Abstract
Hydrurus foetidus is a geographically widespread alga commonly detected as 2–10 cm thalli in mountain streams in early spring, and also in lowland rivers at latitudes where seasonal conditions are appropriate for this cold-water species. Reaching macroscopic dimensions but with a pronounced phenotypic plasticity, this species is not typical for the golden algae (Chrysophyceae) – they are almost exclusively microscopic organisms, best known as micro- or nanoplankton in fresh water. Other sessile multicellular members are less conspicuous in nature, and rarely detected and sampled for further investigation. Therefore, the phylogenetic position of Hydrurus within the Chrysophyceae is not clear and has been disputed. We determined the 18S and 28S rDNA subunit sequences from a typical H. foetidus sampled at Finse, Hardangervidda mountain plateau, in south Norway. Phylogenetic trees inferred from concatenated 18S and 28S sequences, including representatives of all known groups of heterokonts, safely confirmed Hydrurus as a chrysophyte. Extending the taxon sampling to include nearly all available 18S chrysophyte sequences from cultured species and environmental DNA, our analysis placed H. foetidus within a separate, well-defined clade (here named the Hydrurus-clade) dominated by environmental sequences and poorly defined strains of chrysophytes in culture, mostly from cold environments. The environmental sequences derived from other mountainous regions showed high similarity and may represent homologous or closely related species. However, the phylogenetic relationship to the closest morphologically described chrysophyte clades remains unresolved. Genetic tools for investigating the Hydrurus complex are now available. An increased sampling of Hydrurus-like heterokont species as well as chrysophytes in general is crucial for understanding the evolution of this lineage and its relations to other chrysophyte clades.
Introduction
Few members of the class Chrysophyceae (Chromista, Heterokonta) are large and visible without optical magnification. Most species within the class are planktonic, either unicellular or colonial, consisting of a limited number of cells and still requiring a microscope to be identified – examples are the unicellular Chromulina or the colonial Dinobryon. There are also a number of sessile or benthic species – either unicellular, like Lepochromulina, or colonial, as in Anthophysa – and a few multicellular species whose inconspicuous life-form and rarity may have prevented a closer acquaintance, such as Phaeodermatium (see Kristiansen & Preisig, Citation2001, or Nicholls & Wujek, Citation2003, for more information). The golden algae are also diverse ecologically, encompassing a few marine and many freshwater species with varying lifestyles, including heterotrophy. The planktonic Dinobryon was the first phototrophic chrysophyte also observed to engulf bacteria under natural conditions (Bird & Kalff, Citation1986). A life cycle has been described for Dinobryon that involves the formation of a zygote in the shape of a silicified binucleate statospore after fusion of cells from + and − strains (cf. Sandgren, Citation1988). The silicified resting stages of chrysophytes (stomatocysts) are characteristic and serve as valuable indicators for palaeolimnologists (e.g. Smol et al., Citation2005).
Hydrurus foetidus (Villars) Trevisan (Citation1848) is a large chrysophyte algae, and prominently visible during the late winter as ‘an exclusive inhabitant of cold mountain streams… [that] is distributed worldwide’ (Wehr & Sheath, Citation2003, p. 12). The thalli are firmly attached to hard rock or large stones in turbulent water, and easy to recognize due to their colour and the scarcity of other algae under such cold conditions. They may reach a few cm in unpolluted, soft-water rivers, but can reach lengths of 30 cm or more under more favourable conditions (Bursa, Citation1934). In fast-running and turbulent rivers, the thalli are arbuscular, with more or less well-developed central axes and branches made up of elongated, ellipsoid to ovoid cells in a firm polysaccharide matrix, carrying numerous minor branches of spherical and compressed cells; each axis or branch terminates in a larger triangular to conical apical cell (cf. Klaveness & Lindstrøm, Citation2011). The entire thallus is embedded within a gel, which gives it a smooth and slippery surface. In slowly seeping water films, Hydrurus may appear as a slimy coating upon rock and pebble surfaces (e.g. Klaveness, Citation1992, ). The apparent phenotypic plasticity within this morphospecies may reflect ecotypes or different species – a question to be solved by wider sampling and discussion of relevant species concepts, and by obtaining genetic sequences from several natural strains. A wide range of ‘species’ are also available from herbaria in Europe. Here, we follow the recommendations by recognized authorities, from Pascher & Lemmermann (Citation1913) to Kristiansen & Preisig (Citation2001), treating the genus as monotypic until modern evidence points to different conclusions.
Due to its size and prominent morphology and its rather spectacular occurrence under seasonal climate regimes, Hydrurus has been the focus for many investigations. The occurrence of Hydrurus in central European alpine regions and the Tatra mountains has inspired a number of benchmark papers (e.g. Rostafinski, Citation1882; Klebs, Citation1893, Citation1896; Lagerheim, Citation1888; Geitler, Citation1927; Avel & Avel, Citation1932; Bursa, Citation1934; Kann, Citation1978), where further references may be found. Early records from the UK are listed by Whitton et al. (Citation1978), and the distribution there outlined by Kristiansen (Citation2002). Records in North America go back to 1862 (Setchell & Gardner, Citation1903). In Scandinavia, Hydrurus is well known from mountain streams, sometimes favoured in watersheds of sedimentary origin (e.g. Wille, Citation1885) or in regions influenced by settlements (e.g. Ström, Citation1926) and at times present also during summer in environments where water temperatures remain low. Massive development of this alga is known (e.g. Wille, Citation1885; Geitler, Citation1927; Bursa, Citation1934; Skuja, Citation1964; Skulberg & Lillehammer, Citation1984). Early blooms of Hydrurus in affected rivers have ecological significance as food for water insects (Ward, Citation1994; Milner et al., Citation2001) and microbial life (e.g. Rott et al., Citation2006).
Hydrurus foetidus has been carefully investigated by light (e.g. Klebs, Citation1893, Mack, Citation1953; Fukushima, Citation1962; Joyon, Citation1963; Klaveness & Lindstrøm, Citation2011) and electron microscopy (e.g. Hovasse & Joyon, Citation1960; Vesk et al., Citation1984; Hoffman et al., Citation1986; Andersen, Citation1991), and the details of its peculiar silicified stomatocyst have been outlined (Hovasse & Joyon, Citation1960). The motile zoospores have attracted attention, since their tetrahedral shape appears to be unique to Hydrurus and genera identified as related, partly due to the morphology of motile cells (e.g. Phaeodermatium). A full life cycle involving phase transitions has not been outlined, since efforts to culture Hydrurus have failed until recently (Klaveness & Lindstrøm, Citation2011).
The few studies where molecular phylogenies have been inferred for a considerable number of chrysophyte species show little resolution of the group (see for example Lavau et al., Citation1997; Caron et al., Citation1999; Andersen, Citation2007; Patil et al., Citation2009), with Hydrurus yet to be investigated with 18S and/or 28S rDNA. Only 5S rDNA gene sequences were available for Hydrurus, and the corresponding phylogeny did not resolve the position of the genus (Lim et al., Citation1986; Hori & Osawa, Citation1987). It was thus uncertain as to where the genus would be placed in a phylogenetic analysis inferred from more sequence characters.
The goal of this study was to shed light upon the phylogenetic position of H. foetidus using molecular phylogenetic techniques. We performed phylogenetic analyses of 18S and 28S rDNA genes to investigate the identification and classification of the species as well as the more general problem of the evolution and relationships of Hydrurus in a wider context.
Materials and methods
Materials
Hydrurus foetidus was collected at the outlet of Lake Finsevatn (‘Garpefossen’) in the vicinity of Finse Alpine Research Center, at 60° 36′ N, 7° 30′ E and 1215 m altitude (for map of locality, see Klaveness & Lindstrøm, Citation2011). Microscopy confirming identity and absence of foreign organisms (epiphytes etc.), and photographic documentation were done immediately or within hours of sampling; samples were stored at 0°C. The first collection (February 2006) included 20–30 mm thalli, which stuck easily to white card left to dry in an upright position. Sampling was repeated in March 2007, specimens being dried on card and also used for sequencing rDNA genes. The sample sheet from which sequenced material was cut is deposited in the herbarium of the Natural History Museum, University of Oslo, under the species name (‘Hydrurus foetidus (Villars) Trevisan Citation1848’) and dated 7 March 2007. Stock cultures of this strain (named G 070301) are also kept in algal culture collections in Oslo and Göttingen.
DNA isolation and PCR amplification
Material on the cardboard paper was cut into small pieces and collected in a microfuge tube. 600 µl of POWERlyse buffer (Nordiag ASA, Norway) was added and incubated at 65°C for 1 min. The lysate was then centrifuged briefly, supernatant transferred to a new tube and DNeasy Plant mini kit (Qiagen GmbH, Hilden, Germany) used following the manufacturer's protocol. The Hydrurus 18S rDNA gene was amplified using universal 18S primers (Medlin et al., Citation1988). The 28S rDNA gene was amplified using the 28S primers; 5.8S F, LSU 4256R and LSU 15R (Riisberg et al., Citation2009). PCR amplification was performed using Finnzymes Phusion (Finnzyme Oy, Finland) high-fidelity DNA polymerase. Cycling conditions for 18S amplification were: (1) 98°C for 30 s, (2) 98°C for 10 s, (3) 50°C for 20 s, (4) 72°C for 35 s, (5) repeat steps (2)–(4) 34 times, and (6) 72°C for 7 min. For the 28S amplification, the conditions were: (1) 98°C for 30 s, (2) 98°C for 10 s, (3) 67°C for 30 s, (4) 72°C for 2.5 min, (5) repeat steps (2)–(4) 34 times, and (6) 72°C for 7 min. Negative PCR controls absent for DNA were run to exclude any possible contamination.
Cloning and sequencing
PCR products were cloned into pCR4 Blunt-TOPO vector using a Zero Blunt cloning kit (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. Plasmid DNA was extracted from E. coli TOPO10 chemically competent cells using Promega Plasmid Wizard kit. Plasmids were analysed confirming positive insertion using EcoRI. A minimum of five positive clones was sequenced with a big dye terminator system (v 3.1 Applied Biosystems) using the primers M13F, M13R for 18S sequences. All five clones contained the same insert sequence. The 28S sequencing primers used were LSU4F, LSU4R, LSU5F and LSU5R (Riisberg et al., Citation2009). The sequences generated here are deposited at and available from NCBI Entrez with accession numbers FM955256 (18S rDNA) and FM955257 (28S rDNA).
Data mining and phylogenetic analyses
The 18S and 28S rDNA alignment of Heterokonta and Chrysophyceae was the same as in Riisberg et al. (Citation2009) with sequences from Hydrurus generated in this study added to it. Publicly available 18S rDNA sequences of Chrysophyceae for the generation of the 18S rDNA Chrysophyceae alignment were acquired from GenBank via BLAST searches using Hydrurus query sequences. Sequence alignments were constructed with MAFFT (Katoh et al., Citation2002) and subsequently edited manually. After removing ambiguously aligned sites, the 18S alignment consisted of 94 taxa and 1627 nucleotide characters, whereas the 28S had 2367 nucleotide characters and 48 taxa. The concatenated (18S + 28S) alignment contained 48 taxa and 3904 nucleotide characters. The alignments are available in the supplementary material, accessible via the Supplementary Content tab on the article's online page at http://dx.doi.org.10.1080/09670262.2011.598950.
The alignments were subjected to maximum-likelihood (ML) and Bayesian analyses. ML analyses were performed with RaxML v7.2.6 (Stamatakis et al., Citation2005) using the General Time Reversible (GTR) model considering the proportion of invariable sites (I) and gamma (G) distributed site rates, as suggested by the program Modeltest (Posada & Buckley, Citation2004). The gamma distribution was approximated with four rate categories and the analyses were run from a random starting tree. The topology with the highest likelihood score out of 100 heuristic searches with a randomly selected starting tree for each search was chosen. Non-parametric bootstrap scores were calculated from 500 pseudo-replicates using the best topology as starting tree. The Bayesian inferences were performed using MrBayes v3.1.2 (Ronquist & Huelsenbeck, Citation2003), applying the GTR + G + I model as selected by MrModeltest (Nylander, Citation2004). Ten independent analyses were run, each from a random starting tree and with four Monte Carlo Markov Chain (MCMC) chains that lasted for 4 000 000 generations. The MCMC chains included three heated and one cold chain. The harmonic mean likelihood values, posterior probability and tree topology were calculated from the sampled trees after burn-in. Burn-in was set at 25% after visual inspection of the marginal likelihood scores of the sampled trees. Harmonic mean values and posterior probability values for the internal nodes were almost identical in each run, suggesting convergence of the MCMC chains.
Fast-evolving sites were identified using PAML implemented in the AIR package (Kumar et al., Citation2009) on the 18S rDNA dataset. From 10% to 90% (with 10% intervals) of the fastest evolving sites were removed and ML analyses on each reduced alignment were run as before.
To test for possible outgroup artifacts we analysed the 18S dataset with different sets of closely related outgroups [Picophagus, Chlamydomyxa, Synchroma, Leukarachnion and a closely related environmental sequence (AB534476); for details see Andersen (Citation2007), Patil et al. (Citation2009) and Grant et al. (Citation2009)], as well as without any outgroup. For the final analyses Leukarachnion and sequence AB534476 were chosen as outgroup, because they had the shortest branches of the outgroup taxa.
All phylogenetic analyses were performed on University of Oslo's Bioportal Platform (http://www.bioportal.uio.no cf. Kumar et al., Citation2009).
Results
Morphology
The strain from the outlet of Lake Finsevatn is documented in . Specific characters include the arbuscular thalli, consisting of a firm central axis and peripheral branches () containing characteristic cells within a viscous gelatinous coat (). By March thalli averaged 3–5 cm in length, with a maximum of 7 cm. Zoospores were released from the periphery of the thallus; they were rounded at first, before attaining a tetrahedral shape () with a single visible flagellum within minutes of release. The thallus, and the cells in the periphery in particular, were sensitive to temperature and quickly changed morphology (eventually decomposing) as they warmed to room temperature. Comparable morphologies and details have been documented and discussed by Rostafinski (Citation1882), Lagerheim (Citation1888), Klebs (Citation1893), Mack (Citation1953), Hovasse & Joyon (Citation1960), Hoffmann et al. (Citation1986), Canter-Lund & Lund (Citation1995) and Graham & Wilcox (Citation2000).
Figs 1–5. Morphology of Hydrurus foetidus collected at Finse, Norway. 1. Specimen dried on cardboard paper and depicted by optical scanning at 600 dpi showing a small, richly branched specimen, collected late February 2006. 2. Individual thalli collected March 2007, in good growth. 3. Specimen sampled in March 2007, apex of two individual branches in good growth. 4. Detail of branch apex in good growth, showing the dominant apical cell and adjacent vegetative cells. 5. Zoospore released from cells a few hours after collection. The zoospore is released as a spherical cell but develops rapidly (within minutes) into the tetrahedal zoospore (shown here) characteristic for this species. Scale bars: 10 mm (: bar in ), 100 µm () or 10 µm (: bar in ).

Phylogenetic placement of H. foetidus within the heterokont group from 18S + 28S rDNA phylogeny
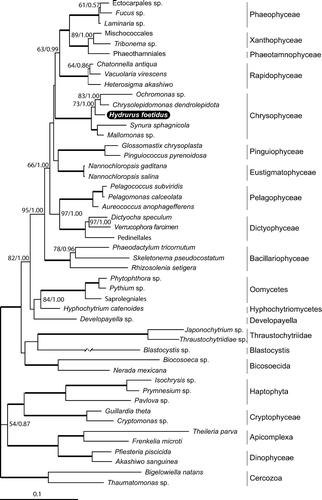
In an attempt to place H. foetidus within the phylogeny of heterokonts, we amplified both 18S and 28S rDNA sequences from the species and performed phylogenetic inferences of the concatenated sequences from the entire heterokont clade (). The analysis recovered the photosynthetic classes of heterokonts as a monophyletic clade with high ML bootstrap support and Bayesian posterior probability values [95% bootstrap support (BS) and 1.00 Bayesian posterior probability (PP)]. The heterotrophic lineages comprised two clades, though these were without significant support (Oomycetes + Hyphochytridiomycetes + Developayella and Thraustochytridae + Blastocystis +Bicosoecida). In accordance with other reports on the rDNA phylogeny of heterokonts (Cavalier-Smith & Chao, Citation2006; Riisberg et al., Citation2009), the heterotrophic clades were not supported as monophyletic. The Chrysophyceae, however, was recovered as monophyletic with maximum support. Within this, H. foetidus was the closest relative to Ochromonas sp. and Chrysolepidomonas dendrolepidota (73% BS/1.00 PP) ().
Fig. 6. Bayesian phylogenetic tree based on the concatenated alignments of 18S and 28S rDNA sequences (3904 bp and 48 taxa) for Heterokonta, rooted with Haptophyceae, Cryptophyceae, Apicomplexa, Dinophyceae and Cercozoa. Maximum-likelihood bootstrap (BS, in %) and posterior probability values (PP) at the branches are separated by slashes. Support values are only shown for branches that received maximum-likelihood BS support >50% and Bayesian support >0.80 PP. Thick lines indicate maximum support values (100% BS/1.00 PP) An asterisk (*) indicates that the branch length is divided by two. The Hydrurus 18S and 28S rDNA sequences are deposited in Genbank under the accession numbers FM955256 (18S) and FM955257 (28S). For the other accession numbers see table in Riisberg et al. (Citation2009).
Phylogeny of H. foetidus related to Chrysophyceae: 18S rDNA phylogeny
The 18S + 28S rDNA phylogeny did not unequivocally reveal the placement of Hydrurus within the Chrysophyceae clade, due to the low taxon sampling in the concatenated alignment (only five chrysophyte species including Hydrurus). Therefore, we inferred an 18S phylogeny with an extended sampling, based on the sequences already available, including those originating from environmental samples. The tree was rooted with Leukarachnion sp. and a closely related environmental clone (AB534476) () and H. foetidus was recovered among the Chrysophyceae with high support (100% BS/1.00 PP). The nearest sister to H. foetidus was the environmental sequence AJ867745 (97% BS/1.00 PP). These two taxa clustered together with the undescribed strain CCMP1899 and several other environmental sequences in a highly supported clade (85% BS/1.00 PP), which is referred to here as the Hydrurus-clade (). The Hydrurus-clade was in turn contained within a larger group that included several sequences from environmental samples, as well as the species Phaeoplaca thallosa and the culture collection strains CCCM41 and CCMP2296 (88% BP/1.00 PP). Additional information about the related sequences in the Hydrurus clade is summarized in . No further resolution of the position of Hydrurus and its closest relatives was given by the 18S rDNA phylogeny as there was no support for the backbone nodes in the tree. Removing fast-evolving sites and testing different outgroups (not shown) did not result in a different topology or improved support values than in the phylogeny presented in .
Fig. 7. Bayesian phylogenetic tree of Chrysophyceae based on an 18S rDNA alignment of 1627 characters and 97 taxa, rooted with Leukarachnion sp. and an uncultured eukaryote (AB534476). Maximum likelihood bootstrap values and Bayesian posterior probability values as in Fig. 6.
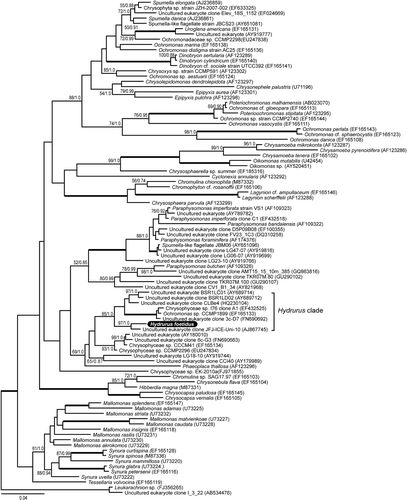
Table 1. Accession numbers, origin of samples and sequences clustering within the Hydrurus clade (AY689714 to AJ867745) and their closest neighbours in the 18S rDNA tree (). The unpublished author information is from the NCBI-Entrez pages for the accession numbers given in the first column.
Discussion
In Pascher's keystone publications (Pascher & Lemmermann, Citation1913; Pascher, Citation1914), Hydrurus was assigned to the class Chrysophyceae, a policy continued in subsequent classifications and supported by more recent light and transmission electron microscopy (e.g. Preisig, Citation1995; Kristiansen & Preisig, Citation2001; Andersen, Citation2007). The 18S + 28S rDNA phylogeny presented here () confirms that Hydrurus has an evolutionary origin among the golden algae. When it came to understanding whether Hydrurus is a primitive or advanced member among the golden algae, traditional viewpoints diverged. While Klebs (Citation1893) depicted the cellular organelles in detail, including contractile vacuoles within the vegetative cells, and treated Hydrurus as a member of the flagellates, Fritsch (Citation1935, p. 546) pointed to the fact that Hydrurus ‘in its marked division of labour far surpasses any of the palmelloid forms found in other classes and may in some respect be ranked as high as Draparnaldia’. Although electron microscopy revealed a wealth of new information and resolved important structural and functional differences between classes of heterokonts (see Andersen, Citation2007, for recent developments), this laborious method contributed little to an understanding of evolutionary relationships among chrysophytes. A problem with the morphological evidence at the transmission electron microscopy level is the uneven availability of information concerning characters of possible evolutionary significance, like mitosis, meiosis, cytoskeletal construction and function, and the ultrastructure of the flagellar bases. Mitosis in Hydrurus has been investigated by electron microscopy (Vesk et al., Citation1984) and exhibits a variant of orthomitosis (Raikov, Citation1994), involving a symmetrical metaphase within the boundaries of a more or less deconstructed nuclear membrane. This is known also from other chrysophytes (Raikov, Citation1994). The bases of two vestigial flagella on the vegetative cell of Hydrurus were first shown by Vesk et al. (Citation1984) and in more detail by Hoffman et al. (Citation1986; see also Andersen, Citation1991) – gained by a very laborious (and expensive) method only available in dedicated laboratories. We are confronted with the fact that molecular methods are the most efficient tools for evaluation of evolutionary relationships, across diverging morphologies, incommensurable information, and different environments.
The 5S rDNA sequences analysed by Lim et al. (Citation1986) placed Hydrurus at the base of the chromophyte phylogeny. However, the resolution and support provided by the short 5S rDNA sequence was poor. Generally, the concatenation of 18S and 28S rDNA has improved reconstruction of the heterokont phylogeny (Ben Ali et al., Citation2002; Edvardsen et al., Citation2007; Riisberg et al., Citation2009). In our concatenated analysis, many of the deep branches are well supported, but there are few taxa for which data on both genes are available and this hinders any further resolution of the placement of Hydrurus, other than its allocation to the Chrysophyceae.
In our 18S rDNA phylogeny, there is essentially no support for the backbone nodes and it is therefore not possible to confidently determine the evolutionary position of the Hydrurus-clade and its allies (see ) among the Chrysophyceae. This is also seen in other 18S phylogenies of the Chrysophyceae (Andersen, Citation2007). The Hydrurus sequence does not cluster with any other described chrysophyte species. Instead, its closest relatives are environmental samples from snow and ice in alpine or arctic areas, or from Baltic Sea ice (). These form the highly supported ‘Hydrurus-clade’ (AY689714 to AJ867745). Closely related to this clade are unclassified and uncultured chrysophytes (AY180010 to EU247834), of which at least two are from cold environments. Of interest is also the affinity to Phaeoplaca thallosa (AF123296), a pseudoparenchymatous chrysophyte. In the comparable phylogenetic tree of Andersen (Citation2007), this species grouped (96% BS) with EF165134 (=CCCM41) and EF165133 (=CCMP1899), the latter being a member of the Hydrurus clade here. The link with P. thallosa suggests that it will be rewarding to generate new phylogenies that include colonial or multicellular species, such as Celloniella palensis (Pascher, Citation1929), the presumably more closely related Chrysonebula holmesii (cf. Hibberd, Citation1977) and the ‘advanced’, pseudoparenchymatous Phaeodermatium rivulare (see e.g. chapter 12 in Wehr & Sheath, Citation2003, for an overview of chrysophyte diversity); addressing this goal will require additional taxon and gene sampling.
In his paper on the systematics of the Chrysophyceae and Synurophyceae, Andersen (Citation2007) has shown that traditional classifications based upon characters like flagellar numbers or the formation of colonies or thalli are not compatible with phylogenetic trees constructed from 18S rDNA and/or rbcL genes. Unicellular genera with either a single or two flagella (visible in the light microscope) may have appeared several times during the evolution of chrysophytes and therefore do not constitute natural clades in the phylogenetic trees. Similarly, groupings of life forms with capsoid thalli (with individual non-motile cells embedded in mucilage) seem also not to be natural.
Since the 18S rDNA phylogenetic tree shown by us here reveals environmental sequences from high-altitude watersheds with snow and ice, and other cold localities as closest relatives to our Hydrurus, it is likely that a clade of Hydrurus-related species exists on a geographically widespread scale. Unfortunately, the winter and early spring seasons traditionally have had low priority for sampling in lakes and rivers (cf. Salonen et al., Citation2009). However, since the recent emergence of a Working Group of Winter Limnology within the International Limnological Society, and because symposia on winter limnology are arranged biannually, there is now a realistic hope of more samples and in situ identifications.
tejp_a_598950_sup_19797735.nxs
Download (159.5 KB)tejp_a_598950_sup_19797734.nxs
Download (184.7 KB)Acknowledgements
We are grateful to Ingvild Riisberg and Russell Orr for providing the heterokont 28S alignment, and the latter also for improving the language. The Research Council of Norway by a grant to KSJ funded the project (Project # 172572/S40). The Bioportal platform (http://www.bioportal.uio.no) is acknowledged for providing computer resources. KST thank University of Oslo for a fellowship to JB.
References
- Andersen , RA . 1991 . The cytoskeleton of chromophyte algae . Protoplasma , 164 : 143 – 159 .
- Andersen , RA . 2007 . “ Molecular systematics of the Chrysophyceae and Synurophyceae ” . In Unravelling the Algae , Edited by: Brodie , J and Lewis , J . 285 – 313 . Boca Raton, Florida : CRC Press .
- Andersen , RA , Van de Peer , Y , Potter , D , Sexton , JP , Kawachi , M and LaJeunesse , T . 1999 . Phylogenetic analysis of the SSU rRNA from members of the Chrysophyceae . Protist , 150 : 71 – 84 .
- Avel , MME and Avel , M . 1932 . Sur l’existence dans le Massif Central da la Chrysomonadine Hydrurus foetidus Kirchner . Rev. Algol. , 11 : 347 – 349 .
- Ben Ali , A , de Baere , R , de Wachter , R and Van de Peer , Y . 2002 . Evolutionary relationships among heterokont algae (the autotrophic stramenopiles) based on combined analysis of small and large subunit ribosomal RNA . Protist , 153 : 123 – 132 .
- Bird , DF and Kalff , J . 1986 . Bacterial grazing by planktonic lake algae . Science , 231 : 493 – 495 .
- BURSA, A. (1934). Hydrurus foetidus Kirch. in der Polnischen Tatra. Oekologie, Morphologie. I. Phenologie. II. Bull. Acad. Pol. Sci. Lettr. 1934. B: Sciences Naturelles I : 69–84, 113–131
- Canter-Lund , H and Lund , JWG . 1995 . Freshwater Algae: Their Microscopic World Explored , Bristol : Biopress .
- Caron , DA , Lim , EL , Dennet , MR , Gast , RJ , Kosman , C and DeLong , EF . 1999 . Molecular phylogenetic analysis of heterotrophic chrysophyte genus Paraphysomonas (Chrysophyceae), and the design of rRNA-targeted oligonucleotide probes for two species . J. Phycol. , 35 : 824 – 837 .
- Cavalier-Smith , T and Chao , EE . 2006 . Phylogeny and megasystematics of phagotrophic heterokonts (Kingdom Chromista) . J. Mol. Evol. , 62 : 388 – 420 .
- Edvardsen , B , Eikrem , W , Shalchian-Tabrizi , K , Riisberg , I , Johnsen , G , Naustvoll , L and Throndsen , J . 2007 . Verrucophora farcimen gen. et sp. nov (Dictyochophyceae, Heterokonta) – a bloom-forming ichthyotoxic flagellate from the Skagerrak, Norway . J. Phycol. , 43 : 1054 – 1070 .
- Fritsch , FE . 1935 . The Structure and Reproduction of the Algae , Vol. 1 , Cambridge : Cambridge University Press .
- Fukushima , H . 1962 . Preliminary report on the life history of Hydrurus foetidus . Acta Phytotax. Geobot. , 20 : 290 – 295 .
- Geitler , L . 1927 . Über Vegetationsfärbungen in Bächen . Biol. Gen. , 3 : 791 – 814 .
- Graham , LE and Wilcox , LW . 2000 . Algae , New Jersey : Prentice-Hall .
- Grant , J , Tekle , YI , Anderson , OR , Patterson , DJ and Katz , LA . 2009 . Multigene evidence for the placement of a heterotrophic amoeboid lineage Leukarachnion sp. among photosynthetic stramenopiles . Protist , 160 : 376 – 385 .
- Hamilton , AK , Lovejoy , C , Galand , PE and Ingram , RG . 2008 . Water masses and biogeography of picoeukaryote assemblages in a cold hydrographically complex system . Limnol. Oceanogr. , 53 : 922 – 935 .
- Hibberd , DJ . 1977 . The cytology and ultrastructure of Chrysonebula holmesii Lund (Chrysophyceae), with special reference to the flagellar apparatus . Br. Phycol. J. , 12 : 369 – 383 .
- Hoffman , LR , Vesk , M and Pickett-Heaps , JD . 1986 . The cytology and ultrastructure of zoospores of Hydrurus foetidus (Chrysophyceae) . Nord. J. Bot. , 6 : 105 – 122 .
- Hori , H and Osawa , S . 1987 . Origin and evolution of organisms as deduced from 5S ribosomal RNA sequences . Mol. Biol. Evol. , 4 : 445 – 472 .
- Hovasse , R and Joyon , L . 1960 . Contribution à l'étude de la Chrysomonadine Hydrurus foetidus . Rev. Algol. (N.S.) , 5 : 66 – 83 .
- Joyon , L . 1963 . Contribution a l'etude cytologique de quelques protozoaires flagellés . Ann. Fac. Sci. Univ. Clermont , 22 : 1 – 96 .
- Kann , E . 1978 . Systematik und Ökologie der Algen der österreichischer Bergbäche . Arch. Hydrobiologie , Suppl. 53 : 405 – 643 .
- Katoh , K , Misawa , K , Kuma , K and Miyata , T . 2002 . MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform . Nucleic Acid Res. , 30 : 3059 – 3066 .
- Klaveness , D . 1992 . Ferskvanns-algene i Norge: en forskningsoppgave “for leg og lærd” . Blyttia , 50 : 121 – 140 .
- Klaveness , D and Lindstrøm , E-A . 2011 . Hydrurus foetidus (Chromista, Chrysophyceae) – a large freshwater chromophyte alga in laboratory culture . Phycol. Res. , 59 : 105 – 112 .
- Klebs , G . 1893 . Flagellatenstudien, Theil I + II . Z. wiss. Zool. , 55 : 265 – 445 .
- KLEBS, G. (1896). Ueber die Fortpflanzungs-Physiologie der niederen Organismen, der Protobionten. Specieller Theil. Die Bedingungen der Fortpflanzung bei einigen Algen und Pilzen. Gustav Fischer, Jena
- Kristiansen , J . 2002 . “ Phylum Chrysophyta (Golden Algae) ” . In The Freshwater Algal Flora of the British Isles , Edited by: John , DM , Whitton , BA and Brook , AJ . 214 – 244 . Cambridge : Cambridge University Press .
- KRISTIANSEN, J. & PREISIG, H.R. (2001). Encyclopedia of Chrysophyte Genera. Bibliotheca Phycologica. Vol. 110. J. Cramer, Berlin & Stuttgart
- Kumar , S , Skjaeveland , A , Orr , R , Enger , P , Ruden , T , Mevik , BH , Burki , F , Botnen , A and Shalchian-Tabrizi , K . 2009 . AIR: a batch-oriented web program package for construction of supermatrices ready for phylogenomic analyses . BMC Bioinformatics , 10 : 357
- Lagerheim , G . 1888 . Zur Entwickelungsgeschichte des Hydrurus . Ber. Deutsch. Bot. Ges. , 6 : 73 – 85 .
- Lavau , S , Saunders , GW and Wetherbee , R . 1997 . A phylogenetic analysis of Synurophyceae using molecular data and scale case morphology . J. Phycol. , 33 : 135 – 151 .
- Lim , B-L , Kawai , H , Hori , H and Osawa , S . 1986 . Molecular evolution of 5S ribosomal RNA from red and brown algae . Jap. J. Genet. , 61 : 169 – 176 .
- Mack , B . 1953 . Untersuchungen an Chrysophyceen IV. Zur Kenntnis von Hydrurus foetidus . Österr. Bot. Z. , 100 : 579 – 582 .
- Medlin , L , Elwood , HJ , Stickel , S and Sogin , ML . 1988 . The characterisation of enzymatically amplified eukaryotic 16S-like rRNA-coding regions . Gene , 71 : 491 – 499 .
- Milner , AM , Brittain , JE , Castellas , E and Petts , GE . 2001 . Trends of macroinvertebrate community structure in glacier-fed rivers in relation to environmental conditions: a synthesis . Freshw. Biol. , 46 : 1833 – 1847 .
- Nicholls , KH and Wujek , DE . 2003 . “ Chrysophycean algae ” . In Freshwater Algae of North America – Ecology and Classification , Edited by: Wehr , JD and Sheath , RG . 471 – 507 . San Diego : Academic Press .
- NYLANDER, J.A.A. (2004). MrModeltest v2. Distributed by the author. Evolutionary Biology Centre, Uppsala University
- Pascher , A . 1914 . Über Flagellaten und Algen . Ber. Deutsch. Bot. Ges. , 32 : 136 – 160 .
- Pascher , A . 1929 . Über die Beziehungen zwischen Lagerform und Standortsverhältnissen bei einer Gallertalge (Chrysocapsale) . Arch. Protistenk. , 68 : 637 – 668 .
- PASCHER, A. & LEMMERMANN, E. (1913). Chrysomonadinae. In Die Süsswasserflora Deutschlands, Österreichs und der Schweiz, Heft 2: Flagellatae II, 7–95 (Pascher, A. editor). G. Fischer, Jena
- Patil , V , Bråte , J , Shalchian-Tabrizi , K and Jakobsen , KS . 2009 . Revisiting the phylogeny of Synchroma grande . J. Euk. Microbiol. , 56 : 394 – 396 .
- Posada , D and Buckley , TR . 2004 . Modeltest: testing the model of DNA substitution . Bioinformatics , 14 : 817 – 818 .
- Preisig , H . 1995 . “ A modern concept of chrysophyte classification ” . In Chrysophyte Algae. Ecology, Phylogeny and Development , Edited by: Sandgren , CD , Smol , JP and Kristiansen , J . 46 – 74 . Cambridge : Cambridge University Press .
- Raikov , IB . 1994 . The diversity of forms of mitosis in protozoa: a comparative review . Eur. J. Protistol. , 30 : 253 – 269 .
- Richards , TA , Vepritskiy , AA , Gouliamova , DE and Nierzwici-Bauer , SA . 2005 . The molecular diversity of freshwater picoeukaryotes from an oligotrophic lake reveals diverse, distinctive and globally dispersed lineages . Environ. Microbiol. , 7 : 1413 – 1425 .
- Riisberg , I , Orr , RJS , Kluge , R , Shalchian-Tabrizi , K , Bowers , HA , Patil , V , Edvardsen , B and Jakobsen , KS . 2009 . Seven gene phylogeny of heterokonts . Protist , 160 : 191 – 204 .
- Ronquist , F and Huelsenbeck , JP . 2003 . MrBayes 3: Bayesian phylogenetic inference under mixed models . Bioinformatics , 19 : 1572 – 1574 .
- Rostafinski , MJ . 1882 . L'Hydrurus et ses Affinités . Ann. Sci. Nat., ser. 6, Bot. , 14 : 5 – 25 .
- Rott , E , Füreder , L , Schütz , C , Sonntag , B and Wille , A . 2006 . A conceptual model for niche differentiation of biota within an extreme stream microhabitat . Verh. Internat. Verein. Limnol. , 29 : 2321 – 2323 .
- Salonen , K , Leppäranta , M , Viljanen , M and Gulati , RD . 2009 . Perspectives in winter limnology: closing the annual cycle of freezing lakes . Aquat. Ecol. , 43 : 609 – 616 .
- Sandgren , CD . 1988 . “ The ecology of chrysophyte flagellates: their growth and perennation strategies as freshwater phytoplankton ” . In Growth and Reproductive Strategies of Freshwater Phytoplankton , Edited by: Sandgren , CD . 9 – 104 . Cambridge : Cambridge University Press .
- Setchell , WA and Gardner , NL . 1903 . Algae of Northwestern America . Univ. California Publ. (Botany) , 1 : 165 – 418 .
- Skuja , H . 1964 . Grundzüge der Algenflora und Algenvegetation der Fjeld-Gegenden um Abisko in Schwedisch-Lappland . Nova Acta Regiae Soc. Sci. Upsal., Ser. 4 , 18 ( 3 ) : 1 – 465 .
- Skulberg , OM and Lillehammer , A . 1984 . “ Glåma ” . In Ecology of European Rivers , Edited by: Whitton , BA . 469 – 498 . London : Blackwell Scientific Publishers .
- SMOL, J.P., WOLFE; A.P., BIRKS, H.J.B., DOUGLAS, M.S.V., JONES, V.J., KORHOLA. A., PIENITZ, R., RÜHLAND, K., SORVARI, S., ANTONIADES, D., BROOKS, S.J., FALLU, M.A., HUGHES, M., KEATLEY, B.E., LAING, T.E., MICHELUTTI, N., NAZAROVA, L., NYMAN, M., PATERSON, A.M., PERREN, B., QUINLAN, R., RAUTIO, M., SAULNIER-TALBOT, E., SIITONEN, S., SOLOVIEVA, N. & WECKSTRÖM, J. (2005). Climate-driven regime shifts in the biological communities of arctic lakes. Proc. Natl. Acad. Sci. USA, 102: 4397–4402
- Stamatakis , A , Ludwig , T and Meier , H . 2005 . RAxML-III: a fast program for maximum likelihood-based inference of large phylogenetic trees . Bioinformatics , 21 : 456 – 463 .
- Stoeck , T and Epstein , S . 2003 . Novel eukaryotic lineages inferred from small-subunit rRNA analyses of oxygen-depleted marine environments . Appl. Environ. Microbiol. , 69 : 2657 – 2663 .
- Ström , KM . 1926 . Norwegian mountain algae. An account of the biology, ecology and distribution of the algae and pelagic invertebrates in the region surrounding the mountain crossing of the Bergen railway . Skr. Det Norske Videnskaps-Akad. i Oslo I. Mat.-Naturv. Klasse , 1926 ( 6 ) : 1 – 263 .
- TREVISAN, V.B.A. (1848). Saggio di una monografia delle Alghe Coccotalle. Padua. (Cited from http://www.algaebase.org; searched on 18 May 2011)
- Vesk , M , Hoffman , LR and Pickett-Heaps , JD . 1984 . Mitosis and cell division in Hydrurus foetidus (Chrysophyceae) . J. Phycol. , 20 : 461 – 470 .
- Ward , JV . 1994 . Ecology of alpine streams . Freshw. Biol. , 32 : 277 – 294 .
- Wehr , JD and Sheath , RG . 2003 . “ Freshwater habitats of algae ” . In Freshwater Algae of North America – Ecology and Classification , Edited by: Wehr , JD and Sheath , RG . 11 – 57 . San Diego : Academic Press .
- WHITTON, B.A., HOLMES, N.T.H. & SINCLAIR, C. (1978). A Coded List of 1000 Freshwater Algae of the British Isles. Water Archive Manual Series 2. Reading, UK
- Wille , N . 1885 . Bidrag til Algernes Physiologiske Anatomi . Kungl. Svenska Vetenskaps-Akad. Handl. , 21 ( 12 ) : 1 – 104 .
Supplementary material
The following supplementary material is available for this article, via the Supplementary Content tab of the article's online page at http://dx/doi.org/10.1080/09670262.2011.598950:
Hydrurus18S-28S-alignment.nxs
Hydrurus18S-alignment.nxs