Abstract
Morphological and molecular studies have been performed on Laurencia dendroidea derived from Brazil and the Canary Islands. This species possesses all of the characters that are typical of the genus Laurencia, including the production of the first pericentral cell underneath the basal cell of the trichoblast; the production of tetrasporangia from particular pericentral cells without the formation of additional fertile pericentral cells; spermatangial branches that are produced from one of two laterals on the suprabasal cell of the trichoblasts; and a procarp-bearing segment that possesses five pericentral cells. The phylogenetic position of L. dendroidea was inferred by analysing the chloroplast-encoded rbcL gene sequences of 51 taxa. Phylogenetic analyses revealed that the taxa previously identified and cited in Brazil as Laurencia filiformis, L. majuscula and L. obtusa and in the Canary Islands as L. majuscula all represent the same taxonomic entity and examination of type material allowed us to identify this entity as L. dendroidea, whose type locality is in Brazil. Laurencia obtusa from the Northern Atlantic is confirmed to represent a distinct species, which displays high genetic divergence with respect to western and eastern Atlantic samples. The phylogenetic analyses also supported the nomenclatural transfer of Chondrophycus furcatus (Cordeiro‐Marino & M.T. Fujii) M.T. Fujii & Sentíes to Palisada furcata (Cordeiro‐Marino & M.T. Fujii) Cassano & M.T. Fujii comb. nov.
Introduction
The red alga Laurencia and related genera comprise a very diverse group of species that has undergone several taxonomic changes in recent decades, culminating in the recognition of five genera: Laurencia J.V. Lamouroux, Osmundea Stackhouse, Chondrophycus (Tokida & Saito) Garbary & J.T. Harper, Palisada (Yamada) K.W. Nam and Yuzurua (K.W. Nam) Martin‐Lescanne. The changes made were based on both morphological and molecular approaches and involved the resurrection of the genus Osmundea (Nam et al., Citation1994); the elevation of subgenus Chondrophycus to generic rank (Garbary & Harper, Citation1998); new delineations of the genera Chondrophycus, Laurencia and Osmundea (Nam, Citation1999); proposal and subsequent validation by Nam (Citation2006, Citation2007) of the genus Palisada, based on Yamada's (Citation1931) section Palisadae; and the establishment of the genus Yuzurua, based on Nam's (Citation1999) subgenus Yuzurua (Martin‐Lescanne et al., Citation2010).
Several morphological, anatomical and reproductive characters that are used in the taxonomy of the Laurencia complex of genera have been shown to possess diagnostic value only at the generic level (Saito, Citation1967; Nam et al., Citation1994; Garbary & Harper, Citation1998; Nam, Citation1999, Citation2006). Likewise, many species are poorly defined and exhibit extensive morphological plasticity, which complicates their taxonomic delimitation. This complication is reflected by the large number of species and infraspecific taxa that have been assigned to the complex: Guiry & Guiry (Citation2011) list 430 taxa but consider only 134 to be currently accepted taxonomically. The use of molecular markers has proved to be useful in delimiting taxa and inferring their phylogenetic relationships, and has corroborated the current generic classification (Nam et al., Citation2000; McIvor et al., Citation2002; Abe et al., Citation2006; Fujii et al., Citation2006; Díaz‐Larrea et al., Citation2007; Cassano et al., Citation2009; Gil‐Rodríguez et al., Citation2009; Martin‐Lescanne et al., Citation2010; Rocha‐Jorge et al., Citation2010; Sentíes et al., Citation2011).
The Laurencia dendroidea–obtusa complex exemplifies the morphological plasticity of Laurencia species, which has led to many misidentifications. This complex includes the type species of the genus Laurencia, L. obtusa (Hudson) J.V. Lamouroux, which was described as Fucus obtusus by Hudson (Citation1778). Its type locality was originally unspecified but was probably Devon or Sussex, England, according to Maggs & Hommersand (Citation1993) and Silva et al. (Citation1996). Laurencia obtusa has long been one of the most commonly identified Laurencia species and has been reported from tropical, temperate and cold seas, assuming a cosmopolitan character. Many infraspecific taxa have been described within it (37 according to Guiry & Guiry, Citation2011). Laurencia dendroidea was originally described by J. Agardh (Citation1852) and was collected from an unspecified site in Brazil. It was later included in the Brazilian species list by Martens (Citation1870). Crouan & Crouan (in Mazé & Schramm, Citation1878) proposed two varieties and one form of L. dendroidea from Guadeloupe, namely var. corymbifera Crouan P.L. & H.M. Crouan, var. tenuifolia Crouan P.L. & H.M. Crouan and f. denudata P.L. Crouan & H.M. Crouan. They provided a minimal description for each of the three names, including only a brief phrase regarding the colours of the thalli to distinguish them. These taxa are considered to be nomina subnuda in P.C. Silva's on line Index Nominum Algarum (http://ucjeps.berkeley.edu/INA.html).
Yamada (Citation1931) reduced Laurencia dendroidea to a variety of L. obtusa; he separated them on the basis that var. dendroidea had more slender branches than other varieties, and it lacked projecting surface cells. Taylor (Citation1931) reported L. dendroidea from Brazil and also questioned its validity as an independent species. Following Yamada (Citation1931), Taylor (Citation1960) later considered vars corymbifera and tenuifolia and f. denudata to be synonyms of L. obtusa.
Until recently, Laurencia obtusa has been widely reported for the Brazilian coast (Joly, Citation1965; Oliveira Filho, Citation1969, Citation1977; Pedrini, Citation1980; Yoneshigue, Citation1985; Paes e Mello & Pereira, Citation1990; Figueiredo Creed & Yoneshigue‐Valentin, Citation1997; Pinheiro‐Joventino et al., Citation1998; Da Gama et al., Citation2002; Paradas et al., Citation2010). In addition, specimens have been reported from Brazil that have similar morphology to L. obtusa but have been identified as L. microcladia Kützing (Joly, Citation1965; Oliveira Filho, Citation1969; Cordeiro‐Marino, Citation1978; Figueiredo et al., Citation2004), L. heteroclada Harvey (Oliveira Filho, Citation1969), L. composita Yamada (Oliveira Filho, Citation1969; Paes e Mello & Pereira, Citation1990), L. arbuscula Sonder (Cordeiro‐Marino et al., Citation1983; Fujii, Citation1990), L. scoparia J. Agardh (Cordeiro‐Marino et al., Citation1983; Fujii, Citation1990), L. majuscula (Harvey) A.H.S. Lucas (Széchy & Nassar, Citation2005) and L. filiformis (Fujii, Citation1998; Fujii & Sentíes, Citation2005).
Fujii (Citation1990) considered that Laurencia heteroclada sensu Oliveira Filho (Citation1969), L. microcladia sensu Joly (Citation1965) and Cordeiro‐Marino (Citation1978) and L. obtusa sensu Joly (Citation1965) represented misidentifications of L. arbuscula or L. scoparia, although the latter species has been considered to be conspecific with L. filiformis by Rodríguez de Ríos & Saito (Citation1983). Fujii (Citation1998) argued that the taxa that were named as L. arbuscula, L. scoparia, L. filiformis f. filiformis, L. filiformis f. dendritica Saito & Womersley and L. filiformis f. heteroclada Saito & Womersley from Brazil form a species complex, but nevertheless maintained them as distinct entities. Later, Fujii & Sentíes (Citation2005) recognized that specimens reported from Brazil as L. arbuscula, L. scoparia and L. filiformis and its varieties correspond to morphological variations of a single species, with the epithet L. filiformis taking priority over the others. This observation was confirmed when Fujii et al. (Citation2006) analysed rbcL gene sequences and illustrates the great difficulty of taxonomic delimitation in Laurencia based solely on morphological features.
In this paper, our aim was to use detailed morphological observations and molecular analyses to establish whether L. dendroidea is distinct from L. obtusa and to determine the true identities of specimens from Brazil and the Canary Islands previously referred to the various Laurencia species listed above. Our phylogenetic analyses also allowed us to resolve the taxonomic position of Chondrophycus furcatus (Cordeiro‐Marino & M.T. Fujii) Sentíes & M.T. Fujii, supporting transfer to the genus Palisada (Yamada) K.W. Nam.
Materials and methods
Morphological observations
Samples of Laurencia dendroidea were collected from Brazil and the Canary Islands (Spain). Voucher specimens and material for morphological study were fixed in 4% formalin–seawater or pressed as herbarium sheets. Transverse and longitudinal hand sections were made with a stainless‐steel razor blade and stained with 0.5% aqueous aniline blue solution, acidified with 1 N HCl (Tsuda & Abbott, Citation1985). Living specimens were also examined to check for presence of corps en cerise. Measurements are given as length × diameter. Vouchers are deposited in HRJ, SP, SPF, UAMIZ, TFC and BCM. Additional specimens from the Botanical Museum Herbarium, Lund, Sweden (LD), the State Herbarium of South Australia (AD), Trinity College Dublin, Ireland (TCD), Nationaal Herbarium Nederland, Leiden University, the Netherlands (L), the Herbarium of the University of São Paulo, Brazil (SPF), and the Herbarium of the Botanical Garden of Rio de Janeiro, Brazil (RB), were analysed for comparison. Specimens examined morphologically are listed in Appendix S1 (Supplementarymaterial). Photomicrographs were taken with a Sony W5 digital camera (Tokyo, Japan) coupled to a Nikon microscope. Herbarium abbreviations follow the online Index Herbariorum (http://www.nybg.org/bsci/ih/ih.html).
Molecular analyses
Samples used for molecular analysis were dried in silica gel. Total DNA was extracted, after grinding in liquid nitrogen, using the DNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA) following the manufacturer's instructions. A total of 51 rbcL sequences were used in this study, including 14 newly generated sequences; the rest were obtained from GenBank (TableS1,Supplementarymaterial). For each sequence generated a total of 1467 base pairs of the rbcL gene were amplified in three overlapping parts with the primer pairs: FrbcLstart–R753, F492–R1150 and F993–RrbcS (Freshwater & Rueness, Citation1994), using PCR master mix (Promega, Madison, WI, USA). All PCR products were analysed by electrophoresis in 1% agarose to check product size and were purified with MicroSpinTMS‐300 HR Columns (GE Healthcare Life Sciences, Piscataway, USA) in accordance with the manufacturer's instructions. Sequencing was carried out with BigDye Terminator Cycle Sequencing Reaction Kits (Applied Biosystems, NJ, USA) on an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems). The primers used for sequencing were those used for the PCR amplification. The full sequence was obtained from both DNA strands. Multiple alignments for rbcL sequences were constructed using the computer program BioEdit 7.0.4.1 (Hall, Citation1999) and are given in the Supplementaryfile.
Phylogenetic analyses
Phylogenetic relationships were inferred with PAUP 4.0b10 (Swofford, Citation2002) and MrBayes v.3.0 beta 4 (Huelsenbeck & Ronquist, Citation2001). Maximum‐parsimony trees (MP) were constructed using the heuristic search option, tree–bisection–reconnection branch‐swapping, and unordered and unweighted characters. Support values for the relationships discovered in each analysis were calculated by performing bootstrap analyses (Felsenstein, Citation1985), as implemented in PAUP. Ten thousand heuristic search replicates were executed using the TBR branch‐swapping algorithm. The model used in the Bayesian analysis was the general‐time‐reversible model of nucleotide substitution with invariant sites and gamma distributed rates for the variable sites (GTR + I + G). This model was selected based on maximum‐likelihood ratio tests implemented in Modeltest version 3.06 (Posada & Crandall, Citation1998) with a significance level of 0.01 by the Akaike Information Criterion. For Bayesian analysis, four chains of the Markov chain Monte Carlo (one hot and three cold) were used, sampling one tree every 10 generations for 1 000 000 generations starting with a random tree. Log‐likelihood values stabilized at around 50 000 generations, which were discarded as ‘burn in’. A 50% consensus tree (majority rule as implemented by PAUP) was computed after the ‘burn in’ point. The range of rbcL divergence values within and among species was calculated using uncorrected ‘p’ distances using PAUP.
Results
Molecular analyses
We sequenced the rbcL gene for 14 specimens: eleven of Laurencia sensu stricto, two of Palisada, and Chondria collinsiana M.A. Howe. By adding sequences from GenBank, an alignment of 51 sequences was assembled, including Ceramium brevizonatum, Bostrychia radicans, Chondria collinsiana, C. dasyphylla and C. californica as outgroups (Table S1). A total of 257 nucleotides was removed from the alignment at the beginning and end, as many sequences from the GenBank were incomplete, producing a data set of 1210 base pairs (Supplementaryfile3).
Specimens of Laurencia from Brazil and the Canary Islands collected under various names – L. arbuscula, L. cf. catarinensis, L. filiformis, L. majuscula and L. obtusa – showed low levels of genetic variation for the rbcL gene. Based on the 1210 bp alignment, the Brazilian samples exhibited 0–0.90% genetic divergence, whereas among sequences for both Brazilian and Canaries specimens, there was 0.66–0.90% divergence. In contrast, Laurencia obtusa from the North Atlantic (Ireland) differed from the Laurencia specimens from Brazil and the Canary Islands (‘L. majuscula’) by 5.6–6.0%.
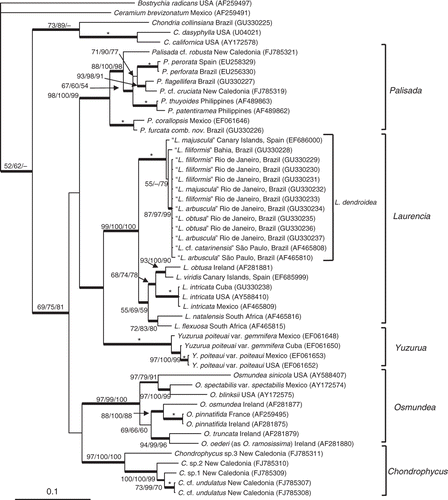
The data set consisted of 751 constant characters, 366 parsimony‐informative sites and 93 parsimony non‐informative sites. The analyses showed that Laurencia and related genera comprise a monophyletic group containing five clades, each with high support and corresponding to one of the currently recognized genera (). Within Laurencia sensu stricto, rbcL sequences of Laurencia specimens from Rio de Janeiro and Bahia resembling L. arbuscula, L. filiformis, L. majuscula and L. obtusa, together with Genbank sequences under the names L. cf. catarinensis and L. arbuscula from São Paulo (Fujii et al., Citation2006) and L. majuscula from Tenerife, Canary Islands, Spain (Gil‐Rodríguez et al., 2009), formed a fully supported clade in all of the analyses. In all analyses, Chondrophycus furcatus was phylogenetically distant from the genus Chondrophycus, and joined Palisada with high support.
Fig. 1. Consensus tree derived from Bayesian analyses of rbcL sequences. The posterior probabilities (when > 95%) are shown as thicker branches. Bootstrap supports for MP/NJ (2000 replicates)/ML (100 replicates) are shown at the nodes; – indicates lack of bootstrap support; * indicates bootstrap support = 100%.
Morphological comparisons
Given the rbcL evidence indicating conspecificity of Brazilian and Canaries specimens previously identified under a variety of different names, we studied type specimens in order to discover their correct name, which proved to be L. dendroidea. We also re‐examined herbarium specimens from Brazil and the Canaries to provide correct determinations for them and to establish the range of form within this species. Below, we give a unified, emended description for L. dendroidea, document its type, and make comparisons with species previously confused with it.
Laurencia dendroidea J. Agardh ( Citation 1852 , p. 753)
(Figs )
Figs 2–5. Habit of Laurencia dendroidea. 2, 3. Habit of specimens from protected areas. 4. Habit of specimen from exposed area, with detail of ultimate branchlets (inset). Note detail of ultimate branchlets markedly curved towards the axis. 5. Detail of basal portion of the thallus showing stolon‐like and descending branches formed from the lower portions (arrow). Scale bars = 2 cm (Figs , ), 1 cm (Figs , ), and 1 mm (, inset).

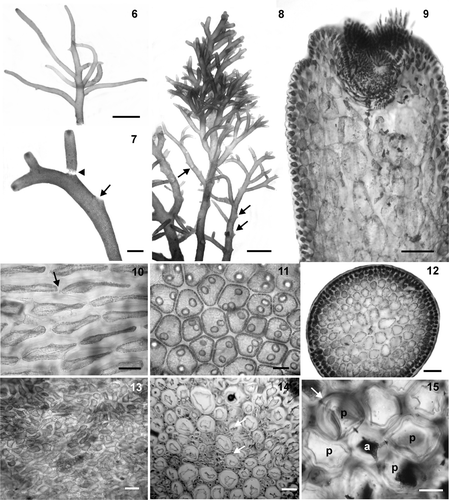
Figs 6–15. Branchlets and vegetative structures of Laurencia dendroidea. 6. Detail of long and sinuous ultimate branchlets. 7. Detail of a branch showing scar of released branchlet (arrow). Note detachment of a branchlet (arrowhead). 8. Upper portion of the thallus showing branches with many scars of released branchlets (arrows). 9. Longitudinal section through a branchlet showing projecting cortical cells. 10. Cortical cells in surface view, showing a secondary pit‐connection (arrow). 11. Cortical cells in surface view, showing corps en cerise in living material. 12. Transverse section of the thallus. 13. Old portion of the thallus in surface view showing secondary cortication. 14. Detail of medulla of older thallus in transverse section, showing filling cells (arrows). 15. Transverse section of the upper portion of a branch showing an axial cell (a) with four pericentral cells (p). Note lenticular thickening in pericentral cell (arrow). Scale bars = 2 mm (Figs , ), 500 µm (), 100 µm (Figs , , ), 50 µm (Figs , ) and 25 µm (Figs , ).
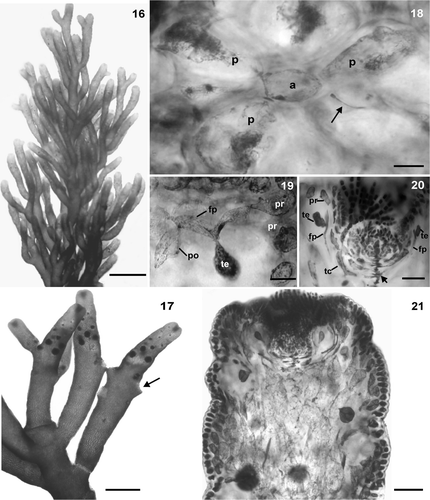
Figs 16–21. Tetrasporangial structures of Laurencia dendroidea. 16. Tetrasporangial branches. 17. Detail of tetrasporangial branchlets. Note scars of released branchlets (arrow). 18. Transverse section of tetrasporangial axial segment showing an axial cell (a) and one fertile pericentral cell, the fourth (arrow); the other pericentral cells remain vegetative (p). 19. Detail of a fertile pericentral cell (fp) with two pre‐sporangial cover cells (pr), tetrasporangium (te) and one post‐sporangial cover cell (po). 20. Longitudinal section through an apical portion of tetrasporangial branchlet, showing origin of tetrasporangia (te) from axial cell (arrow), fertile pericentral cells (fp), and pre‐sporangial cover cells (pr). Note trichoblast (tc) from an axial cell. 21. Longitudinal section through a tetrasporangial branchlet showing parallel arrangement of the tetrasporangia. Scale bars = 2 mm (), 500 µm (), 100 µm (), 50 µm (Figs , ), and 25 µm ().
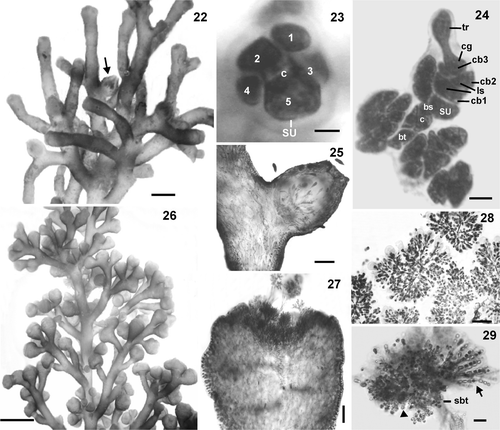
Figs 22–29. Female and male structures of Laurencia dendroidea. 22. Female branches. Note cystocarp (arrow). 23. Procarp‐bearing segment with five pericentral cells, the fifth becoming the supporting cell (su) of the carpogonial branch; central cell of procarp‐bearing segment (c). 24. Procarp before fertilization with four‐celled carpogonial branch (cb), carpogonium (cg), trichogyne (tr), lateral sterile group (ls), basal sterile group (bs), supporting cell (su), central cell of procarp‐bearing segment (c), basal cell of trichoblast (bt). 25. Longitudinal section through a female branchlet with prominent cystocarp without protuberant ostiole. 26. Male branches. 27. Longitudinal section through a male branchlet showing spermatangial branches in cup‐shaped tips. 28. Detail of trichoblast‐type spermatangial branches with terminal vesicular sterile cells. 29. Detail of spermatangial branches on trichoblast with two laterals, sterile (arrow) and spermatangial (arrowhead) branches on its suprabasal cell (sbt). Note spermatangia with an apical nucleus. Scale bars = 2 mm (), 1 mm (), 200 µm (), 100 µm (), 25 µm (Figs , ), 10 µm () and 5 µm ().
HOMOTYPIC SYNONYM : Laurencia obtusa (Hudson) J.V. Lamouroux var. dendroidea (J. Agardh) Yamada, Citation1931: 224.
HETEROTYPIC SYNONYM: Laurencia scoparia J. Agardh, Citation1852: 746–747 (syntype localities: La Guaira, Venezuela; Brazil [Silva et al., Citation1996: 509]).
LECTOTYPE: Botanical Museum Herbarium, Lund, Sweden (LD 36669)!
TYPE LOCALITY: Brazil (unspecified site).
DISTRIBUTION: Widespread in the Atlantic Ocean.
PREVIOUS MISAPPLIED NAMES FOR BRAZIL: Laurencia filiformis sensu Fujii (Citation1998, pp. 26–29, figs 3–33); Pinheiro‐Joventino et al. (Citation1998, p. 35); Pereira et al. (Citation2002, p. 122); Fujii & Sentíes (Citation2005, pp. 84–85, figs 26–35); Széchy & Nassar (Citation2005, p. 384); Pereira et al. (Citation2005, p. 80); as Laurencia sp. 4 (Fujii Citation1990, pp. 101–102, figs 140–149); Laurencia filiformis f. dendritica sensu Fujii (Citation1998, p. 29); Pereira et al. (Citation2002, p. 122); Fujii & Sentíes (Citation2005, p. 85); as Laurencia sp. 2 (Fujii Citation1990, pp. 87–88, figs 119–129); Széchy & Paula (Citation1997, p. 6); Laurencia filiformis f. heteroclada sensu (Fujii, Citation1998, p. 29); Fujii & Sentíes (Citation2005, p. 86); as Laurencia sp. 3 (Fujii Citation1990, pp. 94–95, figs 130–139); Laurencia heteroclada sensu Oliveira‐Filho (Citation1969, pp. 178–179, pl. 29, figs 167, 168); Laurencia arbuscula sensu Cordeiro‐Marino et al. (Citation1983, pp. 29–39, figs 1–13); Fujii (Citation1990, pp. 36–38, figs 48–58); Figueiredo‐Creed & Yoneshigue‐Valentin (Citation1997, p. 36); Gestinari et al. (Citation1998, p. 73); Pedrini et al. (Citation1994, p. 729); Nunes (Citation1998, p. 13); Széchy & Nassar (Citation2005, p. 384); Laurencia composita sensu Oliveira‐Filho (Citation1969, pp. 176–177, pl. D, , pro parte); Laurencia majuscula sensu Széchy & Nassar (Citation2005, p. 384); Laurencia microcladia sensu Joly (Citation1965, p. 234, pls 56, 58, 59, figs 670, 688, 695); Oliveira Filho (Citation1969, p. 179); Cordeiro‐Marino (Citation1978, pp. 130, figs 395, 396); Pinheiro‐Joventino et al. (Citation1998, p. 35); Figueiredo et al. (Citation2004, p. 13); Laurencia obtusa sensu Joly (Citation1965, p. 244, pl. 56, figs 671, 672, pl. 58, fig. 689, pl. 59, figs 696, 697); Pedrini (Citation1980, pp. 232–233); Yoneshigue (Citation1985, pp. 328–329); Figueiredo‐Creed & Yoneshigue‐Valentin (1997, p. 36, pro parte); Pinheiro‐Joventino et al. (Citation1998, p. 35); Laurencia obtusa var. densa [‘L. densa’] sensu Oliveira‐Filho (Citation1969, p. 180, pl. 31, fig. 180); Pedrini (Citation1980, p. 233).
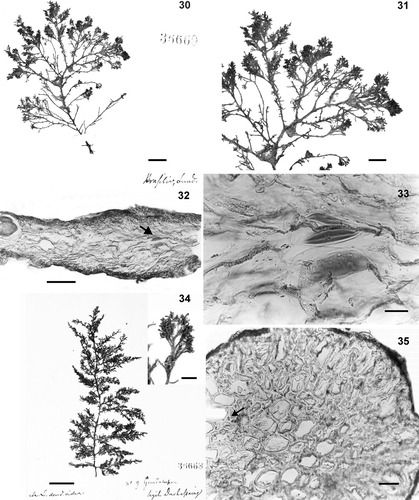
Figs 30–35. Type material of Laurencia dendroidea. . Lectotype (LD 36669). 30. Habit of the thallus. 31. Detail of branches. 32. Transverse section of ultimate branchlet showing lenticular thickenings (arrow). 33. Detail of lenticular thickenings. Figs , . Isolectotype (LD 36668). 34. Habit of the thallus. 35. Transverse section of old portion of the thallus showing filling cells (arrow). Scale bars = 2 cm (), 2 mm (, inset). 1 cm (), 5 mm (), 100 µm (), 40 µm () and 25 µm ().
PREVIOUS MISAPPLIED NAMES FOR THE CANARY ISLANDS, SPAIN: Laurencia majuscula sensu Gil‐Rodríguez & Haroun (1993, pp. 114–115); Masuda et al. (Citation1998, pp. 266–268, figs 1–6); Haroun et al. (Citation2002, p. 152); Haroun et al. (Citation2003, p. 156); Gil‐Rodríguez et al. (Citation2003, p. 30); Haroun et al. (Citation2008, p. 170); Haroun et al. (Citation2009, p. 164).
Habit: Plants erect, forming dense tufts 4–20 cm high, brown‐purple or violet‐greenish in colour (usually greenish in the main axes and primary lateral branches and violet in remaining portions of the plant). The thalli are terete, are cartilaginous in texture, adhere to herbarium paper when dry, and are 0.9–2.8 mm in diameter in the middle portion of the main axis (). Erect axes arise from a single discoid holdfast or from an aggregation of discoid holdfasts; stolon‐like branches and descending branches are formed from the lower portions () and are secondarily attached by smaller holdfasts. The branching is alternate‐spiral to irregular, uniformly dense throughout the thallus to sparse, with 3–5 orders of branches. The first‐order branches are long (up to 17 cm) and display the same branching pattern as the main axes; they become shorter upwards, resulting in a thallus with a pyramidal outline. The ultimate branchlets are simple or compound, and are usually cylindrical with truncated tips; however, in some populations they narrow towards the apex, which results in spine‐like branchlets (). The branchlets are short and straight or long and sinuous and arise at a wide to very narrow angle. They can be parallel to the axis, turned slightly to markedly outwards, or curved in towards the axis, and measure (0.35−) 0.6–3.2 (−6.3) × (0.17−) 0.4–0.6 (−0.75) mm. The branchlets and second‐, third‐ and fourth‐order branches are sometimes deciduous, so that the axes display scars following detachment (Figs , ). The main axes and first‐order branches can bear many adventitious branchlets, which can produce tetrasporangia.
Vegetative structure: In median longitudinal sections through a branchlet, the outer cortical cell walls near the apices sometimes do not project beyond the surface, or they may project slightly or even markedly (). In surface view, the cortical cells are regularly arranged in longitudinal rows throughout the thalli and are connected to each other by longitudinally oriented secondary pit‐connections (). There are one or two (or three) corps en cerise per cortical cell () but only one in each trichoblast cell. In surface view, the cortical cells are isodiametric and polygonal in the upper portions of the thalli, rounded to longitudinally elongate in the middle portions [(28−) 43–150 (−188) × (25−) 33–62 (−83) µm] and elongate‐polygonal in the lower portions. In transverse section, the thalli can be seen to possess one layer of pigmented cortical cells and four or five layers of colourless medullary cells (). In transverse section the cortical cells are quadratic, cuneiform to rectangular, are neither radially elongated nor arranged as a palisade, and measure (20−) 27.5–50 (−77.5) × (20−) 30–42.5 (−65) µm in the middle portions of the thalli. Secondary cortication is present in the lower portions of the thalli (). Medullary cells are rounded or slightly radially elongated, measure (55−) 80–150 (−182.5) × (40−) 55–100 (−125) µm in the middle portions of the thalli, and become larger toward the thallus centre. Intercellular spaces are present in the younger portions, and many filling cells are present in the older portions of the main axes (). Each vegetative axial segment cuts off four pericentral cells (). Lenticular thickenings can be abundant, rare, or absent. When they are present, they occur in medullary cell walls of the main axes or lateral branches (), and especially at the bases of the branches or cystocarps. The first pericentral cell is produced underneath the basal cell of the trichoblast.
Reproductive structures: Tetrasporangial branchlets are cylindrical, simple or compound, and measure (0.6−) 1.3 − 4.5 (−7.5) × 0.3 − 0.6 mm (Figs , ). At the apex of the fertile branches, each axial segment produces only one fertile pericentral cell (the fourth) (Figs , ), which cuts off two pre‐sporangial cover cells distally and abaxially to the tetrasporangial initial (Figs , ). Subsequently, one post‐sporangial cover cell is produced (); it continues to divide and contributes to the production of cortication around the tetrasporangium. The pre‐sporangial cover cells do not divide and display a transverse‐type alignment relative to the fertile axis in surface view. Tetrasporangial maturation occurs in a clockwise spiral, and the final arrangement is a parallel pattern with respect to the fertile branchlets (). Mature tetrasporangia are tetrahedrally divided, measuring 60–170 µm in diameter.
In the female thalli (), each procarp‐bearing segment produces five pericentral cells (), the fifth of which becomes the supporting cell of a four‐celled carpogonial branch containing two groups of sterile cells (basal and lateral) (). The fully developed cystocarps are conical without a protuberant ostiole; they are subapical and prominent in the thallus, and measure 570–1120 µm in diameter (). Carposporangia are clavate, 112.5– 245 × 20–80 µm.
Male branches are clavate, characteristically swollen, and simple or compound, and measure (850–) 960–1280 (−1360) µm in diameter (). In longitudinal section through a fertile branchlet, the spermatangial pits are cup-shaped, and an axial cell row is discernible at the base (). Spermatangial trichoblasts arise from the axial cell, and consist of fertile and sterile branches (Figs , ); the fertile branches produce many ovoid spermatangia measuring 7.5 10 × 5–7.5 µm, and terminate in vesicular sterile cells, of 17.5–30 × 12.5–22.5 µm; each spermatium possesses an apical nucleus.
Habitat: The specimens were collected from the intertidal to subtidal zone at a 3 m depth, and they were protected from or exposed to wave action sites.
Observations on the type of Laurencia dendroidea
LECTOTYPE: LD 36669 ().
ISOLECTOTYPE: LD 36668 (, ).
The lectotype (LD 36669) is a tetrasporophyte c. 8 cm high, with irregularly spiral branching and ultimate branchlets that are short and predominantly compound (Figs , ). Lenticular thickenings were sparsely observed in the ultimate branchlets (Figs , ). The isolectotype specimen (LD 36668) consists of a plant 14 cm high with dense, alternate‐spiral to irregular branching (). In a cross‐section of the thallus of the isolectotype, the basal portion contained little secondary growth and cell filling (). Adventitious branches were observed to be distributed along the thallus from the base to the upper portion. Lateral branches were up to 4 cm long and were distributed throughout the plant with the same branching pattern as the main axis. The ultimate branchlets were long and mostly simple.
Comparison between Laurencia dendroidea and L. filiformis, L. arbuscula and L. obtusa
Comparison between our material and the lectotype of L. filiformis from the Agardh herbarium in Lund, Sweden (LD 36488), revealed that L. filiformis is morphologically distinct from Brazilian and Canarian L. dendroidea, having narrow thalli with irregular and sparse branching. Laurencia filiformis was originally described from Western Australia as Chondria filiformis C. Agardh and its great morphological plasticity was recognized by Saito & Womersley (Citation1974), who therefore referred to it as the ‘filiformis complex’. Based on morphological and anatomical characters, especially on differences related to the habitat, Saito & Womersley (Citation1974) recognized two other forms of L. filiformis in addition to the typical form, namely L. filiformis f. heteroclada, based on L. heteroclada Harvey, and L. filiformis f. dendritica. The typical form was characterized by Saito & Womersley (Citation1974) as growing in protected sites with calm waters, with one or few axes arising from a small discoid holdfast, narrow thalli of up to 1 mm in diameter, and lenticular thickenings often present in the walls of the medullary cells. Laurencia filiformis f. heteroclada was characterized as growing in areas exposed to wave action; it presents numerous fastigiate axes originating from a stoloniferous base, a broader thallus (1–2 mm in diameter) than in the typical form, and lenticular thickenings are absent or occasionally present. The third form, Laurencia filiformis f. dendritica, was described as typical of deeper waters, growing in areas with moderate to strong wave action; it has a larger and more robust thallus (up to 3 mm in diameter) and lenticular thickenings are absent (Saito & Womersley, Citation1974). However, Masuda (Citation1997) recognized f. filiformis and f. heteroclada as separate species, justifying this by the presence of numerous fastigiated axes from a stoloniferous base, the occasional presence of lenticular thickenings in heteroclada, and the production of unrelated sets of halogenated secondary metabolites by filiformis and heteroclada. His treatment was followed by Wynne et al. (Citation2005) in their study of the Laurencia complex of the Sultanate of Oman, although Womersley (Citation2003) did not himself recognize the forms of L. filiformis he had previously described (in Saito &Womersley, Citation1974).
Our examination of the type specimen of L. heteroclada (Herb. Harvey, Alg. Aust. Exsic. 210) revealed that it differs from all of the non-fastigiate material of L. dendroidea from Brazil and the Canaries. Likewise, material of L. arbuscula from the type locality in Australia (SPF8500), previously cited by Womersley (Citation2003, p. 467, fig. 208A) as representative of this species, proved to have a different habit from Brazilian specimens of L. dendroidea previously identified as L. arbuscula: the Australian material has long axes covered by abundant short branchlets, which are spaced at regular intervals.
Laurencia microcladia sensu Joly (Citation1965) and sensu Cordeiro‐Marino (1978) was placed in synonymy with L. arbuscula or L. scoparia by Fujii (Citation1990), based on an analysis of herbarium material (SP and SPF). Later, both were regarded as L. filiformis in Brazil by Fujii & Sentíes (Citation2005) and Fujii et al. (Citation2006). The specimens of ‘L. filiformis’ were proven to be L. dendroidea according to our results. Likewise, analysis of herbarium specimens (SPF) from Brazil identified as L. composita revealed that these taxa also correspond to L. dendroidea.
The Laurencia obtusa specimen that we analysed (from Guernsey, Channel Islands, off the coast of France (xi.1990, C. Maggs, SP 400.116) was morphologically distinct from L. dendroidea, especially regarding the habit and the very flaccid thallus, which was not cartilaginous in texture. The cortical cells projected slightly near the apex of the branches, and the specimen lacked lenticular thickenings in the walls of the medullary cells. However, lenticular thickenings were found in the type specimen (‘Fucus obtusus’: LD36708) that we examined.
Discussion
Molecular circumscription and relationships of Laurencia dendroidea
The rbcL sequences of Laurencia specimens from Rio de Janeiro and Bahia resembling L. arbuscula, L. filiformis, L. majuscula and L. obtusa, were compared with each other and with Genbank sequences under the names L. cf. catarinensis and L. arbuscula from São Paulo (Fujii et al., Citation2006) and L. majuscula from Tenerife, Canary Islands, Spain (Gil‐Rodríguez et al., Citation2009). The twelve Brazilian populations (Rio de Janeiro, São Paulo and Bahia) and the Canarian population formed a fully supported clade in all of the analyses, with intraclade divergences ranging from 0 to 0.90%. The results confirm that all of the morphological variants belong to a single, extremely plastic taxonomic entity, and they corroborate the results obtained by Fujii et al. (Citation2006) for L. arbuscula, L. filiformis and L. scoparia from Brazil.
Comparisons of the Brazilian specimens with the type material of L. filiformis and reference material from the type locality show that these taxa are morphologically distinct, especially with respect to the habit of the plants. However, the characteristics of our specimens are consistent with the characteristics of L. dendroidea, which is a species that was originally described from Brazil. Therefore, based on morphological and molecular studies, we confirmed that the species that were previously attributed to the Brazilian coast as L. arbuscula, L. filiformis, L. majuscula and L. obtusa must all be identified as L. dendroidea. The phylogenetic analysis that was performed in this study and the analysis by Fujii et al. (Citation2006) using the rbcL gene sequences showed that L. obtusa from the North Atlantic (Ireland, a region near the type locality) is a distinct species that is not grouped with any L. dendroidea samples from Brazil and the Canary Islands. Therefore, L. dendroidea should not be treated as a variety of L. obtusa and should instead be treated as an independent species.
The characteristics of Laurencia dendroidea
Laurencia dendroidea possesses all of the characters that are typical of the genus, such as the production of the first pericentral cell underneath the basal cell of the trichoblast, the production of tetrasporangia from particular pericentral cells, without the formation of additional fertile pericentral cells, spermatangial branches that are produced from one of two laterals on the suprabasal cell of the trichoblasts, and a procarp‐bearing segment with five pericentral cells. The species is recognizable primarily by its thallus, which is usually densely branched from the base to the upper portions, pyramidal in outline, and contains one fertile pericentral cell (the fourth) in its tetrasporangial segments.
Studies of the type material and/or reference materials of L. dendroidea, L. filiformis, L. majuscula and L. obtusa from different herbaria were made by Fujii (Citation1990) and again in this study. Our examination of the type specimen of L. dendroidea (LD 36669) revealed the presence of lenticular thickenings, which were not observed by Yamada (Citation1931). Yamada treated L. dendroidea as a variety of L. obtusa and characterized it as lacking lenticular thickenings and surface cell projections, and having branches that are thinner than in other varieties of L. obtusa.
Relationship between Laurencia dendroidea, L. majuscula and L. filiformis
Laurencia dendroidea is closest morphologically to L. majuscula. This was noted long ago by J. Agardh (Citation1876), who considered the species to be a synonym of L. dendroidea. Saito & Womersley (Citation1974) considered L. majuscula to be clearly different from other Australian species. It is readily recognized by its habit, is densely branched, has a relatively soft texture, contains distinctly projected cortical cells (especially near the apices of the axes and branches) and lacks lenticular thickenings. The isotype (MEL 1006997) and additional material of L. majuscula that were examined in this study revealed specimens that are morphologically close to some Brazilian and Canarian L. dendroidea plants, with (1) a thallus that is pyramidal in outline, (2) dense branching, (3) development of adventitious branchlets, and (4) projecting cortical cells. However, due to the broad morphological and habit plasticity displayed in many of the populations we examined, further judgement regarding the relationship between L. dendroidea and L. majuscula must await molecular studies of the latter. Likewise, sequence data are needed for L. filiformis to clarify its taxonomic position and phylogenetic relationships.
Morphological plasticity of Laurencia dendroidea and causes of confusion with other Laurencia species
Morphological variation of L. dendroidea with regard to the basal system, the presence or absence of lenticular thickenings, and the degree of projection of the cortical cell walls, and also differences in habitat, have misled previous research and led to incorrect citation of this taxon under different names for the Brazilian coast, such as L. arbuscula (Cordeiro‐Marino et al., Citation1983), L. composita (Oliveira Filho, Citation1969), L. filiformis f. filiformis, L. filiformis f. heteroclada, L. filiformis f. dendritica (Fujii & Sentíes, Citation2005), L. majuscula (Széchy & Nassar, Citation2005), L. microcladia (Joly, Citation1965; Cordeiro‐Marino, Citation1978; Pinheiro‐Joventino et al., Citation1998; Figueiredo et al., Citation2004) and L. obtusa (Joly, Citation1965; Pedrini, Citation1980; Yoneshigue, Citation1985; Figueiredo‐Creed & Yoneshigue‐Valentin, Citation1997). In our study, specimens with the characteristics of these other taxa showed morphological features that can be related to environmental conditions. For example, specimens that grew in the intertidal zone and the fringe of the subtidal zone in moderately exposed areas or areas exposed to high‐energy wave action had smaller thalli (4–7 cm high) and several erect axes arising from a basal portion consisting of an aggregation of discoid holdfasts; lenticular thickenings were absent or rare, sometimes found only in the bases of cystocarps. Specimens from Brazil with such characteristics (though some may have longer thalli) have previously been referred to L. arbuscula or L. filiformis f. dendritica (Fujii, Citation1990, Citation1998). However, some specimens with the same characters but completely lacking lenticular thickenings have previously been identified as L. obtusa (Joly, Citation1965), or L. obtusa var. densa if the branches were densely arranged, covering the axes (Oliveira Filho, Citation1969); both authors followed Yamada (Citation1931), the main monographic study of Laurencia sensu lato available at that time. Specimens growing in the subtidal zone down to 3‐m depth in calm areas, such as bays and inlets, had longer thalli (up to 20 cm), a basal portion consisting of a single discoid holdfast, stolon‐like branches, and lateral descending branches formed from the lower portions, and abundant to rare or absent lenticular thickenings. These specimens have previously been identified as the typical form of L. filiformis. Similar plants growing in the lower intertidal zone in areas with high water movement and always possessing lenticular thickenings have been identified as L. filiformis f. heteroclada (Fujii, Citation1998), or even as L. majuscula, if the cortical cell walls projected markedly near the apices (Széchy & Nassar, Citation2005).
Since Yamada published his Citation1931 analysis, morphological characters, such as the projection of the cortical cell walls, and the presence, absence and frequency of lenticular thickenings, have been considered to be valuable for separating sections, species and varieties in Laurencia sensu lato. However, in L. dendroidea, the degree of projection of the cortical cell walls was extremely variable in all of the populations we studied, and varied among individuals within the same population. Similarly, the presence, absence and frequency of lenticular thickenings varied among different populations; they are found more frequently at the bases of the cystocarps or younger lateral branches. In some populations, the plants displayed many vegetative or tetrasporangial deciduous branches and branchlets, which left scars on the axes following detachment. The specimens presented characteristically long, sinuous and spiny‐like branchlets. These deciduous branchlets may function in the vegetative propagation of the species.
Transfer of Chondrophycus furcatus to Palisada
Chondrophycus furcatus was described as Laurencia furcata Cordeiro‐Marino & M.T. Fujii from Brazil (Cordeiro‐Marino et al., Citation1994) and transferred to Chondrophycus by Fujii & Sentíes (Citation2005), primarily due to the presence of two pericentral cells per vegetative axial segment. The authors commented that due to the presence of fertility at the second pericentral cells in the tetrasporangial segments, this species was more closely related to species currently assigned to the genus Palisada rather than Chondrophycus. Our phylogenetic analyses strongly support transfer of C. furcatus to Palisada and the nomenclatural change is proposed here:
Palisada furcata (Cordeiro‐Marino & M.T. Fujii) Cassano & M.T. Fujii, comb. nov.
BASIONYM: Laurencia furcata Cordeiro‐Marino & M.T. Fujii in Cordeiro‐Marino et al., (Citation1994, p. 374, figs 1–19).
HOMOTYPIC S YNONYM: Chondrophycus furcatus (Cordeiro‐Marino & M.T. Fujii) M.T. Fujii & Sentíes (Citation2005, p. 109, figs 69–77).
tejp_a_647334_sup_23793626.zip
Download Zip (56 KB)Acknowledgements
We are very grateful to Michael Wynne for his helpful comments, to the curators of the herbaria cited in this study for loans and information, to Patrik Frödén for providing the scanned image of the type of Laurencia dendroidea, and to our colleagues listed in the Supplementary file section titled ‘Specimens Examined Morphologically’ for help with field material. We also thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes) to V.C. and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Proc. 301438/2009‐9 to M.T.F, 470417/2006‐4 to M.C.O. A.S. thanks Secretaría de Educación Pública‐PROMEP (UAM‐I‐CA‐117). M.T.F. and M.C.O. thank CNPq for a Research Productivity Fellowship (Proc. 308426/2006‐1, 301217/2007‐6). This work was partially supported by the Ministry of Sciences and Innovation (MICINN) of the Spanish Government (CGL 2010‐14881), CNPq (Proc. 474021/2007‐6) and by FAPESP (Proc. 2007/51270‐7, Proc. 2010/52244‐2).
References
- Abe , T , Kurihara , A , Kawaguchi , S , Terada , R and Masuda , M . 2006 . Preliminary report on the molecular phylogeny of the Laurencia complex (Rhodomelaceae) . Coastal Marine Science , 30 : 209 – 213 .
- Agardh, J.G. (1852). Species genera et ordines algarum, seu descriptiones succinctae specierum, generum et ordinum, quibus algarum regnum constituitur, volumen secundum: algas florideas complectens, part 3, fasc. 1, pp. 701–786. C.W.K. Gleerup, Lund
- Agardh, J.G. (1876). Species genera et ordines algarum, seu descriptiones succinctae specierum, generum et ordinum, quibus algarum regnum constituitur, volumen tertium: de florideis curae posteriores, part 1, pp. 1–724. C.W.K. Gleerup, Leipzig
- Cassano , V , Díaz-Larrea , J , Sentíes , A , Oliveira , MC , Gil-Rodríguez , MC and Fujii , MT . 2009 . Evidence for the conspecificity of Palisada papillosa with P. perforata (Ceramiales, Rhodophyta) from the western and eastern Atlantic Ocean on the basis of morphological and molecular analyses . Phycologia , 48 : 86 – 100 .
- Cordeiro-Marino , M . 1978 . Rodofíceas bentônicas marinhas do estado de Santa Catarina . Rickia , 7 : 1 – 243 .
- Cordeiro-Marino , M , Fujii , MT and Yamaghishi-Tomita , N . 1983 . Morphological and cytological studies on Brazilian Laurencia. 1: L. arbuscula Sonder (Rhodomelaceae, Rhodophyta) . Rickia , 10 : 29 – 39 .
- Cordeiro-Marino , M , Fujii , MT and Pinheiro-Joventino , F . 1994 . Morphological and cytological studies of Laurencia furcata Cordeiro-Marino and Fujii (Rhodophyta, Rhodomelaceae): a new species from Brazil . Cryptogamic Botany , 4 : 373 – 380 .
- Da Gama , BAP , Pereira , RC , Carvalho , AGV , Coutinho , R and Yoneshigue-Valentin , Y . 2002 . The effects of seaweed secondary metabolites on biofouling . Biofouling , 18 : 13 – 20 .
- Díaz-Larrea , J , Sentíes , A , Fujii , MT , Pedroche , FF and Oliveira , MC . 2007 . Molecular evidence for Chondrophycus poiteaui var. gemmiferus comb. et stat. nov. (Ceramiales, Rhodophyta) from the Mexican Caribbean Sea: implications for the taxonomy of the Laurencia complex . Botanica Marina , 50 : 250 – 256 .
- Felsenstein , J . 1985 . Confidence limits on phylogenies: an approach using the bootstrap . Evolution , 39 : 783 – 791 .
- Figueiredo‐Creed, M.A. de O. & Yoneshigue‐Valentin, Y. (1997). Chlorophyta, Phaeophyta e Rhodophyta. In Mapeamento da cobertura vegetal e listagens das espécies ocorrentes na Área de Proteção Ambiental de Cairuçu, Município de Parati, RJ (Marques, M.C.M., editor), 30–36. Série Estudos e Contribuições, Instituto de Pesquisas do Jardim Botânico do Rio de Janeiro, Rio de Janeiro, Brasil
-
Figueiredo
,
M.A. de O.
,
Barros-Barreto
,
MBB
and
Reis
,
RP
.
2004
.
Caracterização das macroalgas nas comunidades marinhas da Área de Proteção Ambiental de Cairuçú, RJ – subsídios para futuros monitoramentos
.
Revista Brasileira de Bot
nica , 27 : 11 – 17 .
- Freshwater , DW and Rueness , J . 1994 . Phylogenetic relationships of some European Gelidium (Gelidiales, Rhodophyta) species, based on rbcL nucleotide sequence analysis . Phycologia , 33 : 187 – 194 .
- Freshwater , DW , Fredericq , S , Bradley , SB , Hommersand , MH and Chase , MW . 1994 . A gene phylogeny of the red algae (Rhodophyta) based on rbcL . Proceedings of the National Academy of Science of the United States of America , 91 : 7281 – 7285 .
- Fujii , MT . 1990 . Gênero Laurencia (Rhodomelaceae, Rhodophyta) no Estado de São Paulo: aspectos biológicos e taxonômicos. Master's dissertation, Universidade Estadual Paulista Rio Claro, Brazil
- FUJII, M.T. (1998). Estudos morfológicos, quimiotaxonômicos e citológicos em quatro espécies selecionadas de Laurencia (Ceramiales, Rhodophyta) do litoral brasileiro. Ph.D. dissertation, Universidade Estadual Paulista, Rio Claro, Brazil
- FUJII, M.T. & SENTÍES, A. (2005). Taxonomia do complexo Laurencia (Rhodomelaceae, Rhodophyta) do Brasil, com ênfase nas espécies dos estados de São Paulo e do Espírito Santo. In Monografias Ficológicas II (Sentíes, A. & Dreckmann, K.M., editors), 69–135. Universidad Autônoma Metropolitana–Iztapalapa, México and Instituto de Botânica, São Paulo, Brazil
- Fujii , MT , Guimarães , SMPB , Gurgel , CF and Fredericq , S . 2006 . Characterization and phylogenetic affinities of the red alga Chondrophycus flagelliferus (Rhodomelaceae, Ceramiales) from Brazil on the basis of morphological and molecular evidence . Phycologia , 45 : 432 – 441 .
- Garbary , DJ and Harper , JT . 1998 . A phylogenetic analysis of the Laurencia complex (Rhodomelaceae) of the red algae . Cryptogamie, Algologie , 19 : 185 – 200 .
- Gestinari , LMS , Nassar , CAG and Arantes , PVS . 1998 . Algas marinhas bentônicas da Reserva Biológica Estadual da Praia do Sul, Ilha Grande, Angra dos Reis, Rio de Janeiro, Brasil . Acta Botanica Brasilica , 12 : 67 – 76 .
- Gil-Rodríguez , MC and Haroun , R . 1993 . The genus Laurencia (Rhodomelaceae, Rhodophyta) in the Canary Islands . Courier Forschungsinstitut Senckenberg , 159 : 113 – 117 .
- GIL-RODRÍGUEZ, M.C., HAROUN, R., OJEDA RODRÍGUEZ, A., BERECIBAR ZUGASTI, E., DOMÍNGUEZ SANTANA, P. & HERRERA MORÁN, B. (2003). Div. Protoctistas. In Lista de especies marinas de Canarias (algas, hongos, plantas y animales) (Moro, L., Martín, J.L., Garrido, M.J. & Izquierdo, I., editors), 5–30. Consejería de Política Territorial y Medio Ambiente del Gobierno de Canarias, Spain
- Gil-Rodríguez , MC , Sentíes , A , Díaz-Larrea , J , Cassano , V and Fujii , MT . 2009 . Laurencia marilzae sp. nov. (Ceramiales, Rhodophyta) from the Canary Islands, Spain, based on morphological and molecular evidence . Journal of Phycology , 45 : 264 – 271 .
- Guiry, M.D. & Guiry, G.M. (2011). AlgaeBase version 4.2. World‐wide electronic publication, National University of Ireland, Galway. Available at: http://www.algaebase.org, accessed 6 April 2011
- Hall , TA . 1999 . BioEdit: a user-friendly biological sequence alignment and analysis and analysis program for Windows 95/98/NT . Nucleic Acids Symposium Series , 41 : 95 – 98 .
- Haroun , RJ , Gil-Rodríguez , MC , Díaz de Castro , J and Prud’homme Van Reine , WF . 2002 . A checklist of the marine plants from the Canary Islands (Central Eastern Atlantic Ocean) . Botanica Marina , 45 : 139 – 169 .
- Haroun, R.J., Gil-Rodríguez, M.C. & Wildpret de La Torre, W. (2003). Plantas marinas de las Islas Canarias. Consejería de Política Territorial y Medio Ambiente del Gobierno de Canarias, GESPLAN. Tenerife, Canarias, Spain
- Haroun , RJ , Gil-Rodriguez , MC , Wildpret de La Torre , W and Schnetter , R . 2008 . Meerespflanzen der Kanarischen Inseln Las Palmas de Gran Canaria, Spain
- Haroun , RJ , Gil-Rodríguez , MC , Wildpret de La Torre , W and Prud’homme Van Reine , WF . 2009 . Marine Plants of the Canary Islands. BlaBla Ediciones, Las Palmas de Gran Canaria Spain
- Hudson , W . 1778 . Flora anglica. Editio altera, emendata et aucta London
- Huelsenbeck , JP and Ronquist , FR . 2001 . MrBayes. Bayesian inference of phylogeny . Biometrics , 17 : 754 – 755 .
- Joly , AB . 1965 . Flora marinha do litoral norte do estado de São Paulo e regiões circunvizinhas . Boletim da Faculdade de Filosofia, Ciências e Letras da Universidade de São Paulo, série Botânica , 21 : 1 – 393 .
- Index Nominum Algarum, University Herbarium, University of California, Berkeley. Compiled by Paul Silva. Available at: http://ucjeps.berkeley.edu/INA.html, accessed 2 March 2010
- Lin , S , Fredericq , S and Hommersand , MH . 2001 . Systematics of the Delesseriaceae (Ceramiales, Rhodophyta) based on LSU rDNA and rbcL sequences, including the Phycodryoideae, subfam . nov. Journal of Phycology , 37 : 881 – 899 .
- Maggs , CA and Hommersand , MH . 1993 . Seaweeds of the British Isles. Volume 1. Rhodophyta. Part 3A. Ceramiales HMSO, London
- Martens , G. Von . 1870 . Conspectus algarum Brasiliae hactenus detectarum . Videnskabelige Meddelelser fra den Naturhistoriske Forening i Kjøbenhavn , 2 : 297 – 314 .
- Martin-Lescanne , J , Rousseau , F , De Reviers , B , Payri , C , Couloux , A , Cruaud , C and Le Gall , L . 2010 . Phylogenetic analyses of the Laurencia complex (Rhodomelaceae, Ceramiales) support recognition of five genera: Chondrophycus, Laurencia, Osmundea, Palisada and Yuzurua stat nov . European Journal of Phycology , 45 : 51 – 61 .
- Masuda , M . 1997 . A new species of Laurencia: L. omaezakiana (Ceramiales, Rhodophyta) from Japan . Phycological Research , 45 : 123 – 131 .
- Masuda , M , Kogame , K , Arisawa , S and Suzuki , M . 1998 . Morphology and halogenated secondary metabolites of three Gran Canarian species of Laurencia (Ceramiales, Rhodophyta) . Botanica Marina , 41 : 265 – 277 .
- Mazé , H and Schramm , A . 1878 . Essai de classification des algues de la Guadeloupe Imprimerie du gouvernement, Basse-Terre, Guadeloupe
- McIvor, L., Maggs, C.A., Guiry, M.D. & Hommersand, M.H. (2002). Phylogenetic analysis of the geographically disjunct genus Osmundea Stackhouse (Rhodomelaceae, Rhodophyta). Constancea 83.9 [online publication of the Jepson Herbarium, UC, Berkeley]: http://ucjeps.berkeley.edu/constancea/83/mcivor_etal/osmundea.html
- Nam , KW . 1999 . Morphology of Chondrophycus undulatus and C. parvipapillata and its implications for the taxonomy of the Laurencia (Ceramiales, Rhodophyta) complex . European Journal of Phycology , 34 : 455 – 468 .
- Nam , KW . 2006 . Phylogenetic re-evaluation of the Laurencia complex (Rhodophyta) with a description of L. succulenta sp. nov. from Korea . Journal of Applied Phycology , 18 : 679 – 697 .
- Nam , KW . 2007 . Validation of the generic name Palisada (Rhodomelaceae, Rhodophyta) . Algae , 22 : 53 – 55 .
- Nam , KW , Maggs , CA and Garbary , DJ . 1994 . Resurrection of the genus Osmundea with an emendation of the generic delineation of Laurencia (Ceramiales, Rhodophyta) . Phycologia , 33 : 384 – 395 .
- Nam , KW , Maggs , CA , McIvor , L and Stanhope , MJ . 2000 . Taxonomy and phylogeny of Osmundea (Rhodomelaceae, Rhodophyta) in Atlantic Europe . Journal of Phycology , 36 : 759 – 772 .
- Nunes , JMC . 1998 . Catálogo de algas marinhas bentônicas do estado da Bahia, Brasil . Acta Botanica Malacitana , 23 : 5 – 21 .
- Oliveira Filho , EC . 1969 . Algas marinhas do sul do estado do Espirito Santo (Brasil) . I-Ceramiales. Boletim da Faculdade de Filosofia, Ciências e Letras da Universidade de São Paulo, série Botânica , 26 : 1 – 277 .
- Oliveira Filho , EC . 1977 . Algas marinhas bentônicas do Brasil Thesis, Universidade de São Paulo, São Paulo, Brazil
- Paes e Mello , LB and Pereira , SMB . 1990 . Estudos taxonômicos sobre a Família Rhodomelaceae (Rhodophyta – Ceramiales) no litoral Oriental do Estado do Rio Grande do Norte. I. Gênero Laurencia Lamouroux . Trabalhos Oceanográficos da Universidade Federal de Pernambuco , 21 : 165 – 185 .
- Paradas , WC , Salgado , LT , Sudatti , DB , Crapez , MAC , Fujii , MT , Coutinho , R , Pereira , RC and Amado Filho , GM . 2010 . Induction of halogenated vesicle transport in cells of the red seaweed Laurencia obtusa . Biofouling , 26 : 277 – 286 .
- Pedrini, A.G. de. (1980). Algas marinhas bentônicas da Baía de Sepetiba e arredores. Masters’ dissertation, Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil
- Pedrini, A.G. de, Cassano, V., Coelho, L.G. & Labronici, G.J. (1994). Macroalgas marinhas da região sob influência da Central Nuclear Almirante Álvaro Alberto, Angra dos Reis, RJ, Brasil, I – Composição taxonômica. In V Congresso Geral de Energia Nuclear, vol. 2, 733–736. Associação Brasileira de Energia Nuclear, Rio de Janeiro
- Pereira, S.M.B., Pedrosa, M.E.B. & Burgos, D.C. (2005). Macroalgae from the Saint Peter's and Saint Paul's Archipelago (Brazil). Phycologia, 36, Supplement: 80
- Pereira, S.M.B., Carvalho, M.F.O., Angeiras, J.A., Pedrosa, M.E.B., Oliveira, N.M.B., Torres, J., Gestinari, L.M.S., Concentino, A.M., Santos, M.D., Nascimento, P.R.F. & Cavalcanti, D.R. (2002). Algas marinhas bentônicas do estado de Pernambuco. In Diagnóstico da biodiversidade de Pernambuco (Tabarelli, M. & Silva, J.M.C., organizers), vol. 1, 97–124. Secretaria de Ciência, Tecnologia e Meio Ambiente, Editora Massangana, Recife
- Pinheiro-Joventino , F , Dantas , NP and Maraschin , CDH . 1998 . Distribuição de algas marinhas no litoral de Fortaleza, Ceará, Brasil . Arquivos de Ciências do Mar , 31 : 29 – 40 .
- Posada , D and Crandall , KA . 1998 . Modeltest: testing the model of DNA substitution . Bioinformatics , 14 : 817 – 818 .
- Rocha-Jorge , R , Cassano , V , Oliveira , MC and Fujii , MT . 2010 . The occurrence of Laurencia marilzae (Ceramiales, Rhodophyta) in Brazil based on morphological and molecular data . Botanica Marina , 53 : 143 – 152 .
- Rodriguez de Ríos , N and Saito , Y . 1983 . Laurencia scoparia J. Agardh, nuevo sinonimo de Laurencia filiformis (C. Agardh) Montagne (Rhodophyta, Ceramiales) . Ernstia , 21 : 19 – 28 .
- Saito , Y . 1967 . Studies on Japanese species of Laurencia, with special reference to their comparative morphology . Memoirs of the Faculty of Fisheries, Hokkaido University , 15 : 1 – 81 .
- Saito , Y and Womersley , HBS . 1974 . The southern Australian species of Laurencia (Ceramiales, Rhodophyta) . Australian Journal of Botany , 22 : 815 – 874 .
- Sentíes , A , Díaz-Larrea , J , Cassano , V , Gil-Rodriguez , MC and Fujii , MT . 2011 . Laurencia marilzae (Ceramiales, Rhodophyta) from the Mexican Caribbean: a new record for the tropical western Atlantic . Bulletin of Marine Science , 87 : 681 – 686 .
- Silva , P , Basson , PW and Moe , RL . 1996 . Catalogue of the benthic marine algae of the Indian Ocean . University of California Publications in Botany , 79 : 1 – 1259 .
- Swofford , DL . 2002 . PAUP. Phylogenetic Analysis Using Parsimony (*and other Methods) , Sunderland , MA : version 4. Sinauer Associates .
- Széchy , MTM and Paula , E.J. de. 1997 . Macroalgas epífitas em Sargassum (Phaeophyta-Fucales) do litoral dos estados do Rio de Janeiro e São Paulo, Brasil . Leandra , 12 : 1 – 10 .
- Széchy, M.T.M. & Nassar, C.A.G. (2005). Flora ficológica bentônica da Baía da Ribeira, sul do estado do Rio de Janeiro: Avaliação após duas décadas de operação da Central Nuclear Almirante Álvaro Alberto. In Anais da X Reunião Brasileira de Ficologia, Formação de Ficólogos: um compromisso com a sustentabilidade dos recursos aquáticos (Pereira, R.C. et al., organizers), 373–397. Museu Nacional, Rio de Janeiro
- Taylor , WR . 1931 . A synopsis of the marine algae of Brasil . Revue Algologique , 5 : 1 – 35 .
- Taylor , WR . 1960 . Marine Algae of the Eastern Tropical and Subtropical Coasts of the Americas University of Michigan Press, Ann Arbor , MI
- Tsuda , RT and Abbott , IA . 1985 . “ Collecting, handling, preservation and logistics ” . In Handbook of Phycological Methods, Ecological Field Methods: Macroalgae , Edited by: Littler , M.M. and Littler , D.S. 67 – 86 . Cambridge : Cambridge University Press .
- Womersley , HBS . 2003 . The Marine Benthic Flora of Southern Australia. Part IIID, Australian Biological Resources Study and the State Herbarium of South Australia Canberra
- Wynne , MJ , Serio , D , Cormaci , M and Furnari , G . 2005 . The species of Chondrophycus and Laurencia (Rhodomelaceae, Ceramiales) occurring in Dhofar, the Sultanate of Oman . Phycologia , 44 : 497 – 509 .
- Yamada , Y . 1931 . Notes on Laurencia, with special reference to the Japanese species . University of California Publications in Botany , 16 : 185 – 310 .
- Yoneshigue, Y. (1985). Taxonomie et ecologie des algues marines dans la region de Cabo Frio (Rio de Janeiro), Brésil. Ph.D. dissertation, Faculté des Sciences de Luminy, Uníversité d' Aix‐Marseille II, Marseille, France
Supplementary material
The following supplementary material is available for this article, accessible via the Supplementary Content tab on the article's online page at http://dx.doi.org/10.1080/09670262.2011.647334.
TEJP 647334 supplementary material.docx