Abstract
Clarification of chromosome number and behaviour during meiosis is important for genetic investigations of the unicellular alga Chlamydomonas reinhardtii. However, the chromosome number and their morphology remain uncertain in this alga. We stained living zygotes of C. reinhardtii with the highly sensitive DNA fluorochrome SYBR Green I and observed chromosomal behaviour during meiosis. Zygotes with two and four cells, resulting from the first and second meiotic divisions, appeared approximately 16 and 20 h, respectively, after mature zygotes were exposed to light under experimental conditions. In prophase I, chromosomes in the nucleus changed from long, thread-like structures to bivalents. At metaphase I and anaphase I, chromosomes assumed raft-like configurations but chromosomal configurations were less distinct at metaphase II and anaphase II. The nuclei were always localized near the zygotic surface. By observing bivalents at diakinesis we determined the total number of chromosomes to be 18. Chromosome lengths ranged from 0.5 to 2 µm. Using approximate fluorescence intensities, we estimated DNA content of the largest chromosome to be 3–6 times greater than that of the smallest.
Introduction
The observation of chromosomes during meiosis is important in genetic studies for determining chromosome number. However, in the unicellular alga Chlamydomonas reinhardtii, the observation of chromosomes during meiosis has been difficult because of its solid zygospore walls (Harris, Citation1989), resulting in few observations of chromosomes or nuclear morphology during meiosis.
Using light microscopy, Levine & Folsome (Citation1959) observed meiotic nuclei stained with either Azure A or Feulgen and reported a possible chromosome number of eight. McVittie & Davies (Citation1971) observed meiotic zygotes in prophase I and reported 16 bivalents at diakinesis, but could not elucidate the chromosomal configuration and behaviour because of the low resolution of the chromosomes and fragmentary evidence of events during meiosis.
Electron microscopy revealed that prophase leptotene was marked by chromosomal condensation and the appearance of axial cores that were associated with the nuclear envelope in early zygotene (Triemer & Brown, Citation1976). Storms & Hastings (Citation1977) analysed the synaptonemal complexes in pachytene by reconstructing images of serial sections. They found 16 individual bivalents plus four bivalent arms attached to a common mass of condensed chromatin, suggesting a haploid chromosome number of 18 or 20. However, the chromosomal configurations and behavioural details of meiosis, especially after metaphase I, have not been fully elucidated, and the number of chromosomes in C. reinhardtii remains unclear.
We stained zygotes undergoing meiosis with the highly sensitive, DNA-specific fluorochrome SYBR Green I, allowing us to observe nuclei in living cells (Nishimura et al., Citation1998; Aoyama et al., Citation2006; Hiramatsu et al., Citation2006). We obtained high-resolution images of the morphological behaviour of meiotic cell chromosomes using fluorescence microscopy, observing chromosomes during zygotic meiosis and discovering 18 chromosomes.
Materials and methods
Cell strains and culture
We examined wild-type mt + and mt − 137c strains of C. reinhardtii. Cells were cultured on 1.2% agar plates at 22°C under a 12:12 h light–dark cycle, as described previously (Nakamura et al., Citation1986), and gametes were induced by incubating vegetatively growing cells in nitrogen-free medium (Nakamura et al., Citation1991). The methods of zygote formation, zygote maturation, and zygote germination have been described previously (Nakamura et al., Citation1991). Zygotes were maintained for 7 days in darkness to mature.
Cell staining
Mature zygotes were exposed to light for 12–22 h. To stain nuclei of living zygotes, SYBR Green I (FMC Bioproducts, Rockland, ME, USA) was added to the cell suspension at a final dilution of 1:1000 (Aoyama et al., Citation2006) and the zygotes incubated for 30–60 min. The cells were then washed by gentle centrifugation and observed under a fluorescence microscope, as described previously (Aoyama et al., Citation2006; Nishimura et al., Citation1998).
Chromosome number counts
Mature zygotes were exposed to light for 16–18 h and then treated with fixative (3:1 methanol:acetic acid) for 1 min, after which 100 µl of the zygote solution was placed on a glass slide and steam fixed for 2–5 min. The chromosomes were then stained with SYBR Green I solution (diluted 1:1000 with distilled water) and observed under a fluorescence microscope.
Relative fluorescence intensity and estimation of DNA quantity of each chromosome
After rearranging the chromosomes in order of size, the relative fluorescence intensity of each chromosome was estimated using NIH Image 1.63 software with the gel plotting macro (http://rsb.info.nih.gov/nih-image/). The strongest chromosome intensity was used as the intensity standard for that group of chromosomes.
To estimate DNA content (Mbp) of each chromosome, we used a value of 121 Mbp for total haploid chromosome DNA (Grossman et al., Citation2007). We also assumed that fluorescence intensity corresponded to DNA quantity.
Results
Frequencies of zygotes completing the first and second meiotic divisions
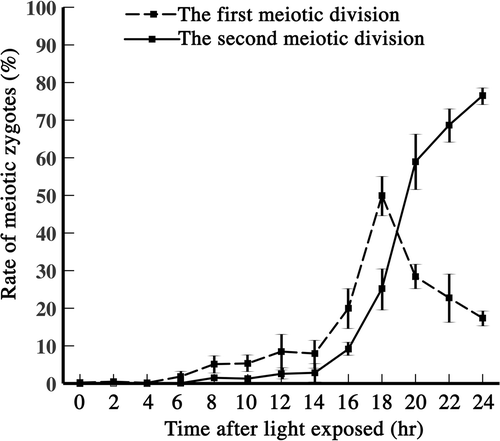
To estimate the time course of meiosis, we measured the frequencies of zygotes with two and four cells that had completed the first and second meiotic divisions, respectively, after exposing mature zygotes to light (). Two-celled zygotes began to increase in number at about 14 h, reaching approximately 50% at around 18 h (, dashed line). Four-celled zygotes began to increase at about 16 h, reaching approximately 80% by 24 h. The time between the occurrence of zygotes with two and four cells was approximately 2 h, judging from the two curves at 14–18 h. Meiosis I probably occurred after 16–18 h.
Chromosomal behaviour in meiosis
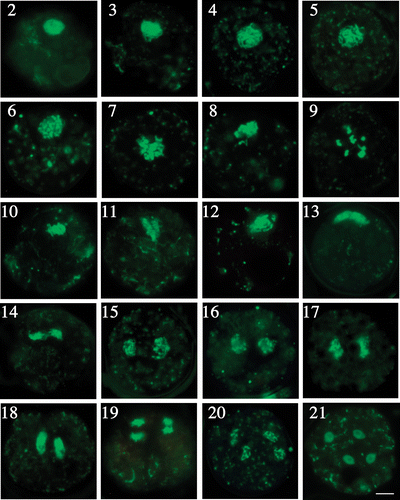
We observed morphological changes from prophase I to telophase II in the zygote cell nucleus stained with SYBR Green I using fluorescence microscopy (). The dye stained DNA in the nucleus and in nucleoids of chloroplasts and mitochondria. In probable early prophase I, the nucleus was about 5 µm in diameter and located near the surface of the zygote (). At leptotene, chromosomes first became visible as long, thread-like structures (), at zygotene and pachytene, the thread-like structures thickened and cleared () as the chromosomes formed sister chromatids and paired, but we could not distinguish these structures because of the resolution limitations of fluorescence microscopy. At diplotene and diakinesis, the homologous chromosomes became shorter and thicker (), but we could not observe chiasmatic structures. At diakinesis, each bivalent chromosome appeared as a large chromosomal mass () and bivalents expanded to about 10 µm in diameter. At metaphase I, the bivalent chromosomes were arranged in parallel, like a raft (, ). The number of chromosomes appeared to decrease compared with that at diakinesis because of some overlapping as the chromosomes were rearranged in the same plane. At anaphase I, each homologous chromosome was quite distinct and separated from the others (), and the chromosomes appeared short and rod-like compared with the metaphase bivalents (, ). At telophase I, the chromosomes moved to the opposite poles (), which were about 10 µm apart. The nuclear envelope can form briefly around each nucleus in telophase I and prophase II, but our staining did not reveal this (). All these events occurred in the surface region of the cell ().
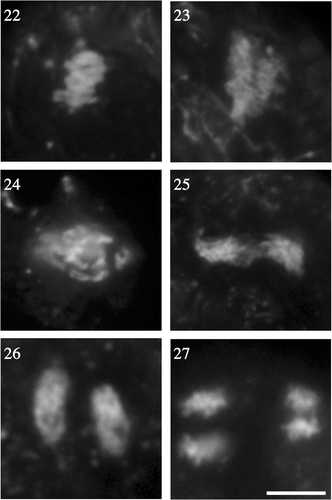
Figs 2–21. Fluorescence microscopy of chromosome behaviour during meiosis. . Chromosome morphology in prophase I. . Leptotene. . Zygotene. . Pachytene. . Diplotene and diakinesis at approximately 16 h. . Metaphase I to telophase II. . Metaphase I. . Anaphase I. . Telophase I. . The side view shows chromosomes situated at the surface of the zygotes. . Prophase II. . Metaphase II. . Anaphase II. . Telophase II. . Formation of four daughter cells. Scale bar: 5 µm.
The second meiotic division occurred similarly to the first meiotic division (). In anaphase II, the direction of chromosome movement was perpendicular to that of anaphase I (), but the chromosomal images were not as clear as those at the first meiotic division, probably because of the smaller size of the chromosomes (, ). Finally, four nuclei were formed at telophase II (). Throughout meiosis, the nucleus was always located near the cell surface.
Number of chromosomes at diakinesis
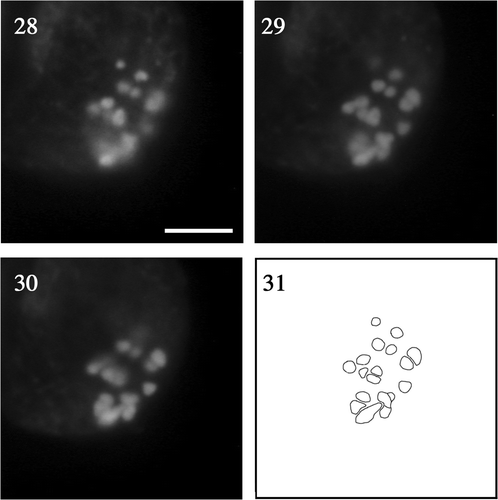
We counted the number of chromosomes stained with SYBR Green I in cells undergoing the first meiotic division. We first observed zygotes with incompletely broken walls with all the chromosomes still within the zygote () to capture entire chromosome images. At diakinesis, we observed large, bivalent chromosomes (), and counting the number of chromosomes was easier at this than at any other meiotic stage (, ). We constructed a composite diagram from three different focal planes to separate overlapped chromosomes (). Despite some overlapping (), we were able to identify 18 chromosomes, 0.5–2 µm in length.
Figs 28–31. Fluorescence micrographs of chromosomes at meiotic diakinesis at different focal planes of the same zygote. . Low. . Middle. . Upper. . Composite image based on the superimposition of views . Scale bar: 5 µm.
To resolve the overlapping problem, after breaking the zygote wall we spread chromosomes on glass slides () and then rearranged them in order of size (), observing a total of 18 chromosomes, ∼0.5–1 µm long. The largest chromosome was about twice as long as the smallest. Our results suggest that C. reinhardtii has 18 chromosomes.
Figs 32–35. Fluorescent photomicrographs of spread chromosomes at diakinesis. . Total chromosomes spread on glass slide. . Chromosomes arranged in order of fluorescence intensity. Scale bar: 5 µm.
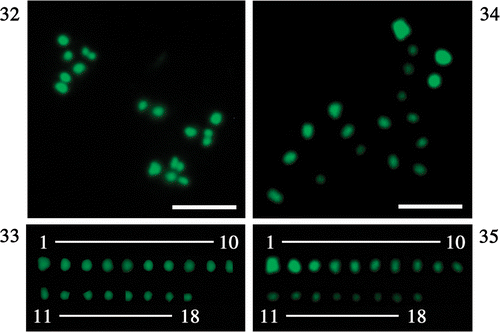
Chromosomes lengths ranged between 0.5 and 2 µm in fluorescence images of unbroken and incompletely broken zygotes (, ) at diakinesis, and the chromosome size and configuration did not differ between incompletely and completely broken zygotes (Figs , ; respectively). Under fluorescence microscopy, nuclear chromosomes were larger than mitochondrial (0.1 µm) and chloroplast nucleoids (0.4 µm; Nishimura et al., Citation1998), although we could not reliably distinguish between mitochondrial and chloroplast nucleoids.
Relative fluorescence intensity and estimation of DNA quantity of chromosomes
Four chromosome images were used for the relative fluorescence measurements (), in two of which the chromosomes were completely spread (images I and II). Images of completely spread chromosomes were rare because the period of diakinesis () was short and most chromosomes were inseparable at other stages.
Table. 1. Relative fluorescence intensity and estimation of DNA quantity of each chromosome.
Assuming that fluorescence correlated with DNA content, the intensity ratios () showed that the quantity of DNA in the largest chromosome was 3–6-fold greater than that of the smallest chromosome. DNA quantities decreased gradually from the largest to the smallest chromosome. Using a value of 121 Mbp for DNA content of total haploid chromosomes (Grossman et al., Citation2007), the largest chromosome was estimated to be 12.8 Mbp and the smallest chromosome 3.2 Mbp.
Discussion
We observed changes in chromosome morphology and configuration during meiosis in C. reinhardtii using fluorescence microscopy and the fluorochrome SYBR Green I to stain DNA. To our knowledge, this is the first report of chromosomal behaviour throughout the meiosis of live zygotes in the green algae. We estimate that C. reinhardtii has 18 chromosomes.
The first meiotic division occurred between 16 and 18 h, and the second meiotic division occurred at about 20 h (), by which time meiosis was complete. Previously, we reported that cell nuclear DNA was synthesized at approximately 4–6 h (Aoyama et al., Citation2006), therefore, the G phase probably occurred between 7 and 12 h.
The nucleus was localized at the surface of the zygote during meiosis because a large chloroplast occupied the central part (Triemer et al., Citation1976). We did not observe a spindle or the breakdown of the nuclear envelope at the end of prophase, but a comparison of the diameter of the cell nucleus between early prophase I and the end of prophase showed that bivalents were more widely expanded at diakinesis, indicating that the nuclear envelope was broken at this stage. In anaphase, chromosomes were drawn to the spindle poles, which were located approximately 10 µm apart.
In meiotic prophase, telomeres attach to the inner nuclear envelope and cluster to form the so-called bouquet arrangement observed in most organisms (Dernburg et al., Citation1995; Scherthan, Citation2001), suspected to be involved in homologous chromosome pairing and synapsis (Zickler & Kleckner, Citation1998). We observed that thread-like chromosomes formed a spherical structure in prophase I (), but we did not observe a clear bouquet arrangement.
Chromosome number in C. reinhardtii has been reported from observations of mitosis and meiosis. Schaechter & Delamater (Citation1955) counted 18 ± 2 chromosomes per cell in mitosis, while Wetherell & Krauss (Citation1956) found approximately 16 chromosomes in normal mitotic nuclei. Buffaloe (Citation1958) described the nucleus of C. reinhardtii in late mitotic prophase as containing a variable number of condensing chromatic bodies, which finally coalesced into a ring of eight chromosomes at metaphase. McVittie & Davies (Citation1971) observed meiotic zygotes using light microscopy and reported 16 bivalents at diakinesis. Storms & Hastings (Citation1977) suggested a haploid chromosome number of 18 or 20 by electron microscopy. Harris (Citation1989) reported possible chromosome numbers of 8, 16, and 18–20. Electrophoretic separation of chromosomes did not resolve the question of the number of chromosomes (Hails et al., Citation1993) because most of the chromosomes co-migrate and are resolved into four bands on pulsed-field gels. Our observations of chromosomes stained with the highly sensitive, DNA-specific fluorochrome SYBR Green I suggest that the number of chromosomes is 18.
Storms & Hastings (Citation1977) estimated chromosome length using electron microscopy. They suggested a length difference of four to eight between the longest and shortest chromosome. We found this length difference to be three to six times. These values are similar, but less than the fifteen-fold difference found by chromosome mapping (Harris, Citation1993).
Acknowledgements
This work was supported by Grant-in-Aid for Scientific Research A (No.19207004), and by grants from Frontier Project “Adaptation and Evolution of Extremophile” from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and from the Program for the Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN), and by the 21st century COE program of the University of the Ryukyus from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Aoyama , H , Hagiwara , Y , Misumi , O , Kuroiwa , T and Nakamura , S . 2006 . Complete elimination of maternal mitochondrial DNA during meiosis resulting in the paternal inheritance of the mitochondrial genome in Chlamydomonas . Protoplasma , 228 : 231 – 242 .
- Buffaloe , ND . 1958 . A comparative cytological study of four species of Chlamydomonas . Bull. Torrey Bot. Club , 85 : 157 – 178 .
- Dernburg , AF , Sedat , JW , Cande , WZ and Bass , HW . 1995 . “ Cytology of telomeres ” . In Telomeres , Edited by: Blackburn , EH and Greider , CW . 295 – 338 . New York, , USA : Laboratories Press .
- Grossman , AR , Croft , M , Gladyshev , VN , Merchant , SS , Posewitz , MC , Prochnik , S and Spalding , MH . 2007 . Novel metabolism in Chlamydomonas through the lens of genomics . Curr. Opin. Plant Biol. , 10 : 190 – 198 .
- Hails , T , Jobling , M and Day , A . 1993 . Large arrays of tandemly repeated DNA sequences in the green alga Chlamydomonas reinhardtii . Chromosoma , 102 : 500 – 507 .
- Harris , EH . 1989 . The Chlamydomonas Sourcebook , San Diego, , USA : Academic Press .
- Harris , EH . 1993 . “ Chlamydomonas reinhardtii ” . In Genetic Maps. Locus Maps of Complex Genomes, , 6th , Edited by: O’Brien , SJ . 2.157 – 2.169 . Cold Spring Harbor, , USA : Cold Spring Harbor Laboratory .
- Hiramatsu , T , Misumi , O , Kuroiwa , T and Nakamura , S . 2006 . Morphological changes in mitochondrial and chloroplast nucleoids and mitochondria during the Chlamydomonas reinhardtii cell cycle . J. Phycol. , 42 : 1048 – 1058 .
- Levine , RP and Folsome , CE . 1959 . The nuclear cycle in Chlamydomonas reinhardi . Zeitschrift fur Vererbungslehre , 90 : 215 – 222 .
- Mcvittie , A and Davies , DR . 1971 . The location of the Mendelian linkage groups in Chlamydomonas reinhardtii . Mol. Gen. Genetics , 112 : 225 – 228 .
- Nakamura , S , Chibana , H and Kuroiwa , T . 1991 . Domination by female cells over preferential digestion of chloroplast nucleoids in Chlamydomonas reinhardtii . Plant Cell Physiol. , 32 : 359 – 364 .
- Nakamura , S , Ito , S and Kuroiwa , T . 1986 . Behavior of chloroplast nucleus during chloroplast development and degeneration in Chlamydomonas reinhardtii . Plant Cell Physiol. , 27 : 775 – 784 .
- Nishimura , Y , Higashiyama , T , Suzuki , R , Misumi , O and Kuroiwa , T . 1998 . The biparental transmission of the mitochondrial genome in Chlamydomonas reinhardtii visualized in living cells . Eur. J. Cell Biol. , 77 : 124 – 133 .
- Schaechter , M and Delamater , ED . 1955 . Mitosis of Chlamydomonas . Am. J. Bot. , 42 : 417 – 422 .
- Scherthan , H . 2001 . A bouquet makes ends meet . Nat. Rev. Mol. Cell Biol. , 2 : 621 – 627 .
- Storms , R and Hastings , PJ . 1977 . A fine structure analysis of meiotic pairing in Chlamydomonas reinhardii . Exp. Cell Res. , 104 : 39 – 46 .
- Triemer , RE and Brown , RM . 1976 . Ultrastructure of meiosis in Chlamydomonas reinhardtii . Br. Phycol. J. , 12 : 23 – 44 .
- Wetherell , DF and Krauss , RW . 1956 . Colchicine–induced polyploidy in Chlmydomonas . Science , 124 : 25 – 26 .
- Zickler , D and Kleckner , N . 1998 . The leptotene-zygotene transition of meiosis . Ann. Rev. Genet. , 32 : 619 – 697 .