Abstract
Despite its widespread distribution in the Indo-West Pacific Ocean, Sarcodia is one of the least understood genera among the red algae. Samples investigated from different parts of the Indo-West Pacific Ocean were well separated phylogenetically, based on rbcL base pair distances, and probably represent separate species. In this report we investigate the developmental morphology of two species from New Zealand: the type species, Sarcodia montagneana, and S. grandifolia (including S. ‘flabellata’). Molecular studies indicate that S. montagneana is restricted to the north-eastern part of the North Island and that S. grandifolia occurs from Wellington southwards as far as the Snares Islands. Thalli are multiaxial and consist of three primary layers: a surface layer of uninucleate cells, a cortex of five or six layers of polygonal multinucleate cells, and a medulla of multinucleate stellate cells. Secondary rhizoidal filaments are frequent to abundant in the medulla. Spermatangia occur in chains and release spermatia through a pore. The female reproductive system is procarpic and consists of a supporting cell that bears two side branches and a one-celled terminal carpogonium. The auxiliary cell is the basal cell of one of the side branches and is undifferentiated prior to fertilization. After presumed fertilization, the carpogonium separates into a terminal cell and a subterminal hypogynous cell that fuses with the auxiliary cell and deposits in it a single nucleus. The auxiliary cell cuts off a gonimoblast initial obliquely that first forms a short linear chain of gonimoblast cells. Cystocarp formation proceeds rapidly with the outward development of linear files derived from surface cells that differentiate into a terminal ostiole and inner and outer pericarp layers separated by a central cavity. The mature gonimoblasts consist of an inner gonimoblast reticulum of laterally fused cells that attach secondarily to outer cells of the inner pericarp and outer gonimoblast filaments that terminate in short chains of carposporangia. Tetrasporangia are zonately divided and are scattered over the thallus surface with new initials formed continuously. The morphological and molecular observations reported here contrast sharply with those of previous studies and are largely new to the Sarcodia literature.
Introduction
J.G. Agardh (Citation1852, p. 622) established Sarcodia in the tribe Sphaerococcoideae based on a single species, Rhodomenia (Rhodymenia) montagneana, which J.D. Hooker & Harvey (Citation1845) described from the Bay of Islands, New Zealand. Agardh characterized thalli of the genus as flat, fleshy–membranous, 3–5 times dichotomously branched, and consisting of three layers: a surface layer of small vertically rounded cells, an intermediate layer of angular rounded cells, and an interior layer of laxly intertwining hyaline filiform cells. The cystocarps were described as being hemispherical, raised along the margins and surfaces of the dichotomous branches, and bearing obovate-elongate carpospores on dichotomously branched filaments. Gonimoblast filaments were said to surmount a raised central placenta inside an ostiolate pericarp of concentric layers of radiating cells. Tetrasporangia were described as being oblong, zonately quadripartite and not aggregated in sori but distributed individually over the frond. The key distinguishing characters given by Agardh that separated Sarcodia from other members of the Sphaerococcoideae were the tripartite layering of the vegetative thallus and the scattered, zonately divided tetrasporangia.
Kylin (Citation1932, 1956) established the family Sarcodiaceae to contain Sarcodia J. Agardh, Chondrymenia Zanardini, Dicurella Harvey, Trematocarpus Kützing, and provisionally Nizymenia Sonder. Dicurella has been merged with Trematocarpus (see Searles, Citation1969) and Nizymenia is placed in its own family (see Chiovitti et al., Citation1995). In establishing the Sarcodiaceae, Kylin emphasized the outward orientation of the gonimoblasts, its development from an irregular fusion cell, and the zonate division of the tetrasporangia. Rasmussen (Citation1964) reported that the carpogonial branch system of S. montagneana (J.D. Hooker & Harvey) J. Agardh consists of a supporting cell, one or two carpogonial branches of three cells each, and two or three subsidiary branches, and also that the hypogynous cell links with a nearby undifferentiated auxiliary cell after presumed fertilization by means of a connecting filament. He referred to the gonimoblast fusion cell as a reticulum. Similarly, Norris (Citation1987) described a connecting filament in S. dentata (Suhr) R.E. Norris that linked the carpogonial branch to a nearby auxiliary cell, and also referred to the inner gonimoblast region as a reticulate fusion cell. Norris suggested that vegetative cells might play a role in the formation of the fusion cell. More recently, Kraft (in Liao et al., Citation1993) illustrated a three-celled carpogonial branch in S. montagneana that was similar to the one described earlier by Searles (Citation1968, Citation1969) in species of Trematocarpus.
Kylin (Citation1932, 1956) placed his family Sarcodiaceae in the order Gigartinales; however, Saunders et al. (Citation2004) specifically excluded the family from the Gigartinales and instead inferred a weak relationship between the Sarcodiaceae and the Plocamiales based on a phylogenetic analysis of nuclear small-subunit ribosomal DNA sequences. Guiry & Guiry (Citation2011) list 20 species (and infraspecific) names in their current database under Sarcodia, of which 12 are flagged as currently accepted taxonomically. Even so, S. montagneana is the name commonly chosen by the majority of phycologists when reporting a Sarcodia species from localities in the Indo-West Pacific Ocean (Silva et al., Citation1996; Guiry & Guiry, Citation2011) and from Antarctica and subantarctic waters (Wiencke & Clayton, Citation2002, but see Hommersand et al., Citation2009). This is not surprising, since morphological variation overlaps among many collections and the habit of most falls within the range of forms reported for S. montagneana from New Zealand.
In the present study we investigate the structure and distribution of the two species of Sarcodia found in New Zealand, S. montagneana and S. grandifolia (including S. ‘flabellata’) based on a detailed analysis of their vegetative and reproductive development. A phylogeny of representative species of Sarcodia from New Zealand, Australia and regions of the Indo-West Pacific Ocean is presented and evaluated based on analyses of rbcL sequences.
Materials and methods
Morphological observations
Specimens of Sarcodia montagneana and S. grandifolia were collected from the New Zealand coast subtidally from –0.5 m to c. –25 m depth, or from the drift. Material was observed in surface view or sectioned with a freezing microtome, and preparations were stained either with 1% acidified aqueous aniline blue or with a solution of haematoxylin according to the method of Rodríguez-Prieto & Hommersand (Citation2009). Habit pictures were taken with a Canon EOS 350D (Canon, Tokyo, Japan) and photomicrographs were made with an AxioCam MRc attached to an Axioskop 2 plus microscope (Zeiss, Berlin, Germany). Habit scans of historically important collections were provided by the Natural History Museum, London, UK (BM) and Trinity College, Dublin, Ireland (TCD). Voucher specimens were deposited in the herbaria of the Museum of New Zealand Te Papa Tongarewa, Wellington, NZ (WELT); Auckland Institute and Museum, Auckland, NZ (AK); Herbarium of the University of Girona, Spain (HGI); and herbarium of the University of North Carolina, Chapel Hill, USA (NCU). Herbarium abbreviations follow Thiers (Citation2011, continuously updated) Index Herbariorum: http://sweetgum.nybg.org/ih/.
Molecular observations
DNA from silica gel dried specimens was extracted using DNeasy Plant Mini Kits (Qiagen, Valencia, CA, USA) following the manufacturer's instructions. DNA sequencing procedures were as described in Lin et al. (Citation2001). New sequence data and those available from GenBank were compiled and aligned with Sequencher (Gene Codes, Ann Arbor, MI, USA). Thirteen rbcL sequences were newly generated. Locality data for each sample analysed are provided in .
Table 1. List of species used in rbcL analysis with GenBank accession numbers.
Phylogenetic analyses were performed using maximum parsimony (MP), maximum likelihood (ML) and Bayesian analyses (BA) using PAUP* v4.0 (Swofford, Citation2002), GARLI 1.0 (Zwickl, Citation2006) and MrBayes 3.1.2 (Ronquist & Huelsenbeck, Citation2003), respectively, as described in Lin et al. (2001, 2004, 2011). Support values for MP and ML trees were assessed by calculating 1000 and 100 bootstrap resamplings using PAUP* v4.0 and GARLI 1.0, respectively. The general-time-reversible model for ML was used as the default in GARLI 1.0. A Bayesian analysis was performed in MrBayes 3.1.2 (Ronquist & Huelsenbeck, Citation2003) using a GTR + I + Γ model, which allowed for rate variation among different codon positions. The analysis used four chains, one cold and three incrementally heated. Each run started with a random tree and consisted of 106 generations with sampling every 100 generations. Stationarity was reached at generation 5600. Thus, trees saved until generation 5600 were the ‘burn-in’ of the chain, and inferences about the phylogeny were based on those trees sampled after generation 5600. A 50% consensus tree (majority rule as implemented by PAUP* v4.0) was computed from the 9944 + 1 trees saved after the burn-in point.
Results
Morphological observations
Sarcodia montagneana (J.D. Hooker & Harvey) J. Agardh Citation 1852 , p. 623
(–)
-
BASIONYM: Rhodymenia montagneana J.D. Hooker & Harvey Citation1845, p. 544–545 (‘Rhodomenia').
-
LECTOTYPE (designated here): Cystocarpic specimen in Herb. BM, BM001032008 labelled ‘Bay of Islands, New Zealand, September L/27’ collected by David Lyall, during the Antarctic Expedition of the Erebus and Terror 1839–1843 ().
-
TYPE LOCALITY: New Zealand, North Island, Bay of Islands (Silva et al., Citation1996).
-
SYNTYPES: Samples of S. montagneana collected by either David Lyall or J.D. Hooker in the Bay of Islands, North Island, New Zealand, between August and November 1841, during the Antarctic Expedition of the Erebus and Terror 1839–1843. TCD0012148 labelled ‘N. Zeal., R. montagneana’ (this is the reverse of the image published without comment by Harvey in Nereis Australis Citation1849, pl. 48) (); TCD0012150, labelled ‘New Zealand J.D.H., R. Montagneana H & H’; BM000044273 labelled ‘Rhod. Montagnei, H & H, New Zealand, Antarct. Exped. 1839–1843 J.D.H.’; BM000044274 labelled ‘Bay of Islands, New Zealand, September JDH’ (cystocarpic); BM001032007 labelled ‘R. ornata? Mont., Bay of Islands, New Zealand, September 1841’; BM000044272 with added label ‘Rhod. Montagnei, H & H, New Zealand, Antarct. Exped. 1839–1843 J.D.H’; BM001032001 labelled ‘R. Montagnei H & H, Bay of Islands, New Zealand, September 1841’; BM 00103200 labelled ‘Rhod. Montagnena H & Harv, New Zealand, Herb. Dickie’; BM00004271 labelled ‘Rhodomenia Montagnei H & H, N. Zealand’ (cystocarpic); BM001032006 labelled ‘Bay of Islands, New Zealand, September 1841’; BM001032005 labelled ‘Bay of Islands, New Zealand, September 1841’. (Note: not all the specimens in the type folder at BM are confirmed to be Sarcodia montagneana.)
-
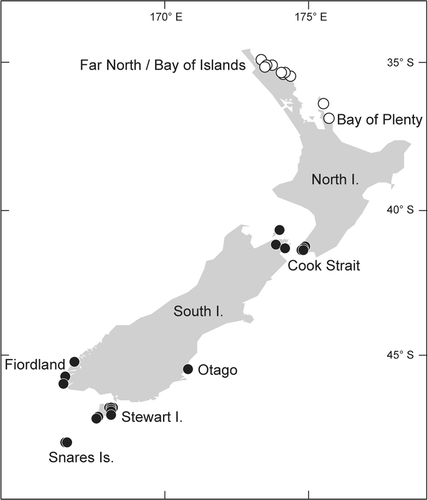
DISTRIBUTION: Northern North Island, east coast to the Bay of Plenty, New Zealand ().
-
HABITAT: From the intertidal region to −11 m depth, collected throughout the year.
-
SPECIMENS EXAMINED: North Island, New Zealand: Doubtless Bay, Mangonui, 34°59′S, 173°32′E (V.W. Lindauer, 05 December 1942, WELT A020586). Bay of Islands: Poraenui Point, 35°11.4′S 174°04.2′E (R. D’Archino, 30 November 2009, WELT A030196, tetrasporangial); Moturoa (S point), 35°13′S, 174°05′E, –5 m (K.A. Johnson, 15 November 1974, WELT A008920, tetrasporangial); Te Miko Reef (between Moturua and Motuarohia), 35°13.4′S, 174°10.5′E, −10.4 m (R. D’Archino, 21 September 2010, WELT A030869, female); East of Motuarohia, 35°13.9′S 174°10.9′E, −11 m, on rhodolith bed (R. D’Archino, 02 December 2009, HGI-A 9545, female); Long Beach, 35°14.8′S 174°07.3′E, drift (R. D’Archino, 18 September 2010, WELT A030870, female); Oneroa Bay, 35°15′S 174°07′E, −5 to −8 m, on forest of Carpophyllum flexuosum (Esper) Greville, sheltered site (R. D’Archino, 02 December 2009, WELT A030191, female); Paihia, 35°17′S, 174°06′E, drift (W.A. Nelson, 07 November 1991, WELT A020101, female); Paihia, 35°17′S, 174°06′E, drift (W.A. Nelson, 27 April 1990, WELT A019414, male); Kahuwhera Point, 35°26′S, 174°18′E, −6.3 m (R. D’Archino, 19 September 2010, WELT A030867, female; WELT A030868, tetrasporangial); Whitianga, Buffalo Beach, 36°49′S, 175°42′E, drift (U.V. Dellow, 10 December 1949, WELT A002706, female).
-
Habit and vegetative structure: Thallus erect, flattened, up to 25 (–40) cm in length, attached to the substratum by a discoid holdfast, shortly stipitate, complanate, subdichotomously branched with rounded axils and apices (). Axis up to 2 (–6) cm wide. Margins smooth or occasionally proliferous (), slightly thicker than the lamina. Thallus moderately firm and rosy to dark red in colour.
Figs 2–13. Sarcodia montagneana, habit and vegetative development. , : aniline blue; : haematoxylin. 2. Lectotype: Rhodomenia (Rhodymenia) montagneana J.D. Hooker & Harvey Citation1845, Herb. BM001032008. Label reads: Bay of Islands, New Zealand, September 28 L/27. This specimen was collected by David Lyall and corresponds to the description and discussion of a female plant in Hooker & Harvey, Citation1845, pp. 544–545. 3. Rhodomenia (Rhodymenia) montagneana J.D. Hooker & Harvey Citation1845, Herb. TCD0012148. The label reads: N. Zeal. R. Montagneana. The figure published by Harvey in Nereis Australis, Citation1849, pl. XLVIII is the reverse image of this plant. 4. Sarcodia montagneana (Hook. fil. & Harvey) J. Agardh. Female plant, WELT A020101, drift, Northland, Bay of Islands, Paihia, coll. W.A. Nelson, 07 November 1991. 5. Part of a young branch of plant in showing early development of cystocarps along thallus margins and surface. 6. Transverse section near apex of thallus (HGI-A 9545). 7. Transverse section showing stellate cells in young medulla (arrow) (WELT A030191). 8. Transverse section of surface layer and outer cortex (WELT A030191). 9. Detail of cortex and surface layer in cross section, showing nucleus and plastid in surface cells (WELT A030870). 10. Surface view showing nucleus and plastid in surface cells (HGI-A 9545). 11. Inner multinucleate subcortical cell showing the small nuclei and dissected ribbon-like plastid (HGI-A 9545). 12. Multinucleate stellate cell in medulla compared to cortical cell in (HGI-A 9545). 13. Transverse section of cortex and medulla showing distribution of cortical and stellate medullary cells and secondary rhizoidal filaments (HGI-A 9545). Abbreviations: n: nucleus; p: plastid. Scale bars: 5 cm (), 100 µm (, ) or 20 µm ().
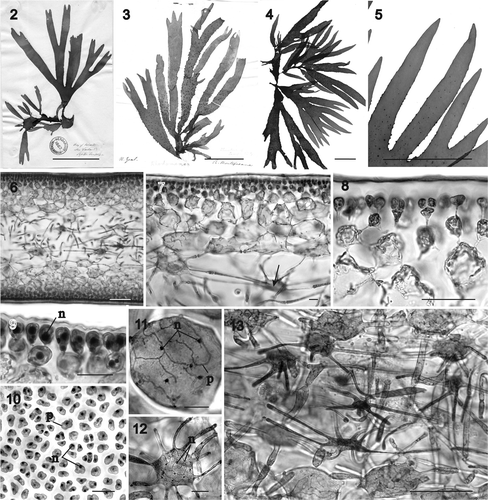
Figs 14–24. Sarcodia montagneana. Spermatangial and early stages of female reproductive development. Haematoxylin. 14. Transverse section of a spermatangial sorus derived from surface layer showing elongated spermatangial parent cells (WELT A019414). 15. Spermatangia aligned in rows (WELT A019414). 16. Transverse section of female plant showing a potentially fertile surface area (WELT A030191). 17. Supporting cell undergoing nuclear division (WELT A030870). 18. Supporting cell bearing paired unicellular, uninucleate sterile branches (WELT A030869). 19. Supporting cell bearing two 2-celled sterile branches (WELT A030191). 20. Carpogonium with short trichogyne partly covered by a 2-celled sterile branch(WELT A030191). 21. Supporting cell bearing a carpogonium and two sterile branches. The arrow points to the pit connection between the supporting cell and a sterile branch (WELT A030870). 22. Supporting cell bearing an elongating carpogonium and two sterile branches (WELT A030870). 23. Supporting cell bearing a carpogonium and sterile branch in which the basal cell has differentiated into an auxiliary cell (WELT A030870). 24. Supporting cell with two side branches in which the basal cell of one has differentiated into an auxiliary cell. The carpogonium has divided into a hypogynous cell and a terminal cell (WELT A030870). Abbreviations: ac: auxiliary cell; cp: carpogonium; hy: hypogynous cell; n: nucleus; sb: sterile branches; sc: supporting cell; sg: spermatangium; spc: spermatangial parent cell; t: trichogyne. Scale bars: 20 µm.
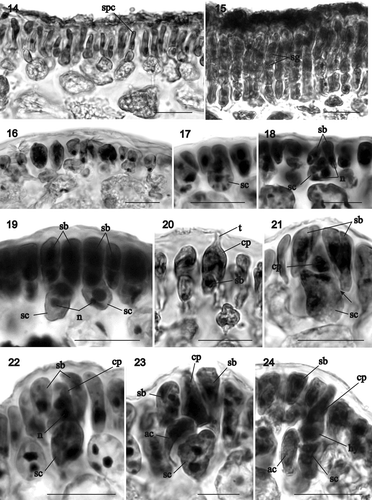
Figs 25–33. Sarcodia montagneana. Postfertilization stages. : haematoxylin; : aniline blue. 25. Supporting cell, sterile side branch and carpogonial remnant. The enlarged auxiliary cell has cut off the first gonimoblast cell (WELT A030191). 26–28. Two-celled stages in gonimoblast development from the auxiliary cell (WELT A030870). 29. Early stage in cystocarp development showing differentiation of the darkly staining inner pericarp (inside circle) from outer pericarp (WELT A030191). 30. Initiation of the central cavity above the developing gonimoblasts, and separation of the inner and outer pericarp regions with some cells elongating (arrow) between the two (WELT A030191). 31. Radiation of gonimoblast filaments at the centre of inner pericarp and gonimoblast expansion into central cavity (HGI-A 9545). 32. Cystocarp with ostiole and differentiation of gonimoblasts into an inner gonimoblast reticulum and outer covering of carposporangia-bearing filaments. Cells adjacent to and above the inner pericarp link to inner pericarp cells without exchanging nuclei (arrow) (HGI-A 9545). 33. Organization of gonimoblast reticulum composed of fused uninucleate cells and transition to outer carposporangia-bearing layers (HGI-A 9545). Abbreviations: ac: auxiliary cell; c: carposporangia; cp: carpogonium; g1: gonimoblast initial; g2: gonimoblast cell developed from g1; gf: gonimoblast filaments; gr: gonimoblast reticulum; ip: inner pericarp; n: nucleus; o: ostiole; op: outer pericarp; sb: sterile branches; sc: supporting cell. Scale bars: 20 µm.
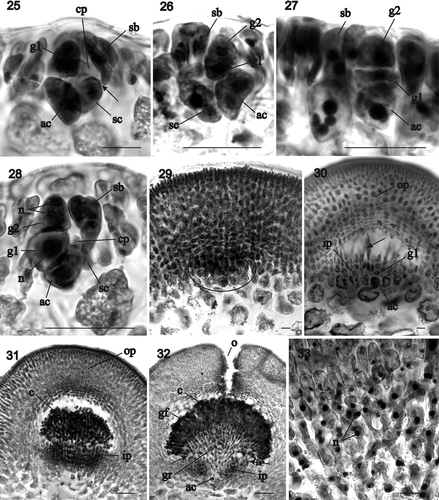
Figs 34–44. Sarcodia montagneana. Postfertilization stages and tetrasporangia development. , , : haematoxylin; , : aniline blue. 34. Portion of a fresh specimen with cystocarps borne along margins and over thallus surface (HGI-A 9545). 35–36. Detail of the ostiole in surface view and in cross section (WELT A030191). 37. Surface and inner cells of the outer pericarp (HGI-A 9545). 38. Mature outer pericarp showing the inner layer of slightly stellate cells (arrow) surrounding cystocarp cavity (HGI-A 9545). 39. Uninucleate mature carposporangia (HGI-A 9545). 40. Group of carposporangia in which one of them is germinating in situ (arrow) (WELT A030191). 41–44. Development of the tetrasporangial initials and zonately dividing tetrasporangia. Note that each tetrasporangium is pit-connected basally with a subcortical cell (arrows), and that the mature tetrasporangia are surrounded by elongated cortical filaments (arrowheads) (WELT A030196). Abbreviation: n: nucleus. Scale bars: 20 µm (), 1 cm () or 100 µm ().
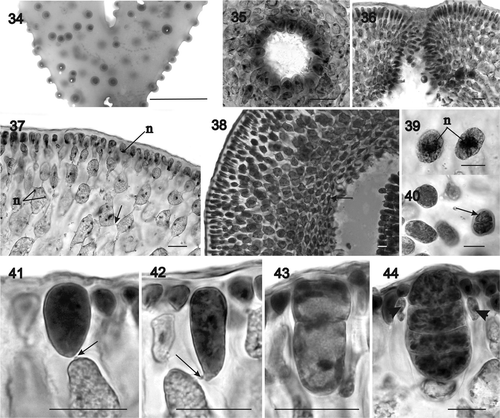
Figs 45–54. Sarcodia grandifolia, habit and vegetative development. : aniline blue; , , : haematoxylin. 45. Lectotype, Levring No. 7585, WELT A022926, Stewart Island, New Zealand, V.W. Lindauer No. 7585, 24 June 1946. 46. A broad, twice-forked female plant, The Neck, beach facing Cow Island, Stewart Island, J.F. Herrick, 07 October 1994 (AK220918). 47. Female plant. Attached, lower littoral, Princess Bay, Wellington, Auckland, New Zealand, coll. D.W. Freshwater (NCU 591801, upper left). 48. Longitudinal section through tip (NCU 591799-802). 49. Surface layer and outer cortex in cross section (NCU 591803-811). 50. External view of uninucleate surface cells with parietal plastids (NCU 591799-802). 51. Multinucleate inner cortical cell with dissected ribbon-shaped plastid (NCU 591803-811). 52. Stellate medullary cell with a dissected ribbon-shaped plastid (NCU 591799-802). 53. Cross section of a thick thallus showing densely packed secondary medullary rhizoidal filaments filling the medulla (NCU 591803-811). 54. Longitudinal section showing a vacuolated multinucleate stellate cell on right and multinucleate secondary medullary rhizoidal filaments on left (NCU 591799-802). Abbreviations: mc: medullary cell; n: nucleus; p: plastid. Scale bars: 5 cm (), 200 µm () or 20 µm ().
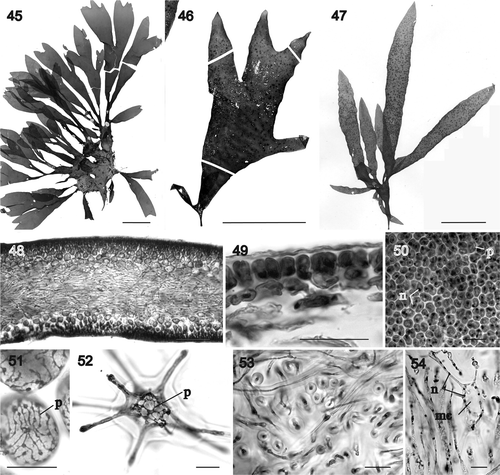
Figs 55–67. Sarcodia grandifolia. Male and young female reproductive stages. , , , : aniline blue; : haematoxylin. 55. Transverse section of a mature spermatangial sorus (WELT A029965). 56. Developing elongated spermatangial parent cells forming tubes (WELT A029965). 57. As above, with differentiating spermatia inside spermatangial parent cells which function as spermatangia (WELT A029965). 58. Seriate spermatangia (WELT A029965). 59. Mature spermatangial sorus showing elongated spermatangial parent cells, seriate spermatangia, terminal spermatia and released spermatia (WELT A029965). 60. Uninucleate supporting cell bearing a pair of one-celled, uninucleate sterile branches and a young carpogonium (NCU 591803-811). 61. Supporting cell bearing a carpogonium flanked by a pair of two-celled sterile branches (NCU 591803-811). 62. Supporting cell bearing an elongated carpogonium and trichogyne that has cut off a hypogynous cell (NCU 591803-811). 63. Supporting cell bearing a carpogonium and hypogynous cell in which the basal cell of the adjacent sterile branch has differentiated into an auxiliary cell (NCU 591803-811). 64. Supporting cell bearing a carpogonium and hypogynous cell, in which the hypogynous cell has fused with the adjacent auxiliary cell (NCU 591803-811). 65. As above, but with the carpogonium bearing a remnant trichogyne (NCU 591803-811). 66. Auxiliary cell situated at the base of a side branch has cut off the first gonimoblast cell laterally which, in turn, bears two additional gonimoblast cells in a row (NCU 591803-811). 67. As above, with the auxiliary cell and gonimoblast cells seen in face view (NCU 591803-811). Abbreviations: ac: auxiliary cell; cp: carpogonium; g: gonimoblast; g1: gonimoblast initial; g2 and g3: second and third gonimoblast cells; hy: hypogynous cell; n: nucleus; s: spermatium; sb: sterile branches; sc: supporting cell; sg: spermatangium; spc: spermatangial parent cell; t: trichogyne. Scale bars: 20 µm.
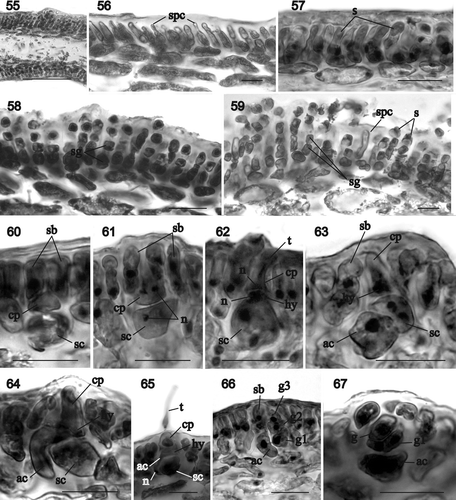
Figs 68–76. Sarcodia grandifolia. Development of cystocarp. Haematoxylin. 68. Young cystocarp showing inner and outer pericarp composed of longitudinal files of cells. The auxiliary cell sits at the base of the gonimoblasts flanked by darkly staining inner pericarp filaments (NCU 591799-802). 69. Enlarged view of the inner pericarp. A uninucleate gonimoblast cell (arrow) is prominent inside the inner pericarp (NCU 591803-811). 70. Developing cystocarp showing gonimoblasts filling the cavity between the inner and outer pericarps. Some inner cells of the gonimoblast reticulum have linked up with cells of the inner pericarp (arrows) (NCU 591799-802). 71. As above, but a later stage in which the outer gonimoblasts have produced terminal chains of carposporangia (NCU 591803-811). 72. Enlarged view of inner pericarp and inner gonimoblast reticulum showing the linkage between the two (arrows) (NCU 591803-811). 73. Inner gonimoblast reticulum at higher magnification (NCU 591799-802). 74. Transition between the inner gonimoblast reticulum and outer gonimoblast files that will bear the carposporangia (NCU 591799-802). 75. Segment of a fresh specimen showing cystocarps scattered over thallus surface and along margins (NCU 591803-811). 76. Surface view of an ostiole (NCU 591799-802). Abbreviations: ac: auxiliary cell; c: carposporangia; gf: gonimoblast filaments; ip: inner pericarp; n: nucleus; op: outer pericarp; gr: gonimoblast reticulum. Scale bars: 20 µm (–, ) or 200 µm (, ).
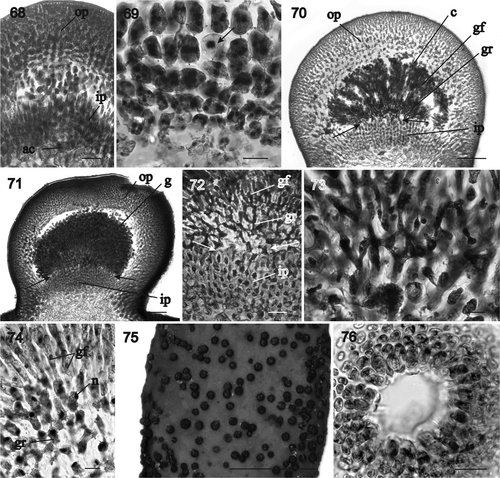
Figs 77–84. Sarcodia grandifolia. Development of cystocarp and tetrasporangia. Haematoxylin. 77. Surface layer and inner cells of the outer pericarp (NCU 591799-802). 78. Mature cystocarp showing the slightly stellate cells (arrow) in the outer pericarp surrounding the gonimoblasts (NCU 591799-802). 79. Basal part of a cystocarp showing the relationships between inner and outer pericarp and the gonimoblast filaments (arrow) (NCU 591803-811). 80. Mature uninucleate carposporangia (NCU 591799-802). 81. Carposporangia and in situ germinating carposporangia (arrows) (NCU 591803-810). 82. In situ germinating carposporangia (NCU 591803-811). 83. Tetrasporangium linked by a basal pit connection (arrow) to a subcortical cell (WELT A030183). 84. Mature zonately divided tetrasporangium pit-connected basally (arrow) to a subcortical cell and flanked by elongated cortical filaments (arrowheads) (WELT A030183). Abbreviations: c: carposporangia; ip: inner pericarp; n: nucleus; gr: gonimoblast reticulum. Scale bars: 20 µm (, ), 50 µm () or 10 µm ().
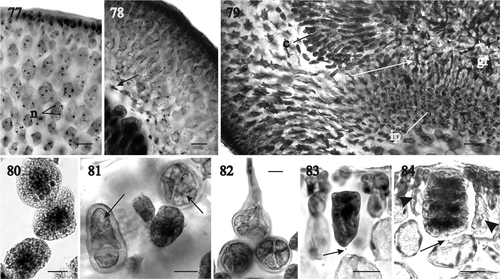
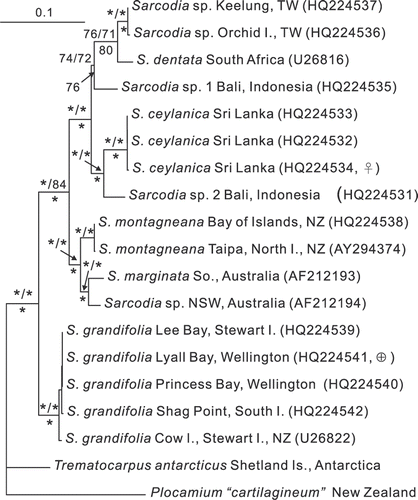
Fig. 85. RbcL phylogeny: ML tree (–lnL = 9560.8708) of the genus Sarcodia from the Indian and Pacific Oceans. Numbers above branches are ML and MP bootstrap values, respectively, whereas numbers below branches are Bayesian posterior probabilities in %. Asterisks indicate support values >99%.
Structure multiaxial with a lax medulla (, ). Outer cortex bilayered, composed of anticlinal once-dichotomously branched filaments that are usually two cells long, without secondary pit connections (, ). Outer cortical cells uninucleate with a parietal plastid that is ovoid to elongate in transverse section and rounded to polygonal in surface view, up to 9 (–14) µm high and 6 (–12) µm in diameter (). Subcortex composed of a multilayered network of cells connected to neighbouring cells by secondary pit connections, the outermost ovoid and small, becoming stellate and increasing in size inwardly, with the largest up to 95 µm in diameter (, ). Subcortical cells multinucleate (, ), the inner ones with dissected ribbon-like plastids (). Medulla composed of stellate cells with the rounded central portion up to 55 µm in diameter and entangled slender rhizoidal filaments, some of them longitudinally orientated and up to 12 µm in diameter (, , ). Stellate cells multinucleate with dissected ribbon-like plastids (). Medullary rhizoidal filaments multinucleate, originating secondarily from inner cortical and stellate medullary cells.
-
Life cycle: Triphasic, with isomorphic gametophytes and tetrasporophytes. Gametophytes dioecious.
-
Male reproductive structures: Spermatangial parent cells initiated in sori from surface cortical cells that elongate toward the thallus surface forming a tube (). Spermatangia ultimately formed in rows with the terminal cells differentiating spermatia (). Upper part of the tube fusing with the thallus surface and releasing spermatia.
-
Female reproductive structures: Fertile areas clearly distinguishable in cross section by their darkly staining cells, which are slightly larger than neighbouring cells (). Such cells undergo division () to produce a basal cell (=the supporting cell) that bears two sterile uninucleate one-celled branches () which later divide to form two-celled branches (). The uninucleate supporting cell cuts off a one-celled carpogonium transversely with a straight trichogyne that elongates toward the thallus surface, flanked by the sterile branches (). A pit connection links the sterile branches to the supporting cell (, arrow). After presumed fertilization, the first cell of one of the sterile branches enlarges and differentiates into an auxiliary cell () and the carpogonium divides into a two-celled filament, with the basal cell becoming the hypogynous cell adjacent to the auxiliary cell (). The carpogonial remnant degenerates and the auxiliary cell cuts off a gonimoblast initial (), which in turn gives rise to a row of cells that comprise the earliest gonimoblasts flanked by the sterile branches ().
Cystocarp development begins when the outer cortex resumes growth and forms files of cells adjacent to and above the developing gonimoblasts (). The cells adjacent to the gonimoblasts become darkly staining and will form the inner pericarp (, circle), while those above are lighter staining and will form the outer pericarp (). The auxiliary cell sits basally between the inner pericarp filaments and the original outer cortex (, ). The outer pericarp separates from the inner pericarp, forming the central cavity, with some cells elongating and remaining interconnected between the outer and inner pericarps (, arrow). During cystocarp development, the gonimoblast filaments branch and progressively fill the cavity between the inner and outer pericarp layers (, ). Gonimoblast cells adjacent to the inner pericarp and those in the inner part of the central cavity are initially uninucleate, containing large nuclei (5–9 µm diameter). Inner gonimoblast cells fuse with one another to form a gonimoblast reticulum of multinucleate filaments with nuclei 5–9 µm in diameter (, ), and those adjacent to and above the inner pericarp link to inner pericarp cells (, arrow). The region above the gonimoblast reticulum is composed of elongated cells which give rise to the gonimoblast filaments that differentiate into terminal chains of carposporangia (, ). Mature gonimoblasts reach up to 650 µm in diameter ().
Cystocarps are sessile and hemispherical. They initially form along the margins but progressively become scattered over both surfaces of the blade (, , , ). An ostiole forms apically in the outer pericarp (, , ) and is a translucent rose colour, through which the enclosed deep-red gonimoblasts can be seen in both fresh and dried specimens (). The outer pericarp consists of a compact surface layer of small ovoid or slightly elongated uninucleate cells that are not linked by secondary pit connections () and an inner cortex of larger multinucleate cells that are secondarily pit-connected (, arrow). The subcortex of the outer pericarp is lax, with rounded or ovoid cells that are more elongated at the base of the cystocarp, whereas the cells surrounding the gonimoblasts are stellate and more compact (). Mature carposporangia are uninucleate and 18–30 µm in diameter (). They occasionally germinate in situ (, arrow).
-
Tetrasporangia: Tetrasporangia are scattered over the thallus surface. They are not produced simultaneously but differentiate continuously and tetrasporangial initials can form adjacent to mature tetrasporangia. Tetrasporangial initials originate laterally from a subcortical cell (). Tetrasporangia are ovoid when young and elongated and enlarged with age (), with the pit connection between the tetrasporangium and the subcortical cell situated close to the base of the tetrasporangium (, , arrows), flanked by elongated cortical filaments (, arrowheads). Mature tetrasporangia are zonately divided and up to 48 µm broad by 78 µm long ().
Sarcodia grandifolia Levring Citation 1949 , p. 395, pl. 42, fig. 3
(–)
-
HETEROTYPIC SYNONYM: Sarcodia flabellata Levring, p. 396, pl. 42, .
-
LECTOTYPE: Specimen collected in Stewart Island (New Zealand), 24 June 1946, WELT A022966 (VWL No. 7585) (Nelson & Phillips, Citation2001). Collector unnamed but probably Mrs Eileen Willa ().
-
ISOTYPE: WELT A022967.
-
TYPE LOCALITY: Stewart Island, New Zealand.
-
DISTRIBUTION: New Zealand: North Island (shores of Cook Strait), South Island (Marlborough, Otago, Fiordland), Stewart Island and Snares Islands ().
-
HABITAT: Intertidal up to −25 m depth collected all year round.
-
SPECIMENS EXAMINED: North Island, New Zealand: Wellington, Lyall Bay, 40°20′S, 174°48′E (M.H. Hommersand, 19 September 1974, NCU 591803-811, female, tetrasporangial); Wellington, West Lyall Bay, 41°21′S, 174°48′E, low intertidal on rock, partially buried in gravel (W.A. Nelson, 07 December 2009, WELT A030183, tetrasporangial); Wellington, Princess Bay, 41°20′S, 174°47′E, attached at low tide (D.W. Freshwater NZ04-559, 17 November 2005, NCU 591799-802, female); Wellington, Island Bay, Taputerenga Island, 41°21′S, 174°46′E, low water (N.M. Adams, 29 September 1976, WELT A009499, female, male). South Island, New Zealand: Shag Point, 45°29′S, 170°49′E, attached (D.W. Freshwater, NZ04-056, 24 October 2004, NCU 591798, female); Fiordland, Chalky Inlet, Passage I. (SE point), 46°20′ S, 166°32′E, −12 to −15 m (C.H. Hay, 23 April 1991, WELT A022576, female). Stewart Island, New Zealand: Lee Bay, 46°52′S, 168°07′E, −1.5 to −3.5 m (D.W. Freshwater NZ04-186, 30 October 2004, NCU591797, female); Halfmoon Bay, Lonneker's Nugget, 46°54′S, 168°8′E, low intertidal (W.A. Nelson, 07 September 1990, WELT A029965, male); Port Pegasus, Blind Passage, 47°13′S, 167°40′E (20 August 1946, WELT A001089 (=ANZE 289), female); The Neck, facing Cow Island, 46°57′S, 168°10′E, low intertidal (J.F. Herrick, 07 October 1994, AK220918, female); Halfmoon Bay, Harrolds Bay, 46°53′S, 168°90′E (A.L. Stewart & C.D. Roberts, 04 March 1992, WELT A029918, tetrasporangial). Snares Islands, New Zealand: NW corner of HoHo Bay, 48°1′S, 166°37′E, −22 m (G.D. Fenwick, 22 February 1977, WELT A009701, female).
-
Habit and vegetative structure: Thallus erect, flattened, up to 19 (–31) cm in length, attached to the substratum by a discoid holdfast, shortly stipitate, complanate, subdichotomously branched and with rounded axils and apices (). Axis up to 4 (–7.5) cm wide. The lectotype () is a cystocarpic plant that is eroded towards the base and has regenerated some 20 or so new branches. Plants that are sparingly and shallowly () or deeply dichotomously branched () have commonly been referred to S. montagneana or S. flabellata in various herbaria and publications (Levring, Citation1949; Adams, Citation1994; Miller, Citation2003). Margins smooth or sometimes proliferous in older individuals. Texture moderately firm, the colour variable but often deep red.
Structure multiaxial with abundant intercellular medullary rhizoidal filaments in mature plants (). Outer cortex composed of anticlinal, dichotomously branched filaments, usually two cells long and lacking secondary pit connections (). Surface cells up to 12 (–19) µm high and 8 (–12) µm in diameter, rectangular in transverse section and rounded to polygonal in surface view, uninucleate, and with a parietal plastid (, ). Inner cortex multilayered, the cells connected to neighbouring cells by secondary pit connections, the outermost ovoid and small, increasing in size inwardly and becoming stellate, with the largest reaching 65 µm in diameter (, ). Subcortical cells multinucleate with the innermost containing dissected ribbon-like plastids (). Medulla consisting of multinucleate stellate cells with dissected ribbon-like plastids in which the rounded central portion is up to 40 µm in diameter (, ). An abundance of entangled slender rhizoidal filaments is usually present, some of which are clearly longitudinally orientated (, , ). The medullary rhizoidal filaments originate near the apex but are of secondary origin, arising from inner cortical and possibly stellate medullary cells.
-
Life cycle: Triphasic, with isomorphic gametophytes and tetrasporophytes. Gametophytes dioecious.
-
Male reproductive structures: Spermatangia develop in large, pale sori on both sides of the thallus (), the earliest stages consisting of spermatangial parent cells which form elongate tubes that extend to the thallus surface (, ). Such cells may function initially as spermatangia that form a single spermatium inside the tube. The upper part of the tube fuses with the thallus surface and releases the uninucleate spermatium. In mature sori a row of rectangular spermatangia appears to differentiate serially inside the tubular spermatangial parent cells (). The walls of mature terminal spermatangia dissolve and the liberated spermatia appear rounded and vacuolate at the ends of the rows, each with a terminal nucleus and proximal vesicle ().
-
Female reproductive structures: Immature female reproductive structures develop from darkly staining cortical cells that undergo divisions to produce a basal intercalary cell (=the supporting cell) and two uninucleate sterile branches. Subsequently, the supporting cell cuts off a carpogonium to the outside, flanked by the sterile branches (, ). These remain pit-connected to the supporting cell and are initially one-celled but later become two-celled. Both the supporting cell and the carpogonium are uninucleate at this stage (). The carpogonium expands, becomes conical, and initiates a straight trichogyne that elongates to the thallus surface. The carpogonium divides after presumed fertilization and gives rise to a hypogynous cell (). At the same time, the basal cell of one of the sterile branches borne on the supporting cell differentiates into an auxiliary cell (). The hypogynous cell fuses with the auxiliary cell (, ) and deposits a conspicuous nucleus inside it (). At this stage the remnant carpogonium and trichogyne can occasionally still be seen (). Finally, the carpogonial remnant degenerates and the auxiliary cell that has fused with the hypogynous cell divides to form a gonimoblast initial, which in turn gives rise to a file of gonimoblast cells (, ).
Cystocarp development is initiated when cells of the outer cortex divide to form files of aligned cells that differentiate into inner and outer pericarps (). Occasionally, a gonimoblast cell may be distinguished from inner pericarp cells by the presence of a single large nucleus (, arrow). A central cavity is initiated by the separation of outer and inner pericarps that soon becomes filled with highly branched gonimoblast filaments (, ). The inner gonimoblasts cells unite laterally to form a gonimoblast reticulum (), which expands into a conspicuous network inside the central cavity (, ), whereas the outer gonimoblasts consist of branched files of gonimoblast filaments () that terminate in short chains of carposporangia (). The innermost cells of the gonimoblast reticulum may fuse with the outermost cells of the inner pericarp (, , arrows). Mature gonimoblasts reach 600 µm in diameter (, ).
Cystocarps are hemispherical and sessile, and are borne along the margins and scattered over both sides of the thallus surface (, , ); they possess a small terminal ostiole (). The outer pericarp and gonimoblasts are dark red in both fresh and dried specimens (, seen as black). The outer pericarp consists of a compact outer cortex composed of small ovoid or slightly elongated uninucleate cells that are not connected by secondary pit connections, and a subcortex of larger multinucleate cells connected by secondary pit connections (). Subcortical cells are rounded or ovoid (, ) becoming elongate at the base of the cystocarp (). The cells adjacent to the gonimoblasts are slightly stellate and more compact (). Mature carposporangia are uninucleate and 28–50 µm in diameter (). A few were occasionally seen germinating in situ (, ).
-
Tetrasporangia: Tetrasporangia are scattered over the thallus surface and are produced more or less continuously with sporangia of different ages adjacent to one another. Tetrasporangial initials develop laterally from subcortical cells and are basally pit-connected to them (, , arrows). Tetrasporangia are ovoid when young and later become broadly elongated and zonately divided, flanked by sterile branches (, arrowheads). Mature tetrasporangia are up to 55 µm broad and 75 µm long ().
Molecular analyses
Thirteen rbcL sequences were newly generated for species of Sarcodia, along with five sequences available from GenBank (). A species of Plocamium and one of Trematocarpus were selected as the outgroup. The data matrix analysed included 1388 base pairs with 120 parsimony-informative sites. The topologies of the ML, MP and BA trees were largely congruent and only the ML rbcL tree is shown (). The species of Sarcodia that were analysed formed a well-supported clade. Two collections of Sarcodia (sp. 1 and sp. 2) from Bali, Indonesia, two of Sarcodia sp. from Taiwan, S. dentata (Suhr) R.E. Norris from South Africa and three collections of S. ceylanica Harvey ex Kützing from Sri Lanka clustered together with 100% support. On the other hand, the two samples of S. montagneana, the generitype from New Zealand, are closely related to S. marginata J. Agardh and Sarcodia sp. from Australia and are only distantly related to the five samples of S. grandifolia. The rbcL sequences of S. ‘flabellata’ (see ) were nearly identical to those of S. grandifolia and the former is treated as a synonym of the latter in the rbcL phylogenetic tree ().
Discussion
Sarcodia montagneana is currently treated as a widespread, morphologically variable species that is distributed throughout the Indo-West Pacific Ocean (Silva et al., Citation1996; Guiry & Guiry, Citation2011). In reality, the type species of Sarcodia, S. montagneana, appears to be limited in its distribution to the northern part of the North Island of New Zealand (). Our molecular data () place S. montagneana closest to S. marginata from southern Australia, which is a subdichotomously branched species, and to a small, undescribed species from New South Wales, Australia. Perhaps it may also be related to the little known species, S. marginalis (Kützing) Millar, in Millar & Prud’homme van Reine (Citation2005) from New Caledonia. Thus, the forms that superficially resemble S. montagneana are not the ones most closely related to it. A second species, S. grandifolia Levring (Citation1949) is widely distributed, occurring in the Wellington area around Cook Strait in the North Island and throughout the South Island, Stewart Island and the Snares Islands. A third species, S. flabellata, was also described by Levring (Citation1949) from New Zealand, but, in our opinion, this species is a smaller, variably branched flabellate form of S. grandifolia, as suggested previously by Adams (Citation1994). Indeed, Levring (Citation1949) already stated that S. grandifolia is variable in shape, with younger specimens clearly flabellate and larger specimens with flabellately divided branches and an attenuated base. The New Zealand species of Sarcodia have long been confused due to their similarity in habit and gross morphology. Vegetative plants can be readily distinguished in cross section by the form and height of their outer cortical cells, which are rounded or ovoid and up to 9 (–14) µm in length in S. montagneana (), and rectangular up to 12 (–19) µm in length in S. grandifolia (). The most conspicuous character distinguishing the two is the colour of the gonimoblasts and outer pericarp as seen in surface view in both fresh and dried specimens. The gonimoblasts are conspicuously red inside a transparent rosy outer pericarp in S. montagneana, as reported in the original description by Hooker & Harvey (Citation1845), whereas in S. grandifolia both the gonimoblasts and the outer pericarp are dark red and indistinguishable in surface view. Other taxonomic characters have been suggested to differentiate the taxa, such as the length of adult specimens (Levring, Citation1949; Adams, Citation1994) and the presence of stalked cystocarps (Adams, Citation1994), but these were found to be unreliable in the present study.
Spermatangia have been described as developing in surface patches in S. montagneana (Adams, Citation1994; Liao et al., Citation1993) and S. dentata (Norris, Citation1987), whereas those of S. grandifolia were unknown. We observed that the development of the spermatangial sori is initiated by the differentiation of the outer cortical cells into spermatangial parent cells with tubular extensions that reach the thallus surface. Paraphyses are absent. Spermatangial parent cells are initially non-septate and function as spermatangia, cutting off a single spermatium. A mature spermatium contains a terminal nucleus and a proximal vesicle and is released through a pore formed by the interaction of the spermatangial tube with the outer wall and cuticle. Subsequently the spermatangial parent cells produce spermatangia in rows, the terminal ones differentiating into spermatia that are later released. Similarly, Searles reported the presence of spermatangia cut off in two-celled chains from elongated spermatangial parent cells in Trematocarpus flabellatus (J. Agardh) De Toni [Searles, Citation1968, as Dicurella scutellata (Hering) Papenfuss], and Womersley (1994) recorded spermatia in chains in T. concinnus (R. Brown ex Turner) De Toni. On the other hand, Liao et al. (Citation1993) reported that the spermatangia are formed singly from surface cortical cells in S. montagneana.
The development and structure of the female apparatus is quite similar in S. montagneana and S. grandifolia and differs significantly from the descriptions by Rasmussen (Citation1964) and Liao et al. (Citation1993), the only authors to have investigated female development in detail. Rasmussen (Citation1964) reported the presence of polycarpogonial systems composed of three-celled carpogonial branches and flattened trichogynes in S. montagneana, whereas Liao et al. (Citation1993) reported monocarpogonial systems with three-celled carpogonial branches in the same species. Norris (Citation1987) described a monocarpogonial system in S. dentata in which the carpogonial branch was two or possibly three cells long. Three-celled carpogonial branches were illustrated by Searles (Citation1968) in Trematocarpus fragilis (as Dicurella fragilis) and T. flabellatus (as Dicurella scutellata) and in T. dichotomus (Searles, Citation1969) and were confirmed by Barrientos & Alveal (Citation2005) and by one of us (Rodríguez-Prieto, pers. observ.) for the Chilean T. dichotomus. The published evidence thus suggests that a three-celled recurved carpogonial branch is the usual condition in most members of the Sarcodiaceae.
Our observations of a one-celled carpogonium cut off terminally from the supporting cell before fertilization in two New Zealand species is in sharp contrast to the expected behaviour. We never saw three-celled carpogonial branches in our material and the few extended trichogynes we saw were tubular. If we interpreted our observations correctly, then the Sarcodiaceae have evolved a second pathway for the development of the female reproductive system, something that is virtually unheard-of in the Rhodophyta. All of our evidence hinges on relating the one-celled terminal carpogonium to postfertilization events. We observed that the carpogonium cuts off a hypogynous cell basally after fertilization at the same time that the basal cell of one of the side branches differentiates into an auxiliary cell, and that this cell cuts off a gonimoblast initial obliquely or laterally that gives rise to an initially filamentous gonimoblast followed by the elaboration of a massive cystocarp. Fusion between the hypogynous cell and the auxiliary cell took place directly indicating that the female reproductive apparatus is procarpic. We did not see a connecting filament linking the hypogynous cell with a nearby cortical cell functioning as an auxiliary cell, as reported by Rasmussen (Citation1964) in S. montagneana or by Norris (Citation1987) working on South African S. dentata. Norris concluded that S. dentata was non-procarpic with the auxiliary cell being a neighbouring cell identical in size and position to the supporting cell. The validity of our contrasting interpretation of postfertilization events in New Zealand species of Sarcodia hinges on the correctness of our observations of the sequence of nuclear events leading to gonimoblast formation. These should be examined again in New Zealand material and in other species of Sarcodia.
Later stages in the development of the cystocarp are not so controversial. Soon after gonimoblast initiation, the surrounding cortical cells initiate parallel files of cells that form the pericarp. Cells immediately below the gonimoblast filaments are densely filled with cytoplasm and darkly staining. We referred to these darkly staining inner files of cells as the inner pericarp. Files of cells above this area were lightly staining and produced the outer pericarp and a central ostiole. Some of the filaments between the two regions elongated or separated to initiate the central cavity, which in turn was filled with branched gonimoblast filaments. Gonimoblast cells were initially uninucleate and contained enlarged nuclei making them readily distinguishable from both inner and outer pericarp cells. The gonimoblast filaments differentiated into inner and outer gonimoblast regions, with the inner gonimoblast filaments fusing laterally to form a gonimoblast reticulum in which the fused cells were multinucleate, whereas the outer filaments grew linearly in branched files that ultimately bore short chains of uninucleate carposporangia. As Norris (Citation1987) noted, gonimoblast cells and cells of the inner pericarp interact. Our observations confirm that cells of the gonimoblast reticulum do attach to the outermost cells of the inner pericarp filaments. The fusion that takes place does not seem to involve an exchange of nuclei between the two regions, inasmuch as nuclei of different sizes were never seen in the same cell. Where we have called the basal filaments ‘inner pericarp’, Norris (Citation1987) referred to them as ‘a ring or partial platform of large, deeply staining cells originated from the vegetative cells.’ Kylin (Citation1956, in Sarcodia), Norris (Citation1987, in S. dentata) and Womersley (1994, in S. marginata) also reported the presence of a reticulate fusion cell at the base of the gonimoblasts. Outer gonimoblast filaments do not differentiate cells or filaments that connect to the outer pericarp, as occurs in Gracilaria and some other genera in the Gracilariaceae. The similarity between the outwardly developed cystocarp with a central ostiole and the structure seen in most Gracilariaceae is a parallel development and the two groups show no phylogenetic relationship.
Molecular studies suggest a weak relationship among the families Plocamiaceae, Pseudoanemoniaceae and Sarcodiaceae (Saunders et al., Citation2004). All three are procarpic, with the carpogonium situated adjacent to the auxiliary cell, and all three produce outwardly developed gonimoblasts surrounded by an external pericarp with a central ostiole. The supporting cell functions as the auxiliary cell in Plocamium (Plocamiaceae) (Kylin, Citation1923; Hommersand & Fredericq, Citation1990) and Hummbrella (Pseudoanemoniaceae) (Hawkes & Johnson, Citation1981) and is thought to serve the same function in the sarcodiacean genus Trematocarpus (Searles, Citation1968, Citation1969). These similarities need to be reinvestigated in Trematocarpus and clarified more fully in the other two families. Sarcodia is multiaxial with an elaborate, highly differentiated cystocarp, whereas Plocamium and Hummbrella are uniaxial with simple filamentous organization and a much simpler cystocarp. All three are ancient groups with few genera that are widely separated phylogenetically. The basis for the ancestral relationship among them remains to be determined. The study by Norris (Citation1987) suggests that important characters may be discovered that distinguish the species of Sarcodia. The present work may be sufficiently detailed to permit new comparisons to be made between species and genera. Likewise, further studies of the Sarcodiaceae, the Plocamiaceae and the Pseudoanemoniaceae may illuminate a basis for evaluating the developmental and phylogenetic similarities and differences among these three seemingly related families.
Acknowledgements
We thank Roberta D’Archino for generously collecting specimens of Sarcodia montagneana. Scans of specimens in the type collection at BM were kindly provided by J. Wilbraham and from TCD by J. Parnell. We thank Sarah Pene Eftonga for additional photographs of W.H. Harvey's collection. We thank Dr D.W. Freshwater and Dr G. Kraft for their reviews and positive criticisms, which have improved the quality of the article. This project was supported by two grants from the Spanish Ministry of Science and Technology (CGL2004-05556-C02-01 and CGL2008-00932); by grants from Taiwan's National Science Council (NSC 99-2621-B-019-003-MY3) and NTOU's Center of Excellence for Marine Bioenvironment and Biotechnology (99529001 G) to S.-M. Lin; by Foundation for Research Science & Technology contract C01X0502 (New Zealand) grant to W.A. Nelson, and by NSF grant DEB0937978 to J.M. Lopez Bautista, M.H. Hommersand and S. Fredericq.
References
- Adams , NM . 1994 . Seaweeds of New Zealand. An Illustrated Guide , Christchurch : Canterbury University Press .
- AGARDH, J.G. (1852). Species genera et ordines algarum. Volumen secundum: algas florideas complectens. Part 2, fasc. 2. C.W.K. Gleerup, Lundae [Lund]
- Barrientos , E and Alveal , K . 2005 . Morphological and reproductive evidence for a new circumscription of the genus Trematocarpus (Rhodophyta, Sarcodiaceae) . Cienc. Mar. , 31 : 399 – 412 .
- Chiovitti , A , Kraft , GT , Saunders , GW , Liao , M-L and Bacic , A . 1995 . A revision of the systematics of the Nizymeniaceae (Gigartinales, Rhodophyta) based on polysaccharides, anatomy and nucleotide sequences . J. Phycol. , 31 : 153 – 166 .
- GUIRY, M.D. & GUIRY, G.M. (2011). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 29 October 2010
- Harvey , WH . 1849 . Nereis Australis. Part 2. Reeve Brothers London
- Hawkes , MW and Johnson , KA . 1981 . Vegetative and reproductive morphology of Hummbrella hydra Earle (Rhodophyta, Gigartinales) . Phycologia , 20 : 321 – 332 .
- Hommersand , MH and Fredericq , S . 1990 . “ Sexual reproduction and cystocarp development ” . In Biology of the Red Algae , Edited by: Cole , KM and Sheath , RG . 305 – 345 . Cambridge : Cambridge University Press .
- HOMMERSAND, M.H. & FREDERICQ, S. 2003. Biography of the marine red algae of the South African west coast: a molecular approach. Proceedings of the International Seaweed Symposium 17: 325–336
- Hommersand , MH , Moe , RL , Amsler , CD and Fredericq , S . 2009 . Notes on the systematics and biogeographical relationships of Antarctic and sub-Antarctic Rhodophyta with descriptions of four new genera and five new species . Bot. Mar. , 52 : 509 – 534 .
- Hooker , JD and Harvey , WH . 1845 . Algae Novae Zelandiae; being a catalogue of all of the species of algae yet recorded as inhabiting the shores of New Zealand, with characters and brief descriptions of the new species discovered during the voyage of H.M. discovery ships ‘Erebus’ and ‘Terror’ and of others communicated to Sir W. Hooker by D. Sinclair, the Rev. Colenso, and M. Raoul . London J. Bot. , 4 : 521 – 551 .
- Kylin , H . 1923 . Studien über die Entwicklungsgeschichte der Florideen . K. Svenska Vetensk-Akad. Handl. , 63 (11) : 1 – 139 .
- Kylin , H . 1932 . Die Florideenordung Gigartinales . Lunds Univ. Årsskr., N.F., Avd. , 28 (8) : 1 – 88 .
- KYLIN, H. (1956). Die Gattungen der Rhodophyceen. C.W.K. Gleerups, Lund
- Levring , T . 1949 . Six marine algae from New Zealand . Trans. Roy. Soc. New Zealand , 77 : 391 – 397 .
- Liao , M-L , Kraft , GT , Munro , SLA , Craik , DJ and Bacic , A . 1993 . Beta/kappa-carrageenans as evidence for continued separation of the families Dicranemataceae and Sarcodiaceae (Gigartinales, Rhodophyta) . J. Phycol. , 29 : 833 – 844 .
- Lin , S-M , Fredericq , S and Hommersand , MH . 2001 . Systematics of the Delesseriaceae (Ceramiales, Rhodophyta) based on LSU rDNA and rbcL sequences, including the Phycodryoideae subfam. nov . J. Phycol. , 37 : 881 – 899 .
- Lin , S-M , Fredericq , S and Hommersand , MH . 2004 . Two new species of Martensia (Delesseriaceae, Rhodophyta) from Kenting National Park, southern Taiwan . Phycologia , 43 : 13 – 25 .
- LIN, S.-M., YANG, S.-Y. & HUISMAN, J.M. (2011). Systematic revision of the genera Liagora and Izziella (Liagoraceae, Rhodophyta) from Taiwan based on molecular analyses and carposporophyte development, with the description of two new species. J. Phycol., 47: 352–365
- Miller , IJ . 2003 . The chemical structure of galactans from Sarcodia montagneana and from Sarcodia flabellata . Bot. Mar. , 46 : 392 – 399 .
- Millar , AJK and Prud’homme Van Reine , WF . 2005 . Marine benthic macroalgae collected by Vieillard from New Caledonia and described as new species by Kützing . Phycologia , 44 : 536 – 549 .
- Nelson , WA and Phillips , LE . 2001 . Locating the type specimens of New Zealand marine algae described by Levring . New Zealand J. Bot. , 39 : 349 – 353 .
- Norris , RE . 1987 . Reproduction in Sarcodia dentata (Suhr) comb. nov. (Gigartinales, Rhodophyceae), with comments on the Sarcodiaceae . Br. Phycol. J. , 22 : 147 – 155 .
- Rasmussen , RA . 1964 . The structure and reproduction of Sarcodia montagneana (Rhodophyta) . Phycologia , 4 : 1 – 7 .
- Rodríguez-Prieto , C and Hommersand , MH . 2009 . Behaviour of the nuclei in pre- and post-fertilization stages in Kallymenia (Kallymeniaceae, Rhodophyta) . Phycologia , 48 : 138 – 155 .
- Ronquist , F and Huelsenbeck , JP . 2003 . MRBAYES 3: Bayesian phylogenetic inference under mixed models . Bioinformatics , 19 : 1572 – 1574 .
- Saunders , GW , Chiovitti , A and Kraft , GT . 2004 . Small-subunit rDNA sequences from representatives of selected families of Gigartinales and Rhodymeniales (Rhodophyta) 3. Delineating the Gigartinales sensu stricto . Can. J. Bot. , 82 : 43 – 74 .
- Searles , RB . 1968 . Morphological studies of red algae of the order Gigartinales . Univ. Calif. Publ. Bot. , 43 : 1 – 100 .
- Searles , RB . 1969 . Observations on the morphology of Trematocarpus dichotomus Kützing and the status of the genus Dicurella . Phycologia , 8 : 21 – 25 .
- Silva , PC , Basson , PW and Moe , RL . 1996 . Catalogue of the benthic marine algae of the Indian Ocean . Univ. Calif. Publ. Bot. , 79 : 1 – 1259 .
- SWOFFORD, D.L. (2002). PAUP* Phylogenetic Analyses Using Parsimony (*and Other Methods) Version 4 b. Sinauer Associates, Sunderland
- THIERS, B. (2011). (continuously updated) Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden's Virtual Herbarium. http://sweetgum.nybg.org/ih/
- WIENCKE, C. & CLAYTON, M.N. 2002 . Antarctic seaweeds. In Synopsis of the Antarctic Benthos (Wagle J.W., editor), 159 pp, A.R.G. Gantner, Ruggell, Liechtenstein
- WOMERSLEY, H.B.S. (1994). The Marine Benthic Flora of Southern Australia – Part IIIA – Bangiophyceae and Florideophyceae (Acrochaetiales, Nemaliales, Gelidiales, Hildenbrandiales and Gigartinales sensu lato). Australian Biological Resources Study, Canberra
- ZWICKL, D.J. (2006). Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. Ph.D. dissertation, The University of Texas at Austin