Abstract
The wavelength dependency of xanthophyll cycling in two marine microalgae (Thalassiosira weissflogii and Dunaliella tertiolecta) was studied by establishing biological weighting functions (BWFs) during exposure to natural ultraviolet radiation. High-(HL) and low-(LL) light-acclimated cultures of both species were exposed outdoors for up to 60 min under a series of UVR (280–400 nm) cut-off filters, after which the de-epoxidation state of xanthophyll cycle pigments, radiocarbon assimilation and photochemical quantum yield were measured. Exposures were repeated 4–8 times during the daily cycle to create exposure–response curves for each wavelength condition. UVR affected the three target processes significantly in both species and biological weights increased with decreasing wavelength, particularly in the UVBR region (280–315 nm). Minor wavelength dependency was observed between 315 and 360 nm. After BWF normalization to 300 nm, the LL cultures showed highly similar responses when comparing the three target processes, while the BWFs for the HL cultures differed significantly. The observed enhanced xanthophyll cycling activity in the UVR region implied that xanthophylls had an active role in diminishing UVR stress. However, this enhancement seems to be an indirect effect of damage within the dark reactions of photosynthesis. Hence, another vital target process further downstream in the photosynthetic process, possibly involved in the dark reactions, seems to be responsible for the high similarity in BWFs.
Introduction
Ultraviolet radiation exposure may cause adverse effects in marine microalgae, ultimately leading to reduced growth or viability loss. Natural ultraviolet A radiation (UVAR; 315–400 nm) and ultraviolet B radiation (UVBR; 280–315 nm) reduce primary productivity in a vast range of phytoplankton assemblages, as shown by in situ radiocarbon incorporation measurements. Other cellular targets that are studied to monitor adverse UVR effects include DNA damage or the reduction in PSII efficiency (Villafañe et al., Citation2003). Direct comparisons between the alleged responsible target processes for UVR stress and their possible functional relationships are rare. At the same time, comparison of studies addressing UVR effects is frustrated by differences in species-specificity, quantitative and qualitative irradiance conditions or experimental duration. Yet, whatever the underlying target processes, natural UVR is indisputably a common stress factor in marine microalgal assemblages. Photosynthetic organisms may respond to excess irradiance, including excess PAR (400–700 nm), by enhancing D1 protein turnover, inducing DNA repair mechanisms, or by synthesizing protective pigments. At the regulatory level, damage to PSII is avoided by utilizing quenching pathways that may prevent accumulation of reactive oxygen species (ROS). Non photochemical quenching (NPQ) refers to processes such as state transitions, representing a change in the relative antenna size of PSII and PSI, and high energy state quenching by heat dissipation via xanthophyll cycle activity (Ting & Owens, Citation1993; Horton et al., Citation1999; Finazzi et al., Citation2006). Some microalgae are thought to use state transitions or changes in the PSII core as their major form of NPQ (van Leeuwe et al., Citation2005; Finazzi et al., Citation2006; Eisenstadt et al., Citation2008). For other marine algae NPQ was found to be strongly linearly related with xanthophyll cycle activity (Olaizola & Yamamoto, Citation1994; Schofield et al., Citation1998; Dijkman & Kroon, Citation2002; Ruban et al., Citation2004). In plants and green algae, the process of energy dissipation by xanthophyll cycling is associated with the reversible de-epoxidation of violaxanthin to its active heat-dissipating forms zeaxanthin and antheraxanthin (VAZ). For algae in the golden-brown lineage de-epoxidation from diadinoxanthin to diatoxanthin (DD/DT) occurs. In plants, the xanthophyll cycle is thought to be activated when the acidification of the lumen, by the proton pump, is sufficiently high and the pH has dropped below a critical point (Yamamoto et al., Citation1999; Muller et al., Citation2001). Conversely, epoxidation would occur during low irradiance or darkness, when ΔpH is reduced.
Information on the role of xanthophyll cycling in preventing or reducing UVR damage is contradictory. First of all, UVBR was found to induce new synthesis of xanthophyll pigments, increasing xanthophyll/photosynthetic (xan/phot) pigment ratios, thereby indicating an active role of xanthophyll pigments in UVBR protection (Buma et al., Citation1996, 2000; Prezelin et al., Citation1998; Goss et al., Citation1999; Moisan & Mitchell, Citation1999; Zudaire & Roy, Citation2001). Secondly, it was suggested that UVBR-induced viability loss in marine microalgae was prevented by pre-acclimating the cultures to high irradiance that induced increased xan/phot ratios (van de Poll et al., Citation2005, Citation2006). Thirdly, enhanced de-epoxidation was described for a picophytoplankter exposed to UVR (Sobrino et al., Citation2005). In contrast, other studies showed a negative impact of UVR on xanthophyll cycling, in particular the enzymes involved in xanthophyll conversion (Pfundel et al., Citation1992; Bischof et al., Citation2002; Mewes & Richter, Citation2002). Consequently, a rather unclear view exists of the significance and effectiveness of xanthophyll cycle activity in preventing UVR stress in microalgae.
Biological weighting functions (BWFs) are an essential tool to understand and predict the UVR wavelength dependency of biological processes. Until recently, BWFs based on inhibition of carbon assimilation showed variability due to species specificity, habitat origin, nutrient status or irradiance history (Cullen et al., Citation1992; Boucher & Prezelin, Citation1996; Neale et al., Citation1998; Litchman et al. Citation2002; Villafañe et al., Citation2004). Andreasson & Wangberg (Citation2006) compared BWFs (<360 nm) of PSII quantum yield and inhibition of carbon assimilation in three microalgal species. This study showed differences in UVBR responses for both parameters, with a lower sensitivity for PSII photochemical quantum yield as compared with carbon assimilation (Andreasson & Wangberg, Citation2006). In addition, Sobrino et al. (Citation2005) presented a BWF for xanthophyll de-epoxidation in the marine picoplankter Nannochloropsis gaditana, demonstrating enhanced de-epoxidation in the UVR region.
In the present study we compared the impact of solar UVR on three target processes: xanthophyll cycle activity, carbon assimilation and PSII fluorescence (e.g. photochemical quantum yield) by constructing BWFs for all three parameters simultaneously. To facilitate realistic wavelength ratios and thus realistic relative proportions of damage/repair process, the cultures were exposed to natural solar irradiance under relevant UVR cut-off filters. We used two microalgae with different xanthophyll cycles: a green flagellate (Dunaliella tertiolecta: VAZ cycle) and a diatom (Thalassiosira weissflogii: DD/DT cycle). Finally, in order to examine the impact of the state of conversion of the xanthophyll cycling on the wavelength dependency of the other two targets, xan/phot ratios were varied by pre-acclimating both species to high (HL) and low (LL) irradiance.
Materials and methods
Cultures
Dunaliella tertiolecta (Dunal) Teodoresco (Chlorophyceae) and Thalassiosira weissflogii (Grunow) G. Fryxell et Hasle (Bacillariophyceae) were obtained from the Algal Culture Collection of Estación de Fotobiología Playa Unión (EFPU). Between 1 and 2 l of Thalassiosira weissflogii and Dunaliella tertiolecta were acclimated to low (60 µmol m−2 s−1), or high (200 µmol m−2 s−1), photosynthetically active radiation (PAR) for at least 1 week prior to the outdoor exposures. Pre-acclimation was in culture chambers at 20°C, in a 16-h : 8-h light–dark cycle. Cultures were diluted daily with F/2 medium (Guillard & Ryther, Citation1962), based on pre-filtered, sterilized natural seawater from the Argentinean Sea, in order to maintain maximal growth under the imposed conditions.
Experiments and sampling
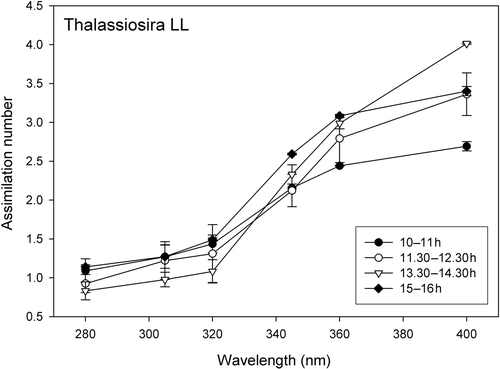
Experiments were conducted outside the EFPU, Playa Unión, Chubut, Argentina (43°S; 65°W) during January 2006. Outdoor exposure experiments were performed on January 16 and 18 for D. tertiolecta LL and HL, respectively. Experiments with T. weissflogii LL were performed on January 20, while T. weissflogii HL was incubated on January 24 and January 26. On the day of the outdoor exposures, the cultures were diluted four- to five-fold (v/v) with fresh F/2 medium. Samples were then exposed 4–8 times (30 and 60 min) over a 24 h period, exposing cultures under all wavelength conditions simultaneously, followed by simultaneous sampling for carbon assimilation, effective quantum yield and xanthophyll cycling. Just prior to each outdoor incubation (t0), samples for HPLC, chlorophyll a analyses and Pulse Amplitude Modulated (PAM) fluorescence measurements were taken from the pre-cultures. In addition, 2 ml of culture was fixed with formalin (final concentration 0.5%) and stored at 4°C, until flow cytometric analysis of cell density which was carried out at the University of Groningen – RuG (see below). All outdoor exposures were in screw-capped quartz tubes; 20 ml for carbon incorporation and PAM measurements and 50 ml for xanthophyll pigment measurements. For each wavelength treatment six tubes were placed in a black aluminum frame, and covered with Schott cut-off filters (WG280, WG305, WG320, WG345, WG360) and Plexiglas UF-3 (cut-off at 400 nm) thereby creating in total six spectral UV conditions. Subsequently, the tubes were exposed outdoors in a temperature controlled water bath (20.0 ± 1.5°C). Two out of the six tubes per wavelength treatment were ‘spiked’ with radiocarbon to measure carbon incorporation (see below). After 30 min two tubes of each wavelength treatment were collected and processed for HPLC and PAM fluorescence measurements. After 60 min, the remaining four tubes were harvested for HPLC, PAM fluorescence measurements and carbon incorporation. At all times, the cultures were immediately taken inside and processed. To establish exposure–response curves for BWF construction, the above described exposure experiments were done for each species and acclimation condition (i.e. LL or HL). In order to ascertain the largest possible range in irradiance levels, these experiments were evenly distributed over the daily cycle (first incubation starting ca. 10:00 h and last incubation starting ca. 16:00 h). An example of a dataset for carbon assimilation, obtained during four 1 h exposures is given in (T. weissflogii LL culture), with the starting times for the incubations indicated in (arrows, lowest panel UVBR irradiance). In order to keep the sample processing time to a minimum, we chose the above-described design, instead of performing simultaneous incubations, using neutral density screens (all irradiance levels simultaneously). This would increase the number of samples beyond the capability of rapid processing (see below under HPLC analysis), required for all the parameters under study. It should be noted that in our experimental set-up the ratio UVB/UVA/PAR changed throughout the day and thus from one incubation to the other. On the other hand, this approach accounted for the natural spectral variability experienced by the cells without disturbing BWF calculations (based on dose–response curves of measured irradiances). Furthermore, the initial conditions of the cultures changed slightly during the day (mean and standard deviation are presented in ). However, these changes were taken into account as BWF data were normalized to t = 0 values for each incubation series.
Carbon assimilation
Samples were inoculated with 5 µCi (0.185 MBq) of labeled sodium bicarbonate (Steeman-Nielsen, Citation1952). After the incubation, the cultures were filtered onto Whatman GF/F filters (25 mm) and exposed to HCl fumes overnight. The filters were dried and subsequently counted using liquid scintillation techniques. Carbon incorporation was then determined from c.p.m. values (Holm-Hansen & Helbling, Citation1995). Assimilation numbers were calculated by normalization to the chl a concentration.
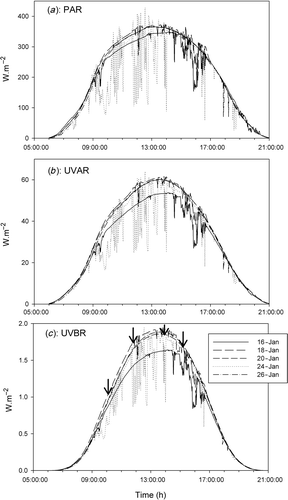
Fig. 2. Incident photosynthetically active radiation (PAR) (a), ultraviolet A radiation (UVAR) (b), and ultraviolet B radiation (UVBR) (c) during the experimental days.
Samples for chl a determination (50 ml) were taken prior to the experiments, filtered onto Whatman GF/F filters (25 mm) and stored at –20°C until analysis after the last exposure series on an experimental day. Pigments were extracted in the dark (1 h, 4°C) in 7 ml of absolute methanol (Holm-Hansen & Riemann, Citation1978) after sonication for 15 min (20°C). Then the samples were centrifuged and the supernatants scanned between 250 and 750 nm using a spectrophotometer (Hewlett Packard model HP-8453E). The same extract was used to determine chl a concentration fluorometrically (Turner Designs model TD 700) from the readings before and after acidification with 1N HCl (Holm-Hansen et al., Citation1965). Both determinations were in excellent agreement with HPLC based chl a determinations (results not shown).
Table 1. Pre-experimental cell characteristics.
Fluorescence parameters
PAM fluorescence parameters were determined within 1 min after sample collection, using a portable pulse amplitude modulated fluorometer (PAM - WATER-ED, Walz, Germany). The photosynthetic quantum yield (Y) was determined by measuring the instant maximal fluorescence () and the steady state fluorescence (Ft) of light-adapted cells using a saturating white light pulse (∼5300 μmol photons m−2 s−1 in 0.8 s) in the presence of a weak measuring actinic light. The yield was calculated according to van Kooten & Snel (Citation1990) and Genty et al. (Citation1989) as:
Pigments
For HPLC analysis of pigments, aliquots of 50 ml of culture were filtered onto Whatman GF/F filters (25 mm) and immediately stored in liquid nitrogen, typically within 2 minutes after sampling, before transportation to The Netherlands. Here, filters were freeze-dried (48 h) and pigments extracted in 4 ml 90% cold acetone (v/v, 48 h, 5°C). All handling steps were done under dim light. Pigments were resolved using HPLC (Waters 2690 separation module, 996 photodiode array detector) with a C18 5 µm DeltaPak reversed-phase column (Milford, MA, USA) and identified by retention time and diode array spectroscopy (Van Leeuwe et al., Citation2006).
Pigment quantification was done using standard dilutions of chlorophyll a, b, c2, c3, fucoxanthin, diadinoxanthin (DD), diatoxanthin (DT), zeaxanthin (Zea), lutein, antheraxanthin (Anthera), and violaxanthin (Viola). Cell counts, as determined on a Coulter Epics MXL flow cytometer [Beckman Coulter (van de Poll et al., Citation2005)] were used to calculate cellular pigment concentrations. De-epoxidation states (DEPS) were calculated as DT/(DT + DD) for T. weissflogii and as (Zea + Anthera)/(Zea + Anthera + Viola) for D. tertiolecta (Qui et al., Citation2003). No correction was done for DD and Violaxanthin pools not involved in xanthophyll cycling, since maximal xanthophyll conversion might differ between LL and HL cultures, or change during UV exposure.
BWF calculation
BWFs for inhibition of carbon fixation and yield, as well as xanthophyll cycle activity, were calculated using an exposure–response curve based on irradiance. The biological responses for each wavelength interval over the incubation period were expressed as a function of the average irradiance (over incubation time) in the exposure interval. The irradiance over the UVR interval and between each filter interval was determined with nanometer resolution using the STAR software (Ruggaber et al., Citation1994). The wavelength integrated irradiance values for UVBR (280–315 nm) and UVAR (315–400 nm) were then adjusted to the irradiance level according to the actual exposure of the samples using the data from a broad band filter radiometer (ELDONET, see below). In addition, during some incubations we used a USB diode array spectroradiometer (HR 2000CG-UV-NIR, Ocean Optics, Duneclin, USA) with a 10 m fiber optics and cosine diffuser to determine the actual solar spectra. A good agreement was found between the measured spectral energy and the spectra determined as described above with the STAR and ELDONET adjustment (data not shown). Thus, the spectral data obtained with the STAR program (adjusted as mentioned above) was used for each determination of BWF.
The spectral dependence of the BWF in the wavelength intervals used in our experiments was extracted using the method of Rundel (Citation1983). A third degree polynomial function was used to fit the data in each experimental series: the best fit was then obtained by iteration (r 2>0.95). Since four or eight different exposures were carried out for each culture, sufficient datapoints for each wavelength interval were provided. Our calculations were based on six spectral wavelength intervals (as mentioned above) that had a bandwidth of 15–40 nm over the UVR region of the spectra. Narrower bandwidth intervals could not be created due to experimental constraints. However, this would not compromise the data in any way as the shape of the BWFs would not be affected and thereby the relationship between the three targeted processes would not change.
Irradiance measurements
Incident solar radiation was measured continuously using a broadband filter radiometer (ELDONET, Real Time Computers, Inc., Germany) installed on the roof of the EFPU. The instrument records irradiance in the UVBR, UVAR and PAR (400–700 nm) wavebands with a 1-min frequency. Regular measurements using a USB diode array spectroradiometer (HR 2000CG-UV-NIR, Ocean Optics, Duneclin, USA) with a 10-m fiber optics and cosine diffuser were made for calibration purposes of the continuous measurements, as described above.
Results
Irradiance conditions were favourable for the outdoor exposures (), with high natural levels of UVAR, UVBR and PAR throughout the experimental period. Furthermore, conditions were similar for the experimental days, except for January 24th. On this day (T. weissflogii HL experiments) clouds were present, which caused variability in incident irradiance on short time-scales (). The overall stable weather conditions and few clouds facilitated a large range of irradiance conditions during the daily exposures.
The pre-acclimation conditions resulted in different pigment pool sizes in both species (). For T. weissflogii, acclimation to HL caused a strong reduction in the cellular content of the major photosynthetic pigments (chl a and fucoxanthin) as compared with the LL conditions. Pool sizes of the xanthophyll cycle pigments did not differ between HL and LL conditions. Due to the decrease in photosynthetic pigments the ratio xan/phot showed a three-fold increase between HL and LL in T. weissflogii. A similar response was found for D. tertiolecta, with significantly lower photosynthetic pigments (chl a and chl b) under the HL conditions, while no significant changes in xanthophyll pigments were observed. In D. tertiolecta HL acclimation induced a two-fold increase in the xan/phot ratio. For both species xanthophyll cycle activity was already present under the pre-acclimation conditions: D. tertiolecta exhibited the largest variability, showing a six-fold difference in DEPS between LL and HL (0.107 vs 0.665 respectively), while this difference was smaller for the T. weissflogii culture (0.237 for LL vs 0.414 for HL).
The transition from the pre-acclimation conditions to the high outdoor PAR conditions (>400 nm, UF-3 cut-off filter, approximating eight times the HL PAR level), did not affect the photochemical quantum yield in both HL cultures with mean Y values of 0.63 (SD 0.013) and 0.56 (SD 0.04) for D. tertiolecta and T. weissflogii, respectively, at the end of the outdoor incubations. In contrast, mean decreases in Y of 21% (D. tertiolecta) and 40% (T. weissflogii) as compared with pre-acclimation conditions were found for the LL cultures. With respect to carbon assimilation under natural PAR only (UF-3 filter), D. tertiolecta LL had a 72% inhibition (2.72 mg C (mg chl a)−1 h−1), as compared with the HL culture that had a mean assimilation number of 9.19 mg C (mg chl a)−1 h−1. T. weissflogii LL had 41% inhibition (3.36 mg C (mg chl a)−1 h−1) relative to the HL culture that had a mean assimilation number of 5.66 mg C (mg chl a)−1 h−1.
BWFs for DEPS showed a similar general shape for all four cultures (, upper panel). Above 360 nm a strong reduction in DEPS was found. Between 360 nm and 315 nm the wavelength dependency was relatively minor, although for both HL cultures a small optimum was observed around 360 nm. LL cultures from both species showed a significantly (p<0.05) lower DEPS between 315 nm and 360 nm, as compared with the HL cultures. In this same region D. tertiolecta showed a lower DEPS than T. weissflogii. In the UVBR region (<315 nm), a sharp increase in DEPS was observed for both species and pre-acclimation conditions. The BWFs for photochemical quantum yield (, middle panel) showed similar general shapes as those for DEPS, with minor wavelength dependency roughly between 360 and 315 nm. Similarly, steep increases were found in the UVBR region as well as sharp decreases above 360 nm. Thalassiosira weissflogii showed little differences between the HL and LL cultures. For D. tertiolecta the inhibition was significantly (p<0.05) lower for the HL culture as compared with the LL culture.
Fig. 3. Biological Weighting Functions (BWFs) for D. tertiolecta (Dun) and T. weissflogii (Thal) (low-light-[LL] and high-light- [HL] acclimated); upper panel; de-epoxidation state (DEPS); middle panel: yield; lower panel: assimilation number. Bold lines refer to BWFs, weak lines with similar style to their respective 95%-confidence intervals.
![Fig. 3. Biological Weighting Functions (BWFs) for D. tertiolecta (Dun) and T. weissflogii (Thal) (low-light-[LL] and high-light- [HL] acclimated); upper panel; de-epoxidation state (DEPS); middle panel: yield; lower panel: assimilation number. Bold lines refer to BWFs, weak lines with similar style to their respective 95%-confidence intervals.](/cms/asset/d805ff49-638d-4fd5-87ed-c1b52a731fd5/tejp_a_397361_o_f0003g.gif)
The BWFs for assimilation number (, lower panel) showed very little difference between the four cultures, especially when considering both T. weissflogii cultures. Again UVBR caused a strong inhibition, while exposure to the 315–360 nm region showed little wavelength dependency. Although not significant, D. tertiolecta LL showed the highest inhibition throughout. Wavelength responses for the three parameters were subsequently normalized to 1, at 300 nm for each culture ().
Fig. 4. Biological Weighting Functions for all four cultures and parameters after normalization to 1 at 300 nm. Abbreviations: DEPS: de-epoxidation state; HL: high light; LL: low light.
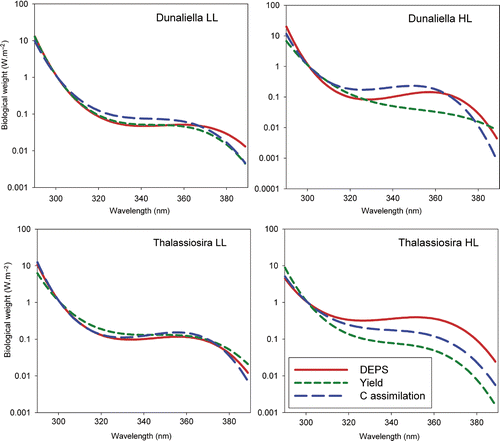
Normalization allowed comparison between wavelength responses of the different processes and previously published 300 nm normalized BWFs (Cullen et al., Citation1992; Neale et al., Citation1998; Neale & Kieber, Citation2000; Villafañe et al. Citation2003; Andreasson & Wangberg, Citation2006). The normalized BWFs for both LL cultures were remarkably similar. However, for this pre-acclimation condition, the inhibition in carbon assimilation was slightly greater in the low UVAR region (315–360 nm) for D. tertiolecta (), while in the same region T. weissflogii showed more inhibition of photochemical quantum yield (). In contrast, large differences were observed after normalization for both HL cultures: here, the photochemical quantum yield was least inhibited in the low UVAR region, while DEPS showed increased activity. For both species, inhibition of carbon assimilation was more pronounced in the UVAR region as compared with inhibition of the yield.
Discussion
BWFs obtained under solar radiation are preferred over those using artificial irradiance conditions (Sobrino et al., Citation2005; Andreasson & Wangberg, Citation2006), since UVR effects are the net result of damage and repair, or protective processes that are influenced by different wavelength regions, including PAR. On the other hand, natural irradiance exposure might cause problems due to short-term irradiance variability resulting from passing clouds such as occurring in our experiments on the 24th of January. During that day, T. weissflogii HL may have responded differently, because irradiance fluctuations may induce a more low irradiance response as opposed to constant irradiance levels in T. weissflogii (van de Poll et al., Citation2007). More likely, the similarity between T. weissflogii HL and LL BWFs, as compared with those for D. tertiolecta could be related with the smaller difference in DEPS under the HL and LL pre-acclimation conditions, as compared with the D. tertiolecta cultures ().
It is well recognized that xan/phot ratios change in microalgae and higher plants, when acclimating to higher irradiance. Thalassiosira weissflogii is known to decrease PSII reaction centres with increasing growth irradiance (Behrenfeld et al., Citation1998). In our study, pigment changes were caused by changes in photosynthetic pigment content and not by altered xanthophyll contents (), as observed previously (Prezelin et al., Citation1998; Goss et al., Citation1999; Moisan & Mitchell, Citation1999; Buma et al., Citation2000, Citation2006). It was possible that the LL irradiance level (60 µmol m−2 s−1) could have been too high to induce different cellular xanthophyll levels between LL and HL, since LL induced xanthophyll cycle activity in both species (). Therefore the differences in BWFs between the HL and LL cultures could become more pronounced, if differences in pre-acclimation irradiances had been greater.
The outdoor exposure under PAR alone caused reduction of photochemical quantum yield, as well as carbon assimilation, (D. tertiolecta 72%, T. weissflogii 41%) in the LL cultures, while for the HL cultures no reduction in the yield was observed. This indicated that the HL cultures were better equipped for the outdoor treatments than the LL cultures. In addition, the LL culture was shown to be most vulnerable to UVR (), whilst the D. tertiolecta HL culture was most UV resistant. D. tertiolecta therefore seems more flexible than T. weissflogii in adjusting to a variety of irradiance conditions (Van Leeuwe et al., Citation2005). On the other hand, sudden transfers to excess irradiance would pose more risks to this species, when acclimated to low irradiance, than it does to the diatom examined.
The general shapes of the described BWFs specified three wavelength regions: UVBR (<315 nm), low UVAR (315–360 nm) region and high UVAR (>360) region. Other published BWFs for photoinhibition (radiocarbon incorporation) also displayed relatively minor wavelength dependency in the low UVAR (Villafañe et al., Citation2003). In addition, the maxima around 360 nm, observed for all three parameters (, ) were also observed by other researchers when cut-off filters within the UVAR region were used (e.g. WG 320, 335, 345, 360, 375 nm) (Villafañe et al., Citation2003).
It has been proposed that xanthophyll cycling exhibits decreased activity during UVR exposure, for example, due to increased activity of epoxidase enzymes. Also, de-epoxidation could be reduced when the ΔpH is disturbed by UVR induced thylakoid membrane disruption (Jacob et al., Citation2001). Similarly, UVR exposure was shown to enhance de-epoxidation as a result of the loss of the pH gradient across the thylakoid membrane (Mewes & Richter, Citation2002). Our BWFs for xanthophyll cycle activity do not support these observations (, ). Therefore, the exposures to natural solar irradiance in this study did not seem to cause thylakoid disruption that could lead to decreased xanthophyll function. Instead, the data support those obtained by Sobrino et al. (Citation2005), who demonstrated enhanced xanthophyll cycle activity in response to UVR, using semi-realistic exposures under a solar simulator.
The strong similarity between the BWFs of DEPS, inhibition of yield and assimilation number in the UVBR region observed for the LL cultures would imply a strong causal relationship between these parameters. Yet, when considering the HL cultures () this did not seem to hold: the increased overall DEPS activities and reduced photochemical quantum yield did not automatically result in a lower inhibition of carbon assimilation in T. weissflogii, but was instead highly similar to the LL culture (). It is known that the fraction available for de-epoxidation varies with growth conditions, increasing under conditions of high irradiance (Yamamoto et al., Citation1999; Lavaud et al., Citation2003). Given the observation that DEPS was increased in the HL cultures () and that simultaneously the increased xan/phot ratios in the HL cultures () would favour enhanced protection against excess irradiance, lower photosynthetic inhibition under HL would be expected. However, this was not observed (, ) and thus the wavelength-dependent effect on carbon assimilation does not seem to depend on the effectiveness of heat dissipation, as indicated by DEPS.
Our results imply that another target process is primarily affected at wavelengths <360 nm, possibly further downstream in the photosynthetic process. As clearly demonstrated for oilseed rape (Brassica napus) (Keiller et al., Citation2003), UVBR-mediated reduction in photosynthesis was primarily correlated with the reduction in abundance as well as expression of the large and small RuBisCO subunits, in agreement with earlier studies on plants (Mackerness et al., Citation1997; Jordan et al., Citation1998). Also, Dunaliella tertiolecta exhibited different repair dynamics related to excess irradiance exposure (Heraud & Beardall, Citation2000), with inhibition of PSII and carbon assimilation linearly related in the PAR, but not in the UVR region. It was concluded that some factors that are affected by UVR, such as RuBisCO activity, would directly affect carbon fixation but only indirectly affect PSII efficiency. As suggested by Bischof et al. (Citation2002) for marine macroalgae, (partial) inactivation of RuBisCO could cause a down-regulation of carbon assimilation in the UVR, thereby causing over-reduction of the electron transport chain, which could indirectly affect quantum yield and DEPS. Hence, xanthophyll de-epoxidation would then result from the destruction of another target, without initially being effective in UVR protection. Given the indications that ROS themselves function as cell signals for protective responses against excess irradiance (Mackerness et al., Citation1999), accumulation of ROS, due to the (partial) inactivation of the Calvin cycle, could induce photoprotective responses such as increased heat dissipation. In other words, xanthophyll de-epoxidation could be stimulated during UVBR exposure as a result of damage that has already occurred instead of effectively functioning in the prevention of damage.
In summary, our study has demonstrated that: (i) natural UVBR stimulates de-epoxidation in both species; (ii) this stimulation is not effective in prevention of UVR-mediated reduction in carbon assimilation; (iii) BWFs for carbon assimilation, inhibition of yield and xanthophyll cycling are very similar, especially in LL cells; and (iv) little wavelength dependency occurs between 315 and 360 nm.
Acknowledgements
We thank Rodrigo Gonçalves for general support at EFPU and Regina Flores for assistance with the PAM measurements. This work was made possible by a NWO/MEERVOUD grant to Buma, NWO/NAAP grant to W.H. van de Poll and by the Global Environmental Funding, United Nations Development Program (PNUD-BC39) to E.W. Helbling and V.E. Villafañe.
References
- Andreasson , KIM and Wangberg , S-A . 2006 . Biological weighting functions as a tool for evaluating two ways to measure UVB radiation inhibition on photosynthesis . J. Photochem. Photobiol. B: Biol. , 84 : 111 – 118 .
- Behrenfeld , MJ , Prasil , O , Kolber , ZS , Babin , M and Falkowski , PG . 1998 . Compensatory changes in PSII electron turnover rates protect photosynthesis from photoinhibition . Photosynth. Res. , 58 : 259 – 268 .
- Bischof , K , Kraebs , G , Wiencke , C and Hanelt , D . 2002 . Solar ultraviolet radiation affects the activity of ribulose-1,5-bisphosphate carboxylase oxygenase and the composition of photosynthetic and xanthophyll cycle pigments in the inter-tidal green alga Ulva lactuca L . Planta , 215 : 502 – 509 .
- Boucher , NP and Prezelin , BB . 1996 . Spectral modeling of UV inhibition of in situ Antarctic primary production using a field derived biological weighting function . Photochem. Photobiol. , 64 : 407 – 418 .
- Buma , AGJ , Van Oijen , T , Van De Poll , WH , Veldhuis , MJW and Gieskes , WWC . 2000 . The sensitivity of Emiliania huxleyi (Prymnesiophyceae) to ultraviolet-B radiation . J. Phycol. , 36 : 296 – 303 .
- Buma , AGJ , Wright , SW , Van Den Enden , R , Van De Poll , WH and Davidson , AT . 2006 . PAR acclimation and UVBR-induced DNA damage in Antarctic marine microalgae . Mar. Ecol. Prog. Ser. , 315 : 33 – 42 .
- Buma , AGJ , Zemmelink , HJ , Sjollema , K and Gieskes , WWC . 1996 . UV-B radiation modifies protein and photosynthetic pigment content, volume and ultrastructure of marine diatoms . Mar. Ecol. Prog. Ser. , 142 : 47 – 54 .
- Cullen , JJ , Neale , PJ and Lesser , MP . 1992 . Biological weighting function for the inhibition of phytoplankton photosynthesis by ultraviolet radiation . Science , 258 : 646 – 650 .
- Dijkman , NA and Kroon , BMA . 2002 . Indications for chlororespiration in relation to light regime in the marine diatom Thalassiosira weissflogii . J. Photochem. Photobiol. B: Biol. , 66 : 179 – 187 .
- Eisenstadt , D , Ohad , I , Keren , N and Kaplan , A . 2008 . Changes in the photosynthetic reaction centre II in the diatom Phaeodactylum tricornutum result in non-photochemical fluorescence quenching 468 . Environ. Microbiol. , 10 : 1997 – 2007 .
- Finazzi , G , Johnson , GN , Dall’ósto , L , Zito , F , Bonente , G , Bassi , R and Wollmann , F-A . 2006 . Nonphotochemical quenching of chlorophyll fluorescence in Chlamydomonas reinhardtii . Biochem. , 45 : 1490 – 1498 .
- Genty , BE , Briantais , JM and Baker , NR . 1989 . The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence . Plant Physiol. Biochem. , 28 : 1 – 10 .
- Goss , R , Mewes , H and Wilhelm , C . 1999 . Stimulation of diadinoxanthin cycle in Phaeodactylum tricornutum by mild UVR . Photosynth. Res. , 59 : 73 – 80 .
- Guillard , RRL and Ryther , JH . 1962 . Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve . Can. J. Microbiol. , 8 : 229 – 239 .
- Heraud , P and Beardall , J . 2000 . Changes in chlorophyll fluorescence during exposure of Dunaliella tertiolecta to UV radiation indicate a dynamic interaction between damage and repair processes . Photosynth. Res. , 63 : 123 – 134 .
- Holm-Hansen , O and Helbling , EW . 1995 . “ Técnicas para la medición de la productividad primera en el fitoplancton ” . In Manual de Métodos Ficológicos , Edited by: Alveal , K , Ferrario , ME , Oliveira , EC and Sar , E . 329 – 350 . Concepción, , Chile : Universidad de Concepción .
- Holm-Hansen , O , Lorenzen , CJ , Holmes , RW and Strickland , JDH . 1965 . Fluorometric determination of chlorophyll . Journal de Conseil pour l' Exploration de la Mer , 30 : 3 – 15 .
- Holm-Hansen , O and Riemann , B . 1978 . Chlorophyll-a determination: Improvements in methodology . Oikos , 30 : 438 – 447 .
- Horton , P , Ruban , AV and Young , AJ . 1999 . “ Regulation of the structure and function of the light harvesting complexes of photosystem II by the xanthophyll cycle ” . In The Photochemistry of Carotenoids , Edited by: Frank , HA , Young , AJ , Britton , G and Cogdell , RJ . 271 – 291 . Dortrecht, , The Netherlands : Kluwer Academic Publishers .
- Jacob , T , Goss , R and Wilhelm , C . 2001 . Unusual pH-dependence of diadinoxanthin de-epoxidase activation causes chlororespiratory-induced accumulation of diatoxanthin in the diatom Phaeodactylum tricornutum . J. Plant Physiol. , 158 : 383 – 390 .
- Jordan , BR , James , PE and Mackerness , SA-H . 1998 . Factors affecting UV induced changes in Arabidopsis thaliana gene expression: the role of development, protective pigments and the chloroplast signal . Plant Cell Physiol. , 39 : 769 – 778 .
- Keiller , D , Mackerness , SA-H and Holmes , MG . 2003 . The action of a range of supplementary ultraviolet (UV) wavelengths on photosynthesis in Brassica napus L. in the natural environment: effects on PS II, CO2 assimilation and level of chloroplast proteins . Photosynth. Res. , 75 : 139 – 150 .
- Lavaud , J , Rousseau , B and Etienne , A-L . 2003 . Enrichment of the light harvesting complex in diadinoxanthin and implications for the nonphotochemical fluorescence quenching in diatoms . Biochem. , 42 : 5802 – 5808 .
- Litchman , E , Neale , PJ and Banaszak , AT . 2002 . Increased sensitivity to ultraviolet radiation in nitrogen-limited dinoflagellates: photoprotection and repair . Limnol. Oceanogr. , 47 : 86 – 94 .
- Mackerness , SA-H , Jordan , BR and Thomas , B . 1999 . Reactive oxygen species in the regulation of photosynthetic genes by ultraviolet radiation (UV-B 280–320 nm) in green and etiolated buds of pea (Pisum sativum L.) . J. Photochem. Photobiol. B: Biol. , 48 : 180 – 188 .
- Mackerness , SA-H , Thomas , B and Jordan , BR . 1997 . The effects of supplementary UV-B radiation on mRNA transcripts translation and stability of chloroplast proteins and pigment formation in Pisum sativum L . J. Exp. Bot. , 48 : 729 – 738 .
- Mewes , H and Richter , M . 2002 . Supplementary ultraviolet-B radiation induces a rapid reversal of the diadinoxanthin cycle in the strong light exposed diatom Phaeodactylum tricornutum . Plant Physiol. , 130 : 1527 – 1535 .
- Moisan , TA and Mitchell , BG . 1999 . Photophysiological acclimation of Phaeocystis antarctica Karsten under light limitation . Limnol. Oceanogr. , 44 : 247 – 258 .
- Muller , P , Li , X-P and Niyogi , K . 2001 . Non-photochemical quenching. A response to excess light energy . Plant Physiol. , 125 : 1558 – 1566 .
- Neale , PJ , Cullen , JJ and Davis , RF . 1998 . Inhibition of marine photosynthesis by ultraviolet radiation: variable sensitivity of phytoplankton in the Weddell-Scotia Confluence during the austral spring . Limnol. Oceanogr. , 43 : 433 – 448 .
- Neale , PJ and Kieber , DJ . 2000 . “ Assessing biological and chemical effects of UV in the marine environment: Spectral weighting functions ” . In Causes and Environmental Implications of Increased UVB Radiation , Edited by: Hester , RE and Harrison , RM . 61 – 83 . Cambridge, , UK : The Royal Society of Chemistry .
- Olaizola , M and Yamamoto , HY . 1994 . Short-term responses of the diadinoxanthin cycle and fluorescence yield to high irradiance in Chaetoceros muelleri (Bacillariophyceae) . J. Phycol. , 30 : 606 – 612 .
- Pfundel , EE , Pan , RS and Dilley , RA . 1992 . Inhibition of violaxanthin de epoxidation by ultraviolet-B radiation in isolated chloroplasts and intact leaves . Plant Physiol. , 98 : 1372 – 1380 .
- Prezelin , BB , Moline , MA and Matlick , HA . 1998 . Icecolors’ 93: spectral UV radiation effects on Antarctic frazil ice algae . Antarctic Res. Ser. , 73 : 45 – 83 .
- Qui , N , Lu , Q and Lu , C . 2003 . Photosynthesis, photosystem II efficiency and the xanthophyll cycle in the salt-adapted halophyte Atriplex centralasiatica . New Phytol. , 159 : 479 – 486 .
- Ruban , AV , Lavaud , J , Rousseau , B , Guglielmi , G , Horton , P and Etienne , A-L . 2004 . The super-excess energy dissipation in diatom algae: comparative analysis with higher plants . Photosynth. Res. , 82 : 165 – 175 .
- Ruggaber , A , Dlugi , R and Nakajima , T . 1994 . Modeling of radiation quantities and photolysis frequencies in the troposphere . J. Atmospheric Chem. , 18 : 171 – 210 .
- Rundel , RD . 1983 . Action spectra and estimation of biologically effective UV radiation . Physiol. Plant. , 58 : 360 – 366 .
- Schofield , O , Evens , TJ and Millie , DF . 1998 . Photosystem II quantum yields and xanthophyll cycle pigments of the macroalga Sargassum natans (Phaeophyceae): responses under natural sunlight . J. Phycol. , 34 : 104 – 112 .
- Sobrino , C , Neale , PJ , Montero , O and Lubian , LM . 2005 . Biological weighting function for xanthophyll de-epoxidation induced by ultraviolet radiation . Physiologia Plantarum , 125 : 41 – 51 .
- Steeman-Nielsen , E . 1952 . The use of radioactive carbon (C14) for measuring organic production in the sea . Journal de Conseil Pour l' Exploration de la Mer. , 18 : 117 – 140 .
- Ting , CS and Owens , TG . 1993 . Photochemical and non-photochemical fluorescence quenching processes in the diatom Phaeodactylum tricornutum . Plant Physiol. , 101 : 1323 – 1330 .
- Van De Poll , WH , Alderkamp , A-C , Janknegt , PJ , Roggeveld , J and Buma , AGJ . 2006 . Acclimation to a dynamic irradiance regime changes excessive irradiance sensitivity of Emiliania huxleyi and Thalassiosira weissflogii . Limnol. Oceanogr. , 53 : 1239 – 1248 .
- Van De Poll , WH , Van Leeuwe , MA , Roggeveld , J and Buma , AGJ . 2005 . Nutrient limitation and high irradiance acclimation reduce PAR and UV induced viability loss in the Antarctic diatom Chaetoceros brevis (Bacillariophyceae) . J. Phycol. , 41 : 840 – 850 .
- Van De Poll , WH , Visser , RJW and Buma , AGJ . 2007 . Acclimation to a dynamic irradiance regime changes excessive irradiance sensitivity of Emiliania huxleyi and Thalassiosira weissflogii . Limnol. Oceanogr. , 52 : 1430 – 1438 .
- Van Kooten , O and Snel , JFH . 1990 . The use of chlorophyll fluorescence nomenclature in plant stress physiology . Photosynth. Res. , 25 : 147 – 150 .
- Van Leeuwe , MA , Sikkelerus , B , Gieskes , WWC and Stefels , J . 2005 . Taxon-specific differences in photoacclimation to fluctuating irradiance in an Antarctic diatom and a green flagellate . Mar. Ecol. Prog. Ser. , 288 : 9 – 10 .
- Van Leeuwe , MA , Villerius , LA , Roggeveld , J , Visser , RJW and Stefels , J . 2006 . An optimized method for automated analysis of algal pigments by HPLC . Mar. Chem. , 102 : 267 – 275 .
- Villafañe , VE , Barbieri , ES and Helbling , EW . 2004 . Annual patterns of Ultraviolet radiation effects in temperate marine phytoplankton off Patagonia, Argentina . J. Plankton Res. , 26 : 167 – 174 .
- Villafañe , VE , Sundback , K , Figueroa , FL and Helbling , EW . 2003 . “ Photosynthesis in the aquatic environment as affected by UVR ” . In UV Effects in Aquatic Organisms 589 and Ecosystems , Edited by: Helbling , EW and Zagarese , HE . 357 – 397 . Cambridge, , UK : The Royal Society of Chemistry .
- Yamamoto , Y , Bugos , RC and Hieber , AD . 1999 . “ Biochemistry and molecular biology of the xanthophyll cycle ” . In The Photochemistry of Carotenoids , Edited by: Frank , HA , Young , AJ , Britton , G and Cogdell , RJ . 293 – 303 . Dortrecht, , The Netherlands : Kluwer Academic Publishers .
- Zudaire , L and Roy , S . 2001 . Photoprotection and long-term acclimation to UV radiation in the marine diatom Thalassiosira weissflogii . J. Photochem. Photobiol. B: Biol. , 62 : 26 – 34 .