Abstract
Cyanobacteria frequently form mass developments in surface waters. Populations consist of strains that differ in the production of bioactive peptides, e.g. microcystins (MC) inhibiting protein phosphatases 1 and 2A and anabaenopeptins (APN) inhibiting carboxypeptidases. Forty-nine strains (18 green-pigmented (phycocyanin-rich) P. agardhii strains and 31 red-pigmented (phycoerythrin-rich) P. rubescens strains) of the filamentous cyanobacterium Planktothrix (Anagnostidis et Komarék) were analysed for their MC and APN net production rates. These rates were compared with (i) the pigmentation, (ii) the proportion of extra- and intracellular peptide concentrations and (iii) the cellular growth rates under standardized laboratory conditions. Excluding the strains lacking MC and APN, the MC and APN contents varied up to 14-fold and 12-fold, each. The variation in minimum and maximum peptide content (0.32–4.51 µg MC mg−1 dry weight; 0.85–10.32 µg APN mg−1 dry weight) exceeded the variation found for chlorophyll a (4.8–16.9 µg mg−1 dry weight). The extracellular proportions of MC (0–62%) and APN (0–58%) varied among strains, however, on average, proportions of extracellular MC and APN were low (MC: 8.8 ± (1 SE) 1.9%, APN: 8.4 ± 1.8%). Among all strains cellular growth rates showed a 5-fold variation (0.07–0.33 doublings of dry weight.day−1) and were found independent of the pigmentation and the peptide net production rate. It is concluded that the MC and APN net production rate is not causally related to the cell-division cycle and the synthesis of highest amounts of MC and APN does not constrain cell division.
Introduction
Because of their frequent occurrence in fresh water, toxin-producing cyanobacteria are considered to be a general threat to drinking water quality (Codd et al., Citation1999; WHO, Citation2004). Many of the studies dealing with toxin production have focused on microcystins (MC), cyclic heptapeptides inhibiting eukaryotic proteinphosphatase 1 and 2A. MCs are synthesized via non-ribosomal peptide synthetases (NRPS) and polyketide synthases (PKS) assembled into large multifunctional proteins encoded by the mcy gene cluster (Tillett et al., Citation2000). The chemical structure of MC is cyclo (D-Ala1-X2-D-MeAsp3-Z4-Adda5-D-Glu6-Mdha7), where D-MeAsp is the non-proteinogenic amino acid D-erythro-β-iso-aspartic acid (methyl aspartate), Mdha is N-methyl-dehydroalanine and Adda is a β-amino acid with a C10-chain: (2S, 3S, 8S, 9S)-3-amino-9-methoxy-2,6,8-trimethyl-10-phenyldeca-4,6-dienoic acid. X and Z represent variable L-amino acids in positions 2 and 4, respectively (Carmichael, Citation1992). Similar to MCs, anabaenopeptins (APN) have been reported to inhibit protein phosphatase 1 (APN A and B; Gkelis et al., Citation2006) as well as proteases such as carboxypeptidase A (APN G and H, Itou et al., Citation1999). APNs contain six amino acids: five of them form a cyclic structure with a sixth amino acid that is attached. The pentapeptide moiety has the common structure cyclo (D-Lys5- L-Val4- L-Hty3- L-MeAla2- L-Phe1) with L-Arg6 (or L-Tyr) bound to D-Lys5 through an ureido linkage. L-Hty is L-homotyrosine and MeAla is N-methyl-alanine (Harada et al., Citation1995; Fujii et al., Citation2002). D-Lys5 is the only non-variable amino acid in APN. APNs are synthesized by NRPS analogously to the MCs (Fujii et al., Citation2002).
Regulation of MC net production in single isolates (strains) has been addressed by a number of studies quantifying the effects of various environmental conditions on MC content in the laboratory, e.g. effects of temperature, pH, irradiance, macronutrients (N, P), and trace elements (Sivonen & Jones, Citation1999). Contrary to expectations the production of MCs was not inducible and different environmental factors were found to induce moderate changes in MC content only. These results are in contrast to the regulation of antibiotics and sporulation factors via NRPS, which is induced or increased as growth declines (Marahiel et al., Citation1993). For example during the transition from the logarithmic to the stationary phase of growth in batch culture, Bacillus subtilis shows increased production of the peptide antibiotics tyrocidine and gramicidine S and the lipopeptide surfactin. Orr & Jones (Citation1998) suggested that MC production is coupled primarily to cell division of the organism and concluded that MC–although it is clearly a secondary metabolite–rather displays the attributes of essential intracellular nitrogenous compounds. According to this theory the major influence of all possible environmental factors is indirect–through their effect on cell division and growth, whereas the direct effects on MC biosynthesis are of minor importance. The specific growth rate has been used to predict the intracellular MC content under nitrogen-limiting conditions in culture (Long et al., Citation2001). The model of Long et al. (Citation2001) implies that MC production is constitutive and MC-producing strains will always contain at least a minimal intracellular MC content and a maximum content determined by the nutrient saturated growth rate (for a given temperature and light growth conditions).
In contrast to the regulation of MC synthesis within a strain, very few studies have investigated the phenotypic variation in MC content between strains. In a few cases large differences in MC content related to the dry weight of the strains have been reported (Bolch et al., Citation1997; Saker et al., Citation2005). For Planktothrix 10-fold and 16-fold differences in MC content related to dry weight have been reported (Kurmayer et al., Citation2005; Welker et al., Citation2004). While dry weight is usually considered rather inaccurate to estimate the MC content it is completely unclear how the MC production rate relates to the differences in growth rates among strains as well as to the differences in the production of other peptides.
In this study 49 strains of Planktothrix were analysed for their MC and APN contents and their specific peptide net production rates during conditions of maximum specific growth (µm, sensu Kohl & Nicklisch, Citation1988). The genus Planktothrix (Oscillatoria) (Anagnostidis & Komarek, Citation1988) occurs frequently in lakes and reservoirs in the temperate zone of the Northern Hemisphere. The species P. rubescens and P. agardhii differ in pigmentation and planktonic life-form: P. rubescens is typically found in deep, stratified and oligo- to mesotrophic waters, while P. agardhii occurs in shallow and more eutrophic waters. Chromatic adaptation has not been observed within Planktothrix (Skulberg & Skulberg, Citation1985) and the hydrostatic pressure and the underwater light climate act as factors leading to the ecological divergence between both life forms (Beard et al., Citation2000; Davis et al., Citation2003). In some ecosystems both species have been reported to co-occur for years, for example in Blelham Tarn, United Kingdom (Davis et al., Citation2003) or in Lake Steinsfjorden, Norway (Halstvedt et al., Citation2007).
Material and methods
Origin and strain cultivation
Forty-nine strains (18 P. agardhii, 31 P. rubescens) were isolated from different lakes as described previously (Kurmayer et al., Citation2004, see supplementary material available at http://www.informaworld.com/mpp/uploads/downloadfile.pdf) and assigned to P. agardhii (green-pigmented) or P. rubescens (red-pigmented) according to Suda et al. (Citation2002). All strains were grown in BG11 medium under sterile conditions (Rippka, Citation1988) at 20°C and 40–60 µmol m−2 s−1 (Philips LTD, 36W/965, Vienna, Austria). A few strains have been purified axenic prior to the growth experiments (No. 70/1a, No. 160/1a, No. 12/1a, PCC 7821, No. 14/1a, No. 90/1i, No. 113/1b). In order to adapt the cells to the experimental conditions pre-cultures for a minimal duration of 3 months were established and their growth was monitored by measuring optical density (OD) at 880 nm (5-cm light path). At the start of each growth experiment pre-cultures were diluted down to OD880 = 0.01 (3 × 104 − 16 × 104 cells ml−1) in a total volume of 800 ml and aliquots of 100 ml were distributed over eight Erlenmeyer flasks (250 ml) to achieve optimal growth. Cultures were grown semi-continuously and growth was monitored by OD readings every other day. Because not all strains could be grown simultaneously one strain (P. agardhii strain No. 32) was included as a control strain to standardize for uncontrolled factors. Each strain was tested in duplicate at two consecutive sampling dates.
Cell harvesting
Cells were harvested at OD = 0.1–0.15 at two consecutive sampling dates, usually between days 4 and 19 depending on the strain-specific growth rate. Growth rates (r) (day−1) and peptide net production rates (day−1) were calculated using the formula, r = (ln N2 − ln N1)/Δt, where N1,2 were the cell concentration/peptide concentrations at consecutive sampling days and Δt was the interval in days (3 days). Cells were fixed in 2% formaldehyde and filtered onto Isopore™ membrane filters (0.2 µm GTBP Ø 25 mm, Millipore), stained with DAPI and enumerated using epifluorescence microscopy. A minimum of 400 specimens was counted. As individual cells in the filaments of Planktothrix spp. could not always be distinguished, the filament length was estimated using the boxes of the counting grid (at 400× magnification). For 20 randomly selected filaments the cell number per box of the counting grid was determined and the average cell number per box was used to calculate cell numbers. Analogously biovolume was determined by measuring the diameter of 20 randomly selected filaments at 1125× magnification and calculating biovolume by assuming a cylindrical shape.
Samples were harvested by filtering onto pre-weighed glass fibre filters (BMC Ederol, Vienna), dried in a vacuum centrifuge, weighed and stored frozen at −20°C. A minimum of 1.0 mg of dry weight of biomass was collected. Dissolved peptides were collected from the filtrate using solid phase extraction (SPE) via tC18 cartridges [Waters, Sep-Pak Vac 1cc (100 mg)] according to standard techniques. Cartridges were stored at −20°C. After acclimation to room temperature the cartridges were washed with 10 ml H2O and 10 ml 20% (v/v) methanol and peptides were eluted in 1 ml 90% MeOH and dissolved extracts were stored at −20°C until HPLC analysis. During pilot experiments the spiking of BG 11 medium (300 ml) with 1 µg MC-LR standard revealed an average recovery rate of 85.6 ± 12.7% (95% confidence limit, n = 6) as measured by HPLC. For 1 µg of APN B the recovery rate was 99 ± 0.4%.
Peptide extraction and HPLC analysis
To extract MC and APN filters were cut into pieces and extracted in 50% MeOH (v/v). Extracts were shaken for 30 min, centrifuged at 16,000 g and the clear supernatant was evaporated to dryness in a vacuum centrifuge. This procedure was repeated twice. Pilot experiments showed 99% extraction efficiency after three repetitions and optimal yields with extracting on ice and without sonification compared with extraction at room temperature with sonification (strain No. 40).
The extracts were analysed for MCs and APNs by high performance liquid chromatography (HPLC) coupled to diode array detection (HPLC-DAD). For HPLC analysis the samples were re-dissolved in 600 µl MeOH (50/50, v/v), centrifuged at 16,000 g (10 min) and the cleared supernatant was injected. MCs and APNs were quantified at 240 nm and 210 nm, each using a linear gradient from 20% acetonitrile (0.05% TFA) to 50% acetonitrile on a LiChrosper® 100, ODS, 5 µm, LiChroCART® 250-4 cartridge system (Agilent, Vienna, Austria). Structural variants of MCs and APNs were assigned using the information obtained from UV spectra and retention times and identified by sensitive matrix assisted desorption ionization-time of flight (MALDI-TOF) mass spectrometry (MS) as described (Kurmayer et al., Citation2004, 2005). In order to evaluate the reproducibility of the detection of MCs and APNs from aqueous-methanolic extracts via the HPLC-DAD technique the following pilot experiment was performed (Welker & Erhard, Citation2007): the biomass of ten strains (Nos 40, 67, 77, 82, 83, 95, 101, 110, 173, 208, CCAP 1460/5) was (i) extracted as described above and analysed by HPLC-DAD coupled to MALDI-TOF MS and (ii) directly analysed by single filament MALDI-TOF MS as described (Kurmayer et al., Citation2004). This comparison revealed that all of the MCs and APNs present in a specific strain were detected using the linear gradient in HPLC-DAD as described above. Further, the retention time and the UV-spectra were useful to identify the elution of APN B (16.3–17 min), APN F (18.9–19.5 min), APN A (20.7–21.3 min), oscillamide (OSC) Y (23.4–23.7 min) and those of MCs from ([Asp, Mdha]-MC-RR (25.8–26.2 min), [Asp, Dhb]-MC-RR (26.5–26.9 min), [Asp]-MC-HtyR (32.6–33.3 min), [Asp]-MC-LR (33.2–34 min).
In addition standards have been used to identify novel MCs (35–36.1 min), the structure of which has been elucidated as [Asp, Dhb]-MC-HtyY, m/z 1074 and [Asp, Dhb]-MC-HtyHty, m/z 1088 (R. Kurmayer & T. Hemscheidt, unpublished). Standards of the variants APN 908 (m/z 909) and APN 915 (m/z 919) were used to identify the more rare variants APN J (18.4–18.9 min) and APN I (22.8–23.2 min), respectively (H. Okumura, B. Philmus & T. Hemscheidt, unpublished). The concentrations of MCs were determined as concentration equivalents of [MeAsp, Mdha]-MC-LR (Calbiochem, Schwalbach, Germany). The linear regression was y = 1.9525x (R 2 = 1), where y was the peak area at 240 nm and x was the injected concentration of microcystin-LR standard (µg). The concentrations of APNs were determined as concentration equivalents of APN B using a purified standard (Judith Blom, University of Zürich, Institute of Plant Biology, Limnological Station) and quantification via the molar extinction coefficient (λmax = 225 nm, ε = 8833, Harada et al., Citation1995). The linear calibration regression was y = 1.3169 x (R 2 = 1), where y was the area recorded at 210 nm and x was the concentration of APN injected (µg). Under the conditions as described above the detection limit was 10 ng both for the MC-LR and the APN B standard.
For chlorophyll a analysis, filters containing aliquots of the cells that were harvested for peptide analysis, were extracted in 1 ml of 90% acetone (v/v) and grinded with a mortar under dim light. Four ml of 90% acetone were added and the extraction was performed in the refrigerator over night. Two ml of the suspension were centrifuged (10 min, 16,000 g, 4°C) and 100 µl of the clear supernatant were injected into HPLC-DAD using a linear gradient of three solvents: (A) methanol (80 : 20 methanol: 0.5 M ammoniumacetate, v/v), (B) acetonitrile (90%) and (C) acetic acid ethyl ester, as developed by Wright et al. (Citation1991). A Superspher® 100 RP-18, 4 µm, 250-4 cartridge system (Agilent, Vienna, Austria) was used. Concentrations in µg per mg dry weight were estimated for chlorophyll a with calibration curves using a standard supplied by DHI Water & Environment (Hørsholm, Denmark). For determination of the acid ratio A/Aa (the ratio of chlorophyll a to magnesium-free phaeophytine) samples were acidified with 0.1 M HCl according to standard protocols (Lorenzen, Citation1967). A/Aa ∼ 1.7, if only undegraded chlorophyll a is present and A/Aa ∼ 1.0, if only degradation products of chlorophyll a are present.
Results
Variation of peptide contents within and among strains
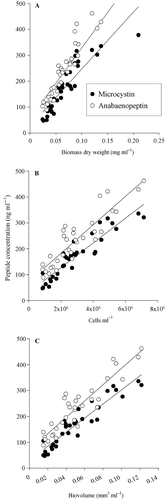
Three methods of biovolume estimation were compared in their ability to predict the concentration of MC and APN in cells of the control strain No. 32: cellular dry weight (DW), cell counting and biovolume estimated from the microscope. All three methods gave highly significant correlations between peptide concentrations and the estimated biovolume (). The regressions were for dry weight: y = 2193x + 28 (n = 35, R 2 = 0.8, MC) and y = 2718x + 57 (n = 35, R 2 = 0.83, APN), for cell numbers: y = 5 × 10−5 x + 31.7 (n = 35, R 2 = 0.87, MC) and y = 6 × 10−5 x + 72.7 (n = 35, R 2 = 0.82, APN), for biovolume: y = 2716x + 31 (n = 35, R 2 = 0.89, MC) and y = 3154x + 71 (n = 35, R 2 = 0.85, APN), where y was the peptide concentration (ng ml−1) and x was the biovolume concentration.
Fig. 1. Relationship between microcystin (MC; black circles) and anabaenopeptin (APN; white circles) concentrations and biovolume of the filamentous cyanobacterium Planktothrix strain No. 32. Biovolume was estimated by (A) dry weight (regression coefficients for MC: R 2 = 0.8 and APN R 2 = 0.83) (B) cell counting (MC: R 2 = 0.87 and APN R 2 = 0.82) and (C) calculating biovolume from filaments by assuming a cylindrical shape (MC: R 2 = 0.89 and APN R 2 = 0.85).
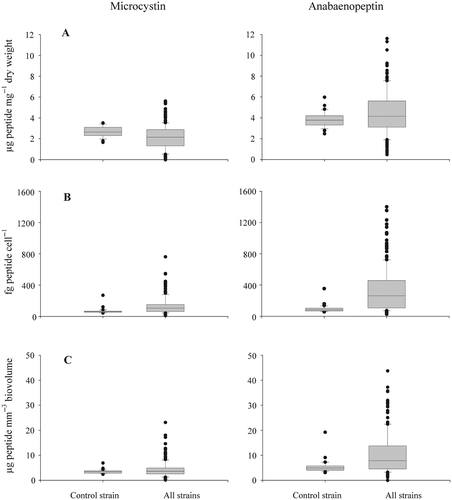
In total 35 strains produced MCs, while 46 strains contained APNs. The cellular concentration of both MC and APN was variable between strains, i.e. the MC and APN contents varied 14-fold and 12-fold, each (excluding the strains lacking MC or APN). In order to find out whether this variability was due to analytical errors or due to strain-specific differences the variability in peptide contents found within the control strain was compared with the variability found between strains (). The variation found between strains was significantly higher when compared with the variation found within the control strain (Mann–Whitney rank sum test, p < 0.001).
Fig. 2. Variability of microcystin (MC) and anabaenopeptin (APN) contents within the control strain P. agardhii No. 32 and between strains of P. agardhii and P. rubescens (n = 49). The control strain was used as a reference during all experiments to standardize for within strain variation. MC/APN content per (A) dry weight (three outliers of APN contents not shown), (B) cells and (C) biovolume (two outliers of APN contents not shown).
Peptide contents and peptide composition
In general all three biovolume estimates (dry weight, microscopical determination of cell numbers and microscopical determination of cyanobacterial biovolume) showed highly consistent results (). The counting of cells under the microscope did not improve the errors of the estimates, which was attributed to the unequal distribution of aggregate forming filaments on the filters as mentioned earlier (Repka et al., Citation2004). Only the contents related to dry weight are reported in the following.
Table 1. Strains of P. agardhii and P. rubescens analysed for their microcystin (MC), anabaenopeptin (APN) and chlorophyll a contents (mean ± 1SE), estimated per dry weight (DW), per cell and per mm3 of biovolume.
The contents of MC and APN were found in the same order of magnitude and were 15-fold (MC) or 19-fold (APN) lower (excluding the strains with zero peptide content) when compared with the chlorophyll a content (). The two species differed significantly in chlorophyll a content (P. argardhii: mean ± SE, 13.1 ± 2.4 µg mg−1 DW; P. rubescens: 8.0 ± 1.8 µg mg−1 DW; t-test, p < 0.001). No significant difference (t-test, p = 0.124) in MC content between species was found: Among P. agardhii strains the MC content (mean ± SE, 1.7 ± 0.3 µg mg−1 DW) varied between zero (four strains) and 4.5 ± 0.4 µg mg−1 DW (No. 31/1), while in P. rubescens the MC content (mean ± SE, 1.2 ± 0.2 µg mg−1 DW) was found to vary between zero (nine strains) and 4.5 ± 0.4 µg mg−1 DW (No. 82). In contrast the APN content differed significantly between species (P. argardhii: mean ± SE, 2.9 ± 0.4 µg mg−1 DW; P. rubescens: 5.0 ± 0.4 µg mg−1 DW; t-test, p < 0.001), while the APN content ranged from zero (two strains) to 7.2 ± 0.7 µg mg−1 DW (No. 66) in P. agardhii and from zero (CCAP 1459/14) to 10.3 ± 1.3 µg mg−1 DW (No. 64, ) in P. rubescens.
In general, strains contained one or two structural variants of MC, in two strains of P. agardhii three variants were found (). Strains either contained [Asp, Mdha]-MC-RR/[Asp, Dhb]-MC-RR only or [Asp, Mdha]-MC-RR/[Asp, Dhb]-MC-RR paired with lower amounts of [Asp]-MC-LR, and [Asp]-MC-HtyR. A number of strains did not contain any MC-RR but contained [Asp]-MC-HtyR paired with [Asp]-MC-LR. The new MCs [Asp, Dhb]-MC-HtyY and [Asp, Dhb]-MC-HtyHty were never paired with the other MC variants.
Table 2. Proportion of intracellular microcystin (MC) and anabaenopeptin (APN) variants (mean ±1 SE) in P. agardhii and P. rubescens strains.
Among APNs six different variants were observed (). The majority of the strains (25 strains) contained APN B, A, F and OSC Y. A few strains contained APN B only (four strains). Ten strains produced two APN variants (APN B and F), while six strains produced three APN variants [APN B, F, A (8%), APN B, A, OSC Y (4%)]. APN I and J were found in strain CYA 126/8 only.
Extracellular peptide concentrations
All MC-producing Planktothrix sp. strains were analysed for extracellular MCs in the culture media (n = 144). Seventy seven % of the samples were found to contain less than 10% dissolved MCs of total MC content, 91% of the samples contained less than 20% dissolved MCs. On average 8.8 ± 1.9% of MC were found extracellular. A few strains (No. 70/1a, No. 108, No. 90/1i, CCAP 1459/11A, CCAP 1459/21) showed a higher proportion of dissolved MCs.
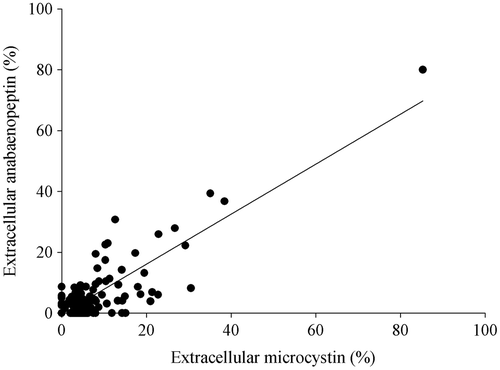
As for the MCs, the majority of the APN-producing strains (n = 168) contained less than 10% of the dissolved fraction (83%). In 90% of the strains the percentage of the dissolved fraction was <20%. On average 8.4 ± 1.8% of APN were found extracellular. The amount of extracellular MC and APN showed a significant positive correlation: y = 0.82x − 0.36 (R 2 = 0.77, ). The acid ratio as determined for the chlorophyll a content was used to infer the physiological status and a possible peptide leakage of deteriorating cells in the cultures. Some strains (No. 70/1a, No. 82) showed high extracellular peptide concentrations paired with a high A/Aa>1.7 and no correlation was found between the amount of extracellular peptide concentration and the acid ratio. Therefore a passive leakage of MC and APN in consequence to cell death was considered to be of minor importance.
Microcystin content in dependence on the anabaenopeptin content
The majority of the strains (41/49) contained significantly higher amounts of APN when compared with MC (Mann-Whitney rank sum test, p < 0.001). One strain produced neither MC nor APN, five strains contained equal amounts of MC and APN (no statistically significant difference), while two strains contained MC only. With the exception of strain PCC 7805 all strains without detectable MC contained APN.
MC-producing strains were divided into five groups according to their MC content (). Those groups were found to differ significantly in MC content (Kruskal-Wallis One Way Analysis of Variance on Ranks, p < 0.001). The APN content (intracellular and extracellular) varied independently from the MC content (Kruskal-Wallis One Way Analysis of Variance on Ranks, p = 0.62).
Cellular growth rate and peptide net production rate
The growth rates among all the strains varied 5-fold as calculated from dry weight (min-mean ± SE-max: 0.07 − 0.2 ± 0.01 − 0.33), however, 21-fold as calculated from cell numbers (0.03 − 0.29 ± 0.02 − 0.64) and 24-fold as calculated from biovolume (0.03 − 0.29 ± 0.02 − 0.64). The growth rates of the 18 P. agardhii strains (DW: 0.07 − 0.18 ± 0.01 − 0.28; cell numbers: 0.11 − 0.27 ± 0.03 − 0.64; biovolume: 0.12 − 0.27 ± 0.03 − 0.64) did not differ significantly from the growth rates as measured for the 31 P. rubescens strains (DW: 0.13 − 0.20 ± 0.01 − 0.33; cell numbers: 0.03 − 0.30 ± 0.02 − 0.6; biovolume: 0.03 − 0.30 ± 0.02 − 0.61).
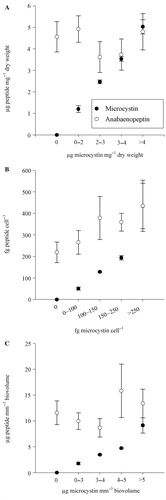
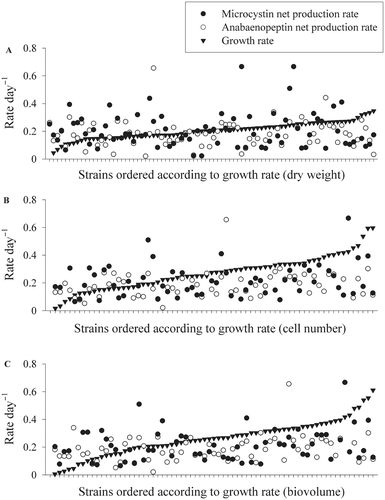
The MC and APN net production rates varied up to 19-fold (min-mean ± SE-max, MC: 0.02 − 0.25 ± 0.01 − 0.42, APN: 0.03 − 0.18 ± 0.01 − 0.66). There was no difference found between the peptide net production rates of P. agardhii and P. rubescens. Among all strains the peptide net production rates varied independently from the variation in cellular growth rates (), i.e. fast growing strains did not show higher peptide production rates when compared with slow growing strains or vice versa. There was also no difference found between the growth rates of MC/APN-producing strains and those containing no MC/APN.
Fig. 5. Growth rates (black triangles) and corresponding peptide production rates (microcystin, black circles and anabaenopeptin, white circles) for 49 Planktothrix strains measured under maximum growth rate conditions. Cell growth rates were estimated from (A) dry weight, (B) cell numbers and (C) biovolume.
Discussion
In this study an attempt was made to find a relationship between peptide net production and cell division rates between strains. The growth conditions at the temperature and light conditions used in this study (20°C, 40–60 µmol m−2 s−1) were not optimal for all the strains and a significant intraspecific variation in maximal-specific growth rates has been observed. While such an intraspecific variation has been found earlier (Van Liere & Mur, Citation1980; Davis & Walsby, Citation2002) the general understanding of factors causing this intraspecific variation in growth under identical experimental conditions in the laboratory is not complete. Mikkola & Kurland (Citation1992) explained a threefold intraspecific variation in growth rates (0.48–1.43 doublings h−1) among natural isolates of Escherichia coli by strain-specific differences in translational efficiency. The same authors were able to select for highest growth rates (1.33 doublings h−1) under continuous culture conditions over 280 generations implying that selection for faster growth rates under nutrient poor conditions in nature is of minor importance when compared with the nutrient rich conditions in the laboratory.
However, to accept or to reject the hypothesis that the growth rate is related to peptide net production not only within a strain but between strains it is not necessary to find out the maximum intrinsic growth rate for each strain. According to the conclusions proposed by Orr & Jones (Citation1998) a slow growing strain can be expected to show an increase in peptide net production if the real maximum intrinsic growth rate would have been found. In contrast to the results observed by Orr & Jones (Citation1998), in this study a linear relationship between the peptide net production rate and the cellular growth rate obtained from all strains could not be found. Indeed strains growing fast had both low and high MC and APN contents and MC and APN production rates (). It is known that NRPS are large multi-enzyme complexes synthesizing peptides by the thiotemplate-directed mechanism, independent of the ribosomal peptide synthesis pathway (Marahiel et al., Citation1997). Typically, secondary metabolites such as antibiotics synthesized via NRPS are then actively exported out of the cell and thought to enhance the competitiveness or confer resistance to the producer itself (see Pearson et al., Citation2004 for a review). In contrast for MCs export has not been observed (Wiedner et al., Citation2003; Tonk et al., Citation2005; Rohrlack & Hyenstrand, Citation2007) and higher extracellular concentrations have only been reported from dense cultures making it impossible to differentiate between passive leakage and active export of MC. Accordingly in this study the average dissolved peptide concentrations were found to make up less than 10% of the total peptide contents. Instead cyanobacteria seem to accumulate MC and other peptides in certain cell regions, i.e. immunogold labelling revealed preferential localization of MCs in thylakoids and in the periphery of polyphosphate bodies (Shi et al., Citation1995; Young et al., Citation2005). Recently a binding of MCs and cyanopeptolins to phycobiliproteins situated onto the thylakoid membranes has been reported (Jüttner & Lüthi, 2008). It is concluded that the frequently observed relationship between cell division and MC net production rate is rather the result of an accumulation of MCs in specific cell compartments rather than due to a direct dependence of MC synthesis on the processes involved in the cell-division cycle.
From the results of this study it follows that the variation in the cellular growth rate is independent from the increase/decrease of the MC/APN production. Indeed the insertional inactivation of the MC synthesis by a gene knock-out experiment in Microcystis did not lead to a measurable difference in growth rate under various light conditions (4–110 µmol m−2 s−1) as compared with the wild type (Hesse et al., Citation2001). Correspondingly, the knock-out mutant lacking anabaenopeptilide in Anabaena strain 90 did not show increased growth rates of the mutant when compared with the wild type (Repka et al., Citation2004). In this study, several strains containing the total mcy gene cluster (CCAP 1459/36, No. 40, No. 67, No. 91/1, No. 110) but inactive in MC synthesis due to the insertion of a transposable element (Christiansen et al., Citation2006), did not show higher growth rates when compared with the other MC-producing strains. Consequently, costs related to MC production and of other NRS peptides at the post-translational level should not be considered to be a factor influencing cellular growth rates and potential competitive replacement between MC-producing and non-MC-producing strains. Rather the deletion of NRPS gene clusters or larger parts of it may provide sufficient physiological advantage by reducing the genome size, as the selection coefficient on small deletions is considered negligible (Mira et al., Citation2001). In fact subcultures of strains of Microcystis that lost a larger part of the MC synthesis gene cluster (>34 kbp) have been observed to succeed the MC-producing original strain (Schatz et al., Citation2005).
In summary, peptide contents and growth rates between strains are highly variable and independent of each other. No relationship of MC net production with regard to the life-form of both species P. agardhii and P. rubescens could be found, implying that MC is not directly related with the ecological divergence observed (Reynolds et al., Citation2002). Most studies so far were based on the genus Microcystis, however, as argued by one referee, there is no reason to assume principal differences between the two genera with regard to the results obtained in this study. For the purpose of comparing estimates of MC cell contents between partners of the EU-project PEPCY (www.pepcy.de, Nov 2002–June 2006), Microcystis strain PCC 7806 has been analysed when grown in O2 medium (Van Liere & Mur Citation1978) but under otherwise identical conditions as for Planktothrix. For three parallels the results were 3.6 ± 0.3 µg MC mg−1 DW, 71 ± 12 fg MC cell−1, 1.9 ± 0.3 µg MC mm−3 biovolume with 10 ± 1.4% of dissolved MC. This result compares with other studies on Microcystis cellular MC contents (Wiedner et al., Citation2003, Saker et al., Citation2005).
It is emphasized that the intraspecific differences in cellular growth rate have been observed in nutrient-rich media in the laboratory and the relevance of those differences in strain-specific growth rates under natural conditions has not been documented. To date, the influence of strain-specific growth rates on the varying abundance of MC-producing and non-MC-producing genotypes in the environment remains speculative (Sivonen & Jones, Citation1999) and aside from genotype-specific growth rates as a consequence of ecological divergence other factors such as genotype-specific losses due to bacteriophages or algae-lysing bacteria need to be considered. First results, however, rather point to a coexistence of genotypes differing in peptide production that can last for years (Christiansen et al., Citation2006) or even decades (Guljamow et al., Citation2007).
Variation in peptide net production and growth among strains of the toxic cyanobacterium Planktothrix spp. Supplementary material
Download Zip (62 KB)Acknowledgements
We thank Judith Blom (University of Zürich, Institute of Plant Biology) and Benjamin Philmus, Thomas Hemscheidt (University of Hawaii at Manoa, Department of Chemistry) for kindly providing standards. We are grateful to Guntram Christiansen for helpful discussions. We also thank Martin Welker (TU Berlin) for MALDI-TOF analysis of fractionated HPLC peaks. Inge Flieger (Federal Environmental Agency, Berlin) gave advice for the recovery experiments of dissolved MC. The text has been improved by the comments of two anonymous referees. This study was financed by grants of the Austrian Science Funds (P15709, P18185).
References
- Anagnostidis , K and Komarek , J . 1988 . Modern approach to the classification system of cyanophytes, 3-Oscillatoriales . Algol. Stud. , 50–53 : 327 – 472 .
- Beard , SJ , Davis , PA , Iglesias-Rodriguez , D , Skulberg , OM and Walsby , AE . 2000 . Gas vesicle genes in Planktothrix spp. from Nordic lakes: strains with weak gas vesicles possess a longer variant of gvpC . Microbiology , 146 : 2009 – 2018 .
- Bolch , CJS , Blackburn , SI , Jones , GJ , Orr , PT and Grewe , PM . 1997 . Plasmid content and distribution in the toxic cyanobacterial genus Microcystis Kützing ex Lemmermann (Cyanobacteria: Chroococcales) . Phycologia , 36 : 6 – 11 .
- Carmichael , WW . 1992 . Cyanobacteria secondary metabolites–the cyanotoxins . J. Appl. Bacteriol. , 72 : 445 – 459 .
- Christiansen , G , Kurmayer , R , Liu , Q and Börner , T . 2006 . Transposons inactivate biosynthesis of the nonribosomal peptide microcystin in naturally occurring Planktothrix spp . Appl. Environ. Microbiol. , 72 : 117 – 123 .
- Codd , GA , Bell , SG , Kaya , K , Ward , CJ , Beattie , KA and Metcalf , JS . 1999 . Cyanobacterial toxins, exposure routes and human health . Eur. J. Phycol. , 34 : 405 – 415 .
- Davis , PA , Dent , M , Parker , J , Reynolds , CS and Walsby , AE . 2003 . The annual cycle of growth rate and biomass change in Planktothrix spp. in Blelham Tarn, English Lake District . Freshwat. Biol , 48 : 852 – 867 .
- Davis , PA and Walsby , AE . 2002 . Comparison of measured growth rates with those calculated from rates of photosynthesis in Planktothrix spp. isolated from Blelham Tarn, English Lake District . New Phytol. , 156 : 225 – 239 .
- Fujii , K , Sivonen , K , Nakano , T and Harada , K . 2002 . Structural elucidation of cyanobacterial peptides encoded by peptide synthetase gene in Anabaena species . Tetrahedron , 58 : 6863 – 6871 .
- Gkelis , S , Lanaras , T and Sivonen , K . 2006 . The presence of microcystins and other cyanobacterial bioactive peptides in aquatic fauna collected from Greek freshwaters . Aquat. Toxicol. , 78 : 32 – 41 .
- Guljamow , A , Jenke-Kodama , H , Saumweber , H , Ouillardet , P , Frangeul , L , Castets , AM , Bouchier , C , Tandeau De Marsac , N and Dittmann , E . 2007 . Horizontal gene transfer of two cytoskeletal elements from a eukaryote to a cyanobacterium . Curr. Biol. , 17 : R757 – R759 .
- Halstvedt , CB , Rohrlack , T , Andersen , T , Skulberg , O and Edvardsen , B . 2007 . Seasonal dynamics and depth distribution of Planktothrix spp. in Lake Steinsfjorden (Norway) related to environmental factors . J. Plankton Res. , 29 : 471 – 482 .
- Harada , K , Fujii , K , Shimada , T and Suzuki , M . 1995 . Two cyclic peptides, anabaenopeptins, a third group of bioactive compounds from the cyanobacterium Anabaena flos-aquae NRC 525–17 . Tetrahedron Lett. , 36 : 1511 – 1514 .
- Hesse , K , Dittmann , E and Börner , T . 2001 . Consequences of impaired microcystin production for light-dependent growth and pigmentation of Microcystis aeruginosa PCC 7806 . FEMS Microbiol. Ecol. , 37 : 39 – 43 .
- Itou , Y , Suzuki , S , Ishida , K and Murakami , M . 1999 . Anabaenopeptins G and H, potent carboxypeptidase A inhibitors from the cyanobacterium Oscillatoria agardhii (NIES-595) . Bioorg. Med. Chem. Lett. , 9 : 1243 – 1246 .
- Jüttner , F and Lüthi , H . Topology and enhanced toxicity of bound microcystins in Microcystis PCC 7806 Toxicon , 51 388 – 397 .
- Kohl , J and Nicklisch , A . 1988 . Ökophysiologie der Algen. Wachstum und Ressourcennutzung , Stuttgart, , Germany : Urban & Fischer .
- Kurmayer , R , Christiansen , G , Fastner , J and Börner , T . 2004 . Abundance of active and inactive microcystin genotypes in populations of the toxic cyanobacterium Planktothrix spp . Environ. Microbiol. , 6 : 831 – 841 .
- Kurmayer , R , Christiansen , G , Gumpenberger , M and Fastner , J . 2005 . Genetic identification of microcystin ecotypes in toxic cyanobacteria of the genus Planktothrix . Microbiology , 151 : 1525 – 1533 .
- Long , BM , Jones , GJ and Orr , PT . 2001 . Cellular microcystin content in N-limited Microcystis aeruginosa can be predicted from growth rate . Appl. Environ. Microbiol. , 67 : 278 – 283 .
- Lorenzen , CJ . 1967 . Determination of chlorophyll and pheo-pigments – spectrophotometric equations . Limnol. Oceanogr. , 12 : 343 – 346 .
- Marahiel , MA , Nakano , MM and Zuber , P . 1993 . Regulation of peptide antibiotic production in Bacillus . Mol. Microbiol. , 7 : 631 – 636 .
- Marahiel , MA , Stachelhaus , T and Mootz , HD . 1997 . Modular peptide synthetases involved in nonribosomal peptide synthesis . Chem. Rev. , 97 : 2651 – 2673 .
- Mikkola , R and Kurland , CG . 1992 . Selection of laboratory wild-type phenotype from natural isolates of Escherichia coli in chemostats . Mol. Biol. Evol. , 9 : 394 – 402 .
- Mira , A , Ochman , H and Moran , NA . 2001 . Deletional bias and the evolution of bacterial genomes . Trends Genet. , 17 : 589 – 596 .
- Orr , PT and Jones , GJ . 1998 . Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures . Limnol. Oceanogr. , 43 : 1604 – 1614 .
- Pearson , LA , Hisbergues , M , Börner , T , Dittmann , E and Neilan , BA . 2004 . Inactivation of an ABC transporter gene, mcyH, results in loss of microcystin production in the cyanobacterium Microcystis aeruginosa PCC7806 . Appl. Environ. Microbiol. , 70 : 6370 – 6378 .
- Repka , S , Koivula , M , Harjunpä , V , Rouhiainen , L and Sivonen , K . 2004 . Effects of phosphate and light on growth of and bioactive peptide production by the cyanobacterium Anabaena strain 90 and its anabaenopeptilide mutant . Appl. Environ. Microbiol. , 70 : 4551 – 4560 .
- Reynolds , C , Huszar , V , Kruk , C , Naselli-Flores , L and Melo , S . 2002 . Towards a functional classification of the freshwater phytoplankton . J. Plankton Res. , 24 : 417 – 428 .
- Rippka , R . 1988 . Isolation and purification of cyanobacteria . Meth. Enzymol. , 167 : 3 – 27 .
- Rohrlack , T and Hyenstrand , P . 2007 . Fate of intracellular microcystins in the cyanobacterium Microcystis aeruginosa (Chroococcales, Cyanophyceae) . Phycologia. , 46 : 277 – 283 .
- Saker , M , Fastner , J , Dittmann , E , Christiansen , G and Vasconselos , V . 2005 . Variation between strains of the cyanobacterium Microcystis aeruginosa isolated from a Portuguese river . J. Appl. Microbiol. , 99 : 749 – 757 .
- Schatz , D , Keren , Y , Hadas , O , Carmeli , S , Sukenik , A and Kaplan , A . 2005 . Ecological implications of the emergence of non-toxic subcultures from toxic Microcystis strains . Environ. Microbiol. , 7 : 798 – 805 .
- Shi , L , Carmichael , WW and Miller , I . 1995 . Immuno-gold localization of hepatotoxins in cyanobacterial cells . Arch. Microbiol. , 163 : 7 – 15 .
- Sivonen , K and Jones , G . 1999 . “ Cyanobacterial toxins ” . In Toxic Cyanobacteria in Water. A Guide to their Public Health Consequences, Monitoring and Management , Edited by: Chorus , I and Bartram , J. 41 – 112 . London, , UK : E & FN Spon .
- Skulberg , OM and Skulberg , R . 1985 . Planktic species of Oscillatoria (Cyanophyceae) from Norway. Characterisation and classification . Algol. Stud. , 38/39 : 157 – 174 .
- Suda , S , Watanabe , MM , Otsuka , S , Mahakahant , A , Yongmanitchai , W , Nopartnaraporn , N , Liu , Y and Day , JG . 2002 . Taxonomic revision of water-bloom-forming species of oscillatorioid cyanobacteria . Int. J. Syst. Evol. Microbiol. , 52 : 1577 – 1595 .
- Tillett , D , Dittmann , E , Erhard , M , Von Döhren , H , Börner , T and Neilan , BA . 2000 . Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: an integrated peptide-polyketide synthetase system . Chem. Biol. , 7 : 753 – 764 .
- Tonk , L , Visser , P , Christiansen , G , Dittmann , E , Sneldfer , E , Wiedner , C , Mur , L and Huisman , J . 2005 . The microcystin composition of the cyanobacterium Planktothrix agardhii changes toward a more toxic variant with increasing light intensity . Appl. Environ. Microbiol. , 71 : 5177 – 5181 .
- Van Liere , L and Mur , LR . 1978 . Light-limited cultures of the blue-green alga Oscillatoria agardhii . Mitt. Internat. Verein. Limnol. , 21 : 158 – 167 .
- Van Liere , L and Mur , LR . 1980 . Occurrence of Oscillatoria agardhii and some related species, a survey . Develop. Hydrobiol. , 2 : 67 – 77 .
- Welker , M , Christiansen , G and Von Döhren , H . 2004 . Diversity of coexisting Planktothrix (cyanobacteria) chemotypes deduced by mass spectral analysis of microystins and other oligopeptides . Arch. Microbiol. , 182 : 288 – 298 .
- Welker , M and Erhard , M . 2007 . Consistency between chemotyping of single filaments of Planktothrix rubescens (Cyanobacteria) by MALDI-TOF and the peptide patterns of strains determined by HPLC-MS . J. Mass Spec. , 42 : 1062 – 1068 .
- WHO . 2004 . Guidelines for Drinking-Water Quality, Vol. 1, Recommendations , 3rd , Geneva, , Switzerland : World Health Organization .
- Wiedner , C , Visser , P , Fastner , J , Metcalf , JS , Codd , GA and Mur , LR . 2003 . Effects of light on the microcystin content of Microcystis strain PCC 7806 . Appl. Environ. Microbiol. , 69 : 1475 – 1481 .
- Wright , SW , Jeffrey , SW , Mantoura , RFC , Llewellyn , CA , Bjornland , T , Repeta , D and Welschmeyer , N . 1991 . Improved HPLC method for the analysis of chlorophylls and carotenoids from marine phytoplankton . Mar. Ecol. Prog. Ser. , 77 : 183 – 196 .
- Young , F , Thompson , C , Metcalf , J , Lucocq , J and Codd , G . 2005 . Immunogold localisation of microcystins in cryosectioned cells of Microcystis . J. Struct. Biol. , 151 : 208 – 214 .