Abstract
Diatoms are used as indicators of water quality as they respond predictably to many water chemistry variables and their ecology is relatively well known. However, temporal variation in diatom assemblages remains poorly resolved and studies covering large temporal and spatial scales are urgently needed. Here, we studied how diatom assemblages vary within and between three years at eight boreal stream sites located in Finland using Mantel tests and ordination analyses. Our study streams differed greatly in their trophic level and size: Vantaanjoki is mesotrophic to eutrophic and large, whereas Luutajoki and Evojoki are oligotrophic and smaller. We found that species composition reflected well the status of the sampled streams. In Vantaanjoki, the most common diatoms were all typical species for mesotrophic to eutrophic streams, whereas oligotrophic streams were characterized by species with narrow ecological niches confined to nutrient-poor water. According to Non-metric Multidimensional Scaling (NMDS), the between-year variation was nearly comparable at all sites, eutrophic sites generally showing slightly higher between-year variation. The reasons for large between-year differences in assemblages were probably related to different weather conditions between the years. The Mantel tests also showed a notable degree of within-year variation in all assemblages. Therefore, we suggest that ecosystem size and productivity act in different directions in the data, resulting in no major differences in the turnover rates at sampled sites. However, NMDS analyses conducted simultaneously for the whole diatom data showed that temporal variation does not confound the large differences in assemblages between eutrophic and oligotrophic streams and that temporal variation may add slightly less noise in spatial assemblage variation in oligotrophic streams. This suggests that site classifications based on diatoms may be overall slightly more robust in oligotrophic waters, whereas in mesotrophic to eutrophic waters, misclassifications may be more likely due to large temporal variation in assemblages.
Introduction
The assessment of ecological conditions in fresh waters is usually based on biomonitoring programmes that use various biological indicators. However, factors such as reliability of ecological indicators and lack of sufficient monitoring data can hamper biological monitoring (Treweek, Citation1999). Moreover, intra-annual and inter-annual variation in occurrence and abundance of aquatic taxa is typically large, which can render the water quality assessment difficult. Therefore, the quantification of such temporal variation in assemblages living in different ecosystems is an important prerequisite for the reliable monitoring of fresh waters.
Diatoms are locally abundant and widespread in various kinds of aquatic ecosystems and they are one of the most important producer groups in streams. Diatoms are also good indicators of water quality as they respond predictably to many water chemistry variables and their ecology is relatively well known (Stevenson et al., Citation1996; Soininen, Citation2007). Several factors affect the variation of diatom assemblage composition in space such as the physico-chemical conditions of the water body, biological interactions and dispersal history, although diatoms primarily reflect the chemical quality of the water. Besides varying spatially, assemblages also change in time.
Diatom studies in natural lotic ecosystems have mostly focused on the use of diatoms in biological monitoring (e.g. Lavoie et al., Citation2008). Some pioneer studies have been conducted in lacustrine ecosystems (e.g. King et al., Citation2006; Kelly et al., Citation2009), but temporal variation in stream ecosystems remains poorly resolved. Seasonal and interannual succession of the assemblages have been studied separately (Duncan & Blinn, Citation1989; Garnier et al., Citation1995; Soininen & Eloranta, Citation2004), but not simultaneously by collecting multiple samples per year repeatedly over several years. Thus, it is still unclear if diatom assemblages vary more within the year or between years.
In general, there are at least three factors that affect the magnitude of temporal variation in assemblage composition. First, the degree of temporal variation may be related to assemblage diversity. Thus, in highly diverse assemblages, abundances of some of the species may vary strongly but total biomass and assemblage composition may vary less (Tilman, Citation1996; Lehman & Tilman, Citation2000; Mykrä et al., Citation2011). High diversity may buffer the system against perturbation. In species-poor assemblages, however, changes in abundance of only one or two species can have a strong relative effect on assemblage composition. Second, temporal turnover is affected by the productivity of the ecosystem. In highly productive systems, assemblage composition may vary more due to multiple stable states in assemblages, as suggested by Chase (Citation2003). For example, Korhonen et al. (Citation2010) found that turnover was faster in lower latitudes (which had higher productivity) at short timescales across a wide range of aquatic assemblages. Third, assemblage variation is affected by ecosystem size. Adler & Lauenroth (Citation2003) and Adler et al. (Citation2005) provided evidence for a general species–time–area relationship (STAR), showing that the temporal variation decreases as the area increases and vice versa. Other studies have suggested that this relationship may not be valid generally: Korhonen et al. (Citation2010) found that species turnover was actually faster in larger ecosystems.
Here, we study how diatom assemblages vary within and between three years at eight boreal stream sites located in three different stream systems in Finland. Our study streams differ greatly in their trophic levels: Vantaanjoki is mesotrophic to eutrophic and Luutajoki and Evojoki are oligotrophic. We thus studied if the degree of temporal variation is related to trophic status of the water and expected that assemblages at eutrophic sites may be more variable in time. Our study streams differ also in size, as Vantaanjoki is much larger than the Evojoki and Luutajoki. We thus studied whether assemblage variation may be related to ecosystem size. If so, we expected the variation to be larger in the smaller ecosystems, i.e. Evojoki and Luutajoki, due to the general STAR relationship (Adler & Lauenroth, Citation2003).
Materials and methods
Sampling
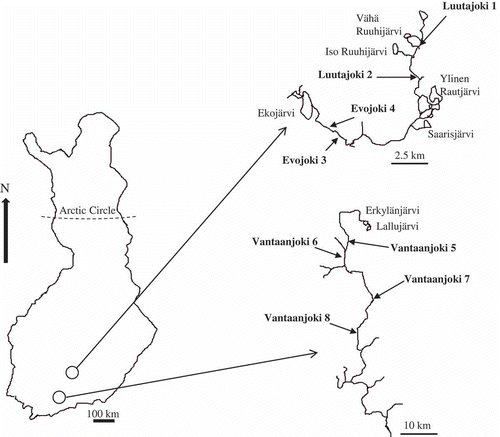
Diatoms were sampled at eight stream sites located in southern Finland (). Four of the sampling sites were located in the mesotrophic to eutrophic Vantaanjoki and the four others were located in the oligotrophic Evojoki and Luutajoki.
The samples were collected at each site nine times per summer in years 2006–2008. Sampling days were chosen so that, starting from 1 June, the summer was divided into three periods of 20 days (see details in Virtanen & Soininen, Citation2012). Between every 20 days sampling period, there was a gap of 20 days in the sampling. For each of the three 20-day periods, three sampling days were picked at random (Underwood, 1997). However, this sampling scheme had one limitation. There were at least three days between all the samplings and hence sampling did not occur on consecutive days. However, this sampling scheme allowed us to detect changes in assemblages at several temporal scales and suited our research plan better, compared with evenly spaced sampling.
For sampling, a riffle was divided into ten cross-sections at intervals of one metre. Ten stones were picked from these sections, one per section. Diatom samples were collected semi-quantitatively from the stones by using a toothbrush and a soft rubber template, in which a hole of 9 cm2 had been cut. From every stone, a sample of the same size was brushed, and finally all the ten samples were pooled into one container.
During the first sampling in summer 2006, pH, conductivity and temperature were also measured at the stream sites. In addition, we determined average current velocity, water depth, stream width, slope and shading by the canopy. Depth (cm) and current velocity (cm s−1) were measured at 40 random locations along the same transects where we picked up the sampling stones. To measure the current velocity we used a flowtracker (SonTek, FlowTracker Handheld-ADV®). Stream slope (cm/10 m) was determined using a carpenter's level. Shading (% canopy cover) was estimated visually using a cylinder (diameter 7.5 cm) at 20 random locations along transects. At each sampling site, we also collected one 500-ml water sample for total phosphorus (P) and nitrogen (N) analyses and one 250-ml sample for the water colour analyses. The results of these analyses are summarized in .
Table 1. The main physicochemical factors in the Luutajoki, Evojoki and Vantaanjoki streams in 2006
Organic material was removed from the diatom samples by boiling with hydrogen peroxide (30% H2O2). Cleaned diatoms were mounted in Naphrax® (Brunel Microscopes, Chippenham, Wiltshire, UK). A total of 500 frustules per sample were identified using Krammer & Lange-Bertalot (1986–1991) and counted using phase contrast light microscopy (magnification 1000 ×). We retain Krammer & Lange-Bertalot's nomenclature.
Data analyses
Non-metric Multidimensional Scaling (NMDS) was used to illustrate the temporal variation in diatom assemblage structure between the years. NMDS is an ordination method highly suitable for ecological data containing numerous zero values (Minchin, Citation1987). A two-dimensional solution was chosen since stress values changed only slightly with subsequent dimensions. We used Sorensen's distance measure for all analyses. Along with the Jaccard index, Sorensen index has been one of the most widely used similarity indices among ecologists and it is highly recommended, for example, by Southwood & Henderson (Citation2000) and Magurran (Citation2004). The statistical significance of differences between the assemblage compositions between the sampling years was tested using Multi-Response Permutation Procedures (MRPP; Berry et al., Citation1983). This is a non-parametric procedure for testing the significance of possible differences between classified groups set a priori. MRPP has the advantage of not requiring assumptions such as multivariate normality and homogeneity of variances and is easily applied to multivariate space. Within-group homogeneity was evaluated using the chance-corrected within-group agreement ‘A’. If the value of A > 0, the within-group homogeneity is larger than expected by chance. NMDS and MRPP were conducted using the program PC-ORD version 4 (Mccune & Mefford, Citation1999).
We then calculated the relationship between assemblage similarity and distance in time for each pair of observations (samples). This was done to examine how fast assemblage similarity decayed in time at each site within the sampling years. The statistical significance of the relationships was determined using a Mantel test (Mantel, Citation1967) with 999 random permutations (see also Korhonen et al., Citation2010). We used the correlation coefficient (Mantel r) as an indicator temporal variation. We used a t-test to compare the correlation coefficients between the streams. As a similarity metric, we used the Sorensen coefficient.
For one site, Vantaanjoki 8, we also studied if changes in assemblage similarity were related to corresponding changes in water chemistry in time. We used water chemistry data (pH, total nitrogen, conductivity, turbidity, total phosphorus and temperature) collected by the Finnish Environment Institute and related the environmental distance (Euclidean distance based on water chemistry variables) to pairwise assemblage similarity in time separately for each year. The statistical significance of the relationships was determined using a Mantel test (Mantel, Citation1967) with 999 random permutations.
Results
Species richness and composition
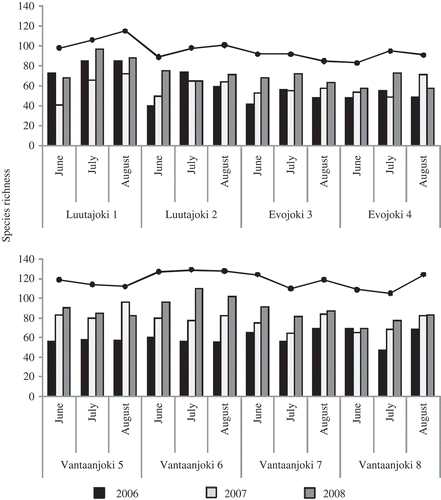
In total, we detected 241 diatom species from the samples collected at eight sampling sites. The most species-rich sampling sites were located in Vantaanjoki. The differences in mean species richness (averaged over the three years) between Vantaanjoki on the one hand, and Luutajoki and Evojoki on the other, were statistically significant (ANOVA, P = 0.04, Fig. 2). Also, the total number of species detected during the three years sampling in Vantaanjoki (i.e. at four sites) compared with Luutajoki and Evojoki (at four sites in total) were significantly higher (t-test, P = 0.002).
In Luutajoki, Achnanthes minutissima was the most dominant species in all of the samples with relative abundances of 25.6–32.3% ( ). Gomphonema angustatum was abundant in early summer, but sparse later in the summer. Other common species in Luutajoki were, for example, Achnanthes subatomoides, Eunotia implicata and Fragilaria capucina var. gracilis. Achnanthes minutissima was also the most common species in Evojoki (), its relative abundance varying from 44.6% to 59.5%; it was dominant in July and August during the three sampling years and all other species had low abundances. Other frequently occurring diatoms in Luutajoki were A. linearis, E. bilunaris, F. capucina var. gracilis, G. angustatum and Tabellaria flocculosa.
Table 2. The most common diatom species in 2006–2008 in Evojoki, Luutajoki and Vantaanjoki. The proportions (%) of total species composition are given for the most abundant species, illustrating within- and between-year variation in the diatom assemblages; values for the years and months are means
In Vantaanjoki, there were several almost equally abundant species (). However, Cocconeis placentula and Cyclotella atomus were somewhat more abundant than the other species. Cocconeis placentula was more abundant in July and August in all years while Cyclotella atomus dominated in July but was rather sparse in August. Other frequently occurring diatoms were A. lanceolata, A. minutissima, Navicula lanceolata and N. minima ().
Between-year variation in assemblages
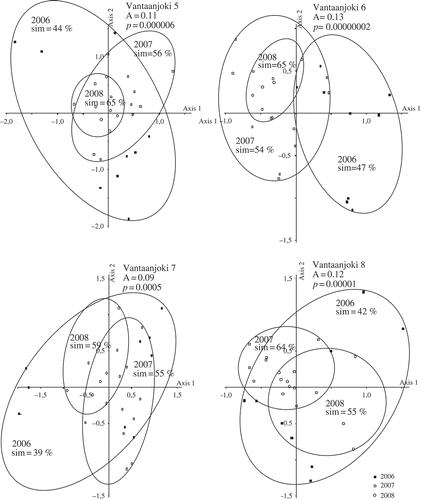
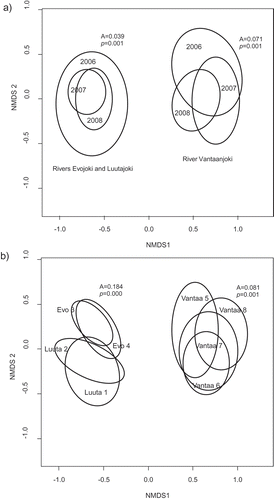
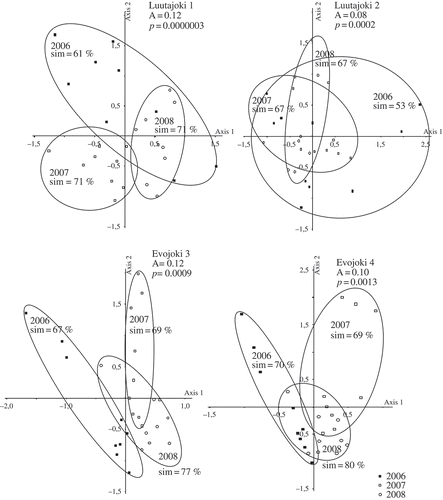
According to NMDS analyses, assemblages varied widely between the sampling years (–). Based on NMDS analysis conducted simultaneously for the whole diatom data, the oligotrophic Evojoki and Luutajoki were clearly separated from the mesotrophic to eutrophic Vantaanjoki in ordination space (). When samples were grouped according to year, the higher A value (A = 0.071) indicated that Vantaanjoki showed more different assemblage compositions between the years than Evojoki and Luutajoki (A = 0.039, ). However, when grouping was based on sampling sites, Evojoki and Luutajoki showed more distinct assemblage compositions (A = 0.184), whereas sites in Vantaanjoki showed a higher degree of overlap (A = 0.081, ).
Figs 2. Diatom species richness at eight sampling locations during the sampling periods in 2006–2008. The solid line gives total species richness in the sampling periods during the three years. Bars indicate the number of species detected within one month (including three sampling occasions).
Figs 3. NMDS plots based on the whole diatom data. In panel (a) samples are grouped according to years with the distinct groups for Vantaanjoki, Evojoki and Luutajoki. Larger ellipses indicate larger changes in diatom assemblages within one year. In panel (b) samples are grouped according to sampling sites. Here the ellipses include all samples (n = 27) collected from a site. Shown are also A values indicating chance-corrected within-group agreements as well as P values based on MRPP analyses.
At most of the sites, the samples collected in 2006 seemed to show larger variation than the other samples (, ). MRPP analyses indicated that the assemblage composition differed statistically significantly among the three years at all sites (P < 0.001) (, ). The diatom assemblages showed greatest temporal variation among the years at site Vantaanjoki 6 (A = 0.13, P < 0.001). However, the differences in the degree of temporal variation between sampling sites in Vantaanjoki, Luutajoki and Evojoki seem to be minor, as the between-year temporal variation was almost equally strong, for example at sites Luutajoki 1 (A = 0.12, P < 0.001 ) and Evojoki 3 (A = 0.12, P < 0.001), as in Vantaanjoki.
Figs 4. Results of NMDS ordination analysis in Luutajoki and Evojoki, illustrating assemblage similarity in 2006–2008. Sim = similarity in species composition. Value A is calculated using MRPP and indicates similarity between years contrasted with a randomized expected value. A can have values between –1 and +1 and values larger than zero indicate higher within-group similarity than expected by chance.
Within-year variation in assemblages
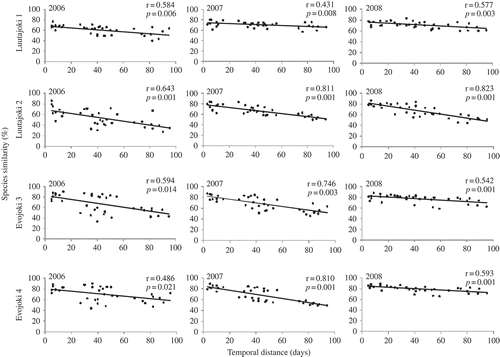
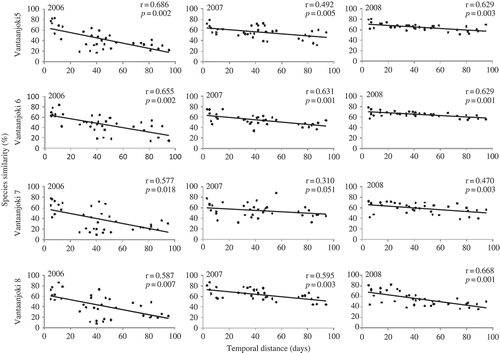
According to Mantel tests, there were no statistical differences in within-year variation in diatom assemblages between Vantaanjoki on the one hand, and Evojoki and Luutajoki on the other, as Mantel r values did not differ from each other (ANOVA, P = 0.8). Overall, however, the r values were slightly higher in Evojoki and Luutajoki (). The greatest temporal variation was detected in 2006. In Vantaanjoki, diatom assemblage composition changed c. 80% during the summer (Mantel r = 0.626, P = 0.007, ) and in Evojoki and Luutajoki c. 50% of the assemblage were replaced by different species during the sampling campaign in 2006 (Mantel r = 0.577, P = 0.01, ). In 2007 and 2008, species composition in Evojoki and Luutajoki changed 36–48% and in Vantaanjoki 30–64%.
Figs 6. The relationship between assemblage similarity (%) and temporal distance (in days) in Luutajoki and Evojoki during 2006–2008. r = Mantel correlation coefficient; P value indicates the statistical significance of the decrease of assemblage similarity. Black dots indicate pairwise similarity between two sampling occasions and temporal distance.
Figs 7. The relationship between assemblage similarity (%) and temporal distance (days) in Vantaanjoki during 2006–2008. For details, see legend for Fig. 6.
For sampling site Vantaanjoki 8, we also tested for the relationship between pairwise assemblage similarity and environmental distance based on water chemistry variables and found that only in 2007 was there a significant relationship (Mantel r = −0.586, P = 0.004) between the temporal change in assemblage similarity and the corresponding change in water chemistry. However, in 2006 (Mantel r = 0.071, P = 0.548) and 2008 (Mantel r = −0.169, P = 0.259) the relationship was non-significant.
Discussion
In this study, species composition reflected well the nutrient status of the streams. In Vantaanjoki, the most common diatoms, such as Cyclotella atomus, Cocconeis placentula and Gomphonema parvulum, are all typical species for mesotrophic and eutrophic streams and streams affected by organic loading (Soininen, Citation2002). In Luutajoki and Evojoki, the species present were mainly typical oligotrophic species, such as Achnanthes linearis, Eunotia ssp. and Gomphonema angustatum (Soininen, Citation2002; Soininen et al., Citation2004). In Luutajoki and Evojoki, only one or few species dominated, whereas in Vantaanjoki several species were co-dominant. Overall, species richness was somewhat higher in Vantaanjoki than in the other two, likely reflecting the higher productivity of mesotrophic to eutrophic streams.
According to NMDS analyses conducted separately for each site, diatom assemblage composition varied substantially between the years at all sampling locations. This is in line with several papers showing that diatoms vary strongly among years (Lavoie et al., Citation2008). Overall, the between-year variation within a site was nearly the same in eutrophic and oligotrophic streams suggesting either that the difference in productivity was not large enough to result in large differences in temporal variation or that it was counteracted by other variables such as differences in ecosystem size.
The reasons for large between-year variation in assemblages are probably related to differences in weather between the years. Summer 2006 was warm and dry, while the summers of 2007 and 2008 were more rainy and colder. The mean temperature in summer 2006 (June–August) was 20.9°C whereas in 2007 and 2008 it was 19.3°C and 17.5°C, respectively. Mean discharge (MQ) in summer 2006 was 5.2 m3 s−1. In summers 2007 and 2008, MQ was 9.5 m3 s−1 and 23.0 m3 s−1, respectively. The fact that the three sampling years differed notably in weather conditions thus possibly contribute greatly to the degree of between-year variation in species composition in diatom assemblages. Other reasons could be, for example, priority effects or stochastic variation in communities (Jenkins & Buikema, Citation1998). For example, Jenkins & Buikema (Citation1998) documented that different zooplankton assemblages developed at environmentally similar sites due to priority effects related to variation in dispersal processes among sites.
When the whole diatom data were analysed simultaneously, NMDS and associated MRPP analyses first showed that assemblage composition varied more between oligotrophic vs. mesotrophic-eutrophic sampling sites than in time, because samples collected in Vantaanjoki were clearly separated in ordination space from samples collected in Evojoki and Luutajoki (). This shows that although local diatom assemblage composition typically varies greatly in time, it does not overwhelm the great distinction between assemblages growing in oligotrophic vs. mesotrophic to eutrophic waters. This suggests that the use of assemblage composition as an indicator of nutrient status is robust even if assemblages vary in time. Second, our analyses showed that between-site assemblage variation was slightly larger in oligotrophic streams than in mesotrophic to eutrophic streams (). In other words, this analysis revealed that the oligotrophic streams Evojoki and Luutajoki comprised very different assemblages, which were separated clearly in NMDS space, whereas in Vantaanjoki the assemblages were typically quite similar at the four sampling sites, showing high degree of overlap. This has important implications for biomonitoring. We showed that misclassifications of sites may be more likely at mesotrophic to eutrophic sites than at oligotrophic sites, where spatial variation in assemblages more likely overwhelms the temporal noise in the assemblages.
We emphasize that potentially there are multiple underlying drivers for temporal assemblage variation, yet we found only a weak relationship between changes in water chemistry and corresponding changes in assemblage similarity (see also Soininen & Eloranta, Citation2004). Eutrophic ecosystems can have higher turnover rates in species composition because of higher numbers of multiple stable states in these ecosystems – this means that assemblages can evolve in many different directions (Chase, Citation2003; Korhonen et al., Citation2010). In species-rich assemblages, there are a great number of species capable of reacting to changing environmental conditions (Lavoie et al., Citation2008). Further, in highly productive systems, interspecific competition is common, perhaps causing severe assemblage fluctuations (Tilman, Citation1996). Highly productive streams, such as Vantaanjoki, should thus have more variable species composition than oligotrophic streams. However, our results did not show much greater within-year variation in Vantaanjoki. The reason could be that Vantaanjoki is also the largest ecosystem studied and ecosystem size is known to be correlated negatively with the rate of species turnover in time (Adler & Lauenroth Citation2003; Adler et al. Citation2005; but see Korhonen et al., Citation2010). Therefore, we suggest that these forces act in different directions in these data, resulting in no major changes in patterns of turnover within years. We further speculate that the results may have been slightly affected by the differences in species richness between the sampled streams. The diatom assemblages were most diverse in Vantaanjoki and, according to Tilman (Citation1996) and Mykrä et al. (Citation2011), individual species abundances can fluctuate rapidly in highly diverse assemblages while the assemblage composition stays relatively stable. As we did not find major differences in the within-year species turnover between these streams, however, the effect of species richness does not appear to be strong.
To conclude, our study shows that it is important to acknowledge that several factors can affect temporal variation of stream diatoms simultaneously, resulting often in contradictory results. Therefore, we encourage ecologists to study further the factors crucial to temporal variation using, for example, novel experimental approaches. We have shown, however, that temporal variation may not overwhelm the strong distinction in diatom assemblages between oligotrophic and mesotrophic to eutrophic streams and that within oligotrophic streams, temporal variation adds perhaps less noise to between-site variation in assemblages, making site classifications perhaps more robust in time than in highly productive streams, where assemblages at different sites may resemble each other more.
Acknowledgements
Financial support was provided by Academy of Finland to Janne Soininen and University of Helsinki and Otto A. Malm Foundation to Jenni J. Korhonen. We want to thank two anonymous reviewers and Laura Virtanen for their fruitful comments.
References
- Adler , P.B. and Lauenroth , W.K. 2003 . The power of time: spatiotemporal scaling of species diversity . Ecology Letters , 6 : 749 – 756 .
- Adler , P.B. , White , E.P. , Lauenroth , W.K. , Kaufman , D.M. , Rassweiler , A. and Rusak , J.A. 2005 . Evidence for a general species–time–area relationship . Ecology , 86 : 2032 – 2039 .
- Berry , K.J. , Kvamme , K.L. and Mielke , P.W. 1983 . Improvements in the permutation test for the spatial analysis of the distribution of artifacts into classes . American Antiquity , 48 : 547 – 553 .
- Chase , J.M. 2003 . Community assembly: when should history matter? . Oecologia , 136 : 489 – 498 .
- Collins , S.L. , Micheli , F. and Hartt , L. 2000 . A method to determine rates and patterns of variability in ecological communities . Oikos , 91 : 285 – 293 .
- Duncan , S.W. and Blinn , D.W. 1989 . Importance of physical variables on the seasonal dynamics of epilithic algae in a highly shaded canyon stream . Journal of Phycology , 25 : 455 – 461 .
- Garnier , J. , Billen , G. and Coste , M. 1995 . Seasonal succession of diatoms and Chlorophyceae in the drainage network of the Seine River: observations and modeling . Limnology and Oceanography , 40 : 750 – 765 .
- Jenkins , D.G. and Buikema , A.L. 1998 . Do similar communities develop in similar sites? A test with zooplankton structure and function . Ecological Monographs , 68 : 421 – 443 .
- Kelly , M. , Bennion , H. , Burgess , A. , Ellis , J. , Juggins , S. , Guthrie , R. , Jamieson , J. , Adriaenssens , V. and Yallop , M. 2009 . Uncertainty in ecological status assessments of lakes and rivers using diatoms . Hydrobiologia , 633 : 5 – 15 .
- King , L. , Clarke , G. , Bennion , H. , Kelly , M. and Yallop , M. 2006 . Recommendations for sampling littoral diatoms in lakes for ecological status assessments: a literature review . Journal of Applied Phycology , 18 : 15 – 25 .
- Korhonen , J.J. , Soininen , J. and Hillebrand , H. 2010 . A quantitative analysis of temporal turnover in aquatic species assemblages across ecosystems . Ecology , 91 : 508 – 517 .
- Krammer , K. and Lange-Bertalot , H. 1986–1991 . “ Bacillariophyceae ” . In Süßwasserflora von Mitteleuropa , Edited by: Ettl , H. , Gerloff , J. , Heynig , H. and Mollenhauer , D. Vol. 2 , parts 1 – 4 . Stuttgart, Germany : Gustav Fischer .
- Lavoie , I. , Campeau , S. , Darchanbeau , F. , Cabana , G. and Dillon , P.J. 2008 . Are diatoms good integrators of temporal variability in stream water quality? . Freshwater Biology , 53 : 827 – 841 .
- Lehman , C. and Tilman , D. 2000 . Biodiversity, stability, and productivity in competitive communities . American Naturalist , 156 : 534 – 552 .
- Magurran , A. 2004 . Measuring biological diversity , Oxford : Blackwell .
- Mantel , N. 1967 . The detection of disease clustering and a generalized regression approach . Cancer Research , 27 : 209 – 220 .
- McCune , B. and Mefford , M.J. 1999 . PC-ORD. Multivariate analysis of ecological data, Version 4 , Lincoln Beach, Oregon : MjM Software Design .
- Minchin , P.R. 1987 . An evaluation of the relative robustness of techniques for ecological ordination . Vegetatio , 69 : 89 – 107 .
- Mykrä , H. , Heino , J. , Oksanen , J. and Muotka , T. 2011 . The stability–diversity relationship in stream macroinvertebrates: influences of sampling effects and habitat complexity . Freshwater Biology , 56 : 1122 – 1132 .
- Soininen , J. 2002 . Responses of epilithic diatom communities to environmental gradients in some Finnish rivers . International Review of Hydrobiology , 87 : 11 – 24 .
- Soininen , J. 2007 . Environmental and spatial control of freshwater diatoms – a review . Diatom Research , 22 : 473 – 490 .
- Soininen , J. and Eloranta , P. 2004 . Seasonal persistence and stability of diatom communities in rivers: are there habitat specific differences? . European Journal of Phycology , 39 : 153 – 160 .
- Soininen , J. , Paavola , R. and Muotka , T. 2004 . Benthic diatom communities in boreal streams: community structure in relation to environmental and spatial gradients . Ecography , 27 : 330 – 342 .
- Southwood , R. and Henderson , P.A. 2000 . Ecological methods , Vol. 3 , Oxford : rd ed. Blackwell .
- Stevenson , R.J. , Bothwell , M.L. and Lowe , R.L . 1996 . Algal ecology , San Diego, USA : Academic Press .
- Tilman , D. 1996 . Biodiversity: population versus ecosystem stability . Ecology , 77 : 350 – 363 .
- Treweek , J. 1999 . Ecological impact assessment , Oxford : Blackwell Science .
- Underwood , A.J. 1991 . Beyond BACI: experimental designs for detecting human environmental impacts on temporal variation in natural populations . Australian Journal of Marine and Freshwater Research , 42 : 569 – 587 .
- Virtanen , L. and Soininen , J. 2012 . The roles of environment and space in shaping stream diatom communities . European Journal of Phycology , 47 : 160 – 168 .