Abstract
Although benthic diatoms are used to assess river water quality, there are few data on the rate at which diatom assemblages react to changes in water quality. The aim of this study was to assess the reaction time of diatoms and to discuss the changes occurring during water quality improvement on the basis of their autecological characteristics. In order to simulate this improvement, diatom-dominated biofilms grown on artificial sandstone substrata were transferred from several polluted rivers to an unpolluted river. They were sampled three times: before transfer and 1 and 2 months after transfer. The ecology and growth-forms of the taxa explained most of the changes in species composition observed during the experiment. Adnate diatoms gradually replaced motile and stalked taxa. Gomphonema parvulum, a stalked diatom positioned vertically in the biofilm, is adapted for light and space competition in high-density algal biofilms. When transferred to an unpolluted site, this growth-form is less competitive and does not tolerate the high grazing pressure. Fistulifera saprophila is a single celled motile diatom, living in organic matrices. When the artificial substrata were transferred to the unpolluted site, this particular ecological niche disappeared quickly. On the other hand, Achnanthidium minutissimum, which is considered to be cosmopolitan and an early colonizer, increased during the first month of transfer and then decreased. It was gradually replaced by A. biasolettianum, which was the taxon best suited to this pristine stream. The changes observed differed between treatments depending on the species composition and architecture of the biofilms. In particular, biofilms dominated by stalked and motile diatoms were more quickly modified than those dominated by small motile diatoms. The diatom index reflects these changes, and its values showed that about 60 days following a water quality improvement were necessary for transferred diatom assemblages to reach diatom index values similar as those at the unpolluted river.
Introduction
Diatoms are frequently dominant in freshwater biofilms in rivers (Blinn et al., Citation1980). The specific composition of diatom assemblages is affected by, amongst other factors, chemical parameters such as acidification (Mulholland et al., Citation1986), nutrient concentrations (Kelly, Citation2003) and organic load (Van Dam et al., Citation1994). Furthermore, the generation time of diatoms is short (Rott, Citation1991) and thus they can indicate quickly a change in the water quality (Stevenson & Pan, Citation1999). For these reasons, benthic diatoms are used routinely for river quality assessment (Ector et al., Citation2004) alongside macrophytes, macroinvertebrates and fish. Nevertheless, few studies relate diatom-based water quality indices to ecological processes occurring in the biofilms, or consider the dynamics within biofilms undergoing changes in response to water quality fluctuations. Data on the time needed for an assemblage to reflect a change in environmental conditions are few and variable. Iserentant & Blancke (Citation1986) demonstrated that diatom assemblages transferred from a polluted site to an unpolluted site were still different from the assemblages of the native site after 45 days, whereas Wendker (Citation1992 a) reported responses in the order of 3 to 7 days. It has been reported that the integration period of diatom indices varies between 2 to 5 weeks, as a function of trophic status (Lavoie et al., Citation2008). Other studies showed that it was different depending on the diatom index considered (Rimet et al., Citation2005) and was also dependent on the studied habitat (Soininen & Eloranta, Citation2004). In the present report, the changes in diatom assemblages were studied by simulating a water quality improvement after the transfer of diatom-dominated biofilms from polluted sites to an unpolluted site.
The objective of this study was to determine the time needed for the translocated diatom assemblages, with varying species composition and life forms, to become similar to a native assemblage. To assist in the interpretation of the results, quantitative data on the relative abundance of taxa and major functional groups were supplemented by a descriptive account of the evolution of the biofilms, supported by scanning electron microscopy.
Materials and methods
Study area
Six sites in the south region of Luxembourg, which is characterized by a homogenous geology (sandstone), were chosen in four rivers with different water qualities and diatom assemblages. Sites #1 (Alzette river in Esch-sur-Alzette), #2 (Alzette river in Hespérange) and #5 (Chiers river in Rodange) were characterized by a significant organic load (biological oxygen demand [BOD], ). Sites #2 and #3 (Alzette river in Ettelbruck) and #5 were enriched with nutrients (
,
). Site #4 (Attert river in Colmar) was less polluted than the other sites. Site R (Rollingerbaach stream in Rollingen) was unpolluted with very low nutrient concentrations and organic load (). The polluted sites were approximately 7–35 km from site R.
Table 1. Chemical parameters measured during the experiment (block deposit: 06/06/2003, D0: 26/06/2003, D29: 25/07/2003, D60: 25/08/2003).
Experimental design
Water temperature, dissolved oxygen, conductivity and pH were measured in situ. Water samples were collected and analysed in the laboratory for BOD, ,
, total phosphorus (Ptot),
,
,
, Cl−, K+, and Na+ following standard procedures (APHA, Citation1995).
The transfer experiments were performed using artificial substrata similar to the most frequently encountered substratum in the rivers, i.e. sandstone. At each of the six sites, five sandstone blocks (10 cm × 10 cm × 6 cm) were deposited (06/06/2003). After 20 days of colonization (26/06/2003), three blocks from each site were sampled and fixed with 4% formalin (Prygiel & Coste, Citation2000; AFNOR, Citation2007) in order to analyse the diatom community and to check the homogeneity of the assemblages between blocks on the same site. The two remaining blocks were transferred to the reference site R (Fig. S1a, see supplemental material available at: http://www.informaworld.com/mpp/uploads/tejp_a_420025_supp.pdf) and left for 2 months. One of the two blocks from each polluted site, as well as a block from the reference site, were sampled after 29 days (D29: 25/07/2003) and a second set of samples were collected after 60 days (D60: 25/08/2003) ().
Table 2. Summary of transfer dates and codes given to each block sampled.
The biofilms were sampled by scraping the upper surface of the blocks with clean toothbrushes. The samples were then treated with 40% hydrogen peroxide in order to eliminate organic matter and with hydrochloric acid to dissolve calcium carbonate. Clean diatom frustules were mounted in a synthetic resin (Naphrax) and at least 400 valves counted in each sample (AFNOR, Citation2007) by light microscopy with 1000× magnification. The floras of Krammer & Lange-Bertalot (Citation1986–1991) were used as the basis for diatom identification.
In order to assess the relative abundance of dead frustules versus live cells in the diatom communities, samples were homogenized by manual shaking for several minutes to disintegrate the biofilms. Then sub-samples were mounted on a Bürker slide and dead frustules (those without a chloroplast) as well as living frustules were distinguished by light microscopy with 400× magnification. For each sample, frustules were identified to genus level and four counts made to elucidate the average genera composition.
Simultaneously with the deposition of the sandstone blocks, three small sandstone cubes (2 cm × 1 cm × 1 cm) were placed near the blocks in each experimental site for colonization and were later observed using Scanning Electron Microscopy (SEM) (Fig. S1b, see supplemental material available at: http://www.informaworld.com/mpp/uploads/tejp_a_420025_supp.pdf). After 20 days of colonization, they were also transferred to the reference site R. The small cubes were sampled at the beginning of the transfer (D0), as well as 29 days (D29) and 60 days (D60) after the transfer. They were immediately fixed in a 5% glutaraldehyde-phosphate buffer for 4 h. In the laboratory, the samples were rinsed two times with phosphate buffer, before being processed in a graded acetone series, finishing with three changes in 100% acetone. Finally, the samples were air-dried, stored in a dessicator, fixed on SEM stubs and vacuum-coated with gold (40 nm). A Leica Stereoscan 430i, operated at 20 kV was used for biofilm examination.
Data analysis
In order to assess the changes in the transferred assemblages, a Detrented Correspondence Analysis (DCA) was calculated with PC-Ord software (McCune & Mefford, Citation1999). In addition, a Multi-Response Permutation Procedure (MRPP) was used to analyse differences among groups. The changes in the abundance of the dominant taxa were estimated by calculating the difference of percentage during the first month, and during the second month of the transfer in each diatom assemblage. Box-plots were used to represent these differences of abundances for the most abundant taxa.
To assess the changes of growth form abundances during the transfers, the diatoms were clustered into six groups (Table S1, see supplemental material available at: http://www.informaworld.com/mpp/uploads/tejp_a_420025_supp.pdf) according to Whitford (Citation1956), Stevenson (Citation1983), Roemer et al. (Citation1984), Planas et al. (Citation1989), Rieter (Citation1989), Tanaka & Watanabe (Citation1990), Katoh (Citation1992), Molloy (Citation1992) and to SEM photos of the dehydrated biofilms: adnate () (Achnanthidium, Amphora, Cocconeis, Planothidium, Psammothidium), stalked () (Cymbella, Encyonema, Encyonopsis, Gomphonema, Reimeria, Rhoicosphenia), motile (, ) (among others Navicula sensu lato, most Nitzschia, Surirella), planktonic (Nitzschia acicularis, centric diatoms, except Melosira varians), filamentous (Diatoma vulgaris, Melosira varians, Staurosirella pinnata) and rosette forming (Ulnaria ulna, Meridion circulare). This classification should not be considered as adaptable to every situation, but at least corresponded to what was observed with light and electron microscopy in the samples of the present study.
The OMNIDIA software (Lecointe et al., Citation1993) was used in order to calculate the diatom index SPI (Specific Pollution sensitivity Index: Cemagref, Citation1982), which is routinely used in Luxembourg to assess water quality (Descy & Ector, Citation1999).
Results
Changes in species composition and life forms
The dominant diatoms in the reference site were Achnanthidium biasolettianum, A. minutissimum, Amphora pediculus and Gomphonema pumilum. Whereas the diatom community composition of the reference site R remained quite stable for the duration of the experiment, the assemblages of the transferred sites underwent marked changes (Table S2, see supplemental material available at: http://www.informaworld.com/mpp/uploads/tejp_a_420025_supp.pdf). The assemblages of site #1 were initially dominated by Gomphonema parvulum, Eolimna minima and Nitzschia palea; these three taxa disappeared quickly and Achnanthidium minutissimum was observed in significant numbers after 29 days. The pollution-tolerant diatom Fistulifera saprophila was the most abundant taxon at site #2, but was replaced after 29 days by the taxa dominant in the reference assemblage, i.e. A. minutissimum and G. pumilum, E. subminuscula and Mayamaea atomus var. permitis were also present at site #2, but disappeared after 60 days. This was also the case for the transferred assemblages of site #3. For site #4, the initially-dominant Fistulifera saprophila was still abundant after 29 days, but only formed a negligible proportion of the assemblage after 60 days. By contrast, A. biasolettianum and especially A. minutissimum, both typical of reference conditions, were the most abundant taxa after 29 days and replaced E. subminuscula, M. atomus var. permitis and G. parvulum.
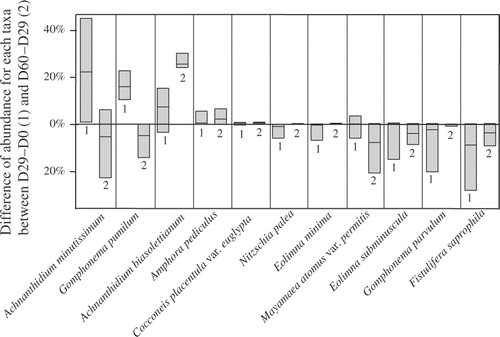
Overall, A. minutissimum and G. pumilum showed a marked increase in the transferred diatom assemblages during the first month of the experiment (). The relative abundance of A. biasolettianum increased over the duration of the experiment and especially during the second month after the transfer. On the other hand, F. saprophila and G. parvulum disappeared during the first month. The abundance of living cells of N. palea, E. minima and E. subminuscula also decreased, although to a lesser extent. M. atomus var. permitis disappeared during the second month after the transfer.
Fig. 1. Changes in relative abundance of the dominant taxa in the transferred biofilms. For each taxon, the 1st box (1) corresponds, for each taxon, to the difference of cells density between the 29th and the 1st day, and the 2nd box (2) corresponds to the difference between the 60th and the 29th day of the experiment. The differences are calculated from the transferred biofilms: boxes indicate the 25th and the 75th percentile and the bar in the box the median value.
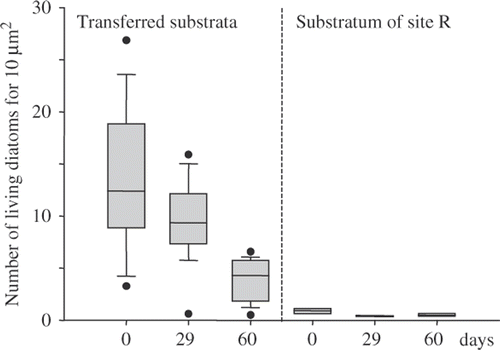
The transfer of diatom assemblages from polluted sites to the reference site also resulted in changes in the life forms of diatoms and in the density of living cells. The taxonomical changes resulted in a shift from dominance by stalked and motile diatoms (site #1), or of motile diatoms (sites #2, #3, #4, #5) to dominance by adnate ones (). Other life forms (planktonic, filamentous, rosette forming) were of minor importance in these assemblages (maximum abundance of 1.7%) and were not represented in . The density of living diatoms decreased in the transferred biofilms, but still remained higher after 60 days in the transferred biofilms when compared with the reference site R (). MRPP showed no significant differences (p = 0.259) between counts carried out on non-treated samples, excluding dead diatoms (Bürker slide), and counts carried out on permanent slides (Naphrax-mounted slides).
Fig. 2. Changes in the relative abundances of the growth forms in the biofilms after 29 and 60 days of transfer. Boxes indicate the 25th and the 75th percentiles and the bar in the box the median value.
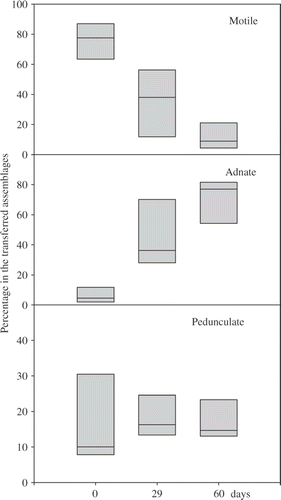
Fig. 3. Diatom densities (living cells) on the substrata during the experiment. Boxes indicate the 25th and the 75th percentiles, the bar in the box the median value, the whiskers the 10th and 9th percentiles. Dots represent maximum and minimum values.
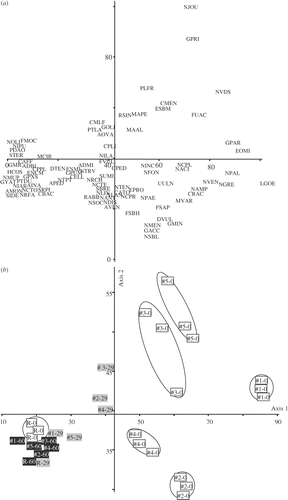
As demonstrated on the DCA plot (), the transferred diatom assemblages became similar to the assemblage of the reference site R over the time course of the experiment. However, the composition of the assemblages converged at different rates; thus, after one month diatom assemblages of site #1 were similar to (albeit still statistically different from) the assemblage of the unpolluted site R. After 2 months, all transferred diatom assemblages were more similar to the assemblage at the reference site R, although they still remained statistically different (MRPP, p < 0.001).
Fig. 4. Detrended Correspondence Analysis (DCA) calculated on the basis of the diatom assemblages counted in the treated samples (Chi2: axis 1: 65.7%, axis 2: 5.1%). (a) Species score plots–codes are given in the Table S1, see supplemental material available at: http://www.informaworld.com/mpp/uploads; (b) sample score plots–codes are given in .
Rate of changes
In the present study, the speed of response of diatom-dominated assemblages was variable; up to 60 days were necessary to develop assemblages comparable to those of the native site for some of the assemblages, whereas others already had similar assemblages after 29 days. A relationship was observed between the assemblages’ architecture and the rate of change. Two main types of assemblages can be defined.
The first type, characteristic of site #1, was dominated by stalked (G. parvulum) and motile diatoms (E. minima and N. palea) and changed rather quickly (). Their transfer to the unpolluted site led to a decrease in diatom density in the biofilm after 29 days () and to the rapid appearance of adnate forms of the genus Achnanthidium. After 60 days, diatom densities were much lower, but were still higher in the transferred biofilm than in the biofilms of R.
The second assemblage type, found in sites #2, #3, #4 and #5, was dominated by small motile diatoms: F. saprophila, M. atomus var. permitis and E. subminuscula. These small-sized Naviculales are considered to be α-meso-polysaprobous and eutraphentic taxa (Van Dam et al., Citation1994). Biofilms from the latter four sites had a rather heterogeneous structure due to the presence of filamentous algae (), organic mucilage (), and diatoms mixed with bacteria and detritus ().
At sites #2, #3 and #4, this motile assemblage was replaced completely by adnate Achnanthidium and stalked G. pumilum after 60 days. G. parvulum was only present at sites #1 and #5 and the loss of this stalked diatom 29 days after transfer may explain the quicker speed of change of these assemblages.
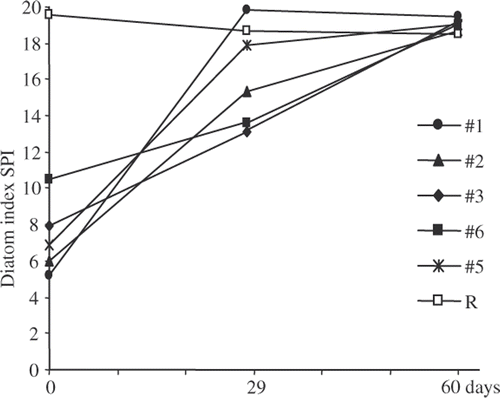
Changes in water quality index SPI
The changes in the composition of the transferred assemblages resulted in changes of the SPI values (). At the beginning of the experiment the SPI values at the six sites were different from each other (three replicates for each site, Kruskal-Wallis One Way Analysis of Variance on Ranks showed a significant difference p < 0.01). Furthermore a pairwise comparisons (Student-Newman-Keuls Method, p < 0.05) indicated the following hierarchy for SPI values: #1<#2 =#5<#3<#4<R. SPI values of all the transferred diatom assemblages increased during the experiment, albeit at different rates. Assemblages from sites #1 and #5 evolved very quickly to reach values close to the reference site R after 1 month. The other assemblages #2, #3 and #4 changed more slowly. After 2 months, all the diatom assemblages had attained a SPI value similar to the reference site R.
Discussion
Translocation experiments described in the literature showed varying reaction rates to changes in the ecological conditions. In rather short experiments, Ivorra (Citation2000) and Tolcach & Gómez (Citation2002) demonstrated that after 2 weeks, the assemblages of biofilms transferred from polluted to unpolluted sites were still different. Experiments performed over a longer period (45 days) have come to the same conclusion (Iserentant & Blancke, Citation1986). In contrast, rapid changes in the diatom community structure were observed after only three days, or one week, in a small softwater stream (Wendker, Citation1992 a).
In general, colonization of new substrata by epilithic diatom communities goes through the following stages: accrual of bacteria and organic slime, development of an adnate diatom layer, development of vertically positioned algal layer (stalked, or rosette forming) and the development of filamentous algal layer, leading in some cases to stromatolithic structures (Winsborough & Golubić, Citation1987). Mature epilithic communities are characterized by having a multi-layered structure: adnate species inhabit the lower layer, with stalked, rosette-forming and filamentous species forming the upper layers. However, processes that take place when a well-established diatom biofilm faces a change in environmental conditions are different from those occurring during the colonization of new substrata. For example, both the competition between species for already occupied space and the effects of changing abiotic parameters on the growth rates of the different taxa play a crucial role. In the present study, the initial complex three-dimensional assemblages changed to a two-dimensional assemblage dominated by adnate taxa. As observed in another study, the cell densities of these assemblages were negatively correlated with the relative abundance of adnate species (Katoh, Citation1992).
The pollution-sensitive diatoms dominating in the reference site (A. biasolettianum, A. minutissimum, A. pediculus and G. pumilum) increased in relative abundance in the translocated assemblages, whereas the relative abundance of the pollution-tolerant taxa of the initial assemblages declined. Achnanthidium minutissimum was the dominant diatom after 1 month of transfer and then decreased after 2 months. This cosmopolitan taxon is an early colonizer that quickly occupies free space (Sabater, Citation2000) when inter-specific competition decreases (Rodríguez, Citation1994). Achnanthidium minutissimum abundance can be used to assess disturbance intensity (Stevenson & Bahls, Citation1999). Transfers of diatom communities originating from the polluted sites to the unpolluted stream are comparable to abrupt physical and chemical disturbances (temperature decrease, reduction of nutrient concentration and organic load). These changes lead to a sloughing of some parts of the biofilm and to colonization by A. minutissimum in these free spaces. Achnanthidium biasolettianum increased in abundance throughout the experiment. It was the dominant and best-adapted taxon to this oligosaprobous and oligotraphentic stream. Its ecological optimum corresponds to oligotrophic limestone and sandstone rivers (Van Dam et al., Citation1994; Rimet et al., Citation2003, Citation2004). This diatom has an adnate lifestyle and seemed to be attached to the substratum by mucilage secreted from the raphe slits () as are C. placentula and A. pediculus (see illustrations in Round & Lee, Citation1989; Pantazidou et al., Citation2001). Small Achnanthidium are often observed adnately attached (Whitford, Citation1956; Roemer et al., Citation1984; Planas et al., Citation1989). They can also be observed attached to the substrate by stalks when current speed decreases, or if the substrata have been colonized for a long time (Roemer et al., Citation1984; Tanaka & Watanabe, Citation1990). Adnate attachment enables diatoms to resist the strong physical disturbances caused by water turbulences and also grazing pressure (Luttenton et al., Citation1986; Katoh, Citation1992). In the case of the reference site R, water turbulence was similar to the one of the polluted rivers, but grazing was probably a determining factor. Gomphonema pumilum increases in abundance at the same rate as A. minutissimum. Gomphonema pumilum is short-stalked, like G. parvulum (), but it was mainly observed in depressions in the substratum (), with only a small part of the frustule jutting out. This characteristic probably enabled it to resist to high grazing pressure by freshwater gastropods such as Ancylus, which were often observed on the blocks in the reference site. These snails are known to scrape benthic diatoms out of the epilithic biofilm (Tachet et al., Citation2000). Ancylus fluviatilis is a stone-dwelling herbivore, which usually ingests periphyton. After field observation and laboratory experiments, Calow (Citation1973) suggested that Ancylus prefers diatoms and that within this group Gomphonema is apparently the most favoured genus. In our transfer experiments, G. parvulum disappeared quickly, mainly during the first month after the transfer. Nutrient depletion was probably a determining negative factor for this eutraphentic taxon (Van Dam et al., Citation1994). Furthermore, G. parvulum is a short-stalked diatom (Hoagland et al., Citation1982), adapted to biofilms in which competition for space is significant. According to Katoh (Citation1992) and Luttenton et al. (Citation1986), vertically positioned diatoms loose their advantage because competition for space is less crucial in oligotrophic and oligosaprobic rivers. Thus, the diatom density decreases after translocation to unpolluted sites may have led to the decline of G. parvulum populations. Moreover, the stalked diatoms were subject to higher grazing pressure than adnate diatoms in unpolluted conditions where scrapers are abundant (Luttenton et al., Citation1986; Katoh, Citation1992).
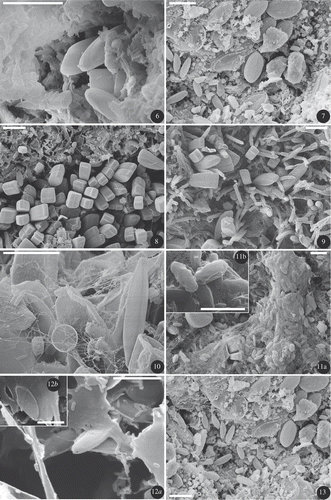
Figs 6–13. SEM photos of dehydrated biofilms, : site R (day 29), Gomphonema pumilum, attached with short stalks, always observed in depressions. : site #1 transferred in R (day 29), Achnanthidium biasolettianum and Cocconeis placentula var. euglypta, adnate attachment. : site #2 (day 0), colony of Gomphonema parvulum, attached with short stalks. : site #5 (day 0), Gomphonema parvulum attached with short stalks among filamentous bacteria. : site #3 (day 0), Nitzschia palea, non-attached, motile diatom with a small centric on the left. : site #3 (day 0), colony of the motile Fistulifera saprophila in a mucilaginous organic matrix. : site #4 (day 0), the motile Mayamaea atomus var. permitis in an organic matrix. : site #1 transferred in R (day 29), Achnanthidium minutissimum and Cocconeis placentula var. euglypta, with adnate attachment. Scale bars: 10 µm (); 5 µm ().
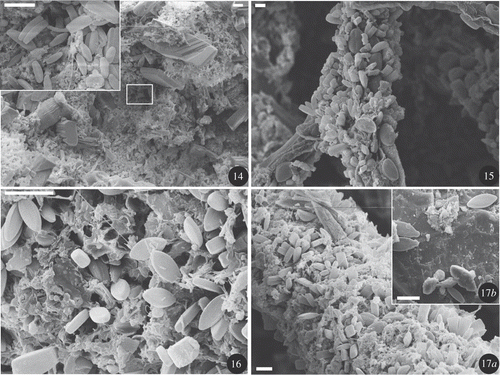
Figs 14–17. SEM photos of dehydrated biofilms. : site #4 transferred in R (day 60), diatoms are observed mainly in depressions. : site #2 (day 0), epiphytic diatoms on filamentous algae. : site #2 (day 0), Eolimna subminuscula, non-attached, motile diatoms present in a mixture of detritus with bacteria. a: site #3 transferred in R (day 29), filamentous algae colonized by diatoms. b: site #3 transferred in R (day 29), sandstone substratum presenting a lower density of diatoms than on filamentous algae. Scale bars: 10 µm (Figs 15, 16, 17); 20 µm ().
Motile diatoms such as Nitzchia palea also disappeared quickly. N. palea was mainly observed in the biofilm at the most polluted site #1 () that was characterized by an important organic load and was often deoxygenated. Motility offers a selective advantage on silty substrata, but can be associated with other ecophysiological traits such as tolerance to dissolved oxygen. Such traits also allow taxa to move and live inside oxygen-depleted biofilms. These competitive traits were less advantageous in the thin, oxygen-saturated, biofilms that developed at the reference site (). Moreover, Kawamura et al. (Citation2004) demonstrated that Nitzschia species are affected by grazing pressure of gastropods. Taxa such as E. subminuscula () and E. minima are also solitary motile cells that showed similar changes in their abundances as N. palea.
Fistulifera saprophila was present in high abundance in thick organic matrices (). Fistulifera saprophila is known to live in dense populations enclosed in mucilaginous layers (Lange-Bertalot, Citation2001). Wendker (Citation1992 b) hypothesized that these gelatinous layers may shield it from high current velocities. These organic matrices were never observed within one month of transfer in the unpolluted site R. The loss of this particular ecological niche probably explains the quick disappearance of F. saprophila, which is considered to be α-meso-polysaprobous and eutraphentic (Van Dam et al., Citation1994). Mayamaea atomus var. permitis has a similar niche in the biofilms as F. saprophila, since it has solitary cells and is often enclosed in mucilage (). It is very abundant in the wastewater of sewage plants (Lange-Bertalot, Citation2001). Nevertheless, it seemed to be capable of resisting the change in environmental conditions better than the former species (E. subminuscula, E. minima, F. saprophila, G. parvulum and N. palea), because its decrease mainly occurred during the second month of the experiment.
In the work of Passy (Citation2007), different ecological guilds were defined on the basis of the potential of diatoms to tolerate nutrient limitation and physical disturbance. For instance, the so-called low-profile guild (Passy, Citation2007), which encompass small, slow-moving and non-motile taxa (e.g. Achnanthidium, Cocconeis), is observed in nutrient poor and high disturbance habitats. This is in accordance with the increase of Achnanthidium species observed in the unpolluted site of this translocation experiment. Passy (Citation2007) defined a motile-guild (Nitzschia, Navicula), which is affected by physical disturbance (e.g. current velocity, grazing pressure), this is also is accordance with our findings.
Implications for diatom-based water-quality monitoring
Diatoms are valuable indicators of environmental conditions in rivers and streams, because they respond directly and sensitively to many physical, chemical, and biological changes, such as temperature, current, nutrient concentrations, and herbivory, in river and stream ecosystems. Because of their capacity to divide frequently, they can respond rapidly to changes in water quality (Stevenson & Pan, Citation1999). This experiment demonstrated that, when transferred from polluted to unpolluted sites, a period of 29 to 60 days was necessary to reach values of diatom index (SPI) similar to the unpolluted site, depending on the initial assemblage. This is directly comparable to the reports by Iserentant & Blancke (Citation1986) and Kelly (Citation2003). The results of the present study indicate that the time needed for diatom assemblage to react to changed environmental conditions, not only depends on the reaction time of the different species to changes in water chemistry and other environmental parameters (like grazing), but also on the architecture of the assemblages and thus on the life-forms of the dominant diatoms.
These results cannot be directly transferred to non-simulated water quality improvement, since biofilms are not transferred directly to a reference condition with a completely different diatom community available as inoculum for a change. Under natural conditions, it would be expected that these new species would originate from upstream sites (by natural drift), where the conditions are usually less polluted. If these species (charactering low polluted waters) were not present in upstream stretches, other dispersal processes, as water birds, fishes (Coste & Ector, Citation2000), or wind would presumably play an important role. Almost certainly these ‘natural’ processes would give slower reaction times than translocations of biofilms observed in this study.
Nevertheless our results will allow water managers to develop a ‘view’ of good ecological status that goes beyond taxonomy and thus start to address issues of the structure and functioning of aquatic ecosystems that are closer to the definition of ecological status given in the Water Framework Directive.
Rimet et al. supplementary material
Download Zip (1,008.3 KB)Acknowledgements
We thank M.G. Kelly, as well as the anonymous reviewers for the scientific and English revision of the manuscript.
References
- AFNOR . 2007 . Norme Française NF T 90-354. Qualité de l’Eau-Détermination de l’Indice Biologique Diatomées (IBD). Décembre 2007 , Saint-Denis La Plaine, , France : Association Française de Normalisation .
- APHA . 1995 . Standard Methods for Examination of Water and Wastewater, , 19th , Washington (DC), , USA : American Public Health Association .
- Blinn , DW , Fredericksen , A and Korte , V . 1980 . Colonization rates and community structure of diatoms on three different rock substrata in a lotic system . Br. Phycol. J. , 15 : 303 – 310 .
- Calow , P . 1973 . The food of Ancylus fluviatilis (Müll.), a littoral stone-dwelling herbivore . Oecologia , 13 : 113 – 133 .
- Cemagref . 1982 . Etude des Méthodes Biologiques d’Appréciation Quantitative de la qualité des eaux , Lyon, , France : Rapport Q.E. Lyon, Agence de l’Eau Rhône-Méditerranée-Corse–Cemagref .
- Coste , M and Ector , L . 2000 . Diatomées invasives exotiques ou rares en France: principales observation effectuées au cours des dernières décennies . Syst. Geogr. Pl. , 70 : 373 – 400 .
- Descy , JP and Ector , L . 1999 . “ Use of diatoms for monitoring rivers in Belgium and Luxemburg ” . In Use of Algae for Monitoring Rivers III , Edited by: Prygiel , J , Whitton , BA and Bukowska , J . 128 – 137 . Douai, , France : Agence de l’Eau Artois-Picardie .
- Ector, L. Kingston, J.C. Charles, D.F. Denys, L. Douglas, M.S.V. Manoylov, K. Michelutti, N. Rimet, F. Smol, J.P. Stevenson, R.J. et al. (2004). Freshwater diatoms and their role as ecological indicators. In Proceedings of the 17th International Diatom Symposium (Poulin, M., editor), 469–480. Biopress Limited, Bristol, UK.
- Hoagland , KD , Roemer , SC and Rosowski , JR . 1982 . Colonization and community structure of two periphyton assemblages, with emphasis on the diatoms (Bacillariophyceae) . Am. J. Bot. , 69 : 188 – 213 .
- Iserentant, R. & Blancke, D. (1986). A transplantation experiment in running water to measure the response rate of diatoms to changes in water quality. In Proceedings of the 8th International Diatom Symposium (Ricard, M., editor), 347–354. Koeltz Scientific Books, Koenigstein, Germany.
- Ivorra, N. (2000). Metal Induced Succession in Benthic Diatom Consortia. Ph.D., Universiteit van Amsterdam, Amsterdam, The Netherlands.
- Katoh , K . 1992 . Correlation between cell density and dominant growth form of epilithic diatom assemblages . Diatom Res. , 7 : 77 – 86 .
- Kawamura , T , Takami , H and Yamashita , Y . 2004 . Effect of grazing by a herbivorous gastropod Homalopoma amussitatum, a competitor for food with post-larval abalone, on a community of benthic diatoms . J. Shellfish Res. , 23 : 989 – 993 .
- Kelly , MG . 2003 . Short term dynamics of diatoms in an upland stream and implications for monitoring eutrophication . Environ. Pollut. , 125 : 117 – 122 .
- Krammer , K and Lange-Bertalot , H . 1986–1991 . “ Bacillariophyceae 1. Teil: Naviculaceae; 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae; 3. Teil: Centrales, Fragilariaceae, Eunotiaceae; 4. Teil: Achnanthaceae. Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema ” . In Süsswasserflora von Mitteleuropa , Edited by: Ettl , H , Gärtner , G , Gerloff , J , Heynig , H and Mollenhauer , D . Stuttgart, , Germany : Gustav Fischer Verlag .
- Lange-Bertalot , H . 2001 . “ Navicula sensu stricto, 10 genera separated from Navicula sensu lato, Frustulia ” . In Diatoms of Europe. Diatoms of the European Inland Waters and Comparable Habitats , Edited by: Lange-Bertalot , H . Ruggell, , Liechtenstein : Gantner Verlag .
- Lavoie , I , Campeau , S , Darchambeau , F , Cabana , G and Dillon , PJ . 2008 . Are diatoms good indicators of temporal variability in stream water quality? . Freshwat. Biol , 53 : 827 – 841 .
- Lecointe , C , Coste , M and Prygiel , J . 1993 . “OMNIDIA”: software for taxonomy, calculation of diatom indices and inventories management . Hydrobiologia , 269/270 : 509 – 513 .
- Luttenton , MR , Vansteenburg , JB and Rada , RG . 1986 . Phycoperiphyton in selected reaches of the Upper Mississippi River: community composition, architecture, and productivity . Hydrobiologia , 136 : 31 – 46 .
- McCune , B and Mefford , J . 1999 . Multivariate analysis of ecological data version 4.01 , Gleneden Beach (OR), , USA : MjM software .
- Molloy , JM . 1992 . Diatom communities along stream longitudinal gradients . Freshwater Biol , 28 : 59 – 69 .
- Mulholland , PJ , Elwood , JW , Palumbo , AV and Stevenson , RJ . 1986 . Effect of stream acidification on periphyton composition, chlorophyll, and productivity . Can. J. Fish. Aquat. Sci. , 43 : 1846 – 1858 .
- Pantazidou, A. Belegratis, M.R. Luvrou, I. & Economou-Amili, A. (2001). Euendolithic algae and patterns of diatom colonization on biotic carbonate substrates. In Proceedings of the 16th International Diatom Symposium (Economou-Amilli, A., editor), 381–393. University of Athens, Athens, Greece.
- Passy , SI . 2007 . Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters . Aquat. Bot. , 86 : 171 – 178 .
- Planas , D , Lapierre , L , Moreau , G and Allard , M . 1989 . Structural organization and species composition of a lotic periphyton community in response to experimental acidification . Can. J. Fish. Aquat. Sci. , 46 : 827 – 835 .
- Prygiel , J and Coste , M . 2000 . Guide méthodologique pour la mise en œuvre de l’Indice Biologique Diatomées. NF T90-354 , Bordeaux, , France : Agences de l’eau–Cemagref .
- Rieter , MA . 1989 . Development of benthic algal assemblages subjected to differing near-substrate hydrodynamic regimes . Can. J. Fish. Aquat. Sci. , 46 : 1375 – 1382 .
- Rimet , F , Cauchie , HM , Hoffmann , L and Ector , L . 2005 . Response of diatom indices to simulated water quality improvements in a river . J. Appl. Phycol. , 17 : 119 – 128 .
- Rimet , F , Ector , L , Cauchie , HM and Hoffmann , L . 2004 . Regional distribution of diatom assemblages in the headwater streams of Luxembourg . Hydrobiologia , 520 : 105 – 117 .
- Rimet , F , Tudesque , L , Peeters , V , Vidal , H and Ector , L . 2003 . Assemblages-types de diatomées benthiques des rivières non polluées du bassin Rhône-Méditerranée-Corse (France) . Bull. Soc. Nat. Ouest de la France, 2ème supplément hors série , : 272 – 287 .
- Rodríguez , MA . 1994 . Succession, environmental fluctuations, and stability in experimentally manipulated microalgae communities . Oikos , 70 : 107 – 120 .
- Roemer , SC , Hoagland , KD and Rosowski , JR . 1984 . Development of a freshwater periphyton community as influenced by diatom mucilages . Can. J. Bot. , 62 : 1799 – 1813 .
- Rott , E . 1991 . “ Methodological aspects and perspectives in the use of periphyton for monitoring and protecting rivers ” . In Use of Algae for Monitoring Rivers , Edited by: Whitton , BA , Rott , E and Friedrich , G . 9 – 16 . Innsbruck, , Austria : Universität Innsbruck, Rott E. Publisher .
- Round , FE and Lee , K . 1989 . Studies on freshwater Amphora species IV. The Amphora epiphytic on other diatoms . Diatom Res. , 4 : 345 – 349 .
- Sabater , S . 2000 . Diatom communities as indicators of environmental stress in the Guadiamar River, S-W. Spain, following a major mine tailings spill . J. Appl. Phycol. , 12 : 113 – 124 .
- Soininen , J and Eloranta , P . 2004 . Seasonal persistence and stability of diatom communities in rivers: are there habitat specific differences? . Eur. J. Phycol. , 39 : 153 – 160 .
- Stevenson , RJ . 1983 . Effects of current and conditions simulating autogenically changing microhabitats on benthic diatom immigration . Ecology , 64 : 1514 – 1524 .
- Stevenson , RJ and Bahls , LL . 1999 . “ Periphyton protocols ” . In Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates, and Fish , Edited by: Gerristen , M , Snyder , J and Stribling , B . 601 – 623 . Washington (DC), , USA : US Environmental Protection Agency .
- Stevenson , RJ and Pan , Y . 1999 . “ Assessing environmental conditions in rivers and streams with diatoms ” . In The Diatoms. Applications for the Environmental and Earth Sciences , Edited by: Stoermer , EF and Smol , JP . 11 – 40 . Cambridge, , UK : Cambridge University Press .
- Tachet, H. Richoux, P. Bournaud, M. & Usseglio-Polatera, P. (2000). Invertébrés d'Eau Douce: Systématique, Biologie, Ecologie. CNRS éditions, Paris, France.
- Tanaka , S and Watanabe , T . 1990 . The colonization process of a typical epilithic algal community–Homoeothrix janthina-Achnanthes japonica community–in a less polluted river in Japan . Jpn J. Phycol. , 38 : 167 – 177 .
- Tolcach , ER and Gómez , N . 2002 . The effect of translocation of microbenthic communities in a polluted lowland stream . Verh. Internat. Verein. Theor. Angew. Limnol. , 28 : 254 – 258 .
- Van Dam , H , Mertens , A and Sinkeldam , J . 1994 . A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands . Neth. J. Aquat. Ecol. , 28 : 117 – 133 .
- Wendker , S . 1992a . Diatom community response to translocation in a small softwater stream . Nova Hedwig. , 55 : 397 – 406 .
- Wendker , S . 1992b . Influence of current velocity on diatoms of small softwater stream . Diatom Res. , 7 : 387 – 396 .
- Whitford , LA . 1956 . The communities of algae in the springs and spring streams in Florida . Ecology , 37 : 433 – 442 .
- Winsborough , BM and Golubić , S . 1987 . The roles of diatoms in stromatolite growth: two examples from modern freshwater settings . J. Phycol. , 23 : 195 – 201 .