Abstract
Benthic dinoflagellates of the genera Coolia, Ostreopsis and Prorocentrum isolated from coastal waters of the south-eastern Bay of Biscay were identified morphologically by means of light microscopy (LM) including epifluorescence microscopy, and scanning electron microscopy (SEM). To identify the strains to species level, molecular phylogenetic analyses using the nuclear large subunit rDNA (LSU) were performed for 16 strains of the three genera. These morphological and phylogenetic analyses revealed the presence of the following species: Coolia canariensis S. Fraga, Coolia monotis Meunier, Ostreopsis cf. siamensis Schmidt, Prorocentrum emarginatun Fukuyo, P. lima (Ehrenberg) Dodge, P. rhathymum Loeblich III, Sherley & Schmidt, and two as yet unidentified species, which in the phylogenetic tree were grouped with different strains of Prorocentrum emarginatun and P. fukuyoi Murray et Nagahama from GenBank. A strain from Minorca (Balearic Islands, western Mediterranean Sea) analysed in this study for comparative purposes and fitting morphologically into the P. emarginatum/P. fukuyoi group also appeared in this cluster, which seems to include morphologically cryptic or semicryptic species. The most common taxa were Coolia monotis, Ostreopsis cf. siamensis and Prorocentrum lima, which appeared at most sampling sites. Only the strains corresponding to Ostreopsis cf. siamensis and Prorocentrum lima were toxic to Artemia franciscana.
Introduction
Dinoflagellates of the genera Coolia, Ostreopsis and Prorocentrum can produce a variety of toxins with harmful effects on other marine organisms and human health. Although primarily benthic or epiphytic, they can become freely motile, forming blooms in the water column. These genera are widely distributed in tropical and subtropical marine areas where they co-occur with species of the genus Gambierdiscus, which seems to be responsible for most cases of ciguatera fish poisoning (Richlen et al., Citation2008; Litaker et al., Citation2009). Gambierdiscus was first found in tropical and subtropical waters but has recently been reported from temperate waters of the Mediterranean Sea (Aligizaki & Nikolaidis, Citation2008) and the Canary Islands (Aligizaki et al., Citation2008b ). Outside tropical and subtropical areas, Ostreopsis is very frequent and well-documented in the Mediterranean Sea (Vila et al., Citation2001; Turki, Citation2005; Aligizaki & Nikolaidis, Citation2006; Ciminiello et al., Citation2006; Mangialajo et al., Citation2008; Totti et al., Citation2010) and reports of blooms in temperate waters have increased in recent years (Shears & Ross, Citation2009; Rhodes, Citation2010). Coolia and Prorocentrum appear to be more widely distributed, extending to colder waters than Gambierdiscus and Ostreopsis (Faust, Citation1991; Bravo et al., Citation2001; Levasseur et al., Citation2003; Nascimento et al., Citation2005; Maranda et al., Citation2007; Vale et al., Citation2009).
Potentially toxic benthic algae have been the subject of increased research effort over the last few years; molecular methods and electron microscopy have allowed us to gain an insight into their taxonomy over broad geographical ranges (Nagahama & Fukuyo, Citation2005; Murray et al. Citation2007; Faust et al., Citation2008; Fraga et al., Citation2008; Hoppenrath & Leander, Citation2008; Litaker et al., Citation2009; Chomerat et al., Citation2010). Several species are widely distributed and may be more abundant in warmer periods (Aligizaki & Nikolaidis, Citation2006; Mangialajo et al., 2008; Ingarao et al., Citation2009), indicating either a preference for warm waters or a positive correlation with factors associated with summer conditions such as the presence of higher macroalgal biomass or calm waters.
The south-eastern Bay of Biscay is characterized by warmer sea temperatures than the rest of the Atlantic coast of Spain, ranging between 10 and 25°C (Revilla et al., Citation2010). The absence of large brown algae, which are present elsewhere along the Atlantic coast of Spain, reveals the Mediterranean quality of this area, which could therefore constitute a suitable habitat for benthic dinoflagellates of the genera Coolia, Ostreopsis and Prorocentrum. The aim of the present study was to investigate the presence and abundance of these genera both in the benthos and in the water column in beach areas of the south-eastern Bay of Biscay and to investigate the morphology and phylogenetic relationships of selected isolates of these three genera as inferred from the nuclear large subunit (LSU) rDNA.
Materials and methods
Study area and sampling strategy
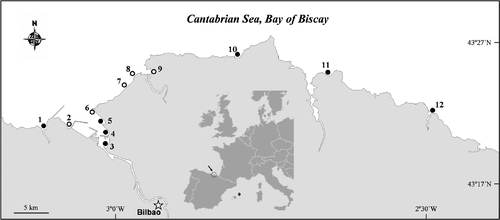
Seven fixed stations (1, 3, 4, 5, 10, 11, 12; ) along the beaches of the south-eastern Bay of Biscay were sampled two to four times each summer from 2007 to 2009. In addition, samples were collected several times in other non-fixed stations along the coast (sites 0 [100 km to the west of the study area and not shown in ], 2, 6, 7, 8, 9) (the latitude and longitudes for all stations are given in a supplementary table, available via the supplementary tab on the article's online page at http://dx.doi.org/10.1080/09670262.2010.550387). A sample taken in Minorca (Balearic Islands, Western Mediterranean Sea) in summer 2009 was analysed for comparative purposes. Samples were obtained from macroalgae collected by hand from depths of 0.5–5 m at low tide. Dominant macroalgae included representatives of the genera Cladostephus, Corallina, Cystoseira, Dictyota, Gelidium, Plocamium and Stypocaulon. After shaking the macroalgae vigorously in plastic bottles to detach epiphytic dinoflagellates, the samples were filtered through 200 µm mesh sieves to remove large particles and then on 20 µm mesh sieves to concentrate the microalgae. Samples were also taken from the water column with tow nets (20 µm mesh size) and bottles. Only bottle samples were used for counting the algae.
Isolation and cultures
Clonal, non-axenic cultures were obtained by micropipetting cells under a Nikon Eclipse T2000-UT inverted microscope. Pipetted cells were incubated in 24-well tissue culture plates (Iwaki Microplate, Japan) filled with 1 ml of f/2 at 35 psu. When the cell concentration was sufficient, the culture was transferred to 6-well tissue culture plates and eventually to 100 ml Erlenmeyer flasks containing 50 ml culture medium. Cultures for taxonomic study and molecular analyses were maintained in 30 or 35 psu in f/2 medium (Guillard, Citation1975) prepared with filter-sterilized (0.22 µm Millipore) seawater, at a temperature of 17 or 22°C and an irradiance of 60 µmol m−2 s−1 in a 12 : 12 h light : dark regime.
Microscopy
Live cells and cells stained in calcofluor (0.15 mg ml−1) were viewed and photographed under a Leica™ DMRB light microscope. Lugol's-fixed bottle samples were settled in sedimentation chambers (10–50 ml) and identified and counted under a Nikon Eclipse T2000-UT inverted microscope. For scanning electron microscopy (SEM), cultures were fixed either with Lugol's or glutaraldehyde (final concentration 4%) at room temperature. Fixed cultures were either filtered on polycarbonate filters (5 µm) or left to settle onto polylysine-coated coverslips. Mounts were rinsed in culture media, postfixed in 1% OsO4, rinsed in distilled water and dehydrated in a graded ethanol series (30–100%) for 10 min at each concentration. Concentrated cultures of Prorocentrum could also be fixed directly in 70% ethanol and then by following the protocol after that dehydration point. The last bath in ethanol was followed by two baths in hexamethyldisilazane (HMDS). Then mounts were left to dry, mounted on a stub, coated with gold-palladium and observed with a Hitachi S-4800 SEM. Cell dimensions of cultured cells were measured under the LM, except for Ostreopsis which was measured from wild specimens since cultured cells were not representative of the cell size and shape observed in natural samples. Measurements of other structures such as thecal pores were made under the SEM. Plate nomenclature follows kofoidian plate tabulation. Cell size and shape as well as thecal plate structure and ornamentation were used for species identification. Platelets of the periflagellar area in Prorocentrum are labelled a–h following Taylor (Citation1980) and Fensome et al. (Citation1993) and those without an equivalent letter are labelled ‘*’.
Toxicity tests
Toxicity of the strains was tested on the crustacean Artemia franciscana (ARTOKIT M™). Artemia cysts were hatched in filtered sea water at 25°C at an irradiance of 60 µmol m−2 s−1. About 1000 cells from each algal strain in exponential phase were put into wells of tissue culture plates (24-well, Iwaki Microplate, Japan) containing 1.5 ml medium. About 15 Artemia individuals 30 h old were added to this medium, except for the test with Ostreopsis, in which instars of 30, 54 and 78 h had to be used because it was observed that the 30 h instars did not contain algae in the gut. Three replicates and a control (without addition of dinoflagellates) were used for each experiment. Nauplii showing no movement of appendages during 10 s of observation after 24 h of incubation were considered dead and the results are expressed as percentage of dead nauplii after 24 h.
DNA extraction, amplification and sequencing
Phylogenetic relationships were inferred from sequences of the nuclear large subunit (LSU) rDNA. DNA was extracted from centrifuged cultures using the DNeasy Plant Mini DNA extraction kit (Qiagen, Hilden, Germany). Polymerase chain reaction (PCR) was carried out using the primers D1R and D2C (Scholin et al., Citation1993). Sequencing used the ABI PRISM™ BIGDYE v3.1® Terminator Sequencing Reaction® (Applied Biosystems) and an automatic sequencer ABI PRISM 3130xl Genetic Analyzer. The sequences were edited using BioEdit v7.0.9 software (Hall, Citation1999).
Alignment and phylogenetic analyses
The alignment comprised LSU rDNA sequences of 16 strains obtained in this study, including a strain morphologically similar to the group Prorocentrum emarginatum/P. fukuyoi isolated from Minorca. In addition, sequences from GenBank of 21 other taxonomically related strains were used to make comparisons with the local strains, aligned using ClustalW2 software (Larkin et al., Citation2007). GenBank accession numbers for our sequences are provided in .
Table 1. Strains of Coolia, Ostreopsis and Prorocentrum isolated during this study (in bold) together with those from different geographical areas included in the phylogenetic analyses.
Phylogenetic analyses were performed separately for the genera Coolia and Ostreopsis and for Prorocentrum. The evolutionary history of the 22 taxa of Coolia and Ostreopsis and the 18 taxa of Prorocentrum was inferred using Neighbour Joining (NJ), Maximum Parsimony (MP) and Maximum Likelihood (ML) methods. NJ and MP analyses were conducted with MEGA4 (Tamura et al., Citation2007) and ML analysis with phyML 3.0 (Guindon & Gascuel, Citation2003). The percentage of replicate trees in which the associated taxa were grouped by bootstrapping (1000 replicates) is shown next to the branches (Felsenstein, Citation1985). Branch lengths are proportional to evolutionary distances. The NJ algorithm was carried out with the Kimura 2-parameter substitution model (Kimura, Citation1980). The MP tree was obtained using the Close-Neighbour-Interchange algorithm (Nei & Kumar, Citation2000) with search level 3 in which the initial trees were obtained with the random addition of sequences (10 replicates). All positions containing gaps and missing data were eliminated from the dataset. In the final data set, there were a total of 536 and 492 positions for Coolia–Ostreopsis data set and Prorocentrum data set respectively, out of which 348 and 220 were parsimony-informative.
Prior to ML analyses, jMODELTEST v0.1.1 software (Posada, Citation2008) was used to choose with the Akaike information criterion (AIC) the DNA substitution model best fitting our data. The TrN + I + G model was selected as the best fit for the Coolia and Ostreopsis data set (A = 0.29, C = 0.15, G = 0.21, T = 0.34; Gamma distribution = 1.872) and the TrN+G model best fit the Prorocentrum data (A = 0.25, C = 0.20, G = 0.31, T = 0.24; Gamma distribution = 0.669). ML searches were conducted using the chosen model.
Results
Morphological features
In the study area, we distinguished two morphospecies of Coolia, C. canariensis and C. monotis, one Ostreopsis corresponding to O. cf. siamensis, and five Prorocentrum, corresponding to P. emarginatum, P. lima, P. rhathymum, and two forms (strains Dn32EHU and Dn33EHU) morphologically similar to P. emarginatum/P. fukuyoi (). A strain from Minorca (Dn34EHU) was very similar to local P. emarginatum/P. fukuyoi.
Cells of C. canariensis are almost spherical, 28–43 µm long, 28–44 µm width and with an anteroposterior axis of 37–39 µm (). As in other species in the genus, the thecal plates are unequal in size and shape, and arranged in an irregular pattern (). Plate formula is: Po, 3′, 7″, 6C, ?S, 5″′, 2″″. The apical plate 1′, which is the largest of the epithecal plates, occupies the central part of the epitheca and is bordered by the apical pore (Po), which is nearly 8 µm long, and by plates 2′, 3′, 1″, 2″, 6″ and 7″. Plate 2′ is hexagonal, elongated and overlapped by plates 3′, 2″, 3″ and 4″ (). Plate 3′ is pentagonal, slightly wedge-shaped, and touches plates Po, 1′, 2′, 4″, 5″ and 6″ (). Plate 6″ is pentagonal and the biggest of the precingular plates (). Plate 7″ is quadrate, has a width/length ratio of 1.7–2.2 (). The thecal surface is smooth in the epitheca but presents numerous shallow depressions in the hypothecal plates ().
Figs 2–9. Coolia canariensis. 2–5. SEM. 2. Ventral view showing plate 7″, which is two times wider than long. Note the ornamented hypothecal plates that contrast with the smooth epitheca. 3. Antapical view showing all hypothecal plates. 4. Dorso-apical view showing apical pore (Po). 5. Apical view showing the pore complex and the central plate 1′, the largest plate of the epitheca. 6–9. Calcofluor-stained cells showing thecal plates. Scale bars: 10 µm.
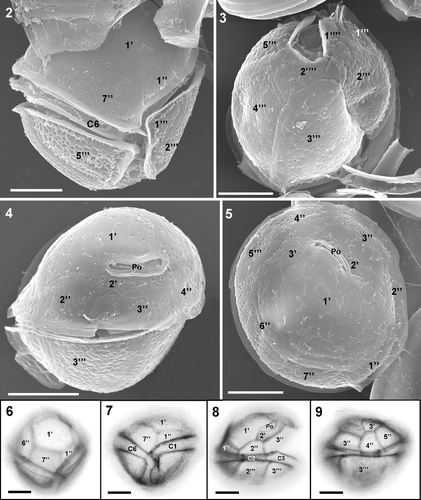
Table 2. Morphological measurements (ranges, means and standard deviations) of identified species determined by means of light and electron microscopy. All data are from cultured cells, except for specimens of Ostreopsis cf. siamensis, whose measurements were taken from natural samples.
Coolia monotis is round in apical view and anteroposteriorly compressed (). The anterior-posterior axis is characteristically oblique, with the thickest point dorsal in the epitheca and ventral in the hypotheca (). Cells range in length from 26 to 37 µm, and 23 to 37 µm in width and the anteroposterior axis is 22–33 µm in length (). Plate formula is: Po, 3′, 7″, 6C, ?S, 5′′′, 2′′′′. The apical pore plate (Po) is located among the second (2′), third (3′) and first (1′) apical plates, 7–9 µm long, with a 5.5–7 µm opening (). Plate 1′ is oblong, hexagonal with its right side located in the middle of the dorso-ventral part of the cell, touching plate 6″, which is the largest plate of the epitheca (). Plate 3′ varies between pentagonal and wedge-shaped and touches Po, 1′, 2′, 4″, 5″ and 6″. Plate 2″ is wider than its adjacent 1″ and 3″ plates. Plate 7″ has a width/length ratio of 1.1–1.5. The cingulum and sulcus are deeply excavated and the sulcus is bordered by the wing-like extensions of plates 5′′′ and 1′′′′ (). The thecal surface is smooth with scattered large round pores (). Additionally, there are a few small pores, at least in plate 3′′′ (). Small pores or perforations are located inside the large pores ().
Figs 10–18. Coolia monotis. 10–13. SEM. 10. Ventral-right view of the antero-ventral side showing cingulum, sulcus and thecal plate architecture. 11. Right-lateral view. 12. View of the wide 3′′′ plate showing some small pores (arrows) in addition to the usual large pores which perforate the smooth surface. 13. Apical view. 14. LM of a vegetative cell in lateral view showing the oblique axis. 15. Ultrastructural detail of the large plate pores viewed from outside the cell. Note the small perforations inside the pores. 16–18. Calcofluor-stained cells. 16. Antapical plates. 17–18. Apical plates. Note the variability in plate shape between cells. Scale bars: 10 µm (); 1 µm ().
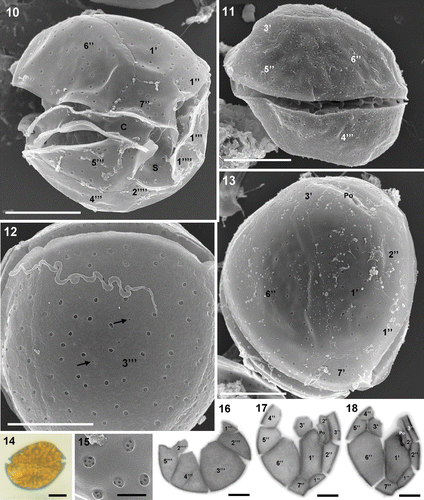
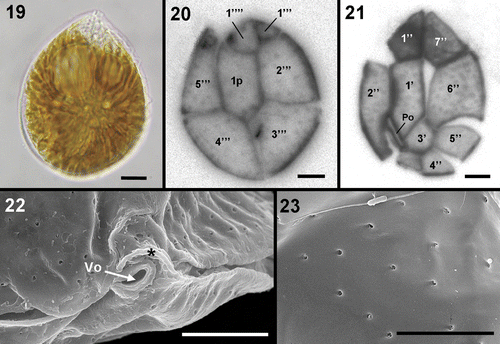
Ostreopsis cells, when cultured, were observed to develop aberrant morphologies that precluded their use for morphological identification. Occasionally, characteristic tear-shaped forms similar in size to wild cells and significantly smaller cells were also observed in culture. Field cells are anteroposteriorly compressed, tear-shaped and pointed towards the ventral side (). They have a dorsoventral diameter of 51–67 µm and a width of 33–56 µm (). They display the plate formula Po, 3′, 7″, 5′′′, 2′′′′ and 1p, which is common to all described Ostreopsis species (). Plate 1′ is long and hexagonal and occupies the left side of the epitheca (). Plate 5″ is quadrangular and does not touch plate 1′, which is bordered by plates Po, 2′, 3′, 1″, 2″, 6″ and 7″. Plate 6″, the largest of the precingular plates, is pentagonal and touches the apical plates 1′ and 3″ and the precingular plates 5″ and 7″. Plate 4′′′ is the larger of the postcingular plates and the suture between it and plate 3′′′ is located on the dorso-ventral axis of the cell. The thecal surface is smooth and covered with scattered round pores predominantly 0.15–0.31 µm in diameter (). The length of the Po, which is slightly curved, is 7–9 µm.
Figs 19–23. Ostreopsis cf. siamensis. . LM. 19. Bright field of a vegetative cell from a field sample. 20, 21. Calcofluor-stained cells. 20. Hypothecal plates. 21. Epithecal plates. Note that plate 3″ is not well preserved. 22, 23. SEM. 22. Detail of the ventral opening (Vo) and the rigid plate (*). 23. Detail of thecal pores. Note that only one pore class is present. Scale bars: 5 µm (); 10 µm ().
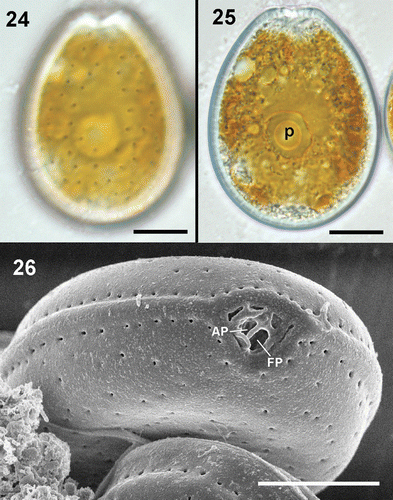
Prorocentrum lima cells are oblong in shape, with the anterior end narrower than the posterior end (). Plates are smooth and covered, except in the middle of the cell, with ovoid to oblong pores more densely distributed on the valve margins (). The right valve has a V-shaped depression at the anterior end corresponding to the periflagellar area (). The left valve exhibits a flat ridge at the anterior end (). Cells in culture varied in length from 38 to 49 µm, 26 to 37 µm in width, and had a length/width (L/W) ratio between 1.28 and 1.48 ().
Figs 24–26. Prorocentrum lima. 24, 25. LM. 24. Surface focus on a vegetative cell showing the pore pattern. 25. Vegetative cell with pyrenoid (p). 26. SEM. Periflagellar area showing flagellar (FP) and apical (AP) pores and detail of the pores along the valve margin, which are more closely spaced than the rest of the valve pores. Scale bars: 10 µm.
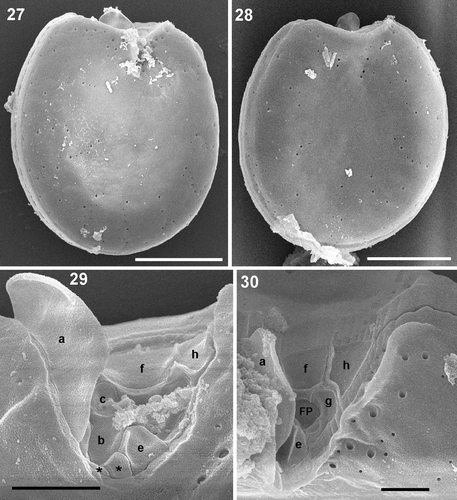
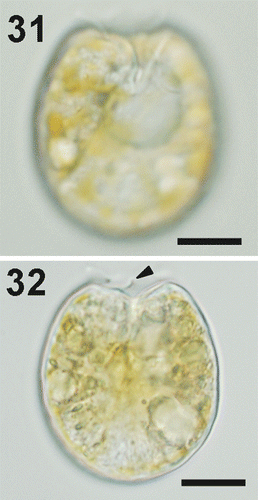
Four cultured strains fitting morphologically the P. emarginatum/P. fukuyoi group (three from the study area and the one from Minorca) have been examined in this study. Cells of local strain Dn31EHU are ovoid in valve view, 31–39 µm long and 24–30 µm wide, with a mean length/width (L/W) ratio of 1.18 (). The left valve is concave at the anterior end and the right valve has a deeply excavated periflagellar area with a prominent flange or spine (). The thecal surface is smooth and covered with many small pores scattered on the valve and large pores lying in rows perpendicularly from the margin to the centre of the valve, which lacks pores (). No pyrenoids were observed (). Strain Dn32EHU () is notably narrower than Dn31EHU and Dn33EHU. Cells are oblong, 39.5–47 µm long and 29–34 µm wide, with a mean L/W ratio of 1.4 (). The right valve has an anterior V-shaped and slightly curved indentation that contains the periflagellar area, which bears a wing-like flange on the right side (). The valve surface is smooth with two sizes of pores. The smaller pores are evenly distributed around the periphery of the valve and scattered in the centre. The larger pores are scattered over the valve. The intercalary band is transversely striated. No pyrenoids were observed in this strain. A third strain from the study area (Dn33EHU) and the strain from Minorca (Dn34EHU) are broadly ovate (Figs 38–50), similar to strain Dn32EHU in length (43.5 µm and 41.3 µm, respectively) but intermediate between Dn31EHU and Dn32EHU in terms of valve length to width ratio (L/W 1.25 and 1.22, respectively) (). Anterior valve margins are excavated and the right valve presents the characteristic periflagellar area of this group in a V-shaped indentation. The thecal surface of both strains is smooth and covered with scattered small pores and large pores arranged in perpendicular rows from the margin to the valve centre (, ). No pyrenoids were observed in those strains (, ). Cells dividing to form hyaline cysts were observed in all four strains but were more frequent in Dn32EHU ().
Figs 27–30. Prorocentrum emarginatum, strain Dn31EHU. 27–30. SEM. 27. Right valve view showing the V-shaped excavation of the periflagellar area and the large flange. 28. Left valve view. 29. Periflagellar area with the platelets not hidden by platelet ‘a’. 30. Detail of the two sizes of thecal pores which border the periflagellar area. Scale bars: 2 µm (); 10 µm ().
Figs 31–32. Prorocentrum emarginatum, strain Dn31EHU, LM. 31. Right valve of a vegetative cell showing the deep indentation. 32. Cell showing the flange or spine in the anterior excavation (arrowhead). Scale bars: 10 µm.
Figs 33–37. Prorocentrum sp. strain Dn32EHU. 33, 34. SEM. 33. Right valve showing the V-shaped indentation of the periflagellar area. 34. Detail of some platelets and flagellar pore (FP) of the periflagellar area and two sized thecal pores. 35–37. LM. 35. Cells dividing into a transparent division cyst. 36. Focus on the right valve showing the V-shaped indentation of the periflagellar area. 37. Cell showing the nucleus (n). Scale bars: 2 µm (); 5 µm (); 10 µm ().
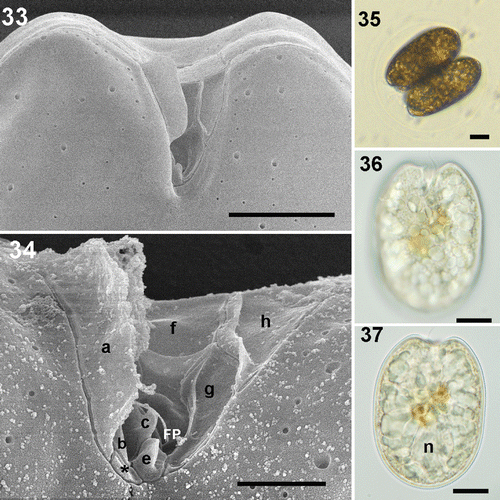
Figs 38–43. Prorocentrum sp. strain Dn33EHU. 38–41. SEM. 38. Left valve showing marginal and radial thecal pores on the smooth surface, which is convex in the centre. 39. Right valve with the anterior end deeply indented. 40. Detail of the periflagellar area showing two small protrusions (arrows). 41. Anterior end of the cell with the striated intercalary band and the periflagellar area. 42, 43. LM. 42. Left valve with the anterior end markedly excavated. 43. Right valve showing the nucleus (n) and the indented anterior end. Scale bars: 2 µm (); 5 µm (); 10 µm (; ).
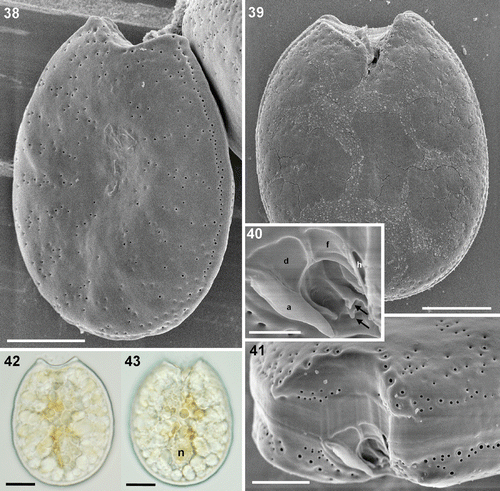
Figs 44–50. Prorocentrum sp. strain Dn34EHU. 44, 45. SEM. 44. Right valve showing the anterior indented end and the smooth surface. 45. Left valve. 46–49. LM. 46, 47. Left and right valve views of living cells. 48. Left and right valves of an empty cell. 49. Detail of the platelets that limit the anterior periflagellar area. 50. SEM. Detail of the periflagellar area with the platelets and the flagellar pore (FP) as well as the accessory pore (arrow). Scale bars: 2 µm (); 5 µm (); 10 µm ().
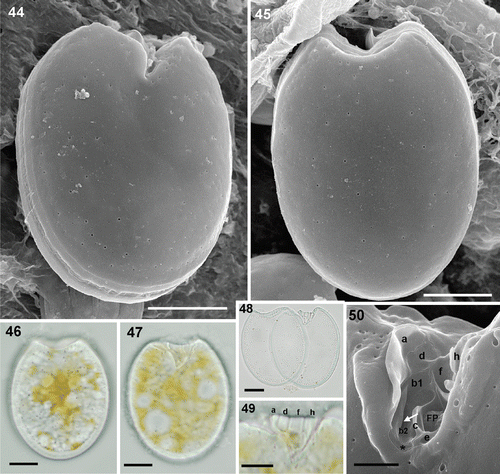
In the periflagellar area, the following ultrastructural details were observed in the local strains. The periflagellar area of strain Dn31EHU could not all be observed since the flange of platelet ‘a’ hides part of the right side. Therefore, the exact number of platelets could not be determined; nevertheless, at least ten platelets were recognized, nine of them shown in ; the tenth platelet ‘d’ is not shown. Platelet ‘a’ covers most of the right side of the periflagellar area except the posterior part (). The anterior margin is covered by the largest platelets ‘d’, ‘f’ and ‘h’, together with platelet ‘a’, called a spine in some descriptions. The left side in the figure is bordered by platelet ‘h’, followed by platelets ‘g’ and ‘e’ (). Two small platelets appear in the posterior end and platelets ‘b’ and ‘c’ border the right side in the figure of the flagellar pore, between platelets ‘e’ and ‘f’. At least three of the platelets bear ridges or flanges. The largest is that of platelet ‘a’, which can be observed by light microscopy (). Platelet ‘h’ shows a long ridge along the border with platelets ‘f’ and ‘g’. In the posterior part of platelet ‘f’ a horizontal ridge divides a posterior portion of the platelet with a different angle delimiting the flagellar pore. A fourth small ridge appears at the right-hand border of platelet ‘e’. This periflagellar area is very similar to that of strain Dn32EHU (). At least nine platelets were observed and labelled in Dn32EHU, six of them with ridges or flanges of different magnitude. The flange of platelet ‘a’ is the largest and can sometimes be observed with the light microscope. Platelets ‘h’ and ‘f’ have ridges like those described in strain Dn31EHU. Platelet ‘g’ bears a less conspicuous ridge along its right border, and platelets ‘e’ and ‘c’ bear smaller flanges or protrusions. At least 10 platelets were observed in the periflagellar area of strain Dn34EHU (). The anterior margin is covered by platelets ‘a’, ‘d’, ‘f’ and ‘h’ (). Platelets ‘a’, ‘f’, ‘h’, ‘g’, ‘b’ and ‘c’ bear flanges or ridges of different dimensions (). The ridges of platelets ‘a’, ‘h’ and ‘f’ are like those described for Dn31EHU and Dn32EHU. A small unlabelled platelet appears in the posterior end, between platelets ‘a’ and ‘e’. The accessory pore is bordered by platelets ‘b1’ and ‘b2’ and the flagellar pore by platelets ‘f’, ‘g’, ‘e’ and ‘c’. The periflagellar area structure of strain Dn33EHU was only partially resolved (). Platelet ‘f’ shows the same horizontal ridge described in the other strains (, ), which is shown to be continued in platelet ‘d’. In the posterior part, a longitudinal flange extends to the left of platelet ‘a’, presumably belonging to platelet ‘b’ (compare with Dn34EHU, ), whereas two other small protrusions can also be seen.
Prorocentrum rhathymum has a simple small spine in the apical side (). The periflagellar area, located at the anterior end of the right valve, is wide and V-shaped. Cells are oval with a cell size of 30–37 µm long, 21–26 µm wide and an anteroposterior diameter of 18–23 µm (). Cells do not show any perceptible pyrenoid. The valve contains three classes of pores (). Large pores form parallel short rows located in small depressions emerging from the margin of the valve. The large pores of the posterior side run parallel to the valve margin. Small pores are unevenly distributed and occupy even the centre of the valve which contains only a few large pores. Clumps of three or four very small pores appear close to the valve margin (). The intercalary band shows both horizontal and transverse striations ().
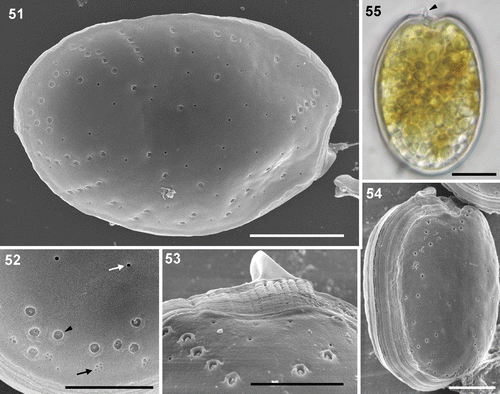
Figs 51–55. Prorocentrum rhathymum. 51–54. SEM. 51. Left valve showing the anterior spine-like prolongation and the pore pattern. 52. Detail of a valve portion containing three classes of pores: large (arrowhead), small (white arrow) and the clumps of smaller pores (black arrow) on the valve margin. 53. Anterior end showing the arrangement of the large pores in this area, the spine or collar and the longitudinal intercalary band, which appears transversally striated. 54. Side view of the left valve showing longitudinal striations of the intercalary band. 55. LM. Oval shaped cultured cell showing the spine at the anterior end. Scale bars: 5 µm (); 10 µm ().
Abundance and distribution
Coolia monotis was the species more commonly found, followed by Ostreopsis cf. siamensis and Prorocentrum lima (). Coolia monotis appeared at all sampling sites except 3, 4 and 5, which are located in an estuarine area. All species were very scarce in the water column, not exceeding 300 cells l−1 (). The most abundant species were also the most widely distributed. Prorocentrum rhathymum was found in only one sample. Coolia canariensis appeared in two consecutive years at the same locality (site 2, ) but it was not found at adjacent sampling points (sites 1, 4–6). Cells resembling P. emarginatum and P. fukuyoi are treated as a species group for distributional observations because they can hardly be distinguished to species level under LM. Cells of this group were observed at low concentrations in several but not all sites. Sampling sites under the influence of the plume of the largest river in the area (sites 3–5) were the poorest both in species diversity and abundance.
Table 3. Presence of identified species in the different places of the study area and date of sampling (07, 08, 09: years 2007, 2008 and 2009).
Table 4. Highest cell densities (cells l−1) of the most abundant species in the water column with sampling site and date.
Phylogenetic relationships
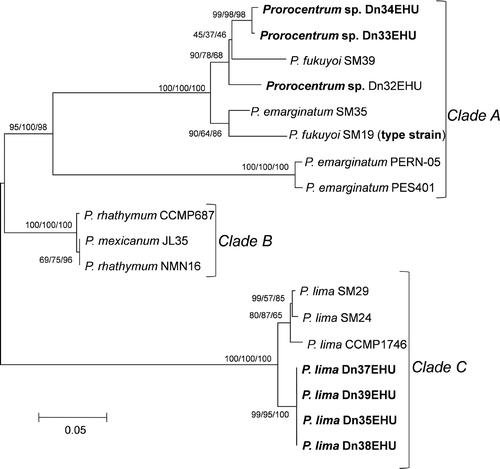
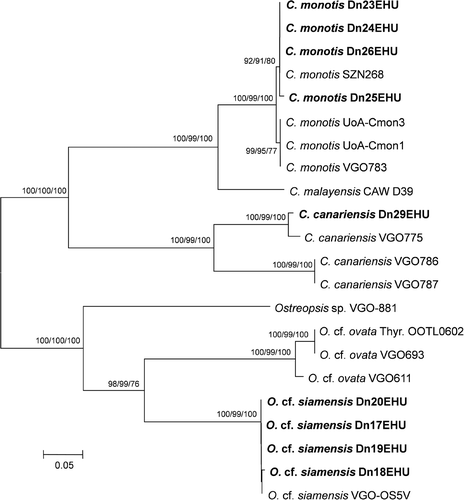
Phylogenetic analysis of the 22 taxa of Coolia and Ostreopsis () delineated two major clades within the genus Coolia, one containing strains from C. canariensis, and the other including European strains of C. monotis plus C. malayensis. The four strains of C. canariensis formed a well-supported clade with two separate subclades, one of them containing the local isolate. Strains of C. monotis clustered together with strong bootstrap support. Three lineages were delineated in the genus Ostreopsis, one composed of a single Ostreopsis sp. strain from Canary Islands, and two sister clades, one containing O. cf. ovata strains and the other with the local O. cf. siamensis strains together with a strain from the western Mediterranean Sea.
Fig. 56. Phylogenetic analysis of the 536-bp long 28S rDNA LSU sequences of 22 taxa of Coolia and Ostreopsis (neighbour joining tree, Kimura 2-parameter substitution model). Values on branches are bootstrap values for ML/MP/NJ (1000 iterations).
The evolutionary history of 18 taxa of Prorocentrum is depicted in , showing three major clades. Clade A included strains of the P. emarginatum/P. fukuyoi group, which was split into two subclades, one with two strains of P. emarginatum, supported by high bootstrap values, and the other including the three local strains of this group plus several strains from GenBank identified either as P. emarginatum or P. fukuyoi. This phylogenetic analysis reveals the difficulty in assigning strains to one of these species solely on the basis of morphology. Clade B consisted of two strains of P. rhathymum and a third strain named P. mexicanum in GenBank but most likely belonging to P. rhathymum. No sequence was obtained for the local strain identified by its morphology as P. rhathymum which could not be used for phylogenetic analysis. The third clade (C) was formed of two subclades, one containing the four local strains of P. lima and the other three tropical strains of this species.
Toxicity
For all the tested species, algae were observed in the gut content of Artemia. Only Prorocentrum lima and Ostreopsis cf. siamensis were toxic to Artemia nauplii. Prorocentrum lima showed the highest toxicity, with a mortality of 86.9% after 24 h. Results of the toxicity tests on 1–3 day instars of Artemia for O. cf. siamensis showed an increase in mortality from 55.7% for the 1-day instars to 85.3% for the 3-day instars.
Discussion
Distribution
Several benthic dinoflagellate species were identified in the south-eastern Bay of Biscay. They were found attached to macroalgae and less commonly in the water column. The most abundant and frequently occurring species were Coolia monotis, Ostreopsis cf. siamensis and Prorocentrum lima. Coolia monotis is frequently found both in temperate as well as in tropical areas (Meunier, Citation1919; Penna et al., Citation2005; Aligizaki & Nikolaidis, Citation2006; Dolopsakis et al., Citation2006) and the P. lima complex has also been reported from temperate areas (e.g. Faust, Citation1991; Bravo et al., Citation2001; Levasseur et al., Citation2003; Foden et al., Citation2005; Maranda et al., 2007; Aligizaki et al., Citation2009) in addition to tropical ones. This study is the second report of C. canariensis, recently described by Fraga et al. (Citation2008) from the Canary Islands, although in our study area it has been found at only one locality. Ostreopsis cf. siamensis, which was considered to have a pantropical distribution, has also been recently found in many warm temperate areas, as reviewed by Shears & Ross (Citation2009), and at higher latitudes in the Sea of Japan (Selina & Orlova, Citation2010). The observation of Ostreopsis in the present study is the northernmost record of this genus in the north-east Atlantic. Ostreopsis cf. siamensis was found at most of the sites sampled indicating that it is frequent in the area during late summer. It was also the most abundant epibenthic dinoflagellate species in the water column, although it has not yet been observed in bloom proportions. Given the strong positive correlation between the abundance of these dinoflagellates as epiphytes and in the water column (Vila et al., Citation2001; Aligizaki & Nikolaidis, 2006; Mangialajo et al., 2008; Aligizaki et al., Citation2009), it could be inferred that Ostreopsis cf. siamensis is also the most abundant benthically. The possibility that O. cf. siamensis could proliferate in sheltered parts of the south-eastern Bay of Biscay where water temperatures in hot summers can reach 25°C should not be excluded. Water motion seems to be a key factor in triggering blooms of Ostreopsis as the greatest abundances have been observed in sheltered places and after periods of calm water conditions (Shears & Ross, 2009; Totti et al., Citation2010). Some authors consider temperature to be a factor in bloom development (Aligizaki & Nikolaidis, Citation2006; Mangialajo et al., 2008), whereas for others this relationship is not so clear (Vila et al., Citation2001; Monti et al., Citation2007; Totti et al., Citation2010). Prorocentrum emarginatum is a cosmopolitan species, whereas its sister taxon, P. fukuyoi, has been recently described from samples taken in Australia, and it was previously reported under different names as reviewed by Murray et al. (Citation2007). More recently, P. fukuyoi has been found in Kuwait Bay (Saburova et al., Citation2009). While in this study it was only occasional, P. rhathymum has been observed in high densities in warmer localities (Ismael & Aida, Citation1997; Pearce et al., Citation2005; Aligizaki et al., Citation2009), indicating its preference for higher temperatures.
Taxonomic remarks
Two species of Coolia have been found in the study area: C. canariensis and C. monotis. The morphology of C. canariensis corresponds well with the original description of Fraga et al. (2008), except for the morphology of thecal pores of plate 1′, which are round in our strain but described as oval in the original description, and the cell dimensions, which are slightly larger in the local strains. The tabulation pattern of C. canariensis is very similar to that of C. areolata, but thecal ornamentation allows differentiation of the two species. Cell sizes of C. monotis strains fall within published values (Penna et al., Citation2005; Dolapsakis et al., Citation2006). The strains have morphological features similar to previous descriptions of the species, although with some differences from Meunier's (Citation1919) original description and other later descriptions, especially regarding some plates of the epitheca. Plates 6″ and 5″ are elongated in the original description and the suture between them runs in a nearly dorso-ventral direction, while in our strains it runs to the right side, resulting in a wider, not elongated 6″ plate and a smaller 5″, similar to other later descriptions (Fukuyo, Citation1981; Penna et al., Citation2005; Dolapsakis et al., Citation2006). Differences are also found in plates 1″ and 2″. In the original description those plates are of similar size, while in some of our strains plate 2″ is double the size of 1″, wider and slightly longer, similar to the Greek strain in Dolapsakis et al. (Citation2006). Additionally, strains with a narrow plate 1′, similar to that described by Dolapsakis et al. (Citation2006), are found together with wider 1′ plates fitting the original description or that shown in Penna et al. (Citation2005). Dolapsakis et al. (Citation2006) describe plate 3′ of their strain as unlike any other reported in the literature because of its wedge shape, while in our strains both 3′ plates fit well with the original illustration in that hexagonal morphology in addition to wedge-shaped plates are found. Molecular analysis of the local C. monotis strains shows that the variability in the shape of some plates is not related to geographical genetic variability. Morphology of plate 7″ was proposed by Fraga et al. (2008) as one of the discriminating features among Coolia species, distinguishing a plate with a width/length ratio of ∼1 for C. monotis, 2 for C. areolata and C. canariensis and 4 for C. tropicalis. Later, C. malayensis was described with a width/length ratio for plate 7″ ranging from 1.2 to 1.5 (Leaw et al., Citation2010). The range of this ratio in local strains is 1.1–1.5 for C. monotis and 1.7–2.2 for C. canariensis. One of the main constraints when applying the morphological species concept is to judge whether the observed organisms are sufficiently morphologically different to be considered separate species, if differences are due to intraspecific polymorphism, or if they represent cryptic or semicryptic species. The newest Coolia species, C. malayensis, was described from strains isolated in Malaysian waters (Leaw et al., Citation2010). This species is morphologically very similar to C. monotis, but the authors showed it to represent a different species based on the secondary structure of rRNA Internal Transcribed Spacer (ITS). Some slight morphological differences were also pointed out, but these are not very marked, and the local C. monotis strains would be difficult to assign to one of the two species in the absence of molecular analyses. The small perforations inside large thecal pores, which have been described as a specific character of C. malayensis, have also been observed in the local strains of C. monotis, invalidating this character as distinctive for C. malayensis.
Nine species of the genus Ostreopsis have been described to date. All of them exhibit the same plate pattern, except O. heptagona, which is the only species in which plates 1′ and 5″ touch each other. Identification to the species level within the genus is a difficult task because the main differential feature is cell size, which is very variable within species. The size of the local strains is below the range of most described Ostreopsis species. It overlaps at its upper limit with O. lenticularis and at its lower limit with O. ovata and fits best with some O. cf. siamensis descriptions. The main morphometric difference between O. cf. siamensis and O. cf. ovata is the anteroposterior diameter, which is lower (much flatter) in O. cf. siamensis, than in O. cf. ovata (Penna et al., Citation2005; Aligizaki & Nikolaidis, Citation2006). Ostreopsis cf. siamensis differs from O. lenticularis in its cell dimensions and the presence of only one size of trichocyst pore (Fukuyo, Citation1981; Mohammad-Noor et al., Citation2006). Taking into account cell shape and dimensions, thecal pores and Po size in different species of the genus Ostreopsis, the local strains best fit the Mediterranean O. cf. siamensis strains given by Penna et al. (Citation2005) and Aligizaki & Nikolaidis (Citation2006), an affinity confirmed by the molecular analysis. The morphology of the local strains of Ostreopsis also fits the description given by Fukuyo (Citation1981) for O. siamensis, except in cell size (smaller in our strains), and differs from that given by Faust et al. (Citation1996) and Chang et al. (Citation2000) in having only one size of thecal pores.
According to Murray et al. (2007), slight morphological features differentiate P. emarginatum and P. fukuyoi. Prorocentrum emarginatum is more rounded, with a wider apical region and with a left valve less deeply indented than P. fukuyoi. Both have a smooth valve surface with small and large pores but the pattern generally radiates more clearly from the centre in P. emarginatum than in P. fukuyoi. The presence in P. fukuyoi of two small protrusions in the periflagellar area has been proposed by Murray et al. (2007) as another distinguishing feature. However, these authors recognize that these structures cannot be resolved in the original description of P. emarginatum due to the lack of detailed figures of the apical area. Murray et al. (2007) stated that both species have very similar apical plate patterns, with a main collar or flange in the right boundary of the apical region and a second smaller flange to the left. The four strains of this group analysed in this study bear those two flanges, which correspond to projections from platelets ‘a’ and ‘h’ following the terminology of Fensome et al. (Citation1993), and, additionally, smaller flanges or ridges have been observed in platelets ‘b’, ‘c’, ‘e’ and ‘g’. The presence of at least five projections in the periflagellar area was presented as a novel characteristic in P. tsawwassenense (Hoppenrath & Leander, 2008), but a more detailed examination of strains of P. emarginatum/P. fukuyoi group in this study has revealed the presence of projections in several platelets of the species of this group, although of much smaller dimensions. The protrusions observed by Murray et al. (2007) in P. fukuyoi are most likely two of those projections of the small platelets in the posterior periflagellar area. Ridges observed in platelets ‘b’, ‘c’ or ‘e’ of our strains could correspond to those protrusions, but comparisons with figures from Murray et al. (2007) are difficult. The distribution of the flanges in our strains coincides with those described in P. tsawwassenense (Hoppenrath & Leander, 2008), a species that also displays a radiating distribution pattern of large pores, denoting that this species has an overall similarity to P. emarginatum. The morphological differences between the strains of the P. emarginatum/ P. fukuyoi group analysed in this study compromise cell size, shape (length to width ratio, L/W), pore pattern and shape of the anterior excavation. The smallest and roundest (L/W 1.18) strain is Dn31EHU, the one that more closely matches the dimensions of the original description of P. emarginatum (Fukuyo, Citation1981), which was described as being 35–36 µm long and 32 µm wide (L/W 1.11). It also matches the description of P. emarginatum from the western Indian Ocean (Hansen et al., Citation2001), from Greek waters (Aligizaki et al., Citation2009) and from Malaysia (Mohammad-Noor et al., Citation2006), although a pyrenoid was observed in some cells in the latter two studies. The most elongated strain is Dn32EHU (L/W 1.38), which shows a thecal surface with scarce pores without a clear radiating pattern. From these features, this strain morphologically fits P. fukuyoi, which was described as having a L/W of 1.3–1.5, and P. cf. marinum, reported from the Wadden Sea by Elbrächter & Hoppenrath (Citation2009), which differs from P. fukuyoi by the presence of a conspicuous pyrenoid surrounded by a starch sheath. These authors consider the possibility that P. cf. marinum is conspecific with P. fukuyoi. Strains Dn33EHU and Dn34EHU have clearly radiating pores, resembling P. emarginatum, but are larger and less rounded, being similar to the specimens from Belize identified as P. emarginatum (Faust, Citation1990; Faust et al., Citation1999). Other similarities with specimens from Belize are: the valve centre, which is clearly concave in Dn33EHU; the swollen margins of the right valve near the periflagellar area [compare our with from Faust (Citation1990)]; the shelf-like valve edging visible in Faust's ; and the acute anterior edges of the valve excavation [compare our with Faust (Citation1990, ) or Faust et al. (Citation1999, )].
The local strains of P. lima fit the description given by Nagahama & Fukuyo (Citation2005), who redescribed the species, and also fall within the range described for other strains (e.g. Nascimento et al., Citation2005; Mohammad-Noor et al., Citation2006; Aligizaki et al., Citation2009). Great variability in cell size and shape was observed by Aligizaki et al. (Citation2009) for P. lima strains from the North Aegean coastal waters, who considered them to be members of a P. lima species complex.
Based on SEM observations, Cortés-Altamirano & Sierra-Beltrán (Citation2003) reinstated the species P. rhathymum, which had been considered synonymous with P. mexicanum by Steidinger (Citation1983), defining the morphological features that distinguish them. Prorocentrum mexicanum has the same number of trichocyst pores (about 80) as P. rhathymum but in addition it has about 1000 poroids, which are lacking in P. rhathymum. Our strain lacks poroids and fits P. rhathymum for most morphological features. Cell dimensions are slightly larger than in the original description by Loeblich et al. (Citation1979) and the reports of Mohammad-Noor et al. (Citation2006) and Aligizaki et al. (2009), but fit well with those given by Fukuyo (Citation1981). The intercalary band of P. rhathymum is described as horizontally striated in Aligizaki et al. (2009) and as smooth in Mohammad-Noor et al. (Citation2006), while both horizontal and transverse striations have been observed in this study.
Phylogeny
Phylogenetic analyses confirmed the identity of the local strains of C. canariensis and C. monotis. Two main clades of Coolia were consistently well supported in the phylogenetic tree, one including the strains of C. monotis plus C. malayensis as sister taxon, and the other the strains of C. canariensis. In agreement with the results of Penna et al. (Citation2005), only slight geographical differences can be observed among the different strains from the Mediterranean and the local isolates of C. monotis, which appear to belong to a single evolutionary lineage, whereas the New Zealand strain constitutes a genetically distinct entity described as Coolia malayensis (Leaw et al., Citation2010). Similar relationships were inferred by Fraga et al. (2008) who found three different lineages within selected strains of the genus Coolia, one of them including strains of C. canariensis, a second with C. monotis from Indonesia and Belize and a third containing two sister groups, one corresponding to C. monotis from European localities and the other to C. monotis and C. malayensis from New Zealand, Malaysia and Florida. The local strains morphologically identified as Ostreopsis cf. siamensis clustered with a strain of this species from the Mediterranean (VGO-OS5V) in a subclade separated from the subclade containing strains of O. cf. ovata. These results agree well with those of Penna et al. (Citation2010) which showed a clear genetic differentiation between strains of O. cf. siamensis and O. cf. ovata from several geographical areas.
Strains of the Prorocentrum emarginatum/fukuyoi group appeared as two sister clades. In one were two GenBank sequences of strains from Reunion Island in the tropical Indian Ocean identified as P. emarginatum. The other well-supported clade was constituted of the local strains Dn32EHU, Dn33EHU and DN34EHU together with three strains of P. emarginatum and P. fukuyoi analysed by Murray et al. (2007). These authors based the new species P. fukuyoi on strains SM19 and SM39 plus some wild cells, designating as holotype a SEM micrograph of strain SM19. Given the similarities between the new species and P. emarginatum, they also analysed strain SM35, identified as P. emarginatum, for comparative purposes. Phylogenetic analysis including the LSU rDNA sequence of the type strain (SM19) showed P. fukuyoi to be a sister taxon of P. emarginatum strain SM35 while the sequence of a strain identified as P. emarginatum was more distantly located in the phylogenetic tree. The sequence of the strain SM39 described as P. fukuyoi was only available later (Murray et al., Citation2009), showing that it was more distantly related to the type strain SM19 of P. fukuyoi than P. emarginatum strain SM35. The phylogenetic analysis of those strains, together with the sequences obtained in this study, reveals the existence of a complex of morphologically similar species. Even if the description of P. fukuyoi was based on specimens of different species, the type strain was molecularly characterized allowing an unambiguous circumscription of the species. Based on morphology, our strain Dn32EHU fits the description of P. fukuyoi, but phylogenetic analysis reveals that it is genetically too distant to be considered conspecific with strain SM19, the type strain of P. fukuyoi, being more closely related to strain SM39, described by the same authors also as P. fukuyoi. Prorocentrum lima strains form a well-supported clade, which is subdivided into two groups, one containing the local strains, and the other formed of the three strains from tropical areas, in agreement with the high genetic variability observed by other authors in strains of this species from different geographical areas.
Toxicity
Among the local strains, only Ostreopsis cf. siamensis and Prorocentrum lima were toxic to Artemia franciscana. The toxicity of both species is well documented (Murakami et al., Citation1982; Rhodes et al., Citation2000; Bravo et al., Citation2001; Foden et al., Citation2005; Ciminiello et al., Citation2006, 2008; Mohammad-Noor et al., Citation2006; Aligizaki et al., Citation2008a, Citation2009; Vale et al., Citation2009). Differences in the toxic character of a species can be due to the presence of multiple cryptic species within a morphospecies, as reported by Richlen et al. (Citation2008) for Gambierdiscus, or because the same species of microalgae may present varying toxicity depending on physiological status, environmental conditions or even between populations in different areas (Guerrini et al., Citation2009). Our C. monotis strains are not toxic, in agreement with the observations on other European Atlantic and Mediterranean strains (Penna et al., Citation2005). The only Coolia strains observed to be toxic are those identified as C. monotis by Nakajima et al. (Citation1981) from Okinawa; that from Northeastern Australia analysed and identified as C. monotis by Holmes et al. (Citation1995); those from New Zealand (Rhodes et al., Citation2000) originally identified as C. monotis; and those from Malaysia identified as Coolia sp. by Mohammad-Noor et al. (Citation2006). However, the identification of some of these strains should be revised. Thus, plate 7″ of the strain of Coolia reported by Holmes et al. (Citation1995) was more than three times wider than long, better fitting C. tropicalis than C. monotis. The analysis of the LSU rDNA of the strain CAW D39 analysed by Rhodes et al. (Citation2000) showed this strain belongs to C. malayensis, and the morphology of Coolia sp. from Malaysia (Mohammad-Noor et al. Citation2006) shows many morphological similarities to C. malayensis, which together with their geographical co-occurrence, suggests they could be the same species. No morphological characterization is given for the C. monotis strain analysed by Nakajima et al. (Citation1981), but interestingly this strain, like other toxic strains, came from the western Pacific Ocean. Taking into account the high morphological similarity between C. monotis and C. malayensis, the posibility that the strain analysed by Nakajima et al. (Citation1981) belongs to C. malayensis cannot be excluded. It can be hypothesized that part of the discrepancy concerning toxicity of different C. monotis strains is due to the presence of a second toxic semicryptic species (C. malayensis). Nevertheless, more molecular studies of toxic strains of Coolia are needed to gain insight into their taxonomy.
The toxicity of P. rhathymum is also controversial. In agreement with the results of Mohammad-Noor et al. (Citation2006), our strain was non-toxic to Artemia. However, P. rhathymum has been considered toxic by several authors (Nakajima et al., Citation1981; Tindall et al., Citation1989; Aligizaki et al., Citation2009). Pearce et al. (Citation2005) observed that Artemia did not display any adverse effects of exposure to P. rhathymum although the crustacean experienced mortality when exposed to lipid-soluble extracts of the algae. The nature of the toxins of P. rhathymum remained unknown until the study of Caillaud et al. (Citation2010), which revealed the production of low levels of okadaic acid by this species. Prorocentrum emarginatum cells were not toxic for Artemia, in agreement with other reports (Mohammad-Noor et al., Citation2006; Escoffier et al., Citation2007; Aligizaki et al., Citation2009). Its sister taxon, P. fukuyoi, is considered to be a non-toxic species.
In conclusion, the epibenthic dinoflagellate community from the south-eastern Bay of Biscay comprises typically temperate species such as Prorocentrum lima and Coolia monotis, others with a pantropical distribution such as Ostreopsis cf. siamensis and Prorocentrum rhathymum, and others with no clear biogeographical pattern. This study also shows the existence of a geographical pattern of genetic diversity among strains of the same morphospecies, such as P. lima, and the difficulties in identification of the local strains of the group P. emarginatum/P. fukuyoi. It is important to gain insight into the morphological differences among the members of this group, some of which remain morphologically cryptic or semicryptic. It will be interesting to determine the extent to which biogeographical and genetic differences found in similar morphospecies are also shown in the physiology of the algae including the production of toxins and the response to climatic factors.
tejp_a_550387_sup_17764780.pdf
Download PDF (45.5 KB)Acknowledgements
Financial support for this research was provided by the Department for Environment of Bizkaiko Foru Aldundia, the Bilbao-Bizkaia Water Consortium, and the Basque Government (projects IT-417-07 and Etortek 2007: K-egokitzen). This work was also supported by a specialization fellowship for PhD researchers awarded by the University of the Basque Country to A. Laza-Martínez. We wish to thank Fernando Ugalde for his assistance with the toxicity tests. We also thank the reviewers for very helpful comments and suggestions.
References
- Aligizaki , K , Katikou , P , Nikolaidis , G and Panou , A . 2008a . First episode of shellfish contamination by palytoxin-like compounds from Ostreopsis species (Aegean Sea, Greece) . Toxicon , 51 : 418 – 427 .
- Aligizaki , K and Nikolaidis , G . 2006 . The presence of the potentially toxic genera Ostreopsis and Coolia (Dinophyceae) in the North Aegean Sea, Greece . Harmful Algae , 5 : 717 – 730 .
- Aligizaki , K and Nikolaidis , G . 2008 . Morphological identification of two tropical dinoflagellates of the genera Gambierdiscus and Sinophysis in the Mediterranean Sea . J. Biol. Res.-Thessalon. , 9 : 75 – 82 .
- Aligizaki , K , Nikolaidis , G and Fraga , S . 2008b . Is Gambierdiscus expanding to new areas? . Harmful Algae News , 36 : 6 – 7 .
- Aligizaki , K , Nikolaidis , G , Katikou , P , Baxevanis , AD and Abatzopoulos , TJ . 2009 . Potentially toxic epiphytic Prorocentrum (Dinophyceae) species in Greek coastal waters . Harmful Algae , 8 : 299 – 311 .
- Bravo , I , Fernández , ML , Ramilo , I and Martínez , A . 2001 . Toxin composition of the toxic dinoflagellate Prorocentrum lima isolated from different locations along the Galician coast (NW Spain) . Toxicon , 39 : 1537 – 1545 .
- Caillaud , A , de la Iglesia , P , Campas , M , Elandaloussi , L , Fernandez , M , Mohammad-Noor , N , Andree , K and Diogene , J . 2010 . Evidence of okadaic acid production in a cultured strain of the marine dinoflagellate Prorocentrum rhathymum from Malaysia . Toxicon , 55 : 633 – 637 .
- Ciminiello , P , Dell’Alversano , C , Fattorusso , E , Forino , M , Magno , GS , Tartaglione , L , Grillo , C and Melchiorre , N . 2006 . The Genoa 2005 outbreak. Determination of putative palytoxin in Mediterranean Ostreopsis ovata by a new liquid chromatography tandem mass spectrometry method . Anal. Chem. , 78 : 6153 – 6159 .
- Ciminiello , P , Dell’Aversano , C , Fattorusso , E , Forino , M , Tartaglione , L , Grillo , C and Melchiorre , N . 2008 . Putative palytoxin and its new analogue, ovatoxin-a, in Ostreopsis ovata collected along the Ligurian coasts during the 2006 toxic outbreak . J. Am. Soc. Mass Spectr. , 19 : 111 – 120 .
- Chang , FH , Shimizu , Y , Hay , B , Stewart , R , Mackay , G and Tasker , R . 2000 . Three recently recorded Ostreopsis spp. (Dinophyceae) in New Zealand: temporal and regional distribution in the upper North Island from 1995 to 1997 . N.Z. J. Mar. Freshwater Res. , 34 : 29 – 39 .
- Chomerat , N , Sellos , DY , Zentz , F and Nézan , E . 2010 . Morphology and molecular phylogeny of Prorocentrum consutum sp. nov. (Dinophyceae), a new benthic dinoflagellate from south Brittany (northwestern France) . J. Phycol. , 46 : 183 – 194 .
- Cortés-Altamirano , R and Sierra-Beltrán , AP . 2003 . Morphology and taxonomy of Prorocentrum mexicanum and reinstatement of Prorocentrum rhathymum (Dinophyceae) . J. Phycol. , 39 : 221 – 225 .
- Dolapsakis , NP , Kilpatrick , MW , Economou-Amilli , A and Tafas , T . 2006 . Morphology and rDNA phylogeny of a Mediterranean Coolia monotis (Dinophyceae) strain from Greece . Sci. Mar. , 70 : 67 – 76 .
- Elbrächter , M and Hoppenrath , M . 2009 . “ Dinoflagellates/Dinophyceae ” . In Marine Phytoplankton. Selected Microphytoplankton Species from the North Sea around Helgoland and Sylt , Edited by: Hoppenrath , M , Elbrächter , M and Drebes , G . 114 – 206 . Stuttgart : Kleine Senckenberg-Reihe 49 .
- Escoffier , N , Gaudin , J , Mezhoud , K , Huet , H , Chateau-Joubert , S , Turquet , J , Crespeau , F and Edery , M . 2007 . Toxicity to medaka fish embryo development of okadaic acid and crude extracts of Prorocentrum dinoflagellates . Toxicon , 49 : 1182 – 1192 .
- Faust , MA . 1990 . Morphologic details of six benthic species of Prorocentrum (Pyrrophyta) from a mangrove island, Twin Cays, Belize, including two new species . J. Phycol. , 26 : 548 – 558 .
- Faust , MA . 1991 . Morphology of ciguatera-causing Prorocentrum lima (Pyrrophyta) from widely differing sites . J. Phycol. , 27 : 642 – 648 .
- Faust , MA , Larsen , J and Moestrup , O . 1999 . “ Leaflet No. 184. Potentially Toxic Phytoplankton, 3. Genus Prorocentrum (Dinophyceae) ” . In ICES Identification Leaflets for Plankton , Edited by: Lindley , JA . Copenhagen : International Council for the Exploration of the Sea .
- Faust , MA , Morton , SL and Quod , JP . 1996 . Further SEM study of marine dinoflagellates: the genus Ostreopsis (Dinophyceae) . J. Phycol. , 32 : 1053 – 1065 .
- Faust , MA , Vandersea , MW , Kibler , SR , Tester , PA and Litaker , RW . 2008 . Prorocentrum levis, a new benthic species (Dinophyceae) from a mangrove island, Twin Cays, Belize . J. Phycol. , 44 : 232 – 240 .
- Felsenstein , J . 1985 . Confidence limits on phylogenies: an approach using the bootstrap . Evolution , 39 : 783 – 791 .
- Fensome , RA , Taylor , FJR , Norris , G , Sarjeant , WAS , Wharton , DI and Williams , GL . 1993 . A Classification of Living and Fossil Dinoflagellates , Micropaleontology Spec. Publ. no. 7 Hanover, PA : Sheridan Press .
- Foden , J , Purdie , DA , Morris , S and Nascimento , S . 2005 . Epiphytic abundance and toxicity of Prorocentrum lima populations in the Fleet Lagoon, UK . Harmful Algae , 4 : 1063 – 1074 .
- Fraga , S , Penna , A , Bianconi , I , Paz , B and Zapata , M . 2008 . Coolia canariensis sp. nov. (Dinophyceae), a new nontoxic epiphytic benthic dinoflagellate from the Canary Islands . J. Phycol. , 44 : 1060 – 1070 .
- Fukuyo , Y . 1981 . Taxonomical study on benthic dinoflagellates collected in coral reefs . B. Jpn. Soc. Sci. Fish. , 47 : 967 – 978 .
- Guerrini , F , Pezzolesi , L , Feller , A , Riccardi , M , Ciminiello , P , Dell’Aversano , C , Tartaglione , L , Dello Iacovo , E , Fattorusso , E , Forino , M and Pistocchi , R . 2009 . Comparative growth and toxin profile of cultured Ostreopsis ovata from the Tyrrhenian and Adriatic Seas . Toxicon , 55 : 211 – 220 .
- Guillard , RRL . 1975 . “ Culture of phytoplankton for feeding marine invertebrates ” . In Culture of Marine Invertebrate Animals , Edited by: Smith , WL and Chanley , MH . 26 – 60 . New York : Plenum Press .
- Guindon , S and Gascuel , O . 2003 . A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood . Syst. Biol. , 52 : 696 – 704 .
- Hall , TA . 1999 . BioEdit: a user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT . Nucleic Acids Symp. Ser. , 41 : 95 – 98 .
- HANSEN, G., TURQUET, J., QUOD, J. P., TEN-HAGE, L., LUGOMELA, C., KYEWALYANGA, M., HURBUNGS, M., WAWIYE, P., OGONGO, B., TUNJE, S. & RAKOTOARINJANAHARY. (2001). Potentially Harmful Microalgae of the Western Indian Ocean. A Guide Based on a Preliminary Survey. IOC Manuals and Guides No. 41, Intergovernmental Oceanographic Commission of UNESCO.
- Holmes , MJ , Lewis , RJ , Jones , A and Wong Hoy , AW . 1995 . Cooliatoxin, the first toxin from Coolia monotis (Dinophyceae) . Nat. Toxins , 3 : 355 – 362 .
- Hoppenrath , M and Leander , BS . 2008 . Morphology and molecular phylogeny of a new marine sand-dwelling Prorocentrum species, P. tsawwassenense (Dinophyceae, Prorocentrales), from British Columbia, Canada . J. Phycol. , 44 : 451 – 466 .
- Ingarao , C , Lanciani , G , Verri , C and Plagliani , T . 2009 . First record of Prorocentrum lima (Dinophyceae) inside harbor areas and along the Abruzzo region coast, W Adriatic . Mar. Pollut. Bull. , 58 : 596 – 600 .
- Ismael , GE and Aida , M . 1997 . First record of a Prorocentrum rhathymum (Prorocentraceae) red tide in the Gulf of California . Rev. Biol. Trop. , 45 : 1263
- Kimura , M . 1980 . A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences . J. Mol. Evol. , 16 : 111 – 120 .
- Larkin , MA , Blackshields , G , Brown , NP , Chenna , R , McGettigan , PA , McWilliam , H , Valentin , F , Wallace , IM , Wilm , A , Lopez , R , Thompson , JD , Gibson , TJ and Higgins , DG . 2007 . Clustal W and Clustal X version 2.0 . Bioinformatics , 23 : 2947 – 2948 .
- Leaw , CP , Lim , PT , Cheng , KW , Ng , BK and Usup , G . 2010 . Morphology and molecular characterization of a new species of thecate benthic dinoflagellate, Coolia malayensis sp. nov. (Dinophyceae) . J. Phycol. , 46 : 162 – 171 .
- Levasseur , M , Couture , J-Y , Weise , AM , Michaud , S , Elbrächter , M , Sauvé , G and Bonneau , E . 2003 . Pelagic and epiphytic summer distributions of Prorocentrum lima and P. mexicanum at two mussel farms in the Gulf of St. Lawrence, Canada . Aquat. Microb. Ecol. , 30 : 283 – 293 .
- Litaker , RW , Vandersea , MW , Fasut , MA , Kibler , SR , Chinain , M , Holmes , MJ , Holland , WC and Tester , PA . 2009 . Taxonomy of Gambierdiscus including four new species, Gambierdiscus caribaeus, Gambierdiscus carolinianus, Gambierdiscus carpenteri and Gambierdiscus ruetzleri (Gonyaulacales, Dinophyceae) . Phycologia , 48 : 344 – 390 .
- Loeblich , AR III , Sherley , JL and Schmidt , RJ . 1979 . The correct position of flagellar insertion in Prorocentrum and description of Prorocentrum rhathymum sp. nov. (Pyrrhophyta) . J. Plankton Res. , 1 : 113 – 120 .
- Mangialajo , L , Bertolotto , R , Cattaneo-Vietti , R , Chiantore , M , Grillo , C , Lemee , R , Melchiorre , N , Moretto , P , Povero , P and Ruggieri , N . 2008 . The toxic benthic dinoflagellate Ostreopsis ovata: quantification of proliferation along the coastline of Genoa, Italy . Mar. Poll. Bull. , 56 : 1209 – 1214 .
- Maranda , L , Corwin , S and Hargraves , PE . 2007 . Prorocentrum lima (Dinophyceae) in northeastern USA coastal waters. I. Abundance and distribution . Harmful Algae , 6 : 623 – 631 .
- Meunier , A . 1919 . Microplancton de la mer Flamande . Mem. Mus. R. Hist. Nat. Bruxelles , 8 : 1 – 116 .
- Mohammad-Noor , N , Daugbjerg , N , Moestrup , O and Anton , A . 2006 . Marine epibenthic dinoflagellates from Malaysia – a study of five cultures and preserved samples based on light and scanning electron microscopy . Nord. J. Bot. , 24 : 629 – 690 .
- Monti , M , Minocci , M , Beran , A and Ibesa , L . 2007 . First record of Ostreopsis cfr ovata on macroalgae in Northern Adriatic Sea . Mar. Pollut. Bull. , 54 : 598 – 601 .
- Murakami , Y , Oshima , Y and Yasumoto , T . 1982 . Identification of okadaic acid as a toxic component of a marine dinoflagellate Prorocentrum lima . B. Jpn. Soc. Sci. Fish. , 48 : 69 – 72 .
- Murray , S , Nagahama , Y and Fukuyo , Y . 2007 . Phylogenetic study of benthic, spine-bearing prorocentroids, including Prorocentrum fukuyoi sp. nov . Phycol. Res. , 55 : 91 – 102 .
- Murray , S , Camilla , L-C , Moore , R , Nagahama , Y and Fukuyo , Y . 2009 . Are prorocentroid dinoflagellates monophyletic? A study of 25 species based on nuclear and mitochondrial genes . Protist , 160 : 245 – 264 .
- Nagahama , Y and Fukuyo , Y . 2005 . Redescription of Cryptomonas lima, collected from Sorrento, Italy, the basionym of Prorocentrum lima . Plank. Biol. Ecol. , 52 : 107 – 109 .
- Nakajima , I , Oshima , Y and Yasumoto , T . 1981 . Toxicity of benthic dinoflagellates in Okinawa . Bull. Jpn. Soc. Sci. Fish. , 47 : 1029 – 1033 .
- Nascimento , SM , Purdie , DA and Morris , S . 2005 . Morphology, toxin composition and pigment content of Prorocentrum lima strains isolated from a coastal lagoon in southern UK . Toxicon , 45 : 633 – 649 .
- Nei , M and Kumar , S . 2000 . Molecular Evolution and Phylogenetics , New York : Oxford University Press .
- Pearce , I , Handlinger , JH and Hallegraeff , GM . 2005 . Histopathology in Pacific oyster (Crassostrea gigas) spat caused by the dinoflagellate Prorocentrum rhathymum . Harmful Algae , 4 : 61 – 74 .
- Penna , A , Fraga , S , Battocchi , C , Casabianca , S , Giacobbe , MG , Riobo , P and Vernesi , C . 2010 . A phylogeographical study of the toxic benthic dinoflagellate genus Ostreopsis Schmidt . J. Biogeogr. , 37 : 830 – 841 .
- Penna , A , Vila , M , Fraga , S , Giacobbe , MG , Andreoni , F , Riobo , P and Vernesi , C . 2005 . Characterization of Ostreopsis and Coolia (Dinophyceae) isolates in the western Mediterranean Sea based on morphology, toxicity and internal transcribed spacer 5.8s rDNA sequences . J. Phycol. , 41 : 212 – 225 .
- Posada , D . 2008 . jModelTest: Phylogenetic Model Averaging . Mol. Biol. Evol. , 25 : 1253 – 1256 .
- Revilla , M , Borja , A , Fontán , A , Franco , J , González , M and Valencia , V . 2010 . A two-decade record of surface chlorophyll ‘a’ and temperature in offshore waters of the Basque country (southeastern Bay of Biscay) . Rev. Inv. Mar. , 17 : 13 – 20 .
- RHODES, L.L. (2010). World-wide occurrence of the toxic dinoflagellate genus Ostreopsis Schmidt. Toxicon, in press, doi: 10.1016/j.toxicon.2010.05.010.
- Rhodes , L , Adamson , J , Suzuki , T , Briggs , L and Garthwaite , I . 2000 . Toxic marine epiphytic dinoflagellates, Ostreopsis siamensis and Coolia monotis (Dinophyceae), in New Zealand . N.Z. J. Mar. Freshwater Res. , 34 : 371 – 383 .
- Richlen , ML , Morton , SL , Barber , PH and Lobel , PS . 2008 . Phylogeography, morphological variation and taxonomy of the toxic donoflagellate Gambierdiscus toxicus . Harmful Algae , 7 : 614 – 629 .
- Saburova , M , Al-Yamani , F and Polikarpov , I . 2009 . Biodiversity of free-living flagellates in Kuwait's intertidal sediments . BioRisk , 3 : 97 – 110 .
- Scholin , CA , Anderson , DM and Sogin , ML . 1993 . Existence of two distinct small-subunit rRNA genes in the North American toxic dinoflagellate Alexandrium fundyense (Dinophyceae) . J. Phycol. , 29 : 209 – 216 .
- Selina , MS and Orlova , TY . 2010 . First occurrence of the genus Ostreopsis (Dinophyceae) in the Sea of Japan . Bot. Mar. , 53 : 243 – 249 .
- Shears , NT and Ross , PM . 2009 . Blooms of benthic dinoflagellates of the genus Ostreopsis; an increasing and ecologically important phenomenon on temperate reefs in New Zealand and worldwide . Harmful Algae , 8 : 916 – 925 .
- Steidinger , KA . 1983 . “ A re-evaluation of toxic dinoflagellate biology and ecology ” . In Progress in Phycological Research, Vol. 2 , Edited by: Round , FE and Chapman , D . 147 – 188 . Amsterdam : Elsevier .
- Tamura , K , Dudley , J , Ner , M and Kumar , S . 2007 . MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0 . Mol. Biol. Evol. , 24 : 1596 – 1599 .
- Taylor , FJR . 1980 . On dinoflagellate evolution . Biosystems , 13 : 65 – 108 .
- Tindall , DR , Miller , DM and Bomber , JW . 1989 . Culture and toxicity of dinoflagellates from ciguatera endemic regions of the world . Toxicon , 27 : 83
- Totti , C , Accoroni , S , Cerino , F , Cucchiari , E and Romagnoli , T . 2010 . Ostreopsis ovata bloom along the Conero Riviera (northern Adriatic Sea): relationships with environmental conditions and substrata . Harmful Algae , 9 : 233 – 239 .
- Turki , S . 2005 . Distribution of toxic dinoflagellates along the leaves of seagrass Posidonia oceanica and Cymodocea nodosa from the Gulf of Tunis . Cah. Biol. Mar. , 46 : 29 – 34 .
- Vale , P , Veloso , V and Amorim , A . 2009 . Toxin composition of a Prorocentrum lima strain isolated from the Portuguese coast . Toxicon , 54 : 145 – 152 .
- Vila , M , Garces , E and Maso , M . 2001 . Potentially toxic epiphytic dinoflagellate assemblages on macroalgae in the NW Mediterranean . Aquat. Microb. Ecol. , 26 : 51 – 60 .
Supplementary material
The following supplementary material is available for this article, accessible via the Supplementary Content tab on the article's online page at http://dx.doi.org/10.1080.09670262.2010.550387
Supplementary Table. Geographical locations of sampling sites.