Abstract
The transmitted double-beam interference microscope was used to determine the dry weight per unit biovolume of single living cells, trichomes and mucous sheaths of eight mainly terrestrial species of cyanobacteria from cultures and in situ samples. The minimum dry weight was 131.7 fg µm−3 whereas the maximum was 459.2 fg µm−3 from single cell measurements. The average (±SD) of all 72 measurements was 265 ± 46 fg µm−3. This value is lower than the average calculated from literature data by a factor of 1.8. Additional elemental measurements of the amount of carbon resulted in an average value (±SD) of 48 ± 3% of dry weight, which corresponds with literature data. Thus we recommend a new conversion factor of 0.127 for biovolume (mm3) of cells to mg carbon, which could be used for cyanobacteria in respect to overall biomass calculations. Dry weight measurements were also carried out on the mucous sheaths of both trichomes (Phormidium) and coenobia (Gloeocapsa). Dry weights per unit volume of the sheaths varied greatly, ranging from 28 fg µm−3 (Phormidium) to 70 fg µm−3 and even 210 fg µm−3 (Gloeocapsa). In Gloeocapsa the dry mass of sheath material of a single coenobium exceeded the cellular dry weight 6-fold. As the interference microscopical technique is unique in its ability to determine dry masses of single living untreated cells, even in complex environmental samples, we intended to develop this method to make it available to a broad range of applications.
Introduction
Ecological studies of the flow and cycling of organic matter in aquatic and terrestrial systems require the quantification of biomass, e.g. of microorganisms. Microscopic enumeration, measurements of microbial cell sizes and calculations of biovolumes are the first steps in determining biomasses as dry weight or organic carbon.
In general, group-specific and constant conversion factors for bacteria, fungi and algae are used for calculating biomass from biovolumes. The conversion factors represent average values of several species, measured by different methods and published by different authors. In particular the calculation of both cellular microbial dry weight and cellular carbon remain problematic. In addition to methodological uncertainties, the physiological conditions of cells cause fluctuations in cellular dry weight and therefore cellular carbon (Reynolds, Citation1984; Paul & Clark, Citation1989; Montagnes et al., Citation1994; Paul et al., Citation1999; Stramski, Citation1999; Bölter et al., Citation2006).
The use of constant factors may be acceptable with respect to large scale investigations in time and space. However, if biocoenotic analyses or in vitro ecotoxicological test systems on a meso- to micro-scale over a defined time period are to be realised with high resolution, conversion factors have to be highly accurate. Small changes of biomass over short time intervals that are statistically significant must be determined. Thus species-specific conversion factors should be determined regardless of time costs, using minimal amounts of sample material in the course of the experiment. For example knowing the dry weight or carbon per unit biovolume of a single species or morphological type of cells at the actual sampling time could be of immense importance.
Stramski (Citation1999) discussed the concept of using the refractive index of single cells of phytoplankton organisms as a measure of intracellular carbon concentration. He suggested the use of flow cytometry, microspectrophotometry or optical tweezers to realise such measurements routinely. However, to date this approach has not been followed up. Thus, our motivation was to find a simple method that could be used routinely to determine the cellular dry weight of a single, living specimen microscopically, even when mixed with soil or detritus particles, in order to minimise the disturbance of the experimental system by frequent sampling.
The transmitted light double-beam interference microscope is a suitable instrument for analysing single, living cells (Walter, Citation1962). In the 1950s to the 1970s interference microscopy was a common and widely-used method for the quantitative determination of dry mass in living cells, tissues and other biological objects (Davies, Citation1954; Sandritter, 1960; Beyer, Citation1974). One reason that this technology vanished from the market, was the limited applicability of electronic image analysis and video detection at that time (Dunn, Citation1998). The possibility of using interference microscopy in ecology e.g. to quantify the dry mass content of microalgal cells was not considered.
With this instrument the optical path difference can be measured. This depends on the physical path length (thickness), the refractive index of the optically isotropic specimen and the refractive index of the surrounding medium. Measuring the optical path length enables either one of these quantities to be determined if the other is known.
Equal masses of any of the cellular substances, protein, carbohydrates etc, when dissolved in water to the same final volume of solution, produce approximately the same increase in the refractive index (Davies, Citation1954). The refractive index depends directly on the dry mass content of the cell. Thus, no erroneous results can be produced by calculating cellular dry masses, otherwise caused by weighing large algal masses and of parallel enumeration of cells, particularly of filamentous or mucilagenous species.
Apart from the dry mass, the optical path difference alone could be a characteristic cellular parameter during the development of single cells and the whole population correlated with the nutritional status, abiotic physico-chemical parameters or even xenobiotica. Data of dry mass or carbon per unit biovolume of cyanobacteria are rare, particularly those of mucilagenous morphotypes and terrestrial ones. Our aim is to improve the available dry mass data, presenting measurements on eight mainly terrestrial cyanobacterial species. The dry mass of mucilagenous sheaths of cyanobacterial species was calculated by measuring the optical path difference of the mucilagenous sheaths separately. In addition, to make a comparison with literature data, the carbon content of some of the cultivated species was measured.
Materials and methods
Cyanobacteria
Phormidium autumnale (Agardh) Trevisan ex Gomont, Leptolyngbya foveolarum (Rabenhorst ex Gomont) Anagnostidis et Komárek and Oscillatoria c.f. rupicola Hansgirg were isolated from the surface of agricultural soils (Luvisol, FAO) near Aachen, Rhineland, Germany. Anabaena c.f. thermalis Vouk was isolated from sandy soil from the Aral Sea region (Russia). The isolates were cultivated, non-axenic, on Basal-Medium (BM, Bischoff & Bold, Citation1963) agar. Axenic Spirulina platensis (Norstedt) Geitler (strain 85.79, SAG, Göttingen) was cultivated in Spirulina-Medium (SM, Aiba & Ogawa, Citation1977). The cultures were incubated in a plant cabinet with overhead irradiation at temperatures of 18 ± 1°C and 25°C (Spirulina) respectively. Fluorescent tubes (Philips TL 33 and TL 54 (1 : 1) were used as a light source with a 14 : 10-h light–dark cycle and a quantum flux density of 0.4 × 1016 Q s−1 cm−2 (QSL 100 Biospherical Instruments Inc.). Gloeocapsa spec. Kützing, Calothrix parietina Thuret and Cylindrospermum muscicola Kützing were collected from terrestrial habitats in Aachen and measured one day after sampling, these species were not cultivated.
Biovolume and organic carbon
To calculate biovolumes, cell sizes were determined using both calibrated (stage micrometer) eyepiece graticule and 2000-fold microscopic magnification (ZEISS Plan 100/1.25 Oil, eyepiece 10× and ZEISS Optovar magnification 2×) and calibrated (digital image of a stage micrometer) pixel sizes of digital images of the specimen. Biovolumes were calculated by assuming simple geometric solids such as cylinder, sphere or ellipsoid (Hillebrand et al., Citation1999).
Total organic carbon as a percentage of dry weight of the cultivated species was determined using a VARIO EL III elemental analyser (elementar, Hanau). The respective cell material was removed from the plates and suspended in BM or SM. The cyanobacteria were gently concentrated by membrane filtration (cellulose nitrate, pore size 0.65 µm). The respective cell material, still moist, was immediately taken off from the filter and filled in pre-weighed (±0.001 mg) tin foil containers (3–6 replicates) used for elemental analysis. To determine the cyanobacterial dry weight, the containers including cell material were weighed again after drying at 105°C for 24 hours.
Interference microscopy
The physical basis of interference microscopy is the division of the illuminating light wave front in two partial coherent waves, where one of these waves passes through the object (object wave) while the other remains undisturbed (reference wave). The object waves become phase retarded due to the thickness and the refractive index of the specimen. In comparison to the reference wave this is a measure of the optical path difference (OPD) introduced by the object, as long both optical pathways only differ by the presence of the object. By combining the object wave with the reference wave an interference stripe field could be observed in the view field. By analysing the interference stripes the OPD can be measured directly according to the formula,
If object and reference wave are recombined at a steep angle, the interference pattern appears in stripes of maxima where the OPD causes constructive interference and minima with total attenuation where the OPD causes destructive interference. Between the extremes the intensity is modulated in a cosine shape. The distance between adjacent maxima or minima in the interference pattern depends on the angle of recombination and the wavelength of the light. In the case of monochromatic illumination the interference pattern appears with equidistant and periodic stripes, where the maxima have the colour of the illuminating light and the minima are totally black.
The contrast between minima and maxima depends on the coherency of the illumination light source and is highest for stripes with low interference order. The lateral distance between two maxima or minima observed in the view field represents the optical path difference of one wavelength (Grehn, Citation1959, Citation1960). If white light sources are used for illumination, the interference pattern appears in coloured stripes, because the distance of minima and maxima in the interference space depend on the wavelength.
The specimen causes a path difference in the object wave if the index of refraction differs from that of the surrounding medium. The OPD then causes a curvature in the interference stripe that seems to cross the object in the field of view. After adjusting the lateral distance of undisturbed stripes the actual OPD can be determined by measuring the lateral shift of a stripe in the object (). The angle of recombination of the two waves can be adjusted to a flat angle, so that the interference stripes appear widened in the field of view, until the whole field is homogeneous.
Fig. 1. Phormidium sp. in the monochromatic (λ = 546 nm) stripe field of the Leitz Dualbeam microinterferometer. The optical path difference (OPD) caused by the object leads to a curvature in the interference stripe. The distance between the undisturbed stripes is the direct measure of the OPD and can be read out directly from the image.
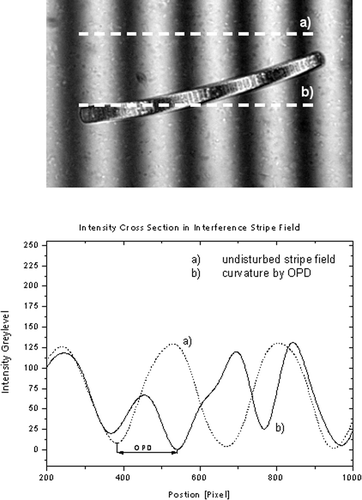
Objects inducing an OPD then appear bright on a dark background or the inverse, depending on which interference order was chosen for the homogenous field. The homogeneous field offers a different method to measure the OPD that is suitable for objects where the curvature of a stripe is too complex. In the case of white light illumination a certain interference colour could be chosen to adjust a homogeneous field in the background of the object. The interference colours in the object are specific for a certain OPD. With this method the OPD of different structures in a single image could be determined simultaneously.
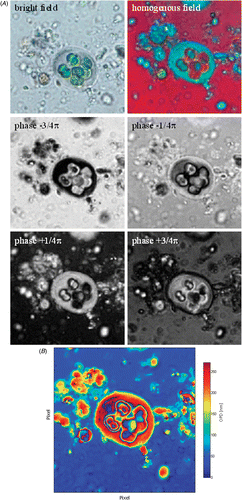
One established method in reflexion interferometry is the automatic phase shifting algorithms (Schwider, Citation1983; Creath, Citation1988). So far, this type of measurement has only been applied by Dunn (1995) for a transmission microinterferometer. This method requires at least four images of the object in a homogenous interference field, while each picture differs by a phase shift of π/2 (a quarter of the illumination wavelength). The pictures are processed as described by Creath (Citation1988) and provide the lateral distribution of the OPD as result ().
Fig. 2. (A) Determination of OPD by phase-stepping algorithm. This method is suitable for coloured or complex objects like Gloeocapsa. The bright field image shows that the algae have a green colour due to chlorophyll content, so the specific interference colour of the cell in the homogenous field is a mixture in the chlorophyll specific colour. By shifting the overall phase between reference and object wave in four different positions, which differ by a quarter of a wavelength or π/2 (where 2π is a full wavelength) the OPD distribution in the image plane can be calculated by a phase stepping algorithm (Schwider, Citation1983). (B) The result is a false coloured picture, in which OPD can be determined.
Double-beam interference microscope
The best adaptation to the physical principle of interference microscopy was achieved with the dual beam interference microscopes developed by Horn, Citation1958 and manufactured by Leitz (Wetzlar). This instrument has the optical arrangement of a Mach-Zehnder interferometer combined with, wave optical identical, two microscope optics in each pathway. With this complex but very accurate arrangement of the optical system and by using low aperture condensers with a numerical aperture (NA) of 0.3, the best compromise between spatial and phase resolution could be achieved. The instrument used for this work can be equipped with pairs of identical objects for object and reference beam path (20×/0.35, Fl 50×/0.85, Fl 100×/1.36) and a 100 W halogen lamp, a mercury lamp or a Helium-Neon Laser. For further analysis pictures of specimen were taken with a Canon PowerShot S80 Camera (8 mega-pixels, 1/1.8 inch chip size) with a 4-fold magnification optical zoom objective and a projective microscope adaptation.
Determination of the optical path difference
To measure the OPD introduced by the object, two different methods were used. In the case of a simple, cylindrical geometry such as an alga or a mucous sheath, the curvature of a stripe was measured. Therefore a steep angle between object- and reference-wave was chosen, so that ten minima and maxima filled the field of view. The object of interest was placed, so that it was cantered to the lowest interference order and the curvature of the stripe was still clearly visible. For further analysis the object in the interference-stripe-field was photographed with a digital camera. The lateral curvature was measured with the Analysis Pro image-analysing software. As monochromatic illumination was chosen the OPD between two undisturbed interference stripes are given by the wavelength of the light. In comparison to the curvature in the interference stripe that is caused by the object the OPD can directly be determined in nanometers or fractions of wavelength ().
In the case of a more complex geometry e.g. for Spirulina, the measurement was made with white light interference. As in the previous situation, a stripe field was set up and a picture of both the coloured and the monochromatic interference was taken. When comparing these two pictures, the lateral distance for the OPD could be adjusted and the specific interference colour sorted to a specific OPD. By spreading the angle of recombination every specific OPD then appears with its characteristic colour in the observed object. The specific RGB level could be studied with the same image analysing software and compared to the colour in the striped field.
The highest precision in measuring the OPD is given by the use of phase-shifting algorithms described by Schwider (Citation1983) and Creath (Citation1988). This method gives the lateral distribution of the OPD of a whole image. The OPD of each image pixel is calculated from four intensity pictures, which differ by a quarter of the illumination wavelength in the global phase between object and reference wave ().
Calculation of concentration and dry mass
The index of refraction of a specimen depends directly on the dry mass concentration, i.e. the mass without water:
The increment of cells is assumed to be α = 0.0017 (mainly protein) and of sheaths α = 0.0014 (mainly carbohydrates) (Gerlach, Citation1976; Weast, Citation1976).
To obtain the dry weight (dw) the biovolume must be multiplied by % dry mass:
Results
Measurement of thickness d
In order to calculate dry weights of algal cells from OPD, it is necessary to determine the thickness or diameter of the relevant cells. shows the data measured with the two different methods presented here. The respective pairs of values agree with each other. For accurate calculations it would be better to use those values of d determined by pixel counts of the digital images that were used for measuring OPD. For the average of all measurements on cells of one species, the standard deviations were calculated to be ±10%. However, for species with different cell sizes within the trichome (Anabaena) or with trichomes of different diameters (Leptolyngbya), standard deviations of ±20% were calculated (). So when measuring Leptolyngbya, the two trichome types were calculated separately. The standard deviations then decreased to 7 and 5%.
Table 1. Trichome and cell sizes (diameter) of eight cyanobacterial species – comparison of two methods.
Cellular dry weight
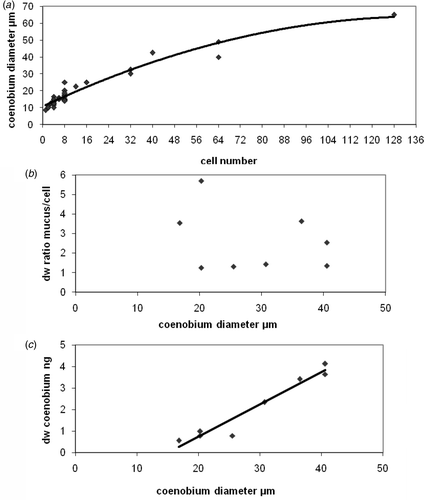
The average values of measured OPD and calculated values of dry weight per unit biovolume of the individual species are listed in Tables and . Average values range from 193 fg µm−3 for Cylindrospermum to 330 fg µm−3 for Leptolyngbya. The sheaths of P. autumnale were measured separately and the mucilage contributes 11% of the dry weight of the trichome. The large dry weight value of the basal region of the C. parietina trichome is remarkable, and the influence of the thick lamellated sheaths is obvious compared to the slender middle part of the same trichome with its thin sheaths. Roughly calculated, dry weight reaches 20%. The mucilagenous aggregates, the coenobia, of Gloeocapsa sp. are more complex. Cells and the mucilage sheaths under water saturated conditions, were measured separately with the phase-stepping method. The coenobia of the in situ samples were roughly ball-shaped and contained up to 128 cells, corresponding to a coenobial diameter of 65 µm (). Coenobia containing up to 40 cells were selected for OPD measurements and dry mass calculations. In the average values of both, dry weight per unit cellular biovolume and of unit sheath volume are listed. The sheath mucilage is characterized by a high dry mass content, fluctuating by a factor of three. There is no correlation between sheath dw:per cell dw ratios and coenobial diameters (B) in the ratio of 1 : 6. Whereas cell numbers and total coenobial dry weights (cells and mucilage) correlate with coenobial diameters ().
Fig. 3. Parameters related to coenobial diameters of Gloecapsa spec. Points represent single coenobia. (a) Cell numbers of single coenobia; (b) dry weight ratio mucous material per cell; (c) the total (cells and mucilage) dry weight of the coenobia.
The average (±SD) of all 72 single measurements calculated as dry weight per unit biovolume is 264.8 ± 45.9 fg µm−3. The lowest value (192.6 fg µm−3) was measured for C. muscicola. The standard deviation of mean dry weights varies for most species for 8% and 14%, whereas the standard deviations of A. cf. thermalis and S. platensis are more than 30%.
Table 2. The optical path difference (OPD) of eight cyanobacterial species in relation to the means of trichome and cell diameters.
Carbon content
In order to calculate the amount of carbon per dry mass, cultured cyanobacterial material was sampled from agar plates, dried and analysed. The data are presented in and the carbon content of dry mass of S. platensis was lowest, 48.1% (±3.3 SD).
Table 3. Mean values of the dry weight per unit biovolume of eight cyanobacterial species, calculated from OPD and size measurements; min–max values are from single cells and trichomes respectively.
Table 4. Carbon content of 8 cyanobacterial species as a percentage of dry mass.
Discussion
Cellular and sheath dry weight
The dry weight determinations of trichomes and coenobia, both simple geometrical and complex shapes, support the use of interference microscopy as a method to determine dry weight. Comparing average values of cellular dry weight per unit biovolume with the limited literature data on cyanobacteria, it is obvious, that our average of 72 measurements, 264.8 ± 45.9 fg µm−3, is lower by a factor of 1.8 than the average of 486 fg µm−3 from the literature (). Thus, for cyanobacteria, the conversion factor for overall calculations from biovolume to cellular dry weight should be lowered.
Table 5. Dry weight per unit biovolume of six cyanobacterial species calculated by different authors.
The measurement of single cells makes the problem of calculating an average evident. In this case the standard deviation reflects the heterogeneity of the biological material and does not reflect the methodological error. On the one hand it is an advantage of the method that developmental changes in dry weight of a single cell could be accurately followed. However, one needs species-specific, or overall, conversion factors for biomass calculations. In the latter case standard deviations of ca ±10% would be acceptable, e.g. homogenous trichomes like Phormidium. However, species characterized by different cells within one trichome e.g. akinetes in genera such as Anabaena are problematic, resulting in large standard deviations. Spirulina was sampled at the end of exponential growth. The differences in dry weight reflect the developmental phase of cells, some were obviously stationary others still growing. In the case of Gloeocapsa, differences in dry weight can be explained by the arrangement of cells inside the coenobium and the mode of cell division. Within the coenobia, cells were often packed one upon the other. Thus it may be sometimes difficult to decide, whether there are two or more cells. In addition to the visual examination, measurement of the differences in OPDs can help to decide whether cells are overlapping. Before cell division occurs cells become elongated, so that sometimes measurements of cell sizes may be not correct, resulting in an extraordinary high dry weight per unit biovolume. The highest value measured, 459 fg µm−3 for a Gloeocapsa cell (, min–max), points to that and suggests that this situation has occurred. That cell development causes changes in OPD has previously been documented for yeast cells by Davies (Citation1958).
Species-specific literature, reporting on the amount of mucilage sheath material as dry weight are rare, although as a component of bacterial biofilms the quantities of EPS (Extra Polymeric Substances) may constitute 50–90% of the total organic matter (Wingender et al., Citation1999). EPS:chlorophyll a ratios of Microcoleus (Mazor et al., Citation1996) and of algal biofilms (Barranguet et al., Citation2005) can be converted to EPS:algal dry weight ratios (C/Chl a = 40 and C = 50% of dry weight, Jörgensen, Citation1979) resulting in EPS:algal dw ratios of <0.2 (biofilms), to a maximum of 1.0 (Microcoleus). In the case of old cultures of Phormidium uncinatum, Hoiczyk (Citation1998) measured sheath material as 21% of the total culture dry weight. This species is similar to P. autumnale. Our value of 11% sheath material is considerably lower because the cultures were still growing.
The coenobia of Gloeocapsa were stable in shape and the mucilagenous outer envelopes were typically lamellated, due to the individual gelatinous sheaths of the single cells. The sheaths were slightly grey-violet in colour. The mucilage is characterized by high dry mass per unit sheath volume, up to 210 fg µm−3 suggesting an adaptation to habitats such as concrete walls and rocks that are sometimes dry and hot. These conditions are reflected by both cell dw: EPS ratios between 1 and 6 and coenobial EPS volume per biovolume of coenobial cells between 3.4 and 17.3. It must be remembered that dry mass is not evenly distributed within the sheath. The material forms a hollow structure, similar to the marine planktonic haptophyte Phaeocystis sp., which produces a muco-polysaccharide matrix that forms a hollow spherical and stable structure in young colonies. Alderkamp et al. (Citation2006) calculated the contribution of mucopolysaccharides to the carbon content in Phaeocystis to be less than 5% during exponential growth, increasing up to 13% during the stationary phase.
Carbon content
The carbon content of dry mass should only vary over a small range, as the carbon contents of proteins, fats or carbohydrates are similar. Thus variations in these cell components will have only small effects on the carbon:dry weight ratio. The average of 47.9% ± 1.64% (SD) reported by Jörgensen (Citation1979) for cyanobacteria agrees with the average of our data, indicating that conversion of dry weight to carbon and vice versa using a value of 48% may be appropriate. Thus, for our data, we recommend an average (±SD) conversion factor of 0.127 ± 0.022, for total biomass calculations, cyanobacterial biovolume (mm3) to carbon (mg). The standard deviation of ±18% (our conversion factor) is comparatively low, as Hagmeier (Citation1961) calculated a total error of ±28% when estimating the dry weight of marine phytoplankton cells from their biovolume. Differences between species, physiological cellular condition, inorganic incrustion of cell walls, physical and chemical environmental factors may cause large fluctuations in cellular dry weight or carbon, as discussed and summarized by Montagnes et al. (Citation1994). He analysed the cell volume to carbon, relationship of 29 marine phytoplankton species (not including cyanobacteria). Cellular carbon ranged between 37.3 to 800 pg µm−3 and the standard deviation of the average was ±60%.
Our conversion factor lies within the range of 0.09–0.15 suggested for algae by Strickland (Citation1960) and Montagnes et al. (Citation1994). It is lower than the conversion factor of 0.22 by 1.7 suggested by Reynolds (Citation1984), and (by a factor of 2) than the conversion factor of 0.255 (<100 µm3 cell volume) reported by Strathman (Citation1967). In the case of bacteria, conversion factors between 0.15 and 0.32 are recommended, and for fungal hyphae factors between 0.13 and 0.15 are commonly used (Paul et al., Citation1999). Further measurements of individual cells growing under different light, temperature and substrate regimes will reveal the species-specific total range of cellular dry weight.
Methodological errors and limits
The average OPD values and the corresponding standard deviations are listed in (). The standard deviations do not reflect the reproducibility of repeated measurements, but demonstrate the heterogeneity of the biological material. On discussing the reproducibility of repeated measurements Sandritter (1960) reported a reading error of only 1%. In the case of environmental or other perturbations, e.g. when cells are nutrient limited, the protein:lipid ratio may be lowered significantly. When lipid content increased from 10 to 30%, with a reduction in protein content, the refractive increment decreased from 0.0017 to ca 0.0016 and an error of 6% to 10% could be possible (Sandritter, 1960).
The measurement of OPDs of single cells within aggregates will always be problematic. In the case of Gloeocapsa it becomes difficult to measure single cells if the coenobium contains more than 40 relatively densely aggregated cells. However, measuring the sheath material remains possible, even for large coenobia. Whether it is feasible accurately to determine dry weight in large and heavy pigmented algal cells will be determined in further investigations. In comparison to, e.g. green algae, cyanobacterial cells are relatively homogenous, due to the lack of chloroplasts. The non-homogeneity of objects, diffraction and cell size could have effects on the accuracy of OPD measurements obtained; this is discussed in detail in Davies (Citation1958). With the cyanobacterial cells tested, these effects may be negligible, because they are relatively large, the structure of the cells is homogeneous and the contrast is not noticeably lowered by pigmentation. In most cases diffraction should be negligible in interference microscopy (Davies, Citation1958).
Conclusions
Double-beam interference microscopy interferometry is undoubtedly worth further development, particularly combined with the currently available powerful imaging and software tools. With new and specifically adapted software, the methodological problems, e.g. for complex cell shapes, could be solved. Automation would also increase its applicability in many fields of ecology, environmental sciences and biotechnology. However, even using basic microscopy, as outlined above, the method proved to be accurate, easy and rapid in routine work. The method should be explored by measuring other groups of algae and microorganisms, including green- and yellow-green algae, fungal hyphae and more problematic cell types including; diatoms, dinoflagellates, protozoa and even heterotrophic bacteria.
Acknowledgements
We are grateful to Prof M. Sernetz and Prof H. Korr who made the Mach-Zehnder microscopes available to us. B. Kosier and two anonymous reviewers are acknowledged for critically reading and correcting the manuscript.
References
- Aiba , S and Ogawa , T . 1977 . Assessment of growth yield of a blue-green alga Spirulina platensis, in axenic and continuous culture . J. Gen. Microbiol. , 102 : 179 – 182 .
- Alderkamp , A-C , Nejstgaard , JC , Verity , PG , Zirbel , MJ , Sazhin , AF and Van Rijssel , M . 2006 . Dynamics in carbohydrate composition of Phaeocystis pouchetii colonies during spring blooms in mesocosms . J. Sea Res. , 55 : 169 – 181 .
- Barranguet , C , Veuger , B , Van Beusekom , SAM , Marvan , P , Sinke , JJ and Admiraal , W . 2005 . Divergent composition of algal-bacterial biofilms developing under various external factors . Eur. J. Phycol. , 40 : 1 – 8 .
- Beyer , H . 1974 . Theorie und Praxis der Interferenzmikroskopie , Leipzig, , Germany : Akademische Verlagsgesellschaft Geest & Portig K.G. .
- Bischoff , HW and Bold , HC . 1963 . Phycological studies IV. Some soil algae from enchanted rock and related algae species . University of Texas Publication , 6318 : 1 – 95 .
- Bölter , M , Bloem , J , Meiners , K and Möller , R . 2006 . “ Enumeration and biovolume determination of microbial cells ” . In Microbiological Methods for Assessing Soil Quality , Edited by: Bloem , J , Hopkins , DW and Benedetti , A . 93 – 113 . Wallingford, , UK : CAB International .
- Creath , K . 1988 . “ Phase-Measurement Interferometry Techniques ” . In Progress in Optics , Edited by: Wolf , E . 349 – 393 . Amsterdam, , The Netherlands : Elsevier .
- Davies , HG . 1954 . The use of the interference microscope to determine dry mass in living cells and as a quantitative cytochemical method . Quart. J. Micr. Sci. , 95 : 271 – 304 .
- Davies , HG . 1958 . The determination of mass and concentration by microscope interferometry . Gen. Cytochem. Meth. , 1 : 55 – 161 .
- Dunn , G . 1998 . Transmitted-light interference microscopy: a technique born before its time . Proc. Royal Micr. Soc. , 33 : 189 – 196 .
- Dunn , G and Zicha , D . 1995 . An image processing system for cell behaviour studies in subconfluent cultures . J. Micros. , 179 : 11 – 21 .
- Gerlach , D . 1976 . Das Lichtmikroskop , Stuttgart, , Germany : Thieme .
- Grehn , J . 1959 . Das durchlicht-interferenz-mikroskop – ein instrument des biologen . LEITZ-Mitt. g. Wiss. u. Techn. , I/2 : 35
- Grehn , J . 1960 . Das interferenz-durchlicht-mikroskop von E. Leitz . Act. Hist. , 9 : 204
- Hagmeier , E . 1961 . Plankton-Aequivalente . Kieler Meeresforsch. , 17 : 32 – 47 .
- Hillebrand , H , Dürselen , CD , Kirschtel , D , Pollingher , U and Zohary , T . 1999 . Biovolume calculation for pelagic and benthic microalgae . J. Phycol. , 35 : 403 – 424 .
- Hoiczyk , E . 1998 . Structural and biochemical analysis of the sheath of Phormidium uncinatum . J. Bact. , 180 : 3923 – 3932 .
- Horn , W . 1958 . Mikro-Interferenz II, in Jahrbuch für Optik und Feinmechanik 3 , Wetzlar, , Germany : Pegasus-Verlag .
- Jörgensen , SE . 1979 . “ Handbook of environmental data and ecological parameters. Environmental Sciences and Applications Vol. 6 ” . In International Society for ecological Modelling, , 1st edition , Oxford, , UK : Pergamon Press .
- Mazor , G , Kidron , GJ , Vonshak , A and Abeliovich , A . 1996 . The role of cyanobacterial exopolysaccharise in structuring desert microbial crusts . FEMS Microbiol. Ecol. , 21 : 121 – 130 .
- Montagnes , DJS , Berges , JA , Harrison , PJ and Taylor , FJR . 1994 . Estimating carbon, nitrogen, protein, and chlorophyll a from volume in marine phytoplankton . Limnol. Oceanogr. , 39 : 1044 – 1060 .
- Paul , EA and Clark , FE . 1989 . Soil microbiology and biochemistry , San Diego, , USA : Academic Press Inc. .
- Paul , EA , Harris , D , Klug , MJ and Ruess , RW . 1999 . “ The determination of microbial biomass ” . In Standard Soil Methods for Long-Term Ecological Research , Edited by: Robertson , GP , Coleman , DC , Bledsoe , CS and Sollins , P . 291 – 317 . New York, , USA : Oxford University Press .
- Reynolds , CS . 1984 . The Ecology of Freshwater Phytoplankton , Cambridge, , UK : Cambridge University Press .
- Sandritter , W , Schiemer , HG and Alt , W . 1960 . Das Interferenzmikroskop im Dienste der Cytologie und Krebsforschung . Klin. Wschr. , 12 : 590 – 595 .
- Schwider , J . 1983 . Digital wave-front measuring interferometry: some systematic error sources . Appl. Opt. , 22 : 3421 – 3432 .
- Stramski , D . 1999 . Refractive index of planktonic cells as a measure of cellular carbon and chlorophyll a content . Deep-Sea Res. , 46 : 335 – 351 .
- Strathmann , RR . 1967 . Estimating the organic carbon content of phytoplankton from cell volume or plasma volume . Limnol. Oceanogr. , 12 : 411 – 418 .
- Strickland , JDH . 1960 . Measuring the production of marine phytoplankton . Fish. Res. Bd. Can., Bull. , 122 : 172
- Walter , F . 1962 . Die Mikrointerferometrie als quantitative Methode in der biologischen Forschung (I) . LEITZ-Mitt. g. Wiss. u. Techn. , II/2 : 41 – 48 . 77–85
- Weast , RC . 1976 . Handbook of Chemistry and Physics, , 57th edition , Cleveland, , USA : CRC Press .
- Wingender , J , Neu , TR and Flemming , H-C . 1999 . “ What are bacterial extracellular polymeric substances? ” . In Microbial extracellular Polymeric Substances, Characterization, Structure and Function , Edited by: Wingender , J , Neu , TR and Flemming , H-C . 1 – 19 . Berlin, , Germany : Springer .