Abstract
The genus Liagora is broadly defined and incorporates species with diverse carposporophyte developmental patterns, including species having compact gonimoblasts with discrete involucral filaments and species having diffuse gonimoblasts with intermingling involucral filaments and fused or unfused carpogonial branches. In order to clarify the phylogenetic significance of these patterns of cystocarp development, we inferred the species relationships of Liagora with diffuse gonimoblasts and related genera from the northwestern Pacific Ocean, based on rbcL sequence analysis. Molecular analyses demonstrated that Liagora is polyphyletic and the species currently recognized are clustered in three distinct clades. We revise the taxonomy by presenting new genera for two of these clades. The clade containing the generitype, L. viscida retains the name Liagora. The second clade, containing Liagora perennis, is described as Macrocarpus gen. nov. The third clade, for which we propose the new genus Neoizziella, contains Neoizziella asiatica sp. nov. and N. divaricata comb. nov. (basionym: Liagora divaricata C.K. Tseng). Neoizziella is characterized by morphologically similar, intermingling involucral and gonimoblast filaments, carpogonial branch cells that remain discrete, and small, undivided carposporangia. In contrast, Macrocarpus has larger, divided carposporangia, in addition to diffuse gonimoblasts with unfused carpogonial branches. The genera Akalaphycus and Stenopeltis, which also possess diffuse gonimoblasts, can be separated from these genera by a combination of cortical and carpogonial features.
Introduction
The current concept of the genus Liagora J.V. Lamouroux (Liagoraceae) is rather broadly defined and includes species displaying at least three types of carposporophyte development, including: (1) a diffuse gonimoblast with fused carpogonial branches; (2) a compact gonimoblast with fused carpogonial branches; and (3) a diffuse gonimoblast with unfused carpogonial branches. In the first and the third types, the gonimoblasts intermingle with the involucral filaments, whereas they remain separate in the second type (Abbott, Citation1990; Huisman, Citation2002, Citation2006). Several authors (e.g. Huisman, Citation2002; Huisman & Schils, Citation2002; Lin et al., Citation2011) have commented on the apparent inequity of Liagora amongst the numerous precisely defined, related genera in the Liagoraceae (e.g. Yamadaella I.A. Abbott, Citation1970), suggesting that, based on these reproductive differences, the genus should be subdivided. This process has now been underway for some time. Earlier studies were based on morphology (e.g. Huisman & Kraft, Citation1994; Huisman & Schils, Citation2002), but more recently DNA sequence analyses have been incorporated, these generally supporting interpretations based on morphology (e.g. Huisman et al., Citation2006). Thus far, three genera (Ganonema K.C. Fan & Yung-C. Wang, Izziella Doty and Titanophycus Huisman, G.W. Saunders & A.R. Sherwood) have been segregated from (or resurrected from the synonymy of) Liagora.
Lin et al. (Citation2011) undertook a morphological and molecular study of the species of Liagora and the closely related Izziella from Taiwan and reinforced the importance of carposporophyte developmental patterns, noting that in Izziella the involucral filaments lie beneath the lower portion of the gonimoblast, in contrast to the condition in Liagora, in which the involucral filaments either loosely envelop or intermingle with the gonimoblasts. They also noted that the fusion cell in Izziella is composed of cells of the carpogonial branch and some inner gonimoblast cells, whereas the fusion cell consists of just the cells of the carpogonial branch in both the generitype, Liagora viscida, and in L. harveyana Zeh.
In order to clarify the phylogenetic significance of the patterns of carposporophyte development found in Liagora, we inferred the relationships among species with diffuse gonimoblast filaments (e.g. L. perennis I.A. Abbott, Citation1995, L. julieae I.A. Abbott & Huisman, Citation2003, L. divaricata C.K. Tseng, Citation1941 and L. harveyana Zeh, Citation1912) and several other Liagora species that possess compact gonimoblasts from Taiwan, along with the related genera [e.g. Stenopeltis Itono & Yoshizaki (1992), Akalaphycus Huisman, I.A. Abbott & A.R. Sherwood (Citation2004)], based on rbcL sequence analysis from the northwestern Pacific Ocean. We also undertook a morphological examination of L. perennis and L. divaricata from several localities in the western Pacific Ocean, as well as L. viscida from Mediterranean Sea and L. harveyana from New Zealand and southern Australia.
Materials and methods
Specimens were collected intertidally or subtidally by snorkelling. Samples used in morphological studies were preserved in 5% formalin–seawater or pressed on herbarium sheets. Type and voucher specimens have been deposited in the herbarium of the National Taiwan Ocean University, Taiwan (NTOU). Herbarium abbreviations follow Thiers (Citation2011, continuously updated). Hand sections were stained with 1% aniline blue acidified with 1% HCl and mounted in 25–30% Karo® syrup (Englewood Cliffs, NJ, USA) or treated with Wittmann's aceto-iron-haematoxylin–chloral hydrate (Wittmann, Citation1965) and mounted in 50% Hoyer's mounting medium (Lin et al., Citation2004). Photomicrographs were taken on an Olympus BX51 microscope with a Q-imaging digital camera (Burnaby, BC, Canada), and habit views were reproduced with an Epson scanner (Tokyo, Japan).
DNA from silica gel-dried specimens was extracted using the DNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA) following the manufacturer's instructions. DNA sequencing procedures were as described in Lin et al. (Citation2001). New sequence data and those available from GenBank were compiled and aligned with Sequencher (Gene Codes, Ann Arbor, MI, USA). Thirteen rbcL sequences of ‘L.’ perennis, ‘L.’ divaricata and L. albicans from Taiwan and its neighbouring islands were newly generated (see ). The families Galaxauraceae and Scinaiaceae were selected as outgroups, based on Lin et al. (Citation2011).
Table 1. List of species of the Liagoraceae used for rbcL analysis in this study and their GenBank accession numbers.
Phylogenetic analyses were performed using maximum-parsimony (MP) and calculation of bootstrap percentage values (BP) were conducted as followed in Lin et al. (Citation2001, Citation2008) using heuristic searches. MP heuristic searches were consisting of 500 random sequence additions, MULPARS (but holding five trees at each step), and tree-bisection-reconnection (TBR), whereas the MP bootstrap analysis was conducted by using simple sequence addition. MP and Maximum Likelihood (ML) analyses and bootstrapping methods were performed using PAUP* v4.0 (Swofford, Citation2003) and GARLI 1.0 (Zwickl, Citation2006), with 1000 and 100 bootstrap replicates completed for the MP and ML analyses, respectively. The model used for ML was the general-time-reversible with gamma distributed rate heterogeneity as the default in GARLI 1.0. A Bayesian analysis (BA) was performed in MrBayes 3.1.2 (Ronquist & Huelsenbeck, Citation2003) using a GTR + I + Γ model, which allowed for rate variation among different codon positions. The analysis was applied in four chains of the Markov chain Monte Carlo (one hot and three cold). Each run started with a random tree and consisted of 106 generations with sampling every 100 generations. Stationarity was reached at generation 140 000. Thus, trees saved until generation 140 000 were the ‘burn-in’ of the chain, and inferences about the phylogeny were based on those trees sampled after generation 140 000. A 50% consensus tree (majority rule as implemented by PAUP* v4.0) was computed from the 8600 + 1 trees saved after the burn-in point.
Results
Molecular analyses
The 36 rbcL sequences analysed in this study included the genera Liagora, Izziella, Titanophycus, Stenopeltis, Akalaphycus, Ganonema, Helminthocladia and Dermonema in the Liagoraceae, and included 13 rbcL sequences that were newly generated for the species L. perennis, L. divaricata, L. albicans and Dermonema virens from Taiwan, Pratas Island, Cebu, the Philippines and Bali, Indonesia (see ). The analysed data matrix included 1383 base pairs for rbcL, with 471 parsimony-informative sites.
The topologies of the ML, MP and BA trees were largely congruent and only the ML tree is shown in . The genera of Liagoraceae analysed formed a strongly supported assemblage in the rbcL tree, in which the genus Liagora was split into three evolutionary clades: a ‘Liagora divaricata/Liagora sp.’ clade, for which we are describing the new genus Neoizziella (Clade I) including N. divaricata (C.K. Tseng) comb. nov. (basionym: L. divaricata) and a new species, N. asiatica, from the northwestern Pacific Ocean; a ‘Liagora’ perennis clade from Taiwan (Clade II), which is treated as a second new genus, Macrocarpus, from Hawaii, China and Taiwan; and a Liagora sensu stricto clade (Clade III) containing the generitype and the species possessing fused carpogonial branches, each of which has a compact carposporophyte or gonimoblasts intermingled with their involucral filaments (see ). The genus Stenopeltis is weakly associated with the Liagora and Izziella clades, whereas the Titanophycus, Akalaphycus, Neoizziella and Macrocarpus clades clustered together to form a larger clade with strong to no support based on posterior probability and bootstrapping analyses, respectively.
Fig. 1. RbcL phylogeny: ML tree (ln L = –10165.9564) of the proposed new genera Neoizziella and Macrocarpus from the northwestern Pacific Ocean, using a selection of the related genera within the families Liagoraceae, Scinaiaceae and Galaxauraceae. Numbers above branches are ML bootstrap values, MP bootstrap values and Bayesian posterior probabilities in %, respectively. A dash indicates support values <50%.
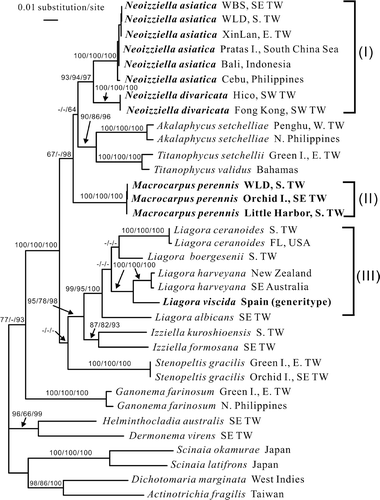
Neoizziella asiatica was shown by our analyses to have a wide distribution in the western Pacific Ocean (eastern and southern Taiwan), South China Sea [Pratas Island and Cebu (the Philippines)] and northern Indian Ocean (Bali, Indonesia). The intraspecific genetic distance of N. asiatica from different sample sites ranged from 0% to 0.29% (0–4 bases pairwise difference). There was no genetic difference between the populations of N. divaricata from the two collecting sites (Hico and Fong Kong) in southwestern Taiwan. Although we were not able to obtain an rbcL sequence of ‘L.’ perennis (treated as M. perennis herein) from its Hawaiian type locality, the LSU sequence of ‘L.’ perennis (from Pingtung County in southern Taiwan (coll. S.-M. Lin, 23.Dec.2007) possessed 99.27% similarity (1648 of 1660 base pairs, JF710637) with the published LSU sequence of ‘L.’ perennis (AY523822) from Hawaii.
Morphological observations
Our molecular analyses clearly show that, as currently circumscribed, the genus Liagora is polyphyletic and we therefore propose two new genera: Neoizziella for Clade I and Macrocarpus for Clade II (see ). The alternative would be to recognize a broadly defined Akalaphycus, including the taxa herein recognized as Neoizziella, Titanophycus and Macrocarpus. This, however, would result in an unacceptably broad range of morphologies and reproductive development in a single genus, and would be counter to most recent interpretations of the Liagoraceae. Morphologically, Neoizziella differs from the Liagora clade (Clade III, containing the generitype L. viscida) and the Macrocarpus clade (Clade II), both in producing involucral filaments that are morphologically similar to and intermingle with the gonimoblast filaments, and also in its relatively small carposporangia. Macrocarpus can be characterized by its divided and relatively large carposporangia, in addition to having diffuse gonimoblasts.
A key to Liagora and the closely related genera (as detected from rbcL sequence analyses) Neoizziella, Macrocarpus, Izziella, Stenopeltis, Akalaphycus and Titanophycus, can be constructed using morphological characteristics, as follows:
Key to Neoizziella, Macrocarpus, Liagora and related genera
1a Gonimoblast filaments not associated with paraphyses2
1b Gonimoblast filaments associated with paraphyses6
2a Cells of carpogonial branches unfused after fertilization3
2b Cells of carpogonial branches fused after fertilization5
3a Compact gonimoblasts deeply embedded inside a distinct cluster of involucral filaments, which have barrel-shaped terminal cells Titanophycus
3b Diffuse gonimoblasts intermingling with involucral filaments4
4b Carposporangia not divided and relatively small, involucral filaments produced from the cortical cells in the vicinity of the support cell Neoizziella
4b Carposporangia divided and relatively large, involucral filaments produced only from the subcortical cell below the support cell Macrocarpus
5a Gonimoblasts diffuse to compact, surrounding by or intermingling with involucral filaments, fusion cells relatively small and seldom incorporating inner gonimoblast cells Liagora
5b Gonimoblasts always compact, involucral filaments forming a distinct layer at the basal part of the carposporophyte, fusion cells large and incorporating inner gonimoblast cells Izziella
6a Carpogonial branches borne on inflated inner cortical cells Stenopeltis
6b Carpogonial branches borne on slender cells, paraphyses derived from outer cortical filaments Akalaphycus
New taxa
Descriptions and morphological observations of new taxa are given below, and also an examination of critical stages of Liagora viscida (the generitype) and L. harveyana for comparison with Neoizziella and Macrocarpus.
Neoizziella Showe-Mei Lin, Shi-Yuan Yang & Huisman, gen. nov.
GENERITYPE: Neoizziella asiatica Showe-Mei Lin, Shi-Yuan Yang & Huisman, sp. nov.
DESCRIPTION: Thalli erecti fruticosi, ex axibus principalibus 3–5 constantes, subdichotome ramosi, ordine ramificationis 3–9, 3–7 cm longi, ex haptero discoideo orientes, ramis parce ut graviter calcificatis; cellulae filamentorum assimilantorum subsphaericae ad ellipsoideae, in filamentis pallidis medullosis portatae; gametophyta dioecia; cellulae parentales spermatangiorum e cellulis apicalibus vel subapicalibus abscissae; spermatangia matura 3–4 µm diametro, 2 vel 3 in catenas seriatim facta; ramus carpogonialis rectus vel leviter pandus, 4- vel- 5-cellularis, secundum fecundationem (sumptam) cellulis rami carpogonialis cellulisque sustinentibus propriis manentibus, conjunctionicellula non facta; filamenta involucralia ex cellulis ramum carpogonialem cingentibus orientia, ordine usque ad 5 ramificationis, inter filamenta gonimoblastis miscentia; carposporangia pyriformia uninucleata, deinceps differentiata in catenis terminalibus; ubi matura, filamenta involucralia filamentis gonimoblasti carposporangiferis respectu formam similia.
Thalli erect and bushy, consisting of three to five main axes, subdichotomously branched, with 3–9 orders of branching, 3–7 cm in length, arising from a discoid holdfast, branches moderately to heavily calcified; cells of assimilatory filaments subspherical to ellipsoidal, borne on colourless medullary filaments; gametophytes dioecious; spermatangial parent cells cut off from apical and subapical cells; mature spermatangia 3–4 µm in diameter, 2 or 3 formed sequentially in chains; carpogonial branches straight or slightly curved, 4- or 5-celled, cells of the carpogonial branch and supporting cells remaining distinct after fertilization and no fusion cell formed; involucral filaments initiated from the cells surrounding the carpogonial branch, branched up to 5 orders and intermingling with the gonimoblast filaments, carposporangia pyriform and uninucleate, differentiating sequentially in terminal chains; when mature, involucral filaments morphologically similar to carposporangia-bearing gonimoblast filaments.
ETYMOLOGY: Named for Isabella (Izzie) Abbott, for her numerous contributions to our knowledge of the Liagoraceae.
Consistent with the results of the molecular analysis, we describe a new species, Neoizziella asiatica, and propose a new combination, Neoizziella divaricata.
Key to the two species of Neoizziella
1a. Thalli relatively large, 4–6 cm in height, bushy, subdichotomously branched to 7–9 orders; distal ends of branches not tapering, with blunt apices Neoizziella asiatica
1b. Thalli relatively small, 3–4 cm in height, subdichotomously and sparsely branched to 3–5 orders, distal ends of branches tapering Neoizziella divaricata
Neoizziella asiatica Showe-Mei Lin, Shi-Yuan Yang & Huisman, sp. nov. (Figs )
DESCRIPTION: Thalli erecti fruticosi, ex axibus principalibus 3–5 teretibus constantes, subdichotome ramosi, ordine ramificationis 7–9, plerumque 4–7 cm longi, ex haptero discoideo orientes, subrosei vel dilute purpurei vel fuscorubres, ramis parce ad graviter calcificatis; cellulae filamentorum assimilantorum subsphaericae ad ellipsoideae; gametophyta dioecia; cellulae parentales spermatangiorum e cellulis apicalibus subapicalibusque abscissae; spermatangia matura 3–4 µm diametro, 2 vel 3 in catenis seriatim facta; rami carpogoniales recti vel leviter pandi, 4- vel 5-cellulares; filamenta involucralia ordine usque ad 5 ramificationis, inter filamenta gonimoblastis miscentia; carposporangia pyriformia uninucleata 5–6 µm lata × 9–11 µm longa, terminalia aut 2 vel 3 deinceps differentiata in catenis terminalibus.
Thalli erect and bushy, consisting of three to five terete axes, subdichotomously branched 7–9 orders, mostly 4–7 cm in length, arising from a discoid holdfast, pinkish to pale purple to reddish brown in colour; branches moderately to heavily calcified; cells of assimilatory filaments subspherical to ellipsoidal; gametophytes dioecious, spermatangial parent cells cut off from apical and subapical cells, the mature spermatangia 3–4 µm in diameter, 2 or 3 formed sequentially in chains; carpogonial branches straight or slightly curved, 4- or 5-celled, involucral filaments branched up to 5 orders and intermingling with the gonimoblast filaments, carposporangia pyriform and uninucleate, 5–6 µm wide by 9–11 µm long, terminal or differentiated sequentially in terminal chains of 2 or 3.
HOLOTYPE: NTOU-23xii2007-WLD-holo-1.
ISOTYPES: NTOU-23xii2007-WLD -iso-2∼ NTOU-23xii2007-WLD -iso-6.
TYPE LOCALITY: Wan-Li-Dong, Pintung County, S. Taiwan (N 22°02′, E 120°50′).
ETYMOLOGY: ‘asiatica’ refers to the alga being found in various places in Asia.
DISTRIBUTION: south and east Taiwan, the South China Sea (Pratas Island & Cebu, Philippines) and Bali (Indonesia).
SPECIMENS EXAMINED : Taiwan: Pintung County, S. Taiwan – Won-Li-Dong, coll. S.-M. Lin, 7.Jan.2002 (NTOU-WLD7i02Na), 23.Dec.2007 (NTOU-23xii2007-WLD -isoNa) (females, males); Kenting National Park, Wind Blow Sand, coll. S.-M. Lin, 18.Mar.2003 (NTOU-WBS18iii03Na) (females, males). Taitung County, E. Taiwan – coll. S.-M. Lin, 28.Apr.2002 (NTOU-TT28iv02Na) (females). South China Sea: Pratas Island (Taiwan) – coll. S.-M. Lin, 25 April 2004 (NTOU-DS25iv04Na); Cebu, The Philippines – coll. S.-L. Liu, 07 November 2002 (NTOU-CB7xi02Na). Indonesia: Serangan Island, Bali – coll. S.-M. Lin, 24 July 2008 (NTOU-Bl24vii08Na).
HABITAT AND SEASONALITY: Gametophytic plants grow at 1–2 m depth on rocky substrata or coral reefs from early winter (November) to late summer (April) in Taiwan and the South China Sea (Cebu and Pratas Island). In Bali (Indian Ocean), however, gametophytic plants were found in July.
Habit
The thalli are erect, 4–7 cm in length, and composed of 3 to 5 terete axes branched subdichotomously to 7–9 orders, arising from a small discoid holdfast (, ). The colour ranges from pinkish or pale grey to reddish brown. The main axes are moderately to heavily calcified, whereas terminal branchlets are lightly calcified and mucilaginous ().
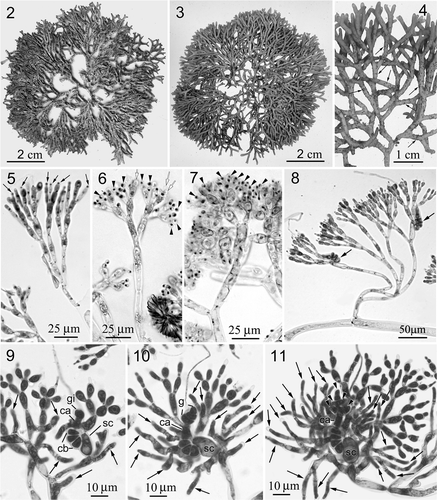
Figs 2–11. Neoizziella asiatica, sp. nov. 2. Holotype, a female plant. 3. Habit, a liquid preserved, female plant. 4. Close-up of a female plant showing branching pattern and cystocarps (arrows). 5. Young cortex showing spermatangial initials. 6. Differentiating spermatangia (arrowheads) and the discharged spermatangia (white arrows). 7. Mature spermatangia (arrowheads) in cluster. 8. Close-up of assimilatory filaments and carpogonial branches (arrows). 9. An early post-fertilization stage showing supporting cell (sc), carpogonial branch (cb), fertilized carpogonium (ca), gonimoblast initial (gi) and initials of involucral filaments (arrows). 10. Another post-fertilization stage showing supporting cell (sc), carpogonial branch (cb), fertilized carpogonium (ca), early gonimoblasts (g) and branched involucral filaments (arrows). 11. A later post-fertilization stage showing supporting cell (sc), elongated carpogonium (ca), developing gonimoblasts (arrowheads) and elongated involucral filaments (arrows).
Vegetative morphology
The thalli are multiaxial, composed of pigmented, assimilatory filaments borne on colourless medullary filaments (). The upper parts of the assimilatory filaments are subdichotomously branched 3 or 4 times and have subspherical to ellipsoidal cells, 5–8 µm wide by 10–18 µm long, whereas the lower parts are unbranched or once branched, with elongated cells, 4–7 µm wide by 30–60 µm long (, , ). The terminal cells of assimilatory filaments rarely bear hairs.
Reproductive morphology
Gametophytes are dioecious and isomorphic. Spermatangial parent cells are initiated from apical and subapical cells () and spermatangia are cut off terminally (, arrowheads) and discharged sequentially (, white arrows). Each spermatangial parental cell may cut off 2 or 3 spermatangia, 3–3.5 µm in diameter, in a terminal cluster (). Carpogonial branches () are straight or slightly curved, 4- or 5-celled, situated in the upper-middle parts of assimilatory filaments in young branchlets.
After presumed fertilization, the carpogonium divides transversely to produce a gonimoblast initial (). Meanwhile, the cortical cells surrounding the supporting cell and the carpogonial branch cut off many involucral filament initials (). At an early stage of gonimoblast development, the gonimoblast initial cuts off primary gonimoblast cells obliquely (), and the cells of the involucral filaments elongate and branch several times (). As gonimoblast development continues, the involucral filaments elongate and divide transversely 4 or 5 times, surrounding the young gonimoblast (, ). Afterwards, the initially globular gonimoblast cells become filamentous and intermingle with the involucral filaments (). The pit connections between the cells of the carpogonial branch do not break down during maturation of the gonimoblasts (, ). At maturity, the growth of the gonimoblasts overtakes the involucral filaments and carposporangia are produced sequentially in terminal chains; these are uninucleate and pyriform, 4–6 µm wide by 8–10 µm long ().
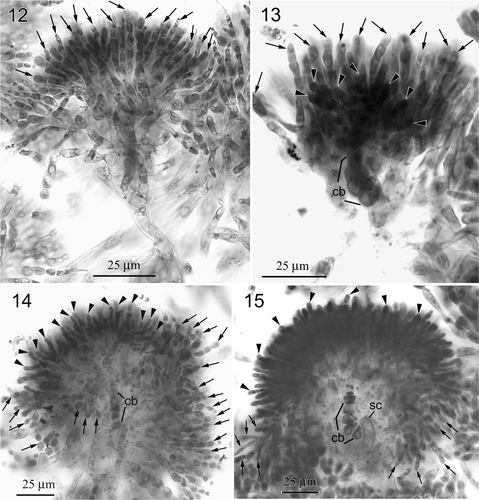
Figs 12–15. Neoizziella asiatica, gen. et sp. nov. , . Two views of a young carposporophyte. 12. Focal plane of the involucral filaments (arrows), which surround the gonimoblasts completely. 13. Focal plane of distinct carpogonial branch (cb), the gonimoblasts (arrowheads) and the rear involucral filaments (arrows). 14. Nearly mature carposporophyte showing the gonimoblasts (arrowheads) intermingling with involucral filaments (arrows). Note that the cells of carpogonial branch (cb) are not fused. 15. Fully developed gonimoblasts bearing terminal, mature carposporangia (arrowheads). Note that the cells of carpogonial branch (cb) and the supporting cell (sc) remain distinct.
Neoizziella divaricata (C.K. Tseng) Showe-Mei Lin, Shi-Yuan Yang & Huisman, comb. nov. ()
BASIONYM: Liagora divaricata C.K. Tseng. Bulletin of the Fan Memorial Institute of Biology, Botany, 10: 268 (1941).
TYPE LOCALITY: Hainan Island, southern China.
SPECIMENS EXAMINED: Southern Taiwan: Hico, coll. S.-M. Lin, 18 Dec. 2002 & 12 Feb. 2009 (females, male); Fongkang, A. Liu, 12 Jan. 2006 & 16 Mar. 2006 (females).
Morphology
The specimens we examined fit Tseng's (Citation1941) and Abbott & Huisman's (Citation2003) descriptions and illustrations closely. Gametophytic thalli are dioecious, 3–5 cm high, and moderately to heavily calcified (). The main axes are subdichotomously branched to 3–5 orders and the branches gradually taper. The carpogonial branches are slightly curved and 4 or 5 cells long, borne in the mid-region of the supporting cells (). The involucral filaments are produced from the subcortical cells neighbouring the supporting cell and flank the gonimoblast initials (). At an early stage of gonimoblast development, growth of the involucral filaments overtakes the gonimoblast filaments and branches several times (). At maturity, the gonimoblasts are embedded in and intermingled with the involucral filaments (). As seen in N. asiatica, the pit connections between the cells of the carpogonial branch do not break down during maturation of the gonimoblasts. Carposporangia are uninucleate and pyriform, 5–6 µm wide by 9–11 µm long, produced sequentially in terminal or subterminal chains and are morphologically similar to the involucral filaments ().
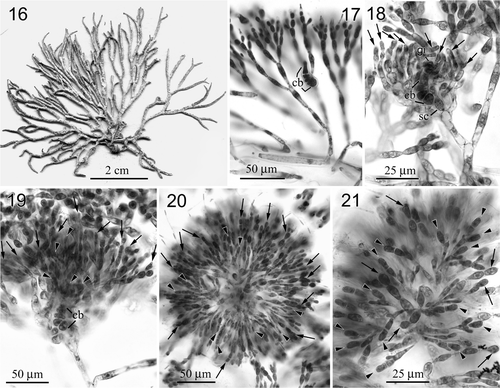
Figs 16–21. Neoizziella divaricata, comb. nov. (Hico, Pintung County, Taiwan). 16. Habit, a female plant. 17. Close-up of assimilatory filaments and a carpogonial branch (arrow). 18. An early post-fertilization stage showing supporting cell (sc), carpogonial branch (cb), gonimoblast initial (gi) and young involucral filaments (arrows). 19. A later post-fertilization stage showing carpogonial branch (cb), young gonimoblasts (arrowheads) and elongated involucral filaments (arrows). 20. Fully developed gonimoblasts (arrowheads) intermingling with involucral filaments (arrows). 21. Close of another mature cystocarp showing undivided carposporangia (arrowheads) and involucral filaments (arrows).
Macrocarpus Showe-Mei Lin, Shi-Yuan Yang & Huisman, gen. nov. ()
DESCRIPTION: Thalli erecti parce ad graviter calcificati, subdichotome ramosi, ordine ramificationis 5–6, 3–6 cm longi, ex haptero discoideo per stipitem brevem orientes; apices ramorum obtusi; cellulae filamentorum assimilantorum ovoideae ad ellipsoideae, in filamentis pallidis medullosis portatae, pilis terminalibus deciduis vulgaribus; gametophyta monoecia vel dioecia, ramo carpogoniali recta vel leviter panda, 4 vel 5 cellulari; ubi gonimoblasti maturi, rami carpogoniales obturamenta fovearum dilatata; filamenta involucralia tantum ex cellula subcorticali sub cellula sustinenti initiata, inter filamenta gonimoblastis miscentia; carpotetrasporangia ad extrema distalia filamentorum gonimoblasti facta.
Thalli erect and heavily calcified, subdichotomously branched to 5–6 orders, 3–6 cm in length, arising from a discoid holdfast with a short stipe, branches with blunt tips; cells of assimilatory filaments ovoid to ellipsoidal, borne on colourless medullary filaments, terminal deciduous hairs common; gametophytes monoecious or dioecious, carpogonial branches straight or slightly curved, 4- or 5-celled, pit-plugs between cells of the carpogonial branch broadened when the gonimoblasts fully mature, involucral filaments initiated only from the subcortical cell below the supporting cell and intermingling with the gonimoblast filaments, carpotetrasporangia formed at the distal ends of gonimoblast filaments.
GENERITYPE: Macrocarpus perennis (I.A. Abbott) Showe-Mei Lin, Shi-Yuan Yang & Huisman, comb. nov.
BASIONYM: Liagora perennis I.A. Abbott. Chinese Journal of Oceanology and Limnology, 13 (4): 343 (1995).
TYPE LOCALITY: Ala Moana Beach Park, Honolulu, O’ahu, Hawaiian Islands.
ETYMOLOGY: Named for its large carposporangia.
SPECIMENS EXAMINED: Taiwan: Kenting National Park, Pintung County (S Taiwan): Wan Li Tong coll. S.-M. Lin, 23 December 2007; Xian Gang Kou, coll. S.-M. Lin, 12 February 2009. Hougtou, Orchid Island (SE Taiwan), coll. S.-M. Lin, 28 April 2002.
Morphology
The specimens we examined fit Abbott's (Citation1995) and Abbott & Huisman's (Citation2003) descriptions and illustrations closely. Gametophytic thalli are monoecious or dioecious, heavily calcified, and 3–6 cm in length, arising from a discoid holdfast with a short stipe (). Thalli are subdichotomously branched to 5 or 6 orders and the tips of the branches are blunt. The cells of assimilatory filaments are ovoid to ellipsoidal and borne on colourless medullary filaments, and terminal deciduous hairs are very common (). Carpogonial branches are straight or slightly curved, 4- or 5-celled. After fertilization, cells of the carpogonial branch remain rounded and unchanged in shape, but their pit-plugs broaden when the gonimoblasts are fully developed. The involucral filaments are initiated mostly from the subcortical cell below the supporting cell () and eventually intermingle with the gonimoblast filaments (). Mature carposporangia were not observed in this study. Nevertheless, based on the observations of Abbott (Citation1995), quadripartite carposporangia are formed at the ends of gonimoblast filaments.
Figs 22–25. Macrocarpus perennis, gen. et comb. nov. (, : Won-Li-Dong, PinTung County, Taiwan; , : Houtou, Orchid Island, Taiwan). 22. Habit of a female plant. 23. Close-up of assimilatory filaments and carpogonial branches (cb). 24. An early post-fertilization stage showing carpogonial branch (cb), gonimoblast initial (gi), primary gonimoblasts (arrowheads) and young involucral filaments (arrows). 25. A later post-fertilization stage showing carpogonial branch (cb), gonimoblast initial (gi), young gonimoblasts (arrowheads) and involucral filaments (arrows).

Liagora viscida (Forsskål) C. Agardh Citation1822: 395 ()
NEOTYPE LOCALITY: Anse du Troc, Banyuls-sur-Mer, France (Abbott, Citation1990).
SPECIMENS EXAMINED: Spain: El Golfet, Calella de Palafrugell, coll. C. Rodriguez-Prieto, 12 August 2005. Italy: Capraia, Tuscan Islands, coll. O. De Clerck, 30 May 2004.
Morphology
The specimens we examined fit the descriptions and illustrations of Abbott (Citation1990) and Huisman (Citation2002). Gametophytic thalli are monoecious, 2–5 cm high, bushy, and moderately to heavily calcified. Carpogonial branches are 3- or 4-celled, borne in the middle to lower parts of the assimilatory filaments (). After presumed fertilization, the fertilized carpogonium divides transversely to produce a gonimoblast initial. At the same time, many involucral filament initials are rapidly produced from the neighbouring cells connected to the supporting cell (). At an early stage of gonimoblast development, the involucral filaments elongate and form a dense involucre that envelops the developing gonimoblast (). At this time, the pit-connections between the cells of the carpogonial branch start to break down and a fusion cell is formed, consisting only of the cells of the carpogonial branch. When mature, the involucral filaments form a distinct cluster intermingling with the gonimoblast filaments. The gonimoblast filaments are diffuse and the carposporangia are obovate to clavate, 12–18 µm wide by 25–30 µm long, and differentiate sequentially terminally or subterminally ().
Figs 26–33. Liagora spp. . Liagora viscida (, : Cabrera, Illes Balears, Spain; , : Capraia, Tuscan Islands, Italy). 26. Close-up of assimilatory filaments and a carpogonial branch (cb). 27. An early post-fertilization stage showing carpogonial branch (cb), gonimoblast initial (gi) and young involucral filaments (arrows). 28. A later post-fertilization stage showing young gonimoblasts (arrowheads) and elongated involucral filaments (arrows). 29. Fully developed gonimoblasts (arrowheads) showing mature carposporangia, fusion cell (fc) and surrounding involucral filaments (arrows). Note that some gonimoblasts are squeezed out (white arrows). . Liagora harveyana, carposporophyte development (, , : Port Fairy, Victoria, southern Australia; : Leigh, Auckland Island, New Zealand). 30. An early post-fertilization stage showing carpogonial branch (cb), gonimoblast initial (gi) and young involucral filaments (arrows). 31. A later post-fertilization stage showing young gonimoblasts (arrowheads) and elongated involucral filaments (arrows). 32. Fully developed gonimoblasts (arrowheads) showing undivided carposporangia (arrowheads), fusion cell (fc) and involucral filaments (arrows). Note that the involucral filaments loosely surround the gonimoblasts. 33. Mature cystocarp showing divided (white arrowheads) and undivided (black arrowheads) carposporangia, fusion cell (fc) and involucral filaments (arrows). Note that the involucral filaments intermingle with the gonimoblasts.
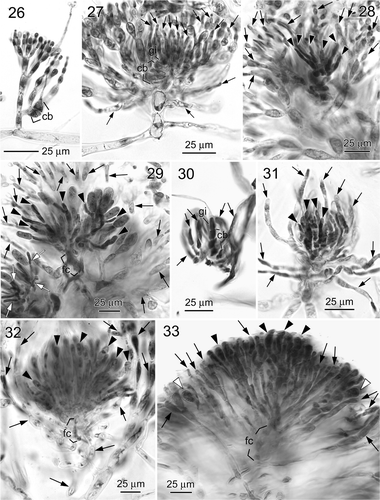
Liagora harveyana Zeh Citation1912: 270 ()
TYPE LOCALITY: King Georges Sound, Western Australia, Australia.
SPECIMENS EXAMINED: New Zealand: Marine Station of the University of Auckland, Leigh, coll. M.H. Hommersand, 21 March 1975. S Australia: Port Fairy, Victoria, coll. A.H.S. Lucas, January 1932 (NSW 401401, NSW 401399).
Morphology
The specimens we examined fit the description and illustrations of Huisman (Citation2002, Citation2006). Gametophytic thalli are monoecious or dioecious, up to 7 cm (plants from New Zealand) or 14 cm (plants from Australia) high, bushy, heavily calcified. Carpogonial branches are 3- to 5-celled, borne in lower part of the assimilatory filaments (). At an early stage of gonimoblast development, the involucral filaments elongate and initially form a loose involucre that envelops the developing gonimoblasts (). When mature, the cells of the carpogonial branch form a small fusion cell and the gonimoblasts are loosely surrounded by the involucral filaments (New Zealand plants, ), or become moderately intermingled with the gonimoblasts (Australian plants, ). Carposporangia are undivided, obovate to clavate, 9–11 µm wide by 20–24 µm long (New Zealand plants, ), or quadripartite, 11–14 µm wide by 18–23 µm long (Australian plants, ).
Discussion
Lin et al. (Citation2011) successfully resolved the taxonomic confusion regarding species of Izziella and Titanophycus that are superficially similar to some species of Liagora. Their conclusions were based on rbcL sequence analysis and a combination of morphological characters relating to the behaviour of the carpogonial branch (whether or not it forms a fusion cell) and the characteristics of the involucral filaments (their length and morphology) after fertilization. In this study, we further analysed relationships among Liagora species having diffuse gonimoblasts, again based on rbcL sequence analysis and carposporophyte development. Our molecular analysis suggest that the species of Liagora are polyphyletic and cluster in three clades: Clade I containing Liagora divaricata and an unidentified species, Clade II containing Liagora perennis, and Clade III containing the generitype, L. viscida, and some species having compact gonimoblasts (see ). In species included in Clades I and II, the cells of the carpogonial branches do not fuse together, in contrast to the fused carpogonial branch seen in Liagora sensu stricto (Clade III). In order to resolve this clearly polyphyletic Liagora, we propose two new genera: Neoizziella for Clade I, including the new species N. asiatica and the new combination N. divaricata, and Macrocarpus for Clade II, containing the new combination M. perennis.
The two new genera Neoizziella and Macrocarpus can be separated from Liagora morphologically by a combination of characters, including whether or not the gonimoblasts are similar to the involucral filaments, whether the cells of carpogonial branches fuse during carposporophyte development, and whether the carposporangia remain undivided or become quadripartite. Neoizziella can be characterized by: (1) gonimoblast filaments that are morphologically similar to and intermingling with involucral filaments, (2) cells of the carpogonial branch remaining discrete and not forming a fusion cell after fertilization, and (3) carposporangia that are undivided and small (4–6 µm wide by 8–11 µm long). On the other hand, Macrocarpus differs from Neoizziella in having larger quadripartite carposporangia, 6–12 µm wide by 17–30 µm long (Abbott & Huisman, Citation2003). In contrast, in L. viscida (the generitype) and L. harveyana, the cells of the carpogonial branch fuse to form a small fusion cell during carposporophyte development and the carposporangia (9–18 µm wide by 20–30 µm long) are larger than those in the two new genera.
The new species recognized here, N. asiatica, can be difficult to distinguish from Macrocarpus perennis in the field in Taiwan, as they often grow in the same habitat (tidal pools or reefs) and their thalli are morphologically similar, being of similar size and calcification. However, N. asiatica can often be separated from M. perennis by its larger size (4–7 cm in height, rather than 3–6 cm), and more reliably by its highly branched thalli (up to 9 orders) and small and undivided carposporangia. Both rbcL (see ) and LSU sequence analyses support the separation of N. asiatica from M. perennis from Hawaii and Taiwan. Neoizziella divaricata has been recorded (as Liagora divaricata) from numerous locations, including Australia (Huisman, Citation2002, Citation2006), but these records require confirmation based on sequence analyses.
We expect that future studies and sequence analyses of Liagora species from other regions will see the transfer of several additional species to Neoizziella and Macrocarpus, and suggest that the taxonomic scheme proposed here will provide a sound framework for further revision. Of particular interest will be testing our emphasis on characters relating to carposporophyte development, such as the behaviour of the carpogonial branches and the development and morphology of the involucral filaments after fertilization, which we feel are useful for delineating genera in the Liagoraceae.
Acknowledgements
This project was largely supported by a grant from National Science Council (Taiwan) (NSC 99-2621-B-019 -003 -MY3) and from NTOU's Center of Excellence for Marine Bioenvironment and Biotechnology (99529001G) to S.-M. Lin. J.M.H. acknowledges the support of the ‘Australian Biological Resources Study’. The authors sincerely thank Dr Max H. Hommersand at University of North Carolina at Chapel Hill, Dr Wendy Nelson at the National Institute of Water & Atmospheric Research Ltd. and Dr Conxi Rodríguez-Prieto at the University of Girona for sending Liagora specimens used in this study.
References
- Abbott , IA . 1970 . Yamadaella, a new genus in the Nemaliales (Rhodophyta) . Phycologia , 9 : 115 – 123 .
- Abbott , IA . 1990 . A taxonomic assessment of the species of Liagora (Nemaliales, Rhodophyta) recognized by J. Agardh, based upon studies of type specimens . Cryptog. Bot. , 1 : 308 – 322 .
- Abbott , IA . 1995 . A new ‘tetrasporangial’ species of Liagora (Rhodophyta, Nemaliales) from Hawai’i . Chinese J. Oceanol. Limnol. , 13 : 343 – 347 .
- Abbott , IA and Huisman , JM . 2003 . The Liagoraceae (Nemaliales, Rhodophyta) of the Hawaiian Islands II: the species of Liagora with quadripartite carposporangia, including descriptions of L. donaldiana sp. nov. and L. julieae sp. nov . Phycologia , 42 : 594 – 605 .
- Agardh , CA . 1822 . Species Algarum , Vol. 1, part 2 , Berlin : Lund .
- Huisman , JM . 2002 . The type and Australian species of the red algal genera Liagora and Ganonema (Liagoraceae, Nemaliales) . Aust. Syst. Bot. , 15 : 773 – 838 .
- Huisman, J.M. (2006). Algae of Australia – Nemaliales. ABRS, Melbourne: Canberra & CSIRO Publishing. viii+153 pp
- Huisman , JM and Kraft , GT . 1994 . Studies of the Liagoraceae (Rhodophyta) of Western Australia: Gloiotrichus fractalis gen. et sp. nov. and Ganonema helminthaxis sp. nov . Eur. J. Phycol. , 29 : 73 – 85 .
- Huisman , JM and Schils , T . 2002 . A re-assessment of the genus Izziella Doty (Liagoraceae, Rhodophyta) . Cryptog. Algol. , 23 : 237 – 249 .
- Huisman , JM , Abbott , IA and Sherwood , AR . 2004 . Large subunit rDNA gene sequences and reproductive morphology reveal Stenopeltis to be a member of the Liagoraceae (Nemaliales, Rhodophyta), with a description of Akalaphycus gen. nov . Eur. J. Phycol. , 39 : 257 – 272 .
- Huisman , JM , Saunders , GW and Sherwood , AR . 2006 . “ Recognition of Titanophycus, a new genus based on Liagora valida Harvey (Liagoraceae, Nemaliales) ” . In Algae of Australia: Nemaliales , Edited by: Huisman , J.M. 116 – 119 . Melbourne : Australian Biological Resources Study, Canberra & CSIRO Publishing .
- Lin , S-M , Fredericq , S and Hommersand , MH . 2001 . Systematics of the Delesseriaceae (Ceramiales, Rhodophyta) based on LSU rDNA and rbcL sequences, including the Phycodryoideae subfam. nov . J. Phycol. , 37 : 881 – 899 .
- Lin , S-M , Fredericq , S and Hommersand , MH . 2004 . Two new species of Martensia (Delesseriaceae, Rhodophyta) from Kenting National Park, southern Taiwan . Phycologia , 43 : 13 – 25 .
- Lin , S-M , Liang , H-Y and Hommersand , MH . 2008 . Two types of auxiliary cell ampullae in Grateloupia (Halymeniaceae, Rhodophyta), including G. taiwanensis Lin et Liang sp. nov. and G. orientalis Lin et Liang sp. nov. from Taiwan based on rbcL sequence analysis and cystocarp development . J. Phycol. , 44 : 196 – 214 .
- Lin , S-M , Yang , S-Y and Huisman , JM . 2011 . Systematic revision of the genera Liagora and Izziella (Liagoraceae, Rhodophyta) from Taiwan based on molecular analyses and carposporophyte development, with the description of two new species . J. Phycol. , 47 : 352 – 365 .
- Ronquist , F and Huelsenbeck , JP . 2003 . MrBayes 3: Bayesian phylogenetic inference under mixed models . Bioinformatics , 19 : 1572 – 1574 .
- Swofford, D.L. (2003). PAUP*: Phylogenetic Analysis using Parsimony (*and other Methods). Version 4.0b10. Sunderland, MA: Sinauer Associates
- Thiers, B. (2011, continuously updated). Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium, http://sweetgum.nybg.org/ih/
- Tseng , CK . 1941 . Studies on the Chinese species of Liagora . Bull. Fan Mem. Inst. Biol., Bot. , 10 : 265 – 282 .
- Wittmann , W . 1965 . Aceto-iron-haematoxylin-chloral hydrate for chromosome staining . Stain Tech. , 40 : 161 – 164 .
- Zeh , W . 1912 . Neue Arten der Gattung Liagora . Notizbl. K. Berlin Bot. Gart. Mus. , 5 : 268 – 273 .
- Zwickl, D.J. (2006). Genetic Algorithm Approaches for the Phylogenetic Analysis of Large Biological Sequence Datasets under the Maximum Likelihood Criterion. Ph.D. dissertation, University of Texas at Austin