Abstract
The carotenoid biosynthesis pathway catalyses the synthesis of essential pigments that are crucial for light harvesting and photoprotection in photosynthetic organisms. It allows the production of several commercially important compounds and is the target of many herbicides. In the present work we studied the influence of light on the carotenoid composition and the expression of genes encoding the main steps of the pathway in the freshwater microalga Chlamydomonas reinhardtii. We observed that there is an activation of the xanthophyll cycle in response to high light, but also in response to other stress conditions, such as nitrogen starvation, which has not been reported previously. We analysed the expression level of (1) genes encoding the two first enzymes of the pathway, phytoene synthase and phytoene desaturase; (2) the enzymes responsible for the cyclization of lycopene, lycopene β-cyclase and lycopene ε-cyclase; (3) zeaxanthin epoxidase, which catalyses the epoxidation of zeaxanthin; and (4) the three known carotene hydroxylases, directly involved in the synthesis of xanthophylls from α and β-carotene. Measurements of carotenoid content in the presence of inhibitors of protein and carotenoid synthesis suggest that only one of the two possible routes for the synthesis of zeaxanthin upon transference to high light, either the de novo synthesis of carotenoids or the interconversion of violaxanthin and zeaxanthin, is dependent on protein synthesis. The high increase in the transcript levels of the cytochrome-dependent carotene β- and ε-hydroxylases in response to high light suggests an important role of these enzymes in regulation of xanthophyll synthesis upon light stress. These conclusions may be of high interest if efficient engineering of the pathway is to be accomplished.
Abbreviations
| bkt | = |
β-carotene ketolase gene |
| CPTA | = |
2-(4-chlorophenylthio) triethylamine hydrochloride |
| DW | = |
dry weight |
| NPQ | = |
non-photochemical quenching |
| LHCII | = |
light harvesting complex II |
| PSII | = |
photosystem II |
| HPLC | = |
high performance liquid chromatography |
| LHC | = |
light harvesting complex |
| psy | = |
phytoene synthase gene |
| pds | = |
phytoene desaturase gene |
| zep | = |
zeaxanthin epoxidase gene |
| vde | = |
violaxanthin de-epoxidase gene |
| lycb | = |
lycopene β-cyclase gene |
| lcye | = |
lycopene ε-cyclase gene |
| chyb | = |
carotene β-hydroxylase gene |
| cyp97a5 | = |
P450 cytochrome-dependent carotene β-hydroxylase gene |
| cyp97c3 | = |
P450 cytochrome-dependent carotene ε-hydroxylase gene |
Introduction
Carotenoids are isoprenoid pigments with high commercial value and important physiological functions, due to their antioxidant, colourant and provitamin properties. In plant cells, this pathway is the target of many herbicides and catalyses the synthesis of essential pigments for light harvesting, maintenance of photosystem structure, and photoprotection mechanisms. Animals and other non-photosynthetic organisms that do not synthesize carotenoids de novo must include them in their diet to acquire their characteristic colours or act as precursors for essential compounds such as vitamin A or its derivative, the visual pigment retinal. Furthermore, the intake of carotenoids has proved to offer protection against macular degeneration, UV-induced skin damage and some age-related degenerative diseases (Guerin et al., Citation2003; Fraser & Bramley, Citation2004). The reviews by Cunningham & Gantt (Citation1998), Lohr et al. (Citation2005), Botella-Pavía & Rodriguez-Concepción (Citation2006) and Sandmann et al. (Citation2006), and also those by Lu & Li (Citation2008), Giuliano et al. (Citation2008) and Takaichi (Citation2011), offer a good general view of this metabolic pathway in plants and microorganisms.
The nutritional benefits of these terpenoids have stimulated work to obtain genetically modified plants with new carotenoids or higher quantities of those already synthesized. However, in many cases, unexpected collateral effects, low functionality or silencing of the exogenous genes has reduced the productivity or viability of the new transgenic strains (Ye et al., Citation2000; Fraser et al., Citation2002; Paine et al., Citation2005; Diretto et al., Citation2007). Despite the physiological and economic importance of this metabolic route in microalgae, many regulatory aspects are not clear and several genes remain unidentified. It is essential to get a deep understanding of the enzymes involved in this pathway and to identify the key regulatory steps before it will be possible to achieve efficient metabolic engineering of this pathway and improve the production of the different added-value carotenoids (Stephanopoulos, Citation2002). Furthermore, it has been shown that the synthesis of carotenoids can be increased to an extraordinary extent under certain environmental conditions, such as high light, nutrient starvation or other stress conditions. Changes in the expression of the key enzymatic steps of the pathway as a response to stress conditions are also essential to understand the response of the cells and eventually to find the best conditions to synthesize carotenoids.
Microalgae are one of the main sources of natural carotenoids. They combine the fast and easy growth of bacteria and other unicellular microorganisms with an active isoprenoid metabolism, which ensures enough precursors for the carotenogenic pathway, and adequate storage capacity. All these reasons make microalgae ideal cell factories for the biotechnological production of high added-value carotenoids (León et al., Citation2004). Unfortunately, the nuclear transformation of microalgae is limited to a small number of species. Chlamydomonas was the first genetically modified microalga (Fernández et al., Citation1989) and, even now, is one of the easiest and most stable eukaryotic systems for genetic manipulation. Indeed, this microalga has become a powerful model system, due to its easy growth in the laboratory under different environmental and nutritional conditions, short generation time (about 6 h) and the availability of molecular and bioinformatic tools, including plenty of specific promoters and marker genes and a draft of the complete genome sequence (http://www.phytozome.net/chlamy) (Merchant et al., Citation2007).
Chlamydomonas could be an excellent biotechnological system for the massive production of high added-value carotenoids, if adequately engineered (León et al., Citation2004; Couso et al., Citation2011). It has already been shown that the expression of an exogenous β-carotene oxygenase (bkt) gene in C. reinhardtii allows the synthesis of new ketocarotenoids that the microalga did not previously produce (León et al., Citation2007), and the over-expression of foreign phytoene synthase (psy) genes causes the production of higher levels of carotenoids (Cordero et al., Citation2011; Couso et al., Citation2011). But there are still serious difficulties in achieving high productivity. Limited knowledge about the mechanisms and signals that control carotenoid biosynthesis and storage is the main challenge to obtain higher levels of carotenoids through metabolic engineering of the carotenoid biosynthetic pathway in Chlamydomonas and other microalgae.
In this work we try, using the model species C. reinhardtii, to gain knowledge about the regulation of carotenoid biosynthesis pathway in green algae. We studied changes in the carotenoid profile when Chlamydomonas cells are subjected to high light stress in the presence of protein and carotenoid synthesis inhibitors, and we analysed the expression level of the genes encoding the enzymes that catalyse the main steps of the pathway.
Materials and methods
Organisms and standard culture conditions
Chlamydomonas reinhardtii wild strain 21 gr from Dr R. Sager (Sidney Faber Cancer Center, Boston, MA) was grown at 25°C in 15 mM K-phosphate (pH 7) buffered medium (Sueoka et al., Citation1967) under continuous white light irradiation of 100 µE m−2 s−1, except where indicated below. The cultures were bubbled with air containing 5% CO2. Inhibitors used were cycloheximide (4-[(2R)-2-[(1S, 3S, 5S)-3,5-Dimethyl-2-oxocyclohexyl]-2-hydroxyethyl]piperidine-2,6-dione) or norflurazon (4-chloro-5-methylamino-2-(α, α,α-trifluoro-m-tolyl) pyridazin-3(2H)-one), supplied by SIGMA (St. Louis, MO, USA); these were added to the culture medium. The Escherichia coli strain used for in vivo amplification of DNA was DH5α, cultured in LB medium as previously described by Sambrook et al. (Citation1989).
High-irradiance experiments
For high-irradiance experiments, C. reinhardtii cultures contained in 0.5-l flasks were illuminated with an adjustable halogen lamp (Philips 500W). The flasks were immersed in a glass thermostatic bath. The bath was connected to a refrigerated circulator (LAUDA E300, Germany) to ensure culture temperatures of 25°C even at very high light intensity. Light intensity was measured by a Delta OHM (Padova, Italy) quantum photo radiometer equipped with a PAR sensor.
Photosynthetic activity
For light-dependent photosynthetic activity determinations 1 ml of microalga culture was placed in the reaction chamber of a Hansatech CBI Clark-type electrode (Norfolk, UK) and the light-dependent O2 evolution was measured. Measurements were made at 25°C under saturating white light (1500 µE m−2 s−1). All data are the average of at least three replicates.
RNA isolation and retrotranscription reaction
Isolation of total RNA was performed with the RNAeasy plant MiniKit of Qiagen (Hilden, Germany) and residual genomic DNA was removed by incubation with the DNAase (Quiagen) for 10 min, as indicated by the manufacturer. The RNA concentrations were determined at 260 nm with a Nanodrop 1000 spectrophotometer (Thermo Scientific, MA, USA). Single-strand cDNA was synthesized from total RNA with oligodT (0.5 µM) and the SuperScript II RNaseH- reverse transcriptase (Invitrogen, CA, USA) in 20 µl of reaction mixture, at 25°C for 10 min, followed by 50 min incubation at 42°C. The reaction was stopped by heat inactivation at 70°C for 15 min, and RNA was removed with RNase H treatment at 37°C for 20 min. The obtained cDNA was used as substrate for real time (RT-) PCR reactions.
Quantitative RT-PCR
Real-time PCR was performed on a Mx3000P Multiplex Quantitative PCR System from Stratagene using the Brilliant SYBR Green QPCR Master Mix (Stratagene, Agilent Technologies, La Jolla, CA, USA). Each determination was carried out in triplicate using as template the cDNA synthesized from total RNA, as previously described, and 10 pmol of the primers indicated (see ) in a final volume of 25 µl. Cycling conditions were: 10 min at 95°C for activation of the hot start Taq polymerase and 40 cycles for melting (30 s at 95°C), annealing (30 s at 60°C) and extension (30 s at 72°C). The fluorescence measurement was made at the end of the annealing step. A dissociation curve (30 s at 95°C, 30 s at 55°C and 30 s at 95°C) was applied at the end of the amplification reaction to check possible formation of dimers.
Table 1. Sequence of the primers used for real-time PCR experiments.
All primers were designed to yield amplicons between 150 and 280 bp () and in such a way that they anneal to regions of the mRNA encoded by different exons. Eventual amplification of genomic DNA with these primers yielded a longer PCR product that could be easily detected, both in agarose gels and in the dissociation curve of RT-PCR. This avoided interference by possible genomic contaminations in the mRNA quantification. All primer pairs designed had efficiencies of 100–110%.
The cblp gene (GenBank X53574), which encodes a protein that shows sequence similarity to the beta subunit of G-binding proteins from mammals, was used as the housekeeping gene (Im & Grossman, Citation2002). Expression of this gene was previously checked to be constitutive under the different conditions used. Primers cblpfor and cblprev amplify a 219 bp fragment. Eventual amplification of genomic DNA with these primers generated a fragment of 449 bp.
Dry weight determination
Dry weight was determined by filtering an exact volume of macroalgal culture (10 ml) on pre-tared glass-fibre filters (1 µm pore size). The filter was washed with a solution of ammonium formate (0.5 M) to remove salts and dried at 100°C for 24 h. The dried filters were weighed in an analytical balance and the dry weight calculated by difference between the pre-tared and the cell-containing dried filters. All measurements were done in triplicate.
Pigment extraction and analysis
Carotenoids and chlorophylls were extracted with 80% acetone. The separation and chromatographic analysis of pigments were performed on a Merck Hitachi LaCrom Elite HPLC (Darmstadt, Germany) equipped with a diode-array detector, as described by Young et al. (Citation1997), using a RP-18 column and a flow rate of 1 ml min−1. The mobile phase consisted of ethyl acetate as solvent A and 9 : 1 (v/v) acetonitrile : water as solvent B and the gradient programme applied was: 0–16 min, 0–60% A; 16–30 min, 60% A; and 30–35 min 100% A. The injection volume was 100 µl. Pigment detection was carried out at 450 nm, excepting phytoene, which was detected at 288 nm. Pigment standards were supplied by SIGMA or DHI (Hoersholm, Denmark). The results are the average of at least two replicates.
Results
Activation of the xanthophyll cycle in response to high irradiance and other stress conditions
To check the ability of several stress factors to trigger the xanthophyll cycle, we subjected Chlamydomonas cells to increasing light intensities between 50 and 1000 µE m−2 s−1, to nitrogen starvation and to the herbicide norflurazon. The content of zeaxanthin, antheraxanthin and violaxanthin after several hours of growth were measured in these conditions. In , the concentrations ofthe xanthophyll cycle components and the de-epoxidation state observed after 6 h of growth are plotted versus light intensity. The de-epoxidation state is expressed as the (ant + zea)/(ant + zea + viol) ratio. Accumulation of zeaxanthin and antheraxanthin, and the corresponding decrease in violaxanthin are correlated with high light intensity. After only 6 hours of exposure to very high irradiance (1000 µE m−2 s−1), almost half of the total xanthophyll pool was de-epoxidized.
Fig. 1. De-epoxidation state of the xanthophyll cycle in Chlamydomonas reinhardtii cells after 6 h exposure to light of different intensities. The concentrations of violaxanthin (•), zeaxanthin (▴), and antheraxanthin (♦), and the de-epoxidation state (□), expressed as the ratio [(ant + zea)/(ant + zea + viol)], are shown.
![Fig. 1. De-epoxidation state of the xanthophyll cycle in Chlamydomonas reinhardtii cells after 6 h exposure to light of different intensities. The concentrations of violaxanthin (•), zeaxanthin (▴), and antheraxanthin (♦), and the de-epoxidation state (□), expressed as the ratio [(ant + zea)/(ant + zea + viol)], are shown.](/cms/asset/f278ac67-b0c2-47eb-b513-56561278d1fc/tejp_a_692816_o_f0001g.gif)
No zeaxanthin was found in control cells cultured at light intensities of 100 µE m−2 s−1, but when these moderate-light-cultured cells were submitted to other stressing conditions an activation of the xanthophyll cycle and accumulation of zeaxanthin was also observed. In , the intracellular zeaxanthin concentrations are given for C. reinhardtii cells cultured for 48 h at moderate light in the presence of the herbicide norflurazon (5 µg ml−1) and with nitrogen starvation. The presence of zeaxanthin started to be detectable only after 24 h of growth at the indicated conditions and practically no antheraxanthin was found. Thus we showed that high light intensity and depletion of nitrogen were able to trigger the synthesis of zeaxanthin. Since the response to high-light stress was much faster than the response to nitrogen depletion, we used high irradiance (800 µE m−2 s−1) to induce the xanthophyll cycle in the presence of protein and carotenoid synthesis inhibitors, as described in the following experiments.
Table 2. Concentration of the xanthophyll cycle components and the de-expoxidation state of the cycle in C. reinhardtii cells grown in different conditions. Cells grown in standard conditions were harvested at the exponential phase of growth and resuspended in fresh complete medium, low-nitrogen medium, or complete medium containing 5 µg ml−1 of the herbicide norflurazon. All cultures were kept at normal irradiance (100 µE m−2 s−1), except the high light treatment, which was subjected to 1000 µE m−2 s−1. Analysis of violaxanthin, zeaxanthin and antheraxanthin was performed at the times indicated in parentheses and are expressed as mean ± SD.
Time-course evolution of the carotenoid content in the presence of protein and carotenoid synthesis inhibitors
Upon transfer of Chlamydomonas cells to high light, there were changes not only in the concentrations of xanthophyll cycle components, but also in the content of other intermediates of the pathway. We analysed the time-course evolution of the main carotenoid pigments in C. reinhardtii cells cultured at different light intensities, and we studied the effect of norflurazon and cycloheximide on this evolution ().
Fig. 2. Time-course evolution of the main carotenoid pigments in C. reinhardtii cells cultured at high light in the presence of carotenoid and protein synthesis inhibitors. Chlamydomonas reinhardtii cells harvested at the beginning of the exponential phase were resuspended in fresh medium and cultured at high irradiance (800 µE m−2 s−1) in the presence of 2 µg ml−1 of the protein translation inhibitor, cycloheximide (▴); in the presence of 5 µg ml−1 of the carotenoid synthesis inhibitor, norflurazon (♦); and with no inhibitor (▪). The pigment content was also analysed in control cells cultured at a light intensity of 100 µE m−2 s−1 (□). After 2, 4, 6 and 8 h growth in these conditions, zeaxanthin, antheraxanthin, neoxanthin, β-carotene, lutein and violaxanthin were quantified by HPLC.
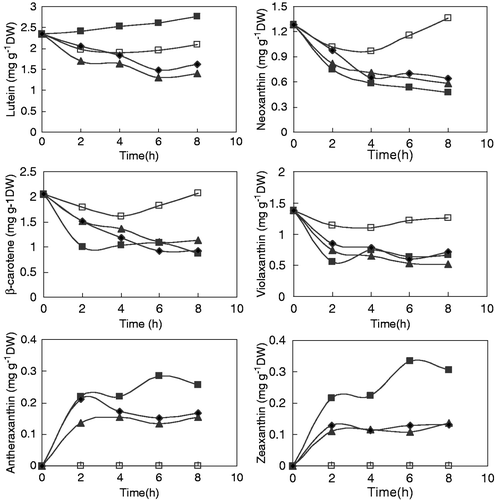
Cycloheximide is an inhibitor of eukaryotic protein translation and has previously been used successfully for inhibition of protein expression in Chlamydomonas (León & Galván, Citation1995). Norflurazon is a well-known bleaching herbicide that inhibits carotenoid biosynthesis in higher plants (Simkin et al., Citation2000) and microalgae (Shaish et al., Citation1990; León et al., Citation2005). To optimize the concentration of norflurazon able to inhibit the synthesis of carotenoids in C. reinhardtii, the microalga was cultured with increasing quantities of the herbicide for 24 h (). Norflurazon concentrations of 1, 2, 5 and 10 µg ml−1 caused a decrease in the β-carotene content and an increase in the concentration of phytoene. The highest accumulation of phytoene was observed at a norflurazon concentration of 5 µg ml−1. Higher concentrations of norflurazon caused certain inhibition of photosynthetic activity and lower production of carotenoids. So, for the time-course evolution experiments a norflurazon concentration of 5 µg ml−1 was used.
Table 3. Effect of the herbicide norflurazon on the carotenoids content in Chlamydomonas reinhardtii. The content of phytoene and the major carotenoids, violaxanthin, lutein and β-carotene, was determined in C. reinhardtii cells grown for 24 h in standard conditions (see Materials and methods) in the presence of different quantities of norflurazon. Photosynthetic activity is also shown. All figures are mean ± SD.
Chlamydomonas reinhardtii cells grown in the standard conditions described in Materials and methods were harvested at the beginning of the exponential phase of growth, resuspended in fresh medium and cultured at high irradiance (800 µE m−2 s−1) with either (1) cycloheximide (2 µg ml−1), (2) norflurazon (5 µg ml−1) or (3) no inhibitor. After 2, 4, 6 and 8 h of growth in these conditions, the carotenoids lutein, zeaxanthin, antheraxanthin, neoxanthin, β-carotene and violaxanthin were quantified by HPLC (). The content of carotenoids was also analysed in control cells cultured at a photon irradiance of 100 µE m−2 s−1. Zeaxanthin and antheraxanthin were undetectable in Chlamydomonas cells grown at low light (50 µE m−2 s−1) intensity, whereas under high irradiance the concentration of both zeaxanthin and the intermediate antheraxanthin was about 0.3 µg g−1 DW (). At this high irradiance, zeaxanthin and antheraxanthin were, at least partially, synthesized at the expense of violaxanthin, whose internal concentration decreased drastically in high-light stressed cells but stayed constant or increased in control cells illuminated at low intensity.
The intracellular levels of zeaxanthin and antheraxanthin in high-light stressed cells treated with norflurazon were much higher than in cells grown at low irradiance, but not as high as in the stressed cells without inhibitor. This means that, although the zeaxanthin is mainly synthesized by de-epoxidation of violaxanthin, a proportion is synthesized from β-carotene. The increase in the concentration of zeaxanthin observed at high irradiance in Chlamydomonas in the presence of norflurazon was due exclusively to the interconversion of violaxanthin and zeaxanthin, since de novo synthesis of carotenoids from their precursors is inhibited in the presence of the herbicide. Accumulation of zeaxanthin on transfer to high light in the presence of cycloheximide was not as high as in the absence of the antibiotic, but was not completely prevented (). In the light-stressed cultures, β-carotene level showed a certain decrease, while lutein increased slightly. Norflurazon and cycloheximide caused a major decrease in the content of lutein but had no influence on the evolution of β-carotene during the 8 hours of exposure ().
Expression of key genes of the carotenoid biosynthesis pathway upon exposure to high irradiance
Microarray (Im et al., Citation2003; Ledford et al., Citation2004; Fischer et al., Citation2006) and proteomic approaches (Förster et al., Citation2006) have been used to identify genes and proteins induced by high light in Chlamydomonas. These studies have given a global picture of changes in gene expression in response to high light, but the genes and proteins involved in carotenoid synthesis, which are very important in the response to light- and oxidative stress, have been poorly represented.
We studied the transcription level of a wide range of carotenoid synthesis genes following transfer from the dark to high light intensity. Chlamydomonas reinhardtii cells were collected at the beginning of the exponential phase of growth and cultured in the dark for 16 h, then transferred to high irradiance (800 µE m−2 s−1) where they were cultured for 6 h. Samples were taken after 1, 3 and 6 h of exposure. Total RNA was extracted from each of the samples and transcript levels of the enzymes catalysing the main steps of the pathway were analysed by qPCR. We studied the first steps of the pathway (PSY and PDS), the expression of the genes encoding the enzymes responsible for cyclization of lycopene (LCYE and LCYB), the hydroxylases involved in synthesis of the xanthophylls zeaxanthin and lutein (CHYB, CYP97C3 and CYP97A5), and zeaxanthin epoxidase (ZEP). Levels of each transcript were normalized to the housekeeping gene cblp level and expressed relative to the normalized level of the corresponding gene at zero time. Data about the transcription of vde would complete the picture of the synthesis of xanthophylls but no orthologue to this gene has been found in the last version of the genome of C. reinhardtii.
Exposure of dark-grown cells to high light intensity (800 µE m−2 s−1) seemed to trigger induction of both psy and pds, whose transcript levels were increased 2- and 4-fold, respectively, after 1 hour of exposure to very high light. LCYE and LCYB, involved in the cyclization of lycopene to yield ε-carotene and/or β-carotene, showed a slight decrease in their mRNA level. For ZEP, which catalyses the synthesis of violaxanthin and is directly involved in the xanthophyll cycle, we observed a very slight decrease of its transcript during exposure to high light (). Regarding carotene β-hydroxylase (CHYB), a non-heme di-iron monoxygenase, which is supposed to be involved in the conversion of β-carotene into zeaxanthin, we observed that its expression was slightly and progressively induced during the first 3 hours of exposure to light stress.
Fig. 3. Transcript levels profile of key carotenoid biosynthetic genes in C. reinhardtii cells cultured in the dark for 16 h and then transferred to high irradiance (800 µE m−2 s−1). Total RNA was extracted at the times indicated and the transcript levels of phytoene synthase (psy), phytoene desaturase (pds), β- and ε-lycopene cyclases (lcyb and lcye), the non-heme di-iron enzyme carotene β-hydroxylase (chyb), the cytochrome P450 β- and ε-hydroxylases (cyp97a5 and cyp97c3) and zeaxanthin epoxidase (zep) were analysed by qPCR. Levels of each transcript are normalized to the housekeeping gene cblp level and expressed as a relative fold to the normalized level of the corresponding gene at zero time in the dark. All qPCR data are the mean of measures obtained in triplicate and the result of two independent identical experiments.
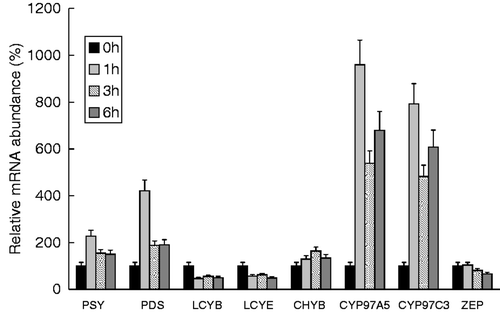
The most interesting results concerned the expression of the P450 cytochrome–dependent ε- and β-ring carotene hydroxylase genes. The transcript levels of both cytochrome-dependent hydroxylases, CYP97C3 and CYP97A5, were much higher in high-light stressed cells, being respectively 10- and 8-fold the levels in control cells. The maximum induction for both genes was observed after 1 hour of exposure to high light, but a high level, of about 5-fold the initial value, was maintained even after 6 hours.
Discussion
In some green microalgae, nutrient limitation, high light, or other abiotic stresses induce major alterations in the carotenoid pattern and even the synthesis of new carotenoids. Dunaliella or Haematococcus are good examples, where the content of β-carotene or astaxanthin increases extraordinarily, to reach almost 10% of the algal dry weight (Ben-Amotz & Avron, Citation1983; Salguero et al., Citation2003). In C. reinhardtii, such high carotenoid increases in response to environmental stress have not been observed and the synthesis of new carotenoids, such as ketocarotenoids, has been reported only in mature zygospores of Chlamydomonas (Lohr, Citation2009).
The most significant alteration in the carotenoid content of vegetative Chlamydomonas cells is, as in other higher plants and microalgae, the induction of the xanthophyll cycle in response to photo-oxidative stress, which causes the reversible conversion of violaxanthin into zeaxanthin. Activation of the xanthophyll cycle in response to high-light stress has been widely studied, and has been shown to be triggered by an acidification of the thylakoid lumen that occurs during illumination with excess light (Anwaruzzaman et al., Citation2004; Rohacek, Citation2010). The response of the cycle to other stress factors, such as nutrient limitation, has been poorly studied.
Nitrogen deprivation has been demonstrated to induce carotenogenesis in Dunaliella (Salguero et al., Citation2003) and to regulate other important metabolic and physiological processes, such as gametogenesis (Abe et al., Citation2004). In C. reinhardtii there is no significant alteration of the β-carotene content in response to nitrogen starvation, but there is an increase in zeaxanthin content (), due to the activation of the xanthophyll cycle. These data are consistent with the previous results obtained by Wycoff et al. (Citation1998), following phosphorus and sulphur limitation, and suggest that Chlamydomonas is able to activate the xanthophyll cycle under any nutrient deprivation conditions. Nutrient limitation or other environmental stresses can cause a decrease in the photosynthetic capacity and an imbalance between the absorbed light and the photosynthetic capacity, even at low light intensities (Li et al., Citation2009; Niyogi, Citation2009). This could be the cause of the activation of the xanthophyll cycle that we observed under nitrogen limitation or in the presence of norflurazon, which also inhibits photosynthetic activity ().
In , we show changes in zeaxanthin content during the shift to high light in the presence of the herbicide norflurazon, which inhibits the pathway at the step catalysed by phytoene desaturase. The response is similar to that reported by Depka et al. (Citation1998) in the presence of the lycopene cyclase inhibitor, CPTA, confirming that part of the zeaxanthin synthesized at high light intensities comes from the hydroxylation of β-carotene. Furthermore, the results of cycloheximide treatment () indicate that only one of the two possible routes for the synthesis of zeaxanthin upon transfer to high light – either the de novo synthesis of carotenoids or the interconversion of violaxanthin and zeaxanthin – is dependent on protein synthesis.
Our observations show that synthesis of lutein, not directly involved in the xanthophyll cycle, is also induced under light stress and that the transcription of the two hydroxylases involved in lutein synthesis is extraordinarily strongly induced after transfer to high light. In Arabidopsis, the synthesis of lutein has been reported to be mediated by the hydroxylases CYP97A and CYP97C1 (Kim et al., Citation2009) and the double mutant cyp97a cyp97c1, which is unable to produce normal levels of lutein, exhibited high-light susceptibility. Niyogi et al. (Citation1997) showed that mutants of C. reinhardtii defective in either alpha or beta carotenoids were able to tolerate high light, but not the double mutant npq1lor1, which is defective in both lutein and zeaxanthin, suggesting a possible role of lutein in photoprotection. The extraordinary induction of these two hydroxylases that we observed in C. reinhardtii cells exposed to high light, indicates that they are key steps in regulating the response to high light and support a possible role of lutein in photoprotection.
Although there is extensive information about the main pathways involved in the synthesis of carotenoids in higher plants, and although comparative genomic studies have allowed putative orthologues for many of the genes encoding the enzymes of the pathway to be identified in Chlamydomonas (Grossman et al., Citation2003; Lohr et al., Citation2005), the control mechanisms are not yet clear. Here we have been able to document the complete pattern of carotenoid changes when dark-grown C. reinhardtii are transferred to high light and to relate this pattern to the expression of practically all the genes of the pathway (). To our knowledge, this is the first detailed study of the regulation of carotenoid biosynthesis genes in response to light stress in Chlamydomonas. The few reports that have focused on the molecular response of carotenogenic pathway to light, either by qPCR or northern-blot analysis, have studied only a limited subset of genes. Bohne & Linden (Citation2002), and more recently Sun et al. (Citation2010), studied psy and pds expression when transferring dark- or low-light-grown Chlamydomonas cells to moderate (200 µE m−2 s−1) white or blue light. They observed a transient induction of psy and pds expression, reaching a maximum increase 1–2 h after transfer to light and recovery of the original levels after 4 h. Similar up-regulation was observed when cells were exposed to very low intensity light (0.01 µE m−2 s−1) (Lohr et al., Citation2005; Im et al., Citation2006) after a dark period. It is interesting to note that the up-regulation induced by such a low light intensity is similar to the increase that we have observed for these two genes, suggesting that their control is at least partially dependent on specific photoreceptors and confirming their important regulatory role.
Regarding ZEP, which catalyses the synthesis of violaxanthin and is directly involved in the xanthophyll cycle, both a slight up-regulation (Lohr et al., Citation2005) and a down-regulation (Im et al., Citation2006) upon exposure to light have previously been described. The small decrease that we have observed in the zep mRNA level makes us think that regulation at expression level of zep cannot be critical when cells are submitted to a high-light shock. Regulation of the synthesis of the other component of the xanthophyll cycle, violaxanthin de-epoxidase, would be of great interest but, as we have already indicated, the sequence corresponding to this gene is still unknown in Chlamydomonas. Incomplete sequencing could explain the lack of an orthologue of vde in the Chlamydomonas genome, but the fact that it has not been found in other related algae whose genomes have been sequenced, such as Volvox, suggests that a structurally unrelated enzyme is responsible for the epoxidation of zeaxanthin. This hypothesis is supported by the fact that the common plant violaxanthin de-epoxidase inhibitor, dithiothreitol, is not effective on Chlamydomonas (Lohr et al., Citation2005).
The non-heme di-iron hydroxylase of Chlamydomonas, CHYB, has an identity of 76% with its orthologue in Arabidopsis (Sun et al., Citation1996). On the other hand, the CYP97C3 and CYP97A5 hydroxylases found in Chlamydomonas have high similarity with the corresponding ε- and β-hydroxylases of Arabidopsis, CYP97C1 and CYP97A3, respectively (Tian et al., Citation2004; Kim & DellaPenna, Citation2006), with identities/similarities of 56%/72% for the CYP97As and 61%/74% for the CYP97Cs (Lohr et al., Citation2005; Lohr, Citation2009). In Arabidopsis, the function of these two unrelated hydroxylases families has been well studied using multiple mutants and performing substrate specificity studies by complementation experiments (Kim et al., Citation2009). It is generally considered that the CYP97 hydroxylases are mainly responsible for the hydroxylation of the ε and β rings of α-carotene to yield lutein, while the non-heme di-iron hydroxylase is mainly involved in the hydroxylation of β-carotene to yield zeaxanthin (Inoue, Citation2004), although a degree of functional overlap between the different hydroxylases seems to exist (Sun et al., Citation1996; Kim et al., Citation2010). In Chlamydomonas there are no data about the activity and substrate preference of these hydroxylases, which have been assigned a function by assuming homology with their corresponding orthologues in higher plants. The increase in the transcript levels of the CYP97 hydroxylases and the moderate increase in lutein levels would also support the idea of an important role for lutein in photoprotection. Our results suggest the importance of the regulation that these enzymes have under high-light stress and open up new possibilities for efficient manipulation of the pathway, once we know where the main control points are in this metabolic pathway.
Acknowledgements
We thank the Andalusian government for financial support (P09-CVI-5053).
References
- Abe , J , Kubo , T , Takagi , Y , Saito , T , Miura , K , Fukuzawa , H and Matsuda , Y . 2004 . The transcriptional program of synchronous gametogenesis in Chlamydomonas reinhardtii . Current Genetics , 46 : 304 – 315 .
- Anwaruzzaman , M , Chin , BL , Li , XP , Lohr , M , Martinez , DA and Niyogi , KK . 2004 . Genomic analysis of mutants affecting xanthophyll biosynthesis and regulation of photosynthetic light harvesting in Chlamydomonas reinhardtii . Photosynthesis Research , 82 : 265 – 276 .
- Ben-Amotz , A and Avron , M . 1983 . On the factors which determine the massive beta-carotene accumulation in the halotolerant algae Dunaliella bardawil . Plant Physiology , 72 : 593 – 597 .
- Bohne , F and Linden , H . 2002 . Regulation of carotenoid biosynthesis in response to light in Chlamydomonas reinhardtii . Biochimica et Biophysica Acta , 1579 : 26 – 34 .
- Botella-Pavia , P and Rodríguez-Concepción , M . 2006 . Carotenoid biotechnology in plants for nutritionally improved foods . Physiologia Plantarum , 126 : 369 – 381 .
- Couso , I , Vila , M , Rodríguez , H , Vargas , MA and León , R . 2011 . Overexpression of an exogenous phytoene synthase gene in the unicellular alga Chlamydomonas reinhardtii leads to an increase in the content of carotenoids . Biotechnology Progress , 27 : 54 – 60 .
- Cordero , BF , Couso , I , León , R , Rodríguez , H and Vargas , MA . 2011 . Enhancement of carotenoids biosynthesis in Chlamydomonas reinhardtii by nuclear transformation using a phytoene synthase gene isolated from Chlorella zofingiensis . Applied Microbiology and Biotechnology , 91 : 341 – 351 .
- Cunningham , FX and Gantt , E . 1998 . Genes and enzymes of carotenoid biosynthesis in plants . Annual Review of Plant Physiology and Plant Molecular Biology , 49 : 557 – 583 .
- Depka , B , Jahns , P and Trebst , A . 1998 . ß-Carotene to zeaxanthin conversion in the rapid turnover of the D1 protein of photosystem II . FEBS Letters , 424 : 267 – 270 .
- Diretto , G , Welsch , R , Tavazza , R , Mourgues , F , Pizzichini , D , Beyer , P and Giuliano , G . 2007 . Silencing of beta-carotene hydroxylase increases total carotenoid and beta-carotene levels in potato tubers . BMC Plant Biology , 7 : 11 – 19 .
- Fernández , E , Schnell , R , Ranum , LP , Hussey , SC , Silflow , CD and Lefebvre , PA . 1989 . Isolation and characterization of the nitrate reductase structural gene of Chlamydomonas reinhardtii . Proceedings of the National Academy of Sciences of the United States of America , 86 : 6449 – 6453 .
- Fischer , BB , Wiesendanger , M and Eggen , RIL . 2006 . Growth condition-dependent sensitivity, photodamage and stress response of Chlamydomonas reinhardtii exposed to high light conditions . Plant Cell Physiology , 37 : 1135 – 1145 .
- Förster , B , Mathesius , U and Pogson , BJ . 2006 . Comparative proteomics of high light stress in the model alga Chlamydomonas reinhardtii . Proteomics , 6 : 4309 – 4320 .
- Fraser , PD and Bramley , PM . 2004 . The biosynthesis and nutritional uses of carotenoids . Progress in Lipid Research , 43 : 228 – 265 .
- Fraser , PD , Römer , S , Shipton , CA , Mills , PB , Kiano , JW , Misawa , N , Drake , RG , Schuch , W and Bramley , PM . 2002 . Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner . Proceedings of the National Academy of Sciences of the United States of America , 99 : 1092 – 1097 .
- Giuliano , G , Tavazza , R , Diretto , G , Beyer , P and Taylor , MA . 2008 . Metabolic engineering of carotenoid biosynthesis in plants . Trends in Biotechnology , 26 : 139 – 145 .
- Grossman , AR , Harris , EE , Hauser , C , Lefebvre , PA , Martinez , D , Rokhsar , D , Shrager , J , Silflow , CD , Stern , D , Vallon , O and Zhang , Z . 2003 . Chlamydomonas reinhardtii at the crossroads of genomics . Eukaryotic Cell , 2 : 1137 – 1150 .
- Guerin , M , Huntley , ME and Olaizola , M . 2003 . Haematococcus astaxanthin: applications for human health and nutrition . Trends in Biotechnology , 21 : 210 – 216 .
- Im , CS and Grossman , AR . 2002 . Identification and regulation of high light-induced genes in Chlamydomonas reinhardtii . The Plant Journal , 30 : 301 – 313 .
- Im , CS , Zhang , Z , Shrager , J , Chang , CW and Grossman , AR . 2003 . Analysis of light and CO2 regulation in Chlamydomonas reinhardtii using genome-wide approaches . Photosynthesis Research , 75 : 111 – 125 .
- Im , CS , Eberhard , S , Huang , K , Beck , CF and Grossman , AR . 2006 . Phototropin involvement in the expression of genes encoding chlorophyll and carotenoid biosynthesis enzymes and LHC apoproteins in Chlamydomonas reinhardtii . The Plant Journal , 48 : 1 – 16 .
- Inoue , K . 2004 . Carotenoid hydroxylation – P450 finally! . Trends in Plant Science , 9 : 515 – 517 .
- Kim , J and DellaPenna , D . 2006 . Defining the primary route for lutein synthesis in plants: the role of Arabidopsis carotenoid β-ring hydroxylase CYP97A3 . Proceedings of the National Academy of Sciences of the United States of America , 103 : 3474 – 3479 .
- Kim , JE , Kimberly , M , Cheng , N , Craft , E , Hamberger , B and Douglas , CJ . 2010 . Over-expression of Arabidopsis thaliana carotenoid hydroxylases individually and in combination with a β-carotene ketolase provides insight into in vivo functions . Phytochemistry , 71 : 168 – 178 .
- Kim , J , Smith , JJ , Tian , L and DellaPenna , D . 2009 . The evolution and function of carotenoid hydroxylases in Arabidopsis . Plant Cell Physiology , 50 : 463 – 479 .
- Ledford , HK , Baroli , I , Shin , JW , Fischer , BB , Eggen , RIL and Niyogi , KK . 2004 . Comparative profiling of lipid soluble antioxidants and transcripts reveals two phases of photooxidative stress in a xanthophyll-deficient mutant of Chlamydomonas reinhardtii . Molecular Genetics and Genomics , 272 : 470 – 479 .
- León , R and Galván , F . 1995 . Metabolic pathway for glycerol synthesis under osmotic stress in the freshwater green alga Chlamydomonas reinhardtii . Plant Physiology and Biochemistry , 33 : 213 – 218 .
- León , R , Gónzalez , D , Galván , A and Férnandez , E . 2004 . Transgenic microalgae as green cell factories . Trends in Biotechnology , 22 : 45 – 52 .
- León , R , Vila , M , Herranz , D and Vilches , C . 2005 . Production of phytoene by herbicide-treated microalga Dunaliella bardawil in two phase systems . Biotechnology and Bioengineering , 92 : 695 – 701 .
- León , R , Couso , I and Fernández , E . 2007 . Metabolic engineering of ketocarotenoids biosynthesis in the unicellular microalga Chlamydomonas reinhardtii . Journal of Biotechnology , 130 : 143 – 152 .
- Li , Z , Wakao , S , Fischer , BB and Niyogi , KK . 2009 . Sensing and responding to excess light . Annual Review of Plant Biology , 60 : 239 – 260 .
- Lohr , M . 2009 . “ Carotenoids ” . In The Chlamydomonas Sourcebook: Organellar and Metabolic Processes , Edited by: Stern , D . Vol. 2 , 799 – 817 . San Diego : Elsevier .
- Lohr , M , Im , CS and Grossman , AR . 2005 . Genome-based examination of chlorophyll and carotenoid biosynthesis in Chlamydomonas reinhardtii . Plant Physiology , 138 : 490 – 515 .
- Lu , S and Li , L . 2008 . Carotenoid metabolism: biosynthesis, regulation, and beyond . Journal of Integrative Plant Biology , 50 : 778 – 785 .
- Merchant , SS , Prochnik , SE , Vallon , O , Harris , EH , Karpowicz , SJ , Witman , GB , Terry , A , Salamov , A , Fritz-Laylin , LK , Maréchal-Drouard , L , Marshall , WF , Qu , LH , Nelson , DR , Sanderfoot , AA , Spalding , MH , Kapitonov , VV , Ren , Q , Ferris , P , Lindquist , EM , Shapiro , H , Lucas , SM , Grimwood , J , Schmutz , J , Cardol , P , Cerutti , H , Chanfreau , G , Chen , CL , Cognat , V , Croft , MT , Dent , R , Dutcher , S , Fernández , E , Fukuzawa , H , González-Ballester , D , González-Halphen , D , Hallmann , A , Hanikenne , M , Hippler , M , Inwood , W , Jabbari , K , Kalanon , M , Kuras , R , Lefebvre , PA , Lemaire , SD , Lobanov , AV , Lohr , M , Manuell , A , Meier , I , Mets , L , Mittag , M , Mittelmeier , T , Moroney , JV , Moseley , J , Napoli , C , Nedelcu , AM , Niyogi , K , Novoselov , SV , Paulsen , IT , Pazour , G , Purton , S , Ral , JP , Riaño-Pachón , DM , Riekhof , W , Rymarquis , L , Schroda , M , Stern , D , Umen , J , Willows , R , Wilson , N , Zimmer , SL , Allmer , J , Balk , J , Bisova , K , Chen , CJ , Elias , M , Gendler , K , Hauser , C , Lamb , MR , Ledford , H , Long , JC , Minagawa , J , Page , MD , Pan , J , Pootakham , W , Roje , S , Rose , A , Stahlberg , E , Terauchi , AM , Yang , P , Ball , S , Bowler , C , Dieckmann , CL , Gladyshev , VN , Green , P , Jorgensen , R , Mayfield , S , Mueller-Roeber , B , Rajamani , S , Sayre , RT , Brokstein , P , Dubchak , I , Goodstein , D , Hornick , L , Huang , YW , Jhaveri , J , Luo , Y , Martínez , D , Ngau , WC , Otillar , B , Poliakov , A , Porter , A , Szajkowski , L , Werner , G , Zhou , K , Grigoriev , IV , Rokhsar , DS and Grossman , AR . 2007 . The Chlamydomonas genome reveals the evolution of key animal and plant functions . Science , 318 : 245 – 250 .
- Niyogi , KK . 2009 . “ Photoprotection and high light responses ” . In The Chlamydomonas Sourcebook: Organellar and Metabolic Processes , Edited by: Stern , D . Vol. 2 , 847 – 870 . San Diego : Elsevier .
- Niyogi , KK , Bjirkman , O and Grossman , AR . 1997 . Chlamydomonas xanthophyll cycle mutants identified by video imaging of chlorophyll fluorescence quenching . The Plant Cell , 9 : 1369 – 1380 .
- Paine , JA , Shipton , CA , Chaggar , S , Howell , RM , Kennedy , MJ , Vernon , G , Susan , Y , Wright , SY , Hinchliffe , E , Adams , JL , Aron , L , Silverstone , AL and Drake , R . 2005 . Improving the nutritional value of Golden Rice through increased pro-vitamin A content . Nature Biotechnology , 23 : 482 – 487 .
- Rohacek , K . 2010 . Method for resolution and quantification of components of the non-photochemical quenching (qN) . Photosynthesis Research , 105 : 101 – 113 .
- Salguero , A , De La Morena , B , Vigara , J , Vega , JM , Vilchez , C and & León , R . 2003 . Carotenoids as protective response against oxidative damage in Dunaliella bardawil . Biomolecular Engineering , 20 : 249 – 253 .
- Sambrook , J , Fritsch , EF and Maniatis , T . 1989 . Molecular Cloning: a Laboratory Manual , 2nd , New York : Cold Spring Harbor Laboratory Press .
- Sandmann , G , Römer , S and Fraser , PD . 2006 . Understanding carotenoid metabolism as a necessity for genetic engineering of crop plants . Metabolic Engineering , 8 : 291 – 302 .
- Shaish , A , Avron , A and Ben-Amotz , A . 1990 . Effect of inhibitors on the formation of stereoisomers in the biosynthesis of β-carotene in D . bardawil. Plant and Cell Physiology , 31 : 689 – 696 .
- Simkin , AJ , Breitenbach , J , Kuntz , M and Sandmann , G . 2000 . In vitro and in situ inhibition of carotenoid biosynthesis in Capsicum annuum by bleaching herbicides . Journal of Agricultural and Food Chemistry , 48 : 4676 – 4680 .
- Stephanopoulos , G . 2002 . Metabolic engineering: perspective of a chemical engineer . AlChE Journal , 48 : 920 – 926 .
- Sueoka , N , Chiang , KS and Kates , JR . 1967 . Deoxyribonucleic acid replication in meiosis of Chlamydomonas reinhardtii . Journal of Molecular Biology , 25 : 47 – 66 .
- Sun , H , Liu , CQ , Hui , YY , Wu , WK , Zhou , ZG and Lu , S . 2010 . Coordinated regulation of gene expression for carotenoid metabolism in Chlamydomonas reinhardtii . Journal of Integrative Plant Biology , 52 : 868 – 878 .
- Sun , Z , Gantt , E and Cunningham , FX . 1996 . Cloning and functional analysis of the β-carotene hydroxylase of Arabidopsis thaliana . Journal of Biological Chemistry , 271 : 24349 – 24352 .
- Takaichi , S . 2011 . Carotenoids in algae: distributions, biosyntheses and functions . Marine Drugs , 9 : 1101 – 1118 .
- Tian , L , Musseti , V , Kim , J , Magallanes-Lundback , M and Della Penna , D . 2004 . The Arabidopsis LUT1 locus encodes a member of the cytochrome p450 family that is required for carotenoid ε-ring hydroxylation activity . Proceedings of the National Academy of Sciences of the United States of America , 101 : 402 – 407 .
- Wycoff , DD , Davies , JP , Melis , A and Grossman , AR . 1998 . The regulation of photosynthetic electron transport during nutrient deprivation in Chlamydomonas reinhardtii . Plant Physiology , 117 : 129 – 139 .
- Ye , X , Al-Babili , S , Klöti , A , Zhang , J , Lucca , P , Beyer , P and Potrykus , I . 2000 . Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm . Science , 287 : 303 – 305 .
- Young , A , Orset , S and Tsavalos , A . 1997 . “ Methods for carotenoid analysis ” . In Handbook of Photosynthesis , Edited by: Pessarakli , M . 597 – 622 . New York : Marcel Dekker .