Abstract
The acclimation to high light of Phaeodactylum tricornutum cultures grown outdoors both in an open pond and in tubular photobioreactors (PBRs) was studied by means of chlorophyll fluorescence, pigment analysis and growth. Cultures grown in PBRs (5-cm tube diameter) at two biomass concentrations (0.3 and 0.6 g l−1) were compared with a culture grown in a 10-cm deep open pond (0.3 g l−1). Therefore, the performance of the cultures was compared on the basis of both the same biomass concentration and areal density. Cultures grown at 0.3 g l−1 in PBRs experienced the highest light stress, which resulted in dramatic changes in both chlorophyll fluorescence and photosynthesis parameters, and in low areal productivity. In this culture, the Fv/Fm ratio was 0.5 in the morning and dropped to about 0.1 within 1 h of exposure to bright sunlight. Similar behaviour was observed with the measurements of rETR, and the initial slope (αETR) of the photosynthesis curve, while the saturation irradiance parameter (Ik) increased about four-fold compared to the morning value. These changes were accompanied by a higher induction of the diadinoxanthin-cycle pigments, evidenced by an increasing ratio of diatoxanthin to diadinoxanthin during the day (up to 104% at 1400 h), and a higher value of non-photochemical quenching (NPQ) that rose to up to 1.2 in the high stress culture. The culture grown in the open pond at the same biomass concentration (0.3 g l−1) did not show sizeable changes either in pigments or in chlorophyll fluorescence parameters. Denser cultures in PBRs (0.6 g l−1) showed less dramatic changes in the photochemical parameters. Changes in the areal productivity of the cultures correlated well with changes in the photochemical parameters. Areal productivity was about 27% higher in the open pond than in PBRs when cultivated at the same concentration of 0.3 g l−1.
Introduction
Among microalgae, the diatom Phaeodactylum tricornutum is one of the most studied species. This diatom is considered an important potential source of eicosapentanoic acid (EPA), an essential polyunsaturated fatty acid, and also of carotenoids for human consumption and aquaculture animal feeds (Chrismadha & Borowitzka, Citation1994; Lebeau & Robert, Citation2003a , Citation2003b ). Recently, Phaeodactylum has been considered among potential candidates for biodiesel production (Chisti, Citation2007; Mata et al., Citation2010).
The organization of the photosynthetic apparatus of diatoms differs in some respects from that of green algae and higher plants. In diatoms, the thylakoid membrane arrangement differs remarkably from that of higher plants because there is no differentiation into PSII-rich grana and PSI-rich stroma thylakoids (Owens, Citation1988). Instead, the thylakoids span the whole length of the plastids in bundles of three thylakoids each, and no segregation of PSs has been detected so far. Moreover, no special PSI or PSII antenna has been found (Owens & Wold, Citation1986; Brown, Citation1988; Berkaloff et al., Citation1990; Wilhelm et al., Citation2006). Diatoms have a specific set of pigments, including chlorophyll c, fucoxanthin and diadinoxanthin; diadinoxanthin can be de-epoxidized to diatoxanthin. Relative to chlorophyll a, the xanthophyll concentration can be two to four times higher than in higher plants (Wilhelm, Citation1990). In the protection of plants and microalgae from excess light, the conversion of epoxy-xanthophylls plays an important role. There is evidence that de-epoxidized xanthophylls dissipate excess excitation energy, mainly in the light-harvesting antenna of PSII (Demmig-Adams & Adams, Citation1996). Two different types of reversible de-epoxidation have been described: (1) the so-called violaxanthin cycle, in which violaxanthin (Vx) is de-epoxidized to antheraxanthin (Ax) and finally to zeaxanthin (Zx) in a two-step, high light-induced reaction (Yamamoto et al., Citation1962); and (2) the diadinoxanthin cycle, which includes the de-epoxidation of diadinoxanthin (Ddx) to diatoxanthin (Dtx) in a single-step process (Hager, Citation1980). The de-epoxidation of xanthophylls leads to enhanced dissipation of excess excitation energy as heat in the photosystem II (PSII) antenna, thereby preventing inactivation and damage to the photosynthetic apparatus (Demmig-Adams, Citation1990; Masojídek et al., Citation1999). The process can be monitored as non-photochemical quenching of chlorophyll fluorescence. The violaxanthin cycle is present in higher plants, green algae and brown algae, whereas the diadinoxanthin cycle can be found in diatoms, dinophytes and haptophytes. However, Phaeodactylum possesses both the Ddx and Vx cycles (Lohr & Wilhelm, Citation1999). De-epoxidation of epoxy-xanthophyll molecules takes place under conditions of high irradiance and reverses under low light intensities or in darkness.
The switching from the heat-dissipation state back to the light-harvesting state is regulated differently in violaxanthin- and diadinoxanthin-cycle organisms. In green algae and vascular plants, disaggregation of the light-harvesting complex (i.e. the return to the light absorption state) is triggered by the fast breakdown of the high light-driven proton gradient, whereas in diatoms fucoxanthin-chlorophyll protein (FCP) aggregation (i.e. the heat-dissipating state) is reverted by the fast epoxidation of Dtx to Ddx, which is mandatory due to the high quenching capacity of Dtx even in the absence of the ΔpH (Goss et al., Citation2006; Wilhelm et al., Citation2006; Goss & Jakob, Citation2010). This means that a fast conversion of the PSII antenna from the heat-dissipation state back to the light-harvesting state can be attained only by efficient removal of the quenching pigment diatoxanthin (Goss & Jakob, Citation2010).
Since the mid-1990s, a significant contribution to microalgal biotechnology has been achieved by the introduction of modern photobiochemical methods, mainly measurement of chlorophyll (Chl a) fluorescence. By using this method, it is possible to monitor the photosynthetic activity of microalgae cultures in outdoor systems (Torzillo et al., Citation1998; Kromkamp et al., Citation2009; Sukenik et al., Citation2009): it is fast, non-invasive and easy to measure. At present, two main chlorophyll-fluorescence approaches have been used to monitor PSII photosynthetic performance in microalgal mass cultures: the fast fluorescence induction kinetics and the saturation-pulse method (Strasser et al., Citation1995; Schreiber, Citation2004).
In this work we carried out experiments outdoors with mass cultures of Phaeodactylum grown at various biomass concentrations, comparing two outdoor cultivation systems – a closed tubular photobioreactor and an open circular pond – with the aim of following changes in photosynthetic activity, photoprotective mechanisms and productivity.
Materials and methods
Organism and culture conditions
The diatom Phaeodactylum tricornutum UTEX 640 was obtained from the culture collection of the University of Texas, Austin. The cells were grown in artificial sea water (Adriatic Sea Aquarium & Equipment, Rimini, Italy) supplemented with f/2 salts (Guillard & Ryther, Citation1962), while for mass culture the supplements were increased to: 1 g l−1 NaNO3, 36 mg l−1 NaH2PO4, 247 mg l−1 Na2HPO4 and 60 mg l−1 Na2SiO3.9H2O. The inoculum for the outdoor facilities was grown in the laboratory under a photon irradiance of 70 µmol m−2 s−1 supplied from both sides of glass columns (i.d. = 50 mm; 400 ml working volume). Mixing of the cultures was provided by bubbling with a mixture of air and CO2 (97 : 3, v/v) at a continuous flow rate of 5 l min−1. Subsequently, cultures were scaled up in 5- and 8-l Pyrex bottles (20-cm diameter), illuminated from both sides with a photon irradiance of 300 µmol m−2 s−1. About 25 l of laboratory culture were used to inoculate the outdoor systems. The culture medium for dilution of outdoor cultures was prepared from deionized water, and sterilized by means of both filtration and UV treatment.
Outdoor culture management
The photobioreactor (PBR) used for growth experiments with P. tricornutum consisted of 10 parallel Pyrex glass tubes (length 2 m, i.d. 4.85 cm) connected by PVC U-bends to a 23-m loop (Torzillo et al., Citation1996). The area occupied by the photobioreactor (i.e. diameter by length) was 1 m2 and the corresponding surface-to-volume ratio was 19.4 m−1. The tubes were placed horizontally in a stainless-steel basin for temperature control. Culture temperature was maintained at 20°C ± 1°C. The culture was circulated by a centrifugal PVC pump equipped with three stainless-steel flat blades at an angle of 120° to each other on the propeller shaft. The distance between the blades and the casing was 0.5 cm. At the end of the loop, the culture flowed into a 2.2-l transparent PVC cylindrical degasser (i.d. 10 cm, height 28 cm). The degasser contained several inputs for fresh medium addition, air and CO2 bubbling, and culture sampling.
A PVC circular pond of 1 m diameter was used for outdoor experiments. The culture depth was maintained at 10 cm and a centrally pivoted rotating PVC arm (96 cm long; 6 rpm) was used for mixing the culture and supply of air/CO2 mixture. The surface-to-volume ratio was 10 m−1. The culture temperature was maintained at 20°C ± 1°C by means of cold water circulated in a stainless steel coil placed in the bottom of the pond and connected to the water cooling system.
Cultures were grown in a semi-continuous regime. Each morning, a part of the culture volume was withdrawn from the outdoor units and replaced with fresh medium to adjust biomass concentration to the set values of 0.3 and 0.6 g DW l−1, for the photobioreactors, and 0.3 g l−1 for the open pond. The pH of the cultures was maintained at a constant value of 7.2 by automatic CO2 addition through sand-stone spargers.
Analytical procedures
Dry weight (DW) was measured in duplicate using 10-ml samples taken from the outdoor cultures in the morning and at the end of the day. Chlorophyll and total carotenoids were determined spectrophotometrically in 90% acetone (Lichtenthaler, Citation1987). The concentrations of individual carotenoids were assessed using a reversed-phase Beckman System Gold HPLC (module 125 solvent) equipped with a diode array detector, model 168 Nouveau (Beckman Instruments, CA, USA), with a C8, 100 A column (model Luna, Phenomenex Inc., Torrance, CA, USA), according to Van Heukelem & Thomas (Citation2001). Daily growth rate (µ, d−1) of cultures was calculated as: ln x 1 − ln x 0 /Δt, where x 1 and x 0 (g l−1), are the dry weights measured at the end and at the beginning of the day, and Δt is the time interval between the two measurements (days). The areal yield (g m−2 d−1) was calculated as (x 1 − x 0) × V/S, where x 1 and x 0 (g l−1) are as above, V (m3) is the culture volume, and S (m2) the surface area occupied by the culture. For the tubular photobioreactors as well as circular pond, S was 1 m2.
Fluorescence measurements
Chlorophyll fluorescence of culture samples taken at 1-h intervals from the outdoor cultures was measured using a portable pulse-amplitude-modulation fluorometer (PAM-2500, H. Walz, Effeltrich, Germany) and expressed following the nomenclature by van Kooten & Snel (Citation1990). For Fv/Fm determination, 0.5-ml samples were taken from the outdoor units (PBR and pond), and dark-adapted for 15 min. Minimum PSII fluorescence (F0) was measured with a low-intensity modulated measuring beam (red LEDs; 0.3 µmol m−2 s−1). Measurements of chlorophyll fluorescence were carried out in an ED-101US/M optical compartment (H. Walz, Effeltrich, Germany).
For the in-situ fluorescence measurements the fibre-optics of the fluorometer (PAM-2100, H. Walz, Effeltrich, Germany) were placed perpendicular to the glass wall of the culture tube in the illuminated section of the photobioreactor, as reported by Torzillo et al. (Citation1998). For the measurement of fluorescence in the culture pond, the fibre-optic tip was inserted in a glass test-tube and placed in the culture close to the surface at an angle of 45°. Prior to the F0 and Fm measurements the cultures were covered with a black plastic sheet, and the spot of culture involved in the fluorescence measurement was previously exposed to far-red light provided by the PAM-2100. Measurements of steady-state (Fs) and maximum fluorescence in the light-state, using ambient light (), were performed at 1-h intervals. The fraction of absorbed light utilized in electron transport was given by the effective PSII quantum yield, ΔF/
= (
− Fs)/
(Genty et al., Citation1989). The relative electron transfer rate (rETR) of the cultures during the day was calculated as:
The non-photochemical quenching coefficient (NPQ) was calculated according to the Stern–Volmer equation, NPQ = (Fm − )/
(Bilger & Björkman, Citation1990). In order to compensate for the increase in Fm due to the increase in chlorophyll concentration during the day, the Fm values were recalculated according to Fm = F0/(1 − Fv/Fm), where Fv/Fm is the maximum value measured in the morning, and F0 is that recorded at different times of the day (Torzillo et al., Citation1998).
We used the non-linear least-squares regression model by Eilers & Peeters (1988) to fit the rETR/PFD curves performed on culture samples taken from the outdoor cultures, and to estimate the maximum electron transport rate (rETRmax), αETR (i.e. the initial slope of the curve), which is proportional to the maximum light utilization efficiency. The Ik (i.e. the saturation irradiance) was given as an intercept between αETR and rETRmax.
Chlorophyll a fluorescence transients were recorded with a Handy-PEA (Hansatech Instruments, UK) in 2-ml dark-adapted samples measured under continuous light (650 nm peak wavelength, 3500 µmol photons m−2 s−1 light intensity), which was provided by light-emitting diodes (LEDs). Each chlorophyll-a fluorescence induction curve was analysed using Biolyzer HP3 software in accordance with the so-called JIP-test (Strasser et al., Citation1995). Normalized chlorophyll fluorescence data for both F0 and Fm, were calculated as relative variable fluorescence Vt = (Ft − F0)/(Fm − F0) at all times. From the fluorescence measurements, the variable fluorescence at the J step, VJ = (FJ − F0)/(Fm − F0) was calculated. This parameter is useful to evaluate the level of the PQ pool reduction, as the increase of the fluorescence intensity at the J-step level is related to the accumulation of electrons at the QA level (Strasser et al., Citation1995; Appenroth et al., Citation2001).
Using a dual-modulation fluorometer (FL-200/PS, P.S.I., Brno, Czech Republic) (Trtílek et al., Citation1997), the relaxation kinetics of the variable fluorescence yield (QA reoxidation) were measured in the 10 µs to 10 s time range after single-turnover pulse excitation to obtain information on PSII donor- and acceptor-side processes. The instrument was fitted with red LEDs for both actinic (20 µs) and measuring (2.5 µs) flashes with cells diluted to a concentration of 2 µg Chl ml−1. Analysis of the fluorescence relaxation kinetics was based on the widely used model of the two-electron gate (Diner, Citation1998). According to this model, the fast (few hundred microseconds) decay component reflects QA reoxidation via forward electron transport in the centres that contain bound PQ (in the oxidized or semi-reduced form) at the QB site before the flash. The middle phase (few milliseconds) component arises from QA reoxidation in the centres that have an empty QB site at the time of the flash and have to fill the pocket from the PQ pool. Finally, the slow-phase (few seconds) component reflects QA reoxidation via back reaction with the S2 state of the water-oxidizing complex. Both the open pond and the PBRs were shaded in the evening in order to start morning measurements with dark-acclimated cultures.
Results
The photoacclimation of P. tricornutum cultures grown in both the open pond and in tubular photobioreactors was studied during the day by means of growth, chlorophyll fluorescence and pigment analysis. The behaviour of cultures grown in photobioreactors (PBRs) at two biomass concentrations (0.3 and 0.6 g l−1) was compared with the one in an open pond (0.3 g l−1).
Chlorophyll fluorescence quenching changes during the day
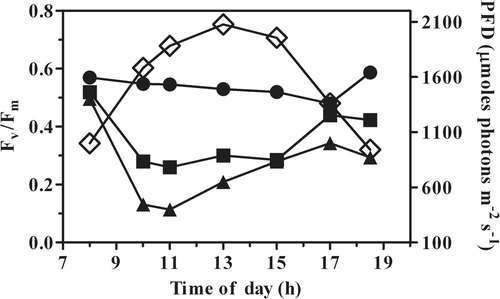
shows the daily time course of the Fv/Fm ratio of the cultures. In the morning, it was about 0.5 in the culture grown in the PBRs, whereas the value was 0.58 in the open pond. In the open pond, the Fv/Fm ratio showed only small changes during the day, whereas in the PBR-grown cultures it dropped significantly as soon as the cultures were exposed to solar light, particularly in the culture grown at the lower biomass concentration (0.3 g l−1). In this culture, the Fv/Fm ratio decreased up to 0.1 in about one hour of exposure to high light. By 1900 h, the pond culture fully recovered the morning Fv/Fm value, the higher-biomass PBR (0.6 g l−1) culture recovered up to 81%, while the other PBR culture (0.3 g l−1) recovered only 60%, indicating that this culture was subjected to a substantial inhibition of PSII activity.
Fig. 1. Time course of the Fv/Fm ratio in P. tricornutum outdoor cultures, in an open pond and tubular photobioreactors (PBR) grown at different biomass concentrations (•, pond 0.3 g l−1; ▪, PBR 0.6 g l−1; ▴, PBR 0.3 g l−1). ◊, PFD (photon flux density, µmol m−2 s−1).
Samples were taken at different times of the day and light-response curves were measured using chlorophyll fluorescence in order to calculate basic parameters, such as the relative rETRmax, the initial slope αETR, and the saturating irradiance IK. The values of rETRmax recorded in the morning were generally lower, due to the well-known downregulation of enzyme activity during darkness. However, during the course of the day the rETRmax of the pond culture was significantly higher than those measured in the PBR cultures (). Moreover, in the open-pond culture, rETR showed a clear increase at midday, when light availability for cells was higher. The lowest rETRmax was attained by the PBR culture grown at the lower biomass concentration (0.3 g l−1), whereas the culture grown at 0.6 g l−1 showed a course similar to the open-pond culture.
Fig. 2. Changes in the maximum relative electrons transport rate (rETR) in P. tricornutum grown outdoors at different biomass concentrations (•, pond 0.3 g l−1; ▪, PBR 0.6 g l−1; ▴, PBR 0.3 g l−1). Samples were taken from the outdoor cultures at the time of day indicated.
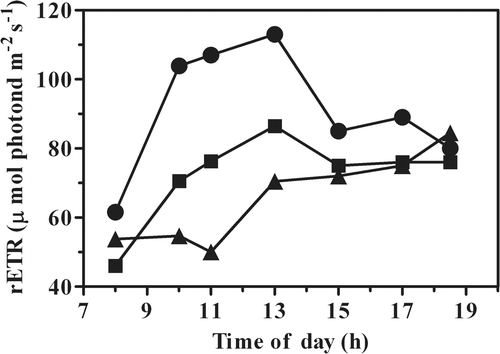
The initial slopes αETR calculated from the light-response curves during the day showed behaviour similar to that observed with Fv/Fm ( vs ): the highest values of maximum photochemical efficiency were attained by the culture in the open pond; the lowest in the PBR culture at cell density of 0.3 g l−1. The highest daily values in the initial slope of all the cultures were found during the morning and late afternoon, when the light intensity was lower ().
Fig. 3. Changes in the initial slope of the light response curve, α ETR, in P. tricornutum outdoor cultures, in an open pond and tubular photobioreactors (PBR) at different biomass concentrations (•, pond 0.3 g l−1; ▪, PBR 0.6 g l−1; ▴, PBR 0.3 g l−1).
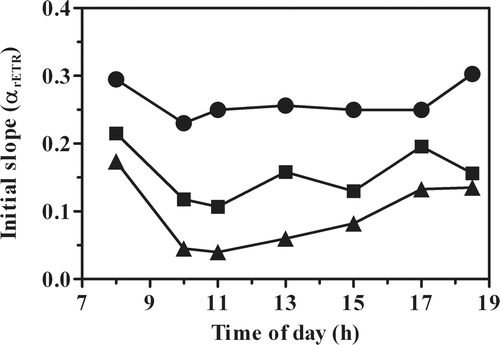
The behaviour of the light saturation parameter IK was opposite to Fv/Fm and the initial slope (compare with and ). In fact, IK was highest in the cultures grown in the PBR at lower density, showing a four-fold increase within 1 h, just after the cultures were exposed to bright morning light. In this culture, IK remained high until 1500 h (between 1000 and 1200 µmol photons m−2 s−1) and declined by half in the late afternoon. In the culture grown in the open pond, IK kept steady between 200 and 400 µmol photons m−2 s−1, while in the cultures grown in the PBR at higher biomass concentration, Ik increased three-fold. In the afternoon the Ik of the cultures generally recovered. However, at the time of the last daily measurement (1900 h), recovery was incomplete, except for the open-pond culture, as the incident light irradiance was still high enough to saturate photosynthesis (about 900 µmol m−2 s−1).
Fig. 4. Time course of the saturation irradiance, Ik, in P. tricornutum outdoor cultures, in an open pond and tubular photobioreactors (PBR) at different biomass concentrations(•, Pond 0.3 g l−1; ▪, PBR 0.6 g l−1; ▴, PBR 0.3 g l−1).
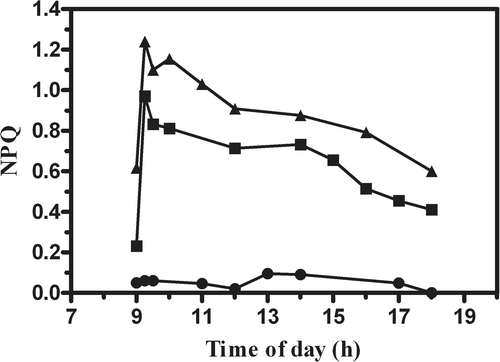
The daily time course of NPQ in the outdoor cultures is shown in . A sharp increase in the NPQ value was observed as soon as the cultures were exposed to sunlight, particularly those grown in the PBRs. In fact, particularly in the one maintained at the lower biomass concentration, NPQ increased from values close to zero (dark condition) up to 1.24 within 25 min of exposure to sunlight. Similar behaviour was also observed in the higher-density PBR culture, in which NPQ increased to 1.0. NPQ remained high in the PBR cultures, particularly in the one at lower density, while no relevant changes were observed for the pond culture.
Chlorophyll a fluorescence transient changes during the day
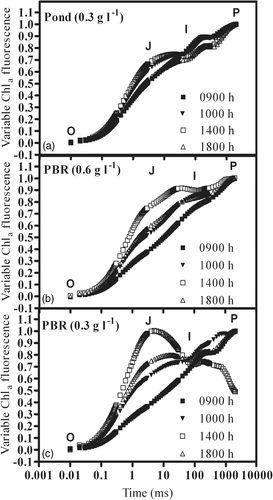
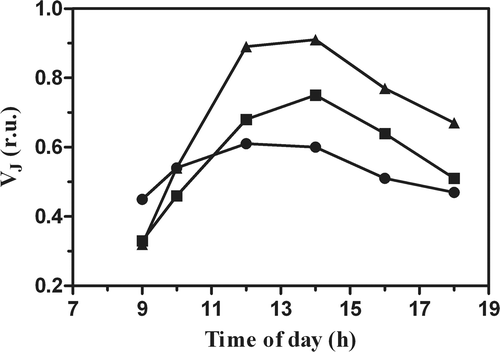
During the exposure to bright light, the chlorophyll a fluorescence transients and the analysis of the JIP-test parameter, VJ, revealed differences in the PSII performance, exhibiting strong effects at 1400 h, particularly in the cultures grown in photobioreactors (). In particular, the transients of the PBR cultures at 0.6 g l−1 () and 0.3 g l−1 () exhibited an increase at the J-step level, indicating that the exposure to the sunlight reduced the capability to transfer electrons behind QA, due to the accumulation of reduced QA in the PSII core. This was reflected by changes in the VJ parameter, which increased in all the cultures during exposure to bright light (). The highest values of VJ were observed at 1400 h, increasing by 184% and 127% in the culture grown at 0.3 g l−1, and 0.6 g l−1 in the PBR, respectively, whereas it increased by only 25% in the pond culture. In agreement with the transients, at the end of the day the VJ of the culture in the pond exhibited a full recovery, declining to the value of the morning, whereas the PBR cultures showed only a partial recovery, by 65% and 40%, respectively, for cultures grown at 0.6 g l−1 and 0.3 g l−1.
QA reoxidation changes
The QA reoxidation kinetics measurements carried out in Phaeodactylum cultures taken from the PBRs showed significant differences compared to those taken from the pond where the course of the QA relaxation kinetics did not change during the day, showing the course of low-irradiance acclimated cultures (). In contrast to the pond culture, the course of the relaxation kinetics in the 0.6 g l−1 PBR showed a significant inhibition of QA reoxidation at 1100 h, with a partial recovery at 1700 h as compared to the morning sample at 0800 h (). In the low biomass density culture (0.3 g l−1), an even greater inhibition of electron transport processes was observed at 1100 h and recovery of QA reoxidation activity at 1700 h was much less than in the denser culture ( vs ). In the morning (0800 h) the QA relaxation kinetics were similar in all samples and dominated by the fast (315–348 µs) component (). The contribution of the middle (3 ms) phase was 10–16% and that of the slow phase was 7–16% (). At 1100 h, the analysis of QA reoxidation kinetics of both PBR cultures showed mainly an increase of the t 1/2 of the fast phase, to about 407 and 435 µs, respectively, indicating a slower electron transfer from QA to bound QB, which might be the consequence of QB site modification. The amplitude of the fast phase was much higher (64%) in the denser PBR culture, compared to 27% in the more dilute one. In contrast, the amplitude of the middle phase was doubled (44%) in the dilute culture, compared to the denser one (18%). In the pond, the culture was least influenced by high irradiance at midday, since the t 1/2 of the fast phase increased only slightly, from 325 to about 360 µs, with a relative amplitude of 78%. The QA reoxidation kinetics showed that the reaction centres were photoinhibited in the cultures exposed in both PBRs, since the complete recovery of electron transport did not happen by 1700 h in all cultures.
Table 1. Changes in QA reoxidation parameters in outdoor cultures of P. tricornutum in an open pond and tubular photobioreactor (PBR) grown at different biomass concentrations.
Pigment changes
The pigment composition at the start of the experiment, after acclimation to ambient irradiance, appeared different among the cultures. The highest initial pool of diadinoxanthin (Ddx) and diatoxanthin (Dtx) was found in the PBR cultures, while the highest amounts of the main carotenoid, fucoxanthin, were found in the pond culture, most likely as a result of increased light-harvesting capacity under low-light acclimation (). Fucoxanthin showed a decline of about 20% during the day in all the cultures, which might be the result of acclimation to high irradiance. This would agree with the low content of Dtx, which is a part of the irradiance-protective cycle, in the morning. No detectable amounts of zeaxanthin (Zx) and antheraxanthin (Ax) were found in the morning cultures.
Fig. 8. Time course of QA reoxidation kinetics during the day in P. tricornutum cultures grown outdoors in an open pond and tubular photobioreactors.
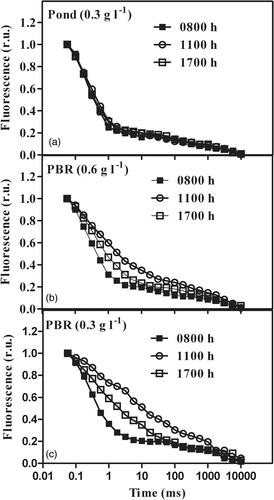
Table 2. Changes in pigment composition in P. tricornutum outdoor cultures at different times in an open pond and in tubular photobioreactors (PBR) at two biomass concentrations. The pigment content is reported as mmol mol−1 Chl a.
As soon as the cultures were exposed to bright sunlight, a rapid increase occurred in the conversion of Ddx to Dtx, particularly in the cultures grown in the PBRs. During the first 15 min of exposure to high irradiance, Dtx increased at a rate of 2.9 mmol (mol Chl a)−1 min−1 in the more dilute PBR culture (0.3 g l−1), and at a rate of 0.62 mmol (mol Chl a)−1 min−1 in the denser one (0.6 g l−1), while in the pond culture, the rate of increase of Dtx was only 0.34 mmol (mol Chl a)−1 min−1. In all the cultures, the maximum amount of Dtx was found at around 1400 h, particularly in the PBR culture grown at low biomass density, where it reached 285 mmol (mol Chl a)−1 (). It was also observed that, during the day, the Ddx+Dtx pool increased considerably, particularly in the low concentration culture, by about 40% compared with the morning value. A sizeable increase in the pool was also observed in the denser PBR culture (by about 17% compared with morning value), while in the pond culture the pool remained almost unchanged ().
The de-epoxidation status (DES), expressed as the Dtx : (Ddx + Dtx) ratio, increased up to 0.51 at midday, most significantly in the less dense PBR culture, indicating that more than a half of the Ddx + Dtx pool was represented by Dtx (). The lowest DES ratio was observed in the pond culture, where it was about one order of a magnitude lower than in PBR cultures.
Although at a much lower extent, Ax and Zx, which are part of the violaxanthin cycle, were detected at midday, particularly in the less dense PBR culture, at 21.5 mmol (mol Chl a)−1, while no detectable amounts of Zx were found in the pond culture. At noon, the ratio between the Vx + Ax + Zx and Ddx + Dtx pools was fairly constant, ranging between 3.2% and 4.8%, and the interconversion trends of both cycles were similar. The DES of violaxanthin-cycle pigments (Ax + 0.5 Zx/Vx + Ax + Zx), was higher than that of the diadinoxanthin cycle, but did not show any appreciable change over the day, most likely as a result of a simultaneous synthesis of Vx. Higher values were found in the low-density PBR culture. The results indicated that de novo synthesis of Vx and of its de-epoxidized forms occurred in all the cultures exposed to high irradiance.
Productivity of cultures
The productivity of cultures in PBRs at two biomass concentrations (i.e. 0.3 and 0.6 g l−1) was compared to that of a culture grown in an open pond (0.3 g l−1). However, in the latter culture, being 10-cm deep, the areal density (30 g DW m−2) corresponded to the culture grown in PBR at 0.6 g l−1 (5-cm tube diameter). Therefore, the cultures grown in photobioreactors could be compared to that grown in an open pond, both on the basis of biomass concentration (0.3 g l−1), as well as on the basis of areal density (30 g DW m−2).
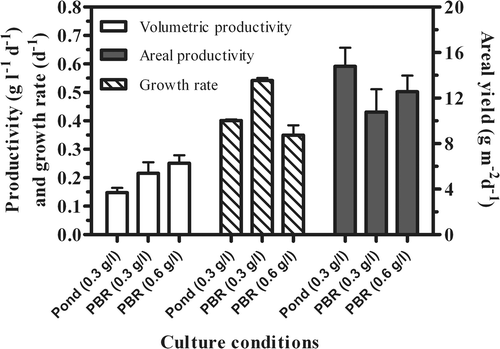
Areal productivity (based on footprint area occupied by cultivation unit) of the cultures grown in PBRs decreased (by 27% and 15%, respectively, for the lower and higher densities) compared to the pond, where it reached 14.8 g DW m−2 d−1 (). However, higher areal productivity (18 g DW m−2 d−1) was attained in the PBR when the initial biomass concentration was increased to 1.2 g l−1, i.e. at its optimum value (results not shown). Instead, the highest growth rate (0.542 d−1) was attained by the low-density PBR culture, and was 26% and 35% higher than in the open pond and denser PBR cultures.
Fig. 9. Comparison of volumetric and areal productivities and growth rates of P. tricornutum outdoor cultures grown in an open pond and in photobioreactors at different biomass concentrations.
Comparison between areal biomass yield and daily integrated relative electron transport rate measured in situ is shown in . Changes in the daily integrated values of the rETR correlated well with analogous changes in the daily areal productivity of the pond and denser PBR cultures, while the discrepancy between rETR and areal productivity increased in the more dilute PBR culture.
Table 3. Comparison between areal biomass yields and daily integrated values of the relative electron transport rates (ΔF/ × PFD) measured in situ in P. tricornutum cultures grown outdoors in an open pond and photobioreactors. Data are means ± SD (n = 3).
× PFD) measured in situ in P. tricornutum cultures grown outdoors in an open pond and photobioreactors. Data are means ± SD (n = 3).
Biochemical composition of the cells
The biochemical composition of the biomass grown in the open pond and PBRs did not differ much. Protein content was above 50% in both pond and PBR cultures. Lipid content was close to 25%, while carbohydrates were about 17%. On the whole, because the cultures were nutrient-replete, the chemical composition of the resulting biomass was well balanced.
Analyses of fatty acid composition were performed on biomass samples harvested from the open-pond and PBR cultures (). As expected, the predominant fatty acid was EPA, which reached 35% of total fatty acids. Biomass grown in the open pond showed a slightly higher ratio of saturated to unsaturated fatty acids, compared to that grown in PBRs. The difference was due to a higher presence of myristic and stearic acids in the biomass grown in the open pond.
Table 4. Fatty acid composition of P. tricornutum biomass grown outdoors in an open pond and in tubular photobioreactors at different biomass concentrations.
Discussion
In outdoor conditions microalgae have to cope with continuously changing environmental conditions, particularly irradiance, which can fluctuate from limiting to photoinhibitory conditions within hours or days. Hence, to cope with rapid changes in irradiance, microalgae have developed fast photoprotective mechanisms against excess light. In mass culture of microalgae, the necessity for acclimation to high light is very dependent on their biomass concentration, culture depth and rate of mixing (Vonshak & Torzillo, Citation2004). Contrary to what has been usually reported (Torzillo et al., Citation1996, Citation1998; Vonshak et al., Citation2001), the Fv/Fm pattern was not symmetric to that of the solar irradiance recorded during the day. In fact, minima of the Fv/Fm ratio were found between 1000 h and 1100 h in PBR cultures, i.e. within a couple of hours of the peak daily light irradiance. This hysteresis in the Fv/Fm pattern must be related to the sudden increase of irradiance to which cultures were exposed in the morning: the black covers of the cultures were removed at 0900 h, and at this time the photon irradiance was already about 1200 µmol m−2 s−1. The cultures experienced photodamage to PSII, particularly in PBR cultures, before they could mobilize substantial amounts of photoprotective pigments. Thereafter, the cultures started to recover as they acclimated to the increasing photon irradiance.
In general it was observed that the changes in photosynthetic parameters monitored by chlorophyll fluorescence measurements were in line with culture productivity. However, some influence on photosynthetic parameters due to variation in the chlorophyll optical cross-section cannot be ruled out (Kromkamp et al., Citation2009). Indeed, during the acclimation to high light, cells may increase their chlorophyll optical cross-section, particularly in dilute cultures, and this may reduce the differences between the pond, in which we can expect a lower chlorophyll optical cross-section, and PBR cultures, although the daily pattern would not be substantially modified. An increase in the chlorophyll optical cross-section, particularly in the 0.3 g l−1 PBR cultures, may have affected the correlation between changes in areal productivity and the integrated electron transport rate. Indeed, the correlation between the pond and 0.6 g l−1 PBR cultures was high, whereas the integrated rETR of the 0.3 g l−1 PBR culture overestimated the reduction in the areal productivity by about 10%. Comparisons of the Fv/Fm, QA reoxidation and induction curves of the pond and low-density PBR cultures indicate that the former was photolimited while the latter was oversaturated by light. The denser PBR culture showed an intermediate behaviour. However, the midday depression of maximum PSII photochemical yield (Fv/Fm) of about 20% in the open-pond culture (compared to morning values) was characteristic of cultures that were performing well, while lower or higher depression of Fv/Fm indicated low-light acclimated or photoinhibited cultures, respectively (Masojídek et al., Citation2011).
The violaxanthin cycle plays a major role in the photoprotection mechanisms of higher plants and some microalgae. The diatoms (Bacillariophyceae) operate a different xanthophyll cycle, the so-called diadinoxanthin cycle (Goss & Jacob, Citation2010). This cycle represents the most important photoprotection mechanism of diatoms and leads to an efficient dissipation of excessively absorbed energy as heat, which is evidenced by a strong increase in NPQ (Lavaud et al., Citation2002; Goss et al., Citation2006). However, strong NPQ induced by diatoxanthin, can pose a serious problem in diatoms grown in mass culture once the light-harvesting system has to be switched back from a heat-dissipating state to a light-harvesting state. In vascular plants and green algae, this rearrangement of the antenna system is regulated by ΔpH, and the immediate decrease of the proton gradient after change from high light to low light leads to a very fast relaxation of NPQ; Zx loses its ability to quench non-photochemically and does not interfere with light-harvesting. Conversely, in diatoms, diatoxanthin remaining after transfer of cells to low light (or darkness) is still able to quench non-photochemically, even in the complete absence of a proton gradient (Goss et al. Citation2006; Goss & Jakob, Citation2010). Diatoxanthin epoxidation, although very fast, may require 2–3 min. In outdoor mass cultures, the maintenance of high non-photochemically quenching capacity may be advantageous upon transfer of cells from the bottom layers (in low light or darkness) to upper ones during re-exposure of cells to strong irradiance, but it may cause loss of photosynthetic capacity during the opposite movement, when cells are subjected to increasing photo-limitation. This sort of de-synchronization between light irradiance and the energy state of the antenna system may cause loss of productivity. It is therefore necessary to find an optimal combination of cell concentration and mixing rate that does not activate photoprotection mechanisms but at the same time prevents acclimation of cells to low light, which can occur in poorly mixed high biomass concentration grown cultures. On either side of this optimum reduced productivity will result (Masojídek et al., Citation2011).
In our experiments, the Phaeodactylum cultures showed a clear induction of both violaxanthin and diadinoxanthin cycles. This was particularly evident in the PBR cultures, which showed clear evidence of a situation of stress (especially in the less dense one), resulting in a rapid and significant reduction in the Fv/Fm ratio, which was mirrored by a lower light-use efficiency (lower initial slope αETR of photosynthesis curve), lower rETR and high light-saturation irradiance (Ik). These modifications were also accompanied by changes in the shape of the chlorophyll fluorescence transients. In fact, the transients of the cultures measured after exposure of cultures for 1 h to bright light revealed an increased J-step in the curve of the PBR low-density culture, with respect to the one grown in the pond. This resulted in a reduced capability to transfer electrons beyond QA and thus in an accumulation of QA, in the photosynthetic apparatus. This finding was well reflected by a higher value of VJ. Indeed, inflection at the J-step corresponds to the double reduction of the electron carriers Ph, QA and QB; due to the limitation of electron acceptance by QB, the increase of the fluorescence intensity at the J-step level usually occurs when cells are exposed to excessive light, which increases the degree of reduction of the PQ pool (Strasser et al., Citation1995; Kromkamp et al., Citation2009). The cultures grown in the open pond did not exhibit relevant changes.
We have previously shown that modified QA reoxidation curves due to prolonged over-excitation of the microalga Haematococcus can occur during the diel cycle when dilute cultures are grown outdoors (Torzillo et al., Citation2003). In the present experiments, we found similar behaviour of outdoor Phaeodactylum cultures, where the photobiochemical activity of diluted cultures was strongly inhibited due to the over-excitation of QA in the PSII centres. The slowing-down of the fast decay constant reflected a blocking of QA reoxidation via forward electron transport to the QB site. The strong increase in PSII sensitivity to high light, particularly in the cultures subjected to high excitation pressure (when grown at low cell concentration and reduced depth), corresponded well to the massive induction of the diadinoxanthin cycle, with increased conversion to diatoxanthin in this culture. In the pond culture, the diadinoxanthin cycle was only slightly induced, in accordance with lesser changes in the fluorescence transients and JIP-test parameters. The violaxanthin cycle was also significantly induced in the cultures experiencing high light stress, particularly in the dilute PBR culture, although to a lower extent than the diadinoxanthin cycle. This finding confirms the observation that Phaeodactylum possesses both photoprotective cycles (Lohr & Wilhelm, Citation1999). However, results for two diatoms (including Phaeodactylum: Olaizola &Yamamoto, Citation1994; Olaizola et al., Citation1994) and energetic calculations performed on isolated pigments (Frank et al., Citation1996), indicate that diatoxanthin mediates energy quenching less efficiently than zeaxanthin, but the lower efficiency might be compensated by the larger pool size of the diadinoxanthin cycle () compared to the violaxanthin cycle in higher plants. As a result, accumulation of zeaxanthin in Phaeodactylum may be an unavoidable byproduct, since the violaxanthin cycle pigments are obligatory precursors for synthesis of diadinoxanthin/diatoxanthin and the main light-harvesting pigment fucoxanthin (Lohr & Wilhelm, Citation1999; Wilhelm et al., Citation2006; Goss & Jakob, Citation2010). The fact that the violaxanthin cycle changed in proportion to the diadinoxanthin cycle during the day in our cultures supports this opinion. Further confirmation of the predominance of the diadinoxanthin cycle in the photoprotection of Phaeodactylum cells is given by the much faster activation of diatoxanthin (within 5 min or less) than of the violaxanthin cycle. In our outdoor experiments, the amount of zeaxanthin detected was an order of magnitude lower than in laboratory cultures exposed to constant high light (Lohr & Wilhelm, Citation1999), and the zeaxanthin cycle pool (Vx + Ax + Zx) was much lower (about a quarter), of that found in Scenedesmus quadricauda and other microalgal species (Masojídek et al., Citation2004). Moreover, it has also been suggested that diadinoxanthin is a better substrate for the de-epoxidase than violaxanthin, making it more favourable for short-term regulation (Lohr & Wilhelm, Citation1999; Jakob et al., Citation2001). These observations taken together allow us to conclude that the role of the violaxanthin cycle in outdoor cultures of Phaeodactylum may be negligible with respect to the diadinoxanthin cycle.
Exposure of the cultures to increasing light during the day caused an increased accumulation of diatoxanthin, resulting not from de-epoxidation of diadinoxanthin but rather from de novo diatoxanthin synthesis; this was particularly evident in the cultures experiencing high light stress (Olaizola et al., Citation1994). Correspondingly, the level of NPQ was higher in the cultures grown in the PBRs and exposed to higher light, compared to the open pond. Higher NPQ was found in cultures with higher diatoxanthin content. However, the highest level of NPQ was registered within 0.5 h of exposure to bright light in the morning, during which the Ddx + Dtx pool remained almost constant indicating that diatoxanthin was the result of diadinoxanthin de-epoxidation (Lavaud et al., Citation2004). A 40% increase in the Ddx + Dtx pool was observed after midday in the high stress culture which did not correspond to a proportional increase in NPQ. A similar situation has been found by Schumann et al. (Citation2007) during the shift from low light to high light of Phaeodactylum cultures. They proposed that additional Ddx + Dtx molecules, which are protein-bound but do not participate in NPQ, may serve as a sink for the synthesis of the light-harvesting pigment fucoxanthin in low-light periods following high-light illumination, as proposed by Lohr & Wilhelm (Citation1999). However, even in the culture subjected to high excitation pressure (PBR 0.3 g l−1), NPQ did not reach values above 1.25, which is not much different from the level recorded in microalgae grown under similar conditions by Masojídek et al. (Citation2004) and Sukenik et al. (Citation2009), but much lower than was reported by Lavaud et al. (Citation2002), who found values up to 10. However, such extreme values have been observed only under specific growth conditions using an intermittent light regime. Therefore, it is conceivable that such high values of NPQ are attained only with diluted cultures under strong light, probably in combination with fluctuating light patterns, e.g. a fast transition from high light to low light and vice versa.
Volumetric productivity was higher in the PBR cultures than in the open pond, but lower when the productivity of the cultures was compared on the basis of areal productivity (i.e. on the basis of the ground area occupied), because the PBR cultures received a higher amount of light per unit of volume, as their surface area to volume ratio was considerably higher. Growth rate is influenced by both cell concentration and culture depth, since these two parameters influence the light available per cell, and therefore the growth rates attained by the low density culture (PBR 0.3 g l−1) were higher than in the other cultures. The growth rates of the cultures grown in the open pond and in the PBR at 0.6 g l−1 slightly differed (by 14% in favour of the open-pond cultures), although the amount of biomass per unit of surface was similar (30 g m−2). It is conceivable that this difference was due to the fact that PBR cultures experienced stress because of their higher S/V ratio. The results of the fluorescence measurements and pigment composition support this conclusion. The areal biomass yield attained by the open pond surpassed that attained by the equivalent PBR culture (operated at the same concentration of 0.3 g l−1) by about 27%. The difference in areal productivity between the two units remained (about 15% higher in the open pond) even when the cultures were compared on the same areal densities (about 60 g DW m−2). However, the performance of the PBR cultures surpassed that of the open pond by 18% when the concentration was increased to 1.2 g DW l−1, that is, once the initial concentration in the PBR was close to optimal.
In conclusion our results show that Phaeodactylum cultures exposed to bright sunlight develop an efficient photoprotection system based substantially on the rapid induction of the diadinoxanthin cycle, which was quantitatively much higher than the violaxanthin cycle. The performance of the cultures was strongly affected by the biomass concentration used for their growth. High induction of the diadinoxanthin cycle was associated with high-irradiance stress, correlated with the increase of non-photochemical quenching, which resulted in lower productivity. We confirmed that chlorophyll fluorescence measurements provide timely information on the physiological status of microalgal cultures and useful indications of their biomass productivity.
Acknowledgement
The work was supported mostly by bilateral scientific agreement between the Czech Academy of Sciences and the National Research Council of Italy (CNR). Partial support was provided by the Czech Science Foundation, project 521/09/0656 and by the Ministry of Education, Youth and Health of the Czech Republic, project Algatech CZ.1.05/2.1.00/03.0110 (J.M., J.K., J.P.). Ana M. Silva is indebted to Consejo Nacional para Investigaciones Científicas y Tecnológicas (CONICIT) for a travel grant and to the University of Costa Rica (UCR) for enabling her to spend a year visiting the ISE-CNR of Florence (Italy) within the framework of the agreement between UCR and ISE-CNR.
References
- Appenroth , KJ , Stöckel , J , Srivastava , A and Strasser , RJ . 2001 . Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probed by OJIP chlorophyll a fluorescence measurements . Environmental Pollution , 115 : 49 – 64 .
- Berkaloff , C , Caron , L and Rousseau , B . 1990 . Subunit organization of PSI particles from brown algae and diatoms: polypeptide and pigment analysis . Photosynthesis Research , 23 : 181 – 193 .
- Bilger , W and Björkman , O . 1990 . Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis . Photosynthesis Research , 25 : 173 – 185 .
- Brown , J . 1988 . Photosynthetic pigment organization in diatoms . Journal of Phycology , 24 : 96 – 102 .
- Chisti , Y . 2007 . Biodiesel from microalgae . Biotechnology Advances , 25 : 294 – 306 .
- Chrismadha , T and Borowitzka , MA . 1994 . Effect of cell density and irradiance on growth, proximate composition and eicopentaenoic acid production of Phaeodactylum tricornutum grown in a tubular photobioreactor . Journal of Applied Phycology , 6 : 67 – 74 .
- Demmig-Adams , B . 1990 . Carotenoids and photoprotection in plants. A role for the xanthophyll zeaxanthin . Biochimica et Biophysica Acta , 1020 : 1 – 24 .
- Demmig-Adams , B. and Adams , W.W. 1996 . Xanthophyll cycle and light stress in nature: uniform response to excess direct sunlight among higher plant species . Planta , 198 : 460 – 470 .
- Diner , BA . 1998 . Photosynthesis: molecular biology of energy capture . Methods in Enzymology , 297 : 337 – 360 .
- Eilers , PH and Peeters , JCH . 1988 . A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton . Ecological Modelling , 42 : 199 – 215 .
- Frank , HA , Cua , A , Chynwat , V , Young , A , Gosztola , D and Wasielewki , MR . 1996 . The lifetimes and energies of the first excited singlet states of diadinoxanthin and diatoxanthin: the role of these molecules in excess energy dissipation in algae . Biochimica et Biophysica Acta , 1277 : 243 – 252 .
- Genty , B , Briantais , JM and Baker , NR . 1989 . The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence . Biochimica et Biophysica Acta , 990 : 87 – 92 .
- Goss , R and Jakob , T . 2010 . Regulation and function of xanthophyll cycle-dependent photoprotection in algae . Photosynthesis Research , 106 : 103 – 122 .
- Goss , R , Pinto , EA , Wilhelm , C and Richter , M . 2006 . The importance of a highly active and ΔpH-regulated diatoxanthin epoxidase for regulation of the PSII antenna function in diadinoxanthin cycle containing algae . Journal of Plant Physiology , 163 : 1008 – 1021 .
- Guillard , RRL and Ryther , JH . 1962 . Studies of marine planktonic diatoms. I Cyclotella nana Hustedt and Detonula confervacea Cleve . Canadian Journal of Microbiology , 8 : 229 – 239 .
- Hager , A . 1980 . “ The reversible, light-induced conversion of xanthophylls in the chloroplast ” . In Pigments in plants , Edited by: Czygan , FC . 57 – 79 . Stuttgart : Fisher .
- Jakob , T , Goss , R and Wilhelm , C . 2001 . Unusual pH-dependence of diadinoxanthin de-epoxidase activation causes chlororespiratory induced accumulation of diatoxanthin in the diatom Phaeodactylum tricornutum . Journal of Plant Physiology , 158 : 383 – 390 .
- Kromkamp , J , Beardall , J , Sukenik , A , Kopecký , J , Masojídek , J , Bergeijk , SV , Gabai , S , Shaham , E and Yamshon , A . 2009 . Short-term variations in photosynthetic parameters of Nannochloropsis cultures grown in two types of outdoor mass cultivation systems . Aquatic Microbial Ecology , 56 : 309 – 322 .
- Lavaud , J , Rousseau , B , van Gorkom , HJ and Etienne , AL . 2002 . Influence of the diadinoxanthin pool size on photoprotection in the marine planktonic diatom Phaeodactylum tricornutum . Plant Physiology , 129 : 1398 – 1406 .
- Lavaud , J , Rousseau , B and Etienne , AL . 2004 . General features of photoprotection by energy dissipation in planktonic diatoms (Bacillariophyceae) . Journal of Phycology , 40 : 130 – 137 .
- Lichtenthaler , HK . 1987 . Chlorophyll and carotenoids: pigments of photosynthetic membranes . Methods in Enzymology , 148 : 350 – 382 .
- Lebeau , T and Robert , JM . 2003a . Diatom cultivation and biotechnologically relevant products. Part I: Cultivation at various scales . Applied Microbiology and Biotechnology , 60 : 612 – 623 .
- Lebeau , T and Robert , JM . 2003b . Diatom cultivation and biotechnologically relevant products. Part II: Current and putative products . Applied Microbiology and Biotechnology , 60 : 624 – 632 .
- Lohr , M and Wilhelm , C . 1999 . Algae displaying the diadinoxanthin cycle also possess the violaxanthin cycle . Proceedings of the National Academy of Sciences USA , 96 : 8784 – 8789 .
- Masojídek , J , Torzillo , G , Koblížek , M , Kopecký , J , Bernardini , P , Sacchi , A and Komenda , J . 1999 . Photoadaptation of two members of the Chlorophyta (Scenedesmus and Chlorella) in laboratory and outdoor cultures: changes in chlorophyll fluorescence quenching and the xanthophyll cycle . Planta , 209 : 126 – 135 .
- Masojídek , J , Kopecký , J , Koblížek , M and Torzillo , G . 2004 . The xanthophyll cycle in green algae (Chlorophyta): its role in the photosynthetic apparatus . Plant Biology , 6 : 1 – 8 .
- Masojídek , J , Kopecký , J , Giannelli , L and Torzillo , G . 2011 . Productivity correlated to photochemical performance of Chlorella mass cultures grown outdoors in thin-layer cascades . Journal of Industrial Microbiology and Biotechnology , 38 : 307 – 317 .
- Mata , TM , Martins , A and Caetano , N . 2010 . Microalgae for biodiesel production and other applications: a review . Renewable and Sustainable Energy Review , 14 : 217 – 232 .
- Olaizola , M and Yamamoto , HY . 1994 . Short-term response of the diadinoxanthin cycle and fluorescence yield to high irradiance in Chaetoceros muelleri (Bacillariophyceae) . Journal of Phycology , 30 : 606 – 612 .
- Olaizola , M , LaRoche , J , Kolber , Z and Falkowski , PG . 1994 . Non-photochemical fluorescence quenching and the diadinoxanthin cycle in a marine diatom . Photosynthesis Research , 41 : 357 – 370 .
- Owens , TG . 1988 . “ Light-harvesting antenna systems in the chlorophyll a/c-containing algae ” . In Light-energy Transduction in Photosynthesis , Edited by: Stevens , CLR and Bryant , DA . 122 – 136 . Rockville , , Maryland : American Society of Plant Physiologists .
- Owens , TG and Wold , ER . 1986 . Light-harvesting function in the diatom Phaeodactylum tricornutum: isolation and characterization of pigment–protein complexes . Plant Physiology , 62 : 516 – 521 .
- Schreiber , U . 2004 . “ Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: an overview ” . In Chlorophyll a Fluorescence: A Signature in Photosynthesis. Advances in Photosynthesis and Respiration , Edited by: Papageorgiu , GC and Govindjee . 279 – 319 . the Netherlands : Springer .
- Schumann , A , Goss , R , Jakob , T and Wilhelm , C . 2007 . Investigation of the quenching efficiency of diatoxanthin in cells of Phaeodactylum tricornutum (Bacillariophyceae) with different pool sizes of xanthophyll cycle pigments . Phycologia , 46 : 113 – 117 .
- Sukenik , A , Beardall , J , Kromkamp , J , Kopecký , J , Masojídek , J , Bergeijk , SV , Gabai , S , Shaham , E and Yamshon , A . 2009 . Photosynthetic performance of outdoor Nannochloropsis mass cultures under a wide range of environmental conditions . Aquatic Microbial Ecology , 56 : 297 – 308 .
- Strasser , RJ , Srivastava , A and Govindjee . 1995 . Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria . Photochemistry and Photobiology , 61 : 33 – 42 .
- Torzillo , G , Accolla , P , Pinzani , E and Masojídek , J . 1996 . In-situ monitoring of chlorophyll fluorescence to assess the synergistic effect of low temperature and high irradiance stresses in Spirulina cultures grown outdoors in photobioreactors . Journal of Applied Phycology , 8 : 283 – 291 .
- Torzillo , G , Bernardini , P and Masojídek , J . 1998 . On-line monitoring of chlorophyll fluorescence to assess the extent of photoinhibition of photosynthesis induced by high oxygen concentration and low temperature and its effect on the productivity of outdoor cultures of Spirulina platensis (cyanobacteria) . Journal of Phycology , 34 : 504 – 510 .
- Torzillo , G , Goksan , T , Faraloni , C , Kopecký , J and Masojídek , J . 2003 . Interplay betwen photochemical activities and pigment composition in an outdoor culture of Haematococcus pluvialis during the shift from the green to red stage . Journal of Applied Phycology , 15 : 127 – 136 .
- Trtílek , M , Kramer , DM , Koblížek , M and Nedbal , L . 1997 . Dual modulation LED kinetic fluorometer . Journal of Luminescence , 72–74 : 597 – 599 .
- Van Heukelem , L and Thomas , CS . 2001 . Computer-assisted high performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments . Journal of Chromatography A , 910 : 31 – 49 .
- Van Kooten , O and Snel , JFH . 1990 . The use of chlorophyll fluorescence nomenclature in plant stress physiology . Photosynthesis Research , 25 : 145 – 147 .
- Vonshak , A and Torzillo , G . 2004 . “ Environmental stress physiology ” . In Handbook of Microalgal Cultures: Biotechnology and Applied Phycology , Edited by: Richmond , A . 57 – 82 . Oxford : Blackwell Publishing .
- Vonshak , A , Torzillo , G , Masojidek , J and Boussiba , S . 2001 . Sub-optimal morning temperature induces photoinhibition in dense outdoor cultures of the alga Monodus subterraneus (Eustigmatophyta) . Plant Cell and Environment , 24 : 1113 – 1118 .
- Wilhelm , C . 1990 . The biochemistry and physiology of light-harvesting processes in chlorophyll c-containing algae . Plant Physiology and Biochemistry , 28 : 293 – 306 .
- Wilhem , C , Büchel , C , Fisahn , J , Goss , R , Jakob , T , LaRoche , J , Lavaud , J , Lohr , M , Riebesell , U , Stehfest , K , Valentin , K and Kroth , PG . 2006 . The regulation of carbon and nutrient assimilation in diatoms is significantly different from green algae . Protist , 157 : 91 – 124 .
- Yamamoto , H , Nakayama , O and Chichester , CO . 1962 . Studies on the light and dark interconversions of leaf xanthophylls . Archives of Biochemistry and Biophysics , 97 : 168 – 173 .