Abstract
Biological invasions have attracted particular attention since they often result in serious consequences for natural ecosystems. One planktonic invasive species is Cylindrospermopsis raciborskii, a cyanobacterium originally reported to occur exclusively in the tropics. Over the last few decades its range has extended to temperate regions and it occupies shallow highly eutrophic lakes previously dominated by other cyanobacteria. The purpose of this study was to examine the ecology of C. raciborskii during Planktothrix agardhii blooms in two shallow lakes in western Poland and to determine whether these species have different environmental preferences. Multiple linear regression showed that the biomass of P. agardhii was significantly negatively related to Secchi depth in Lake Bnińskie. In Lake Bytyńskie, P. agardhii was significantly positively related to concentrations of , chlorophyll a and total phosphorus and negatively related to Secchi depth,
, and total nitrogen. Cylindrospermopsis raciborskii was significantly positively related only to concentrations of
. There was a negative correlation between the biomass of P. agardhii and C. raciborskii perhaps showing different responses to environmental variables. Moreover, the biomass of P. agardhii was negatively correlated with Shannon–Wiener diversity of the phytoplankton assemblages. Our results support the concept that these cyanobacterial species have different environmental preferences and their niches differ from each other. These results suggest that light is an important driver of phytoplankton community structure resulting in shifts from a community dominated by P. agardhii in very turbid waters to more diverse communities perhaps including the invasive C. raciborskii in clearer waters.
Introduction
Biological invasions are considered a major threat to global biodiversity and are an important part of ongoing global climate change. The number of invasions has grown considerably in recent decades, many of them driven by human activities that diminish natural barriers to dispersal (Wells et al., Citation1986; Dukes & Mooney, Citation1999). At present only a few habitats remain free of species introduced by humans (Fridriksson & Magnusson, Citation1992). Ecological consequences of biotic invasions vary enormously, but they often result in the altered identity and functioning of natural communities (Mack et al., Citation2000). Invaders may severely affect the ecosystems by modifying the species dominance structure, nutrient dynamics and levels of primary productivity (Bertness, Citation1984; Vitousek, Citation1990).
Successful invasions accompany the major components of global change that include increasing temperatures (Walther, Citation2003), nitrogen deposition, and CO2 concentration, plus changing patterns of land use or disturbance regimes (Dukes & Mooney, Citation1999). Aquatic ecosystems are especially sensitive to these changes, which often include more frequent cyanobacterial blooms. Rising temperatures favour cyanobacteria both directly, as they grow faster at higher temperatures during periods of water stratification, and indirectly by changing patterns of precipitation and drought (Paerl & Huisman, Citation2008). These changes may also allow for the establishment of invasive cyanobacterial species.
Among the many examples of biological invasions is the expansion of the cyanobacterium Cylindrospermopsis raciborskii (Woloszyńska) Seenayya & Subba Raju in temperate aquatic ecosystems. This species, common in the tropics, has been observed in temperate regions over the last two decades (Pádisak, Citation1997; Wiedner et al., Citation2002; Saker et al., Citation2003; Manti et al., Citation2005). Recently, it was found in shallow, eutrophic, polymictic lakes of the Wielkopolska region in western Poland, often co-occurring with the native species Planktothrix agardhii (Gomont) Anagnostidis & Komárek (Stefaniak & Kokociński, Citation2005). These species are planktonic, filamentous cyanobacteria belonging to the Nostocales and Oscillatoriales, respectively. Both species are of great concern due to their ability to produce toxins harmful to humans and animals. Planktothrix agardhii is known for producing a variety of microcystins (Carmichael, Citation1994; Chorus, Citation2001), while C. raciborskii is able to synthesize the neurotoxin saxitoxin (Lagos et al., Citation1999; Castro et al., Citation2004) and the alkaloid hepatotoxin cylindrospermopsin (Ohtani et al., Citation1992; Li et al., Citation2001).
Although C. raciborskii and P. agardhii are able to grow under varied conditions, their environmental requirements probably differ. Temperature has been considered the major limiting factor for growth of C. raciborskii (Pádisak, Citation1997; Wiedner et al., Citation2007), with its optimal growth rate in laboratory experiments being at 25–35°C (Saker & Griffiths, Citation2000; Briand et al., Citation2004; Castro et al., Citation2004; Chonudomkul et al., Citation2004). Furthermore, its akinete germination is temperature dependent, and germination occurs primarily from 22–23.5°C (Pádisak, Citation1997). In contrast, P. agardhii can grow well at much lower temperatures, 6–16°C (Berger, Citation1989; Dokulil & Teubner, Citation2000) and long-lasting populations have been observed in highly eutrophic reservoirs even during winter (Berger, Citation1975; Post et al., Citation1985; Nixdorf, Citation1994; Pouličkova et al., Citation2004). Nonetheless, it is well known that P. agardhii populations develop optimally in late summer or autumn, because it prefers warm waters above 20°C (Van Liere & Mur, Citation1980; Ramberg, Citation1988).
Population densities of phytoplankton species are also affected by light intensity. Based on earlier findings, it was suggested that C. raciborskii can benefit from low light intensities (Dokulil & Mayer, Citation1996; Pádisak & Reynolds, Citation1998; Bouvy et al., Citation1999; Briand et al., Citation2002b ; Mischke & Nixdorf, Citation2003). However, recent experimental studies on tropical and temperate C. raciborskii isolates showed no significant differences in growth rates at different light intensities (Briand et al., Citation2004). Planktothrix agardhii is also associated with turbid waters, and is a common species in shallow, polymictic lakes with high disturbance frequency (Rücker et al., Citation1997; Scheffer et al., Citation1997; Nixdorf et al., Citation2003). Light and temperature and their interactions are currently gaining attention since global warming has increased water temperatures in lakes during spring in addition to altering physical conditions (George et al., Citation2000; Gerten & Adrian, Citation2000). Moreover, relationships between temperature and photoperiod linked to climate warming may influence phytoplankton composition (Nicklisch et al., Citation2008).
Nutrient concentrations also have a role in structuring phytoplankton communities. Cylindrospermopsis raciborskii is characterized by its association with high TN/TP ratios (Briand et al., Citation2002b ), while P. agardhii appears to be favoured by low TN/TP ratios (Rücker et al., Citation1997). Moreover, both species are able to develop very abundant populations or even form intense blooms (Berger, Citation1975, Citation1989; De Souza et al., Citation1998; Fonseca & De M. Bicudo, Citation2008). Occurrence of C. raciborskii with mass development of P. agardhii is known in Polish lakes (Stefaniak & Kokociński, Citation2005; Pełechata et al., Citation2006; Kokociński et al., Citation2009) but documented examples of abundant coexistence of these species are sparse (Mischke & Nixdorf, Citation2003; Crossetti & De M. Bicudo, Citation2008).
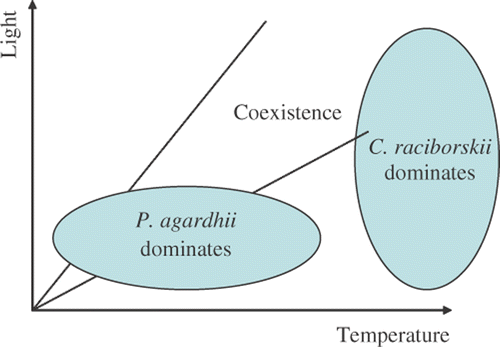
Although the autecology of these species is now better known, the relationships between their environment and their occurrence in temperate lakes is less understood. Therefore, the objectives of this study are: (i) to examine which environmental factors drive the temporal patterns in phytoplankton communities in the two lakes studied; (ii) to examine which environmental factors control C. raciborskii and P. agardhii concentrations and whether the species respond differently to environmental factors; and (iii) determine whether these two species coexist or show clear negative correlations in their abundances. We predict that growth of C. raciborskii will be favoured by high temperatures and high light intensities, while P. agardhii is associated with reduced light intensities and lower temperatures ().
Materials and methods
Sampling sites
The study was conducted in two lakes, Bnińskie (hereafter ‘Lake BN’) and Bytyńskie (hereafter ‘Lake BY’), located near the city of Poznań in the Wielkopolska region of western Poland. Both lakes are shallow (maximum depth <8.5 m), polymictic and highly eutrophic, surrounded by agricultural catchments, with public recreational usage. Their average depths are 4.2 and 3.5 m respectively, with other features given in .
Table 1. Physical characteristics of Lake Bnińskie (BN) and Lake Bytyńskie (BY) in western Poland.
Sampling
Sampling was carried out twice a month from July to October each year from 2004 to 2007. Altogether 32 samples were collected from each lake during the study. As no thermal or oxygenation stratification was observed, and following previous studies in shallow lakes (Berger, Citation1975; Romo & Miracle, Citation1993, Citation1994; Briand et al., Citation2002a ; Ott et al., Citation2003) phytoplankton samples were collected from 0.5 m below the surface of the water. Samples for phytoplankton and chemical analysis were collected using a 5 l Limnos water sampler at the deepest point of the reservoir. The phytoplankton samples were fixed with acidified Lugol's solution (Wetzel & Likens, Citation2000) immediately after sampling. Then, formaldehyde was added as a preservative and samples were stored under cool and dark conditions until counted. Before counting, samples were sedimented in a 1 l glass cylinder for 48 h, after which the overlying water was gently decanted off and the lower layer (volume 40 ml) was used for phytoplankton analyses.
Phytoplankton analysis
Phytoplankton identification and counts were conducted using a Zeiss light microscope (magnification 400×). The phytoplankton counts were carried out in 160 fields of the Fuchs-Rosenthal chamber, which ensured that at least 400 specimens were counted to reduce the error to less than 10% (P = 0.05; Javornicky, Citation1958). Single cells, coenobia or filaments were considered as one specimen in this study. We assumed 100 µm to be the standard length for filaments and 100 cells the size of large spherical colonies. The biovolume of each species was determined via volumetric analysis of cells using geometric approximation and expressed as wet weight following Hindak (Citation1978) and Wetzel & Likens (Citation2000). For each species geometrical dimensions of at least 30 individuals were measured to obtain a mean value. The diversity was calculated using the Shannon–Wiener diversity index (Shannon, Citation1948) based on phytoplankton biomass.
Chemical and physical analysis
Water samples for chemical analyses were collected at the same time as the phytoplankton samples, and were analysed for ammonium nitrogen (), nitrate nitrogen (
), nitrite nitrogen (
), total nitrogen (TN), orthophosphate phosphorus (
) and total phosphorus (TP) by using a HACH Spectrophotometer. For chlorophyll a (chl a) analysis, 200 ml of water was filtered through a GF/C Whatman filter. The concentration was determined spectrophotometrically following the Lorenzen method after 90% acetone extraction and corrected for phaeopigments (Wetzel & Likens, Citation2000). During field sampling, water temperature, pH and conductivity were determined using a multiparameter Elmetron CPC-401 probe. Water transparency was measured each time using a Secchi disc.
Statistical analyses
Prior to analyses, rare phytoplankton taxa, i.e. those that occurred in only one sample, were excluded from the ordinations. Environmental variables (except pH) were log-transformed to better approximate multivariate normality. We first used canonical correspondence analysis (CCA) to examine the temporal patterns of the entire phytoplankton community along major environmental gradients. CCA is a direct gradient analysis that uses both species and environmental data by combining ordination and regression techniques (Ter Braak, Citation1986; Legendre & Legendre, Citation1998). We used forward selection of environmental variables. At each step, only variables significantly (P < 0.05; Monte Carlo randomization test with 499 permutations) related to assemblage structure were included in the model. CCA was run using CANOCO version 4.0 (Ter Braak & Smilauer, Citation1998).
We then analysed more closely which of the environmental variables were controlling the P. agardhii and C. raciborskii populations by using stepwise multiple regression. We used the probability level of 0.05 as the limit for entry into the model. Before analysis, we excluded variables that showed strongest (r > 0.80) co-linearity with other variables.
Spearman correlation analysis was carried out using Statistica 7.1 to examine the correlation between biomass of P. agardhii and C. raciborskii. We then related the biomass of P. agardhii to phytoplankton species richness using linear regression analysis.
Results
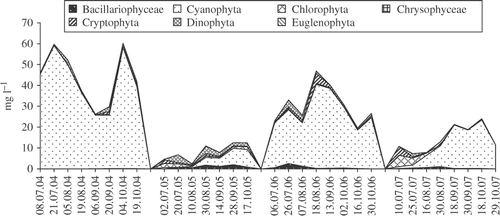
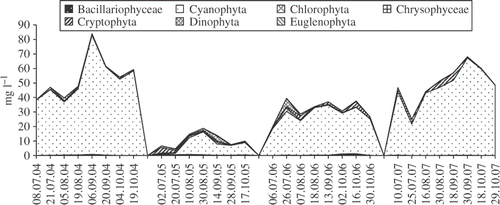
Throughout the study in both lakes the phytoplankton communities were dominated by cyanobacteria which contributed over 80% of the total phytoplankton biomass in most of the samples (Figs ). Planktothrix agardhii was the dominant species in both lakes with Limnothrix redekei (Van Goor) Meffert being subdominant. Beside these two species, other common cyanobacteria in the samples were Pseudanabaena limnetica (Lemmermann) Komárek (Oscillatoriales) and Aphanizomenon flos-aquae Brébisson ex Bornet & Flahault, Aphanizomenon gracile Lemmermann and C. raciborskii (Nostocales).
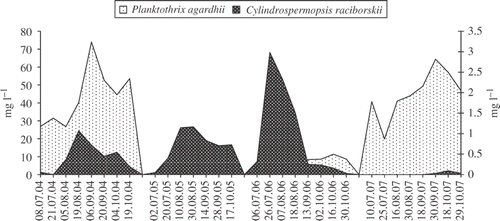
Fig. 3. Biomass of Planktothrix agardhii and Cylindrospermopsis raciborskii in Lake Bnińskie from July to October in the years 2004–2007.
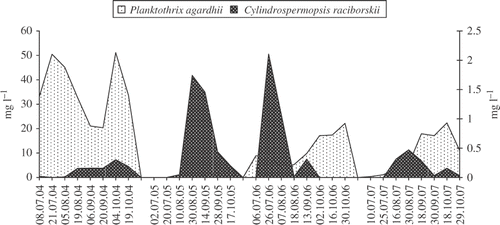
Fig. 5. Biomass of Planktothrix agardhii and Cylindrospermopsis raciborskii in Lake Bytyńskie from July to October in the years 2004–2007.
Chlorophyta, Bacillariophyceae and other algal groups were less abundant, never dominating the communities. Higher concentrations of algae other than cyanobacteria were observed only in 2005 when the total phytoplankton biomass was the lowest over the study period. During the beginning of the sampling period, there was an increased total biomass of Chlorophyta and Dinophyta in Lake BN and of Chlorophyta and Cryptophyta in Lake BY. Similarly in 2007, Chlorophyta and Cryptophyta were relatively common in Lake BN. The dominant species were Coelastrum microporum Näg. in A. Braun, Oocystis lacustris Chodat, Scenedesmus ecornis (Ehr. ex Ralfs) Chodat, Monoraphidium griffithii (Berk.) Kom.-Legn. (Chlorophyta), Cryptomonas erosa Ehrenberg, Cryptomonas reflexa (M. Marsson.) Skuja, Cryptopmonas marsonii Skuja, Rhodomonas minuta Skuja (Cryptophyta) and Peridiniopsis berolinense (Lemm.) Bourrelly (Dinophyta).
The biomass of C. raciborskii in Lake BY was generally slightly higher than in Lake BN during the period studied, with the exception of 2007. The highest concentration of C. raciborskii in both lakes occurred in 2006 (Figs ) but the highest contribution of C. raciborskii to the total phytoplankton biomass was in 2005 when it contributed 18.5% of the total biomass in Lake BN and 9.3% in Lake BY.
In Lake BN, the eigenvalues of the first two CCA axes were 0.399 and 0.338 and significant (P < 0.05, ). The first two CCA axes jointly explained 31.8% of the total variance (2.00) in the phytoplankton communities. The species–environment correlations for CCA axes were relatively high, indicating a strong relationship between phytoplankton and the environmental variables. Secchi depth, chl a, and the TN/TP ratio were the most significant contributors to CCA axis 1 (). CCA axis 2 was most strongly correlated with concentrations, temperature and water pH.
Fig. 6. Ordination diagram for Canonical Correspondence Analysis of the phytoplankton assemblages in Lake Bnińskie.
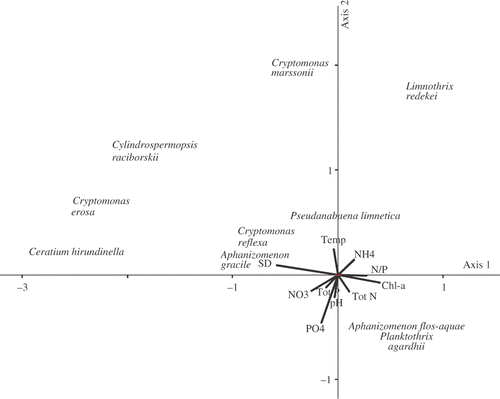
Table 2. The results of Canonical Correspondence Analyses (CCA) with forward selected variables (P < 0.05) for Lake Bnińskie (BN) and Lake Bytyńskie (BY).
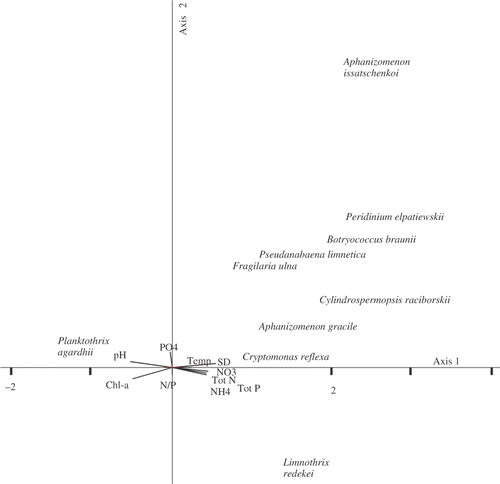
In Lake BY the eigenvalues of the first two CCA axes were 0.462 and 0.208 and significant (P < 0.05, ). The first two axes jointly explained 35.2% of the total variance (1.53) in the communities. The species-environment correlations for CCA axes were also high in Lake BY, indicating a relatively strong relationship between phytoplankton and the environmental variables. Secchi depth, pH, chl a, and the amount of total nutrients were the most significant contributors to CCA axis 1, with , chl a and TN/TP ratio contributing most to CCA axis 2 ().
Fig. 7. Ordination diagram for Canonical Correspondence Analysis of the phytoplankton assemblages in Lake Bytyńskie.
According to multiple regression analyses, biomass of P. agardhii was significantly (P < 0.001) negatively related to Secchi depth in Lake BN (). In Lake BN, C. raciborskii was not related to any of the measured environmental variables. In Lake BY P. agardhii was significantly (P < 0.001) positively related to , chl a and TP; and negatively related to Secchi depth,
, and TN. C. raciborskii was also significantly (P < 0.001) positively related to
.
Table 3. The results of multiple regression analyses for Lake Bnińskie (BN) and Lake Bytyńskie (BY) showing the main environmental factors related to the abundance of P. agardhii and C. raciborskii.
According to the Spearman correlation analysis, P. agardhii was significantly negatively correlated (r = 0.149, p < 0.05) with C. raciborskii in Lake BN. Planktothrix agardhii was significantly negatively correlated (r = 0.438, P < 0.05) with C. raciborskii in Lake BY. In addition, P. agardhii was significantly negatively correlated (r = 0.942, P < 0.05) with Shannon–Wiener diversity in Lake BN and in Lake BY (r = 0.781, P < 0.05). Cylindrospermopsis raciborskii was significantly positively correlated (r = 0.542) with Shannon–Wiener diversity in Lake BY. In Lake BN, the correlation of C. raciborskii with Shannon–Wiener diversity was non-significant (r = 0.208).
Discussion
Oscillatoriales dominated the phytoplankton communities in both lakes. Although communities were strongly dominated by P. agardhii, L. redekei was also abundant, thus supporting findings by Rücker et al. (Citation1997) that both species are often present in shallow and eutrophic waters, and compete successfully with other filamentous cyanobacteria since they tolerate very low light conditions. In addition to the Oscillatoriales, Nostocales such as Aphanizomenon flos-aquae, Aphanizomenon gracile and C. raciborskii were relatively abundant. Cylindrospermopsis raciborskii was often present and its abundance increased slightly after 2003 (Stefaniak & Kokociński, 2005). Its increased abundance is typical for many eutrophic temperate lakes (Nixdorf et al., Citation2003; Stüken et al., Citation2006). Cylindrospermopsis raciborskii reached its highest biomass during 2005, but biomass was nonetheless relatively low in comparison with other groups of algae.
The CCA indicated that turnover of the phytoplankton composition was controlled most strongly by changes in Secchi depth (suggesting differences in light intensity), water temperature, water pH and nutrient concentrations. The results are highly congruent with other studies where the importance of nutrient concentrations, temperature and light has been documented (Forsström et al., Citation2005; Grover & Chrzanowski, Citation2006; Kokociński & Soininen, Citation2008). Although P. agardhii and C. raciborskii often co-occurred, multiple regression analyses confirmed that these species responded differently to several environmental variables. For example, the importance of light intensity for regulating P. agardhii was supported given that Secchi depth was a significant explanatory variable of its biomass in both lakes. More specifically, the biomass of P. agardhii was negatively related to Secchi depth supporting earlier findings that it is able to grow exceedingly well under low light intensities (Rücker et al., Citation1997; Scheffer et al., Citation1997; Nixdorf et al., Citation2003). Moreover, the finding that the biomass of C. raciborskii was not related to Secchi depth was congruent with our initial expectation that C. raciborskii can grow under different light intensities and at high temperature (). Our findings also concur with Briand et al. (Citation2004) since there were no significant differences in growth rates of C. raciborskii at different light intensities.
Both species preferred nutrient-rich waters, with P. agardhii being positively related to TP and concentrations (in Lake BY), and C. raciborskii to
concentrations. The fact that C. raciborskii was not related to phosphorus may suggest that it has a high uptake and high storage capacity for phosphorus allowing it to grow under relatively low phosphate supply (Branco & Senna, Citation1994; Présing et al., Citation1996; Isvánovics et al., Citation2000; Briand et al., Citation2002b
). Recent experimental studies also showed that C. raciborskii is able to grow faster and sustain a high biomass under phosphorus limitation during a sufficient supply of
or
(Kenesi et al., Citation2009). In addition,
was shown to be effectively assimilated by C. raciborskii in previous experimental studies by Sprőber et al. (Citation2003). A positive relationship between C. raciborskii and
in our study seems to support these findings. In contrast, the growth of P. agardhii is favoured by higher concentrations of phosphorus and its dominance is especially marked in shallow, turbid lakes with intensive interaction between the sediment and water column (Nixdorf et al., Citation2003; Nöges & Jensen, Citation2003).
The different environmental requirements of C. raciborskii and P. agardhii were indicated by the regression analyses where their abundances had a negative relationship. At low light intensities and low temperature P. agardhii dominated the community, whereas C. raciborskii was favoured by higher temperatures and greater light intensities. The latter was evident, however, only in CCA where C. raciborskii was positively related to Secchi depth and water temperature. These findings are congruent with our initial predictions (). The finding that P. agardhii grows best in turbid waters is also in agreement with studies by Nixdorf et al. (Citation2003) who place P. agardhii in the phytoplankton functional group S1 (sensu Reynolds et al., Citation2002) of photoadapting filamentous cyanobacteria common in shallow, wind-exposed lakes with reduced light availability and intense sediment–water interactions. Although C. raciborskii never reached dominance, it increased in abundance when P. agardhii was less abundant.
It is feasible that in this situation C. raciborskii was able to out-compete P. agardhii, and the increasing number of successful invasions by C. raciborskii in temperate regions supports this view. This finding supports the hypothesis of Reynolds et al. (Citation2002) that P. agardhii and C. raciborskii belong to different functional groups. According to Pádisak & Reynolds (Citation1998) and Reynolds et al. (Citation2002) C. raciborskii has been allocated to the SN phytoplankton functional group characteristic of warm, mixed waters enriched with phosphorus. The Shannon–Wiener diversity was also consistently low when there was a high biomass of P. agardhii. In contrast, C. raciborskii was positively correlated with Shannon–Wiener diversity in Lake BY. These results indicate that P. agardhii can be considered as a steady-state species during the Oscillatoriaceae-dominated steady state in shallow lakes (Dokulil & Teubner, Citation2000; Scheffer et al., Citation1997; Scheffer, Citation1998) when few species are able to coexist (Nixdorf et al., Citation2003).
To conclude, the abundances of P. agardhii and the invasive C. raciborskii are negatively related, possibly because they have different environmental niches. As we initially predicted, P. agardhii is favoured by turbid conditions with elevated phosphorus concentrations, while the abundance of C. raciborskii is directly related to ammonium nitrogen and it prefers less turbid warmer waters. Our results may therefore shed more light on mechanisms promoting the invasion of C. raciborskii in temperate regions. Future studies are needed to examine if C. raciborskii is able to sustain large populations in temperate lakes and to out-compete the native cyanobacteria.
Acknowledgements
Financial support was provided by the Polish Ministry of Science and Higher Education through research grant No. N 304 051 31/1855 and by the Academy of Finland and Kone foundation (to JS). We thank Dr Harold G. Marshall for his English language revision and helpful comments on the manuscript.
References
- Berger , C . 1975 . Occurrence of Oscillatoria agardhii Gom. in some shallow eutrophic lakes . Verh. Internat. Verein. Limnol. , 19 : 2689 – 2697 .
- Berger , C . 1989 . In situ primary production, biomass and light regime in the Wolderwijd, the most stable Oscillatoria agardhii lake in the Netherlands . Hydrobiologia , 188 : 233 – 344 .
- Bertness , MD . 1984 . Habitat and community modification by an introduced herbivorous snail . Ecology , 65 : 370 – 381 .
- Bouvy , M , Molica , R , De , Oliveira , S , Marinho , M and Beker , B . 1999 . Dynamics of a toxic cyanobacterial bloom (Cylindrospermopsis raciborskii) in a shallow reservoir in the semi-arid region of northern Brazil . Aquat. Microb. Ecol. , 20 : 285 – 297 .
- Branco , CWC and Senna , PAC . 1994 . Factors influencing the development of Cylindrospermopsis raciborskii and Microcystis aeruginosa in the Paranoa Reservoir, Brasilia, Brazil . Algol. Stud. , 75 : 85 – 96 .
- Briand , JF , Robillot , C , Quiblier-Llobéras , C and Bernard , C . 2002a . A perennial bloom of Planktothrix agardhii (Cyanobacteria) in a shallow eutrophic French lake: limnological and microcystin production studies . Arch. Hydrobiol. , 153 : 605 – 622 .
- Briand , JF , Robillot , C , Quiblier-Liobéras , C , Humbert , JF , Couté , A and Bernard , C . 2002b . Environmental context of Cylindrospermopsis raciborskii (Cyanobacteria) blooms in shallow pond in France . Water Res. , 36 : 3183 – 3192 .
- Briand , JF , Leboulanger , C , Humbert , JF , Bernard , C and Dufour , P . 2004 . Cylindrospermopsis raciborskii (Cyanobacteria) invasion at mid-latitudes: selection, wide physiological tolerance, or global warming? . J. Phycol. , 40 : 231 – 238 .
- Carmichael , WW . 1994 . The toxins of Cyanobacteria . Sci. Am. , 270 : 64 – 70 .
- Castro , D , Vera , D , Lagos , N , Garcĭa , C and Vásquez , M . 2004 . The effect of temperature on growth and production of paralytic shellfish poisoning toxins by the cyanobacterium Cylindrospermopsis raciborskii C10 . Toxicon , 44 : 483 – 489 .
- Chonudomkul , D , Yongmanitchai , W , Theeragool , G , Kawachi , M , Kasai , F , Kaya , K and Watanabe , MM . 2004 . Morphology, genetic diversity, temperature tolerance and toxicity of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) strains from Thailand and Japan . FEMS Microbiol. Ecol. , 48 : 345 – 355 .
- Chorus, I. (2001). Cyanotoxins: Occurrence, Causes, Consequences. Springer-Verlag KG, Berlin.
- Crossetti , LO and De M. Bicudo , C . 2008 . Adaptations in phytoplankton life strategies to imposed change in a shallow urban tropical eutrophic reservoir, Garças Reservoir, over 8 years . Hydrobiologia , 614 : 91 – 105 .
- De Souza , RCR , Carvalho , MC and Truzzi , AC . 1998 . Cylindrospermopsis raciborskii (Wolosz.) Seenaya and Subba Raju (Cyanophyceae) dominance and a contribution to the knowledge of Rio Pequeno arm, Billings Reservoir, Brazil . Environ. Toxicol. Water Qual. , 13 : 73 – 81 .
- Dokulil , MT and Mayer , J . 1996 . Population dynamics and photosynthetic rates of a Cylindrospermopsis–Limnothrix association in highly eutrophic urban lake, Alte Donau, Austria . Algol. Stud. , 83 : 179 – 195 .
- Dokulil , MT and Teubner , K . 2000 . Cyanobacterial dominance in lakes . Hydrobiologia , 438 : 1 – 12 .
- Dukes , JS and Mooney , HA . 1999 . Does global change increase the success of biological invaders? . Trends Ecol. Evol. , 14 : 135 – 139 .
- Fonseca , BM and De M. Bicudo , CE . 2008 . Phytoplankton seasonal variation in a shallow stratified eutrophic reservoir (Garças Pond, Brazil) . Hydrobiologia , 600 : 267 – 282 .
- Forsström , L , Sorvari , S and Korhola , A . 2005 . Seasonality of phytoplankton in subarctic Lake Saanajärvi in NW Finnish Lapland . Polar Biol. , 28 : 846 – 861 .
- Fridriksson , S and Magnusson , B . 1992 . Development of the ecosystem on Surtsey with references to Anak Krakatau . GeoJournal , 28 : 287 – 291 .
- George , DG , Talling , JF and Rigg , E . 2000 . Factors influencing the temporal coherence of five lakes in the English Lake District . Freshwat. Biol. , 43 : 449 – 461 .
- Gerten , D and Adrian , R . 2000 . Climate-driven changes in spring plankton dynamics and the sensitivity of shallow polymictic lakes to the North Atlantic Oscillation . Limnol. Oceanogr. , 45 : 1058 – 1066 .
- Grover , JP and Chrzanowski , TH . 2006 . Seasonal dynamics of phytoplankton in two warm temperate reservoirs: association of taxonomic composition with temperature . J. Plankton Res. , 28 : 1 – 17 .
- Hindak, F., editor (1978). Freshwater Algae. Slovenské Pedagogické Nakladelstvo, Bratislava.
- Isvánovics , V , Shafik , HM , Présing , M and Juhos , S . 2000 . Growth and phosphate uptake kinetics of the cyanobacterium, Cylindrospermopsis raciborskii (Cyanophyceae) in throughflow cultures . Freshwat. Biol. , 2 : 257 – 275 .
- Javornicky, P. (1958). Revise některých method pro zjišt’ovănĭkvantity fitoplantonu (Revision of some methods for the phytoplankton quantity estimation). Scientific Paper 2, Faculty of Technology of Fuel and Water, Institute of Chemical Technology, Prague, pp. 283–267.
- Kenesi , G , Shafik , HM , Kovács , AW , Herodek , S and Présing , M . 2009 . Effect of nitrogen forms on growth, cell composition and N2 fixation of Cylindrospermopsis raciborskii in phosphorus-limited chemostat cultures . Hydrobiologia , 623 : 191 – 202 .
- Kokociński , M , Dziga , D , Spoof , L , Stefaniak , K , Jurczak , T , Mankiewicz-Boczek , J and Meriluoto , J . 2009 . First report of the cyanobacterial toxin cylindrospermopsin in the shallow, eutrophic lakes of western Poland . Chemosphere , 74 : 669 – 675 .
- Kokociński , M and Soininen , J . 2008 . Temporal variation in phytoplankton in two lakes with contrasting disturbance regimes . Fundam. Appl. Limnol. , 171 : 39 – 48 .
- Lagos , N , Onodera , H , Zagatto , PA , Andrinolo , D , Azevedo , SMFQ and Oshima , Y . 1999 . The first evidence of paralytic shellfish toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii, isolated from Brazil . Toxicon , 37 : 1359 – 1373 .
- Legendre , P and Legendre , L . 1998 . Numerical ecology.,, , 2nd English , Elsevier Science BV, Amsterdam .
- Li , R , Carmichael , WW , Brittain , S , Eaglesham , GK , Shaw , GR , Noparatnaraporn , AMN , Yongmanitchai , W , Kaya , K and Watanabe , MM . 2001 . Detection of cylindrospermopsin from a strain of Cylindrospermopsis raciborskii (Cyanobacteria) isolated from Thailand . Toxicon , 39 : 973 – 980 .
- Mack , RN , Simberloff , D , Lonsdale , WM , Evans , H , Clout , M and Bazzaz , FA . 2000 . Biotic invasions: causes, epidemiology, global consequences and control . Ecol. Appl. , 10 : 689 – 710 .
- Manti , G , Mattei , D , Messineo , V , Melchiorre , S , Bogialli , S , Sechi , N , Casiddu , P , Luglio , L , Di Brizio , M and Bruno , M . 2005 . First report of Cylindrospermopsis raciborskii (Nostocales, Cyanophyta) in Italy . Harmful Algae News , 28 : 8 – 9 .
- Mischke , U and Nixdorf , B . 2003 . Equilibrium phase conditions in shallow German lakes: how Cyanoprocaryota species establish a steady state phase in late summer . Hydrobiologia , 502 : 123 – 132 .
- Nicklisch , A , Shatwell , T and Köhler , J . 2008 . Analysis and modelling of the interactive effects of temperature and light on phytoplankton growth and relevance for the spring bloom . J. Plankton Res. , 30 : 75 – 91 .
- Nixdorf , B . 1994 . Polymixis of a shallow lake (Großer Müggelsee, Berlin) and its influence on seasonal phytoplankton dynamics . Hydrobiologia , 275/276 : 173 – 186 .
- Nixdorf , B , Mischke , U and Rücker , J . 2003 . Phytoplankton assemblages and steady state in deep and shallow eutrophic lakes – an approach to differentiate the habitat properties of Oscillatoriales . Hydrobiologia , 502 : 111 – 121 .
- Nőges , P and Jensen , JP . 2003 . Occurrence, coexistence and competition of Limnothrix redekei and Planktothrix agardhii: analysis of Danish–Estonian lake database . Algol. Stud. , 109 ( Cyanobacterial Research 4 ) : 429 – 441 .
- Ohtani , I , Moore , RE and Runnegar , MTC . 1992 . Cylindrospermopsin: a potent hepatotoxin from the blue-green alga Cylindrospermopsis raciborskii . J. Am. Chem. Soc. , 114 : 7941 – 7942 .
- Ott , I , Nõges , P , Laugaste , R and Kõiv , T . 2003 . Occurrence of Limnothrix redekei Van Goor in Estonian lakes . Algol. Stud. , 109 ( Cyanobacterial Research 4 ) : 455 – 468 .
- Pádisak , J . 1997 . Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju, an expanding, highly adaptative cyanobacterium: worldwide distribution and review of its ecology . Arch. Hydrobiol., Suppl. , 107 : 563 – 593 .
- Pádisak , J and Reynolds , CS . 1998 . Selection of phytoplankton associations in Lake Balaton, Hungary, in response to eutrophication and restoration measures, with special reference to the cyanoprokaryotes . Hydrobiologia , 384 : 41 – 53 .
- Paerl , HW and Huisman , J . 2008 . Blooms like it hot . Science , 320 : 57 – 58 .
- Pełechata , A , Pełechaty , M and Pukacz , A . 2006 . Cyanoprokaryota of shallow lakes of Lubuskie Lakeland (mid-western Poland) . Ocean. Hydrob. Stud. , 35 : 3 – 14 .
- Post , A , de Wit , R and Mur , LR . 1985 . Interactions between temperature and light intensity on growth and photosynthesis of the cyanobacterium Oscillatoria agardhii . J. Plankton Res , 7 : 487 – 495 .
- Pouličkova , A , Hašler , P and Kitner , M . 2004 . Annual cycle of Planktothrix agardhii (Gommont) Anagnostidis & Komárek nature population . Int. Rev. Hydrobiol , 89 : 278 – 288 .
- Présing , M , Herodek , S , Vörös , L and Kóbor , I . 1996 . Nitrogen fixation, ammonium and nitrate uptake during a bloom of Cylindrospermopsis raciborskii in Lake Balaton . Arch. Hydrobiol , 136 : 553 – 562 .
- Ramberg , L . 1988 . Relations between planktic blue-green algal dynamics and environmental factors in four eutrophic Swedish lakes . Arch. Hydrobiol , 112 : 161 – 175 .
- Reynolds , CS , Huszar , V , Kruk , C , Naselli-Flores , L and Melo , S . 2002 . Towards a functional classification of the freshwater phytoplankton . J. Plankton Res. , 24 : 417 – 428 .
- Romo , S and Miracle , MR . 1993 . Long-term periodicity of Planktothrix agardhii, Pseudanabaena galeata and Geitlerinema sp. in a shallow hypertrophic lagoon, the Albufera of Valencia (Spain) . Arch. Hydrobiol. , 126 : 469 – 486 .
- Romo , S and Miracle , MR . 1994 . Long-term phytoplankton changes in a shallow hypertrophic lake, Albufera of Valencia (Spain) . Hydrobiologia , 275 : 153 – 164 .
- Rücker , J , Wiedner , C and Zippel , P . 1997 . Factors controlling the dominance of Planktothrix agardhii and Limnothrix redekei in eutrophic shallow lakes . Hydrobiologia , 342/343 : 107 – 115 .
- Saker , ML and Griffiths , DJ . 2000 . The effect of temperature on growth and cylindrospermopsin content of seven isolates of the cyanobacterium Cylindrospermopsis raciborskii (Woloszyńska) Seenayya and Subba Raju from water bodies in northern Australia . Phycologia , 39 : 349 – 354 .
- Saker , ML , Nogueira , ICG , Vasconcelos , VM , Neilan , BA , Eaglesham , GK and Pereira , P . 2003 . First report and toxicological assessment of the cyanobacterium Cylindrospermopsis raciborskii from Portuguese freshwaters . Ecotoxicol. Environ. Safety , 55 : 243 – 250 .
- Scheffer , M . 1998 . Ecology of Shallow Lakes , Chapman & Hall, London .
- Scheffer , M , Rinaldi , S , Gragnami , A , Mur , LR and Van Nes , EH . 1997 . On the dominance of filamentous Cyanobacteria in shallow, turbid lakes . Ecology , 78 : 272 – 282 .
- Shannon , CE . 1948 . A mathematical theory of communication . Bell System Technical Journal , 27 : 626 – 656 .
- Sprőber , P , Shafik , MH , Présing , M , Kovács , AW and Herodek , S . 2003 . Nitrogen uptake and fixation in the cyanobacterium Cylindrospermopsis raciborskii under different nitrogen conditions . Hydrobiologia , 506–509 : 169 – 174 .
- Stefaniak , K and Kokociński , M . 2005 . Occurrence of invasive Cyanobacteria species in polimictic lakes of the Wielkopolska region (Western Poland) . Ocean. Hydrob. Stud., 34, Suppl , 3 : 137 – 148 .
- Stüken , A , Rücker , J , Endrulat , T , Preussel , K , Hemm , M , Nixdorf , B , Karsten , U and Wiedner , C . 2006 . Distribution of three alien cyanobacterial species (Nostocales) in northeast Germany: Cylindrospermopsis raciborskii, Anabaena bergii and Aphanizomenon aphanizomenoides . Phycologia , 45 : 696 – 703 .
- Ter Braak , CJF . 1986 . Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis . Ecology , 67 : 1167 – 1178 .
- Ter Braak, C.J.F. & Smilauer, P. (1998). Program CANOCO, version 4.0. Centre for Biometry, Wageningen.
- Van Liere , L and Mur , LR . 1980 . “ Occurrence of Oscillatoria agardhii and some related species, a survey ” . In Developments in Hydrobiology , Edited by: Barica , J and Mur , LE . Vol. 2 , 67 – 77 . The Hague : Dr. W. Junk BV .
- Vitousek , PM . 1990 . Biological invasions and ecosystem process – towards an integration of population biology and ecosystem studies . Oikos , 57 : 7 – 13 .
- Walther , G-R . 2003 . Plants in a warmer world . Perspect. Plant Ecol. Evol. System. , 6 : 169 – 185 .
- Wells , MJ , Poynton , AA , Balsinhas , KJ , Musil , H , van Hoepen , JE and Abbott , SK . 1986 . “ The history of introduction of invasive alien plants to southern Africa ” . In The Ecology and Management of Biological Invasions in Southern Africa , Edited by: Macdonald , AW , Kruger , FJ and Ferrar , AA . 21 – 35 . Cape Town : Oxford University Press .
- Wetzel , RG and Likens , GF . 2000 . Limnological Analyses.,, , 3rd , Springer-Verlag, New York .
- Wiedner , C , Nixdorf , B , Heinze , R , Wirsing , B , Neumann , U and Weckesser , J . 2002 . Regulation of Cyanobacteria and microcystin dynamics in polymictic shallow lakes . Arch. Hydrobiol. , 155 : 383 – 400 .
- Wiedner , C , Rücker , J , Brüggemann , R and Nixdorf , B . 2007 . Climate change affects timing and size of populations of invasive cyanobacterium in temperate regions . Oecologia , 152 : 473 – 484 .