageing appears to enhance the quality of Laminaria POM, before it decreases again (Stuart, Citation1982; Cranford & Grant, Citation1990; Fredriksen, Citation2003; Norderhaug et al., Citation2003). Bacteria may also aggregate on the DOC released by macroalgal fragmentation and, thus, direct dissolved components towards the benthic detrital food chain (see citations in Rieper-Kircher, Citation1990). Even when L. longicruris was consumed by Strongylocentrotus droebachiensis, 67% of the biomass was defecated and entered the detritus pool as POM (Mamelona & Pelletier, Citation2005). Generally, most of the Laminaria production enters detritus pathways (Miller et al., Citation1971; Mann, Citation1977). Webster et al. (Citation1975) found that the major portion was exported to nearby planktonic and benthic communities. For Arctic L. solidungula, Dunton et al. (Citation1982) proposed that more than 90% of the production entered the detrital food chain.
The influence of kelps on suspension feeders may be immense. In the Aleutian Islands, growth rates of suspension feeders were several-fold higher on islands which had kelp beds (Laminaria groenlandica, L. longipes and Alaria fistulosa) than on islands where barren grounds predominated (Duggins et al., Citation1989). Here, the contribution of kelp to higher trophic levels was shown to be considerable by stable carbon isotope analysis. An earlier isotopic study by Dunton and Schell (Citation1987) documented the assimilation of kelp throughout an Arctic food web, and demonstrated the increased incorporation of Laminaria carbon into key crustacean species during the dark winter period when phytoplankton production was minimal.
In a series of papers, Newell and co-workers reported on the detrital pathway of the fragmentation of Laminaria pallida in South Africa. They found that 20–30% of the annual production is lost as DOM (Newell et al., Citation1980; Newell & Lucas, Citation1981). The various components of DOM and POM (Newell et al., Citation1980) showed different rates of decomposition (Lucas et al., Citation1981; Stuart et al., Citation1981), with the primary photosynthate D-mannitol used most rapidly, followed by sugars and by alginates, which constitute 45% of POM DW per unit DW, consumption of DOM permitted a higher bacterial production (42 mg g−1) than POM (16.5 mg g−1; Newell & Lucas, Citation1981), and dissolved compounds were more readily utilizable for bacteria than particulate matter (Stuart et al., Citation1981). Annual conversion from kelp production to bacterial biomass had an efficiency of 14% (for details see Newell & Lucas, Citation1981). Bacteria were grazed by protozoans (Linley et al., Citation1981; Newell & Lucas, Citation1981; Stuart et al., Citation1981). Water analysis from the vicinity of a mussel bed of Aulacomya ater showed that, in the natural environment, only 15% of POM was phytoplankton, and 85% was detritus particles. In laboratory feeding studies with L. pallida debris (Stuart, Citation1982), A. ater grew better if fed with L. pallida detritus than with Dunalliella promolecta cells. Populations of the clam Donax serra on the same coast may also live mainly on kelp-derived detritus (including L. pallida) rather than on phytoplankton. Kelp detritus in general may be the more important energy source for coastal benthic systems in upwelling regions (Soares et al., Citation1997, and references therein). On the other hand, Cranford & Grant (Citation1990) reported that phytoplankton rather than L. longicruris detritus was the most important food source for the sea scallop Placopecten magellanicus in Nova Scotia.
In laboratory studies with Laminaria longicruris detritus, 54% of detrital carbon leached out as DOC in initial washing, while a further 20–25% was converted to bacterial biomass with an efficiency of 21–43% (Robinson et al., Citation1982). The remaining 20–25% of the material had a surprisingly low C:N ratio, but was relatively refractory and broken down only slowly. The authors proposed that this was due to the high polyphenol content of the remaining matter. Contrary to expectations based on aquatic plants with high structural tissue content, such as seagrasses, consumption by macrofauna inhibited rather than accelerated the decomposition of subtidal accumulations of L. saccharina debris in Scotland because microbes at the margins of decaying Laminaria particles were consumed selectively, and this delayed decomposition in comparison to removal of macrofauna (Bedford & Moore, Citation1984). The detrital food chain is mimicked in aquaculture, as ground-up material of Laminaria together with accumulated bacteria may be used as a food source for suspension feeders (Uchida et al., Citation1997; Camacho et al., Citation2004).
Conclusion
Kelp beds (reviewed here: Laminaria) constitute enormous energy sources for coastal benthic secondary production. Their importance often exceeds that of phytoplankton for many suspension feeders. The destruction of kelp beds by the sea urchin genus Strongylocentrotus has been documented at many sites along the distribution range of Laminaria. Future research should shift its focus to the role of mesograzers, which are often abundant on Laminaria spp. but whose effects are still largely unknown. For a complete assessment of grazing effects on kelp population dynamics, grazing impacts on all life history stages, including meiospores and gametophytes, should be included, which will require more sophisticated experimental techniques in the field.
15. Competition
Interspecific competition may be divided into ‘resource competition’ and ‘interference competition’. Resource competition between photosynthetic organisms is considered to occur mainly for space, light and nutrients, while interference competition addresses more direct interactions, such as effects of allelochemicals and whiplashing on other species. Equilibrium models regard the spatial organization of species to be a product of their intrinsic relative competitive abilities at a certain set of environmental conditions, while the dynamic views include the destruction of competitively dominant species by disturbance. Disturbance may lead to a succession of communities in which Laminaria spp. may represent either transitional or final stages. The equilibrium view is often applied, when species zonation along the depth gradient is discussed. There exist two general models: (i) In the first model, species are considered to occupy the range in which they are physiologically most competent. It is hypothesized that at zone boundaries, the competitive dominance together with the physiological competence switches from one species to the next (see e.g. Chapman, Citation1973b, 1974b) so that fundamental and realized niches show more or less the same pattern. Fundamental niches are those niches without the influence of other species and realized niches those with the influence of other species (sensu Hutchinson, Citation1957). (ii) The second model was described by Keddy (Citation1989) as a ‘Competitive hierarchy model’ and was further developed for seaweeds by Chapman (e.g. Chapman, Citation1990; Karez & Chapman, Citation1998). Here, all species compete for the more favourable end of the gradient, but are successively excluded by more dominant species towards less benign ranges of the gradient. This means that species have a trade-off in competitive ability and tolerance to unfavourable conditions. Fundamental niches of inferior species include those of competitively superior species (see Karez & Chapman, Citation1998, for details). These authors proposed a ‘relaxed’ version of Keddy's model to be more realistic. Here, the intrinsic dominant species together with its physiological competence increasingly loses its dominance at the border of its fundamental niche.
For Laminaria, resource competition seems to be mainly for light (see also Section 5: Growth and photosynthetic performance of sporophytes). Often those kelp species with maximum final lengths eventually dominate the community. This was described in a study by Dayton et al. (Citation1984) where upper canopy layer species invaded stands of smaller species but not vice versa. In a Californian kelp forest, the long Macrocystis plants finally remained and Laminaria was only a transient successional stage unless there was sufficient disturbance to the surface canopy (Dayton et al., Citation1984, 1992; Tegner et al., Citation1997). In other areas, such as the NW Atlantic (e.g. Scheibling, Citation1986; Chapman & Johnson, Citation1990; but see Himmelman et al., Citation1983) and the NE Atlantic (Markham & Munda, Citation1980; Leinaas & Christie, Citation1996), Laminaria species constitute the largest and thus final successional stage. On the east coast of Nova Scotia, L. longicruris is superior to L. digitata except in extremely exposed shallow sites (Johnson & Mann, Citation1988) but, on the southwestern coast, L. digitata is reported to be superior to L. longicruris (Smith, Citation1986). In SE Alaska, Laminaria species dominate the kelp communities; here, L. groenlandica dominates over annual kelp species in the second year of succession (e.g. Duggins, Citation1980). In some situations, Laminaria species finally outgrow other kelp species by higher longevity. For example, L. digitata with higher maximum life expectancy finally replaces L. longicruris (Smith, Citation1985). Lapointe et al. (Citation1981) interpreted Norton & Burrows’ (1969) data as indicating that longer living L. hyperborea and L. saccharina out-compete Saccorhiza by being perennial.
Laminaria species often form distinct zones along a depth gradient. The underlying competitive interactions have mainly been revealed by removal experiments with release from competition by removing one of the zone builders and surveying if adjacent species invaded the empty space. In Britain, L. digitata was able to extend its upward range, when Fucus serratus was removed, but showed only low growth and survival. In contrast, F. serratus was clearly able to invade the lower zone, if L. digitata was removed (Hawkins & Harkin, Citation1985; Hawkins & Hartnoll, Citation1985) showing that the competitive strength depends on the environment and supporting the relaxed model of Keddy proposed by Karez & Chapman (Citation1998). L. saccharina was displaced by L. digitata through competition on moderately exposed shores and L. hyperborea out-competed Alaria, Saccorhiza and Desmarestia (Hawkins & Harkin, Citation1985). Exclusion of Saccorhiza may depend on water current velocity (Kitching & Thain, Citation1983). By transplant experiments, John (Citation1970) showed that L. digitata grew successfully at greater depths than normal where it is probably outcompeted by L. hyperborea. In SW Nova Scotia, the upper limit of L. longicruris is set by superior red algae such as Chondrus crispus (Johnson & Mann, Citation1988).
In several sites, only one Laminaria species remains dominant at greater depths even if shallow water areas contain stands of several kelp species (e.g. L. pallida replaces Ecklonia maxima in South Africa: Velimirov & Griffiths, Citation1979; L. solidungula replaces Agarum cribrosum at 30 m in Newfoundland: Whittick et al., Citation1982; L. hyperborea replaces L. digitata and L. saccharina in the North Sea: Lüning, Citation1970). Other studies report an increase in the relative competitive dominance of Laminaria at greater depths (Santos, Citation1993; Tegner et al., Citation1997; Dayton et al., Citation1999). In Alaska, however, Laminaria spp. excluded Agarum cribrosum from areas shallower than 6 m, but encountered suboptimal conditions due to low light or grazing further down. Here, with less intense Laminaria spp. competition, Agarum becomes more abundant (Estes et al., Citation1978; see also Estes & Steinberg, Citation1988). In addition to competition and light availability, grazing can limit the downward extension of Laminaria spp. (Witman, Citation1987; Estes & Steinberg, Citation1988).
Interspecific competition does not only take place among different kelp species. Recently Laminaria beds in Nova Scotia and Maine are threatened by the introduction of new competitors - the green macroalga Codium fragile ssp. tomentosoides and the European bryozoan Membranipora membranacea (Harris & Tyrrell, Citation2001). Codium is not able to invade intact Laminaria beds. However, after disturbances such as sea urchin grazing, this species persistently dominates local algal assemblages of shallow subtidal and intertidal habitats. Once established, it inhibits recruitment of kelps by pre-emption of space (Levin et al., Citation2002). Furthermore, it is less preferred by herbivorous sea urchins than Laminaria (Scheibling & Anthony, Citation2001; Sumi & Scheibling, Citation2005). The bryozoan Membranipora covers large areas of the Laminaria blade and thereby drastically reduces spore liberation of L. longicruris and thus its recolonization potential (Saier & Chapman, Citation2004). In addition, Membranipora makes the blade brittle and may lead to the destruction of whole kelp beds during storms (Scheibling et al., Citation1999; Levin et al., Citation2002). The negative influence of invasive species was also shown elsewhere. In the Limfjord (Denmark), L. saccharina populations declined significantly during the establishment of the invasive Sargassum muticum (Staer et al., Citation2000). In Washington State, L. groenlandica was less abundant when S. muticum was present (Britton-Simmons, Citation2004). Other Laminaria competitors include Desmarestia ligulata. This species inhibited the re-establishment of kelps including L. farlowii for two years after kelp destruction by storms (Dayton et al., Citation1992). There are also indications that Laminaria is dominant in resource competition for nutrients due to its ability to store nitrogen (Johnson & Mann, Citation1988, and citations therein).
Inside Laminaria populations, there is also intraspecific competition. As in higher plant communities, there are biomass–density (B–D) effects, which lead to skewed size class distributions and reductions in individual biomass. Finally, when mortality is driven by competitive suppression, self-thinning may take place. However, although a lot of studies give biomass and density data for natural Laminaria stands (see Section 3: Demography of Laminaria Communities, ), there have been few explicit studies of B–D relations and even less of the dynamics of self-thinning. Only Creed et al. (Citation1998) showed these mechanisms for L. digitata and Kang & Koh (Citation1999) for L. japonica. The effects of density on the biomass of stands and survival along a depth gradient are generally unexplored.
Interference competition is known from coralline red algal allelochemicals that may inhibit recruitment of Laminaria religiosa and L. japonica on barren grounds (Japan: Suzuki et al., Citation1998; Nova Scotia: Denboh et al., Citation1997). Coralline red algae also seem able to inhibit Laminaria recruitment on their surfaces by shedding their epidermal cells (Masaki et al., Citation1981, 1984). Both phenomena further stabilize sea urchin dominated barren grounds (see Section 14: Trophic interactions). Dayton et al. (Citation1984) proposed a whiplash effect of Laminaria and Cystoseira as a reason for the inhibition of Macrocystis recruitment.
Conclusion
There have been several studies considering the competition of Laminaria spp. with other seaweeds. However, statements have often been derived from observations rather than from experimental evidence. Systematic research exploring the competitive abilities of certain Laminaria species and their putative competitors under a full range of environmental conditions (e.g. over the full depth gradient) is largely lacking. As depth gradient studies may include conditions ranging from high light stress through optimal irradiation conditions to low light stress, control of all factors is extremely difficult. Nevertheless, the fundamental niches of all species should be explored by transplant experiments and their competitive abilities analysed with experiments as described, for example, by Underwood (Citation1986). This would enable us to verify the models discussed in community ecology and the mechanisms affecting the zonation of subtidal seaweeds.
16. Recent developments in aquaculture: Resources and uses
Seaweed aquaculture has enjoyed an unprecedented rate of development during the last two decades, and constituted 93.25% of the worldwide commercial harvest of seaweeds in 2004, representing a value of US $6.8 billion (Chopin et al., Citation1998; FAO, Citation2007). The utilization of algal products plays an important role in many fields of modern everyday life. Algae, and in particular the Laminariales, have a wide variety of uses in human and animal consumption, in industrial products and in bioremediation.
Production from fisheries and aquaculture
The various existing publications and databases on seaweed production, as well as the statistical records of both fisheries and aquaculture collected by different authorities, are locked in personal files or obscure governmental publications (Critchley & Ohno, Citation1998), and are therefore not easily accessible. The prospective user of these data must undertake a time-consuming search for records. Sometimes access to data is expensive and they may not even be calibrated (McHugh, Citation1991). Compilation of these diverse statistics leads to incomplete or inconsistent statements on the production outputs. Several attempts have been made by various authors and organizations to throw light on the available datasets. In this review, we have mainly used data originating from the Food and Agriculture Organization (FAO) of the United Nations containing data up to 2005, but also from publications and reviews by McHugh (Citation1991, 2003), Critchley & Ohno (Citation1998), Zemke-White & Ohno (Citation1999), Wikfors & Ohno (Citation2001), Lüning & Pang (Citation2003) and Feng et al. (Citation2004). In spite of their shortcomings, these data have been useful in guiding commercial interests and giving insight into the development of seaweed cultivation (McHugh, Citation1991).
In 2004, 8 million tonnes of wet seaweed, either harvested from wild resources or farmed, went into industrial use. Seaweed farming has expanded rapidly because the demand long ago outstripped the available supply from natural resources (FAO, Citation2006). Commercial harvesting is recorded in 35 countries from both hemispheres, and in waters ranging from cold through temperate to tropical conditions.
Harvesting of natural stocks of aquatic plants including brown seaweeds is subsumed under the term ‘fisheries’. For the year 2005, this is mainly reported for Norway with a production of approx. 154,000 tonnes (including Laminaria hyperborea), France with approx. 75,000 tonnes (mainly L. digitata), and Japan with approx. 78,575 tonnes (L. japonica; FAO, Citation2007). The cultivation of algae represents 23% of global aquaculture production by weight and almost 10% by value (Lowther, Citation2006). Nearly all (99.8%) cultured aquatic plants (approx. 14 million tonnes, worth US $6.8 billion) come from Asia and the Pacific region (FAO, Citation2006). Even though the emphasis in aquaculture is on protein-rich species of finfish, molluscs and crustaceans, the species with the highest annual production was L. japonica (‘kombu’); 4.5 million tonnes of kombu were produced in 2004, mainly grown in China, where the species is not native but was introduced in 1927 (Tseng, Citation1987; Lowther, Citation2006).
In the past 30 years, several investigations have explored the utilization of Laminaria resources. While uses of laminarian seaweeds in Western countries have been mainly based on exploiting natural beds, Asian countries have been cultivating Laminaria species since the early 1950s. In China, the breakthrough came in 1952 when artificial substrata were introduced and improved cultivation techniques allowed the production of summer seedlings (Tseng, Citation1987), thus leading to a quick development of this aquaculture sector (Tseng, Citation1958, 1962, 1984). North Korea is the second largest producer of L. japonica in the world (Zemke-White & Ohno, Citation1999; FAO, Citation2007). In Japan, the demand from domestic consumers was met by increasing the harvest of naturally growing L. japonica. Cultivation has been mainly impeded by the fact that Laminaria sporophytes took as long as 2 years to grow into a desirable market product for Japanese consumers (Chen, Citation2006). Nowadays, L. japonica is mainly cultivated in China (4 million tonnes, ≈US $2.4 billion), in North Korea (0.44 million tonnes, ≈US $0.24 billion), and Japan (0.05 million tonnes, ≈US $0.1 billion; FAO, Citation2007). In the late 1980s, when better culture techniques for shrimps were developed, the area for Laminaria mariculture and total yield of Laminaria declined temporarily, because most seaweed farmers switched to the more lucrative but risky farming of shrimps (McHugh, Citation2003; Feng et al., Citation2004). In addition to the Asian countries mentioned above, the far east of Russia is a recent producer of L. japonica. For 2004, the average Russian production was estimated to be about 0.01 million tonnes wet weight (Chen, Citation2006). Other species of Laminaria, such as L. saccharina (Kain, Citation1991; CRM, Citation2001; Buck & Buchholz, Citation2004), L. digitata (Perez et al., Citation1992) or L. longicruris (Chapman, Citation1987), have been cultivated in Europe and North America, but at a scale almost not worth mentioning in comparison with Japanese kelp in Asia. However, a longline cultivation system for kelp has been developed in British Columbia, Canada, whose production is equivalent to that reported elsewhere (3–20 kg wet weight kelp per meter farm rope; Druehl et al., Citation1988b). At present, there are four 1-acre farms (1 acre ≈ 0.4 ha, 64 × 64 m) in British Columbia, cultivating L. groenlandica and L. saccharina for sea urchin feed, sea vegetables and raw materials for cosmetics (Druehl, pers. comm.). Moreover, since 2002, roughly one tonne per year of L. saccharina has been farmed using an extensive fixed off-bottom longline technique in the Baltic Sea (CRM, Citation2001) and in land-based cultivation tanks on the North Sea island of Sylt in Germany (Sylter Algenfarm, Citation2006).
Cultivation techniques and system designs
Worldwide, there are quite a number of technical variations in cultivating seaweeds, even if considering only Laminaria. Cultivation methods comprise single species cultures and co-cultures. Basically, Laminaria meiospores are ‘seeded’ on ropes, which are subsequently fixed to various suspended or floating culture devices. Critchley et al. (Citation2006) attribute the success of Laminaria cultivation to the scientific control of growth and maturation of the plant throughout its entire life cycle (see ) as well as to the efficiency of production systems. Currently, cultivation is successfully accelerated by the so-called ‘forced-cultivation’ technique (Hasegawa, Citation1971; Ohno, Citation1993; Critchley & Ohno, Citation1997). This technique is labour and cost-intensive (McHugh, Citation2003; Chen, Citation2006) as the development of zygotes and the attachment of young sporophytes on ropes require land-based indoor tank facilities. According to Tseng (Citation1987), the cultivation procedure for L. japonica can be divided into two steps: (i) in the ‘seedling phase’, meiospores are artificially released from mature sporophytes and seeded onto a substrate (ropes fixed to plastic frames), where they germinate into gametophytes, reach sexual maturity, develop zygotes and finally form juvenile sporophytes; (ii) in the ‘grow-out phase’, culture ropes with juvenile sporophytes are transferred to the open sea where they grow in one season to a frond length of 1.0–2.5 m, depending on the species. This ‘forced cultivation’ method shortens the cultivation period from 2 years to 12 months.
Since Buchholz & Lüning (Citation1999) discovered that separating Laminaria blade portions from the meristem induces them to become sporogenous far ahead of their natural reproductive season, mature sporophytes may be available all year round providing independence of naturally available seedstock (see also Lüning et al., Citation2000; Pang & Lüning, Citation2004). This method of ‘meristem-free fronds’ has so far been applied to several Laminaria species (see Section 5: Sporogenesis and meiospore release).
Ohno (Citation1993) described two further methods besides ‘forced cultivation’. One is the conventional ‘2-year cultivation’ procedure including biannual plants, which are left at sea for 18 months. This method takes more than 20 months from seeding, thereby increasing the price for the product. The other method is called ‘cultivation by transplanting’, which uses natural Laminaria sporophytes either washed ashore or manually thinned out. As the activity of the meristem increases in late winter to early spring (see Section 4: Growth and photosynthetic performance of sporophytes), new haptera are easily formed and allow a new attachment on ropes during this time. The time from transplantation to harvest is 12–18 months.
The commonest system for grow-out of Laminariales at sea is a longline or raft construction, first used in China in 1952 (Tseng, Citation1984, 1987). Longline cultivation involves a system of horizontal ropes with anchoring weights to stabilize the entire system and with buoys to provide flotation. The seeded ropes either hang vertically from the longline (‘vertical hanging method’) or are horizontally attached between several longlines (‘longline method’) to allow better light harvest (e.g. Holt & Kain, Citation1983; Kawashima, Citation1984; Kain & Dawes, Citation1987; Kain, Citation1991; Critchley & Ohno, Citation1997). The ‘mixed culture method’ combines both systems, thereby overcoming the disadvantages of each (Chen, Citation2006); the ropes are first hung vertically and then suspended between two supporting ropes into a horizontal position. The mixed method has now been widely adopted by Laminaria sea farming enterprises in China. A third method, but rarely used today, is the ‘Dragon line raft’ (Jia & Chen, Citation2001), which is well suited for turbid inshore or deep open waters with strong currents. The entire system with the seeded ropes is submersed to a depth of about 1.5 m. The system dimensions are specific to site conditions. L. saccharina was grown on ropes off the Isle of Man in the Irish Sea in the 1980s (Holt & Kain, Citation1983; Holt, Citation1984; Kain & Dawes, Citation1987; Kain, Citation1991) eventually using various longlines in parallel to form a large grid of 250 × 250 m. Seeded strings were tied to adjacent longline ropes parallel to the water surface thus ensuring optimal light harvest and spacing for increased production per unit area (Dawes, Citation1988; Kain, Citation1991).
A convenient technique is the land-based tank cultivation of free-floating Laminaria sporophytes agitated by air (Lüning & Pang, Citation2003). The tanks allow cultivation at a density of about 10 kg m−2. Circulation of the water body is driven by aeration, which brings all plants to the water surface at intervals to allow photosynthesis. In addition, the relatively high density of thalli prevents the fronds from being over-grown by epiphytes, such as was seen with some tank-grown red algae, because the light penetration to the bottom of the tank is almost zero (Ryther et al., Citation1979; Bidwell et al., Citation1985). Further reduction of epiphytes can be induced by continuous short day treatment. L. digitata cultivated in outdoor tanks with automatic blinds limiting daylength to 8 h of light per day in summer maintained high growth activity throughout the summer months (Gómez & Lüning, Citation2001). Continuous growth activity also seemed to prevent the fronds from being settled by epiphytes.
Since there is a worldwide interest in moving aquaculture activities offshore, various technical structures have been suggested (Polk, Citation1996; Hesley, Citation1997; Stickney, Citation1998; Bridger & Costa-Pierce, Citation2003). The major difficulties in the development of suitable techniques for open-ocean aquaculture are the harsh environmental conditions, which place an enormous stress on materials and algae (Buck, Citation2004). Longlines installed in exposed environments did not survive offshore conditions. The-state-of-the-art is an offshore construction developed by Buck & Buchholz (Citation2004). A ring design, which moved below the surface due to shearing forces, withstood strong currents and wind-generated waves ().
Fig. 2. Preparation of Laminaria harvest from a ring-system after growth in the sea near Helgoland (Germany; North Sea). The ring was lifted from the water by a land-based crane (from Buck & Buchholz, Citation2004 with kind permission of Springer Science and Business Media; original figure in colour).
In order to examine the biological and technical potential of offshore kelp cultures, the physical forces experienced by the attached algae were studied in more detail. The degree of exposure influenced the shape of the algae and their resistance to environmental forcing. Gerard (Citation1987), Koehl & Alberte (Citation1988) and Johnson & Koehl (Citation1994) demonstrated that seaweeds grown at exposed sites show morphological differences to those originating from sheltered conditions. While the latter had wider blades with thick undulated margins, offshore sporophytes were thin and streamlined. Buck & Buchholz (Citation2005) showed that Laminaria saccharina sporophytes pre-cultivated onshore but transferred to the sea at very early stages developed a streamlined blade and resisted current velocities up to 2.5 m s−1. One of the interesting prospects of Laminaria aquaculture is the combination with offshore wind farms, since these would provide stable fixed structures for the cultivation systems (e.g. Buck, Citation2002; Krause et al., Citation2003; Buck et al., Citation2004). In this context, technical investigations should include culture constructions designed to hold the algae and also to anchor the structure for supporting seaweed cultivation to the offshore wind generator (Buck et al., Citation2006). So far, the high cost of infrastructure for offshore aquaculture systems is one of the major drawbacks for their development (Buck, Citation2004).
Utilization of Laminaria species
The seaweed industry provides a wide variety of products with an estimated value of US $5.5–6.0 billion in 2004 (FAO, Citation2004). Food products for human consumption contribute about US $5 billion. Extracted substances from seaweeds, such as hydrocolloids, account for a large part of the remainder, while smaller, miscellaneous uses, such as fertilizers and animal feed additives, make up the rest. The most important and traditional sectors of Laminaria utilization comprise food and alginate production but, during the past three decades, several new applications have emerged. Compounds derived from Laminaria are found in cosmetics, health food, drugs and fertilizers. Moreover, Laminaria can be used for bioremediation to abate pollution, eutrophication, global warming and coastal erosion, as a bioreactor in molecular biotechnology or as an alternative to fossil fuel. In the following, the different uses and their development are described in more detail:
Food products
Food made out of seaweeds has a long tradition in Asia and can be traced back to the 4th century in Japan and the 6th century in China (Tseng, Citation1987; McHugh, Citation2003). During the past decades, cultivation of seaweeds for food purposes has increased in South America and in Africa (Critchley et al., Citation1991; Zemke-White & Ohno, Citation1999; Wikfors & Ohno, Citation2001; McHugh, Citation2002). Known in Japan as Kombu (Laminaria sp.) or Wakame (Undaria pinnatifida), the kelps are processed into dried sheets, flakes or powder. These are used as additives to various meals, such as salads, soups or confectioneries, or as an essence to make up beverages (Brault & Briand, Citation1987; McHugh, Citation1991; Ohno, Citation1993; McHugh, Citation2003; FAO, Citation2004). The worldwide production of L. japonica, economically the most important edible seaweed, increased from 0.8 million tonnes in 1976 to 4.5 million tonnes in 2004 (FAO, Citation2007). Most of the harvest of L. angustata, L. coriacea, L. japonica, L. longissima, and L. ochotensis enters the marketing chain as dried ‘Suboshi Kombu’ (McHugh, Citation2003). While people from China, Japan and North Korea prefer the genus Laminaria as part of their cuisine, Undaria is preferred by South Koreans (McHugh, Citation1991). In Alaska and Canada, L. groenlandica and L. saccharina are used to produce ‘roe on kelp’, a dried raw kelp coated with accumulations of herring roe (Zemke-White & Ohno, Citation1999).
Animal feed and fertilizer
While sheep or cattle have been traditionally grazed on alluvial seaweeds washed onto their pastures, modern animal fodder may include seaweed powder (McHugh, Citation2003). Although Laminaria species contain only about 10% protein and 2% fat, adding seaweed to animal feeds or human food may generally improve the nutrition of mammals because of the useful amounts of iodine, minerals, trace elements, and vitamins (Fleurence, Citation1999; He et al., Citation2002; Rupérez, Citation2002; McHugh, Citation2003; Schmid et al., Citation2003). Among seven seaweeds investigated, Laminaria had the highest content of iodine with 734 mg kg−1 wet weight, 99.2% of the iodine being water soluble (Hou et al., Citation1997). It has long been known that iodine deficiency in humans leads to serious health disorders (see e.g. Delange, Citation1994) and the 400 million people in China alone, who live in areas deficient in iodine, require appropriate iodine-enriched food (Chen, Citation2006). Investigations of livestock breeding with a Laminaria-enhanced diet revealed that pigs fed with L. digitata showed a significantly increased iodine content in adipose tissue, heart, liver and kidneys in addition to beneficial titres of thyroid hormones and a daily 10% increase in body weight (He et al., Citation2002; Schmid et al., Citation2003). Similar results were found when the feed of freshwater fish (Salvelinus sp.) was supplemented with L. digitata (Schmid et al., Citation2003). Their iodine content, especially in the skin, was significantly higher and comparable to that of marine fish. A bioavailability experiment conducted in the same study further proved that the iodine transfer from seaweed to man via fish can be successful. Laminaria seems to be a more effective source of iodine than inorganic salts, because the element is contained in different water-soluble forms, or even as iodo-aminoacids in L. japonica (Hou et al., Citation1997). Rupérez (Citation2002) demonstrated that L. digitata contains considerably more minerals and trace elements, especially potassium (0.116 mg g−1 DW) and calcium (10.05 mg g−1 DW), than most edible land plants. Compared with iodine and minerals, the protein content of L. digitata is rather low (8–15% of DW) and thus less interesting for animal or human diet complementation (Fleurence, Citation1999). In summary, Laminaria species have the potential to become an important component within the world food industry, either as feed-supplement for livestock or as an additive in health food products for direct human consumption. Another traditional application of Laminaria in Europe has been as fertilizers in agriculture, especially in France and Iceland (Kain & Dawes, Citation1987; Blunden, Citation1991; McHugh, Citation2003), but other brown seaweeds are more widely used in horticulture (McHugh, Citation2003).
Alginates and new compounds
The best known seaweed products, apart from traditional foods, are the three classes of hydrocolloids–alginates, agar and carrageenan–which achieved commercial importance because of their physical features as emulsifying, gelling or water retention agents (Indergaard & Østgaard, Citation1991). Of these, only alginates are present in the Phaeophyceae. They are located in the cell walls of Laminariales. The alginate industry became important during the late 1930s (Percival & McDowell, Citation1967; Kain & Dawes, Citation1987). The spectrum of alginate applications is large: they are used in the food industry as emulsifiers and suspension agents in salad dressings, as stabilizers in ice creams or thickener for sauces and syrups, and in brewing to achieve a creamy long-lasting froth on keg beer. They also serve as thickening agents for printing dye and for improving material adsorptivity in textiles, in commercial chemical synthesis for encapsulating biocatalysts, in pharmaceuticals as a smoothing agent in ointments or for regulating the humidity of wound dressings (Thomas, Citation2000a–c), in dietary food and capsules, in creams, lotions or shampoos and facial masks for cosmetic purposes, not to forget the complete algal body-wrap for overall beauty (e.g. De Roeck-Holtzhauer, Citation1991; McHugh, Citation2003). The worldwide alginate industry relies on temperate species, which contain better alginates, and largely on the harvest of wild stocks, since the labour intensive cultivation procedure is too expensive, even if conducted in low cost countries. Only the surplus production of Chinese Laminaria japonica farms goes into alginate extraction (Wikfors & Ohno, Citation2001; McHugh, Citation2003). Alginate production reached a value of approximately US $213 million in 2003 (McHugh, Citation2003; FAO, Citation2004), the hydrocolloid being mainly extracted from L. japonica (China), L. digitata (France, Iceland), L. hyperborea (France, Ireland, Norway, Scotland) and L. schinzii (Namibia, South Africa; Critchley et al., Citation1991; McHugh, Citation1991; Zemke-White & Ohno, Citation1999; McHugh, Citation2003).
Besides hydrocolloids and fermentation products, which are used as ingredients of cosmetics and as health drinks, seaweeds are potential suppliers of new compounds: antiviral, antibiotic, antitumour, anti-cancer or anti-inflammatory substances are of interest for medical purposes and as new drugs (e.g. Teas, Citation1983; Stein & Borden, Citation1984; Mayer & Hamann, Citation2002, 2005; Smit, Citation2004). Natural pesticides, antifouling and agrochemical compounds are attracting interest for agrochemistry (Smit, Citation2004). In this context, molecular biotechnology of marine algae has developed as a new branch in science, aiming to improve the quality and features of cultivated algae. Genetic investigations of Laminaria japonica have been carried out in China since the 1960s (Tseng, Citation1987), and have recently led to research in the genetic transformation of macroalgae into marine bioreactors. Qin et al. (Citation2004, 2005) established a genetic transformation model system for L. japonica based on modulating the seaweed life cycle and using the technology applied in land plant transformation. Transgenic kelp is a potential candidate for producing high value products such as oral vaccines or drugs at low costs (Qin et al., Citation2004, 2005).
Energy crop
Another important feature of seaweeds is their high carbohydrate content, which makes them of interest as an energy crop. The main carbohydrates are laminaran and mannitol, which are suitable for anaerobic digestion (fermentation). In the mid 1970s, Troiano et al. (Citation1976) observed that anaerobic digestion of Laminaria saccharina results in methane. Further experiments showed that fermentation of brown algae, especially L. saccharina and L. hyperborea, can provide useful end-products, such as biogas (methane) or binding materials in peat products (Hanssen et al., Citation1987; Østgaard et al., Citation1993). Even though laminaran and mannitol are the main reagents for the fermentation process, the total methane yield depends on the accumulated carbohydrate content of raw materials, including alginates (Østgaard et al., Citation1993). Horn et al. (Citation2000) used a different approach showing that L. hyperborea extracts can also be fermented to ethanol.
The fermentation products described are attractive as potential contributors to alleviating global energy problems and current concerns about CO2 emissions to the atmosphere. Alternative energy supplies become increasingly important. Marine macroalgae are potential candidates as sources for methane, methanol or ethanol. If macroalgae were used as alternatives to fossil fuel, they would be carbon-neutral since they would have bound CO2 while growing and would not add to the CO2-concentration of the atmosphere. Global warming and other environmental impacts might, therefore, be abated (Jensen, Citation1993; Gao & McKinley, Citation1994; Chynoweth et al., Citation2001; McHugh, Citation2003; Muraoka, Citation2004). This approach is supported by the high productivity of seaweeds (max. 1.8 kg C m−2 yr−1), which is comparable to that of dense terrestrial forests and up to 10-times higher than phytoplankton production (Jensen, Citation1993; Chynoweth et al., Citation2001; Lüning & Pang, Citation2003). Gao & McKinley (Citation1994) compared the productivity of macroalgae to that of sugarcane, which is regarded as the top cultivated energy crop. Their calculations indicate that naturally grown seaweeds are 2.8-times as productive as sugarcane, if the maximum values of both crops are compared. The projected productivity of cultivated Laminaria japonica was even 6.5-times higher than the maximum projected yield for sugarcane (Gao & McKinley, Citation1994). Thus, along with other macroalgae, Laminaria species can be considered as new high-energy crops, which additionally do not compete for farmland with terrestrial crops.
Nutrient and heavy metal uptake systems
Large scale Laminaria cultivation is thought to offer further advantages for the environment. Apart from their CO2 consumption, kelps are also able to absorb large amounts of combined nitrogen and phosphate, thus helping to abate coastal eutrophication (McHugh, Citation2003; Lüning & Pang, Citation2003; Fei, Citation2004). This feature of Laminaria and other seaweeds qualifies them for use in sustainable mariculture. ‘Integrated Farming Systems’ or ‘polycultures’ have a long history in Asia, where various systems such as grass-fish, rice-fish or animal-fish systems have been developed (FAO, ICLARM & IIRR, 2001). From these traditional setups, modern integrated aquaculture systems have been developed and are constantly being improved. The combination of seaweed culture with land-based fish culture or open marine cage culture has found great acceptance (Subandar et al., Citation1993; Petrell & Alie, Citation1996; Ahn et al., Citation1998; Troell et al., Citation1999; Chopin et al., Citation2001; Neori et al., Citation2004). Macroalgae, including Laminaria, act as biofilters for the aquaculture of finfish, removing part of their egesta and surplus nutrients and providing extra oxygen and biomass. There are also publications describing the ability of Laminaria biomass to adsorb heavy metals such as Cd, Cu or Zn and, based on laboratory-scale trials, to be used for wastewater treatment (e.g. Sandau et al., Citation1996; Figueira et al., Citation2000a,b; Nigro et al., Citation2002), but there seems to have been no implementation on a large scale (McHugh, Citation2003). Even a potentially economic method using waste products from the production of liquid fertilizer from Ecklonia maxima (Kelpak waste, Stirk & van Staden, Citation2000) has not yet been adopted at the industrial level.
Coastal protection
The effect of kelp harvesting on coastal erosion has been examined in a few studies. Sivertsen (Citation1985) and Berg & Munkejord (Citation1991) investigated the influence of Laminaria hyperborea harvesting on dune erosion along the Jæren coast of Norway, but neither of these studies addressed the physical oceanography of wave damping by kelp forests nor the effects of unusually high waves on dune erosion. Early investigations by Price et al. (Citation1968) with artificial seaweed showed that macroalgae can assist in the build-up of beaches by promoting an onshore transport of material. Beavis & Charlier (Citation1987) suggested large-scale experiments on Belgian coasts to prevent sand erosion. The actual effect of L. hyperborea upon wave motion has been demonstrated in a 1:10 laboratory model showing that kelp vegetation has a substantial impact on wave damping, an important prerequisite for shoreline protection (Dubi & Tørum, Citation1995; Løvås & Tørum, Citation2001).
Conclusion
A well-founded knowledge of the physiological characteristics underlying the response of Laminaria to the various environmental factors is an indispensable basis for successful large-scale aquaculture. The present economic importance of Laminaria crops in Asian countries may find a growing counterpart in European countries provided that algal farms can be established in the proposed offshore wind farms. The multitude of current and potential uses of Laminaria will surely stimulate a progression towards an intensified aquaculture.
Acknowledgements
Many thanks for helpful advice and valuable discussions go to C.E. Lane, L.D. Druehl and N. Yotsukura (recent species concept), to J. Imhoff (epiphytic microorganisms) and to A.R.O. Chapman (trophic interactions and competition). Special thanks go to M.J. Dring and the reviewers for considerable work with editing and reviewing this long manuscript.
Notes
Notes
The sections were authored as follows: Introduction. I. Bartsch, C. Wiencke & K. Valentin; Section 1. Recent developments in taxonomy and phylogeny. K. Valentin & I. Bartsch; Section 2. Morphotypes, ecotypes and population dynamics. H. Schubert & P. Feuerpfeil; Section 3. Demography of Laminaria communities. H. Schubert & P. Feuerpfeil; Section 4. Growth and photosynthetic performance of sporophytes. D. Hanelt, C. Wiencke, K. Bischof & I. Bartsch; Section 5. Sporogenesis and meiospore release. I. Bartsch; Section 6. Biology of microstages: Meiospores, gametophytes and gametes. C. Wiencke, M.Y. Roleda & I. Bartsch; Section 7. Endogenous rhythms controlling metabolism and development. S. Jacobsen; Section 8. Macro- and micronutrient metabolism. R. Schumann & U. Karsten; Section 9. Storage compounds and growth substances. U. Karsten; Section 10. Salinity tolerance and osmotic acclimation. U. Karsten; Section 11. Physiological defences against abiotic stress. F. Weinberger & K. Bischof; Section 12. Defence against biotic stress factors. M. Molis & F. Weinberger; Section 13. Laminaria as habitat for epi- and endobionts. J. Wiese, A. Eggert & M. Molis; Section 14. Trophic interactions. R. Karez; Section 15. Competition. R. Karez; Section 16. Recent developments in aquaculture: resources and uses. B.H. Buck & C.M. Buchholz.
References
- Abe , E , Matsuyama , K and Tsuji , Y . 1982 . On the recolonization of Laminaria religiosa Miyabe, in Oshoro Bay, Hokkaido . Sci. Rep. Hokkaido Fish. Exp. Stn. , 24 : 41 – 50 .
- Agardh , JG . 1868 . De Laminarieis symbolas offert . Lunds Universitets Årsskrift , 4 : 1 – 36 .
- Agatsuma , Y . 1998 . Aquaculture of the sea urchin Strongylocentrotus nudus transplanted from coralline flats in Hokkaido, Japan . J. Shellfish Res. , 17 : 1541 – 1547 .
- Aguilera , J , Bischof , K , Karsten , U , Hanelt , D and Wiencke , C . 2002 . Seasonal variation in ecophysiological patterns in macroalgae from an Arctic fjord. II. Pigment accumulation and biochemical defence systems against high light stress . Mar. Biol. , 140 : 1087 – 1095 .
- Ahn , O , Petrell , RJ and Harrison , PJ . 1998 . Ammonium and nitrate uptake by Laminaria saccharina and Nereocystis luetkeana from a salmon seacage farm . J. Appl. Phycol. , 10 : 333 – 340 .
- Akaike , S , Yoshida , H , Matsuda , T , Yagi , H and Tomiyama , M . 1999 . Year-to-year variation of areas of macroalgal and crustose coralline algal communities interpreted from aerial photographs and SCUBA along the western coast of the Shakotan Peninsula, Hokkaido, Japan . Sci. Rep. Hokkaido Fish. Exp. Stn. , 56 : 125 – 135 . (In Japanese, English abstract.)
- Akiyama , K . 1965 . Studies of ecology and culture of Undaria pinnatifida (Harv.) Sur. II. Environmental factors affecting the growth and maturation of gametophyte . Bull. Tohoku Reg. Fish. Res. Lab. , 25 : 143 – 170 .
- Allen , JC and Griffiths , CL . 1981 . The fauna and flora of a kelp bed canopy . South Afr. J. Zool. , 16 : 80 – 84 .
- Almeida , M , Filipe , S , Humanes , M , Maia , MF , Melo , R , Severino , N , DA Silva , JAL , DA Silva , J and Wever , R . 2001 . Vanadium haloperoxidases from brown algae of the Laminariaceae family . Phytochemistry , 57 : 633 – 642 .
- Al-Ogily , SM and Knight-Jones , EW . 1977 . Anti-fouling role of antibiotics produced by marine algae and bryozoans . Nature , 265 : 728 – 729 .
- Amado Filho , GMA , Andrade , LR , Karez , CS , Farina , M and Pfeiffer , WC . 1999 . Brown algal species as biomonitors of Zn and Cd at Sepetiba Bay, Rio de Janeiro, Brazil . Mar. Environ. Res. , 48 : 213 – 224 .
- Amat , MA and Srivastava , LM . 1985 . Translocation of iodine in Laminaria saccharina (Phaeophyta) . J. Phycol. , 21 : 330 – 333 .
- Amsler , CD . 2001 . Induced defenses in macroalgae: The herbivore makes a difference . J. Phycol. , 37 : 353 – 356 .
- Amsler , CD and Fairhead , VA . 2006 . Defensive and sensory chemical ecology of brown algae . Adv. Bot. Res. , 43 : 1 – 91 .
- Amsler , CD and Neushul , M . 1989a . Diel periodicity of spore release from the kelp Nereocystis luetkeana (Mertens) Postels et Ruprecht . J. Exp. Mar. Biol. Ecol. , 134 : 117 – 127 .
- Amsler , CD and Neushul , M . 1989b . Chemotactic effects of nutrients on spores of the kelps Macrocystis pyrifera and Pterygophora californica . Mar. Biol. , 102 : 557 – 564 .
- Amsler , CD and Neushul , M . 1990 . Nutrient stimulation of spore settlement in the kelps Pterygophora californica and Macrocystis pyrifera . Mar. Biol. , 107 : 297 – 304 .
- Amsler , CD and Neushul , M . 1991 . Photosynthetic physiology and chemical composition of spores of the kelps Macrocystis pyrifera, Nereocystis luetkeana, Laminaria farlowii, and Pterygophora californica (Phaeophyceae) . J. Phycol. , 27 : 26 – 34 .
- Anderson , BS and Hunt , JW . 1988 . Bioassay methods for evaluating the toxicity of heavy metals, biocides and sewage effluent using microscopic stages of giant kelp Macrocystis pyrifera (Agardh): a preliminary report . Mar. Environ. Res. , 26 : 113 – 134 .
- Andersson , S , Kautsky , L and Kalvas , A . 1994 . Circadian and lunar gamete release in Fucus vesiculosus in the atidal Baltic Sea . Mar. Ecol. Prog. Ser. , 110 : 195 – 201 .
- Ang , PO . 1987 . Use of projection matrix models in the assessment of harvesting strategies for Sargassum . Hydrobiologia , 151/152 : 335 – 339 .
- Ang , PO . 1991 . Natural dynamics of a Fucus distichus (Phaeophyceae, Fucales) population: reproduction and recruitment . Mar. Ecol. Prog. Ser. , 78 : 71 – 85 .
- Ang , PO and De Wreede , RE . 1990 . Matrix models for algal life history stages . Mar. Ecol. Prog. Ser. , 59 : 171 – 181 .
- Apt , KE . 1988 . Etiology and development of hyperplasia induced by Streblonema sp. (Phaeophyta) on members of the Laminariales (Phaeophyta) . J. Phycol. , 24 : 28 – 34 .
- Ar Gall , E , Asensi , A , Marie , D and Kloareg , B . 1996 . Parthenogenesis and apospory in the Laminariales: a flow cytometry analysis . Eur. J. Phycol. , 31 : 369 – 380 .
- Ar Gall , E , Küpper , FC and Kloareg , B . 2004 . A survey of iodine content in Laminaria digitata . Bot. Mar. , 47 : 30 – 37 .
- Arnold , TM and Targett , NM . 2002 . Marine tannins: the importance of a mechanistic framework for predicting ecological roles . J. Chem. Ecol. , 28 : 1919 – 1934 .
- Arroyo , NL , Maldonado , M , Perez–Portela , R and Benito , J . 2004 . Distribution patterns of meiofauna associated with a sublittoral Laminaria bed in the Cantabrian Sea (north-eastern Atlantic) . Mar. Biol. , 144 : 231 – 242 .
- Asano , M , Kikuchi , S and Kawamura , T . 1990 . Effect of small herbivorous sea-snails on survival rates of the young Laminariales plants . Bull. Tohoku Natl. Fish. Res. Inst. , 52 : 65 – 71 .
- Axelsson , L , Mercato , JM and Figueroa , FL . 2000 . Utilization of at high pH by the brown macroalga Laminaria saccharina . Eur. J. Phycol. , 35 : 53 – 59 .
- Bai , FW and Qin , S . 1998 . Study on parthenogenesis of filamentous gametophytes of Laminaria japonica (Phaeophyta) . Marine Sciences (Qingdao) , 6 : 32 – 35 .
- Baldauf , SL . 2003 . The deep roots of eukaryotes . Science , 300 : 1703 – 1706 .
- Ballschmiter , K . 2003 . Pattern and sources of naturally produced organohalogens in the marine environment: biogenic formation of organohalogens . Chemosphere , 52 : 313 – 324 .
- Barton , NH . 1988 . “ Speciation ” . In Analytical Biogeography: an Integrated Approach to the Study of Animal and Plant Distribution , Edited by: Myers , AA and Giller , PS . 185 – 218 . London, , UK : Chapman & Hall .
- Beavis , A and Charlier , RH . 1987 . An economic appraisal for the onshore cultivation of Laminaria spp . Hydrobiologia , 151/152 : 387 – 398 .
- Bedford , AP and Moore , PG . 1984 . Macrofaunal involvement in the sublittoral decay of kelp debris: the detritivore community and species interactions . Estuar. Coast. Shelf Sci. , 18 : 97 – 111 .
- Benet , H , Ar Gall , E , Asensi , A and Kloareg , B . 1994 . Protoplast regeneration from gametophytes and sporophytes of some species in the order Laminariales (Phaeophyceae) . Protoplasma , 199 : 39 – 48 .
- Berdar , A , Conato , V , Cavallaro , G and Giacobbe , S . 1978 . First contribution to the knowledge of the epiphyte and associated organisms of the Laminariales of the Straits of Messina . Mem. Biol. Mar. Oceanogr. , 8 : 77 – 89 .
- Berg , BS and Munkejord , AA . 1991 . Forsvinner Jærstrendene? . Årsrapport for miljøvernavdelingen ved Fylkesmannen i Rogaland , 1 : 19 – 26 . Rogaland country Environmental Department
- Berglin , M , Delage , L , Potin , P , Vilter , H and Elwing , H . 2004 . Enzymatic cross-linking of a phenolic polymer extracted from the marine alga . Fucus serratus. Biomacromolecules , 5 : 2376 – 2383 .
- Berman , J , Harris , L , Lambert , WJ , Buttrick , M and Dufresne , M . 1992 . Recent invasions of the Gulf of Maine: three contrasting ecological histories . Cons. Biol. , 6 : 435 – 441 .
- Bernstein , BB and Mann , KH . 1982 . Changes in the nearshore ecosystem of the Atlantic coast of Nova Scotia, 1968-81 . Sci. Coun. Stud. , 5 : 101 – 105 .
- Bernstein , BB , Williams , BE and Mann , KH . 1981 . The role of behavioral responses to predators in modifying urchins’ (Strongylocentrotus droebachiensis) destructive grazing and seasonal foraging patterns . Mar. Biol. , 63 : 39 – 49 .
- Bernstein , BB , Schroeter , SC and Mann , KH . 1983 . Sea urchin (Strongylocentrotus droebachiensis) aggregating behavior investigated by a subtidal multifactorial experiment . Can. J. Fish. Aquat. Sci. , 40 : 1975 – 1986 .
- Bhadury , P and Wright , PC . 2004 . Exploitation of marine algae: biogenic compounds for potential antifouling applications . Planta , 219 : 561 – 578 .
- Bhattacharya , D , Mayes , C and Druehl , LD . 1991 . Restriction endonuclease analysis of ribosomal DNA sequence variation in Laminaria (Phaeophyta) . J. Phycol. , 27 : 624 – 628 .
- Bidwell , RGS , Mclachlan , J and Lloyd , NDH . 1985 . Tank cultivation of Irish Moss, . Chondrus crispus. Bot. Mar. , 28 : 87 – 97 .
- Billot , C , Rousvoal , S , Estoup , JT , Saumitou-Laprade , P , Valero , M and Kloareg , B . 1998 . Isolation and characterization of microsatellite markers in the nuclear genome of the brown alga Laminaria digitata (Phaeophyceae) . Mol. Ecol. , 7 : 1778 – 1780 .
- Billot , C , Boury , S , Benet , H and Kloareg , B . 1999 . Development of RAPD markers for parentage analysis in Laminaria digitata . Bot. Mar. , 42 : 307 – 314 .
- Billot , C , Engel , CR , Rousvoal , S , Kloareg , B and Valero , M . 2003 . Current patterns, habitat discontinuities and population genetic structure: the case of the kelp Laminaria digitata in the English Channel . Mar. Ecol. Prog. Ser. , 253 : 111 – 121 .
- Bischof , K , Hanelt , D , Tüg , H , Karsten , U , Brouwer , PEM and Wiencke , C . 1998 . Acclimation of brown algal photosynthesis to ultraviolet radiation in Arctic coastal waters (Spitsbergen, Norway) . Polar Biol. , 20 : 388 – 395 .
- Bischof , K , Hanelt , D , Aguilera , J , Karsten , U , Vögele , B , Sawall , T and Wiencke , C . 2002 . Seasonal variation in ecophysiological patterns in macroalgae from an Arctic fjord. I. Sensitivity of photosynthesis to ultraviolet radiation . Mar. Biol. , 140 : 1097 – 1106 .
- Bischof , K , Gómez , I , Molis , M , Hanelt , D , Karsten , U , Lüder , U , Roleda , MY , Zacher , K and Wiencke , C . 2006 . Ultraviolet radiation shapes seaweed communities . Rev. Environ. Sci. Biotechnol. , 5 : 141 – 166 .
- Black , WAP . 1950 . The seasonal variation in weight and chemical composition of the common British Laminariaceae . J. Mar. Biol. Assoc. UK , 29 : 45 – 72 .
- Blunden , G . 1991 . “ Agricultural use of seaweeds and seaweed extracts ” . In Seaweed Resources in Europe. Uses and Potential , Edited by: Guiry , MD and Blunden , G . 65 – 81 . Chichester, , UK : John Wiley & Sons .
- Bold , HC and Wynne , MJ . 1985 . Introduction to the Algae, ed. 2 , Engelwood Cliffs, , USA : Prentice Hall Inc. .
- Bologna , PAX and Steneck , RC . 1993 . Kelp beds as habitat for American lobster Homarus americanus . Mar. Ecol. Prog. Ser. , 100 : 127 – 143 .
- Bolton , JJ and Lüning , K . 1982 . Optimal growth and maximal survival temperatures of Atlantic Laminaria species (Phaeophyta) in culture . Mar. Biol. , 66 : 89 – 94 .
- Bolton , JJ , Germann , I and Lüning , K . 1983 . Hybridization between Atlantic and Pacific representatives of the Simplices section of Laminaria (Phaeophyta) . Phycologia , 22 : 133 – 140 .
- Boo , SM , Lee , WJ , Yoon , HS , Kata , A and Kawai , H . 1999 . Molecular phylogeny of Laminariales (Phaeophyceae) inferred from small subunit ribosomal DNA sequences . Phycol. Res. , 47 : 109 – 114 .
- Borchardt , SA , Allain , EJ , Michels , JJ , Stearns , GW , Kelly , RF and Mccoy , WF . 2001 . Reaction of acylated homoserine lactone bacterial signaling molecules with oxidized halogen antimicrobials . Appl. Environ. Microbiol. , 67 : 3174 – 3179 .
- Borum , J , Pedersen , MF , Krause-Jensen , D , Christensen , PB and Nielsen , K . 2002 . Biomass, photosynthesis and growth of Laminaria saccharina in a high-arctic fjord, NE Greenland . Mar. Biol. , 141 : 11 – 19 .
- Bradley , PM . 1991 . Plant hormones do have a role in controlling growth and development of algae . J. Phycol. , 27 : 317 – 321 .
- Brady-Campbell , MM , Campbell , DB and Harlin , MM . 1984 . Productivity of kelp (Laminaria spp.) near the southern limit in the northwestern Atlantic Ocean . Mar. Ecol. Prog. Ser. , 18 : 79 – 88 .
- Braga , AC and Yoneshigue-Valentin , Y . 1996 . Nitrogen and phosphorus uptake by the Brazilian kelp Laminaria abyssalis (Phaeophyta) in culture . Hydrobiologia , 326/327 : 445 – 450 .
- Brault , D and Briand , X . 1987 . L’algue alimentaire humaine, perspectives de développement en France . Equinoxe , 16 : 4 – 13 .
- Breuer , G and Schramm , W . 1988 . Changes in macroalgal vegetation of Kiel Bight (Western Baltic Sea) during the past 20 years . Kieler Meeresforsch. Sonderh. , 6 : 241 – 255 .
- Bridger , CJ and Costa-Pierce , BA . 2003 . Open Ocean Aquaculture: from Research to Commercial Reality , 351 p Baton Rouge, , USA : World Aquaculture Society .
- Brinkhuis , BH , Levine , HG , Schlenk , CG and Tobin , S . 1987 . “ Laminaria cultivation in the Far East and North America ” . In Seaweed Cultivation for Renewable Resources , Edited by: Bird , KT and Benson , PH . 107 – 146 . Amsterdam, , The Netherlands : Elsevier .
- Britton-Simmons , KH . 2004 . Direct and indirect effects of the introduced alga Sargassum muticum on benthic, subtidal communities of Washington State, USA . Mar. Ecol. Prog. Ser. , 277 : 61 – 78 .
- Britz , SJ and Briggs , WR . 1976 . Circadian rhythms of chloroplast orientation and photosynthetic capacity in . Ulva. Plant Physiol. , 58 : 22 – 27 .
- Broadgate , WJ , Malin , G , Küpper , FC , Thompson , A and Liss , PS . 2004 . Isoprene and other non-methane hydrocarbons from seaweeds: a source of reactive hydrocarbons to the atmosphere . Mar. Chem. , 88 : 61 – 73 .
- Brown , A and Simpson , J . 1972 . Water relations of sugar-tolerant yeasts: the role of intracellular polyols . J. Gen. Microbiol. , 72 : 589 – 591 .
- Brown , VB , Davies , SA and Synnot , RN . 1990 . Long-term monitoring of the effects of treated sewage effluent on the intertidal macroalgal community near Cape Schanck, Victoria, Australia . Bot. Mar. , 33 : 85 – 98 .
- Bruhn , J and Gerard , VA . 1996 . Photoinhibition and recovery of the kelp Laminaria saccharina at optimal and superoptimal temperatures . Mar. Biol. , 125 : 639 – 648 .
- Brumbaugh , DR , West , JM , Hintz , JL and Anderson , FE . 1994 . “ Determinants of recruitment by an epiphytic marine bryozoan: field manipulations of flow and host quality ” . In Reproduction and Development of Marine Invertebrates , Edited by: Wilson , WH , Stricker , SA and Shinn , GL . Baltimore, , USA : Johns Hopkins University Press .
- Bryan , GW and Hummerstone , LG . 1973 . Brown seaweed as an indicator of heavy metals in estuaries in south-west England . J. Mar. Biol. Assoc. UK , 53 : 705 – 720 .
- Brzezinski , MA , Reed , DC and Amsler , CD . 1993 . Neutral lipids as major storage products in zoospores of the giant kelp Macrocystis pyrifera (Phaeophyceae) . J. Phycol. , 29 : 16 – 23 .
- Buchholz , C and Lüning , K . 1999 . Isolated, distal blade discs of the brown alga Laminaria digitata form sorus, but not discs, near to the meristematic transition zone . J. Appl. Phycol. , 16 : 579 – 584 .
- Buck , BH . 2002 . Open Ocean Aquaculture und Offshore Windparks: Eine Machbarkeitsstudie über die multifunktionale Nutzung von Offshore-Windparks und Offshore-Marikultur im Raum Nordsee . Ber. Polarforsch. Meeresforsch. , 412 : 1 – 252 .
- Buck , BH . 2004 . Farming in a high energy environment: potentials and constraints of sustainable offshore aquaculture in the German Bight (North Sea) , Germany : University of Bremen . PhD thesis
- Buck , BH and Buchholz , CM . 2004 . The offshore-ring: A new system design for the open ocean aquaculture of macroalgae . J. Appl. Phycol. , 16 : 355 – 368 .
- Buck , BH and Buchholz , CM . 2005 . Response of offshore cultivated Laminaria saccharina to hydrodynamic forcing in the North Sea . Aquaculture , 250 : 674 – 691 .
- Buck , BH , Krause , G and Rosenthal , H . 2004 . Extensive open ocean aquaculture development within wind farms in Germany: the prospect of offshore co-management and legal constraints . Ocean Coastal Manag. , 47 : 95 – 122 .
- Buck , BH , Berg-Pollack , A , Assheuer , J , Zielinski , O and Kassen , D . 2006 . Technical realization of extensive aquaculture constructions in offshore wind farms: consideration of the mechanical loads . Proc. 25th International Conference on Offshore Mechanics and Arctic Engineering, Omae , : 1 – 7 .
- Buggeln , RG . 1983 . Photoassimilate translocation in brown algae . Prog. Phycol. Res. , 2 : 283 – 332 .
- Burkhardt , E and Peters , AF . 1998 . Molecular evidence from nrDNA ITS sequences that Laminariocolax (Phaeophyceae, Ectocarpales sensu lato) is a worldwide clade of closely related kelp endophytes . J. Phycol. , 34 : 682 – 691 .
- Burrows , EM . 1964 . An experimental assessment of some of the characters used for specific delimitation in the genus . Laminaria. J. Mar. Biol. Ass. UK , 44 : 137 – 143 .
- Busdosh , M , Beehler , CL , Robilliard , GA and Tarbox , KR . 1985 . Distribution and abundance of kelp in the Alaskan Beaufort Sea near Prudhoe Bay . Arctic , 38 : 18 – 22 .
- Bustamante , RH , Branch , GM and Eekhout , S . 1995 . Maintenance of an exceptional intertidal grazer biomass in South Africa: subsidy by subtidal kelps . Ecology , 76 : 2314 – 2329 .
- Cabello-Pasini , A and Alberte , RS . 1997 . Seasonal patterns of photosynthesis and light-independent carbon fixation in marine macrophytes . J. Phycol. , 33 : 321 – 329 .
- Cabello-Pasini , A and Alberte , RS . 2001 . Expression of carboxylating enzymes in Laminaria setchelli (Phaeophyceae) . Phycologia , 40 : 351 – 358 .
- Calvin , N and Ellis , RJ . 1978 . Quantitative and qualitative observations on Laminaria dentigera and other subtidal kelps of southern Kodiak island, Alaska . Mar. Biol. , 47 : 331 – 336 .
- Calvin , N and Ellis , RJ . 1981 . Growth of subtidal Laminaria groenlandica in southeastern Alaska related to season and depth . Bot. Mar. , 24 : 107 – 114 .
- Camacho , P , Salinas , JM , Fuertes , C and Delgado , M . 2004 . Preparation of single cell detritus from Laminaria saccharina as a hatchery diet for bivalve molluscs . Mar. Biotechnol. , 6 : 642 – 649 .
- Carpenter , EJ and Liss , PS . 2000 . On temperate sources of bromoform and other reactive organic bromine gases . J. Geophys. Res. , 105D : 20539 – 20547 .
- Carpenter , LJ , Malin , G , Liss , PS and Küpper , FC . 2000 . Novel biogenic iodine-containing trihalomethanes and other short-lived halocarbons in the coastal East Atlantic . Glob. Biogeochem. Cycl. , 14 : 1191 – 1204 .
- Caswell , H . 1986 . Life cycle models for plants . Lect. Math. Life Sci. , 18 : 171 – 233 .
- Cavalier-Smith , T . 1998 . A revised six-kingdom system of life . Biol. Rev. , 73 : 203 – 266 .
- Cetrulo , GL and Hay , ME . 2000 . Activated chemical defenses in tropical versus temperate seaweeds . Mar. Ecol. Prog. Ser. , 207 : 243 – 253 .
- Chapman , ARO . 1973a . Phenetic variability of stipe morphology in relation to season, exposure, and depth in the non-digitate complex of Laminaria Lamour. (Phaeophyta, Laminariales) in Nova Scotia . Phycologia , 12 : 53 – 57 .
- Chapman , ARO . 1973b . A critique of prevailing attitudes towards the control of seaweed zonation on the sea shore . Bot. Mar. , 16 : 80 – 82 .
- Chapman , ARO . 1974a . The genetic basis of morphological differentiation in some Laminaria populations . Mar. Biol. , 24 : 85 – 91 .
- Chapman , ARO . 1974b . The ecology of macroscopic marine algae . Ann. Rev. Ecol. Syst. , 5 : 65 – 80 .
- Chapman , ARO . 1975 . Inheritance of mucilage canals in Laminaria in eastern Canada . Br. Phycol. J. , 10 : 219 – 223 .
- Chapman , ARO . 1981 . Stability of sea urchin dominated barren grounds following destructive grazing of kelp in St. Margaret's Bay, eastern Canada . Mar. Biol. , 62 : 307 – 311 .
- Chapman , ARO . 1984 . Reproduction, recruitment and mortality in two species of Laminaria in southwest Nova Scotia . J. Exp. Mar. Biol. Ecol. , 18 : 99 – 109 .
- Chapman , ARO . 1986 . Age versus stage: an analysis of age- and size-specific mortality and reproduction in a population of Laminaria longicruris Pyl . J. Exp. Mar. Biol. Ecol. , 97 : 113 – 122 .
- Chapman , ARO . 1987 . “ The wild harvest and culture of Laminaria longicruris in Eastern Canada ” . In Case Studies of seven Commercial Seaweed Resources , Edited by: Doty , MS , Caddy , JF and Santelices , B . 239 – 263 . Rome, , Italy : FAO Fisheries Technical Paper 281, Food and Agriculture Organisation of the United Nations .
- Chapman , ARO . 1990 . Competitive interaction among Fucus spiralis L. and F. vesiculosus L. (Fucales, Phaeophyta) . Hydrobiologia , 204/205 : 205 – 209 .
- Chapman , ARO . 1993 . “Head” data for matrix modelling of Laminaria digitata (Laminariales, Phaeophyta) populations . Hydrobiologia , 260/261 : 263 – 267 .
- Chapman , ARO and Craigie , JS . 1977 . Seasonal growth in Laminaria longicruris: relations with dissolved inorganic nutrients and internal reserves of nitrogen . Mar. Biol. , 40 : 197 – 205 .
- Chapman , ARO and Craigie , JS . 1978 . Seasonal growth in Laminaria longicruris: relations with reserve carbohydrate storage and production . Mar. Biol. , 46 : 209 – 213 .
- Chapman , ARO and Lindley , JE . 1980a . Productivity of Laminaria solidungula J. Ag. in the Canadian high Arctic: a year round study . Proc. Int. Seaweed Symp. , 10 : 247 – 252 .
- Chapman , ARO and Lindley , JE . 1980b . Seasonal growth of Laminaria solidungula in the Canadian High Arctic in relation to irradiance and dissolved nutrient concentrations . Mar. Biol. , 57 : 1 – 5 .
- Chapman , ARO and Johnson , CR . 1990 . Disturbance and organization of macroalgal assemblages in the northwest Atlantic . Hydrobiologia , 192 : 77 – 121 .
- Chapman , ARO , Markham , JW and Lüning , K . 1978 . Effects of nitrate concentration on the growth and physiology of Laminaria saccharina (Phaeophyta) in culture . J. Phycol. , 14 : 195 – 198 .
- Chen , J . 2006 . “ Cultured aquatic species information programme–Laminaria japonica ” . In Cultured Aquatic Species Fact Sheets , FAO Inland Water Resources and Aquaculture Service (FIRI) .
- Chess , JR . 1993 . Effects of the stipe-boring amphipod Peramphithoe stypotrupetes (Corophioidea: Ampithoidae) and grazing gastropods on the kelp . Laminaria setchelli. J. Crustacean Biol. , 13 : 638 – 646 .
- Chi , EY and Neushul , M . 1972 . Electron microscopic studies of sporogenesis in Macrocystis . Proc. Int. Seaweed Symp. , 7 : 181 – 187 .
- Cho , GY , Yoon , HS , Boo , SM and Yarish , C . 2000 . Atlantic kelp species Laminaria longicruris and L. saccharina (Laminariales) are conspecific . J. Phycol. , 36 ( suppl. ) : 12 – 13 .
- Cho , GY , Klochkova , NG , Krupnova , TN and Boo , SN . 2006 . The reclassification of Lessonia laminarioides (Laminariales, Phaeophyceae) . Pseudolessonia gen. nov. J. Phycol. , 42 : 1289 – 1299 .
- Chopin , T , Yarish , C , Levine , I and Van Patten , V . 1998 . Seaweed aquaculture . World Aquaculture Magazine , 29 : 17
- Chopin , T , Buschmann , AH , Halling , C , Troell , M , Kautsky , N , Neori , A , Kraemer , GP , Zertuche-González , JA , Yarish , C and Neefus , C . 2001 . Integrating seaweeds into marine aquaculture systems: a key toward sustainability . J. Phycol. , 37 : 975 – 986 .
- Christie , H and Rinde , E . 1995 . Changes in sea urchin abundance, sea urchin parasite and benthic algal vegetation along the coast of mid-Norway . Norsk Institutt for Naturforskning, Opptragsmelding , (in Norwegian, English abstract)
- Christie , H , Rinde , E , Fredriksen , S and Skadsheim , A . 1994 . Ecological consequences of kelp trawling: re-establishment of kelp forest, epiphytes and holdfast fauna after kelp trawling at the Rogaland coast . NINA Oppdragsmelding , 295 : 1 – 29 .
- Christie , H , Frederiksen , S and Rinde , E . 1998 . Regrowth of kelp and colonization of epiphyte and fauna community after kelp trawling at the coast of Norway . Hydrobiologia , 375/376 : 49 – 58 .
- Christie , H , Jørgensen , NM , Norderhaug , KM and Waage-Nielsen , E . 2003 . Species distribution and habitat exploitation of fauna associated with kelp (Laminaria hyperborea) along the Norwegian coast . J. Mar. Biol. Assoc. UK , 83 : 687 – 699 .
- Chung , IK and Brinkhuis , BH . 1986 . Copper effects in early life stages of the kelp, Laminaria saccharina . Mar. Pollut. Bull. , 17 : 213 – 218 .
- Chynoweth , DP , Owens , JM and Legrand , R . 2001 . Renewable methane from anaerobic digestion of biomass . Renewable Energy , 22 : 1 – 8 .
- Clayton , MN . 1992 . Propagules of marine macroalgae: structure and development . Br. Phycol. J. , 27 : 219 – 232 .
- Coelho , SM , Rijstenbil , JW and Brown , MT . 2000 . Impacts of anthropogenic stresses on the early development stages of seaweeds . J. Aquat. Ecosyst. Stress Recovery , 7 : 317 – 333 .
- Colin , C , Leblanc , C , Wagner , E , Delage , L , Leize-Wagner , E , Van Dorsselaer , A , Kloareg , B and Potin , P . 2003 . The brown algal kelp Laminaria digitata features distinct bromoperoxidase and iodoperoxidase activities . J. Biol. Chem. , 278 : 23545 – 23552 .
- Collén , J and Davison , IR . 1999 . Reactive oxygen production and damage in intertidal Fucus spp. (Phaeophyceae) . J. Phycol. , 35 : 54 – 61 .
- Connan , S , Goulard , F , Stiger , V , Deslandes , E and Ar Gall , E . 2004 . Interspecific and temporal variation in phlorotannin levels in an assemblage of brown algae . Bot. Mar. , 47 : 410 – 416 .
- Conolly , NJ and Drew , EA . 1985 . Physiology of Laminaria III. Effect of a coastal eutrophication gradient on seasonal patterns of growth and tissue composition in L. digitata Lamour. and L. saccharina (L.) Lamour . P. S. Z. N. I. Mar. Ecol. , 6 : 191 – 195 .
- Corre , S and Prieur , D . 1990 . Density and morphology of epiphytic bacteria on the kelp Laminaria digitata . Bot. Mar. , 33 : 515 – 523 .
- Correa , JA and Mclachlan , JL . 1994 . Endophytic algae of Chondrus crispus (Rhodophyta): 5. Fine structure of the infection by Acrochaete operculata (Chlorophyta) . Eur. J. Phycol. , 29 : 33 – 47 .
- Cosson , J . 1976 . Evolution de la fertilité des populations de Laminaria digitata (L.) Lamouroux (Phéophycée, Laminariale) au cours de l'année . Soc. Phycol. de France , 21 : 28 – 34 .
- Cosson , J . 1987 . Croissance des sporophytes résultant d'hybridations interspécifiques et intergénériques chez les Laminariales . Cryptogam. Algol. , 8 : 61 – 72 .
- Cosson , J . 1999 . Sur la disparition progressive de Laminaria digitata sur les côtes du Calvados (France) . Cryptogam. Algol. , 20 : 35 – 42 .
- Cosson , J and Gayral , P . 1983 . Les bases experimentales des hybridations realisées chez des Laminariales de côte francaises . IFREMER Actes de Colloques , 1 : 15 – 18 .
- Cosson , J and Olivari , R . 1982 . Premiers résultats concernant les possibilités d’hybridation interspécifiques et intergénériques chez les Laminariales des côtes de la Manche . C.R. Acad. Sci. Paris , t295 : 381 – 384 . Série III
- Cosson , J , Gayral , P and Olivari , R . 1984 . On specimens of Laminaria digitata with bifurcate stipes: a hypothesis as to their origin . Cryptogam. Algol. , 5 : 15 – 20 .
- Cota , GF and Sturges , WT . 1997 . Biogenic bromine production in the Arctic . Mar. Chem. , 56 : 181 – 192 .
- Cracraft , J . 1989 . “ Speciation and its ontology: the empirical consequences of alternative species concepts for understanding patterns and processes of differentiation ” . In Speciation and its Consequences , Edited by: Otte , D and Endler , JA . 28 – 59 . Sunderland, , USA : Sinauer Association .
- Cranford , PJ and Grant , J . 1990 . Particle clearance and absorption of phytoplankton and detritus by the sea scallop Placopecten magellanicus (Gmelin) . J. Exp. Mar. Biol. Ecol. , 137 : 105 – 121 .
- Creed , JC , Kain , JM and Norton , TA . 1998 . An experimental evaluation of density and plant size in two large brown seaweeds . J. Phycol. , 34 : 39 – 52 .
- Crepineau , F , Roscoe , T , Kaas , R , Kloareg , B and Boyen , C . 2000 . Characterisation of complementary DNAs from the expressed sequence tag analysis of life cycle stages of Laminaria digitata (Phaeophyceae) . Plant Mol. Biol. , 43 : 503 – 513 .
- Critchley , AT and Ohno , M . 1997 . “ Cultivation and Farming of Marine Plants. Biodiversity of Expert Centre for Taxonomic Identification (ETI). CD-ROM Version 1.0 ” . New York, , USA : Springer Verlag Electronic Media Dept .
- Critchley , AT and Ohno , M . 1998 . Seaweed Resources of the World , Edited by: Critchley , AT and Ohno , M . Yokosuka, , Japan : Japan International Cooperation Agency .
- Critchley , AT , Rotmann , KWG and Molloy , FJ . 1991 . The Namibian seaweed industry: present and potential . Bioresour. Technol. , 38 : 137 – 143 .
- Critchley , AT , Ohno , M and Largo , DB . 2006 . World Seaweed Resources. An Authoritative Reference System , ETI BioInformatics. DVD-ROM
- CRM . 2001 . “ Wirtschaftliches und ökologisches Potential einer Laminarien-Farm in Deutschland (Economic and ecologic potentials of algae farming in Germany) ” . In Coastal Research & Management 37 Kiel, , Germany
- Dā Costa Braga , A and Yoneshigue-Valentin , Y . 1996 . Nitrogen and phosphorus uptake by the Brazilian kelp Laminaria abyssalis (Phaeophyta) in culture . Hydrobiologia , 327 : 445 – 450 .
- Davison , IR . 1987 . Adaptation of photosynthesis in Laminaria saccharina (Phaeophyta) to changes in growth temperature . J. Phycol. , 23 : 273 – 283 .
- Davison , IR . 1991 . Environmental effects on algal photosynthesis: temperature . J. Phycol. , 27 : 2 – 8 .
- Davison , IR and Davison , JO . 1987 . The effect of growth temperature on enzyme activities in the brown alga Laminaria saccharina . Br. Phycol. J. , 22 : 77 – 87 .
- Davison , IR and Reed , RH . 1985a . Osmotic adjustment in Laminaria digitata (Phaeophyta) with particular reference to seasonal changes in internal solute concentrations . J. Phycol. , 21 : 41 – 50 .
- Davison , IR and Reed , RH . 1985b . The physiological significance of mannitol accumulation in brown algae: the role of mannitol as a compatible cytoplasmic solute . Phycologia , 24 : 449 – 457 .
- Davison , IR and Stewart , WDP . 1983 . Occurrence and significance of nitrogen transport in the brown alga Laminaria digitata . Mar. Biol. , 77 : 107 – 112 .
- Davison , IR and Stewart , WDP . 1984 . Studies on nitrate reductase activity in Laminaria digitata (Huds.) Lamour. 1. Longitudinal and transverse profiles of nitrate reductase activity within the thallus . J. Exp. Mar. Biol. Ecol. , 74 : 201 – 210 .
- Davison , IR , Andrews , M and Stewart , WDP . 1984 . Regulation of growth in Laminaria digitata: use of in-vivo nitrate reductase activities as an indicator of nitrogen limitation in field populations of Laminaria spp . Mar. Biol. , 84 : 207 – 217 .
- Davison , IR , Greene , RM and Podolak , EJ . 1991 . Temperature acclimation of respiration and photosynthesis in the brown alga . Laminaria saccharina. Mar. Biol. , 110 : 449 – 454 .
- Dawes , CP . 1988 . “ Seaweed culture technology ” . In Feasibility Study on the Technology of Mariculture. Vol. II: Review of Technologies and Services , Edited by: Mackay Consultants and Munro , A . 107 – 116 . Aberdeen, , UK : University Marine Studies .
- Day , RW and Cook , P . 1995 . Bias towards brown algae in determining diet and food preferences: the South African abalone Haliotis midae . Mar. Freshwater Res. , 46 : 623 – 627 .
- Dayton , PK , Currie , V , Gerrodette , T , Keller , BD , Rosenthal , R and Ven Tresca , D . 1984 . Patch dynamics and stability of some California kelp communities . Ecol. Monogr. , 54 : 253 – 289 .
- Dayton , PK , Tegner , MJ , Parnell , PE and Edwards , PB . 1992 . Temporal and spatial patterns of disturbance and recovery in a kelp forest community . Ecol. Monogr. , 62 : 421 – 445 .
- Dayton , PK , Tegner , MJ , Edwards , PB and Riser , KL . 1999 . Temporal and spatial scales of kelp demography: the role of oceanographic climate . Ecol. Monogr. , 69 : 219 – 250 .
- Deal , MS , Hay , ME , Wilson , D and Fenical , W . 2003 . Galactolipids rather than phlorotannins as herbivore deterrents in the brown seaweed Fucus vesiculosus . Oecologia , 136 : 107 – 114 .
- DE Boer , E , Tromp , MGM , Plat , H , Krenn , GE and Wever , R . 1986 . Vanadium (V) as an essential element for haloperoxidase activity in marine brown algae (Laminaria saccharina): purification and characterization of a vanadium (V)-containing bromoperoxidase from Laminaria saccharina . Biochim. Biophys. Acta , 872 : 104 – 115 .
- Deevey , ES . 1947 . Life tables for natural populations of animals . Q. Rev. Biol. , 22 : 283 – 314 .
- Delange , F . 1994 . Disorders induced by iodine deficiency . Thyroid , 4 : 107 – 128 .
- Denboh , T , Suzuki , M , Mizuno , Y and Ichimura , T . 1997 . Suppression of Laminaria sporelings by allelochemicals from coralline red algae . Bot. Mar. , 40 : 249 – 256 .
- De Reviers , B and Rousseau , F . 1999 . Towards a new classification of the brown algae . Prog. Phycol. Res. , 13 : 107 – 201 .
- De Roeck-Holtzhauer , Y . 1991 . “ Uses of seaweeds in cosmetics ” . In Seaweed Resources in Europe: Uses and Potential , Edited by: Guiry , MD and Blunden , G . 84 – 95 . Chichester, , UK : Wiley & Sons Ltd. .
- Devinny , J and Volse , L . 1978 . Effects of sediments on the development of Macrocystis pyrifera gametophytes . Mar. Biol. , 48 : 343 – 348 .
- De Wreede , RE . 1984 . Growth and age class distribution of Pterygophora californica (Phaeophyta) . Mar. Ecol. Prog. Ser. , 19 : 93 – 100 .
- De Wreede , RE and Klinger , T . 1988 . “ Reproductive strategies in algae ” . In Plant Reproductive Ecology. Patterns and Strategies , Edited by: Lovett Doust , J and Lovett Doust , L . 267 – 284 . New York, , USA : Oxford University Press .
- Dieckmann , GS . 1980 . Aspects of the ecology of Laminaria pallida (Grev.) J. Ag. off the Cape Peninsula (South Africa) . Bot. Mar. , 13 : 579 – 585 .
- Ding , M . 1992 . The effects of the environmental factors on Laminaria disease caused by alginic acid decomposing bacteria . Acta Oceanol. Sinica , 11 : 123 – 130 .
- Dimitrieva , GYU and Dimitriev , SM . 1996 . Symbiotic microflora of brown algae of genus Laminaria as bioindicator of ecological condition of coastal laminarian biocenoses . Russ. J. Mar. Biol. , 22 : 276 – 281 .
- Dimitrieva , GY , Crawford , RL and Yüksel , GÜ . 2006 . The nature of plant growth-promoting effects of a pseudoalteromonad associated with the marine algae Laminaria japonica and linked to catalase excretion . J. Appl. Microbiol. , 100 : 1159 – 1169 .
- Dixon , J , Schroeter , SC and Kastendiek , J . 1981 . Effects of the encrusting bryozoan, Membranipora membranancea, on the loss of blades and fronds by the giant kelp Macrocystis pyrifera (Laminariales) . J. Phycol. , 17 : 341 – 345 .
- Doblin , MA and Clayton , MN . 1995 . Effects of secondarily-treated sewage effluent on the early life-history stages of two species of brown macroalgae: Hormosira banksii and Durvillaea potatorum . Mar. Biol. , 122 : 689 – 698 .
- Dobretsov , SV . 1999 . Effects of macroalgae and biofilm on settlement of blue mussel (Mytilus edulis L.) larvae . Biofouling , 14 : 153 – 165 .
- Dobretsov , S and Wahl , M . 2001 . Recruitment preferences of blue mussel spat (Mytilus edulis) for different substrata and microhabitats in the White Sea (Russia) . Hydrobiologia , 445 : 27 – 35 .
- Dotsu , K , Nomura , H , Ohta , M and Iwakura , Y . 1999 . Factors causing formation of Laminaria religiosa bed on coralline flats along the southwest coast of Hokkaido . Nippon Suisan Gakkaishi , 65 : 216 – 222 . (In Japanese, English abstract.)
- Draisma , SGA , Prud’Homme van Reine , WF , Stam , WT and Olsen , JL . 2001 . A reassessment of phylogenetic relationships within the Phaeophyceae based on rubisco large subunit and ribosomal DNA sequences . J. Phycol. , 37 : 586 – 603 .
- Drew , EA . 1972 . Growth of a kelp forest at 60 metres in the Straits of Messina . Mem. Biol. Mar. Ocean N.S. , 2 : 135 – 157 .
- Drew , EA . 1983 . Physiology of Laminaria. II. Seasonal variation of photosynthesis and respiration in Laminaria digitata Lamour., L. hyperborea (Gunn.) Fosl. and L. saccharina (L.) Lamour. and a model for calculation of annual carbon budgets . P. S. Z. N. I: Mar. Ecol. , 4 : 227 – 250 .
- Drew , EA , Ireland , JF , Muir , C , Robertson , WAA and Robinson , JD . 1982 . Photosynthesis, respiration and other factors influencing the growth of Laminaria ochroleuca Pyl. below 50 metres in the Straits of Messina . P. S. Z. N. I: Mar. Ecol. , 3 : 335 – 355 .
- Dring , MJ . 1984 . Photoperiodism and phycology . Prog. Phycol. Res. , 3 : 159 – 192 .
- Dring , MJ . 1986 . Pigment composition and photosynthetic action spectra of sporophytes of Laminaria (Phaeophyta) grown in different light qualities and irradiances . Br. Phycol. J. , 21 : 199 – 207 .
- Dring , MJ . 1987 . “ Light climate in intertidal and subtidal zones in relation to photosynthesis and growth of benthic algae: a theoretical model ” . In Plant Life in Aquatic and Amphibious Habitats , Edited by: Crawford , RMM . 23 – 34 . Oxford, , UK : Blackwell .
- Dring , MJ . 1988 . Photocontrol of development in algae . Ann. Rev. Plant Physiol. Plant Mol. Biol. , 39 : 157 – 174 .
- Dring , MJ . 2006 . Stress resistance and disease resistance in seaweeds: the role of reactive oxygen metabolism . Adv. Bot. Res. , 43 : 175 – 207 .
- Dring , MJ , Forster , RM and Schmid , R . 1994 . Ecological significance of blue-light stimulation of photosynthetic capacity in Laminaria spp. and other brown-algae . Mar. Ecol. Prog. Ser. , 113 : 271 – 277 .
- Dring , MJ , Makarov , V , Schoschina , E , Lorenz , M and Lüning , K . 1996 . Influence of ultraviolet-radiation on chlorophyll fluorescence and growth in different life-history stages of three species of Laminaria (Phaeophyta) . Mar. Biol. , 126 : 183 – 191 .
- Dring , MJ , Wagner , A and Lüning , K . 2001 . Contribution of the UV component of natural sunlight to photoinhibition of photosynthesis in six species of subtidal brown and red seaweeds . Plant, Cell Environ. , 24 : 1153 – 1164 .
- Drobyshev , VP . 1971 . Acclimatisation of marine algae when maintained in media of differing salinities . Ekologiya , 2 : 96 – 98 .
- Druehl , LD . 1967 . Distribution of two species of Laminaria as related to environmental factors . J. Phycol. , 3 : 103 – 108 .
- Druehl , LD . 1968 . Taxonomy and distribution of northeast Pacific species of Laminaria . Can. J. Bot. , 46 : 539 – 547 . vii Plates
- Druehl , LD . 1969 . The northeast Pacific rim distribution of the Laminariales . Proc. Int. Seaweed Symp. , 6 : 161 – 170 .
- Druehl , LD and Boal , R . 1981 . Manipulation of the laminarialean life-cycle and its consequences for kombu mariculture . Proc. Int. Seaweed Symp. , 10 : 575 – 580 .
- Druehl , LD and Hsiao , SIC . 1977 . Intertidal kelp response to seasonal environmental changes in a British Columbia inlet . J. Fish. Res. Board Can. , 34 : 1207 – 1211 .
- Druehl , LD and Masuda , M . 1973 . The description of Laminaria yendoana Miyabe . Bull. Jap. Soc. Phycol. , 21 : 133 – 138 .
- Druehl , LD , Cabot , EL and Lloyd , KE . 1987 . Seasonal growth of Laminaria groenlandica as a function of plant age . Can. J. Bot. , 65 : 1599 – 1604 .
- Druehl , L , Footit , RG and Masuda , M . 1988a . Morphological affinities of Japanese species of Laminaria (Phaeophyta) . Phycologia , 27 : 405 – 412 .
- Druehl , L , Baird , R , Lindwall , A , Lloyd , KE and Pakula , S . 1988b . Longline cultivation of some Laminariaceae in British Columbia, Canada . Aquacult. Fish. Manag. , 19 : 253 – 263 .
- Druehl , LD , Robertson , BR and Button , DK . 1989a . Characterizing and sexing laminarialean meiospores by flow cytometry . Mar. Biol. , 101 : 451 – 456 .
- Druehl , LD , Harrison , PJ , Lloyd , KE and Thompson , PA . 1989b . Phenotypic variation in N uptake by Laminaria groenlandica Rosenvinge (Laminariales, Phaeophyta) . J. Exp. Mar. Biol. Ecol. , 127 : 155 – 164 .
- Druehl , LD , Mayes , C , Tan , IH and Saunders , GW . 1997 . Molecular and morphological phylogenies of kelp and associated brown algae . Plant Syst. Evol. , 11 ( suppl. ) : 221 – 235 .
- Druehl , LD , Collins , JD , Lane , CE and Saunders , GW . 2005 . An evaluation of methods used to assess intergeneric hybridization in kelp using Pacific Laminariales (Phaeophyceae) . J. Phycol. , 41 : 250 – 262 .
- Duan , D , Liu , X , Pan , F , Liu , H , Chen , N and Fei , X . 1995 . Extraction and identification of cytokinin from Laminaria japonica Aresch . Bot. Mar. , 38 : 409 – 412 .
- Dubi , A and Tørum , A . Wave damping by kelp vegetation . Proceedings of the 24th International Conference on Coastal Engeneering (by ASCE) . Edited by: Edge , BL . pp. 142 – 156 . Kobe, , Japan
- Duggins , DO . 1980 . Kelp beds and sea otters: an experimental approach . Ecology , 61 : 447 – 453 .
- Duggins , DO . 1981 . Sea urchins and kelp: the effects of short term changes in urchin diet . Limnol. Oceanogr. , 26 : 391 – 394 .
- Duggins , DO . 1983 . Starfish predation and the creation of mosaic patterns in a kelp-dominated community . Ecology , 64 : 1610 – 1619 .
- Duggins , DO and Eckman , JE . 1997 . Is kelp detritus a good food for suspension feeders? Effects of kelp species, age and secondary metabolites . Mar. Biol. , 128 : 489 – 495 .
- Duggins , DO , Simenstad , CA and Estes , JA . 1989 . Magnification of secondary production by kelp detritus in coastal marine ecosystems . Science , 245 : 170 – 173 .
- Dummermuth , AL , Karsten , U , Fischer , KM , Koenig , GM and Wiencke , C . 2003 . Responses of marine macroalgae to hydrogen-peroxide stress . J. Exp. Mar. Biol. Ecol. , 289 : 103 – 121 .
- Dunlap , JC and Loros , JJ . 2004 . The Neurospora circadian system . J. Biol. Rhythms , 19 : 414 – 424 .
- Dunton , KH . 1984 . “ An annual carbon budget for an arctic kelp community ” . In The Alaskan Beaufort Sea: Ecosystems and Environments , Edited by: Barnes , PW , Schell , DM and Reimnitz , E . 311 – 325 . Orlando, , USA : Academic Press .
- Dunton , KH . 1985 . Growth of dark-exposed Laminaria saccharina (L.) Lamour. and Laminaria solidungula J. Ag. (Laminariales: Phaeophyta) in the Alaskan Beaufort Sea . J. Exp. Mar. Biol. Ecol. , 94 : 181 – 189 .
- Dunton , KH and Dayton , PK . 1995 . “ The biology of high latitude kelp ” . In Ecology of Fjords and Coastal Waters , Edited by: Skojdal , H.R. , Hopkins , C , Erikstad , K.E. and Leinaas , H.P. 499 – 507 . Amsterdam, , The Netherlands : Elsevier Science .
- Dunton , KH and Jodwalis , CM . 1988 . Photosynthetic performance of Laminaria solidungula measured in situ in the Alaskan High Arctic . Mar. Biol. , 98 : 277 – 285 .
- Dunton , KH and Schell , DM . 1986 . A seasonal carbon budget for the kelp Laminaria solidungula in the Alaskan high Arctic . Mar. Ecol. Prog. Ser. , 31 : 57 – 66 .
- Dunton , KH and Schell , DM . 1987 . Dependence of consumers on macroalgal (Laminaria solidungula) carbon in an arctic kelp community: δ13C evidence . Mar. Biol. , 93 : 615 – 625 .
- Dunton , KH , Reimnitz , E and Schonberg , S . 1982 . An arctic kelp community in the Alaskan Beaufort Sea . Arctic , 35 : 465 – 484 .
- Ebeling , AW , Laur , DR and Rowley , RJ . 1985 . Severe storm disturbance and reversal of community structure in a southern California kelp forest . Mar. Biol. , 84 : 287 – 294 .
- Edwards , A . 1980 . Ecological studies of the kelp Laminaria hyperborea, and its associated fauna in south-west Ireland . Ophelia , 19 : 47 – 60 .
- Egan , B and Yarish , C . 1990 . Productivity and life history of Laminaria longicruris at its southern limit in the Western Atlantic Ocean . Mar. Ecol. Prog. Ser. , 67 : 263 – 273 .
- Ellertsdottír , E and Peters , AF . 1997 . High prevalence of infection by endophytic brown algae in populations of Laminaria spp. (Phaeophyceae) . Mar. Ecol. Prog. Ser. , 146 : 135 – 143 .
- Elner , RW and Vadas , RL . 1990 . Inference in ecology: the sea urchin phenomenon in the northwestern Atlantic . Am. Nat. , 136 : 108 – 125 .
- Erting , L , Daugbjerg , N and Pedersen , PM . 2004 . Nucleotide diversity within and between four species of Laminaria (Phaeophyceae) analysed using partial LSU and ITS rDNA sequences and AFLP . Eur. J. Phycol. , 39 : 243 – 256 .
- Espinoza , J and Chapman , ARO . 1983 . Ecotypic differentiation of Laminaria longicruris in relation to seawater nitrate concentration . Mar. Biol. , 74 : 213 – 218 .
- Estes , JA and Duggins , DO . 1995 . Sea otters and kelp forests in Alaska: generality and variation in a community ecological paradigm . Ecol. Monogr. , 65 : 75 – 100 .
- Estes , JA and Palmisano , JF . 1974 . Sea otters: their role in structuring nearshore communities . Science , 185 : 1058 – 1060 .
- Estes , JA and Steinberg , PD . 1988 . Predation, herbivory, and kelp evolution . Paleobiol. , 14 : 19 – 36 .
- Estes , JA , Mith , NS and Palmisano , JF . 1978 . Sea otter predation and community organization in the western Aleutian Islands, Alaska . Ecology , 59 : 822 – 833 .
- Estes , JA , Tinker , MT , Williams , TM and Doak , DF . 1998 . Killer whale predation on sea otters linking oceanic and nearshore ecosystems . Science , 282 : 473 – 476 .
- Estes , JA , Danner , EM , Doak , DF , Konar , B , Springer , AM , Steinberg , PD , Tinker , MT and Williams , TM . 2004 . Complex trophic interactions in kelp forest ecosystems . Bull. Mar. Sci. , 74 : 621 – 638 .
- Evans , VL . 1963 . A large chromosome in the laminarean nucleus . Nature , 198 : 215
- Evans , LV . 1965 . Cytological studies in the Laminariales . Ann. Bot. , 29 : 541 – 562 .
- Fain , SR , Druehl , LD and Baillie , DL . 1988 . Repeat and single copy sequences are differently conserved in the evolution of kelp chloroplast DNA . J. Phycol. , 24 : 292 – 302 .
- Fang , TC , Tai , JH , Ou , YL , Tui , CC and Chen , TC . 1978 . Some genetic observations on the monoploid breeding of Laminaria japonica . Scientia Sinica , 21 : 401 – 408 .
- FAO . 2004 . The State of World Fisheries and Aquaculture (SOFIA) , Rome, Italy : Food and Agriculture Organisation of the United Nations .
- FAO . 2006 . “ State of World Aquaculture: 2006 ” . In FAO Fisheries Technical Paper, 500 , Rome, Italy : Food and Agriculture Organisation of the United Nations .
- FAO . 2007 . “ Fishery Information, Data and Statistics Unit. Aquaculture production: values 1984-2005 ” . In FISHSTAT Plus - Universal software for fishery statistical time series , Rome, Italy : Food and Agriculture Organization of the United Nations .
- FAO, ICLARM & IIRR . 2001 . “ Integrated agriculture–aquaculture: a primer ” . In FAO Fisheries Technical Paper, T407 , Rome, Italy : Food and Agriculture Organisation of the United Nations .
- Fei , X . 2004 . Solving the coastal eutrophication problem by large scale seaweed cultivation . Hydrobiologia , 512 : 145 – 151 .
- Feng , YY , Hou , LC , Ping , NX , Ling , TD and Kyo , CI . 2004 . Development of mariculture and its impacts in Chinese coastal waters . Rev. Fish Biol. Fisheries , 14 : 1 – 10 .
- Figueira , MM , Volesky , B , Azarian , K and Ciminelli , VST . 2000a . Biosorption column performance with a metal mixture . Envir. Sci. Technol. , 34 : 4320 – 4326 .
- Figueira , MM , Volesky , B and Ciminelli , VST . 2000b . Biosorption of metals in brown seaweed biomass . Wat. Res. , 34 : 196 – 204 .
- Fletcher , RL . 1980 . Studies of the recently introduced brown alga Sargassum muticum (Yendo) Fensholt. III. Periodicity in gamete release and ‘incubation’ of early germling stages . Bot. Mar. , 31 : 425 – 432 .
- Fleurence , J . 1999 . Seaweed proteins: biochemical, nutritional aspects and potential uses . Trends Food Sci. Technol. , 10 : 25 – 28 .
- Fortes , MD and Lüning , K . 1980 . Growth rates of North Sea macroalgae in relation to temperature, irradiance and photoperiod . Helgoländer Meeresunters. , 34 : 15 – 29 .
- Fralick , RA , Turgeon , KW and Mathieson , AC . 1974 . Destruction of kelp populations by Lacuna vincta (Montagu) . Nautilus , 88 : 112 – 114 .
- Franklin , LA , Osmond , CB and Larkum , AWD . 2003 . “ Photoinhibition, UV–B and algal photosynthesis ” . In Photosynthesis in Algae , Edited by: Larkum , AWD , Douglas , SE and Raven , JA . 351 – 384 . Dordrecht, , Germany : Kluwer Academic .
- Fredj , G . 1972 . Compte rendu de plongées en SP300 sur les fonds à Laminaria rodriguezii Bornet de la Pointe de revellata (Corse) . Bull. Inst. Océanogr. Monaco , 71 : 1 – 42 .
- Fredriksen , S . 2003 . Food web studies in a Norwegian kelp forest based on stable isotope (δ13C and δ15N) analysis . Mar. Ecol. Prog. Ser. , 260 : 71 – 81 .
- Fredriksen , S , Sjøtun , K , Lein , TE and Rueness , J . 1995 . Spore dispersal in Laminaria hyperborea (Laminariales, Phaeophyceae) . Sarsia , 80 : 47 – 54 .
- Fukuhara , Y , Mizuta , H and Yasui , H . 2002 . Swimming activities of zoospores in Laminaria japonica (Phaeophyceae) . Fish. Sci. , 68 : 1173 – 1181 .
- Funano , T . 1980 . Crossing experiments between several species of Laminaria in Hokkaido . Hokusuishi–Geppo , 37 : 181 – 207 .
- Funano , T . 1983 . The ecology of Laminaria religiosa Miyabe. I. The life history and the alternation of nuclear phases of Laminaria religiosa, and the physiological ecology of the gametophytes and the embryonal sporophytes . Sci. Rep. Hokkaido Fish. Exp. Stat. , 25 : 61 – 109 .
- Gabrielson , PW , Widdowson , TB and Lindstrom , SC . 2006 . Keys to the seaweeds and seagrasses of southeast Alaska. British Columbia, Washington and Oregon . Phycological Contribution , 7 : 1 – 209 .
- Gagné , JA , Mann , KH and Chapman , ARO . 1982 . Seasonal patterns of growth and storage in Laminaria longicruris in relation to differing patterns of availability of nitrogen in the water . Mar. Biol. , 69 : 91 – 101 .
- Gagnon , P , Himmelman , JH and Johnson , LE . 2003 . Algal colonization in urchin barrens: defense by association during recruitment of the brown alga Agarum cribrosum . J. Exp. Mar. Biol. Ecol. , 290 : 179 – 196 .
- Gagnon , P , St-Hilaire-Gravel , LV , Himmelman , JH and Johnson , LE . 2006 . Organismal defenses versus environmentally mediated protection from herbivores: Unraveling the puzzling case of Desmarestia viridis (Phaeophyta) . J. Exp. Mar. Biol. Ecol. , 334 : 10 – 19 .
- Gao , K and McKinley , KR . 1994 . Use of macroalgae for marine biomass production and CO2 remediation: a review . J. Appl. Phycol. , 6 : 45 – 60 .
- Garbary , DJ , Kim , KY , Klinger , T and Duggins , D . 1999 . Red algae as hosts for endophytic kelp gametophytes . Mar. Biol. , 135 : 35 – 40 .
- Gardner , MJ , Hubbard , KE , Hotta , CT , Dodd , AN and Webb , AA . 2006 . How plants tell the time . Biochem. J. , 397 : 15 – 24 .
- Garman , GD , Pillai , M and Cherr , GN . 1994 . Inhibition of cellular events during early algal gametophyte development: effects of selected metals and an aqueous petroleum waste . Aquatic Toxicol. , 28 : 127 – 144 .
- Gerard , VA . 1987 . Hydrodynamic streamlining of Laminaria saccharina Lamour. in response to mechanical stress . J. Exp. Mar. Biol. Ecol. , 107 : 237 – 244 .
- Gerard , VA . 1988 . Ecotypic differentiation in light related traits of the kelp Laminaria saccharina . Mar. Biol. , 97 : 25 – 36 .
- Gerard , VA . 1997 . The role of nitrogen nutrition in high-temperature tolerance of the kelp Laminaria saccharina (Chromophyta) . J. Phycol. , 33 : 800 – 810 .
- Gerard , VA and Du Bois , KR . 1988 . Temperature ecotypes near the southern boundary of the kelp Laminaria saccharina . Mar. Biol. , 97 : 575 – 580 .
- Gerard , VA and Mann , KH . 1979 . Growth and production of Laminaria longicruris (Phaeophyta) populations exposed to different intensities of water movement . J. Phycol. , 15 : 33 – 41 .
- Gerard , VA , DuBois , K and Greene , R . 1987 . Growth responses of two Laminaria saccharina populations to environmental variation . Hydrobiologia , 151/152 : 229 – 232 .
- Gévaert , F , Davoult , D , Creach , A , Kling , R , Janquin , MA , Seuront , L and Lemoine , Y . 2001 . Carbon and nitrogen content of Laminaria saccharina in the eastern English Channel: biometrics and seasonal variations . J. Mar. Biol. Assoc. UK , 81 : 727 – 734 .
- Gévaert , F , Creach , A , Davoult , D , Migne , A , Levavasseur , G , Arzel , P , Holl , A and Lemoine , Y . 2003 . Laminaria saccharina photosynthesis measured in situ: photoinhibition and xanthophyll cycle during a tidal cycle . Mar. Ecol. Prog. Ser. , 247 : 43 – 50 .
- Giaccone , G . 1972 . Struttura, ecologia e corologia dei popolamenti a Laminarie dello stretto di Messina e del mare di Alboran . Mem. Biol. Mar. Ocean NS , 2 : 37 – 49 .
- Givernaud , T , Cosson , J and Givernaud-Mouradi , A . 1991 . “ Étude des populations de Sargassum muticum (Yendo) Fensholt sur les côtes de Basse-Normandie (France) ” . In Estuaries and Coasts: Spatial and Temporal Intercomparisons. ECSA 19 Symposium, Caen, September 1989 , Edited by: Elliott , M and Ducrotoy , JP . 129 – 132 . Fredensborg, , Denmark : Olsen & Olsen .
- Gómez , I and Lüning , K . 2001 . Constant short–day treatment of outdoor–cultivated Laminaria digitata prevents summer drop in growth rate . Eur. J. Phycol. , 36 : 391 – 395 .
- Goodwin , KD , North , WJ and Lidstrom , ME . 1997 . Production of bromoform and dibromomethane by giant kelp: factors affecting release and comparison to anthropogenic bromine sources . Limnol. Oceanogr. , 42 : 1725 – 1734 .
- Gordillo , FJL , Dring , MJ and Savidge , G . 2002 . Nitrate and phosphate uptake characteristics of three species of brown algae cultured at low salinity . Mar. Ecol. Prog. Ser. , 234 : 111 – 116 .
- Gordillo , FJL , Aguielra , J and Jiménez , C . 2006 . The response of nutrient assimilation and biochemical composition of Arctic seaweeds to a nutrient input in summer . J. Exp. Bot. , 57 : 2661 – 2671 .
- Gorgula , SK and Connell , SD . 2004 . Expansive covers of turf–forming algae on human-dominated coast: The relative effects of increasing nutrient and sediment loads . Mar. Biol. , 145 : 613 – 619 .
- Goulard , F , Lüning , K and Jacobsen , S . 2004 . Circadian rhythm of photosynthesis and concurrent oscillations of transcript abundance of photosynthetic genes in the marine red alga . Grateloupia turuturu. Eur. J. Phycol. , 39 : 431 – 437 .
- Graham , LE and Wilcox , LW . 1999 . Algae , Englewood Cliffs, , USA : Prentice Hall Inc .
- Granbom , M , Pedersen , M and Lüning , K . 2001 . Circadian rhythm of photosynthetic oxygen evolution in Kappaphycus alvarezii (Rhodophyta): dependence on light quantity and quality . J. Phycol. , 37 : 1020 – 1025 .
- Granbom , M , Chow , F , Lopes , PF , De Oliveira , MC , Colepicolo , P , De Paula , EC and Pedersen , M . 2004 . Characterisation of nitrate reductase in the marine macroalga Kappaphycus alvarezii (Rhodophyta) . Aquat. Bot. , 78 : 295 – 305 .
- Graeve , M , Kattner , G , Wiencke , C and Karsten , U . 2002 . Fatty acid composition of Arctic and Antarctic macroalgae: indicator of phylogenetic and trophic relationships . Mar. Ecol. Prog. Ser. , 231 : 67 – 74 .
- Greene , RM and Gerard , VA . 1990 . Effect of high-frequency light fluctuations on growth and photoacclimation of the red alga Chondrus crispus . Mar. Biol. , 105 : 337 – 344 .
- Grzymski , J , Johnsen , G and Sakshaug , E . 1997 . The significance of intracellular self-shading on the biooptical properties of brown, red, and green macroalgae . J. Phycol. , 33 : 408 – 414 .
- Guiry , MD and Guiry , GM . 2007 . AlgaeBase version 4.2 , Worldwide electronic publication, National University of Ireland, Galway Available at: http://www.algaebase.org, accessed 26 October 2006
- Gwinner , E . 1989 . Photoperiod as a modifying and limiting factor in the expression of avian circannual rhythms . J. Biol. Rhythms , 4 : 237 – 250 .
- Gwinner , E . 2003 . Circannual rhythms in birds . Curr. Opin. Neurobiol. , 13 : 770 – 778 .
- Hagen , NT . 1983 . Destructive grazing of kelp beds by sea urchins in Vestfjorden, northern Norway . Sarsia , 68 : 177 – 190 .
- Hagen , NT . 1987 . Sea urchin outbreaks and nematode epizootics in Vestfjorden, northern Norway . Sarsia , 72 : 213 – 229 .
- Hagen , NT . 1995 . Recurrent destructive grazing of successionally immature kelp forests by green sea urchins in Vestfjorden, northern Norway . Mar. Ecol. Prog. Ser. , 123 : 95 – 106 .
- Hallam , ND , Clayton , MN and Parish , D . 1980 . Studies on the association between Notheia anomala and Hormosira banksii (Phaeophyta) . Aust. J. Bot. , 28 : 239 – 248 .
- Hammerstrom , K , Dethier , MN and Duggins , DO . 1998 . Rapid phlorotannin induction and relaxation in five Washington kelps . Mar. Ecol. Progr. Ser. , 165 : 293 – 305 .
- Han , L , Fan , X and Li , X . 2001 . Study on organic iodine in seaweed. 2. The states and content of organic iodine in seaweed . Stud. Mar. Sin./Haiyang Kexue Jikan , 43 : 129 – 135 .
- Han , T . 1993 . Wavelength dependent effect of high irradiance on early sporophytes of Laminaria hyperborea (Phaeophyta) . Kor. J. Phycol. , 8 : 199 – 205 .
- Han , T and Kain , JM . 1996 . Effect of photon irradiance and photoperiod on young sporophytes of four species of the Laminariales . Eur. J. Phycol. , 31 : 233 – 240 .
- Hanelt , D . 1998 . Capability of dynamic photoinhibition in Arctic macroalgae is related to their depth distribution . Mar. Biol. , 131 : 361 – 369 .
- Hanelt , D , Wiencke , C , Karsten , U and Nultsch , W . 1997a . Photoinhibition and recovery after high light stress in different developmental and life–history stages of Laminaria saccharina (Phaeophyta) . J. Phycol. , 33 : 387 – 395 .
- Hanelt , D , Wiencke , C and Nultsch , W . 1997b . Influence of UV radiation on the photosynthesis of Arctic macroalgae in the field . J. Photochem. Photobiol. B. , 38 : 40 – 47 .
- Hanelt , D , Tüg , H , Bischof , K , Groß , C , Lippert , H , Sawall , T and Wiencke , C . 2001 . Light regime in an Arctic fjord: a study related to stratospheric ozone depletion as a basis for determination of UV effects on algal growth . Mar. Biol. , 138 : 649 – 658 .
- Hanelt , D , Wiencke , C and Bischof , K . 2003 . “ Photosynthesis in marine macroalgae ” . In Photosynthesis in Algae , Edited by: Larkum , WA , Douglas , E and Raven , JA . 413 – 435 . Dordrecht, , Germany : Kluwer Academic .
- Hanssen , JF , Indergaard , M , Østgaard , K , Baevre , OA , Pedersen , TA and Jensen , A . 1987 . Anaerobic digestion of Laminaria spp. and Ascophyllum nodosum and application of end products . Biomass , 14 : 1 – 13 .
- Harmer , SL , Hogenesh , JB , Straume , M , Chang , HS , Han , B , Zhu , T , Wang , X , Kreps , JA and Kay , SA . 2000 . Orchestrated transcription of key pathways in Arabidopsis by the circadian clock . Science , 290 : 2110 – 2113 .
- Harries , R . 1932 . An investigation by cultural methods of some of the factors influencing the development of the gametophytes and the early stages of the sporophytes of Laminaria digitata, L. saccharina and L. cloustonii . Ann. Bot. , 46 : 893 – 928 .
- Harris , LG and Tyrrell , MC . 2001 . Changing community states in the Gulf of Maine: synergism between invaders, overfishing and climate change . Biol. Invasions , 3 : 9 – 21 .
- Harrison , PJ , Druehl , LD , Lloyd , KE and Thompson , PA . 1986 . Nitrogen uptake kinetics in three year–classes of Laminaria groenlandica (Laminariales, Phaeophyta) . Mar. Biol. , 93 : 29 – 35 .
- Harrold , C and Reed , DC . 1985 . Food availability, sea urchin grazing, and kelp forest community structure . Ecology , 66 : 1160 – 1169 .
- Hasegawa , Y . 1971 . Forced cultivation of Laminaria . Bull. Hokkaido Reg. Fish. Res. Lab. , 37 : 49 – 52 .
- Hastings , JW , Rusak , B and Boulos , Z . 1991 . “ Circadian rhythms: the physiology of biological timing ” . In Neural and Integrative Animal Physiology: Comparative Animal Physiology , Edited by: Prosser , CL . 435 – 546 . New York, , USA : Wiley–Liss Inc. .
- Hastings , MH and Follett , BK . 2001 . Towards a molecular biological calendar? . J. Biol. Rhythms , 16 : 424 – 430 .
- Hatcher , BG , Chapman , ARO and Mann , KH . 1977 . An annual carbon budget for the kelp Laminaria longicruris . Mar. Biol. , 44 : 85 – 96 .
- Haug , A and Jensen , A . 1954 . Seasonal variation in the chemical composition of Alaria esculenta, Laminaria saccharina, L. hyperborea and L. digitata from northern Norway . Norsk inst. F. tang–og tareforskning , 4 : 1 – 14 .
- Hawkins , JS and Harkin , E . 1985 . Preliminary canopy removal experiments in algal dominated communities low on the shore and in the shallow subtidal on the Isle of Man . Bot. Mar. , 28 : 223 – 230 .
- Hawkins , JS and Hartnoll , RG . 1985 . Factors determining the upper limits of intertidal canopy–forming algae . Mar. Ecol. Prog. Ser. , 20 : 265 – 271 .
- He , ML , Hollwich , W and Rambeck , WA . 2002 . Supplementation of algae to the diet of pigs: a new possibility to improve the iodine content in the meat . J. Anim. Physiol. A. Anim. Nutr. , 86 : 97 – 104 .
- Heesch , S and Peters , AF . 1999 . Scanning electron microscopy observation of host entry by two brown algae endophytic in Laminaria saccharina (Laminariales, Phaeophyceae) . Phycol. Res. , 47 : 1 – 5 .
- Hellebust , JA and Haug , A . 1972 . Photosynthesis, translocation, and alginic acid synthesis in Laminaria digitata and Laminaria hyperborea . Can. J. Bot. , 50 : 169 – 176 .
- Henley , WJ and Dunton , KH . 1995 . A seasonal comparison of carbon, nitrogen, and pigment content in Laminaria solidungula and L. saccharina (Phaeophyta) in the Alaskan arctic . J. Phycol. , 31 : 325 – 331 .
- Henley , WJ and Dunton , KH . 1997 . Effects of nitrogen supply and continuous darkness on growth and photosynthesis of the arctic kelp Laminaria solidungula . Limnol. Oceanogr. , 42 : 209 – 216 .
- Henry , BE and Van Alstyne , KL . 2004 . Effects of UV radiation on growth and phlorotannins in Fucus gardneri (Phaeophyceae) juveniles and embryos . J. Phycol. , 40 : 527 – 533 .
- Henry , EC and Cole , K . 1982a . Ultrastructure of swarmers in the Laminariales (Phaeophyceae). I. Zoospores . J. Phycol. , 18 : 550 – 569 .
- Henry , EC and Cole , K . 1982b . Ultrastructure of swarmers in the Laminariales (Phaeophyceae). II. Sperm . J. Phycol. , 18 : 570 – 579 .
- Hepburn , CD and Hurd , CL . 2005 . Conditional mutualism between the giant kelp Macrocystis pyrifera and colonial epifauna . Mar. Ecol. Prog. Ser. , 302 : 37 – 48 .
- Hepburn , CD , Hurd , CL and Frew , RD . 2006 . Colony structure and seasonal differences in light and nitrogen modify the impact of sessile epifauna on the giant kelp Macrocystis pyrifera (L.) C. Agardh . Hydrobiologia , 560 : 373 – 384 .
- Hesley , C . 1997 . Open Ocean Aquaculture: Charting the Future of Ocean Farming . Proceedings of an International Conference . April 23–25 1997 . pp. 353 Hawaii, , USA : Maui .
- Himmelman , JH , Cardinal , A and Bourget , E . 1983 . Community development following removal of urchins, Strongylocentrotus droebachiensis, from the rocky subtidal zone of the St. Lawrence Estuary, eastern Canada . Oecologia , 59 : 27 – 39 .
- Hjörleifsson , E , Kaasa , Ö and Gunnarsson , K . 1995 . “ Grazing of kelp by green sea urchin in Eyjafjördur, North Iceland ” . In Ecology of Fjords and Coastal Waters , Edited by: Hopkins , C , Erikstad , KE and Leinaas , HP . 593 – 597 . Amsterdam, , The Netherlands : Elsevier Science .
- Holt , TJ . 1984 . The development of techniques for the cultivation of Laminariales in the Irish Sea , Liverpool, , UK : University of Liverpool . PhD thesis
- Holt , TJ and Kain , JM . 1983 . “ The cultivation of large brown algae as an energy crop ” . In Energy from Biomass , Edited by: Strup , A , Chartier , P and Schleser , G . 319 – 323 . London, UK/New York, , USA : Applied Science Publishers . 2nd E.C. Conference.
- Honya , M , Nishizawa , Y and Kashiwabara , K . Seasonal variation of fatty acids and their metabolism in Laminaria japonica of forced cultivation . Abstract, 1. International Marine Biotechnology Conference . Tokyo, , Japan
- Honya , M , Kinosita , T , Ishikawa , M , Mori , H and Nisizawa , K . 1993 . Monthly determination of alginate, M/G ratio, mannitol, and minerals in cultivated Laminaria japonica . Nippon Suisan Gakkaishi/Bull. Jap. Soc. Sci. Fish. , 59 : 295 – 299 .
- Honya , M , Kinosita , T , Ishikawa , M , Mori , H and Nisizawa , K . 1994 . Seasonal variation in free and bound amino acids of cultured Laminaria japonica . J. Mar. Biotechnol. , 2 : 19 – 22 .
- Hooper , RG . 1984 . Functional adaptations to the polar environment by the arctic kelp Laminaria solidungula . Br. Phycol. J. , 19 : 194
- Hopkin , R and Kain , JM . 1978 . The effects of some pollutants on the survival, growth and respiration of Laminaria hyperborea . Estuar. Coast. Mar. Res. , 7 : 531 – 553 .
- Horn , SJ , Aasen , IM and Østgaard , K . 2000 . Ethanol production from seaweed extract . J. Ind. Microbiol. Biotechnol. , 25 : 249 – 254 .
- Hou , X , Chai , XC , Qian , Q , Yan , X and Fan , X . 1997 . Determination of chemical species of iodine in some seaweeds . Sci. Total Environ. , 204 : 215 – 221 .
- Hu , Y and Zhou , Z . 2001 . Extraction of RAPD–friendly DNA from Laminaria japonica (Phaeophyta) after enzymatic dissociation of the frozen sporophyte tissues . J. Appl. Phycol. , 13 : 415 – 422 .
- Huang , J , Tang , X , Liu , T and Li , Y . 2002 . Alteration of activated oxygen and antioxidant system in kelp during alginic acid decomposing bacteria infection . J. Ocean Univ. Qingdao/Qingdao Haiyang Daxue Xuebao , 32 : 574 – 578 .
- Huovinen , PS , Oikari , AOJ , Soimasuo , MR and Cherr , GN . 2000 . Impact of UV radiation on the early development of the giant kelp (Macrocystis pyrifera) gametophytes . Photochem. Photobiol. , 72 : 308 – 313 .
- Hurd , CL , Durante , KM and Harrison , PJ . 2000 . Influence of bryozoan colonization on the physiology of the kelp Macrocystis integrifolia (Laminariales, Phaeophyta) from nitrogen–rich and–poor sites in Barkley Sound, British Columbia, Canada . Phycologia , 39 : 435 – 440 .
- Hutchinson , GE . 1957 . Concluding remarks. Cold Spring Harbor Symposium . Quant. Biol. , 22 : 415 – 427 .
- Huvé , H . 1955 . Présence de Laminaria rodriguezii Bornet sur les côtes francaises de Mediterranée . Rec. Trav. Stat. Mar. Endoume , 15 : 73 89 + 11 plates
- Ikawa , T , Watanabe , T and Nisizawa , K . 1972 . Enzymes involved in the last steps of the biosynthesis of mannitol in brown algae . Plant Cell Physiol. , 13 : 1017 – 1029 .
- Indergaard , M and Minsaas . 1991 . “ Animal and human nutrition ” . In Seaweed Resources in Europe. Uses and Potential , Edited by: Guiry , MD and Blunden , G . 21 – 64 . Chichester, UK : John Wiley & Sons .
- Indergaard , M and Østgaard , K . 1991 . “ Polysaccharides for food and pharmaceutical uses ” . In Seaweed Resources in Europe. Uses and Potential , Edited by: Guiry , MD and Blunden , G . 169 – 183 . Chichester, , UK : John Wiley & Sons .
- Ireland , CD and Horn , MH . 1991 . Effects of macrophyte secondary chemicals on food choice and digestive efficiency of Cebidichthys violaceus (Girard), and herbivorous fish of temperate waters . J. Exp. Mar. Biol. Ecol. , 153 : 179 – 194 .
- Iwamoto , K and Shiraiwa , Y . 2005 . Salt–regulated mannitol metabolism in algae . Mar. Biotechn. , 7 : 407 – 415 .
- Izquierdo , JL , Pérez–Ruzafa , I and Gallardo , T . 2002 . Effect of temperature and photon fluence rate on gametophytes and young sporophytes of Laminaria ochroleuca Pylaie . Helgol. Mar. Res. , 55 : 285 – 292 .
- Jacobsen , S , Lüning , K and Goulard , F . 2003 . Circadian changes in relative transcript abundance of two photosynthetic transcripts in the marine macroalga Kappaphycus alvarezii (Rhodophyta) . J. Phycol. , 39 : 888 – 896 .
- Jennings , DB , Ehrenshaft , M , Pharr , DM and Williamson , JD . 1998 . Roles for mannitol and mannitol dehydrogenase in active oxygen–mediated plant defense . Proc. Natl. Acad. Sci. USA , 95 : 15129 – 15133 .
- Jennings , JG and Steinberg , PD . 1997 . Phlorotannins versus other factors affecting epiphyte abundance on the kelp Ecklonia radiata . Oecologia , 109 : 461 – 473 .
- Jensen , A . 1993 . Present and future needs for algae and algal products . Hydrobiologia , 260/261 : 15 – 23 .
- Jerlov , NG . 1968 . Optical Oceanography , Amsterdam : Elsevier .
- Jia , J and Chen , J . 2001 . “ Sea farming and sea ranching in China ” . In FAO Fisheries Technical Paper, 418 , Rome, Italy : Food and Agriculture Organisation of the United Nations .
- John , DM . 1970 . Differences in the growth of three species of Laminaria along a depth gradient . Nova Hedwigia. , 19 : 789 – 798 .
- Johnson , AS and Koehl , MAR . 1994 . Maintenance of dynamic strain similarity and environmental stress factor in different flow habitats: thallus allometry and material properties of a giant kelp . J. Exp. Biol. , 195 : 381 – 410 .
- Johnson , CR and Mann , KH . 1986 . The importance of plant defence abilities to the structure of subtidal seaweed communities: The kelp Laminaria longicruris de la Pylaie survives grazing by the snail Lacuna vincta (Montagu) at high population densities . J. Exp. Mar. Biol. Ecol. , 97 : 231 – 267 .
- Johnson , CR and Mann , KH . 1988 . Diversity, patterns of adaptation, and stability of Nova Scotian kelp beds . Ecol. Monogr. , 58 : 129 – 154 .
- Johnson , CR and Mann , KH . 1993 . Rapid succession in subtidal understorey seaweeds during recovery from overgrazing sea urchins in eastern Canada . Bot. Mar. , 36 : 63 – 77 .
- Johnson , CH and Kondo , T . 2001 . “ Circadian rhythms in unicellular organisms ” . In Handbook of Behavioral Neurobiology , Edited by: Takahashi , JS , Turek , FW and Moore , RY . Vol. 12 , 61 – 77 . New York, , USA : Plenum Press .
- Joly , AB and De Oliveira Filho , EC . 1967 . Two Brazilian Laminarias . Publ. Inst. Pesq. Mar. , 4 : 1 – 13 .
- Jordan , P , Kloareg , B and Vilter , H . 1991 . Detection of vanadate–dependent bromoperoxidases in protoplasts from the brown algae Laminaria digitata and L. saccharina . J. Plant Physiol. , 137 : 520 – 524 .
- Jorde , I and Klavestad , N . 1963 . The natural history of the Hardangerfjord. 4. The benthonic algal vegetation . Sarsia , 9 : 1 – 99 .
- Jormalainen , V , Honkanen , T , Koivikko , R and Eränen , J . 2003 . Induction of phlorotannin production in a brown alga: defense or resource dynamics . Oikos , 103 : 640 – 650 .
- Jørgensen , NM and Christie , H . 2003 . Diurnal, horizontal and vertical dispersal of kelp–associated fauna . Hydrobiologia , 503 : 69 – 76 .
- Kai , T , Nimura , K , Yasui , H and Mizuta , H . 2006 . Regulation of sorus formation by auxin in Laminariales sporophytes . J. Appl. Phycol. , 18 : 95 – 101 .
- Kain , JM . 1971 . Synopsis of biological data on Laminaria hyperborea . FAO Fisheries Synopsis , 87 : 1 – 68 .
- Kain , JM . 1975 . The biology of Laminaria hyperborea VII. Reproduction of the sporophyte . J. Mar. Biol. Ass. UK , 55 : 567 – 582 .
- Kain , JM . 1976 . The biology of Laminaria hyperborea VIII. Growth on cleared areas . J. Mar. Biol. Ass. UK , 56 : 267 – 290 .
- Kain , JM . 1979 . A view of the genus Laminaria . Ann. Rev. Oceanogr. Mar. Biol. , 17 : 101 – 161 .
- Kain , JM . 1989 . The seasons in the subtidal . Br. Phycol. J. , 24 : 203 – 215 .
- Kain , JM . 1991 . “ Cultivation of attached seaweeds ” . In Seaweed Resources in Europe. Uses and Potential , Edited by: Guiry , MD and Blunden , G . 309 – 377 . Chichester, , UK : John Wiley & Sons .
- Kain , JM and Dawes , CP . 1987 . Useful European seaweeds: past hopes and present cultivation . Hydrobiologia , 151/152 : 173 – 181 .
- Kang , RS and Koh , CH . 1999 . Growth and survival of Laminaria japonica Areschoug at different densities . J. Korean Fish. Soc. , 32 : 444 – 451 . (In Korean, English abstract.)
- Karez , R and Chapman , ARO . 1998 . A competitive hierarchy model integrating roles of physiological competence and competitive ability does not provide a mechanistic explanation for the zonation of three intertidal Fucus species in Europe . Oikos , 81 : 471 – 494 .
- Karsten , U . 2007 . Salinity tolerance of Arctic kelps from Spitsberen . Phycol. Res. , (in press)
- Karsten , U , Barrow , KD , Nixdorf , O , West , JA and King , RJ . 1997 . Characterization of mannitol metabolism in the mangrove red alga Caloglossa leprieurii (Montagne) J. Agardh . Planta , 201 : 173 – 178 .
- Kawai , H and Sasaki , H . 2000 . Molecular phylogeny of the brown algal genera Akkesiphycus and Halosiphon (Laminariales), resulting in the circumscription of the new families Akkesiphycaceae and Halosiphonaceae . Phycologia , 39 : 416 – 428 .
- Kawai , T , Kaneta , T and Kuwahara , H . 2003 . Optimum removed timing of the sea urchin and mean benthic velocity for afforestation of the kelp in Isoyake area using fence . Fisheries engineering (Japan)/Suisan Kogaku (Japan) , 39 : 197 – 204 .
- Kawamata , S . 1997 . Modelling the feeding rate of the sea urchin Strongylocentrotus nudus (A. Agassiz) on kelp . J. Exp. Mar. Biol. Ecol. , 210 : 107 – 127 .
- Kawamata , S . 1998 . Effect of wave–induced oscillatory flow on grazing by a subtidal sea urchin Strongylocentrotus nudus (A. Agassiz) . J. Exp. Mar. Biol. Ecol. , 224 : 3 – 48 .
- Kawamata , S . 2001a . Adaptive mechanical tolerance and dislodgement velocity of the kelp Laminaria japonica in wave–induced water motion . Mar. Ecol. Prog. Ser. , 211 : 89 – 104 .
- Kawamata , S . 2001b . Effect of waves on grazing by sea urchins and abalone on the coast of northern Japan . Bull. Fish. Res. Agency , 1 : 59 – 107 . (In Japanese, English abstract.)
- Kawashima , S . 1983 . Sporangial sorus formation of Laminaria angustata Kjellman . Jpn. J. Phycol. , 31 : 208 – 216 .
- Kawashima , S . 1984 . Kombu cultivation in Japan for human foodstuff . Jpn. J. Phycol. , 32 : 379 – 394 .
- Keats , DW , South , GR and Steele , DH . 1982 . Experimental assessment of the effect of Strongylocentrotus droebachiensis on subtidal algal communities in Newfoundland, Canada . Br. Phycol. J. , 17 : 234 – 235 .
- Keats , DW , South , GR and Steele , DH . 1990 . Effects of an experimental reduction in grazing by green sea urchins on a benthic macroalgal community in eastern Newfoundland . Mar. Ecol. Prog. Ser. , 68 : 181 – 193 .
- Keddy , PA . 1989 . Competition , London, , UK : Chapman & Hall .
- King , RJ and Schramm , W . 1976 . Determination of photosynthetic rates for the marine algae Fucus vesiculosus and Laminaria digitata . Mar. Biol. , 37 : 209 – 213 .
- Kirst , GO . 1990 . Salinity tolerance of eukaryotic marine algae . Ann. Rev. Plant Physiol. Plant Mol. Biol. , 41 : 21 – 53 .
- Kitching , JA and Thain , VM . 1983 . The ecological impact of the sea urchin Paracentrotus lividus (Lamarck) in Lough Ine, Ireland . Phil. Trans. R. Soc. Lond., Ser. B. , 300 : 513 – 552 .
- Klenell , M , Snoeijs , P and Pedersén , M . 2002 . The involvement of a plasma membrane H +–ATPase in the blue–light enhancement of photosynthesis in Laminaria digitata (Phaeophyta) . J. Phycol. , 38 : 1143 – 1149 .
- Klenell , M , Snoeijs , P and Pedersen , M . 2004 . Active carbon uptake in Laminaria digitata and L. saccharina (Phaeophyta) is driven by a proton pump in the plasma membrane . Hydrobiologia , 514 : 41 – 53 .
- Klinger , T . 1984 . Allocation of blade surface area to meiospore production in annual and perennial representatives of the genus Laminaria , Vancouver, , Canada : University of British Columbia . MSc thesis
- Klinger , T and De Wreede , RE . 1988 . Stipe rings, age, and size in populations of Laminaria setchellii Silva (Laminariales, Phaeophyta) in British Columbia, Canada . Phycologia , 27 : 234 – 240 .
- Klotchkova , NG and Berezovskaya , VA . 2000 . Influence of anthropogenic pollution on macrophytobenthos of the Avacha Bay (southeast Kamchatka, Russia) . J. Phycol. , 36 : 37
- Koehl , MAR and Alberte , RS . 1988 . Flow, flapping and photosynthesis of Nereocystis luetkeana: A functional comparison of undulate and flat blade morphologies . Mar. Biol. , 99 : 435 – 444 .
- Kohlmeyer , J . 1979 . Marine fungal pathogens among Ascomycetes and Deuteromycetes . Experientia , 35 : 437 – 439 .
- Koivikko , R , Loponen , J , Honkanen , T and Jormalainen , V . 2005 . Contents of soluble, cell–wall–bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implication on their ecological functions . J. Chem. Ecol. , 31 : 195 – 212 .
- Konar , B and Estes , JA . 2003 . The stability of boundary regions between kelp beds and deforested areas . Ecology , 84 : 174 – 185 .
- Korb , RE and Gerard , VA . 2000a . Nitrogen assimilation characteristics of polar seaweeds from differing nutrient environments . Mar. Ecol. Prog. Ser. , 198 : 83 – 92 .
- Korb , RE and Gerard , VA . 2000b . Effects of concurrent low temperature and low nitrogen supply on polar and temperate seaweeds . Mar. Ecol. Progr. Ser. , 198 : 73 – 82 .
- Kornfeldt , R-A . 1984 . Variation in distribution and biomass of marine benthic algae of Kullen, S Sweden . Nord. J. Bot. , 4 : 563 – 584 .
- Kostetsky , EY , Goncharova , SN , Sanina , NM and Shnyrov , VL . 2004 . Seasonal influence on lipid composition of marine macrophytes . Bot. Mar. , 47 : 134 – 139 .
- Kraan , S and Guiry , MD . 2000 . Sexual hybridization experiments and phylogenetic relationships as inferred from Rubisco spacer sequences in the genus Alaria . J. Phycol. , 36 : 190 – 198 .
- Krause , G , Buck , BH and Rosenthal , H . Multifunctional use and environmental regulations: Potentials in the offshore aquaculture development in Germany . Proceedings of the Multidisciplinary Scientific Conference on Sustainable Coastal Zone Management “Rights and Duties in the Coastal Zone” . Edited by: Sjoestroem , A . Stockholm, , Sweden Management Beijer Occasional Paper Series CD ROM
- Kremer , BP . 1980 . Transversal profiles of carbon assimilation in the fronds of three Laminaria species . Mar. Biol. , 59 : 95 – 103 .
- Kremer , BP . 1984 . Carbohydrate reserve and dark carbon fixation in the brown macroalga Laminaria hyperborea . Plant Physiol. , 117 : 233 – 242 .
- Kremer , BP . 1985 . Aspects of cellular compartmentation in brown marine macroalgae . J. Plant Physiol. , 120 : 401 – 407 .
- Kremer , BP and Küppers , U . 1977 . Carboxylating enzymes and pathway of photosynthetic carbon assimilation in different marine algae–evidence for the C4–pathway? . Planta , 133 : 191 – 196 .
- Kremer , BP and Markham , JW . 1979 . Carbon assimilation by different developmental stages of Laminaria saccharina . Planta , 144 : 497 – 501 .
- Kubanek , J , Lester , SE , Fenical , W and Hay , ME . 2004 . Ambiguous role of phlorotannins as chemical defenses in the brown alga Fucus vesiculosus . Mar. Ecol. Prog. Ser. , 277 : 79 – 93 .
- Kucho , K , Okamoto , K , Tabata , S , Fukuzawa , H and Ishiura , M . 2005 . Identification of novel clock–controlled genes by cDNA macroarray analysis in Chlamydomonas reinhardtii . Plant Mol. Biol. , 57 : 889 – 906 .
- Kumura , T , Yasui , H and Mizuta , H . 2006 . Nutrient requirement for zoospore formation in two alariaceous plants Undaria pinnatifida (Harvey) Suringar and Alaria crassifolia Kjellman (Phaeophyceae: Laminariales) . Fish. Science , 72 : 860 – 869 .
- Kühl , M , Glud , RN , Borum , J , Roberts , R and Rysgaard , S . 2001 . Photosynthetic performance of surface–associated algae below sea ice as measured with a pulse–amplitude–modulated (PAM) fluorometer and O2 microsensors . Mar. Ecol. Prog. Ser. , 223 : 1 – 14 .
- Küpper , FC , Schweigert , N , Ar Gall , E , Legendre , JM , Vilter , H and Kloareg , B . 1998 . Iodine uptake in Laminariales involves extracellular, haloperoxidase–mediated oxidation of iodide . Planta , 207 : 163 – 171 .
- Küpper , FC , Kloareg , B , Guern , J and Potin , P . 2001 . Oligoguluronates elicit an oxidative burst in the brown algal kelp Laminaria digitata . Plant Physiol. , 125 : 278 – 291 .
- Küpper , FC , Müller , DG , Peters , AF , Kloareg , B and Potin , P . 2002 . Oligoalginate recognition and oxidative burst play a key role in natural and induced resistance of sporophytes of Laminariales . J. Chem. Ecol. , 28 : 2057 – 2081 .
- Küpper , FC , Gaquerel , E , Boneberg , E-M , Morath , S , Salaün , J-P and Potin , P . 2006 . Early events in the perception of lipopolysaccharides in the brown alga Laminaria digitata include an oxidative burst and activation of fatty acid oxidation cascades . J. Exp. Bot. , 57 : 1991 – 1999 .
- Küppers , U and Weidner , M . 1980 . Seasonal variation of enzyme activities in Laminaria hyperborea . Planta , 148 : 222 – 230 .
- Kuwahara , H . 2003 . Studies on mechanisms for kelp (Laminaria religiosa) forest development on the barren ground, along the southwest coast of Hokkaido, Japan . Fisheries engineering (Japan)/Suisan Kogaku (Japan) , 39 : 213 – 218 . (In Japanese, English abstract.)
- La Barre , SL , Weinberger , F , Kervarec , N and Potin , P . 2004 . Monitoring defensive responses in macroalgae: Limitations and perspectives . Phytochem. Rev. , 3 : 371 – 379 .
- Lambert , W , Levin , PS and Berman , J . 1992 . Changes in the structure of a New England (USA) kelp bed: The effects of an introduced species? . Mar. Ecol. Prog. Ser. , 88 : 303 – 307 .
- Lamouroux , JVF . 1813 . Essai sur les genres de la famille des thalassiophytes non articulées . Annales du Muséum d'Histoire Naturelle, Paris , 20 : 21 – 47 . 115–139, 267–293, Plates 7–13
- Lane , CE , Mayes , C , Druehl , LD and Saunders , GW . 2006 . A multi–gene molecular investigation of the kelp (Laminariales, Phaeophyceae) supports substantial reorganisation . J. Phycol. , 42 : 493 – 512 .
- Lapointe , BE , Niell , FX and Fuentes , JM . 1981 . Community structure, succession, and production of seaweeds associated with mussel–rafts in the Ria de Arosa, N. W. Spain . Mar. Ecol. Prog. Ser. , 5 : 243 – 253 .
- Larson , BR , Vadas , RL and Keser , M . 1980 . Feeding and nutritional ecology of the sea urchin Strongylocentrotus droebachiensis in Maine, USA . Mar. Biol. , 59 : 49 – 62 .
- Larsson , C and Axelsson , L . 1999 . Bicarbonate uptake and utilization in marine macroalgae . Eur. J. Phycol. , 34 : 79 – 86 .
- Laturnus , F . 1996 . Volatile halocarbons released from Arctic macroalgae . Mar. Chem. , 55 : 359 – 366 .
- Laturnus , F . 2001 . Marine macroalgae in polar regions as natural sources for volatile organohalogens . Environ. Sci. Poll. Res. , 8 : 103 – 108 .
- Lawrence , JM . 1975 . On the relationship between marine plants and sea urchins . Oceanogr. Mar. Biol. Ann. Rev. , 13 : 213 – 286 .
- Laycock , RA . 1974 . The detrital food chain based on seaweeds. I. Bacteria associated with the surface of Laminaria fronds . Mar. Biol. , 25 : 223 – 231 .
- Lee , JA and Brinkhuis , BH . 1986 . Reproductive phenology of Laminaria saccharina (L.) Lamour. (Phaeophyta) at the southern limit of its distribution in the northwestern Atlantic Ocean . J. Phycol. , 22 : 276 – 285 .
- Lee , JA and Brinkhuis , BH . 1988 . Seasonal light and temperature interaction effects on development of Laminaria saccharina (Phaeophyta) gametophytes and juvenile sporophytes . J. Phycol. , 24 : 181 – 191 .
- Lein , TE , Sjøtun , K and Wakili , S . 1991 . Mass–occurrence of a brown filamentous endophyte in the lamina of the kelp Laminaria hyperborea (Gunnerus) Foslie along the southwestern coast of Norway . Sarsia , 76 : 187 – 193 .
- Leinaas , HP and Christie , H . 1996 . Effects of removing sea urchins (Strongylocentrotus droebachiensis): stability of the barren state and succession of kelp forest recovery in the east Atlantic . Oecologia , 105 : 524 – 536 .
- Levin , PS , Coyer , JA , Petrik , R and Good , TP . 2002 . Community–wide effects on nonindegenous species on temperate rocky reefs . Ecology , 83 : 3182 – 3193 .
- Lewis , RJ . 1996 . Phycological Reviews 16. Chromosomes of the brown algae . Phycologia , 35 : 19 – 40 .
- Lewis , RJY , Jiang , B , Neushul , M and Fei , XG . 1993 . Haploid parthenogenetic sporophytes of Laminaria japonica (Phaeophyceae) . J. Phycol. , 29 : 363 – 369 .
- Linley , EAS , Newell , RC and Bosma , SA . 1981 . Heterotrophic utilisation of mucilage released during fragmentation of kelp (Ecklonia maxima and Laminaria pallida). I. Development of microbial communities associated with the degradation of kelp mucilage . Mar. Ecol. Prog. Ser. , 4 : 31 – 41 .
- Lippert , H , Iken , K , Rachor , E and Wiencke , C . 2001 . Macrofauna associated with macroalgae in the Kongsfjord (Spitsbergen) . Polar Biol. , 24 : 512 – 522 .
- Liu , C , Yang , Z and Tang , X . 2002a . The relationship between resistance against alginic acid decomposing bacteria and the SOD activity in Laminaria japonica . Mar. Sci./Haiyang Kexue , 26 : 1 – 2 .
- Liu , C , Yang , Z and Tang , X . 2002b . Generality of production of reactive oxygen species under infection of alginic acid decomposing bacteria in Laminaria japonica . Mar. Fish. Res./Haiyang Shuichan Yanjiu , 23 : 33 – 36 .
- Lopes , PF , De Oliveira , MC and Colepicolo , P . 1997 . Diurnal fluctuations of nitrate reductase activity in the marine red alga Gracilaria tenuistipitata (Rhodophyta) . J. Phycol. , 33 : 225 – 231 .
- Lopes , PF , Santa-Maria , UR and Colepicolo , P . 2002 . Effect of light quality on the circadian expression of nitrate reductase in the red macroalga Gracilaria tenuistipitata . Biol. Rhythm Res. , 33 : 391 – 400 .
- Løvås , SM and Tørum , A . 2001 . Effect of the kelp Laminaria hyperborea upon sand dune erosion and water particle velocities . Coastal Engineering , 44 : 37 – 63 .
- Lowther , A . 2006 . Highlights from the FAO database on Aquaculture Statistics . FAO Aquaculture Newsletter , 35 : 32 – 33 .
- Lucas , MI , Newell , RC and Velimirow , B . 1981 . Heterotrophic utilisation of mucilage released during fragmentation of kelp (Ecklonia maxima and Laminaria pallida). II. Differential utilisation of dissolved organic components from kelp mucilage . Mar. Ecol. Prog. Ser. , 4 : 43 – 55 .
- Lüder , UH and Clayton , MN . 2004 . Induction of phlorotannins in the brown macroalga Ecklonia radiata (Laminariales, Phaeophyta) in response to simulated herbivory: the first microscopic study . Planta , 218 : 928 – 937 .
- Lüning , K . 1969 . Growth of amputated and dark–exposed individuals of the brown alga Laminaria hyperborea . Mar. Biol. , 2 : 218 – 223 .
- Lüning , K . 1970 . Tauchuntersuchungen zur Vertikalverteilung der sublitoralen Helgoländer Algenvegetation . Helgoländer wiss. Meeresunters. , 21 : 271 – 291 .
- Lüning , K . 1975 . Kreuzungsexperimente an Laminaria saccharina von Helgoland und von der Isle of Man . Helgol. wiss. Meeresunters. , 27 : 108 – 114 .
- Lüning , K . 1979 . Growth strategies of three Laminaria species (Phaeophyceae) inhabiting different depth zones in the sublittoral region of Helgoland (North Sea) . Mar. Ecol. Prog. Ser. , 1 : 195 – 207 .
- Lüning , K . 1980 . Critical levels of light and temperature regulating the gametogenesis of three Laminaria species . J. Phycol. , 16 : 1 – 15 .
- Lüning , K . 1981 . Egg release in gametophytes of Laminaria saccharina: induction by darkness and inhibition by blue light and UV . Br. Phycol. J. , 16 : 579 – 593 .
- Lüning , K . 1982 . “ Seasonality of larger brown algae and its possible regulation by the environment ” . In Synthetic and Degradative Processes in Marine Macrophytes , Edited by: Srivastava , LM . 47 – 67 . Berlin, , Germany : Walter de Gruyter .
- Lüning , K . 1984 . Temperature tolerance and biogeography of seaweeds: the marine algal flora of Helgoland, North Sea, as an example . Helgoländer Meeresunters. , 38 : 305 – 317 .
- Lüning , K . 1985 . Meeresbotanik: Verbreitung, Ökophysiologie und Nutzung der marinen Makroalgen , Stuttgart, , Germany : Thieme Verlag .
- Lüning , K . 1986 . New frond formation in Laminaria hyperborea (Phaeophyta): A photoperiodic response . Br. Phycol. J. , 3 : 269 – 273 .
- Lüning , K . 1988 . Photoperiodic control of sorus formation in the brown alga Laminaria saccharina . Mar. Ecol. Prog. Ser. , 45 : 137 – 144 .
- Lüning , K . 1990 . Seaweeds: Their Environment, Biogeography, and Ecophysiology , New York, , USA : Wiley & Sons .
- Lüning , K . 1991 . Circannual growth rhythm in a brown alga, Pterygophora californica . Bot. Acta , 104 : 157 – 162 .
- Lüning , K . 1992 . Day and night kinetics of growth rate in green, brown and red seaweeds . J. Phycol. , 28 : 794 – 803 .
- Lüning , K . 1993 . Environmental and internal control of seasonal growth in seaweeds . Hydrobiologia , 260/261 : 1 – 14 .
- Lüning , K . 1994 . Circadian growth rhythm in juvenile sporophytes of Laminariales (Phaeophyta) . J. Phycol. , 30 : 193 – 199 .
- Lüning , K . 2001 . Circadian growth in Porphyra umbilicalis (Rhodophyta): spectral sensitivity of the circadian system . J. Phycol. , 37 : 52 – 58 .
- Lüning , K . 2005 . “ Endogenous rhythms and daylength effects in macroalgal development ” . In Algal Culturing Techniques , Edited by: Andersen , RA . 347 – 364 . Elsevier, , London, UK : Academic Press .
- Lüning , K and Dring , MJ . 1972 . Reproduction induced by blue light in female gametophytes of Laminaria saccharina . Planta , 104 : 252 – 256 .
- Lüning , K and Dring , M . 1975 . Reproduction, growth and photosynthesis of gametophytes of Laminaria saccharina grown in blue and red light . Mar. Biol. , 29 : 195 – 200 .
- Lüning , K and Dring , MJ . 1979 . Continuous underwater light measurement near Helgoland (North Sea) and its significance for characteristic light limits in the sublittoral region . Helgol. Meeresunters. , 32 : 403 – 424 .
- Lüning , K and Dring , MJ . 1985 . Action spectra and spectral quantum yield of photosynthesis in marine macroalgae with thin and thick thalli . Mar. Biol. , 87 : 119 – 129 .
- Lüning , K and Freshwater , W . 1988 . Temperature tolerance of northeast Pacific marine algae . J. Phycol. , 24 : 310 – 315 .
- Lüning , K and Kadel , P . 1993 . Daylength range for circannual rhythmicity in Pterygophora californica (Alariaceae, Phaeophyta) and synchronization of seasonal growth by daylength cycles in several other brown algae . Phycologia , 32 : 379 – 387 .
- Lüning , K and Neushul , M . 1978 . Light and temperature demands for growth and reproduction of laminarian gametophytes in southern and central California . Mar. Biol. , 45 : 297 – 309 .
- Lüning , K and Pang , S . 2003 . Mass cultivation of seaweeds: current aspects and approaches . J. Appl. Phycol. , 15 : 115 – 119 .
- Lüning , K and Tom Dieck , I . 1989 . Environmental triggers in algal seasonality . Bot. Mar. , 32 : 389 – 397 .
- Lüning , K and Tom Dieck , I . 1990 . “ The distribution and evolution of the Laminariales: North Pacifc–Atlantic relationships ” . In NATO ASI Series, Evolutionary Biogeography of the Marine Algae of the North Atlantic , Edited by: Garbary , DJ and South , GR . 187 – 204 . Berlin, , Germany : Springer Verlag .
- Lüning , K , Schmitz , K and Willenbrink , J . 1972 . Translocation of 14C labelled assimilates in two laminarian species . Proc. Int. Seaweed Symp. , 7 : 420 – 425 .
- Lüning , K , Schmitz , K and Willenbrink , J . 1973 . CO2–fixation and translocation in benthic marine algae. III. Rates and ecological significance of translocation in Laminaria hyperborea and L. saccharina . Mar. Biol. , 23 : 275 – 281 .
- Lüning , K , Chapman , ARO and Mann , KH . 1978 . Crossing experiments in the non–digitate complex of Laminaria from both sides of the Atlantic . Phycologia , 17 : 293 – 298 .
- Lüning , K , Wagner , A and Buchholz , C . 2000 . Evidence for inhibitors of sporangium formation in Laminaria digitata (Phaeophyceae) during the season of rapid growth . J. Phycol. , 36 : 1129 – 1134 .
- Lyngby , JE and Mortensen , SM . 1996 . Effects of dredging activities on growth of Laminaria saccharina . Mar. Ecol. , 17 : 345 – 354 .
- Maberly , SC . 1990 . Exogenous sources of inorganic carbon for photosynthesis by marine macroalgae . J. Phycol. , 26 : 439 – 449 .
- Macaya , EC , Rothäusler , E , Thiel , M , Molis , M and Wahl , M . 2005 . Induction of defenses and within–alga variation of palatability in two brown algae from the northern–central coast of Chile: Effects of mesograzers and UV radiation . J. Exp. Mar. Biol. Ecol. , 325 : 214 – 227 .
- Machalek , KM , Davison , IR and Falkowski , PG . 1996 . Thermal acclimation and photoacclimation of photosynthesis in the brown alga Laminaria saccharina . Plant Cell Environ. , 19 : 1005 – 1016 .
- Machiguchi , Y , Sanbonsuga , Y and Okada , Y . 2006 . Nitrogen uptake and growth of Laminaria angustata var. longissima in the blade–renewal stage . Bull. Hokkaido Reg. Fish. Res. Lab. , 50 : 45 – 61 .
- Maier , I . 1995 . Brown algal pheromones . Prog. Phycol. Res. , 11 : 51 – 102 .
- Maier , I and Müller , DG . 1986 . Sexual pheromones in algae . Biol. Bull. , 170 : 145 – 175 .
- Maier , I , Müller , DG , Schmid , C , Boland , W and Jaenicke , L . 1988 . Pheromone receptor specificity and threshold concentrations for spermatozoid release in Laminaria digitata . Naturwiss. , 75 : 260 – 263 .
- Makarov , MV and Voskoboinikov , GM . 2001 . The influence of ultraviolet–B radiation on spore release and growth of the kelp Laminaria saccharina . Bot. Mar. , 44 : 89 – 94 .
- Makarov , VN , Schoschina , EV and Lüning , K . 1995 . Diurnal and circadian periodicity of mitosis and growth in marine macroalgae. I. Juvenile sporophytes of Laminariales (Phaeophyta) . Eur. J. Phycol. , 30 : 261 – 266 .
- Malin , G , Küpper , FC , Carpenter , L , Baker , A , Broadgate , W , Kloareg , B and Liss , PS . 2001 . Trace gas production by seaweeds: Defense, oxidative stress, signalling and atmospheric significance . J. Phycol. , 37 : 32 – 33 .
- Mamelona , J and Pelletier , É . 2005 . Green urchin as a significant source of fecal particulate organic matter within nearshore benthic ecosystems . J. Exp. Mar. Biol. Ecol. , 314 : 163 – 174 .
- Manley , SL . 2002 . Phytogenesis of halomethanes: A product of selection or a metabolic accident? . Biogeochemistry , 60 : 163 – 180 .
- Manley , SL , Goodwin , K and North , WJ . 1992 . Laboratory production of bromoform, methylene bromide, and methyl iodide by macroalgae and distribution in nearshore southern California waters . Limnol. Oceanogr. , 37 : 1652 – 1650 .
- Mann , KH . 1971 . Relation between stipe length, environment and the taxonomic characters of Laminaria . J. Fish. Res. Can. , 28 : 778 – 780 .
- Mann , KH . 1977 . Destruction of kelp–beds by sea urchins: a cyclical phenomenon or irreversible degradation? . Helgoländer wiss. Meeresunters. , 30 : 455 – 467 .
- Markager , S and Sand–Jensen , K . 1994 . The physiology and ecology of light–growth relationships in macroalgae . Prog. Phycol. Res. , 10 : 209 – 298 .
- Markham , JW . 1973 . Observations on the ecology of Laminaria sinclairii on three northern Oregon beaches . J. Phycol. , 9 : 336 – 341 .
- Markham , JW and Munda , IM . 1980 . Algal recolonization in the rocky eulittoral at Helgoland, Germany . Aquat. Bot. , 9 : 33 – 71 .
- Marshall , W . 1960 . An underwater study of the epiphytes of Laminaria hyperborea (Gunn.) Fosl . Br. Phycol. Bull. , 2 : 18 – 19 .
- Martinez , EA . 1996 . Micropopulation differentiation in phenol content and susceptibility to herbivory in the Chilean kelp Lessonia nigrescens (Phaeophyta, Laminariales) . Hydrobiologia , 326/327 : 205 – 211 .
- Masaki , T , Fujita , D and Akioka , H . 1981 . Observations on the spore germination of Laminaria japonica on Lithophyllum yessoense (Rhodophyta, Corallinaceae) in culture . Bull. Fac. Fish., Hokkaido Univ. , 32 : 349 – 356 .
- Masaki , T , Fujita , D and Hagen , NT . 1984 . The surface ultrastructure and epithallium shedding of crustose coralline algae in an ‘Isoyake’ area of southwestern Hokkaido, Japan . Hydrobiologia , 116/117 : 218 – 223 .
- Maxwell , DP , Falk , S , Trick , CG and Huner , NPA . 1994 . Growth at low temperature mimics high–light acclimation in Chlorella vulgaris . Plant Physiol. , 105 : 535 – 543 .
- Mayes , C . 1984 . Molecular phylogeny of the kelp genus Laminaria (Laminariaceae: Phaeophyta) , Vancouver, , Canada : Simon Fraser University . MSc thesis
- Mayer , AMS and Hamann , MT . 2002 . Marine pharmacology in 1999: compounds with antibacterial, anticoagulant, antifungal, anthelmintic, anti–inflammatory, antiplatelet, antiprotozoal and antiviral activities affecting the cardiovascular, endocrine, immune and nervous systems, and other miscellaneous mechanisms of action . Comp. Biochem. Physiol. C: Pharmacol. Toxicol. , 132 : 315 – 339 .
- Mayer , AMS and Hamann , MT . 2005 . Marine pharmacology in 2001–2002: Marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti–inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action . Comp. Biochem. Physiol. C: Pharmacol. Toxicol. , 140 : 265 – 286 .
- Mazure , HGF and Field , JG . 1980 . Density and ecological importance of bacteria on kelp fronds in an upwelling region . J. Exp. Mar. Biol. Ecol. , 43 : 173 – 182 .
- McClung , CR . 2001 . Circadian rhythms in plants . Ann. Rev. Physiol. Plant Mol. Biol. , 52 : 139 – 162 .
- McHugh , DJ . 1991 . Worldwide distribution of commercial resources of seaweeds including Gelidium . Hydrobiologia , 221 : 19 – 29 .
- McHugh , DJ . 2002 . Prospects for seaweed production in developing countries . FAO Fisheries Circular , 968 : FIIU/C968
- McHugh , DJ . 2003 . “ A guide to the seaweed industry ” . In FAO Fisheries Technical Papers T441 , Food and Agriculture Organization of the United Nations .
- McKenzie , R , Smale , D and Kotkamp , M . 2004 . Relationship between UVB and erythemally weighted radiation . Photochem. Photobiol. Sci. , 3 : 252 – 256 .
- McPeak , RH . 1981 . Fruiting in several species of Laminariales from southern California . Proc. Int. Seaweed Symp. , 8 : 404 – 409 .
- Mehrtens , G and Laturnus , F . 1997 . Halogenating activity in an arctic population of brown macroalga Laminaria saccharina (L.) Lamour . Polar Research , 16 : 19 – 25 .
- Michler , T , Aguilera , J , Hanelt , D , Bischof , K and Wiencke , C . 2002 . Long–term effects of ultraviolet radiation on growth and photosynthetic performance of polar and cold–temperate macroalgae . Mar. Biol. , 140 : 1117 – 1127 .
- Miller , RJ . 1985a . Seaweeds, sea urchins, and lobsters: a reappraisal . Can. J. Fish. Aquat. Sci. , 42 : 2061 – 2072 .
- Miller , RJ . 1985b . Succession in sea urchin and seaweed abundance in Nova Scotia, Canada . Mar. Biol. , 84 : 275 – 286 .
- Miller , RJ and Mann , KH . 1973 . Ecological energetics of the seaweed zone in a marine bay on the Atlantic coast of Canada. III. Energy transformations by sea urchins . Mar. Biol. , 18 : 99 – 114 .
- Miller , RJ , Mann , KH and Scarratt , DJ . 1971 . Production potential of a seaweed–lobster community in eastern Canada . J. Fish. Res. Bd. Can. , 28 : 1733 – 1738 .
- Mittag , M . 2001 . Circadian rhythms in microalgae . Int. Rev. Cytol. , 206 : 213 – 247 .
- Mittag , M , Kiaulehn , S and Johnson , CH . 2005 . The circadian clock in Chlamydomonas reinhardtii. What is it for? What is it similar to? . Plant Physiol. , 137 : 399 – 409 .
- Miyabe , K . 1957 . On the Laminariaceae of Hokkaido . J. Sapporo Agr. Coll. , 1 : 1 (English edition.), 50 + 29 plates.
- Mizuta , H , Torii , K and Yamamoto , H . 1997 . The relationship between nitrogen and carbon contents in the sporophytes of Laminaria japonica (Phaeophyceae) . Fish. Sci. , 63 : 553 – 556 .
- Mizuta , H , Nimura , K and Yamamoto , H . 1999a . Sorus development on median and marginal parts of the sporophyte of Laminaria japonica Areschoug (Phaeophyceae) . J. Appl. Phycol. , 11 : 585 – 591 .
- Mizuta , H , Nimura , K and Yamamoto , H . 1999b . Inducible conditions for sorus formation of the sporophyte discs of Laminaria japonica Areschoug (Phaeophyceae) . Fish. Sci. , 65 : 104 – 108 .
- Mizuta , H , Ogawa , S and Yasui , H . 2003 . Phosphorus requirement of the sporophyte of Laminaria japonica (Phaeophyceae) . Aquat. Bot. , 76 : 117 – 126 .
- Molis , M , Körner , J , Ko , Y-W , Kim , JH and Wahl , M . 2006 . Inducible responses in the brown seaweed Ecklonia cava: the role of grazer identity and season . J. Ecol. , 94 : 243 – 249 .
- Molis , M , Ko , J , Kim , YW and Körner , JH . 2008 . Specificity of inducible seaweed anti–herbivory defences depends on identity of macroalgae and herbivores . Mar. Ecol. Prog. Ser , in press
- Morizur , Y . 2001 . Changements climatiques ou surexploitation? Gros temps sur les algues brunes . Les nouvelles de l’Ifremer , 25 : 1
- Motomura , T . 1989 . Ultrastructural study of sperm in Laminaria angustata (Laminariales, Phaeophyta), especially on the flagellar apparatus . Jpn. J. Phycol. , 37 : 105 – 116 .
- Motomura , T . 1990 . Ultrastructure of fertilization in Laminaria angustata (Phaeophyta, Laminariales) with emphasis on the behaviour of centrioles, mitochondria and chloroplast of the sperm . J. Phycol. , 26 : 80 – 89 .
- Motomura , T . 1991 . Immunofluorescence microscopy of fertilization and parthenogenesis in Laminaria angustata (Phaeophyta) . J. Phycol. , 27 : 248 – 257 .
- Motomura , T . 1993 . Ultrastructure and immunofluorescence studies of zoosporogenesis in Laminaria angusta . Algol. Res. Fac. Sci. , 9 : 1 – 32 .
- Motomura , T and Sakai , Y . 1981 . Effect of chelated iron in culture media on oogenesis in Laminaria angustata . Bull. Japan Soc. Sci. Fish. , 47 : 1535 – 1540 .
- Motomura , T and Sakai , Y . 1984 . Ultrastructural studies of gametogenesis in Laminaria angustata (Laminariales, Phaeophyta) regulated by iron concentration in the medium . Phycologia , 23 : 331 – 343 .
- Motomura , T and Sakai , Y . 1988 . The occurrence of flagellated eggs in Laminaria angustata (Phaeophyta, Laminariales) . J. Phycol. , 24 : 282 – 285 .
- Motomura , T , Ichimura , T and Melkonian , M . 1997 . Coordinative nuclear and chloroplast division in unilocular sporangia of Laminaria angustata (Laminariales, Phaeophyceae) . J. Phycol. , 33 : 266 – 271 .
- Moy , F , Dahl , J , Green , E , Johnsen , N , Lømsland , TM , Magnusson , ER , Omli , J , Olsgaard , L , Oug , F , Pedersen , E , Rygg , A , Walday , B and Aure , M . 2003 . Landtidsovervåking av miljøkvaliteten i kystområdene av Norge . Årsrapport for 2002 , : 1 – 69 .
- Müller , DG . 1962 . Über jahres–und lunarperiodische Erscheinungen bei einigen Braunalgen . Bot. Mar. , 2 : 387 – 419 .
- Müller , DG . 1981 . “ Sexuality and sex attraction ” . In The Biology of Seaweeds , Edited by: Lobban , CS and Wynne , MJ . 661 – 673 . Oxford, , UK : Blackwell Scientific Publications .
- Müller , DG . 1989 . “ Sexuality and sexual attraction ” . In Algae as Experimental Systems , Edited by: Coleman , AW , Goff , LJ and Stein-Taylor , JR . 201 – 213 . New York, , USA : A.R. Liss .
- Müller , DG , Gassman , G and Lüning , K . 1979 . Isolation of a spermatozoid–releasing and attracting substance from female gametophytes of Laminaria digitata . Nature , 279 : 430 – 431 .
- Müller , DG , Peters , AF , Gassman , G , Boland , W , Marner , F–J and Jaenicke , L . 1982 . Identification of a sexual hormone and related substances in the marine brown alga Desmarestia . Naturwissenschaften , 69 : 290
- Müller , DG , Boland , W , Becker , U and Wahl , T . 1988 . Caudoxirene, the spermatozoid–releasing and attracting factor in the marine brown alga Perithalia caudate (Phaeophyceae, Sporochnales) . Biol. Chem. H.–S. , 369 : 655 – 659 .
- Muraoka , D . 2004 . Seaweed resources as a source of carbon fixation . Bull. Fish. Res. Agen. , 1 : 59 – 63 .
- Nabata , S , Abe , E and Kakiuchi , M . 1992 . On the ‘Isoyake’ condition in Taisei–cho, southwestern Hokkaido . Sci. Rep. Hokkaido Fish. Exp. Stn. , 38 : 1 – 14 . (In Japanese, English abstract.)
- Nakahara , H and Nakamura , Y . 1973 . Parthenogenesis, apogamy and apospory in Alaria crassifolia (Laminariales) . Mar. Biol. , 18 : 327 – 332 .
- Neefus , CD , Allen , BP , Baldwin , HP , Mathieson , AC , Eckert , RT , Yarish , C and Miller , MA . 1993 . An examination of the population genetics of Laminaria and other brown algae in the Laminariales using starch gel electrophoresis . Hydrobiologia , 260–261 : 67 – 69 .
- Neori , A , Chopin , T , Troell , M , Buschmann , AH , Kraemer , GP , Halling , C , Shpigel , M and Yarish , C . 2004 . Integrated aquaculture: rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture . Aquaculture , 231 : 361 – 391 .
- Neumann , D . 1981 . “ Tidal and lunar rhythms ” . In Handbook of Behavioral Neurobiology , Edited by: Aschhoff , J . 351 – 380 . New York, , USA : Plenum Press .
- Newell , RC and Lucas , MI . 1981 . The quantitative significance of dissolved and particulate organic matter released during fragmentation of kelp in coastal waters . Kieler Meeresforsch., Sonderheft , 5 : 356 – 369 .
- Newell , RC , Lucas , MI , Velimirow , B and Seiderer , LJ . 1980 . Quantitative significance of dissolved organic losses following fragmentation of kelp (Ecklonia maxima and Laminaria pallida) . Mar. Ecol. Prog. Ser. , 2 : 45 – 59 .
- Niemeyer , R . 1976 . Cyclic condensed metaphosphates and linear polyphosphates in brown and red algae . Arch. Microbiol. , 108 : 243 – 247 .
- Nightingale , PD , Malin , G and Liss , PS . 1995 . Production of chloroform and other low–molecular–weight halocarbons by some species of macroalgae . Limnol. Oceanogr. , 40 : 680 – 689 .
- Nigro , SA , Stirk , WA and Van Staden , J . 2002 . Optimising heavy metal adsorbance by dried seaweeds . S. Afr. J. Bot. , 68 : 333 – 341 .
- Niihara , Y . 1975 . Physiological studies of Laminaria japonica var. ochotensis. The effect of temperature, light intensity and salinity upon photosynthesis and respiration of young sporophytes . Sci. Rep. Hokkaido Fish. Exp. Stn. , 17 : 11 – 18 .
- Nimura , K and Mizuta , H . 2002 . Inducible effects of abscisic acid on sporophyte discs from Laminaria japonica Areschoug (Laminariales, Phaeophyceae) . J. Appl. Phycol. , 14 : 159 – 163 .
- Nimura , K , Mizuta , H and Yamamoto , H . 2002 . Critical contents of nitrogen and phosphorus for sorus formation in four Laminaria species . Bot. Mar. , 45 : 184 – 188 .
- Nishibayashi , T and Inoh , S . 1956 . Morphogenetical studies on Laminariales I. The development of zoosporangia and the formation of zoospores in Laminaria angusta Kjellmann . Biol. J. Okayama Univ. , 2 : 147 – 158 .
- Norderhaug , KM , Christie , H and Rinde , E . 2002 . Colonisation of kelp imitations by epiphyte and holdfast fauna: A study of mobility patterns . Mar. Biol. , 141 : 965 – 973 .
- Norderhaug , KM , Fredriksen , S and Nygaard , K . 2003 . Trophic importance of Laminaria hyperborea to kelp forest consumers and the importance of bacterial degradation to food quality . Mar. Ecol. Prog. Ser. , 255 : 135 – 144 .
- Norton , TA . 1978 . The factors influencing the distribution of Saccorhiza polyschides in the region of Lough Ine . J. Mar. Biol. Ass. UK , 58 : 527 – 536 .
- Norton , TA . 1992 . Dispersal by macroalgae . Br. Phycol. J. , 27 : 293 – 301 .
- Norton , TA and Burrows , EM . 1969 . Studies on marine algae of the British Isles. 7. Saccorhiza polyschides (Lightf.) Batt . Br. Phycol. J. , 4 : 19 – 53 .
- Norton , TA , Hiscock , K and Kitching , JA . 1977 . The ecology of Lough Ine: XX. The Laminaria forest at Carrigathorna . J. Ecol. , 65 : 919 – 941 .
- Novaczek , I and Mclachlan , J . 1986 . Recolonization by algae of the sublittoral habitat of Halifax county, Nova Scotia, following the demise of sea urchins . Bot. Mar. , 29 : 69 – 73 .
- Ohmori , T . 1967 . Morphogenetical studies on Laminariales . Biol. J. Okayama Univ. , 13 : 23 – 84 .
- Ohno , M . 1972 . The periodicity of gamete liberation in Monostroma . Proc. Int. Seaweed Symp. , 7 : 405 – 409 .
- Ohno , M . 1993 . Cultivation methods and physiological aspect for edible seaweeds in Japan . Serie Ocasional , 2 : 163 – 170 .
- Ohsawa , N , Ogata , Y , Okada , N and Itoh , N . 2001 . Physiological function of bromoperoxidase in the red marine alga, Corallina pilulifera: production of bromoform as an allelochemical and the simultaneous elimination of hydrogen peroxide . Phytochemistry , 58 : 683 – 692 .
- Okada , M , Inoue , M and Ikeda , T . 1978 . Circadian rhythm in photosynthesis of the green alga . Bryopsis maxima. Plant Cell Physiol. , 19 : 197 – 202 .
- Okada , Y , Sanbonsuga , Y and Machiguchi , Y . 1985 . The effects of temperature on the growth and shape of the early sporophytes of Laminaria japonica, L. ochotensis, L. diabolica, L. religiosa and L. angustata var. longissima in culture . Bull. Hokkaido Reg. Fish. Res. Lab. , 50 : 27 – 44 .
- Okamura , B and Partridge , JC . 1999 . Suspension feeding adaptations to extreme flow environments in a marine bryozoan . Biol. Bull. , 196 : 205 – 215 .
- Olivera , L , Walker , DC and Bisalputra , T . 1980 . Ultrastructural, cytochemical, and enzymatic studies on the adhesive “plaques” of the brown algae Laminaria saccharina (L.) Lamour. and Nereocystis luetkeana (Mert.) Post. et Rupr . Protoplasma , 104 : 1 – 15 .
- Østgaard , K , Indergaard , M , Markussen , S , Knutsen , SH and Jensen , A . 1993 . Carbohydrate degradation and methane production during fermentation of Laminaria saccharina (Laminariales, Phaeophyceae) . J. Appl. Phycol. , 5 : 333 – 342 .
- Oudot–Le Secq , MP , Kloareg , B and Loiseaux–de Goer , S . 2002 . The mitochondrial genome of the brown alga Laminaria digitata: a comparative analysis . Eur. J. Phycol. , 37 : 163 – 172 .
- Ozaki , A , Mizuta , H and Yamamoto , H . 2001 . Physiological differences between the nutrient uptakes of Kjellmaniella crassifolia and Laminaria japonica (Phaeophyceae) . Fish. Sci. , 67 : 415 – 419 .
- Palmer , CJ , Anders , TL , Carpenter , LJ , Küpper , FC and McFiggans , GB . 2005 . Iodine and halocarbon response of Laminaria digitata to oxidative stress and links to atmospheric new particle production . Environ. Chem. , 2 : 282 – 290 .
- Palmer , JD . 1995 . The Biological Rhythms and Clocks of Intertidal Animals , New York, , USA : Oxford University Press .
- Pang , SJ and Lüning , K . 2004 . Breaking seasonal limitation: year–round sporogenesis in the brown alga Laminaria saccharina by blocking the transport of putative sporulation inhibitors . Aquaculture , 240 : 531 – 541 .
- Paul , AJ , Paul , JM , Hood , DW and Nevé , RA . 1977 . Observations on food preferences, daily ration requirements and growth of Haliotis kamtschatkana Jonas in captivity . Veliger , 19 : 303 – 309 .
- Paul , NA , De Nys , R and Steinberg , PD . 2006 . Chemical defence against bacteria in the red alga Asparagopsis armata: linking structure with function . Mar. Ecol. Progr. Ser. , 306 : 87 – 101 .
- Pavia , H , Cervin , G , Lindgren , A and Aberg , P . 1997 . Effects of UV–B radiation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodosum . Mar. Ecol. Prog. Ser. , 157 : 139 – 146 .
- Pearse , JS and Hines , AH . 1979 . Expansion of a central California kelp forest following the mass mortality of sea urchins . Mar. Biol. , 51 : 83 – 91 .
- Pedersen , A . 1980 . “ Fenolinnhold i brunalger (Phaeophycea) som funksion av vekstype og salinitet ” . In Inst. Marinbiol , 138 Bergen, , Norway : University of Bergen .
- Percival , E . 1979 . The polysaccharides of green, red and brown seaweeds: their basic structure, biosynthesis and function . Br. Phycol. J. , 14 : 103 – 117 .
- Percival , E and McDowell , RH . 1967 . Chemistry and Enzymology of Marine Algal Polysaccharids , London, , UK : Academic Press .
- Perez , P , Kaas , R , Campello , F , Arbault , S and Barbaroux , O . 1992 . La Culture des Algues Marines dans le Monde , Nantes, , France : Ifremer .
- Peters , AF . 1991 . Field and culture studies of Streblonema macrocystis sp. nov. (Ectocarpales, Phaeophyceae) from Chile: A sexual endophyte of giant kelp . Phycologia , 30 : 365 – 377 .
- Peters , AF . 2003 . Molecular identification, taxonomy and distribution of brown algal endophytes, with emphasis on species from Antarctica . Proc. Int. Seaweed Symp. , 17 : 293 – 302 .
- Peters , AF and Burkhardt , E . 1998 . Systematic position of the kelp endophyte Laminarionema elsbetiae (Ectocarpales sensu lato, Phaeophyceae) inferred from nuclear ribosomal DNA sequences . Phycologia , 37 : 114 – 120 .
- Peters , AF and Ellertsdottír , E . 1996 . New record of the kelp endophyte Laminarionema elsbetiae (Phaeophyceae, Ectocarpales) at Helgoland and its life history in culture . Nova Hedwigia , 62 : 341 – 349 .
- Peters , AF and Schaffelke , B . 1996 . Streblonema (Ectocarpales, Phaeophyceae) infection in the kelp Laminaria saccharina (Laminariales, Phaeophyceae) in the western Baltic . Hydrobiologia , 327 : 111 – 116 .
- Petrell , RJ and Alie , SY . 1996 . Integrated cultivation of salmonids and seaweeds in open systems . Hydrobiologia , 326/327 : 67 – 73 .
- Petrov , JE . 1972 . De systemate specierum nonnullarum Laminariae Lamour. ex oriente extremo . Novit. System. Plant. non Vasc. , 9 : 47 – 58 .
- Petrov , JE , Suchovejeva , MV and Avdejev , GV . 1973 . Species generis Laminaria Lam. e mari philippinensi nova . Novit. System. Plant. non Vasc. , 10 : 59 – 61 .
- Phillipps , DJH . 1977 . The use of biological indicator organisms to monitor trace metal pollution in marine and estuarine environments–a review . Environ. Poll. , 13 : 281 – 317 .
- Pittendrigh , CS . 1993 . Temporal organization: reflections of a Darwinian clock-watcher . Annu. Rev. Physiol. , 55 : 17 – 54 .
- Pohnert , G . 2004 . Chemical defense strategies of marine organisms . Topics Curr. Chem. , 239 : 179 – 219 .
- Pohnert , G and Boland , W . 2002 . The oxylipin chemistry of attraction and defense in brown algae and diatoms . Nat. Prod. Rep. , 19 : 108 – 122 .
- Polk , M . Open Ocean Aquaculture . Proceedings of an International Conference . May 8-10 1996 , Portland, Maine, USA. pp. 642 UNHMP-CP-SG-96-9, Portland, New Hampshire/Maine Sea Grant College Program
- Potin , P , Bouarab , K , Salaün , J-P , Pohnert , G and Kloareg , B . 2002 . Biotic interactions of marine algae . Curr. Opin. Plant Biol. , 5 : 308 – 317 .
- Price , WA , Hunt , KW and Tomlinson , JN . The effect of artificial seaweed in promoting the build-up of beaches . Proc. 11th Conference on Coastal Engineering, London, England, Sept. 1968 . pp. 570 – 578 .
- Pringle , JD , Sharp , GJ and Caddy , JF . 1982 . Interactions in kelp bed ecosystems in the northwest Atlantic: review of a workshop . Can. Spec. Publ. Fish. Aquat. Sci. , 59 : 108 – 115 .
- Pueschel , CM and Korb , RE . 2001 . Storage of nitrogen in the form of protein bodies in the kelp Laminaria solidungula . Mar. Ecol. Prog. Ser. , 218 : 107 – 114 .
- Pybus , C . 1973 . Effects of anionic detergent on the growth of Laminaria . Mar. Pollut. Bull. , 4 : 73 – 77 .
- Qin , S , Jiang , P and Tseng , CK . 2004 . Molecular biotechnology of marine algae in China . Hydrobiologia , 512 : 21 – 26 .
- Qin , S , Jiang , P and Tseng , CK . 2005 . Transforming kelp into a marine bioreactor . Trends Biotechnol. , 23 : 264 – 268 .
- Ragan , M and Glombitza , K-W . 1986 . Phlorotannins, brown algal polyphenols . Progr. Phycol. Res. , 4 : 129 – 241 .
- Raven , JA , Wollenweber , B and Handley , LL . 1992 . A comparison of ammonium and nitrate as nitrogen sources for photolithotrophs . New Phytol. , 121 : 19 – 32 .
- Raven , JA , Johnston , MA , Kübler , JE , Korb , R , McInroy , SG , Handley , LL , Scrimgeour , CM , Walker , DI , Beardall , J , Vanderklift , M , Fredriksen , S and Dunton , KH . 2002 . Mechanistic interpretation of carbon isotope discrimination by marine macroalgae and seagrasses . Funct. Plant. Biol. , 29 : 355 – 378 .
- Reed , DC , Laur , DR and Ebeling , AW . 1988 . Variation in algal dispersal and recruitment: the role of episodic events . Ecol. Monogr. , 58 : 321 – 335 .
- Reed , DC , Brzezinski , MA , Coury , DA , Graham , WM and Petty , RL . 1999 . Neutral lipids in macroalgal spores and their role in swimming . Mar. Biol. , 133 : 737 – 744 .
- Rees , TAV . 2003 . Safety factors and nutrient uptake by seaweeds . Mar. Ecol. Prog. Ser. , 263 : 29 – 42 .
- Rensing , L and Ruoff , P . 2002 . Temperature effect on entrainment, phase shifting, and amplitude of circadian clocks and its molecular bases . Chronobiol. Int. , 19 : 807 – 864 .
- Richter , M , Rühle , W and Wild , A . 1990 . Studies on the mechanism of photosystem II photoinhibition I. A two–step degradation of D1 protein . Photosynth. Res. , 24 : 229 – 235 .
- Rieper-Kirchner , M . 1990 . Macroalgal decomposition: laboratory studies with particular regard to microorganisms and meiofauna . Helgoländer Meeresunter. , 44 : 397 – 410 .
- Rinde , E and Sjøtun , K . 2005 . Demographic variation in the kelp Laminaria hyperborea along a latitudinal gradient . Mar. Biol. , 146 : 1051 – 1062 .
- Robinson , JD , Mann , KH and Novitsky , JA . 1982 . Conversion of the particulate fraction seaweed detritus to bacterial biomass . Limnol. Oceanogr. , 27 : 1072 – 1079 .
- Roeder , V , Collén , J , Rousvoal , S , Corre , E , Leblanc , C and Boyen , C . 2005 . Identification of stress gene transkripts in Laminaria digitata (Phaeophyceae) protoplast cultures by expressed sequence tag analysis . J. Phycol. , 41 : 1227 – 1235 .
- Roenneberg , T and Merrow , M . 2003 . The network of time: understanding the molecular circadian system . Curr. Biol. , 13 : R198 – R207 .
- Rodrigues , MA , Dos Santos , CP , Yoneshigue-Valentin , Y. Strbac , D and Hall , DO . 2000 . Photosynthetic light-response curves and photoinhibition of the deep-water Laminaria abyssalis and the intertidal Laminaria digitata (Phaeophyceae) . J. Phycol. , 36 : 97 – 106 .
- Rodrigues , AM , Dos Santos , CP , Young , AJ , Strbac , D and Hall , DO . 2002 . A smaller and impaired xantophyll cycle makes the deep sea macroalgae Laminaria abyssalis (Phaeophyceae) highly sensitive to daylight when compared with shallow water . Laminaria digitata. J. Phycol. , 38 : 939 – 947 .
- Rogerson , A . 1991 . On the abundance of marine naked amoebae on the surfaces of five species of macroalgae . FEMS Microbial Ecol. , 85 : 301 – 312 .
- Roland , WG . 1984 . Resource management biology for the edible kelp Cymathere triplicata . Can. J. Fish. Aquat. Sci. , 41 : 271 – 277 .
- Roleda , MY . 2006 . Effects of ultraviolet radiation on early life stages of cold temperate and Arctic macroalgae: Implications for recruitment and vertical depth distribution . Ber. Polarforsch. Meeresforsch. , 526 : 1 – 158 .
- Roleda , MY , Hanelt , D , Kräbs , G and Wiencke , C . 2004 . Morphology, growth, photosynthesis and pigments in Laminaria ochroleuca (Laminariales, Phaeophyta) under UV radiation . Phycologia , 43 : 603 – 613 .
- Roleda , MY , Wiencke , C , Hanelt , D , Van de Poll , WH and Gruber , A . 2005 . Sensitivity of Laminariales zoospores from Helgoland to ultraviolet and photosynthetically active radiation: implications on depth distribution and reproductive season . Plant Cell Environ. , 28 : 466 – 479 .
- Roleda , MY , Hanelt , D and Wiencke , C . 2006a . Growth and DNA damage in young Laminaria sporophytes exposed to ultraviolet radiation: implication for depth zonation of kelps on Helgoland (North Sea) . Mar. Biol. , 148 : 1201 – 1211 .
- Roleda , MY , Clayton , MN and Wiencke , C . 2006b . Screening capacity of UV-absorbing compounds in spores of Arctic Laminariales . J. Exp. Mar. Biol. Ecol. , 338 : 123 – 133 .
- Roleda , MY , Wiencke , C and Hanelt , D . 2006c . Thallus morphology and optical characteristics affect growth and DNA damage by UV radiation in juvenile Arctic Laminaria sporophytes . Planta , 223 : 407 – 417 .
- Roleda , MY , Hanelt , D and Wiencke , C . 2006d . Exposure to ultraviolet radiation delays photosynthetic recovery in Arctic kelp zoospores . Photosyn. Res. , 88 : 311 – 322 .
- Roleda , MY , Wiencke , C and Lüder , UH . 2006e . Impact of ultraviolet radiation on cell structure, UV-absorbing compounds, photosynthesis, DNA damage and germination in zoospores of Arctic Saccorhiza dermatodea . J. Exp. Bot. , 57 : 3847 – 3856 .
- Rothäusler , E , Macaya , EC , Molis , M , Wahl , M and Thiel , M . 2005 . Laboratory experiments examining inducible defense show variable responses of temperate brown and red macroalgae . Rev. Chil. Hist. Nat. , 78 : 603 – 614 .
- Rupérez , P . 2002 . Mineral content of edible marine seaweeds . Food Chem. , 79 : 23 – 26 .
- Rusanowski , PC and Vadas , RL . 1974 . Localization of laminaran and a model for cell metabolite utilization in the Laminariales . Proc. Int. Seaweed Symp. , 8 : 232 – 243 .
- Russell , G . 1983a . Formation of an ectocarpoid epiflora on blades of Laminaria digitata . Mar. Ecol. Prog. Ser. , 11 : 181 – 187 .
- Russell , G . 1983b . Parallel growth patterns in algal epiphytes and Laminaria blades . Mar. Ecol. Prog. Ser. , 13 : 303 – 304 .
- Ryland , JS and Hayward , PJ . 1977 . “ British anascan bryozoans. ” . In Synopses of the British Fauna (new series) , London, , UK : Academic Press .
- Ryther , JD , DeBoer , JA and Lapointe , BE . 1979 . Cultivation of seaweeds for hydrocolloids, waste treatment and biomass for energy conversion . Proc. Int. Seaweed Symp. , 9 : 1 – 16 .
- Saenko , GN , Kravtsova , YY , Ivanenko , VV and Shedludko , SI . 1978 . Concentration of iodine and bromine by plants in the seas of Japan and Okhotsk . Mar. Biol. , 47 : 243 – 250 .
- Saier , B and Chapman , AS . 2004 . Crusts of the alien bryozoan Membranipora membranacea can negatively impact spore output from native kelps (Laminaria longicruris) . Bot. Mar. , 47 : 265 – 271 .
- Saito , Y . 1972 . “ On the effects of environmental factors on morphological characteristics of Undaria pinnatifida and the breeding of hybrids in the genus Undaria ” . In Contributions to the Systematics of Benthic Marine Algae of the North Pacific , Edited by: Abott , IA and Kurogi , M . 117 – 133 . Kobe, , Japan : Japanese Society of Phycology .
- Sakai , Y . Stock enhancement of short-spined sea urchin Strongylocentrotus intermedius and its effect on Laminaria fishery in eastern Hokkaido, Japan . Proceedings of Aquaculture Conference, Lake Buena Vista, FL (USA), Book of abstracts . pp. 563
- Sakanishi , Y , Yokohama , Y and Aruga , Y . 1990 . Seasonal changes in photosynthetic capacity of Laminaria longissima Miyabe (Phaeophyta) . Jpn J. Phycol. , 38 : 147 – 153 .
- Sanbonsuga , Y and Neushul , MC . 1978 . Hybridization of Macrocystis (Phaeophyta) with other float-bearing kelps . J. Phycol. , 14 : 214 – 224 .
- Sandau , E , Sandau , P , Pulz , O and Zimmermann , M . 1996 . Heavy metal sorption by marine algae by-products . Acta Biotechnol. , 16 : 103 – 119 .
- Santos , R . 1993 . A multivariate study of biotic and abiotic relationships in a subtidal algal stand . Mar. Ecol. Prog. Ser. , 94 : 181 – 190 .
- Saunders , GW and Druehl , LD . 1992 . Nucleotide sequences of the small subunit ribosomal RNA genes from selected Laminariales (Phaeophyta): Implications for kelp evolution . J. Phycol. , 28 : 544 – 549 .
- Saunders , GW and Druehl , LD . 1993 . Revision of the kelp family Alariaceae and the taxonomic affinities of Lessoniopsis Reincke (Laminariales, Phaeophyta) . Hydrobiologia , 260/261 : 689 – 697 .
- Sauvageau , C . 1918 . Recherches sur les laminaires des côtes de France . Mémoires de l’Academie des Sciences de France , 56 : 1 – 240 .
- Sawabe , T , Ohtsuka , M and Ezura , Y . 1997 . Novel alginate lyases from marine bacterium Alteromonas sp. H-4 . Carbohydr. Res. , 304 : 69 – 76 .
- Sawabe , T , Makino , H , Tatsumi , M , Nakano , K , Tajima , K , Iqbal , MM , Yumoto , I , Ezura , Y and Christen , R . 1998a . Pseudoalteromonas bacteriolytica sp. nov., a marine bacterium that is the causative agent of red spot disease of Laminaria japonica . Int. J. Syst. Bacteriol. , 48 : 769 – 774 .
- Sawabe , T , Sawada , C , Suzuki , E and Ezura , Y . 1998b . Intracellular alginate-oligosaccharide degrading enzyme activity that is incapable of degrading intact sodium alginate from a marine bacterium Alteromonas sp . Fish. Sci. , 64 : 320 – 324 .
- Sawabe , T , Tanake , R , Iqbal , MM , Tajima , K , Ezura , Y , Ivanova , EP and Christen , R . 2000 . Assignment of Alteromonas elyakovii KMM 162T and five strains isolated from spot-wounded fronds of Laminaria japonica to Pseudoalteromonas elyakovii comb. nov. and the extended description of the species . Int. J. Syst. Evol. Microbiol. , 50 : 265 – 271 .
- Schaffelke , B . 1993 . Circannuale Rhythmik der Brauntange Laminaria hyperborea (Gunn.) Fosl. und L. digitata (Huds.) Lamour. bezüglich Wachstumsaktivität und jahreszeitlichem Gehalt an Abscisinsäure, Laminaran sowie Mannit , Hamburg, , Germany : University of Hamburg . PhD thesis
- Schaffelke , B . 1995 . Storage carbohydrates and abscisic acid contents in Laminaria hyperborea are entrained by experimental daylengths . Eur. J. Phycol. , 30 : 313 – 317 .
- Schaffelke , B and Lüning , K . 1994 . A circannual rhythm controls seasonal growth in the kelps Laminaria hyperborea and L. digitata from Helgoland (North Sea) . Eur. J. Phycol. , 29 : 49 – 56 .
- Schaffelke , B , Peters , AF and Reusch , TBH . 1996 . Factors influencing depth distribution of soft–bottom inhabiting Laminaria saccharina (L) Lamour. in Kiel Bay, western Baltic . Hydrobiologia , 327 : 117 – 123 .
- Schaffer , R , Landgraf , J , Accerbi , M , Simon , V , Larson , M and Wisman , E . 2001 . Microarray analysis of diurnal and circadian-regulated genes in Arabidopsis . Plant Cell , 13 : 113 – 123 .
- Schatz , S . 1980 . Degradation of Laminaria saccharina by higher fungi: a preliminary report . Bot. Mar. , 23 : 617 – 622 .
- Schatz , S . 1984a . Degradation of Laminaria saccharina by saprobic fungi . Mycologia , 76 : 426 – 432 .
- Schatz , S . 1984b . The Laminaria-Phycomelaina host-parasite association - Seasonal patterns of infection, growth and carbon and nitrogen storage in the host . Helgoländer Meeresunters. , 37 : 623 – 631 .
- Scheibling , RE . 1986 . Increased macroalgal abundance following mass mortalities of sea urchins (Strongylocentrotus droebachiensis) along the Atlantic coast of Nova Scotia . Oecologia , 68 : 186 – 198 .
- Scheibling , RE and Anthony , SX . 2001 . Feeding, growth and reproducing of sea urchins (Strongylocentrotus droebachiensis) on single and mixed diets of kelp (Laminaria spp.) and the invasive alga Codium fragile ssp. tomentosoides . Mar. Biol. , 139 : 139 – 146 .
- Scheibling , RE and Raymond , BG . 1990 . Community dynamics on a subtidal cobble bed following mass mortalities of sea urchins . Mar. Ecol. Prog. Ser. , 63 : 127 – 145 .
- Scheibling , RE , Hennigar , AW and Balch , T . 1999 . Destructve grazing, epiphytism, and disease: the dynamics of sea urchin–kelp interactions in Nova Scotia . Can. J. Fish. Aquat. Sci. , 56 : 2300 – 2314 .
- Schmid , R and Dring , MJ . 1992 . Circadian rhythm and fast responses to blue light of photosynthesis in Ectocarpus (Phaeophyta, Ectocarpales). I. Characterization of the rhythm and the blue-light response . Planta , 187 : 53 – 59 .
- Schmid , R , Mills , JA and Dring , MJ . 1996 . Influence of carbon supply on the stimulation of light-saturated photosynthesis by blue light in Laminaria saccharina: implications for the mechanism of carbon acquisition in higher brown algae . Plant Cell Environ. , 19 : 383 – 391 .
- Schmid , S , Ranz , D , He , ML , Burkard , S , Lukowicz , MV , Reiter , R , Arnold , R , Le Diet , H , David , M and Rambeck , WA . 2003 . Marine algae as natural source of iodine in the feeding of freshwater fish–a new possibility to improve iodine supply of man . Revue Med. Vet. , 154 : 645 – 648 .
- Schmitz , K . 1981 . “ Translocation ” . In Biology of Seaweeds , Edited by: Lobban , CS and Wynne , MJ . 534 – 558 . Oxford, UK : Blackwell .
- Schmitz , K and Lobban , CS . 1976 . A survey of translocation in Laminariales (Phaeophyceae) . Mar. Biol. , 36 : 207 – 216 .
- Schmitz , K , Lüning , K and Willenbrink , J . 1972 . CO2-Fixierung und Stofftransport in benthischen marinen Algen. 2. Zum Ferntransport 14C-markierter Assimilate bei Laminaria hyperborea und L. saccharina . Z. Pflanzenphysiol. , 67 : 418 – 429 .
- Schoenwaelder , MEA . 2002a . Physode distribution and the effects of ′Thallus Sunburn′ in Hormosira banksii (Fucales, Phaeophyceae) . Bot. Mar. , 45 : 262 – 266 .
- Schoenwaelder , MEA . 2002b . The occurence and cellular significance of physodes in brown algae . Phycologia , 41 : 125 – 139 .
- Schoenwaelder , MEA and Clayton , MN . 1998a . The secretion of phenolic compounds following fertilization in Acrocarpia paniculata (Fucales, Phaeophyta) . Phycologia , 37 : 40 – 46 .
- Schoenwaelder , MEA and Clayton , MN . 1998b . Secretion of phenolic substances into the zygote wall and cell plate in embryos of Hormosira and Acrocarpia (Fucales, Phaeophyta) . J. Phycol. , 34 : 969 – 980 .
- Schoenwaelder , MEA , Wiencke , C , Clayton , MN and Glombitza , KW . 2003 . The effect of elevated UV radiation on Fucus spp. (Fucales, Phaeophyta) zygote and embryo development . Plant Biol. , 5 : 366 – 377 .
- Schoschina , EV . 1997 . On Laminaria hyperborea (Laminariales, Phaeophyceae) on the Murman coast of the Barents Sea . Sarsia , 82 : 371 – 373 .
- Schramm , W and Nienhuis , PH . 1996 . Marine benthic vegetation. Recent changes and the effects of eutrophication . Ecol. Stud. Anal. Synth. , 123 : 1 – 470 .
- Schreiber , E . 1930 . Untersuchungen über Partenogenesis, Geschlechtsbestimmung und Bastardisierungsvermögen bei Laminarien . Planta , 12 : 331 – 353 .
- Schultz , TF and Kay , SA . 2003 . Circadian clocks in daily and seasonal control of development . Science , 301 : 326 – 328 .
- Schultze , K , Janke , K , Krüß , A and Weidemann , W . 1990 . The macrofauna and macroflora associated with Laminaria digitata and L. hyperborea at the island of Helgoland (German Bight, North Sea) . Helgoländer Meeresunters. , 44 : 39 – 51 .
- Sears , JR and Wilce , RT . 1975 . Sublittoral, benthic marine algae of southern Cape Cod and adjacent islands: seasonal periodicity, associations, diversity, and floristic composition . Ecol. Monographs , 45 : 337 – 365 .
- Seed , R . 1976 . Observations on the ecology of Membranipora (Bryozoa) and a major predator Doridella steinbergae (Nudibranchiata) along the fronds of Laminaria saccharina at Friday Harbor, Washington . J. Exp. Mar. Biol. Ecol. , 24 : 1 – 17 .
- Seed , R and Harris , S . 1980 . The epifauna of the fronds of Laminaria digitata Lamour in Strangford Lough, Northern Ireland . Proc. R. Irish Acad. , 80 : 91 – 106 .
- Setchell , WA . 1893 . On the classification and geographical distribution of the Laminariaceae . Trans. Conn. Acad. Arts , 9 : 333 – 375 .
- Setchell , WA . 1900 . Critical notes on the New England species of Laminaria . Rhodora , 2 : 143 – 146 .
- Setlow , RB . 1974 . The wavelengths in sunlight effective in producing skin cancer: a theoretical analysis . Proc. Nat. Acad. Sci. USA , 71 : 3363 – 3366 .
- Sheppard , CRC , Bellamy , DJ and Sheppard , ALS . 1977 . The fauna associated with the Laminaria ochroleuca Pyl. in the straits of Messina . Mem. Biol. Mar. , 7 : 1 – 9 .
- Shibata , T , Kawaguchi , S , Hama , Y , Inagaki , M , Yamaguchi , K and Nakamura , T . 2004 . Local and chemical distribution of phlorotannins in brown algae . J. Appl. Phycol. , 16 : 291 – 296 .
- Simenstad , CA , Estes , JA and Kenyon , KW . 1978 . Aleuts, sea otters, and alternate stable-state communities . Science , 200 : 403 – 411 .
- Sitte , P , Weiler , EW , Kadereit , JW , Bresinsky , A and Körner , C . 2002 . Strasburger Lehrbuch der Botanik, ed. 35 , Heidelberg, , Germany : Spektrum Verlag .
- Sivertsen , K . 1985 . Taretråling en mulig årsak til økt erosjon av sandstrender på Jærkysten, Nordland Distriktshøgskole . NDH-rapport , 6 : 1 – 17 .
- Sivertsen , K . 1997 . Geographic and environmental factors affecting the distribution of kelp beds and barren grounds and changes in biota associated with kelp reduction at sites along the Norwegian coast . Can. J. Fish. Aquat. Sci. , 54 : 2872 – 2887 .
- Sivertsen , K and Bjoerge , A . 1980 . Reduction of algal vegetation in Helgoland coastal waters . Fisk. Havet , 4 : 1 – 9 .
- Sjøtun , K . 1993 . Seasonal lamina growth in two age groups of Laminaria saccharina (L.) Lamour. in Western Norway . Bot. Mar. , 36 : 433 – 441 .
- Sjøtun , K and Fredriksen , S . 1995 . Growth allocation in Laminaria hyperborea (Laminariales, Phaeophyceae) in relation to age and wave exposure . Mar. Ecol. Progr. Ser. , 126 : 213 – 222 .
- Sjøtun , K and Schoschina , EV . 2002 . Gametophytic development of Laminaria spp. (Laminariales, Phaeophyta) at low temperature . Phycologia , 41 : 147 – 152 .
- Sjøtun , K , Fredriksen , S , Lein , TE , Rueness , J and Sivertsen , K . 1993 . Population studies of Laminaria hyperborea from its northern range of distribution in Norway . Hydrobiologia , 260/261 : 215 – 221 .
- Sjøtun , K , Rueness , S , Lein , J and Fredriksen , TE . 1995 . “ Ecological studies of the kelp Laminaria hyperborea (Gunnerus) Foslie in Norway ” . In Ecology of Fjords and Coastal Waters , Edited by: Skjoldal , H.R. , Hopkins , C , Erikstad , K.E. and Leinaas , H.P. 525 – 536 . Amsterdam, , The Netherlands : Elsevier Science .
- Sjøtun , K , Fredriksen , S and Rueness , J . 1996 . Seasonal growth and carbon and nitrogen content in canopy and first-year plants of Laminaria hyperborea (Laminariales, Phaeophyceae) . Phycologia , 35 : 1 – 8 .
- Sjøtun , K , Fredriksen , S and Rueness , J . 1998 . Effect of canopy biomass and wave exposure on growth in Laminaria hyperborea (Laminariaceae: Phaeophyta) . Eur. J. Phycol. , 33 : 337 – 343 .
- Sjøtun , K , Christie , H and Fossaa , JH . 2000 . Kelp resources and regrowth after an experimental trawling in the county of Soer-Troendelag (Norway) . Fisken Havet , 6 : 27 (In Norwegian, English abstract.)
- Skriptsova , AV and Titlyanov , EA . 2003 . The effect of meristem on sporulation in Laminaria cichorioides . Biol. Morya , 29 : 419 – 423 .
- Smit , AJ . 2004 . Medical and pharmaceutical uses of seaweed natural products: A review . J. Appl. Phycol. , 16 : 245 – 262 .
- Smith , BD . 1985 . Recovery following experimental harvesting of Laminaria longicruris and L. digitata in southwestern Nova Scotia . Helgoländer Meeresunters. , 39 : 83 – 101 .
- Smith , BD . 1986 . Implications of population dynamics and interspecific competition for harvest management of the seaweed Laminaria . Mar. Ecol. Prog. Ser. , 33 : 7 – 18 .
- Smith , GM . 1947 . On the reproduction of some Pacific coast species of Ulva . Amer. J. Bot. , 34 : 80 – 87 .
- Soares , AG , Schlacher , TA and Mclachlan , A . 1997 . Carbon and nitrogen exchange between sandy beach clams (Donax serra) and kelp beds in the Benguela coastal upwelling region . Mar. Biol. , 127 : 657 – 664 .
- Staer , PA , Pedersen , MF , Thomsen , MS , Wernberg , T and Krause-Jensen , D . 2000 . Invasion of Sargassum muticum in Limfjorden (Denmark) and its possible impact on the indigenous macroalgal community . Mar. Ecol. Prog. Ser. , 207 : 79 – 88 .
- Stam , WT , Bot , PVM , Boele-Bos , SA , Van Rooij , JM and Van Den Hoek , C . 1988 . Single-copy DNA-DNA hybridizations among five species of Laminaria (Phaeophyceae): Phylogenetic and biogeographic implications . Helgoländer Meeresunters. , 42 : 251 – 267 .
- Stegenga , H , Anderson , JJ and Bolton , RJ . 1997 . Seaweeds of the South African West coast , Cape Town, , South Africa : University of Cape Town, Bolus Herbarium . Contributions from the Bolus Herbarium 18.
- Stein , JR and Borden , CA . 1984 . Causative and beneficial algae in human disease conditions: a review . Phycologia , 23 : 485 – 501 .
- Steinberg , PD . 1985 . Feeding preferences of Tegula funebralis and chemical defenses of marine brown algae . Ecol. Monogr. , 55 : 333 – 349 .
- Steinberg , PD . 1994 . Lack of short-term induction of phlorotannins in the Australasian brown algae Ecklonia radiata and Sargassum vesitum . Mar. Ecol. Progr. Ser. , 112 : 129 – 133 .
- Stengel , DB , McGrath , H and Morrison , LJ . 2005 . Tissue Cu, Fe and Mn concentrations in different-aged and different functional thallus regions of three brown algae from western Ireland . Estuar. Coast. Shelf Sci. , 65 : 687 – 696 .
- Stephan , FK . 2002 . The “other” circadian system: food as a Zeitgeber . J. Biol. Rhythms , 17 : 284 – 292 .
- Stickney , RR . Joining forces with industry–Open Ocean Aquaculture . Proceedings of the Third Annual International Conference . May 10–15 . pp. 152 Texas : Corpus Christi . TAMU-SG-99-103, Corpus Christi, Texas Sea Grant College Program
- Stirk , WA and Van Staden , J . 2000 . Removal of heavy metals from solution using dried brown seaweed material . Bot. Mar. , 43 : 467 – 473 .
- Stuart , V . 1982 . Absorbed ration, respiratory costs and resultant scope for growth in the mussel Aulacomya ater (Molina) fed on a diet of kelp detritus of different ages . Mar. Biol. Lett. , 3 : 289 – 306 .
- Stuart , V , Lucas , MI and Newell , RC . 1981 . Heterotrophic utilisation of particulate matter from the kelp Laminaria pallida . Mar. Ecol. Prog. Ser. , 4 : 337 – 348 .
- Suárez-López , P , Wheatley , K , Robson , F , Onouchi , H , Valverde , F and Coupland , G . 2001 . CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis . Nature , 410 : 1116 – 1120 .
- Subandar , A , Petrell , RJ and Harrison , PJ . 1993 . Laminaria culture for reduction of dissolved inorganic nitrogen in salmon farm effluent . J. Appl. Phycol. , 5 : 455 – 463 .
- Sukhoveeva , MV . 1975 . Epiphytes on Laminaria of the Far East seas . Izv. TINRO , 98 : 184 – 192 .
- Sumi , CBT and Scheibling , RE . 2005 . Role of grazing by sea urchins Strongylocentrotus droebachiensis in regulating the invasive alga Codium fragile ssp. tomentosoides in Nova Scotia . Mar. Ecol. Prog. Ser. , 292 : 203 – 212 .
- Sundene , O . 1958 . Interfertility between forms of Laminaria digitata . Nytt Magasin for Botanikk , 6 : 121 – 128 .
- Suzuki , L and Johnson , CH . 2001 . Algae know the time of day: circadian and photoperiodic programs . J. Phycol. , 37 : 933 – 942 .
- Suzuki , Y , Takabayshi , T , Kawaguchi , T and Matsunaga , K . 1998 . Isolation of an allelopathic substance from the crustose coralline algae, Lithophyllum spp., and its effect on the brown alga, Laminaria religiosa Miyabe (Phaeophyta) . J. Exp. Mar. Biol. Ecol. , 225 : 69 – 77 .
- Swanson , AK and Druehl , LD . 2000 . Differential meiospore size and tolerance of ultraviolet light stress within and among kelp species along a depth gradient . Mar. Biol. , 136 : 657 – 664 .
- Swanson , AK and Druehl , LD . 2002 . Induction, exudation and the UV protection role of kelp phlorotannins . Aquat. Bot. , 73 : 241 – 253 .
- Sylter , Algenfarm . 2006 . Available at: http://www.algenfarm.de
- Takami , H and Kawamura , T . 2003 . Dietary changes in the abalone, Haliotis discus hannai, and relationships with the development of the digestive organ . Jap. Agricult. Res. Quart. , 37 : 89 – 98 .
- Targett , NM and Arnold , TM . 2001 . “ Chemical mediation of colonization of seaweed surfaces ” . In Marine Chemical Ecology , Edited by: McClintock , JB and Baker , BJ . 391 – 411 . Boca Raton, , USA : CRC Press .
- Teas , J . 1983 . The dietary intake of Laminaria, a brown seaweed, and breast cancer prevention . Nutr. Cancer , 4 : 217 – 222 .
- Tegner , MJ and Dayton , PK . 1981 . Population structure, recruitment and mortality of two sea urchins (Strongylocentrotus franciscanus and S. purpuratus) in a kelp forest . Mar. Ecol. Prog. Ser. , 5 : 255 – 268 .
- Tegner , MJ and Dayton , PK . 1991 . Sea urchins, El Niño, and the long term stability of southern California kelp forest communities . Mar. Ecol. Prog. Ser. , 77 : 49 – 63 .
- Tegner , MJ , Dayton , PK , Edwards , PB and Riser , KL . 1997 . Large-scale, low-frequency oceanographic effects on kelp forest succession: A tale of two cohorts . Mar. Ecol. Prog. Ser. , 146 : 117 – 134 .
- Thomas , S . 2000a . Alginate dressings in surgery and wound management: Part 1 . J. Wound Care , 9 : 56 – 60 .
- Thomas , S . 2000b . Alginate dressings in surgery and wound management: Part 2. J . Wound Care , 9 : 115 – 119 .
- Thomas , S . 2000c . Alginate dressings in surgery and wound management: Part 3. J . Wound Care , 9 : 163 – 166 .
- Thornton , DCO . 2004 . Formation of transparent exopolymeric particles (TEP) from macroalgal detritus . Mar. Ecol. Prog. Ser. , 282 : 1 – 12 .
- Titlyanov , EA , Titlyanova , T and Lüning , K . 1996 . Diurnal and circadian periodicity of mitosis and growth in marine macroalgae. II. The green alga Ulva pseudocurvata . Eur. J. Phycol. , 31 : 181 – 188 .
- Tokida , J , Nakamura , Y and Druehl , LD . 1980 . Typification of species of Laminaria (Phaeophyta, Laminariales) described by Miyabe, and taxonomic notes on the genus in Japan . Phycologia , 19 : 317 – 328 .
- Tom Dieck , I . 1989 . Vergleichende Untersuchungen zur Ökophysiologie und Kreuzbarkeit innerhalb der digitaten Sektion der Gattung Laminaria , Hamburg, , Germany : University of Hamburg . PhD thesis
- Tom Dieck , I . 1991 . Circannual growth rhythm and photoperiodic sorus induction in the kelp Laminaria setchellii (Phaeophyta) . J. Phycol. , 27 : 341 – 350 .
- Tom Dieck , I . 1992 . North Pacific and North Atlantic digitate Laminaria species (Phaeophyta): Hybridization experiments and temperature responses . Phycologia , 31 : 147 – 163 .
- Tom Dieck , I . 1993 . Temperature tolerance and survival in darkness of kelp gametophytes (Laminariales, Phaeophyta): ecological and biogeographical implications . Mar. Ecol. Prog. Ser. , 100 : 253 – 264 .
- Tom Dieck , I and De Oliveira , EC . 1993 . The section Digitatae of the genus Laminaria (Phaeophyta) in the northern and southern Atlantic: Crossing experiments and temperature responses . Mar. Biol. , 115 : 151 – 160 .
- Toth , GB and Pavia , H . 2002a . Lack of phlorotannin induction in the kelp Laminaria hyperborea in response to grazing by two gastropod herbivores . Mar. Biol. , 140 : 403 – 409 .
- Toth , GB and Pavia , H . 2002b . Intraplant habitat and feeding preference of two gastropod herbivores inhabiting the kelp Laminaria hyperborea . J. Mar. Biol. Assoc. UK , 82 : 243 – 247 .
- Troell , M , Rönnbäck , P , Halling , C , Kautsky , N and Buschmann , A . 1999 . Ecological engineering in aquaculture: use of seaweeds for removing nutrients from intensive mariculture . J. Appl. Phycol. , 11 : 89 – 97 .
- Troiano , RA , Wise , DL , Augenstein , DC , Kispert , RG and Kooney , CL . 1976 . Fuel gas production by anaerobic digestion of kelp . Res. Recovery Conserv. , 2 : 171 – 176 .
- Tseng , CK . Laminaria cultivation and research in China . Proceedings of the Second Conference of the Western Pacific Fisheries Research Commission . pp. 31 – 43 . Beijing, , China : Science Press .
- Tseng , CK . 1962 . “ The haidai (Laminaria japonica) cultivation industry of China ” . In Manual of Cultivation of Haidai (Laminaria japonica) , Edited by: Tseng , C.K. and Wu , C.Y. 99 – 112 . Beijing, , China : Science Press .
- Tseng , CK . 1984 . Phycological research in the development of the Chinese seaweed industry . Hydrobiologia , 116/117 : 7 – 18 .
- Tseng , CK . 1987 . “ Laminaria mariculture in China ” . In Case Studies of seven Commercial Seaweed Resources , Edited by: Doty , M.S. , Caddy , J.F. and Santelices , B . 239 – 263 . Rome, Italy : FAO Fisheries Technical Paper 281, Food and Agriculture Organisation of the United Nations .
- Tseng , CK , Ren , KZ and Wu , CY . 1959 . On the discharge of eggs and spermatozoids of Laminaria japonica and the morphology of the spermatozoids . Kexue Tongbao , 4 : 129 – 130 . (In Chinese.)
- Tugwell , S and Branch , GM . 1989 . Differential polyphenolic distribution among tissues in the kelps Ecklonia maxima, Laminaria pallida and Macrocystis angustifolia in relation to plant-defence theory . J. Exp. Mar. Biol. Ecol. , 129 : 219 – 230 .
- Tugwell , S and Branch , GM . 1992 . Effects of herbivore gut surfactants on kelp polyphenol defenses . Ecology , 73 : 205 – 215 .
- Tzetlin , AB , Mokievsky , VO , Melnikov , AN , Saphonov , MV , Simdyanov , TG and Ivanov , IE . 1997 . Fauna associated with detached kelp in different types of subtidal habitats in the White Sea . Hydrobiologia , 355 : 91 – 100 .
- Uchida , M . 1996 . Formation of single cell detritus densely covered with bacteria during experimental degradation of Laminaria japonica thalli . Fish. Sci. , 62 : 731 – 736 .
- Uchida , M , Nakata , K and Maeda , M . 1997 . Introduction of detrital food webs into an aquaculture system by supplying single cell algal detritus produced from Laminaria japonica as a hatchery diet for Artemia nauplii . Aquaculture , 154 : 123 – 135 .
- Underwood , AJ . 1986 . “ The analysis of competition by field experiments ” . In Community Ecology: Pattern and Process , Edited by: Kikkawa , J and Anderson , DJ . 240 – 268 . Melbourne, , Australia : Blackwell Scientific Publications .
- Vadas , RL and Steneck , RS . 1995 . “ Overfishing and inferences in kelp-sea urchin interactions ” . In Ecology of Fjords and Coastal Waters , Edited by: Hopkins , C , Erikstad , KE and Leinaas , HP . 509 – 524 . Amsterdam, , The Netherlands : Elsevier Science .
- Vadas , RL , Elner , RW , Garwood , PE and Babb , IG . 1986 . Experimental evaluation of aggregation behavior in the sea urchin Strongylocentrotus droebachiensis . Mar. Biol. , 90 : 433 – 448 .
- Vairappan , CS , Suzuki , M , Motomura , T and Ichimura , T . 2001 . Pathogenic bacteria associated with lesions and thallus bleaching symptoms in the Japanese kelp Laminaria religiosa Miyabe (Laminariales, Phaeophyceae) . Hydrobiologia , 445 : 183 – 191 .
- Valentin , K and Zetsche , K . 1990 . Rubisco genes indicate a close phylogenetic relationship between the plastids of Chromophyta and Rhodophyta . Plant Mol. Biol. , 15 : 575 – 584 .
- Valentin , K , Cattolico , RA and Zetsche , K . 1992 . “ Phylogenetic origin of the plastids ” . In Origins of Plastids , Edited by: Lewin , RA . 193 – 222 . New York, , USA : Chapman & Hall .
- Van Alstyne , KL , McCarthy , JJ , Hustead , CL and Kearns , LJ . 1999 . Phlorotannin allocation among tissues of northeastern pacific kelps and rockweeds . J. Phycol. , 35 : 483 – 492 .
- Van Alstyne , KL , Dethier , MN and Duggins , DO . 2001a . “ Spatial patterns in macroalgal chemical defenses ” . In Marine Chemical Ecology , Edited by: McClintock , JB and Baker , BJ . 301 – 324 . New York, , USA : CRC Press .
- Van Alstyne , KL , Whitman , SL and Ehlig , JM . 2001b . Differences in herbivore preferences, phlorotannin production, and nutritional quality between juvenile and adult tissues from marine brown algae . Mar. Biol. , 139 : 201 – 210 .
- Van den Hoek , C , Mann , C and Jahns , HM . 1995 . Algae: An Introduction to Phycology , Cambridge, , UK : Cambridge University Press .
- Van Patten , MS and Yarish , C . 1993 . Allocation of blade surface to reproduction in Laminaria longicruris of Long Island Sound (USA) . Hydrobiologia , 260/261 : 173 – 181 .
- Velimirov , B and Griffiths , CL . 1979 . Wave-induced kelp movements and its importance for community structure . Bot. Mar. , 22 : 169 – 172 .
- Véliz , K , Edding , M , Tala , F and Gómez , I . 2006 . Effects of ultraviolet radiation on different life cycle stages of the south Pacific kelps, Lessonia nigrescens and Lessonia trabeculata (Laminariales, Phaeophyceae) . Mar. Biol. , 149 : 1015 – 1024 .
- Vreeland , V and Laetsch , WM . 1990 . “ A gelling carbohydrate in algal cell wall formation ” . In Organisation and Assembly of Plant and Animal Extracellular Matrix , Edited by: Adair , WS and Mecham , RP . 137 – 171 . San Diego, , USA : Academic Press .
- Waaland , SD and Cleland , R . 1972 . Development in the red alga Griffithsia pacifica: control by internal and external factors . Planta , 105 : 196 – 204 .
- Wahl , M and Hay , ME . 1995 . Associational resistance and shared doom: Effects of epibiosis on herbivory . Oecologia , 102 : 329 – 340 .
- Wahl , M and Mark , O . 1999 . The predominantly facultative nature of epibiosis: Experimental and observational evidence . Mar. Ecol. Prog. Ser. , 187 : 59 – 66 .
- Wakefield , RL and Murray , SN . 1998 . Factors influencing food choice by the seaweed-eating marine snail Norrisia norrisi (Trochidae) . Mar. Biol. , 130 : 631 – 642 .
- Wang , X , Lou , Q and Yan , X . 1996 . Contents and Distribution of Iodine in Fresh Laminaria japonica , Beijing, , China : Science Press .
- Wang , X-L , Yang , Y-X , Cong , Y-Z and Duan , D-L . 2004 . DNA fingerprinting of selected Laminaria (Phaeophyta) gametophytes by RAPD markers . Aquaculture , 238 : 143 – 153 .
- Wang , Y , Tang , XX , Yang , Z and Yu , ZM . 2006 . Effect of alginic acid decomposing bacterium on the growth of Laminaria japonica (Phaeophyceae) . J. Environ. Sci. , 18 : 543 – 551 .
- Webster , TJ , Paranjape , MA and Mann , KH . 1975 . Sedimentation of organic matter in St. Margaret's Bay, Nova Scotia . J. Fish. Res. Bd. Can. , 32 : 1399 – 1407 .
- Wharton , WG and Mann , KH . 1981 . Relationship between destructive grazing by sea urchin, Strongylocentrotus droebachiensis, and the abundance of American lobster, Homarus americanus, on the Atlantic coast of Nova Scotia . Can. J. Fish. Aquat. Sci. , 38 : 1339 – 1349 .
- Whittick , A . 1983 . Spatial and temporal distributions of dominant epiphytes on the stipes of Laminaria hyperborea (Gunn.) Fosl. (Phaeophyta: Laminariales) in S.E. Scotland . J. Exp. Mar. Biol. Ecol. , 73 : 1 – 10 .
- Whittick , A , Knight , K and Hooper , RG . 1982 . Fouling algae on steel structures in the Newfoundland inshore . Br. Phycol. J. , 17 : 241
- Wiencke , C and Fischer , G . 1990 . Growth and stable carbon isotope composition of cold-water macroalgae in relation to light and temperature . Mar. Ecol. Prog. Ser. , 65 : 283 – 292 .
- Wiencke , C , Bartsch , I , Bischoff , B , Peters , AF and Breeman , AM . 1994 . Temperature requirements and biogeography of Antarctic, Arctic and amphiequatorial seaweeds . Bot. Mar. , 37 : 247 – 259 .
- Wiencke , C , Gómez , I , Pakker , H , Flores-Moya , A , Altamirano , M , Hanelt , D , Bischof , K and Figueroa , F-L . 2000 . Impact of UV radiation on viability, photosynthetic characteristics and DNA of brown algal zoospores: implications for depth zonation . Mar. Ecol. Prog. Ser. , 197 : 217 – 229 .
- Wiencke , C , Clayton , MN and Schoenwaelder , MEA . 2004 . Sensitivity and acclimation to UV radiation of zoospores from five species of Laminariales from the Arctic . Mar. Biol. , 145 : 31 – 39 .
- Wiencke , C , Roleda , MY , Gruber , A , Clayton , MN and Bischof , K . 2006 . Susceptibility of zoospores to UV radiation determines upper depth distribution limit of Arctic kelps: evidence through field experiments . J. Ecol. , 94 : 455 – 463 .
- Wiencke , C , Lüder , UH and Roleda , MY . 2007 . Impact of ultraviolet radiation on physiology and development of zoospores of the brown alga Alaria esculenta from Spitsbergen . Physiol. Plant. , 130 : 601 – 612 .
- Wikfors , GH and Ohno , M . 2001 . Impact of algal research in aquaculture . J. Phycol. , 37 : 968 – 974 .
- Wilce , RT . 1960 . Studies in the genus Laminaria. II. Laminaria groenlandica L. K. Rosenvinge . Bot. Not. , 113 : 203 – 209 .
- Wilce , RT . 1965 . Studies in the genus Laminaria. III. A revision of the north Atlantic species of the Simplices section of Laminaria . Bot. Gothoburg , 3 : 247 – 256 .
- Wildgoose , PB , Blunden , G and Jewers , K . 1978 . Seasonal variations in gibberellin activity of some species of Fucaceae and Laminariaceae . Bot. Mar. , 21 : 63 – 65 .
- Winter , FC and Estes , JA . 1992 . Experimental evidence for the effects of polyphenolic compounds from Dictyoneurum californicum Ruprecht (Phaeophyta: Laminariales) on feeding rate and growth in the red abalone Haliotus rufescens Swainson . J. Exp. Mar. Biol. Ecol. , 155 : 263 – 277 .
- Witman , JD . 1987 . Subtidal coexistence: storms, grazing, mutualism, and the zonation of kelps and mussels . Ecol. Monogr. , 57 : 167 – 187 .
- Woelfle , MA and Johnson , CH . 2006 . No promoter left behind: global circadian gene expression in cyanobacteria . J. Biol. Rhythms , 21 : 419 – 431 .
- Xia , P and Wang , X-L . 2005 . Genetic study of Kelp “901” strain . Chin. J. Oceanol. Limnol. , 23 : 152 – 157 .
- Yabu , H . 1964 . Early development of several species of Laminariales in Hokkaido . Mem. Fac. Fish., Hokkaido University , 12 : 1 – 54 .
- Yabu , H and Yasui , H . 1991 . Chromosome numbers in four species of Laminaria (Phaeophyta) . Jpn. J. Phycol. , 39 : 185 – 187 .
- Yamaguchi , T , Ikawa , T and Nisizawa , K . 1966 . Incorporation of radioactive carbon from . Plant Cell Physiol. , 7 : 217 – 229 .
- Yamaguchi , T , Ikawa , T and Nisizawa , K . 1969 . Pathway of mannitol formation during photosynthesis in brown algae . Plant Cell Physiol. , 10 : 425 – 440 .
- Yancey , P . 2005 . Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses . J. Exp. Biol. , 208 : 2819 – 2830 .
- Yasui , H . 1992 . Chromosome numbers and a sex chromosome of Laminaria yendoana Miyabe (Phaeophyta) . Nippon Suisan Gakkaishi , 58 : 1385
- Yarish , C , Penniman , CA and Egan , B . 1990 . Growth and reproductive responses of Laminaria longicruris (Laminariales, Phaeophyta) to nutrient enrichment . Hydrobiologia , 204/205 : 505 – 511 .
- Yoneshigue-Valentin , Y . 1990 . The life cycle of Laminaria abyssalis (Laminariales, Phaeophyta) in culture . Hydrobiologia , 204/205 : 461 – 466 .
- Yoon , HS and Boo , SM . 1999 . Phylogeny of Alariaceae (Phaeophyta) with special reference to Undaria based on sequences of the RuBisCo spacer region . Hydrobiologia , 398/399 : 47 – 55 .
- Yoon , HS , Lee , JL , Boo , SM and Bhattacharya , D . 2001 . Phylogeny of Alariaceae, Laminariaceae, and Lessoniaceae (Phaeophyceae) based on plastid-encoded RuBisCo spacer and nuclear-encoded ITS sequence comparisons . Mol. Phylogenies Evol. , 21 : 231 – 243 .
- Yoshida , T . 1980 . Distribution of Streblonema aecidioides around Japan and its host . Jpn. J. Phycol. , 27 : 182
- Yoshida , T , Yoshinaga , K and Nakajima , Y . 2000 . Check list of marine algae of Japan (Revised in 2000) . Jpn. J. Phycol. , 48 : 113 – 166 . (In Japanese.)
- Yoshimura , A , Yoshikawa , K and Oishi , K . 1992 . Iodine distribution in blades of several Laminarias grown in the same sea area . Nippon Suisan Gakkaishi , 58 : 1373 – 1379 .
- Yoshimori , A , Kono , T and Iizumi , H . 1998 . Mathematical models of population dynamics of the kelp Laminaria religiosa, with emphasis on temperature dependence . Fish. Oceanogr. , 7 : 136 – 146 .
- Yotsukura , N , Denboh , T , Motomura , T , Horiguchi , T , Coleman , AW and Ichimura , T . 1999 . Little divergence in ribosomal DNA internal transcribed spacer -1 and -2 sequences among non-digitate species of Laminaria (Phaeophyceae) from Hokkaido . Phycol. Res. , 47 : 71 – 80 .
- Yotsukura , N , Kawai , T , Motomura , T and Ichimura , T . 2001 . Random amplified polymorphic DNA markers for three Japanese laminarian species . Fish. Sci. , 67 : 857 – 862 .
- Yotsukura , N , Kawai , T , Motomura , T and Ichimura , T . 2002 . Tandem 5S ribosomal RNA genes and the spacer region sequences of three Japanese Laminaria species . J. Appl. Phycol. , 14 : 233 – 239 .
- Yotsukura , N , Kawai , T , Kawashima , S , Ebata , H and Ichimura , T . 2006 . Nucleotide sequence diversity of the 5S rDNA spacer in the simple blade kelp genera Laminaria, Cymathaere and Kjellmaniella (Laminariales, Phaeophyceae) from northern Japan . Phycol. Res. , 54 : 269 – 279 .
- Zemke-White , WL and Ohno , M . 1999 . World seaweed utilisation: An end-of-century summary . J. Appl. Phycol. , 11 : 369 – 376 .
- Zvereva , LV . 1998 . Mycobioty of the cultivated brown alga Laminaria japonica . Russ. J. Mar. Biol. , 24 : 19 – 23 .