Abstract
A new diatom genus Conticribra is erected to accommodate C. tricircularis, described from a freshwater Pliocene deposit in Trout Creek, Oregon (USA). The genus accommodates species possessing: (i) loculate areolae with (semi-) continuous cribra; (ii) non-plicated valve face; (iii) rimoportula located on the valve mantle, replacing a fultoportula. Conticribra tricircularis has no valve face fultoportulae and can easily be distinguished by its marginal fultoportulae with four satellite pores arranged in three rings. Three species are transferred to the new genus from Thalassiosira sensu lato. Using evidence from the fossil record and recent molecular data, a hypothesis concerning the freshwater origin of Conticribra is discussed.
Introduction
In 1873 the genus Thalassiosira was erected by Cleve (Cleve, Citation1873). There are now at least 300 species in the genus (Catalogue of Diatom Names, Citation2009), of which the majority have been reported from marine modern and fossil assemblages. All species in the genus Thalassiosira have processes, both rimoportula(e) and fultoportulae. The presence of the fultoportula (the strutted process) is a character of the family Thalassiosiraceae Lebour emend. Hasle, to which the genus Thalassiosira belongs (Hasle, Citation1973). In most species of Thalassiosira the usually loculate areolae are open to the outside by circular foramina, while internally they are occluded by individual, slightly raised, cribra (e.g. Round et al., Citation1990, p. 133, figs h–k). However, several species in this genus possess a (semi-)continuous cribra structure, which forms radial rows separated by a hyaline area (e.g. Hasle & Lange, Citation1989). Many Thalassiosira species have tangentially undulated valves. These belong to the so-called ‘plicated’ group in contrast to the ‘non-plicated’ species, which possess non-undulated valves (e.g. Hasle & Lange, Citation1989).
In 1989, Hasle & Lange reported on brackish and freshwater species of Thalassiosira, pointing out that “this exceptional group … is differentiated in two groups by the cribra structure and partly by the habitat” (Hasle & Lange, Citation1989, p. 131). They noted that the valves of species with continuous cribra – that is, aligned in radial ‘zig-zag’ paths – are known from brackish and freshwater environments. One group of species they describe as ‘undulated’, includes the following species: T. lacustris (Grunow) Hasle, T. australiensis (Grunow) Hasle in Hasle & Lange and T. gessneri Hustedt. The group was contrasted with the ‘non-undulated’ species, which are known only in the freshwater/brackish water species T. weissflogii (Grunow) G. Fryxell & Hasle and the marine species T. lepotopus (Grunow) Hasle & G. Fryxell. Since the publication of Hasle & Lange (Citation1989) more species of Thalassiosira (both plicated and non-plicated) with continuous cribra have been described: T. kamczatica (Lupikina) Lupikina & Khursevich (these data require confirmation by additional SEM studies), T. kilarskii Kaczmarska (see emended description in Kociolek & Khursevich, Citation2001), T. nevadica Khursevich & VanLandingham, and T. inlandica Hayashi in Hayashi, Tanimura & Sakai (see also, Thalassiosira A in Julius & Tanimura, Citation2001, ; Thalassiosira sp., Ognjanova-Rumenova, unpublished data). All are freshwater and extinct (see ). From modern freshwater populations, two species possessing continuous cribra have recently been described: T. rudis Tremarin, Ludwig, Becker & Torgan and Spicaticribra kingstonii Johansen, Kociolek & Lowe, the latter being placed in a new genus (Johansen et al., Citation2008). Interestingly, there are also species of Thalassiosira that possess loculate areolae with individual cribra, known from freshwater, both extinct (e.g. Serieyssol et al., Citation1998; Kociolek & Khursevich, Citation2001) and living (e.g. Cassie & Dempsey, Citation1980; Pienaar & Pieterse, Citation1990). Thalassiosira patagonica Maidana is an extant freshwater species of Thalassiosira with areolae possessing “flat cribra” (Maidana, Citation1999: p. 325). However, from the published illustrations, it is unclear whether the cribra are continuous or individual (Maidana, Citation1999: figs 13, 14).
Figs 1–6. Conticribra tricircularis sp. nov., SEM, external view of valve. Specimens from the Pliocene sediment from Trout Creek (Oregon, USA). Figs 1, 2. Whole valve surface with non-plicated valve face; valve surface with radiate areolation pattern. Note external openings of marginal fultoportulae forming three regular rings (arrow). Figs 3, 4. Valve surface with loculate areolae arranged in radiate rows, and external openings of marginal fultoportulae usually forming three rings (arrow). Figs 5, 6. Partly dissolved valves, with details of external openings of marginal fultoportulae (arrow) and network of ribs. Scale bars: 5 µm (Figs 1, 2): 2 µm (Figs 3, 4); 1 µm (Figs 5, 6).
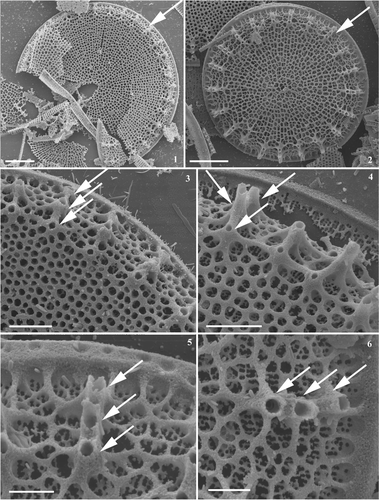
Specimens investigated herein belong to the group characterized by loculate areolae with continuous cribra and a non-plicated valve face. In addition, a rimoportula replaces a marginal fultoportula on the valve surface. They are separated from Thalassiosira s.l., and a new genus, Conticribra, is erected for two extinct and two extant species.
Table 1. Fossil record of extinct species in Conticribra and Thalassiosira s.l. with continuous cribra.
Materials and methods
The sample studied was from a freshwater Pliocene deposit in Trout Creek, Oregon, USA, donated to the Natural History Museum, London, UK (BM) from the Brigger Collection, California Academy of Sciences (CAS), San Francisco, USA. Specimens were mounted on strips of mica to facilitate examination with SEM, performed using a Field Emission microscope (Philips XL30) at BM. Terminology follows Ross et al. (Citation1979), Hasle & Lange (Citation1989), Round et al. (Citation1990) and Theriot & Serieyssol (Citation1994).
Figs 7–14. Conticribra tricircularis sp. nov., SEM, internal view of valve. Specimens from the Pliocene sediment from Trout Creek (Oregon, USA). Figs 7, 8. Internal view of valve surface, showing non-plicated valve face, semi-continuous cribra and three regular rings of marginal fultoportulae. Note absence of valve face fultoportula(e). . Processes (arrow R: rimoportula; arrow: marginal fultoportulae), semi-continuous cribra on valve face and continuous cribra on valve mantle. . Raised rimoportula replacing marginal fultoportulae, externally opened by elongated, spine-like tube, internally oriented along valve radius. . Details of marginal fultoportulae: slightly elongated central tube possessing satellite pore cover (arrow C) and four cowlings. Note hyaline area in process zone. . Partly dissolved valves with processes (arrow R: rimoportula; arrow: marginal fultoportulae), semi-continuous cribra on valve face and continuous cribra on valve mantle. Scale bars: 5 µm (Figs 7, 8); 1 µm (Figs 9, 11, 12, 14); 0.5 µm (Figs 10, 13).
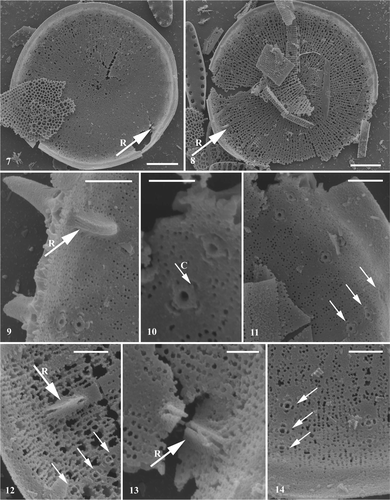
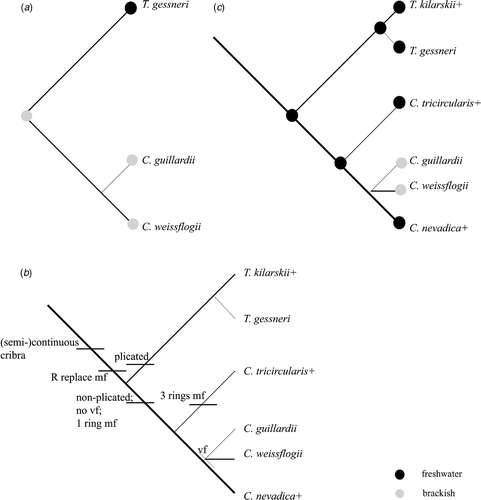
Fig. 15. Diagrams to show phylogenetic relationships and hypothesis of the freshwater origin of Conticribra. (a) Modified from Alverson et al. (Citation2007, ) to include only species relevant to this paper; (b) phylogenetic relationships from a with addition of extinct species of Conticribra and Thalassiosira kilarskii (based on morphology). Abbreviations: mf: marginal fultoportulae; R: rimoportula; vf: valve face fultoportulae; +: extinct species. (c) Phylogenetic relationships from a with addition of extinct species of Conticribra and Thalassiosira kilarskii (based on morphology) showing freshwater origin of the genus and recent brackish water colonizations.
Observations
Species description
Conticribra gen. nov.
DIAGNOSIS: Valvae circulares, pariete non plicato, areolis loculatis, foraminis externis circularibus; cribris (semi-)continuis e poris lineatis, etiam presentibus in limbo; fultoportulis centralibus interdum presentibus varie dispositis, marginalibus in annulis dispositis, fultoportularum foraminibus externis elevatis in processo tubuliforme; rimoportulis singulis foramine interna, pro fultoportula marginale substitutis.
Valves circular, with non-plicated (flat) valve face; areolae loculate, open to outside by circular foramina. Areolae on valve face covered internally by (semi-)continuous cribra, consisting of linearly arranged pores; areolae on valve mantle, covered internally by continuous cribra. Valve face fultoportulae sometimes present. Marginal fultoportulae arranged in regular ring(s), external opening usually as an elongated tube; internal opening a slightly elongated central tube; single rimoportula replaces a marginal fultoportula.
AGE RANGE: Miocene–Recent.
HABITAT: Freshwater–brackish water.
ETYMOLOGY: The name refers to the diagnostic feature of the genus: (semi-)continuous cribra.
GENERITYPE: Conticribra tricircularis
Conticribra tricircularis sp. nov.
DIAGNOSIS: Valvae circulares, pariete non plicato, 22–29 µm diametric; areolis externis rotundatis, 19 in 10 µm, cibris internis continuis in limbo valvae et semicontinuis in facie; fultoportulis centralibus absentibus, ad marginem superficiei valvae et limbo annul marginalibus fultoportularum (2)3 instructae, fultoportularum poris satelliticis 4; rimoportulis singulis in limbo, pro fultoportula substitutis, processis tubuliformibus externis fultoportularum et rimoportularum.
Valves circular, with non-plicated (flat) valve face; valve diameter 22–29 µm. Areolae loculate, opening to outside by circular foramina, arranged in radial rows, 19 in 10 µm. Areolae on valve face covered internally by semi-continuous cribra, consisting of linearly arranged pores; on internal valve mantle, by continuous cribra. Valve face fultoportulae absent. Three rings of marginal fultoportulae located on mantle (one ring), mantle/valve face junction (one ring), near mantle/valve face junction (one ring). Two fultoportulae may be present among the rings. Each marginal fultoportula possesses four satellite pores. External opening of marginal fultoportula an elongated tube; internal opening a slightly elongated central tube, with satellite pore cover and cowlings. A single rimoportula, replacing a marginal fultoportula, opens externally by an elongated, spine-like tube, internally by a radially oriented slit.
HOLOTYPE: Slide BM 11344 deposited in BM, London, UK (BM).
TYPE LOCALITY: Lacustrine sediment in Oregon, USA.
AGE RANGE: Pliocene.
ETYMOLOGY: The name refers to the three rings of marginal fultoportulae (three – lat. tres, ring – lat. circulus), the diagnostic feature of this new species.
Ultrastructural observations
Externally, the loculate areolae (Figs ) develop within the network of ribs, visible on partly dissolved valves (Figs 5, 6). Internally, semi-continuous cribra occur on the valve face (Figs ), and continuous cribra on the mantle (). The cribra are arranged in several linear rows, more or less parallel to each other, separated by distinct hyaline areas (Figs ). Hyaline areas between the cribra are also distinct, among the parallel zones of mantle fultoportulae (). The three rings of mantle fultoportulae usually occur on the mantle, mantle/valve face junction, and near mantle/valve face junction (). Each fultoportula possesses four satellite pores (). Externally, the fultoportulae open by elongated tubes (); internally, the tube and four cowlings are slightly elongated (), with distinct satellite pore coverings (). Valve face fultoportulae are absent (), a single rimoportula is present, at the mantle/valve face junction, replacing one triplet of marginal fultoportulae (). However, it is not clear from the valves examined whether the rimoportula replaces two fultoportulae (located towards valve centre) leaving just one (Figs 9, 12, 13). Externally, the rimoportula has an elongated, spine-like tubular opening (); internally, it appears raised ().
The new species C. tricircularis is characterized by (semi-)continuous cribra, a non-plicated valve face, one marginal rimoportula replacing a fultoportula and the absence of the valve face fultoportulae. Contibribra tricircularis shares these features with the extinct species, T. nevadica, and, additionally, both possess rimoportulae that open externally by an elongated tube. From the illustrations in Khursevich & VanLandingham (Citation1993), it is unclear if there are two or four satellite pores in T. nevadica. However, whereas C. tricircularis possesses three rings of mantle fultoportulae, T. nevadica has only one ring. Extant taxa sharing these valve characters include freshwater to brackish water species, such as T. guillardii Hasle and T. weissflogii. Conticribra tricircularis shares a number of features with T. guillardii: a single rimoportula replacing the marginal fultoportula; absence of valve face fultoportulae in freshwater populations; marginal fultoportulae with four satellite pores. Interestingly, the extinct species are characterized by a lack of valve face fultoportulae whereas extant species have either a few or none at all. Detailed morphological comparisons are given in .
Table 2. Comparison of morphological characters of species in Conticribra and two Thalassiosira species with continuous cribra and non-plicated valve face.
The molecular data analysed by Alverson et al. (Citation2007, diagrams 2–4) yield a sister-group relationship between T. weissflogii and T. guillardii. Thalassiosira pseudonana Hasle & Heimdal (rimoportula between fultoportulae), T. gessneri (plicated), and Thalassiosira CCMP1065 (individual cribra, Alverson's unpublished data) are paraphyletic with respect to the T. guillardii–T. weissflogii group. Moreover, all these species form a monophyletic group separate from all other species of Thalassiosira.
Evidence from morphology, the fossil record and molecules suggests that C. tricircularis, as well as other species with continuous cribra, non-plicated valve face and rimoportula replacing the fultoportula, form a monophyletic group relative to Thalassiosira s.l. (). Three taxonomic changes are therefore required, transferring T. nevadica, T. guillardii and T. weissflogii to Conticribra.
Conticribra nevadica (Khursevich & VanLandingham) comb. nov.
BASIONYM: Thalassiosira nevadica Khursevich & VanLandingham, Citation1993, Nova Hedwigia 56: 390, 392, figs .
TYPE LOCALITY: c. 5 km NW of Crow Spring, between Cedar Mountains and Monte Cristo Range in sec. 30, T. 5 N., R. 39 E., Esmeralda County, Nevada, USA.
Conticribra guillardii (Hasle) comb. nov.
BASIONYM: Thalassiosira guillardii Hasle, Citation1978, Phycologia 17: 274, figs 28–47, 49, 50.
TYPE LOCALITY: Helsinki Fölisöfjord, Gulf of Finland, 14.v.1970, leg. Åki Niemi.
Conticribra weissflogii (Grunow) comb. nov.
BASIONYM: Micropodiscus weissflogii Grunow in Van Heurck, 1885, Synopsis des Diatomées de Belgique: 210.
SYNONYMS: Eupodiscus weissflogii Grunow in Van Heurck, 1882–1885, Types du Synopsis des Diatomées de Belgique: 3 (No. 11), 100 (No. 416), nom. invalid.
Micropodiscus weissflogii Grunow in Van Heurck, 1882–1885, Types du Synopsis des Diatomées de Belgique: 3 (No. 11), 100 (No. 416), nom. invalid.
Eupodiscus weissflogii (Grunow in Van Heurck) De Toni, 1894, Sylloge algarum omnium hucusque cognitarum. Vol. II. Bacillarieae; sectio III. Cryptoraphideae. Typis Seminarrii: 1087.
Thalassiosira weissflogii (Grunow) G. Fryxell & Hasle, Citation1977, Beih. Nova Hedwigia, 54: 68.
Thalassiosira fluviatilis Hustedt, 1926, Ber. Deutsch. Bot. Ges. 43: 565.
Discussion
There have been two approaches to the subdivision of species in Thalassiosira. The first, proposed by Makarova (Citation1988), distinguished four sections based mainly on areolation pattern: Tangentales (type: T. eccentrica (Ehrenberg) Cleve); Fasciculigera (type: T. baltica (Grunow in Cleve & Grunow) Ostenfeld); Thalassiosira (type: T. nordenskioeldii Cleve); Inconspicuae (type: T. condensata Cleve). Conticribra guillardii and C. weissflogii, together with 44 other Thalassiosira species were placed in the section Thalassiosira based on the following five characters: (i) primarily flat valves; (ii) radial rows of areolae; (iii) one ring of marginal fultoportulae; (iv) one-to-many valve face fultoportulae in the central zone; and (v) one rimoportula, usually situated on the valve face/mantle junction.
The second approach, proposed by Hasle & Lange (Citation1989) and developed by Julius & Tanimura (Citation2001) and Hayashi et al. (Citation2007), divided the species into plicated versus non-plicated, or a marine versus brackish water/freshwater group (Hasle, Citation1978; Hasle & Lange, Citation1989; Tanimura, Citation1996; Julius & Tanimura, Citation2001), based on two characters: valve face ‘undulation’ and cribrum structure.
Recent molecular evidence, based on analysis of sequence data from nuclear SSU rRNA, suggests that the genus Thalassiosira is either paraphyletic (Kaczmarska et al., Citation2006) or, using a multiple gene approach (SSU rRNA, partial LSU rDNA, psbC and rbcL), polyphyletic (Alverson et al., Citation2007; but see Theriot, Citation2008). Alverson et al. (Citation2007) used species of Thalassiosira from several of the subgroups, e.g. with a tangentially undulated valve face or linear areolar array. Species that possess continuous cribra are non-monophyletic relative to species with individual cribra Alverson et al. (Citation2007,). Furthermore, modern species with continuous cribra, non-plicated valve face and rimoportula replacing fultoportula form a monophyletic group (Alverson et al., Citation2007, ).
Combining data on morphology (for both extinct and extant species) and molecules (Alverson et al., Citation2007), the concept of Thalassiosira proposed by Hasle & Lange (Citation1989) can be modified. Species with (semi-)continuous cribra, non-plicate valve face and rimoportula replacing fultoportula(e) belong to the new genus Conticribra.
Of the other species of Thalassiosira, T. inlandica (Pleistocene) has a non–plicated valve face and lacks valve face fultoportulae, their rimoportula(e) being located on the valve mantle, but the presence of multiple rimoportulae (opening externally by a pore), which may be situated near the mantle fultoportula, differentiates it from species of Conticribra. Additionally, T. inlandica has its mantle fultoportulae opening externally as a pore with conical thickenings and its internal structure has three satellite pores. Specimens of Thalassiosira s.l. from a freshwater Neogene deposit in the Balkan Peninsula are characterized by non-plicated valve face, continuous cribra and a rimoportula located between mantle fultoportulae. Valve face fultoportulae have not been observed (Ognjanova-Rumenova, unpublished data).
The extinct (Miocene) marine species Thalassiosira manifesta Sheshukova-Poretzkaja (illustrations in Makarova, Citation1988: XLVII, ; Glezer et al., Citation1988: ), has continuous cribra and a non-plicated valve face. However, the position of a rimoportula in the submarginal zone of the valve face differentiates it from species in Conticribra. Additionally, T. manifesta possesses several central valve face fultoportulae.
Extant taxa, such as Thalassiosira pseudonana, T. rudis and T. salvadoriana Hustedt with continuous cribra and a non-plicated valve face are found in fresh (T. salvadoriana and T. rudis) to brackish water (T. pseudonana). However, like T. inlandica, T. pseudonana has its rimoportula located between fultoportulae (but not replacing any). Molecular data also suggest it is not monophyletic with Conticribra guillardii and C. weissflogii (Alverson et al., Citation2007). Thalassiosira rudis has been diagnosed by having 1–3 rimoportulae between fultoportulae (Ludwig et al., Citation2008), and a similar rimoportula position was noted in Spicaticribra kingstonii (Johansen et al., Citation2008). According to their original description of Spicaticribra, this genus is characterized by spicate cribra, the absence of fultoportulae on the valve face and lack of external extensions on the marginal fultoportulae (Johansen et al., Citation2008). Morphological observations on T. salvadoriana (Friedel, unpublished data) require investigation before its relationship to other species can be established; evidence from molecular data would be useful.
Kaczmarska & Ehrman (Citation2008) hypothesised relationships for a group of early thalassiosiroid diatoms, including the genera Poloniasira Kaczmarska & Ehrman (Oligocene), Lomonycus Komura and Nephrodiscus Komura (the last two both Miocene) and the species Thalassiosira fraga Schrader in Schrader & Fenner and T. spumellaroides Schrader (early Miocene). This group is defined by the lack of central valve fultoportulae and few, if any, valve face fultoportulae. Kaczmarska & Ehrman (Citation2008) also noted that all these taxa have fultoportulae subtended by 5–7 satellite pores, as opposed to 2–4 in recent species. The presence of marginal fultoportulae only, subtended by four satellite pores, found in the extinct representatives of Conticribra, expands the number of fossil thalassiosiroids without valve face fultoportulae. The extinct and extant species of Conticribra are relevant to the interpretation of the evolution of fultoportulae (Kaczmarska et al., Citation2006; Theriot, Citation2008). Observations on Conticribra species support the location of rimoportulae, whose position has been used to differentiate between Tertiarius Håkansson & Khursevich and Pliocaenicus Round & Håkansson emend. Khursevich & Stachura-Suchoples (Håkansson & Khursevich, Citation1997; Khursevich & Stachura-Suchoples, Citation2008) as a diagnostic character (synapomorphy).
Tanimura (Citation1996) compiled important data on the fossil record and phylogeny of the marine, fossil species of plicated Thalassiosira. Tanimura (Citation1996) suggested that the distinctive plication of the valves, single prominent marginal rimoportula, scattered fultoportulae on the valve face and one row of marginal fultoportulae were all common defining characters of the extinct species. All species in Tanimura's (Citation1996) study (extinct and extant), have individual cribra. With the exception of T. temperei (Brun) Akiba & Yanagisawa (Miocene-Pliocene) and T. sancettae Akiba (Miocene-Pleistocene) the extinct species are generally restricted to the Miocene; all those species studied by Tanimura (Citation1996) have an age range of 17 to 0 Ma. Species of Conticribra and Thalassiosira s.l. with continuous cribra (non-plicated and plicated) are known from the Miocene to Recent (see also ).
There are several hypotheses on the origin of freshwater and marine diatoms. It has been suggested that a primary group originated: (i) in shallow muddy areas with unstable salinity from which freshwater and marine groups evolved independently (Round & Sims, Citation1980); (ii) as marine species, later being introduced into continental waters (Skabitchevsky, Citation1981); (iii) in brackish waters, from which the continental forms were first derived, followed by marine diatoms (Strelnikova & Lastivaka, Citation1999). Here, we contribute a few comments to the general discussion on colonization events in diatoms, our evidence derived from Thalassiosira s.l. and Conticribra.
With the exception of Thalassiosira sp. A (Julius & Tanimura, Citation2001; 17 Ma) and T. manifesta, all extinct species mentioned here have continuous cribra and have been reported from freshwater deposits (). Alverson et al. (Citation2007, p. 204 and 207, respectively) wrote that ‘likelihood mapping favored a total of three independent colonizations of fresh waters in the lineages leading to T. gessneri, Clade B + T. pseudonana, and Clade C ()’, commenting further that ‘[O]ne freshwater colonization involved T. gessneri, the sole representative of a presumably larger clade of marine, brackish, and freshwater Thalassiosira species with a tangentially undulated valve face’. Sims et al. (Citation2006, p. 388) noted that one of the major flooding events (particularly high sea levels) occurred in the middle Miocene (c. 18–13 Ma), and suggested that major flooding events ‘must have had a considerable effect on diatom evolution, creating and then destroying large numbers of freshwater bodies’. Sims et al. (Citation2006) also suggested that in the early to middle Miocene (c. 22–15 Ma) freshwater colonization events occurred, replacing species of Thalassiosira by species from other genera, such as Cyclotella Kütz., Cyclostephanos Round, Mesodictyon Theriot & Bradbury and Stephanodiscus Ehrenb.; the oldest record for the genus Pliocaenicus is the middle Miocene (Stachura-Suchoples et al., Citation2009). One might add Conticribra () to this evolutionary scenario, with the implication that the freshwater to brackish water–marine colonization event is relatively recent, as indicated by both the extant and extinct members. Moreover, it may be possible to relate T. kilarski to the extant species of plicated Thalassiosira via T. gessneri.
Considering the fossil record, the colonization proposed by Alverson et al. (Citation2007, diagram 5; ) could be modified. We suggest that the freshwater origin of species should be placed between their clades A and B (Alverson et al., Citation2007, diagram 5). However, there is a limited fossil evidence documenting species with continuous cribra, making it difficult to establish a definitive link, and the possible colonization and potential evolutionary scenarios may be considerably more complicated.
Acknowledgements
We would like to thank Drs Galina Khursevich and Regine Jahn for reading and commenting on the manuscript. Ms Friedel Hinz, Dr Nadja Ognjanova-Rumenova and Dr Andrew Alverson kindly provided access to unpublished SEM observations. K.S. is grateful to the staff of the Botany and Mineralogy Departments for their warm hospitality and wonderful scientific atmosphere during a Synthesys visit to the Natural History Museum, in London, the UK. We thank Dr Harrie Sipman for his help in improving the Latin diagnosis. This research received support from the SYNTHESYS Project: http//www.synthesys.info/, which was financed by European Community Research Infrastructure Action under the FP 6 ‘Structuring the European Research Area’ Programme (to K.S., application GB-TAF-3994).
References
- Alverson , AJ , Jansen , RK and Theriot , EC . 2007 . Bridging the Rubicon: phylogenetic analysis reveals repeated colonizations of marine and fresh waters by thalassiosiroid diatoms . Mol. Phylogen. Evol. , 45 : 193 – 210 .
- Cassie , V and Dempsey , GP . 1980 . A new freshwater species of Thalassiosira from some small oxidation ponds near Auckland, New Zealand, and its ultrastructure . Bacillaria , 3 : 273 – 292 .
- Catalogue of Diatom Names> (2009). California Academy of Sciences, On-line Version. Compiled by Elisabeth Fourtanier & J. Patrick Kociolek. Available online at http://www.calacademy.org/research/diatoms/names/index.asp
- Cleve , PT . 1873 . On diatoms from the Arctic Sea . Bih. K. Svenska Ve-. tensk. Akad., Handl. , 1 : 1 – 28 .
- Fryxell , GA and Hasle , GR . 1977 . The genus Thalassiosira: Some species with a modified ring of central strutted processes . Nova Hedwig., Beih. , 54 : 67 – 98 .
- Glezer, Z.I., Makarova, I.V., Moisseeva, A.I., & Nikolaev, V.A. (1988). The diatoms of the USSR. Fossil and recent, 2(I): Pyxidiculaceae, Thalassiosiropsidaceae, Triceratiaceae, Thalassiosiracea. “Nauka”, Leningrad. (In Russian)
- Håkansson , H and Khursevich , GK . 1997 . Tertiarius gen. nov., a new genus in the Bacillariophyceae, the transfer of some cyclotelloid species and a comparison to closely related genera . Diatom Res. , 12 : 19 – 33 .
- Hasle , GR . 1973 . Thalassiosiraceae, a new diatom family . Norw. J. Bot. , 20 : 67 – 69 .
- Hasle , GR . 1976 . Examination of diatom type material: Nitzschia delicatissima Cleve, Thalassiosira minuscula Krasske, and Cyclotella nana Hustedt . Br. Phycol. J. , 11 : 101 – 110 .
- Hasle , GR . 1978 . Some freshwater and brackish water species of the diatom genus Thalassiosira Cleve . Phycologia , 17 : 263 – 292 .
- Hasle , GR and Lange , CB . 1989 . Freshwater and brackish water Thalassiosira (Bacillariophyceae): taxa with tangentially undulated valves . Phycologia , 28 : 120 – 135 .
- Hayashi , T , Tanimura , Y and Sakai , H . 2007 . A fossil freshwater Thalassiosira, T. inlandica sp. nov. (Bacillariophyta), with semicontinuous cribra and elongated marginal fultoportulae . Phycologia , 46 : 353 – 362 .
- Johansen , J , Kociolek , P and Lowe , R . 2008 . Spicaticribra kingstonii, gen. nov. et sp. nov. (Thalassiosirales, Bacillariophyta) from Great Smoky Mountains National Park, U.S.A . Diatom Res. , 23 : 367 – 375 .
- Julius , ML and Tanimura , Y . 2001 . Cladistic analysis of plicated Thalassiosira. (Bacillariophyceae) . Phycologia , 40 : 111 – 122 .
- Kaczmarska , I . 1985 . The diatom flora of Miocene lacustrine diatomites from the Harper Basin, Oregon, USA . Acta Paleobotanica , 25 : 33 – 100 .
- Kaczmarska , I , Beaton , M , Benoit , AC and Medlin , LK . 2006 . Molecular phylogeny of selected members of the order Thalassiosirales (Bacillariophyta) and evolution of the fultoportula . J. Phycol. , 42 : 121 – 138 .
- Kaczmarska , I and Ehrman , JM . 2008 . Poloniasira fryxelliana Kaczmarska, a new thalassiosiroid diatom (Bacillariophyta) from the Early Oligocene diatomites in Polish Flysch Carpathians, southeast Poland . Nova Hedwig., Beih. , 133 : 217 – 230 .
- Khursevich , GK and Stachura-Suchoples , K . 2008 . The genus Pliocaenicus Round & Håkansson (Bacillariophyta): morphology, taxonomy, classification and biogeography . Nova Hedwig. , 86 : 419 – 444 .
- Khursevich , GK and VanLandingham , S . 1993 . Frustular morphology of some diatom species from Miocene freshwater sedimentary rocks of western U.S.A. and Canada . Nova Hedwig. , 56 : 389 – 400 .
- Kociolek , JP and Khursevich , GK . 2001 . Valve ultrastructure of new and rare fossil freshwater species of Thalassiosira Cl. (Bacillariophyta) from China and USA . Int. J. Algae , 3 : 86 – 98 .
- Ludwig , TAV , Tremarin , PI , Becker , V and Torgan , LC . 2008 . Thalassiosira rudis sp. nov. (Coscinodiscophyceae): a new freshwater species . Diatom Res. , 23 : 389 – 400 .
- Lupikina , EG and Khursevich , GK . 1992 . A new freshwater species of Thalassiosira (Bacillariophyta) from Miocene deposits of Kamchatka . Paleontologichesky Zhurnal , 1 : 136 – 138 .
- Maidana , NI . 1999 . Thalassiosira patagonica sp. nov. (Thalassiosiraceae, Bacillariophyceae), a new lacustrine centric diatom from Santa Cruz, Argentina . Diatom Res. , 14 : 323 – 329 .
- Makarova, I.V. (1988). Diatomovie vodorosli morei SSSR: rod Thalassiosira Cl. USSR. Acad. Sci. “Nauka”, Leningrad. (In Russian)
- Pienaar , C and Pieterse , AJH . 1990 . Thalassiosira duostra. sp. nov., a new freshwater centric diatom the Vaal River, South África . Diatom Res. , 5 : 105 – 111 .
- Ross , R , Cox , EJ , Karayeva , NI , Mann , DG , Paddock , TBB , Simonsen , R and Sims , PA . 1979 . An amended terminology for siliceous components of the diatom cell . Nova Hedwig., Beih. , 64 : 513 – 533 .
- Round , F and Sims , P . 1980 . “ The distribution of diatom genera in marine and freshwater environment and some evolutionary considerations ” . In Proceedings of 6th International Diatom Symposium, Budapest , Edited by: Ross , R . 301 – 320 . Germany : O. Koeltz, Koenigstein .
- Round , FE , Crawford , RM and Mann , DG . 1990 . The Diatoms: Biology & Morphology of the Genera , Cambridge, , UK : Cambridge University Press .
- Serieyssol , KK , Israde Garduno , I and Gasse , F . 1998 . Thalassiosira dispar comb. nov. and T. cuitzeonensis spec. nov. (Bacillariophyceae) found in Miocene sediments from France and Mexico . Nova Hedwig. , 66 : 177 – 186 .
- Sims , PA , Mann , DG and Medlin , LK . 2006 . Evolution of the diatoms: insights from fossil, biological and molecular data . Phycologia , 45 : 361 – 402 .
- Skabitchevskii , AP . 1981 . Vselienie diatomovih vodoroslei v presnie vodi. Bjulleten Moskovskogo obshestva ispitatelei prirodi . Otdelenie biologii , 86 : 115 – 125 .
- Stachura-Suchoples , K and Jahn , R . 2009 . Middle Miocene record of Pliocaenicus chanbaiense sp. nov. from Changbai Shan (Jilian Province, China) . Acta Bot. Croat. , 68 : 211 – 220 .
- Strelnikova , NI and Lastivka , TV . 1999 . “ The problem of the origin of marine and freshwater diatoms ” . In Proceedings of 14th International Diatom Symposium, Tokyo, Japan , Edited by: Mayama , S , Idei , M and Koizumi , I . 113 – 123 . Koenigstein, , Germany : Koeltz Scientific Books .
- Tanimura , Y . 1996 . Fossil marine plicated Thalassiosira: taxonomy and an idea on phylogeny . Diatom Res. , 11 : 165 – 202 .
- Theriot , E . 2008 . Application of phylogenetic principles to testing evolutionary scenarios: a comment on Kaczmarska et al. “Molecular phylogeny of selected members of the order Thalassiosirales (Bacillariophyta) and evolution of the fultoportula” . J. Phycol. , 44 : 821 – 833 .
- Theriot , E and Serieyssol , K . 1994 . Phylogenetic systematics as a guide to understanding features and potential morphological characters of the centric diatom family Thalassiosiraceae . Diatom Res. , 9 : 429 – 450 .