Abstract
Primary production and calcification responses to irradiance were investigated in Lithophyllum cabiochae, a crustose coralline alga from Mediterranean coralligenous communities, collected at c. 25 m depth in the Bay of Villefranche. Algae were maintained in aquaria at temperature and irradiance levels close to in situ conditions. Physiological measurements were performed using incubation chambers in the dark and in the light at different irradiance levels within the range of those measured in situ. Both photosynthesis and calcification rates in L. cabiochae were strongly related to irradiance. Dark respiration averaged 0.2–0.3 µmol cm−2 thallus h−1 in terms of both O2 consumption and CO2 release and maximal gross photosynthesis averaged 1.0 µmol cm−2 h−1 in terms of both O2 production and CO2 uptake. Mean rate of net calcification was 0.1 µmol CaCO3 cm−2 h−1 in the dark and reached 0.4 µmol CaCO3 cm−2 h−1 in the light. Diel net and gross organic C productions were estimated to be 3 and 7 µmol C cm−2 thallus d−1, respectively. Diel net inorganic C production was estimated to be 3 µmol CaCO3 cm−2 thallus d−1. Despite the low light conditions experienced by the algae at c. 25 m depth, L. cabiochae can be considered as a major contributor to primary productivity and calcium carbonate deposition, making coralligenous communities a major carbon and carbonate producer in the Mediterranean Sea.
Introduction
Red calcareous coralline algae (Corallinales, Rhodophyta) are abundant and widespread in coastal areas throughout the world, from polar regions to the tropics (Johansen, Citation1981; Steneck, Citation1986). They grow from the surface to depths below 200 metres (Littler et al., Citation1991); some under intense illumination, others in very dim light conditions. Coralline algae are of particular ecological importance in shallow waters and the ecosystem services they provide, which include provision of nursery habitat for commercially important invertebrate species, have been recognized (Kamenos et al., Citation2004). They produce chemicals or harbour specific bacteria that promote larval settlement and recruitment of numerous invertebrates (Morse et al., Citation1980; Morse & Morse, Citation1984; Johnson et al., Citation1991) and their rigid structure provides microhabitats for a high diversity of associated organisms (Cabioch, Citation1969; Bosence, Citation1976, Citation1979; Foster, Citation2001). Coralline algae are also of significant importance in the carbon and carbonate cycles of shallow coastal ecosystems, being major contributors to CO2 fluxes through high community photosynthesis (CO2 sink) and respiration (CO2 source) (Martin et al., Citation2005, Citation2006, Citation2007) and through high CaCO3 production (CO2 source) and dissolution (CO2 sink) (Barrόn et al., 2006; Martin et al., Citation2006, Citation2007).
Coralline algae are major constructors of biogenic habitats in benthic marine environments. For example, crustose coralline algae are the main binding agent in coral reefs (Littler, Citation1973) and free-living coralline algae may occur at high concentrations over large areas by forming rhodolith (maerl) beds (Foster, Citation2001). In the Mediterranean Sea, the accumulation of encrusting coralline algae produces a typical habitat known as ‘coralligenous’. This habitat represents a unique calcareous formation of biogenic origin in the Mediterranean Sea (Ballesteros, Citation2006) and is considered a key benthic ecosystem in the Mediterranean, second only to Posidonia oceanica meadows in terms of biodiversity (Boudouresque, Citation2004). The complex structure of the calcareous coralligenous concretions allows the development of a patchwork of communities dominated by living algae, suspension feeders, borers and even soft-bottom fauna (in the sediment deposited in cavities and holes) (Ballesteros, Citation2006).
The coralligenous communities thrive exclusively in dim light conditions mainly in deep waters (20–120 m depth) but also in shady crevices and overhangs (from 5 m depth; Ballesteros, Citation2006). Irradiance is probably the most important environmental factor for the build-up and maintenance of the coralligenous community, as algae and invertebrates living in this habitat are adapted to low light levels and the coralligenous communities develop where irradiance is reduced to between 0.05 and 3% of that at the surface (Ballesteros, Citation2006). Mediterranean coralligenous-forming crustose coralline algae are thus photosynthetic organisms adapted to grow in low light conditions; however, their response to irradiance is, to our knowledge, unknown. In this study, we investigated the effects of irradiance on photosynthesis and calcification in Lithophyllum cabiochae, which is a common crustose coralline alga in north-western Mediterranean coralligenous communities. This study provides the first quantification of respiration, primary production and calcification in crustose coralline algae from Mediterranean coralligenous habitats and thus an insight into their role in coastal carbon and carbonate budgets.
Materials and methods
Biological material
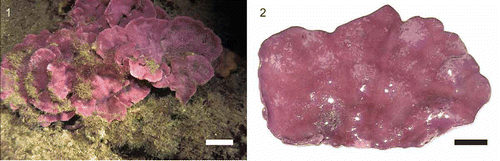
Specimens of the crustose coralline alga, Lithophyllum cabiochae (Boudouresque & Verlaque) Athanasiadis (, ) were collected by SCUBA divers from the coralligenous community at c. 25 m depth in the Bay of Villefranche (NW Mediterranean Sea, France; 43°40.73′N, 07°19.39′E) on 27 April 2006 and transported to the Laboratoire d'Océanographie de Villefranche. The algae were thoroughly cleaned of epiphytic organisms without causing any damage to the thalli. Flat thalli of c. 30 cm2 () were selected for the experiments. The algal surface area was determined from photographs using the software Image J Version 1.37v (http://rsb.info.nih.gov/ij/). The algal fresh weight was determined at the end of the experiment. Dry weight was measured to the nearest mg after drying at 60°C until weight was constant. Carbonate content was estimated as the dry weight of the thallus after organic material had been removed via combustion for 6 h at 550°C.
Experimental setup
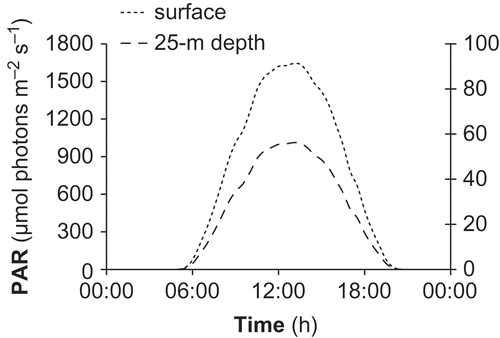
The algal thalli were maintained in a 26-l aquarium continuously supplied with unfiltered Mediterranean seawater, pumped from 10 m depth in the Bay of Villefranche to prevent any change in seawater composition, at a rate of 13 l h−1. The parameters of the seawater carbonate system in the aquarium () were representative of those measured in the upper 50 m of the Bay of Villefranche (J.-P. G., unpublished data). Temperature and irradiance in the aquarium corresponded to those at the depth of algal collection in the Bay of Villefranche in May. Temperature was adjusted to 16.0°C, which was the mean temperature recorded in May 2006 at 30 m depth in the Bay of Villefranche by the Service d'Observation Rade de Villefranche, SO-Rade, of the Observatoire Océanologique and the Service d'Observation en Milieu Littoral, SOMLIT/CNRS-INSU. Temperature was controlled in the aquarium to within ± 0.1°C using a temperature controller (Corema) connected to a 150 W submersible heater. Water motion in the aquarium was ensured by two submersible pumps. Photosynthetically available radiation (PAR; µmol photons m−2 s−1) in the Bay of Villefranche was calculated from a vertical irradiance profile obtained from the RV Sagitta on 26 April 2006 using an underwater flat quantum sensor (LI-COR, LI-192SA). The photosynthetic photon flux density was 50 µmol photons m−2 s−1 at c. 25 m depth at noon. The attenuation coefficient of PAR (KPAR, 0.13 m−1) was calculated according to Kirk (Citation1983) from the vertical profile and used in combination with the incident PAR at the surface to determine the daily cycle of irradiance at c. 25 m depth (). The incident PAR at the surface was measured using a flat quantum sensor (LI-COR, LI-192SA) set on the top of the semaphore of Saint-Jean-Cap-Ferrat, located near the sampling station. The experimental photosynthetic photon flux density was adjusted to a mean daily value of c. 25 µmol photons m−2 s−1 using neutral density filters. The light source consisted of two 39 W fluorescent tubes (JBL Solar Ultra Marin Day) above the aquarium. The photoperiod was adjusted to the light : dark ratio for May (15 : 9).
Figs 4. Dark respiration and calcification rates of Lithophyllum cabiochae measured in May 2006 during daytime after exposure to ambient light and during night-time after exposure to darkness. Data are means ± SE (n = 5).
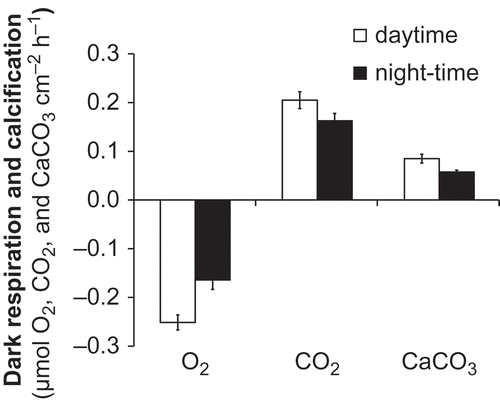
Table 1. Parameters of the carbonate system in the aquarium during daytime and night-time. Results are expressed as mean ± SE (n = 48 for daytime between 10:00 and 16:00 and n = 5 for night-time between 22:00 and 02:00). The CO2 partial pressure (pCO2), the concentrations of CO2, CO3 2–, HCO3– and dissolved inorganic carbon (C T), and the saturation state of seawater with respect to calcite (Ωc) and aragonite (Ωa) were calculated from measurements of pH on the total scale (pHT), total alkalinity (A T), temperature (16.0 °C) and salinity (38), using the R package seacarb (Proye & Gattuso, Citation2003)
Physiological measurements
Physiological measurements were taken after a one-week acclimation period under laboratory conditions. Five algal thalli were incubated individually in closed Perspex chambers filled with c. 200 ml of seawater from the aquarium and continuously stirred with a magnetic stirring bar. The chambers were placed inside the aquarium in order to control temperature. Clear chambers were used to assess net production (P n) and calcification (G) in the light, while chambers with a dark plastic cover were used to assess dark respiration (R d) and calcification (G d). The algae were incubated at five irradiance levels (5–6, 11–12, 24–25, 36–37 and 51 µmol photons m−2 s−1) , all of which were in the range of those occurring at c. 25 m depth in the Bay of Villefranche in May. The irradiance levels were adjusted using neutral density filters and monitored with a flat quantum sensor (LI-COR, LI-192SA). Incubations took place between 10:00 and 18:00 and lasted 2 h. The algae were acclimated at the desired irradiance level for at least 4 h prior to the incubation and incubated only once a day. The algae were incubated in the dark during daytime between 10:00 and 17:00 after exposure to the ambient irradiance (R d and G d) and during night-time between 22:00 and 04:00 after exposure to darkness (R D and G D). Reference incubations without the algae were performed in light and dark conditions.
The concentration of dissolved oxygen (O2, µmol l−1) was continuously measured inside the chamber using Clark-type Strathkelvin 1302 oxygen electrodes connected to a Strathkelvin 782 oxygen meter (Strathkelvin Instrument Ltd). O2 concentration increased (light) or decreased (dark) linearly with time. Water samples were taken at the beginning and at the end of the incubations for measurements of pH and total alkalinity (A T). pH was measured using a glass combination electrode (Orion 8103SC) calibrated on the total scale (pHT) using Tris/HCl and 2-aminopyridine/HCl buffer solutions with a salinity of 38.0 and prepared according to DOE (Citation1994). The accuracy of the pH-meter (Metrohm 826 pH mobile) was ± 0.003 pH units. Seawater samples for A T were filtered through Whatman GF/F membranes, immediately poisoned with mercuric chloride and stored in a cool dark place pending analyses (DOE Citation1994). A T was determined on 20-ml sub-samples using a home-made titration system comprising a 20 ml open thermostated titration cell (kept at 25.0°C), an Orion 8103SC pH electrode calibrated on the National Bureau of Standards scale, and a computer-driven Metrohm 665 Dosimat titrator. The seawater samples and the acid titrant (0.1 N HCl) were kept at a constant temperature of 25.0°C. The samples were weighed (± 0.01 g) prior to the titration to determine their exact volume from temperature and salinity. A T was calculated from the Gran function applied to pH variations from 4.2 to 3.0 as the function of added volume of HCl. The A T measurements had a reproducibility of ± 3 µmol kg−1. The concentration of dissolved inorganic carbon (C T) was determined from pH, A T, temperature and salinity (38) using the R package seacarb (Proye & Gattuso, Citation2003).
The rates of net photosynthesis (P n) and dark respiration (R d) (in µmol O2 cm−2 thallus h−1) were calculated from the slope of O2 change over time as follows:
The changes in C T during the incubations are controlled by the metabolism of organic carbon (photosynthesis and respiration) and inorganic carbon (calcification and dissolution). The precipitation of 1 mol of CaCO3 decreases C T by 1 mol and A T by two equivalents according to:
The calcification (G, µmol CaCO3 cm−2 thallus h−1) rate was calculated by the difference between initial and final A T values by using the alkalinity anomaly technique (Smith & Key, Citation1975; Chisholm & Gattuso, Citation1991) as follows:
P n and R d expressed in terms of carbon (µmol C cm−2 thallus h−1) were calculated by the difference between the initial and final C T values as follows:
P n and G versus irradiance (E, µmol photons m−2 s−1) curves were established by fitting non-linear exponential models to P n and G data using an exponential function (Chalker, Citation1981):
The compensation irradiance (E c, µmol photons m−2 s−1) is the irradiance at which P n = 0 (or P g = R d).
Diel carbon and carbonate production
Diel (24 h) P n (or G) was calculated by summing daily P n (or G) during the daylight period and R D (or G D) during the night-time period. Diel P g was equal to daily P g during the daylight period. Daily P n (P g or G) was calculated by integrating the P n (P g or G) versus E curves against the incident irradiance at 25 m depth in May 2006 in the Bay of Villefranche during the daylight period (). Diel P n, P g and G were converted to mmol C or CaCO3 m−2 coralligen d−1 by using the carbonate content in living coralline algae in Mediterranean coralligenous concretions of c. 1.5 kg CaCO3 m−2 (Canals & Ballesteros, Citation1997).
Statistical analyses
Dark respiration and calcification rates measured during daytime (R d and G d) and night-time (R D and G D) were compared using paired t-tests. The slopes of the functional regressions (PQ and RQ) were compared between treatments using a Z-test (Scherrer, Citation1984). Results are expressed as mean ± standard error of the mean (SE).
Results
Respiration
Dark respiration (R d) measured during daytime averaged 0.25 µmol cm−2 thallus h−1 in terms of O2 consumption and 0.20 µmol cm−2 thallus h−1 in terms of CO2 release (), corresponding to 0.85 µmol O2 g−1 dry wt thallus h−1 and 0.69 µmol CO2 g−1 dry wt thallus h−1, respectively (). Dark respiration measured during night-time (R D) averaged 0.16 µmol cm−2 h−1 in terms of both O2 consumption and CO2 release (). Comparison between R d and R D revealed lower rates of respiration during the night after exposure to the dark, with a decline of R D of c. 20 to 30 % relative to R d. Differences were significant only for O2 fluxes (Paired t-test, t = –7.90, P = 0.001) but not for CO2 fluxes (Paired t-test, t = 2.20, P = 0.09). The RQ was 1.01 ± 0.2, which was not significantly different from 1 (Z-test, Z = 0.03, P = 0.98). The intercept did not differ significantly from 0 (Z-test, Z = 0.38, P = 0.70).
Figs 5. Net photosynthesis versus irradiance curves for Lithophyllum cabiochae in May 2006. Data are means ± SE (n = 5).
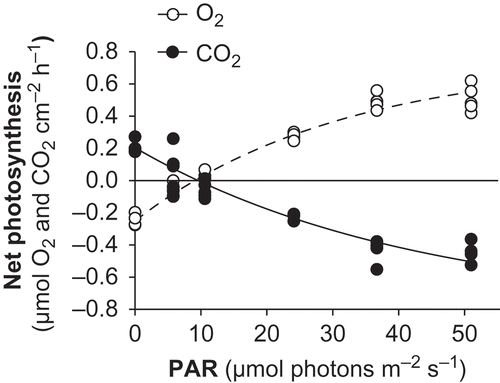
Table 2. Parameters of P n–E and G–E curves for Lithophyllum cabiochae in May 2006. Results are expressed as mean ± SE (n = 5). R d (or G d) = dark respiration (or calcification) rates (µmol O2, CO2 or CaCO3 cm−2 thallus h−1); P g max (or G g max) and P n max (or G max) = maximum rates of gross and net photosynthesis (or calcification) (µmol O2, CO2 or CaCO3 cm−2 thallus h−1); E k = the saturating irradiance (µmol photons m−2 s−1); and E c = the compensation irradiance (µmol photons m−2 s−1)
Photosynthesis
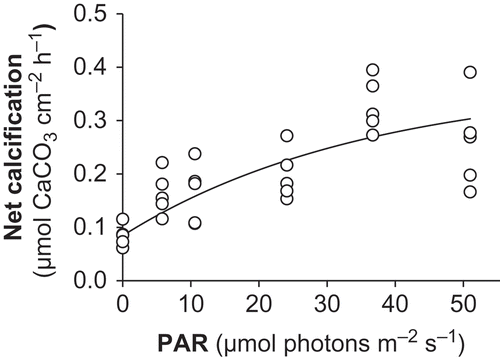
The response of L. cabiochae net photosynthesis to irradiance is presented in . The P n–E curves for individual specimens showed a close dependence of both net O2 production (r 2 of 0.97–1.00) and CO2 uptake (r 2 = 0.88–1.00) on PAR. When the five specimens were considered together, the proportion of data variability explained by the P n–E model was equal or higher when P n data were normalized to thallus surface (r 2 = 0.97 and 0.92 for O2 and CO2 fluxes, respectively) relative to thallus biomass (r 2 = 0.97 and 0.90 for O2 and CO2 fluxes, respectively). Maximal gross photosynthesis (P g max) averaged 1.0 µmol cm−2 h−1 in terms of both O2 production and CO2 uptake, corresponding to 3.3 µmol g−1 dry wt h−1 (). The mean ratio of P g max : R d was 4 ± 1 and 5 ± 1 for O2 and CO2 fluxes, respectively. The maximal net photosynthesis (P n max) averaged 0.75 µmol O2 cm−2 h−1 and 0.81 µmol CO2 cm−2 h−1. The mean saturating irradiances (E k) were 31 and 43 µmol photons m−2 s−1 and the mean compensation irradiances (E c) were 8 and 9 µmol photons m−2 s−1 for O2 and CO2 fluxes, respectively. The PQ was 1.08 ± 0.01, which was not significantly different from 1 (Z-test, Z = 1.10, P = 0.27). The intercept did not differ significantly from 0 (Z-test, Z = 0.66, P = 0.51).
Calcification
Dark calcification measured during daytime (G d) averaged 0.08 µmol CaCO3 cm−2 h−1, corresponding to 0.28 µmol g−1 dry wt h−1 (). Dark calcification measured during night-time (G D) was 25% lower and averaged 0.06 µmol cm−2 h−1. The difference between G d and G D only just failed to meet the 0.05 level chosen to assess significance (Paired t-test, t = –2.75, P = 0.052). The response of net calcification to irradiance is illustrated in . G–E curves for individual specimens illustrate a close dependence on PAR (r 2 = 0.47–0.88). When the five specimens were considered together, the proportion of variability explained by the G–E model was higher when G data were normalized to thallus surface (r 2 = 0.60) relative to thallus biomass (r 2 = 0.46). The mean maximum rate of net calcification (G max) was 0.37 µmol CaCO3 cm−2 h−1, corresponding to 1.26 µmol g−1 dry wt h−1. The mean ratio of G max : G d was 3.5 ± 1. E k averaged 36 µmol photons m−2 s−1. Highly significant correlations were found between G and P n (r = 0.79, P < 0.0001).
Diel organic and inorganic carbon production
Diel (24-h) net organic C production (P n) was estimated to be 2.6 µmol C cm−2 thallus d−1 (8.5 µmol C g−1 dry wt thallus d−1), while gross organic C production (P g) was estimated to be 7.1 µmol C cm−2 thallus d−1 (23.8 µmol C g−1 thallus d−1; ). Carbonate content of the thallus after removal of organic material via combustion averaged 90% (0.90 ± 0.01) of thallus dry weight. Diel P n and P g were thus estimated to be 10 and 27 µmol C g−1 CaCO3 d−1, respectively. Using an average value for the carbonate content in living coralline algae in Mediterranean coralligenous concretions (1500 g CaCO3 m−2: Canals & Ballesteros, Citation1997), diel P n and P g averaged 14 and 40 mmol C m−2 coralligen d−1. Diel net inorganic C production (G) was estimated to be 3.1 µmol CaCO3 cm−2 thallus d−1 (13.7 µmol CaCO3 g−1 dry wt thallus d−1; ). Average G was 15 µmol CaCO3 g−1 thallus CaCO3 d−1 and 23 mmol CaCO3 m−2 coralligen d−1.
Table 3. Diel net and gross photosynthesis and net calcification by Lithophyllum cabiochae estimated at 25 m depth in the Bay of Villefranche in May 2006. Results are expressed as mean ± SE (n = 5)
Discussion
Respiration
The rate of respiration found in the present study for L. cabiochae is in the low range of values reported in the literature for tropical species of crustose coralline algae (Chisholm, Citation2003) but more than 10-fold higher than those of polar species (Roberts et al., Citation2002; Schwarz et al., Citation2005; ). Metabolic rates of crustose coralline algae are usually expressed per unit of crust surface to avoid any artefact due to the thickness of skeletal carbonate below the living coralline layer (Chisholm, Citation2003). Accordingly, normalization of our metabolic data by surface area explained more variability than normalization by biomass. Respiration rate expressed per unit of biomass for L. cabiochae (R d of 0.8 µmol O2 g−1 dry wt thallus h−1 or 0.7 µmol C g−1 h−1) is, however, consistent with those reported for temperate species of coralline algae, such as the free-living coralline alga (maerl), Lithothamnion corallioides (0.1–0.3 µmol O2 or C g−1 dry wt h−1: Martin et al., Citation2006).
Table 4. Photosynthetic parameters measured for selected crustose coralline algae from polar, temperate and tropical regions. Values have been normalized to µmol O2 cm−2 thallus h−1 for comparison. E k and E c are in μmol photons m−2 s−1; – = not reported
Dark respiration (non-photorespiratory mitochondrial respiration) occurs both in the light and in darkness and is essential for maintenance as well as growth of cells, since it generates much of the usable energy (ATP), reducing power (e.g. NADPH and NADH) and carbon skeleton from complex organic compounds (Atkin et al., 2005). Here, dark respiration measured during daytime after exposure to ambient light is 20–30% higher than that measured during the night-time after exposure to darkness. Stimulation of dark respiration after a preceding period of photosynthesis has already been described by Furbank & Rebeille (Citation1986), who found that respiration may be stimulated up to two-fold by a preceding period of photosynthesis. This photosynthesis-dependent enhancement of respiratory O2 consumption after a period of photosynthesis (termed ‘light-enhanced dark respiration’) is frequently observed in microalgae and plants (Raghavendra et al., Citation1994), and is mainly attributed to the mitochondrial oxidation of malate produced by chloroplasts (Raghavendra et al., Citation1994; Xue et al., Citation1996).
Photosynthesis
Data on the production rates of crustose coralline algae are lacking for Mediterranean species, despite their important role as dominant primary producers in dim light conditions in coralligenous communities in the Mediterranean Sea (Ballesteros, Citation2006). More generally, data are scarce for all temperate species, although they appear to be highly productive relative to tropical species (Ichiki et al., Citation2001). Lithophyllum cabiochae has maximum rates of net and gross photosynthesis (P n max and P g max) that are higher than those reported for the temperate crustose coralline alga, L. yessoense from the sublittoral zone in south-western Hokkaido, Japan (Ichiki et al., Citation2001) but comparable to the rates of tropical crustose corallines of the genera Hydrolithon and Neogogniolithon (Chisholm, Citation2003; ). Photosynthetic rates of L. cabiochae are several times higher than those reported for polar crustose corallines living at 15–20 m depth (Roberts et al., Citation2002; Schwarz et al., Citation2005; ). Lithophyllum cabiochae has considerable photosynthetic capacity for an alga living at 25 m depth but it exhibited specific shade-adapted photosynthetic characteristics as shown by low values of E k and E c. These values are several times lower than those reported for coralline algae living at shallower depths and exposed to higher light levels, such as the temperate species of maerl Lithothamnion corallioides (E k ranging between 123 and 179 µmol photons m−2 s−1 and E c ranging from 12 and 40 µmol photons m−2 s−1; Martin et al., Citation2006) and tropical species (Chisholm, Citation2003; ). Payri et al. (Citation2001) reported E k values for Hydrolithon onkodes of 110 µmol photons m−2 s−1 for specimens exposed to high light (1600 µmol photons m−2 s−1), reduced to 27 µmol photons m−2 s−1 for specimens exposed to lower light levels (< 200 µmol photons m−2 s−1). Roberts et al. (Citation2002) and Schwarz et al. (Citation2005) also reported low values of E k and E c in Arctic and Antarctic crustose corallines living at 15–20 m depth in very dim light conditions. In spite of the low light levels (≤50 µmol photons m−2 s−1 in May) experienced by L. cabiochae at 25 m depth in the Bay of Villefranche, its ability to make a net gain from photosynthesis at very low light levels (≥8–9 µmol photons m−2 s−1) during most of the daylight period (12/15 h in May) permits L. cabiochae to be an active primary producer in coralligenous communities.
Calcification
Few data are available for calcification rates in crustose coralline algae and none exist for Mediterranean species in response to irradiance. However, the calcification rate of Lithophyllum cabiochae at saturated light (G max of 0.4 µmol CaCO3 cm−2 thallus h−1) is high, and comparable to those reported for tropical crustose corallines of the genera Hydrolithon and Neogoniolithon (G max of 0.2–1.0 µmol CaCO3 cm−2 h−1; Chisholm, Citation2000) using the same methodology (the alkalinity anomaly technique). The rate of net calcification in the dark of L. cabiochae (G d of 0.08 µmol CaCO3 cm−2 thallus h−1) is high, even relative to those reported for Hydrolithon and Neogoniolithon, which were found to range between –0.2 (net dissolution) and 0.1 µmol CaCO3 cm−2 h−1 (Chisholm, Citation2000). Calcification in the dark in L. cabiochae represents 30% of the light-saturated rate (G max : G d ratio of 3.5 : 1). The light/dark calcification ratio of L. cabiochae is similar to those reported for the Mediterranean coralline alga, Corallina elongata (3.6 : 1; El Haïkali et al., 2004) and for C. officinalis (3–4 : 1; Pentecost, 1978), but lower than those reported for the temperate species Lithothamnion corallioides (c. 10 : 1) or the tropical species studied by Chisholm (Citation2000: c. 10 : 1 or negative values). El Haïkali et al. suggested that dark calcification corresponds to a belated biological activity after the transition from light to dark. However, the dark calcification rates found here during the night, after a long period of exposure to darkness (G D), remained high (0.06 µmol CaCO3 cm−2 thallus h−1).
The strong relationship between calcification and irradiance (G–E curves) found in Lithophyllum cabiochae is consistent with those previously reported for other species of coralline algae (Chisholm, Citation2000; Martin et al., Citation2006, Citation2007). The G–E curves followed the same trend as the P–E curves, reinforcing the hypothesis that calcification and photosynthetic processes are tightly linked (Pentecost, 1978). Photosynthesis influences calcification through the formation of the fibrous organic matrix of the cell walls of coralline algae, which is required for the deposition of calcite crystals, and through changes in internal pH. Changes in pH that occur in the cell wall at the site of calcification are affected by both photosynthesis and respiration; as a result calcification is largely regulated by these metabolic activities (Smith & Roth, Citation1979; Gao et al., Citation1993; Hurd et al., Citation2011). Increased (or decreased) pH due to photosynthesis (or respiration) leads to increased (or decreased) concentrations of CO3 2–and therefore can promote (or hinder) the precipitation of CaCO3 by increasing (or decreasing) the saturation state of CaCO3. The 25% decrease in dark calcification rate in L. cabiochae during the night relative to the day is likely to be due to a more pronounced decrease in pH at the site of calcification, as a result of respiration during the night. Changes in pH related to the metabolic processes of photosynthesis or respiration may also occur in the diffusion boundary layer between the algal surface and external seawater. Microelectrode data show that the pH at the surface of the crustose coralline alga Sporolithon durum can increase by 0.5 units in the light and decrease by 0.35 units in darkness, relative to the external seawater pH (Hurd et al., Citation2011). Calcification rates may also have been affected by the changes in pH that occurred in the incubation chambers but these were limited to –0.1 to +0.1 pH units in the light and –0.1 to –0.2 pH units in the dark after 2 h incubation.
Diel C and CaCO3 budgets
While the ecological role of coralline algae in coastal ecosystems is well known (Nelson, Citation2009), their role in the biogeochemical cycles of carbon and calcium carbonate is still poorly studied. To our knowledge, photosynthesis, respiration and calcification rates have not been investigated simultaneously in Mediterranean crustose coralline algae. Here, diel estimates of organic and inorganic carbon production were calculated using dark respiration and calcification values measured during the night. This is of particular importance in the calculations of diel C and CaCO3 budgets, as there was a decline in the rates during night-time. Although these changes may have particular implications for C and CaCO3 budgets, most studies to date have assumed constant dark respiration and calcification rates in the light and dark, which may lead to an overestimation of diel rates when using daytime values of dark respiration and calcification.
The mean diel net organic carbon production estimated here for L. cabiochae growing at c. 25 m depth in the Bay of Villefranche (3 µmol C cm−2 thallus d−1, corresponding to 9 µmol C g−1 dry wt d−1), is comparable to those reported for tropical crustose corallines (1–10 and 1–11 µmol C cm−2 thallus d−1 for laboratory and in situ measurements, respectively: Chisholm, Citation2003) and in the high range of those reported for the temperate maerl Lithothamnion corallioides growing at 1–5 m depth (3–11 µmol C g−1 dry wt d−1 in summer at 16.5°C: Martin et al., Citation2006). The gross production of Lithophyllum cabiochae (7 µmol C cm−2 thallus d−1, corresponding to 24 µmol C g−1 dry wt d−1) is in the mean range of those reported for tropical species (6–19 µmol C cm−2 thallus d−1: Chisholm, Citation2003) and higher than that of Lithothamnion corallioides (maximum of 18 µmol C g−1 dry wt d−1 at 1 m depth in summer). The coralline algal net productivity estimated here for the coralligenous communities at 25 m depth (14 mmol C m−2 coralligen d−1 or 0.17 g C m−2 d−1) is, however, in the low range of those reported for the highly productive Lithothamnion corallioides populations (maerl beds, 0.06–1.7 g C m−2 d−1 in summer: Martin et al., Citation2006) or for coral reefs (0.1–1.3 g C m−2 d−1or 0.6–5 g C m−2 d−1 with adjustment to reef surface relief, given 100% cover by crustose coralline algae: Chisholm, Citation2003). Coralline algae in coralligenous communities, however, are one of the major carbon producers in the Mediterranean Sea, with gross primary production (40 mmol C m−2 coralligen d−1) comparable to those reported for the highly productive NW Mediterranean P. oceanica meadows (25 and 173 mmol C m−2 d−1: CitationBarrón et al., 2006).
Diel carbonate production estimated here for L. cabiochae growing at 25 m depth under irradiance of between 0 and 56 µmol photons m−2 s−1 (3 µmol CaCO3 cm−2 thallus d−1, corresponding to 0.3 mg CaCO3 cm−2 thallus d−1) using the total alkalinity method is consistent with the rates measured in the laboratory under constant irradiance (35 µmol photons m−2 s−1) using the buoyant weight method (0.3 to 0.4 mg CaCO3 cm−2 d−1 in summer; Martin & Gattuso, Citation2009). As was mentioned above for light and dark calcification, the diel calcification rate in L. cabiochae is high and comparable to those reported for tropical crustose corallines (0.8–9 µmol CaCO3 cm−2 d−1; Chisholm, Citation2000). This rate (14 µmol CaCO3 g−1 dry wt d−1) is even higher than the maximal rate (10 µmol CaCO3 g−1 dry wt d−1) reported for the temperate maerl species Lithothamnion corallioides at 1 m depth in summer (Martin et al., Citation2006). In the coralligenous communities, the coralline algal carbonate deposition rate reached 23 mmol CaCO3 m−2 d−1 (2.3 g CaCO3 m−2 d−1). This value is only half that of the lowest values observed in coral reefs between the surface and 18 m depth (4–28 g CaCO3 m−2 d−1 assuming 100% cover of the reef by crustose coralline algae and considering relief factors; Chisholm, Citation2000) and in the low range of those reported for maerl beds growing at between 1 and 5 m depth (1.4 to 12.6 g CaCO3 m−2 d−1 in summer). Coralline algal carbonate production in the coralligenous is, however, in the mean range of that of maerl beds developing at 10 m depth (0.2–4 g CaCO3 m−2 d−1). Despite its moderate diel net community carbonate production relative to shallower systems, the coralligenous community can be considered as a major contributor to calcium carbonate deposition in the Mediterranean Sea. For comparison, diel carbonate production is several times higher than that of NW Mediterranean infralittoral communities dominated by the articulated coralline alga, Corallina elongata (8 mmol CaCO3 m−2 d−1: Bensoussan & Gattuso, Citation2007) and that of Posidonia oceanica meadows (1.5 mmol CaCO3 m−2 d−1: CitationBarrón et al., 2006).
This study confirms that coralline algae and coralligenous habitat are major contributors to biogenic carbonate production in the NW Mediterranean Sea (Canals & Ballesteros, Citation1997) and that they may significantly influence regional carbon and carbonate budgets. However, coralline algae are highly vulnerable to ocean acidification and global warming and net dissolution is likely to exceed net calcification in the Mediterranean crustose coralline alga L. cabiochae in the near future (Martin & Gattuso, Citation2009). These changes may have dramatic consequences for the sustainability of Mediterranean coralligenous habitats, which are carbonate bioconstructions built by these algae, thus causing major habitat loss for numerous associated species and major implications for the budgets of carbon and calcium carbonate in coastal Mediterranean ecosystems.
Acknowledgements
We thank D. Luquet, J.-Y. Carval and J.-L. Prevost for assistance in the field and M.-D. Pizay for assistance in the laboratory. We also thank M. Verlaque and A. Meinesz for their help with species determination. The Service d'Observation en Milieu Littoral, INSU-CNRS is gratefully acknowledged for its kind permission to use in situ temperature data. The Service National d'Analyse des Paramètres Océaniques du CO2 performed the weekly measurements of A T and C T in the bay. The two anonymous referees as well as the editor are gratefully acknowledged for their constructive comments and suggestions. This work was supported by the CarboOcean IP of the European Commission (grant 511176-2) and is a contribution to the ‘European Project on Ocean Acidification’ (EPOCA) and MedSeA which received funding from the European Community (grant agreements 211384 and 265103).
References
- Atkin , O.K. , Bruhn , D. , Hurry , V.M. and Tjoelker , M.G. 2005 . The hot and the cold: unraveling the variable response of plant respiration to temperature . Functional Plant Biology , 32 : 87 – 105 .
- Ballesteros , E. 2006 . Mediterranean coralligenous assemblages: a synthesis of present knowledge . Oceanography and Marine Biology: an Annual Review , 44 : 123 – 195 .
- Barrón , C. , Duarte , C.M. , Frankignoulle , M. and Borges , A.V. 2006 . Organic carbon metabolism and carbonate dynamics in a Mediterranean seagrass (Posidonia oceanica) meadow . Estuaries and Coasts , 29 : 417 – 426 .
- Bensoussan , N. and Gattuso , J.-P. 2007 . Community primary production and calcification in a NW Mediterranean ecosystem dominated by calcareous macroalgae . Marine Ecology Progress Series , 334 : 37 – 45 .
- Bosence , D.J. 1976 . Ecological studies on two unattached coralline algae from western Ireland . Paleontology , 19 : 365 – 395 .
- Bosence , D.J. 1979 . Live and dead faunas from coralline algal gravels . Paleontology , 22 : 449 – 478 .
- Boudouresque , C.F. 2004 . Marine biodiversity in the Mediterranean: status of species, populations and communities . Scientific Reports of Port-Cros National Park , 20 : 97 – 146 .
- Cabioch , J. 1969 . Les fonds de maerl de la baie de Morlaix et leur peuplement végétal . Cahier de Biologie Marine , 9 : 139 – 161 .
- Canals , M. and Ballesteros , E. 1997 . Production of carbonate particles by photobenthic communities on the Mallorca–Menorca shelf, northwestern Mediterranean Sea . Deep Sea Research II , 44 : 611 – 629 .
- Chalker , B.E. 1981 . Simulating light-saturation curves for photosynthesis and calcification by reef-building corals . Marine Biology , 63 : 135 – 141 .
- Chisholm , J.R.M. 2000 . Calcification by crustose coralline algae on the northern Great Barrier Reef, Australia . Limnology and Oceanography , 45 : 1476 – 1484 .
- Chisholm , J.R.M. 2003 . Primary productivity of reef-building crustose coralline algae . Limnology and Oceanography , 48 : 1376 – 1387 .
- Chisholm , J.R.M. and Gattuso , J.-P. 1991 . Validation of the alkalinity anomaly technique for investigating calcification and photosynthesis in coral reef communities . Limnology and Oceanography , 36 : 1231 – 1239 .
- DOE . 1994 . “ Handbook of methods for the analysis of the various parameters of the carbon dioxide system in sea water ” . In version 2 Edited by: Dickson , A.G. and Goyet , C. AC – 74 . ORNL/CDI-
- El Haïkali , B. , Bensoussan , N. , Romano , J.-C. and Bousquet , V. 2004 . Estimation of photosynthesis and calcification rates of Corallina elongata Ellis and Solander, 1786, by measurements of dissolved oxygen, pH and total alkalinity . Scientia Marina , 68 : 45 – 56 .
- Foster , M.S. 2001 . Rhodoliths: between rocks and soft places . Journal of Phycology , 37 : 659 – 667 .
- Furbank , R.T. and Rebeille , F. 1986 . Dark respiration in the marine macroalga . Chondrus crispus (Rhodophyceae). Planta , 168 : 267 – 272 .
- Gao , K. , Aruga , Y. , Asada , K. , Ishihara , T. , Akano , T. and Kiyohara , M. 1993 . Calcification in the articulated coralline alga Corallina pilulifera, with special reference to the effect of elevated CO2 concentration . Marine Biology , 117 : 129 – 132 .
- Hurd , C.L. , Cornwall , C. , Currie , K. , Hepburn , C.D. , McGraw , C.M. , Hunter , K.A. and Boyd , P. 2011 . Metabolically induced pH fluctuations by some coastal calcifiers exceed projected 22nd century ocean acidification: a mechanism for differential susceptibility? . Global Change Biology , 17 : 3254 – 3262 .
- Ichiki , S. , Mizuta , H. , Yasui , H. and Yamamoto , H. 2001 . Effects of irradiance and water temperature on the photosynthesis and growth of the crustose coralline alga . Lithophyllum yessoense Foslie (Corallinales, Rhodophyceae). Bulletin of Fisheries Sciences , 52 : 103 – 109 .
- Johansen , H.W. 1981 . Coralline algae, a first synthesis , Boca Raton, Florida : CRC Press .
- Johnson , C.R. , Sutton , D.C. , Olson , R.R. and Giddins , R. 1991 . Settlement of crown-of-thorns starfish: role of bacteria on surfaces of crustose coralline algae and a hypothesis for deepwater recruitment . Marine Ecology Progress Series , 71 : 143 – 162 .
- Kamenos , N.A. , Moore , P.G. and Hall-Spencer , J.M. 2004 . Nursery-area function of maerl grounds for juvenile queen scallops . Aequipecten opercularis and other invertebrates. Marine Ecology Progress Series , 274 : 183 – 189 .
- Kirk , J.T.O. 1983 . Light and photosynthesis in aquatic ecosystems , Cambridge : Cambridge University Press .
- Littler , M.M. 1973 . The population and community structure of Hawaiian fringing-reef crustose Corallinaceae (Rhodophyta, Cryptonemiales . Journal of Experimental Marine Biology and Ecology , 11 : 103 – 120 .
- Littler , M.M. , Littler , D.S. and Hanisak , M.D. 1991 . Deep-water rhodolith distribution, productivity, and growth history at sites of formation and subsequent degradation . Journal of Experimental Marine Biology and Ecology , 150 : 163 – 182 .
- Martin , S. and Gattuso , J.-P. 2009 . Response of Mediterranean coralline algae to ocean acidification and elevated temperature . Global Change Biology , 15 : 2089 – 2100 .
- Martin , S. , Clavier , J. , Guarini , J.-M. , Chauvaud , L. , Hily , C. , Grall , J. , Thouzeau , G. , Jean , F. and Richard , J. 2005 . Comparison of . Zostera marina and maerl community metabolism. Aquatic Botany , 83 : 161 – 174 .
- Martin , S. , Castets , M.-D. and Clavier , J. 2006 . Primary production, respiration and calcification of the temperate free-living coralline alga . Lithothamnion corallioides. Aquatic Botany , 85 : 121 – 128 .
- Martin , S. , Clavier , J. , Chauvaud , L. and Thouzeau , G. 2007 . Community metabolism in temperate maerl beds. I. Carbon and carbonate fluxes . Marine Ecology Progress Series , 335 : 19 – 29 .
- Morse , A.N.C. and Morse , D.E. 1984 . Recruitment and metamorphosis of Haliotis larvae induced by molecules uniquely available at the surface of crustose red algae . Journal of Experimental Marine Biology and Ecology , 75 : 191 – 215 .
- Morse , D.E. , Tegner , M. , Duncan , H. , Hooker , N. , Trevelyan , G. and Cameron , A. 1980 . “ Induction of settling and metamorphosis of planktonic molluscan (Haliotis) larvae. III. Signaling by metabolites of intact algae is dependent on contact ” . In Chemical Signals , Edited by: Muller-Schwartze , D. and Silverstein , R.M. 67 – 86 . New York : Plenum Press .
- Nelson , W.A. 2009 . Calcified macroalgae – critical to coastal ecosystems and vulnerable to change: a review . Marine and Freshwater Research , 60 : 787 – 801 .
- Pentecost , A. 1978 . Calcification and photosynthesis in Corallina officinalis L. using 14CO2 method . British Phycological Journal , 13 : 383 – 390 .
- Payri , C.E. , Maritorena , S. , Bizeau , M. and Rodière , M. 2001 . Photoacclimation in the tropical coralline alga Hydrolithon onkodes (Rhodophyta, Corallinaceae) from a French Polynesian reef . Journal of Phycology , 37 : 223 – 234 .
- Proye, A. & Gattuso, J.-P. (2003). Seacarb: seawater carbonate chemistry with R. Available at (accessed January 2006). http://CRAN.R-project.org/package=seacarb (http://CRAN.R-project.org/package=seacarb)
- Raghavendra , A.S. , Padmasree , K. and Saradedevi , K. 1994 . Interdependence of photosynthesis and respiration in plant cells: interactions between chloroplasts and mitochondria . Plant Science , 97 : 1 – 14 .
- Ricker , W.E. 1973 . Linear regression in fishery research . Journal of the Fisheries Research Board of Canada , 30 : 409 – 434 .
- Roberts , R.D. , Kühl , M. , Glud , R.N. and Rysgaard , S. 2002 . Primary production of crustose coralline red algae in a high arctic fjord . Journal of Phycology , 38 : 273 – 283 .
- Scherrer , B. 1984 . Biostatistique , Chicoutimi : Gaëtan Morin .
- Schwarz , A.M. , Hawes , I. , Andrew , N. , Mercer , S. , Cummings , V. and Thrush , S. 2005 . Primary production potential of non-geniculate coralline algae at Cape Evans, Ross Sea, Antarctica . Marine Ecology Progress Series , 294 : 131 – 140 .
- Smith , A. D. and Roth , A.A. 1979 . Effect of carbon dioxide concentration on calcification in the red coralline alga Bossiella orbigniana . Marine Biology , 52 : 217 – 225 .
- Smith , S. and Key , G. 1975 . Carbon dioxide and metabolism in marine environments . Limnology and Oceanography , 20 : 493 – 495 .
- Steneck , R.S. 1986 . The ecology of coralline algal crusts: convergent patterns and adaptative strategies . Annual Review of Ecology and Systematics , 17 : 273 – 303 .
- Xue , X. , Gauthier , D.A. , Turpin , D.H. and Weger , H.G. 1996 . Interactions between photosynthesis and respiration in the green alga . Chlamydomonas reinhardtii – characterization of light-enhanced dark respiration. Plant Physiology , 112 : 1005 – 1014 .