Abstract
The microfilamentous green alga Uronema curvatum is widely distributed along the western and eastern coasts of the north Atlantic Ocean where it typically grows on crustose red algae and on haptera of kelps in subtidal habitats. The placement of this marine species in a genus of freshwater Chlorophyceae had been questioned. Molecular phylogenetic analysis of nuclear-encoded small and large subunit rDNA sequences reveal that U. curvatum is closely related to the ulvophycean order Cladophorales, with which it shares a number of morphological features, including a siphonocladous level of organization and zoidangial development. The divergent phylogenetic position of U. curvatum, sister to the rest of the Cladophorales, along with a combination of distinctive morphological features, such as the absence of pyrenoids, the diminutive size of the unbranched filaments and the discoid holdfast, warrants the recognition of a separate genus, Okellya, within a new family of Cladophorales, Okellyaceae. The epiphytic Urospora microscopica from Norway, which has been allied with U. curvatum, is revealed as a member of the cladophoralean genus Chaetomorpha and is herein transferred to that genus as C. norvegica nom. nov.
Introduction
Green algae display a wide diversity of thallus organization, ranging from flagellate or coccoid unicells to colonial forms and various levels of multicellular organization. This morphological variation has been the basis for conventional green algal classification (Round, Citation1984). For example flagellates were commonly grouped in the order Volvocales, coccoids in the Chlorococcales, and unbranched filaments in the Ulotrichales (Bold & Wynne, Citation1985). Ultrastructural work, comparative biochemistry and life-history studies have demonstrated that a filamentous nature (and various other vegetative features) are independently derived in different lineages within the green algae (Mattox & Stewart, Citation1984). Molecular systematics have largely corroborated these findings, showing that convergent evolution is responsible for the presence of unbranched filaments in distantly related green algal lineages, such as the chlorophytan classes Ulvophyceae (Ulothrix Kützing, Urospora Areschoug and Chaetomorpha Kützing) and Chlorophyceae (Microspora Thuret, Oedogonium Link, Uronema Lagerheim), and the streptophytan classes Klebsormidiophyceae (Klebsormidium Silva, Mattox & Blackwell) and Zygnematophyceae (Spirogyra Link and other genera) (Lewis & McCourt, Citation2004; Pröschold & Leliaert, Citation2007). Most notably, the genus Ulothrix, which is often regarded as the morphological archetype of the unbranched uniseriate filamentous morphology, has been shown to be polyphyletic, with its various members belonging to different green algal classes (O’Kelly, Citation2007).
Although the phylogenetic position of numerous unbranched filamentous species has now been resolved based on ultrastructural and molecular evidence (e.g. Booton et al., Citation1998; Leliaert et al., Citation2003; O’Kelly et al., Citation2004), several taxa have remained largely unstudied. Amongst them are the marine microfilamentous species Uronema curvatum Printz and Urospora microscopica Levring.
The genus Uronema (Lagerheim, Citation1887) is characterized by unbranched filaments of uninucleate cells with a single chloroplast and 1–4 pyrenoids (Chaudhary, Citation1979). The filaments are attached by a basal discoid gelatinous holdfast, and apical cells are typically acuminate. Thalli reproduce asexually by zoospores, one (sometimes two) being formed per cell. The genus is currently placed in the chlorophycean order Chaetophorales, based on ultrastructural evidence and 18S rDNA sequence data (Booton et al., Citation1998). Uronema includes about 17 species (Guiry & Guiry, Citation2009), all but two restricted to freshwater or damp terrestrial habitats. The only marine species are the North Atlantic U. curvatum and the south-west Pacific U. marinum Womersley, both inconspicuous algae that have probably passed unnoticed in many investigations. Uronema curvatum was originally described from Trondheim Fjord, Norway (Printz, Citation1926), and has since been reported from scattered localities along the eastern and western coasts of the north Atlantic Ocean (Feldmann, Citation1954; Rueness, Citation1977; South & Tittley, Citation1986; Rueness, Citation1992; Kornmann & Sahling, Citation1994; Maggs & O’Kelly, Citation2007). The species grows in subtidal habitats on non-calcified crustose red algae (such as Peyssonnelia Decaisne and Cruoria Fries), crustose cyanobacteria on pebbles, and on haptera of kelps. Uronema curvatum differs from the freshwater representatives of the genus in zoosporangial and apical cell morphology, and its generic placement has been debated by Rueness (Citation1992) and Kornmann & Sahling (Citation1994), who suggested a relationship with the Cladophorales or Ulotrichales (Acrosiphoniales).
Urospora (Areschoug, Citation1866) is a genus of cold water, marine green algae characterized by an unbranched filamentous gametophyte composed of multinucleate cells with a parietal reticulate chloroplast, and a unicellular, club-shaped, uninucleate sporophyte (Codiolum phase). The uniseriate gametophytes are attached to the substratum by multicellular rhizoids arising from the basal cells. Urospora has a complex nomenclatural history and the taxonomic position of the genus has long been uncertain (Lokhorst & Trask, Citation1981). The genus has been placed in the Cladophorales or Siphonocladales based on the multinucleate cells (Wille, Citation1890; Rosenvinge, Citation1893; Setchell & Gardner, Citation1920; Printz Citation1932) but is now recognised as a close relative of Acrosiphonia J. Agardh and Spongomorpha Kützing in the ulvophycean order, Ulotrichales, based on morphological, ultrastructural and life-history features, and molecular data (Jorde, Citation1933; Kornmann, Citation1963; Floyd & O’Kelly, Citation1984; van Oppen et al., Citation1995; Jónsson, Citation1999; Lindstrom & Hanic, Citation2005). The genus includes about 12 species worldwide (Guiry & Guiry, Citation2009), four of which are common along western European shores (Lokhorst & Trask, Citation1981). The epiphytic U. microscopica from Norway has been distinguished from other species in the genus by its minute filaments (Levring, Citation1937). Since its description, the species has remained largely unnoticed and its systematic position uncertain (Rueness, Citation1992; Lein et al., Citation1999).
In the present study we assess the phylogenetic position of the enigmatic microfilamentous species, U. curvatum and U. microscopica, by molecular phylogenetic analysis of nuclear-encoded small and large subunit rDNA sequences.
Materials and methods
Specimens of U. curvatum, growing as epiphytes on Peyssonnelia dubyi P.L. Crouan & H.M. Crouan, were collected from Vega (county of Nordland, Norway) on 26 October 1990 (Rueness, Citation1992). Specimens of U. microscopica, growing epiphytically on Cystoclonium purpureum (Hudson) Batters at a depth of 3–5 m, were collected from Busepollen in Austevoll (county of Hordaland, Norway) in September 1994. Unialgal cultures of both species were obtained as described in Rueness (Citation1992), and have been deposited in the Culture Collection of Algae and Protozoa (CCAP) as CCAP 455/1 (U. curvatum) and CCAP 504/1 (U. microscopica). Specimens were examined with a Nikon Eclipse TE 300 (Nikon Co., Tokyo, Japan) and Olympus BX51 (Olympus Co., Tokyo, Japan) bright field light microscopes. Photographs were taken with a Nikon DS-5 M or Olympus E410 digital camera mounted on the microscope. Pyrenoids were stained with Lugol's iodine. DAPI nuclear staining was performed as described by Rueness (Citation1992).
Molecular phylogenetic analyses were based on nuclear-encoded small subunit (SSU) and partial large subunit (LSU) rDNA sequences. DNA extraction, PCR amplification and sequencing were performed as described in Leliaert et al. (Citation2007). Taxa for which new sequences were generated are listed in Table S1 (see supplemental material available at http://www.informaworld.com/mpp/uploads/leliaert_et_al._supplementary_material.pdf). Sequences have been deposited in EMBL/GenBank under accession numbers FN257507- FN257512.
Two alignments were created for phylogenetic analyses. The first one was assembled to assess the phylogenetic position of U. curvatum and U. microscopica within the Chlorophyta. This alignment consisted of 30 SSU sequences, including other Uronema and Urospora representatives and exemplar taxa from a broad representation of chlorophytan classes for which SSU sequences have been deposited in GenBank. Two prasinophycean algae were used as outgroup taxa. Although no sequence data are available for the type species of Uronema (U. confervicola Lagerheim) and Urospora (U. mirabilis Areschoug), there is indirect evidence that Uronema belkae Lokhorst and Urospora neglecta (Kornmann) Lokhorst & Trask (included in our phylogenetic analyses) are closely related to the types of the respective genera. Schlösser (Citation1987) showed that the autolysin of U. confervicola reacts in bioassays on strains of U. belkae, suggesting that the two species are closely allied (Pröschold & Leliaert, Citation2007). Urospora mirabilis is currently regarded as a synonym of Urospora penicilliformis (Roth) J.E. Areschoug, which has been found to be related to U. neglecta based on 18S rRNA gene sequence data (Lindstrom & Hanic, Citation2005).
Based on the results of the phylogenetic analysis inferred from the SSU alignment, a second dataset of partial LSU sequences was assembled and analysed to examine the phylogenetic position of the two species within the Cladophorales with more confidence. In the Cladophorales, partial LSU sequences (first ca. 500 bp) are known to be more phylogenetically informative than SSU sequences (Leliaert et al., Citation2003). The LSU alignment consisted of 20 cladophoralean sequences with Caulerpa Lamouroux and Chlorodesmis Harvey & Bailey (Bryopsidales), Acrosiphonia (Ulotrichales) and Ulva Linnaeus (Ulvales) as outgroup taxa. Sequences were aligned using MUSCLE (Edgar, Citation2004), and inspected visually.
Evolutionary models for the two alignments were determined by the Akaike Information Criterion in PAUP/Modeltest 3.6 (Posada & Crandall, Citation1998; Swofford, Citation2002). Both datasets were analysed with maximum likelihood (ML) and Bayesian inference (BI), using PhyML v2.4.4 (Guindon & Gascuel, Citation2003) and MrBayes v3.1.2 (Ronquist & Huelsenbeck, Citation2003) respectively. The SSU dataset was analysed under a general time-reversible model with a proportion of invariable sites and gamma distribution split into four categories (GTR+I+G4). The LSU alignment was analysed under a general time-reversible model with gamma distribution, split into four categories and no separate rate class for invariable sites (GTR+G4). BI analyses consisted of two parallel runs of four incrementally heated chains each, and 4 000 000 generations with sampling every 1000 generations. A burnin sample of 2000 trees was removed before constructing the majority rule consensus tree. For the ML trees, the reliability of internal branches was evaluated with non-parametric bootstrapping (1000 replicates).
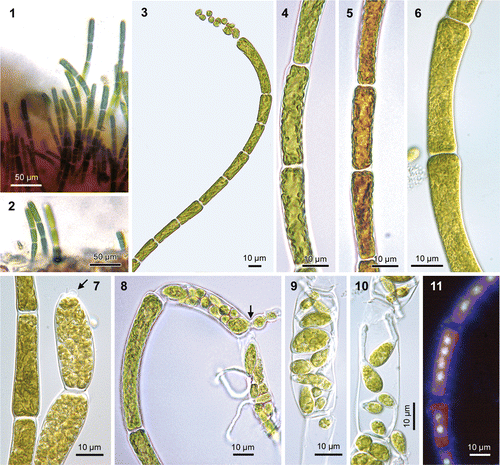
Figs 1–11. Uronema curvatum (=Okellya curvata, comb. nov.). Figs 1, 2. Field-collected sample, growing as an epiphyte on a red crust, on the haptera of Laminaria hyperborea (diameter of filaments 7–8 µm). Figs 3–11
Figs 12–21. Urospora microscopica (=Chaetomorpha norvegica, nom. nov.), culture. Fig. 12. Filament with basal cell with attachment disc. Fig. 13. Apical portion of filament with sporangia and lateral exit pores. Fig. 14. Parietal, lobed chloroplast. Fig. 15. Cell showing pyrenoids following staining with iodide solution. Figs 16, 17. The same filaments after DAPI-staining as seen under light field and fluorescence microscopy, showing multinucleate cells with four nuclei in axial arrangement (note one spore attached outside the filament and a few spores left in sporangium). Fig. 18. Lobed attachment disc with germinating spores. Fig. 19. Sporangia with exit pore (top filament) and start of exit pore formation (bottom filament, arrow) (fixed material, without cell contents). Figs 20, 21. Sporangia with spores, red eye spot visible (arrows).
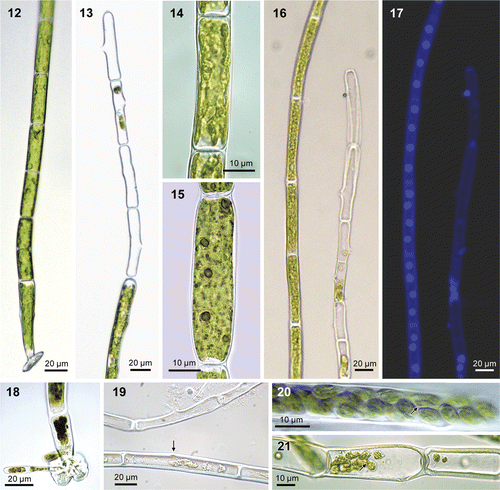
Fig. 22. ML tree of the core chlorophytes (Ulvophyceae, Trebouxiophyceae, Chlorophyceae), rooted with two prasinophytes, inferred from SSU nrDNA sequences. The phylogenetic position of Uronema curvatum (=Okellya curvata, comb. nov.) and Urospora microscopica (=Chaetomorpha norvegica, nom. nov.), along with other members of the two genera are shown. Maximum likelihood bootstrap values (>50) and Bayesian inference posterior probabilities (>0.90) are indicated at branches. The branches leading to Acetabularia and Caulerpa are scaled 50% (*) and 25% (**).
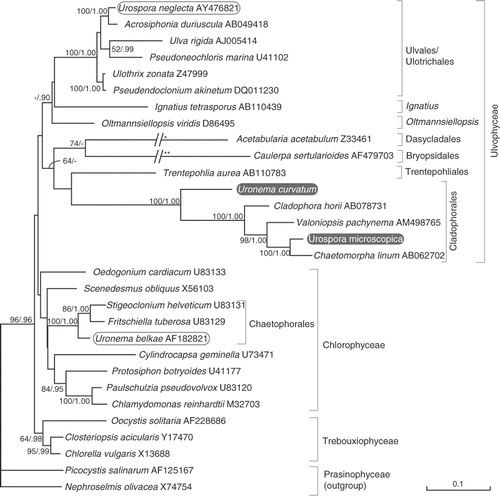
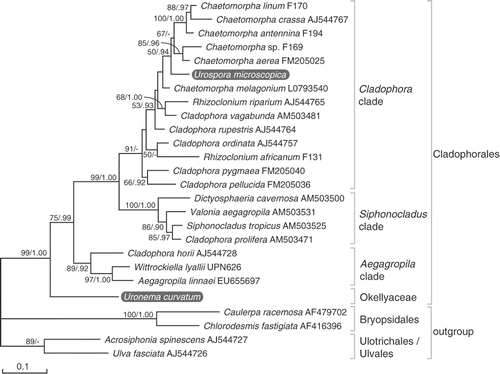
Fig. 23. Maximum likelihood (ML) tree of the Cladophorales inferred from partial large subunit nrDNA sequences, showing the phylogenetic position of Uronema curvatum (=Okellya curvata, comb. nov.) and Urospora microscopica (=Chaetomorpha norvegica, nom. nov.). ML bootstrap values (>50) and Bayesian inference posterior probabilities (>0.90) are indicated at branches.
Results
Morphology
Thalli of U. curvatum form minute epiphytic turfs of curved, uniseriate, unbranched filaments, composed of 3–10 cells, 100–180 µm long (in culture, filaments may grow up to 100 cells and 700 µm long), diameter increasing towards the apex (, ). Thallus is dull, yellowish green in colour. Filaments are attached to the substratum by a basal discoid holdfast. Vegetative cells are subcylindrical, 3.5–6.0 µm in diameter at the base, increasing to 7–10 µm at the apex, 1.5–6.0 times as long as broad, up to 21 µm long. The chloroplast is parietal and lobed, and occupies most of the cell wall (); transmission electron microscopy showed that more than one chloroplast might be present per cell (Rueness, Citation1992); pyrenoids are absent (). Cells are multinucleate, containing (1−) 2−4 (−8) nuclei (). Thalli become reproductive before the filaments reach about 10 cells (in culture, unattached filaments may become longer). Prior to differentiation into sporangia, cells contain 8–16 (−32) nuclei (Rueness, Citation1992). Zoids develop by transformation of apical and subapical cells into slightly swollen zoosporangia (, ); (8−) 16−32 zoids are formed per cell, which emerge through a domed pore in the upper part of the cell, on the outer face relative to the curvature of filaments (, ) (Rueness, Citation1992). In culture, spores may germinate within the parent cell (, ). Filaments that were isolated into unialgal culture in 1990 have since been reproducing asexually by spores.
Thalli of Urospora microscopica () form straight or curved, uniseriate, unbranched filaments up to 1750 µm long, composed of cylindrical cells, 10–20 µm in diameter (). Thallus is bright, grass green in colour. Filaments are attached to the substratum by a basal, hyaline, lobed holdfast (, ). Cells contain a parietal, lobed chloroplast with several pyrenoids (ca. 5) (, ). Cells are multinucleate, containing four axially arranged nuclei (, ). Zoids develop by transformation of apical and subapical cells into zoosporangia; 10–35 zoids are formed per cell, which emerge through a domed pore in the middle part of the cell (sometimes subapical or sub-basal) (). Filaments that were isolated into unialgal culture in 1994 have since been reproducing asexually by spores.
Molecular phylogeny
Specifications of the SSU and LSU sequence alignments and evolutionary models applied are given in Table S2 (http://www.informaworld.com/mpp/uploads/leliaert_et_al._supplementary_material.pdf).
Phylogenetic analysis of the SSU dataset resulted in a chlorophytan tree with a poorly resolved backbone, in which the monophyly of the Ulvophyceae, Chlorophyceae and Trebouxiophyceae, and the relationships among these classes were weakly supported (). Even so, the phylogenetic positions of U. curvatum and U. microscopica could be determined with high support. Uronema curvatum is unrelated to the freshwater U. belkae (or any other member of the chlorophycean order Chaetophorales), but instead sister to the Cladophorales. Urospora microscopica is not allied with U. neglecta or any other member of Ulvales but is placed within the cladophoralean clade.
Phylogenetic analysis of the Cladophorales LSU alignment resulted in three well–supported clades, termed the Cladophora, Siphonocladus and Aegagropila clades (). Concordant with the SSU tree, U. curvatum is sister to the Cladophorales with high support. Urospora microscopica falls within the Cladophora clade. It is most closely related to Chaetomorpha, although its exact phylogenetic position could not be determined with satisfactory statistical support.
Discussion
The placement of the marine species U. curvatum in a genus of freshwater Chlorophyceae had been questioned. Rueness (Citation1992) examined the species in culture, and suggested a relationship with the cladophoralean genera Chaetomorpha and Rhizoclonium, or with the ulotrichalean Urospora, based on the multinucleate cells. Kornmann & Sahling (Citation1994) formally transferred the species to Urospora based on zoospore morphology, but this transfer was not widely adopted (Bartsch & Kuhlenkamp, Citation2000; Nielsen & Gunnarsson, Citation2001; Maggs & O’Kelly, Citation2007). The present study shows that U. curvatum is closely allied to the ulvophycean order, the Cladophorales.
Uronema curvatum shares a number of ecological and morphological features with the green macroalgal order, the Cladophorales. Like most members of this order, U. curvatum occurs in benthic marine coastal habitats. The assumption that the Cladophorales are an originally marine clade, which successfully invaded freshwater habitats at least twice independently (Hanyuda et al., Citation2002), is reinforced by the phylogenetic position of U. curvatum, sister to the rest of the Cladophorales.
Morphologically, U. curvatum shares the typical siphonocladous level of organization of the Cladophorales, i.e. multicellular thalli composed of multinucleate cells. The number of nuclei in cladophoralean species is highly variable and generally proportional to cell size. Most cladophoralean taxa have relatively large cells (ranging from several µm to several mm across) with hundreds or even thousands of nuclei, arranged in cytoplasmic domains (Kapraun & Nguyen, Citation1994). The cells of U. curvatum typically contain 2–4 nuclei (Rueness, Citation1992; Maggs & O’Kelly, Citation2007), comparable to numbers found in Rhizoclonium riparium (Roth) Harvey, which has cell dimensions of the same order of magnitude (mostly 5–20 µm in diameter) (Leliaert & Boedeker, Citation2007).
Thallus organization in the Cladophorales ranges from branched or unbranched uniseriate filaments to more complex architectural types (one notable exception being the coccoid Spongiochrysis in the Aegagropila clade, Rindi et al., Citation2006). Unbranched filamentous thalli, assigned to Chaetomorpha or Rhizoclonium, have evolved from branched forms several times independently within the Cladophorales (Hanyuda et al., Citation2002; Leliaert et al., Citation2003). Uronema curvatum thus represents another unbranched filamentous lineage of the Cladophorales. It attaches to the substratum by a basal discoid holdfast and hence differs from most Cladophorales, which are attached to the substratum by branched or unbranched rhizoids that develop from basal or intercalary cells. The diminutive Cladophora pygmaea Reinke also attaches by a similar basal discoid holdfast. Based on this feature it was placed in a separate section of the genus by van den Hoek (Citation1963), but a molecular phylogenetic study refuted the separate placement of C. pygmaea and showed that mode of attachment is not an evolutionarily conserved character and has little taxonomic value above the species level (Leliaert et al., Citation2009).
Uronema curvatum shares the typical zoidangial development and exit aperture of the Cladophorales: after vegetative growth ceases, the apical cells swell slightly and the cytoplasm is divided into zoids that are dispensed through a domed pore at the upper end of the cell. Some cladophoralean taxa display variation in zoidangial morphology. For example, in Wittrockiella Wille (Aegagropila clade) the spores are released through extremely elongated exit tubes, resembling colourless hairs (Leliaert & Boedeker, Citation2007), and many taxa of the Siphonocladus clade have large cells that form numerous, lateral exit pores (Hori, Citation1994).
The chloroplast of U. curvatum is parietal and lobed, and lines most of the cell wall. In contrast, the cells of most other Cladophorales contain numerous chloroplasts interconnecting by delicate strands to form a continuous layer or a parietal network. In some taxa, such as Rhizoclonium riparium (Roth) Harvey, cells contain a single or few parietal, lobed chloroplasts, similar to U. curvatum (Leliaert & Boedeker, Citation2007). Uronema curvatum differs from other Cladophorales by the lack of pyrenoids, although TEM observations by Rueness (Citation1992) suggest the presence of starch grains inside the chloroplasts. In other Cladophorales most of the chloroplasts in a cell contain a single pyrenoid. The majority of species have bilenticular pyrenoids, i.e. each pyrenoid consists of two hemispheres, separated by a single thylakoid and each hemisphere is capped by a bowl-shaped starch grain. This pyrenoid structure was initially thought to be uniform within the order (Jónsson, Citation1962; van den Hoek et al., Citation1995), but several exceptions to this pattern have been reported, mainly in species of the Aegagropila clade (Matsuyama et al., Citation1998; Miyaji, Citation1999; Hanyuda et al., Citation2002).
Another marine species of Uronema, U. marinum, has been described as an epiphyte on green and (non-crustose) red seaweeds in shallow subtidal habitats from southern and western Australia, the Great Barrier Reef, Lord Howe Island, Micronesia and Hawaii (Womersley, Citation1984; Abbott & Huisman, Citation2004; Kraft, Citation2007). This species resembles U. curvatum in size, cell dimensions and mode of attachment, but differs in having one or two pyrenoids per cell. The taxonomic position of U. marinum remains undecided at this stage, and the name is retained pending molecular investigations.
The micro-filamentous species, U. microscopica, was described from Osund, Norway, growing epiphytically on Nitophyllum Greville and Cystoclonium Kützing (Levring, Citation1937). Since its original description, the species has remained largely unnoticed (Lein et al., Citation1999). Rueness (Citation1992), who re-examined the type material, found that U. microscopica differed from U. curvatum in cell dimensions, straight filaments, and the position of the sporangial pore, which is lateral or sub-basal in U. microscopica versus subapical in U. curvatum. In the present study we examined recent collections and cultures of U. microscopica, which made it possible to investigate this species in more detail and assess its phylogenetic position based on molecular data. The phylogenetic analysis clearly shows that U. microscopica is a member of the Cladophora clade. The species seems to be most closely related to Chaetomorpha, although the presence of four nuclei and sporangia with a single lateral pore would suggest a relationship with the R. riparium complex (Leliaert & Boedeker, Citation2007).
Chaetomorpha is a marine genus of attached or unattached unbranched macro-filaments. More than 200 species and infraspecific taxa have been described worldwide (Index Nominum Algarum), of which only about 50 are currently accepted (Guiry & Guiry, Citation2009). Morphological features used to delimit species within the genus are growth form, cell dimensions and shape of the basal attachment cell. However, the extensive variability of these morphological characters, depending on environmental conditions, accounts for a great deal of taxonomic confusion, and the genus is clearly in need of revision (Leliaert & Boedeker, Citation2007).
Urospora microscopica differs from other members of the genus Chaetomorpha in the diminutive growth form, small cell size (10–20 µm in diameter) and low number of nuclei per cell. Most Chaetomorpha species are much more robust, forming macroscopic thalli with cells ranging from ca. 60 µm in diameter in C. ligustica (Kützing) Kützing to one or several mm across (e.g. C. melagonium (Weber & Mohr) Kützing, C. coliformis (Montagne) Kützing). Only a few other minute Chaetomorpha species have been described. Chaetomorpha sphacelariae Foslie (Citation1881) is a diminutive epiphyte on Sphacelaria Lyngbye from Norway, which has remained unnoticed since its original description. Chaetomorpha minima Collins & Hervey (Citation1917) has been morphologically associated with C. sphacelariae. This species from the north-west Atlantic Ocean and Caribbean Sea also grows epiphytically on algae, seagrasses and salt-marsh plants, and forms inconspicuous filaments, composed of long cells, 10–27 µm across (Schneider & Searles, Citation1991; Dawes & Mathieson, Citation2008; Littler et al., Citation2008). Another minute species, C. recurva Scagel (Citation1966), is found along the Pacific coast of North America and forms minute thalli with filaments 6–10 µm across. Given that morphological features, especially cell dimensions, are now known to be poor indicators of phylogenetic relationships in the Cladophorales (and green algae in general) (Leliaert et al., Citation2007), these species will need to be further examined using molecular tools to assess their phylogenetic affinity.
Taxonomic conclusions
The divergent phylogenetic position of U. curvatum, sister to the rest of the Cladophorales, along with a combination of distinctive morphological features such as the absence of pyrenoids, the diminutive size of the unbranched filaments and a discoid holdfast, warrants the recognition of a separate genus within a new family of Cladophorales:
Okellyaceae Leliaert et Rueness, familia nov.
Algae benthicae marinae, filamentibus simplicibus erectis curvatis, disco basali ad substratum affixae. Cellularum divisio intercalaris. Filamenta 3–8 cellulibus, usque 200 μm longa (in cultura usque 100 cellulibus, 1 mm longa), diametro dilatato apicem versus. Cellulae apicales cylindricae obtusae, cellulae intercalares cylindricae, 3–20 μm latae, (1−) 2–4 (−8) nucleatae. Chloroplastus parietalis, pyrenoide absenti autem granis amyloideis. Zoosporangia apicalia et subapicalia, zoosporibus pluribus (8–32), e sporangioporo singulari emergentibus. Thalli epiphytici super algas crustosas in zona sublitorali.
Marine benthic algae forming minute tufts of slightly curved, erect unbranched uniseriate filaments, attached by a basal discoid holdfast. Growth by intercalary cell divisions. Filaments composed of 3–8 cells, up to 200 µm long (in culture sometimes up to 100 cells and 1 mm or more), diameter increasing towards the apex. Apical cell cylindrical with obtuse tip, intercalary cells cylindrical, 3–20 µm in diameter, containing (1−) 2−4 (−8) nuclei. Chloroplast parietal, lacking pyrenoids but including starch grains. Multiple zoospores (8–32) developing by transformation of apical and subapical cells into zoosporangia, emerging through a single pore in the upper part of the cell. Thalli epiphytic on various crustose algae in the subtidal zone.
TYPE GENUS: Okellya Leliaert et Rueness, gen. nov.
Cum characteribus familia. Characters as for family.
TYPE SPECIES: Okellya curvata (Printz) Leliaert et Rueness, comb. nov.
BASIONYM: Uronema curvatum Printz, Algenveg. Trondhjemsfj.: 233, pl. VII: figs 105–114 (1926).
ETYMOLOGY: Named in honour of Charles J. O’Kelly for his pioneering and influential work on green algal systematics.
Urospora microscopica is most closely related to Chaetomorpha (type: Chaetomorpha linum (O.F. Müller) Kützing) in the Cladophorales and is therefore transferred to that genus. Since the combination Chaetomorpha microscopica has already been made by Meyer (Citation1927) for a freshwater filamentous cladophoralean species (later transferred to Cladochaete Meyer & Skabitschevsky and Chaetocladiella Meyer & Skabitschevsky), a new specific epithet must be chosen.
Chaetomorpha norvegica Leliaert et Rueness, nom. nov.
BASIONYM: Urospora microscopica Levring, Lunds Univ. Årsskr. 2, 33(8): 30, fig. (1937).
Leliaert et al. supplementary material
Download Zip (95 KB)Acknowledgements
We are grateful to Caroline Vlaeminck for generating the sequence data. We thank Paul Goetghebeur for help with the Latin diagnosis and Olga Gerasymova for supplying old Russian literature. Funding was provided by FWO-Flanders (research grant G.0142.05, and post-doctoral fellowships to HV and FL) and the Ghent University BOF (doctoral fellowship to EC).
References
- Abbott , IA and Huisman , JM . 2004 . Marine Green and Brown Algae of the Hawaiian Islands , Honolulu, , USA : Bishop Museum Press .
- Areschoug , JE . 1866 . Observationes phycologicae. Particula prima. De Confervaceis nonnullis . Nov. Act. Reg. Soc. Scient. Ups., Ser 3 , 6 : 1 – 26 .
- Bartsch , I and Kuhlenkamp , R . 2000 . The marine macroalgae of Helgoland (North Sea): an annotated list of records between 1845 and 1999 . Helgol. Mar. Res. , 54 : 160 – 189 .
- Bold , HC and Wynne , MJ . 1985 . Introduction to the Algae, , 2nd , New Jersey, , USA : Prentice-Hall .
- Booton , GC , Floyd , GL and Fuerst , PA . 1998 . Origins and affinities of the filamentous green algal orders Chaetophorales and Oedogoniales based on 18S rRNA gene sequences . J. Phycol. , 34 : 312 – 318 .
- Chaudhary , BT . 1979 . Some observations on the morphology, reproduction and cytology of the genus Uronema Lagh. (Ulotrichales, Chlorophyceae) (Note) . Phycologia , 18 : 299 – 302 .
- Collins , FS and Hervey , AB . 1917 . The algae of Bermuda . Proc. Am. Acad. Arts Sci. , 53 : 1 – 195 .
- Dawes , CJ and Mathieson , AC . 2008 . The Seaweeds of Florida , Gainesville, , USA : University Press of Florida .
- Edgar , RC . 2004 . MUSCLE: multiple sequence alignment with high accuracy and high throughput . Nucl. Acids Res. , 32 : 1792 – 1797 .
- Feldmann , J . 1954 . Inventaire de la flore marine de Roscoff. Algues, champignons, lichens et spermatophytes . Trav. St. Biol. Roscoff, Nouv. Sér. Suppl. , 6 : 152
- Floyd , GL and O’Kelly , CJ . 1984 . Motile cell ultrastructure and the circumscription of the orders Ulotrichales and Ulvales (Ulvophyceae, Chlorophyta) . Am. J. Bot. , 71 : 111 – 120 .
- Foslie , M . 1881 . Om nogle nye arctiske havalger . Christiania Vidensk.-Selsk. Forh. , 1881 : 1 – 14 .
- Guindon , S and Gascuel , O . 2003 . A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood . Syst. Biol. , 52 : 696 – 704 .
- Guiry, M.D. & Guiry G.M. (2009). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. Available at: http://www.algaebase.org; accessed 23 January 2009
- Hanyuda , T , Wakana , I , Arai , S , Miyaji , K , Watano , Y and Ueda , K . 2002 . Phylogenetic relationships within Cladophorales (Ulvophyceae, Chlorophyta) inferred from 18S rRNA gene sequences, with special reference to Aegagropila linnaei . J. Phycol. , 38 : 564 – 571 .
- Hori , T . 1994 . An Illustrated Atlas of the Life History of Algae. Volume 1. Green Algae , Tokyo, , Japan : Uchida Rokakuho Publishing Co., Ltd .
- Index Nominum Algarum, University Herbarium, University of California, Berkeley. Compiled by Paul Silva. Available at: http://ucjeps.berkeley.edu/INA.html; accessed 23 January 2009
- Jónsson , S . 1962 . Recherches sur des Cladophoracées marines: structure, reproduction, cycles comparés, conséquences systématiques . Ann. Sci. Nat., Bot. , 12 : 25 – 263 .
- Jónsson , S . 1999 . The status of the Acrosiphoniales (Chlorophyta) . Rit Fiskideildar , 16 : 187 – 196 .
- Jorde , I . 1933 . Untersuchungen über den Lebenszyklus von Urospora Aresch. und Codiolum A. Braun . Nyt. Mag. Naturvidensk. , 73 : 1 – 19 .
- Kapraun , DF and Nguyen , MN . 1994 . Karyology, nuclear DNA quantification and nucleus-cytoplasmic domain variations in some multinucleate green algae (Siphonocladales, Chlorophyta) . Phycologia , 33 : 42 – 52 .
- Kornmann , P . 1963 . Die Ulotrichales, neu geordnet auf der Grundlage entwicklungsgeschichtlicher Befunde . Phycologia , 3 : 60 – 68 .
- Kornmann , P and Sahling , P-H . 1994 . Meeresalgen von Helgoland: Zweite Ergänzung . Helgol. Meeresunters. , 48 : 365 – 406 .
- Kraft , GT . 2007 . Algae of Australia. Marine Benthic Algae of Lord Howe Island and the Southern Great Barrier Reef, 1. Green algae , Canberra & Melbourne, , Australia : Australian Biological Resources Study & CSIRO Publishing .
- Lagerheim , G . 1887 . Note sur l'Uronema, nouveau genre des algues d'eau douce . Malpigia , 1 : 517 – 523 .
- Lein , TE , Bruntse , G , Gunnarsson , K and Nielsen , R . 1999 . New records of benthic marine algae for Norway, with notes on some rare species from the Florø district, western Norway . Sarsia , 84 : 39 – 53 .
- Leliaert , F and Boedeker , C . 2007 . “ Cladophorales ” . In Green Seaweeds of Britain and Ireland , Edited by: Brodie , J , Maggs , CA and John , DM . 131 – 183 . London, , UK : British Phycological Society .
- Leliaert, F., Boedeker, C., Pena, V., Bunker, F., Verbruggen, H. & De Clerck, O. (2009). Cladophora rhodolithicola sp. nov. (Cladophorales, Chlorophyta), a diminutive species from European maerl beds. Eur. J. Phycol., 44: 155–169
- Leliaert , F , De Clerck , O , Verbruggen , H , Boedeker , C and Coppejans , E . 2007 . Molecular phylogeny of the Siphonocladales (Chlorophyta: Cladophorophyceae) . Mol. Phylogenet. Evol. , 44 : 1237 – 1256 .
- Leliaert , F , Rousseau , F , de Reviers , B and Coppejans , E . 2003 . Phylogeny of the Cladophorophyceae (Chlorophyta) inferred from partial LSU rRNA gene sequences: is the recognition of a separate order Siphonocladales justified? . Eur. J. Phycol. , 38 : 233 – 246 .
- Levring , T . 1937 . Zur Kenntnis der Algenflora der Norwegischen Westküste . Lunds Univ. Årsskr. , 33 : 1 – 147 .
- Lewis , LA and McCourt , RM . 2004 . Green algae and the origin of land plants . Am. J. Bot. , 91 : 1535 – 1556 .
- Lindstrom , SC and Hanic , LA . 2005 . The phylogeny of North American Urospora (Ulotrichales, Chlorophyta) based on sequence analysis of nuclear ribosomal genes, introns and spacers . Phycologia , 44 : 194 – 201 .
- Littler , DS , Littler , MM and Hanisak , MD . 2008 . Submersed Plants of the Indian River Lagoon: A Floristic Inventory and Field Guide , Washington, , USA : OffShore Graphics, Inc .
- Lokhorst , GM and Trask , BJ . 1981 . Taxonomic studies on Urospora (Acrosiphoniales, Chlorophyceae) in western Europe . Acta Bot. Neerl. , 30 : 353 – 431 .
- Maggs , CA and O’Kelly , CJ . 2007 . “ Uronema ” . In Green Seaweeds of Britain and Ireland , Edited by: Brodie , J , Maggs , CA and John , DM . 209 – 210 . London, , UK : British Phycological Society .
- Matsuyama , K , Matsuoka , T , Miyaji , K , Tanaka , J and Aruga , Y . 1998 . Ultrastructure of the pyrenoid in the family Cladophoraceae (Cladophorales, Chlorophyta) . J. Jap. Bot. , 73 : 279 – 286 .
- Mattox, K.R. & Stewart, K.D. (1984). Classification of the green algae: a concept based on comparative cytology. In The Systematics of Green Algae (Irvine, D.E.G. and John, D.M., editors), 29–72. The Systematics Association Special Volume 27, London, UK: Academic Press
- Meyer , KI . 1927 . Algae of the northern end of Lake Baikal . Russ. Arkh. Protist. , 6 : 93 – 118 . (In Russian, with French summary)
- Miyaji , K . 1999 . A new type of pyrenoid in the genus Rhizoclonium (Cladophorales, Chlorophyta) . Phycologia , 38 : 267 – 276 .
- Nielsen , R and Gunnarsson , K . 2001 . Seaweeds of the Faroe Islands. An annotated checklist . Fródskaparrit , 49 : 45 – 108 .
- O'Kelly , CJ . 2007 . “ The origin and early evolution of green plants ” . In Evolution of Primary Producers in the Sea , Edited by: Falkowski , PG and Knoll , AH . 287 – 309 . Amsterdam, , The Netherlands : Elsevier .
- O'Kelly , CJ , Wysor , B and Bellows , WK . 2004 . Gene sequence diversity and the phylogenetic position of algae assigned to the genera Phaeophila and Ochlochaete (Ulvophyceae, Chlorophyta) . J. Phycol. , 40 : 789 – 799 .
- Posada , D and Crandall. , KA . 1998 . MODELTEST: testing the model of DNA substitution . Bioinformatics , 14 : 817 – 818 .
- Printz , H . 1926 . Die Algenvegetation des Trondhjemsfjordes . Skr. Norske Vidensk.-Akad. Oslo, Mat.-naturv. Kl. , 1926 : 1 – 273 .
- Printz , H . 1932 . Observations on the structure and reproduction in Urospora Aresch . Nyt. Mag. Naturvidensk. , 70 : 274 – 287 .
- Pröschold, T. & Leliaert, F. (2007). Systematics of the green algae: Conflict of classic and modern approaches. In Unravelling the Algae: The Past, Present, and Future of Algal Systematics (Brodie J. and Lewis J.M., editors), 123–153. The Systematics Association Special Volume Series, 75. Boca Raton, USA: CRC Press
- Rindi , F , López-Bautista , JM , Sherwood , AR and Guiry , MD . 2006 . Morphology and phylogenetic position of Spongiochrysis hawaiiensis gen. et sp. nov., the first known terrestrial member of the order Cladophorales (Ulvophyceae, Chlorophyta) . Int. J. Syst. Evol. Microbiol. , 56 : 913 – 922 .
- Ronquist , F and Huelsenbeck , JP . 2003 . MrBayes 3: Bayesian phylogenetic inference under mixed models . Bioinformatics , 19 : 1572 – 1574 .
- Rosenvinge , LK . 1893 . Grønlands Havalger . Medd. Grønland , 3 : 763 – 981 .
- Round , FE . 1984 . “ The systematics of the Chlorophyta: an historical review leading to some modern concepts ” . In The Systematics of Green Algae , The Systematics Association Special Volume 27 Edited by: Irvine , DEG and John , DM . 1 – 28 . London, , UK : Academic Press .
- Rueness , J . 1977 . Norsk Algeflora , Universitetsforlaget : Oslo .
- Rueness , J . 1992 . Field and culture observations on Uronema curvatum Printz (Chlorophyta) . Acta Phytogeogr. Suec. , 78 : 125 – 130 .
- Scagel , RF . 1966 . Marine algae of British Columbia and northern Washington, Part I: Chlorophyceae (green algae) . Bull. Natl. Mus. Can. , 207 : 1 – 257 .
- Schlösser , UG . 1987 . “ Action of cell wall autolysins in asexual reproduction of filamentous green algae: evidence and species specificity ” . In Algal Development: Molecular and Cellular Aspects , Edited by: Wiessner , W , Robinson , DG and Starr , RC . 75 – 80 . Berlin, Heidelberg, , Germany : Springer .
- Schneider , CW and Searles , RB . 1991 . Seaweeds of the Southeastern United States. Cape Hatteras to Cape Canaveral , Durham & London, , UK : Duke University Press .
- Setchell , WA and Gardner , NL . 1920 . The marine algae of the Pacific coast of North America. Part II. Chlorophyceae . Univ. Calif. Publ. Bot. , 8 : 139 – 374 .
- South , GR and Tittley , I . 1986 . A Checklist and Distributional Index of the Benthic Marine Algae of the North Atlantic Ocean , New Brunswick, , Canada : British Museum (Natural History) and Huntsman Marine Laboratory, London and St. Andrews .
- Swofford , DL . 2002 . PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4 , Sunderland, , USA : Sinauer Associates .
- van den Hoek , C . 1963 . Revision of the European Species of Cladophora , Leiden, , The Netherlands : Brill .
- van den Hoek , C , Mann , DG and Jahns , HM . 1995 . Algae. An Introduction to Phycology , Cambridge : Cambridge University Press .
- Van Oppen , MJH , Olsen , JL and Stam , WY . 1995 . Genetic variation within and among North Atlantic and Baltic populations of the benthic alga Phycodrys rubens (Rhodophyta) . Eur. J. Phycol. , 30 : 251 – 260 .
- Wille , N . 1890 . “ Cladophoraceae ” . In Die natürlichen Pflanzenfamilien. I.Teil, Abt. 2 Edited by: Engler , A and Prantl , K . 114 – 119 . Leipzig, , Germany
- Womersley , HBS . 1984 . The Marine Benthic Flora of southern Australia. Part I , Adelaide, , South Australia : Government Printer .