Abstract
Investigations were carried out to evaluate lipid class and fatty acid composition of Chlorella vulgaris in relation to several combinations of cadmium (2 × 10−8; 10−7 M Cd) and nitrogen (2.9 × 10−6 to 1.1 × 10−3 M N) concentrations. Triacylglycerols (TAG), acetone mobile polar lipids (AMPL) and phospholipids (PL) were the major lipid classes of C. vulgaris under all the tested conditions. TAG was the lipid class accumulated in the highest amount in N-limited C. vulgaris. The AMPL lipid class was lowest at the lowest N and highest Cd treatment. High proportions of saturated fatty acids (SAFA) and monounsaturated fatty acids (MUFA) in the total lipid extracts were recorded under N limitation in the presence of Cd. 16:1(n-11) was an exception among the MUFA as its levels decreased at low N. Total polyunsaturated FA (PUFA), ω3 PUFA levels, and PUFA:SAFA ratios increased with increasing N concentrations, but when exposed to Cd, their levels were significantly reduced. Unlike the other PUFA, 18:2(n-6) increased with increasing N limitation. Significant negative relationships between TAG, MUFA and SAFA with N were recorded. Cd had a positive correlation with TAG, while N was with AMPL, PL and PUFA. In conclusion, a combination of N limitation and Cd stress significantly altered the lipid composition of C. vulgaris, and N limitation had the most significant overall effect on lipid class and fatty acid composition of the microalga.
Introduction
Nitrogen is one of the major nutrients required for algal growth, and changes in its availability controls phytoplankton community structures in aquatic ecosystems (e.g. Reynolds, Citation2006; Chia et al., Citation2009, Citation2011a , Citation2011b ; Zhao et al., Citation2009; Kotut et al., Citation2010). Microalgae can use nitrogen in the form of ammonium, nitrate or nitrite ions; and over-abundance of this nutrient can cause algal blooms (Kotut et al., Citation2010). As a response to environmental variability, including nitrogen availability, microalgal cells can undergo a series of metabolic acclimations, which often result in variations in the cellular composition of macromolecules. Among these, carbohydrates, lipids, amino acids, nucleic acids, pigments and proteins have been reported to vary with the nutritional status of algae (Zhao et al., Citation2009). Nitrogen is an important nutrient required in major amounts for protein synthesis. According to Reynolds (Citation2006), the nitrogen content of replete cells, relative to cell phosphorus, is generally in the range 13–19 mol N (mol P)−1. It is known that nitrogen affects lipid metabolism of members from virtually all algal groups (Hu et al., Citation2008).
Unusual biomolecules can be produced by microalgae as a result of metabolic modification in response to the wide range of environmental conditions to which they are exposed (Guschina & Harwood, Citation2006a ). Algae synthesize fatty acids as building blocks for the formation of various types of lipids. The commonly synthesized fatty acids in green algae have chain lengths from C16 to C18 (Petkov & Garcia, Citation2007). Fatty acids are either saturated or unsaturated, and unsaturated fatty acids may vary in number and position of double bonds on the carbon chain backbone (Hu et al., Citation2008). Hu et al. (Citation2008) reported that the degree of lipid saturation is affected by algal physiological state and nutrient concentrations (nitrogen, phosphorus and silicon) to which microalgae are exposed. It is well documented that N-limited microalgae increase their lipid content and that this is especially due to triacylglycerol (TAG) lipid class increase (Parrish & Wangersky, Citation1987; Gardner et al., Citation2011). Not only are the amounts of lipid affected by nitrogen concentration in microalgae, but qualitative variations are reported as well. Under nitrogen limitation, an increase in the proportion of saturated fatty acids (SAFAs) and monounsaturated fatty acids (MUFAs), and a decrease in the proportion of polyunsaturated fatty acids (PUFAs) in relation to total lipids, have been observed (Griffith et al., Citation2012).
In natural environments, microalgae are not exposed to environmental conditions varying singly and in isolation, but to all the compounds present (nutrients, trace metals and other ions, organic and inorganic pollutants), and these may interact either synergistically or antagonistically. Consequently, microalgae are exposed to rather complex conditions, and the regulation of their growth and physiology is the result of a multitude of factors. Cadmium concentrations have increased worldwide due to increased industrial activities (Stoeppler, Citation1991), and because metal ions are not biodegradable, Cd accumulates in the environment and it has been shown to be transferred through food webs (Chan et al., Citation2003). Another aspect to be considered with respect to Cd and phytoplankton, is the low affinity of this metal to binding sites in naturally occurring dissolved organic materials (Gouvêa et al., Citation2005). This can lead to increased availability to microalgae, either in the form of free Cd2+ ions or labile species. So, investigations of the interactions of Cd ions with other dissolved nutrients are not only of great ecological significance, but are also important for understanding the physiological responses of microalgae to multiple stress factors.
Although usually investigated as a toxic metal (Morin et al., Citation2008), a biological function for Cd has been demonstrated for marine diatoms. Lane & Morel (Citation2000) showed that a positive effect of Cd on the growth rate of Zn-limited diatoms is mediated through an increase in cellular carbonic anhydrase (CA) activity, and that the increase is due to the expression of a Cd-specific CA. Based on previous results (Lane & Morel, Citation2000; Lane et al., Citation2005), Park et al. (Citation2007) explored the distribution and diversity of Cd-CA in 21 marine phytoplankton species under pure culture conditions and showed that 12 of them (all diatoms) expressed the Cd-specific CA. The existence of a Cd enzyme in marine phytoplankton may indicate that there is a unique selection pressure for metallo-enzymes in the marine environment by diatoms (Lane et al., 2005).
Investigations of the effects of combinations of Cd and nutrients on the growth and physiology of microalgae have been reported. Singh & Yadav (Citation1984) showed that Cd inhibited the uptake of phosphorus by phytoplankton. Awasthi & Rai (Citation2005) examined the effects of Cd on nitrate uptake by Scenedesmus quadricauda and showed a 45% reduction in nitrate uptake in the presence of 2.3 × 10−6 M Cd, which corresponded to the LC50 concentration; Devriese et al. (Citation2001) showed 75% inhibition of nitrate uptake in Chlamydomonas reinhardtii at concentrations exceeding 1.5 × 10−4 M, but no effect was observed below 10−4 M Cd ions. Because both nitrogen and Cd are known to affect lipid synthesis, and Cd ions induce oxidative stress in microalgae (Pinto et al., Citation2003), different metabolic routes are likely to be affected with such combinations. However, at sublethal and environmentally relevant concentrations of Cd such as those used here, 2 × 10−8 to 10−7 M Cd, the effects can be different. These values are similar to the maximum contamination levels (MCL) permitted in water for Cd by the Food and Agriculture Organization/World Health Organization (Citation1984) and the United States Environmental Protection Agency (Citation2012), the permitted levels being 8.92 × 10−8 M and 4.46 × 10−8, respectively. The interactive effects of trace metals and nitrogen in the form of nitrate at various levels have been shown to influence the physiology of microalgae, controlling the cellular uptake and toxicity of the metals (Shun-Xing et al., Citation2007; Sivakumar et al., Citation2010). Information about patterns of variation in lipids is a contribution to understanding the mechanisms that microalgae use to adapt themselves to different environmental stresses, such as Cd/N levels.
Land application of sewage sludge is becoming a common practice to improve soil properties and reduce disposal in landfills. However, because sewage sludges are often metal contaminated, such practices increase the content of metal ions in soils (Lombardi & Garcia, Citation1999), which end up washed into adjacent aquatic ecosystems. In wetlands, where nitrogen and phosphorus are enough to support a diverse biological community, the input of metals can cause severe system disturbances. In addition, tertiary sewage treatments, such as artificial wetlands, that are promising for restricting eutrophication problems in natural waters are vulnerable to runoff waters and other contamination sources (Breitholtz et al., 2012). In areas where there are no combinations of sewer waters and fertilizers, the amounts of nutrients such as nitrogen found in association with Cd also vary. Furthermore, secondary effluents usually contain relatively low inorganic nutrients (around 10–15 mg l−1 N and 0.5–1 mg l−1 P), while they may still contain environmentally relevant concentrations of heavy and trace metals. Cd like other heavy metals is capable of causing essential nutrient deficiency, and even changing the concentrations of basic nutrients such as N and P in plants (Siedlecka, Citation1995; Pankovic et al., Citation2000). So a better understanding of how Cd and other trace metals affect microalgal physiology and lipid composition under varying nitrogen concentrations is important and can aid the design and management of artificial wetlands.
This study evaluates lipid composition of Chlorella vulgaris exposed to different Cd and nitrogen concentrations. To avoid the problem of uncertainties of the physiological status of phytoplankton cells that are inherent to batch culture systems, we performed all the experimental cultures in semi-continuous mode after cells were acclimated to each specific experimental treatment. In general, our results showed that a combination of N limitation and Cd stress significantly altered the lipid composition of C. vulgaris.
Materials and methods
Culture conditions
The strain of Chlorella vulgaris used in this study was obtained from the freshwater microalgae culture collection of the Botany Department at the Federal University of São Carlos (UFSCar), Brazil. Stock cultures of Chlorella vulgaris were maintained in LC Oligo medium (AFNOR, Citation1980) using batch systems. Growth medium pH was adjusted to 7.0 ± 0.2 prior to autoclaving and was maintained throughout the experimental period due to the semi-continuous culture system, which consisted of daily culture medium replacement. Sterilization of culture media was performed 24 h before use by autoclaving (121°C, 20 min). Experimental conditions were kept constant at a light intensity of 150 µmol m−2 s−1, a light:dark cycle of 16:8 h and a temperature of 20 ± 2°C. Media preparation reagents were of analytical grade.
Nitrogen (N) was provided as KNO3 at 2.9 × 10−6, 1.1 × 10−5 and 1.1 × 10−3 M (control), which represent limiting and replete environmentally relevant N concentrations in aquatic ecosystems (Larned, Citation1998; Reynolds, Citation2006). At each N concentration, Cd was added, as Cd(NO3)2, at 2.0 × 10−8 and 1.0 × 10−7 M, while the control had no Cd addition. The chemical equilibrium software MINEQL+ 4.62.3 (Environmental Research Software, Hallowell, ME, USA) was used to calculate the concentration of free Cd2+ ion in the culture media. This was done considering the culture conditions of pH, temperature, ionic strength and open system for gas exchange. The results showed that at least 98% of the added Cd remained as free Cd2+ ions in the cultures at the beginning of the experiments ().
Table 1. Cadmium (Cd) speciation in the growth media at the beginning of the experiments as calculated using MINEQL software. pCd and pLabile are negative natural log values for free Cd ion and labile Cd concentrations.
All experiments were carried out in 1-litre polycarbonate bottles (Nalgene, Penfield, New York, USA) containing 250 ml of sterile culture media. Even though the cultures were not axenic, we used sterile techniques throughout to minimize contaminations; thus culture manipulations were done under a flow of filtered and sterile air using PA-PCR laminar flow hood (Pachane, Piracicaba, Sao Paulo, Brazil).
Before beginning the experiments, C. vulgaris cells were acclimated to each treatment in a batch culture system. This acclimation consisted of culture transfers at exponential growth phase and monitoring each new culture for its growth rate. After three statistically similar consecutive growth rates, the microalgae were considered to be acclimated to each treatment concentration in the culture media to be tested. An initial cell density of ∼105 cells ml−1 was used for all treatments. Daily dilutions through culture removal and replacement with fresh and sterile culture media containing the specific treatment condition were made in line with the growth rates. On a daily basis before the dilutions of the semi-continuous cultures, aliquots of the culture were taken to determine cell density and consequently growth rates. This guaranteed a cell density within a known range throughout the experiments. Daily monitoring was performed by measuring optical density at 684 nm using a HACH DR 5000 (HACH, Loveland, Colorado, USA) spectrophotometer. Based on equations (1 and 2 below) described in Lombardi & Maldonado (Citation2011) specific growth rates (μ) were obtained.
Cell counts were done under an optical microscope using an improved bright lined Neubauer haemocytometer. The daily dilutions continued for 16 days, when samples were taken for lipid analysis. Three experimental replicates were performed for each treatment.
Chemical analyses
Chlorophyll a determination was performed according to Shoaf & Lium (Citation1976), while its concentration was computed from the equation of Jeffrey & Humphrey (Citation1975). Nitrogen (as nitrate) was determined according to APHA (Citation1998).
Lipid extraction was performed according to Parrish (Citation1999), with dichloromethane:methanol (2:1) as the extraction solvent. Glass fibre filters (GF/C; BOECO, Hamburg, Germany) previously baked overnight at 400°C were used to retain the algal biomass. Immediately after filtration of culture samples and prior to extraction, the filters were placed in screw-capped Teflon centrifuge tubes (Nalgene) containing 6 ml dichloromethane (CH2Cl2) : methanol (MeOH) (2:1) and spiked with 10 µg of internal standard (hexadecane-3-one). This was then sonicated using a UNIQUE sonicator (Unique Group, Indaiatuba, Brazil) for 3 min at a setting of ‘60’ on the power control, using three cycles of 1 min each in an ice bath. Afterwards 1.5 ml ultrapure water (Barnstead EASYpure II, Thermo Scientific, Dubuque, IA, USA) was added to backwash the methanol and non-lipid materials. Centrifugation at 1846 × g for 3 min was used to separate the two phases, an upper aqueous layer containing methanol and a lower organic layer containing dichloromethane and lipids. All subsequent extractions were done with 100% CH2Cl2 to minimize the amount of MeOH in the final concentrate. MeOH should be removed to reduce its interference with the quantification of ME/WE content. Samples were concentrated and maintained under an atmosphere of ultrapure N2 gas to ∼20 µl.
Lipid samples were spotted onto silica gel-coated rods (Chromarods-SIII), focused three times in acetone and analysed for lipid class composition using an Iatroscan TLC-FID (Iatron Laboratories, Tokyo, Japan) according to Parrish (Citation1999). Lipid classes were identified and quantified from calibration curves made with nine lipid standards with loads from 0.2 to 20 µg. The nine standards for identification of the lipid classes were n-nonadecane for aliphatic hydrocarbon (HC), cholesteryl palmitate for wax esters/steryl ester (WE/SE), n-hexdecan-3-one for ketone (KET), glyceryl tripalmitate for triacylglycerol (TAG), palmitic acid for free fatty acids (FFA), 1-hexadecanol for free aliphatic alcohol (ALC), cholesterol for free sterol (ST), 1-monopalmitoyl-rac-glycerol for acetone mobile polar lipids (AMPL), and 1,2,di-0-hexadecyl-sn-glycerol-3-phosphatidylcholine for phospholipids (PL) that were bought from Sigma–Aldrich (St Louis, Missouri, USA).The analytical conditions for the TLC-FID runs were: hydrogen flow 173 ml min−1, air flow 2 l min−1 and scan speed 4 mm s−1. The composition of lipid classes given according to their peak areas were recorded and processed by PeakSimple software version 6.78 (SRI Instruments, Menlo Park, California, USA) for windows. TLC-FID results were generally reproducible to + 10% of individual classes.
Transesterification of samples from acyl lipid to fatty acid methyl esters was carried out with 14% boron trifluoride at 85°C for 1.5 h followed by analysis with a Hewlett Packard Agilent 6890 gas chromatograph (GC) (Santa Clara, California, USA). The GC is equipped with a 7683 autosampler and a flexible fused-silica column (30 m × 0.32 mm internal diameter × 0.25 µm film thickness) coated with polyethylene glycol (ZBwax+, Phenomenex, Agilent Technologies, Santa Clara, California, USA). Column temperature started at 65°C for 0.5 minutes, it was then increased to 195°C at a rate of 40°C min−1 where it was held for 15 min. The temperature was then ramped to 220°C at a rate of 2°C min−1 and held for 3.25 min. Injector temperature started at 150°C and increased at a rate of 200°C min−1 to a final temperature of 250°C, while the detector remained fixed at 260°C. Retention times of fatty acids were obtained from a Supelco 37 (Sigma Aldrich, St Louis, Missouri, USA) component FAME mix (product number 47885-U), bacterial acid methyl ester mix (product number 47080-U), PUFA 1 (product number 47033) and PUFA 3 (product number 47085-U; Sigma Aldrich). All GF/C filters, glassware and metal materials that came into contact with the samples were previously baked overnight at 400°C. Prior to use, all materials were further rinsed with acetone and dichloromethane to reduce contaminations. Reagents used for the lipid class analysis were of chromatographic grade.
Data analyses
To determine the appropriate test of variance to be used on the obtained data, Levene's test for homogeneity of variances was employed. Factorial analysis of variance (ANOVA) and Tukey's HSD multiple range comparison tests were used to test for significant differences between means of analysed parameters. Principal components analysis (PCA) with a correlation matrix was used to determine the relationship between analysed parameters. A combination of the first two axes gives the best plane of the relationship between factors as exhibited by their distribution from the barycentre. PCA scores were grouped by cluster analysis using a single linkage. All analyses were done at the 95% confidence interval. ANOVA and post hoc analyses were done using Statistica 8.0 (Stat Soft, Tulsa, Oklahoma, USA) software, PCA was done with PAST 2.09 (University of Oslo, Oslo, USA) and cluster analysis with Minitab version 16.0 (Minitab, State College, Pennsylvania, USA).
Results
Chlorella vulgaris growth rates decreased under N limitation and Cd addition in N-replete conditions, and a combination of high (10−7 M) Cd and N limitation further inhibited the growth rate. Chlorophyll a concentrations on a per cell basis decreased with decreasing N in the cultures, but increased with Cd increase under N limitation conditions (). Percentage chlorophyll a contribution to total lipids decreased with decreased N and the presence of Cd. Total lipids increased with the increase of Cd in the cultures and with the decrease of N.
Table 2. Specific growth rate (Growth: divisions day−1), chlorophyll a concentration (chl a: pg cell−1), per cent chlorophyll a of total lipids (Chl a: %) and total lipids (TL: pg cell−1) of Chlorella vulgaris as a function of different cadmium and nitrogen concentrations. Values are means ± SD for n = 3. Rows with same superscript letters are not significantly different (P > 0.05).
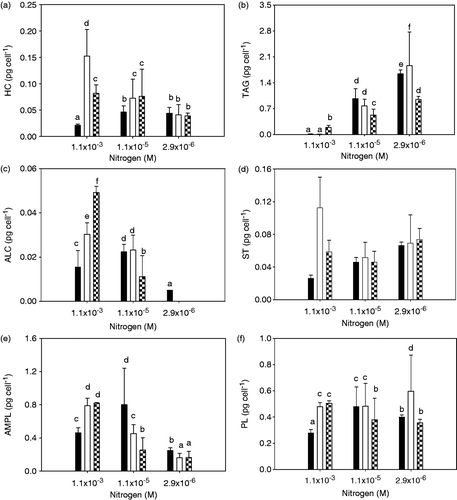
Lipid class results are shown in . In N-replete conditions (control), the addition of Cd resulted in an increase of all lipid classes with no clear trend at the two Cd concentrations tested. HC and ST were 6 and 3.7 times higher at a sublethal Cd concentration in relation to the control. The important lipid classes AMPL and PL were 1.8 and 2.0 times higher than the control at both of the Cd concentrations tested. TAG concentrations increased with Cd increase in N-replete conditions, while a higher increase was obtained with N limitation and no Cd addition. ALC increased with Cd increase under N-replete conditions, but decreased when N was limiting in the presence of Cd.
Fig. 1. Lipid class composition of Chlorella vulgaris at different nitrogen (N) and cadmium (Cd) concentrations. Black bars represent treatments without Cd, white bars 10−8 M Cd and checked bars 10−7 M Cd. Error bars represent standard deviation for n = 3. HC = aliphatic hydrocarbon; TAG = triacylglycerol; ALC = aliphatic alcohol; ST = free sterol; AMPL = acetone mobile polar lipids; PL = phospholipids. Bars with the same letters are not statistically different.
Both total saturated fatty acids (SAFA) and monounsaturated fatty acids (MUFA) increased under N limitation and Cd stress (). Contrary to the results for SAFA and MUFA, total polyunsaturated fatty acids (PUFA), ω3 PUFA and PUFA/SAFA ratios all decreased under N limitation and Cd stress ( and , ANOVA P < 0.05). The presence of Cd stimulated higher production of SAFA and MUFA. The 18:1(n-9) and 18:1(n-7) fatty acid proportions were lowest in the control but increased when N was limited. The combination of N limitation and Cd stress resulted in higher production of 14:0, 18:0 and 18:1(n-7) fatty acids. However, the proportion of 16:1(n-11) was higher in unstressed culture conditions (high N and no Cd), opposite to the behaviour in relation to the production of the other MUFAs. Most of the PUFA members decreased under N limitation and Cd stress. However, the accumulation of 18:2(n-6) was stimulated under N limitation.
Table 3. Fatty acid composition (per cent total fatty acids in total lipid extract) of Chlorella vulgaris as a function of different cadmium (Cd) and nitrogen concentrations. Columns with same letters are not significantly different (P > 0.05). MUFA = monounsaturated fatty acids; PUFA = polyunsaturated fatty acids; ω3 = omega 3 fatty acids; SAFA = saturated fatty acids.
Table 4. Analysis of variance results for the effect of nitrogen (N) and cadmium (Cd) on lipid composition of Chlorella vulgaris. Values represent F values and those in parentheses represent P values. Note: values with P < 0.05 are statistically significant and marked in bold. Abbreviations: HC = aliphatic hydrocarbon; TAG = triacylglycerol; ALC = aliphatic alcohol; ST = free sterol; AMPL = acetone mobile polar lipids; PL = phospholipids; MUFA = monounsaturated fatty acids; PUFA = polyunsaturated fatty acids; ω3 = omega 3 fatty acids.
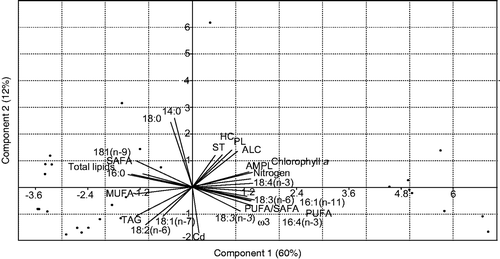
PCA analysis showed that the first two components were responsible for 72% of the total data variation (). The levels of ST, HC, PL, ALC, AMPL and PUFA (principal component 1 loadings of c. 0.16 to 0.25) presented significant positive correlations with N (0.24 PC1 loading), whereas TAG, MUFA, SAFA, 18:1(n-9), 16:0, 18:2(n-6) and 18:1(n-7) (PC loadings of c. −0.22 in PC1) were negatively correlated with N. Cd (PC loading of −0.32 in PC1) was positively correlated with 18:2(n-6) and 18:1(n-7) (−0.26 and −0.19 PC2 loadings, respectively), while it showed negative correlations with PL, HC, ST, 14:0 and 18:0 (c. 0.22 to 0.47 PC2 loadings).
Fig. 2. PCA biplot showing the relationship of the lipid composition of Chlorella vulgaris with the different nitrogen and cadmium concentrations investigated. Parameters grouped together on the same orthogonal axis are positively correlated, while those on opposite axes have a negative correlation. MUFA = monounsaturated fatty acids (FA); SAFA = Saturated FA; PUFA = Polyunsaturated FA; AMPL = Acetone mobile polar lipids; PL = Phospholipids; HC = Aliphatic hydrocarbons; ALC = Free aliphatic alcohols; ST = Free sterols; TAG = Triacylglycerol; the others represent individual FA group members.
Discussion
The increased total lipid concentration per cell obtained for Chlorella vulgaris under N limitation and Cd stress, and under Cd stress in N-replete conditions, is consistent with other investigations on microalgae grown under nutrient and Cd stresses (Einicker-Lamas et al., Citation1996; Guschina & Harwood, Citation2006a ; Bhola et al., Citation2011). These studies have shown that under N limitation or Cd stress, there is an increase in total lipid concentration and a decrease in growth rate. But, contrary to the findings of Bajguz (Citation2011) that Cd at 10−6 to 10−4 M caused a decrease in chlorophyll a concentration of C. vulgaris, our findings show that at the environmentally relevant concentrations studied here (2 × 10−8 to 10−7 M), chlorophyll a content per cell increased. This shows that the behaviour of Chlorella when exposed to environmentally relevant concentrations of Cd may differ from that shown by Chlorella exposed to very high Cd concentrations. Converti et al. (2009) demonstrated that a combination of temperature and nitrogen limitation caused the growth rate of C. vulgaris to decrease while total lipid content tripled, which agrees with our findings with the alga under combined Cd and N stress.
The role lipid metabolism plays as a tool for evaluating Cd stress in C. vulgaris at different N concentrations is explored here in terms of lipid class composition. The HC, ALC and ST lipid classes constituted a minor component (<5%) of total lipid concentration. This agrees with literature results for other microalgae (Lombardi & Wangersky, Citation1991, Citation1995; Guschina & Harwood, Citation2006b ). Accumulation of triacylglycerol (TAG) in response to N limitation has also been observed in a variety of algal species (Parrish & Wangersky, Citation1987; Basova, Citation2005; Merzlyak et al., Citation2007; Gardner et al., Citation2011). However, the neutral lipid class TAG did not change significantly in concentration under the combination of Cd/N stress tested here. This differs from the results of Pal et al. (Citation2011), who demonstrated that combinations of stress factors can, under certain circumstances, inhibit neutral lipid synthesis in Nannochloropsis sp., while Gardner et al. (Citation2011) showed increased TAG production in C. vulgaris under pH stress and N limitation. This means that the response of C. vulgaris, like any other species, can vary under a combination of stress factors. Solovchenko et al. (2009) proposed that the increase in neutral lipids, predominantly TAGs, under N limitation plays a protective role and renders microalgae less prone to oxidative damage. So, it might be expected that Cd stress would induce TAG synthesis, but this did not in fact occur in C. vulgaris. The inverse relationship we obtained between TAG and PL has been reported previously and suggested to be due to changes in de novo synthesis of both lipid classes, with modification of their metabolic pathways towards the formation and accumulation of neutral lipids; this occurs via the sequestering of de novo-produced fatty acid moieties in the form of TAG in C. vulgaris (Stephenson et al., 2010) and Nannochloropsis sp. (Pal et al., Citation2011). Accumulation of TAG under these stress conditions may reflect a preferential use of diacylglycerol for triacylglycerol formation rather than use of phosphatidylcholine (PC) and glycosylglycerides for membrane lipid production. The significant positive relationship between AMPL and chlorophyll a is associated with the fact that a proportion of this lipid class usually comprises microalgae pigments (Parrish & Wangersky, Citation1987; Lombardi & Wangersky, Citation1991; Hu et al., Citation2008). A reduction in AMPL under N limitation can be explained in the context of a reduction in the membrane lipid content of C. vulgaris, which can be related to the glycosylglycerides, monogalactosyldiacylglycerol (MGDG), digalactosyldiacylglycerol (DGDG) and sulphoquinovosyldiacylglycerol (SQDG) being reduced under N limitation and Cd stress, even though they were not individually resolved. These lipid classes migrate together when AMPL is separated via TLC (Guschina & Harwood, Citation2006a ; Harwood & Guschina, Citation2009). Khozin-Goldberg & Cohen (Citation2006) showed that nutrient starvation of Monodus subterraneus resulted in an increase proportion of TAG, while collectively MGDG, DGDG and SQDG and PL decreased in relation to total lipids. In addition, Bigogno et al. (Citation2002) demonstrated that, in the green oleaginous alga Parietochloris incisa, changes in growth phase and conditions that resulted in TAG increases also resulted in a simultaneous decrease in the proportions of MGDG, DGDG, SQDG and PL, which agrees with the changes in AMPL at the lowest N treatment and Cd stress in this study.
Fatty acid production by C. vulgaris exhibited a high degree of plasticity with changing culture conditions, with most fatty acids having long chain length groups of 16 and 18 carbon atoms (Brett et al., Citation2006; Petkov & Garcia, Citation2007; Villar-Argaiz et al., Citation2009). Pinto et al. (Citation2011) showed that SAFA and MUFA content of Gracilaria tenuistipitata increased with increasing Cd concentration, which is in agreement with our study showing increased SAFA and MUFA production by C. vulgaris in the presence of Cd. The accumulation of these fatty acids groups is thought to be dependent on oxidative stress and the production of reactive oxygen species (Pinto et al., Citation2003, Citation2011; Rocchetta et al., Citation2006). Griffith et al. (Citation2012) reported that C. vulgaris responds to N limitation by increasing its SAFA and MUFA and decreasing its PUFA content. This increase in SAFA and MUFA (primarily 18:1) in cultures with reduced nitrogen may correspond to a reduction in the synthesis activity of PUFAs. In our experiments the fatty acid tentatively identified as 16:1(n-11) did not increase with N depletion and/or Cd stress; instead it decreased with increasing either stress condition. Therefore, we suggest that it may be considered a stress biomarker. The significant association of TAGs with most MUFAs and SAFAs is in accordance with literature data, which show that these fatty acid groups are the main contributors to TAG content in chlorophytes (Rezanka et al., Citation2011; Spijkerman & Wacker, Citation2011) and in Chlorella specifically (Mendoza Guzmán et al., Citation2011; Laurens et al., Citation2012). Liu et al. (Citation2011) further demonstrated that the neutral lipid content of Chlorella contains almost 70% of the total 16:0 and 18:0.
PUFA/SAFA ratios are an important physiological index of stress because they indicate the degree of fatty acid saturation (Mendoza Guzmán et al., Citation2011). In this study, N-replete concentrations supported higher ω3 and PUFA content in C. vulgaris than did N-limited treatments, which agrees with the results of Griffith et al. (Citation2012) for PUFA production in C. vulgaris grown at different N concentrations. Griffith et al. reported 50% PUFA increase, which is in agreement with the 40% we obtained for N-replete conditions. Mendoza Guzmán et al. (Citation2011) reported that variations in MUFA and PUFA content and especially the increase in 18:1 can be a response to N starvation and activation strategies. In addition, Choi et al. (Citation2011) showed a 2.6-fold increase under N limitation, relative to N-sufficient conditions, in the expression of the stearoyl-ACP desaturase gene (sad) encoding a stearoly-ACP desaturase involved in the synthesis of oleic acid by Botryococcus braunii. Their findings can be used to explain the MUFA increase and PUFA decrease in cultures under N limitation in our study. Our finding that PUFA contents are significantly affected and their levels reduced in the presence of trace metals is also in accordance with other studies (Rocchetta et al., Citation2006; Pinto et al., Citation2011). The fatty acid 18:2(n-6) can be used as a nutrient stress indicator in C. vulgaris because its concentration increases under N limitation and Cd stress, unlike other PUFAs. The significant association of PL and AMPL with individual PUFAs and total PUFA is because these lipid classes are principally made up of PUFAs (Hill et al., Citation2011). All these groups are important in thylakoid membranes: PUFAs are the predominant fatty acids in the composition of structures in chloroplast membranes and their role in membrane structural integrity is critical in green algae (Yokthongwattana et al., Citation2005; Wu et al., Citation2009; Mendoza Guzmán et al., Citation2011). The simultaneous decrease in PL, AMPL and PUFA content in this microalga could be associated with the greater impact of nitrogen starvation on these fatty acids via the strong effect of nitrogen limitation on the photosynthesis system in Chlorella spp. (Mendoza Guzmán et al., Citation2011).
From the present results, we conclude that the combination of N limitation and Cd stress has a significant influence on total lipid production, lipid class and fatty acid composition in C. vulgaris. TAGs were most accumulated under N stress, while the presence and increasing concentrations of Cd caused the TAG levels to decrease. The degree of fatty acid desaturation increased with increasing N concentration in the medium, which was demonstrated by the individual and total PUFA, ω3 and PUFA/SAFA levels in the different treatments. However, 16:1(n-11) and 18:2(n-6) exhibited changes in their concentrations that were opposite to the general trends shown by the MUFAs and PUFAs under N limitation and Cd stress, respectively.
Acknowledgements
The authors ATL and MGGM are grateful to FAPESP (2008/02078-9; 2011/09694-0), CNPq (556588/2009-6; 314361/2009-0); MAC is grateful to TWAS, CNPq (190034/2009-2) and ABU Zaria for research funding; CCP acknowledges the Natural Sciences and Engineering Research Council of Canada.
References
- AFNOR – ASSOCIATION FRANÇAISE DE NORMALISATION (1980). Essais des eaux. Determination de l'inhibition de Scenedesmus subspicatus par une substance. Norme experimentale T90-304. Association Française de Normalisation, Paris, France
- American Public Health Association (APHA) . 1998 . Standard Methods for the Examination of Water and Wastewater, , 20th American Public Health Association, American Water Works Association/Water Environmental Federation, Washington, DC
- Awasthi , M and Rai , LC . 2005 . Toxicity of nickel, zinc, and cadmium to nitrate uptake in free and immobilized cells of Scenedesmus quadricauda . Ecotoxicology and Environmental Safety , 61 : 268 – 272 .
- Bajguz , A . 2011 . Suppression of Chlorella vulgaris growth by cadmium, lead, and copper stress and its restoration by endogenous Brassinolide . Archives of Environmental Contamination and Toxicology , 60 : 406 – 416 .
- Basova , MM . 2005 . Fatty acid composition of lipids in microalgae . International Journal on Algae , 7 : 33 – 57 .
- Bhola , V , Desikan , R , Santosh , SK , Subburamu , K , Sanniyasi , E and Bux , F . 2011 . Effects of parameters affecting biomass yield and thermal behaviour of Chlorella vulgaris . Journal of Bioscience and Bioengineering , 111 : 377 – 382 .
- Bigogno , C , Khozin-Goldberg , I , Boussiba , S , Vonshak , A and Cohen , Z . 2002 . Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid . Phytochemistry , 60 : 497 – 503 .
- BREITHOLTZ, M., NÄSLUND, M., STRÅE, D., BORG, H., GRABIC, R. & FICK, J. (2012). An evaluation of free water surface wetlands as tertiary sewage water treatment of micro-pollutants. Ecotoxicology and Environmental Safety, 78: 63–71
- Brett , MT , Muller-Navarra , DC , Ballantyne , AP , Ravet , JL and Goldman , CR . 2006 . Daphnia fatty acid composition reflects that of their diet . Limnology and Oceanography , 51 : 2428 – 2437 .
- Chan , SM , Wang , W and Ni , I . 2003 . The uptake of Cd, Cr, and Zn by the macroalga Enteromorpha crinite and subsequent transfer to the marine herbivorous rabbitfish, Sigunus canaliculatus . Archives of Environmental Contamination and Toxicology , 44 : 298 – 306 .
- Chia , AM , Oniye , SJ , Ladan , Z , Lado , Z , Pila , EA , Inekwe , VU and Mmerole , JU . 2009 . A survey for the presence of microcystins in aquaculture ponds in Zaria, northern Nigeria: possible public health implication . African Journal of Biotechnology , 8 : 6282 – 6289 .
- Chia , AM , Bako , SP , Alonge , S and Adamu , AK . 2011a . Green algal interactions with physicochemical parameters of some manmade ponds in Zaria, northern Nigeria . Revista Brasileira de Botanica , 34 : 285 – 295 .
- Chia , AM , Iortsuun , DN , Stephen , BJ , Ayobamire , AE and Ladan , Z . 2011b . Phytoplankton responses to changes in macrophyte density in a tropical artificial pond in Zaria, Nigeria . African Journal of Aquatic Sciences , 36 : 34 – 46 .
- Choi , GG , Kim , BH , Ahn , CY and Oh , HM . 2011 . Effect of nitrogen limitation on oleic acid biosynthesis in Botryococcus braunii . Journal of Applied Phycology , 23 : 1031 – 1037 .
- CONVERTI, A., CASAZZA, A.A., ORTIZ, E.Y., PEREGO, P. & DEL BORGHI, M. (2009). Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chemical Engineering and Processing: Process Intensification, 48: 1146–1151
- Devriese , M , Tsakaloudi , V , Garbayo , I , León , R , Vilchez , C and Vigara , J . 2001 . Effect of heavy metals on nitrate assimilation in the eukaryotic microalga Chlamydomonas reinhardtii . Plant Physiology and Biochemistry , 39 : 443 – 448 .
- Einicker-Lamas , M , Soares , MJ , Soares , MS and Oliveira , MM . 1996 . Effects of cadmium on Euglena gracilis membrane lipids . Brazilian Journal of Medical and Biological Research , 29 : 941 – 948 .
- FOOD AND AGRICULTURE ORGANIZATION/WORLD HEALTH ORGANIZATION (1984). List of Maximum Levels Recommended for Contaminants by the Joint FAO/WHO Codex Alimentarius Commission. Second Series. CAC/FAL, Rome. 3: 1–8
- Gardner , R , Peters , P , Brent , P and Cooksey , KE . 2011 . Medium pH and nitrate concentration effects on the accumulation of triacylglycerol in two members of the Chlorophyta . Journal of Applied Phycology , 23 : 1005 – 1016 .
- Gouvêa , SP , Vieira , AAH and Lombardi , AT . 2005 . Copper and cadmium complexation by high molecular weight materials of dominant microalgae and of water from a eutrophic reservoir . Chemosphere , 60 : 1332 – 1339 .
- Griffith , MJ , Van Hille , RP and Harrison , STL . 2012 . Lipid productivity, settling potential and fatty acid profile of 11 microalgal species grown under nitrogen replete and limited conditions . Journal of Applied Phycology , 24 : 989 – 1001 .
- Guschina , IA and Harwood , JL . 2006a . Lipids and lipid metabolism in eukaryotic algae . Progress in Lipid Research , 45 : 160 – 186 .
- Guschina , IA and Harwood , JL . 2006b . Lead and copper effects on lipid metabolism in cultured lichen photobionts with different phosphorus status . Phytochemistry , 67 : 1731 – 1739 .
- Harwood , JL and Guschina , IA . 2009 . The versatility of algae and their lipid metabolism . Biochimie , 91 : 679 – 684 .
- Hill , WR , Rinchard , J and Czesny , S . 2011 . Light nutrients and fatty acid composition of stream periphyton . Freshwater Biology , 56 : 1825 – 1836 .
- Hu , Q , Sommerfeld , M , Jarvis , E , Ghirardi , M , Posewitz , M , Seibert , M and Darzins , A . 2008 . Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances . The Plant Journal , 54 : 621 – 639 .
- Jeffrey , SW and Humphrey , GF . 1975 . New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae, and natural phytoplankton . Biochemie und Physiologie der Pflanzen , 167 : 191 – 194 .
- Khozin-Goldberg , I and Cohen , Z . 2006 . The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus . Phytochemistry , 67 : 696 – 701 .
- Kotut , K , Ballot , A , Wiegand , C and Krienitz , L . 2010 . Toxic cyanobacteria at Nakuru sewage oxidation ponds – a potential threat to wildlife . Limnologica , 40 : 47 – 53 .
- Lane , T and Morel , FFM . 2000 . A biological function for cadmium in marine diatoms . Proceedings of the National Academy of Sciences USA , 97 : 4627 – 4631 .
- Lane , T , Saito , MA , George , GN , Pickering , IJ , Prince , RC and Morel , FFM . 2005 . Isolation and preliminary characterization of a cadmium carbonic anhydrase from a marine diatom . Nature , 435 : 42
- Larned , ST . 1998 . Nitrogen-versus phosphorus-limited growth and sources of nutrients for coral reef macroalgae . Marine Biology , 132 : 409 – 421 .
- Laurens , LML , Quinn , M , Van Wychen , S , Templeton , DW and Wolfrum , EJ . 2012 . Accurate and reliable quantification of total microalgal fuel potential as fatty acid methyl esters by in situ transesterification . Analytical and Bioanalytical Chemistry , 403 : 167 – 178 .
- Liu , J , Huang , J , Sun , J , Zhong , Y , Jiang , Y and Chen , F . 2011 . Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel production . Bioresource Technology , 102 : 106 – 110 .
- Lombardi , AT and Garcia , O . 1999 . An evaluation into the potential of biological processing for the removal of metals from sewage sludges . Critical Reviews in Microbiology , 25 : 275 – 288 .
- Lombardi , AT and Maldonado , MT . 2011 . The effects of copper on the photosynthetic response of Phaeocystis cordata . Photosynthesis Research , 108 : 77 – 87 .
- Lombardi , AT and Wangersky , PJ . 1991 . Influence of phosphorus and silicon on lipid class production by the marine diatom Chaetoceros gracilis grown in turbidostat cage cultures . Marine Ecology Progress Series , 77 : 39 – 47 .
- Lombardi , AT and Wangersky , PJ . 1995 . Particulate lipid class composition of three marina phytoplankters Chaetoceros gracilis, Isochrysis galbana (Tahiti) and Dunaliella tertiolecta grown in batch culture . Hydrobiologia , 306 : 1 – 6 .
- Mendoza Guzmán , H , Valido , AJ , Duarte , LC and Presmanes , KF . 2011 . Analysis of interspecific variation in relative fatty acid composition: use of flow cytometry to estimate unsaturation index and relative polyunsaturated fatty acid content in microalgae . Journal of Applied Phycology , 23 : 7 – 15 .
- Merzlyak , MN , Chivkunova , OB , Gorelova , OA , Reshetnikova , IV , Solovchenko , AE , Khozin-Goldberg , I and Cohen , Z . 2007 . Effect of nitrogen starvation on optical properties, pigments, and arachidonic acid content of the unicellular green alga Parietochloris incisa (Trebouxiophyceae, Chlorophyta) . Journal of Phycology , 43 : 833 – 843 .
- Morin , S , Duong , TT , Herlory , O , Feurtet-Mazel , A and Coste , M . 2008 . Cadmium toxicity and bioaccumulation in freshwater biofilms . Archives of Environmental Contamination and Toxicology , 54 : 173 – 186 .
- Pal , D , Khozin-Goldberg , I , Cohen , Z and Boussiba , S . 2011 . The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp . Applied Microbiology and Biotechnology , 90 : 1429 – 1441 .
- Pankovic , D , Plesnicar , M , Arsenijevic-Maksimovic , I , Petrovic , N , Sakac , Z and Kastori , R . 2000 . Effects of nitrogen nutrition on photosynthesis in Cd-treated sunflower plants . Annals of Botany , 80 : 841 – 847 .
- Park , H , Song , B and Morel , FMM . 2007 . Diversity of the cadmium-containing carbonic anhydrase in marine diatoms and natural waters . Environmental Microbiology , 9 : 403 – 413 .
- Parrish , CC . 1999 . “ Determination of total lipid, lipid classes, and fatty acids in aquatic samples ” . In Lipids in Freshwater Ecosystems , Edited by: Arts , MT and Wainman , BC . 4 – 20 . New York : Springer-Verlag .
- Parrish , CC and Wangersky , PJ . 1987 . Particulate and dissolved lipid classes in cultures of Phaeodactylum tricornutum grown in cage culture turbidostats with a range of nitrogen supply rates . Marine Ecology Progress Series , 35 : 119 – 128 .
- Petkov , G and Garcia , G . 2007 . Which are fatty acids of the green alga Chlorella? . Biochemical Systematics and Ecology , 35 : 281 – 285 .
- Pinto , E , Sigaud-Kutner , TCS , Leitão , MAS , Okamoto , OK , Morse , D and Colepicolo , P . 2003 . Heavy metal induced oxidative stress in algae . Journal of Phycology , 39 : 1008 – 1018 .
- Pinto , E , Carvalho , AP , Cardozo , KHM , Malcata , FX , Maria Dos Anjos , F and Colepicolo , P . 2011 . Effect of heavy metals and light levels on the biosynthesis of carotenoids and fatty acids in the macroalgae Gracilaria tenuistipitata (var liui Zhang & Xia) . Brazilian Journal of Pharmacognosy , 21 : 349 – 354 .
- Reynolds , CS . 2006 . Ecology of phytoplankton Cambridge University Press, Cambridge
- Rezanka , T , Lukavsky , J , Nedbalová , L and Sigler , K . 2011 . Effect of nitrogen and phosphorus starvation on the polyunsaturated triacylglycerol composition, including positional isomer distribution, in the alga Trachydiscus minutus . Phytochemistry , 72 : 2342 – 2351 .
- Rocchetta , I , Mazzuca , M , Conforti , V , Ruiz , L , Balzaretti , V and De Molinna , MDR . 2006 . Effect of chromium on the fatty acid composition of two strains of Euglena gracilis . Environmental Pollution , 141 : 353 – 358 .
- Shoaf , WT and Lium , BW . 1976 . Improved extraction of chlorophyll a and b from algae using dimethyl sulfoxide . Limnology and Oceanography , 21 : 926 – 928 .
- Shun-Xing , L , Hua-Sheng , H , Feng-Ying , Z , Nan-Sheng , D and Fang , L . 2007 . Influence of nitrate on metal sorption and bioaccumulation in marine phytoplankton, Dunaliella salina . Environmental Toxicology , 22 : 582 – 586 .
- Siedlecka , A . 1995 . Some aspects of interactions between heavy metals and plant mineral nutrients . Acta Societatis Botanicorum Poloniae , 64 : 265 – 272 .
- Singh , SP and Yadav , V . 1984 . Cadmium induced inhibition of ammonium and phosphorus uptake in Anacystis nidulans: interaction with other divalent cations . Journal of General Microbiology , 30 : 79 – 86 .
- Sivakumar , S , Song , YC , Park , IS , Cho , SH , Lee , CY and Kim , BG . 2010 . Short-term influence of phosphate and nitrate on heavy metal accumulation by red alga Acrosorium uncinatum . Environmental Monitoring and Assessment , 165 : 449 – 460 .
- Solovchenko , A , Khozin-Goldberg , I , Cohen , Z and Merzlyak , M . 2009 . Carotenoid-to-chlorophyll ratio as a proxy for assay of total fatty acids and arachidonic acid content in the green microalga Parietochloris incisa . Journal of Applied Phycology , 21 : 361 – 366 .
- Spijkerman , E and Wacker , A . 2011 . Interactions between P-limitation and different C conditions on the fatty acid composition of an extremophile microalga . Extremophiles , 15 : 597 – 609 .
- STEPHENSON, A.I., DENNIS, J.S., HOWE, C.J., SCOTT, S.A. & SMITH, A.G. (2010). Influence of nitrogen limitation regime on the production by Chlorella vulgaris of lipids for biodiesel feedstocks. Biofuels, 1: 47–58
- Stoeppler , M . 1991 . Metals and their compounds in the environment: occurrence, analysis and biological relevance VCH Publishers, Weinheim, Germany
- UNITED STATES ENVIRONMENTAL PROTECTION ANGENCY (2012). Water: Drinking Water Contaminants. http://www.epa.gov/safewater/contaminants/index.html. Accessed 12/11/2012
- Villar-Argaiz , M , Medina-Sánchez , JM , Bullejos , FJ , Delgado-Molina , JA , Pérez , OR , Navarro , JC and Carrillo , P . 2009 . UV radiation and phosphorus interact to influence the biochemical composition of phytoplankton . Freshwater Biology , 54 : 1233 – 1245 .
- Wu , Q , Liu , T , Liu , H and Zheng , GC . 2009 . Unsaturated fatty acid metabolism, synthesis and gene regulation . African Journal of Biotechnology , 8 : 1782 – 1785 .
- Yokthongwattana , K , Savchenko , T , Polle , JEW and Melis , A . 2005 . Isolation and characterization of a xanthophylls-rich fraction from the thylakoid membrane of Dunaliella salina (green algae) . Photochemical and Photobiological Sciences , 4 : 1028 – 1034 .
- Zhao , YF , Yu , ZM , Song , XX and Cao , XH . 2009 . Biochemical compositions of two dominant bloom-forming species isolated from the Yangtze River Estuary in response to different nutrient conditions . Journal of Experimental Marine Biology and Ecology , 368 : 30 – 36 .