Abstract
Recent changes in kelp distribution along the north coast of Spain are described and analysed through a long-term population study focused on Saccorhiza polyschides. The main purpose of this work was to understand which population processes are more sensitive to increased sea surface temperatures and reductions in the intensity of upwelling episodes in the current scenario of global warming. Data on the distribution of kelp species (old and recent data) were obtained from the literature and compared with current species distributions assessed by field sampling between 2007 and 2010 and covering a transition coastline of 200 km. The long-term population study of Saccorhiza polyschides was conducted at a site close to the edge of its current distribution. Data for recruitment, growth and survival, as well as density and supported biomass of populations collected since the 1990s were analysed using data from the late 1970s for comparison. Kelps on the north coast of Spain have shown a westward retreat since the 1980s. Dense populations of Saccorhiza polyschides, the most important species, and Laminaria ochroleuca have been reduced to small patches and isolated individuals east of Peñas Cape (43° 39.4′ N; 5° 50.8′ W). The long-term study of Saccorhiza polyschides populations showed a collapse in the growth of the sporophyte and very low recruitment from the beginning of this century. The possible causal factors of this population decline and its consequences are considered, suggesting that long warm summer periods (more than 30 consecutive days of seawater temperature > 20°C) could alter kelp performance.
Introduction
Kelps are large brown seaweeds colonizing hard substrates in the low intertidal or shallow subtidal zones of temperate and polar coastal waters. They are ‘foundation species’ (Dayton, Citation1972), which create complex ecosystems (‘kelp forests’) that are highly diverse in terms of structure and function and provide important goods and services (Steneck et al., Citation2002).
Several species of laminarian kelps dominate low intertidal and shallow subtidal communities along the Atlantic coast of Europe and show a continuous distribution from the Arctic to Brittany, but southwards they are only present associated with areas of intense upwelling (Lüning, Citation1990). This is the case, for example, in the coastal waters of the Iberian Peninsula (Atlantic coasts of Spain and Portugal), in which Saccorhiza polyschides (Lightfoot) Batters, Laminaria hyperborea (Gunnerus) Foslie, Laminaria ochroleuca Bachelot de la Pylaie and, sporadically, Saccharina latissima (Linnaeus) C.E. Lane, C. Mayes, Druehl & G.W. Saunders are the most important kelps.
The north coast of Spain is an interesting area where the distribution limits of cold temperate species, especially brown seaweeds, have moved eastwards or westwards by hundreds of kilometres during the last century (Sauvageau, Citation1897; Fischer-Piette, Citation1957, Citation1963; Anadón & Niell, Citation1980, Fernández & Niell, Citation1982; Fernández & Anadón, Citation2008). Upwelling events on this coast are frequent in summer (Fraga et al., Citation1982; Botas et al., Citation1990), lowering surface temperatures and increasing nutrient availability. From 1970 onwards the superficial seawater temperature (SST) in the coastal waters of northern Spain has increased (Planque et al., Citation2003) especially the maximum temperature (Anadón et al., 2009). There has also been a reduction in the seasonality and intensity of summer upwelling (Llope et al., Citation2006) and a decreasing trend in nutrient content (Llope et al., Citation2007). The above-mentioned changes are more intense west to Peñas Cape (43° 39.4′ N; 5° 50.8′ W) and could be responsible for the shifts in the distributional limits of the species along the coast.
Long-term studies of the abundance and distribution of kelps allow us to identify spatial and temporal changes as well as to predict the future of these communities (Steneck et al., Citation2002). Many studies have been carried out in North America and some kelp forests have been regularly monitored over the last century (Tegner et al., Citation1996). Unfortunately, this is not the case for European kelps: there are very good synopses of biological data published in the 1970s by the FAO (Norton, Citation1970; Kain, Citation1971; Gayral & Cosson, Citation1973) and more recently a review by Bartsch et al. (Citation2008), but there are no long-term data that can be used for a large-scale time-series analysis.
The main focus of this paper is Saccorhiza polyschides, the most abundant kelp in southern Europe (Raffaelli & Hawkins, Citation1996). Sacchoriza polyschides is an annual alga whose sporophytes grow very quickly during spring and summer and decay in autumn, before being detached by winter storms (Norton, Citation1970). Young sporophytes arising at the end of summer usually overwinter, but towards the south of S. polyschides’ geographical distribution sporophytes are completely removed in winter and no overwintering individuals remain the next year (‘meridional populations’: Ardré, Citation1971). This type of population is found in the north of Spain, where S. polyschides is one of the largest common brown seaweeds growing in the low intertidal and on subtidal rocky reefs (Fernández & Niell, Citation1982). At the end of the 1970s the general aspects of its biological cycle were studied by Fernández (Citation1980).
The aim of this work was to use a long-term monitoring study to determine which population processes are more sensitive to an increase in seawater temperature and a reduction in the upwelling intensity. Data for recruitment, growth and survival, as well as density and supported biomass were analysed for populations collected since the 1990s, using data from the late 1970s for comparison. As a complementary study, some recent changes in the distribution of kelps along the north coast of Spain are documented, using recent data from several surveys as well as personal observations.
Materials and methods
The study of kelp distributions was conducted on the mid northern coast of Spain (in Asturias, ), where there is a transition between cold and warm-temperate floras (Anadón & Niell, Citation1980; Lüning, Citation1990). Twenty localities, the same as those surveyed in 1977–78 by Anadón & Niell (Citation1980), covering 200 km of coastline () from Arnao (43° 33′ 28″ N; 07° 01′ 20″ W) to Buelna (43° 23′ 48″ N; 4° 36′ 51″ W) were re-surveyed in 2007–10. Abundance of kelps was estimated by collecting samples of 50 × 50 cm2 from the low intertidal. Sampling consisted of complete removal of kelps present in two randomly selected plots of 50 × 50 cm2, according the methodology used by Anadón and Niell (Citation1980), and five randomly selected plots of 50 × 50 cm2 in which percentage cover was estimated.
Fig. 1. Map of the northern Iberian Peninsula showing the 20 intertidal sites sampled between Buelna (1) and Arnao (20). Site 11 is Aramar beach.
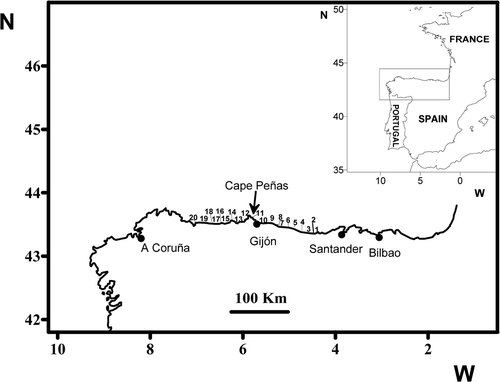
The study of Saccorhiza polyschides was carried out at Aramar (43° 36′ N, 5° 46′ W), a semi-exposed rocky shore in the Peñas Cape region () The substratum consisted of a gently-sloping rocky platform, the upper intertidal being dominated by invertebrates (barnacles and limpets), the lower intertidal by macroalgae. Saccorhiza polyschides was co-dominant with Cystoseira baccata in the lowest intertidal (Fernández & Niell, Citation1982).
Biological data
Kelp distributions. Two sets of data were considered: (1) data from recent literature reports (Alcock, Citation2003; J. Juanes, personal communication) and (2) data collected by sampling along the Asturias coast between 2007 and 2010. Data from 1977–78, as well as other historical data, were used as a benchmark.
Saccorhiza long-term study. Three sets of data were collected between 1977 and 2010 were used to study the population changes of Saccorhiza polyschides. First, three annual cycles (1977–78, 1978–79 and 1979–80) of S polyschides biomass measurements were made from monthly collections of 2–5 samples from 50 × 50 cm quadrats. In each sample all individuals were removed, bagged and carried to the laboratory, where the algae were carefully examined for the presence of sori, measured for length, and assigned to a class according to the degree of development of the attachment structures. At first the holdfast is simple, with a single ‘ridge’ above it; later on the ‘ridge’ expands, producing a ‘bell’ structure obscuring the holdfast and, finally a large hollow ‘bulb’ is formed. To facilitate the attachment to the substrate, rows of haptera are produced around the ‘bulb’ as it continues expanding and it is easy to distinguish the separately rows until 5–7 are produced (Sauvageau, Citation1918; Norton & Burrows, Citation1969; Fernández & Niell, Citation1981). After morphological examination, the plants were weighed after drying for 48 h at 60°C.
The second set of data was collected from 1990 onwards and involved removal if three to five samples from 50 × 50 cm quadrats in October each year, which is the month when S. polyschides reached its maximum biomass (Fernández, Citation1980). The processing and analysis of samples was as described above.
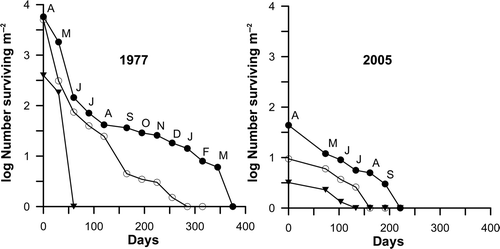
Third, one permanent quadrat of 1 m2 was established for demographical purposes at three different low intertidal levels, namely in the Bifurcaria bifurcata community (0.70 m above the Lowest Astronomical Tide [LAT]), in the Gelidium spinosum community (0.40 m above LAT) and in the Saccorhiza–Cystoseira community (0.20 m above LAT). All young sporophytes were counted monthly in each quadrat with the exception of the first two months, in which 10 subsamples of 15 × 15 cm were used due to the high density of small individuals. The sampling was repeated in 1977, 1978 and 2005. Counts were used to construct survivorship curves.
Oceanographic data
Superficial Seawater Temperature (SST) data were obtained from Advanced Very High Resolution Radiometer sensors from the NOAA-12 to NOAA-18 satellites. SST time series from the NOAA Optimum interpolation 0.25° daily sea-surface temperature analysis (OISST version 2) were used. The series is based on the methods described in Reynolds et al. (Citation2007), and it is produced and maintained by C. Liu and R.W. Reynolds at NCDC (www.ncdc.noaa.gov/oa/climate/research/sst/oi-daily.php).
‘In situ’ SSTs were obtained using a Hobo Temperature Logger (Onset Computer Corporation, Pocasset, MA, USA) permanently fixed to the substratum in the study area from 1996 to 2004. These data were used to calibrate the satellite SST data, which can be inaccurate in areas very close to land (Smale & Wernberg, Citation2009).
Results
Kelp distributions
Saccorhiza polyschides has experienced two periods of retreat from 1895 onwards. At that time the species was very common on the Basque coast and all along the north coast of Spain (Sauvageau, Citation1897) but by the 1950s it had moved westwards to Santander (Fischer-Piette, Citation1963; Crisp & Fischer-Piette, Citation1959). In the 1970s it was one of the most important species characterizing the low intertidal and shallow subtidal of the north Spanish coast (Anadón & Niell, Citation1980; Fernández, Citation1980) and at the beginning of the 1980s it re-colonized the Spanish Basque coast (Borja & Gorostiaga, Citation1990) (). From this decade onwards, however, populations suffered a sharp demise and the survey by Alcock (Citation2003) in 2000–2001 showed the species to be rare east of Peñas Cape, where it was found only in damp and shady sites, e.g. at Mundaka (43° 24′ 43″ N, 2° 41′ 59″ W), the easternmost locality at which the species was present. Data collected in 2007–2008 along the coast of Asturias and personal observations from 2010 show the same tendency, with dense populations only west of Peñas Cape ().
Fig. 2. North coast of Spain: kelp distribution maps in the 1980s and 2000s. Dense populations (dark) and small patches or isolated individuals (grey) are indicated.
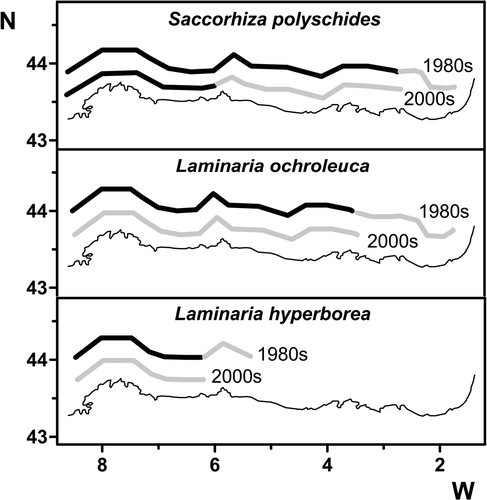
The situation is similar for other kelps. Laminaria ochroleuca was very common west of Peñas Cape in the 1970s and 1980s, forming dense stands (40–50 plants m−2, my observations) in the low intertidal or the shallow subtidal (McNeill, Citation1992). East of Peñas Cape intertidal populations of L. ochroleuca disappeared but subtidal populations – replacing S. polyschides at depth (up to 15 m.) – were frequent in several places on the Asturias and Cantabrian coasts (McNeill, Citation1992; J. Juanes, personal communication). Some plants were even observed on the inner part of the Basque coast (Casares, Citation1987; Gorostiaga et al., Citation1981) (). Nowadays, 90% of these kelp forests have disappeared and only small isolated individuals are found west of Peñas Cape (Rico et al., Citation2009; J. Juanes, personal communication). The same trend has occurred in the two remaining kelp species, Laminaria hyperborea (), which is a subtidal species that was very common in the west part of the north coast of Spain in the 1970s and 1980s (McNeill, Citation1992) but also present east of Peñas Cape in the 1990s (my observations), and finally Saccharina latissima, which is a rare kelp on the north coast of Spain (Miranda, Citation1931; Anadón & Niell, Citation1980; Weber-Peukert & Schnetter, Citation1982; Bárbara et al., Citation2005).
Saccorhiza long-term study
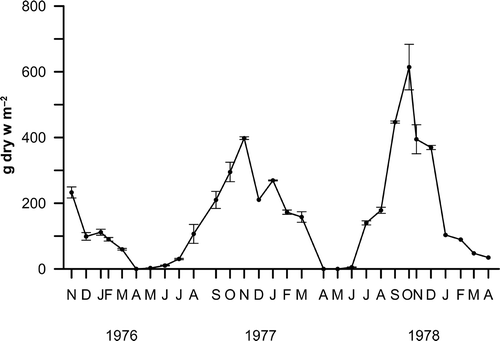
Seasonal cycle, standing-crop and density. Each new generation of sporophytes arises between March and April. From May onwards, the young sporophytes grow very fast, reaching a maximum of biomass (around 500 g dry weight m−2) in the autumn (). Then they begin senescence, followed by the gradual decay and shedding of the lamina, the stipe, and finally the bulb, which is the last structure to remain, persisting until the end of winter.
Fig. 3. Annual pattern of biomass of Saccorhiza polyschides at the end of the 1970s (mean values ± S.D.).
The autumn standing crop, which can be used as a proxy for annual net production, showed inter-annual differences as expected in an annual alga but there has been a sharp decrease since c. 1998 (). There was a significant linear negative relationship for the period 1992–2010 (r 2 = 0.59, P < 0.01, n = 17). This trend agrees with data on algal density (, r 2 = 0.65, P < 0.01, n = 16). In the last 10 years, algae have been rare and have seemed to stop growth before completing their development (plants with more than three rows of haptera have even rarer). There was initially no clear trend in the lengths of plants, but in the last five years there seems to have been a decline (, though no statistical significance can be attached to this: r 2 = 0.12, n.s., n = 16).
Fig. 4. Long-term changes in the standing crop (a), density (b), thallus length (c) and density of fertile specimens (d) of Saccorhiza polyschides (mean values ± S.D. in each case).
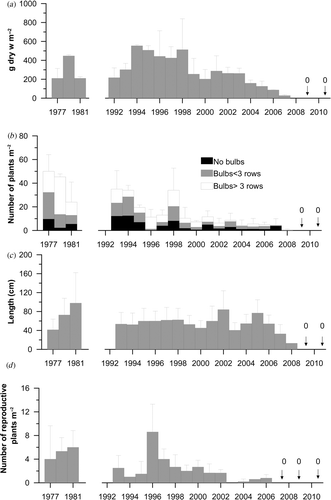
Fertility. The first reproductive plants appeared in September but the onset of fertility is not simultaneous for all the plants. They have to reach a size of development that could be estimated from the number of rows of haptera of the bulb: five (Norton & Burrows, Citation1969) or at least more than three (Fernández, Citation1980). The density of fertile plants has varied from year to year but there was a sharp decrease from a peak in 1996. From 2002 onwards, fertile plants have been extremely rare (, linear negative relationship, r 2 = 0.32, P < 0.05, n = 16) and no reproductive plants have been found since 2006.
Demography of the sporophytes. Young sporophytes became conspicuous in spring. The first individuals appeared in March and April but they were rare and the highest density of small plants (less than 5 cm long) occurred in May. Five cohorts were identified in the 1970s (Fernández & Niell, Citation1981) but the most important in terms of biomass (92% of the overall biomass) were those appearing in May and June.
Despite the fact that small sporophytes were distributed throughout the low intertidal, only those growing at the lowest level became adults. shows the survivorship of sporophytes growing at three low intertidal levels in 1977 and 2005. Between these years there are strong differences, including a reduction in the number of recruits (by two orders of magnitude: 5700 individuals m−2 in 1977 vs. 44 individuals m−2 in 2005) and in the number of cohorts (5 vs. 1) and also a shortening of the sporophyte's life-span (12 vs. 6 months).
Discussion
Kelp distributions: the case of the north coast of Spain
The regression of kelps along the north coast of Spain corresponds to a general trend seen elsewhere, involving the retreat of temperate species northwards (Parmesan and Yohe, Citation2003), but it is unique because the retreat is taking place on a long E–W coastline. Similar retreats of kelps, but along ± N–S coastlines, have been observed elsewhere, e.g. regression of Laminaria digitata on the Atlantic coast of France (Cosson, Citation1999) and of Saccharina latissima in southern Norway (Husa, Citation2007). An increase in seawater temperature is the common causal factor that predictive models use to explain the northward retreat of European kelps (Breeman Citation1990; Hiscock et al., Citation2004; Müller et al., Citation2009).
The possibility of recovery is difficult to predict because climate-driven thermal effects impacting kelp forests operate at different scales in space and time (Steneck et al., Citation2002). But if we consider that, from Brittany southwards, the European kelps are already only present in isolated patches associated with upwelling (Lüning, Citation1990), then it seems likely that the kelps of the north coast of Spain, including Saccorhiza polyschides, are at risk of disappearance.
The case of Saccorhiza polyschides
The evidence provided by this paper suggests that the recent decline of Saccorhiza polyschides on the north coast of Spain is likely to have been caused by (1) a failure in the growth and development of the sporophyte, preventing most of the plants from becaming reproductive; and/or (2) a failure in the microscopic phase (from the settlement of the spores to the embryonic sporophytes). In either case, the result is poor recruitment, possibly becoming too low to maintain a viable population. Oceanographic anomalies in temperature, salinity or nutrients might cause physiological stress, affecting processes like recruitment and growth and finally causing kelp deforestation at latitudes of c. 40° or less (Steneck et al., Citation2002).
Looking at the macroscopic phase, what could cause young sporophytes that became visible in May not to complete their development? Before 2000, young sporophytes on the north coast of Spain grew very fast in summer (1–2 cm day−1), became reproductive in 4–5 months – like those in the British Isles (Norton & Burrows, Citation1969) – and survived to 10–12 months (Fernández, Citation1980). Nowadays sporophytes do not survive for more than 5–6 months and most do not reach maturity.
Traditionally, temperature has been invoked as a main factor affecting several biological processes during the life cycle of kelps (see Bartsch et al., Citation2008 for a summary). In the case of S. polyschides, temperature has been considered a critical factor affecting the viability of spores, the fertility of the gametophytes and the survival of the young sporophytes (Norton, Citation1977), and has been suggested to be the factor limiting the geographical distribution of the species (van den Hoeck, 1982) ().
Table 1. Critical temperatures for different phases and processes of the life history of Saccorhiza polyschides, based on data of Kain (Citation1969) and Norton (Citation1977). The months when the process takes place, according to Fernández (Citation1980), are given in parentheses.
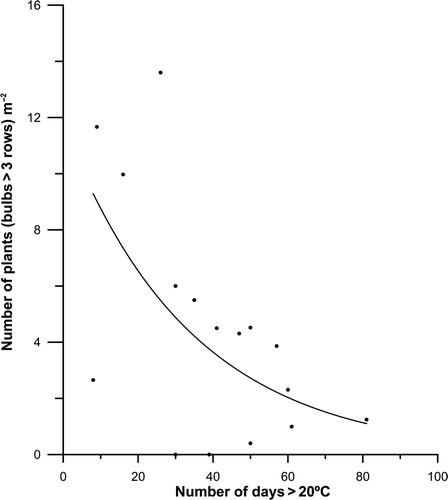
Summer superficial waters on the north coast of Spain have shown significant warming in recent years (Llope et al., 2006; Anadón et al., Citation2009). This trend is supported by the satellite SST data as well as by ‘in situ’ logger data (). Although Saccorhiza polyschides has been considered a warm-temperate kelp with a survival interval between 5 and 23°C (van den Hoek, Citation1982), summer periods longer than 30 consecutive days of SST > 20°C (see ) may be considered as a stressor causing a decrease in organism performance (Pörtner & Farrel, Citation2008; Wernberg et al. Citation2010) and hence a collapse in growth, judging by the data in . Unfortunately, there are no available data about the effects of temperature on the rate of growth of the macroscopic sporophytes. http://www.ukmarinesac.org.uk/infralittorial-reefs.htm.
Fig. 6. (a) Mean annual sea surface temperature (SST) and (b) number of consecutive days with SST > 20°C recorded by in situ loggers (open circles) and satellite (dark circles) on the north coast of Spain at Aramar beach (43° 36′ N, 5° 46′ W9) and 43° 37′N, 5° 38′W, respectively.
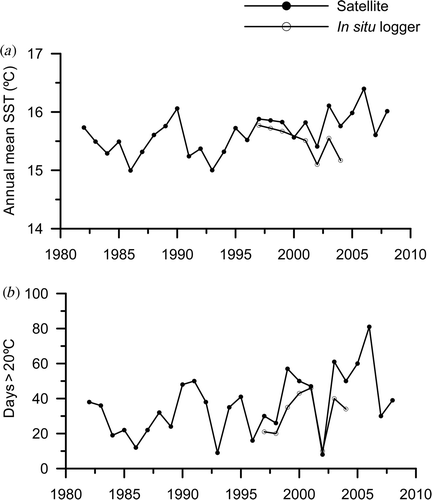
Fig. 7. Saccorhiza polyschides: relationship between the density of algae with developed bulbs (>3 rows of haptera) and the number of consecutive days with SST > 20°C.
It is also important to consider other factors, such as the shifts in the timing and strength of the summer upwelling, which have contributed to an increase in the period of summer stratification and a decreasing tendency for nitrate availability (Llope et al., Citation2007), which is a critical factor affecting the growth of kelps (Chapman & Craigie, Citation1977). It is difficult to determine how abiotic factors interact and contribute to the growth performance of a seaweed, since most studies focus on the effects of a single factor and the interactive effects of several factors are less well known. However, there is some evidence that an interaction between high temperatures and low availability of nitrate is responsible for a decline in the photosynthetic performance of Saccharina latissima (Gerard, Citation1997) and Ulva lactuca (Rivers & Peckol, Citation1995), as well as in the reproductive allocation and spore standing-stock in Macrocystis pyrifera (Reed et al., Citation1996) and in the distribution, abundance and size of Californian kelps (Dayton et al., Citation1999). Something similar could be affecting the growth of Saccorhiza polyschides but it is difficult to find a straightforward relationship between ecological responses and changes in the abiotic environment. The pattern of change in the distribution and abundance of a species can be gradual or punctuated, depending on the way the abiotic factors change and whether there is a critical threshold or ‘tipping point’ for species performance (Harley & Paine, Citation2009).
As the period of temperature stress happens in summer, the microscopic stages (from the spore release to the growth of sporophytes to a macroscopic size) that are present from autumn to spring should not be affected (see ), but many other factors influencing the supply and the establishment of propagules need to be investigated (Norton, Citation1978; Reed et al., Citation2004; Graham et al., Citation2007), despite the difficulty of making in situ studies of these microscopic stages (Dayton, Citation1985).
No data about grazing pressure from sea urchins or alternative predators (crabs and fishes) are available. However, herbivory by urchins, which is the main biological factor responsible for the decline of kelp populations at latitudes of 40–60° (Steneck et al., Citation2002), is unlikely to be important along the north coast of Spain because urchin species (mainly Paracentrotus lividus) are a fishery resource, regularly and intensively harvested (Catoira, Citation2004). Nowadays the population of sea urchins in this area has been reduced to small patches and harvesting is regulated (http://tematico.asturias.es/dgpesca/index.php).
Finally, the consequences of this kelp demise should be considered. A single plant of S. polyschides forms a particularly complex habitat, in which up to 50 species of algae and 100 species of animals can be found (Norton & Burrows, Citation1969), most of them especially associated with the hollow bulbous holdfast, which can reach up to 30 cm wide (http://www.ukmarinesac.org.uk/infralittorial-reefs.htm). The disappearance of this kelp could have dramatic effects in the subtidal, where it is the dominant species, growing up to 3–4 m long. The vertical component of the three-dimensional structure will be severely reduced as well as resources in terms of food and shelter, resulting in a cascading effect on the community (Dayton et al., Citation1999) and a considerable loss of biodiversity. In the low intertidal, where Saccorhiza polyschides co-dominates with several species of Cystoseira, the effects might be smaller because some of the canopy and vertical structure will be maintained.
Acknowledgements
This work was partially supported by the project “Caracterización y modelización de los patrones de variación espacial y temporal de las comunidades costeras en Asturias” (CTM 2006-05588). I thank the NOAA NESDIS and NCDC, and especially C. Liu and R.W. Reynolds, for the production, availability and maintenance of NOAA Optimum interpolation 0.25° daily sea surface temperature analysis. I also thank my colleagues, R. Anadón, J.L. Acuña, F. González-Taboada, J.M. Rico and J.Arrontes for their valuable suggestions and comments. J. Juanes provided personal information about current kelp distributions on the coast of Cantabria. The manuscript profited from the helpful comments of Dr G. Pearson and two anonymous reviewers.
References
- ALCOCK, R. (2003). The effects of climate change on rocky shore communities in the Bay of Biscay, 1895–2050. PhD Thesis, University of Southampton
- ANADÓN, R., FERNÁNDEZ, C., GARCÍA FLÓREZ, L., LOSADA, I. & VALDÉS, L. 2009. Evidencias y efectos potenciales del cambio climático en Asturias. 5. Costas y Océanos. In Evidencias y efectos potenciales del cambio climático en Asturias, 126–170. Consejería de Medio Ambiente, Ordenación del Territorio e Infraestructuras, Gobierno del Principado de Asturias [http://ria.asturias.es/RIA/bitstream/123456789/365/1/LIBRO%20COMPLETO_baja.pdf]
- Anadón , R and Niell , FX . 1980 . Distribución longitudinal de macrófitos en la costa asturiana (N. de España) . Invest. Pesq. (Spain) , 45 : 143 – 156 .
- Ardré , F . 1971 . Contribution à l’étude des algues marines du Portugal.II. Ecologie et chorologie . Bull. Cent. Étud. Rech. Sci., Biarritz , 8 : 359 – 374 .
- Bárbara , I , Cremades , J , Calvo , S , López-Rodriguez , MC and Dosil , J . 2005 . Checklist of the benthic marine and brackish Galician algae (NW Spain) . An. Jard. Bot., Madrid , 62 : 69 – 100 .
- Bartsch , I , Wiencke , C , Bischof , K , Buchholz , CM , Buck , BH , Eggert , A , Feuerpfeil , P , Hanelt , D , Jacobsen , S , Karez , R , Karsten , U , Molis , M , Roleda , MY , Schumann , R , Schubert , H , Valentin , K , Weinberger , F and Wiese , J . 2008 . The genus Laminaria sensu lato: recent insights and developments . Eur. J. Phycol. , 43 : 1 – 86 .
- Borja , A and Gorostiaga , JM . 1990 . Distribución geográfica de Saccorhiza polyschides (Ligthf.) Batt. en la costa vasca. Su posible relación con la temperatura . Bentos , 6 : 1 – 8 .
- Botas , JA , Fernández , E , Bode , A and Anadón , R . 1990 . A persistent upwelling off the central Cantabrian Coast (Bay of Biscay) . Est. Coast. Shelf Sci. , 30 : 185 – 199 .
- Breeman , AM . 1990 . “ Expected effects of changing seawater temperatures on the geographic distribution of seaweed species ” . In Expected Effect of Climate Change on Marine Coastal Ecosystems , Edited by: Beukema , J.J. , Wolff , W.J. and Brouns , J.J.W.M. 69 – 76 . Dordrecht : Kluwer Academic Press .
- CASARES, C. (1987). Estudio de la flora bentónica marina de la costa de Guipúzcoa. PhD Thesis, Universidad de Barcelona
- CATOIRA, J.L. (2004). History and current state of sea urchin Paracentrotus lividus Lamarck, 1816, fisheries in Galicia, NW Spain. In Sea Urchins: Fisheries and Ecology (Lawrence, J.D., editor), Proc. Int. Conf. on Sea-Urchins Fisheries and Aquaculture, Puerto Varas, Chile, 64–73. Lancaster, USA
- Chapman , ARO and Craigie , JS . 1977 . Seasonal growth in Laminaria longicruris: Relation with dissolved inorganic nutrients and internal reserves of nitrogen . Mar. Biol. , 40 : 197 – 205 .
- Cosson , J . 1999 . Sur la disparition progressive de Laminaria digitata sur les côtes du Calvados (France) . Cryptog. Algol. , 20 : 35 – 42 .
- Crisp , D and Fischer-Piette , E . 1959 . Répartition dès principales espèces intercotidales de la côte atlantique française en 1954–55 . Ann. Inst. Oceanogr. , 36 : 276 – 381 .
- DAYTON, P.K. (1972). Toward an understanding of community resilience and the potential effects of enrichment to the benthos at McMurdo Sound, Antarctica. In Proceedings of the Colloquium on Conservation Problems in Antarctica (Parker, B.C., editor), 81–95. Allen Press, Lawrence, Kansas
- Dayton , PK . 1985 . The ecology of kelp communities . Ann. Rev. Ecol. Syst. , 16 : 215 – 245 .
- Dayton , PK , Tegner , MJ , Edwards , PB and Riser , KL . 1999 . Temporal and spatial scales of kelp demography: The role of oceanographic climate . Ecol. Monogr. , 69 : 219 – 250 .
- FERNÁNDEZ, C. (1980). Estudios estructurales y dinámica del fitobentos intermareal (facies rocosa) de la región de Cabo Peñas con especial atención a la biología de Saccorhiza polyschides (Ligthf.) Batt. PhD Thesis, Universidad de Oviedo, Oviedo
- Fernández , C and Anadón , R . 2008 . La cornisa cantábrica: un escenario de cambios de distribución de comunidades intermareales . Algas, , 39 : 30 – 31 .
- Fernández , C and Niell , FX . 1981 . Discusión sobre los métodos usados en la estimación de la producción en macrófitos intermareales . Oecol. Aquat. , 5 : 43 – 52 .
- Fernández , C and Niell , FX . 1982 . Zonación del fitobentos intermareal de la región de Cabo Peñas (Asturias) . Invest. Pesq. (Spain) , 46 : 121 – 141 .
- Fischer-Piette , E . 1957 . Sur le déplacement des frontiers biogéographiques observées au large des côtes iberiques dans le domaine intercotidale . Publ. Inst. Biol. Apl., XXVI Simposio de Biogeografía Ibérica , 26 : 35 – 40 .
- Fischer-Piette , E . 1963 . La distribution des principaux organismes intercotidaux Nord-Iberiques . Ann. Inst. Oceanogr. , 40 : 165 – 311 .
- Fraga , F , Mouriño , C and Manríquez , M . 1982 . Las masas de agua en la costa de Galicia: Junio-Octubre . Result. Exped. Cient. , 10 : 51 – 77 .
- Gayral , P and Cosson , J . 1973 . Exposé synoptique des données biologiques sur la laminaire digitée Laminaria digitata . FAO Fish. Synops. , 89
- Gerard , VA . 1997 . The role of nitrogen nutrition in high-temperature tolerance of the kelp Laminaria saccharina (Chromophyta) . J. Phycol. , 33 : 800 – 810 .
- Graham , MH , Vásquez , JA and Buschmann , AH . 2007 . Global ecology of the giant Kelp Macrocystis: From ecotypes to ecosystems . Oceanogr. Mar. Biol., Annu. Rev. , 45 : 39 – 88 .
- Gorostiaga , JM , Angulo , R and Ibañez , M . 1981 . Nueva cita de Saccorhiza polyschides y Laminaria ochroleuca en la costa vasca . Lurralde , 4 : 265 – 270 .
- Harley , CDG and Paine , RT . 2009 . Contingencies and compounded rare perturbations dictate sudden distributional shifts during periods of gradual climate change . Proc. Nat. Acad. Sci. USA , 106 : 11172 – 11176 .
- Hiscock , K , Southward , A , Tittley , I and Hawkins , S . 2004 . Effect of changing temperature on benthic marine life in Britain and Ireland . Aquat. Conserv. , 14 : 333 – 362 .
- Hoek , C. van den . 1982 . The distribution of benthic marine algae in relation to the temperature regulation of their life histories . Biol. J. Linn. Soc. , 18 : 81 – 144 .
- Husa , V . 2007 . Effects of increased sea temperatures on macro algae . Marine Research News , 14 : 1 – 2 .
- Kain , JM . 1969 . The biology of Laminaria hyperborea. Comparison with early stages of competitors . J. Mar. Biol. Ass. U.K. , 49 : 455 – 473 .
- Kain , JM . 1971 . Synopsis of biological data on Laminaria hyperborea . FAO Fish. Synops. , 87
- Llope , M , Anadón , R , Sostres , JA and Viesca , L . 2007 . Nutrients dynamics in the southern Bay of Biscay (1993–2003): Winter supply, stochiometry, long-term trends, and their effects on the phytoplankton community . J. Geophys. Res. , 112 C07029, doi: 10.1029/2006JC003573
- Llope. , M , Anadón , R , Viesca , L , Quevedo , M , González-Quirós , R and Stenseth , NC . 2006 . Hydrographic dynamics in the Southern Bay of Biscay: Integrating multi-scale physical variability over the last decade (1993–2003) . J. Geophys. Res. , 111 C09021, doi: 10.1029/2005JC002963
- Lüning , K . 1990 . Seaweeds: Their Environment, Biogeography and Ecophysiology Wiley & Sons, New York
- McNeill , SE . 1992 . Criterios para el establecimiento de zonas protegidas en la costa asturiana , Spain : Universidad de Oviedo .
- Miranda , F . 1931 . Sobre las algas y cianofíceas del Cantábrico, especialmente de Gijón . Trab. Mus. Nac. Cienc. Nat., Madrid , 25 : 4 – 106 .
- Müller , R , Laepple , T , Bartsch , I and Wiencke , C . 2009 . Impact of oceanic warming on the distribution of seaweeds in polar and cold-temperate waters . Bot. Mar. , 52 : 617 – 638 .
- Norton , TA . 1970 . Synopsis of the biological data on Saccorhiza polyschides (Lightf.) Batt . FAO Fish. Synops. , 83
- Norton , TA . 1977 . Experiments on the factors influencing the geographical distributions of Saccorhiza polyschides and Saccorhiza dermatodea . New Phytol. , 78 : 625 – 635 .
- Norton , TA . 1978 . The factors influencing the distribution of Saccorhiza polyschidesin the region of Lough Ine . J. Mar. Biol. Ass. U.K. , 58 : 527 – 536 .
- Norton , TA and Burrows , EM . 1969 . Studies on marine algae of the British Isles. 7. Saccorhiza polyschides (Lightf.) Batt . Br. Phycol. J. , 4 : 19 – 53 .
- Parmesan , C and Yohe , G . 2003 . A globally coherent fingerprint of climate change impacts across natural systems . Nature , 421 : 37 – 42 .
- Planque , B , Beillois , P , Jégou , P , Lazure , PM , Petitgas , P and Puillat , I . 2003 . Large scale hydroclimatic variability in the Bay of Biscay. The 1990s in the context of interdecadal changes . ICES Mar. Sci. Symp. , 219 : 61 – 70 .
- Pörtner , HO and Farrel , AP . 2008 . Physiology and climate change . Science , 322 : 690 – 692 .
- Raffaelli , D and Hawkins , SJ . 1996 . Intertidal Ecology , London : Chapman & Hall .
- Reed , DC , Ebeling , AW , Anderson , TW and Anghera , M . 1996 . Differential reproductive responses to fluctuating resources in two seaweeds with different reproductive strategies . Ecology , 77 : 300 – 316 .
- Reed , DC , Schroeter , SC and Raimondi , PT . 2004 . Spore supply and habitat availability as sources of recruitment limitation in the giant kelp Macrocystis pyrifera (Phaeophyceae) . J. Phycol. , 40 : 275 – 284 .
- Reynolds , RW , Smith , TM , Liu , C , Chelton , DB , Casey , KS and Schlax , MG . 2007 . Daily high-resolution-blended analyses for sea surface temperature . J. Climate , 20 : 5473 – 5496 . doi: doi: 10.1175/2007JCLI1824.1
- Rico , JM , Álvarez Raboso , J and Llera , EM . 2009 . Desaparición de los bosques de Laminarias en la costa de Asturias . Asturnatura , 26 : 25 – 27 .
- Rivers , JS and Peckol , P . 1995 . Summer decline of Ulva lactuca (Chlorophyta) in a eutrophic embayment: Interactive effcets of temperature and nitrogen availability? . J. Phycol. , 31 : 223 – 228 .
- Sauvageau , C . 1918 . Réchérches sur les Laminaires des côtes de France . Mém. Acad. Sci., Paris , 56 : 1 – 240 .
- Sauvageau , C . 1897 . Note préliminaire sur les algues marines du golfe de Gascogne . J. Botanique , 11 : 166 – 230 .
- Smale , DA and Wernberg , T . 2009 . Satellite-derived SST data as a proxy for water temperature in nearshore benthic ecology . Mar. Ecol. Prog. Ser. , 387 : 27 – 37 .
- Steneck , RS , Graham , MH , Bourque , BJ , Corbett , D , Erlandson , JM , Estes , JA and Tegner , MJ . 2002 . Kelp forest ecosystems: Biodiversity, stability, resilience and future . Environ. Conserv. , 29 : 436 – 459 .
- Tegner , MJ , Dayton , PK , Edwards , PB and Riser , KL . 1996 . Is there evidence for long-term climatic change in southern California kelp forests? . Calif. Coop. Ocean. Fish. Invest. Rep. , 37 : 111 – 126 .
- Weber-Peukert , G and Schnetter , R . 1982 . Floristische und ökologische Untersuchungen über benthische Meeresalgengesellschaften der Küste Asturiens (Spanien) . Nova Hedwigia , 36 : 65 – 80 .
- Wernberg , T , Thomsen , MS , Tuya , F , Kendrick , GA , Staehr , PA and Toohey , BD . 2010 . Decreasing resilience of kelp beds along a latitudinal temperature gradient: Potential implications for a warmer future . Ecol. Lett. , 13 : 685 – 694 .