Abstract
The freshwater cyanobacterium Cylindrospermopsis raciborskii spreads from tropical to temperate regions worldwide. This entails acclimation to varied light and temperature conditions. We studied the thermal and light acclimation of the photosynthetic machinery of C. raciborskii by monitoring alteration of the chlorophyll a and carotenoid content in German strains of C. raciborskii, in African and Australian strains of C. raciborskii, and in German strains of Aphanizomenon gracile, a native cyanobacterium belonging to the same order (Nostocales). Our results showed that temperate and tropical C. raciborskii strains did not differ in pigment acclimation to light and temperature. In contrast, the ratio of photoprotective carotenoids (namely the carotenoid glycoside 4-hydroxymyxol glycoside [aphanizophyll]) to chlorophyll a increased significantly more in C. raciborskii in comparison with A. gracile (1) with decreasing temperatures from 20 to 10°C and a moderate light intensity of 80 µmol photons m−2 s−1 and (2) with increasing light intensities at a suboptimal temperature of 15°C, compared to 20°C. We conclude that below 20°C photoinhibition is avoided by greater photoprotection in the invasive species C. raciborskii compared to the native species A. gracile.
Introduction
For the successful colonization of a new habitat, organisms need to exhibit considerable phenotypic plasticity, enabling rapid acclimation to a wide range of environments, or the capacity to adapt within a few generations to the new environment. Photosynthetic organisms that spread across latitudes are primarily affected by changes in the irradiance level and temperature.
Acclimation to light (photoacclimation) affects different components of the photosynthetic apparatus. It involves changes in the cellular content and in the ratio of light-harvesting and photoprotective pigments, in the ratio of photosystem I to photosystem II (PS I : PS II) reaction centres, and in the catalytic activity and synthesis of Calvin-cycle enzymes, primarily of RuBisCO (MacIntyre et al., Citation1997, Citation2002; Durnford et al., Citation2003; Suggett et al., Citation2007). Thermal acclimation affects similar components of the photosynthetic apparatus as photoacclimation (Maxwell et al., Citation1994; Morgan-Kiss et al., Citation2006). Low temperature in combination with high irradiance tends to increase the susceptibility to photoinhibition (Jensen & Knutsen, Citation1993; Long et al., Citation1994). At low temperatures the susceptibility to photodamage rises and the physiological energy demand for growth decreases as enzymatically controlled processes such as carbon assimilation, carotenoid biosynthesis and repair of reaction centres, as well as membrane-associated electron transfer processes, are slowed down (Falkowski & Raven, Citation1997). Hence, at low temperatures the light levels that cause photodamage or allow optimal growth are lower compared to high temperatures. Thus, acclimation to low temperatures can mimic high light acclimation, resulting in a high ratio of photoprotective carotenoids to light-harvesting pigments (Maxwell et al., Citation1994). Consequently, if a tropical species spreads northwards, it is likely to experience suboptimal temperatures and thus be subject to high light stress or even photoinhibition, thereby triggering pigment changes.
In general, cyanobacteria are well suited to study thermal and light acclimation. They contain two major groups of pigments – the hydrophilic phycobiliproteins, which serve as light-harvesting antenna pigments of PS II, and lipophilic pigments, comprising chlorophyll a and a unique variety of keto- and glycosidic carotenoids. In cyanobacteria, carotenoids are believed to play a minor role in light harvesting (Kana et al., Citation1988) and the carotenoids myxoxanthophyll, zeaxanthin and canthaxanthin are thought instead to have a photoprotective function (Steiger et al., Citation1999; Lakatos et al., Citation2001; MacIntyre et al., Citation2002). Different carotenoid compositions can be found at the species level (Hertzberg et al., Citation1971; Nicklisch & Woitke, Citation1999; Schagerl & Donaubaum, Citation2003) or even the strain level (Aakermann et al., Citation1992; Woitke et al., Citation1997) and cyanobacteria can acclimate their pigment content to varying irradiance (Kana et al., Citation1988; Millie et al., Citation1990; Woitke et al., Citation1997) and temperature (Várkonyi et al., Citation2002).
The freshwater cyanobacterium Cylindrospermopsis raciborskii (order Nostocales) started spreading from the tropics and subtropics to high latitudes in the last decades (Padisák, Citation1997). Nowadays it colonizes many temperate freshwater habitats worldwide (Stüken et al., Citation2006; Kokociński & Soininen, Citation2012). Its successful invasion may be favoured by its ability to acclimate to a wide range of environments, especially to light and temperature (Briand et al., Citation2004) and/or by climate change (Wiedner et al., Citation2007). Aphanizomenon gracile (also Nostocales) is a frequent native cyanobacterium of temperate fresh waters. Both species co-occur and likely compete with each other in temperate lakes (Rücker et al., Citation2007; Mehnert et al., Citation2010). The lipophilic pigments of C. raciborskii and A. gracile have previously been investigated in two separate studies. Várkonyi et al. (Citation2002) reported that a decrease of culture temperature induced the accumulation of myxoxanthophyll in C. raciborskii, and Rücker et al. (Citation1995) noticed that A. gracile responded to an increase of irradiance and to phosphorus limitation with an increased carotenoid to chlorophyll a ratio. In addition, the growth of C. raciborskii and A. gracile under different light supply and temperature has been studied by Mehnert et al. (Citation2010). Below 20°C, A. gracile had higher growth rates than the invasive species, while above 20°C C. raciborskii growth rates exceeded those of the native species. With respect to light-dependent growth, no clear differences were found between the two species.
To test if changes in the pigment composition took place during the northwards spread of C. raciborskii, we compared the lipophilic pigment content of C. raciborskii strains isolated from tropical and temperate lakes. To test our hypothesis that the invasive species is likely to experience suboptimal temperatures in the temperate zone and thus be subject to high light stress or even photoinhibition, we studied lipophilic pigment acclimation and non-photochemical fluorescence quenching (NPQ) of temperate isolates of C. raciborskii and A. gracile. Strains were cultivated at a range of temperatures (10–35°C) and light intensities (20–300 µmol photons m−2 s−1).
Materials and methods
Strains and culture conditions
The study was carried out with three Cylindrospermopsis raciborskii strains (24G7, 19F6, ZIE11CR) and three Aphanizomenon gracile strains (16D11, 30D11, ZIE23AFA), all isolated from lakes in northern Germany, and three C. raciborskii strains from Australia and Africa (AQS, LJ, CYA507). Detailed information on the origin of the strains is given in Haande et al. (Citation2008) and Mehnert et al. (Citation2010). Strains 16D11 and 24G7 were used to study thermal acclimation. Photoacclimation was examined with all German C. raciborskii and A. gracile strains. Comparison of light and thermal acclimation between isolates of different geographical origin was studied for all German and all African and Australian C. raciborskii strains.
Strains were cultivated in Z8-medium with some modification (Kotai, Citation1972). It contained additionally 40 µg l−1 of biotin, vitamin B12 and thiamine hydrochloride. A pH of 8.0–8.8 was adjusted by addition of NaOH. Erlenmeyer flasks (250 ml) were used as culture vessels and filled with 100 ml of suspension. They were continuously mixed at 80–100 rpm on an orbital shaker (IKA, KS 260 basic, Germany). Cultures were grown in a semi-continuous fashion according to the turbidostat principle (Mehnert et al., Citation2010) in growth chambers (BINDER, KBW 400, Germany) with dimmable illumination (OSRAM, Lumilux 18 W/865XT Cool Daylight, Germany) simulating a 12 : 12 h light : dark photoperiod. Light intensity was measured using a LI-250 A light meter (LI-COR, USA) equipped with a microspherical quantum sensor (US-SQS/L Walz, Effeltrich, Germany).
Determination of growth rates and curve fitting of the temperature- and light-dependent growth curves have been described by Mehnert et al. (Citation2010). The growth rates of the A. gracile strains and the German isolates of C. raciborskii have already been published and are taken from Mehnert et al. (Citation2010).
For the study of thermal acclimation, the cultures were grown at seven different temperatures from 10 to 35°C (±0.5°C) at 80 ± 4 µmol photons m−2 s−1. The cultures of A. gracile collapsed immediately at 35°C. The study of light acclimation was conducted at four different light intensities, at 20 ± 0.5°C (with 20 ± 2, 82 ± 11, 169 ± 11, and 282 ± 30 µmol photons m−2 s−1) and at 15 ± 0.5°C (with 25 ± 5, 66 ± 8, 101 ± 11, and 308 ± 31 µmol photons m−2 s−1). For comparison of strains of different geographical origin, the three C. raciborskii strains from Australia and Africa were grown at 20 ± 0.5°C under 21 ± 2 and 173 ± 13 µmol photons m−2 s−1, and at 15 ± 0.5°C under 24 ± 5 and 108 ± 9 µmol photons m−2 s−1, respectively.
Pigment analysis
Cultures were harvested after their acclimation to temperature and light, when growth rate reached steady-state. For studies of pigments during thermal acclimation, cultures were harvested three times during steady-state, while in the case of light acclimation, cultures were harvested once in steady-state.
Aliquots of 10 ml culture volume with an optical density of 0.1 at 750 nm (absorption cell with 10 mm light path: Lambda 2, Perkin Elmer, USA) were filtered through 25-mm diameter Whatman GF/F-filters and frozen at −80°C, freeze-dried, and stored frozen at −25°C in the dark until HPLC analysis. All steps of pigment extraction were performed under dim light conditions and 4°C. Pigments were extracted with 1 ml dimethylformamide (Wright & Jeffrey, Citation1997). The extraction was carried out by vibration shaking (IKA, VIBRAX-VXR, Germany) at a frequency of 2000 min−1 for 45 min in the dark. After the addition of 0.1 ml of 1 M ammonium acetate in high-purity water, the extraction was continued for a further 45 min. The extract was centrifuged for 20 min at 2500 × g and 0.2 ml of the supernatant was filled into vials and stored in an auto-sampler at 4°C in the dark until HPLC analysis.
Separation, identification and quantification of pigments were performed according to Woitke et al. (Citation1994), with some modifications in order to improve peak resolution and to shorten the time of analysis. The HPLC system (Waters Alliance, Millford, MA, USA) included a Waters 2695 separations module and a 2696 photo diode array detector. Pigments were separated at a flow rate of 1.0 ml min−1 at 30°C through a Waters Symmetry C18 column (3 -µm particle size, 15 cm), protected with a Symmetry C-18 pre-column. The gradient from eluent A to eluent B was optimized ().
Table 1. Optimized binary gradient system for the separation of phytoplankton lipophilic pigments on Waters Symmetry C18 column. Eluent A was 45 : 45 : 10 methanol : acetonitril : 1 M ammonium acetate (v/v/v) +3% 0.1 M ammonium acetate (v/v). Eluent B was 45 : 55 acetonitril : acetone (v/v).
A volume of 60 µl pigment extract was injected with a water packing of 20 µl before and 10 µl after the sample (Wright et al., Citation1997; Van Leeuwe et al., Citation2006). The pigment spectra were monitored in the range 350–700 nm and the peak areas were monitored at 440 nm (all pigments) and 410 nm (chlorophyll a and its derivatives only). Pigments were identified by their relative retention times and by their absorption spectra. Unialgal cultures, standards, and literature data were used for comparison. Chlorophyll a was quantified as a mean of the readings at 440 and 410 nm (both readings should give the same value). Chlorophyll a was calculated as the sum of chlorophyll a, its epimer, its allomer, chlorophyllide a and other detectable derivatives with the typical spectrum of chlorophyll a.
Peak area integration allowed quantification with the help of factors determined by Woitke et al. (Citation1994). These factors were checked by standards supplied by Hoffmann-La Roche (Grenzach, Germany) or Carbon 14 Centralen (Hørsholm, Denmark) from time to time. Chlorophyll a from Anacystis nidulans (Sigma–Aldrich) was used as standard for frequent examination of performance. If small corrections (<5%) of the factors were necessary, the relationships to the other pigments were not changed.
Pigment nomenclature
We designated the carotenoid glycosides according to the proposed nomenclature of Takaichi & Mochimaru (Citation2007). When the sugar moieties had not been identified myxoxanthophyll, aphanizophyll and oscillaxanthin were named as follows: myxol, 4-hydroxymyxol and oscillol glycoside.
Analysis of dry weight (DW)
Triplicate aliquots of 40 ml culture volume with an optical density of 0.1 at 750 nm (absorption cell with 10 mm light path; Lambda 2, Perkin Elmer, USA) were filtered through tared 25-mm diameter Whatman GF/F-filters. Filters were dried at 40°C in the dark and their weight was determined with an analytical balance (Sartorius MC1 Analytic AC 120 S, Germany). Dry weight was calculated in relation to the filtered culture volume (mg DW l−1). This was done for each strain grown at 20°C and 82 ± 11 µmol photons m−2 s−1. These strain-specific DW per volume ratios were used to normalize the lipophilic pigment contents from cultures (which were always diluted to the same optical density of 0.1 before filtration) grown at all tested light and temperature conditions. This approach is based on observations of Cirés et al. (Citation2011) showing a linear relationship between optical density (750 nm) and DW, independent of the culture conditions.
Fluorescence measurements
Non-photochemical quenching of fluorescence (NPQ) was quantified according to the Stern–Volmer equation:
Data analysis
To test for differences in the pigment content between C. raciborskii and A. gracile, a t-test for independent samples and a Mann–Whitney U-test were applied using PASW Statistics 17.0 (www.spss.com). Statistical tests were two-tailed. In the light experiment the independent variable consisted of the mean pigment content of three different strains with one replicate each, whereas in the temperature experiment the independent variable consisted of the mean of three pigment analyses of one strain sampled every two days from a steady-state growing culture. Linear Regression analysis was performed with the ‘Linear Fit’ function of the PASW Statistics 17.0 program.
Results
Pigment composition
Both Nostocales species, A. gracile and C. raciborskii (temperate and tropical strains), contained the same lipophilic pigments, except oscillol glycoside (oscillaxanthin), which was only found in trace amounts in A. gracile (maximum 0.12 mg g−1 DW). The major fraction of lipophilic pigments of both species consisted of chlorophyll a, β-carotene, echinenone, 4-hydroxymyxol glycoside (aphanizophyll) and myxol glycoside (myxoxanthophyll). Additionally, minor amounts of canthaxanthin and zeaxanthin were found. The maximum contents of canthaxanthin and zeaxanthin were 0.14 and 0.15 mg g−1 DW, respectively, found under high light conditions (>280 µmol photons m−2 s−1). The canthaxanthin content of A. gracile was significantly higher under all light and temperature conditions compared to C. raciborskii.
Comparison of tropical and temperate C. raciborskii strains
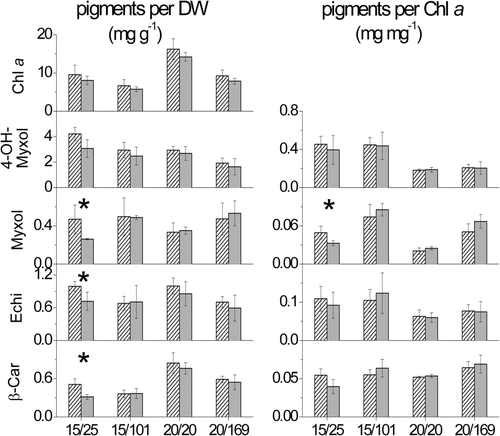
The comparison showed that C. raciborskii strains isolated from German lakes and strains isolated from tropical waters did not differ significantly (P > 0.05) in their pigment contents and carotenoid to chlorophyll a ratios, except at 15°C and low light intensity (25 µmol photons m−2 s−1). Under the latter conditions the content of myxol glycoside, echinenone and β-carotene and the ratio of myxol glycoside to chlorophyll a were significantly higher (P ≤ 0.05) in the temperate C. raciborskii strains, compared to the tropical ones (). In general, all strains responded to enhanced light intensity as well as reduced temperature by a decreased chlorophyll a content ().
Fig. 1. Mean content of the major lipophilic pigments chlorophyll a (Chl a), 4-hydroxymyxol glycoside (4-OH-Myxol), myxol glycoside (Myxol), echinenone (Echi) and β-carotene (β-Car) per dry weight (DW) (mg g−1) and carotenoid to chlorophyll a ratios (mg mg−1) of three temperate (hatched bars) and three tropical C. raciborskii strains (grey bars). Strains were grown at different combination of temperature (°C) and light intensity (µmol photons m−2 s−1) displayed as temperature/light intensity. Pigment content is given as mean and standard deviation of one replicate of three strains each (n = 3). Asterisks mark significant differences between temperate and tropical strains.
Thermal acclimation
The chlorophyll a content per DW at 80 µmol photons m−2 s−1 was significantly higher in A. gracile compared to C. raciborskii (except at 30°C) (). Maximum chlorophyll a content per DW was observed in C. raciborskii at 27.5 and 30°C and in A. gracile between 20 and 27.5°C, when the two species also grew at maximal rates ( and ). At lower and higher temperatures, the chlorophyll a content per DW clearly decreased, whereas the total carotenoid content per DW changed only slightly over the experimental temperature range (). Hence, the ratio of total carotenoids to chlorophyll a increased at suboptimal temperatures (). The increase of total carotenoid : chlorophyll a with declining temperatures was much more pronounced in C. raciborskii than in A. gracile. This ratio was significantly higher in C. raciborskii compared to A. gracile (except at 30°C), which was due mainly to the significantly higher 4-hydroxymyxol glycoside content of the invasive species ().
Fig. 2. Content of major lipophilic pigments per dry weight (DW) (mg g−1) and ratios of carotenoids (Car) to chlorophyll a (Chl a) (mg mg−1) of C. raciborskii strain 24G7 (A, C) and A. gracile strain 16D11 (B, D) grown at 80 µmol photons m−2 s−1 and seven temperatures from 10 to 35°C. Lines represent temperature-dependent growth curves (day−1) (A, B).
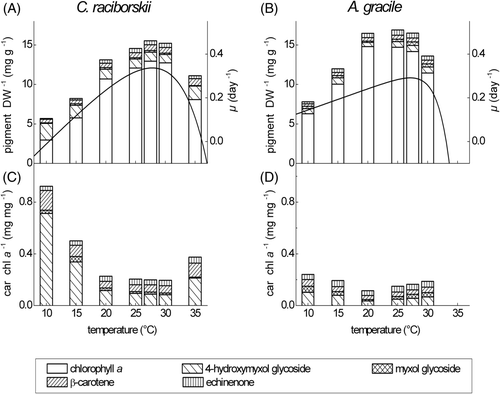
Table 2. Content of major lipophilic pigments per dry weight (DW) (mg g−1) and growth rates (day−1) of C. raciborskii strain 24G7 (C) and A. gracile strain 16D11 (A) acclimated to different temperatures and 80 µmol photons m−2 s−1. Mean and standard deviation of three pigment samples taken at different time-points during steady-state growth are given (n = 3). Differences of the pigment contents between the two species were tested for significance with t-test for independent samples and Mann–Whitney U-test. Abbreviations: ns non significant; *P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001.
Photoacclimation
The content of chlorophyll a per DW (mean of three strains per species) did not differ significantly between C. raciborskii and A. gracile (). The chlorophyll a content declined with increasing light intensities (), while the ratio of total carotenoids to chlorophyll a, as well as single carotenoids, increased as light levels were raised from 80 to 300 µmol photons m−2 s−1; this was true for both species at both 20 and 15 °C (). In C. raciborskii, the ratios of 4-hydroxymyxol and myxol glycoside to chlorophyll a were higher and increased more steeply at 15°C compared to 20°C. In contrast, there was almost no difference between the slopes of the 4-hydroxymyxol and myxol glycoside to chlorophyll a ratios at the different temperatures in A. gracile. For A. gracile, the steepest increase with light was found for the myxol glycoside to chlorophyll a ratio (). The slopes of the β-carotene and echinenone to chlorophyll a ratios of both species were smaller at 15°C compared to 20°C.
Fig. 3. Mean content of the major pigments per dry weight (DW) (mg g−1) and light-dependent growth curves (day−1) of three German C. raciborskii strains (left) and three German A. gracile strains (right) grown at 20 and 15°C and four different light intensities. The growth curve is given as a fit of growth rates of the three strains each.
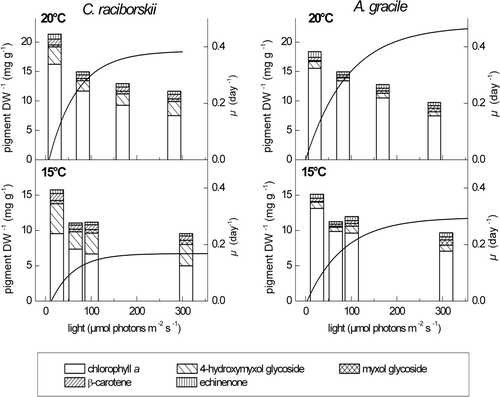
Fig. 4. Ratios of total carotenoids (Total Car), 4-hydroxymyxol glycoside (4-OH-Myxol), myxol glycoside (Myxol), echinenone (Echi) and β-carotene (β-Car) to chlorophyll a (Chl a), given as mean and standard deviation of three German C. raciborskii strains (black boxes) and three German A. gracile strains (open boxes). Cultures were grown at 20°C (left panels) and 15°C (right panels) and four different light intensities. Linear regression was performed without the carotenoid content at the lowest light intensity. The slope b of the regression curve and the coefficient of determination R 2 are displayed in standard font for C. raciborskii and in italics for A. gracile.
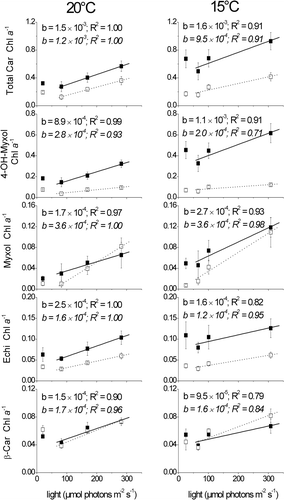
Table 3. Content of major lipophilic pigments per dry weight (DW) (mg g−1) and growth rates (day−1) of three German C. raciborskii (C) and three German A. gracile (A) strains acclimated to 15 and 20°C and different light intensities. Pigment content is given as the mean and standard deviation of one sample for each of three strains of each species (n = 3). Differences of the pigment contents between the two species were tested for significance with t-test for independent samples and Mann–Whitney U-test. Abbreviations: ns non significant; *P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001.
Non-photochemical quenching of fluorescence (NPQ)
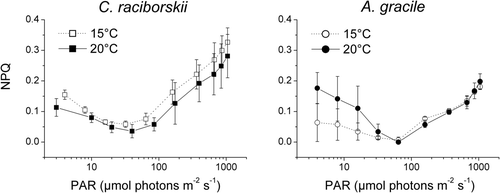
NPQ was high at very low photosynthetically active radiation (PAR) levels, dropping to a minimum at 30–65 µmol photons m−2 s−1 and increasing again with rising PAR (). Moving from the NPQ minimum C. raciborskii strains had clearly higher NPQ values and their NPQ values tended to increase from 20 to 15°C in comparison to those of A. gracile.
Fig. 5. Non-photochemical quenching of fluorescence (NPQ) plotted against photosynthetically active radiation (PAR) emitted by the PHYTO-PAM device. Three C. raciborskii (left panel) and three A. gracile (right panel) strains were grown at 88 µmol photons m−2 s−1 and 15°C (open symbols) and 20°C (closed symbols), respectively. NPQ is given as mean and standard deviation of the measurements of three different strains per species.
Discussion
Comparison of temperate and tropical isolates of C. raciborskii suggests that adaptation of the chlorophyll a and carotenoid content to temperate climate obviously did not take place during the northwards spread. Hence, the species relies on acclimation within the genetic constraints already evolved in the tropics.
We did not find major differences in the pigment composition of the invasive and native cyanobacteria, except that small amounts of oscillol glycoside were found in A. gracile but not in C. raciborskii. Our findings on the pigment composition of A. gracile are in agreement with Rücker et al. (Citation1995). The pigment composition of C. raciborskii is mainly in agreement with Várkonyi et al. (Citation2002). However, Várkonyi et al. (Citation2002) did not detect 4-hydroxymyxol glycoside, perhaps because they analysed pigments from isolated thylakoid membranes, which differ in carotenoid content from cytoplasmic and outer membranes (Omata & Murata, Citation1984; Jürgens et al., Citation1985).
Both the invasive and native species increased their chlorophyll a content with increasing temperatures until the optimal growth rate was reached. Both cyanobacteria apparently improve their light-harvesting capacity to collect more light energy, in order to meet the higher energy demand at higher temperatures. Similarly, with decreasing temperatures, the energy demand decreases and thus the harvested light energy may become excessive. Consequently, antenna pigments must be reduced. Our results show that the reduction of the chlorophyll a content is much more pronounced in C. raciborskii compared to A. gracile, resulting in a steep increase of the carotenoid to chlorophyll a ratio in C. raciborskii, especially of the 4-hydroxymyxol glycoside to chlorophyll a ratio. The high carotenoid to chlorophyll a ratio that we found at suboptimal low and high temperatures has also been observed in polar cyanobacteria (Tang et al., Citation1997; Tang & Vincent, Citation1999). Consistent with the present study, polar cyanobacteria were more prone to photoinhibition at low temperatures as well as at temperatures above the optimum.
Concerning light acclimation, all strains of both species followed the common tendency of photosynthetic organisms to reduce their antenna pigments (e.g. chlorophyll a) with increasing light supply (, ) (Anderson, Citation1986; Anderson et al., Citation1995; MacIntyre et al., Citation2002; Walters, Citation2005). The chlorophyll a content was reduced more than the photoprotective pigments, resulting in rising carotenoid to chlorophyll a ratios. The more pronounced relative increase of 4-hydroxymyxol and myxol glycoside in C. raciborskii, either with increasing light intensities at 15°C () or with decreasing temperatures (), may be an indication that light stress is more likely to occur in the invasive species than in the native A. gracile, at temperatures below 20°C.
To provide a direct evidence of the higher susceptibility of C. raciborskii to photodamage, light response measurements of the non-photochemical quenching (NPQ) of the fluorescence signal were performed at 15 and 20°C. It should be borne in mind that state transitions, rather than thermal energy dissipation, can dominate the NPQ of cyanobacterial fluorescence, at least at low light intensities (Campbell et al., Citation1998; Mullineaux & Emlyn-Jones, 2005). The distribution of excitation energy to PS I in dark- or low-light-adapted cyanobacteria lowers the yield of PS II fluorescence and hence increases NPQ. Thus, to compare the NPQ of the two species we focused on the values determined above 65 µmol photons m−2 s−1 and found that NPQ was generally higher in C. raciborskii than in A. gracile; it was also higher in C. raciborskii strains grown at 15°C compared to strains grown at 20°C. These findings support our assumption that the susceptibility of the invasive species to photodamage will increase if temperature decreases, and that this species is even more subject to light stress at moderate temperatures characteristic of temperate fresh waters than the native species.
Other studies have described different susceptibilities to light stress at lower temperatures within the same species or close relatives originating from different climatic habitats. For example, tropical isolates of a green macrophyte were photoinhibited at suboptimal low temperatures, where they grew at 30% of their maximal growth rate (Eggert et al., Citation2006). In contrast, supraoptimal temperatures of 30°C were not photoinhibiting. The opposite occurred in warm-temperate isolates of the same species: here, supraoptimal temperatures were photoinhibiting but suboptimal temperatures were not. Ursi et al. (Citation2003) reported that, after a lowering of the temperature from 30 to 20°C, tropical rhodophyte (Gracilaria birdiae) populations isolated close to the equator showed a more pronounced increase in their ratio of photoprotective carotenoids to chlorophyll a than populations isolated at higher latitudes. Ursi et al. concluded that the intraspecific differences were genetic and reflected the ambient irradiance and temperature regimes of their native habitat. It would seem likely that the greater susceptibility of C. raciborskii to light stress at low temperatures, compared with A. gracile, is likewise based on genetic differences promoted by their different biogeographical origin. However, both species have a similar high optimum growth temperature of 28°C (Mehnert et al., Citation2010), indicating that A. gracile too might originate from tropical environments.
Our results on the effects of thermal- and photo-acclimation on the two carotenoid glycosides in C. raciborskii and A. gracile are consistent with reports of excess high light, UV and low temperature dependent upregulation of myxol glycoside in various cyanobacteria (Tomaselli et al., Citation1995; Ivanov et al., Citation2000; Várkonyi et al., Citation2002; Schagerl & Müller, Citation2006); no comparable studies exist for 4-hydroxymyxol glycoside. Carotenoid glycosides obviously play an important role in cyanobacterial photoprotection. In a liposome-assay, myxol glycoside has been reported to be superior to all other carotenoids (echinenone, β-carotene, zeaxanthin) in protection against radical and photo-oxidation, whereas echinenone was the least effective but the most stable (Steiger et al., Citation1999). In the present study, there was no obvious alteration in echinenone content with light, but echinenone was the only carotenoid that increased its content continuously from low to high temperature (). Polar carotenoids are known to decrease membrane fluidity (Subczynski et al., Citation1992; Hara et al., Citation1999; Várkonyi et al., Citation2002). Thus, echinenone – which is polar – might have a structural function as well as a role in photoprotection, and may enhance membrane rigidity at high temperatures.
Zeaxanthin occurred in minor amounts in C. raciborskii and A. gracile. Although cyanobacteria are not capable of a xanthophyll cycle, zeaxanthin possesses a photoprotective function in some cyanobacterial species (MacIntyre et al., Citation2002). Cyanobacteria containing large amounts of zeaxanthin possess few carotenoid glycosides or none, and vice versa (Takaichi & Mochimaru, Citation2007). We suggest that myxol and 4-hydroxymyxol glycoside replace the function of zeaxanthin in the two species investigated.
Summary
Overall, the growth of the invasive cyanobacterium Cylindrospermopsis raciborskii was lower at temperatures between 10°C and 20°C compared to the native Nostocales Aphanizomenon gracile, resulting in a higher susceptibility of the invasive species to photoinhibition at low temperatures. To prevent photoinhibition C. raciborskii responds with increasing ratios of photoprotective carotenoids, especially the carotenoid glycosides 4-hydroxymyxol glycoside (aphanizophyll) and myxol glycoside (myxoxanthophyll). The higher susceptibility of C. raciborskii to high light at low temperatures could partly explain why this species mostly inhabits turbid lakes, as reported by Stüken et al. (Citation2006) from a survey in northern Germany.
Acknowledgements
We thank Marén Lentz, Monika Degebrodt and Andrea Launhardt for their reliable work in the laboratory, and Barbara Meinck for her substantial help with the HPLC system. Karina Preußel and Martin Beck kindly supplied German cyanobacterial strains and Sigrid Haande and Martin Saker kindly supplied tropical strains. We also thank Professor Brigitte Nixdorf and Professor Assaf Sukenik for fruitful discussion and Frances Pick for proofreading the English draft of the manuscript. We are grateful to two anonymous reviewers who substantially helped to improve the manuscript. The study was funded by the German Ministry of Education, Science and Research (BMBF project NOSTOTOX, FKZ 0330792 A and B) and the Kompetenz Zentrum Wasser Berlin gGmbH (KWB) with financial support of Veolia Water and Berlin Water Enterprise (BWB).
References
- Aakermann , T , Skulberg , OM and Liaaen-Jensen , S . 1992 . Carotenoids of blue-green algae. Part 12. A comparison of the carotenoids of strains of Oscillatoria and Spirulina (Cyanobacteria) . Biochemical Systematics and Ecology , 20 : 761 – 769 .
- Anderson , JM . 1986 . Photoregulation of the composition, function, and structure of thylakoid membranes . Annual Review of Plant Physiology and Plant Molecular Biology , 37 : 93 – 136 .
- Anderson , JM , Chow , WS and Park , YI . 1995 . The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues . Photosynthesis Research , 46 : 129 – 139 .
- Briand , J-F , Leboulanger , C , Humbert , J-F , Bernard , C and Dufour , P . 2004 . Cylindrospermopsis raciborskii (Cyanobacteria) invasion at mid-latitudes: selection, wide physiological tolerance, or global warming? . Journal of Phycology , 40 : 231 – 238 .
- Campbell , D , Hurry , V , Clarke , AK , Gustafsson , P and Oquist , G . 1998 . Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation . Microbiology and Molecular Biology Reviews , 62 : 667 – 683 .
- Cirés , S , Wörmer , L , Timón , J , Wiedner , C and Quesada , A . 2011 . Cylindrospermopsin production and release by the potentially invasive cyanobacterium Aphanizomenon ovalisporum under temperature and light gradients . Harmful Algae , 10 : 668 – 675 .
- Durnford , DG , Price , JA , Mckim , SM and Sarchfield , ML . 2003 . Light-harvesting complex gene expression is controlled by both transcriptional and post-transcriptional mechanisms during photoacclimation in Chlamydomonas reinhardtii . Physiologia Plantarum , 118 : 193 – 205 .
- Eggert , A , Visser , RJW , Van Hasselt , PR and Breeman , AM . 2006 . Differences in acclimation potential of photosynthesis in seven isolates of the tropical to warm temperate macrophyte Valonia utricularis (Chlorophyta) . Phycologia , 45 : 546 – 556 .
- Falkowski , PG and Raven , JA . 1997 . Aquatic photosynthesis , Malden , MA : Blackwell Science .
- Haande , S , Rohrlack , T , Ballot , A , Røberg , K , Skulberg , R , Beck , M and Wiedner , C . 2008 . Genetic characterisation of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) isolates from Africa and Europe . Harmful Algae , 7 : 692 – 701 .
- Hara , M , Yuan , HQ , Yang , Q , Hoshino , T , Yokoyama , A and Miyake , J . 1999 . Stabilization of liposomal membranes by thermozeaxanthins: carotenoid-glucoside esters . Biochimica et Biophysica Acta – Biomembranes , 1461 : 147 – 154 .
- Hertzberg , S , Liaaen-Jensen , S and Siegelman , HW . 1971 . Carotenoids of blue-green algae. Part 7. The carotenoids of blue-green algae . Phytochemistry , 10 : 3121 – 3127 .
- Ivanov , AG , Miskiewicz , E , Clarke , AK , Greenberg , BM and Huner , NPA . 2000 . Protection of photosystem II against UV-A and UV-B radiation in the cyanobacterium Plectonema boryanum: the role of growth temperature and growth irradiance . Photochemistry and Photobiology , 72 : 772 – 779 .
- Jensen , S and Knutsen , G . 1993 . Influence of light and temperature on photoinhibition of photosynthesis in Spirulina platensis . Journal of Applied Phycology , 5 : 495 – 504 .
- Jürgens , UJ , Golecki , JR and Weckesser , J . 1985 . Characterization of the cell wall of the unicellular cyanobacterium Synechocystis pcc 6714 . Archives of Microbiology , 142 : 168 – 174 .
- Kana , TM , Glibert , PM , Goericke , R and Welschmeyer , NA . 1988 . Zeaxanthin and β-carotene in Synechococcus WH7803 respond differently to irradiance . Limnology and Oceanography , 33 : 1623 – 1627 .
- Kokociński , M and Soininen , J . 2012 . Environmental factors related to the occurrence of Cylindrospermopsis raciborskii (Nostocales, Cyanophyta) at the north-eastern limit of its geographical range . European Journal of Phycology , 47 : 12 – 21 .
- KOTAI, J. (1972). Instructions for Preparation of Modified Nutrient Solution Z8 for Algae. Publication B-11/69. Norwegian Institute for Water Research, Oslo
- Lakatos , M , Bilger , W and Büdel , B . 2001 . Carotenoid composition of terrestrial cyanobacteria: response to natural light conditions in open rock habitats in Venezuela . European Journal of Phycology , 36 : 367 – 375 .
- Long , SP , Humphries , S and Falkowski , PG . 1994 . Photoinhibition of photosynthesis in nature . Annual Review of Plant Physiology and Plant Molecular Biology , 45 : 633 – 662 .
- MacIntyre , HL , Sharkey , TD and Geider , RJ . 1997 . Activation and deactivation of ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco) in three marine microalgae . Photosynthesis Research , 51 : 93 – 106 .
- MacIntyre , HL , Kana , TM , Anning , T and Geider , RJ . 2002 . Photoacclimation of photosynthesis irradiance response curves and photosynthetic pigments in microalgae and cyanobacteria . Journal of Phycology , 38 : 17 – 38 .
- Maxwell , DP , Falk , S , Trick , CG and Huner , NPA . 1994 . Growth at low temperature mimics high-light acclimation in Chlorella vulgaris . Plant Physiology , 105 : 535 – 543 .
- Mehnert , G , Leunert , F , Cires , S , Jöhnk , KD , Rücker , J , Nixdorf , B and Wiedner , C . 2010 . Competitiveness of invasive and native cyanobacteria from temperate freshwaters under various light and temperature conditions . Journal of Plankton Research , 32 : 1009 – 1021 .
- Millie , DF , Ingram , DA and Dionigi , CP . 1990 . Pigment and photosynthetic responses of Oscillatoria argardhii (Cyanophyta) to photon flux density and spectral quality . Journal of Phycology , 26 : 660 – 666 .
- Morgan-Kiss , RM , Priscu , JC , Pocock , T , Gudynaite-Savitch , L and Huner , NPA . 2006 . Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments . Microbiology and Molecular Biology Reviews , 70 : 222 – 252 .
- Mullineaux , CW and Emlyn-Jones , D . 2005 . State transitions: an example of acclimation to low-light stress . Journal of Experimental Botany , 56 : 389 – 393 .
- Nicklisch , A and Woitke , P . 1999 . Pigment content of selected planktonic algae in response to natural light fluctuations and a short photoperiod . International Review of Hydrobiology , 84 : 479 – 495 .
- Omata , T and Murata , N . 1984 . Isolation and characterization of three types of membranes from the cyanobacterium (blue-green alga) Synechocystis PCC 6714 . Archives of Microbiology , 139 : 113 – 116 .
- Padisák , J . 1997 . Cylindrospermopsis raciborskii (Wolozynska) Seenayya et Subba Raju, an expanding, highly adaptive cyanobacterium: worldwide distribution and review of its ecology . Archiv für Hydrobiologie, Supplement , 107 : 563 – 593 .
- Rücker , J , Kohl , JG and Kaiser , K . 1995 . Responses of carotenoids and chlorophylls to variation of growth-limiting factors in three filamentous blue-green algae . Algological Studies , 77 : 51 – 65 .
- Rücker , J , Stüken , A , Nixdorf , B , Fastner , J , Chorus , I and Wiedner , C . 2007 . Concentrations of particulate and dissolved cylindrospermopsin in twenty-one Aphanizomenon dominated temperate lakes . Toxicon , 50 : 800 – 809 .
- Schagerl , M and Donabaum , K . 2003 . Patterns of major photosynthetic pigments in freshwater algae. 1. Cyanoprokaryota, Rhodophyta and Cryptophyta . Annales de Limnologie – International Journal of Limnology , 39 : 35 – 47 .
- Schagerl , M and Müller , B . 2006 . Acclimation of chlorophyll a and carotenoid levels to different irradiances in four freshwater cyanobacteria . Journal of Plant Physiology , 163 : 709 – 716 .
- Steiger , S , Schäfer , L and Sandmann , G . 1999 . High-light-dependent upregulation of carotenoids and their antioxidative properties in the cyanobacterium Synechocystis PCC 6803 . Journal of Photochemistry and Photobiology B-Biology , 52 : 14 – 18 .
- Stüken , A , Rücker , J , Endrulat , T , Preußel , K , Hemm , M , Nixdorf , B , Karsten , U and Wiedner , C . 2006 . Distribution of three alien cyanobacterial species (Nostocales) in Northeast Germany: Cylindrospermopsis raciborskii, Anabaena bergii and Aphanizomenon aphanizomenoides . Phycologia , 45 : 696 – 703 .
- Subczynski , WK , Markowska , E , Gruszecki , WI and Sielewiesiuk , J . 1992 . Effects of polar carotenoids on dimyristoylphosphatidylcholine membranes – a spin-label study . Biochimica et Biophysica Acta , 1105 : 97 – 108 .
- Suggett , DJ , Le Floc’H , E , Harris , GN , Leonardos , N and Geider , RJ . 2007 . Different strategies of photoacclimation by two strains of Emiliania huxleyi (Haptophyta) . Journal of Phycology , 43 : 1209 – 1222 .
- Takaichi , S and Mochimaru , M . 2007 . Carotenoids and carotenogenesis in cyanobacteria: unique ketocarotenoids and carotenoid glycosides . Cellular and Molecular Life Sciences , 64 : 2607 – 2619 .
- Tang , EPY and Vincent , WF . 1999 . Strategies of thermal adaptation by high-latitude cyanobacteria . New Phytologist , 142 : 315 – 323 .
- Tang , EPY , Tremblay , R and Vincent , WF . 1997 . Cyanobacterial dominance of polar freshwater ecosystems: are high-latitude mat-formers adapted to low temperature? . Journal of Phycology , 33 : 171 – 181 .
- Tomaselli , L , Margheri , MC and Sacchi , A . 1995 . Effects of light on pigments and photosynthetic activity in a phycoerythrin-rich strain of Spirulina subsalsa . Aquatic Microbial Ecology , 9 : 27 – 31 .
- Ursi , S , Pedersén , M , Plastino , E and Snoeijs , P . 2003 . Intraspecific variation of photosynthesis, respiration and photoprotective carotenoids in Gracilaria birdiae (Gracilariales: Rhodophyta) . Marine Biology , 142 : 997 – 1007 .
- Van Leeuwe , MA , Villerius , LA , Roggeveld , J , Visser , RJW and Stefels , J . 2006 . An optimized method for automated analysis of algal pigments by HPLC . Marine Chemistry , 102 : 267 – 275 .
- VÁRKONYI, Z., MASAMOTO, K., DEBRECZENY, M., ZSIROS, O., UGHY, B., GOMBOS, Z., DOMONKOS, I., FARKAS, T., WADA, H. & SZALONTAI, B. (2002). Low-temperature-induced accumulation of xanthophylls and its structural consequences in the photosynthetic membranes of the cyanobacterium Cylindrospermopsis raciborskii: An FTIR spectroscopic study. Proceedings of the National Academy of Sciences USA, 99: 2410–2415
- Walters , RG . 2005 . Towards an understanding of photosynthetic acclimation . Journal of Experimental Botany , 56 : 435 – 447 .
- Wiedner , C , Rücker , J , Brüggemann , R and Nixdorf , B . 2007 . Climate change affects timing and size of populations of an invasive cyanobacterium in temperate regions . Oecologia , 152 : 473 – 484 .
- Woitke , P , Martin , CD , Nicklisch , S and Kohl , JG . 1994 . HPLC determination of lipophilic photosynthetic pigments in algal cultures and lake water samples using a non-endcapped C-18-RP-column . Fresenius Journal of Analytical Chemistry , 348 : 762 – 768 .
- Woitke , P , Hesse , K and Kohl , JG . 1997 . Changes in the lipophilic photosynthetic pigment contents of different Microcystis aeruginosa strains in response to growth irradiance . Photosynthetica , 33 : 443 – 453 .
- Wright , SW and Jeffrey , SW . 1997 . “ High-resolution HPLC system for chlorophylls and carotenoids of marine phytoplankton ” . In Phytoplankton Pigments in Oceanography , Edited by: Jeffrey , SW , Mantoura , RFC and Wright , SW . 327 – 341 . Paris : UNESCO Publishing .
- Wright , SW , Jeffrey , SW and Mantoura , RFC . 1997 . “ Evaluation of methods and solvents for pigment extraction ” . In Phytoplankton Pigments in Oceanography , Edited by: Jeffrey , SW , Mantoura , RFC and Wright , SW . 261 – 282 . Paris : UNESCO Publishing .