Abstract
This paper provides the first detailed account of sexual reproduction in Caulerpa taxifolia, describing gametogenesis, sex ratios, periodicity of reproductive episodes and breeding system in C. taxifolia from Caloundra on the subtropical east Australian coast. Gametogenesis is similar to published accounts of the process in 11 other Caulerpa spp. Gender is reliably determined late in gametogenesis: mature dark-green male portions of fronds that release gametes without eyespots are distinguished from brownish-green female portions of fronds that release gametes with eyespots. Twenty-one reproductive episodes were observed for C. taxifolia during the 3.5-month fertile period, with peak reproductive activity occurring in February/March when 13 reproductive episodes were observed over a 36-day period. Caulerpa taxifolia is monoecious, but the Caloundra population showed marked variation in phenotypic gender expression during the fertile season, when various proportions of male plants, thalli with bisexual fronds and thalli with bisexual, unisexual male and/or female fronds were observed. Sex allocation in the Caloundra population was markedly male-biased throughout the fertile season with only male plants present, or with male plants dominating the population, for 13 and three reproductive episodes respectively. The implications of these observations are discussed in relation to the reported lack of sexual reproduction in the invasive Mediterranean populations of the species.
Introduction
Many species of macroalgae combine various modes of asexual and sexual reproduction in their life histories, but the relative importance of these reproductive modes, which may vary widely between and within species, remains poorly investigated. In higher plants, variation in reproductive mode is considered to have significant ecological and evolutionary consequences, with respect to genetic variation, colonisation, habitat exploitation, dispersal, maintenance of effective population size and local persistence (Silander, Citation1985; Charlesworth & Charlesworth, Citation1995; Eckert, Citation2002; Dorken et al., Citation2002; Winkler & Fischer, Citation2002). These parameters potentially play a central role in determining whether an exotic species, transported by humans across a major geographical barrier, becomes naturalised or invasive in the new environment.
Caulerpa species spread clonally either by patch expansion or by thallus fragmentation (Sant et al., Citation1996; Ceccherelli & Cinelli, Citation1999; Smith & Walters, Citation1999; Ceccherelli & Piazzi, Citation2001; Renoncourt & Meinesz, Citation2002; Wright, Citation2005; Wright & Davis, Citation2006; Ruesink & Collado-Vides, Citation2006). Eleven species of Caulerpa are known to reproduce sexually (Miyake & Kunieda, Citation1937; Iyengar, Citation1940; Goldstein & Morrall, Citation1970; Kajimura, Citation1977; Meinesz, Citation1979; Ishiwara et al., Citation1981; Enomoto & Ohba, Citation1987; Ohba et al., Citation1992; Clifton, Citation1997; Clifton & Clifton, Citation1999; Panayotidis & Zuljevic, Citation2001). Biflagellate gametes released by the macroscopic thallus fuse to form zygotes which re-establish the macroscopic stage (Price, Citation1972; Ishiwara et al., Citation1981; Enomoto & Ohba, Citation1987; Ohba et al., Citation1992).
Establishing the occurrence of sexual reproduction has important environmental management implications for invasive species, both for zygote dispersal and the potential to generate more invasive genotypes (Ellstrand & Schierenbeck, Citation2000, 2006). Sexual reproduction has not been observed in invasive Mediterranean populations of Caulerpa taxifolia (Vahl) C. Agardh, which are thought to have spread largely by vegetative propagation (Meinesz & Hesse, Citation1991; Olsen, Citation1997; Renoncourt & Meinesz, Citation2002). Sex in macroalgae often tends to be a cryptic process, eluding detection unless populations are closely monitored. Consequently, periodic releases of male gametes by Croatian C. taxifolia is suggestive of sex in this invasive population (Zuljevic & Antolic, Citation2000). Another Mediterranean invader, Caulerpa racemosa (Forsskål) J.Agardh var. occidentalis, (J.Agardh) Boergesen reproduces sexually (Panayotidis & Zuljevic, Citation2001), indicating that invasive Caulerpa spp. generally do not lose the capacity for sex.
Caulerpa taxifolia is indigenous to tropical/subtropical Australia, occurring as both narrow (fronds <5 mm broad) and broad (fronds >10 mm broad) forms on wave-exposed coasts and in sheltered bays, respectively (Phillips & Price, Citation2002). These forms correspond to ‘delicate or small delicate or robust large’ forms in DNA studies of the species (Schaffelke et al., Citation2002; Meusnier et al., Citation2004). Broad (or robust large) C. taxifolia from Moreton Bay is considered the source population for the Mediterranean invasion (Meusnier et al., Citation2001, Citation2002, Citation2004; Fama et al., Citation2002). In the last decade, both narrow and broad forms of C. taxifolia have established invasive populations, presumably from the disposal of aquarium plants, in estuaries on the east and south Australian coasts, well beyond the natural distributional range of the species (Schaffelke et al., Citation2002; Murphy & Phillips, Citation2003; Glasby et al., Citation2005).
This paper provides the first detailed account of sexual reproduction in C. taxifolia and in any species of Caulerpa from Australia. Gametogenesis, sex ratios, periodicity of reproductive episodes and breeding system are documented for narrow C. taxifolia from wave-swept Caloundra on the subtropical east Australian coast. This population undergoes frequent reproductive episodes and has a male-biased sex ratio for most of the fertile season.
Materials and methods
Narrow C. taxifolia thalli were collected at Caloundra (26°48′S; 153°08′E), north of Moreton Bay on the Australian east coast (for locality map see Phillips & Price, Citation2002, ) during the austral summer of 2002–2003 (). Results of DNA studies on this population will be reported elsewhere. To maximise the sampling of different individuals, several clumps (approximately 10 cm in diameter) were collected from widely-separated areas on the wave-swept subtidal rock platform. Thalli were transported to the laboratory in seawater and maintained for at least 3 weeks in aerated seawater aquaria at 25–27°C, under cool-white fluorescent lights at an irradiance of 55 µ mol photons m−2 s−1 and a 14-h:10-h light–dark cycle. Temperature and daylength regimes corresponded to ambient summer conditions in the Moreton Bay region, which records summer sea surface temperatures >25°C (Endean et al., Citation1956; Johnson & Neil, Citation1998). Seawater collected from Caloundra was enriched with 20 ml l−1 of Provasoli's enrichment (PES) added at 2-weekly intervals. During the study, narrow C. taxifolia remained healthy and became fertile in laboratory aquaria.
Figs 1–11. Sexual reproduction in Caulerpa taxifolia. Fig. 1. A mid-green vegetative frond. Fig. 2. Stolons of fertile plants turn white following the transfer of the protoplast into the frond. Fig. 3. The protoplast in mature fronds withdraws from the tips of the lateral branches of the frond and from around the bases of the discharge tubes (arrows). Fig. 4. Mucilage is released from the discharge tubes on the lower part of the frond while the first gametes to be released from the barely visible discharge tube at the frond apex (arrow) are trapped in the mucilage. Fig. 5. The releasing frond loses the reticulate patterning as the gametic masses move to be released from the discharge tube (lower edge of frond). Fig. 6. Gametes are released in a mucilaginous stream which often increases in diameter (arrow) when the stream leaves the tube. Fig. 7. Female gametangia usually contain eight female gametes. Fig. 8. Intact (foreground) and releasing male gametangia. Fig. 9. Male gametes lack an eyespot. Fig. 10. Female gametes have an eyespot (arrow). Fig. 11. Pairing of male and female gametes. Scale bars: 2 mm (Fig 1), 2.6 mm (Fig. 2), 1.2 mm (Fig 3), 1.6 mm (Figs 4, 5), 3 mm (Fig. 6), 7 µm (Figs 7–11).
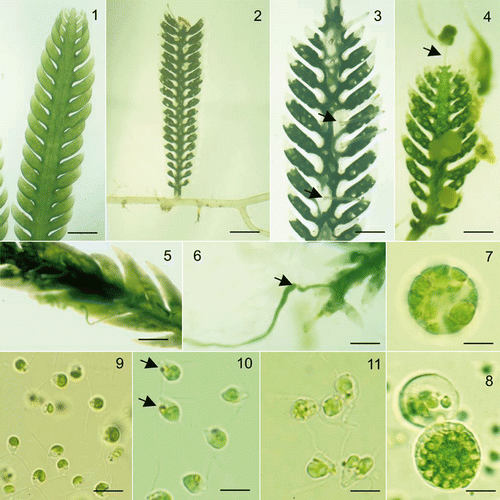
Table 1. Collections of the narrow form of Caulerpa taxifolia from Caloundra.
Observations on fertile thalli
Thalli in aquaria were examined for signs of fertility three times a week from 3rd to 23rd December 2002, and daily from 6th January to 30th April 2003. Fertile thalli with reticulate patterning were transferred to aerated 1-l beakers containing seawater. On the following morning, beakers were removed from cabinets toward the end of the dark cycle but before lights-on, and individual thalli placed in the light in petri dishes containing filtered seawater. After the 31st January 2003, when male and bisexual plants were observed, the colour of fertile fronds was recorded in order to identify male and female portions of fronds, male and female fronds and plants.
Fertilisation experiments
During gamete release, separate suspensions of male and female gametes collected by micropipette from around the end of the discharge tube were pipetted into separate 1.5-ml plastic centrifuge tubes containing 1 ml of filtered seawater. Gamete sex was determined microscopically and correlated to frond colour of mature fertile plants. Drops of freshly-released male and female gametes were mixed on a microscope slide and observed with an Olympus BH2 light microscope under ×1000 magnification. Gamete length and breadth were measured from 20 freshly released gametes at ×1000 magnification using a calibrated eyepiece micrometer and the mean, standard deviation and range of the measurements calculated.
Results
Reproductive episodes
Caulerpa taxifolia thalli became fertile within a month of collection (). Twenty-one reproductive episodes were observed between 12th and 23rd December 2002, and between 6th January and 28th March 2003. The time interval from collection to the first gamete release was 2 days for the February collection, compared with 9–15 days for the other collections. Small numbers of fertile thalli were evident during each reproductive episode, even when aquaria contained many thalli. Peak reproductive period occurred in February/March with 13 gamete releases observed in 36 days, during which successive reproductive episodes were observed on either two, three or four consecutive days or at longer time intervals of 2–7 days.
Table 2. Reproductive episodes and sex ratios in Caulerpa taxifolia from Caloundra.
Onset of gametogenesis
Sterile and fertile fronds of C. taxifolia were easily distinguished by marked changes in frond colour (). With the onset of fertility 48, or less commonly 72, hours before gamete release, the stolon protoplast moved into the erect fronds, giving them a distinctive dark-green colouration and leaving the stolon either partially or completely white (). Concurrently, up to ten discharge tubes formed along one surface of the frond mid-axis and less frequently on the stolons. These tubes were 2–3 mm long, translucent and appeared to have a yellowish or whitish plug at the apex ().
Twenty-four hours before gamete release, the frond protoplast had contracted from the lateral branch apices, from around the base of the discharge tubes and often from the mid-frond, clumping into irregular masses to form a dark-green net-like pattern on the frond (). During gametogenesis, some fronds or upper frond portions remained dark green while other fronds and lower frond portions turned brownish-green up to 12 h before gamete release. This subtle colour differentiation was most reliably distinguished the morning of gamete release. Mature fertile fronds were either bi-coloured, with an upper dark-green and a lower brownish-green portion, or entirely green or entirely brownish-green, while some thalli were composed of either only green fronds, or both green and brownish-green fronds, or bi-coloured fronds, or various combinations of green, brownish-green and bi-coloured fronds.
Sex ratios
Discharges tubes released either male or female gametes from the dark green or brownish-green areas of fronds, respectively. Using this correlation, it was evident that fronds of C. taxifolia were either unisexual or bisexual, and thalli were composed of either male fronds only, or male and female fronds, or bisexual fronds, or bisexual, male and female fronds, or bisexual and male fronds. Male plants dominated throughout the fertile season, being observed during 20 of the 21 reproductive episodes, the only exception being on 4th February 2003 when only one plant with bisexual fronds released gametes (). Only male plants released gametes during 13 reproductive episodes and they dominated in another three reproductive episodes. Thalli with female fronds and/or bisexual fronds were observed in seven of the 21 reproductive episodes and occurred in numbers comparable to male fronds on two occasions (6th and 12th March 2003).
Gamete release
Gamete release in C. taxifolia commenced 20 to 25 min after thalli were exposed to light. At first, a whitish to yellowish mucilaginous material was released from the apical pore of the discharge tubes (), followed by a continuous stream of either green or brownish-green, viscous mucilaginous fluid, spurting from different discharge tubes arising from the green or brownish-green portions/fronds respectively (). The gamete stream increased in diameter as it flowed out of the apical pore (), continuing to flow through the seawater to be deposited onto the Petri dish base. Gentle agitation of Petri dishes dispersed the gametes out of the deposited mucilaginous gametic mass and into the water column. Gametes were released for approximately 15 min, usually leaving parental fronds white from the protoplast loss.
Gametes and gametangia of one sex were released from each discharge tube. Gametangia were membrane-bound spherical structures which usually contained either eight female gametes () or 64–128 male gametes (). Movement of the gametes ruptured the gametangial wall, liberating the gametes into the seawater.
Gamete structure
Gametes of C. taxifolia were pyriform in shape with two equal, anteriorly-inserted flagella (). Female gametes (5–8 µm long [x = 6.8; SD = 0.68] by 4–5 µm broad [x = 4.7; SD = 0.64]) were slightly larger than male gametes (4–5 µm long [x = 4.8; SD = 0.41] by 2–3 µm broad [x = 3.1; SD = 0.34]), had an eyespot, were sluggish swimmers and frequently rotated on the same spot. In contrast, male gametes were active swimmers but lacked the phototactic eyespot and swam without direction. After losing motility, gametes remained suspended in the water column for at least eight hours after release.
Fertilisation
Pairing of C. taxifolia gametes occurred 1–2 min after mixing male and female gametes and was followed by gametic fusion (). The plane of fusion varied, with lateral fusion being more common than apical or posterior fusion. Relatively few quadriflagellate zygotes were observed in the gamete mixtures.
Discussion
This paper presents the first detailed report of sexual reproduction in C. taxifolia. The pattern of gametogenesis is similar to the holocarpic reproductive pattern previously described for 11 species of Caulerpa (Miyake & Kunieda, Citation1937; Iyengar, Citation1940; Goldstein & Morrall, Citation1970; Kajimura, Citation1977; Ishiwara et al., Citation1981; Enomoto & Ohba, Citation1987; Ohba et al., Citation1992; Clifton, Citation1997; Clifton & Clifton, Citation1999; Panayotidis & Zuljevic, Citation2001). Gametogenesis appears to be a relatively invariable process in Caulerpa species with respect to the transfer of protoplast to the erect fronds, formation of discharge tubes and net-like patterns of unspecialized gametangia in the fertile fronds, gamete structure and release. Colour differentiation of mature fertile fronds and female gametes with an eyespot are reliable characters for determining the sex of plants in Caulerpa species.
Relatively few gamete pairs were observed in mixtures of male and female gametes of Caloundra C. taxifolia and Mediterranean C. racemosa (Panayotidis & Zuljevic, Citation2001). Whether this was due to gamete incompatibility or to unfavourable environmental conditions in the laboratory is not known. In contrast, other Caulerpa species have been reported to exhibit higher rates of zygote formation. In Caulerpa cupressiodes (Vahl) C.Agardh, Caulerpa sertularioides (S.G.Gmelin) Howe and Caulerpa serrulata (Forsskål) J.Agardh, intraspecific crosses produced active clumping reactions of 10–50 gametes (Goldstein & Morrall, Citation1970), and under favourable conditions, 30–50% of gametes of Caulerpa brachypus Harvey (Miyake & Kunieda, Citation1937) and Caulerpa scalpelliformis var. denticulata (Decaisne) Weber-van Bosse (Kajimura, Citation1977) formed zygotes.
Monoecy is the predominant breeding system for the genus Caulerpa, known to occur in C. racemosa and varieties, C. prolifera (Forsskål) J.V.Lamour., C. mexicana Sonder ex Kützing., C. serrulata, C. sertularioides, C. cupressiodes, C. verticillata J. Agardh, C. peltata J.V.Lamour. and C. taxifolia (Iyengar, Citation1940; Goldstein & Morrall, Citation1970; Enomoto & Ohba, Citation1987; Ohba et al., Citation1992; Clifton & Clifton, Citation1999; Panayotidis & Zuljevic, Citation2001; this study). Monoecious species of Caulerpa typically have bisexual fronds which, in local C. taxifolia, have upper male and lower female portions, the reverse pattern reported for other Caulerpa species (Goldstein & Morrall, Citation1970; Ishiwara et al., Citation1981; Enomoto & Ohba, Citation1987; Ohba et al., Citation1992). Burr & West (Citation1970) and Rietema (Citation1971) reported similar reversals in gametangial distribution on branch segments on different plants within American and European populations of the monoecious green alga Bryopsis hypnoides J.V. Lamour. More variation in gametangial distribution in Caulerpa spp. may become evident with further studies, particularly since Panayotidis and Zuljevic (Citation2001) reported mosaically-bicoloured fertile fronds for C. racemosa var. occidentalis (J. Agardh) Børgensen.
Combinations of male plants, plants with unisexual male and female fronds and plants with bisexual, unisexual male and/or female fronds observed in local C. taxifolia have not been previously reported for any Caulerpa species. Breeding systems of monoecious macroalgal species have been little investigated, but it would appear that Caloundra C. taxifolia has an andromonoecious breeding system, defined as populations comprising many male plants and some bisexual plants (Willson, Citation1983; Arista & Talavera, Citation1997; Richards, Citation1997; Sarkassian et al., Citation2001). A similar breeding system may also occur in the green algae Bryopsis plumosa (Hudson) J. Agardh (Rietema, Citation1975; Richardson, Citation1982), Codium fragile (Suringar) Hariot (Arasaki et al., Citation1955; Borden & Stein, Citation1969) and Codium decorticatum (Woodward) M. Howe (as C. elongatum (Turner) C. Agardh, Hartmann & Hämmerling, Citation1950) and in some red algae (West, Citation1970; Dixon, Citation1973; Whittick & West, Citation1979; Choi & Lee, Citation1996; Holmes & Brodie, Citation2004) for which both unisexual and bisexual thalli have been reported. In C. decorticatum, the proportion of unisexual to bisexual plants varied temporally from few to many unisexual plants within the population.
Sex ratios in macroalgal species have been poorly studied (DeWreede & Klinger, Citation1988; Holmes & Brodie, Citation2004), and the causal factors responsible for biased sex ratios in algae remain unknown. However, the markedly male-biased sex ratio in C. taxifolia indicates that gender expression in this population has deviated from the 1:1 male:female allocation expected when sex is wholly genetically determined. Similar gender imbalances also occur in monoecious Mediterranean populations of C. prolifera, for which either only male (Meinesz, Citation1979) or female gametes (Dostal, Citation1929) have been observed, and in C. decorticatum (as C. elongatum), which was dominated by female plants on four occasions, by bisexual plants on two occasions or by both female and bisexual plants once (Hartmann & Hämmerling, Citation1950). Male-biased sex ratios may also be a plausible explanation for the observation of only male gametes during the fertile season of Croatian C. taxifolia (Zuljevic & Antolic, Citation2000).
Release of large quantities of gametes (Iyengar, Citation1940; Goldstein & Morrall, Citation1970; Enomoto & Ohba, Citation1987; Ohba et al., Citation1992; Clifton & Clifton, Citation1999; this study), spurting out of discharge tubes in a continuous mucilaginous stream (Clifton & Clifton, Citation1999, ; this study) and dissipating into the water column may increase fertilisation rates and aid in dispersal of Caulerpa zygotes. In the laboratory, gametes were deposited onto the base of containers (Iyengar, Citation1940; Enomoto & Ohba, Citation1987; Ohba et al., Citation1992; this study), but water movements in marine habitats suspend and enhance mixing in large gamete clouds in the water column above high densities of Caulerpa plants (see , Zuljevic & Antolic, Citation2000; Panayotidis & Zuljevic, Citation2001; Clifton & Clifton, Citation1999). Such gamete clouds dissipate within 5–10 mins (Zuljevic & Antolic, Citation2000; Panayotidis & Zuljevic, Citation2001), dispersing zygotes and unfused gametes down-current from released plants on Caribbean reefs (Clifton & Clifton, Citation1999).
Caulerpa species expend considerable effort on frequent sexual episodes, which leads to parental death. Daily monitoring identified 39 reproductive episodes over 125 days in wild populations of tropical C. racemosa (Clifton, Citation1997), similar to 13 episodes in 36 days observed in subtropical C. taxifolia, which presumably has a shorter fertile period. Random sampling detected nine episodes of gamete release over a 6- (C. okamurai, Ishiwara et al., Citation1981) or 8-week period (C. taxifolia, male gametes only, Zuljevic & Antolic, Citation2000). Typically, only a small number of Caulerpa plants in a population become fertile during each reproductive episode, variously estimated to be less than 20% (Meinesz, Citation1979) or usually 5%, but sometimes 15–20% of thalli (Clifton, Citation1997; this study). These observations are consistent with those of Wilson et al. (Citation1996) who concluded that vigorously-spreading clonal plants are best placed to produce greater numbers of sex cells.
There are a number of lines of evidence that support the contention that sex occurs in invasive C. taxifolia. Mediterranean C. taxifolia experiences summer sea surface temperatures >25°C (Meinesz, Citation1979; Zuljevic & Antolic, Citation2000), sufficient to trigger gametogenesis in Mediterranean C. racemosa (Panayotidis & Zuljevic, Citation2001) and in other subtropical/warm temperate populations of Caulerpa spp. (Miyake & Kunieda, Citation1937; Ishiwara et al., Citation1981; this study). Furthermore, Croatian C. taxifolia progressed through a process identical to sexual reproduction in other Caulerpa species, except for the lack of female gametes (Zuljevic & Antolic, Citation2000), including expending considerable resources producing gamete clouds during many successive reproductive episodes. The perceived lack of sex may be related to a markedly male-biased sex allocation which requires close monitoring to find the female gender. Furthermore, the occurrence of two ITS types in invasive C. taxifolia from east Australia (Schaffelke et al., Citation2002) indicates either intraspecific hybridisation or genetic recombination from sexual reproduction (Meusnier et al., Citation2004).
Further studies are urgently required to clarify many aspects of sexual reproduction, breeding system and genetic structure in invasive and non-invasive populations of C. taxifolia. Detailed data on the biology and ecology of indigenous populations of invasive species, of which there is a marked lack in C. taxifolia, are crucial for developing management strategies for biological invasions. It is important to establish what roles sexual reproduction plays in establishing and maintaining invasive C. taxifolia populations, particularly the potential of sexual recombination in generating new genotypes that could produce more successful invaders.
Acknowledgements
This research on reproduction of C. taxifolia was funded for one summer as part of a research grant from National Heritage Trust (Environment Australia) to B. Schaffelke, CSIRO Marine Research (Hobart), Centre for Research on Introduced Marine Pests, and J. A. Phillips. I thank R. Hancock for the preparation of the photographic plate and the valuable and helpful comments on the manuscript from two reviewers.
References
- Arasaki , S , Tokuda , H and Fujiyama , K . 1955 . Reproduction and morphology in Codium fragile . Bot. Mag. (Tokyo) , 69 : 39 – 45 .
- Arista , M and Talavera , S . 1997 . Gender expression in Abies pinsapo Boiss, a Mediterranean fir . Ann. Bot. , 79 : 337 – 342 .
- Borden , CA and Stein , JR . 1969 . Reproduction and early development in Codium fragile (Suringar) Hariot: Chlorophyceae . Phycologia , 8 : 91 – 99 .
- Burr , FA and West , JA . 1970 . Light and electron microscope observations on the vegetative and reproductive structures of Bryopsis hypnoides . Phycologia , 9 : 17 – 37 .
- Ceccherelli , G and Cinelli , F . 1999 . The role of vegetative fragmentation in dispersal of the invasive alga Caulerpa taxifolia in the Mediterranean . Mar. Ecol. Prog. Ser. , 182 : 299 – 303 .
- Ceccherelli , G and Piazzi , L . 2001 . Dispersal of Caulerpa racemosa fragments in the Mediterranean: lack of detachment time effect on establishment . Bot. Mar , 44 : 209 – 213 .
- Charlesworth , D and Charlesworth , B . 1995 . Quantitative genetics in plants: The effect of a breeding system on genetic variability . Evolution , 49 : 911 – 920 .
- Choi , H-G and Lee , IK . 1996 . Mixed phase reproduction in Dasysiphonia chejuensis (Rhodophyta) from Korea . Phycologia , 35 : 9 – 18 .
- Clifton , KE . 1997 . Mass spawning by green algae on coral reefs . Science , 275 : 1116 – 1118 .
- Clifton , KE and Clifton , LM . 1999 . The phenology of sexual reproduction by green algae (Bryopsidales) on Caribbean coral reefs . J. Phycol , 35 : 24 – 34 .
- Dewreede , RE and Klinger , T . 1988 . “ Reproductive strategies in algae ” . In Plant Reproductive Ecology. Patterns and Strategies , Edited by: Doust , JL and Doust , LL . 267 – 284 . Oxford, UK : Oxford University Press .
- Dixon , PS . 1973 . The Biology of the Rhodophyta , Edinburgh, UK : Oliver and Boyd .
- Dorken , ME , Friedman , J and Barrett , SCH . 2002 . The evolution and maintenance of monoecy and dioecy in Sagittaria latifolia (Alismataceae) . Evolution , 56 : 31 – 41 .
- Dostal , R . 1929 . Sur la reproduction de Caulerpa . C. R. Acad. Sci. Paris , 189 : 493 – 494 .
- Eckert , CG . 2002 . The loss of sex in clonal plants . Evol. Ecol , 15 : 501 – 520 .
- Ellstrand , NC and Schierenback , KA . 2000 . Hybridization as a stimulus for the evolution of invasiveness in plants . Proc. Nat. Acad. Sci. USA , 97 : 7043 – 7050 .
- Ellstrand , NC and Schierenback , KA . 2006 . Hybridization between populations and species: Critical evolutionary change that creates an opportunity for increased invasiveness . Euphytica , 148 : 35 – 46 .
- Endean , R , Kenny , R and Stephenson , W . 1956 . The ecology and distribution of intertidal organisms on the rocky shores of the Queensland mainland . Aust. J. Mar. Freshwat. Res , 7 : 88 – 146 .
- Enomoto , S and Ohba , H . 1987 . Culture studies on Caulerpa (Caulerpales, Chlorophyceae). I. Reproduction and development of C. racemosa var. laetevirens . Jpn J. Phycol. , 35 : 167 – 177 .
- Fama , P , Jousson , O , Zanneti , L , Meinesz , A , Dini , R , Di Giuseppe , G , Millar , AJK and Pawlowski , J . 2002 . Genetic polymorphism in Caulerpa taxifolia (Ulvophyceae) chloroplast DNA revealed by a PCR-based assay of the invasive Mediterranean strain . J. Evol. Biol , 15 : 618 – 624 .
- Glasby , T , Creese , RG and Gibson , PT . 2005 . Experimental use of salt to control the invasive marine alga Caulerpa taxifolia in New South Wales, Australia . Biol. Conserv , 122 : 573 – 580 .
- Goldstein , M and Morrall , S . 1970 . Gametogenesis and fertilisation in Caulerpa . Ann. New York Acad. Sci. , 175 : 660 – 672 .
- Hartmann , M and Hämmerling , J . 1950 . Beiträge zur Kenntnis der Befruchtung und Sexualität mariner Algen . Pubblic. Statz. Zool. Napoli , 22 : 129 – 137 .
- Holmes , MJ and Brodie , J . 2004 . Morphology, seasonal phenology and observations on some aspects of the life history in culture of Porphyra dioica (Bangiales, Rhodophyta) from Devon, UK . Phycologia , 43 : 176 – 188 .
- Ishiwara , J , Hirose , H and Enomoto , S . 1981 . The life history of Caulerpa okamurai Weber van-Bosse . Proceedings of the 8th International Seaweed Symposium . 1981 . pp. 112 – 116 .
- Iyengar , MOP . 1940 . On the formation of gametes in Caulerpa . Indian Bot. Soc. , 19 : 191 – 195 .
- Johnson , PR and Neil , DT . 1998 . “ The corals of Moreton Bay: Living with extremes ” . In Moreton Bay and Catchment , Edited by: Tibbetts , IR , Hall , NJ and Dennison , WC . 503 – 524 . Brisbane, , Australia : School of Marine Science, University of Queensland .
- Kajimura , M . 1977 . On dioecious and isogamous reproduction of Caulerpa scalpelliformis (R.Br.) Ag. var. denticulata (Decsn.) Weber-van Bosse from the Oki Islands, Shimane Prefecture . Bull. Jpn Soc. Phycol. , 25 : 27 – 33 .
- Meinesz , A . 1979 . Contribution à l’etude de Caulerpa prolifera (Forsskål) Lamouroux (Chlorophycée, Caulerpale). II La reproduction sexée sur la cotes occidentales de la Méditerranée . Bot. Mar. , 22 : 117 – 122 .
- Meinesz , A and Hesse , B . 1991 . Introduction et invasion de l’algue tropicale Caulerpa taxifolia en Méditerranéan nord-occidente . Oceanol. Acta , 14 : 415 – 426 .
- Meusnier , I , Olsen , JL , Stam , WT , Destombe , C and Valero , M . 2001 . Phylogenetic analyses of Caulerpa taxifolia (Chlorophyta) and of its associated bacterial microflora provides clues to the origin to the Mediterranean introduction . Mol. Ecol , 10 : 931 – 946 .
- Meusnier , I , Valero , M , Destombe , C , Gode , C , Desmarais , E , Bonhomme , F , Stam , WT and Olsen , JL . 2002 . PCR-SSCP analyses of nuclear and chloroplast DNA provide evidence for recombination, multiple introductions and nascent speciation in Caulerpa taxifolia complex . Mol. Ecol , 11 : 2317 – 2325 .
- Meusnier , I , Valero , M , Olsen , JL and Stam , WT . 2004 . Analysis of rDNA ITS1 indels in Caulerpa taxifolia (Chlorophyta) supports a derived, incipient species status for the invasive strain . Eur. J. Phycol , 39 : 83 – 92 .
- Miyake , K and Kunieda , H . 1937 . On sexual reproduction in Caulerpa . Cytologia , 8 : 205 – 207 .
- Murphy , N and Phillips , JA . 2003 . Invasive Macroalga Caulerpa Taxifolia in Australia – Need for Improved Diagnostic Tools to Manage the Problem , Canberra, , Australia : National Heritage Trust, Final Report to Environment Australia .
- Ohba , H , Nashima , H and Enomoto , S . 1992 . Culture studies on Caulerpa (Caulerpales, Chlorophyceae). III. Reproduction, development and morphological variation of laboratory-cultured C. racemosa var. peltata . Bot. Mag. Tokyo , 105 : 589 – 600 .
- Olsen , JL . 1997 . Caulerpa taxifolia in the Mediterranean: Damage control and opportunities for new research . J. Phycol. , 33 : 1068 – 1069 .
- Panayotidis , P and Zuljevic , A . 2001 . Sexual reproduction of the invasive green alga Caulerpa racemosa var. occidentalis in the Mediterranean Sea . Oceanol. Acta , 24 : 199 – 203 .
- Phillips , JA and Price , IR . 2002 . How different is Mediterranean Caulerpa taxifolia (Caulerpales, Chlorophyta) to other populations of the species? . Mar. Ecol. Prog. Ser , 238 : 61 – 71 .
- Price , IR . 1972 . Zygote development in Caulerpa (Chlorophyta, Caulerpales) . Phycologia , 11 : 217 – 218 .
- Renoncourt , L and Meinesz , A . 2002 . Formation of propagules on an invasive strain of Caulerpa racemosa (Chlorophyta) in the Mediterranean Sea . Phycologia , 41 : 533 – 535 .
- Richards , AJ . 1997 . Plant Breeding Systems , London, UK : Chapman & Hall .
- Richardson , JP . 1982 . Life history of Bryopsis plumosa (Hudson) Agardh (Chlorophyceae) in North Carolina, USA . Bot. Mar , 25 : 177 – 183 .
- Rietema , H . 1971 . Life history studies in the genus Bryopsis (Chlorophyceae) iv. Life histories in Bryopsis hypnoides from different points along European coasts . Acta Bot. Neerl , 20 : 291 – 298 .
- Rietema , H . 1975 . Comparative Investigations on the Life Histories and Reproduction in some Species in the Siphonous Green Algal Genera Bryopsis and Derbesia , Groningen, , The Netherlands : Rijksuniversitiet Te Groningen Verenigde Reproduktie Bedrijven .
- Ruesink , JF and Collado-Vides , L . 2006 . Modelling the increase and control of Caulerpa taxifolia, an invasive marine macroalga . Biological Invasions , 8 : 309 – 325 .
- Sant , N , Delgado , O , Rodriguez-Prieto , C and Ballesteros , E . 1996 . The spreading of the introduced seaweed Caulerpa taxifolia (Vahl) C. Agardh in the Mediterranean Sea: Testing the boat transportation hypothesis . Bot. Mar. , 39 : 427 – 430 .
- Sarkissian , TS , Barrett , SCH and Harder , LD . 2001 . Gender variation in Sagittaria latifolia (Alismataceae): Is size all that matters? . Ecology , 82 : 360 – 373 .
- Schaffelke , B , Murphy , N and Uthicke , S . 2002 . Using genetic techniques to investigate the sources of the invasive alga Caulerpa taxifolia in three new locations in Australia . Mar. Poll. Bull. , 44 : 204 – 210 .
- Silander , JA . 1985 . “ Microevolution in clonal plants ” . In Population Biology and Evolution of Clonal Organisms , Edited by: Jackson , JBC , Buss , LW and Cook , RE . 107 – 152 . New Haven, , USA : Yale University Press .
- Smith , CM and Walters , LJ . 1999 . Fragmentation as a strategy for Caulerpa species: Fate of fragments and implications for management of an invasive weed . P.S.Z.N. Mar. Ecol. , 20 : 307 – 319 .
- West , JA . 1970 . A monoecious isolate of Rhodochorton purpureum . J. Phycol , 6 : 368 – 370 .
- Whittick , A and West , JA . 1979 . The life history of a monoecious species of Callithamnion (Rhodophyta, Ceramiaceae) in culture . Phycologia , 18 : 30 – 37 .
- Willson , MF . 1983 . Plant Reproductive Ecology , New York, , USA : John Wiley & Sons .
- Wilson , P , Buonopane , M and Allison , TD . 1996 . Reproductive biology of the monoecious clonal shrub Taxus canadensis . Bull. Torrey Bot. Club , 123 : 7 – 15 .
- Winkler , E and Fischer , M . 2002 . The role of vegetative spread and seed dispersal for optimal life histories of clonal plants: a simulation study . Evol. Ecol , 15 : 281 – 301 .
- Wright , JT . 2005 . Differences between native and invasive Caulerpa taxifolia: a link between asexual fragmentation and abundance in invasive populations . Mar. Biol. , 147 : 559 – 569 .
- Wright , JT and Davis , AR . 2006 . Demographic feedback between clonal growth and fragmentation in an invasive seaweed . Ecology , 87 : 1744 – 1754 .
- Zuljevic , A and Antolic , B . 2000 . Synchronous release of male gametes of Caulerpa taxifolia (Caulerpales, Chlorophyta) in the Mediterranean Sea . Phycologia , 39 : 157 – 159 .