Abstract
Photosynthetic activity of marine macroalgae in the Windmill Islands, East Antarctica, was measured in situ using submersible modulated fluorometers. An empirical relation incorporating terms for respiration and non-linear electron transport was derived from simultaneous in vivo measurements of effective quantum yield () and oxygen evolution. This relation was used with in situ measurements of
and photosynthetic photon flux density acquired over 24-h periods to estimate oxygen evolution rates of algae over the course of the measurement period. Productivity ranged from −8 to 19 µmol O2 g−1 FW h−1 (FW = fresh weight), with daily carbon gain ranging from −1.5 to 3.6 mg C g−1 FW d−1 for midnight ice-covered algae and midday ice-free algae, respectively. These values were similar to published values of productivity of Antarctic species derived from oxygen- and carbon-based techniques. The technique described here provides a simple and rapid means of estimating primary productivity in marine systems.
Introduction
Primary production can be defined as the production of a mass of organic material by photosynthetic organisms using carbon dioxide and sunlight as substrates, while primary productivity refers to the rate at which this mass is produced. Both terms are fundamental parameters describing ecosystem function. The annual primary production and daily (and hourly) rates of primary productivity of phototrophs in an ecosystem describe the efficiency with which solar energy is used to fix carbon and incorporate it into biomass. While productivity varies markedly over diel time periods, alterations in production on an annual basis due to large-scale climatic events may pose significant threats to marine organisms that depend directly on marine plants and algae for food and habitat, and could have far-reaching impacts on higher trophic levels. In environments where annual irradiance is controlled by climate-sensitive processes, such as the duration and timing of sea-ice cover, estimates of hourly and daily primary productivity under current environmental conditions will enable predictions of changes in seasonal and annual primary production that may occur under the various scenarios for projected climate change.
Primary production in Antarctic near-shore waters occurs mostly in summer when the solar angle is high and sea-ice is largely absent. In the Windmill Islands, East Antarctica, the subtidal rocky reefs of islands and coastline subjected to early sea-ice breakout tend to be dominated by macroalgae such as Palmaria decipiens and Monostroma hariotii near the surface, and Desmarestia menziesii and Himantothallus grandifolius in deeper waters. The often dense canopies afforded by these algae are important sources of nutrition and habitat for motile organisms (e.g. amphipods, gastropods: Takeuchi & Watanabe, 2002). For example, Quartino & Boraso de Zaixso (Citation2008) concluded that macroalgae are a necessary and significant contributor to the overall functioning of the near-shore ecosystem of Potter Cove in the Antarctic Peninsula, where they found the transport of macroalgae-derived biomass out of the bay over a summer period (1370 tonnes) to be almost equal to the total macroalgal production in summer. Understanding the rates of renewal of this significant biological resource is necessary if we are to improve our understanding of the dynamics of near-shore Antarctic ecosystems.
Primary productivity estimates of marine algae can be determined from the movement of labelled and stable carbon isotopes into and out of algal thalli, changes in net weight, and variations in intra- and extra-cellular dissolved oxygen and carbon dioxide concentrations where the rate of transport is directly related to photosynthesis and respiration. These measurements are often conducted in laboratory or mesocosm settings where environmental parameters such as photosynthetic photon flux density (PPFD), temperature and nutrient concentrations can be controlled. However, artifacts due to stirring, shading, spectral light quality and recirculating water may adversely affect the extent to which modelled results accurately describe productivity in marine environments. In situ productivity measurements (Drew & Hastings Citation1992; Cheshire et al., Citation1996; Longstaff et al., Citation2002, Silva et al., Citation2005) eliminate some of these problems (temperature and PPFD) but not all (bottle/incubation chamber effects). Modulated fluorescence techniques offer an attractive complementary technique (Enríquez & Borowitzka, Citation2011), in particular due to their minimal intervention and ease of use.
The introduction of modulated chlorophyll fluorometers (e.g. Schreiber et al., Citation1993), variants of which could later be used outside the laboratory, led to an explosion of in situ fluorescence studies. While our multichannel fluorometer (incorporating a Junior-PAM fluorometer, Walz, Germany; see Runcie & Riddle, Citation2004) brought multi-sample fluorescence monitoring to underwater systems, the more recent development and implementation of specialized autonomous submersible fluorometers (e.g. Shutter Fluorometer, Aquation, Australia) has enabled an increase in both the temporal resolution and replication of in situ macroalgal and seagrass fluorescence (Runcie et al., Citation2009; Runcie & Riddle, Citation2011).
The rate of electron transport (ETR) through PSII as obtained from variable fluorescence measurements (Schreiber, Citation2004) is directly related to rates of oxygen evolution (Beer et al., Citation2000; Franklin & Badger, Citation2001; Longstaff et al., Citation2002). Consequently, ETR has been widely used as a proxy for primary productivity (Beer et al., Citation2000; Franklin & Badger, Citation2001). However, the increasingly non-linear relationship at higher PPFDs is less easily characterized and may be due to one or more non-photosynthetic electron-consuming processes. For macroalgae, cycling of electrons around PSII and non-photochemical quenching in PSII centres are processes that likely contribute to the observed non-linearity (Franklin & Badger, Citation2001; Longstaff et al., Citation2002); other processes may also contribute to the observed non-linearity at high PPFDs (see references in Gilbert et al., Citation2000; Franklin & Badger, Citation2001; Longstaff et al., Citation2002; Suggett et al., Citation2011). In this study we use an empirically derived relation between in vivo rates of oxygen evolution and electron transport to convert in situ measurements of ETR (derived from simultaneous measurements of and ambient PPFD) into carbon fixation estimates.
This study aimed to (1) develop a technique for estimating primary productivity in terms of oxygen evolution on an hourly and daily basis for marine algae using in situ modulated fluorescence techniques, and (2) compare the results of this study with those derived using oxygen- or carbon-based techniques. An intention of the study was to provide a simple field technique that can be used to generate baseline estimates of short-term primary productivity and annual primary production in both polar and lower latitude marine coastal marine environments. These estimates can then contribute to the assessment of ecosystem function. We acknowledge that fluorescence techniques cannot supplant carbon-based or gas-exchange based techniques for measuring productivity due in part to their inability to account for respiration and for reasons explained above. However we suggest a fluorescence-based approach as that described here can provide a useful proxy for productivity.
Materials and methods
Study locations and macroalgal species
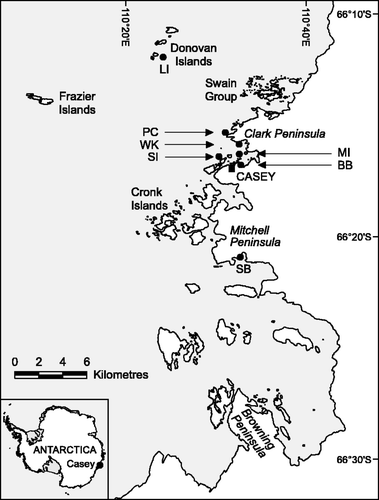
During December 2002, 2005 and 2006, the brown algae Himantothallus grandifolius and Desmarestia menziesii (2002 only) were examined in situ near Casey Station, East Antarctica (66° 17′ S, 110° 32′ E) at various locations at depths of 4–10 m with either low light (under sea-ice; <8 µmol photons m−2 s−1 photosynthetically active radiation [PAR]), or high light (ice-free locations >400 µmol photons m−2 s−1 PAR) (). While the western island locations () are characterized by early sea-ice breakout, rocky substrata with abundant macroalgal cover and wave activity, closer in-shore locations, including Sparkes Bay and Brown Bay, experience later sea-ice breakout around mid December with far less wave activity. Brown Bay is unique amongst locations in this study in that thick snow cover resulted in very low noon PPFD prior to breakout (<1 µmol photons m−2 s−1), and the substratum was dominated by mud. Throughout the year, underwater PPFD at each location is generally determined by the presence of sea-ice and snow cover thickness, and the presence of phytoplankton (generally when sea-ice is absent). The red algal genus Iridaea was also examined during the same sampling periods and at the same locations. Iridaea mawsonii and I. cordata are considered synonymous (Ricker, Citation1987), as the type and/or any specimens pertaining to Lucas's I. mawsonii (Lucas, Citation1919) do not exist (A. Millar, personal communication). They are referred to in this study as Iridaea sp. In situ fluorescence experiments and the collection of voucher specimens were conducted using surface-supply diving techniques.
In situ PPFD measurements
Photosynthetic photon flux density was measured in situ during each field deployment with a cosine-corrected sensor either deployed adjacent to the algae measured (Odyssey Submersible Photosynthetic Irradiance Recording System, Dataflow Systems, New Zealand) or incorporated in each fluorometer (see below). Light sensors were positioned on the substratum at the same depth as the alga being monitored, and were located on patches of substratum relatively clear of thalli in order to minimize or avoid intermittent shading from the algal canopy. Measured PPFD data were assumed to approximate irradiance experienced by the algal sample. Sensors were calibrated against a LiCor 190 sensor (LiCor Biosciences, USA). PPFD is reported in µmol photons m−2 s−1. Continuous PPFD data were obtained from Odyssey units deployed at numerous locations and depths for periods exceeding 12 months; the fluorometers were retrieved immediately after each deployment.
Annual photon dose estimates
The photon dose for each day of the year was calculated for 10, 20, 30, 40 and 50 m depths, assuming the presence or absence of sea ice cover and a phytoplankton bloom. In-air, PPFD values were determined throughout each day for latitude 66° 17′ S using a clear-sky solar irradiance model assuming an air mass of 1, a clear or very clear sky, 9% of the beam irradiance is backscattered from the atmosphere generating the diffuse irradiance, and no corrections are available for attenuation by clouds (see Gueymard, Citation1993, for details of relevant models). Model results were used as the basis for our determinations. We assumed that (1) surface waters remained clear for 4 days after the sea-ice breakout and before the phytoplankton bloom reduced water clarity; (2) the attenuation of light through the sea-ice and reflection from snow cover reduced transmitted PPFD to 1% of incident PPFD (cf. Schwarz et al., Citation2003); (3) the reflection from ice-free water due to the low solar angle was 5% (Kirk, Citation1994); (4) water transparency was very high (Kd = 0.05 m−1, where Kd is the diffuse vertical attenuation coefficient representing a 5% decline in downwelling PPFD per metre: Kirk, Citation1994) when sea-ice was present and for 4 days after break-out; and (5) phytoplankton-induced attenuation of PPFD increased Kd to 0.2 m−1. Attenuation of PPFD by sea-ice was accounted for by reducing the surface PPFD (E0) to 1% of modelled values, while in-water attenuation due to a phytoplankton bloom was calculated according to the Beer–Lambert relation assuming Kd values of 0.05 and 0.2 before and during the bloom event:
In situ fluorescence measurements
The in situ fluorometer-based experiments in this study were made in low light conditions under ice (0–5 µmol photons m−2 s−1), in ice-free turbid waters during relatively overcast days and in ice-free clear waters where midday underwater PPFD exceeded 50 µmol photons m−2 s−1. Generally, several replicate samples of each taxon were examined independently at a single location and depth over a period of not less than 24 h. The choice of location and starting time for each set of measurements were constrained, with the start generally any time between 09:30 and 17:00 h. Algal thalli likely to experience intermittent shading from adjacent algae were carefully removed from the substratum, including as much holdfast as possible, and repositioned at the same depth yet clear of other thalli and boulders to prevent shading. Only thalli that were never more than occasionally shaded before removal were selected to minimize the potential effects of high light stress. Thalli were positioned in plastic leaf clips that ensured a fixed distance between sample and fluorometer (see in Runcie & Riddle, Citation2011). Generally, measurements of the effective quantum yield of PSII fluorescence () were made halfway along the length of the thallus to avoid juvenile and distal necrotic tissue. The fine branches of D. menziesii were positioned close together in the sample clip but care was taken to avoid overlapping of branchlets. The fluorometers and samples were left in situ to take regular measurements for at least 24 h.
Active fluorescence measurements of D. menziesii () were conducted in situ using the multichannel fluorometer described elsewhere (Runcie & Riddle, Citation2004). This custom instrument incorporates a Junior-PAM (Walz, Germany) with an internal carousel that directs the fluorescence signal through eight independent light pipes (hence ‘multichannel’) to each of eight samples held in plastic leaf clips. Active fluorescence measurements of Iridaea sp. and H. grandifolius were conducted in situ using either the multichannel fluorometer, or with ‘sidefire’ or ‘shutter’ custom fluorometers (Runcie et al., Citation2009; text and in Runcie & Riddle, Citation2011). The custom fluorometers were designed independently of the Walz instruments, and later versions (Shutter Fluorometer and Submersible Datalogger) are now manufactured by Aquation, Umina Beach, Australia. These fluorometers employ modulated measuring (blue LED) and continuous actinic/saturating (white LED) lights. F0 and Fm were measured for 0.8 s each. All field experiments included measurements of the effective quantum yield of fluorescence of PSII (
) every 90 min. A subset of these experiments on Iridaea sp. included rapid light curves (RLCs), where
was repeatedly measured after each of eight consecutive 10 s intervals of increasing actinic irradiance, with a mean maximum irradiance of 190 µmol photons m−2 s−1 (White & Critchley, Citation1999). During these measurements the alga was exposed to both ambient and actinic irradiance. The field experiments provided information describing simultaneous values of
and PPFD measured throughout a 24-h interval that could then be used (with the relation derived from the in vivo experiments described above) to calculate productivity (see below).
Calculation of fluorescence parameters
Fluorescence parameters follow the notation of Van Kooten & Snel (Citation1990), where F denotes actual fluorescence intensity at any time, F0 denotes minimum fluorescence of dark acclimated material, and and Fm denote maximal fluorescence obtained during a saturating pulse for samples exposed to ambient light or acclimated to darkness respectively. Photosynthetic parameters were derived from RLCs described by the exponential model of Webb et al. (Citation1974) with no respiration term, and were calculated using non-linear least-squares minimization techniques; the parameters were electron transport rate (ETR), maximum ETR (ETRmax), the initial slope of a PE curve (α), the PPFD corresponding to ETRmax (Ek). ETR is the rate of electron flux through the photochemical apparatus and is generally calculated according to Genty et al. (Citation1989) as the product of the efficiency of photochemical energy conversion (
), ambient PPFD (µmol photons m−2 s−1), absorptance (the fraction of incident flux absorbed by photosynthetic pigments) and the proportion of energy that is allocated to PSI or PSII (generally – and in this study – assumed to be shared equally, i.e. 0.5 each). Here, we regularly measured both ambient PPFD and
of Iridaea sp. in situ for periods of at least 24 h.
Daily primary productivity estimates derived from 
Absorbance of 12 thalli representing Iridaea sp. collected from three different locations were measured with a spectrophotometer (GBC UV/VIS 916 Spectrophotometer) by measuring the transmittance of light (400–700 nm) through mid-thallus sections immersed in a seawater-filled cuvette; the mean value for absorbance was used as a proxy for photosynthetic absorptance. This is likely to be an overestimate as the absorbance measurement includes the absorbance of light absorbed by the thallus that is not used in photosynthesis. The overestimate is somewhat compensated as transmittance was partially reduced by reflectance. As the thalli of both H. grandifolius and D. menziesii examined in situ were generally optically opaque, this technique could not be applied, and the same value of absorbance measured using Iridaea sp. was used.
In theory, ETR can be related directly to the rate of oxygen evolution, where four electrons are required for every oxygen molecule produced (Beer et al., Citation2000) and to the carbon fixed, where one mole of oxygen is produced for every mole of carbon fixed (Sakshaug et al., Citation1997; Gilbert et al., Citation2000). While this linear relation holds true under low light, other electron sinks become active with higher light and the relation between ETR and O2 evolution rate becomes non-linear (Gilbert et al., Citation2000; Franklin & Badger, Citation2001; Longstaff et al., Citation2002; Beer & Axelsson, Citation2004). However, this non-linear relationship can be easily characterized in in vivo experiments where both ETR and O2 evolution are measured together. In a previous study we simultaneously measured in vivo rates of oxygen flux and for Iridaea mawsonii and Himantothallus grandifolius over a range of light intensites (Runcie & Riddle, Citation2006, fig. 6), demonstrating species-specific non-linear responses. These samples were maintained in low light conditions for several days prior to the experiments (Runcie & Riddle, Citation2006). In the present study we use the previous in vivo data to empirically relate the rate of oxygen consumption or evolution with simultaneous
data using an exponential increasing-to-a-maximum model with a term for respiration:
This in vivo derived relation was then used to generate a rate estimate of net oxygen evolution for each field-derived and PAR datum. Daily primary productivity was then calculated assuming these estimates were maintained on average over each 90-min interval, and these average values were then summed for a 24-h period. Where experiments ran for longer than 24 h, daily productivity was calculated as the mean of as many consecutive 24-h intervals as there were in the entire period. Estimates for ETR at each time point were also plotted against PPFD measured in the field, and from this diel light curve (cf. Longstaff et al., Citation2002) values of α (linear model) or ETRmax, α and Ek (saturating non-linear model) were calculated. Values of α obtained from algae measured in low- or high-light environments were separately pooled and then compared using a Student's t-test. The model does not account for potential differences in
values of the in vivo samples acclimated to low light conditions (from which the relation between oxygen production and variable fluorescence was derived) versus the in situ samples examined in this study that were acclimating to naturally increasing PPFD during the day. Daily carbon gain estimates were derived from oxygen evolution estimates summed over 24-h intervals assuming one mole oxygen produced equates to one mole of carbon fixed.
Non-linear least-squares curve fitting routines were conducted using Sigmaplot version 8.0 (Systat Software, Chicago, USA) and Optimiz software (Optimiz.xla v 2.0, Foxes Team, 2006, http://digilander.libero.it/foxes). Data are reported as means and standard deviations.
Results
Annual photon dose
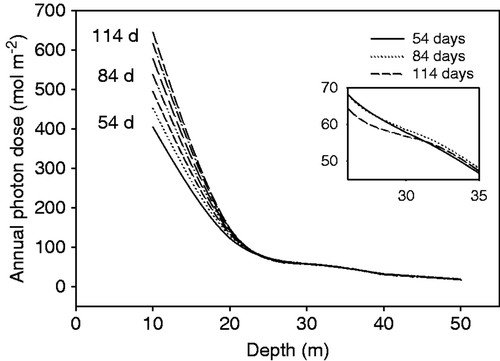
With more ice-free days in a year, the projected annual photon dose in surface waters also increased (). At depths below 20–25 m, these differences were minimal. Interestingly, the relationship reversed at depths below 30 m. Here, the annual photon dose during years with longer ice-free periods was less than the photon dose during years with shorter ice-free periods (, insert). This apparently counter-intuitive finding is a consequence of maximum water clarity occurring directly after sea-ice break-out earlier in the year, when the sun angle is lower and the daylength is shorter.
Fig. 2. Annual photon dose at different depths in near-shore waters at Casey. Values for different numbers of ice free days are presented; there were 84 days at the time of the study. Panel B (inset) shows a crossing over at approximately 30 m: the annual photon dose in shallow waters increases with an increase in the number of ice-free days, whereas at depths below 30 m the annual photon dose decreases with an increase in the number of ice-free days.
In situ diel variation in  and ETR of Iridaea sp.
and ETR of Iridaea sp.
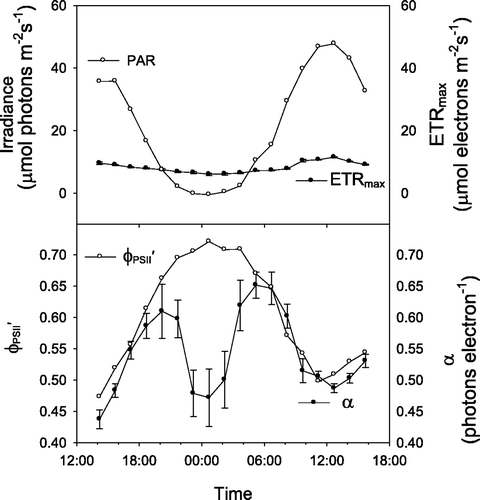
When ambient PPFD at ∼10 m depth under the sea-ice was continuously less than 1 µmol photons m−2 s−1 there was no appreciable diel pattern in any RLC-derived photosynthetic parameters of Iridaea sp. and remained fairly constant at about 0.65 (). This response was consistent for measurements made under the sea-ice. In contrast, photosynthetic parameters varied considerably over 24-h periods in ice-free environments subject to higher midday light (). While RLC-derived values of ETRmax and
both increased predictably during the day or at night (respectively), α declined markedly for several hours around midnight, increasing again to a maximum value around 05:00 ().
Fig. 3. Diel changes in photosynthetic parameters of Iridaea sp. and irradiance in a low light ice-covered environment derived from light response curves conducted every 90 min, where ETRmax represents the maximum rate of electron transport, α represents the initial slope of the PE curve, and represents the effective quantum yield of PSII energy conversion. Irradiance was less than 1 µmol photons m−2 s−1. Error bars are standard deviations.
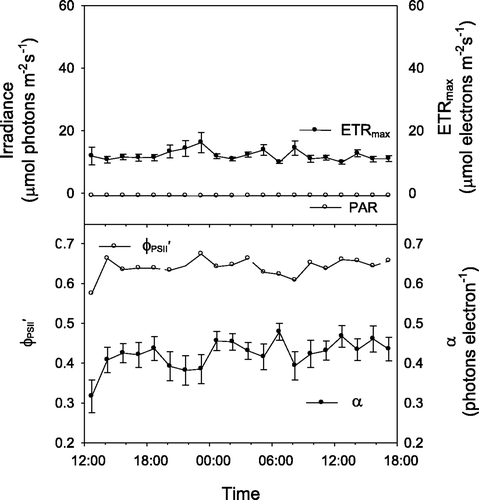
Fig. 4. Diel changes in photosynthetic parameters of Iridaea sp. and irradiance in a high light ice-free environment derived from light response curves conducted every 90 min, where ETRmax represents the maximum rate of electron transport, α represents the initial slope of the PE curve, and represents the effective quantum yield of PSII energy conversion. Error bars are standard deviations.
PE curves for Iridaea sp. obtained by plotting ETR (calculated as the product of ambient PPFD, in situ
, absorptance [0.867], and 0.5) against PAR were generally linear in both low and high light environments. The proportion of electrons transported relative to photons received is described by the slope of these curves (α = 0.269 ± 0.05 electrons photon−1, n = 22) and was similar for all in situ experiments regardless of whether the algae were in a low or high light environment (Students t-test: F = 0.79, P = 0.384). In some instances, the PE curve was curvilinear and ETRmax and Ek could be extrapolated from the data (mean ETRmax = 13 µmol electrons m−2 s−1, mean Ek = 46 µmol photons m−2 s−1).
Primary productivity and production derived from  and PPFD
and PPFD
The absorptance of Iridaea sp. was 0.867 ± 0.04. The empirical relation between electron transport rate and oxygen flux as described by the exponentially increasing function (Webb et al., Citation1974) is provided in , curves are shown in . Use of this relation provided negative oxygen flux estimates at low values of ETR, and was therefore able to account for respiration under low light (). In situ-derived estimates of maximum respiration and photosynthesis varied with species and light. Iridaea sp. demonstrated the greatest range of estimated oxygen evolution rates (−8.22 to 20.29 µmol O2 g−1 FW d−1) over 24-h intervals, while Himantothallus grandifolius (−2.66 and 18.93 µmol O2 g−1 FW d−1) and Desmarestia menziesii (−1.07 to 15.49 µmol O2 g−1 FW d−1) had smaller ranges. Estimates of daily productivity derived from fluorescence measurements of Iridaea sp. in the aquarium and in sea-ice covered Brown Bay were negative, while fluorescence measurements made in higher light locations provided positive oxygen evolution estimates ().
Fig. 5. Oxygen evolution–electron transport rate curves derived from simultaneous in vivo measurements of ambient irradiance, ETR and oxygen concentration. Closed circles represent measurements; open circles represent the best fit line according to the model predictions.
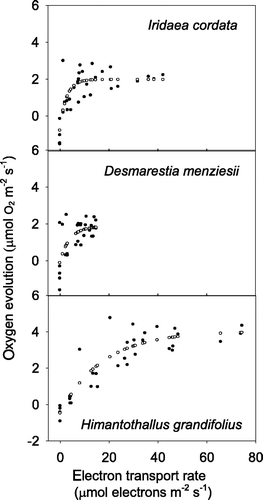
Table 1. Parameters used to describe the empirical non-linear relation between ETR (electrons m−2 s−1, abscissa) and O2 evolution (µmol O2 m−2 s−1, ordinate) for Antarctic macroalgae. Data are estimates derived from a single curve fit of four pooled replicate experiments ± SD; R2 represents the coefficient of determination, ** denotes significance at 99%. Data used in these calculations from fig. 6 of Runcie & Riddle (Citation2006).
Table 2. In vivo- and in situ-derived rates of oxygen evolution and consumption, and carbon fixation rate estimates of Antarctic macroalgae. Algae were either measured at (in situ) or obtained from (in vivo) depths as defined. Values of Pmax were measured in vivo, productivity and production values were derived from in vivo or in situ measurements. Emax represents maximum irradiance during a 24-h interval at the location of collection or in situ measurement. ND = no data.
Discussion
Light availability is of fundamental importance to the survival of Antarctic macroalgae. If the annual photon dose at a location becomes insufficient to sustain growth and reproduction, macroalgae will not survive. Many studies have addressed how light availability influences algal physiology across a range of latitudes and depths; for further detail regarding Antarctic macroalgae the reader is referred to a comprehensive review by Wiencke et al. (Citation2006).
Increasing daylength and annual photon dose
The annual underwater photon dose in the Windmill Islands area is defined not only by how long the fast ice is absent, but also the daylength when this ice breaks out. At Casey Station at 66° 17′ S, the near-shore fast-ice starts to break out early to mid December, some weeks before the longest day (22 December) when the daily photon dose has the potential to be maximal. Our simple model shows that minimal attenuation during the 4-day period after sea-ice break-out accounted for approximately 80% of the PPFD over the 12-month period at depths greater than 30 m. The model also demonstrates that the increase in photon dose afforded by an earlier-than-usual break-out date can be offset by a decrease in water clarity caused by large numbers of phytoplankton in the water column, especially over the longest days in late December. If increasing global temperatures result in earlier break-out dates, then the ice-free days prior to the phytoplankton bloom will accordingly provide less light. This is especially significant for macroalgae at depths exceeding 30 m, where the date of sea-ice break-out – rather than the number of ice-free days – determines the net photon dose.
The annual photon requirements for Antarctic macroalgae are low relative to the requirements of lower latitude species (Wiencke et al., Citation2006). For example, endemic Antarctic macroalgae such as Himantothallus grandifolius and Desmarestia anceps require 31 mol photons m−2 annum, compared with the cold temperate Laminaria hyperborea requiring 71 mol photons m−2 (Wiencke, 1996, and references therein). For H. grandifolius, our projections show this annual photon dose is experienced at 40 m depth (), suggesting this depth to be the limit of this species in near-shore waters at Casey subject to rapidly developing phytoplankton blooms. In contrast H. grandifolius is abundant at much greater depths around offshore islands in the Windmill Islands archipelago, e.g. at least 60 m depth at Lilienthal Island, ∼10 km offshore (data not shown), where the sea-ice breaks out much earlier than locations closer inshore, and the water column remains clear for much longer ().
RLC-derived estimates of diel photosynthesis of Iridaea sp.
Algae exposed to high light at midday responded to artificial light at night differently to algae under the sea-ice. The midnight decline in α of the high-light exposed Iridaea sp. () showed that these algae were less able to respond quickly to the ‘unexpected’ high light of a RLC at midnight, while sea-ice covered algae had no such difficulty (). High-light exposed Iridaea sp. presumably recover during the ‘night’ and are less able then to initiate the rapidly responding component of non-photochemical quenching (qE: Maxwell & Johnson, Citation2000) that is a feature of the RLC treatment. In contrast, low-light exposed Iridaea sp. may be more readily able to respond to a rapid increase in light, e.g. due to sea-ice breakout (Runcie & Riddle, Citation2006). The similarity of ETRmax values derived from diel RLCs of both high and low light Iridaea sp. indicate that these algae are able to regulate their photosynthetic apparatus to operate maximally over a wide range of PPFDs.
Diel PE curves from irradiance and ETR
In situ diel PE curves obtained from Iridaea sp. were mostly linear, and the values of α measured across a wide range of sites and derived from ϕPSII′ estimates measured at ambient light from <1 to 90 µmol photons m−2 s−1 were remarkably similar at 0.197 to 0.342 electrons photon−1 (mean 0.269) which is from 39 to 68% (mean 54%) of the theoretical value of 0.5 electrons photon−1 (eight photons are required to liberate four electrons, which in turn cause one oxygen molecule to be released: Gilbert et al., Citation2000). Thus, under conditions of low to moderate light, approximately 30–40% of light received is directed to pathways other than photosynthetic electron transport. This high value of photon conversion inefficiency may be partly explained by the generally lower values of measured in the red algae, a phenomenon attributed to the different pigment composition of these algae, resulting in reduced fluorescence output at the emission wavelength targeted by chlorophyll a-based fluorometers. Values of Ek and ETRmax derived from the in situ diel PE curves were similar to values reported elsewhere (Gomez et al., Citation1997; Runcie & Riddle, Citation2006).
Primary productivity
Estimates of noon productivity for Iridaea sp., Desmarestia menziesii and H. grandifolius obtained using the in situ fluorescence technique described in this study are similar in magnitude to reported values of Pmax from other in vivo studies on Antarctic macroalgae (). For example, the maximum in situ-derived productivity estimate for Iridaea sp. was 20 µmol O2 g−1 FW d−1, which is of the same order as in vivo-derived measurements of Gigartina skottsbergii (15 ± 2 µmol O2 g−1 FW d−1: Gomez et al., Citation1997) and similar to a general value ascribed to Rhodophyta (19.15 µmol O2 g−1 FW d−1: Weykam et al., Citation1996). In situ-derived productivity estimates of D. menziesii and H. grandifolius also closely approximate published in-vivo derived productivity measurements for the same or closely related Antarctic macroalgal species (; Thomas & Wiencke, Citation1991; Gomez et al., Citation1997, Citation1998). Dark respiration estimates are of a similar order to Pmax: dark respiration of D. menziesii measured at 62° 14′ S in December was less than 4 µmol O2 g−1 FW d−1 (Gomez et al., Citation1998), compared with our in situ-derived estimates of 1.1 µmol O2 g−1 FW d−1.
Daily productivity estimates were similar to reported values of other Antarctic macroalgae. The highest daily productivity estimate of 3.65 mg C g−1 FW d−1 for Iridaea sp. at 4 m depth is very close to a maximum measured value of 3.5 mg C g−1 FW d−1 reported for upper subtidal red algae (Gómez, Citation2001). Lower estimates of 0.88 and 0.56 mg C g−1 FW d−1 for H. grandifolius at 6 m depth () compare well with measured values of 0.66 mg C g−1 FW d−1 for this species (Gómez et al., Citation1997), although the algae examined by these authors were subject to more light prior to the experiments. Differences between daily productivity estimates estimated in this study and those published elsewhere are partly due to differences in light over the course of a 24-h period. Potential differences in fluorescence-derived estimates may also be due to spatial variability in photosynthetic response across a single thallus, where distal exposed portions may experience greater photoinhibition (cf. Colombo-Pallotta et al., Citation2006). However, direct comparison between Macrocystis pyrifera of that study and the morphologically similar taxon H. grandifolius is less straightforward, due to the prostrate nature of the latter species. We suggest that minimizing shading and standardizing the position of the thallus for measurement will lead to a more homogeneous view of the photosynthetic capacity of the taxa examined in this study (cf. Enríquez et al., Citation2002). Equally important is our assumption that 50% of the light energy is distributed between PSII and PSI. Any deviation from this (widely assumed) value will necessarily modify ETR, and we suggest this issue should continue to be the focus of further study (e.g. Sharon et al., Citation2011).
In situ-derived daily productivity estimates for Iridaea sp. demonstrated net respiration (−1.53 mg C g−1 FW d−1) when the daily photon dose was less than 1 mol photons m−2 (), and high values of net productivity (to 3.65 mg C g−1 FW d−1) with higher light. The upper and lower (negative) bounds of oxygen evolution estimates reported here (i.e. photosynthesis and respiration) are defined by the in vivo experiments, where respiration was derived from oxygen consumption in the dark measured over intervals of several minutes. This short interval used to measure respiration, the time of day of measurement, and the pre-acclimation treatment of the sample prior to our in vivo fluorescence-oxygen evolution experiments, are important factors influencing the outcome of in vivo experiments. For example, higher values of light-acclimated in situ material relative to in vivo material exposed to the same PPFD would result in field-based extrapolations providing underestimates of in situ productivity. Hence these conditions directly affect estimates derived from the empirical in vivo relationship determined between oxygen evolution and electron transport rate. Ideally, this relationship should be determined in situ over the course of a 24-h period under both low and high light conditions. An empirically derived relationship between in situ measurements of oxygen evolution and electron transport (via
) could then be applied to in situ fluorescence (
) and PPFD data, eliminating sources of error intrinsic to in vivo measurements.
The results of this study support the usefulness of an empirical relationship between O2 evolution and for estimating in situ productivity with logging chlorophyll fluorometers. An important advantage of this approach is that the relationship used to compare O2 evolution rates and
incorporates (1) the respiratory component under very low lights, (2) the linear low-light component that describes electron transport and is ascribed to photosynthesis, and (3) the non-linear high-light component that comprises both photosynthetic and non-photosynthetic electron sinks. The technique does not describe the nature of these non-photosynthetic sinks.
Using the method described here, one can estimate primary productivity wherever one can deploy a fluorometer and light meter. With additional information describing water clarity and insolation (cf. Kirk, Citation1994; Ritchie, Citation2010 respectively), one can also calculate the annual photon dose at different depths, and map both depth limits and annual production rates across a depth profile for algal species. Larger-scale models in polar systems that predict more ice-free days can then be used to calculate changes in annual light at different depths, and a map of predicted changes in distribution of shallower algae (increasing distribution) and deeper algae (decreasing distribution) can be created. The use of automated submersible data-gathering devices such as modulated fluorometers and PPFD sensors aid this approach.
Acknowledgements
The authors thank divers (Andrew Cawthorne, Norm Bracken and diving expeditioners of ANARE 2002/03, 05/06 and 06/07) and engineers (Martin Buggeln, Peter Kernebone, Steven Whiteside) for their assistance. The authors also wish to acknowledge two anonymous reviewers who contributed significantly, and David Smith for his cartographic contribution. This study was supported by grants awarded to Australian Antarctic Science projects 2201, 2256 and 2656.
References
- Beer , S and Axelsson , L . 2004 . Limitations in the use of PAM fluorometry for measuring photosynthetic rates of macroalgae at high irradiances . European Journal of Phycology , 39 : 1 – 7 .
- Beer , S , Larsson , C , Poryan , O and Axelsson , L . 2000 . Photosynthetic rates of Ulva (Chlorophyta) measured by pulse amplitude modulated (PAM) fluorometry . European Journal of Phycology , 35 : 69 – 74 .
- Cheshire , AC , Westphalen , G , Wenden , A , Scriven , LJ and Rowland , BC . 1996 . Photosynthesis and respiration of phaeophycean-dominated macroalgal communities in summer and winter . Aquatic Botany , 55 : 159 – 170 .
- Colombo-Pallota , MF , García-Mendoza , E and Ladah , LB . 2006 . Photosynthetic performance, light absorption, pigment composition of Macrocystis pyrifera (Laminariales, Phaeophyceae) blades from different depths . Journal of Phycology , 42 : 1225 – 1234 .
- Drew , EA and Hastings , RM . 1992 . A year-round ecophysiological study of Himantothallus grandifolius (Desmarestiales, Phaeophyta) at Signy Island, Antarctica . Phycologia , 31 : 262 – 277 .
- Enríquez , S and Borowitzka , MA . 2011 . “ The use of fluorescence signal in studies of seagrasses and macroalgae ” . In Chlorophyll Fluorescence in Aquatic Sciences: Methods and Applications. Developments in Applied Phycology , Edited by: Suggett , D.J. , Prášil , O. and Borowitzka , M.A. 187 – 208 . Springer : Dordrecht .
- Enríquez , S , Merino , M and Iglesias-Prieto , R . 2002 . Variations in the photosynthetic performance along leaves of the tropical seagrass Thalassia testudinum . Marine Biology , 140 : 891 – 900 .
- Franklin , LA and Badger , MR . 2001 . A comparison of photosynthetic electron transport rates in macroalgae measured by pulse amplitude modulated chlorophyll fluorometry and mass spectrometry . Journal of Phycology , 37 : 756 – 767 .
- Genty , B , Briantais , J-M and Baker , NR . 1989 . The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence . Biochimica et Biophysica Acta , 990 : 87 – 92 .
- Gilbert , M , Wilhelm , C and Richter , M . 2000 . Bio-optical modelling of oxygen evolution using in vivo fluorescence: comparison of measured and calculated photosynthesis/irradiance (P-I) curves in four representative phytoplankton species . Journal of Plant Physiology , 157 : 307 – 314 .
- Gómez , I . 2001 . Ecophysiology of Antarctic macroalgae: effects of environmental light conditions on photosynthetic metabolism . Revista Chilena de Historia Natural , 74 : 251 – 271 .
- Gómez , I , Weykam , G , Kloser , H and Wiencke , C . 1997 . Photosynthetic light requirements, metabolic carbon balance and zonation of sublittoral macroalgae from King George Island (Antarctica) . Marine Ecology Progress Series , 148 : 281 – 293 .
- Gómez , I , Weykam , G and Wiencke , C . 1998 . Photosynthetic metabolism and major organic compounds in the marine brown alga Desmarestia menziesii from King George Island (Antarctica) . Aquatic Botany , 60 : 105 – 118 .
- Gueymard , C . 1993 . Critical analysis and performance assessment of clear sky irradiance models using theoretical and measured data . Solar Energy , 51 : 121 – 138 .
- Kirk , JTO . 1994 . Light and Photosynthesis in Aquatic Ecosystems , Cambridge University Press, Cambridge .
- Longstaff , BJ , Kildea , T , Runcie , JW , Cheshire , A , Dennison , WC , Hurd , C , Kana , T , Raven , JA and Larkum , AW . 2002 . An in situ study of photosynthetic oxygen exchange and electron transport rate in the marine macroalga Ulva lactuca (Chlorophyta) . Photosynthesis Research , 74 : 281 – 293 .
- Lucas , AHS . 1919 . The algae of Commonwealth Bay . Australian Antarctic Expedition 1911–1914. Scientific Reports Series C: Zoology & Botany , 7 ( 2 ) : 1 – 8 . pls 1–9
- Maxwell , K and Johnson , GN . 2000 . Chlorophyll fluorescence – a practical guide . Journal of Experimental Botany , 51 : 659 – 668 .
- Quartino , ML and Boraso De Zaixso , AL . 2008 . Summer macroalgal biomass in Potter Cove, South Shetland Islands, Antarctica: its production and flux to the ecosystem . Polar Biology , 31 : 281 – 294 .
- Ricker , RW . 1987 . Taxonomy and Biogeography of Maquarie Island Seaweeds , British Museum (Natural History), London .
- Ritchie , RJ . 2010 . Modelling photosynthetic photon flux density and maximum potential gross photosynthesis . Photosynthetica , 48 : 596 – 609 .
- Runcie , JW and Riddle , MJ . 2004 . Measuring variability in chlorophyll-fluorescence derived photosynthetic parameters in situ with a programmable multi-channel fluorometer . Functional Plant Biology , 31 : 559 – 562 .
- Runcie , JW and Riddle , MJ . 2006 . Diel variability in photosynthesis of marine macroalgae in ice-covered and ice-free environments in East Antarctica . European Journal of Phycology , 41 : 223 – 233 .
- Runcie , JW and Riddle , MJ . 2011 . Distinguishing downregulation from other non-photochemical quenching of an Antarctic benthic macroalga using in situ fluorometry . European Journal of Phycology , 46 : 171 – 180 .
- Runcie , JW , Paulo , D , Santos , R , Sharon , Y , Beer , S and Silva , J . 2009 . Photosynthetic responses of Halophila stipulacea to a light gradient: I – In situ energy partitioning of non-photochemical quenching . Aquatic Biology , 7 : 143 – 152 .
- Sakshaug , E , Bricaud , A , Dandonneau , Y , Falkowski , PG , Kiefer , DA , Legendre , L , Morel , A , Parslow , J and Takahashi , M . 1997 . Parameters of photosynthesis – definitions, theory and interpretation of results [review] . Journal of Plankton Research , 19 : 1637 – 1670 .
- Schreiber , U . 2004 . “ Pulse-Amplitude (PAM) fluorometry and saturation pulse method ” . In Chlorophyll Fluorescence: A Signature of Photosynthesis , Edited by: Papageorgiou , G. and Govindjee . 279 – 319 . Springer : Dordrecht .
- Schreiber , U , Neubauer , C and Schuwa , U . 1993 . PAM fluorometer based on medium-frequency pulsed Xe-flash measuring light: a highly sensitive new tool in basic and applied photosynthesis research . Photosynthesis Research , 36 : 65 – 72 .
- Schwarz , A-M. , Hawes , I , Andrew , N , Norkko , A , Cummings , V and Thrush , S . 2003 . Macroalgal photosynthesis near the southern global limit for growth; Cape Evans, Ross Sea, Antarctica . Polar Biology , 26 : 789 – 799 .
- Sharon , Y , Levitan , O , Spungin , D , Berman-Frank , I and Beer , S . 2011 . Photoacclimation of the seagrass Halophila stipulacea to the dim irradiance at its 48-meter depth limit . Limnology and Oceanography , 56 : 357 – 362 .
- Silva , J , Santos , R , Calleja , ML and Duarte , CM . 2005 . Submerged versus air-exposed intertidal macrophyte productivity: from physiological to community-level assessments . Journal of Experimental Marine Biology and Ecology , 317 : 87 – 95 .
- Suggett , DJ , Moore , CM and Geider , RJ . 2011 . “ Estimating aquatic productivity from active fluorescence measurements ” . In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Developments (Developments in Applied Phycology 4) , Edited by: Suggett , D.J. , Prášil , O. and Borowitzka , M.A. 103 – 128 . Springer : Dordrecht .
- Takeuchi , I and Watanabe , K . 2002 . Mobile epiphytic invertebrates inhabiting the brown macroalga, Desmarestia chordalis, under the coastal fast ice of Lützow-Holm Bay, East Antarctica . Polar Biology , 25 : 624 – 628 .
- Thomas , DN and Wiencke , C . 1991 . Photosynthesis, dark respiration and light independent carbon fixation of endemic Antarctic macroalgae . Polar Biology , 11 : 329 – 337 .
- Van Kooten , O and Snel , JFH . 1990 . The use of chlorophyll fluorescence nomenclature in plant stress physiology . Photosynthesis Research , 27 : 121 – 133 .
- Webb , WL , Newton , MDS and Starr , D . 1974 . Carbon dioxide exchange of Alnus rubra: a mathematical model . Oecologia , 17 : 281 – 291 .
- Weykam , G , Gómez , I , Wiencke , C , Iken , K and Klöser , H . 1996 . Photosynthetic characteristics and C:N ratios of macroalgae from King George Island (Antarctica) . Journal of Experimental Marine Biology and Ecology , 204 : 1 – 22 .
- White , AJ and Critchley , C . 1999 . Rapid light curves: a new fluorescence method to assess the state of the photosynthetic apparatus . Photosynthesis Research , 59 : 63 – 72 .
- Wiencke , C . 1996 . Recent advances in the investigation of Antarctic macroalgae [review] . Polar Biology , 16 : 231 – 240 .
- Wiencke , C , Clayton , MN , Gómez , I , Iken , K , Luder , UH , Amsler , CD , Karsten , U , Hanelt , D , Bischof , K and Dunton , K . 2006 . Life strategy, ecophysiology and ecology of seaweeds in polar waters . Reviews in Environmental Science and Biotechnology , 6 : 95 – 126 .