Abstract
Cylindrotheca is one of the main model diatoms for ecophysiological and silicification research and is among the few diatoms for which a transformation protocol is available. Knowledge of its life cycle is not available, however, although sexual reproduction has been described for several related genera. In this study, 16 Cylindrotheca closterium strains from a single rbcL lineage were used to describe the life cycle of this marine diatom, including the sexual process and mating system. Similar to other Bacillariaceae, sexual reproduction was induced by the presence of a suitable mating partner, with two gametes produced per gametangium, resulting in two auxospores per gametangial pair. Differences with other Bacillariaceae include details of cell pairing, gamete behaviour, auxospore orientation and chloroplast configuration, and perizonium structure. The mating system is heterothallic, since strains fell into two mating type groups, with several strains of one mating type occasionally displaying intraclonal auxosporulation. Initial cell lengths were on average 95–100 µm, the sexual size threshold was at least 66 µm, and the minimal viable cell length c. 11 µm. Sexual reproduction could be synchronized by dark conditions, which allowed us to determine that the whole sexual process is completed in less than 24 h. Furthermore, large percentages of cells at defined sexual stages can easily be obtained and the possibility to experimentally manipulate the life cycle provides a valuable tool for future research on all aspects of the biology of this model diatom.
Introduction
Cylindrotheca is a reportedly small genus (six spp. in the online Catalogue of Diatom Names: Fourtanier & Kociolek, Citation2011) which is very abundant in coastal and estuarine waters worldwide (Round et al., Citation1990), mostly in benthic environments and sometimes in the phytoplankton. They have an only lightly or partly silicified frustule, which is more or less twisted around the apical axis and provides relatively few characters for species delimitation. Molecular phylogenetic analysis suggests that species diversity in the genus is seriously underestimated (Li et al., Citation2007). Cylindrotheca can easily be grown in the laboratory and cryopreserved, and is widely available in culture collections. Its ecological importance and easy culturing have made it a model diatom for (eco)physiological, biochemical and molecular studies (e.g. Hildebrand, Citation2005; Medlin & Mann, Citation2007, and references therein; Araújo et al., Citation2008; McLachlan etal., Citation2009). Moreover, a transformation protocol is available for C. fusiformis, allowing analysis of gene function (Fischer et al., Citation1999; Poulsen & Kröger, Citation2005). Knowledge of the life cycle of Cylindrotheca and the possibility to manipulate it experimentally would allow the use of forward genetics to detect the genetic basis of phenotypic variation, for example using segregant analysis of sexual progeny (Michelmore et al., Citation1994; Staelens et al., Citation2008; Schneeberger et al., Citation2009; Ehrenreich et al., Citation2010), and open up perspectives for diatom life cycle and speciation research. However, as in some other classical model diatoms, such as Phaeodactylum (Chepurnov et al., Citation2008), auxosporulation (i.e. size restitution by a specialized cell – the auxospore – generally formed by the zygote during sexual reproduction) has to date never been reported in Cylindrotheca. For C. fusiformis, it has even been suggested that it entirely lacks the typical diatom cell size reduction–restitution cycle (Mann & Chepurnov, Citation2004).
Auxosporulation has been described for several genera of Bacillariaceae, the diatom family to which Cylindrotheca belongs (Round et al., Citation1990; Lundholm et al., Citation2002), including Fragilariopsis (Assmy et al., Citation2006), Nitzschia (e.g. Geitler, Citation1928, Citation1970; Mann, Citation1986; Trobajo et al., Citation2006, Citation2009; Kaczmarska et al., Citation2007), Denticula (Geitler Citation1953) and Pseudo-nitzschia (e.g. Chepurnov et al., Citation2005; Quijano-Scheggia et al., Citation2009). It is hard to find a feature of the sexual process that doesn't vary in the Bacillariaceae. In most cases, when cell pairing occurs, the paired gametangia produce two gametes each, resulting in the formation of two auxospores. In Nitzschia amphibia however, only one gamete is produced per gametangium (Geitler, Citation1969). This may also be the case for Fragilariopsis kerguelensis, in which single auxospores with attached parental valves of different sizes (suggesting heterothallic auxosporulation) were observed in a natural population (Assmy etal., Citation2006). Additional variation exists among Bacillariaceae in the details of the sexual process, including the mode of cell pairing, gamete morphology and behaviour, auxospore orientation, fine structure of the auxospore casing, auxospore chloroplast organization, and the way in which the initial cell escapes from the perizonium. The mating system, determined for many Pseudo-nitzschia (e.g. Amato et al., Citation2005; Chepurnov et al., Citation2005; CitationD'Alelio et al., 2009; Quijano-Scheggia et al., Citation2009) and several Nitzschia (overview by Chepurnov et al., Citation2004; Trobajo et al., Citation2009), is generally heterothallic, as is common in pennate diatoms (Chepurnov et al., Citation2004). Intraclonal mating and resulting auxosporulation have been reported in P. brasiliana (Quijano-Scheggia etal., Citation2009) and a few Nitzschia (Chepurnov et al., Citation2004). In at least some Nitzschia species, cell pairing doesn't take place, and auxosporulation is achieved in unpaired cells (Geitler, Citation1932); in the three cases investigated in detail auxosporulation occurs through the fusion of both gametes formed by a single unpaired gametangium (i.e. paedogamy, Geitler, Citation1970; Trobajo et al., Citation2006; Mann et al., Citationin press). Furthermore, auxospores and initial cells have been observed in monoclonal cultures of some other Nitzschia spp. implying either intraclonal mating or automixis (Trobajo et al., unpublished). Autogamy, which involves fusion of two haploid nuclei formed by meiosis without accompanying cell division, has been observed in Denticula tenuis (Geitler Citation1953).
Assessing phylogenetic relationships between lineages in the Bacillariaceae would allow variation in the sexual process to be placed in an evolutionary context. Molecular phylogenies (Lundholm et al., Citation2002; Trobajo et al., Citation2006, Citation2009; Brüder & Medlin, Citation2008a, Citationb; Ruck & Theriot, Citation2011) generally suggest (1) monophyly of the Bacillariaceae (but see Sinninghe Damsté et al., Citation2004; Sorhannus, Citation2007), which is also morphologically well-characterized by the structure of its fibulate raphe system (Ruck & Theriot, Citation2011), (2) paraphyly of Nitzschia, which consists of a number of lineages among which the other genera are interspersed, and (3) paraphyly of Pseudo-nitzschia (which includes Fragilariopsis). Finally, the few Cylindrotheca sequences included are often but not always recovered as monophyletic (e.g. Lundholm etal., Citation2002; Sinninghe Damsté et al., Citation2004). However, taxon sampling in the Bacillariaceae is limited and most relationships among lineages are poorly resolved. Together with a lack of data of sexual reproduction from several lineages, this as yet precludes a formal analysis of the evolution of sexual traits. Additional detailed study of the sexual process in molecularly well-characterized members of the Bacillariaceae is therefore needed. Here, we used 16 Cylindrotheca closterium strains belonging to a single rbcL lineage to (1) determine characteristics of the life cycle and auxospore development; (2) investigate the mating system; and (3) obtain high percentages of synchronized sexualized cells in order to facilitate future molecular and biological studies of this model diatom.
Materials and methods
Sixteen Cylindrotheca strains, isolated from intertidal mudflats in the Scheldt estuary (the Netherlands), were used for studying the Cylindrotheca life cycle. Several other strains, mainly from the Scheldt estuary, were included in the rbcL molecular phylogeny and a strain list with details of source locations and sampling and isolation dates is given in . Establishment of most monoclonal cultures (strains) was done by one of us (B.V.) by isolation of single cells with a micropipette under a stereomicroscope. Strain Pt01 was isolated by Joâo Serôdio (Universidade de Aveiro, Portugal) and ROS97005 was obtained from the Roscoff Culture Collection (RCC # 81). Wherever necessary, the strains were reisolated to get rid of contamination (by flagellates). All cultures were kept in 24-well plates (Greiner Bio-One, Frickenhausen, Germany) in sterile filtered (0.2 µm) seawater (33 psu) enriched with f/2 nutrients (Guillard, Citation1975) at 18 ± 0.3 °C with a 16 : 8 or 12 : 12 light : dark period and a photon irradiance of 20–50 µmol photons m−2 s−1 from cool-white fluorescent tubes. Strains were reinoculated every week to 10 days. Nine of the Cylindrotheca strains have been cryopreserved and included in the diatom culture collection (BCCM/DCG) of the Belgian Coordinated Collection of Micro-organisms (http://bccm.belspo.be, accession numbers DCG 0307–0315).
Table 1. Source locations and sampling and isolation dates of Cylindrotheca strains
Light microscopical (LM) photographs of living cells (bright field) were taken using an Olympus BX51 light microscope with an Ach 100 ×/1.25 Oil objective equipped with a Colorview IIIu camera (Olympus). Cultures for LM examination were prepared in the same way as described below for sexualized cells. Cell widths of strains E6, E9, Mid24, and F1(D8 × E9)1 (n = 5) were measured from LM photographs. Fibula densities were counted from scanning electron microscope (SEM) photographs of valves of B6 (n = 2) and VDO5 (n = 2), and from LM photographs of valves of ROS97005 (n = 5) and E9 (n = 3), taken from the crosses made for photographs of the sexual process (see below). Fibulae were counted on the spindle-shaped central part of the cell on one side of the cell centre.
RbcL sequence data were obtained (as described in Souffreau et al., Citation2011) for the 16 strains for which the sexual process was investigated, as well as for a collection of other Cylindrotheca strains from the same region (). All Cylindrotheca rbcL sequences available in GenBank (in January 2012) were added, as well as three outgroup sequences from different lineages in the Bacillariaceae (Trobajo et al., Citation2009). Next, a ClustalW multiple alignment using default parameters was produced in BioEdit version 7.0.3 (Hall, Citation1999) and corrected manually. After exclusion of positions at both ends that were not available for all sequences, the length of the alignment was 1138 positions, of which 274 were variable and 183 parsimony-informative (revealed by a maximum parsimony analysis in PAUP, as in Vanormelingen et al., Citation2007). Phylogeny reconstruction was done with Bayesian Inference using MrBayes version 3.1.1 (Ronquist & Huelsenbeck, Citation2003). A GTR + I + G model was applied with four rate categories. No initial values were assigned to the model parameters. Two runs of four Markov Chains (one cold and three heated) were run for 10 million generations and sampled every 200 generations. This yielded a posterior probability (PP) distribution of 50 001 trees. After exclusion of 25 000 ‘burn-in’ trees, PPs were calculated by constructing a 50% majority-rule consensus tree. All rbcL sequences were deposited in GenBank (accession numbers JX970999–JX971026).
Sexual compatibility of the different strains belonging to clade V in the rbcL phylogeny was tested between October 2009 and January 2010 in a series of crossing experiments involving all possible pairwise combinations. The culture conditions were the same as used for culture growth, but always with a 12 : 12 h light : dark period. Healthy-looking cultures of the strains were re-inoculated 2–4 days before the start of the crossing experiment to ensure that they were in the exponential growth phase. The crossing experiment was set up by mixing the strains in pairwise combinations in wells of 24-well plates. These were checked for the presence of sexual reproduction two or three times during the next 2 or 3 days. Each well was visually scanned for approximately one minute using an inverted microscope at low magnification (200×), and the intensity of sexual reproduction noted via asimple scoring system (no sexualized cell pairs, 1–5 pairs, 5–10 pairs, 10s to 1000s of pairs). To ensure that the absence of sexual reproduction in a particular cross resulted from incompatibility of the strains and not the inability of one of the strains to mate (due to a cell size above the sexual size threshold or an inappropriate physiological state of the culture), a negative outcome was only accepted when both strains displayed sexual reproduction in at least one other combination in the same crossing experiment.
To determine the upper size threshold for sexualization, a progeny strain, named F1(B6 × Ps2)1, was established by isolating an initial cell from a cross between B6 and Ps2. This strain was first grown for several months after which subcultures of different cell size were established by isolating single cells. These subcultures were grown to sufficient density, their cell lengths determined (n = 10), and crossed with original strains Mid24, VDO5, B6, Ps2, F6A and E6 to check whether they were capable of sexual reproduction. Crosses were checked after 1 and 2 days. A second progeny strain, F1(D8 × E9)1, was used for light microscopy of the vegetative cells (see above).
For light microscopy of the sexual process, compatible strains were combined and allowed to settle on a cover slip placed at the bottom of the well of a 6-well plate (Greiner Bio-One). The cover slip with attached cells was then removed and sealed to a glass slide with Vaseline for microscopical examination. Differential Interference Contrast pictures were taken using a Zeiss Axioplan 2 microscope with a Plan-APOCHROMAT 100× or 63×/1.40 Oil DIC objective equipped with an AxioCam Mrm camera. To visualize the nuclei, coverslips with attached cells were fixed in Carnoy's solution (60% absolute ethanol, 30% glacial acetic acid, 10% chloroform) and stored in a fridge until further processing. Next, the coverslip was transferred to phosphate-buffered saline (PBS), stained with DAPI (40, 6-diamidino-2-phenylindole) at a final concentration of 0.5 µg ml−1 and gently shaken for 20 min in the dark, after which the cover slip was washed with PBS to remove excess DAPI, mounted in a drop of low-fluorescence (halogen-free) immersion oil (Zeiss) and examined using an Axioplan 2 fluorescence microscope with a Plan-Neofluar 100×/1.30 Oil objective connected to an AxioCam Mrm camera.
For SEM examination of auxospore ultrastructure, clones B6 and VDO5 were crossed in a Petri dish, fixed using a final concentration of 1% glutaraldehyde and stored at room temperature until further processing. Next, cells were rinsed with distilled water and picked up by Pasteur pipette under LM and transferred into a drop of strong domestic bleach to remove the organic coat of the cells. Cells were washed via several drops of distilled water using the pipette and then mounted on a cover slip. Air-dried cover slips were attached to aluminium stubs using carbon pads, coated with platinum, and examined with a LEO Supra 55VP instrument, usually at 5 kV and a working distance of 4 mm.
Synchronization of the sexual process was achieved as described for Seminavis robusta (Gillard, Citation2009). This involved placing exponentially growing Cylindrotheca cultures of strains E6 and Ps2 in the dark for 24 h at 18 °C. Immediately after re-illumination, the strains were crossed by putting them together in the wells of a 6-well plate and incubated in continuous light (22 µmol photons m−2 s−1) at 18 °C. Two replicate crosses were made. Starting 1 hour after crossing the strains, photographs were taken every 2 hours on a Zeiss Axiovert inverted microscope at 200× magnification equipped with a Canon Powershot G3 digital camera. Based on these images, cells in different stages of the sexual process (vegetative, paired gametangia, gametes, zygotes, auxospores, initial cells) were counted using the Cell Counter plug-in in ImageJ version 1.40g software (http://rsbweb.nih.gov/ij/index.html). For each combination of replicate and time point, between 224 and 1616 cells were counted. Zygotes were considered to become auxospores from the moment they started bipolar expansion and thus lost their round to slightly oval shape. Auxospores and initial cells were distinguished based on the more slender cell ends and clear cell outline resulting from the formation of the initial valves. This was sometimes difficult to judge. In addition, auxospore and initial cell lengths of, wherever possible, 50 cells per time point were measured using ImageJ. The length of only one randomly chosen auxospore per auxospore pair was recorded.
Results
Molecular and morphological characterization
In the Cylindrotheca rbcL phylogeny, five lineages containing strains from the Scheldt estuary were easily distinguishable and were numbered I–V, of which lineage III was represented by only a single strain (). Lineages I–V were differentiated from other lineages containing Cylindrotheca strains, which were mostly from Jiaozhou Bay, China (Li et al., Citation2007). All 16 strains from the best-represented lineage V were used for the study of sexual reproduction. Vegetative cells of strains in this lineage had a slender outline with a spindle-shaped central part and narrow rostrate apices of variable length (–). In the smallest cells, the rostrate apices were extremely short (), or even entirely absent at one end, resulting in a club-like cell shape (, see also below under ‘Cardinal points’). Cell widths varied between 3.4 and 4.9 µm and fibula density between 14 and 19 in 10 µm. The cells contained two plastids lying next to each other along the longitudinal axis of the cell and appressed to one side of the cell with lobes extending onto the other sides, and the nucleus centrally in between (–). Cell division was longitudinal, dividing both plastids without any obvious preceding plastid rearrangement (not shown). The valves were only lightly silicified and largely reduced to the sternum containing the raphe, which was subtended by fibulae (, ). At least in its central part, fine ribs (virgae) extended out from the raphe-sternum (). The many girdle bands consisted of plain narrow strips. The valves were not obviously twisted for most of their length, but there was a clear twist in the rostrate valve apices (), which is typical for C. closterium (Round etal., Citation1990; Hoppenrath etal., Citation2009).
Figs 1–8. Cylindrotheca rbcL phylogeny and the morphology of valves (SEM) and living cells (LM) of strains from C. closterium lineage V. 1. Most likely tree from Bayesian Inference analysis of Cylindrotheca rbcL sequences. Posterior probabilities > 0.5 are shown at the nodes. Accession numbers are given for sequences downloaded from Genbank. The five lineages containing strains isolated for this study are indicated and numbered I to V. 2. Clustered cells of strain E9 close to the minimal viable cell size, LM. 3. Large cells of progeny strain F1(D8 × E9)1. 4. Cells of strain Mid24. 5. Cells of strain E6. 6. Cell of strain E9 close to the minimal viable cell size. 7. Detail of central area of a valve of strain VDO5 in a cross between B6 and VDO5, SEM. 8. Valve of strain B6 from a cross between B6 and VDO5 (as determined based on the size difference between the strains), SEM. Scale bars = 10 µm (Figs 2–6: bar in Fig. 2), 1 µm (Fig. 7) and 2 µm (Fig. 8).
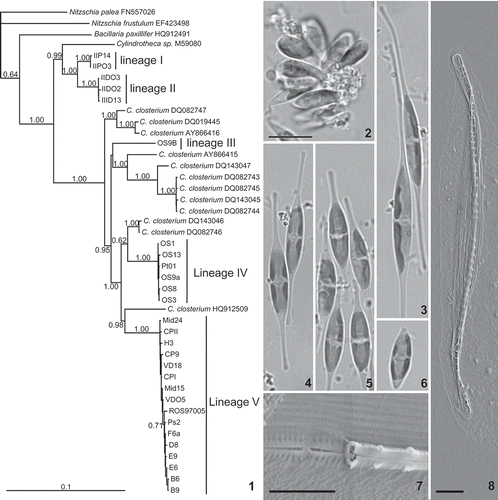
Figs 9–20. Early stages of the sexual process in Cylindrotheca closterium, LM. The crosses are B6 × ROS97005 (Figs 9, 10, 13–15, 18, 19), B9 × Mid15 (Figs 11, 12, 16, 17), and E6 × Ps2 (Fig. 20). 9. Paired gametangia. 10. The gametangium on the right has undergone cytokinesis at the first meiotic division, while the one on the left already contains two rearranged, rounded gametes. 11. DAPI staining of paired gametangia, each with a single nucleus. 12. DAPI staining of a triplet of gametangia. In the gametangium below left, the two nuclei resulting from the first meiotic division are visible. In the other two gametangia, the second meiotic division has taken place, resulting in a total of four nuclei per gametangium (two in each elongate daughter cell resulting from the first meiotic division). 13. The gametangium on the right contains two elongate but contracting cells after the cell division accompanying the first meiotic division; the one on the left contains two rounded gametes. 14. In the right-hand gametangium, the daughter cells are rearranging, shifting over each other while becoming spherical. 15. Rounded gametes in both gametangia. 16. DAPI staining of the four gametes (the four mid-grey bodies) of two paired gametangia. Generally, only one nucleus remains in each gamete, but quite often, a second nucleus can still be observed (top left gamete). 17. DAPI staining of the two zygotes produced by plasmogamy: each seems still to contain two unfused (overlapping) gametic nuclei. 18. Gamete fusion. 19. Zygotes lying between the empty gametangia. 20. Zygotes, each associated with one gametangium. Scale bars = 10 µm (Figs 9, 10, 13–15, 18–20: bar in Fig. 20) and 10 µm (Figs 11, 12, 16, 17: bar in Fig. 12).
Mating system
All strains were capable of sexual reproduction at the first cross when combined with a compatible strain. The mating system was heterothallic: strains fell into two mating type groups with induction of sexual reproduction only when strains of different mating types (termed Cyc1 and Cyc2, following Chepurnov et al., Citation2005) were crossed ( ). The only exception was the presence of rare sexualized cells in crosses between CP1 and several other strains belonging to mating type Cyc1 (). However, observation of sexual stages in paired gametangia in monoclonal CP1 cultures, prepared in the same way as for crosses, indicates that the few sexualized cells in crosses of CP1 with other Cyc1 clones most probably reflected intraclonal sexual reproduction of CP1. In both the monoclonal CP1 cultures and the crosses of CP1 with other Cyc1 clones, only one or a few sexualized cell pairs could be found, compared to 10s to 100s of sexualized cell pairs in crosses between opposite mating types. The appearance of cells of maximal size in cultures of three other strains of mating type Cyc1, being E6, Mid15 and F6A, suggests that they too are capable of very rare intraclonal sexual reproduction.
Table 2. Summary of pairwise crosses between 16 Cylindrotheca closterium strains from rbcL lineage V. The cell length at the first crossing experiment (mean ± S.D.) is given for each strain. Successful crosses between strains of opposite mating type (Cyc1 vs Cyc2) are indicated in the box. 0 = no sexual reproduction observed, S = 1–5 sexualized cell pairs, SS = abundant to vigorous sexual reproduction (10s to 1000s of cases). The presence of sexual reproduction in monoclonal cultures is indicated between square brackets along the diagonal
Sexual process
The first sign of sexual reproduction in mixed cultures was the pairing of vegetative cells, which lay against each other with their long axes more or less parallel (, ). Larger aggregations were also possible, with up to 10 or more cells. The first meiotic division was very similar to a mitotic division and included a longitudinal cell division splitting the parental cell in two, including the chloroplasts and the nucleus (, , 13). The second meiotic nuclear division, occurring in both elongate cells resulting from the first meiotic division (), was not accompanied by a cell division. The elongate cells resulting from the first meiotic division moved to fore and aft positions within each gametangium while becoming shorter (, –15). Thus each gametangium finally contained two spherical gametes. One of the two daughter nuclei in each gamete degenerated during gamete formation (). Gamete formation was not always synchronized (see , , 14). When gametogenesis was complete, each of the two gametes of a gametangium fused with the nearest gamete of the other gametangium (, ). The timing of the fusion of the gamete nuclei in the zygote was not determined. The association of zygotes with the parental gametangia seemed variable. Zygotes could be both associated with one parental gametangium (not shown), one with each gametangium (), or apparently located in between gametangia (), suggesting that gamete behaviour during fusion was variable. There seemed to be some dependency of gamete behaviour on the strain combination used, but this has to be investigated further. The thecae of each gametangium sometimes stayed connected by their ends during gamete and zygote formation (, , 20), possibly due to their apices being twisted.
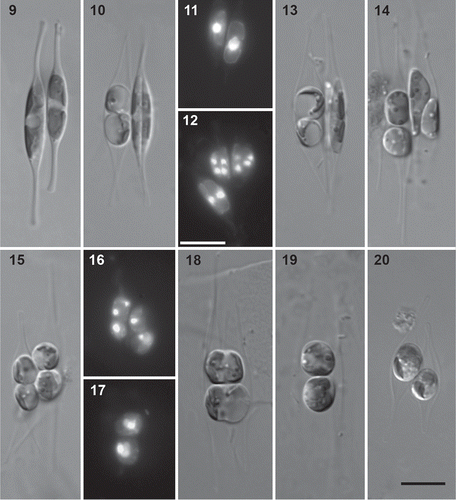
Zygotes started expanding, thereby transforming into auxospores, more or less parallel to the empty parental valves (Figs 21–23), although in some cases one of the auxospores lay almost perpendicular to the parental valves (). Each auxospore contained four chloroplasts, which were usually more or less regularly arranged around the auxospore centre (, ), although sometimes three chloroplasts could be found on one side of the cell centre (, upper auxospore). They remained at the centre during auxospore expansion. In some cases, one of the chloroplasts appeared rounded and reduced in size (, arrow). A single nucleus could sometimes be observed, apparently lying against one side of the auxospore (, arrowhead).
Figs 21–24. Auxospore and initial cell formation in Cylindrotheca closterium, LM. The crosses illustrated are B6 × E9 (Figs 21, 24) and E6 × Ps2 (Figs 22, 23). 21. Young auxospores starting expansion. 22. Expanding auxospores. The bands of the transverse perizonium are visible, as well as a polar cap covering one of the auxospore ends (arrowhead). One of the chloroplasts in the upper auxospore is rounded and reduced in size (arrow). 23. Full-grown auxospores. The arrowhead indicates a single nucleus lying against one side of the auxospore. 24. Initial cell with ruptured perizonium. The rupture zone is indicated by arrowheads. Scale bars = 10 µm.
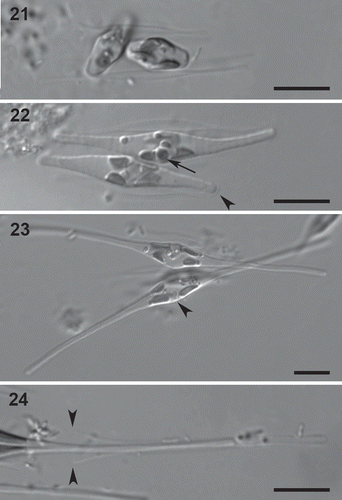
The bands of a transverse perizonium were easily visible using both LM and SEM, though very delicate (, –28). They were wider in the central part of the auxospore but there was no distinct central band (). A ring-like structure was present on each perizonial band one-third of the way along from its proximal margin, i.e. the margin closest to the expanded centre of the auxospore (, arrowheads). Bands were also narrower near their proximal margin, which was widely overlapped by the distal part of the previous band, at least in the distal parts of the perizonium (, ). Underneath the transverse perizonium, longitudinal strips could sometimes be distinguished using SEM (, arrow), but it is unclear whether these represent a longitudinal perizonium, parts of the cell content, or parts of the initial cell wall. A polar cap, presumably representing the ruptured remains of the original zygote wall, covered the auxospore endings (, , arrowhead). Scales (Sato et al., Citation2004; Kaczmarska et al., Citation2007) or other incunabula (e.g. the strip-like elements outside the perizonium in Nitzschia fonticola: Trobajoet al., Citation2006), could not be detected. After expansion was complete, an initial cell was formed which mostly had the same shape as the surrounding auxospore. Sometimes, however, a constriction was visible near the ends of the spindle-shaped part of the perizonium. The perizonium ruptured on one side of the cell centre (), allowing the initial cell to break free.
Figs 25–28. Ultrastructure of Cylindrotheca closterium auxospores, SEM. The strains crossed were B6 and VDO5. 25. Central part of the auxospore. 26, 27. Distal parts of the auxospore. In Fig. 27, the distal end of the auxospore is to the left, the central part to the right. The ring-like structure on each perizonial band is indicated by arrowheads, the longitudinal lines underneath the transverse perizonial bands by an arrow. 28. Auxospore end covered by a polar cap (arrowhead). Scale bars = 5 µm (Figs 25, 26) and 1 µm (Figs 27, 28).
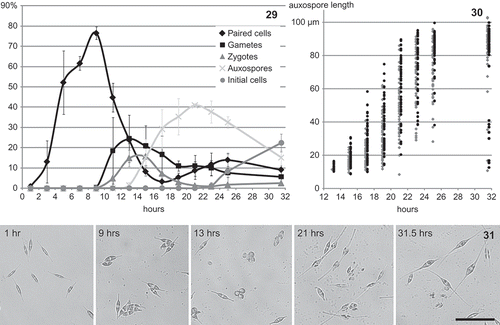
Synchronization and timing of the sexual process
In the cross with dark-synchronized cultures, cells had already started pairing 1 hour after re-illumination andmixing of the strains ( ). The frequency of paired cells increased rapidly and reached a maximum average of 77% 9 hours after re-illumination ().By then, the first gametes could already be observed and the percentage of sexualized cells (all stages) never fell below 50% during the remaining observation period. The maximal percentages of gametes (24%) and zygotes (16%) were reached after 13 and 15 h, respectively. The first auxospores were detected after 13 h, and reached a maximum of 41% after 21 h. The auxospores expanded gradually and the first fully grown auxospores and initial cells were observed 10 h after the first young auxospores were detected (). The maximal auxospore or initial cell length recorded was 100 µm. After reaching a minimum at 17 h, the percentage of paired cells increased again to some extent, presumably because vegetative cells that had not mated previously now again reached the cell cycle phase in which they were susceptible to sexualization (Gillard, Citation2009). Later on this renewed activity resulted in some additional gametes () and, after 31.5 h, a new small cohort of young auxospores ().
Figs 29–31. Sex synchronization in a cross between Cylindrotheca closterium strains E6 and Ps2. 29. Percentages of different sexual stages in continuous light as a function of time after re-illuminating and crossing dark-adapted cultures of both strains. 30. Auxospore and initial cell lengths vs. time for the two replicate crosses. 31. Inverted microscope images of the cross at different times after mixing the strains. Scale bar = 50 µm.
Cardinal points
Both the original and F1 progeny strains gradually diminished in size during vegetative growth, showing the presence of a typical diatom life cycle, in which three so-called ‘cardinal points’ mark key transitions (Geitler, Citation1932).
The first cardinal point is the maximal cell size, achieved in the initial cells. Initial cell lengths in crosses of B6 × ROS97005 and B6 × Mid24 (see for parental cell lengths measured one month earlier) were 100.6 ± 3.6 µm (mean ± S.D., range 94.7–108.4) and 94.7 ± 4.3 µm (84.0–103.0) (n = 20 in both cases), respectively. This agrees well with the lengths of fully grown auxospores and initial cells recorded from the synchronization experiment ().
The second cardinal point is the upper size threshold for sexual reproduction. All original strains with a maximal average cell length of 53 µm () were already capable of sexual reproduction. Furthermore, all subcultures of progeny strain F1(B6 × Ps2)1 with intermediate to long cells, being 31.8 ± 1.4 (29.6–33.5) µm, 57.4 ± 9.5 (45.6–68.1) µm, 61.3 ± 2.8 (57.2–65.6) µm, 61.5 ± 1.2 (58.8–62.6) µm, 66.5 ± 1.9 (62.5–69.2) µm and 67.5 ± 1.0 (65.8–68.8) µm (n = 10 for all subcultures), were capable of sexual reproduction with the original strains B6 and E6. However, sexual reproduction was only observed after 2 days for the four largest strains, compared with 1 day for the smaller strains, and was not vigorous in the crosses with the two largest progeny strains, suggesting that they were close to the upper size threshold. Crosses between F1 progeny and strain F6A were less successful: sexual reproduction was found only with the two smallest F1 progeny cultures and only after 2 days, possibly due to the poorer condition of F6A cells.
The critical minimal size for sexualization is the third cardinal point. This is often the same as the critical minimal size below which cells are not viable any more (‘open’ sexual size range, as opposed to ‘closed’, see Drebes, Citation1977). The minimal viable cell length was about 11 µm; 6 weeks after strain E9 reached an average cell length of 13.2 ± 1.3 µm (11.1–15.2 µm) (n = 13), all cells grew in clusters and so slowly that the culture had to be discarded. In strain F6A too, when it reached an average cell length of 13.5 ± 1.4 µm (10.8–15.4 µm) (n = 20), cells grew very slowly and often in clusters, especially the smallest cells. At that moment, they were gradually replaced by cells having the maximal size (probably resulting from homothallic sexual reproduction: see above). Such small cells had almost completely lost the slender cell apices, with the smallest ones often having one blunt cell end resulting in a club-like shape (). They were still capable of sexual reproduction, however, since crosses between the above-mentioned small-celled F6A and Mid24 or Ps2 were successful. The sexual size range is thus ‘open’.
Discussion
Our rbcL phylogeny confirms the presence of multiple lineages within Cylindrotheca closterium sensu lato (, cf. also Li et al., Citation2007 and Medlin & Mann, Citation2007), suggesting that this morphospecies constitutes a (semi)cryptic species complex rather than a single species, as has been observed in many other diatoms (Mann et al., Citation2010). We selected strains from a single well-represented lineage in a molecular phylogeny with good (i.e. species-level) resolution to increase the chance of having sufficient interbreeding strains of the same species for the study of sexual reproduction. As initial cells in this lineage were never larger than ± 100 µm, they do not correspond to specimens from the lectotype population of C. closterium (Jahn & Kusber, Citation2005), which are all much larger (> 130 µm). Further revision of the C. closterium complex is needed to establish the true identity of our lineage. For the time being, we therefore adopt a wide concept of C. closterium (cf. also Jahn & Kusber, Citation2005).
The C. closterium lineage studied here has an archetypal pennate diatom life cycle, including a size reduction–restitution cycle, with size restitution occurring through the formation of auxospores, which result from sexual reproduction of two mating cells. The presence of two mating types suggests a heterothallic mating system, as is common in pennate diatoms (Chepurnov et al., Citation2004). In some strains, however, restricted intraclonal sexual reproduction was present. This has also been observed in several other heterothallic pennate diatoms (Chepurnov et al., Citation2004, Davidovich et al., Citation2006, Citation2010; Vanormelingen et al., Citation2008). All Cylindrotheca strains displaying intraclonal reproduction were of the same mating type, but whether this is coincidence or not is uncertain due to the limited sample size. In Nitzschia longissima, intraclonal sexual reproduction has been reported to be restricted to male strains (Davidovich etal., Citation2006, who therefore referred to it as andromixis). Also three Tabularia fasciculata strains capable of intraclonal sexual reproduction were male (Davidovich et al., Citation2010). How the normal mate recognition system, with mating only taking place between cells of opposite mating type, is circumvented or changed is unknown and merits further study.
As in most other Bacillariaceae for which there are published data on sexual reproduction, sexual reproduction in C. closterium can be classified as Type I, i.e. with two auxospores produced by a pair of gametangia (Geitler, Citation1973). The sexual process in Cylindrotheca seems most similar to that of a species identified as Nitzschia reversa (Mann, Citation1993), which has a similar valve shape (i.e. with prolonged ends), but for which unfortunately no DNA sequences are available. Similarities with N. reversa include the lack of a copulation jelly (present in e.g. N. angustata, Geitler, Citation1954), and absence of papillae (present in N.amphibia, Geitler, Citation1969) or tubes (present in many Nitzschia, overview in Kaczmarska et al., Citation2007; for N. palea, see also Trobajo et al., Citation2009). Cylindrotheca closterium and N. reversa are also similar in having isogamous gamete morphology and behaviour (although gamete behaviour in C. closterium may be variable), a similar central plastid arrangement in the auxospore, and central rupture of the perizonium wall allowing escape of the initial cell. In this respect the presence in both taxa of a perizonium with a wide centre and narrow ends presumably necessitates a central escape of the initial cell. Differences between C.closterium and N. reversa are the apparent lack of a distinct transverse perizonium in N. reversa, the seemingly more variable direction of auxospore expansion, and the regular degeneration of two of the four auxospore plastids. Fully grown N. reversa auxospores had lengths of c. 183–189 µm (as measured from figs 5–7 in Mann, Citation1993), almost twice as long as those of our C. closterium.
The sexual process of other investigated Bacillariaceae differs in various aspects from that of C. closterium and N. reversa. Despite having a similar vegetative cell shape to C. closterium or N. reversa, N.longissima perizonia do not narrow towards the cell ends, allowing the initial cell to slide out of the end of the perizonium (Kaczmarska et al., Citation2007). In N. dissipata and N. palea, one gamete in a gametangium is active and the other passive (Geitler, Citation1958; Trobajo etal., Citation2009), while in Pseudo-nitzschia and Nitzschia longissima both are either active or passive (see below). Both of these contrast with the isogamy of C. closterium. In Pseudo-nitzschia and Fragilariopsis (Fryxell et al., Citation1991; Davidovich & Bates, Citation1998; Amato et al., Citation2005; Chepurnov et al., Citation2005; Assmy et al., Citation2006), and at least some Nitzschia species (e.g. Geitler, Citation1928, Citation1969; Trobajo et al., Citation2006; Mann et al., Citationin press), at least during part of the auxospore development, there are two plastids in the auxospore ends, with the two other plastids around the cell centre, or all four plastids are arranged linearly.
The Pseudo-nitzschia–Fragilariopsis lineage recovered in LSU rDNA molecular phylogenies (Lundholm et al., Citation2002; Trobajo et al., Citation2006) appears to be well-characterized by details of the sexual process. Pseudo-nitzschia species differ from all other Bacillariaceae investigated thus far, including Cylindrotheca, by the combination of (1) behavioural differentiation between gametes originating from gametangia of opposite mating type (in one mating type the two gametes produced by each gametangium are both active, in the other mating type both passive), (2) the association of the tip of the auxospores with the female frustule and (3) auxospore expansion perpendicular to the parental frustules (Fryxell et al., Citation1991; Amato et al., Citation2005; Chepurnov et al., Citation2005; CitationD'Alelio et al., 2009; Quijano-Scheggia et al., Citation2009). However, in a few Pseudo-nitzschia species, a looser auxospore–parental frustule attachment and an irregular direction of auxospore expansion has been observed (Davidovich & Bates, Citation1998). Observations of auxospores in a natural population of Fragilariopsis kerguelensis show that it shares at least the second and third features with Pseudo-nitzschia (Assmy et al., Citation2006). Auxospores were always single in F. kerguelensis, however, and gamete and initial cell behaviour were not recorded. Additional study of the sexual process of Fragilariopsis is needed. None of the three features mentioned above have been observed in any Nitzschia species thus far, except in N. longissima, which has at least the same gamete behaviour as in Pseudo-nitzschia (Kaczmarska et al., Citation2007). However, in N. longissima, auxospores expand parallel to the parental frustules (perpendicular in Pseudo-nitzschia) and the male and female gametes are also morphologically differentiated. The initial cells of all investigated Pseudo-nitzschia spp. escape by the perizonium end, but this was also observed in N. frustulum var. perpusilla (Geitler, Citation1970) and N. inconspicua (Mann et al., Citationin press). Lack of knowledge of the evolutionary relationships and the sexual process of many representatives in the Bacillariaceae precludes further analysis at the moment.
The sexual size threshold in the investigated C.closterium lineage, at a cell length of at least 66 µm or 68% of the maximal cell length, is relatively close to the maximal cell size. In general the sexual size threshold in diatoms is at 45–55% of the maximal cell length, though with a range of 30–75% (Davidovich, Citation2001). Abrupt size reduction, shortening the life cycle, probably occurs in C. closterium, given the appearance of low numbers of small cells in the F1 progeny strain, but it was not observed.
An extended dark period appears to arrest the vast majority of Cylindrotheca fusiformis cells in the G1 phase, with apparently also a small percentage of cells in G2+M phase arrest (Brzezinski et al., Citation1990). A dark-induced G1 phase arrest has also been found in Seminavis robusta (Gillard et al., Citation2008) and Phaeodactylum tricornutum (Brzezinski et al., Citation1990; Huysman et al., Citation2010). The G1 phase is also the cell cycle phase in which S. robusta cells are susceptible to the presence of opposite mating type cells, and the sexual process in this diatom can consequently be synchronized using a prolonged dark period (Gillard, Citation2009). While experimental evidence is needed to confirm the G1 arrest for C. closterium, large percentages of synchronized sexualized cells were readily obtained with the same protocol. As we made only visual estimates of the numbers of cells of both strains added to the crosses, the percentage of sexualized cells could probably be increased further by optimizing the (relative) cell densities of the strains in the mixed cultures.
The sexual process proceeds rapidly in C. closterium, with the first initial cells appearing 24 h after mixing the two compatible strains, which is similar to Seminavis robusta (Chepurnov et al., Citation2008). The appearance of mating cells is markedly faster in C.closterium than in S. robusta, where mating in mixed cultures starts 5–6 h after reillumination. This difference suggests a much shorter or absent pre-mating induction phase in the sexual process of C. closterium. In S. robusta, such an induction phase arrests vegetative division and sensitizes cells of one mating type so that they can exhibit directed motility towards cells of the other mating type (Gillard, Citation2009). Together with the availability of a genetic transformation protocol for C.fusiformis, an indispensable tool for gene function analysis, and the upcoming genome sequences for two Bacillariaceae, Pseudo-nitzschia multiseries (http://genome.jgi-psf.org/Psemu1/Psemu1.home.html) and Fragilariopsis cylindrus (http://genome.jgi-psf.org/Fracy1/Fracy1.home.html), the easy synchronization of the sexual stages and rapid development make Cylindrotheca an attractive choice for future life cycle, biochemical and ecophysiological research.
Acknowledgements
Pieter Vanormelingen is a postdoctoral research fellow with the Research Foundation – Flanders (FWO). Sofie D'Hondt performed the molecular work. Joâo Serôdio kindly provided one of the Cylindrotheca strains included in the molecular phylogeny.
References
- Amato , A. , Orsini , L. , D'Alelio , D. and Montresor , M . 2005 . Life cycle, size reduction patterns, and ultrastructure of the pennate planktonic diatom Pseudo-nitzschia delicatissima (Bacillariophyceae) . Journal of Phycology , 41 : 542 – 556 .
- Araújo , C.V.M. , Diz , F.R. , Moreno-Garrido , I. , Lubián , L.M. and Blasco , J . 2008 . Effects of cold-dark storage on growth of Cylindrotheca closterium and its sensitivity to copper . Chemosphere , 72 : 1366 – 1372 .
- Assmy , P. , Henjes , J. , Smetacek , V. and Montresor , M . 2006 . Auxospore formation by the silica-sinking, oceanic diatom Fragilariopsis kerguelensis (Bacillariophyceae) . Journal of Phycology , 42 : 1002 – 1006 .
- Brüder , K. and Medlin , L.K . 2008a . Morphological and molecular investigations of naviculoid diatoms. III. Hippodonta and Navicula s.s . Diatom Research , 23 : 331 – 347 .
- Brüder , K. and Medlin , L.K . 2008b . Morphological and molecular investigations of naviculoid diatoms. II. Selected genera and families . Diatom Research , 23 : 283 – 329 .
- Brzezinski , M.A. , Olson , R.J. and Chisholm , S.W . 1990 . Silicon availability and cell-cycle progression in marine diatoms . Marine Ecology Progress Series , 67 : 83 – 96 .
- Chepurnov , V.A. , Mann , D.G. , Sabbe , K. and Vyverman , W . 2004 . Experimental studies on sexual reproduction in diatoms . International Review of Cytology , 237 : 91 – 154 .
- Chepurnov , V.A. , Mann , D.G. , Sabbe , K. , Vannerum , K. , Casteleyn , G. , Verleyen , E. , Peperzak , L. and Vyverman , W . 2005 . Sexual reproduction, mating system, chloroplast dynamicsand abrupt cell size reduction in Pseudo-nitzschia pungens from the North Sea . European Journal of Phycology , 40 : 379 – 395 .
- Chepurnov , V.A. , Mann , D.G. , von Dassow , P. , Vanormelingen , P. , Gillard , J. , Inzé , D. , Sabbe , K. and Vyverman , W . 2008 . In search of new tractable diatoms for experimental biology . BioEssays , 30 : 692 – 702 .
- D'Alelio , D. , Amato , A. , Luedeking , A. and Montresor , M . 2009 . Sexual and vegetative phases in the planktonic diatom Pseudo-nitzschia multistriata . Harmful Algae , 8 : 225 – 232 .
- Davidovich , N.A . 2001 . “ Species-specific sizes and size range of sexual reproduction in diatoms ” . In Proceedings of the 16th International Diatom Symposium , Edited by: Economou-Amilli , A. 191 – 196 . Athens : Amvrosiou .
- Davidovich , N.A. and Bates , S.S . 1998 . Sexual reproduction in the pennate diatoms Pseudo-nitzschia multiseries and P. pseudodelicatissima (Bacillariophyceae) . Journal of Phycology , 34 : 126 – 137 .
- Davidovich , N.A. , Kaczmarska , I. and Ehrman , J.M . 2006 . “ The sexual structure of a natural population of the diatom Nitzschia longissima (Bréb.) Ralfs ” . In Proceedings of the 18th International Diatom Symposium , Edited by: Witkowski , A. 27 – 40 . Bristol : Biopress Limited .
- Davidovich , N.A. , Kaczmarska , I. and Ehrman , J.M . 2010 . Heterothallic and homothallic sexual reproduction in Tabularia fasciculata . Fottea , 10 : 251 – 266 .
- Drebes , G . 1977 . “ Sexuality ” . In The Biology of Diatoms. Botanical Monographs Vol. 13 , Edited by: Werner , D. 250 – 283 . Oxford : Blackwell Scientific Publications .
- Ehrenreich , I.M. , Torabi , N. , Jia , Y. , Kent , J. , Martis , S. , Shapiro , J.A. , Gresham , D. , Caudy , A.A. and Kruglyak , L . 2010 . Dissection of genetically complex traits with extremely large pools of yeast segregants . Nature , 464 : 1039 – 1044 .
- Fischer , H. , Robl , I. , Sumper , M. and Kröger , N . 1999 . Targeting and covalent modification of cell wall and membrane proteins heterologously expressed in the diatom Cylindrotheca fusiformis (Bacillariophyceae) . Journal of Phycology , 35 : 113 – 120 .
- Fourtanier, E. & Kociolek, J.P. [editors] (2011). Catalogue of Diatom Names. California Academy of Sciences, http://research.calacademy.org/research/diatoms/names/index.asp (http://research.calacademy.org/research/diatoms/names/index.asp) (Accessed: 19 September 2011 ).
- Fryxell , G.A. , Garza , S.A. and Roelke , D.L . 1991 . Auxospore formation in an Antarctic clone of Nitzschia subcurvata Hasle . Diatom Research , 6 : 235 – 245 .
- Geitler , L . 1928 . Copulation und Geschlechtsverteilung bei einer Nitzschia-Art . Archiv für Protistenkunde , 61 : 419 – 442 .
- Geitler , L . 1932 . Der Formwechsel der pennaten Diatomeen . Archiv für Protistenkunde , 78 : 1 – 226 .
- Geitler , L . 1953 . Allogamie und Autogamie bei der Diatomee Denticula tenuis und die Geschlechtsbestimmung der Diatomeen . Österreichische Botanische Zeitschrift , 100 : 331 – 352 .
- Geitler , L . 1954 . Paarbildung und Gametenfusion bei Anomoeoneis exilis und einigen anderen pennaten Diatomeen . Österreichische Botanische Zeitschrift , 101 : 441 – 452 .
- Geitler , L . 1958 . Notizen über Rassenbildung, Fortpfanzung, Formwechsel und morphologische Eigentümlichkeiten bei pennaten Diatomeen . Österreichische Botanische Zeitschrift , 105 : 408 – 442 .
- Geitler , L . 1969 . Die Auxosporenbildung von Nitzschia amphibia . Österreichische Botanische Zeitschrift , 117 : 404 – 410 .
- Geitler , L . 1970 . Pädogame Automixis und Auxosporenbildung bei Nitzschia frustulum var . perpusilla. Österreichische Botanische Zeitschrift , 118 : 121 – 130 .
- Geitler , L . 1973 . Auxosporenbildung und Systematik bei pennaten Diatomeen und die Cytologie von Cocconeis-Sippen . Österreichische Botanische Zeitschrift , 122 : 299 – 321 .
- Gillard , J . 2009 . The pennate diatom life cycle: a genetic, physiological and biochemical study using Seminavis robusta as a new experimental model. Ph.D. dissertation , Belgium : Ghent University .
- Gillard , J. , Devos , V. , Huysman , M.J.J. , De Veylder , L. , D'Hondt , S. , Martens , C. , Vanormelingen , P. , Vannerum , K. , Sabbe , K. , Chepurnov , V.A. , Inzé , D. , Vuylsteke , M. and Vyverman , W . 2008 . Physiological and transcriptomic evidence for a close coupling between chloroplast ontogeny and cell cycle progression in the pennate diatom Seminavis robusta . Plant Physiology , 148 : 1394 – 1411 .
- Guillard , R.R.L . 1975 . “ Culture of phytoplankton for feeding marineinvertebrates ” . In Culture of marine invertebrate animals , Edited by: Smith , W.L and Chanley , M.H . 29 – 60 . NewYork : Plenum .
- Hall , T.A . 1999 . BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT . Nucleic Acids Symposium Series , 41 : 95 – 98 .
- Hildebrand , M . 2005 . Cloning and functional characterization of ammonium transporters from the marine diatom Cylindrotheca fusiformis (Bacillariophyceae) . Journal of Phycology , 41 : 105 – 113 .
- Hoppenrath , M. , Elbrächter , M. and Drebes , G . 2009 . Selected microphytoplankton species from the North Sea around Helgoland and Sylt. Kleine Senckenberg-Reihe, Band 49 , Stuttgart : Schweizerbart Science Publishers .
- Huysman , M. , Martens , C. , Vandepoele , K. , Gillard , J. , Rayko , E. , Heijde , M. , Bowler , C. , Inzé , D. , Van de Peer , Y. , De Veylder , L. and Vyverman , W . 2010 . Genome-wide analysis of the diatom cell cycle unveils a novel type of cyclins involved in environmental signaling . Genome Biology , 11 : R17
- Jahn , R. and Kusber , W.-H . 2005 . Reinstatement of the genus Ceratoneis Ehrenberg and lectotypification of its type specimen: C. closterium Ehrenberg . Diatom Research , 20 : 295 – 304 .
- Kaczmarska , I. , Davidovich , N.A. and Ehrman , J.M . 2007 . Sex cells and reproduction in the diatom Nitzschia longissima (Bacillariophyta): discovery of siliceous scales in gamete cell walls and novel elements of the perizonium . Phycologia , 46 : 726 – 737 .
- Li , H. , Yang , G. , Sun , Y. , Wu , S. and Zhang , X . 2007 . Cylindrotheca closterium is a species complex as was evidenced by the variations of rbcL gene and SSU rDNA . Journal of Ocean University of China , 6 : 167 – 174 .
- Lundholm , N. , Daugbjerg , N. and Moestrup , Ø . 2002 . Phylogeny of the Bacillariaceae with emphasis on the genus Pseudo-nitzschia (Bacillariophyceae) based on partial LSU rDNA . European Journal of Phycology , 37 : 115 – 134 .
- Mann , D.G . 1986 . Methods of sexual reproduction in Nitzschia: systematic and evolutionary implications (Notes for a monograph of the Bacillariaceae 3) . Diatom Research , 1 : 193 – 203 .
- Mann , D.G . 1993 . Sexual reproduction in a marine member of the Bacillariaceae . Diatom Research , 8 : 109 – 116 .
- Mann , D.G. and Chepurnov , V.A . 2004 . What have the Romans ever done for us? The past and future contribution of culture studies to diatom systematics . Nova Hedwigia , 79 : 237 – 291 .
- Mann , D.G. , Sato , S. , Trobajo , R. , Vanormelingen , P. and Souffreau , C . 2010 . DNA barcoding for species identification and discovery in diatoms . Cryptogamie, Algologie , 31 : 557 – 577 .
- Mann , D.G , Sato , S. , Rovira , L. and Trobajo , R . 2013 . Paedogamy and auxosporulation in Nitzschia sect. Lanceolatae (Bacillariophyta) . Phycologia , 52 : 204 – 220 .
- McLachlan , D.H. , Brownlee , C. , Taylor , A.R. , Geider , R.J. and Underwood , G.J.C . 2009 . Light-induced motile responses of the estuarine benthic diatoms Navicula perminuta and Cylindrotheca closterium . Journal of Phycology , 45 : 592 – 599 .
- Medlin , L. and Mann , D.G . 2007 . Proposal to conserve the name Cylindrotheca against Ceratoneis (Bacillariophyceae) . Taxon , 56 : 953 – 955 .
- Michelmore , R.W. , Paran , I. and Kesseli , R.V . 1994 . Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomicregions by using segregating populations . Proceedings of the National Academy of Sciences USA , 88 : 9828 – 9832 .
- Poulsen , N. and Kröger , N . 2005 . A new molecular tool for diatoms, Control of mRNA and protein biosynthesis by an inducible promoter-terminator cassette . The FEBS Journal , 272 : 3413 – 3423 .
- Quijano-Scheggia , S. , Garcés , E. , Andree , K. , Fortuňo , J.M. and Camp , J . 2009 . Homothallic auxosporulation in Pseudo-nitzschia brasiliana (Bacillariophyta) . Journal of Phycology , 45 : 100 – 107 .
- Ronquist , F. and Huelsenbeck , J.E . 2003 . MrBayes 3: Bayesian phylogenetic inference under mixed models . Bioinformatics , 19 : 1572 – 1574 .
- Round , F.E. , Crawford , R.M. and Mann , D.G . 1990 . The Diatoms , Cambridge : Biology and Morphology of the Genera. Cambridge University Press .
- Ruck , E.C. and Theriot , E . 2011 . Origin and evolution of the canal raphe system in diatoms . Protist , 162 : 723 – 737 .
- Sato , S. , Nagumo , T. and Tanaka , J . 2004 . Auxospore formation and the morphology of the initial cell of the marine araphid diatom Gephyria media (Bacillariophyceae) . Journal of Phycology , 40 : 684 – 691 .
- Schneeberger , K. , Ossowski , S. , Lanz , C. , Juul , T. , Høgh Petersen , A. , Lehmann Nielsen , K. , Jørgensen , J.-E. , Weigel , D. and Uggerhøy Andersen , S . 2009 . SHOREmap: simultaneous mapping and mutation identification by deep sequencing . Nature Methods , 5 : 550 – 551 .
- Sinninghe Damsté , J.S. , Muyzer , G. , Abbas , B. , Rampen , S.W. , Massé , G. , Allard , W.G. , Belt , S.T. , Robert , J.-M. , Rowland , S.J. , Moldowan , J.M. , Barbanti , S.M. , Fago , F.J. , Denisevich , P. , Dahl , J. , Trindade , L.A.F. and Schouten , S . 2004 . The rise of the rhizosolenid diatoms . Science , 304 : 584 – 587 .
- Sorhannus , U . 2007 . A nuclear-encoded small-subunit ribosomal RNA timescale for diatom evolution . Marine Micropaleontology , 65 : 1 – 12 .
- Souffreau , C. , Verbruggen , H. , Wolfe , A.P. , Vanormelingen , P. , Siver , P.A. , Cox , E.J. , Mann , D.G. , Van de Vijver , B. , Sabbe , K. and Vyverman , W . 2011 . A time-calibrated multi-gene phylogeny of the diatom genus Pinnularia . Molecular Phylogenetics and Evolution , 61 : 866 – 879 .
- Staelens , J. , Rombaut , D. , Vercauteren , I. , Argue , B. , Benzie , J. and Vuylsteke , M . 2008 . High density linkage maps and sex-linked markers for the black tiger shrimp (Penaeus monodon) . Genetics , 179 : 917 – 925 .
- Trobajo , R. , Mann , D.G. , Chepurnov , V.A. , Clavero , E. and Cox , E.J . 2006 . Taxonomy, life cycle, and auxosporulation of Nitzschiafonticola (Bacillariophyta) . Journal of Phycology , 42 : 1353 – 1372 .
- Trobajo , R. , Clavero , E. , Chepurnov , V.A. , Sabbe , K. , Mann , D.G. , Ishihara , S. and Cox , E.J . 2009 . Morphological, genetic and mating diversity within the widespread bioindicator Nitzschia palea (Bacillariophyceae) . Phycologia , 48 : 443 – 459 .
- Vanormelingen , P. , Chepurnov , V.A. , Mann , D.G. , Cousin , S. , Sabbe , K. and Vyverman , W . 2007 . Congruence of morphological, reproductive and ITS rDNA sequence data in some Australasian Eunotia bilunaris . European Journal of Phycology , 42 : 61 – 79 .
- Vanormelingen , P. , Chepurnov , V.A. , Mann , D.G. , Sabbe , K. and Vyverman , W . 2008 . Genetic divergence and reproductive barriers between morphologically heterogeneous sympatric clones of Eunotia bilunaris sensu lato (Bacillariophyta) . Protist , 159 : 73 – 90 .