Abstract
Twelve clonal cultures of the marine pennate diatom Haslea ostrearia (Gaillon) Simonsen were used to study sexual reproduction and auxosporulation. After crossing, compatible gametangia paired girdle to girdle, with no visible surrounding mucilage. Two gametes were formed per gametangium; these were not attached to the thecae of the parental frustule and gametes from one gametangium fused with the gametes from the other gametangium. Sexual reproduction was isogamous, resulting in the production of two zygotes per paired gametangia. The pattern of reproduction in H. ostrearia can be classified as type IB2a according to Geitler's system. The expansion of auxospores, which was bipolar, was accompanied by the formation of a transverse perizonium. Superfluous nuclei resulting from meiosis were not visible. The upper limit of the cell size range suitable for sexualization was c. 68 µm, i.e. about half the maximum species-specific size of about 140 µm. Sexual reproduction has been shown by previous authors to occur in clonal cultures and our observations confirm that two modes of sexual reproduction, homothallic and heterothallic, coexist in H. ostrearia.
Introduction
The pennate diatom Haslea ostrearia (Gaillon) Simonsen (Citation1974) is well known because it produces a blue pigment, marennine, which is responsible for the greening of oyster gills (Gaillon, Citation1820). As far as we are aware, H. ostrearia is the only organism to produce marennine, and this pigment has found applications in aquaculture (the greening phenomenon is exploited to add economic value to oysters), cosmetics, and personal care product industries. The phenol structure of the pigment has recently been ascertained (Pouvreau et al., Citation2006 a, Citation b ), and its allelopathic activity demonstrated (Pouvreau et al., Citation2007). A water-soluble extract from the diatom H. ostrearia has been shown to possess antiviral activities (Bergé et al., Citation1999), while a recent study, Rowland et al. (Citation2001), has reported that highly branched isoprenoid sesterterpenes (haslenes) synthesized by the diatom exhibit cytostatic properties in vitro. In the last few years, some aspects of the ecophysiology of H. ostrearia have been elucidated (Mouget et al., Citation1999, Citation2004, Citation2005; Tremblin et al., Citation2000). Unfortunately, there has not been any corresponding increase in knowledge about the life cycle, breeding system, or other features of the reproductive biology of this species.
Diatoms are basically sexual organisms, and their vegetative cells are diploid (Lewin & Guillard, Citation1963; Drebes, Citation1977; Mann, Citation1988; Round et al., Citation1990; Chepurnov et al., Citation2004). More recent studies indicate that heterothally is common in pennates (e.g. Roshchin, Citation1994; Mann et al., Citation1999; Chepurnov et al., Citation2004) and that sex determination in dioecious pennate species is apparently genotypic (e.g. Mann et al., Citation2003; Davidovich et al., Citation2004), in contrast to the phenotypic sex determination mechanism in centric diatoms (Drebes, Citation1977). Applying this postulate to various pennates has led to rapid progress in our understanding of their mating systems (Chepurnov et al., Citation2004). As a consequence of the peculiar mechanism of diatom cell division, especially in heterothallic species, clonal cultures inevitably wane in the absence of sexual reproduction, as a result of dwindling size. This severely restricts genetic and molecular investigations, as well as posing problems for the mass production of economically useful strains. Unfortunately, sexual reproduction and breeding system have only been investigated in a small proportion of diatom taxa (Mann & Droop, Citation1996; Edlund & Stoermer, Citation1997).
The first accounts of auxosporulation in H. ostrearia (as Navicula ostrearia (Gaillon) Bory) were reported by Neuville & Daste (Citation1975, Citation1979), who described the formation of auxospores in some of their supposedly ‘monoclonal’ cultures, demonstrating homothally in their clones. However, these authors did not perform any crossing experiments and did not investigate whether the species could reproduce heterothallically.
Since the reports by Neuville & Daste (Citation1975, Citation1979), no further data have been published that can assist in unravelling the breeding system of H. ostrearia. However, a closely-related species Haslea subagnita (Proschkina-Lavrenko) Makarova et Karayeva has been demonstrated to be heterothallic (Chepurnov, Citation1993). The work reported here was undertaken to ascertain whether H. ostrearia can reproduce heterothallically.
There can be crucial differences between homo- and heterothallic reproduction in the same species (Geitler, Citation1932), including gamete morphology as well as their behaviour (isogamy vs anisogamy) and fecundity (e.g. Roshchin, Citation1994, Chepurnov et al., Citation2004; Davidovich et al., Citation2004). One of our aims was, therefore, to provide a detailed description of the stages of heterothallic gametogenesis and auxosporulation in H. ostrearia.
Materials and methods
Twelve clones of H. ostrearia, with differing mean cell sizes used in our crossing experiments, were from the Nantes Culture Collection, WDCM 854 (). Ten clones had been isolated by micropipette from samples taken at intervals since 2000 from the oyster-ponds in the Bay of Bourgneuf, France. Two large cells were isolated from S45 in 2004, to establish clones S120 and S121. As mentioned in the Discussion, the origin of the large cells in clone S45 is uncertain, but they may have resulted from intra-clonal reproduction. Stock cultures of all taxa studied have been deposited in the Nantes Culture Collection.
Table 1. Origin of clones of Haslea ostrearia used in crossing experiments.
The cultures were maintained either in artificial seawater (Laboratoire de Physiologie et Biochimie Végétales), as described in Perkins et al. (Citation2006), or in culture media based on natural seawater (Laboratoire de Biologie Marine and Karadag Natural Reserve). Natural seawater was enriched with elements either following the f/2 recipe (Andersen et al., Citation2005), or as recommended by Roshchin (Citation1994) (see also Chepurnov & Mann, Citation1997), plus additional sea-salt to adjust the salinity to c. 30 psu. The algae were transferred between laboratories by mail; this usually took 9–14 days, and on receipt the clones were inoculated into fresh medium. The algae soon adapted to a new medium, and were able to tolerate abrupt changes in salinity, surviving transfer from 30 to 18 psu and vice versa. At Karadag, clones and mixed cultures used for testing sexual compatibility were incubated in glass Petri dishes (90 mm), at 20 ± 2°C in low light under ‘cool-white’ fluorescent tubes (c. 5 µmol photons m–2 s–1; 10-h : 14-h light–dark cycle), or under natural lighting from a north-facing window. In Le Mans, crossing experiments were conducted in a temperature-controlled room at 16 ± 1°C, under low light levels (c. 20 µmol photons m−2 s−1; 6-h : 18-h light–dark cycle), as recommended by Neuville & Daste (Citation1975, Citation1979). Illumination was provided by Philips TLD 36W/965 fluorescent tubes. Crossing experiments were performed by mixing pairs of cultures that had been maintained in the exponential phase of growth by sub-culturing into fresh medium every 5–6 days. Cells divided mitotically at a growth rate of more than 1 division d−1. Each crossing combination was performed at least in duplicate. The mixed cultures were inspected daily for a week for signs of auxosporulation using an MBS-9 light microscope (LOMO, Leningrad, Russia), or a Biolar PI microscope (PZO, Warsaw, Poland) equipped with differential interference contrast optics (Karadag), or under a Nikon TS100 inverted microscope (Le Mans). Images were taken with a digital camera Canon Power Shot A95 by means of ocular projection, or a Sony SSC-C350P colour CCD camera according to Mouget et al. (Citation1999). The size (apical length) of the cells was measured using an ocular-rule and object-micrometer, with a precision of 1.72 µm. Unless specified otherwise, 10 measurements were made to calculate mean values and standard errors (mean ± SE).
Results
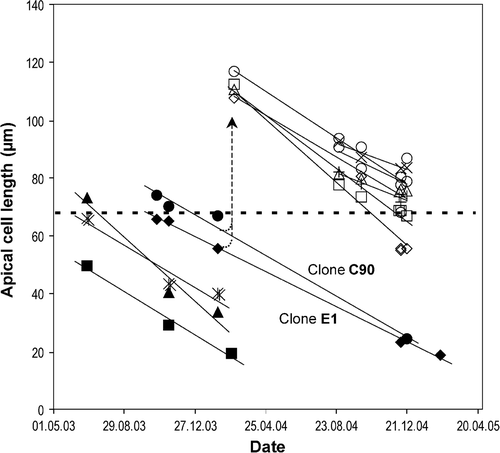
There were no signs of sexual reproduction in any of the clonal cultures in any of the experiments undertaken over a 2-year period. The apical length of cells decreased at an average rate of 3.74 ± 0.33 µm per month (mean ± SE, n = 11), values ranging from 2.4 to 5.4 µm month−1 depending on the clone. The smallest cells capable of sexual reproduction and the largest cells produced as a result of sexual reproduction both decreased in size at similar rates (). Furthermore, cells maintained in culture for a long time often became curved and deformed ().
Fig. 1. Decrease of the apical length of cells in twelve clones of Haslea ostrearia cultivated under stable conditions (see Materials and methods). Horizontal dashed line designates an upper border of the sexually inducible size range. Clones marked with empty markers represent the big-cell descendants arisen as a result of heterothallic sexual reproduction (dashed arrow) of clones E1 (filled diamonds) and C90 (filled circles). Lines were calculated as linear regressions.
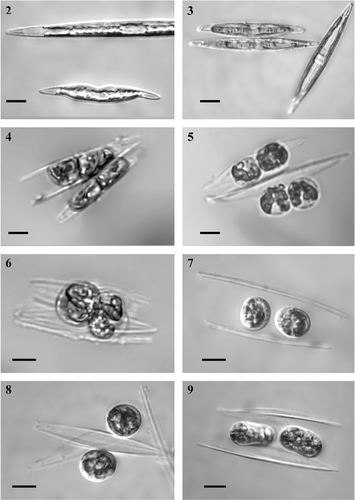
Figs 2–9. Early sexual reproduction stages in Haslea ostrearia, in mixtures of clones ElxC90, differential interference contrast optics. . Small cells, reduced in size during the life cycle (the lower cell) often have various deformities. . Girdle-girdle pairing (on the left); the plastids have moved from the girdle to beneath the valves early in the meiotic prophase, unlike the interphase cell (on the right) where the chloroplasts lie around the girdle. . Two gametes are formed inside each gametangium. . The gametes are morphologically similar. . Allogamous fusion is effected by means of the swelling of the gametes; gametes behave isogamously. . Zygotes slightly contracted after gametic syngamy lie between the thecae of parental cells; note the differing sizes of the thecae. . The zygotes may also be disposed irregularly relatively to gametangial thecae. . Auxospores starting to expand. Scale bars: 10 µm.
Periodically, we mated available clones in various pair-wise combinations (); sometimes three or more clones were mixed together. Sexual reproduction was first recorded in the mixture of clones C90 and E1, when the mean cell apical length of the clones was 66.8 ± 0.3 and 55.6 ± 0.3 µm respectively (means ± SE, n = 10). Sexual reproduction usually involved the pairing and interaction of two cells (gametangia); but occasionally gametangia came together in groups of three or more. The gametangia in pairs always came from different clones, as was demonstrated by the differing lengths of the cells constituting these pairs. As an example, auxospore formation observed in crossing experiment between clones E1 and C90 run on 02.FEB.2003 involved gametangial pairs in which the mean cell lengths were 55.5 ± 0.4 and 65.5 ± 0.4 µm, respectively (mean ± SE, n = 12, ).
Table 2. Results of crossing experiments, apical cell sizes (mean ± SE, n = 10), and cell size ranges (mean ± 2 SD) in Haslea ostrearia clones.
Table 3. Lengths of gametangia in gametangial pairs in the mixture of clones C90 and E1 on 02.02.2003.
Pairs of gametangia aligned side-by-side () and no mucilage was visible around the gametangia. The plastids moved from the girdle to beneath the valves early in the meiotic prophase (). Each gametangium produced two equal sized gametes, which eventually became spherical (). The thecae then moved apart from the rounded gametes (), no longer acting as a barrier preventing the gametes from coming into contact and fusing allogamously (). No differences in morphology, or behaviour, were observed between the two gametes produced by a single gametangium, or between the gametes produced by each of a pair of gametangia. Sexual reproduction was therefore isogamous.
Plasmogamy occurred as a result of close contact between slightly swollen gametes (). After gametic syngamy, the zygotes contracted and became spherical or ellipsoid in shape (). Neither zygotes nor gametes were attached to the gametangial frustules and sometimes lay between (), or near to, the thecae () if their relative position was disturbed by the movement of water, or as a result of contact with moving vegetative cells. The diameter of fully contracted zygotes that determined the diameter of the middle auxospore was on average 12.9 ± 0.5 µm (n = 10). Within a few hours of plasmogamy, the auxospore started to expand (), bursting the primary zygote envelope, which was visible as a thin capsule during the early stage of auxospore growth (). These subsequently persisted as two ‘caps’, one on each tip of the swelling auxo-spore (, ). Growing auxospores were usually approximately parallel to each other and to the gametangia (), but were sometimes positioned asymmetrically relative to the gametangial thecae (). Sometimes single free-floating auxospores occurred in the mixture of parental cultures (). During auxospore expansion, which was bipolar, the two nuclei remained closely associated with one another at the centre of the auxospore, but were still unfused (). No superfluous gametic nuclei were observed. The chloroplasts were usually located at the poles of the swelling auxospore (), which contrasted with the vegetative cells, where the chloroplasts either lay along the girdle during interphase (, a vegetative cell is shown on the right), or shifted to the valve during the stages preceding and during cytokinesis. Perizonia had a transverse structure visible under the light microscope (Figs , ).
Figs 10–17. Auxospore development. . In the early stages of growth, the auxospore is surrounded by a thin, scarcely- visible capsule (arrowheads) that represents remains of primary zygote envelope. Spherical units distended as a result of osmotic pressure (arrow, also ) filled with blue pigment and chloroplasts tended to lie parietally, and may correspond to aborted zygotes, gametes or protoplast remains. . Expanding auxospores, note chloroplasts are usually located at both poles of the swelling auxospore. Growing auxospores are normally more or less parallel to each other and to the gametangia (), but may lie somewhat irregularly in respect to the gametangial thecae (). . Broken zygote envelope halves look like ‘caps’ on each tip of the swelling auxospores (arrowhead, also , ), and remain visible up to the end of initial cell formation. Note the gap (arrow) between the epitheca/hypotheca and the perizonium. . During expansion, the two nuclei remain closely associated with each other at the centre of the auxospore, but are still unfused (arrows, also ). . Single auxospores that have lost their connection with gametangial frustules also occur in the mixture; arrows point to two unfused nuclei. . Perizonium has clearly visible transverse structure (arrows). Note the opening at the end of perizonium (arrowhead, also ). Scale bars, 10 µm.
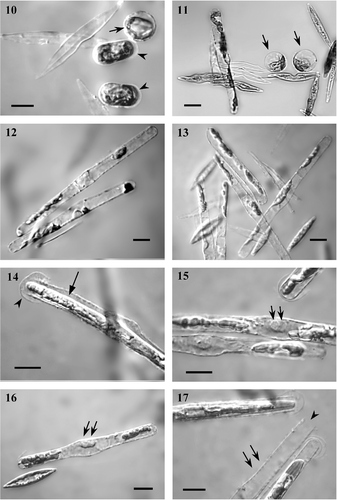
In the mixed cultures where sexual reproduction took place, small pellets resembling zygotes could be seen (); however, they were completely spherical in form, presumably as a result of hyperosmotic pressure. These spheres, which were filled with marennine and chloroplasts, tended to be located parietally () and may have corresponded to aborted zygotes, gametes or protoplast remains.
Figs 18–20. Initial cells. . Initial cells formed inside the perizonium. . The initial cell (arrow) escaping from the perizonium (arrowhead) through the opening at the end (note also ). Fig. 20. If the initial cell cannot escape from the perizonium, the first division occurs inside it. Scale bars, 10 µm.
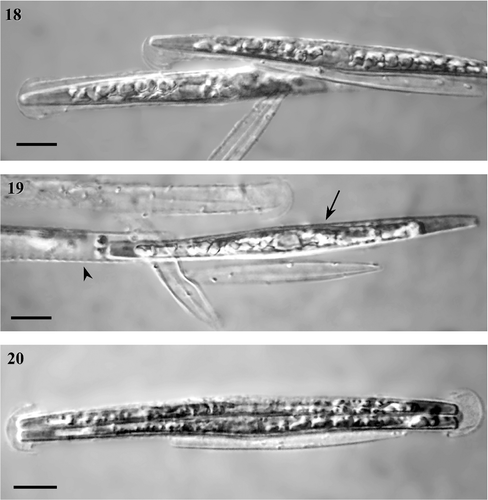
Initial cells were formed within the fully expanded auxospores (). The mean length of the initial cells observed in the E1 × C90 cross was 114.5 ± 0.8 µm (ranging from 101.5 to 127.3 µm, n = 56). We derived 10 new big-cell strains (descendants of crossing E1 and C90) and maintained them in culture for 1 year. We made periodic attempts to mate them with each other, or with the parental clones, but were unsuccessful.
The initial cell was formed inside the auxospore in two steps. First, the epitheca was created, and then the hypotheca. In each case, theca formation was accompanied by contraction of the protoplast, leading to a visible gap between the epitheca or hypotheca and the auxospore wall (). The initial cell slid out of the end of the perizonium, which had probably been lysed (Figs , ). In a few cases, the initial cell did not escape from the perizonium immediately and the first vegetative division occurred inside the perizonium ().
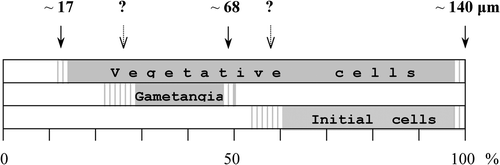
The data obtained during this study allowed us to plot a diagram showing the position of cell length critical thresholds, or ‘cardinal points’ (Geitler, Citation1932), illustrating the life cycle of H. ostrearia ().
Fig. 21. Diagram showing the relative position of the apical size of gametangial, initial, and vegetative cells in the course of the life cycle of Haslea ostrearia. The maximal species-specific size is about 140 µm, the upper border of the cell size range suitable for sexual reproduction is about 68 µm. The position of some cardinal points (marked by question marks) must be ascertained more precisely.
Discussion
Cell size and cardinal points
Haslea ostrearia is incapable of sexual reproduction until the cell length reaches a cardinal point (Geitler, Citation1932). In our experiments this corresponded to about 68 µm in length. The same critical size (c. 65 µm) had previously been reported by Neuville & Daste (Citation1975). These authors also observed that the largest cells produced as a result of auxosporulation measured about 140 µm and that below 50 µm long the cells lost their ability to reproduce sexually as well as acquiring characteristic deformities heralding impending culture decline. The smallest vegetative cells we observed were about 17 µm long. These were deformed, immobile, and clustered on the bottom of the Petri dish. The smallest initial cells observed in the mixtures had an apical size of c. 75 µm, and the biggest measured 138 µm. These data were used to illustrate the position of the cardinal points in the life cycle of H. ostrearia ().
The upper limit of the sexually inducible size range is very close to 50% of the maximum species-specific cell size. This observation is not surprising, given that in most of the diatoms that have been investigated, the upper critical size for sexualization corresponds to about one half the maximum size (Davidovich, Citation2001). Attempts to mate descendants of crossing C90 and E1, which had been maintained in culture for one year, were unsuccessful, apparently because of inappropriate cell sizes (see ).
Neuville & Daste (Citation1975) reported that auxospores appeared in five out of the six clones they studied, the sixth (clone M) may have been incapable of sexual reproduction, because of its small mean cell size (50 µm). The smallest gametangial cells involved in sexual reproduction in our experiments measured 27 ± 0.3 µm. This implies that the size range of gametangia corresponds to about 33% of the full size range. In comparison, in H. subagnital the gametangial size range corresponds to c. 35% of the full size range (Chepurnov, Citation1993; Roshchin, Citation1994). The gametangia size range is thus ‘closed’, i.e. the smallest cells, measuring 17–27 µm, while continuing to divide vegetatively, are unable to enter sexual reproduction, apparently because of their considerable deformities and loss of mobility. This parallels the closed range found in H. subagnita (Chepurnov, Citation1993; Roshchin, Citation1994). The discrepancy between our findings and previous evaluations of the minimum sexual size threshold in H. ostrearia might be attributable to the different ‘size windows’ (corresponding to hetero- and homothallic reproduction), which were narrower in the latter (Roshchin, Citation1994; Chepurnov & Mann, Citation1997). Nevertheless, more data are required to determine more accurately the minimum size of the gametangia and of initial cells corresponding to each type of reproduction behaviour.
Sexual reproduction and auxospore formation
Our findings confirm the original description of sexual reproduction and auxospore formation in H. ostrearia given by Neuville & Daste (Citation1975, Citation1979). As the process was observed in clonal cultures, it must be regarded as homothallic. The mating system of some pennate diatoms is known to combine homo- and heterothallic modes of sexual reproduction. Diatoms such as Nitzschia lanceolata W. Smith, Navicula pennata A. Schmidt var. pontica Mereschkowsky (Roshchin, Citation1994), Achnanthes longipes C.A. Agardh (Chepurnov & Mann, Citation1997) have all been shown to produce gametes that are morphologically isogamous, irrespective of the mode of reproduction. Whereas in others, e.g. Tabularia tabulata (C.A. Agardh) Snoeijs (as Synedra tabulata), Fragilaria delicatissima Proshkina-Lavrenko (Roshchin, Citation1994), Nitzschia longissima (Brébisson) Ralfs (Davidovich et al., Citation2004), intra- and inter-clonal reproduction have been demonstrated to be isogamous and anisogamous respectively.
The pattern of sexual reproduction we observed corresponds to type IB2a in Geitler's classification (Geitler, Citation1973; Round et al., Citation1990; Mann, Citation1993). Briefly, two functional gametes were produced by each laterally-paired gametangium and hence two zygotes were yielded per copulation. Unfortunately, we were unable to observe gamete rearrangement, but judging from final disposition of the gametes inside the gametangial frustules (see ) the process did indeed take place. Morphologically and behaviourally, the gametes were strictly isogamous. The apical axes of the gametes and auxospores were more or less parallel and this was most obvious during the early stages of auxospore expansion. However, in many cases the relative positions of gametangial thecae and auxospores seemed to be disrupted, possibly due to the lack of abundant mucilage, a feature also initially reported by Neuville & Daste (Citation1975), but which was not confirmed in their later publication (Neuville & Daste, Citation1979), and because the zygotes and gametangial thecae were unattached. During auxospore expansion, fusion of the two nuclei could not be observed and further observations are needed, some involving nuclear staining techniques, to trace the superfluous gametic nuclei.
Type IB2a sexual reproduction has also been reported in a related species, H. subagnita (Chepurnov, Citation1993), although in this case there may be a more stable connection between the auxospores and gametangial thecae. Other diatoms that are known to follow the same (IB2a) pattern of sexual reproduction include: Berkeleya micans (Lyngbye) Grunow (Davidovich, Citation1999), B. rutilans (Trentepohl) Grunow (Tschermak-Woess, Citation1973), Navicula cryptocephala Kützing (Geitler, Citation1958), N. directa (W. Smith) Ralf in Pritchard (Mizuno, Citation2000), N. oblonga (Kützing) Kützing (Mann & Stickle, Citation1989), N. radiosa Kützing (Geitler, Citation1952), and Seminavis cf. robusta Danielidis & Mann (Chepurnov et al., Citation2002). In an early description of auxosporulation in another Haslea species, H. crucigera (as Dickieia crucigera), Karsten (Citation1897) described stages of auxospore formation that are generally consistent with the pattern we observed in H. ostrearia. On the whole, most of the features of sexual reproduction in H. ostrearia follow the same pattern as that seen in other raphid diatoms related to the Navicula group (Mann & Stickle, Citation1989), demonstrating that they are closely related.
Protoplasts found in sexually reproduced cultures were divided into two types by Neuville & Daste (Citation1975): spherical protoplasts with cellular material that condensed to form a cap-like shape (Neuville & Daste, Citation1975, p. 1754, cf. our ) and ovoid ones, which were more uniform. We assume that the former were aborted protoplasts, and the latter were zygotes. There are several reasons why these spherical protoplasts arise in cultures. In older cultures (in the late stationary phase of growth), released protoplasts are common. They have a globular form, presumably as a result of osmotic pressure. In sexually-reproducing cultures, unfused gametes floating away from the gametangia are also frequent, and no mucilage capsule is synthesized around the gametes detached from mother frustules (Mann & Stickle, Citation1989; Roshchin & Chepurnov, Citation1994; Chepurnov et al., Citation2002). On the other hand, unfused gametes are sometimes capable of being transformed into auxospores (haploid parthenogenesis) (e.g. Mann, Citation1994; Roshchin & Chepurnov, Citation1994). This is regarded as being quite common in pennate diatoms (Chepurnov et al., Citation2004). We have not studied the progeny of H. ostrearia thoroughly enough to rule out the possibility of haploid parthenogenesis in this alga. However, further observation has shown that the spherical protoplasts subsequently deteriorated without becoming auxospores.
Breeding system
Among the diatoms with type IB2a auxosporulation, heterothallism has been confirmed in H. subagnita (Chepurnov, Citation1993), B. micans (Davidovich, Citation1999), N. directa (Mizuno, Citation2000) and Seminavis cf. robusta (Chepurnov et al., Citation2002). Furthermore, auxospore formation has been shown to occur in both clonal and mixed cultures in N. directa (Mizuno, Citation2000), demonstrating the coexistence of homothallism and heterothallism in this species. In the case of H. ostrearia, it is possible that the strains studied by Neuville & Daste (Citation1975, Citation1979) appeared to reproduce only intra-clonally because no mating experiments were conducted. Apparently these authors did not cross their clones and if their cultures were clonal, then it must be concluded that H. ostrearia is homothallic. In the present study, none of the 12 clones so far examined has shown any signs of undergoing sexual reproduction in clonal culture. Transition to sexual reproduction could only be initiated if two or more sexually compatible clones were mixed, although light and cell density were also important factors controlling sexualization (unpublished). Sexual reproduction involved the pairing and interaction of two cells (gametangia) belonging to different clones, which were then described as being sexually compatible. The fact that this interaction was inter-clonal was easily demonstrated by mixing two clones of differing cell size. Any gametangial pair we observed in such a mixture always consisted of pairs of cells of different sizes.
Intra-clonal reproduction was not detected in any of our experiments, but pair-wise crossings between certain combinations did have a positive outcome (). Therefore, clones E1 and E4 may be regarded as belonging to one mating type, because they did not mate with each other, but did successfully pair with almost all the other clones tested. We can therefore conclude that sexual reproduction is heterothallic in all the H. ostrearia clones so far studied in our laboratories. However, some results are difficult to explain, for instance, clone D3,5 gave a negative result when crossed with E1, or with most other clones, but reproduced when it was crossed with E4. The unsuccessful mating of E4 with S120, S121, D2, and Pl IIA2 could have been due to unfavourable conditions during these experiments. On the other hand, in some combinations, just a few auxospores were observed. It is well known that not every set of experiments gives positive results that can be detected afterwards, particularly as the cells become smaller (e.g. Chepurnov et al., Citation2002).
In conclusion, this work demonstrates that H. ostrearia is not only homothallic, as previously demonstrated by Neuville & Daste (Citation1975, Citation1979), but also heterothallic. Furthermore, in this species, the pattern of inter-clonal sexual reproduction is morphologically isogamous, and does not differ from the intra-clonal pattern. We can therefore conclude that heterothally, even though it is thought to be common in pennate diatoms, is not an obligate breeding mechanism in some species, such as H. ostrearia. Moreover, new and indirect evidence (cell size distribution and abrupt changes in cell size) of clonal reproduction in other clones of H. ostrearia maintained in the Nantes Culture Collection has recently been obtained (Cognie, unpublished). Additional work is therefore required to assess the possible coexistence and relative importance of two alternative breeding patterns in H. ostrearia, as well as the factors which control heterothallism and homothallism in this species. Our increased understanding of the life cycle and mating system of H. ostrearia and of how sexualization and auxospore formation are controlled are clues that offer new perspectives for the maintenance of diatoms in culture collections and for exploring the genetics of these unusual algae. This would also ensure that new clones could be produced ‘on demand’, for industrial exploitation to produce biomass, pigment, or active compounds.
Acknowledgements
We would like to thank Guillaume Massé and Yves Rincé for stimulating discussion. We would also like to thank the two anonymous reviewers for their valuable comments, which have allowed us to improve this manuscript considerably. Some of the funding for this work was provided by the PHC ‘Dnipro-2007’ program.
References
- Andersen , RA , Berges , JA , Harrison , PJ and Watanabe , MM . 2005 . “ Recipes for freshwater and seawater media ” . In Algal Culturing Techniques , Edited by: Andersen , RA . 429 – 538 . Amsterdam, , The Netherlands : Elsevier .
- Bergé , J-P , Bourgougnon , N , Alban , S , Pojer , F , Chermann , J-C , Billaudel , S , Robert , J-M , Durand , P and Franz , G . 1999 . Antiviral and anticoagulant activities of a water soluble compound extracted from the marine diatom Haslea ostrearia . Planta Med. , 65 : 604 – 609 .
- Chepurnov , VA . 1993 . Polovoj protsess u dvudomnoj vodorosli Haslea subagnita (Pr.-Lavr.) Makar. et Kar. (Bacillariophyta) . Algologiya , 3 : 37 – 40 . (in Russian)
- Chepurnov , VA and Mann , DG . 1997 . Variation in the sexual behaviour of natural clones of Achnanthes longipes (Bacillariophyta) . Eur. J. Phycol. , 32 : 147 – 154 .
- Chepurnov , VA , Mann , DG , Sabbe , K and Vyverman , W . 2004 . Experimental studies on sexual reproduction in diatoms . Int. Rev. Cytol. , 237 : 91 – 154 .
- Chepurnov , VA , Mann , DG , Vyverman , W , Sabbe , K and Danielidis , DB . 2002 . Sexual reproduction, mating system, and protoplast dynamics of Seminavis (Bacillariophyceae) . J. Phycol. , 38 : 1004 – 1019 .
- Davidovich , NA . 1999 . Polovoe vosproizvedenie Berkeleya micans (Lyngb.) Grun. (Bacillariophyta) . Algologiya , 9 : 3 – 12 . (in Russian)
- Davidovich , NA . Species specific sizes and size range of sexual reproduction in diatoms . Proceedings of the 16th International Diatom Symposium . 25 August–1 September 2000 , Athens & Aegean Islands. Edited by: Economou-Amilli , A . pp. 191 – 196 . Athens, , Greece : Amvrosiou Press .
- Davidovich , NA , Kaczmarska , I and Ehrman , JM . The sexual structure of a natural population of the diatom Nitzschia longissima . (Bréb.) Ralfs. In Abstracts of the 18th International Diatom Symposium. 2–7 September 2004 , Miedzyzdroje. Edited by: Witkowski , A . Vol. 117 , Poland : University of Szczecin .
- Drebes , G . 1977 . “ Sexuality ” . In The Biology of Diatoms , Edited by: Werner , D . Vol. 13 , 250 – 283 . Oxford, , UK : Blackwell Scientific Publications . Botanical Monographs
- Edlund , MB and Stoermer , EF . 1997 . Ecological, evolutionary, and systematic significance of diatom life histories . J. Phycol. , 33 : 897 – 918 .
- Gaillon , B . 1820 . Des huitres vertes, et des causes de cette coloration . Annales Generales des Sciences Physiques , 7 : 89 – 94 .
- Geitler , L . 1932 . Der Formwechsel der pennaten Diatomeen (Kieselalgen) . Arch. Protistenk. , 78 : 1 – 226 .
- Geitler , L . 1952 . Untersuchungen über Kopulation und Auxosporenbildung pennaten Diatomeen: III. Gleichartigkeit der Gonenkerne und Verhalten des Heterochromatins bei . Navicula radiosa. Österr. Bot. Z. , 99 : 469 – 482 .
- Geitler , L . 1958 . Notizen über Rassenbildung, Fortpflanzung, Formwechsel und morphologische Eigentümlichkeiten bei pennaten Diatomeen . Österr. Bot. Z. , 105 : 408 – 442 .
- Geitler , L . 1973 . Auxosporenbildung und Systematik bei pennaten Diatomeen und die Cytologie von Cocconeis-Sippen . Österr. Bot. Z. , 122 : 299 – 321 .
- von Karsten , G . 1897 . Untersuchungen über Diatomeen. III . Flora. , 83 : 203 – 221 .
- Lewin , JC and Guillard , RRL . 1963 . Diatoms . Ann. Rev. Microbiol. , 17 : 373 – 414 .
- Mann , DG . 1988 . “ Why didn't Lund see sex in Asterionella? A discussion of the diatom life cycle in nature ” . In Algae and the Aquatic Environment , Edited by: Round , FE . 384 – 412 . Bristol, , UK : Biopress Ltd. .
- Mann , DG . 1993 . Patterns of sexual reproduction in diatoms . Hydrobiologia , 269/270 : 11 – 20 .
- Mann , DG . 1994 . Auxospore formation, reproductive plasticity and cell structure in Navicula ulvacea and the resurrection of the genus Dickieia (Bacillariophyta) . Eur. J. Phycol. , 29 : 141 – 157 .
- Mann , DG , Chepurnov , VA and Droop , SJM . 1999 . Sexuality, incompatibility, size variation, and preferential polyandry in natural populations and clones of Sellaphora pupula (Bacillariophyceae) . J. Phycol. , 35 : 152 – 170 .
- Mann , DG , Chepurnov , VA and Idei , M . 2003 . Mating system, sexual reproduction, and auxosporulation in the anomalous raphid diatom Eunotia (Bacillariophyta) . J. Phycol. , 39 : 1067 – 1084 .
- Mann , DG and Droop , SJM . 1996 . 3. Biodiversity, biogeography and conservation of diatoms . Hydrobiologia , 336 : 19 – 32 .
- Mann , DG and Stickle , AJ . 1989 . Meiosis, nuclear cyclosis, and auxospore formation in Navicula sensu stricto (Bacillariophyta) . Br. Phycol. J. , 24 : 167 – 181 .
- Mizuno , M . 2000 . Sexual reproduction of the marine diatom Navicula directa var. directa . Phycological Res. , 48 : 103 – 106 .
- Mouget , J-L , Rosa , P and Tremblin , G . 2004 . Acclimation of Haslea ostrearia to light of different spectral qualities–confirmation of ‘chromatic adaptation’ in diatoms . J. Photochem. Photobiol., B, Biol. , 75 : 1 – 11 .
- Mouget , J-L , Rosa , P , Vachoux , C and Tremblin , G . 2005 . Enhancement of marennine production by blue light in the diatom Haslea ostrearia . J. Appl. Phycol. , 17 : 437 – 445 .
- Mouget , J-L , Tremblin , G , Morant-Manceau , A , Morançais , M and Robert , J-M . 1999 . Long-term photoacclimation of Haslea ostrearia (Bacillariophyta): effect of irradiance on growth rates, pigment content and photosynthesis . Eur. J. Phycol. , 24 : 109 – 115 .
- Neuville , D and Daste , P . 1975 . Observations préliminaires concernant l’auxosporulation chez la diatomée Navicula ostrearia (Gaillon) Bory en culture in vitro . C. R. Acad. Sci. Paris , 281 ( Série D ) : 1753 – 1756 .
- Neuville , D and Daste , P . 1979 . Observations concernant les phases de l’auxosporulation chez la diatomée Navicula ostrearia (Gaillon) Bory en culture in vitro . C. R. Acad. Sci. Paris. , 288 ( Série D ) : 1496 – 1498 .
- Perkins , RG , Mouget , J-L , Lefebvre , S and Lavaud , J . 2006 . Light response curve methodology and possible implications in the application of chlorophyll fluorescence to benthic diatoms . Mar. Biol. , 149 : 703 – 712 .
- Pouvreau , JB , Housson , E , Le Tallec , L , Morançais , M , Rince , Y , Fleurence , J and Pondaven , P . 2007 . Growth inhibition of several marine species induced by the shading effect and allelopathic activity of marennine, a blue-green polyphenolic pigment of the diatom Haslea ostrearia (Gaillon/Bory) Simonsen . J. Exp. Mar. Biol. Ecol. , 352 : 212 – 225 .
- Pouvreau , JB , Morançais , M , Fleury , F , Rosa , P , Thion , L , Cahingt , B , Zal , F , Fleurence , J and Pondaven , P . 2006b . Preliminary characterisation of the blue-green pigment marennine from the thycopelagic diatom H. ostrearia (Gaillon/Bory) Simonsen . J. Appl. Phycol. , 18 : 757 – 767 .
- Pouvreau , JB , Morançais , M , Massé , G , Rosa , P , Robert , J-M , Fleurence , J and Pondaven , P . 2006a . Purification of the blue-green pigment marennine from the marine thycopelagic diatom H. ostrearia (Gaillon/Bory) Simonsen . J. Appl. Phycol. , 18 : 769 – 781 .
- Roshchin , AM . 1994 . Zhiznennye tsikly diatomovykh vodoroslej , 170 Kiev, , Russia : Naukova Dumka . (in Russian)
- Roshchin , AM and Chepurnov , VA . 1994 . Allogamnyj polovoj protsess i gaploidnyj partenogenez u dvudomnoj vodorosli Licmophora ehrenbergii (Kütz.) Grun. (Bacillariophyta) . Algologiya , 4 : 3 – 10. . (in Russian)
- Round , FE , Crawford , RM and Mann , DG . 1990 . The Diatoms. Biology and Morphology of the Genera , Cambridge, , UK : Cambridge University Press .
- Rowland , SJ , Belt , ST , Wraige , EJ , Massé , G , Roussakis , C and Robert , J-M . 2001 . Effects of temperature on polyunsaturation in cytostatic lipids of Haslea ostrearia . Phytochemistry , 56 : 597 – 602 .
- Simonsen , R . 1974 . The diatom plankton of the Indian Ocean Expedition of R/V Meteor 1964-5 . Meteor Forsch.-Ergebnisse Reihe D. , 19 : 1 – 107 .
- Tremblin , G , Cannuel , R , Mouget , J-L , Rech , M and Robert , J-M . 2000 . Change in light quality due to a blue-green pigment, marennine, released in oyster-ponds: effect on growth and photosynthesis in two diatoms, Haslea ostrearia, and Skeletonema costatum . J. Appl. Phycol. , 12 : 557 – 566 .
- Tschermak-Woess , E . 1973 . Die geschlechtliche Fortpflanzung von Amphipleura rutilans und das verschiedene Verhalten der Erstlingszellen von Diatomeen in Gallertschlauchen . Österr. Bot. Z. , 122 : 21 – 34 .