Abstract
The population of an unknown naviculoid diatom from Lake Vrana in Croatia was identified as Navicula hedinii, a species described in 1922 from a small lake in eastern Turkestan (China). This species has some similarities with Navicula pseudocrassirostris, a marine species found in European coastal waters. Based on the ultrastructure of the two species, they can no longer be included within the taxonomical concept of Navicula sensu stricto. Following a comparative morphological analysis of both species with genera bearing similar characters (Adlafia, Veigaludwigia, Kobayasiella, Cavinula, Stenoneis, Climaconeis, Berkeleya, Sellaphora, Cosmioneis), a new genus, Envekadea is proposed for the two species. The new genus is characterized by a sigmoid raphe course with golfclub-like terminal fissures deflected in opposite directions, the areolae covered by external porous hymenes and the presence of one chloroplast, H-shaped in valve view.
Introduction
In 1922, Hustedt described Navicula hedinii from a freshwater lake dominated by Utricularia vulgaris Linnaeus in eastern Turkestan. Although the species has a very characteristic shape and cannot be considered extremely small, it does not seem to have reappeared many times in the literature, at least not under its accepted name N. hedinii. In their comprehensive work on coastal diatoms, Witkowski et al. (Citation2000) presented two images of what we consider to be N. hedinii. However, the authors incorrectly identified the taxon as Navicula (?Stenoneis) pseudocrassirostris Hustedt, a species with similar features but that can easily be distinguished by its narrower valve apices and a different striation pattern. Some of their images represent N. pseudocrassirostris but two should be assigned to N. hedinii (Witkowski et al., Citation2000, plate 156, ; plate 157, ). Apart from a mention of N. hedinii by Xavier Tomás (Citation1987) who found the species in several coastal locations on the Iberian Peninsula, no other records of N. hedinii seem to exist in the literature. Metzeltin (pers. comm.) found the species in Mustang Lo (Nepal) but has not published this record. Recently, it was also found near Buir Nuur, on the eastern border of Mongolia (Mark Edlund, personal comment).
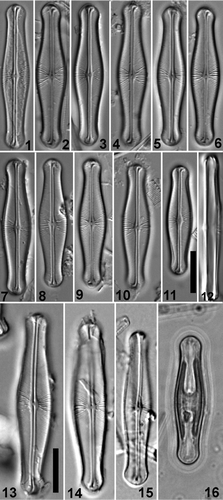
Figs 1–16. Light micrographs of Envekadea (Navicula) hedinii. . Valve views of specimens from Lake Vrana (Croatia). . Girdle view of specimen from Lake Vrana (Cr.). . Valve views of the type population from Mapiek Köll (eastern Turkestan). . Valve view of specimen (Croatian population) showing the chloroplast structure. Scale bars: 10 µm.
Due to the lack of modern data, the correct taxonomic position of N. hedinii was unclear. Over the past few years, following the description and resurrection of a large number of new and ‘forgotten’ genera, more and more species formerly placed in the genus Navicula have been reassigned (e.g. Cox, Citation1987, Citation1988; Mann, Citation1989; Round et al., Citation1990; Mann & Stickle, Citation1991; Lange-Bertalot, Citation1996, Citation2001; Spaulding & Stoermer, Citation1997). If Navicula is restricted to species sharing the characteristics of the typus generis, Navicula tripunctata (O.F. Müller) Bory (Cox, Citation1979), it is clear that many species still placed in Navicula await investigation to assign them to a new, taxonomically correct, position.
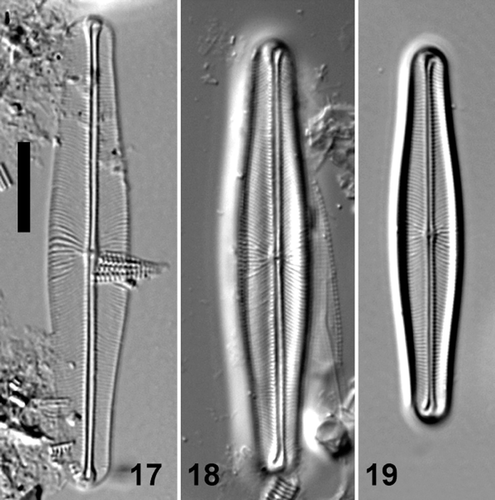
Figs 17–19. Light micrographs of valve views of Envekadea (Navicula) pseudocrassirostris from Eleusis (Athens, Greece). Scale bar: 10 µm.
The occurrence of N. hedinii in some samples from a karst lake in Croatia, collected for a study of the relationship between phytoplankton and environmental lake conditions in Lake Vrana (Gligora et al., Citation2007), allowed a detailed study of the morphological features of this diatom species using both light (LM) and scanning electron microscopy (SEM). Type material collected by Sven Hedin and kept at the Friedrich Hustedt Diatom Collection in Bremerhaven was examined using the same techniques. These results, the possible conspecificity of the Croatian specimens and the implications for its correct taxonomical position are discussed in this paper. In addition, because of its similarity and possible conspecificity, the taxonomic status of N. pseudocrassirostris is also considered.
Materials and methods
The polymictic Lake Vrana (2 m mean depth) is a karstic cryptodepression lake, near the middle of the eastern Adriatic Coast, connected to the Adriatic Sea by a narrow artificial channel at its southern side. The water is clear and dominated by macrophytes that bloom quite luxuriantly during the warm summer months. The water is alkaline (pH 7.7–9.4), with a high conductivity (1638–3960 µS/cm) and a salinity range between 0.7–1.2 psu. More information on Lake Vrana, including its physicochemical and biological characteristics, is given in Gligora et al. (Citation2007).
“Navicula hedinii” was first discovered in Lake Vrana in samples taken for phytoplankton analysis but subsequently, thanks to the shallowness of the lake, benthic and periphytic samples were also studied. The largest populations were found in two samples, V2, from the northwest part of the lake close to a bird sanctuary, and V4, from the middle and deepest part of the lake. This location was covered by vegetation, mainly Chara spp.
For comparison, the type material of N. hedinii and N. pseudocrassirostris, from the Friedrich Hustedt Diatom Collection (BRM) in Bremerhaven, was studied. The holotype of the former is slide 067/84, Tibet, Mapiek-Köll, leg. Sven Hedin, 23/07/1900. Material is available in bottle AS1394. The holotype of N. pseudocrassirostris is slide 199/95, Nordasot, Norway, 87 m, A (Simonsen, Citation1987, plate 727, ). It is also present on slides 308/63, Athen, Teich by Eleusis, b and SIM6/55, Fehmarn, Germany. Material is available in bottle E59218.
Diatom samples were prepared following Hendey (Citation1964). Small parts of the samples were first cleaned of calcium carbonate with hydrochloric acid and then organic matter was removed by adding concentrated sulphuric acid (H2SO4), heated to boiling point with crystals of NaNO3 added. If sodium nitrate addition did not cause discoloration, the sample was heated further until discoloration occurred and all organic matter was destroyed. Cleaned diatom valves were mounted in Naphrax®. Samples and slides are stored at the University of Zagreb (Division of Biology) and the National Botanic Garden of Belgium (Department of Bryophytes and Thallophytes). Light microscope observations were conducted using an Olympus BX51 microscope equipped with Differential Interference Contrast (Nomarski) optics. For scanning electron microscopy (SEM), part of the suspension was filtered onto polycarbonate membrane filters (Millipore®) with a maximum pore diameter of 3 µm, pieces of which were attached to aluminum stubs after air-drying. The stubs were sputter-coated with 50 nm of gold and studied in a JEOL-5800LV at 20 kV. Transmission electron microscopy of the Croatian material was performed using a FEI Morgagni 268D microscope. The type material of N. hedinii and N. pseudocrassirostris was investigated in Bremerhaven using a Zeiss Axioplan LM microscope equipped with a Color View III digital camera and a FEI Quanta FEG 200 scanning electron microscope. Unfortunately, there was insufficient unmounted type material of both species to allow both scanning and transmission electron microscopy, so the decision was taken to perform only SEM analysis on both type populations. Terminology follows Barber & Haworth (Citation1981) and Round et al. (Citation1990).
Results
“Navicula hedinii”, Lake Vrana, Croatia (, 20–34)
Light microscopy
Cells are solitary, frustules rectangular in girdle view with rounded ends (). Valves are linear–lanceolate, 25–38 µm long by 5.5–7.5 µm wide, with a clearly swollen (i.e. convex–elliptical) central part and capitate ends. The width of the poles (4.6–5.5 µm) never exceeds the width of the valve centre. The axial area is very narrow, linear to weakly lanceolate, widening towards the apices and forming a wedge-shaped hyaline area. The central area is relatively small, rather asymmetric with one, more or less rhombic side and one more elliptic side. No fascia is present. The raphe is filiform, straight to weakly undulate with indistinct to weakly expanded, non-deflected central pores. Terminal raphe fissures are hooked, ‘golfclub-like’, towards opposite sides widening to their ends. The central transapical striae differ from those over the rest of the valve. Beside the central area, shorter striae alternate with longer ones presenting a complex striation pattern, (20) 22–25 in 10 µm. Striae are denser over the rest of the valve, 25–27 in 10 µm, radiate to weakly geniculate, suddenly becoming convergent near the narrowest part of the valve. Due to the enlarged apical axial area, striae are much shorter near the apices. Live cells have one plastid, H-shaped in valve view ().
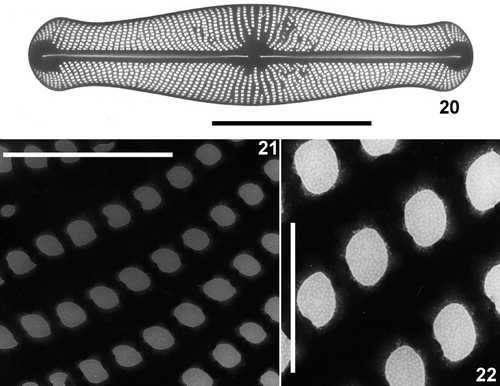
Figs 20–22. Transmission electron micrographs of Envekadea (Navicula) hedinii from Lake Vrana (Croatia). . Entire valve showing the square to polygonal shape of the areolae when the external hymenes have been removed. . Detail of the stria structure with the hymenes covering the areolae. . Detail of the hymenes showing their porous structure. Scale bars: 10 µm (), 1 µm () and 0.5 µm ().
Transmission electron microscopy
Areola shape varies considerably from square to polygonal, but rounded or slit-like areolae are absent (). Square areolae are the most common. Near the axial area, several areolae seem to be transapically subdivided by a very narrow silica bridge, giving the false impression of biseriation. Areolae tend to be smaller around the terminal wedge-shaped hyaline area. Hymenes, perforated by an irregular number of very small rounded pores, cover the areolae ().
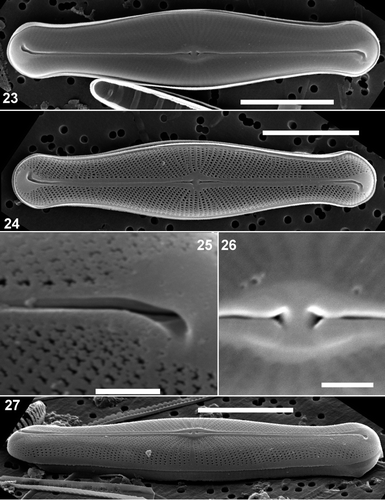
Figs 23–27. Scanning electron micrographs of external views of Envekadea (Navicula) hedinii from Lake Vrana (Croatia). . Presence of the hymenes covering the external areola openings. . An eroded valve showing the raphe path with the fissures deflected into opposite directions and the typical areola structure. . Detail of the terminal raphe fissure. Note the almost straight course of the raphe branch and the irregular areola structure. . Detail of the delta-shaped central raphe pores. . A tilted valve showing the stria structure at the valve face/mantle junction. Scale bars: 10 µm () and 1 µm ().
Scanning electron microscopy
The areolae are usually covered by external hymenes obscuring most external features (). When thoroughly oxidized, the underlying structure of the striae becomes visible. The shape of the areolae varies considerably from square to polygonal but rounded or slit-like areolae are absent (). Striae are uniseriate, never becoming biseriate () although sometimes small silica bridges may divide some areolae close to the axial area (). Areolae tend to be very irregular in shape near the valve apices (). Whether this is the result of partial destruction of the hymenes occluding more regularly shaped areolae, or a real feature remains unclear. However, this type of areola was observed on all specimens (n = 25) from Lake Vrana indicating that it might be a constant feature of this population, not simply a preparation artefact. Striae continue uninterrupted onto the valve mantle (). Shortly before the mantle edge, areolae disappear and there is a wide unperforated zone that narrows around the valve apices (). Towards the apices, striae become much shorter and radiate around the valve apices. Striae are reduced to a single areola at the apices (). The terminal raphe fissures lie in a large, wedge-shaped, unperforated area (). Raphe branches are almost straight but have a very slight deflection to the primary side of the valve immediately distal to the central area (). The external raphe branches are somewhat wider near the central area than in the rest of the valve and lie in a raised siliceous thickening (). The external central raphe endings are not deflected but expanded, forming a delta-like depression (). Terminal raphe endings form a hooked, widening groove (), but the internal raphe ending can be seen diverging from the external fissure, continuing on a straight course (). The external terminal fissures are turned in opposite directions at the two poles giving the raphe a sigmoid path ().
Figs 28–33. Scanning electron micrographs of valve and girdle views of Envekadea (Navicula) hedinii from Lake Vrana (Croatia). . Detail of the external valve apex with the wedge-shaped hyaline area and the clearly deflected terminal raphe fissure. . External detail of the irregularly shaped areolae near the valve apex. . Internal view of an entire valve with a partly detached valvocopula lying in the valve. . Detail of the external valve apex showing the girdle. . Detail of the valvocopula in , showing the lack of perforations and the undulated pars interior. . End of a frustule showing girdle bands and valve apices. Scale bars: 10 µm () and 1 µm ().
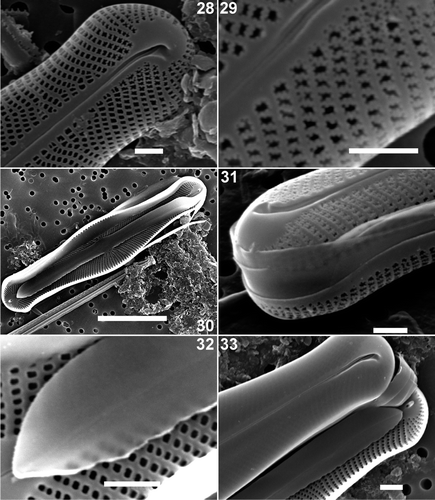
Internally, the central raphe endings are straight or very weakly deflected to the primary side and separated from each other by less than 1 µm (). The axial area corresponds to two straight, weakly raised costae, one on either side of the raphe branches, running form one apex to the other (). The helictoglossae, situated in a large wedge-shaped area, are simple structures, not forming a compound structure with the ribs of the raphe-sternum (). The areolae are rounded, open poroids ().
Figs 34–38. Scanning electron micrographs of internal views of Envekadea (Navicula) hedinii from Lake Vrana (Croatia). . Entire valve. . Detail of the central part of the valve with the raised internal nodule. Note the raised rims between the striae and the round shape of the areolae. . Detail of the central raphe endings. Note the slightly deflected ends. . Detail of the valve apex with the typical, very simple helictoglossa. . Detail of the valve apex with the typical, tunnel-like helictoglossa. Scale bars: 10 µm () and 1 µm ().
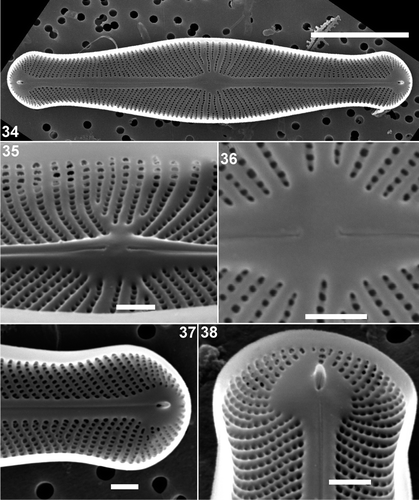
The girdle consists of 2–3 open bands (). Careful examination of intact frustules, both those with the hymenes still present and others in which the hymenes had been oxidized out, did not reveal any kind of perforation in the girdle bands (). Isolated bands that could be assigned with 100% certainty to N. hedinii have not been found due to the presence of girdle bands of many other naviculoid species in the sample. The pars interior of the valvocopula has an undulating edge but is not fimbriate ().
Type material of Navicula hedinii Hustedt (, )
Light microscopy
Valves are linear–lanceolate with an inflated middle part and protracted, broadly rounded, capitate ends. Valves are 35–42 µm long, 5.7–9 µm wide. The axial area is very narrow, a central area barely developed, or only very weakly elliptical. The central raphe endings are slightly expanded, close to each other, the terminal raphe endings curved in opposite directions. Transapical striae are radiate throughout, 26–28 in 10 µm, abruptly convergent near the apices (Hustedt reported approximately 36 in 10 µm). Striae are more widely spaced near the central area, with alternating shorter and longer striae. No information is available on plastid structure.
Figs 39–44. Scanning electron micrographs of Envekadea (Navicula) hedinii from the type population of Mapiek Köll. . External valve view of an entire valve. Presence of the hymenes covering the areola openings. . Detail of the central part of the valve showing the central raphe endings. . Detail of an external valve apex with the typical, deflected terminal raphe fissure. . Internal view of an entire valve. Although broken along the axial area, it is still clear that the central raphe endings are weakly deflected. . Detail of the internal structure of a valve apex with the simple helictoglossa. The presence of the raised rims between the striae and the rounded shape of the areolae is well visible. . Detail of a broken valve showing the presence of external hymenes covering the external areola openings. Inside the valve, it is clear that internal hymenes are completely absent. Scale bars: 10 µm () and 5 µm ().
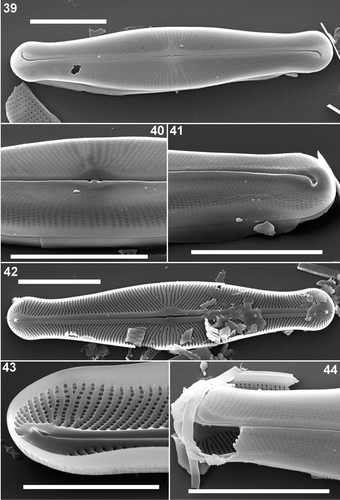
Scanning electron microscopy
The areolae are irregular in shape varying from rectangular to polygonal, and covered externally by hymenes (). The external raphe fissures open in a slightly raised sternum. The central area is rhombic to elliptical (). The central raphe pores are cuneately expanded () and the terminal fissures are ‘golfclub-shaped’ and clearly deflected, although the internal terminal raphe fissures do not entirely follow the external path (). Internally, the areolae are rounded, open poroids () and the striae are separated by weakly raised ribs (). The internal raphe fissures open between two straight slightly thickened longitudinal ribs (). The terminal helictoglossae are rather simple, raised lips (). Observations of the girdle structure were not possible.
Navicula pseudocrassirostris Hustedt (, )
Specimens of N. pseudocrassirostris were examined from three different localities: Fehmarn Germany (; slide SIM6/55), the holotype specimen from Nordasot, Norway (; BRM 199/95), and material from Eleusis (Athens).
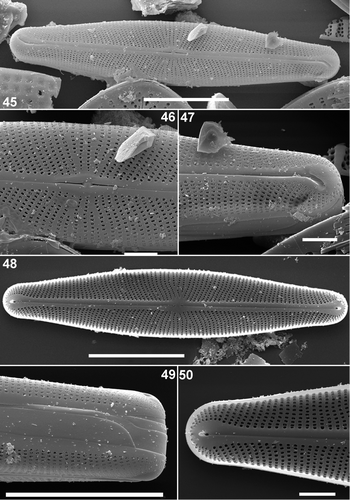
Figs. 45–50. Scanning electron micrographs of Envekadea (Navicula) pseudocrassirostris from Eleusis (Athens, Greece). . External view of an entire valve in a slightly tilted position to show the valve face/mantle margin. Note the sigmoid course of the raphe. . Detail of the external central area with the delta-shaped central raphe pores and the reduced central area. . Detail of an external valve apex with the golfclub-like terminal fissures. The internal raphe branch does not seem to follow the terminal fissure path. . Internal view of an entire valve. . Detail of the girdle. Three unperforated bands can be seen. . Detail of the internal structure of the apex with the simple helictoglossa and the wedge-shaped hyaline area around the terminal ending. Scale bars represent 10 µm () and 1 µm ().
Light microscopy
Valves are linear to linear–lanceolate with weakly convex margins and slightly protracted, broadly rounded apices, 34–52 µm long, 6.5–8.5 µm wide. The axial area is narrow, linear with slightly raised, parallel margins. The central area is rather indistinct, only slightly widened and clearly raised above the rest of the valve face. The raphe is filiform with straight branches. The central raphe endings are straight, slightly drop-like and expanded and the terminal raphe fissures are deflected towards opposite sides with slightly expanded terminal endings. Transapical striae are radiate in the middle, becoming parallel to convergent near the valve apices, 20–24 in 10 µm. Striae are more widely spaced at the centre of the valve.
Scanning electron microscopy
Externally, the striae are composed of irregularly formed, rectangular to polygonal areolae (). The areolae often have small irregular silica outgrowths giving them their irregular appearance (). Near the valve apices, the areolae beside the raphe fissures are subdivided into very small poroids by the silica outgrowths (). Around the valve centre, short and longer striae are interspersed () and the striae are continuous over the valve mantle (). The external raphe fissures are straight, with undeflected central raphe endings terminating in expanded, wedge-shaped depressions (). Externally, the terminal fissures are hooked, lying in a widening groove (). They turn in opposite directions at the poles, creating a sigmoid path () and a large, drop-like unperforated area surrounds the terminal fissures (). The internal raphe slit does not seem to follow the same path as the terminal fissures, but is straight or only weakly deflected ().
Internally, the areola openings are unoccluded, rounded to slightly rectangular throughout the valve (). There was no visible difference between the areolae near the valve apices and elsewhere on the valve (). The internal raphe branches are straight with unilaterally, weakly deflected, central endings and small raised terminal helictoglossae (). The raphe branches are flanked on both sides by slightly thickened longitudinal ribs. The drop-like unperforated area is clearly visible around the helictoglossa (). The girdle comprises three unornamented open bands ().
Ecology of the taxa
The type population of N. hedinii was collected by Dr Sven Hedin on 23 June 1900 from Mapiek Köll, a part of the shallow freshwater lake of Kara-koshun (aka Lop-nor) at an altitude of 816 m (approx. 40°05′N; 90°05′). The lake is now situated in eastern Turkestan, in the autonomous Chinese region of Xinjiang Uygur, and is part of the upper Tarim basin. Hedin (Citation1922) refers to this site as being part of northern Tibet, which is geographically incorrect. Although the maximum depth of the lake is 4.5 m, the mean depth does not exceed 1 m. The entire lake is filled with reeds and is used by the local people (Lopliks) for fishing and watering. According to Hedin (Citation1922), it is perfectly fresh. The sample was taken from an Utricularia vulgaris stand that also contained sterile remains of an unknown Nitella-species.
Navicula hedinii was present in Lake Vrana during the whole year, with a maximum abundance in the northern part of the lake. The maximum development of N. hedinii was in the late autumn and winter period (from November to January) during the dominance of Cosmarium tenue Archer and higher concentrations of inorganic nitrogen.
Navicula pseudocrassirostris is a marine species reported from coastal waters throughout the world (Witkowski et al., Citation2000; Lange & Tiffany, Citation2002; Procopiak et al., Citation2006). Hustedt (1961) described the species from Nordåsot (Norway), but found the largest population in a brackish pool near Eleusis (Athens) in Greece.
Discussion
The Croatian specimens can be unequivocally identified as N. hedinii. All morphological and quantitative features, both in LM and SEM, match the type. Based on Hustedt's (1922) description (38–42 µm long, 8–9 µm wide, with 36 striae in 10 µm), the Croatian population seemed to be somewhat smaller and less densely striate than the type population, but on closer examination it was clear that the original description did not cover the entire range of the type population.
Although N. pseudocrassirostris has similar features, e.g. raphe and areola structure, it differs sufficiently to be excluded from conspecificity with N. hedinii. In N. pseudocrassirostris, the central area is smaller, the valves are less tumid in the middle and the valve apices are less capitate. However, based on their shared ultrastructural morphological features, N. hedinii and N. pseudocrassirostris clearly belong to the same genus, but should, based on outline, raphe structure, not be included within the genus Navicula sensu stricto as delimited by Cox (Citation1979, Citation1999) and Lange-Bertalot (Citation2001). Navicula is characterized by cross-lineate striae, open on the inner side of the valve, a boat-shaped valve, internal raphe fissures deflected to the same (secondary) side and indistinct or weakly curved central raphe endings. It is clear that N. hedinii and N. pseudocrassirostris have a completely different set of features making the transfer to another, more suitable and taxonomically discrete genus inevitable.
The simplest way to classify them would be to transfer both species to Naviculadicta. Although this genus has been typified and described following the International Code for Botanical Nomenclature (Lange-Bertalot & Moser, Citation1994), it is only meant to serve as a kind of ‘waiting room’ for new genera and species that need to be correctly classified since they cannot be included in Navicula s.s. (Kociolek, Citation1996). We therefore firmly opt against putting N. hedinii and N. pseudocrassirostris in this catch-all genus.
The ecology of both species (one marine and one freshwater) makes comparison with similar marine and freshwater genera necessary. At present, only a few freshwater genera show some (although never the entire combination) features that are found in N. hedinii and N. pseudocrassirostris. These are Adlafia Lange-Bertalot, Kobayasiella Lange-Bertalot, Veigaludwigia Lange-Bertalot & Rumrich, Sellaphora Mereschkowsky, Cavinula Mann & Stickle and Cosmioneis Mann & Stickle (). Since Witkowski et al. (Citation2000) questioned whether N. pseudocrassirostris belonged within the marine genus Stenoneis Cleve, the latter should be considered, together with the closely related marine genera, Climaconeis Grunow and Berkeleya Greville (). Based on the data in and , it is clear that all these genera share some resemblance to N. hedinii and N. pseudocrassirostris. However, their discriminating features are the internal and external structure of the areolae (including the hymenes), raphe structure, plastid structure and the sigmoid raphe.
Table 1. Comparison between the new genus Envekadea and some freshwater genera presenting a similar set of features.
The freshwater genus that shows the greatest similarity to our species is Adlafia (). Lange-Bertalot (Citation2001) stated that all Adlafia species had small valve dimensions with a maximum length <25 µm. However, two species have since been found in the sub-Antarctic region with valve lengths exceeding 35 µm (Van de Vijver et al., Citation2002: plates 45, 46): Adlafia bryophiloides (Manguin) Van de Vijver & Beyens and Adlafia linearis (Manguin) Van de Vijver & Beyens, contradicting this size limit. The striation pattern is very dense; distinctly radiate and convergent towards the apices, striae are uniseriate and run continuously over the valve surface, and the areolae are externally occluded by hymenes. However, Adlafia has very small, rounded, simple areolae, whereas N. hedinii and N. pseudocrassirostris have rather large, square or rectangular to even polygonal areolae. This areola type has never been observed in Adlafia. The raphe structure is perhaps the most conflicting feature. Adlafia possesses a filiform, weakly curved raphe with almost indistinct central raphe pores and strongly unidirectionally deflected terminal fissures (Lange-Bertalot, Citation2001, plate 107, ). Navicula hedinii and N. pseudocrassirostris on the contrary present golfclub-like expanded terminal raphe endings, turned in opposite directions at the poles, following a more or less sigmoid course. The girdle bands bear two rows of poroids in Adlafia whereas N. hedinii and N. pseudocrassirostris have non-porous bands. Based on these important differences, it is clear that Adlafia cannot be the host genus for the two species.
Table 2. Comparison between the new genus Envekadea and some marine genera presenting a similar set of features.
Although it contains species with a similar valve outline and comparable light microscopical features, the genus Kobayasiella must also be excluded as a possible host genus for N. hedinii. The main features of Kobayasiella (Lange-Bertalot, Citation1996; Vanhoutte et al., Citation2004) include the presence of an umbilicus (sensu Kobayasi & Nagumo, Citation1988), visible as a slight ‘notch’ at about one third to halfway along the straight raphe (Vanhoutte et al., Citation2004, ) and striae that consist of transapically elongated, finely hymenate areolae extending from the raphe to the valve face/mantle junction where a longitudinal hyaline ridge separates the areolae on the valve face from those on the mantle (Vanhoutte et al., Citation2004, ) (). It is clear that the species discussed in this paper do not have a typical umbilicus although a very weak raphe notch can be seen in certain specimens (). Additionally, the areolae are totally different in Kobayasiella. Navicula hedinii and N. pseudocrassirostris never have transapically elongated areolae.
Veigaludwigia has a similar outline but shows a characteristic set of features () that are absent in N. hedinii and N. pseudocrassirostris (Rumrich et al., 2000). The most important difference is the presence of internal spines along the valve margins (Rumrich et al., 2000, plate 74, ). These spines, that are even visible in LM, have never been observed in our species. Moreover, the raphe in Veigaludwigia has external terminal fissures curved to the same side, lacking the typical sigmoid raphe course of N. hedinii and N. pseudocrassirostris. The shape of the terminal fissures is also different. Internally, the central raphe endings are clearly hooked, unlike N. hedinii (Rumrich et al., 2000, plate 74, ). Therefore, Veigaludwigia cannot be the host genus for our species.
Cavinula () has a similar external sigmoid raphe path with terminal fissures that are deflected in opposite directions (Round et al., Citation1990 p. 524, figs b, c). However, the golfclub-like fissures, present in our two species are absent. Based on the structure of the areolae, Cavinula must be excluded from being the host genus. Areolae in Cavinula are simple, small poroids, occluded internally by hymenes, and external areola coverings have never been reported. The structure of the plastids and girdle bands, with a double row of perforations, complete the separation between Cavinula and the two ‘Navicula’ species.
Cosmioneis () has similar features to Cavinula but lacks oppositely deflected terminal raphe fissures, although the central raphe pores are delta-like, as in N. hedinii and N. pseudocrassirostris. The areolae are relatively large occluded internally by hymenes, without external hymenes. This also applies for Sellaphora, whose areolae and raphe structure differ sufficiently to preclude inclusion of N. hedinii and N. pseudocrassirostris within it.
The marine genus Stenoneis () presents several points of similarity (Poulin, Citation1990; Round et al., Citation1990). Although Witkowski et al. (Citation2000) suggested a possible link between N. pseudocrassirostris and Stenoneis, we believe this genus is not the solution for N. hedinii and N. pseudocrassirostris. The striae in Stenoneis consist of small, round poroids that are usually occluded by an external velum (Poulin, Citation1990). However, the structure of this velum within Stenoneis remains unstudied (Poulin, Citation1990). Enlarged square to polygonal areolae, as in N. hedinii and N. pseudocrassirostris have never been observed in species belonging to Stenoneis. The striation pattern in Stenoneis is interrupted by a large clear, irregular fascia (Poulin, Citation1990: ) in the central area. Small hyaline zones also occur within the striae (Poulin, Citation1990: , ). Cleve (Citation1894) mentioned the presence of a hyaline zone for S. inconspicua (Cleve, Citation1894, plate V, ) although he did not mention this in his original description (Cleve, Citation1894, p. 123). Currently, all Stenoneis species [S. inconspicua (Gregory) Cleve, S. inconspicua var. baculus (Cleve) Cleve and S. obtuserostrata (Hustedt) Poulin], have a hyaline fascia (Poulin, Citation1990). However, similar hyaline zones are always absent from N. hedinii and N. pseudocrassirostris. On the other hand, the internal axial area in Stenoneis is almost identical to that in N. hedinii and N. pseudocrassirostris, with the raphe fissures flanked on both sides by slightly thickened longitudinal ribs. Nevertheless, the external raphe path shows the principal point of difference. Both external terminal fissures are only weakly deflected to the same side (Round et al., Citation1990, p. 523, figs d, e) or are straight (Poulin, Citation1990, , ). They are never bent to opposite sides as is the case in N. hedinii and N. pseudocrassirostris. However, within the genus Placoneis Mereschkowsky, species have terminal raphe fissures hooked towards the same or opposite directions (Round et al., Citation1990), reducing the significance of this feature. Nevertheless, we believe that the difference in raphe structure between Stenoneis and N. hedinii and N. pseudocrassirostris is sufficient to exclude the latter species from Stenoneis. Unfortunately, neither the girdle structure nor the plastids have been studied in Stenoneis. Therefore comparison of these features is not possible at present.
Although the use of ecological features cannot be a conclusive argument for the separation of species and genera, it is additional evidence in this discussion. Almost all Stenoneis species seem to prefer sea ice conditions as their primary habitat (Poulin, Citation1990). There are no freshwater representatives of this genus. On the other hand, N. hedinii and N. pseudocrassirostris show a preference for freshwater to brackish/marine conditions. The combination of the hyaline areas, the round areolae occluded by a velum and the raphe structure is sufficiently different to conclude that Stenoneis, although closely related, cannot be the host genus for N. hedinii and N. pseudocrassirostris.
Both Berkeleya and Climaconeis () show some similarities with N. hedinii and N. pseudocrassirostris but can also be distinguished, based on a large set of differences. Climaconeis has a very characteristic plastid arrangement, with 4–20 plastids, arranged in a line along the cell (Round et al., Citation1990, p. 520, fig. a). Terminal raphe fissures are completely absent. Several Climaconeis-species possess ladder-like craticular bars across the cell (Cox, Citation1982; Round et al., Citation1990, p. 521, figs h, j, k), a feature not observed in N. hedinii and N. pseudocrassirostris. Berkeleya on the other hand has striae composed of poroids that are internally covered by hymenes, and a girdle composed of at least five open bands bearing two rows of areolae (Round et al., Citation1990, p. 519, fig. i). Based on these differences, N. hedinii and N. pseudocrassirostris should not be included within Berkeleya or Climaconeis. Other marine genera such as Parlibellus Cox differ by the presence of internal hymenes and numerous girdle bands (Cox, Citation1988; Round et al., Citation1990, p. 517, fig. h).
In conclusion, it is clear that N. hedinii and N. pseudocrassirostris cannot be assigned to any published genus. One possibility would be to expand the original generic description of the most closely related genus, Stenoneis, including the features observed in N. hedinii and N. pseudocrassirostris. That would mean that the fascia would be ignored as a characterizing feature for Stenoneis, and also that the raphe structure of Stenoneis would no longer be only rather simple, but would also include sigmoid raphe paths. This would create a generic description that could easily include many species now classified in other genera, such as Adlafia. Although this might facilitate the debate on the relationships between genera, we still think that in this case, it will affect largely the typical identity of both Stenoneis and N. hedinii and N. pseudocrassirostris.
Therefore a new genus, Envekadea Van de Vijver, Gligora, Hinz, Kralj & Cocquyt gen. nov., is proposed to accommodate species with large, rectangular to irregularly polygonal areolae occluded externally by porous hymenes, a sigmoid raphe path and non-porous girdle bands.
During the survey, a possible third species was found by Ditmar Metzeltin in material from a freshwater well in Yucatan, identified as Scoliotropis sp. (Metzeltin & Lange-Bertalot, Citation2007, plate 154). The pictures showed similar morphological features to Navicula hedinii i.e. striation pattern, absence of a hyaline zone in the central area and a sigmoid raphe. However, investigations of the Yucatan material did not yield any additional specimens of this species. Although, based on the morphological similarity, this taxon should be described as a new species, we decided not to include it in this paper until we can find a larger population that can be properly investigated. Other species with the same, or very similar, morphological features, include Navicula subcrassirostris Hustedt and Navicula oestrupioides (Oestrup) Hustedt. Further morphological research should clarify whether these species should be assigned to Envekadea or Stenoneis.
Formal description
Envekadea Van de Vijver, Gligora, Hinz, Kralj & Cocquyt gen. nov.
Genus novum in familia Naviculacearum Kützing 1844
TYPUS GENERIS. Envekadea hedinii (Hustedt) Van de Vijver, Gligora, Hinz, Kralj & Cocquyt comb. nov.
BASIONYM. Navicula hedinii Hustedt 1922 in S. Hedin (Ed.) Southern Tibet 6(3) Botany. p. 132, plate 9 .
DIAGNOSIS. Duae species adhuc cognitae sunt notabiliter cum cellulis biraphidis comparate exiguis, maxime 52 µm longis, 9 µm latis. Cellulae solitariae catenas non formantes. Frustula aspectu cingulari anguste rectangularia apicibus leviter rotundatis. Valvae lineares ad lineares-lanceolatas apicibus obtuse rotundatis ad capitatos vel subcapitatos. Area axialis angustissima. In apicibus, area axialis bulbosa. Area centralis paene adest. Systema raphis biraphidea cum ramis raphis filiformibus rectisque. Pori centrales raphis non deflexi distincte expansi in forma litterae delta. Fissurae terminales flexae in lateribus oppositis. Fissurae raphis internae simplices rectae sed poris centralibus leviter deflexa. Helictoglossa simplex adest. Striae transapicales uniseriatae distincte radiantes convergentes ad apices, non interruptae in limbum. Areolae rectangulares ad polygonatas. Hymenes porosae externae obtegentes areolas. Hymenes internae absunt. Cingulum compositum 2–3 copulis apertis, non perforatis. Chromatophorum unum in aspectu valvae adest in forma litterae H. Genus novum satis differt a generibus Kobayasiella, Veigaludwigia, Adlafia, Cavinula, Sellaphora, Stenoneis, Climaconeis, Berkeleya, Cosmioneisque structura raphis, absentia foraminium internorum occlusorum et structura areolarum.
ETYMOLOGY. The genus name Envekadea is derived from the NVKD (Nederlands-Vlaamse Kring van Diatomisten), the association of the Dutch-speaking diatomists. The name was chosen to underline its relationship with Adlafia, a genus named after the Association of the French-speaking diatomists (ADLaF).
New combination
Envekadea pseudocrassirostris (Hustedt) Van de Vijver, Gligora, Hinz, Kralj & Cocquyt comb. nov.
BASIONYM. Navicula pseudocrassirostris Hustedt 1961 in Die Kieselalgen Deutschlands, Österreichs und der Schweiz. Dr L. Rabenhorst's Kryptogamenflora von Deutschland, Band 7, Teil 3, p. 79, fig. 1220.
Acknowledgements
Ing Marcel Verhaegen is acknowledged for his help with the scanning electron microscope. Mrs Petra Peharec and Professor Dr Nikola Ljubesic are thanked for their help with the TEM. Dr Mark Edlund, Dr Sarah Spaulding, Dr R. Crawford, Professor Dr H. Lange-Bertalot, Dr Pierre Compère and Mr Ditmar Metzeltin are thanked for their valuable comments that helped to unravel the identity of the mysterious Croatian diatom.
References
- Barber , HG and Haworth , EY . 1981 . A guide to the morphology of the diatom frustule. , Scientific publication No. 44. Far Sawrey, Ambleside, Cumbria, , UK : The Freshwater Biological Association, The Ferry House .
- Cleve , PT . 1894 . Synopsis of the naviculoid diatoms. Part I. . Kongl. Sv. Vetensk. Akad. Handl. , 26 1–195+5 Plates
- Cox , EJ . 1979 . Taxonomic studies on the diatom genus Navicula Bory. The typification of the genus . Bacillaria , 2 : 137 – 153 .
- Cox , EJ . 1982 . Taxonomic studies on the diatom genus Navicula Bory. IV. Climaconeis Grun., a genus including Okedenia inflexa (Bréb.) Eulenst. ex De Toni and members of Navicula sect. Johnsonieae sensu Hustedt . Br. Phycol. J. , 17 : 147 – 168 .
- Cox , EJ . 1987 . Placoneis Mereschkowsky: the re-evaluation of a diatom genus originally characterized by its chloroplast type . Diatom. Res. , 2 : 145 – 157 .
- Cox , EJ . 1988 . Taxonomic studies on the diatom genus Navicula V. The establishment of Parlibellus gen. nov. for some members of Navicula sect. Microstigmaticae . Diatom Res. , 3 : 9 – 38 .
- Cox , EJ . 1999 . Studies on the diatom genus Navicula Bory. VIII. Variation in valve morphology in relation to the generic diagnosis based on Navicula tripunctata (O.F. Müller) Bory . Diatom Res. , 14 : 207 – 237 .
- Gligora , M , Plenković-Moraj , A , Kralj , K , Grigorszky , I and Peroš-Pucar , D . 2007 . The relationship between phytoplankton species dominance and environmental variables in a shallow lake (Lake Vrana, Croatia) . Hydrobiol. , 584 : 337 – 346 .
- Hedin , S . 1922 . A list of places where plants were collected. In Southern Tibet 6(3) Botany Edited by: Hedin , S . 11 – 24 .
- Hendey , NI . 1964 . An introductory account of the smaller algae of British coastal waters. VI. Bacillariophyceae (Diatoms). , London : Fishery Investigations. Her Majesty's Stationery Office . 317 pp + 45 plates
- Hustedt , F . 1922 . Bacillariales aus Innerasien gesammelt von Dr Sven Hedin. In Southern Tibet 6(3) Botany Edited by: Hedin , S . 107–152+2 plates
- Hustedt , F . 1961 . “ Die Kieselalgen Deutschlands, Osterreichs und der Schweiz unter Berücksichtigung der übrigen Länder Europas sowie der angrenzenden Meeresgebiete ” . In In Dr. L. Rabenhorst Kruptogamen-Flora. Band VII 1 – 160 .
- Kobayasi , H and Nagumo , T . 1988 . Examination of the type materials of Navicula subtilissima Cleve (Bacillariophyceae) . Bot. Mag. Tokyo , 101 : 239 – 253 .
- Kociolek , JP . 1996 . Comment: Taxonomic instability and the creation of Naviculadicta Lange-Bertalot in Lange-Bertalot & Moser, a new catch-all genus of diatoms . Diatom Res. , 11 : 219 – 222 .
- Lange , CB and Tiffany , MA . 2002 . The diatom flora of the Salton Sea, California . Hydrobiol. , 473 : 179 – 201 .
- Lange-Bertalot , H . 1996 . Kobayasia gen. et spec. nov . Icon. Diatom. , 4 : 278 – 287 .
- Lange-Bertalot , H . 2001 . Navicula s.s. 10 genera separated from Navicula sensu lato . Frustulia. Diatoms of Europe , 2 : 1 – 526 .
- Lange-Bertalot , H and Moser , G . 1994 . Brachysira. Monographie der Gattung . Bibl. Diatom. , 29 : 1 – 212 .
- Mann , DG . 1989 . The diatom genus Sellaphora: separation from Navicula . Br. Phycol. J. , 24 : 1 – 20 .
- Mann , DG and Stickle , AJ . 1991 . The genus Craticula . Diatom Res. , 6 : 79 – 107 .
- Metzeltin , D and Lange-Bertalot , H . 2007 . Tropical diatoms of South America II . Icon. Diatom. , 18 : 1 – 877 .
- Poulin , M . 1990 . Sea ice diatoms (Bacillariophyceae) of the Canadian Arctic. I. The genus Stenoneis . J. Phycol. , 26 : 156 – 167 .
- Procopiak , LK , Fernandez , LF and Moreira Filho , H . 2006 . Marine and estuarine diatoms (Bacillariophyta) from Parana, southern Brazil: check-list with emphasis on harmful species. . Biota Neotrop. , 6 Available at: http://www.biotaneotropica.org.br/v6n3/en/abstract?inventory+bn02306032006. Accessed: 28 August 2008
- Round , FE , Crawford , RM and Mann , DG . 1990 . The Diatoms. Biology & Morphology of the Genera , Cambridge, UK : Cambridge University Press .
- Rumrich , U , Lange-Bertalot , H and Rumrich , M . 2000 . Diatoms of the Andes. From Venezuela to Patagonia/Tierre del Fuego. . Icon. Diatom. , 9 : 1 – 649 .
- Simonsen , R . 1987 . Atlas and Catalogue of the Diatom Types of Friedrich Hustedt , Stuttgart, , Germany : J. Cramer Verlag .
- Spaulding , SA and Stoermer , EF . 1997 . Taxonomy and distribution of the genus Muelleria Frenguelli . Diatom Res. , 12 : 95 – 113 .
- Tomas , X . 1987 . Diatomeas de las agues epicontinentales salads del litoral mediterráneo de la Península Ibérica. PhD Thesis. , Barcelona, , Spain : Universidad de Barcelona .
- Van de Vijver , B , Frenot , Y and Beyens , L . 2002 . The freshwater diatom flora of Ile de la Possession (Crozet archipelago, sub-Antarctica) . Bibl. Diatom. , 46 : 1 – 412 .
- Vanhoutte , K , Verleyen , E , Vyverman , W , Chepurnov , V and Sabbe , K . 2004 . The freshwater diatom genus Kobayasiella (Bacillariophyta) in Tasmania, Australia . Aust. Syst. Bot. , 17 : 483 – 496 .
- Witkowski , A , Lange-Bertalot , H and Metzeltin , D . 2000 . Diatom flora of Marine Coasts I . Icon. Diat. , 7 : 1 – 925 .