Abstract
Neodenticula seminae is a very important member of modern diatom assemblages in the Bering Sea and at middle to high latitudes of the North Pacific. In the North Atlantic, this species was considered extinct until it was recorded in high abundance in the 2001 spring phytoplankton bloom of the Gulf of St. Lawrence, Eastern Canada. Here, we discuss some of the most distinctive features of its morphology, including variation in some characters between the subarctic Pacific and the Gulf of St. Lawrence specimens. Most importantly, we observed that the deck and basal ridges, and the solid-walled costae (formerly known as primary pseudosepta) characteristic of N. seminae were present in the subarctic Pacific material, but were absent or vestigial in the Gulf of St. Lawrence material and in cultures from both regions. This morphological variation was most likely due to differences in physico–chemical water properties between the subarctic Pacific and the Gulf of St. Lawrence. Phylogenetic analyses of the internal transcribed spacer regions of the nuclear ribosomal DNA showed that the strains of N. seminae collected in the Gulf of St. Lawrence and the subarctic Pacific clustered in two sister clades, but differed from each other only slightly more than the variation among the subarctic Pacific strains. These results confirmed the reappearance of N. seminae in the NW Atlantic after an absence of ∼0.8 Ma. In addition, the phylogenetic analyses based on the large subunit of the nuclear ribosomal DNA positioned N. seminae firmly within the Bacillariaceae (i.e. diatoms with a fibulate raphe system) and proved a close relationship to species of Fragilariopsis.
Introduction
Diatoms have been widely used as the main biostratigraphic marker for dating and correlating sediments in the major oceans of the world. The North Pacific has received much attention in defining biostratigraphic zonations using marine diatoms, and N. seminae (Simonsen et Kanaya) Akiba et Yanagisawa is no exception, serving as the primary marker for Late Pleistocene marine and continental deposits (Yanagisawa & Akiba, Citation1998). The extant species is also an important autotrophic protist, dominating coastal spring and fall phytoplankton blooms in the subarctic Pacific (Takahashi, Citation1997; Kurihara & Takahashi, Citation2002; Takahashi et al., Citation2002; Onodera et al., Citation2003), where populations have been followed through seasons and from year to year (Takahashi, Citation1994, Citation1997; Takahashi et al., Citation1989, Citation2000, 2002). In the subarctic Pacific, N. seminae is regarded as a productivity indicator (Takahashi, Citation1997; Takahashi et al., Citation1989). N. seminae has been also reported as flourishing in the North Atlantic from the Late Pliocene to Late Pleistocene, roughly 3.3 to 0.8 Ma (Koç & Flower, Citation1998). A high-resolution study of Pleistocene diatom stratigraphy revealed that the first and last occurrences of N. seminae within the Quaternary were 1.26 and 0.84 Ma (Koç et al., Citation1999), indicating that it has been extinct in the North Atlantic since then (Baldauf, Citation1984; Koç et al., Citation1999; Shimada et al., Citation2008).
Routine surveys of phytoplankton populations in the Gulf of St. Lawrence in eastern Canada allowed us to report, for the first time since its extinction, the occurrence of N. seminae in Canadian continental waters (Starr et al., Citation2002; Therriault et al., Citation2002). Since its first massive appearance in the Gulf of St. Lawrence in spring 2001, N. seminae has regularly dominated phytoplankton assemblages in this region. However, accurate recognition of the species has raised some concerns because some of the characteristic valve features, namely the deck and basal ridges, were absent or extremely reduced in specimens from the Gulf. Therefore, we report here on the taxonomy and morphology of this recently reintroduced plankton species, by comparing field material and strains from the Gulf of St. Lawrence and the subarctic Pacific with light microscopy (LM) and electron microscopy (EM).
The taxonomic position of N. seminae has changed several times over the last 50 years. Simonsen & Kanaya (Citation1961) investigated five marine species of the genus Denticula Kützing, including D. seminae Simonsen et Kanaya, which was renamed because the original specific epithet, D. marina (Semina, Citation1956), was already occupied, and thereby nomenclaturally invalid. Later, Simonsen (Citation1979) separated off the marine species of Denticula and erected a new genus, Denticulopsis, for species in which the pseudosepta exhibited peculiar, apically oriented, ridge-like thickenings near the margins. This new genus is by far the most important for the Neogene biostratigraphy of high latitudes in the Northern Hemisphere because it contains many abundant, short-ranging, widely distributed species (Akiba, Citation1986; Akiba & Yanagisawa, Citation1986; Yanagisawa & Akiba, Citation1990). Since its erection, many new taxa have been described, but only one extant species, Denticulopsis seminae (Simonsen et Kanaya) Simonsen. The increased number of species within Denticulopsis led Akiba & Yanagisawa (Citation1986) to undertake a taxonomic re-examination of the described species. They showed and explained the diverse structure of the valves, which was also correlated with stratigraphic and geographic distributions. Consequently, they proposed three generic groups: an amended Denticulopsis, and the establishment of two new genera, Crucidenticula Akiba et Yanagisawa and Neodenticula Akiba et Yanagisawa, the latter including the extant species, N. seminae. All these genera belong to the family Bacillariaceae, which comprises diatoms with a fibulate raphe system. The Bacillariaceae also include Bacillaria Gmelin, Cylindrotheca Rabenhorst, Nitzschia Hassall, Pseudo-nitzschia H. Peragallo and Fragilariopsis Hustedt, the latter morphologically similar to, and possibly evolutionarily closely related to, Neodenticula (Medlin & Sims, Citation1993).
In the present study, we examined the relationships between strains of N. seminae from the Gulf of St. Lawrence and the subarctic Pacific, based on phylogenetic analyses using internal transcribed spacer regions of the nuclear ribosomal DNA (ITS rDNA). We also examined the phylogenetic position of N. seminae within the Bacillariaceae and, more specifically, its relationship to the widespread marine genus, Fragilariopsis, based on the most variable parts (D1–D3) of the large subunit of the nuclear ribosomal DNA (LSU rDNA).
Materials and methods
As part of an ongoing sampling programme at Fisheries and Oceans Canada for the Atlantic Zone Monitoring Program (www.meds-sdmm.dfo-mpo.gc.ca/zmp/main/_zmp_e.html), water samples are routinely collected with Niskin bottles from a network of stations (fixed stations and cross-shelf sections) covering the Gulf of St. Lawrence (NW Atlantic) and preserved with a 1% final concentration of Lugol's solution (Strickland & Parsons, Citation1972). Water samples from the central subarctic Pacific were provided by Dr Kozo Takahashi who collected phytoplankton samples in July 2003 at Station SA (49°N; 174°W).
Microscopical examination
Field samples were examined with light microscopy (LM) with an inverted phase contrast microscope (Zeiss Axiovert 10) to assess cell abundance and colony formation. Examination of valve features was performed after oxidation of organic matter using a mix of nitric and sulphuric acid (1 : 1), followed by several rinses with distilled water. Cleaned diatom material was then mounted permanently on slides with Naphrax and examined in phase contrast and with interference optics (Leica Dialux). Additional cleaned material was prepared on aluminium stubs and coated with gold for observation with scanning electron microscopy (SEM) (Philips ESEM), or on Formvar coated copper grids for observation with transmission electron microscopy (TEM) (JEM–1010). Terminology follows Ross et al. (Citation1979) and Yanagisawa & Akiba (Citation1990). Hence, we used costae to refer to the elongated solid internal thickenings of the valve. Some costae are even further internally developed as transapical walls into the lumen of the frustule; these are referred to as solid-walled costae (formerly known as primary pseudosepta).
Cell cultures
Single cells of N. seminae were isolated from water samples from both sites (
Table 1. Strain designation, origin and accession numbers for Fragilariopsis strains from GenBank and Neodenticula seminae used in the phylogenetic analyses. Strains not mentioned here are found in Lundholm et al. (Citation2002).
DNA extraction, amplification and sequencing
Cultures were concentrated by centrifugation, frozen and extracted following the CTAB method (Doyle & Doyle, Citation1987), with modifications by Lundholm et al. (Citation2002). The ITS1, 5.8S and ITS2 of the nuclear-encoded rDNA (designated ITS) and the D1–D3 regions of the LSU rDNA (designated LSU) were amplified using primers listed in Lundholm et al. (Citation2002, Citation2003). The conditions were: one initial denaturation at 94°C for 2 min, followed by 30–36 cycles each comprising 94°C for 30 s, 60°C for 25 s, 72°C for 25 s, and 72°C for 3 min. PCR-products were purified using QIAquick PCR Purification Kit (Qiagen, Germany). Thirty to 40 ng PCR-product were used in each 20-µl sequencing reaction with the sequencing primers used in Lundholm et al. (Citation2002, Citation2003). Nucleotide sequences were determined using the Dye Terminator Cycle Sequencing Ready Reaction Kit (Perkin Elmer, California) and sequencing was done using an ABI Prism 377 DNA sequencer (Perkin Elmer). GenBank accession numbers of sequences obtained in the present study are listed in ; the remainder were acquired from Lundholm et al. (Citation2002).
Alignment and phylogenetic analyses
LSU rDNA sequences were added to an established alignment (Lundholm et al., Citation2002). The alignment contained 44 taxa, of which three raphid diatoms (Pauliella taeniata (Grunow) Round et Basson, Phaeodactylum tricornutum Bohlin and Navicula cf. erifuga Lange-Bertalot), not belonging to the Bacillariaceae, were used as outgroup. The data set comprised 918 characters of which 872 were considered unambiguous and used in the analyses. The ITS alignment comprised 925 characters and, in addition to ITS1, 5.8S and ITS2, included 97 characters from the terminal end of the SSU gene and 25 characters from the start of the LSU gene. The data set for the ITS analyses contained eight species, and all 925 characters were included in the analyses.
Phylogenetic analyses were performed using PAUP* (version 4.0b.8) (Swofford, Citation2002). Parsimony analyses were done using heuristic searches (1000 replicates) and a branch-swapping algorithm (tree-bisection reconnection, TBR). For the maximum likelihood analyses (ML), the most optimal model was found using Modeltest version 3.06b (Posada & Crandall, Citation1998). The exact parameters were estimated from consecutive heuristic searches (100 and 10 replicates in each search for ITS and LSU, respectively), re-optimizing the parameters until the values of the parameters converged. For the ML analyses on the LSU rDNA data set, a Tamura-Nei substitution model (Tamura & Nei, Citation1993) with equal base frequencies was used, applying among site rate heterogeneity (gamma distribution) between sites after calculation of invariable sites. For the ITS rDNA analyses, a Hasegawa–Kishino–Yano substitution model (Hasegawa et al., Citation1985) with a gamma distribution was applied. Distance analyses were performed on both data sets using neighbor joining analyses (NJ). Bootstrap analyses were used to determine the robustness of the acquired tree topologies. For the LSU data set, 1000 replicates were performed using parsimony and distance analyses, but none using ML. For the ITS data set, 1000 bootstrap replicates were used for all types of analyses.
Results
Morphology
Flourishing populations of N. seminae in the Gulf of St. Lawrence usually occur in long, straight or coiled, ribbon-like colonies of more than 20 cells, joined by the entire valve face (). Short colonies of 8–10 cells, pairs or solitary cells are often observed in both the Gulf of St. Lawrence and the subarctic Pacific (). At low magnification (e.g. ×400), cells of N. seminae can easily be confused with small species of Fragilariopsis which were present in the Gulf of St. Lawrence samples. N. seminae varied in length from 9.5–54.3 µm and in width from 6.9–15.4 µm in the Gulf of St. Lawrence material, and from 11.0–59.6 µm and 5.3–14.5 µm for the subarctic Pacific material (). Nevertheless specimens > 50 µm in length were regularly found in both subarctic Pacific and Gulf of St. Lawrence cultures.
Figs 1–3. Neodenticula seminae from the Gulf of St. Lawrence, Northwest Atlantic, light microscopy. . Straight ribbon-shaped colonies. . Coiled ribbon-shaped colony. . Solitary and paired cells with chloroplasts.
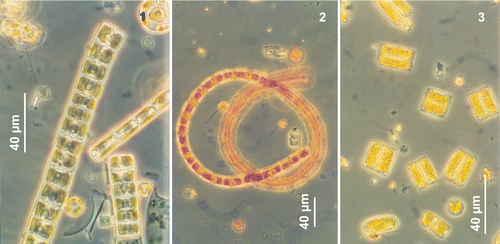
Table 2. Morphometric data on Neodenticula seminae recorded from the subarctic Pacific, the Gulf of St. Lawrence and the literature.
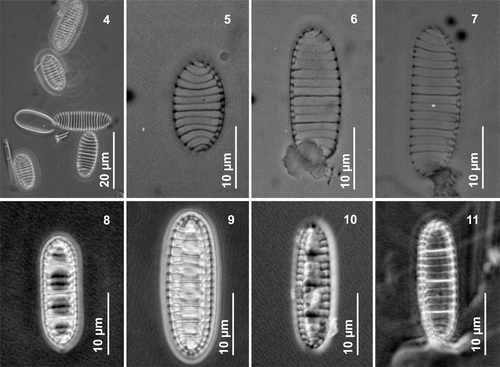
In LM, frustules of N. seminae in all field and cultured material are rectangular in girdle view, with distinctly rounded corners, two plate-like chloroplasts and a distinctive thick ornamentation between cells (). Small-sized cell colonies could be very different in shape, the girdle being deeper than the cell length. This variability in colony outline can render accurate identification difficult in LM. Valve outline ranges from linear, linear–elliptical to almost circular, with broadly rounded apices. A distinct asymmetry, revealed by a more or less straight or convex raphe-supporting margin opposite a slightly convex distal margin, was seen in natural Gulf of St. Lawrence populations as well as in cultured material from both regions (, , ). Conversely, in natural subarctic Pacific specimens, the valves were nearly symmetrical and had more parallel margins (, , ). The asymmetry of the valve was reduced in small, almost circular specimens, independent of their origin (, ). In culture, larger valves (>50 µm), most likely representing initial cells after sexual reproduction, showed slightly constricted or convex margins with very slightly capitate apices (). The apices were usually broadly cuneately rounded in natural subarctic Pacific and Gulf of St. Lawrence specimens.
Figs 4–11. Neodenticula seminae, light microscopy. . Valves of specimens from the Gulf of St. Lawrence () and subarctic Pacific () showing simple and vestigial-walled costae. . Valves of specimens from the subarctic Pacific showing the deck and basal ridges with foramina. . Phase contrast. . Brightfield. . Interference contrast.
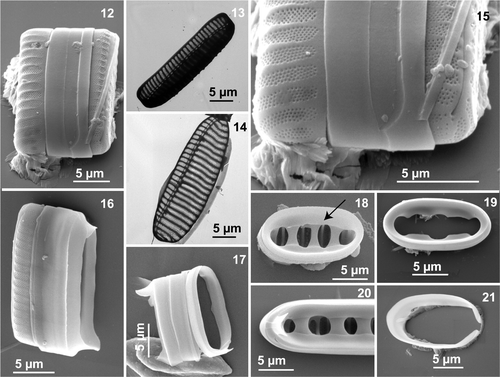
In both LM and EM (both SEM and TEM), all cells of N. seminae (field and cultured material from both regions) possess strongly eccentric, raphe-bearing, fibulate margins diagonally opposite each other within a frustule (). The raphe system consists of a single fissure, running from one apex to the other without any interruption in the middle of the valve (, , , ). Hence, no central nodule was present (, ). Externally, the single raphe fissure runs parallel to the valve margin and is straight at the poles (, ). The raphe is also bordered by a narrow hyaline area (, , ). Internally, the raphe fissure runs through a slightly arched canal (), formed by thick transapical marginal fibulae (7.5–12 in 10 µm; ) and bordered by lightly silicified, apically oriented thin ribs, which open through more or less evenly distributed portulae to the valve interior (, ).
Figs 12–21. Neodenticula seminae, electron microscopy (scanning electron microscopy unless indicated otherwise) of cultured () and natural () Gulf of St. Lawrence specimens and natural subarctic Pacific specimens (). . Whole frustule in girdle view. . Oblique view of valve showing full ornamentation on mantle (transmission electron microscopy). . Valve showing costae, areolate pattern and fibulate raphe system (transmission electron microscopy). . End pole of frustule showing raphe valve opposing non-raphe valve. . Broken frustule with hyaline girdle bands showing ligulate and antiligulate copulae. Note uninterrupted raphe fissure. . Series of girdle bands with ligulate copulae. . Valvocopula (arrow) adhering firmly to the valve. . Closed valvocopula. . Ligulate and antiligulate copulae at end of valve. . Open copula.
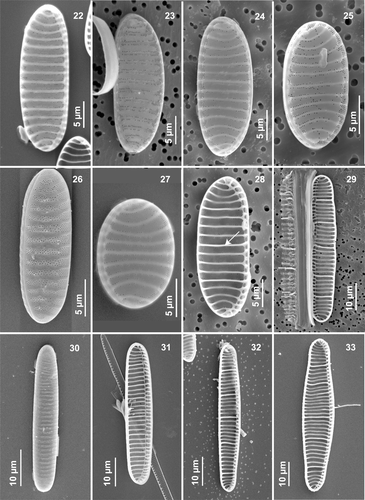
Figs 22–33. Morphological variability in Neodenticula seminae under scanning electron microscopy. . External views of valves (. Natural specimens from the Gulf of St. Lawrence and, from subarctic Pacific). . Internal valve views showing vestigial-walled costae (arrow in ) (. Gulf of St. Lawrence, . Subarctic Pacific; . Cultured subarctic Pacific).
Figs 34–40. Neodenticula seminae, internal views, scanning electron microscopy. . Valve showing simple and vestigial-walled costae (arrow) (subarctic Pacific). . Whole valve (Gulf of St. Lawrence culture). . Apical end showing the raphe canal and curved apical costae (subarctic Pacific and Gulf of St. Lawrence cultures, respectively). . Valve with deck (arrow) and three basal ridges (asterisk) (subarctic Pacific). . Part of valve showing foramina (asterisk), simple costae (long arrow) and solid-walled costae (short arrow) connecting to basal ridges (subarctic Pacific). . Two valves with basal ridges and foramina (subarctic Pacific).
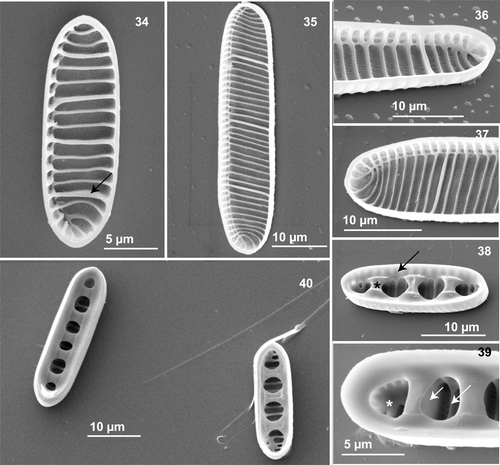
Figs 41–46. Neodenticula seminae, scanning electron microscopy. . Valve showing the external and continuous raphe fissure and full areolate pattern on mantle (Gulf of St. Lawrence). . External views of apex showing raphe ending and ornamentation on mantle (Gulf of St. Lawrence culture and natural material, respectively). Note the very gentle peeling off at the valve–mantle junction in . . External valve centre showing the uninterrupted raphe fissure. Note the fully developed ornamentation on mantle and the very scarce areolae on the valve surface (subarctic Pacific culture). . Internal view of apex (Gulf of St. Lawrence culture). . Apex showing the internal costae and the raphe canal (Gulf of St. Lawrence culture).
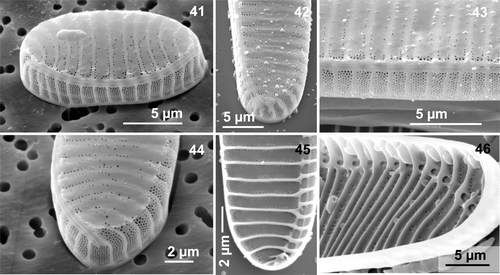
Figs 47–54. Electron microscopy of Neodenticula seminae. . External views, scanning electron microscopy (SEM). . Internal view, SEM. . transmission electron microscopy (TEM). . SEM, external views of apex showing the terminal raphe ending, curved virgae and scarce areolae on the valve surface. . Gulf of St. Lawrence culture, . subarctic Pacific culture. . TEM of almost circular valve with restricted but more complete areolation on the raphe-opposing margin (Gulf of St. Lawrence). . SEM showing spinulose structures (arrow) on the raphe-opposing margin (Gulf of St. Lawrence). . TEM of valve showing costae, reduced ornamentation and raphe canal (Gulf of St. Lawrence). . Part of the valve showing the external raphe fissure, virgae and ornamentation of the valve face and mantle (subarctic Pacific culture). . SEM, internal view of apex showing scarce areolae between costae (Gulf of St. Lawrence culture).
In all specimens examined, the valves were characterized by a series of unevenly spaced and transapically oriented costae (6–12 in 10 µm; ), representing elongated solid internal thickenings. The costae were usually straight ended, sometimes bifurcated into thick fibulae at the raphe-bearing margin, then continuing down the mantle (, , , , , ). Costae were shorter and curved near the apex (, , , ).
With EM, the most obvious feature of the external valve face in all natural and cultured material was the moderate to highly reduced pattern of areolation between the virgae (, , , , ). More complete areola ornamentation was restricted to the distal margin (raphe-opposing side), but was always present over the entire mantle (, , , ). The areolation comprised four to nine short rows of small areolae (5–5.5 in 1 µm; ), disposed more or less in quincunx between the virgae (, , ). The raphe-bearing margin, making up the outer raphe canal wall, was devoid of a multi-areolate pattern (, , ). The reduced ornamentation on the valve face consisted of scattered areolae, usually more or less irregularly lined up on each side of the virgae/costae (, , , ). However, in natural subarctic Pacific material, the areolate ornamentation was sometimes only moderately reduced, consisting of two to three irregular rows ().
A final shared feature was the presence of a few hyaline connective bands (), consisting of a very tightly adhering, closed valvocopula with inner marginal indentations ( [arrow], 19, 20, 40), followed by alternating ligulate and antiligulate copulae (, ). All bands were uniformly silicified without any perforation or ornamentation ().
However, a major morphological difference between the sites was the absence of strongly silicified, internal valve features in the Gulf of St. Lawrence material that are specific to N. seminae. Most valves in natural subarctic Pacific material showed the characteristic internal structure (, ), which consists of a wide deck (, arrow) around the circumference of the valve, which supports the firmly adhering valvocopula (, arrow), and several foramina (, asterisk) delimited by two to five thick basal ridges (, asterisk) originating from thick-walled costae underneath (, short arrow). In natural Gulf of St. Lawrence and a few subarctic Pacific specimens () as well as in culture material from both regions, the deck and basal ridges were entirely absent from the interior valve. The species had (from none to six) vestigial-walled costae (, , arrows), which are slightly thicker, and slightly more silicified, than the (one to nine) intervening costae (, , ). Most valves in natural subarctic Pacific material had a series of unevenly spaced costae (, long arrow) between fully developed and strongly silicified-walled costae (, ).
The last interesting feature differentiating the two populations of N. seminae was the occurrence of minute, blunt external spines (, arrow) at the extremities of the virgae, making the external valve surface look as if it were peeling off slightly (, , ). These spinulose structures were commonly observed in natural Gulf of St. Lawrence material, but only occasionally in Gulf of St. Lawrence cultured material; they were never found in natural and cultured subarctic Pacific material. Such minute blunt spines may play a role in linking the cells closely together when forming colonies.
Phylogenetic inference
In the analyses of the LSU rDNA data set, all the N. seminae strains formed a single clade, without any subdivision, supported by bootstrap values of 100 in both NJ and parsimony analyses (). The Neodenticula clade clustered within a larger clade (B) also containing different Fragilariopsis species, supported by bootstrap values of 86 and 76 for NJ and parsimony analyses, respectively. The position of the Fragilariopsis species in relation to the Neodenticula strains was not supported by bootstrap values above 50% in any of the ML, NJ and parsimony analyses. The relationships within clade B varied slightly between ML and NJ analyses and were unresolved in the parsimony analyses. This clade (Fragilariopsis and Neodenticula) was part of a larger clade (A), which included Pseudo-nitzschia. Clade A was firmly embedded within the Bacillariaceae, with high bootstrap support ().
Fig. 55. Phylogeny of the Bacillariaceae inferred from maximum likelihood analysis of large subunit of the nuclear ribosomal DNA. Numbers show bootstrap values obtained by neighbor joining and maximum parsimony analyses, respectively.
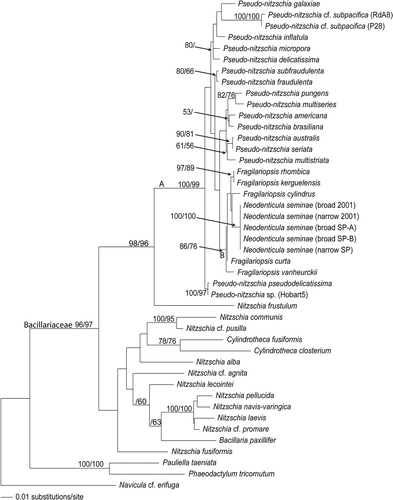
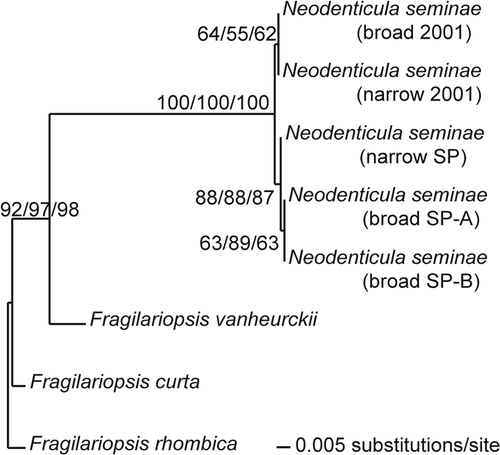
Phylogenetic analyses of the ITS data set of the Neodenticula strains showed that they clustered in one clade, with bootstrap values of 100 in ML, NJ and parsimony analyses (). Within that clade, the subarctic Pacific and Gulf of St. Lawrence strains formed two separate sister clades, with moderate to high bootstrap support (). The difference between the two groups was ascribed to three base pairs (two in ITS1 and one in ITS2), while variation of one or two base pairs was found (in ITS1) among the subarctic Pacific strains. At two ITS1 positions, the two Gulf of St. Lawrence strains and one of the subarctic Pacific strains were identical, whereas the other two Gulf of St. Lawrence strains differed.
Fig. 56. Phylogeny of Neodenticula seminae and Fragilariopsis species inferred from maximum likelihood analysis of internal transcribed spacer regions of the nuclear ribosomal DNA. Numbers show bootstrap values obtained by maximum parsimony, neighbor joining and maximum likelihood analyses, respectively.
Discussion
N. seminae in the North Atlantic
It has hitherto not been considered difficult to identify live cells of N. seminae in plankton samples. However, it became challenging in 2001, when N. seminae was first reported in the spring phytoplankton bloom in the Gulf of St. Lawrence, Northwest Atlantic (Starr et al., Citation2002; Therriault et al., Citation2002). Its small size made solitary cells and colonies appear identical to Fragilariopsis cylindrus (Cleve) Frenguelli and F. oceanica (Cleve) Hasle, which were also abundant during that period following ice break-up. For the first time, in April/May 2001, the spring bloom in the Gulf of St. Lawrence was dominated by N. seminae (up to 1.9 × 106 cells l−1). Neodenticula seminae is important in modern North Pacific diatom assemblages of middle to high latitudes and a productivity indicator in the upper subarctic Pacific (Barron, Citation1981; Sancetta, Citation1982; Taylor & Waters, Citation1982; Takahashi et al., Citation1989, Citation2002; Takahashi, Citation1994, Citation1997; Kang et al., Citation1996; Koç & Flower, Citation1998). Along with N. seminae, other unusually abundant diatom species in the St. Lawrence spring bloom included Corethron hystrix Hensen and Membraneis challengerii (Grunow) Paddock. The latter is usually reported from Antarctica, but was recently observed in the Bering Sea (Kang et al., Citation1996).
N. seminae had been extinct from the North Atlantic phytoplankton since the late Quaternary (0.84 Ma) (Koç & Flower, Citation1998; Koç et al., Citation1999; Shimada et al., Citation2008). It was recorded from high latitudes of the North Atlantic from Neogene and Quaternary sediments, and was associated with the presence of cool, low salinity surface water (Baldauf, Citation1984, Citation1986). This pelagic diatom was considered indicative of an influx of colder polar water into the North Atlantic (Baldauf, Citation1984, Citation1986), and it was postulated that an ice-free polar connection may have existed between the North Atlantic and the Bering Sea during the middle Quaternary (Baldauf, Citation1987). Recently, Koç et al. (Citation1999) interpreted the first occurrence of N. seminae in the North Atlantic as a sign of cooling, starting 1.26 Ma, leading to the establishment of 100 000-year cycles with severe glacial periods (Shimada et al., Citation2008). N. seminae has never been reported from surface sediments in the Canadian High Arctic (Williams, Citation1986; Kravitz et al., Citation1987; Campeau et al., Citation1999, 2000; De Sève, Citation1999). However, Monjanel & Baldauf (Citation1989) recorded it in the sediments from the southern Labrador Sea with an earlier occurrence at about 1.7 Ma and suggested that it dispersed from the Labrador Sea to other regions of the North Atlantic.
N. seminae has never previously been recorded in the phytoplankton or surface sediments from the St. Lawrence ecosystem (Bérard-Therriault et al., Citation1999; Lapointe, Citation2000). There was no indication of its presence from the Fisheries and Oceans Canada's Toxic Algae Monitoring Program from 1989 until its first observation in the Gulf of St. Lawrence in spring 2001 (Bonneau et al., Citation2002; Starr et al., Citation2002; Therriault et al., Citation2002). Lovejoy et al. (Citation2002) did not report this species in the phytoplankton of northern Baffin Bay either. However, it has been observed from the Labrador Sea since 1999 under the Continuous Plankton Recorder survey monitoring plankton in the Northwest Atlantic (Reid et al., Citation2007).
The reappearance of N. seminae in the North Atlantic may be explained partly by recent hydrographic changes which have affected the Arctic circulation, increasing Pacific water inflow into the Northwest Atlantic, and partly by the unprecedented occurrence in 1998 of extensive ice-free water in the Canadian Archipelago (Tremblay et al., Citation2002; Jones et al., Citation2003; Reid et al., Citation2007). Pacific water, which is usually confined to near-surface waters, with salinity typically <∼33.5, flows through the Canadian Archipelago (e.g. Barrow Strait, Lancaster Sound, Nares Strait, Smith Sound and Jones Sound) and exits into the northern North Atlantic along the west coast of the Labrador Sea (Jones et al., Citation2003; Reid et al., Citation2007). In 1998, the Canadian High Arctic experienced an unusually warm year, which contributed to a significant reduction in sea-ice cover and augmentation of the longest sustained period of open water, almost seven months (Arrigo & van Dijken, Citation2004), and an increasing inflow of Pacific water (Jones et al., Citation2003). This atypical event was probably attributable to a large positive anomaly in atmospheric temperature that year (Maslanik et al., Citation1999). The unusually warm year led to higher than normal surface heating and stratification in polar regions, favouring the circulation of near-surface Pacific water through atmospheric forcing (Jones et al., Citation2003). This created a suitable light environment for phytoplankton growth (Arrigo & van Dijken, Citation2004), which may have contributed to the transportation of phytoplankton cells, in this case N. seminae, from the subarctic Pacific through the Canadian Archipelago to the Labrador Sea into the North Atlantic, with episodic intrusions of cold surface water from the Labrador Shelf into the Gulf of St. Lawrence (Therriault et al., Citation2002). These changes coincided with the reappearance of N. seminae in both the Labrador Sea and the St. Lawrence ecosystem in 1999 (Reid et al., Citation2007).
Another possible mechanism for the introduction of N. seminae into the North Atlantic, which cannot be entirely ruled out, is the transfer of shipping ballast water (Reid et al., Citation2007). But, besides ice breakers, because of the severe ice conditions prevailing in the Canadian High Arctic for most of the year, there has been no commercial shipping through the Northwest Passage, from the North Pacific to the North Atlantic. Therefore, the usual route for freighters between the Pacific and the Atlantic coasts and Europe is through the Panama Canal. However, cells of N. seminae would not have survived such a long period of time in ballast waters under warmer temperature conditions when crossing the Canal, based on the death of cultures that we tried to maintain at 15°C.
Morphological comparisons
When N. seminae was first recorded in the spring phytoplankton bloom of the Gulf of St. Lawrence, there was some doubt about its occurrence in Canadian continental waters. When routinely enumerating cells at low magnification, small colonial cells can be easily confused with F. cylindrus and F. oceanica (von Quillfeldt, Citation2001). However, careful attention to the type of colony formation, shape and colour of the chloroplast, and degree of silicification of valve ornamentation allows their differentiation. Moreover, all cleaned cells showed the distinctive valve features of Neodenticula, with its structural asymmetry along the apical axis, marginal fibulae from branching transapical costae, delicate and reduced areolation on the valve face, and the continuous raphe fissure from one pole to the other (Akiba & Yanagisawa, Citation1986; Yanagisawa & Akiba, Citation1990). These features, as well as the presence of walled costae and closed valvocopulae, allow N. seminae to be distinguished from Fragilariopsis species. The morphometric data on subarctic Pacific and Gulf of St. Lawrence specimens were within the published range for N. seminae, except for the larger width of our specimens, but also reported from the North Pacific (Shimada & Tanimura, Citation2006) ().
General valve outline and the deck and basal ridges associated with solid-walled costae (reported as primary pseudosepta in Akiba & Yanagisawa, Citation1986; Yanagisawa & Akiba, Citation1990; Semina, Citation2003) are common to three closely related genera, Neodenticula, Crucidenticula and Denticulopsis. However, basal ridges connected to the deck and the solid-walled costae on the internal valve were only observed in natural subarctic Pacific material (), never in natural Gulf of St. Lawrence material, nor in cultures from both regions grown in natural Gulf of St. Lawrence waters enriched with F/2 culture medium plus silicate (, ); vestigial-walled costae are barely distinguishable from the other costae. Similar morphological differences in the silicification of N. seminae populations from the northwest Pacific (more delicate valves) and the Bering Sea (typically robust valves) have been reported (Shimada et al., Citation2003; Shimada & Tanimura, Citation2006). They concluded that the regional variation in the degree of the valve silicification can be explained by nutrient availability, mainly silicate, perhaps also iron, or both. North Pacific surface water is characterized by high nutrient concentrations and, in particular, silicic acid (Si(OH)4) which is up to three times the concentrations of the Gulf of St. Lawrence and the North Atlantic (Tremblay et al., Citation2002; Reid et al., Citation2007). Silicic acid limitation may have inhibited the morphogenesis of the deck and basal ridges, and the full thickening of the walled costae in the Gulf of St. Lawrence natural and cultured populations (). Si(OH)4 concentration and other environmental variables, such as irradiance, temperature, nutrient concentrations or trace metal ions (iron and zinc), regulate diatom growth rates, including cell division, and are indirectly involved in controlling silicification (Martin-Jézéquel et al., Citation2000). A low supply of silicon across the diatom plasmalemma to the site of polymerization within the silica deposition vesicle may prolong the time spent generating frustules, which may result in thinner or incompletely formed frustules (Brzezinski et al., Citation1990). Brzezinski et al. (Citation1990) also reported a higher percentage of Chaetoceros species with only a single pair of setae under silicic acid-depleted conditions. However, the relationship between limiting levels of iron and zinc and increased frustule silicification is less obvious. Iron limits the productivity of about 30% of the world's oceans and Mock et al. (Citation2008) have provided evidence that diatoms build more heavily silicified cell walls in these environments. Therefore, there is some evidence that Si(OH)4 concentration, and perhaps some trace metal ions, may be responsible for the polymorphism in N. seminae populations between the subarctic Pacific and the Gulf of St. Lawrence.
The phylogenetic analyses did not indicate that the subarctic Pacific and Gulf of St. Lawrence strains represent two populations of N. seminae that have evolved separately for millions of years. Although the strains clustered in two separate clades in the ITS analyses, the between-clade base pair differences were not much larger than those among subarctic Pacific strains. The inclusion of more Neodenticula strains from different areas of the North Pacific would be expected to increase the genetic variation among strains. Despite the morphological differences in the deck and basal ridges and the walled costae between the natural populations from the two regions, the fact that strains from both regions behaved similarly in culture also contradicts the long separation theory.
Reduced ornamentation of the valve, limited to a few irregular rows of areolae between virgae/costae (Yanagisawa & Akiba, Citation1990), was observed in all natural subarctic Pacific material. In natural Gulf of St. Lawrence material and cultures from both regions, the valves always showed further reduction in areolae, disposed unevenly or scattered in a line on either side of the virgae/costae, in contrast with the original diagnosis (Yanagisawa & Akiba, Citation1990; Semina, Citation2003). This reduction in the areola pattern was not reported by Shimada et al. (Citation2003) and Shimada & Tanimura (Citation2006) when they studied polymorphism in N. seminae from the North Pacific and the Bering Sea. This deviant feature may again find some explanation in silicon deficiency, as previously reported by Brzezinski et al. (Citation1990) for Chaetoceros Ehrenberg.
The peculiar external spinulose structures regularly seen on the virgae at the valve–mantle junction in the Gulf of St. Lawrence natural and cultured material have not been previously reported (Akiba & Yanagisawa, Citation1986; Semina, Citation2003; Shimada et al., Citation2003; Shimada & Tanimura, Citation2006). The precise role of these tiny spines is currently unknown, but may be in linking, enabling cells to form ribbon-shaped colonies. External linking spines have often been reported in other colonial species of several diatom genera (Round et al., Citation1990; Siver & Kling, Citation1997). It has also been demonstrated that N. seminae from the North Pacific can produce mucilaginous threads from near the marginal areolae that hold adjacent cells firmly to form colonies (Shimada et al., Citation2003). Such mucilaginous threads were not observed in the present study because of the oxidation protocol used prior to examination in EM, but they are commonly produced by colonial diatoms (Hoagland et al., Citation1993).
The phylogenetic analyses confirmed that Neodenticula belongs to the Bacillariaceae and is phylogenetically most closely related to Fragilariopsis (). Species of both genera produce ribbon-shaped colonies, with cells adhering closely together by the entire valve face. They have linear to slightly lanceolate valves with broadly rounded ends and cells are more or less rectangular in girdle view. The striking reduction of the deck and basal ridges, and the solid-walled costae under certain conditions increases the resemblance between N. seminae and Fragilariopsis. However Fragilariopsis contains some species that possess a central nodule (Hasle, Citation1965). Our results support previous hypotheses that Neodenticula and Fragilariopsis are closely related and have a common ancestor (Medlin & Sims, Citation1993). A close phylogenetic relationship becomes very obvious when N. seminae is compared with Fragilariopsis kerguelensis (O’Meara) Hustedt. The costae in N. seminae are homologous with the highly developed internal costae of F. kerguelensis that penetrate deep into the cell interior (Hasle, Citation1965, Citation1968). The valvocopulae in both species have an indented inner margin and are perforated (Hasle, Citation1965). The reduced ornamentation on the valve surface and the fully developed areola pattern on the mantle are on the other hand a characteristic of N. seminae and F. oceanica (Hasle, Citation1965). To the best of our knowledge, no Fragilariopsis species possesses a closed valvocopula, which may therefore serve as an apomorphic character for Neodenticula differentiating the two genera. In order to determine to which Fragilariopsis species N. seminae is phylogenetically most closely related, analyses including more Fragilariopsis species are necessary.
In summary, the populations of N. seminae from the subarctic Pacific and the Gulf of St. Lawrence shared the characteristic features of the species, except for (i) the deficiency producing the deck and basal ridges, the full and solid-walled costae, and a more complete but reduced ornamentation in natural Gulf of St. Lawrence and cultured material from both regions, and (ii) the occurrence of tiny blunt spines on the valves of natural Gulf of St. Lawrence and cultured material. This variation in the degree of silicification may result from near-surface water Si(OH)4 limitation in the St. Lawrence ecosystem. Specimens from the Gulf of St. Lawrence were still able to flourish in the spring phytoplankton blooms for five consecutive years (2001–2006), but without producing the deck and basal ridges, solid-walled costae and with reduced ornamentation. Phylogenetic analyses showed nearly the same level of genetic variation between N. seminae strains from the two regions as among subarctic Pacific strains, and hence a history of long separation of the two populations is not supported. Further morphological, autecological and molecular studies are needed to understand the variation in some of the valve features, not only between the subarctic Pacific and the St. Lawrence ecosystem, but also in the northwest Pacific, Bering Sea and the Labrador Sea (Reid et al., Citation2007). The phylogenetic position of Neodenticula, closely related to Fragilariopsis, has been established and further studies are needed to assess the finer phylogenetic relationships between the two genera.
Acknowledgements
The research was funded by a Canadian Museum of Nature research grant to M. Poulin, Fisheries and Oceans Canada operating grants to M. Starr, and Carlsberg Foundation (grant 0656/20) to N. Lundholm. We thank F. Akiba (Diatom Minilab Akiba Ltd., Saitama, Japan) and Y. Yanagisawa (Geological Survey of Japan, Tsukuba, Japan) for confirming the identification of N. seminae. We are extremely grateful to K. Takahashi (Kyushu University, Fukuoka, Japan) who kindly provided us with fresh samples of subarctic Pacific phytoplankton that he collected in July 2003. Special thanks to F. Akiba, J.G. Baldauf, J.A. Barron, N. Koç, K. Takahashi and Y. Yanagisawa for very helpful discussions, L. Ley for some SEM work and P.B. Hamilton for producing the figure plates.
References
- Akiba , F . 1986 . Middle Miocene to Quaternary diatom biostratigraphy in the Nankai Trough and Japan Trench, and modified lower Miocene through Quaternary diatom zones for middle-to-high latitudes of the North Pacific . Init. Rep. DSDP , 87 : 393 – 481 .
- Akiba , F and Yanagisawa , Y . 1986 . Taxonomy, morphology and phylogeny of the Neogene diatom zonal marker species in the middle-to-high latitudes of the North Pacific . Init. Rep. DSDP , 87 : 483 – 554 .
- Arrigo, K.R. & van Dijken, G.L. (2004). Annual cycles of sea ice and phytoplankton in Cape Bathurst polynya, southeastern Beaufort Sea, Canadian Arctic. Geophys. Res. Lett., 31: L08304, doi: 10.1029/2003GL018978.
- Baldauf , JG . 1984 . Cenozoic diatom biostratigraphy and paleoceanography of the Rockall Plateau Region, North Atlantic, Deep Sea Drilling Project Leg 81 . Init. Rep. DSDP , 81 : 439 – 478 .
- Baldauf , JG . 1986 . “ Diatom biostratigraphic and paleoceanographic interpretations for the middle to high latitude North Atlantic Ocean ” . In North Atlantic Paleoceanography Edited by: Summerhayes , CP and Schackleton , NJ . 243 – 252 . Geological Society Special Publication No. 21
- Baldauf , JG . 1987 . Diatom biostratigraphy of the middle- and high-latitude North Atlantic Ocean, Deep Sea Drilling Project Leg 94 . Init. Rep. DSDP , 94 : 729 – 762 .
- Barron , JA . 1981 . Late Cenozoic diatom biostratigraphy and paleoceanography of the middle-latitude eastern North Pacific, Deep Sea Drilling Project Leg 63 . Init. Rep. DSDP , 63 : 507 – 538 .
- Bérard-Therriault , L , Poulin , M and Bossé , L . 1999 . Guide d’identification du phytoplancton marin de l’estuaire et du golfe du Saint-Laurent incluant également certains protozoaires . Publ. spéc. can. sci. halieut. aquat. , 128 : 1 – 387 .
- Bonneau , E , Couture , J-Y and Levasseur , M . 2002 . Le programme de monitorage des algues toxiques de la région du Québec: un outil précieux pour le développement des connaissances . AZMP Bull. , 2 : 24 – 26 .
- Brzezinski , MA , Olson , RJ and Chisholm , SW . 1990 . Silicon availability and cell-cycle progression in marine diatoms . Mar. Ecol. Prog. Ser. , 67 : 83 – 96 .
- Campeau , S , Héquette , A and Pienitz , R . 2000 . Late Holocene diatom biostratigraphy and sea-level changes in the southeastern Beaufort Sea . Can. J. Earth Sci. , 37 : 63 – 80 .
- Campeau , S , Pienitz , R and Héquette , A . 1999 . Diatoms as quantitative paleodepth indicators in coastal areas of the southeastern Beaufort Sea, Arctic Ocean . Palaeogeogr. Palaeoclimatol. Palaeoecol. , 146 : 67 – 97 .
- De Sève , M . 1999 . Transfer function between surface sediment diatom assemblages and sea-surface temperature and salinity of the Labrador Sea . Mar. Micropaleontol. , 36 : 249 – 267 .
- Doyle , JJ and Doyle , JL . 1987 . A rapid DNA isolation procedure for small quantities of fresh leaf tissue . Phytochem. Bull. , 19 : 11 – 15 .
- Guillard , RRL and Ryther , JH . 1962 . Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran . Can. J. Microbiol. , 8 : 229 – 239 .
- Hasegawa , M , Kishino , H and Yano , T . 1985 . Dating of the human-ape splitting by a molecular clock of mitochondrial DNA . J. Mol. Evol. , 21 : 160 – 174 .
- Hasle , GR . 1965 . Nitzschia and Fragilariopsis species studied in the light and electron microscopes. III . The genus Fragilariopsis. Skr. Norske Vidensk.–Akad. Oslo I. Mat.–Naturv. Kl. N. S. , 21 : 1 – 49 .
- Hasle , GR . 1968 . Observations on the marine diatom Fragilariopsis kerguelensis (O’Meara) Hust. in the scanning electron microscope . Nytt Mag. Bot. , 15 : 205 – 208 .
- Hasle , GR and Syvertsen , EE . 1997 . “ Marine diatoms ” . In Identifying Marine Phytoplankton , Edited by: Tomas , CR . 5 – 385 . San Diego : Academic Press .
- Hoagland , KD , Rosowski , JR , Gretz , MR and Roemer , SC . 1993 . Diatom extracellular polymeric substances: function, fine structure, chemistry, and physiology . J. Phycol. , 29 : 537 – 566 .
- Jones, E.P., Swift, J.H., Anderson, L.G., Lipizer, M., Civitarese, G., Falkner, K.K., Kattner, G. & Mclaughlin, F. (2003). Tracing Pacific water in the North Atlantic Ocean. J. Geophys. Res., 108, C4, 3116, doi: 10.1029/2001JC001141.
- Kang , S-H , Kang , J-S , Lee , W-C and Chung , C-S . 1996 . Surface phytoplankton in the Bering Sea during summer 1994 and 1995: relationships to physical and chemical environmental parameters . Ocean Res. (Spec.) , 18 : 13 – 16 .
- Koç , N and Flower , BP . 1998 . High-resolution Pleistocene diatom biostratigraphy and paleoceanography of site 919 from the Irminger Basin . Proc. ODP, Sci. Res. , 152 : 209 – 219 .
- Koç , N , Hodell , DA , Kleiven , H and Labeyrie , L . 1999 . High-resolution Pleistocene diatom biostratigraphy of Site 983 and correlations with isotope stratigraphy . Proc. ODP, Sci. Res. , 162 : 51 – 62 .
- Kravitz , JH , Burckle , LH and Bromble , SL . 1987 . Distribution of diatoms in the surface sediments of the Kane Basin . Arct. Alp. Res. , 19 : 89 – 94 .
- Kurihara , M and Takahashi , K . 2002 . Long-term size variation and life cycle patterns of a predominant diatom Neodenticula seminae in the Subarctic Pacific and the Bering Sea . Bull. Plankton Soc. Jpn. , 49 : 77 – 87 .
- Lapointe , M . 2000 . Modern diatom assemblages in surface sediments from the Maritime Estuary and the Gulf of St. Lawrence, Quebec (Canada). . Mar. Micropaleontol. , 40 : 43 – 65 .
- Lovejoy , C , Legendre , L , Martineau , M-J , Bâcle , J and von Quillfeldt , CH . 2002 . Distribution of phytoplankton and other protists in the North Water . Deep-Sea Res. Part II , 49 : 5027 – 5047 .
- Lundholm , N , Daugbjerg , N and Moestrup , Ø . 2002 . Phylogeny of the Bacillariaceae with emphasis on the genus Pseudo-nitzschia (Bacillariophyceae) based on partial LSU rDNA . Eur. J. Phycol. , 37 : 115 – 134 .
- Lundholm , N , Moestrup , Ø , Hasle , GR and Hoef-Emden , K . 2003 . A study of the Pseudo-nitzschia pseudodelicatissima/cuspidata complex (Bacillariophyceae): what is P. pseudodelicatissima? . J. Phycol. , 39 : 797 – 813 .
- Martin-Jézéquel , V , Hildebrand , M and Brzezinski , MA . 2000 . Silicon metabolism in diatoms: implications for growth . J. Phycol. , 36 : 821 – 840 .
- Maslanik , JA , Serreze , MC and Agnew , T . 1999 . On the record reduction in 1998 western Arctic sea-ice cover . Geophys. Res. Lett. , 26 : 1905 – 1908 .
- Medlin , LK and Sims , PA . 1993 . The transfer of Pseudoeunotia doliolus to Fragilariopsis . Nova Hedwigia Beih. , 106 : 323 – 334 .
- Mock , T , Samanta , MP , Iverson , V , Berthiaume , C , Robison , M , Holtermann , K , Durkin , C , Bondurant , SS , Richmond , K Rodesch , M . 2008 . Whole-genome expression profiling of the marine diatom Thalassiosira pseudonana identifies genes involved in silicon bioprocesses . Proc. Natl. Acad. Sci. USA , 105 : 1579 – 1584 .
- Monjanel , AL and Baldauf , JG . 1989 . Miocene to Holocene diatom biostratigraphy from Baffin Bay and Labrador Sea, Ocean Drilling Program Sites 645 and 646 . Proc. ODP, Sci. Res. , 105 : 305 – 322 .
- Onodera , J , Takahashi , K and Honda , MC . 2003 . Diatom fluxes at Station KNOT in the western Subarctic Pacific, 1997–2000 . Bull. Plankton Soc. Jpn. , 50 : 1 – 15 .
- Posada , D and Crandall , KA . 1998 . Modeltest: testing the model of DNA substitution . Bioinformatics , 14 : 817 – 818 .
- Reid , PC , Johns , DG , Edwards , M , Starr , M , Poulin , M and Snoeijs , P . 2007 . A biological consequence of reducing Arctic ice cover: arrival of the Pacific diatom Neodenticula seminae in the North Atlantic for the first time in 800 000 years . Global Change Biol. , 13 : 1910 – 1921 .
- Ross , R , Cox , EJ , Karayeva , NI , Mann , DG , Paddock , TBB , Simonsen , R and Sims , PA . 1979 . An amended terminology for the siliceous components of the diatom cell . Nova Hedwig., Beih. , 64 : 513 – 533 .
- Round , FE , Crawford , RM and Mann , DG . 1990 . The Diatoms , Cambridge, , UK : Cambridge University Press .
- Sancetta , C . 1982 . Distribution of diatom species in surface sediments of the Bering and Okhotsk seas . Micropaleontol. , 28 : 221 – 257 .
- Semina , GI . 1956 . De specie nova generis Denticula Ktz . Notul. Systemat. e Sect. Cryptogam. Inst. Bot. nomine V.L. Komarovii Acad. Sci. URSS , 11 : 82 – 84 .
- Semina , HJ . 2003 . SEM-studied diatoms of different regions of the world ocean . Iconogr. Diatomol. , 10 : 1 – 362 .
- Shimada , C and Tanimura , Y . 2006 . Spatial variability in valve morphology of Neodenticula seminae, an oceanic diatom in the subarctic North Pacific and the Bering Sea . Paleontol. Res. , 10 : 79 – 89 .
- Shimada , C , Burckle , LH and Tanimura , Y . 2003 . Morphological variability in Neodenticula seminae, a marine planktonic diatom in the North Pacific Ocean and Bering Sea . Diatom Res. , 18 : 307 – 322 .
- Shimada , C , Sato , T , Toyoshima , S , Yamasaki , M and Tanimura , Y . 2008 . Paleoecological significance of laminated diatomaceous oozes during the middle-to-late Pleistocene, North Atlantic Ocean (IODP Site U1304) . Mar. Micropaleontol. , 69 : 139 – 150 .
- Simonsen , R . 1979 . The diatom system: ideas on phylogeny . Bacillaria , 2 : 9 – 71 .
- Simonsen , R and Kanaya , T . 1961 . Notes on the marine species of the diatom genus Denticula Kütz . Int. Rev. Gesamten Hydrobiol. , 46 : 498 – 513 .
- Siver , PA and Kling , H . 1997 . Morphological observations of Aulacoseira using scanning electron microscopy . Can. J. Bot. , 75 : 1807 – 1835 .
- Starr, M., St-Amand, L. & Bérard-Therriault, L. (2002). State of phytoplankton in the Estuary and Gulf of St. Lawrence during 2001. DFO Can. Sci. Advisory Sec. Res. Doc. 2002/067, 23 p.
- Strickland , JDH and Parsons , TR . 1972 . A practical handbook of seawater analysis . Bull. Fish. Res. Board Can. , 167 : 1 – 311 .
- Swofford , DL . 2002 . PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4.0 Beta for Linux/UNIX , Sunderland, MA : Sinauer Associates .
- Takahashi , K . 1994 . “ From modern flux to paleoflux: assessment from sinking assemblages to thanatocoenosis ” . In Carbon Cycling in the Glacial Ocean: Constraints on the Ocean's Role in Global Change Edited by: Zahn , R . 413 – 424 . NATO ASI Series 117
- Takahashi , K . 1997 . Siliceous microplankton fluxes in the eastern subarctic Pacific, 1982–1986 . J. Oceanogr. , 53 : 455 – 466 .
- Takahashi , K , Fujitani , N and Yanada , M . 2002 . Long term monitoring of particle fluxes in the Bering Sea and the central subarctic Pacific Ocean, 1990–2000 . Prog. Oceanogr. , 55 : 95 – 112 .
- Takahashi , K , Fujitani , N , Yanada , M and Maita , Y . 2000 . Long-term biogenic particle fluxes in the Bering Sea and the central subarctic Pacific Ocean, 1990–1995 . Deep-Sea Res. Part I , 47 : 1723 – 1759 .
- Takahashi , K , Honjo , S and Tabata , S . 1989 . “ Siliceous phytoplankton flux: interannual variability and response to hydrographic changes in the northeastern Pacific ” . In Aspects of Climate Variability in the Pacific and Western Americas Edited by: Peterson , DH . 151 – 160 . Geophysical Monograph 55.
- Tamura , K and Nei , M . 1993 . Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees . Mol. Biol. Evol. , 10 : 512 – 526 .
- Taylor , FJR and Waters , RE . 1982 . Spring phytoplankton in the Subarctic North Pacific Ocean . Mar. Biol. , 67 : 323 – 335 .
- Therriault , J-C , Galbraith , PS , Starr , M and Harvey , M . 2002 . Intrusions of Labrador Shelf waters into the Gulf of St. Lawrence and their potential influence on the plankton community and higher trophic levels . AZMP Bull. , 2 : 12 – 15 .
- Tremblay, J.-É., Gratton, Y., Carmack, E.C., Payne, C.D. & Price, N.M. (2002). Impact of the large-scale Arctic circulation and the North Water Polynya on nutrient inventories in Baffin Bay. J. Geophys. Res., 107, C8, 3112, doi: 10.1029/2000JC000595.
- von Quillfeldt , CH . 2001 . Identification of some easily confused common diatom species in Arctic spring blooms . Bot. Mar. , 44 : 375 – 389 .
- Williams , KK . 1986 . Recent Arctic marine diatom assemblages from bottom sediments in Baffin Bay and Davis Strait . Mar. Micropaleontol. , 10 : 327 – 341 .
- Yanagisawa , Y and Akiba , F . 1990 . Taxonomy and phylogeny of the three marine diatom genera, Crucidenticula, Denticulopsis and Neodenticula . Bull. Geol. Surv. Jpn. , 41 : 197 – 301 .
- Yanagisawa , Y and Akiba , F . 1998 . Refined Neogene diatom biostratigraphy for the northwest Pacific around Japan, with an introduction of code numbers for selected diatom biohorizons . J. Geol. Soc. Jpn. , 104 : 395 – 414 .