Abstract
Valve and girdle band morphogenesis in a bipolar centric diatom Plagiogrammopsis vanheurckii was examined under SEM. This is the first report demonstrating that the earliest stages of a bipolar centric valve exhibit a ring-shaped pattern centre, the annulus, although the presence of the annulus in mature valves has been known for a long time. Non-synchronous development of sibling valves was observed in the early stages of valve morphogenesis; however, it remains unknown whether this phenomenon is related to the heterovalvate nature of P. vanheurckii because the degree of the heterovalvy is slight in this species. The ocellulus was formed in the final stages of valve morphogenesis. The use of clonal cultures also allowed examination of girdle band formation. In early stages of morphogenesis, the girdle band had a framework similar to araphid diatom valves, in which the primary rib system comprises longitudinal and transverse ribs.
Introduction
The valve morphogenesis of centric diatoms starts with a ring-shaped pattern centre, whereas those of pennates start with a sternum (araphid diatom) or a raphe-sternum (raphid diatoms) (Mann, Citation1984). Over the past 10 years, information on the valve morphogenesis of diatoms has greatly increased (e.g. Cox, Citation1999; Tiffany, Citation2002, 2005, 2008; Cox & Kennaway, Citation2004; Sterrenburg et al., Citation2005; Tiffany & Hernandez-Becerril, Citation2005; Kaluzhnaya & Likhoshway, Citation2007). However, although it has become clear that some aspects of valve morphogenesis have phylogenetic significance at higher taxonomic levels (Mann, Citation1984; Mayama & Kuriyama, Citation2002), there is still insufficient information to reveal how structure and ontogenetic patterns have evolved in the major diatom groups, especially among the lineages of bipolar centrics.
In the present study, I report details of valve and girdle band morphogenesis in a bipolar centric diatom, Plagiogrammopsis vanheurckii (Grunow) Hasle, von Stosch et Syvertsen, using scanning electron microscopy (SEM) of clonal cultures. Because the frustule morphology of P. vanheurckii has been described in detail elsewhere (Hasle et al., Citation1983; Gardner & Crawford, Citation1994), this report focuses on valve and band morphogenesis.
Materials and methods
Sampling and culture conditions
A sample containing P. vanheurckii was collected using a 20-µm mesh plankton net from the Doppelschleuse along the River Weser in Bremerhaven (Northern Germany) on 22 September 2008. Chains of P. vanheurckii were isolated by pasteur pipette into 12-well plates with IMR medium (Eppley et al., Citation1967) to establish clonal cultures. Four strains of P. vanheurckii, s0432 to s0435, were inoculated into 500-ml flasks with IMR medium to obtain large numbers of cells for the observation of valve formation. All cultures were maintained at 15°C, under a 14-h:10-h light–dark photoperiod with cool white fluorescent light at a photon flux density of 30–40 µmol photons m−2s−1. Voucher specimens, which were mounted onto glass slides with Mountmedia (refractive index n20/D 5 1.50; Wako, Osaka, Japan), are available upon request from the author.
Observations
Light microscopy (LM) observations were made using a Zeiss Axioplan (Zeiss, Oberkochen, Germany) microscope with bright field optics on cultures in exponential growth phase. Cells were harvested on filters and cleaned using 30% H2O2 and several drops of HCl, then rinsed with distilled water. Cleaned material was mounted on a glass slide with Mountmedia for LM. For SEM examination, the cleaned material was dropped onto coverslips and air dried. Coverslips were fixed to the SEM stubs with carbon tape, and coated with gold using an SC 500 (Emscope, Ashford, England). Specimens were examined with a QUANTA 200F SEM (FEI Company, Eindhoven, The Netherlands) at an accelerating voltage of 10 kV, and ca. 10 mm working distance. Captured images were adjusted with Adobe Photoshop.
Terminology
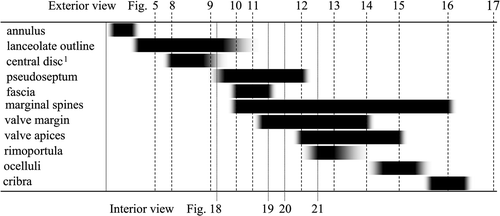
The general terminology follows Anonymous (Citation1975), Round et al. (Citation1990) and Cox (Citation1999). Terms for some morphological structures that are prominent in P. vanheurckii followed Hasle et al. (Citation1983), i.e. fascia: a solid transverse zone underlying central part of the valve; ocellulus: a small ocellus, which is the top plate of an elevation, perforated by pores that are distinctly different from the valve areolae, with a rim devoid of any perforations; pilus: a long proximally flattened hair; pseudoseptum: a transverse internal ridge on the fascia.
Molecular phylogenetic studies of diatoms have revealed that historical diatom classifications do not reflect a natural system, and that centric and araphid pennate diatoms are paraphyletic in most gene phylogenies, e.g. using nuclear 18S rDNA and plastid 16S rDNA (Medlin & Kaczmarska, Citation2004). Nevertheless, I use the terms ‘araphid’ and ‘radial/bipolar centric’ here, because they refer to key morphological features or their absence. I do not imply that this corresponds to a mono- (holo-) phyletic group, nor that it should be accorded any taxonomic status.
Results
Mature frustules
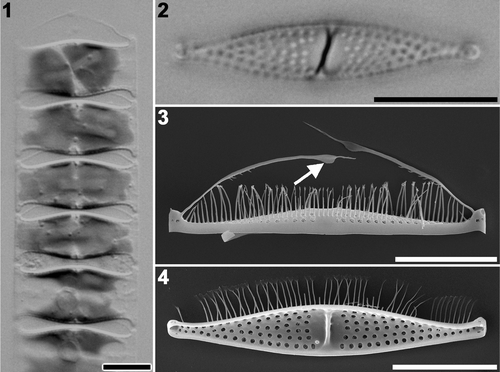
Mature cells are loosely connected to one another to form a chain (), which is slightly twisted around its long axis. Pseudosepta, internal thickenings at the centre of the valves, are recognizable under LM (). Valves are lanceolate with capitate ends (). A slight degree of heterovalvy was observed. Pilus valves lack a rimoportula (). Notably, each tip of the pilus has a prominent broad area, a wing (), but process valves lack pili ().
Figs 1–4. Plagiogrammopsis vanheurckii mature cells. Strain s0435, light microscopy () and strain s0432, scanning electron microscopy (). . Chain of living cells. . Cleaned process valve. . External view of pilus valve. Arrow indicates wing on pilus. . Internal view of process valve. Scale bars: 10 µm.
Pattern centre
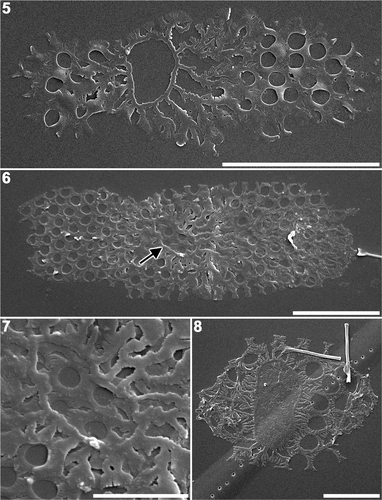
In the earliest stages of morphogenesis, a ring-shaped pattern centre, an annulus, was clearly observed (). Although the centrifugal/bipolar expansion of valve morphogenesis was proceeding at this stage, the inside of the annulus usually remained unsilicified (). However, it should be noted that a single valve at an early stage of morphogenesis was found with slight silicification both within and outside the annulus (). Non-synchronous development of sibling valves was observed in early stages: one valve displayed a prominent annulus and several radiating ribs while the other had a more developed rib structure ().
Figs 5–8. Plagiogrammopsis vanheurckii, strain s0433 () and strain s0435 (). Early stages of valve morphogenesis, scanning electron microscopy. . Valve with a distinct annulus, in which silica deposition has not commenced. . Pair of sibling valves showing different stages of development. Arrow indicates annulus of younger valve. . Enlarged view of the annulus shown in . . Valve with distinct annulus, in which silica deposition is nearly complete. Scale bars: 5 µm () and 2 µm ().
Valve morphogenesis
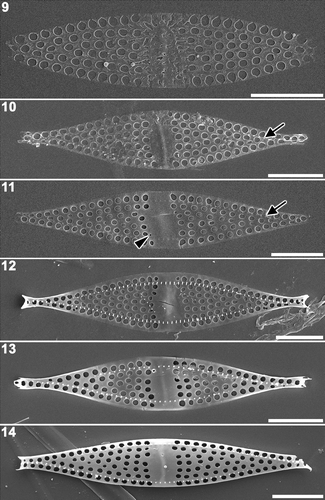
Silica deposition inside the annulus proceeded in a sporadic manner forming an irregular structural pattern (). Once the area within the annulus had become fully silicified, the abutting peripheral regions were also silicified, creating a fascia (). When two-dimensional expansion was accomplished to form the lanceolate valve, three-dimensional development was initiated to form the valve apices, marginal spines, rimoportula and the valve margin (). Both the apices and the marginal spines were formed almost simultaneously, though the bases of the spines were already formed, as small knobs along the valve mantle (). Rimoportula formation was observed at the start of the three-dimensional development of the valve margin (). A valve with fully developed margins had a dome-like valve surface ().
Figs 9–14. Plagiogrammopsis vanheurckii, strain s0432. Middle stages of valve morphogenesis, external view, scanning electron microscopy. . Silica deposition nearly completed inside annulus. . Plain disc at centre. Note presence of pseudoseptum beneath the disk. Arrow indicates row of base of marginal spines. . Fascia at centre. Arrow indicates base of marginal spines. Arrowhead indicates small areola where rimoportula will be formed. . Apex formation. Note distinct row of marginal spines. . Valve margin thickening. . Fully developed valve face and mantle, but apices not yet formed. Scale bars: 5 µm.
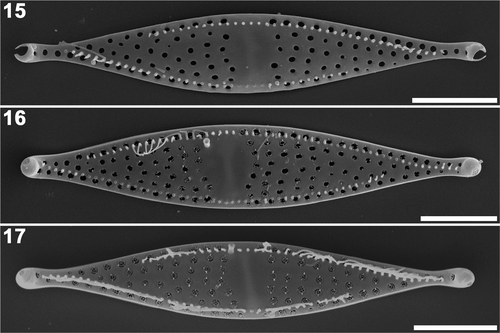
The valve apices were defined by the capitate outline (), after which the ocelluli, the discrete pore areas responsible for mucilage secretion, are formed (). Cribra were also formed in this final stage of valve morphogenesis (). The formation of cribra started near the centre of the valve, spreading toward the apices (). The formation of the pili was not observed in this study.
Figs 15–17. Plagiogrammopsis vanheurckii, strain s0434 () and strain s0432 (). Final stages of valve morphogenesis, external view, scanning electron microscopy. . Valve apex formation, ocelluli still unformed (strain s0434). . Almost mature valve during cribrum formation (strain s0432). . Mature valve (strain s0434). Scale bars: 5 µm.
Pseudoseptum formation
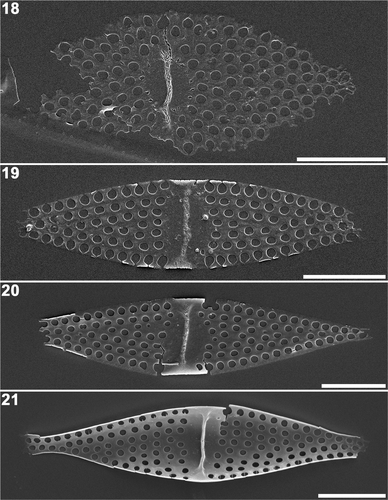
Internal views of the valves revealed that the pseudoseptum had already formed on the plain disc or annulus as the centripetal/bipolar expansion of the valve was proceeding (). As the disc became the fascia, the pseudoseptum expanded transapically (). Thickening of the valve margin first occurred around the valve centre () while the internal process of the rimoportulae was often formed after valve margin development was completed ().
Figs 18–21. Plagiogrammopsis vanheurckii, strain s0433 () and strain s0432 (). Middle stages of valve morphogenesis, internal view, scanning electron microscopy. . Valve during radial expansion with pseudoseptum on the central disc. . Marginal thickening started around fascia. Note the valve margin is incomplete. . Lanceolate valve outline complete. . Three-dimensional valve framework complete. Note rimoportula on right hand side of pseudoseptum. Scale bars: 5 µm.
Areola formation
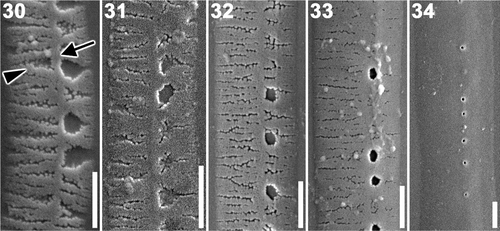
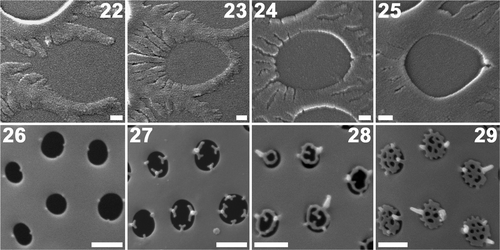
A detailed study of the formation of the areolae and cribra was undertaken (). Centrifugal extension of semicircular arms started at the centripetal side of an areola (). The tips of the arms fused at the opposite side to define the outline of the areola (), after which thickening of the areola margin proceeded centripetally, from the fused end to the starting point (). The bases of cribra were recognizable as knobs at very early stages of cribrum formation (). Cribra developed centripetally from the basal knobs (). Spines, small vertical projections at the junction of the knobs and the cribrum, were sometimes observed ().
Figs 22–29. Plagiogrammopsis vanheurckii, strain s0432. Formation of areolae () and cribra (), scanning electron microscopy. Valve centre lies on the left side in . . Centrifugal extension of two semi-circular arms. . Tips of arms fuse at opposite side to form areola. . Thickening starts at fused site. . Thickening proceeds centripetally. . Bases of cribra seen as knobs. . Centripetal cribrum formation. . Mature cribra. Scale bars: 0.1 () and 0.5 µm ().
Girdle band formation
In the early stages of formation girdle bands consisted of a longitudinal rib and many transverse ones (). One side of the longitudinal rib, where areolae would later form, had shorter transverse ribs than the other side (). As silicification of the transverse ribs proceeded, ribs gradually fused with one another to form the areolae ().
Discussion
Pattern centre
The present observations demonstrate that the bipolar centric P. vanheurckii possesses an annulus as its pattern centre in the early stages of valve morphogenesis. However, the annulus is totally masked by subsequent silica deposition, making it impossible to detect in mature valves of this species.
This is the first observation of an annulus in the immature valve of a bipolar centric, although the presence of an annulus has been noted in mature and/or developing valves of some genera, e.g. Bellerochea Van Heurck and Streptotheca Shrubsole in von Stosch (Citation1977), Papiliocellulus Hasle, von Stosch et Syvertsen in Hasle et al. (Citation1983), Ditylum Bailey in Li & Volcani (Citation1985a ), Chaetoceros Ehrenberg in Li & Volcani (Citation1985b ) and Assmy et al. (Citation2008), Attheya West and Eucampia Ehrenberg in Round et al. (Citation1990).
Based on fossil and molecular phylogenetic studies, the sternum of araphid diatoms presumably evolved from the annulus of bi-/multipolar centrics sometime in the Mesozoic (e.g. Sims et al., Citation2006; Sorhannus, Citation2007). A possible intermediate state would be an elongated annulus. To date, no such pattern centre has been detected in any diatoms, except for some very elongated centrics, i.e. Ardissonea Notaris, Climacosphenia Ehrenberg and Toxarium Bailey. These possess elongated pattern centres (bifacial annuli sensu Mann, Citation1984) but nuclear small subunit rDNA phylogenies suggest that their elongated valve (viz., their elongated pattern centre) evolved independently of the pennate lineage (Medlin et al., Citation2008). Recently, a single chain comprising several rings, which seem homologous to the annulus, was found in the araphid diatom Fragilaria Lyngbye (Mayama, unpubl.). This structure could be an intermediate state, although Synedra acus subsp. radians (Kützing) Skabitch, a closely related species, has a simple sternum (Kaluzhnaya & Likhoshway, Citation2007). The origin and evolution of the sternum remains to be fully explored.
Non-synchronous sibling valve morphogenesis
In this study, non-synchronous development of sibling valves was observed early in valve morphogenesis. However, it remains unknown whether the heterovalvate nature of P. vanheurckii is related to this phenomenon because heterovalvy in this taxon is only determined by the presence or absence of pilus/rimoportula. Non-synchronous development has been reported in some more markedly heterovalvate species, e.g. Gephyria media Arnott (Tiffany, Citation2002), Achnanthes coarctata (Brébisson) Grunow (Boyle et al., Citation1984), A. inflata (Kützing) Grunow (Idei et al., Citation2006) and some species of Cocconeis Ehrenberg, C. convexa Giffen, C. heteroidea Hantzsch and C. scutellum Ehrenberg var. parva (Grunow) Cleve (Suzuki et al., Citation2002 and their unpublished observations).
It is logical that heterovalvate species could exhibit non-synchronicity, because the different structures of the sibling valves probably require different morphogenetic pathways. Slight non-synchronicity of the sibling valve development was also observed in a biraphid diatom, Craspedostauros australis Cox (Cox, Citation1999), suggesting that slight non-synchronicity of the development of sibling valves is not exclusive to heterovalvate diatoms, or that there is subtle heterovalvy in C. australis.
Ocellulus
The ocellulus was formed in the final stages of valve morphogenesis (). On the other hand, the formation of the apical pore field in G. media (Tiffany, Citation2002), Gomphonema truncatum Ehrenberg (Cox, Citation1999) and S. acus subsp. radians (Kaluzhnaya & Likhoshway, Citation2007) took place relatively earlier in their valve morphogenesis. Both structures, the ocellulus and apical pore fields, are responsible for the mucilage secretion, although it is unclear whether these are homologous structures. It should be noted that mucilage secretion from the ocelluli of P. vanheurckii has not been reported despite detailed observations (Hasle et al., Citation1983; Round et al., Citation1990; Gardner & Crawford, Citation1994).
Girdle band formation
In the early stages of morphogenesis girdle bands comprised a longitudinal and transverse rib system. Interestingly, a similar primary rib system can be seen in valve morphogenesis of the araphid diatoms, G. media (Tiffany, Citation2002), S. acus subsp. radians (Kaluzhnaya & Likhoshway, Citation2007) and Rhaphoneis amphiceros (Ehrenberg) Ehrenberg (Sato, unpubl. obs). Most other araphid diatoms possibly have this too. In spite of the morphological similarity between araphid valves and the girdle band formation of centrics, any evolutionary link is unknown.
Unfortunately, diatom girdle band formation has scarcely been reported, partly because it is often difficult to determine which girdle bands belong to which taxon in the assemblages of fragmentary and broken frustules produced by oxidative cleaning of natural populations (Mann & Chepurnov, Citation2004; Tiffany, Citation2005). However, using a sample dominated by a bipolar centric Isthmia nervosa Kützing, Tiffany (Citation2005) successfully observed band formation and showed that early bands comprised a longitudinal rib with transverse ones growing in a unidirectional manner. On the other hand, the longitudinal rib of P. vanheurckii is more or less central along the early bands, and its bidirectional expansion of transverse ribs suggests that band formation is not uniform in diatoms.
Further investigations of other diatom species are clearly needed for a better understanding of the nature of girdle band formation and to elucidate the evolutionary relationship between the valve of araphids and the bands of centrics.
Wing and mantle border of P. vanheurckii
The specimens examined here bore a wing on the tip of each pilus, although previous observations did not detect such a structure (Hasle et al., Citation1983; Gardner & Crawford, Citation1994). Gardner & Crawford (Citation1994) described a second species Plagiogrammopsis, P. mediaequatus Gardner & Crawford, partly based on the fact that the wings were only found on P. mediaequatus in their specimens. They also concluded that the wing shown in Round et al. (Citation1990, p. 301, fig. j) was of P. mediaequatus (Gardner & Crawford, Citation1994) although the other figures were clearly P. vanheurckii.
The present study revealed the morphological variability of pili, supporting the possibility that the wing in Round et al. (Citation1990) could belong to either P. vanheurckii or P. mediaequatus. Alternatively, the presence/absence of the wing may reflect the presence of intraspecific variation within the morphospecies P. vanheurckii, as reported for other diatoms, e.g. Pseudo-nitzschia (Amato et al., Citation2007; Casteleyn et al., Citation2008; Kaczmarska et al., Citation2008), Skeletonema (Medlin et al., Citation1991; Sarno et al., Citation2005, Citation2007; Zingone et al., Citation2005; Kooistra et al., Citation2007), and Sellaphora (Behnke et al., Citation2004; Mann et al., Citation2004; Evans et al., Citation2007), and also in other marine microalgae (e.g. Sáez et al., Citation2003).
Acknowledgements
The author is grateful to Linda K. Medlin for valuable discussion; Masahiko Idei, Shigeki Mayama and Hidekazu Suzuki for sharing their unpublished observations and Eileen J. Cox and two anonymous reviewers for their constructive suggestions.
References
- Amato , A , Kooistra , WHCF , Leviald Ghiron , JH , Mann , DG , Pröschold , T and Montresor , M . 2007 . Reproductive isolation among sympatric cryptic species in marine diatoms . Protist , 158 : 193 – 207 .
- Anonymous . 1975 . Proposals for a standardization of diatom terminology and diagnoses . Nov. Hedwig., Beih. , 53 : 323 – 354 .
- Assmy , P , Hernández-Becerril , DU and Montresor , M . 2008 . Morphological variability and life cycle traits of the type species of the diatom genus Chaetoceros, C. dichaeta . J. Phycol. , 44 : 152 – 163 .
- Behnke , A , Friedl , T , Chepurnov , VA and Mann , DG . 2004 . Reproductive compatibility and rDNA sequence analyses in the Sellaphora pupula species complex (Bacillariophyta) . J. Phycol. , 40 : 193 – 208 .
- Boyle , JA , Pickett-Heaps , DJ and Czarnecki , DB . 1984 . Valve morphogenesis in the pennate diatom Achnanthes coarctata . J. Phycol. , 20 : 563 – 76 .
- Casteleyn , G , Chepurnov , V , Leliaert , F , Mann , DG , Bates , SS , Lundholm , N , Rhodes , L , Sabbe , K and Vyverman , W . 2008 . Pseudo-nitzschia pungens (Bacillariophyceae): a cosmopolitan species? . Harmful Algae , 7 : 241 – 257 .
- Cox , EJ . 1999 . Variation in patterns of valve morphogenesis between representatives of six biraphid diatom genera . J. Phycol. , 35 : 1297 – 1312 .
- Cox , EJ and Kennaway , GM . 2004 . “ Studies of valve morphogenesis in pennate diatoms: investigating aspects of cell biology in a systematic context ” . In Proceedings of the 17th International Diatom Symposium, Ottawa, Canada , Edited by: Poulin , M . 35 – 48 . Bristol, , UK : Biopress Ltd .
- Eppley , RW , Holmes , RW and Strickland , JDH . 1967 . Sinking rates of the marine phytoplankton measured with a fluorochrometer . J. Exp. Mar. Biol. Ecol. , 1 : 191 – 208 .
- Evans , KM , Wortley , AH and Mann , DG . 2007 . An assessment of potential diatom ‘‘barcode’’ genes (cox1, rbcL, 18S and ITS rDNA) and their effectiveness in determining relationships in Sellaphora (Bacillariophyta) . Protist , 158 : 349 – 364 .
- Gardner , C and Crawford , RM . 1994 . A description of Plagiogrammopsis mediaequatus Gardner & Crawford, sp. nov. (Cymatosiraceae, Bacillariophyta) using light and electron microscopy . Diatom Res. , 9 : 53 – 63 .
- Hasle , GR , von Stosch , HA and Syvertsen , EE . 1983 . Cymatosiraceae, a new diatom family . Bacillaria , 6 : 9 – 156 .
- Idei , M , Nagumo , T and Mayama , S . 2006 . “ Morphogenesis in a monoraphid diatom Achnanthes inflata (Kützing) Grunow ” . In 19th International Diatom Symposium, Abstracts Book , Edited by: Likhoshway , YV and Crawford , RM . 59 Russia : Irkutsk .
- Kaczmarska , I , Reid , C , Martin , JL and Moniz , MBJ . 2008 . Morphological, biological, and molecular characteristics of the diatom Pseudo-nitzschia delicatissima from the Canadian Maritimes . Botany , 86 : 1 – 10 .
- Kaluzhnaya , OV and Likhoshway , YV . 2007 . Valve morphogenesis in an araphid diatom Synedra acus subsp. radians . Diatom Res. , 22 : 81 – 87 .
- Kooistra , WHCF , Sarno , D , Balzano , S , Gu , H , Andersen , RA and Zingone , A . 2007 . Global diversity and biogeography of Skeletonema species (Bacillariopyta) . Protist , 159 : 177 – 193 .
- Li , C-W and Volcani , BE . 1985a . Studies on the biochemistry and fine structure of silica shell formation in diatoms. VIII. Morphogenesis of the cell wall in a centric diatom, Ditylum brightwellii . Protoplasma , 124 : 10 – 29 .
- Li , C-W and Volcani , BE . 1985b . Studies on the biochemistry and fine structure of silica shell formation in diatoms. IX. Sequential valve formation in a centric diatom, Chaetoceros rostratum . Protoplasma , 124 : 30 – 41 .
- Mann , DG . 1984 . “ An ontogenetic approach to diatom systematics ” . In Proceedings of the 7th International Diatom Symposium , Edited by: Mann , DG . 113 – 144 . Koenigstein, , Germany : O. Koeltz .
- Mann , DG and Chepurnov , VA . 2004 . What have the Romans ever done for us? The past and future contribution of culture studies to diatom systematics . Nov. Hedwig. , 79 : 237 – 291 .
- Mann , DG , McDonald , SM , Bayer , MM , Droop , SJM , Chepurnov , VA , Loke , RE , Ciobanu , A and Du Buf , JMH . 2004 . Morphometric analysis, ultrastructure and mating data provide evidence for five new species of Sellaphora (Bacillariophyceae) . Phycologia , 43 : 459 – 482 .
- Mayama , S and Kuriyama , A . 2002 . Diversity of mineral cell coverings and their formation processes: a review focused on the siliceous cell coverings . J. Plant. Res. , 115 : 289 – 295 .
- Medlin , LK , Elwood , HJ , Stickel , S and Sogin , ML . 1991 . Morphological and genetic variation within the diatom Skeletonema costatum (Bacillariophyta): evidence for a new species, Skeletonema pseudocostatum . J. Phycol. , 27 : 514 – 524 .
- Medlin , LK and Kaczmarska , I . 2004 . Evolution of the diatoms: V. Morphological and cytological support for the major clades and a taxonomic revision . Phycologia , 43 : 245 – 270 .
- Medlin , LK , Sato , S , Mann , DG and Kooistra , WHCF . 2008 . Molecular evidence confirms sister relationship of Ardissonea, Climacosphenia and Toxarium within the bipolar centric diatoms (Bacillariophyta, Mediophyceae) and cladistic analyses confirms that extremely elongated shape has arisen twice in the diatoms . J. Phycol. , 44 : 1340 – 1348 .
- Round , FE , Crawford , RM and Mann , DG . 1990 . The Diatoms. Biology and Morphology of the Genera , Cambridge, , UK : Cambridge University Press .
- Sáez , AG , Probert , I , Geisen , M , Quinn , P , Young , JR and Medlin , LK . 2003 . Pseudocryptic speciation in coccolithophores . Proc. Natl. Acad. Sci. USA , 100 : 7163 – 7168 .
- Sarno , D , Kooistra , WHCF , Hargraves , PE and Zingone , A . 2007 . Diversity in the genus Skeletonema (Bacillariophyceae). III. Phylogenetic position and morphology of Skeletonema costatum and Skeletonema grevillei, with the description of Skeletonema ardens sp. nov . J. Phycol. , 43 : 156 – 170 .
- Sarno , D , Kooistra , WCHF , Medlin , LK , Percopo , I and Zingone , A . 2005 . Diversity in the genus Skeletonema (Bacillariophyceae). II. An assessment of the taxonomy of S. costatum-like species, with the description of four new species . J. Phycol. , 41 : 151 – 176 .
- Sims , PA , Mann , DG and Medlin , LK . 2006 . Evolution of the diatoms: insights from fossil, biological and molecular data . Phycologia , 45 : 361 – 402 .
- Sorhannus , U . 2007 . A nuclear-encoded small-subunit ribosomal RNA timescale for diatom evolution . Mar. Micropaleontol. , 65 : 1 – 12 .
- Sterrenburg , FAS , Tiffany , MA and Meave del Castillo , ME . 2005 . Valve morphogenesis in the diatom genus Pleurosigma W. Smith (Bacillariophyceae): nature's alternative sandwich . J. Nanosci. Nanotechnol. , 5 : 140 – 145 .
- Suzuki , H , Osada , K and Nagumo , T . 2002 . “ Valve development in Cocconeis ” . In 17th International Diatom Symposium, Abstracts Book , Edited by: Poulin , M . 140 Canada : Ottawa .
- Tiffany , MA . 2002 . Valve morphogenesis in the marine araphid diatom Gephyria media (Bacillariophyceae) . Diatom Res. , 17 : 391 – 400 .
- Tiffany , MA . 2005 . Diatom auxospore scales and early stages in diatoms frustule morphogenesis: their potential for use in nanotechnology . J. Nanosci. Nanotechnol. , 5 : 131 – 139 .
- Tiffany , MA . 2008 . Valve development in Aulacodiscus . Diatom Res. , 23 : 185 – 212 .
- Tiffany , MA and Hernandez-Becerril , DU . 2005 . Valve morphogenesis in the diatom family Asterolampraceae H . L. Smith. Micropaleontology , 51 : 217 – 258 .
- von Stosch , HA . 1977 . Observations on Bellerochea and Streptotheca, including descriptions of three new species of planktonic diatoms . Nov. Hedwig., Beih. , 54 : 113 – 166 .
- Zingone , A , Percopo , I , Sims , PA and Sarno , D . 2005 . Diversity in the genus Skeletonema (Bacillariophyceae). I. A re-examination of the type material of Skeletonema costatum, with the description of S. grevillei sp. nov . J. Phycol. , 41 : 140 – 150 .