Abstract
Cylindrospermopsis raciborskii has recently attracted wide attention owing to its toxigenicity and its capacity for rapid invasion of new geographical regions. We studied the spatial distribution of C. raciborskii in western Poland and the environmental variables related to its occurrence near the border of its current temperate geographical range. We also identified the typical phytoplankton assemblages in which C. raciborskii occurs. Forty-six lakes were sampled once during the summers of 2008 and 2009. C. raciborskii was present in 43% of the samples and its biomass accounted for 0.1–13.9% of the total phytoplankton biomass found in the lakes. A General Linear Model (GLM) analysis and t-tests showed that the lakes in which C. raciborskii occurred were significantly shallower, more turbid and more eutrophic than the lakes from which it was absent. Redundancy Analysis (RDA) and GLM analyses showed that C. raciborskii biomass correlated positively with total nitrogen (TN), total phosphorus (TP) and conductivity. It correlated negatively with temperature, ammonium () and orthophosphate (
). According to Detrended Correspondence Analysis (DCA), C. raciborskii often co-occurred with Aphanizomenon ovalisporum, which is rarely observed in the studied region (common in subtropical regions, like C. raciborskii), rather than with the more common A. aphanizomenoides or the native A. gracile. Moreover, Planktothrix agardhii, the most common species in the studied region, was weakly associated with C. raciborskii. Overall, C. raciborskii was frequently present in the temperate lakes of western Poland, but it was most abundant in eutrophic lakes with high conductivity and high concentrations of TN and TP but low concentrations of
and
.
Introduction
Cylindrospermopsis raciborskii (Order Nostocales) is a filamentous, planktonic, freshwater diazo trophic cyanobacterium common in tropical and subtropical regions (Hooker et al., Citation1991; Fabbro & Duivenvoorden, Citation1996; Bouvy et al., Citation1999; Vidal & Kruk, Citation2008; Burford & Davis, Citation2011). However, it is also known to have invaded temperate lakes since the late 1960s (Padisák, Citation1997). It is of increasing concern owing to its ability to produce toxic alkaloids, including neurotoxic saxitoxins (Lagos et al., Citation1999; Molica et al., Citation2002, Citation2005) and hepatotoxic cylindrospermopsins (Hawkins et al., Citation1985; Ohtani et al., Citation1992; Li et al., Citation2001; Wood & Stirling, Citation2003). Cylindrospermopsin and its analog deoxy-cylindrospermopsin show severe hepatotoxicity, which may affect the liver, kidneys, spleen, heart, thymus, or intestine of humans and animals (Falconer et al., Citation1998; Norris et al., Citation1999; Chorus & Bartram, Citation1999; Carmichael et al., Citation2001). This hepatotoxin is also suspected to be carcinogenic (Falconer & Humpage, Citation2006).
In addition to C. raciborskii`s potential toxigenicity, another concern is its adaptability to different environmental conditions. It has become widely established in freshwater ecosystems (Varkonyi et al., Citation2000; Briand et al., Citation2002, Citation2004). Currently, C. raciborskii is the most widespread species of its genus (Saker & Neilan, Citation2001) and has recently been recorded in temperate lakes and large rivers (Padisák, Citation1997; Fastner et al., Citation2003). Blooms of C. raciborskii have been reported primarily from North and South America, Australia, Asia and several countries of southern Europe (Dokulil & Mayer, Citation1996; Chapman & Schelske, Citation1997; Bouvy et al., Citation1999; McGregor & Fabbro, Citation2000; Chonudomkul et al., Citation2004; Figueredo et al., Citation2007; Figueredo & Giani, Citation2009). However, more recently C. raciborskii has also been reported at higher latitudes (Chapman & Schelske, Citation1997; Fastner et al., Citation2003; Nixdorf et al., Citation2003; Hamilton et al., Citation2005; Hong et al., Citation2006; Messineo et al., Citation2010), including northern Germany (Wiedner et al., Citation2002; Mischke, Citation2003). The first record of C. raciborskii in Poland was in 1973, from lakes that were artificially heated by an electrical power plant (Burchardt, Citation1977). Subsequently, C. raciborskii has been observed in several Polish lakes (Pełechata et al., 2006; Kokociński et al., 2010).
The successful expansion of C. raciborskii in northern European lakes has occurred simultaneously with increasing water temperatures related to climate warming and eutrophication (Kling, Citation2009). The species tolerates wide variation in temperature, with optima between 25°C and 35°C (Saker & Griffiths, Citation2000; Briand et al., Citation2004; Chonudomkul et al., Citation2004). However, several studies also indicate that it is able to grow at much lower temperatures of 15–18°C in temperate regions (Dokulil & Mayer, Citation1996). Studies of tropical and temperate C. raciborskii isolates have shown no differences in light requirements (Briand et al., Citation2004). Moreover, the lower amount of light at higher latitudes does not prevent C. raciborskii from occurring in temperate regions (Dokulil & Teubner, Citation2000; Mischke, Citation2003). C. raciborskii is known to have a high storage capacity for phosphorus (P) (Isvánovics et al., 2000; Shafik et al., 2000; Briand et al., Citation2004; Burford & O’Donohue, Citation2006) and it prefers as a nitrogen (N) source (Présing et al., Citation1996, Shafik et al., Citation2001).
Previous studies have focused on the ecology and toxicity of C. raciborskii in newly colonized regions. However, little is known about the phytoplankton communities within which it most frequently occurs, although it has been shown that C. raciborskii can coexist with various cyanobacteria from different taxonomic groups (Moustaka-Gouni et al., Citation2007; Soares et al., Citation2009; Xie et al., Citation2011). It belongs to the SN functional group recognized by Padisák & Reynolds (Citation1998) and Reynolds et al. (Citation2002). This group consists of species adapted to warm, turbid and well-mixed waters. A previous long-term study confirmed this finding and also indicated a negative correlation between C. raciborskii and P. agardhii, a dominant species in the shallow lakes in western Poland (Kokociński et al., 2010).
The objectives of this study were: (1) to examine the large-scale spatial distribution of C. raciborskii in Polish lakes with different mixing regimes and productivities; (2) to characterize the ecological niche of the species in relation to environmental variables; and (3) to examine the typical phytoplankton assemblages in which C. raciborskii occurs.
Materials and methods
Sampling sites
The study was conducted in 46 randomly selected lakes in three western districts of Poland (). The lakes varied in morphometry (e.g. maximum depth, volume, surface area), mixing regime (dimictic and polymictic) and trophic status, and their limnological characteristics and geographical positions are given in . Most of the lakes have high recreational usage. Based on their transparencies, nutrient levels and chlorophyll a concentrations, they range from meso- to highly eutrophic.
Fig. 1. Study area with the distribution of Cylindrospermopsis raciborskii (black circles indicate lakes with C. raciborskii present).
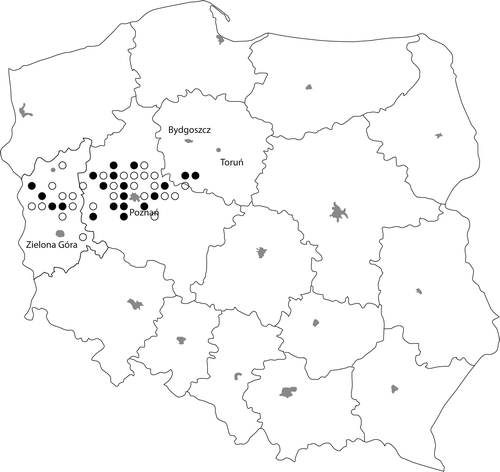
Table 1. Geographical position and morphometric parameters of the investigated lakes, divided into two groups (with and without Cylindrospermopsis raciborskii), selected physico-chemical data, total biomass, and the contribution of cyanobacterial and C. raciborskii biomass to total phytoplankton biomass (P = polymictic, S = stratified, Mean = arithmetic mean value).
Sampling
We sampled 30 lakes in 2008 and 16 lakes in 2009; each was sampled once between July and September. Although the samples were not collected during the same year, we assumed that the sampling of a large number of lakes would furnish a representative and a reliable data set by decreasing the influence of chance error. The sampling protocol used in our study is commonly employed in large-scale ecological studies concerning freshwater organisms (e.g. Stüken et al., Citation2006; Fastner et al., Citation2007, Vidal & Kruk, Citation2008; Soininen et al., Citation2009).
Integrated phytoplankton samples from the water column (in 28 polymictic lakes) or epilimnion (in 18 stratified lakes) were collected from one sampling station in the middle of each lake using a 5 -l ‘Limnos’ water sampler (Limnos. pl, Komorów, Poland). In both cases, subsamples were taken every 1 m of a vertical profile, combined in a plastic bucket, and then mixed. The number of subsamples therefore depended on the maximum depth of the shallow lakes (from 1 to 3 m) and on the depth of the epilimnion in the stratified lakes (from 1 to 4 m). Following the initial collection, pooling and mixing of the samples, a 1-litre subsample was collected and transported to the laboratory. The phytoplankton samples were preserved with acidified Lugol's solution with final concentrations of 1% (APHA, Citation1998; Hötzel & Croome, Citation1999; Hawkins et al., Citation2005) immediately after sampling and stored under cool, dark conditions until they were enumerated. Before counting, the samples were left undisturbed for at least 48 h in 1 -l calibrated glass cylinders (LMS, Germany). The upper layer of the water was then gently decanted and the lower layer (40 ml) containing the settled plankton species was used for the analysis.
Phytoplankton analysis
Phytoplankton were identified and counted using a Fuchs–Rosenthal chamber. At least 400 specimens were counted to reduce the error to <10% (P = 0.05: Javornický, 1958). A single cell, a coenobium, or a filament represented one specimen in the analyses. We used 100 µm as the standard length for filaments and 100 cells for large spherical colonies. Filaments and colonies between 100 µm and 200 µm were counted as two specimens. Filaments and colonies larger than 200 µm were counted as three specimens. This procedure was also factored into the biomass calculations, so that the method of counting specimens would not affect the determination of biomass. Before biomass calculations, we enumerated the number of cells per filaments or colonies, counting at least 30 specimens for each size category, following Laslett et al. (Citation1997) and Hötzel & Croome (Citation1999). The biovolume of each species was determined through volumetric analysis of cells using a geometric approximation and expressed as wet weight following Hindak (Citation1978) and Wetzel & Likens (Citation2000).
Chemical and physical analysis
Simultaneously with the collection of phytoplankton samples and following the same procedures, integrated water samples (one sample for each site) were collected for chemical analyses. The water samples were analysed for by a manual spectrometric method (PN-ISO 7150-1: Citation2002). Nitrate (
) and nitrite (
) were analysed by determining dissolved anions using liquid chromatography (ISO Citation10304-1: Citation2007). TN was calculated according to PN-73/C-04576/14.
and TP were determined by the ammonium molybdate spectrometric method (ISO 6878: Citation2004). For chlorophyll-a (chl-a) analysis, 200 ml of water was filtered through a GF/C Whatman filter. The concentration of chl-a was determined spectrophotometrically after extraction with 90% acetone and calculated using Lorenzen's formula (Wetzel & Likens, Citation2000). On each sampling occasion, the Secchi depth (SD), water temperature, pH and conductivity were determined using a multiparameter probe (Elmetron CPC-401, Elmetron, Zabrze, Poland).
Data analysis
First, t-tests were conducted to determine whether the lakes in which C. raciborskii was present differed physicochemically from the lakes from which it was absent. Prior to analysis, all environmental variables were log-transformed to better approximate normality of the residuals. We also verified that no strong inter-correlations occurred among the environmental variables. The inter-correlations between all pairs of environmental variables had values of r < 0.80. We thus initially included all variables in the ordination and regression analyses, owing to the lack of very strong inter-correlations.
Redundancy Analysis was then used to explore the relationships between the main patterns of phytoplankton community composition and the measured environmental variables. The RDA with forward selection of the environmental variables was run for the entire set of lakes (n = 46).
A General Linear Model was then applied to relate the abundances of C. raciborskii to measured environmental variables. Moreover, we used logistic regression to analyse the probability of occurrence of C. raciborskii in relation to environmental variables measured in the lakes. In both analyses, the most parsimonious models incorporating the environmental variables were identified using Akaike`s Information Criterion (AIC) (Burnham & Anderson, Citation1998). Finally, we used Detrended Correspondence Analysis to examine the main patterns in the phytoplankton assemblages in the lakes.
The statistical analyses were performed using the software package Statistica v. 8 (StatSoft Polska, Krakow, Poland) and the program R (available online at www.r-project.org).
Results
Cylindrospermopsis raciborskii was observed in 20 out of the 46 lakes, but its distribution did not seem to show any distinct spatial pattern for the lakes as a whole (). It accounted for 0.1–13.9% of the total phytoplankton biomass and 0.1–18.3% of the cyanobacterial biomass, but it was never the dominant species in the communities (). Although it occurred in a variety of lakes, the lakes in which it occurred differed significantly from those in which it did not occur. Lakes containing C. raciborskii were generally shallower (t-test, P = 0.01), had a lower Secchi depth (P = 0.001) and had higher (P = 0.01) and TN (P = 0.004) concentrations than the lakes from which it was absent. These lakes were also more eutrophic, based on higher chlorophyll-a concentrations (P = 0.005) and had a higher pH (P = 0.03).
The eigenvalues of the first two RDA axes were 0.20 and 0.13. Both eigenvalues were significant (P < 0.05). These two axes jointly explained 26.7% of the total variance in the phytoplankton communities among the lakes. TP, TN–TP ratio and pH were the most significant contributors to the first RDA axis (). RDA axis 2 was primarily correlated with temperature, TN and conductivity ().
Fig. 2. Plot of the Redundancy Analysis including samples from all investigated lakes. Large dots indicate the samples with C. raciborskii with biomass accounting for less than (white dots) or more than (black dots) 8% of the total phytoplankton biomass. Small dots indicate that C. raciborskii was not detected; max_depth = maximum depth; mean_depth = mean depth; lake_volume = lake volume, NO3 = nitrate; TN_TP = total nitrogen to total phosphorus ratio; cond = conductivity, TN = total nitrogen, TP = total phosphorus, NH4 = ammonium, Temp = temperature of the water, PO4 = phosphate concentration.
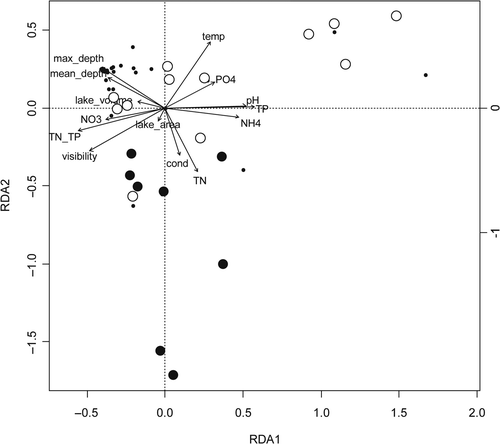
RDA analysis revealed that C. raciborskii occurred in lakes showing variable environmental conditions but was rare in deep reservoirs. The lakes with the highest C. raciborskii biomass showed high levels of TN and high conductivity and had large surface area. Secchi depths of these lakes ranged from 0.2 to 1.0 m ().
According to the GLM analysis using the AIC, the biomass of C. raciborskii was significantly positively related to TN and TP and significantly negatively related to temperature, and
(). Overall, these variables accounted for 52% of the variation in its abundance.
Table 2. Results of the General Linear Models for Cylindrospermopsis raciborskii biomass and probability of occurrence, and explanatory environmental factors (significant variables at P < 0.05 are marked in bold). The variables were selected using Akaike`s Information Criterion. Cond. = conductivity, Temp. = temperature, Chl-a =chlorophyll a, Max. Depth = maximum depth, TP = total phosphorus, TN = total nitrogen,  = ammonium,
= ammonium,  = orthophosphate,
= orthophosphate,  = nitrate, B = regression coefficient, S.E. = standard error, T = test statistic, P = significance.
= nitrate, B = regression coefficient, S.E. = standard error, T = test statistic, P = significance.
The probability of C. raciborskii occurrence increased significantly with water pH and was also weakly positively related to the amount of chlorophyll-a and negatively related to the maximum depth and TP ().
The DCA analysis indicated that C. raciborskii seemed to co-occur often with the following species located close to C. raciborskii in DCA space ():
| • | Cyanobacteria: Aphanocapsa holsatica, Aphanizomenon ovalisporum, Gomphosphaeria compacta | ||||
| • | Chlorophyta: Desmodesmus communis, Tetrastrum glabrum, Pediastrum duplex, Didymocystis planctonica, Micractinium extremum, Monoraphidium circinale, M. komarkovae | ||||
| • | Euglenophyta: Phacus longicauda | ||||
| • | Dinophyta: Peridinium cinctum. | ||||
Fig. 3. Plot of the Detrended Correspondence Analysis of the phytoplankton species. Encircled are phytoplankton species closely associated with Cylindrospermopsis raciborskii. 1 = Cylindrospermopsis raciborskii, 2 = Aphanocapsa holsatica, 3 = Desmodesmus communis, 4 = Aphanizomenon ovalisporum, 5 = Phacus longicauda, 6 = Pediastrum duplex, 7 = Tetrastrum glabrum, 8 = Gomphosphaeria compacta, 9 = Didymocystis planctonica, 10 = Peridinium cinctum, 11 = Monoraphidium komarkovae, 12 = Micractinium extremum, 13 = Monoraphidium circinale, 14 = Aphanizomenon gracile, 15 = Aphanizomenon aphanizomenoides, 16 = Planktothrix agardhii.
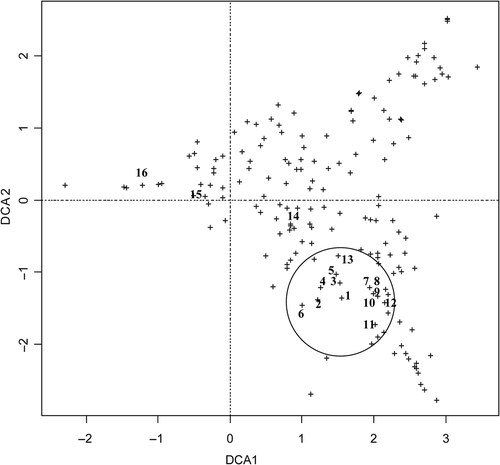
Another potential producer of cylindrospermopsin in these lakes, Aphanizomenon gracile, was more distant from C. raciborskii in DCA space (). Likewise, a further potential cylindrospermopsin producer, invasive A. aphanizomenoides, was also detected in the lakes studied but again not located close to C. raciborskii in DCA space, in contrast to A. ovalisporum. DCA also showed that P. agardhii, a common species of the shallow and turbid lakes in this region, was not located close to C. raciborskii in DCA space (). This result suggested that the two species were not closely associated within the phytoplankton assemblages.
Discussion
Phytoplankton community
To our knowledge, the present study is the first to examine the occurrence of C. raciborskii in a large number of lakes near the current border of its north-eastern distributional range (Padisák, Citation1997; Stüken et al., Citation2006; Vidal & Kruk, Citation2008). It occurred in almost half of the lakes investigated and therefore appears to have a broader distribution in the temperate zone than was previously recognized. It can be considered a common cyanobacterial species in the region. However, in the present study C. raciborskii was never a dominant species in the algal community and typically constituted a smaller fraction of the phytoplankton biomass than in other, comparable studies in temperate zones (Padisák & Reynolds, Citation1998; Briand et al., Citation2002; Mischke, Citation2003; Nixdorf et al., Citation2003; Wiedner et al., Citation2007). However, the maximum values of C. raciborskii biomass found here were higher than those reported by previous studies (Stefaniak & Kokociński, 2005; Kokociński et al., 2010). Nevertheless, its relative contribution to total phytoplankton biomass was similar to previous studies reporting 0.5–10% of the biomass (Stefaniak & Kokociński, 2005) or even less than the 18% of the biomass observed in Lake Bninskie (Kokociński et al., 2010). Therefore, additional studies must be conducted to determine the changes in dynamics of C. raciborskii populations and their relative contribution to the phytoplankton biomass in the assemblages.
Environmental factors
Cylindrospermopsis raciborskii occurred in lakes that varied widely in their environmental characteristics. Our results showed, however, that it was more frequently found in shallow eutrophic lakes than in deep stratified lakes. An exception was the occurrence of C. raciborskii in the deep lakes Szydlowskie, Buszewskie and Kursko. These findings indicated that the species can also maintain populations in the epilimnion of the stratified lakes. However, we must emphasize that the deep lakes in which C. raciborskii occurred could also be categorized as eutrophic and highly productive lakes. The occurrence of C. raciborskii in a wide range of habitats has also been reported in NE Germany (Stüken et al., Citation2006). However, this report also indicated that the lakes in which C. raciborskii occurred were shallow and more turbid than the lakes in which it did not occur. Similarly, Padisák (Citation1997) and Vidal & Kruk (Citation2008) observed that C. raciborskii was more common in shallow, well-mixed lakes. It seems, however, that depth is not per se the primary factor for C. raciborskii occurrence. It seems rather that depth indirectly influences the occurrence of the species, for example through water temperature: deeper lakes warm up more slowly than shallow ones and the temperature required for the akinete germination is therefore reached later. McGregor & Fabbro (Citation2000) reported a high abundance of C. raciborskii in the deep stratified lakes in the tropics, but the cells first started to develop in the shallower parts of the lakes. Our GLM analyses seem to confirm this finding because lake depth was identified as only marginally significant for the occurrence of C. raciborskii, a more important factor being pH. High pH has been correlated with the occurrence of C. raciborskii, both in temperate and tropical regions (Branco & Senna, Citation1994; Padisák, Citation1997; Hamilton et al., Citation2005), but it must be emphasized that high photosynthetic activity elevates water pH, so that primary production might be the ultimate variable driving occurrence. Thus, based on present data, it is difficult to assess the extent to which C. raciborskii actually benefits from higher pH.
With regard to the abundance of C. raciborskii, RDA indicated that the lakes with the highest biomass of C. raciborskii tended to have high levels of TN and high conductivity. GLM further revealed that a higher biomass of C. raciborskii can be expected in lakes with high TP and TN concentrations, but with low levels of ,
and
. Negative relationships with
can be partially explained by the high storage capacity and uptake efficiency of
by C. raciborskii, which is known to be a strong competitor in
limited waters (Isvánovics et al., Citation2000; Shafik et al., Citation2000; Posselt et al., Citation2009). Recent studies have also shown that C. raciborskii can grow in conditions of low P supply as long as sufficient amounts of dissolved inorganic N are supplied (Kenesi et al., Citation2009). In general,
has been shown to be the preferred source of N enhancing the growth of C. raciborskii (Présing et al., Citation1996; Padisák, Citation1997; Saker et al., Citation1999; Hawkins et al., Citation2001; Saker & Neilan, Citation2001; Shafik et al., Citation2001). Nevertheless, the negative relationship between
and C. raciborskii biomass detected here is not surprising, because low concentrations of
also favour C. raciborskii owing to its high uptake affinity for
(Présing et al., Citation1996; Padisák, Citation1997; Burford et al., Citation2006). N2 fixation was not completely inhibited, however, and represented an important source of N after
depletion. The likelihood of N2 fixation is consistent with the observation that filaments of C. raciborskii developed heterocysts in all lakes in which the species occurred.
In contrast to the findings of previous studies (Briand et al., Citation2002; Hamilton et al., Citation2005; Stüken et al., Citation2006; Wiedner et al., Citation2007) temperature was negatively related to the biomass of C. raciborskii in these lakes. This finding was somewhat surprising, as laboratory studies have shown that the optimum temperature for C. raciborskii is generally relatively high, between 25°C and 35°C (Saker & Griffiths, Citation2000; Briand et al., Citation2004; Mehnert et al., Citation2010). However, the species has also been found to occur earlier at lower temperatures of 15°C and 17°C in Austrian and German lakes (Dokulil & Mayer, Citation1996; Mischke, Citation2003), and in tropical regions at temperatures of 19°C and 17°C (Figueredo & Giani, Citation2009; Everson et al., Citation2011). Moreover, a recent study has shown that C. raciborskii is capable of growing at temperatures as low as 11°C in a subtropical lake (Bonilla et al., Citation2011). Wiedner et al. (Citation2007) also showed that C. raciborskii populations may emerge at much lower temperatures than have previously been observed in the northern habitats. Higher temperatures apparently facilitate growth during the germination process at the beginning of the vegetative season (Padisák, Citation1997; Wiedner et al., Citation2002). However, when populations are well developed, growth may continue even at relatively low temperatures (Messineo et al., Citation2010). These contrasting results related to the effect of temperature on C. raciborskii seem to be associated with the occurrence of genetically and ecophysiologically different ecotypes of C. raciborskii (Piccini et al., Citation2011) and its greater phenotypic plasticity in response to environmental factors (Bonilla et al., Citation2011).
Relationship between C. raciborskii and other phytoplankton species
The expansion and successful establishment of C. raciborskii in new habitats is of great concern, because it may induce serious alterations of phytoplankton community structure (Dobberfuhl, Citation2003). However, alterations may also reflect temporal changes in the environment, for example due to climate change (Kling, Citation2009). Even though we cannot evaluate these alternatives directly, because of a lack of phytoplankton data from before the appearance of C. raciborskii, examination of present-day phytoplankton assemblages in the lakes in which C. raciborskii occurs may give insights into the assemblage composition after it colonizes a new habitat. DCA indicated that C. raciborskii most frequently occurs with green algae, e.g. Desmodesmus sp., Pediastrum sp., Monoraphidium sp. and Tetrastrum sp. These algae are common in shallow, eutrophic lakes (Reynolds et al., Citation2002). An interesting finding was that C. raciborskii frequently co-occurred with another invasive species, Aphanizomenon ovalisporum, which is also a potential producer of cylindrospermopsin (Quesada et al., Citation2006), but which, to our knowledge, has never been reported previously from this region of Poland. This finding is in line with recent studies from different geographical regions indicating frequent co-occurrence of these two cyanobacteria (Yilmaz et al., Citation2008; Messineo et al., Citation2010; Orr et al., Citation2010). In contrast, A. aphanizomenoides, another invader in the lakes studied, was not so closely associated with C. raciborskii in the communities. Furthermore, the native A. gracile, commonly reported as co-occurring with C. raciborskii (Padisák & Reynolds, Citation1998; Nixdorf et al., Citation2003; Moustaka-Gouni et al., Citation2007), was seldomly associated with C. raciborskii in our study lakes. These results are surprising as A. ovalisporum is suggested to be only poorly adapted to temperate regions due to its preference for high temperatures (Mehnert et al., Citation2010).
DCA also showed that P. agardhii, a common native cyanobacterium in these lakes, was only weakly associated with C. raciborskii. Other cyanobacteria from Oscillatoriales were also weakly associated with C. raciborskii in the phytoplankton community, a finding that contrasted with Figueredo & Giani (Citation2009) and Orr et al. (Citation2010). This may suggest that these species belong to different phytoplankton functional groups, as indicated earlier by Reynolds et al. (Citation2002). Moreover, the finding that the invasive cyanobacteria often co-occurred indicates that these Nostocales species may have similar environmental requirements and that these requirements differ from those of the native species of Oscillatoriales. Occurrence of these invasive cyanobacteria in phytoplankton communities is of concern due to possible production of cyanotoxins. Therefore, it will be important to continue monitoring their behaviour and spread, together with potentially toxic native cyanobacteria.
Acknowledgements
The financial support was provided by Polish Ministry of Science and Higher Education through research grant No. N N 304 020 437. We thank Dr Harold G. Marshall (Old Dominion University) for his English language revision and helpful comments on the manuscript. The comments of the anonymous reviewers were greatly appreciated.
References
- APHA . 1998 . Standard Methods for the Examination of Water and Wastewater. , 20th , Washington , DC : American Public Health Association .
- Bouvy , M , Molica , R , De Oliveira , S , Marinho , M and Beker , B . 1999 . Dynamics of a toxic cyanobacterial bloom (Cylindrospermopsis raciborskii) in a shallow reservoir in the semi-arid region of northern Brazil . Aquatic Microbial Ecology , 20 : 285 – 297 .
- Bonilla, S., Aubriot, L., Soares, M.C.S., Gonzáles–Piana, M., Fabre, A., Huszar, V.L.M., Lürling, M., Antoniades, D., Padisák, J. & Kruk, C. (2011). What drives the distribution of the bloom forming cyanobacteria Planktothrix agardhii and Cylindrospermopsis raciborskii? FEMS Microbiology Letters. Accepted article DOI: 10.1111/j.1574–6941.2011.01242.x
- Branco , CWC and Senna , PAC . 1994 . Factors influencing the development of Cylindrospermopsis raciborskii and Microcystis aeruginosa in Paranoá Reservoir, Brasilia, Brasil . Algological Studies , 75 : 85 – 96 .
- Briand , JF , Robillot , C , Quiblier-Lloberas , C , Humbert , JF , Couté , A and Bernard , C . 2002 . Environmental context of Cylindrospermopsis raciborskii (Cyanobacteria) blooms in a shallow pond in France . Water Research , 36 : 3183 – 3192 .
- Briand , JF , Leboulanger , C , Humbert , JF , Bernard , C and Dufour , P . 2004 . Cylindrospermopsis raciborskii (Cyanobacteria) invasion at mid-latitudes: selection, wide physiological tolerance, or global warming? . Journal of Phycology , 40 : 231 – 238 .
- Burchardt , L . 1977 . Changes in phytoplankton composition of Lake Pa˛tnowskie, receiver of heated water and waste water from sugar factory (1972/73) . Seria Biologia , 8 : 1 – 119 .
- Burford , MA and Davis , TW . 2011 . Physical and chemical processes promoting dominance of toxic cyanobacterium Cylindrospermopsis raciborskii . Chinese Journal of Oceanology and Limnology , 29 : 883 – 891 .
- Burford , MA and O’Donohue , MJ . 2006 . A comparison of phytoplankton community assemblages in artificially and naturally mixed subtropical water reservoirs . Freshwater Biology , 51 : 973 – 982 .
- Burford , MA , McNeale , KL and McKenzie-Smith , FJ . 2006 . The role of nitrogen in promoting Cylindrospermopsis raciborskii in subtropical water reservoir . Freshwater Biology , 51 : 973 – 982 .
- Burnham , KM and Anderson , DR . 1998 . Model Selection and Inference: A Practical Information-theoretic Approach , New York : Springer .
- Carmichael , WW , Azevedo , SMFO , An , JS , Molica , RJR , Jochmisen , EM , Lau , S , Rinehart , KL , Shaw , GR and Eaglesham , GK . 2001 . Human fatalities from cyanobacteria: chemical and biological evidence for cyanotoxins . Environmental Health Perspectives , 109 : 663 – 668 .
- Chapman , AD and Schelske , CL . 1997 . Recent appearance of Cylindrospermopsis (Cyanobacteria) in five hypereutrophic Florida lakes . Journal of Phycology , 33 : 191 – 195 .
- Chorus , I and Bartram , J . 1999 . Toxic Cyanobacteria in Water. A Guide to their Public Health Consequences, Monitoring and Management , London & New York : E. & F.N. Spon .
- Chonudomkul , D , Yongmanitchai , W , Theeragool , G , Kawachi , M , Kasai , F , Kaya , K and Watanabe , MM . 2004 . Morphology, genetic diversity, temperature tolerance and toxicity of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) strains from Thailand and Japan . FEMS Microbiology Ecology , 48 : 345 – 355 .
- Dobberfuhl , DR . 2003 . Cylindrospermopsis raciborskii in three central Florida lakes: population dynamics, controls and management implications . Lake and Reservoir Management , 19 : 341 – 348 .
- Dokulil , MT and Mayer , J . 1996 . Population dynamics and photosynthetic rates of a Cylindrospermopsis–Limnothrix association in a highly eutrophic urban lake, Alte Donau, Austria . Algological Studies , 83 : 179 – 195 .
- Dokulil , MT and Teubner , K . 2000 . Cyanobacterial dominance in lakes . Hydrobiologia , 438 : 1 – 12 .
- Everson , S , Fabbro , L , Kinnear , S and Wright , P . 2011 . Extreme differences in akinete, heterocyte, and cylindrospermopsin concentrations with depth in successive bloom involving Aphanizomenon ovalisporum (Forti) and Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju . Harmful Algae , 10 : 265 – 276 .
- Fabbro , LD and Duivenvoorden , LJ . 1996 . Profile of a bloom of the cyanobacterium tropical Cylindrospermopsis raciborskii (Woloszynska) Seeneya and Subba Raju in the Fitzroy River in tropical Central Queensland . Marine and Freshwater Research , 47 : 685 – 694 .
- Falconer , IR , Hardy , SJ , Humpage , AR , Froscio , SM , Tozer , GJ and Hawkins , PR . 1998 . Hepatic and renal toxicity of the blue-green alga (cyanobacterium) Cylindrospermopsis raciborskii in male Swiss Albino mice . Environmental Toxicology , 14 : 143 – 150 .
- Falconer , IR and Humpage , AR . 2006 . Cyanobacterial (blue-green algal) toxins in water supplies: Cylindrospermopsins . Environmental Toxicology , 21 : 299 – 304 .
- Fastner , J , Heinze , R , Humpage , AR , Mischke , U , Eaglesham , GK and Chorus , I . 2003 . Cylindrospermopsin occurrence in two German lakes and preliminary assessment of toxicity and toxin production of Cylindrospermopsis raciborskii (cyanobacteria) isolates . Toxicon , 42 : 313 – 321 .
- Fastner , J , Rücker , J , Stüken , A , Preußel , K , Nixdorf , B , Chorus , I , Köhler , A and Wiedner , C . 2007 . Occurrence of the cynobacterial toxin Cylindrospermopsin in northeast Germany . Environmental Toxicology , 22 : 26 – 32 .
- Figueredo , CC and Giani , A . 2009 . Phytoplankton community in the tropical lake of Lagoa Santa (Brazil): conditions favoring a persistent bloom of Cylindrospermopsis raciborskii . Limnologica , 39 : 264 – 272 .
- Figueredo , CC , Giani , A and Bird , DF . 2007 . Does allelopathy contribute to Cylindrospermopsis raciborskii (cyanobacteria) bloom occurrence and geographic expansion? . Journal of Phycology , 43 : 256 – 265 .
- Hamilton , PB , Ley , LM , Dean , S and Pick , FR . 2005 . The occurrence of the cyanobacterium Cylindrospermopsis raciborskii in Constance Lake: an exotic cyanoprocaryote new to Canada . Phycologia , 44 : 17 – 25 .
- Hawkins , PR , Runnegar , MTC , Jackson , ARB and Falconer , IR . 1985 . Severe hepatotoxicity caused by the tropical cyanobacterium Cylindrospermopsis raciborskii (Wołoszyńska) Seenaya and Subba Raju isolated from a domestic water supply reservoir . Applied and Environmental Microbiology , 50 : 1292 – 1295 .
- Hawkins , PR , Putt , E , Falconer , I and Humpage , A . 2001 . Phenotypical variation in a toxic strain of the phytoplankter, Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) during batch culture . Environmental Toxicology , 16 : 460 – 467 .
- Hawkins , PR , Holliday , J , Kathuria , A and Bowling , L . 2005 . Change in cyanobacterial biovolume due to preservation by Lugol's iodine . Harmful Algae , 4 : 1033 – 1043 .
- Hindak , F . 1978 . Freshwater Algae , Bratislava : Slovenské Pedagogické Nakladatelstwo .
- Hong , Y , Steinman , A , Biddanda , B , Rediske , R and Fahnenstiel , G . 2006 . Occurrence of the toxin-producing cyanobacterium Cylindrospermopsis raciborskii in Mona and Muskegon Lakes, Michigan . Journal of Great Lakes Research , 32 : 645 – 652 .
- Hooker , EL , Hernandez , S , Chow , N and Vargas , L . 1991 . Phytoplankton studies in tropical lake (Lake Xolotlán, Nicaragua) . Verhandlungen des Internationalen Verein Limnologie , 24 : 1158 – 1162 .
- Hötzel, G. & Croome, R. (1999). A Phytoplankton Methods Manual for Australian Freshwaters. LWRRDC occasional paper 22/99. Land and Water Resources Research and Development Corporation, GPO Box 2182, Canberra, Australia
- Isvánovics , V , Shafik , HM , Présing , M and Juhos , S . 2000 . Growth and phosphate uptake kinetics of the cyanobacterium, Cylindrospermopsis raciborskii (Cyanophyceae) in throughflow cultures . Freshwater Biology , 43 : 257 – 275 .
- ISO 10304-1: 2007. (2007). In Water Quality – Determination of Dissolved Anions by Liquid Chromatography of Ions. Part 1: Determinations of Bromide, Chloride, Fluoride, Nitrate, Nitrite, Phosphate and Sulfate. International Organization for Standardization. www.iso.org
- ISO 6878: 2004. (2004). In Water Quality – Determination of Phosphorus – Ammonium Molybdate Spectrometric Method. International Organization for Standardization. www.iso.org
- Javornicky, P. (1958). Revise některých method pro zjišt’ovănikvantity fitoplantonu (Revision of some methods for the phytoplankton quantity estimation). Scientific Paper 2, Faculty of Technology of Fuel and Water, Institute of Chemical Technology, Prague
- Kenesi , G , Shafik , HM , Kovács , AW , Herodek , S and Présing , M . 2009 . Effect of nitrogen forms on growth, cell composition and N2 fixation of Cylindrospermopsis raciborskii in phosphorus-limited chemostat cultures . Hydrobiologia , 623 : 191 – 202 .
- Kling , HJ . 2009 . Cylindrospermopsis raciborskii (Nostocales, Cyanophyta): a brief historic overview and recent discovery in the Assiniboine River (Canada) . Fottea , 9 : 45 – 47 .
- Kokociński , M , Stefaniak , K , Mankiewicz-Boczek. , K , Izydorczyk , K and Soininen , J . 2010 . The ecology of the invasive cyanobacterium Cylindrospermopsis raciborskii (Nostocales, Cyanophyta) in two hypereutrophic lakes dominated by Planktothrix agardhii (Oscillatoriales, Cyanophyta) . European Journal of Phycology , 45 : 365 – 374 .
- Lagos , N , Onodera , H , Zagatto , PA , Andrinolo , D , Azevedo , SMFQ and Oshima , Y . 1999 . The first evidence of paralytic shellfish toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii, isolated from Brasil . Toxicon , 37 : 1359 – 1373 .
- Laslett , GM , Clark , RM and Jones , GJ . 1997 . Estimating the precision of filamentous blue-green algae cell concentration from a single sample . Envirometrics , 8 : 313 – 339 .
- Li , R , Carmichael , WW , Brittain , S , Eaglesham , GK , Shaw , GR , Mahakhant , A , Noparatnaraporn , N , Yongmanitchai , W , Kaya , K and Watanabe , MM . 2001 . Isolation and identification of the cyanotoxin cylindrospermopsin and deoxycylindrospermopsin from a Thailand strain of Cylindrospermopsis raciborskii (Cyanobacteria) . Toxicon , 39 : 973 – 980 .
- McGregor , GB and Fabbro , LD . 2000 . Dominance of Cylindrospermopsis raciborskii (Nostocales, Cyanoprocaryota) in Queensland tropical and subtropical reservoirs: Implications for monitoring and management . Lake and Reservoir Management , 5 : 195 – 205 .
- Mehnert , G , Leunert , F , Cirés , S , Jöhnk , K , Rücker , J , Nixdorf , B and Wiedner , C . 2010 . Competitiveness of invasive and native cyanobacteria from temperate freshwaters under various light and temperature conditions . Journal of Plankton Research , 32 : 1009 – 1021 .
- Messineo , V , Melchiorre , S , Di Corcia , A , Gallo , P and Bruno , M . 2010 . Seasonal succession of Cylindrospermopsis raciborskii and Aphanizomenon ovalisporum blooms with cylindrospermopsin occurrence in the volcanic Lake Albano, Central Italy . Environmental Toxicology , 25 : 18 – 27 .
- Mischke , U . 2003 . Cyanobacteria associations in shallow polytrophic lakes: influence of environmental factors . Acta Oecologica , 24 : 11 – 23 .
- Molica , RJR , Onodera , H , Garcia , C , Rvas , M , Andrinolo , D , Nacimento , S , Meguro , H , Oshima , Y , Azevedo , SMFO and Lagos , N . 2002 . Toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii (Cyanophyceae) isolated from Tabocas reservoir in Caruaru, Brazil, including demonstration of a new saxitoxin analogue . Phycologia , 41 : 606 – 611 .
- Molica , RJR , Oliveira , EJA , Carvalho , PVVC , Costa , ANSF , Cunha , MCC , Melo , GL and Azevedo , SMFO . 2005 . Occurrence of saxitoxins and an anatoxin-a(s)-like anticholinesterase in Brazilian drinking water supply . Harmful Algae , 4 : 743 – 753 .
- Moustaka-Gouni , M , Vardaka , E and Tryfon , E . 2007 . Phytoplankton species succession in a shallow Mediterranean lake (L. Kastoria, Greece): steady state dominance of Limnothrix redekei, Microcystis aeruginosa and Cylindrospermopsis raciborskii . Hydrobiologia , 575 : 129 – 140 .
- Nixdorf , B , Mischke , U and Rücker , J . 2003 . Phytoplankton assemblages and steady state in deep and shallow eutrophic lakes – an approach to differentiate the habitat properties of Oscillatoriales . Hydrobiologia , 502 : 111 – 121 .
- Norris , RL , Eaglesham , GK , Pierens , G , Shaw , GR , Smith , MJ , Chiswell , RK , Seawright , AA and Moore , MR . 1999 . Deoxycylindrospermopsin, an analog of cylindrospermopsin from Cylindrospermopsis raciborskii . Environmental Toxicology , 14 : 163 – 165 .
- Ohtani , I , Moore , RE and Runnegar , MTC . 1992 . Cylindrospermopsin: a potent hepatotoxin from the blue-green alga Cylindrospermopsis raciborskii . Journal of the American Chemistry Society , 114 : 7941 – 7942 .
- Orr , PT , Rasmussen , JP , Burford , MA , Eaglesham , GK and Lennox , SM . 2010 . Evaluation of quantitative real-time PCR to characterize spatial and temporal variations in cyanobacteria, Cylindrospermopsis raciborskii (Woloszynska) Seenaya et Subba Raju and cylindrospermopsin concentrations in three subtropical Australian reservoirs . Harmful Algae , 9 : 243 – 254 .
- Padisák , J . 1997 . Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju, an expanding, highly adaptative cyanobacterium: worldwide distribution and review of its ecology . Archiv für Hydrobiologie, Supplement , 107 : 563 – 593 .
- Padisák , J and Reynolds , CS . 1998 . Selection of phytoplankton associations in Lake Balaton, Hungary, in response to eutrophication and restoration measures, with special reference to the cyanoprokaryotes . Hydrobiologia , 384 : 41 – 53 .
- Pełechata , A , Pełechaty , M and Pukacz , A . 2006 . Cyanoprokaryota of shallow lakes of Lubuskie Lakeland (mid-western Poland) . Oceanological and Hydrobiological Studies , 35 : 3 – 14 .
- Piccini , C , Aubriot , L , Fabre , A , Amaral , V , Gonzáles–Piana , M , Gianni , A , Figuerredo , CC , Vidal , L , Kruk , C and Bonilla , S . 2011 . Genetic and eco-physiological differences of South American Cylindrospermopsis raciborskii isolates support the hypothesis of multiple ecotypes . Harmful Algae , 10 : 644 – 553 .
- PN-ISO 7150-1: 2002. (2002). Determination of Ammonia Nitrogen. The Manual Spectrometric Method. International Organization for Standardization. www.iso.org
- PN-73/C-04576/14. (1973). Water and Waste Water. Calculation of Total Nitrogen. International Organization for Standardization. www.iso.org
- Posselt , AJ , Burford , MA and Shaw , G . 2009 . Pulses of phosphate promote dominance of the toxic cyanophyte Cylindrospermopsis raciborskii in a subtropical water reservoir . Journal of Phycology, , 45 : 540 – 546 .
- Présing , M , Herodek , S , Vörös , L and Kóbor , I . 1996 . Nitrogen fixation, ammonium and nitrate uptake during a bloom of Cylindrospermopsis raciborskii in Lake Balaton . Archiv für Hydrobiologie , 136 : 553 – 562 .
- Quesada , A , Moreno , E , Carrasco , D , Paniagua , T , Wormer , L , De Hoyos , C and Sukenik , A . 2006 . Toxicity of Aphanizomenon ovalisporum (Cyanobacteria) in Spanish water reservoir . European Journal of Phycology , 41 : 39 – 45 .
- Reynolds , CS , Huszar , V , Kruk , C , Naselli-Flores , L and Melo , S . 2002 . Towards a functional classification of the freshwater phytoplankton . Journal of Plankton Research , 24 : 417 – 428 .
- Saker , ML , Neilan , BA and Griffiths , DJ . 1999 . Two morphological forms of Cylindrospermopsis raciborskii (cyanobacteria) isolated from Salomon Dam, Palm Island, Queensland . Journal of Phycology , 35 : 599 – 606 .
- Saker , ML and Neilan , BA . 2001 . Variable diazotrophies, morphologies and toxicities of genetically similar isolates of Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) from northern Australia . Applied Environmental Microbiology , 67 : 1839 – 1945 .
- Saker , ML and Griffiths , DJ . 2000 . The effect of temperature on growth and cylindrospermopsin content of seven isolates of the cyanobacterium Cylindrospermopsis raciborskii (Woloszynska) Seenayya and Subba Raju from water bodies in northern Australia . Phycologia , 39 : 349 – 354 .
- Shafik , HM , Presing , M and Juhos , S . 2000 . Growth and phosphate uptake kinetics of the cyanobacterium, Cylindrospermopsis raciborskii (Cyanophyceae) in throughflow cultures . Freshwater Biology , 43 : 257 – 275 .
- Shafik , HM , Herodek , S , Présing , M and Vörös , L . 2001 . Factors affecting growth and cell composition of cyanoprocaryote Cylindrospermopsis raciborskii (Wołoszynska) Seenayya et Subba Raju . Algological Studies , 103 : 75 – 93 .
- Soares , MCS , Rocha , MIA , Marinho , MM , Azevedo , SMFO , Branco , CWC and Huszar , VLM . 2009 . Changes in species composition during annual cyanobacterial dominance in tropical reservoir: physical factors, nutrients and grazing effects . Aquatic Microbial Ecology , 57 : 137 – 149 .
- Soininen , J , Heino , J , Kokociński , M and Muotka , T . 2009 . Local-regional diversity relationship varies with spatial scale in lotic diatoms . Aquatic Microbial Ecology , 36 : 720 – 727 .
- Stefaniak , K and Kokociński , M . 2005 . Occurrence of invasive Cyanobacteria species in polimictic lakes of the Wielkopolska region (Western Poland) . Oceanological and Hydrobiological Studies , 34 ( Supplement 3 ) : 137 – 148 .
- Stüken , A , Rücker , J , Endrulat , T , Preussel , K , Hemm , M , Nixdorf , B , Karsten , U and Wiedner , C . 2006 . Distribution of three alien cyanobacterial species (Nostocales) in northeast Germany: Cylindrospermopsis raciborskii, Anabaena bergii and Aphanizomenon aphanizomenoides . Phycologia , 45 : 696 – 703 .
- Varkonyi , Z , Zsiros , O , Farkas , T , Garab , G and Gombos , Z . 2000 . The tolerance of cyanobacterium Cylindrospermopsis raciborskii to low-temperature photo-inhibition affected by the induction of polyunsaturated fatty-acid synthesis . Biochemical Society Transactions , 28 : 892 – 894 .
- Vidal , L and Kruk , C . 2008 . Cylindrospermopsis raciborskii (Cyanobacteria) extends its distribution to Latitude 34o53′S: taxonomical and ecological features in Uruguayan eutrophic lakes . Pan-American Journal of Aquatic Sciences , 3 : 142 – 151 .
- Wetzel , RG and Likens , GE . 2000 . Limnological Analyses , 3rd , New York : Springer .
- Wiedner , C , Nixdorf , B , Heinze , R , Wirsing , B , Neumann , U and Weckesser , J . 2002 . Regulation of Cyanobacteria and microcystin dynamics in polymictic shallow lakes . Archiv für Hydrobiologie , 155 : 383 – 400 .
- Wiedner , C , Rücker , J , Brüggemann , R and Nixdorf , B . 2007 . Climate change affects timing and size of populations of invasive cyanobacterium in temperate regions . Oecologia , 152 : 473 – 484 .
- Wood , SA and Stirling , DJ . 2003 . First identification of the cylindrospermopsin-producing cyanobacterium Cylindrospermopsis raciborskii in New Zealand . New Zealand Journal of Marine and Freshwater Research , 37 : 821 – 828 .
- Xie , L , Hagar , J , Rediske , RR , O’Keefe , J , Dyble , J , Hong , Y and Steinman , A . 2011 . The influence of environmental conditions and hydrologic connectivity on cyanobacteria assemblages in two drowned river mouth lakes . Journal of Great Lakes Research , 37 : 470 – 479 .
- Yilmaz , M , Philips , EJ , Szabo , NJ and Badylak , S . 2008 . A comparative study of Florida strains of Cylindrospermopsis and Aphanizomenon for cylindrospermopsin production . Toxicon , 51 : 130 – 139 .