Abstract
The aim of our study was to investigate the intracellular toxicity mechanisms of the photoactive, potentially anti-cyanobacterial agent hydrogen peroxide (H2O2) in Microcystis aeruginosa, which represents one of the most significant toxin-producing cyanobacterial species in European water bodies. Metabolic activity and cell membrane integrity were evaluated by flow cytometry in cyanobacteria exposed to H2O2 in the dark or light; the relationships between exposure effects and the kinetics of hydrogen peroxide decomposition were studied. In the light (irradiance 140 µmol m−2 s−1), cyanobacteria were exposed to initial H2O2 concentrations of 0.00 (control), 0.75, 2.00, and 4.00 mg l−1 respectively, while in the dark concentrations were ten times higher. Flow cytometry and chlorophyll a fluorescence measurements suggested that hydrogen peroxide exposure elicits an immediate decline of metabolic (esterase) activity, measured as a decrease in fluorescein fluorescence after hydrolysis of fluorescein diacetate (FDA), and immediate changes of chlorophyll a fluorescence parameters, followed later by an increase in the percentage of membrane-compromised (SYTOX Green positive) cells. When the concentration of H2O2 used was lethal (in the two highly exposed treatments in the light), a significant drop in total cell counts was detected, whereas in other treatments no drop was observed during the entire experimental period (72 h). Our study also confirmed that light is one of the critical factors affecting H2O2 decomposition and thus greatly influences its toxicity. Whereas in the light, M. aeruginosa exposed to 0.75 mg l−1 H2O2 recovered after all the H2O2 had decomposed, in the dark H2O2 decomposed relatively slowly and its toxic effects on the cyanobacteria were observed over the whole 72-h period, though without cell lysis in any experimental concentration.
Introduction
Health risks from human exposure to toxin-producing cyanobacteria have led to efforts to find practical methods for reducing cyanobacterial water-blooms. Due to their high toxicity to cyanobacteria and cost-effectiveness, several metals, or rather, their salts, have been used for water-bloom management, but their toxicity to non-target organisms and their persistence and accumulation in the environment call their suitability into question (Jancula & Marsalek, Citation2011). This is particularly the case with copper, which is toxic to cyanobacteria (Van Hullebusch et al., Citation2002; Hadjoudja et al., Citation2009), but also to other aquatic organisms such as crustaceans or freshwater fish (Murray-Gulde et al., Citation2002; de Oliveira et al., Citation2004).
As an alternative to metals, other methods have been proposed for cyanobacterial water-bloom reduction, including physical treatments such as ultrasound (Zhang et al., Citation2006) and jet cavitation (Xu et al., Citation2006), and biological methods (Jancula et al., Citation2007; Hong et al., Citation2009). Unfortunately, these methods have not yet been studied systematically and thus their selective effect on target organisms remains in question.
Some of our research group's previous studies have suggested a high anti-cyanobacterial potency of photodynamic agents. When activated by light, these substances produce reactive oxygen species (ROS), such as singlet oxygen, hydroxyl and peroxyl radicals. Moreover, they are easily biodegradable. It has been reported that substances primarily producing singlet oxygen (e.g. cationic phthalocyanines substituted with heterocycle) can be used as universal and non-selective algicidal agents, since they possess significantly lower toxicity to aquatic invertebrates than to cyanobacteria or green algae (Jancula et al., Citation2008). Compounds that mainly produce hydroxyl radicals seem to be even more promising for treating cyanobacterial water-blooms, because they have significantly higher toxicity to cyanobacteria than to green algae (Drabkova et al., Citation2007a ). This is especially true of hydrogen peroxide (H2O2), a well-known source of hydroxyl radicals, which has been found to be ten times more toxic to cyanobacteria than to green algae and diatoms (Drabkova et al., Citation2007b , Citation2007 c). Besides direct toxicity to cyanobacteria, it has also been demonstrated that H2O2 exposure may decrease the transcription of the microcystin transport gene in Microcystis aeruginosa cells, which could potentially prevent the transport of this most significant cyanotoxin into the water system (Qian et al., Citation2010). Even though H2O2 has been used in aquaculture as an antifungal agent and disinfectant for several years (Rach et al., Citation1998; Avendano-Herrera et al., Citation2006), data about its interactions with cyanobacteria are still scarce. It has been demonstrated in Anabaena variabilis and Anacystis nidulans that external addition of H2O2 inhibits their growth and photosynthetic electron transport (Samuilov et al., Citation1999, Citation2004) and these parameters can be affected at concentrations of less than 1 mg l−1 (Drabkova et al., Citation2007a , Citation2007 b).
Since data about the intracellular mechanisms of H2O2 actions are still generally lacking and H2O2 toxicity has previously been investigated mainly by using conventional indirect methods (e.g. growth-inhibition tests), we sought to obtain new information about H2O2 toxicity at the single-cell level. For this purpose, metabolic (esterase) activity, as a marker of cell viability, and cell membrane integrity were studied by flow cytometry in the cyanobacterium Microcystis aeruginosa exposed to various concentrations of H2O2. The effects of H2O2 on photosynthetic parameters were also studied. Microcystis aeruginosa was chosen as an experimental organism because it represents one of the most important toxin-producing cyanobacteria in European fresh waters. Because H2O2 is a photoactive and easily biodegradable substance, its toxic effects were investigated in two different experimental regimes, with light or dark exposure. During the experiment, the dynamics of changes in physiological parameters were monitored, as well as actual H2O2 concentrations in the culture medium, to determine the role of cyanobacterial cells in H2O2 degradation and to find relationships between H2O2 degradation rates and H2O2 toxicity for cyanobacteria.
Materials and methods
Culture conditions
The Microcystis aeruginosa strain UTEX B 2667 was obtained from the UTEX Culture Collection of Algae (Austin, Texas, USA) and pre-cultivated in an Erlenmeyer flask in ZBB medium i.e. a 1 : 1 mixture of medium Z (ZEHNDER in Staub, Citation1961) and Bold's Basal medium (BB in Bold, Citation1949) diluted to 50% by sterilized ultrapure water, under standard conditions (temperature 21 ± 2°C, illumination by cool-white fluorescent tubes, PHILIPS 36 W, 140 µmol photons m−2 s−1). After pre-cultivation, cyanobacteria that had reached the exponential growth phase were diluted in glass beakers by freshly prepared sterile ZBB medium to obtain 100 ml of experimental cell suspension with an initial cell density of 1.0 × 106 cells ml−1. Experimental cell suspensions were exposed to various concentrations of H2O2 in each of two different experimental regimes: continuous light (cool-white fluorescent tubes, PHILIPS 36 W, irradiance 140 µmol photons m−2 s−1) or continuous dark. Since preliminary experiments suggested markedly (approximately an order of magnitude) lower toxicity of H2O2 in the darkness when compared with its toxicity under 140 µmol photons m−2 s−1, ten times higher initial H2O2 concentrations were used for testing in the dark. Appropriate amounts of H2O2 (30%, Sigma–Aldrich) were added to cyanobacterial suspensions to obtain nominal concentrations of 0 (control), 0.75, 2.00 and 4.00 mg l−1 (i.e. 0, 22.06, 58.82 and 117.65 µM) for light exposure and 0, 7.50, 20.00 and 40.00 mg l−1 (i.e. 0, 220.60, 588.20 and 1176.50 µM) for dark exposure. The effects of H2O2 were investigated over the course of 72 h and the experiment was performed in four replicates.
Metabolic (esterase) activity
Esterase activity of cyanobacteria was assessed by flow cytometry according to Jochem (Citation1999) (with slight modifications) using fluorescein diacetate (FDA) (Sigma–Aldrich, catalogue number F7378). This non-fluorescent substrate enters the cell, where it is hydrolysed by cellular esterases to green-fluorescent substance fluorescein. The higher the cellular esterase activity is, the higher the fluorescence detected. A stock solution of FDA of 5 mg l−1 was prepared in dimethysulfoxide (DMSO) and stored at 4°C. Before measurement, stock solution was thawed and 10 µl was added to an Eppendorf tube filled with 990 µl of sterilized ultrapure water (filtered through a 0.2 -µm filter). This working solution (50 µg l−1) was mixed carefully on the vortexer and 33 µl was added to the flow cytometric tube containing 1 ml of sample. During analyses, the thawed stock solution of FDA was kept on ice and a new batch of working solution was prepared for each sample, preventing the possibility of substrate precipitation or degradation (Jochem, Citation1999). Cells were analysed using flow cytometry. A flow cytometer CyFlow ML (Partec, Muenster, Germany) equipped with blue laser (488 nm/20 mW) was used for the measurement of esterase activity as well as for other flow cytometric measurements. The results of our preliminary experiments suggested that an incubation period of 13 min is optimal for measurements of M. aeruginosa esterase activity: in spite of the fact that Jochem (Citation1999) used a shorter incubation time of 5 min in his experiment with marine phytoplankton, we found that green fluorescence of Microcystis cells increased for 12 min before becoming stable. Cyanobacterial cells were distinguished from background by means of gating on dot-plots of forward scatter (FSC), side scatter (SSC) and chlorophyll a autofluorescence (band-pass emission filter 675/20 nm). Green fluorescence of fluorescein was measured through a 527/30 nm band-pass filter before the experiment started, as well as after 1, 2, 4, 6, 24, 48 and 72 h of exposure to hydrogen peroxide.
Cell membrane integrity
Membrane integrity was investigated by flow cytometry using a SYTOX Green fluorescent probe as described by Regel et al. (Citation2004) and Daly et al. (Citation2007), with slight modifications. SYTOX Green is a membrane-impermeable fluorescent dye, which is normally unable to reach the intracellular space due to the intact cell membrane of a non-damaged cell. However, if the cell membrane is damaged (e.g. in physically or chemically stressed cells), SYTOX Green enters the cell and binds to its nucleic acids. A stock solution of SYTOX Green (5 mM) (Invitrogen, catalogue number S7020) was diluted by sterile ultrapure water to give a working solution of 50 µM. For the measurement of Microcystis cell membrane integrity, 10 µl of working solution was added to 990 µl of sample in the flow cytometric tube to obtain the final SYTOX concentration of 0.5 µM and the sample was stained for 7 min in the dark. Measurements were performed before the experiment started and after 3, 5, 7, 24, 48 and 72 h of exposure to H2O2. Green fluorescence of cyanobacterial cells was detected using a standard emission band-pass filter (527/30 nm). Heat-treated cyanobacterial cells were used as a positive control. Gating regions representing both intact, i.e. SYTOX-negative (SYTOX–) and membrane-damaged, i.e. SYTOX-positive (SYTOX+) cells were created in two-dimensional dot-plots (side scatter versus green fluorescence). The percentage of SYTOX+ cells in cell suspensions exposed to H2O2 was monitored and compared with controls.
Chlorophyll a (Chl a) fluorescence
Induced Chl a fluorescence was measured at room temperature by means of a high time-resolution fluorometer AOM (Algal Online Monitor, PSI Brno, Czech Republic) equipped with two excitation sources (LEDs with peaks at 455 nm [blue] and 590 nm [amber]). Fluorescence was detected using a PIN photodiode after passing through a long-pass filter (>660 nm). Chl a fluorescence measurements were done six times per sample at the beginning of the experiment and after 1, 2, 3, 4, 5, 6, 7, 24, 48 and 72 h. Dark-exposed samples were kept in the dark and light-exposed samples were dark-adapted for 5 min prior to measurement. Basal chlorophyll fluorescence [F0], maximal efficiency yield of photosystem II FV/FM [FV/FM = (FM − F0)/FM] and performance index per absorption [PIABS] were derived from the fast induction curve (the so-called OJIP curve) and evaluated according to the JIP-test as presented by Appenroth et al. (Citation2001).
Cell counts
Total cyanobacterial counts were measured by flow cytometry at the beginning of the experiment and after 1, 2, 4, 6, 24, 48 and 72 h. The CyFlow ML flow cytometer enables accurate determination of cell counts without the necessity of using beads or other reference particles, due to the so-called ‘volumetric counting function’. During the experiments, cell counts were determined by the measurement of cyanobacterial Chl a autofluorescence. Fluorescent emission was measured after excitation by a blue laser (488 nm/20 mW) using an emission 675/20 nm band-pass filter. Total cyanobacterial counts were also randomly checked by epifluorescence microscopy using an Olympus BX60 microscope.
Kinetics of hydrogen peroxide decomposition
Kinetics of H2O2 decomposition in the cell-free medium as well as in the medium supplemented by cyanobacterial cells (initial density 1.0 × 106 cells ml−1) was measured in both experimental regimes (light and dark) using the same initial concentrations of H2O2 as in toxicity experiments (i.e. 0, 0.75, 2.00 and 4.00 mg l−1 for light and 0, 7.50, 20.00 and 40.00 mg l−1 for dark). H2O2 concentrations were measured before the experiment started and after 2, 4, 6, 8, 24, 48 and 72 h incubation. The spectrophotometric method described by Bader et al. (Citation1988) and modified by Drabkova et al. (Citation2007b ) was used for the measurements. The buffer stock solution was prepared by mixing 0.5 M Na2HPO4 and 0.5 M NaH2PO4 in order to achieve a pH of 6 when 1.2 ml of stock solution was added to 10.8 ml of the sample. The buffered sample (12 ml) was supplemented with 20 µl of DPD reagent (0.1 g of N,N-diethyl-1,4-phenylenediammonium sulphate, Fluka), diluted in 10 ml of 0.1 M H2SO4) and 20 µl of HRP reagent (10 mg of horseradish peroxidase, Sigma–Aldrich, Type II, 181 purpurogallin units mg−1 diluted in 10 ml of deionized water) while continually stirred. The absorbance of the mixture was measured at a wavelength of 551 nm using a DR2800 spectrophotometer (Hach Lange, Düsseldorf, Germany). The whole mixture without HRP addition was used as a blank. The detection limit of this method was 0.03 mg l−1 of H2O2.
Statistics
Data were processed using STATISTICA 8 software (StatSoft, Tulsa, OK, USA). After verification of the normality of the data distribution and homogeneity of variances, a one-way or multiple analysis of variance (ANOVA or MANOVA) was applied and differences evaluated by means of the Scheffe post hoc test at <0.05. Where needed, a Student's t-test or nonparametric Kruskal–Wallis test was used, both at P < 0.05.
Results
Effects of hydrogen peroxide on metabolic (esterase) activity
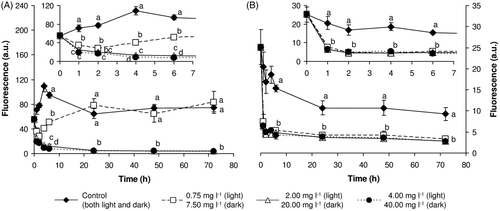
The results of our experiments showed that the metabolic activity of M. aeruginosa is a highly sensitive biomarker of its exposure to H2O2, since in all treatments from both experimental regimes a significant decline in esterase activity (compared with the control) was already apparent after one hour's exposure (see ). In the light, cyanobacteria exposed to 0.75 mg l−1 of H2O2 reacted to the toxic assault by an initial decrease of esterase activity, but this activity subsequently increased again and after 24 h it reached control values. Then and until the end of the experiment with 0.75 mg l−1 of H2O2, esterase activity remained at approximately the same levels as in the control. In contrast, in all other experimental treatments, with both light- and dark-exposure, esterase activity remained significantly below the control level during the entire experimental period and exposure also resulted in changes in other measured parameters. Moreover, since esterase activity of cyanobacteria in the light was generally higher than in the dark, it is clear that darkness itself causes a decline in esterase activity, which can then be further deepened by exposure to H2O2.
Fig. 1. Metabolic (esterase) activity of M. aeruginosa exposed to hydrogen peroxide (A) in the light and (B) in the dark. Results are expressed as the fluorescein fluorescence (mean ± SD) after hydrolysis of FDA. Large plots depict changes over the entire experimental period, inserts highlight the first 8 h of the experiment. Statistical significances (P < 0.05) are indicated using different letters.
Effects of hydrogen peroxide on cell membrane integrity
Flow cytometric measurements proved that SYTOX Green is able to detect changes in membrane integrity of cyanobacteria exposed to H2O2. As shown in the flow cytometric dot-plots in , the percentage of SYTOX+ (i.e. membrane-compromised) cells in highly exposed cyanobacterial suspensions increased with time over the experimental period. Nevertheless, in contrast to the decline of esterase activity, which was immediate, the earliest significant changes in the percentage of membrane-compromised cells took place after 5 h of exposure. In the light, changes occurred slightly more rapidly than in the dark, even though ten times higher concentrations of H2O2 were used for the exposure in the dark regime (). After one day of exposure, more than 95% of cells from the group exposed to 4.00 mg l−1 were membrane-compromised and from the second day, a progressive drop of total cyanobacterial cell counts was also observed (for detailed information see the subsection ‘Total cyanobacterial cell counts’).
Fig. 2. Flow cytometric two-dimensional dot-plots showing effects of hydrogen peroxide (initial concentration 20.00 mg l–1, dark regime) on cell membrane integrity in M. aeruginosa. Side scatter (SSC) and green fluorescence (FL1 – 527/30 nm) were monitored after (A) 7 h, (B) 24 h, (C) 48 h and (D) 72 h of exposure. Membrane-compromised cells were distinguished from the cells with intact membrane using gating regions SYTOX+ and SYTOX– respectively.
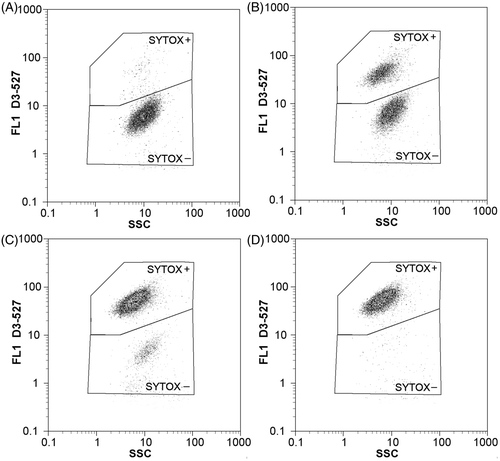
Fig. 3. Cell membrane integrity of M. aeruginosa exposed to hydrogen peroxide in (A) the light and (B) the dark. Results are expressed as the percentage (mean ± SD) of SYTOX+ cells detected by flow cytometry. Statistical significances (P < 0.05) are indicated using different letters.
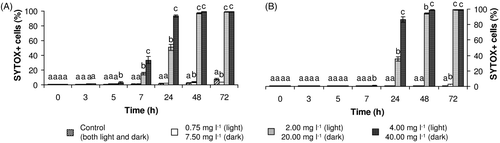
In the dark, on the other hand, the damage to membrane integrity proceeded more slowly. No significant differences in cyanobacterial counts were observed when experimental groups were compared with the control (see the subsection ‘Total cyanobacterial cell counts’). Short-term cultivation of cyanobacteria in the dark also had no significant effect on the SYTOX+ percentage in the control group.
Measurement of parameters of induced chlorophyll a fluorescence
Selected parameters of induced chlorophyll a fluorescence are shown in . H2O2-exposed samples in both the light and the dark demonstrated a concentration-dependent increase in the intensity of the basal fluorescence signal (expressed as F0) during the first 24 h of the exposure (,). After that, in samples exposed to concentrations of 2.00 and 4.00 mg l−1 in the light, the level of fluorescence dropped dramatically. On the other hand, in dark samples exposed to 20.00 and 40.00 mg l−1 the F0 value stayed higher for the whole exposure period than in control and 7.50 mg l−1 samples.
Fig. 4. Changes in selected parameters of induced chlorophyll a fluorescence during exposure of M. aeruginosa to hydrogen peroxide in the light (A, C, E) and dark (B, D, F). The basal chlorophyll a fluorescence level (F0: A, B), maximal quantum yield of photosystem II (FV/FM: C, D) and performance index based on absorption (PIABS: E, F) were monitored for 72 h. Large plots depict changes over the entire experimental period; inserts highlight the first 8 h of the experiment. Results are expressed as the mean ± SD. Statistically significant differences among groups (P < 0.05) are indicated using different letters.
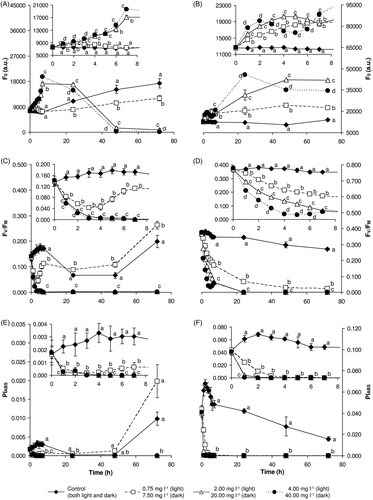
The maximal efficiency yield of photosystem II processes, expressed as the FV/FM ratio, decreased in response to the presence of H2O2 in the light and the dark (,). After 72 h exposure, samples exposed to higher concentrations (2.00, 4.00, 20.00 and 40.00 mg l−1) showed very low FV/FM values, which suggests serious damage to photosystem II. On the other hand, samples exposed in the light to 0.75 mg l−1 reacted by an initial decline of FV/FM values but subsequently recovered and, at the end of the experiment, their FV/FM values were even higher than in the control.
Based on a JIP-test, the decrease of the overall performance index (PIABS) values in H2O2-exposed samples, compared with the control, can be regarded as a serious attack on the photosynthetic apparatus by high concentrations of H2O2 or products of its degradation (,). In the dark, control samples exhibited the highest PIABS values, but the values decreased during the experiment. Conversely, in the light PIABS values decreased in control samples during the first 24 h and recovered towards the end of the experiment, after 72 h. A low concentration of H2O2 (0.75 mg l−1) caused a decrease of the PIABS value already after 1 h, but after 48 h there was a recovery to values even higher than in the control.
Total cyanobacterial cell counts
In the dark, no significant differences were detected, even in the control. Over the entire period of the experiment, counts remained at approximately the same level as at the beginning (i.e. 1.0 × 106 cells ml−1) (see ).
Fig. 5. Total cyanobacterial cell counts of M. aeruginosa exposed to hydrogen peroxide in the light (A) and dark (B). Results are expressed as the mean ±SD. Large plots depict changes over the entire experimental period; inserts highlight the first 8 h of the experiment. Statistically significant differences (if any) among groups (P < 0.05) are marked by different letters.
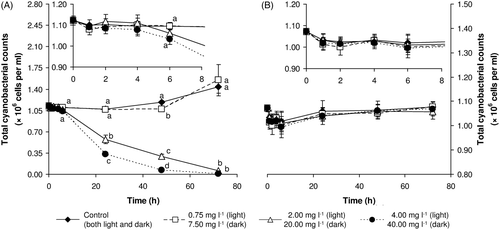
In contrast, significant differences in total cell counts among treatments were detected in the light (see ). In two highly exposed treatments, a significant reduction of total counts was detected from 24 h after the beginning of the exposure. In the highest exposure concentration (4.00 mg l−1) no cells were detected in samples at the end of the experiment. On the other hand, various amounts of cell fragments (i.e. lysed dead cells) were detected in samples from two highly exposed treatments from the second day of exposure. When examined microscopically, these cell fragments were easily distinguished from cyanobacterial cells based on their smaller size and autofluorescence, and it was also possible to gate them out during flow cytometric measurements by using their scatter characteristics. No such fragments were visible in the samples from the dark, agreeing with the lack of change in the cell counts. Whereas total cell counts in two highly exposed groups decreased continuously, cell counts in the control group and in the group exposed to H2O2 at an initial concentration of 0.75 mg l−1 remained relatively stable for the first two days and then increased slightly (see ).
Measurement of hydrogen peroxide decomposition kinetics
Hydrogen peroxide decomposition kinetics can be influenced by several factors (e.g. pH, presence of metal ions, the illumination regime used and presence of the cells in the environment). Therefore, the kinetics were measured (in both experimental regimes: light and dark) in a cell-free medium as well as in a medium supplemented by Microcystis cell suspension. In the light, significant decomposition of H2O2 was detected from the second hour of the experiment (). After 24 h, H2O2 was completely decomposed, or rather its concentrations were below the detection limit (<0.03 mg l−1), in groups exposed to initial concentrations of 0.75 and 2.00 mg l−1. In the treatment with the highest concentration of H2O2, only approximately 5% of H2O2 remained in the medium after 24 h of the exposure. The decomposition of H2O2 in the cell-free medium was significantly slower, compared to that in cyanobacterial cell suspensions, showing that illuminated Microcystis cells play an important role in H2O2 decomposition.
Table 1. Comparison of hydrogen peroxide decomposition in cell suspensions of M. aeruginosa in ZBB medium (initial cell density 1.0 × 106 cells ml–1) (CS) and cell-free ZBB medium (CFM) in light (irradiance 140 µmol m–2 s–1). Results are expressed as the mean ±SD of H2O2 concentration in the medium. After 48 and 72 h, no hydrogen peroxide was detected in any of the treatments. <ld. =below the detection limit of the method (i.e. 0.03 mg l–1); * =statistically significant difference between CS and CFM detected by t-test (P < 0.05).
In the dark the degradation of H2O2 proceeded relatively slowly. At the end of the experiment 21, 68 and 75% of initial H2O2 concentrations were detected in experimental groups exposed to 7.50, 20.00 and 40.00 mg l−1 respectively (). In this case the decomposition rate of H2O2 in the cell-free medium was similar to that detected in medium supplemented with cell suspension, but some significant differences were found. Decomposition of H2O2 was also studied in cell-free deionized water, but even after 72 h no significant changes were found in H2O2 concentrations (data not shown).
Table 2. Comparison of hydrogen peroxide decomposition in cell suspensions of M. aeruginosa in ZBB medium (initial cell density 1.0 × 106 cells ml–1) (CS) and cell-free ZBB medium (CFM) in the dark. Results are expressed as the mean ±SD of H2O2 concentration in the medium. <ld. =below the detection limit of the method (i.e. 0.03 mg l–1); * =statistically significant difference between CS and CFM tested by t-test (P < 0.05).
Discussion
Esterase activity is commonly used for assessing cell viability in ecotoxicity studies of phytoplankton. In the past ten years, esterase activity has been monitored not only in several studies evaluating pollutant toxicity in green algae and cyanobacteria (Franklin et al., Citation2001; Regel et al., Citation2002; Hadjoudja et al., Citation2009; Jamers et al., Citation2009), but also in studies of the influence of environmental factors such as temperature, darkness or nutrient limitation (Jochem, Citation1999; Brookes et al., Citation2000; Wu et al., Citation2008). It has been demonstrated that there are two distinct types of metabolic response among phytoplankton species exposed to prolonged darkness. Whereas some species of marine phytoplankton adapt to the dark by reducing their metabolism to a lower level of activity (type I cells), other species maintain unchanged metabolic activity (type II) (Jochem, Citation1999). As has been suggested by other authors (Zhang et al., Citation2007; Wu et al., Citation2008) and confirmed by our study, M. aeruginosa can be included in the former group, since its metabolic activity (measured as fluorescein fluorescence after FDA hydrolysis) decreases in darkness. The results of our study also proved that the addition of H2O2 to cyanobacterial cell suspensions can inhibit metabolic activity regardless of the experimental regime. Such changes are probably attributable to oxidative damage to enzymatic systems. It seems that the decline in metabolic activity is related to the rate of H2O2 decomposition in the culture medium. Over the entire period of exposure in the dark, mean fluorescence intensity was below control levels in all treatments, because of gradual though relatively slow H2O2 decomposition. The response of cyanobacteria exposed to H2O2 in the light was different. Cyanobacteria exposed to the non-lethal H2O2 concentration of 0.75 mg l−1 reacted to the toxic assault by temporary inhibition of their metabolic (esterase) activity. However, when the decomposition of H2O2 was finished, enzymatic systems of cyanobacteria started to recover and cell metabolic activity increased to the control level. Recovery of M. aeruginosa exposed to non-lethal concentrations of H2O2 after its degradation has previously been described by Qian et al. (Citation2010). The authors reported recovery of growth after 96 h of H2O2 exposure in treatments where the initial concentrations were 25 and 50 µM (0.85 and 1.70 mg l−1 respectively). Although Qian et al. (Citation2010) observed recovery of cyanobacteria in higher initial hydrogen peroxide concentrations than in our study, it should be emphasized that they used a 14 : 10 h light : dark cycle and slightly lower illumination intensity. The results of both studies prove the crucial role of the illumination regime for H2O2 toxicity.
Measurement of cell membrane integrity represents another useful indicator of cell functioning. While cells with intact membranes are presumed to be capable of metabolic activity and of reproduction, unless their DNA is damaged, cells without an intact membrane cannot maintain or generate a membrane potential and can be classified as dead, since their internal structures are freely exposed to the environment (Nebe-von-Caron et al., Citation2000). In phytoplankton, membrane integrity has previously been investigated using membrane-impermeable fluorescent dyes such as propidium iodide (PI) or SYTOX Green (SYTOX). Using PI, Franqueira et al. (Citation2000) investigated the effects of copper and paraquat on freshwater and marine microalgae, demonstrating that the number of cells with damaged membranes increased with the length of exposure (Franqueira et al., Citation2000). SYTOX has previously been used to investigate the effects of experimentally generated turbulence in M. aeruginosa (Regel et al., Citation2004) and also the effects of chlorination (Daly et al., Citation2007). Segovia & Berges (Citation2009) investigated dark-induced apoptosis processes in the unicellular chlorophyte Dunaliella tertiolecta. They reported a significant increase in the number of cells with damaged membranes (i.e. that became stained with SYTOX) after four days of light deprivation. The number rose from less than 5% of cells after three days to almost 90% after four days (Segovia & Berges, Citation2009). In our study, externally added H2O2 caused cell membrane damage in both experimental regimes. While the decline of esterase activity after the addition of H2O2 was immediate, the earliest significant changes in cell membrane integrity were found in highly exposed cyanobacteria (exposed to 4.00 mg l−1 in the light and 40.00 mg l−1 in the dark) after 5 h of exposure. The lethal effect of H2O2 we observed in two highly exposed groups from the light experimental regime was probably connected with so-called programmed cell death (PCD). As reported earlier by Ross et al. (Citation2006), externally added H2O2 can induce caspase activity in vitro, which can precede cellular apoptosis. PCD can also be observed as a consequence of the exposure to other factors such as UV radiation or salinity stress (Ross et al., Citation2006).
Measurements of induced chlorophyll a fluorescence represent a quick, valuable and non-destructive method for photosynthetic status assessment, even though there are some limitations connected to its application to cyanobacteria (for a review see Campbell et al., Citation1998). The level of fluorescence signal is strongly dependent on the actual cellular concentration of photosynthetic pigments – of chlorophylls and especially of the phycobiliproteins. Phycobiliproteins, either free or integrated in phycobilisomes, exhibit their own fluorescence in a similar spectral range to chlorophylls and their spectra partly overlap. The observed increase of basal chlorophyll fluorescence (F0, ,), which is similar to that described by Drabkova et al. (Citation2007b , Citation2007c ), could thus be caused either by (1) inhibition of photosystem II function, or (2) elevated fluorescence yield from phycobilisomes or free phycobiliproteins. According to Campbell et al. (Citation1998), some phycobilisomes are uncoupled to the photosynthetic apparatus. Their fluorescence yield is high, but in favourable conditions their number is very low and their fluorescence contribution is minor; most phycobilisomes are coupled to photosystems I or II. In stress conditions, uncoupling could occur and the fluorescence yield of the phycobilisomes could rise (Campbell et al., Citation1998). Due to its negative effect on cell membrane integrity, H2O2 could probably cause such uncoupling or decomposition of phycobilisomes to autonomous phycobiliproteins.
Maximal chlorophyll fluorescence yield (FV/FM) has been verified as an index of the maximal photochemical efficiency of photosystem II in green algae and higher plants (Rohacek & Bartak, Citation1999). Due to the phycobilisome contribution to fluorescence, this parameter is not fully valid in cyanobacteria, but when compared with control samples, even in cyanobacteria FV/FM can give valuable information. Decrease of photosystem II efficiency, as shown by a drop in FV/FM value (,), is the most common reaction of photosystem II to various kinds of stress, both abiotic and biotic.
The JIP-test has often been utilized to demonstrate the overall performance on fluxes, yields of trapping and electron transport in photosystem II (Appenroth et al., Citation2001). Based on the test, various fluorescence parameters can be calculated and compared. As an overall measure of photosystem II behaviour, the so-called performance index related to the efficiency of energy absorption (PIABS) can be used. In our study, PIABS reached only low values (up to 0.06), even in control samples, compared to values calculated for green algae or higher plants. Nevertheless, the rapid decrease of PIABS after only 1 h in both light and dark H2O2 treatments shows the strong negative effect of H2O2 and the products of its degradation. In the study of Appenroth et al. (Citation2001), Spirodela polyrhiza plants were exposed to chromium and exhibited not only a concentration- but also a time-dependent PIABS response. In our study, the cyanobacterium M. aeruginosa exhibited a similar response to H2O2, but it was faster and bigger.
All of the changes in primary photosynthetic processes described above could also be related to negative effect of H2O2 on the oxygen-evolving complex (OEC). The presence of elevated H2O2 concentration could lead to a release of Mn2+ ions from OEC, lower O2 production and subsequent lack of electrons for reduction of the photosystem II reaction centre and electron transport (Samuilov et al., Citation2004).
The increase in FV/FM and PIABS values found after 48 h in samples exposed in the light to the lowest H2O2 concentration (0.75 mg l−1) proved that this concentration is not lethal for cyanobacteria and, after a period of time that is enough to allow H2O2 decomposition, cells are able to recover their photosynthetic activity.
Our results also confirmed that, when they are illuminated, cyanobacterial cells are involved in H2O2 decomposition, so that the rate of decomposition is significantly higher in cyanobacterial suspensions than in cell-free medium. In the dark, on the other hand, the contribution of M. aeruginosa to H2O2 decomposition is minor and statistically significant differences between cyanobacterial suspensions and cell-free medium were detected only in some treatments and measurement times. This can be explained by differences in cyanobacterial metabolism in the dark and light. In cyanobacteria, several groups of enzymes participate in H2O2 detoxification. Peroxiredoxins represent the only peroxide-degrading enzymes found in all cyanobacterial genomes sequenced so far and they are the only group of peroxide-degrading enzymes whose genes were found in M. aeruginosa genome (reviewed in Bernroitner et al., Citation2009). A complexity of the antioxidant defence has been previously demonstrated in Synechococcus sp. strain PCC 7942. It has been reported that whereas a thioredoxin peroxidase-like enzyme is crucial for growth in high-light conditions, catalase-peroxidase is essential for survival and the elimination of relatively high concentrations of externally added H2O2 (Perelman et al., Citation2003). Little is known about reactive oxygen species defence mechanisms in Microcystis. Based on our results it can be proposed that in M. aeruginosa the expression of peroxiredoxins (or, rather, hydrogen peroxide-degrading enzymes) may be inhibited in the dark.
Conclusions
Our experiments confirmed that changes in H2O2 decomposition kinetics in different illumination regimes strongly influence H2O2 toxicity in M. aeruginosa. The decomposition rate of H2O2 was highly dependent on the illumination intensity. Whereas the decomposition of H2O2 was rapid in continuous light, in the dark it proceeded relatively slowly.
After all H2O2 had decomposed, cyanobacteria exposed in the light (at an irradiance of 140 µmol photons m−2 s−1) to an initial H2O2 concentration of 0.75 mg l−1 recovered. Metabolic activity and parameters of induced chlorophyll a fluorescence represented the most sensitive biomarkers of exposure of cyanobacteria to H2O2. While the earliest changes in these parameters were observed after 1 h of exposure (i.e. during the first measurements after the experiment started), changes in the percentage of membrane-compromised cells were not detected until after 5 h. A loss of membrane integrity was observed, followed by cell lysis, in two highly exposed treatments in the light (with 2.00 and 4.00 mg l−1), but the longest exposure period employed (72 h) was not enough to observe any loss of cells in cyanobacteria exposed to H2O2 in the dark, even though >99.5 % of cells from the two most highly exposed treatments (20.00 and 40.00 mg l−1) were membrane-compromised at the end of the experiment.
The present study is the first detailed experimental assessment of the effects of the potential algicide H2O2 on the metabolic activity and the cell membrane integrity of a M. aeruginosa in different illumination regimes. Future studies should be carried out to investigate properly and in a greater detail the toxic effects of H2O2 on other species of cyanobacteria, as well as on non-target species (fish or aquatic invertebrates).
Acknowledgements
The research was conducted with the financial support from the Ministry of Industry and Trade of the Czech Republic (project FR-TI3/196) and the Technology Agency of the Czech Republic (project TA 01010356). This study was also supported as a long-term research development project no. RVO 67985939 (Institute of Botany of the ASCR).
References
- Appenroth , KJ , Stöckel , J , Srivastava , A and Strasser , RJ . 2001 . Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probed by OJIP chlorophyll a fluorescence measurements . Environmental Pollution , 115 : 49 – 64 .
- Avendano-Herrera , R , Magarinos , B , Irgang , R and Toranzo , AE . 2006 . Use of hydrogen peroxide against the fish pathogen Tenacibaculum maritimum and its effect on infected turbot (Scophthalmus maximus) . Aquaculture , 257 : 104 – 110 .
- Bader , H , Sturzenegger , V and Hoigne , J . 1988 . Photometric-method for the determination of low concentrations of hydrogen-peroxide by the peroxidase catalyzed oxidation of N,N-diethyl-p-phenylenediamine (Dpd) . Water Research , 22 : 1109 – 1115 .
- Bernroitner , M , Zamocky , M , Furtmüller , PG , Peschek , GA and Obinger , C . 2009 . Occurrence, phylogeny, structure, and function of catalases and peroxidases in cyanobacteria . Journal of Experimental Botany , 60 : 423 – 440 .
- Bold , HC . 1949 . The morphology of Chlamydomonas chlamydogama sp. nov . Bulletin of the Torrey Botanical Club , 76 : 101 – 108 .
- Brookes , JD , Geary , SM , Ganf , GG and Burch , MD . 2000 . Use of FDA and flow cytometry to assess metabolic activity as an indicator of nutrient status in phytoplankton . Marine and Freshwater Research , 51 : 817 – 823 .
- Campbell , D , Hurry , V , Clarke , AK , Gustafsson , P and Öquist , G . 1998 . Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation . Microbiology and Molecular Biology Reviews , 62 : 667 – 683 .
- Daly , RI , Ho , L and Brookes , JD . 2007 . Effect of chlorination on Microcystis aeruginosa cell integrity and subsequent microcystin release and degradation . Environmental Science and Technology , 41 : 4447 – 4453 .
- De Oliveira , EC , Lopes , RM and Paumgartten , FJR . 2004 . Comparative study on the susceptibility of freshwater species to copper-based pesticides . Chemosphere , 56 : 369 – 374 .
- Drabkova , M , Marsalek , B and Admiraal , W . 2007a . Photodynamic therapy against cyanobacteria . Environmental Toxicology , 22 : 112 – 115 .
- Drabkova , M , Admiraal , W and Marsalek , B . 2007b . Combined exposure to hydrogen peroxide and light – Selective effects on cyanobacteria, green algae, and diatoms . Environmental Science and Technology , 41 : 309 – 314 .
- Drabkova , M , Matthijs , HCP , Admiraal , W and Marsalek , B . 2007c . Selective effects of H2O2 on cyanobacterial photosynthesis . Photosynthetica , 45 : 363 – 369 .
- Franklin , NM , Adams , MS , Stauber , JL and Lim , RP . 2001 . Development of an improved rapid enzyme inhibition bioassay with marine and freshwater microalgae using flow cytometry . Archives of Environmental Contamination and Toxicology , 40 : 469 – 480 .
- Franqueira , D , Orosa , M , Torres , E , Herrero , C and Cid , A . 2000 . Potential use of flow cytometry in toxicity studies with microalgae . Science of the Total Environment , 247 : 119 – 126 .
- Hadjoudja , S , Vignoles , C , Deluchat , V , Lenain , JF , Le Jeune , AH and Baudu , M . 2009 . Short term copper toxicity on Microcystis aeruginosa and Chlorella vulgaris using flow cytometry . Aquatic Toxicology , 94 : 255 – 264 .
- Hong , Y , Hu , HY , Xie , X , Sakoda , A , Sagehashi , M and Li , FM . 2009 . Gramine-induced growth inhibition, oxidative damage and antioxidant responses in freshwater cyanobacterium Microcystis aeruginosa . Aquatic Toxicology , 91 : 262 – 269 .
- Jamers , A , Lenjou , M , Deraedt , P , Van Bockstaele , D , Blust , R and de Coen , W . 2009 . Flow cytometric analysis of the cadmium-exposed green alga Chlamydomonas reinhardtii (Chlorophyceae) . European Journal of Phycology , 44 : 541 – 550 .
- Jancula , D and Marsalek , B . 2011 . Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms . Chemosphere , 85 : 1415 – 1422 .
- Jancula , D , Suchomelova , J , Gregor , J , Smutna , M , Marsalek , B and Taborska , E . 2007 . Effects of aqueous extracts from five species of the family Papaveraceae on selected aquatic organisms . Environmental Toxicology , 22 : 480 – 486 .
- Jancula , D , Drabkova , M , Cerny , J , Karaskova , M , Korinkova , R , Rakusan , J and Marsalek , B . 2008 . Algicidal activity of phthalocyanines – Screening of 31 compounds . Environmental Toxicology , 23 : 218 – 223 .
- Jochem , FJ . 1999 . Dark survival strategies in marine phytoplankton assessed by cytometric measurement of metabolic activity with fluorescein diacetate . Marine Biology , 135 : 721 – 728 .
- Murray-Gulde , CL , Heatley , JE , Schwartzman , AL and Rodgers , JH . 2002 . Algicidal effectiveness of clearigate, cutrine-plus, and copper sulfate and margins of safety associated with their use . Archives of Environmental Contamination and Toxicology , 43 : 19 – 27 .
- Nebe-von-Caron , G , Stephens , PJ , Hewitt , CJ , Powell , JR and Badley , RA . 2000 . Analysis of bacterial function by multi-colour fluorescence flow cytometry and single cell sorting . Journal of Microbiological Methods , 42 : 97 – 114 .
- Perelman , A , Uzan , A , Hacohen , D and Schwarz , R . 2003 . Oxidative stress in Synechococcus sp. strain PCC 7942: various mechanisms for H2O2 detoxification with different physiological roles . Journal of Bacteriology , 185 : 3654 – 3660 .
- Qian , HF , Yu , SQ , Sun , ZQ , Xie , XC , Liu , WP and Fu , ZW . 2010 . Effects of copper sulfate, hydrogen peroxide and N-phenyl-2-naphthylamine on oxidative stress and the expression of genes involved in photosynthesis and microcystin disposition in Microcystis aeruginosa . Aquatic Toxicology , 99 : 405 – 412 .
- Rach , JJ , Gaikowski , MP , Howe , GE and Schreier , TM . 1998 . Evaluation of the toxicity and efficacy of hydrogen peroxide treatments on eggs of warm and cool water fishes . Aquaculture , 165 : 11 – 25 .
- Regel , RH , Ferris , JM , Ganf , GG and Brookes , JD . 2002 . Algal esterase activity as a biomeasure of environmental degradation in a freshwater creek . Aquatic Toxicology , 59 : 209 – 223 .
- Regel , RH , Brookes , JD , Ganf , GG and Griffiths , R . 2004 . The influence of experimentally generated turbulence on the Mash01 unicellular Microcystis aeruginosa strain . Hydrobiologia , 517 : 107 – 120 .
- Rohacek , K and Bartak , M . 1999 . Technique of the modulated chlorophyll fluorescence: basic concepts, useful parameters, and some applications . Photosynthetica , 37 : 339 – 363 .
- Ross , C , Santiago-Vazquez , L and Paul , V . 2006 . Toxin release in response to oxidative stress and programmed cell death in the cyanobacterium Microcystis aeruginosa . Aquatic Toxicology , 78 : 66 – 73 .
- Samuilov , VD , Bezryadnov , DV , Gusev , MV , Kitashov , AV and Fedorenko , TA . 1999 . Hydrogen peroxide inhibits the growth of cyanobacteria . Biochemistry –Moscow , 64 : 47 – 53 .
- Samuilov , VD , Timofeev , KN , Sinitsyn , SV and Bezryadnov , DV . 2004 . H2O2-induced inhibition of photosynthetic O2 evolution by Anabaena variabilis cells . Biochemistry –Moscow , 69 : 926 – 933 .
- Segovia , M and Berges , JA . 2009 . Inhibition of caspase-like activities prevents the appearance of reactive oxygen species and dark-induced apoptosis in the unicellular chlorophyte Dunaliella tertiolecta . Journal of Phycology , 45 : 1116 – 1126 .
- Staub , R . 1961 . Research on physiology of nutrients of the planktonic cyanobacterium Oscillatoria rubescens . Schweizerische Zeitschrift für Hydrologie , 23 : 82 – 198 .
- Van Hullebusch , E , Deluchat , V , Chazal , PM and Baudu , M . 2002 . Environmental impact of two successive chemical treatments in a small shallow eutrophied lake: Part II. Case of copper sulfate . Environmental Pollution , 120 : 627 – 634 .
- Wu , ZX , Song , LR and Li , RH . 2008 . Different tolerances and responses to low temperature and darkness between waterbloom forming cyanobacterium Microcystis and a green alga Scenedesmus . Hydrobiologia , 596 : 47 – 55 .
- Xu , YF , Yang , J , Wang , YL , Liu , F and Jia , JP . 2006 . The effects of jet cavitation on the growth of Microcystis aeruginosa . Journal of Environmental Science and Health Part A – Toxic/Hazardous Substances and Environmental Engineering , 41 : 2345 – 2358 .
- Zhang , GM , Zhang , PY , Liu , H and Wang , B . 2006 . Ultrasonic damages on cyanobacterial photosynthesis . Ultrasonic Sonochemistry , 13 : 501 – 505 .
- Zhang , M , Kong , FX , Shi , XL , Xing , P and Tan , X . 2007 . Differences in responses to darkness between Microcystis aeruginosa and Chlorella pyrenoidosa . Journal of Freshwater Ecology , 22 : 93 – 99 .