Abstract
Three species of Actinotrichia from Taiwan (Galaxauraceae, Rhodophyta) are described on the basis of morphological and molecular evidence, including A. fragilis, the generitype, A. robusta, a new record for Taiwan, and A. taiwanica sp. nov. Actinotrichia fragilis can be distinguished from the other two species by having a wider branching angle (usually > 40°), persistent and distinctly whorled assimilatory filaments and paraphyses issuing from the pericarp, which do not intermix with the gonimoblasts. Actinotrichia robusta can be distinguished from A. taiwanica by its narrower branches, thinner cortex, more obvious whorled assimilatory filaments and smaller fusion cell that is usually indistinguishable in the mature cystocarp. Recognition of these three species was also supported by rbcL sequence analysis. While A. fragilis is distributed in the pantropical regions of the Indian and Pacific oceans, A. robusta and A. taiwanica sp. nov. are only found along the Kuroshio Current in the northwestern Pacific Ocean.
Introduction
To date, four genera have been delineated and re-assessed within the Galaxauraceae: Actinotrichia Decaisne, Dichotomaria Lamarck, Galaxaura Lamouroux and Tricleocarpa Huisman et Borowitzka (Huisman et al., Citation2004; Wang et al., Citation2005; Huisman, Citation2006). Actinotrichia was originally erected by Decaisne (Citation1842) based on Galaxaura rigida Lamouroux (Citation1816). The species is currently known as A. fragilis (Forsskål) Børgesen (Citation1932) based on the earliest available name Fucus fragilis Forsskål (Citation1775). The genus can be distinguished from other members of the Galaxauraceae by its isomorphic life cycle, the presence of persistent assimilatory filaments that form rings on the thallus surface, the presence of only two sterile branches issuing from the hypogynous cell and the presence of paraphyses enclosed within a well-defined pericarp (Decaisne, Citation1842; Weber-van Bosse, Citation1921; Tseng, Citation1941; Svedelius, Citation1952; Wang & Chiang, Citation2001). The recognition of Actinotrichia as a distinct genus was further supported by molecular evidence using DNA sequence analysis (Huisman et al., Citation2004; Wang et al., Citation2005).
So far, only two species of Actinotrichia have been documented. Actinotrichia fragilis was originally described from Yemen, while A. robusta (Itono, Citation1979) was first described from, and is currently restricted to the Ryukyu Islands in southwestern Japan. Actinotrichia fragilis is widely distributed throughout the Indian Ocean (Silva et al., Citation1996) and in the warmer waters of Australia, Southeast Asia and the Hawaiian Islands (Cordero, Citation1975; Abbott, Citation1999). Only A. fragilis has been documented from Taiwan (Lewis & Norris, Citation1987). Although the development of reproductive structures in A. fragilis is well known, detailed reproductive information for A. robusta is still lacking (Decaisne, Citation1842; Weber-van Bosse, Citation1921; Børgesen, Citation1932; Tseng, Citation1941; Svedelius, Citation1952; Itono, Citation1979; Wang & Chiang, Citation2001). As it is difficult to distinguish cryptic species and morphologically similar species without the help of molecular tools (Zuccarello et al., Citation2002; Zuccarello & West, Citation2003; Saunders, Citation2005), a comprehensive study on the systematics of the genus Actinotrichia using detailed morphological observations and molecular tools should allow us to re-evaluate the biodiversity and taxonomy of this genus. The objective of this study was therefore to present an updated taxonomic account of the genus in Taiwan using detail morphological observations and rbcL sequence analysis.
Materials and methods
Collections were made by snorkeling or SCUBA diving. Immediately after collection, small portions of algal samples were preserved in 95% alcohol for molecular analysis while other portions were fixed in 5–10% formalin/seawater solution and then pressed as dried herbarium samples for morphological examination. Voucher specimens were deposited in the Department of Biology, National Changhua University of Education, Taiwan. Herbarium abbreviations follow Holmgren et al. (Citation1990). For morphological examination, distal branch portions were decalcified in 1–3% HCl solution and either squashed or sectioned by hand or using a Leica CM1850 freezing microtome set at 20–40 µm thickness. Squashed material and sections were either stained with 1% aniline blue and mounted using 50% Karo corn syrup or else treated with Wittmann's aceto-hematoxylin-chloral hydrate (Wittmann, Citation1965) and mounted in 50% Hoyer's mounting medium (Wang et al., Citation2005). Photomicrographs were taken with a Pixera Pro600ES (Tokyo, Japan) digital camera mounted on an Olympus BX51 light microscope. Drawings of female reproductive structures in A. fragilis were made with the aid of a camera lucida based on specimens from this study and specimen slides with fertilised carpogonial branches used by Wang & Chiang (Citation2001) (Specimen number: #SLL NW-04-18-1991-1).
DNA extraction, amplification and sequencing of the rbcL gene follows the description of Wang et al. (Citation2005). Newly generated rbcL sequences are included in . The rbcL alignment initially included 1467 sites, because information was missing for the 5′ end of many sequences and the first 85 sites were excluded from the analyses. Phylogenetic analyses were performed using Maximum Parsimony (MP) and Maximum Likelihood (ML) methods available in the computer programs PAUP* v.4.0 beta 10 (Swofford, 2003). A set of sequences from 18 representative taxa belonging to the Galaxauraceae and Liagoraceae were selected as the ingroup, with two members of the order Acrochaetiales serving as the outgroup (). In the MP analysis, heuristic searches were performed consisting of 500 random stepwise additions, MULPARS (holding only five trees at each step) and a Tree-Bisection-Reconnection (TBR) swapping algorithm until swapping was complete. Consistency (CI) and retention (RI) indices were calculated, excluding uninformative characters (Kluge & Farris, Citation1989). Prior to ML analysis, the nucleotide substitution model for our sequence dataset was tested by MODELTEST (Posada & Crandall, Citation1998). GTR + I + G (general time reversible + proportion of invariable sites + variable site gamma distribution) were selected as optimal settings for the ML analysis. The parameters were as follows: assumed base frequency: A = 0.3120, C = 0.1328, G = 0.2151 and T = 0.3401; substitution model rate matrix with A–C: 2.0372, A–G: 4.0733, A–T: 2.4361, C–G: 0.6629, C–T: 15.6929 and G–T: 1.0000; proportion of invariable sites [I]: 0.5234; variable site [G] gamma distribution shape parameter: 1.2623. Support for nodes was determined by the bootstrap method (Felsenstein, Citation1985) using MP (1000 bootstrap replicates) and ML (100 bootstrap replicates).
Table 1. List of species used in rbcL analysis and their GenBank accession numbers followed by the fraction (%) in sequence.
Results
Species description
Actinotrichia fragilis (Forsskål) Børgesen (Figs )
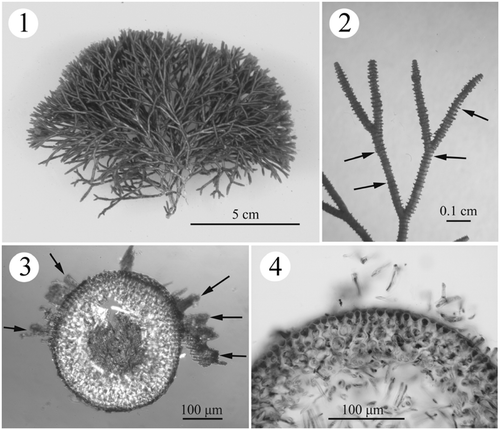
Figs. 1–4. Habit and development of vegetative structures of Actinotrichia fragilis (#SLL WLT-04-13-2002-1). Fig. 1. Cystocarpic plant. Fig. 2. Dichotomous branches showing the distinctly whorled assimilatory filaments (arrows). Fig. 3. Cross-section of branch showing assimilatory filaments arising from the outermost cortical cells (arrows). Fig. 4. Cross-section of branch showing three- to four-cell layered cortex and medullary portion.
BASIONYM: Fucus fragilis Forsskål, Citation1775: 190.
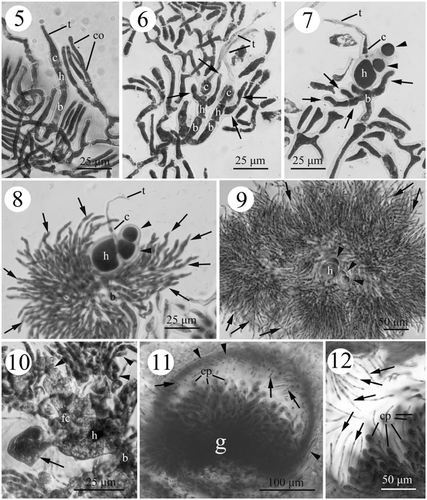
Figs. 5–12. Development of female structures in Actinotrichia fragilis (#SLL WLT-04-13-2002-1). Fig. 5. Initial three-celled carpogonial branch differentiated from the vegetative cortical filaments at the tip of the branch, and consisting of carpogonium, hypogynous and basal cell. An elongate trichogyne is produced from the carpogonium. Fig. 6. Developing three-celled young carpogonial branches consisting of an elongate trichogyne, carpogonium, hypogynous cell, and basal cell showing the production of sterile branches (arrows) from the hypogynous cell. Fig. 7. Sterile branches (arrowheads) derived from the enlarging hypogynous cell (h) with some sterile filaments (arrows) produced from the upper portion of the basal cell. Fig. 8. Further development of the carpogonial branch showing the extension of sterile filaments from the basal cell developing more filaments (arrows). The sterile branches from the hypogynous cell do not develop further. Fig. 9. Sterile filaments (arrows) from the basal cell developing to surround the sterile branches (arrowheads) issued from the hypogynous cell. Fig. 10. Two to four inner gonimoblast cells and the gonimoblast initial cell fusing to form a small fusion cell. Pit connections are visible between the sterile branch (arrow) and the hypogynous cell. The hypogynous cell and the basal cell broaden, but do not fuse. Distal secondary gonimoblast filaments (arrowheads) do not involve the formation of fusion cell. Fig. 11. Cross-section of mature cystocarp showing the centre gonimoblasts that are surrounded by the pericarp (arrowheads). The paraphyses (arrows) derived from the pericarp projecting into the cystocarp cavity. The gonimoblast filaments do not intermix with the paraphyses. Fig. 12. Mature cystocarp showing the paraphyses (arrows) in greater detail. Abbreviations: b, basal cell; c, carpogonium; co, cortical cell; cp, carpospore; fc, fusion cell; g, gonimoblast; h, hypogynous cell; t, trichogyne.
SYNONYMS: See Silva et al. (Citation1996) for detailed synonymy.
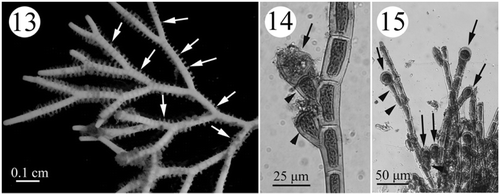
Figs. 13–15. Development of tetrasporangial structures in Actinotrichia fragilis (#SLL HK-07-18-2002-1). Fig. 13. Branches of a tetrasporic plant showing the whorled assimilatory filaments (arrows) where the tetrasporangia develop. Fig. 14. A tetrasporangium-producing (arrow) tetrasporangial mother stalk cell (arrowheads) produced laterally from the assimilatory filament. Fig. 15. Tetrasporangia (arrows) are produced terminally or laterally from assimilatory filaments. Distinct one- to two-celled stalks of lateral tetrasporangial mother cells (arrowheads) are visible.
TYPE LOCALITY: Mokha, Yemen.
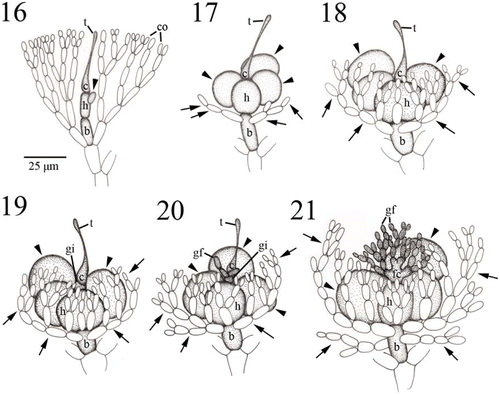
Figs. 16–21. Development of carpogonial branch and gonimoblast in Actinotrichia fragilis (Figs 16–18: #SLL WLT-04-12-2002-1; Figs 19–21: #SLL NW-04-18-1991-1). Fig. 16. Initial carpogonial branch, consisting of a carpogonium with trichogyne, a hypogynous cell, and a basal cell, produced between vegetative cortical filaments. Arrowhead indicates a sterile cell produced from the hypogynous cell. Fig. 17. Developing three-celled young carpogonial branch consisting of an elongate trichogyne, carpogonium, hypogynous cell, and basal cell showing the sterile branches (arrowheads) from the hypogynous cell, and sterile filaments (arrows) from the upper portion of the basal cell. Fig. 18. Mature three-celled young carpogonial branch consisting of an elongate trichogyne, carpogonium, hypogynous cell, and basal cell showing the sterile branches (arrowheads) from the hypogynous cell, and sterile filaments (arrows) from the upper portion of the basal cell. Noted that more secondary sterile filaments are produced from primary sterile filaments on the basal cell. Fig. 19. Fertilised carpogonial branch showing carpogonium divided into two cells, a carpogonium and the gonimoblast initial. At this stage, the enlarged hypogynous cell still consists of two enlarged sterile branches (arrowheads), a one-celled sterile branch and two-celled sterile branch. Fertilised carpogonium and hypogynous cell with its derivatives are surrounded by numerous sterile filaments from the basal cell (arrows). Fig. 20. Further development of fertilised carpogonial branch showing gonimoblast filaments produced from the gonimoblast initial, carpogonium with an elongate trichogyne, hypogynous cell with sterile branches (arrowheads), and numerous sterile filaments (arrows) from the basal cell. Fig. 21. Gonimoblast initial and two to four gonimoblast cells in the basal part of the gonimoblast are incorporated into the formation of a fusion cell, producing numerous gonimoblast filaments. Note that the hypogynous cell does not involve the formation of a fusion cell and its sterile branches (arrowheads) remain distinct. Gonimoblast filaments, fusion cell, and hypogynous cell with its derivatives are surrounded by numerous sterile filaments from the basal cell (arrows). Abbreviations: b, basal cell; c, carpogonium; co, cortical cell; gf, gonimoblast filament; gi, gonimoblast initial; h, hypogynous cell; t, trichogyne.
GEOGRAPHIC DISTRIBUTION: Throughout the Indian Ocean, the Philippines, Taiwan and tropical Australia.
Figs. 22–28. Habit and development of vegetative (#SLL CP-07-21-2002-1) and male structures (#SLL SR-07-22-2002-1) in Actinotrichia taiwanica sp. nov. Fig. 22. Holotype, cystocarpic plant. Fig. 23. Dichotomous branches showing indistinct assimilatory filaments (arrows). Fig. 24. Cross-section of branch. Fig. 25. Cross-section of branch showing details of the cortex and medulla structures. Fig. 26. Spermatangial initial branch arising from the cortical filaments on the tip of a branch producing several primary spermatangial filaments (arrows). Fig. 27. Young male structures showing abundant secondary spermatangial filaments (arrows). Fig. 28. Cross-section of spermatangial conceptacle showing the conceptacle wall (arrows) consisting of the primary spermatangial filaments and numerous secondary spermatangial filaments projecting into the conceptacle cavity (arrowheads). Abbreviation: spb, spermatangial branch.

HABITAT AND SEASONALITY: The collections were made in March, April, May and July. Plants were attached to reef rocks at depths of 1–10 m.
Figs. 29–36. Development of female structures in Actinotrichia taiwanica sp. nov. (#SLL CP-07-21-2002-1). Fig. 29. Initial carpogonial branch produced from the vegetative cortical filaments on the tip of a branch. Fig. 30. Sterile branches produced from the hypogynous cell. Fig. 31. Initials of sterile filaments from the upper portion of the basal cell. Fig. 32. Cell size and nucleus enlarge in the hypogynous cell and its derivatives (arrowheads) but these cells do not develop further. The sterile filaments from the basal cell producing more filaments (arrows). Fig. 33. Well-developed sterile filaments (arrows) from the basal cell surrounding the hypogynous cell and its derivatives (arrowhead). Fig. 34. Two to four inner gonimoblast cells and gonimoblast initial cell fusing to form a small fusion cell. The pit plug is visible between the gonimoblast initial, hypogynous cell, and its derivatives (arrowheads). The basal cell has broadened but is not fused. Fig. 35. Cross-section of mature cystocarp showing the pericarp (arrowheads) and paraphyses (arrow). Fig. 36. Cystocarp showing the intermixing of gonimoblast filaments and paraphyses (arrows). Abbreviations: b, basal cel; c, carpogonium; co, cortical cell; cp, carpospore; fc, fusion cell; h, hypogynous cell; t, trichogyne.

SPECIMENS EXAMINED: Kenting National Park, Taiwan: (1) Nanwan, coll. W.-L. Wang, 18.iv.1991 (female, #SLL NW-04-18-1991-1); (2) Wanlitung, coll. S.-L. Liu, 13.iv.2002 (female, #SLL WLT-04-13-2002-1), coll. S.-L. Liu, 23.vii.2002 (sterile, #SLL WLT-07-23-2002-1), coll. S.-L. Liu, 3.v.2003 (sterile, #SLL WLT-05-03-2003-1); (3) Haikou, coll. S.-L. Liu, 18.xii.2002 (tetrasporic, #SLL HK-07-18-2002-1); 3rd Nuclear Power Plant Outlet, coll. S.-L. Liu and S.-M. Lin, 27.iii.2004 (sterile, #SLL NPPO-03-27-2004-1).
Figs. 37–39. Development of tetrasporangial structures in Actinotrichia taiwanica sp. nov. (#SLL CP-05-11-2002-1). Fig. 37. Branches showing the assimilatory filaments (arrows) where the tetrasporangia are borne. Fig. 38. Young tetrasporangia (arrows) produced terminally from the assimilatory filaments. Fig. 39. Mature tetrasporangia dividing in a cruciate manner (arrow).
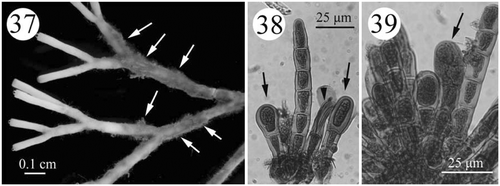
EXTERNAL MORPHOLOGY: Thalli are erect, up to 10 cm tall (), arising from a discoid holdfast, about 0.5 cm in diameter, orange to dark red in colour. The branches are regularly dichotomous, internodes 3–7 mm in length and 400–650 µm wide, branching angles between 40–60°, with distinct whorled assimilatory filaments (). The thalli are filled with numerous calcified crystals in the cortical region, extending into the external portion of medulla, resulting in a very stiff texture. The tetrasporophyte resembles the gametophyte.
Fig. 40. Young carposporophyte of Actinotrichia taiwanica sp. nov. (#SLL CP-07-21-2002-1). Two to four basal gonimoblast cells are incorporated in the formation of a fusion cell. Sterile branches derived from the hypogynous cell (arrowheads) retain their distinct shape. Involucral filaments (paraphyses; arrows) produced by the basal cell intermingle with the gonimoblast filaments. Abbreviations: b, basal cell; fc, fusion cell; h, hypogynous cell; gf, gonimoblast filament.
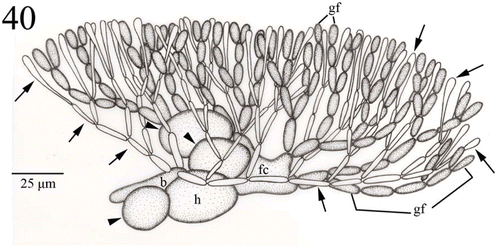
ANATOMICAL STRUCTURE: Growth is apical, initiated by a group of tiny meristematic cells located in the centre of the rounded branch apex. Thalli are multiaxial, composed of a cellular to filamentous cortex and a filamentous medulla (). The cortex is three or four cell layers throughout the thalli (), except for the reproductive regions. Cortex architecture is the same between gametophytes and the tetrasporophyte. The thickness of cortex ranges from 65 to 85 µm. The cells in the outermost cortical layer are tightly arranged (10–15 µm long by 10–15 µm wide) and the innermost layer is composed of oblong cells (25–38 µm wide by 25–38 µm long) (). In some portions of the epidermis, five- to fifteen-celled assimilatory filaments arise from the outermost epidermal cell and form successive whorls on the surface of thallus (). The epidermal cells have four to six sides, are angular in surface view in both the gametophyte and tetrasporophyte and each cell contains one well-developed, stellate chromatophore with a large pyrenoid in the centre.
Figs. 41–47. Habit and development of vegetative (#SLL NPPO-08-11-2001-1) and male structures (#SLL BB-10-01-2002-1) in Actinotrichia robusta. Fig. 41. Cystocarpic plant. Fig. 42. Dichotomous branches showing an indistinct ring of assimilatory filaments (arrows). Fig. 43. Cross-section of branch. Fig. 44. Cross-section of branch showing the cortex and medulla structures in greater detail. Fig. 45. Spermatangial initial branch arising from the cortical filaments on the tip of a branch producing several primary spermatangial filaments (arrows). Fig. 46. Mature male structures showing abundant secondary spermatangial filaments (arrows). Fig. 47. Cross-section of a spermatangial conceptacle showing the conceptacle wall (arrows) formed of primary spermatangial filaments with numerous secondary spermatangial filaments projecting into the conceptacle cavity (arrowheads). Abbreviations: co, cortical cell; spb, spermatangial branch.
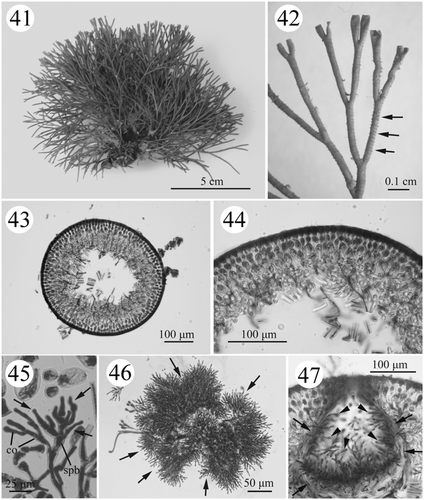
REPRODUCTIVE STRUCTURE: All reproductive structures are scattered between the layers of the cortex and medulla. The gametophytes are dioecious and isomorphic with the tetrasporic thallus. Spermatangia are formed within a conceptacle (see Wang & Chiang, Citation2001) but were not observed in the present study.
Figs. 48–54. Development of female structures in Actinotrichia robusta (#SLL NPPO-08-11-2001-1). Fig. 48. Carpogonial initial branch derived from the vegetative cortical filaments on the tip of a branch. Fig. 49. Young carpogonial branch showing the production of sterile branches (arrows) from the hypogynous cell. Fig. 50. Sterile filaments (arrows) produced from the upper portion of the basal cell. The sterile branches (arrowheads) from the hypogynous cell do not divide further. Fig. 51. The hypogynous cell and its derivatives (arrowheads) enlarge but do not develop further, while the sterile filaments (arrows) from the basal cell produce more filaments. Fig. 52. The sterile filaments (arrows) from the basal cell producing abundant filaments that surround the sterile branches (arrowheads) from the hypogynous cell. Fig. 53. Cross-section of a mature cystocarp with the pericarp (arrowheads) showing the intermixing of gonimoblast filaments and paraphyses (arrows). Fig. 54. Cystocarp showing details of the gonimoblast filaments and paraphyses (arrows). Abbreviations: b, basal cell; c, carpogonium; co, cortical cell; cp, carpospore; h, hypogynous cell; t, trichogyne.
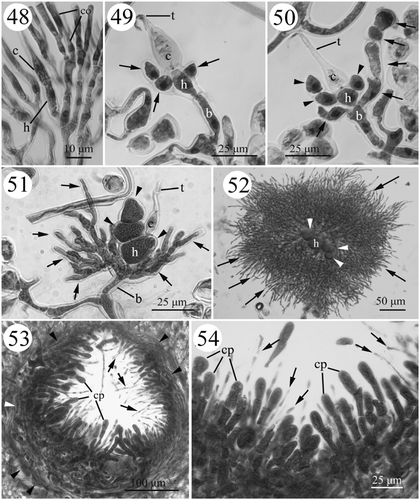
Cystocarps are commonly found in the distal part of the thallus, except the internodal areas. Carpogonial branches are initiated in the place of an ordinary filament () or sometimes issue between the dichotomously branched ordinary filaments towards the apex of a cylindrical branch (). Young carpogonial branches are three-celled, consisting of a flask-shaped carpogonium with a long trichogyne, a cylindrical hypogynous cell and a basal cell (, ">). As the carpogonial branch matures, the hypogynous cell cuts off an initial of the first sterile branch on one side and then produces an initial of a second sterile branch on the opposite side (). Subsequently, the first sterile branch cuts off one cell to form two branches (a one-celled branch and a two-celled branch) on the hypogynous cell (). Occasionally, one to two two-celled branches were observed on the hypogynous cell (). During the development of sterile branches on the hypogynous cell, four to five involucral filaments are simultaneously produced from the basal cell prior to fertilisation (, ).
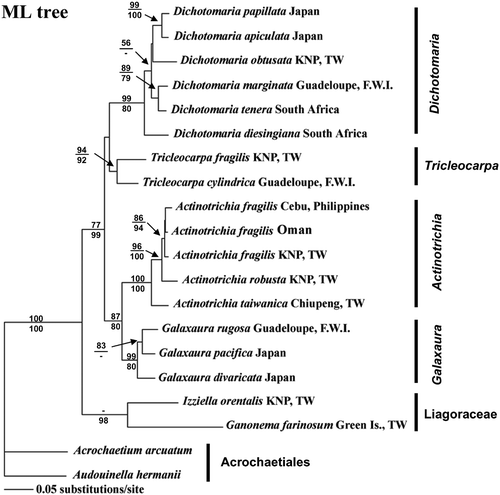
Fig. 55. Phylogenetic tree inferred from the ML analysis showing the MP and ML bootstrap values above and below the nodes of tree, respectively.
A fertilisation event was not seen in our material. However, the slide used by Wang and Chiang (Citation2001) was re-examined for the development of the fertilisation process. Following presumed fertilisation, the fertilised carpogonium divides transversely into two cells (). The lower cell functions as the gonimoblast initial, cutting young, primary gonimoblast cells distally and laterally (). While the nuclei of the hypogynous cell and its derived sterile branches enlarge and become darkly stained, the nuclei of the cells of the involucral filaments derived from the basal cell do not enlarge but remain relatively small (Figs , ). At an early stage of development, pit-connections between the basal gonimoblast cell, the hypogynous cell and the basal cell broaden and the involucral filaments derived from the basal cell divide extensively to form a pericarp surrounding the gonimoblast filaments (, ) while the sterile branches from the hypogynous cell stop dividing but remain distinct throughout cystocarp maturation (, ). Gonimoblasts located in the centre of the cystocarp and numerous paraphyses from the pericarp protrude into the cystocarp cavity (). As cystocarp development progresses, secondary gonimoblast filaments are cut off from the primary gonimoblast filaments inside the cystocarp cavity, and the pit connections linking two to four inner gonimoblast cells to the basal gonimoblast initial break down to form a relatively small, multinucleate fusion cell (Figs , ). Mature cystocarps are hemispherical, 250–400 µm in diameter (). Carposporangia are cut off terminally from the secondary gonimoblast filaments and are oval to obovate in shape, 10–18 µm wide by 20–30 µm long (). New carposporangia are sometimes produced from within old carposporangial walls.
Tetrasporangia (21–27 µm wide by 26–30 µm long) are produced terminally or laterally from the whorled assimilatory filaments (). After maturation, four tetraspores form in a cruciate manner.
Actinotrichia taiwanica sp. nov., S.L. Liu et W.L. Wang (Figs )
DIAGNOSIS: Planta ad 7.5 cm alti; rosacei, grave calcarei; dichotome ramose, ad intervalla 4–7 mm, ad angulum 40°; ex hapteronibus parvis discoideisque exorientes. Axes teretes, ad 1000 μm diam.; constatus cellulosus-filamentous cortex et filamentous medulla. Dioici. Spermantangial conceptaculum hemisphaericus, 250–300 μm diam., filis ramosis 1–3 spermatangia terminaliter. Spermatangia 3–8 μm diam. Rami carpogoniales 3 cellulares, includens carpogonium, cellula hypogynous, et cellula basale. Post-fecundationem carpogonium fila gonimoblastorum producentes. Gonimoblastus moderate diffusus, 250–400 μm diam., filis ramosis carposporangia terminaliter. Carposporangia obovoidea-ovoidea, 10–15 × 25–33 μm. Conjunctio-cellula formata, cellulas gonimoblastas infernas et carpogonium, et cellulas hypogynous includens. Fila sterilia post-fecundationem ex cellula basale rami carpogoniales, filis gonimoblastis intermixta et involucrum. Tetrasporangia 18–23 × 22–30 μm, ferens in cellulis apicalibus et subapicalibus filorum assimilantium.
Plant up to 7.5 cm high, pink, heavily calcified, dichotomously branched every 4–7 mm at an angle of 40° or less, arising from small discoid holdfasts. Axes terete, up to 1000 µm diam., composed of a cellular to filamentous cortex and filamentous medulla. Dioecious. Spermatangial conceptacle hemispherical, 250–300 µm diam., with branched filaments bearing one to three terminal spermatangia, 3–8 µm diam. Carpogonial branches three-celled, consisting of a carpogonium, a hypogynous and a basal cell. Fertilised carpogonium producing gonimoblast filaments directly. Gonimoblast moderately diffuse, 250–400 µm diam., branched, bearing carposporangia terminally. Carposporangia obovoid to ovoid, 10–15 × 25–33 µm. Fusion cell formed by incorporating some proximal gonimoblast cells derived from a fertilised carpogonium. The hypogynous cell remaining distinct without participating in the formation of a fusion cell. Post-fertilisation sterile filaments issue from basal cell of carpogonial branch intermingling with gonimoblast filaments and forming an involucre. Tetrasporangia 18–23 × 22–30 µm, issuing apically from terminal cells and laterally from subapical cells of assimilatory filaments.
HOLOTYPE: Deposited in the Department of Biology, National Changhua University of Education (NCUE), July 21, 2002 (#SLL CP-07-21-2002-1) ().
ETYMOLOGY: “taiwanica” refers to the name of the country, Taiwan, where this new species was found.
TYPE LOCALITY: Chiupeng, Kenting National Park, Taiwan.
DISTRIBUTION: Only known from the southernmost and southeastern coasts of Taiwan.
HABITAT AND SEASONALITY: Collections were made in May, July, August and October. Plants were attached to reef rocks at depths of 1–3 m.
SPECIMENS EXAMINED: Ping Tung, Taiwan: (1) Sail Rock, coll. S.-L. Liu, 22.vii.2002 (male, #SLL SR-07-22-2002-1); (2) Feng Chwei Sha, coll. S.-L. Liu, 21.vii.2002 (tetrasporic, #SLL FCS-07-21-2002-1); (3) Chiupeng, coll. S.-L. Liu, 11.v.2002 (tetrasporic, #SLL CP-05-11-2002-1), coll. S.-L. Liu and C.-S. Lin, 21.vii.2002 (female, #SLL CP-07-21-2002-1, Holotype); (4) Lung-Keng, coll. S.-M. Lin, 17.x.2002 (female, #SLL LKS-10-17-2002-1); Taitung, Taiwan: (1) Jyi Huen, coll. S.-L. Liu and W.-L. Wang, 26.viii.2002 (tetrasporic, #SLL JH-08-26-2002-1); (2) Shan Yuan, coll. S.-L. Liu and W. L. Wang, 27.viii.2002 (tetrasporic, #SLL-SY-07-27-2002-1).
EXTERNAL MORPHOLOGY: Thalli are erect, branched, up to 7.5 cm tall, pink to dark red in colour, have a discoid holdfast, and are 0.5–1 cm in diameter (). The branches are regularly dichotomous, internodes 4–7 mm in length, 600–1000 µm in width, branch angles < 40°, with few assimilatory filaments irregularly distributed around the thalli (). The thalli are calcified and stiff. The tetrasporophyte is morphologically similar to the gametophyte.
ANATOMICAL STRUCTURE: Growth is apical. Thalli consist of a cellular and filamentous cortex and a homogeneously filamentous medulla. Except in reproductive regions, the cortex is 3–4 cell layers thick (), in both gametophytes and tetrasporophytes, 90–150 µm thick. Cells are 8–15 µm long by 8–15 µm wide in the outermost layer and 25–38 µm wide by 38–55 µm long in the innermost layer (). Five- to ten-celled assimilatory filaments usually arise from the outermost epidermal cell and appear patchy on the surface. The surface view of the outermost epidermal cell agrees with that of the generitype.
REPRODUCTIVE STRUCTURE: All reproductive structures are located between cortex and medulla layers. Plants are dioecious. Spermatangial branch initials are derived from the apex of ordinary filaments () and subsequently divide laterally/transversely into primary spermatangial filaments (). They then form a hemispherical conceptacle, 250–300 µm in diameter (). Highly branched secondary spermatangial filaments are produced from the inner side of conceptacle and develop numerous spermatangial mother cells terminally. 1-2-(3) spermatangia, 3–8 µm in diameter, are usually cut off terminally from the spermantangial parent cell.
Cystocarps are frequently found in the distal part of the thallus, except for the internode areas. The structure and developmental pattern of carpogonial branches mostly agree with the generitype (). The three-celled carpogonial branch may replace one of the dichotomous cortical filaments or grow between them (). During cystocarp development, the hypogynous cell and its derivatives enlarge and cease development (). Numerous sterile filaments are derived from the basal cell and surround the carpogonium and hypogynous cell ().
Fertilisation was not seen in our specimen. After fertilisation, the involucral filaments derived from the basal cell form a pericarp surrounding the gonimoblast filaments () and then numerous secondary branched filaments (paraphyses) are formed which intermingle with the gonimoblast filaments (). During the development of the cystocarp, the pit connections that link the two to four inner gonimoblast cells to the basal gonimoblast initial break down to form a multinucleate fusion cell (Figs , ). The basal cell, the hypogynous cell and their derivatives do not participate in the formation of the fusion cell. Mature cystocarps are hemispherical, 250–400 µm in diameter (). Oval or obovate carposporangia, 10–15 µm wide by 25–33 µm long (), are cut off terminally from the secondary gonimoblast filaments. New carposporangia are sometimes produced from within old carposporangial walls.
Tetrasporangia (18–23 µm wide by 22–30 µm long) are produced from assimilatory filaments terminally () to form four cruciate tetraspores.
Actinotrichia robusta Itono (Figs )
BASIONYM: Actinotrichia robusta Itono, Citation1979: 138, figs .
TYPE LOCALITY: Yonaguni Island, Ryukyu Islands, Japan.
ADDITIONAL DISTRIBUTION: Southeastern and southernmost Taiwan, Orchid Island.
HABITAT AND SEASONALITY: Collections were made in January, March, April, August and October. Plants were attached on reef rocks at the depths of 1–3 m.
SPECIMENS EXAMINED: Kenting National Park, Taiwan: (1) Banana Bay, coll. S.-L. Liu and S.-M. Lin, 1.x.2002 (male, #SLL BB-10-01-2002-1); (2) 3rd Nuclear Power Plant outlet, coll. W.-L. Wang, 11.viii.2001 (female, #SLL NPPO-08-11-2001-1), coll. S.-L. Liu and S.-M. Lin, 4.i.2003 (female, #SLL NPPO-01-04-2003-1), coll. S.-L. Liu and S.-M. Lin, 13.iii.2003 (female, #SLL NPPO-03-13-2003); (3) Small Port, coll. S.-L. Liu and S.-M. Lin, 1.iv.2003 (female, #SLL SP-04-01-2003-1); Orchid Island, Taiwan: (1) Hungtou Village, coll. S.-L. Liu and S.-M. Lin, 8.iv.2003 (female, #SLL HV-04-08-2003-1; male, #SLL HV-04-08-2003-2).
EXTERNAL MORPHOLOGY: Thalli are erect, up to 8 cm tall, with a discoid holdfast, 0.5–1 cm in diameter () and orange to dark red in colour. The branches are regularly dichotomous, internodes 3–6 mm in length, 400–600 µm in width, branch angle < 40°, with indistinct whorled assimilatory filaments (). The thalli are calcified and stiff within the cortex and the outer medulla. The tetrasporophyte is morphologically similar to gametophyte.
ANATOMICAL STRUCTURE: Growth is apical. Thalli are multiaxial, composed of a cellular to filamentous cortex and a filamentous medulla. The cortex is three to four cell layers throughout (), 65–100 µm thick, in both gametophytes and tetrasporophytes. Cells of the outermost layer are 10–15 µm long and wide, and the cells of innermost layer are 20–25 µm wide by 25–35 µm long (). The five- to ten-celled assimilatory filaments arise from some outermost epidermal cells and form whorls on the surface. The surface view of epidermal cells in both the gametophyte and tetrasporophyte mostly agrees with the generic description.
REPRODUCTIVE STRUCTURE: Plants are dioecious. Spermatangial branch initials cut off from an ordinary filament () near the apex of branch, subsequently divide into primary spermatangial filaments (), and then develop into a hemispherical conceptacle, which is 250–300 µm in diameter (). Highly branched secondary spermatangial filaments are produced from the inner side of the conceptacle, towards the central cavity, and cut off spermatangial mother cells terminally. There are 1-2-(3) spermatangia, 3–8 µm in diameter, terminally divided from the spermantial parent cell.
Except for the areas near the internodes, cystocarps are found in the upper part of thallus. Structures and development of carpogonial branches are in agreement with generic description (). The three-celled carpogonial branch consists of a carpogonium, the hypogynous cell and the basal cell (). The hypogynous cell produces a one-celled branch and a two-celled branch while the basal cell produces numerous sterile filaments (). The hypogynous cell and its derivates enlarge during development, but the basal cell and its derivates remain small ().
Immediate post-fertilisation events were not seen. The nuclei of the hypogynous cell and its derived sterile branches enlarge while the nuclei of the involucral filaments from the basal cell remain small (). At an early stage of development, pit-connections between the basal gonimoblast cell, the hypogynous cell and the basal cell broaden, and the involucral filaments from the basal cell form a pericarp surrounding the gonimoblast filaments (). The sterile branches from the hypogynous cell, however, stop developing (). Gonimoblast filaments intermix with the pericarp filaments (paraphyses) (). The fusion cell of gonimoblast is derived from the two to four inner gonimoblast cells and indistinct. Mature cystocarps are hemispherical, 250–400 µm in diameter (). Carposporangia are cut off terminally from the secondary gonimoblast filaments, 10–20 µm wide by 20–40 µm long (). Development of female structure resembles that of A. taiwanica.
Tetrasporangia were not observed in this study. Based on the observations of Itono (Citation1979), tetrasporangia were terminally produced from the assimilatory filament and divide cruciately.
Molecular analyses
Because the topologies derived from MP and ML analyses are largely identical, only the ML tree inferred from the GTR + I + G evolutionary model is presented in this study (). For MP analysis, the eight most parsimonious trees were found (Tree length = 1,057, consistency index (CI) = 0.5885, HI = 0.4115, retention index (RI) = 0.6411, and 329 informative characters for the entire rbcL dataset). Bootstrap values from MP (1000 replicates) and ML analysis (100 replicates) were shown above and below the nodes of the tree. With Acrochaetium arcuatum and Audouinella hermannii from the order Acrochaetiales used as outgroups, a monophyletic clade was determined for the Nemaliales with 100% bootstrap support (), which consists of two families, Galaxauraceae and Liagoraceae. The Liagoraceae forms the basal lineage. Four strongly supported clades representing the four genera currently recognised within the Galaxauraceae were found as follows: a Dichotomaria clade, a Tricleocarpa clade, a Galaxaura clade, and an Actinotrichia clade. Phylogenetic relationships between the Actinotrichia/Galaxara complex and the other two genera however remain unresolved. The Actinotrichia clade consists of three species, A. fragilis, A. robusta, and A. taiwanica sp. nov. The phylogenetic relationship between A. fragilis from the Philippines, Taiwan and Oman, on the other hand, remains unresolved. The rbcL sequence divergence within Actinotrichia ranged from 1.1% to 6.2%. Actinotrichia fragilis from the Philippines, Oman and Taiwan exhibited pair-wise differences ranging from 1.1% to 1.7%. Actinotrichia robusta from Taiwan showed some divergence variation of the rbcL sequence ranging from 3% to 3.7% compared with A. fragilis from the Philippines (3.7%), Oman (3%) and Taiwan (3.5%). Actinotrichia taiwanica sp. nov. from Taiwan also showed some variation with percentage divergence pair-wise distances ranging from 4.8% to 6.2% in comparison with A. fragilis (4.8%) and A. robusta (6.2%).
Discussion
Based on molecular evidence, Huisman et al. (Citation2004) recently elucidated the systematic status of calcified genera within the Galaxauraceae and reinstated the genus Dichotomaria for some species, including D. diesingiana (Zanardini) Huisman, Harper et Saunders, D. obtusata (Ellis et Solander) Huisman, Harper et Saunders and D. marginata (Ellis et Solander) Huisman, Harper et Saunders, which were originally assigned to the genus Galaxaura. Their results show that the family Galaxauraceae consists of four genera, namely Actinotrichia, Dichotomaria, Galaxaura and Tricleocarpa. They can be easily distinguished by their peculiar life history and cystocarp development.
Comparing morphological features among these four calcified galaxauraceous genera, Actinotrichia differs from the other three genera in having a pericarp with enclosed paraphyses and an isomorphic life history (Wang et al., Citation2005). Dichotomaria has an isomorphic life history with different cortical structures between the tetrasporophyte and gametophyte, and although pericarps are present, paraphyses are lacking. In contrast, Galaxaura has a dimorphic life history and, lacking pericarps and paraphyses, a gross morphology that differs between tetrasporophytes and gametophytes. Tricleocarpa is characterised by a heteromorphic life history and the presence of both pericarps and paraphyses. Based on our present observations, the type species A. fragilis agrees with the morphological observations of Wang and Chiang (Citation2001). Additionally, the morphological features of A. robusta and the new species A. taiwanica indicate that they clearly belong to the genus Actinotrichia and closely resemble A. fragilis.
Morphological features distinguishing the three Actinotrichia species are summarised in . Primary diagnostic characteristics separating them include assimilatory filaments, branching angles, width of branch or internodes, thickness of the cortex, size and prominence of the fusion cell, and the developmental pattern and fate of the sterile paraphyses (). Actinotrichia fragilis possesses a wider branching angle (usually > 40°), more distinct whorled assimilatory filaments than in A. robusta and A. taiwanica. Actinotrichia robusta has narrower branches and consequently a thinner cortex than in A. taiwanica. Comparing reproductive structures, A. taiwanica has a more distinct fusion cell than either A. fragilis or A. robusta. Additionally, the paraphyses of A. robusta and A. taiwanica intermix with the gonimoblasts and are distributed along the inner walls of the pericarp, while the paraphyses in A. fragilis issue from the inner pericarp wall and project into the cystocarp cavity without intermingling with the gonimoblasts. Intermingling paraphyses were also found in Tricleocarpa cylindrica (Ellis et Solander) Huisman et Borowitzka while non-intermingling ones were found in T. fragilis (Huisman & Borowitzka, Citation1990), providing a reliable interspecific distinction in this genus. Although the developmental sequence of the cystocarp is very similar in Actinotrichia and Tricleocarpa, a close evolutionary relationship is not supported by our molecular analysis. The same interspecific diagnostic characteristics of Tricleocarpa (Huisman & Borowitzka, Citation1990), in addition to the width of branches and the developmental sequence of the cystocarp are used for species discrimination within Actinotrichia.
Table 2. Comparison of the morphological characteristics of Actinotrichia spp. from Taiwan.
Wang et al. (Citation2005) emphasised the significance of the seven to eight inner gonimoblast cells involved in the formation of the fusion cell in Galaxaura, but only three or four of these cells participated in forming the fusion cell in Dichotomaria. As a result, the fusion cell is larger in Galaxaura than in Dichotomaria. In this study, observations made on Actinotrichia species show that two to four inner gonimoblast cells are involved in the formation of the fusion cell. Consequently, their fusion cells are smaller than in Galaxaura, but comparable with Dichotomaria. Within these three genera, both Actinotrichia and Dichotomaria possess a pericarp in the mature cystocarp, which is absent in Galaxaura. Hommersand & Fredericq (Citation1990) suggested that Galaxaura requires a larger fusion cell to provide more nutrition for the development of the growing cystocarp. Alternatively, nutrients are also required for the development of the pericarp, so the smaller fusion cell in genera with a pericarp may represent structural diminution as a result of greater resource allocation to other growing parts. To test this hypothesis further investigation of Tricleocarpa is required, particularly critical examination of the number of inner gonimoblast cells involved in the formation of its fusion cell.
Although A. fragilis is pan-tropical in the Indian and Pacific Oceans (Cordero, Citation1975), current knowledge suggests that A. robusta is restricted to the Ryukyu Islands (Itono, Citation1979) and the coasts of southeastern Taiwan, while A. taiwanica is also found on the southeastern coasts of Taiwan. Clearly the geographical distribution of A. robusta and A. taiwanica is restricted to the region of the warm Kuroshio Current. Both species will likely be found along the cooler tropical waters of eastern Luzon Island in the Philippines and even into the warmer temperate waters of Kyushu in southern Japan. In many cases, the Kuroshio Current effectively disperses warm water species such as the members of Liagoraceae and Galaxauraceae to higher latitudes along East Asia (Yang et al., Citation1994). Consequently, it can be safely assumed that A. robusta and A. taiwanica will also be found near other islands along the path of the Kuroshio Current. Further seasonal studies and additional collections will confirm the strength of this hypothesis.
Key to the species of Actinotrichia in Taiwan
1. Branch angles wide, more than 40°, gonimoblasts in median position within the cystocarp, never mixed with paraphyses.................. A. fragilis
1. Branch angles narrow, less than 40°, gonimoblasts dispersed throughout the cystocarp, mixed with paraphyses....................................................2
2. Branch less than 600 µm wide, fusion cell of gonimoblast less than 25 µm wide....................... A. robusta
2. Branch more than 600 µm wide, fusion cell of gonimoblast more than 25 µm wide.................. A. taiwanica
Acknowledgements
We are grateful to Dr S.-M. Lin (Institute of Marine Biology, National Taiwan Ocean University) for assistance in the molecular analysis, Dr L.M. Liao (Department of Biology, University of San Carlos, Philippines) for organising a collecting trip to Sulpa Island and making critical comments on this manuscript, Dr T. Schils (University of Guam Marine Laboratory) for providing Actinotrichia materials from Oman, Ms Suzanne Huang for her comments and Mr L.C. Wang and Mr C.C. Liao for their valuable field assistance.
References
- Abbott , IA . 1999 . Marine Red Algae of the Hawaiian Islands , Honolulu, , USA : Bishop Museum Press .
- Børgesen , F . 1932 . A revision of Forsskål's algae mentioned in Flora Aegyptiaco-arabica and found in his herbarium in the Botanical Museum of the University of Copenhagen . Dansk. Bot. Ark. , 8 : 1 – 15 .
- Cordero , PA . 1975 . On the occurrence of genus Actinotrichia in the Philippines . Publ. Seto Mar. Biol. Lab. , 22 : 267 – 276 .
- Decaisne , J . 1842 . Essai sur une classification des algues et des polypiers calcifères . Ann. Sci. Nat. Bot., Sér 2 , 17 : 96 – 128 .
- Felsenstein , J . 1985 . Confidence limits on phylogenies: an approach using the bootstrap . Evolution , 39 : 783 – 791 .
- Forsskål , P . 1775 . Flora Aegyptiaco-arabica . Post mortem auctoris edidit Carsten Niebuhr. Havniae [Copenhagen] , : 32 +CXXVI+219 [-220] frontispiece [map]
- Freshwater , DW , Fredericq , S , Butler , BS , Hommersand , MH and Chase , MW . 1994 . A gene phylogeny of the red algae . Proc. Natl. Acad. Sci., USA , 91 : 7281 – 7285 .
- Holmgren , PK , Holmgren , NH and Barnett , LC . 1990 . Index Herbariorum. Part I. The Herbaria of the World , 8th , New York, , USA : International Association for Plant Taxonomy, New York Botanical Garden .
- Hommersand , MH and Fredericq , S . 1990 . “ Sexual reproduction and cystocarp development ” . In The Biology of the Red Algae , Edited by: Cole , KM and Sheath , RG . 305 – 345 . New York, , USA : Cambridge University Press .
- Huisman , JM . 2006 . Algae of Australia: Nemaliales , Melbourne, , Australia : ABRS, Canberra; CSIRO publishing .
- Huisman , JM and Borowitzka , MA . 1990 . A revision of the Australian species of Galaxaura (Rhodophyta. Galaxauraceae), with a description of Tricleocarpa gen. nov . Phycologia , 29 : 150 – 172 .
- Huisman , JM , Harper , JT and Saunders , GW . 2004 . Phylogenetic study of the Nemaliales (Rhodophyta) based on large-subunit ribosomal DNA sequences supports segregation of the Scinaiaceae fam. nov. and resurrection of Dichotomaria Lamarck . Phycol. Res. , 52 : 224 – 234 .
- Itono , H . 1979 . Actinotrichia robusta, a new species of the Chaetangiaceae (Nemaliales, Rhodophyta) . Jpn J. Phycol. , 27 : 137 – 141 .
- Kluge , AG and Farris , JS . 1989 . Quantitative phyletics and the evolution of anurans . Syst. Zool. , 18 : 1 – 32 .
- Kurihara , A , Arai , S , Shimada , S and Masuda , M . 2005 . The conspecificity of Galaxaura apiculata and G. hystrix (Nemaliales, Rhodophyta) inferred from comparative morphology and rbcL and ITS1 sequences . Eur. J. Phycol. , 40 : 39 – 52 .
- Lamouroux , JBD . 1816 . Histoire Naturelle des Animaux Sans Vertebras , Vol. 2 , Paris, , France : Deterville .
- Lewis , JE and Norris , JN . 1987 . A history and annotated account of the benthic marine algae of Taiwan . Smithson. Contrib. Mar. Sci. , 29 : 1 – 38 .
- Posada , D and Crandall , KA . 1998 . Modeltest: testing the model of nucleotide substitution . Bioinformatics , 14 : 817 – 818 .
- Saunders , GW . 2005 . Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications . Philos. Trans. R. Soc. Lond. B Biol. Sci. , 360 : 1879 – 1888 .
- Silva , PC , Basson , PW and Moe , RL . 1996 . Catalogue of the benthic marine algae of the Indian Ocean . Univ. Calif. Publ. Bot. , 79 : 1 – 1259 .
- Svedelius , N . 1952 . Notes on the structure and reproduction of the genus Actinotrichia . Svensk. Bot. Tidskrift. , 46 : 1 – 17 .
- Tseng , CK . 1941 . Studies on the Chaetangiaceae of China . Bull. Fan Mem. Inst. Biol. (Bot.) , 11 : 83 – 118 .
- Vis , ML , Saunders , GW , Sheath , RG , Dunse , K and Entwisle , TJ . 1998 . Phylogeny of the Batrachospermales (Rhodophyta) inferred from rbcL and 18S ribosomal DNA gene sequences . J. Phycol. , 34 : 341 – 350 .
- Wang , WL and Chiang , YM . 2001 . The reproductive development of the red alga Actinotrichia fragilis (Galaxauraceae, Rhodophyta) . Eur. J. Phycol. , 36 : 377 – 383 .
- Wang , WL , Liu , SL and Lin , SM . 2005 . Systematics of the calcified genera of the Galaxauraceae (Nemaliales, Rhodophyta) with an emphasis on Taiwan species . J. Phycol. , 41 : 685 – 703 .
- Weber-Van Bosse , A . 1921 . Liste des algues de Siboga. II. Rhodophyceae: Protoflorideae, Nemalionales, Cryptonemiales . Siboga-Expeditie Monogr. , 59b : 184 – 310 .
- Wittmann , W . 1965 . Aceto-iron-haematoxylin-chloral hydrate for chromosome staining . Stain Technol. , 40 : 161 – 164 .
- Yang , HN , Wang , WL and Liao , LM . 1994 . Marine algal flora of Pengchia Yu and its special place in the marine phytogeography of Taiwan . Bot. Mar. , 37 : 429 – 432 .
- Zuccarello , GC and West , JA . 2003 . Multiple cryptic species: molecular diversity and reproductive isolation in the Bostrychia ranicans/B. moritziana complex (Rhodomelaceae, Rhodophyta) with focus on North American isolates . J. Phycol. , 39 : 948 – 959 .
- Zuccarello , GC , Sandercock , B and West , JA . 2002 . Diversity within red algal species: variation in world-wide samples of Spyridia filamentosa (Ceramiaceae) and Murrayella periclados (Rhodomelaceae) using DNA markers and breeding studies . Eur. J. Phycol. , 37 : 403 – 441 .