Abstract
A new species of Cocconeis has been found growing on thalli of the invasive green alga Caulerpa taxifolia collected from the Croatian Adriatic Sea (Bay of Stari Grad, the Island of Hvar, Central Adriatic, Croatia), the coasts of the Mediterranean (Saint Raphaël, west of Cannes, France) and the eastern coast of Australia (Moreton Bay, southeast Queensland). Additionally, it was observed on samples of Caulerpa racemosa, another invasive alga in the Mediterranean. Preserved thalli of Caulerpa and cleaned material of the new diatom were studied by light and electron microscopy (SEM and TEM). The morphology and fine structure of the new marine epiphytic diatom, for which we propose the name Cocconeis caulerpacola Witkowski, Car & Dobosz, was determined, including the internal and external structure of the raphe and sternum valve, and the cingulum. Comparison between the new species and three closely related species, C. borbonica, C. diruptoides and C. pseudodiruptoides, was made using material from our samples, as well as material from Vis (Adriatic Sea) obtained from the Hustedt collection. Surprisingly, C. caulerpacola is able to colonize Caulerpa taxifolia in very high abundance, but its occurrence seems to be strongly patchy. Indeed, it seems that C. taxifolia is a suitable host for epiphytic diatoms, in particular this tiny Cocconeis, despite its reputation as a ‘killer seaweed’. Cocconeis caulerpacola was observed on Caulerpa species in varying abundance over a wide geographical range.
Introduction
Species of Cocconeis are well known as components of the marine attached diatom community (e.g. Suzuki et al., Citation2001a ), occurring on rocks (epilithic communities), plants (epiphytic), animals (epizoic) or other substrata, where they may constitute communities with very high individual abundances and contribute to species richness (Totti et al., Citation2007). The first species to be described (by Ehrenberg, Citation1837) was C. scutellum, which is the generitype (De Stefano et al., Citation2008; Jahn et al., Citation2009). Cocconeis scutellum and its varieties are still the most commonly reported species in taxonomic and ecological studies due to frequent occurrence, simple valvar morphology and ease of identification under the light microscope (De Stefano et al., Citation2008). Recent studies on the morphology of various marine Cocconeis species have revealed great morphological variability and the genus is among the most structurally complex of diatom genera. Cocconeis exhibits a heterovalvar morphology, each frustule being composed of a rapheless valve [exhibiting a sternum (= pseudoraphe)] and a raphe valve. This type of morphology is described as being ‘monoraphid’ (Kobayasi & Nagumo, Citation1985; Round et al., Citation1990; De Stefano & De Stefano, Citation2005; De Stefano & Romero, Citation2005; Molino & Wetherbee, Citation2008). One of the most important characters of Cocconeis and related genera is the structure of the cingulum (Holmes et al., Citation1982).
Generally, raphid diatoms are among the earliest and most abundant primary colonizers of natural and artificial surfaces (Hoagland et al., Citation1986). The structure of the epiphytic community can be influenced by several environmental factors, such as the age of the leaf, the seasonal cycle of the plant or water depth (De Stefano et al., Citation2000). The epiphytic diatom communities of the endemic Mediterranean seagrass, Posidonia oceanica are some of the most thoroughly investigated (Mazzella et al., Citation1994; De Stefano et al., Citation2000). Mazzella et al. (Citation1994) found that Cocconeis species are the most frequent and abundant diatoms on P. oceanica leaves throughout the seasons and all along the depth range of the seagrass distribution. De Stefano et al. (Citation2000) documented eight species of Cocconeis on the leaves of P. oceanica. Some of these species can be very abundant and can constitute a continuous, almost monospecific layer on the colonized parts of the leaves (Mazzella et al., Citation1994).
In contrast to the Posidonia communities, studies of the diatom communities associated with the marine, green macroalga Caulerpa taxifolia (Bryopsidales, Chlorophyta) are lacking. This alga is out-competing native seaweeds and seagrasses in the Mediterranean by forming dense carpets, leading to a loss of biodiversity. Although C. taxifolia is considered one of the most invasive species in the Mediterranean, it is also indigenous to tropical and subtropical seas worldwide, including Australia (Phillips & Price, Citation2002) and it has been used widely as a decorative plant in the marine aquarium trade. It was accidentally released from the Monaco Aquarium in 1984 (Meinesz & Hesse, Citation1991) and rapidly spread across the western Mediterranean basin (Meinesz et al., Citation2001). Numerous molecular studies strongly support the hypothesis that the invader is a descendant of the so-called ‘aquarium–Mediterranean’ strain of C. taxifolia from Moreton Bay, Australia (e.g. Wiedenmann et al., Citation2001; Famà et al., Citation2002). The ‘aquarium–Mediterranean’ strain also resembles the Moreton Bay population morphologically, both of these having more robust thalli than C. taxifolia found elsewhere (Meinesz et al., Citation1995; Komatsu et al., Citation1997; Phillips & Price, Citation2002). Moreton Bay experiences minimum winter temperatures similar to the Mediterranean Sea and the two populations of C. taxifolia thus exhibit similar cold tolerance thresholds (Komatsu et al., Citation1997; Chisholm et al., Citation2000; Phillips & Price, Citation2002; Burfeind & Udy, Citation2009).
Caulerpa species are characterized by the presence of secondary metabolites, such as caulerpenyne (CYN), the main function of which is a chemical defence mechanism against herbivores and epiphytes (Box et al., Citation2008; Sureda et al., Citation2008, Citation2009). A variety of toxic effects due to CYN have been demonstrated, in particular on the sea urchin Paracentrotus lividus, e.g. killing sea urchin eggs (Lemée et al., Citation1993), or affecting them via regulation of intracellular pH, and also having effects on embryogenesis, larval development and metamorphosis (Galgani et al., Citation1996; Pesando et al., Citation1996, Citation1998; Smit, Citation2004). Maximum concentrations of CYN for the ‘aquarium-Mediterranean’ strain of C. taxifolia were recorded in autumn and a minimum in spring, and reach values that are much higher than are observed in other Caulerpa species (Dumay et al., Citation2002). As the growth and toxicity of C. taxifolia vary greatly within a year (Amade & Lemée, Citation1998; Thibaut et al., Citation2004), the chemical defence of this species may affect the settlement and development of different sessile organisms in invaded systems with different intensities (Prado & Thibaut, Citation2008). The specific composition of diatom communities of C. taxifolia has not been examined, in spite of the potentially important role of epiphytic diatoms in the functioning of the ecosystems influenced by C. taxifolia. This lack of information is of particular importance because of the existence of toxins, and because a new marine species of Cocconeis, C. shikinensis, has also been found growing on another species of Caulerpa, C. racemosa (Suzuki et al., Citation2001b ).
The framework of this research project included studies of the diatom flora epiphytic on Caulerpa taxifolia, and we have focused on the Cocconeis species, which constitute the majority of the diatom population. The epiphytic diatom assemblage on thalli of Caulerpa taxifolia was found to be largely dominated by a species of the genus Cocconeis that could not be identified. The aim of the present paper is to document the morphology of this species, based on light microscopy (LM), scanning electron microscopy (SEM) and transmission electron microscopy (TEM), and to describe it as new following a comparison with similar species, such as Cocconeis diruptoides Hustedt, C. pseudodiruptoides Foged and C. borbonica Riaux-Gobin & Compère.
Materials and methods
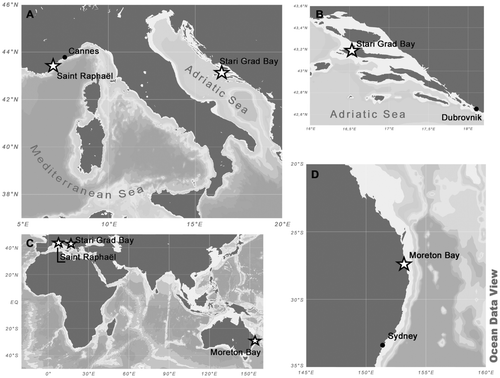
Samples of an unidentified Cocconeis, described here as C. caulerpacola, were obtained from thalli of Caulerpa taxifolia collected during summer and autumn 2008 and in 2010 by SCUBA diving at the following localities ():
| • | The Adriatic Sea, in the Bay of Stari Grad, Island of Hvar, Central Adriatic, Croatia. Sampling was carried out in autumn (October 2008) and summer (June and July 2010). The invasive alga, C. taxifolia, was observed in Stari Grad Bay (43° 10′ 54″ N, 16° 35′ 00″ E) for the first time in 1994 on a hard, sandy and muddy substrate without vegetation or within meadows of Posidonia oceanica (Zuljevic & Antolic, Citation2002). | ||||
| • | The coast of the north-western Mediterranean Sea at Saint Raphaël, west of Cannes, France (approximately 43° 25′ N, 6° 46′ E). Sampling was carried during summer/autumn (August and September 2008). Non-native C. taxifolia is abundant (an established invader) at this location. | ||||
| • | The eastern coast of Australia in Moreton Bay, south-east Queensland (approximately 27.5° S, 153.3° E), where samples of native C. taxifolia were collected during February and March 2010 (Australian summer/autumn). Moreton Bay is a shallow subtropical bay with an average depth of 6.8 m, 45 km from Brisbane. | ||||
All the sampling sites were quite shallow (5–8 m depth) and water temperatures were 19–25°C. Caulerpa taxifolia was carefully detached without damaging the fronds from the seafloor at depth of c. 5 m.
In order to compare the epiphytic diatom community on C. taxifolia with communities reported in previous studies and to provide information about host-dependence, particularly because of the existence of toxins characteristic for Caulerpa species, we also sampled coexisting autochthonous seagrasses and macroalgae. Extensive sampling of the brown alga Padina pavonica and the green alga Halimeda tuna was conducted between October 2008 and October 2010 from Stari Grad Bay. Additional samples of Posidonia oceanica from seagrass meadows affected by C. taxifolia in Stari Grad Bay were obtained in February 2012. Further samples of P. oceanica, together with samples of the invasive Caulerpa racemosa (as part of a larger study of benthic diatom assemblages from areas affected by Caulerpa spp.), had already been taken during autumn 2009 and 2010 by SCUBA diving at depths of 10–15 m in areas affected by Caulerpa racemosa near Dubrovnik (42° 37′ 50″ N, 18° 8′ 2″ E). Caulerpa racemosa was chosen in order to compare the epiphytic diatom communities on two invasive Caulerpa species with different toxin contents. Finally, C. racemosa and brown and green algae were sampled from Mljet National Park (southern Adriatic, Croatia) between October 2009 and October 2010 (approximately 42° 45′ N, 17° 23′ E) ().
Table 1. List of samples from the Adriatic Sea checked for the presence of Cocconeis caulerpacola. Occurrence of C. caulerpacola estimated as + to +++ (+ present in moderate quantities, ++ abundant, +++ extremely abundant) or T (trace); – indicates that the species was not observed in the sample.
Observations of Cocconeis diruptoides and C. pseudodiruptoides were made on material from our samples, as well as on cleaned material obtained from the Hustedt Diatom Collection in Bremerhaven, Germany (material E 74, collected in July 1904 on Ravnik near the island of Vis, Adriatic Sea, Croatia). Cocconeis borbonica was obtained from a sample from the western Indian Ocean (Juan de Nova Island, collected in April 2009).
Samples were cleaned of organic material for LM and electron microscopy (EM) observations by boiling with 30% H2O2 and adding 10% HCl to remove CaCO3.They were then rinsed with deionized water, pipetted onto ethanol-cleaned cover-slips and left to air dry, before mounting in Naphrax®. Specimens of C. caulerpacola were photographed with a Nikon Eclipse 600 light microscope (Nikon, Tokyo, Japan) equipped with differential interference contrast (DIC), and a Zeiss Axioskop (Phase Contrast and DIC) (Zeiss, Jena, Germany), in both cases using a 100× oil immersion objective (n.a. = 1.40). A few images were also taken from our material using advanced light photomicrography by Wulf Herwig: for a detailed description of this method see http://www.microscopy-uk.org.uk/mag/artmar11/Advanced_Light_Photomicrography.pdf.
Ultrastructural analysis was performed with SEM and TEM. A drop of the cleaned sample was filtered onto Whatman Nuclepore polycarbonate membranes (pore sizes 0.015 or 0.030 µm; Whatman International, Maidstone, England) for SEM. Filters were air-dried overnight, mounted on aluminium stubs and coated with gold–palladium or osmium. SEM observations were made primarily at the Warsaw University of Technology, Faculty of Materials Science and Engineering, using a Hitachi S-3500, SU-70 and SEM/STEM S-5500, in which the specimens can be observed simultaneously in scanning and transmission mode. Further SEM observations were done at the University of Frankfurt using a Hitachi S-4500 (Hitachi, Tokyo, Japan). Selected parts of C. taxifolia thalli were dried in liquid carbon dioxide and sputter coated in order to observe diatom assemblages in situ in SEM, using the Hitachi S-4500.
Slides and prepared material have been deposited in the diatom collection (SZCZ) of the Institute of Marine Sciences, University of Szczecin, Szczecin (Poland). Terminology follows Round et al. (Citation1990).
Results
Scanning electron photomicrographs demonstrated the presence of attached diatoms on the surface of the investigated macroalga and seagrass. The community showed high diversity. The diatom assemblage showed a distinct host-dependent difference at the species level. The dominant genera were roughly similar between the two Caulerpa species. Cocconeis comprised the dominant group of epiphytes on the investigated macroalga Caulerpa taxifolia. A new species of Cocconeis was found to be growing both on invasive Mediterranean and Australian native specimens of Caulerpa taxifolia, as well on invasive Caulerpa racemosa, while only a very few specimens, or none at all, were observed on coexisting algae and on the seagrass Posidonia oceanica (). Here we formally describe the new diatom species as Cocconeis caulerpacola Witkowski, Car & Dobosz, sp. nov.
Cocconeis caulerpacola Witkowski, Car & Dobosz, sp. nov.
Figs
DESCRIPTIO: Frustula aspectu cinguli rectangulata. Valvae stricte lineari-ellipticae apicibus late rotundatis. Longitudo 10–18 µm, latitudo 3–4 µm. Raphovalva: raphosternum cum raphe leniter sigmoideum; area axialis angustissima vix visibilis microscopio photonico, area centralis fasciam angustissimam formans margines valvae attigens; striae transapicales non discernendae. Areovalva: area axialis angustissima ut in raphovalva leniter sigmoidea; striae transapicales non discernendae. Facies raphovalvae leviter concava raphosterno angusta elevata. Raphe sigmoidea extremis centralibus parum expansis et dense sitis inter se. Extrema terminalia raphis etiam parum expansa subpolos deflexa ad latera opposita. Fascia angusta formata singularis stria utroque valde abbreviatae. Striae transapicales inter virgas aliquid depressae parallelae in mediis partibus valvae radiantes sub apices, circiter 40 in 10 µm. Foramina areolarum aequalia, isodiametrica, solum serie marginali transapicaliter elongata, 44–46 in 10 µm, occlusa hymenibus. Aspectus internus: facies plana cum sterno leviter elevato; extrema centralia raphis aliquid dilatata et deflexa in directiones oppositas. Extrema terminalia cum helictoglossis simplicibus etiam deflexa in directions oppositas. Facies areovalvae externe leviter convexa in partibus distalibus sed plana in media parte. Sternum leniter sigmoidea impressa in facie apparens interdum cum vestigiis raphis praeditum. Striae transapicales ut in raphovalva ordinatae, externe occlusa ut in raphovalva. Interne facies valvae concava ad margines et plana in mediis partibus. Series areolarum latiores quam virgae ita different a raphovalvis. Cingulum ex paucis (quattuor vel plus) copulis compositum cum valvocopula interne praedita appendicibus reductis inaequalibus valvocopulis fimbriatis in alteris speciebus generis Cocconeis.
HOLOTYPE: Slide no. 16 822 in coll. A. Witkowski, Institute of Marine Sciences University of Szczecin, Poland (SZCZ). Leg. Ana Car, June 2010. Holotype specimen is illustrated in .
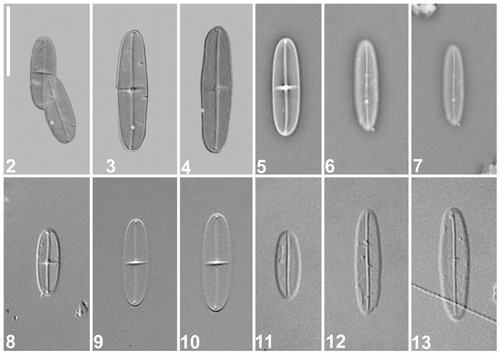
Figs 2–13. Cocconeis caulerpacola, LM. Specimens from the holotype slide. are micrographs taken by means of an advanced photomicrography method (kindly provided by Wulf Herwig); are phase contrast; are differential interference contrast. 2. Holotype specimen, comprising raphe (left) and sternum (right) valves. 3–13. Selected raphe (, ) and sternum () valves. Scale bar = 10 µm.
TYPE HABITAT: On the invasive alga C. taxifolia, collected from sandy bottom of the sublittoral zone in Stari Grad Bay, 43° 10′ 54″ N, 16° 35′ 00″ E.
ETYMOLOGY: The specific epithet refers to generic name of a host plant (Caulerpa taxifolia) on which this species grows abundantly.
Gross morphology
The frustules are rectangular in girdle view (not shown). The valves are strictly linear-elliptical with broadly rounded apices, 10–18 µm long, 3–4 µm wide (Figs ). The raphe valve (RV) has a sigmoid raphe sternum and raphe (). The axial area is barely distinguishable in LM, whereas the central area is fairly distinct, taking the form of a transapically expanded fascia that usually extends almost to the valve margins (). The sternum valve (SV) has a narrow, slightly sigmoid sternum, which is not noticeably enlarged at the centre (). The transapical striae are not resolvable in LM, either on the RV or the SV, unless observed with special methods, such as advanced light photomicrography ().
Specimens of Cocconeis caulerpacola observed with TEM and SEM are illustrated in Figs , including some in situ on Caulerpa taxifolia fronds (, , and ). Cells are attached to Caulerpa by the whole of the valve face of the raphe valve (, and at right). Supplementary SEM observations of Cocconeis caulerpacola are illustrated in Figs S1–S8.
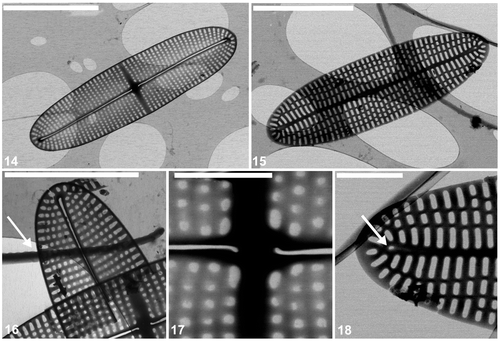
Figs 14–18. Cocconeis caulerpacola, TEM. 14. Raphe valve showing the striation and raphe branches with terminal and central endings. Note that the raphe endings are slightly bent towards opposite sides. 15. Sternum valve with longitudinal ribs and transapically elongate areolae. 16. Detail of raphe valve apex and part of valvocopula with very shallow undulations on one margin (arrow). 17. Centre of raphe valve. 18. Apex of sternum valve. Note the rudiment of a raphe (arrow). Scale bars = 5 µm (), 4 µm (); and 1 µm ().
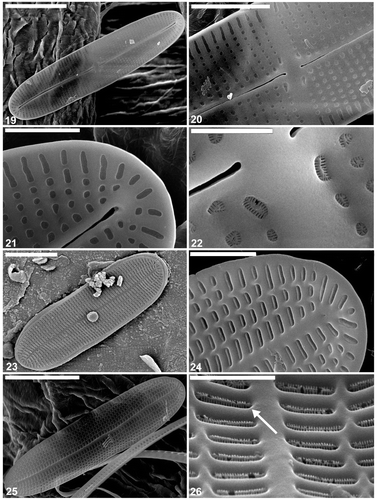
Figs 19–26. Cocconeis caulerpacola, SEM, from the holotype sample, Croatia. 19, 20. Raphe valve exterior, general view. Note sigmoid raphe with external central and terminal endings slightly expanded. 21. Internal view of raphe valve apex. Note the terminal raphe ending. 22. Close up of the centre of the interior of the raphe valve. 23. Sternum valve, external view. 24. Internal view of the sternum valve apex. 25. Sternum valve interior. 26. Close up of sternum valve interior. Note the areolae occlusions (arrow) containing apically oriented slits. Scale bars = 5 µm (); 3 µm (); 2 µm (); 1 µm (); and 500 nm ().
Figs 27–31. Cocconeis caulerpacola, SEM, from France. 27, 28. Specimens photographed in situ on Caulerpa taxifolia. 29, 30. External and internal views of cleaned sample. 31. Raphe valves, internal view. Note the internal central and apical raphe endings. Scale bars = 10 µm (); 6 µm (); 4 µm (); and 3 µm (,).
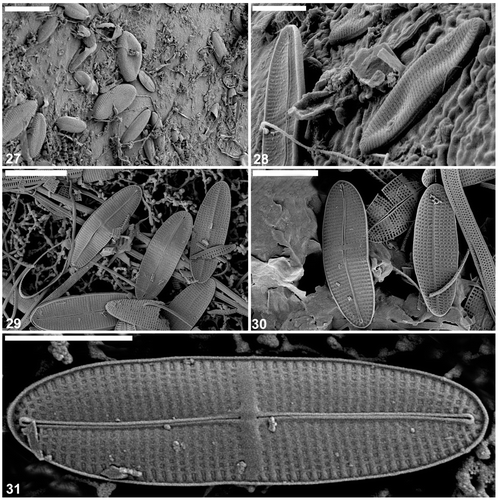
Figs 32–36. Cocconeis caulerpacola from France, SEM. show cells in situ on Caulerpa taxifolia. 32, 33. Cells with sternum valve uppermost, showing generally convex surface along the margins then becoming flat with a slightly depressed sternum. Note that the transapical striae are parallel in the middle, becoming radiate towards the apices. 34–36. Close up and whole () of sternum valve interior showing transapically elongate areolae. The areolae contain the same type of hymenate occlusions as in the raphe valve. Scale bars = 6 µm (); 4 µm (); 3 µm (); and 500 nm ().
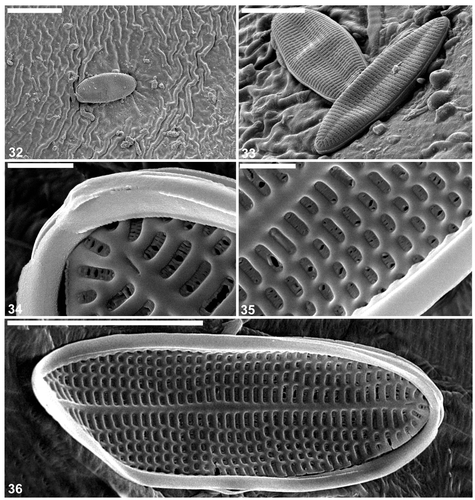
Figs 37–41. Cocconeis caulerpacola from France, SEM. 37. Frustule in oblique view. 38. Valvocopulae detached from frustules of C. caulerpacola. 39, 40. Close ups of valvocopulae: the pars interior has an undulate margin fitting over the valve interstriae. 41. Whole frustule with detached valvocopula. Scale bars = 4 µm (); 3 µm (); and 500 nm ().
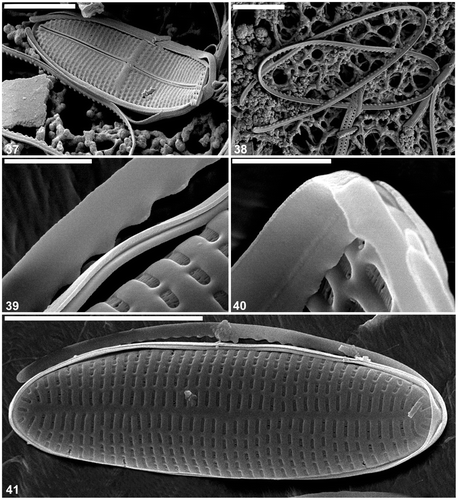
Figs 42–49. Cocconeis caulerpacola from Australia, SEM. 42. Raphe valve, external view. Note the sigmoid raphe and the areola pattern typical for C. caulerpacola from the type habitat. 43–45. Raphe valve internal view. Note the hymenate areolae occlusions. 46, 47. Sternum valve external view. 48, 49. Sternum valve internal view. Note the structure of the areolae hymenate occlusions. Scale bars = 5 µm (); 4 µm (); 3 µm (); 1 µm (); and 500 nm ().
Raphe valve
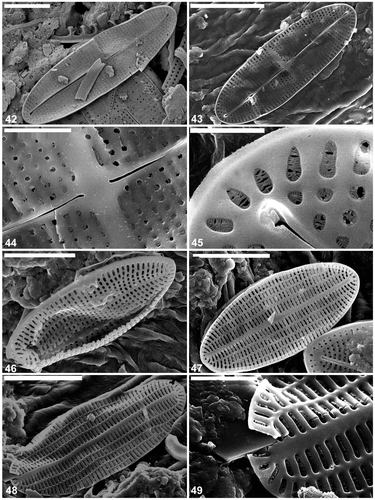
The external surface is slightly concave with an elevated raphe sternum (, ). The axial area is very narrow and symmetrical, with a transapically extended central area in the form of a fascia, which reaches the valve margins (, ). The fascia seems to be created by the absence of a single stria in the middle of the valve (, ). The raphe is sigmoid, with the external central endings usually very slightly expanded (, , , but see ). The external apical endings are slightly expanded, terminate at some distance from the apices (areolae are present between the raphe endings and the valve margin at the pole: ), and are very slightly bent in opposite directions (, , ). The transapical striae are parallel in the middle, becoming radiate towards the apices, and number 40 in 10 µm. The striae are slightly depressed below the coarser interstriae (virgae). All of the valve face areolae are of approximately the same size and shape (square to rounded: , ), the only exceptions being a few larger areolae near the central raphe endings (), and a row of areolae next to the valve margin: these latter are transapically elongate (, , ). The areolae are occluded by hymenate occlusions containing apically oriented slits (). The valve surface is flat internally and the raphe sternum is slightly elevated (). The internal central raphe endings are very slightly expanded and bent in opposite directions (, , , ). The apical internal raphe endings terminate in small, simple helictoglossae which are slightly bent in opposite directions (, , , ). Extra SEM observations of selected raphe valves of C. caulerpacola are illustrated in Figs S1–S4.
Sternum valve
The valve external surface is slightly convex at the margin becoming flat in the middle (, , ). The sternum is slightly sigmoid and depressed below the valve surface (, ) and sometimes bears the remnants of a raphe (, ). The transapical striae are parallel in the middle, becoming radiate towards the apices, and number 44–46 in 10 µm. The striae are crossed by a few longitudinal ribs, resulting in the development of rectangular, transapically elongate areolae (, , ). The areolae are occluded with the same type of hymenate occlusions as in the raphe valve (, , , , ). The SV surface is concave internally along the margin becoming flat in the middle with the sternum elevated above the remainder of the valve interior. The girdle is composed of a few (at least four) plain, open copulae (). The advalvar margin of the valvocopula possesses shallow undulations (, , , ), which appear to match the valve interstriae in their spacing and fit around them in vivo (). Supplementary SEM observations of the selected sternum valves of C. caulerpacola are illustrated in Figs S5–S8.
Differential diagnosis
The newly described species shows some resemblance, in terms of valve outline and the sigmoid raphe (see also Hustedt, Citation1933; Foged, Citation1975; Simonsen, Citation1987; Witkowski et al., Citation2000), to C. diruptoides (Figs S9–S12) and C. pseudodiruptoides (Figs S13–S17). These taxa differ, however, with respect to size range and stria density (), C. caulerpacola being much smaller and more finely striated. Cocconeis caulerpacola also shows some similarities in terms of general valve outline and size to C. borbonica (Riaux-Gobin & Compère, Citation2008). However, they differ with respect to raphe characteristics and stria density. Cocconeis borbonica has a higher stria density, the raphe is not sigmoid but straight, and the fascia does not reach the margin of the raphe valve, in contrast to C. caulerpacola (Fig. S25, contrast Fig. S24).
Table 2. Major morphometric characteristics of Cocconeis caulerpacola and similar taxa, with diagnostic information.
Discussion
A thorough taxonomic survey of the relevant diatom literature (e.g. Hustedt, Citation1933; Foged, Citation1975; Montgomery, Citation1978; Simonsen, Citation1987; Witkowski et al., Citation2000) did not reveal any described species corresponding to C. caulerpacola. It is in some respects close to C. diruptoides and C. pseudodiruptoides, possessing a comparable valve outline and a similar sigmoid appearance of the raphe and the sternum. Like C. caulerpacola, C. diruptoides occurs in the Adriatic Sea as an epiphytic diatom on leaves of Posidonia oceanica (De Stefano & Marino, Citation2001), but C. caulerpacola is much smaller and has a much higher stria density. Similar to C. diruptoides, C. pseudodiruptoides has much lower stria densities than C. caulerpacola, the striae being easily recognizable under LM on both valves (contrast C. caulerpacola), and the raphe and sternum are more strongly sigmoid. Cocconeis caulerpacola and C. borbonica resemble each other in shape and size (both are tiny) and the striation is hardly discernible in LM. However, other features permit them to be differentiated: fascia on the RV does not reach the valve margins in C. borbonica (contrast C. caulerpacola), and the raphe is straight in C. borbonica (sigmoid in C. caulerpacola).
Caulerpa taxifolia is apparently a suitable host for epiphytic diatoms because it tolerates some degree of microbial fouling, despite its common reputation as the ‘killer seaweed’. The major feature of the diatom microflora of C. taxifolia is that epiphytes are composed almost exclusively of diatoms from the genera Cocconeis and Mastogloia, as on the leaves of Posidonia oceanica growing in similar sandy habitats of the Mediterranean. It was therefore expected that the species composition of the epiphytic diatom assemblage on Caulerpa taxifolia might be similar to that on P. oceanica. However, whereas the most abundant taxa on Posidonia are C. scutellum var. posidoniae and C. neothumensis var. marina (De Stefano et al., Citation2000), the most striking characteristic of the epiphytic flora of C. taxifolia – both in the Mediterranean, where it is an invader, and in Australia, where it is native – is the predominance of Cocconeis caulerpacola.
The distribution of Cocconeis caulerpacola, occurring on both Caulerpa taxifolia in Australian marine waters and on invasive C. taxifolia in the Mediterranean, is interesting and in accordance with DNA analyses showing the origin of C. taxifolia to be from Moreton Bay, Queensland, Australia. Allozyme surveys have suggested that populations with a robust form of C. taxifolia in eastern Australia may be genetically more similar to populations with similar morphology in the Mediterranean than to populations with a finer morphology on the central Great Barrier Reef in north-east Australia (Benzie et al., Citation2000). Furthermore, sequencing of the Internal Transcribed Spacer (ITS) region of ribosomal DNA have indicated an aquarium origin of the Mediterranean C. taxifolia and also a genetic similarity between the Mediterranean strain and Australian populations (Jousson et al., Citation1998, Citation2001; Meusnier et al., Citation2001; Famà et al., Citation2002). DNA signatures of C. taxifolia have also provided evidence for the introduction of an aquarium strain into the Mediterranean Sea and showed the close relationship of the ‘aquarium-Mediterranean’ strain to an Australian population from Moreton Bay (Wiedenmann et al., Citation2001). Finally, the presence or absence of a chloroplast intron in the rbcL gene supports the hypothesis that Mediterranean and Australian populations belong to the same lineage. Among natural populations, specimens that possess the intron are restricted to tropical areas (Caribbean, Red Sea, South-East Asia), whereas those that lack it are present from subtropical North-eastern Australia (Queensland) to the temperate waters of South-eastern Australia (New South Wales) (Famà et al., Citation2002). The rbcL intron is lacking in introduced populations from the Mediterranean, as well as in aquarium samples.
Despite different geographical locations and a great difference in CYN values between the two assayed species of Caulerpa, C. taxifolia and C. racemosa, there is a general similarity between their diatom assemblages, including the occurrence of the new Cocconeis. However, a higher number of specimens of C. caulerpacola were observed on C. taxifolia than on C. racemosa. Periods of higher abundance of C. caulerpacola appear to coincide with maximum toxicities observed for C. taxifolia in summer and autumn, while the minimum abundance is observed in winter and spring (Dumay et al., Citation2002). Further research is necessary to determine the influence of seasonal processes, such as the production of CYN and the growth rate of Caulerpa thalli on the occurrence of Cocconeis caulerpacola. The difference in epiphytic diatom community composition between Caulerpa taxifolia and C. racemosa on the one hand, and green and brown macroalgae and seagrass on the other, is striking. Although the same abundant genera are observed (Cocconeis spp. and Mastogloia spp.), different species are present on Caulerpa and Posidonia oceanica, with Cocconeis scutellum dominant on Posidonia oceanica (in agreement with previous examinations: De Stefano et al., Citation2000) collected in areas influenced by invasive Caulerpa sp. (either C. taxifolia or C. racemosa, which is also invasive in the Mediterranean: Klein & Verlaque, Citation2008). Results of analyses of green and brown macroalgae collected at the same time from the same localities as invasive Caulerpa are consistent with a hypothesis of host-specificity of the new Cocconeis species by showing a total absence or the presence of just a few specimens of C. caulerpacola. A future study of the physiology of this new species is therefore justified, to examine why it is almost entirely restricted to colonizing members of the genus Caulerpa and how it is able to colonize C. taxifolia in such great abundance.
supplementary material
Download Zip (7.5 MB)Acknowledgements
This research was supported by the Croatian Ministry of Science, Education and Sports of Croatia (projects 275-0000000-3186 and ‘Jadran’) and the Polish Ministry of Science and Higher Education (N306 468 538), University of Szczecin, Poland. We thank Professor Horst Lange-Bertalot for help with the Latin diagnosis; Dr Oscar Romero and both reviewers for a critical appraisal of the manuscript; Mr Wulf Herwig for LM images of C. caulerpacola taken using advanced photomicrography; Friedel Hinz (Hustedt Diatom Collection) for kindly providing the sample of C. diruptoides; Ruth Nielsen (Botanical Museum, University of Copenhagen) for providing the holotype slide of C. pseudodiruptoides; Dr Colin Archibald and Steve Latham for improving the language of the manuscript; Zoran Jurić and Marko Žarić for their help during sample collection; and Marijeta Čalić for valuable suggestions for improving the manuscript. The sample from Juan De Nova originates from the scientific cruise of R/V Marion Dufrense organized by Terres Australes et Antarctique (TAFF) in April–May 2009.
References
- Amade , P and Lemée , R . 1998 . Chemical defence of the Mediterranean alga Caulerpa taxifolia: variations in caulerpenyne production . Aquatic Toxicology , 43 : 287 – 300 .
- Benzie , JAH , Ballment , E , Chisholm , JRM and Jaubert , JM . 2000 . Genetic variation in the green alga Caulerpa taxifolia . Aquatic Botany , 66 : 131 – 139 .
- Box , A , Sureda , A , Terrados , J , Pons , A and Deudero , S . 2008 . Antioxidant response and caulerpenyne production of the alien Caulerpa taxifolia (Vahl) epiphytized by the invasive algae Lophocladia lallemandii (Montagne) . Journal of Experimental Marine Biology and Ecology , 363 : 24 – 28 .
- Burfeind , DD and Udy , JW . 2009 . The effects of light and nutrients on Caulerpa taxifolia and growth . Aquatic Botany , 90 : 105 – 109 .
- Chisholm , JRM , Marchioretti , M and Jaubert , JM . 2000 . Effect of low water temperature on metabolism and growth of a subtropical strain of Caulerpa taxifolia (Chlorophyta) . Marine Ecology Progress Series , 210 : 189 – 198 .
- De Stefano , M and De Stefano , L . 2005 . Nanostructures in diatom frustules: functional morphology of valvocopulae in Cocconeidacean monoraphid taxa . Journal of Nanoscience and Nanotechnology , 5 : 1 – 10 .
- De Stefano , M and Marino , D . 2001 . Comparison of Cocconeis pseudonotata sp. nov. with two closely related species, C. notata and C. diruptoides, from Posidonia oceanica leaves . European Journal of Phycology , 36 : 295 – 306 .
- De Stefano , M and Romero , OE . 2005 . A survey of alveolate species of the diatom genus Cocconeis (Ehr.) with remarks on the new section Alveolatae . Bibliotheca Diatomologia , 52 : 1 – 133 .
- De Stefano , M , Marino , D and Mazzella , L . 2000 . Marine taxa of Cocconeis on leaves of Posidonia oceanica, including a new species and two new varieties . European Journal of Phycology , 35 : 225 – 242 .
- De Stefano , M , Romero , OE and Totti , C . 2008 . A comparative study of Cocconeis scutellum Ehrenberg and its varieties (Bacillariophyta) . Botanica Marina , 51 : 506 – 536 .
- Dumay , O , Pergent , G , Pergent-Martini , C and Amade , P . 2002 . Variations in caulerpenyne contents in Caulerpa taxifolia and Caulerpa racemosa . Journal of Chemical Ecology , 28 : 343 – 352 .
- Ehrenberg , CG . 1837 . Zusätze zur Erkenntniss grosser organischer Ausbildung in den kleinsten thierischen Organismen . Abhandlungen der Königlichen Akademie der Wissenschaften zu Berlin , 1835 : 150 – 181 .
- Famà , P , Jousson , O , Zaninetti , L , Meinesz , A , Dini , F , Di Giuseppe , G , Millar , AJK and Pawlowski , J . 2002 . Genetic polymorphism in Caulerpa taxifolia (Ulvophyceae) chloroplast DNA revealed by a PCR-based assay of the invasive Mediterranean strain . Journal of Evolutionary Biology , 15 : 618 – 624 .
- Foged , N . 1975 . Some littoral diatoms from the coast of Tanzania . Bibliotheca Phycologica , 16 : 1 – 127 .
- Fourtanier , E and Kociolek , JP . 1999 . Catalogue of the diatom genera . Diatom Research , 14 : 1 – 190 .
- Galgani , I , Pesando , D , Porthe-Nibelle , J , Fossat , B and Girard. , J-P. 1996 . Effect of caulerpenyne, a toxin extracted from Caulerpa taxifolia on mechanisms regulating intracellular pH in sea urchin eggs and sea bream hepatocytes . Journal of Biochemical Toxicology , 11 : 243 – 250 .
- Hoagland , KD , Zlotsky , A and Peterson , CG . 1986 . “ The source of algal colonizers on rock substrates in a freshwater impoundment ” . In Algal Biofouling , Edited by: Evans , L.V. and Hoagland , K.D. 21 – 39 . Amsterdam : Elsevier .
- Holmes , RW , Crawford , RM and Round , FE . 1982 . Variability in the structure of the genus Cocconeis Ehr. (Bacillariophyta) with special reference to the cingulum . Phycologia , 21 : 370 – 381 .
- Hustedt , F . 1933 . “ Die Kieselalgen Deutschlands, Österreichs und der Schweiz unter Berücksichtigung der übrigen Länder Europas sowie der angrenzenden Meeresgebiete ” . In Kryptogamen-Flora von Deutschland, Österreichs und der Schweiz , Edited by: Rabenhorst , L . Vol. 7, part 2 , 323 – 364 . Leipzig : Akademische-Verlagsgesellschaft .
- Jahn , R , Kusber , W-H. and Romero , OE . 2009 . Cocconeis pediculus Ehrenberg and C. placentula Ehrenberg: typification and taxonomy . Fottea , 9 : 275 – 288 .
- Jousson , O , Pawlowski , J , Zaninetti , L , Meinesz , A and Boudouresque , CF . 1998 . Molecular evidence for the aquarium origin of the green alga Caulerpa taxifolia introduced to the Mediterranean Sea . Marine Ecology Progress Series , 172 : 275 – 280 .
- Jousson , O , Pawlowski , J , Zaninetti , L , Meinesz , A and Boudouresque , CF . 2001 . “ Intra-individual genetic polymorphism in Caulerpa taxifolia populations and related Caulerpa species ” . In Fourth International Workshop on Caulerpa taxifolia , Edited by: Gravez , V. , Ruitton , S. , Boudouresque , C.F. , Le Direach , L. , Meinesz , A. , Scabbia , G. and Verlaque , M. 12 – 18 . Marseille , , France : GIS Posidonie Publisher .
- Klein , J and Verlaque , M . 2008 . The Caulerpa racemosa invasion: a critical review . Marine Pollution Bulletin , 56 : 205 – 225 .
- Kobayasi , H and Nagumo , T . 1985 . Observations on the valve structure of marine species of the diatom genus Cocconeis Ehr . Hydrobiologia , 127 : 97 – 103 .
- Komatsu , T , Meinesz , A and Buckles , D . 1997 . Temperature and light responses of the alga Caulerpa taxifolia introduced into the Mediterranean Sea . Marine Ecology Progress Series , 146 : 145 – 153 .
- Lemée , R , Pesando , D , Durand-Clément , M , Dubreuil , A , Meinesz , A , Guerriero , A and Pietra , F . 1993 . Preliminary survey of toxicity of the green alga Caulerpa taxifolia introduced into the Mediterranean . Journal of Applied Phycology , 5 : 485 – 493 .
- MAZZELLA, L., BUIA, M.C. & SPINOCCIA, L. (1994). Biodiversity of epiphytic diatom community on leaves of Posidonia oceanica. In Proceedings of the 13th International Diatom Symposium (Marino D. & Montresor M., editors), 241–251. Biopress, Bristol
- Meinesz , A and Hesse , B . 1991 . Introduction of the tropical alga Caulerpa taxifolia and its invasion of the northwestern Mediterranean . Oceanologica Acta , 14 : 415 – 426 .
- Meinesz , A , Benichou , L , Blachier , J , Komatsu , T , Lemee , R , Molenaar , H and Mari , X . 1995 . Variations in the structure, morphology and biomass of Caulerpa taxifolia in the Mediterranean Sea . Botanica Marina , 38 : 499 – 508 .
- Meinesz , A , Belsher , T , Thibaut , T , Antolic , B , Mustapha , KB , Boudouresque , CF , Chiaverini , D , Cinelli , F , Cotta-Lorda , JM , Djellouli , AS , El Abed , A , Orestano , C , Grau , AM , Ivesa , L , Vaugelas , I , Zavodnik , N and Zuljevic , A . 2001 . The introduced green alga Caulerpa taxifolia continues to spread in the Mediterranean . Biological Invasions , 3 : 201 – 210 .
- Meusnier , I , Olsen , JL , Stam , WT , Destombe , C and Valero , M . 2001 . Phylogenetic analyses of Caulerpa taxifolia (Chlorophyta) and of its associated bacterial microflora provide clues to the origin to the Mediterranean introduction . Molecular Ecology , 10 : 931 – 946 .
- Molino , PJ and Wetherbee , R . 2008 . The biology of biofouling diatoms and their role in the development of microbial slimes . Biofouling , 24 : 365 – 379 .
- MONTGOMERY, R.T. (1978). Environmental and ecological studies of the diatom communities associated with the coral reefs of the Florida Keys. A taxonomic study of Florida Keys: benthic diatoms based on SEM. Ph.D. Dissertation, Florida State University, College of Arts and Sciences, FL
- Pesando , D , Huitorel , P , Dolcini , V , Amade , P and Girard , JP . 1998 . Caulerpenyne interferes with microtubule-dependent events during the first mitotic cycle of sea urchin eggs . European Journal of Cell Biology , 77 : 19 – 26 .
- Pesando , D , Lemée , R , Ferrua , C , Amade , P and Girard , J-P . 1996 . Effects of caulerpenyne, the major toxin from Caulerpa taxifolia on mechanisms related to sea urchin egg cleavage . Aquatic Toxicology , 35 : 139 – 155 .
- Phillips , JA and Price , IR . 2002 . How different is Mediterranean Caulerpa taxifolia (Caulerpales: Chlorophyta) to other populations of the species? . Marine Ecology Progress Series , 238 : 61 – 71 .
- Prado , P and Thibaut , T . 2008 . Differences between epiphytic assemblages on introduced Caulerpa taxifolia and coexisting eelgrass (Zostera capricorni) in Botany Bay (NSW, Australia) . Scientia Marina , 72 : 645 – 654 .
- Riaux-Gobin , C and Compère , P . 2008 . New Cocconeis taxa (Bacillariophyceae) from coral sands off Réunion Island (Western Indian Ocean) . Diatom Research , 23 : 129 – 146 .
- Round , FE , Crawford , RM and Mann , DG . 1990 . The Diatoms. Biology and Morphology of the Genera , Cambridge : Cambridge University Press .
- Simonsen , R . 1987 . Atlas and Catalogue of the Diatom Types of Friedrich Hustedt. 3 volumes , J. Cramer, Berlin .
- Smit , AJ . 2004 . Medicinal and pharmaceutical uses of seaweed natural products: a review . Journal of Applied Phycology , 16 : 245 – 262 .
- Sureda , A , Box , A , Deudero , S and Pons , A . 2009 . Reciprocal effects of caulerpenyne and intense herbivorism on the antioxidant response of Bittium reticulatum and Caulerpa taxifolia . Ecotoxicology and Environmental Safety , 72 : 795 – 801 .
- Sureda , A , Box , A , Terrados , J , Deudero , S and Pons , A . 2008 . Antioxidant response of the seagrass Posidonia oceanica when epiphytized by the invasive macroalgae Lophocladia lallemandii . Marine Environmental Research , 66 : 359 – 363 .
- Suzuki , H , Nagumo , T and Tanaka , J . 2001a . Morphology of the marine epiphytic diatom Cocconeis heteroidea (Bacillariophyceae) . Phycological Research , 49 : 129 – 136 .
- Suzuki , H , Nagumo , T and Tanaka , J . 2001b . A new marine diatom, Cocconeis shikinensis sp. nov. (Bacillariophyceae) from Japan . Phycological Research , 49 : 137 – 144 .
- Thibaut , T , Meinesz , A and Coquillard , P . 2004 . Biomass seasonality of Caulerpa taxifolia in the Mediterranean Sea . Aquatic Botany , 80 : 291 – 297 .
- Totti , C , Cucchiari , E , De Stefano , M , Pennesi , C , Romagnoli , T and Bavestrello , G . 2007 . Seasonal variations of epilithic diatoms on different hard substrates, in the northern Adriatic Sea . Journal of the Marine Biological Association of the United Kingdom , 87 : 649 – 658 .
- Wiedenmann , J , Baumstark , A , Pillen , TL , Meinesz , A and Vogel , W . 2001 . DNA fingerprints of Caulerpa taxifolia provide evidence for the introduction of an aquarium strain into the Mediterranean Sea and its close relationship to an Australian population . Marine Biology , 138 : 229 – 234 .
- Witkowski , A , Lange-Bertalot , H and Metzelin , D . 2000 . “ Diatom flora of marine coasts I ” . In Iconographia Diatomologica , Edited by: Lange-Bertalot , H. Vol. 7 , Ruggell, Liechtenstein : A.R.G. Gantner .
- ZULJEVIC, A. & ANTOLIC, B. (2002). Appearance and eradication of Caulerpa taxifolia in Croatia. In International Caulerpa taxifolia Conference Proceedings (Williams, E. & Grosholz, E., editors), 1–10 (separately paginated). California Sea Grant College Program, University of California, La Jolla, CA. [Available at http://www.csgc.ucsd.edu/BOOKSTORE/Resources/COMP_PUBS/CAULERPA.pdf]
Supplementary Information
The following is available for this article, accessible via the Supplementary Content tab on the article's online page at http://dx.doi.org/10.1080/09670262.2012.735255.
Figs S1–S8. Cocconeis caulerpacola, SEM from sample from France. Figs S1, S2. External view of the selected raphe valves. Note the concave valve surface and the sigmoid raphe. Fig. S3. Raphe valve internal view. Note the internal central and apical raphe endings. Fig. S4. Frustule in oblique view. Fig. S5. Valvocopulae detached from frustules of C. caulerpacola. Fig. S6. Sternum valve photographed in situ on Caulerpa taxifolia. Fig. S7. Sternum valve from prepared material showing generally convex surface along the margins then becoming flat with a slightly depressed sternum. Fig. S8. Internal view of the sternum valve. Scale bars = 4 µm (Fig. S2); 3 µm (Figs S1, S3, S5–S8); and 1 µm (Fig. S4).
Figs S9–S12. Cocconeis diruptoides from Vis (Hustedt Diatom Collection, E74), LM. Scale bar = 10 µm.
Figs S13–S17. Cocconeis pseudodiruptoides, LM. Indian Ocean, Tanzania, Coll. Foged (Citation1975), Holotype slide. Scale bar = 10 µm.
Figs S18–S23. Cocconeis diruptoides and Cocconeis pseudodiruptoides from Vis (Hustedt Diatom Collection, E74), SEM. Figs S18–S20. Cocconeis diruptoides, type material. Raphe valves internal views. Note the internal central and apical raphe endings. Fig. S21. External view of raphe valve. Note the concave valve surface and the sigmoid raphe. Figs S22, S23. Cocconeis pseudodiruptoides. External view of raphe valve. Scale bar = 6 µm (Fig. S19); 5 µm (Figs S18, S21); 4 µm (Figs S22, S23); and 600 nm (Fig. S20).
Figs S24-S25. Comparison of Cocconeis caulerpacola (sample from France) and C. borbonica (sample from Western Indian Ocean, Juan de Nova Island), TEM. Fig. S24. Raphe valve of Cocconeis caulerpacola. Note that both endings are slightly bent into opposite sides. Fig. S25. Raphe valve of Cocconeis borbonica. Note the straight raphe and the presence of a fascia on the RV, which does not reach the valve margins (arrow). Scale bars = 4 µm.