Abstract
The genus Martensia is characterized primarily by its unique thallus morphology, wherein a proximal membranous blade is interrupted distally by one to several bands of net-like tissue (networks). Among the known species, M. fragilis is characterized by fan-shaped, membranous blades with multiple bands of networks. The species has been frequently reported from warm temperate and tropical regions. Collections from various localities in the Indo-Pacific region included many specimens fitting this broad concept of M. fragilis, but differed in several aspects and were difficult to assign to any named species with confidence. In order to delineate the species boundaries within this suite of M. fragilis-like specimens and to clarify the phylogenetic significance of the morphological features used for separating species of Martensia, we used analyses of rbcL sequence to infer phylogenetic relationships among the available species with multiple bands of networks. Molecular analyses revealed that M. fragilis was restricted to its type locality Sri Lanka and that collections of M. ‘fragilis’ from other regions were split into six clades. Martensia ‘fragilis’ collections from Taiwan contained three cryptic species, which were described as new species (M. leeii W.-C. Yang & S.-M. Lin sp. nov., M. kentingii W.-C. Yang & S.-M. Lin sp. nov. and M. taiwanifretensis W.-C. Yang & S.-M. Lin sp. nov.) based on molecular and morphological evidence. RbcL sequences of M. ‘fragilis’ from Korea and Japan, as well as those of the recently described M. bibarii and M. jejuensis, were all virtually identical, indicating that previous records of M. fragilis from those regions should go under the name M. jejuensis, with M. bibarii placed in synonymy. Records of M. ‘fragilis’ from other locations in the Indo-Pacific should be regarded as doubtful until detailed morphological studies and molecular analyses of freshly collected specimens become possible.
Introduction
Martensia (Hering 1841), the largest genus in the subfamily Nitophylloideae within the family Delesseriaceae, is primarily distributed in tropical to subtropical regions and includes 15 species in the western Pacific and Indian Oceans (see Millar, 1990; Lin et al., 2001a, 2004a, 2009; Lee, 2004, 2005, 2006) and only one species, M. pavonia, in the Caribbean Sea and warm water regions of the western Atlantic Ocean (Taylor, 1960, 1969; Littler & Littler, 2000; Wynne, 2011). The genus is primarily characterized by its membranous thalli with one (e.g. M. elegans, M. australis) or multiple bands (e.g. M. fragilis, M. denticulata) of net-like tissue (networks), although in a few species a network is absent (e.g. M. martensii). One of the most commonly recorded species is M. fragilis, which is thought to be widely distributed in both the Indian and western Pacific Oceans (see Guiry & Guiry, 2012).
Lin et al. (2004a, 2009) recently documented the morphology of the generitype, M. elegans, from South Africa, and described three species (M. formosana, M. lewisiae and M. natalensis). They concluded that the developmental pattern of the networks can be used as a taxonomic feature for delineating species, when coupled with rbcL sequence analysis. Our recent collections from the Indo-Pacific regions contained many M. fragilis-like specimens, which were difficult to assign to any named species. We therefore sequenced rbcL from these specimens, which came from the north-western Pacific Ocean (Japan, Korea, Taiwan), along with M. fragilis from Sri Lanka and M. denticulata from Western Australia, in order to infer their phylogenetic relationships, and we evaluated morphological features potentially useful for separating species. We then undertook a detailed morphological examination of M. fragilis-like specimens from Taiwan which, in combination with the rbcL analyses, led to the recognition of three new species.
Materials and methods
Specimens were collected in the shallow subtidal by snorkelling or SCUBA diving. Samples were preserved in 5% formalin in seawater or pressed on herbarium sheets and a fragment of each specimen was preserved in silica gel for subsequent DNA extraction. Voucher specimens have been deposited in the herbarium of the National Taiwan Ocean University, Taiwan (NTOU), the Herbarium of the Ghent University (GENT), and the Western Australian Herbarium (PERTH). Hand sections were stained with 1% aniline blue acidified with 1% HCl and mounted in 25–30% Karo® syrup (Englewood Cliffs, New Jersey, USA) or treated with Wittmann's aceto-iron-haematoxylin–chloral hydrate (Wittmann, 1965) and mounted in 50% Hoyer's mounting medium (Lin et al., 2004b). Photomicrographs were taken on an Olympus BX51 microscope with a Q-imaging digital camera (Burnaby, British Columbia, Canada), and habit images were reproduced with an Epson scanner (Tokyo, Japan) or a Nikon D300 digital camera (Nikon, Japan). DNA from silica gel-dried specimens was extracted using the DNeasy Plant Mini Kit (Qiagen, Valencia, California, USA) following the manufacturer's instructions. DNA sequencing procedures were as described by Lin et al. (2001b).
Phylogenetic analyses were performed using maximum-parsimony (MP), maximum likelihood (ML) and Bayesian analysis. MP and non-parametric bootstrapping followed Lin et al. (2011). We used PAUP* v4.0 (Swofford, 2003) for MP analyses and GARLI 1.0 (Zwickl, 2006) for ML analyses. Bootstrapping involved 1000 and 100 replicates for MP and ML analyses, respectively. The substitution model for ML was the General Time Reversible model with gamma distributed rate heterogeneity, which is the default model in GARLI 1.0. as used in Lin et al. (2009). A Bayesian analysis (BA) was performed in MrBayes 3.1.2 (Ronquist & Huelsenbeck, 2003) using a GTR + I + Γ model, which allowed for rate variation among different codon positions. The analysis consisted of four chains (one hot and three cold), which were run for 106 generations with sampling every 100 generations. The first 1800 generations were discarded as burn-in. Stationarity was reached approximately at generation 18000. A 50% consensus tree (majority rule as implemented by PAUP* v4.0) was computed from the 9820 + 1 trees saved after the burn-in point. The genera Nitophyllum and Augophyllum was selected as outgroups in all analyses (Lin et al., 2004a, 2009).
Results
Molecular analyses
The multiple sequence alignment included 33 taxa of Martensia, including 18 newly generated rbcL sequences of M. fragilis-like specimens, from the Indian Ocean (Sri Lanka, Western Australia and South Africa) and western Pacific Ocean (Japan, Korea, Taiwan, the Philippines) (see Table 1 for the collection and GenBank accession number information). Because information was missing for the 5′ ends of many sequences the first 81 sites were excluded from the analyses, the final data matrix being restricted to 1386 base pairs, 370 of which were informative characters (26.7%).
Table 1. List of species used in rbcL analysis and accession numbers in GenBank. The number after the accession number is the percentage of the gene sequenced
The tree obtained by parsimony analysis via a heuristic search was largely congruent with tree topologies generated by ML and BA. Only the tree topology resulting from the ML is shown (), with the ML and MP bootstrap values and Bayesian posterior probabilities indicated on the branches. The earliest split in Martensia corresponds to the two recognized subgenera: subgenus Martensia which includes the generitype (M. elegans) from South Africa, M. australis from southern Philippines, and M. formosana and M. flabelliformis from Taiwan; and the subgenus Mesotrema, including the species with multiple bands of networks (M. fragilis from Sri Lanka, M. fragilis-like specimens from Hawaii, Japan, Korea, Taiwan and the Philippines, M. denticulata from Western Australia, M. jejuensis and M. bibarii from Korea, M. natalensis from South Africa, and M. pavonia from the Caribbean Sea), or with a weakly developed network (M. lewisiae from Taiwan), or lacking a network altogether (M. martensii from the southern Philippines). Molecular analyses demonstrated that the collections of M. fragilis-like specimens were subdivided into six lineages (see ), comprising specimens from Hawaii (lineage V), Japan and Korea (lineage IV), Taiwan (three lineages: I, II and VII) and the Philippines (lineage III) which were all different from M. fragilis from the type locality, Sri Lanka (, lineage VI). Among the clades of M. ‘fragilis’ from Taiwan, one was from northern Taiwan (I), one from southern Taiwan (II), and one from Penghu Island, Taiwan Strait (VII). The interlineage genetic distances of the seven clades of M. fragilis and M. ‘fragilis’ from the Indo-Pacific region ranged from 1.33% to 11.59% (19–161 pairwise differences, Kimura 2-parameter model), whereas the intraspecific genetic divergence within the clades ranged from 0% to 0.3% (0–4 pairwise differences). The rbcL sequences of M. ‘fragilis’ from Jeju Island, Korea, were identical to M. jejuensis and M. bibarii from the same island and differed only in four base pairs from M. ‘fragilis’ collected from Chiba prefecture, central Japan.
Morphological observations
The rbcL sequence analyses revealed that the collections identified as M. ‘fragilis’ from the Indo-Pacific were split into at least seven evolutionary lineages. We could not unequivocally demonstrate the occurrence of genuine M. fragilis outside its type locality. Accordingly, we recognize three new species, Martensia leeii W.-C. Yang & S.-M. Lin, sp. nov., Martensia kentingii W.-C. Yang & S.-M. Lin, sp. nov., Martensia taiwanifretensis W.-C. Yang & S.-M. Lin, sp. nov., for the three clades detected by molecular analyses from northern, southern and western Taiwan, respectively. We studied the morphology of specimens with multiple bands of networks and summarize the diagnostic features that separate the species in . The other two clades of M. ‘fragilis’ from the southern Philippines and Hawaiian Islands will be documented in detail once enough specimens become available.
Table 2. Morphological comparison of Martensia species with multiple bands of networks from the Indo-Pacific Oceans
A key to the three new species and the closely related species as detected from rbcL sequence analyses can be constructed using morphological characteristics, as follows:
| 1. | Thalli consisting of linear axes bearing flabellate bladelets2 | ||||
| 2. | Thalli flabellate bearing subdichotomously divided, lobed bladelets3 | ||||
| 3. | Thalli bushy, up to 15 (–30) cm long, old networks fragmented or becoming fimbriate, margins of networks covered with few teeth-like protrusions M. jejuensis | ||||
| 4. | Thalli ribbon-like initially, 5–8 cm long, old blade bearing 2–3 bands of network, margins of blades covered with numerous teeth-like or ligulate bladelets M. denticulata | ||||
| 5. | Thalli bushy, 5–9 cm long, each main blade bearing 5–9 bladelets, which also bearing several new marginal lobes, up to 5 orders of alternation of producing marginal bladelets or lobes M. fragilis | ||||
| 6. | Thalli prostrate, 2–4 long, blades bearing conspicuous bands of networks4 | ||||
| 7. | Thalli with thin membranous blades of 2–3 cell layers (less than 80 μm thick), network not fully covering the main blades5 | ||||
| 8. | Thalli with thick membranous blades, 4–5 cell layers (120–170 μm thick), network nearly fully covering the main blades M. kentingii | ||||
| 9. | Main blades slightly to deeply cleft, marginal bladelets mostly arising from the primary longitudinal lamellae M. taiwanifretensis | ||||
| 10. | Main blades mainly flabellate, marginal bladelets mostly arising from the membranous margins of blades M. leeii | ||||
Descriptions of new species
Martensia leeii W.-C. Yang & S.-M. Lin, sp. nov.
(Figs 2–12)
Description: Thalli fan-shaped, consisting of one to several blades, attached to the substratum by a short stipe, 1–3 mm in length; blades flabellate, 1–3 cm wide and 2–3 cm in length, with a conspicuous distal band of network and covered with many marginal, membranous lobes; each lobe may develop a new band of marginal network; network composed of primary longitudinal and cross-connecting lamellae enclosing needle-like, secondary cross-connecting and longitudinal lamellae; cross-connecting strands mostly developing unilaterally and linking unidirectionally to adjacent lamellae on the opposite side; gametophytes not found; tetrasporangial sori borne on primary longitudinal lamellae but not directly on membranous blades; tetrasporangial sori round to oval, 100–500 μm by 200–300 μm in diameter, solitary or aggregated, mature tetrasporangia 75–90 μm in diameter.
Holotype and isotypes: Holotype deposited at the herbarium of the Institute of Marine Biology, National Taiwan Ocean University, #NTOU-LDW-24vi10-05 (, holotype); isotypes #NTOU-LDW-24vi10-01 – #NTOU-LDW-24vi10-04 ().
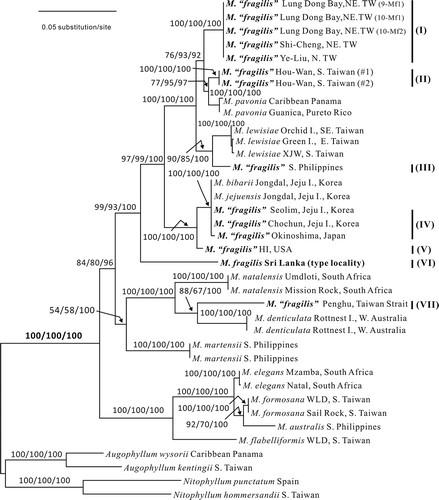
Figs 2. Martensia leeii W.-C. Yang & S.-M. Lin sp. nov. Habit and network morphology (Lung Dong Wan, New Taipei City). 2. Holotype, a tetrasporic plant. Scale bar = 5 mm. 3. Isotype, a young plant. Scale bar = 5 mm. 4. Early stage of network formation showing basal cells (arrows) of longitudinal lamellae. Scale bar = 50 μm. 5. Basal part of a developing network showing connecting strands (arrowheads) and basal cells (arrows) of longitudinal lamellae. Scale bar = 100 μm. 6. Proximal portion of a network showing secondary cross-connecting strands (arrows) and secondary longitudinal lamellae (arrowheads). Scale bar = 250 μm. 7. Basal part of a developing network showing divided basal cells (arrows) of longitudinal lamellae. Scale bar = 100 μm. 8. Proximal portion of thallus showing some lobes from the edge (arrows). Scale bar = 1 mm.
Type locality: Lung Dong Wan, New Taipei County, north-eastern Taiwan (25°07.05′N, 121°54.57′E).
Etymology: The epithet ‘leeii’ honours Professor Yongpil Lee (Cheju National University), who has made a significant contribution to the taxonomy of Martensia from Korea.
Distribution: Found along the coastline of northern Taiwan.
Habitat and seasonality: Plants were found in early summer from June through July; attached to rocky reefs at 1–12 m depths.
Specimens examined: Taiwan: New Taipei City: Yeliu, 1–12 m, coll. S.-M. Lin, sterile, 7 July 1994; Shi Cheng, coll. S.-M. Lin, tetrasporic, 30 June 2009; Lung Dong Wan, coll. L.-C. Liu, tetrasporic, 24 June 2010.
Habit and vegetative morphology. Thalli (, ) are prostrate, pinkish to rose, consisting of one to several flabellate blades, 1–3 cm wide and 2–3 cm in length, attached to rocks by a short stipe, 2–3 mm. Several thalli usually grow together forming a floral-like cluster. Young blades consist of a conspicuous band of network and bear many marginal, membranous lobes (, 8), which eventually become flabellate and bear distal networks (). Growth is diffuse and the network is initiated from a row of transformed marginal cells that divide transversely to form a membranous margin and intercalary longitudinal lamellae (). Cross-connecting strands mostly develop unilaterally and link unidirectionally to adjacent lamellae on the opposite side (). Expansion of the network is by intercalary cell divisions of primary longitudinal lamellae and the continued formation of cross-connecting strands (, 8). Needle-like, secondary longitudinal (, arrowheads) and cross-connecting (, arrows) lamellae are initiated from the middle of the cross-linking lamellae, filling in the space created by the expansion of the primary network. The cells at the base of the network divide once or twice both anticlinally and longitudinally ( , arrows). Primary longitudinal lamellae are sheet-like, 12–18 cells (300–700 μm) wide, and the basal membranous blades are composed of 2–3 cell layers (Fig. 9), 40–80 μm thick. Numerous secondary pit-connections are formed between cells of the membranous parts of the blades (Fig. 10).
Figs 3. Martensia leeii W.-C. Yang & S.-M. Lin sp. nov. Vegetative morphology and tetrasporangial formation (Lung Dong Wan, New Taipei City). 9. Cross-section through basal part of a membranous blade. Scale bar = 100 μm. 10. Close-up of surface cells showing discoid plastids and numerous secondary pit-connections. Scale bar = 50 μm. 11. Close-up of tetrasporangial sori borne on a longitudinal lamella. Scale bar = 250 μm. 12. Close-up of a tetrasporangial sorus showing multinucleate tetrasporangial initials (ti), immature tetrasporagia (it) and tetrahedrally divided tetrasporangia (t). Scale bar = 50 μm.
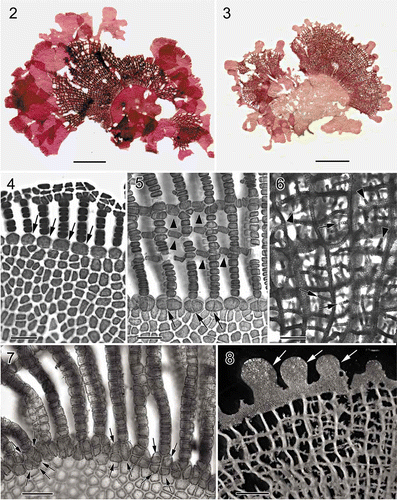
Figs 4. Martensia kentingii W.-C. Yang & S.-M. Lin sp. nov. Habit and network morphology (Hou Wan, Kenting National Park). 13. Holotype, a female plant. Scale bar = 5 mm. 14. Isotype, a young plant. Scale bar = 5 mm. 15. Early stage of network formation showing cross-connecting strand initials (arrowheads) cut off bilaterally from longitudinal lamellae, and basal cells of longitudinal lamellae divided once. Scale bar = 50 μm. 16. Another early development of network showing elongation of cell rows of longitudinal lamellae by intercalary cell divisions, and the formation of cross-connecting strands by pit connections between cells (arrowheads) derived from longitudinal lamellae on opposite sides. Scale bar = 50 μm. 17. Further development of network showing the formation of cross-connecting strands by pit connections between cells (arrowheads) derived from longitudinal lamellae on opposite sides, and further cell division of basal cells (arrows). Scale bar = 125 μm. 18. Basal portion of an older network showing cross-connecting strands (arrowheads) and transversely and oblique cell divisions of basal cells (arrows). Scale bar = 100 μm. 19. Middle portion of an older network showing secondary cross-connecting strands (arrows) and secondary longitudinal lamellae (arrowheads). Scale bar = 500 μm. 20. Close up of upper portion of network bearing lobed, membranous margin. Note that a new network band is already initiated along the margin of the lobe on the right. Scale bar = 1 mm.
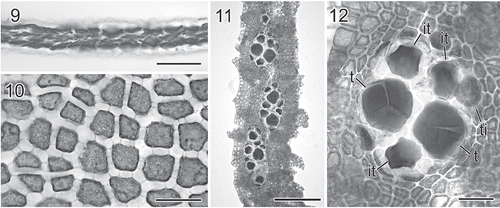
Figs 5. Martensia kentingii W.-C. Yang & S.-M. Lin sp. nov. Vegetative and reproductive morphology (Hou Wan, Kenting National Park). 21. Cross-section through basal part of membranous blade. Scale bar = 50 μm. 22. Close up of a tetrasporangial sorus showing multinucleate tetrasporangial initials (ti), immature tetrasporangia (it) and tetrahedrally divided tetrasporangia (t). Scale bar = 50 μm. 23. Spermatangial sori borne on longitudinal lamellae. Scale bar = 100 μm. 24. Close up of a developing spermatangial sorus showing spermatangial parental cell initials (arrows) and spermatangial parental cells (arrowheads). Scale bar = 25 μm. 25. Close up of cystocarps borne on network. Scale bar = 1 mm. 26. Middle portion of network showing procarp-bearing lobes (arrows) derived from margins of longitudinal lamellae. Scale bar = 100 μm. 27. Close up of a procarp composed of a supporting cell (sc), the one-celled sterile lateral (st), a carpogonial branch (arrowheads) and a cover cell (co). Scale bar = 25 μm. 28. Surface view of a young cystocarp with an ostiole (arrow). Scale bar = 100 μm. 29. Cross-section through a immature carposporophyte showing enlarged nuclei and newly formed basal cell (bc). Scale bar = 100 μm.
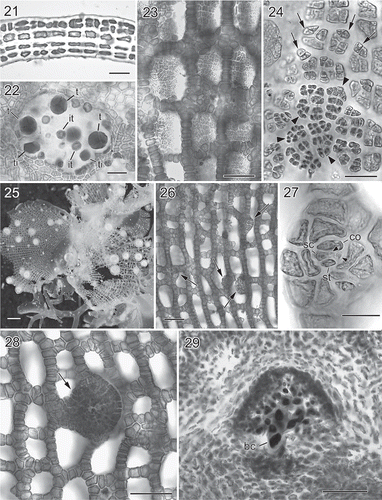
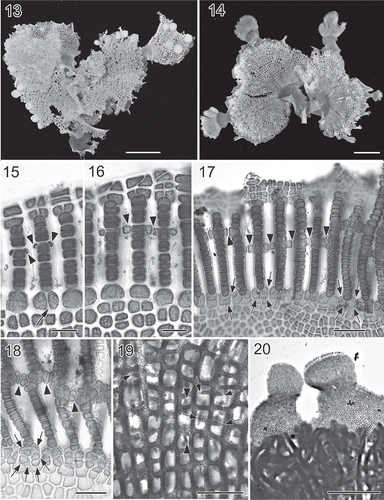
Figs 6. Martensia taiwanifretensis W.-C. Yang & S.-M. Lin sp. nov. Habit, vegetative and reproductive morphology (Makung Island, Penghu County). 30. Holotype, a tetrasporic plant. Note that the network (arrows) at the basal part of the blade is covered by sand. Scale bar = 1 cm. 31. Isotype, another tetrasporic plant with a basal network (arrow). Scale bar = 5 mm. 32. Basal portion of developing network showing newly formed cross-connecting strands (arrowheads) and further cell division of basal cell (arrows). Scale bar = 250 μm. 33. Middle portion of a fully developed network showing primary (arrows) and secondary, spine-like cross-connecting strands (arrowheads). Scale bar = 250 μm. 34. A fully developed network showing the distal ends of longitudinal lamellae developed into wavy bladelets (arrows). Scale bar = 5 mm. 35. Close up of the distal ends of two longitudinal lamellae (arrows) bridged by cross-connecting strands. Scale bar = 500 μm. 36. Close up of cystocarps scattered along the margin of the network. Scale bar = 3 mm. 37. Cross-section through a nearly mature cystocarp showing enlarged nuclei in inner cells (arrowheads) of gonimoblast filaments. Note that this section was made based on a compressed, dry specimen, so that the shape of the cells in the cystocarp is somewhat distorted. Scale bar = 100 μm.
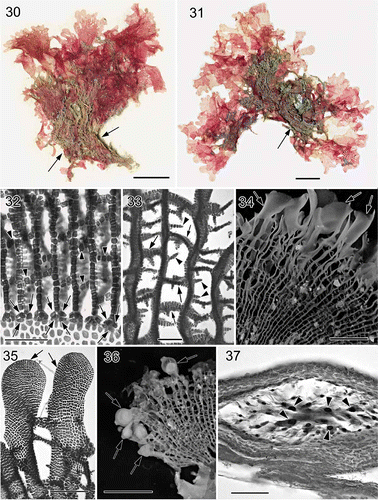
Figs 7. Martensia jejuensis. Thallus morphology. 38–40. Thalli representing M. jejuensis and M. ‘bibarii’ and bearing lobed (arrows) and hairy (arrowheads) networks collected from Chochun, Jeju Island (coll. S.-M. Lin, 28 August 2008). 41–43. Thalli representing M. ‘bibarii’ and M. ‘fragilis’ and bearing lobed (arrows) and hairy (arrowheads) networks collected from Seolim, Jeju Island (coll. S.-M. Lin & W.-J. Lee, 25 June 2005). Scale bars = 15 mm (Fig. 38) and 10 mm (Figs 39–43).
Reproductive morphology. Tetrasporangial sori are round to ovoid in shape, 200–300 μm wide by 100–500 μm long, and are solitary or aggregated on primary longitudinal lamellae (Figs 11, 12). Tetrasporangial initials are transformed from multinucleate lamellar cells into tetrasporocytes, which become uninucleate through nuclear degeneration, leaving behind a single functional nucleus. Mature tetrasporangia are tetrahedrally divided and 70–100 μm in diameter.
Martensia kentingii W.-C. Yang & S.-M. Lin, sp. nov.
(Figs 13–29)
Description: Thalli fan-shaped, consisting of one to five flabellate blades, rose to dark red, attached to the substratum by rhizoidal haptera; blades 1–2 cm wide and 2–3 cm in length, with a conspicuous band of network when old and covered with many marginal, membranous rounded lobes; each lobe may develop a faint band of marginal network; network composed of primary longitudinal and cross-connecting lamellae, which develop bidirectionally and link to adjacent lamellae on the opposite side; needle-like, secondary, cross-connecting strands rare; tetrasporangial sori round to oval, 200–400 μm wide by 300–550 μm long, borne on primary longitudinal lamellae in network, mature tetrasporangia 60–100 μm in diameter; gametophytes dioecious, spermatangial sori irregularly oblong to rectangular and formed on primary longitudinal lamellae, each spermatangial parental cell bearing 1–2 spermatangia, 3–5 μm in diameter; cystocarps scattered over network, 800–1200 μm in diameter; procarps borne on membranous lobes derived from edges of longitudinal lamellae; carposporangia pyriform, 50–70 μm wide by 70–100 μm long, formed terminally.
Holotype and isotypes: Holotype deposited at the herbarium of the Institute of Marine Biology, National Taiwan Ocean University, #NTOU-HW-07ii2002-Mmul-1 (Fig. 13); isotypes #NTOU-HW-07ii2002-Mmul-2 (Fig. 14) to– #NTOU-HW-07ii2002-Mmul-7 and #NTOU-HW-07ii2002-Mmul-7mix.
Type locality: Hou Wan, Kenting National Park, Pintung County, southern Taiwan (22°02.79′N, 120°42.14′E).
Etymology: The epithet ‘kentingii’ refers to Kenting National Park, the type locality.
Distribution: Only found so far in Kenting National Park.
Habitat and seasonality: Plants were found from February through April; attached to rocky reefs at 1–3 m depths.
Specimens examined: Taiwan: Kenting National Park, Pingtung County: Hou Wan, 2–3 m deep, coll. S.-M. Lin, 7 February 2002; Sail Rock, 1–2 m deep, coll. S.-M. Lin, 1 April 2002.
Habit and vegetative morphology. Thalli (Figs 13, 14) are slightly prostrate, pinkish to dark red, consisting of one to five, fan-shaped blades, 1–2 cm high and 2–3 cm wide, attached to rocks by rhizoidal haptera. Old blades consist of a conspicuous band of network, which almost covers the entire blade. Some lobes are produced along the blade margins, each lobe eventually developing into a flabellate blade, bearing a single band of network at the distal end (Figs 13, 14, 20).
Growth is diffuse and the network is initiated from a row of transformed marginal cells that divide transversely to form a membranous margin and intercalary longitudinal lamellae. Cross-connecting strands are initiated bidirectionally from the edges of longitudinal lamellae (Fig. 15) and are pit-connected to one another at the middle to adjacent lamellae on the opposite side (Fig. 16). Expansion of the network is by intercalary cell divisions of primary longitudinal lamellae and the continued formation of cross-connecting strands (Figs 17, 18). Secondary longitudinal lamellae are rare (Fig. 19, arrowheads), whereas cross-connecting strands are relatively common and gradually fill the space created by the expansion of the primary network (Fig. 20, arrows). The cells at the base of the network divide both anticlinally and longitudinally (Figs 17, 18) two or three times. Primary longitudinal lamellae and cross-connecting strands are sheet-like, 15–25 cells (450–900 μm) wide, and the basal membranous blades are composed of 4–5 cell layers (Fig. 21), 120–170 μm thick. Numerous secondary pit-connections are formed between cells of the membranous parts of the blades.
Reproductive morphology. Tetrasporangial sori are formed on primary longitudinal lamellae. Tetrasporangial sori are round to oval in shape (Fig. 22), 200–400 µm wide by 300–550 µm long and mostly solitary but sometimes aggregated. Tetrasporocytes are transformed from multinucleate lamellar cells. Mature tetrasporangia are tetrahedrally divided and measure 60–100 µm in diameter.
Gametophytes are dioecious and are morphologically similar to the tetrasporophytes. Spermatangial sori are irregularly oblong to rectangular and are formed on primary longitudinal lamellae (Fig. 23). Spermatangial parental cell initials are cut off from surface cells of longitudinal lamellae (Fig. 24, arrows) and each parental cell bears one or two spermatangia, 3–5 µm in diameter. Cystocarps are scattered over the network, 800–1200 µm in diameter (Fig. 25). Procarps (Fig. 26) are abundant and borne on membranous lobes that arise from the edges of the longitudinal lamellae within the network. Fully developed procarps (Fig. 27) consist of a supporting cell bearing one four-celled carpogonial branch, a one-celled sterile group and a one-celled cover cell. Young cystocarps develop a central ostiole (Fig. 28). The nuclei of the supporting cell and inner gonimoblasts become enlarged (Fig. 29). Mature carposporangia are 50–70 µm wide by 70–100 µm long and pyriform, and are formed terminally.
Martensia taiwanifretensis W.-C. Yang & S.-M. Lin sp. nov .
(Figs 30–37)
Description: Thalli membranous and prostrate, consisting of several, slightly to deeply cleft blades, 2–3 cm wide and 2–4 cm long, attached to the substratum by rhizoidal haptera; blades with one to three bands of networks and bearing many marginal, membranous lobes derived from the distal ends of primary longitudinal lamellae; each lobe may develop a new band of marginal network; networks composed of primary longitudinal and cross-connecting lamellae enclosing secondary, needle-like filaments borne on primary longitudinal or on cross-connecting strands; tetrasporangial sori, round to oval, 170–200 µm wide by 200–300 µm long, borne on primary longitudinal lamellae in network only, mature tetrasporangia 75–100 µm in diameter; cystocarps, 800–1100 µm in diameter, mostly borne on edges of networks at distal ends of blades, pyriform carposporangia, 40–50 µm wide by 60–90 µm long.
Holotype and isotypes: Holotype deposited at the herbarium of the Institute of Marine Biology, National Taiwan Ocean University, #NTOU-PH-10316-1 (Fig. 30); isotypes #NTOU-PH-10316-2 to #NTOU-PH-10316-13, #NTOU-PH-10316-14 (Fig. 31), #NTOU-PH-10316-21.
Type locality: Li Jhen Gjiao, Penghu County, in the Taiwan Strait, western Taiwan (23°34.55′N, 119°40.20′E).
Etymology: The epithet ‘taiwanifretensis’ refers to the Taiwan Strait, where the type locality is located.
Distribution: Found only at Penghu in the Taiwan Strait.
Habitat and seasonality: Plants were collected from January through November; attached on coral reefs at 1–2 m depth.
Specimens examined: Taiwan: Penghu County in Taiwan Strait: (1) Makung Island: Li Jhen Gjiao, coll. S.-M. Lin & J. Lewis, 15 July 1992 (tetrasporic, females). Jai Shan, coll. S.-L. Liu, tetrasporic, 13 January 2003. Lin Tou Park, coll. S.-M. Lin & J. Lewis, 13 July 1992 (sterile), 13 November 1992 (tetrasporic). Pei Liao, coll. S.-M. Lin & J. Lewis, 16 July 1992 (tetrasporic). Guo Ye, coll. S.-M. Lin & J. Lewis, 16 July 1992 (tetrasporic). Da Liao, coll. S.-M. Lin & J. Lewis, 23 August 1993 (tetrasporic). (2) Won An Island: Won-An, coll. S.-L. Liu, 7 September 2012 (tetrasporic, sterile).
Habit and vegetative morphology. Thalli (Figs 30, 31) are slightly prostrate, pinkish to red, and consist of several, slightly to deeply cleft, membranous blades, 2–3 cm wide and 2–4 cm in length, attached to the substratum by rhizoidal haptera. Main blades consist of one to three bands of networks and bear many marginal bladelets (Fig. 31), which eventually become fan-shaped blades and bear distal networks (Fig. 30). Growth is diffuse and the network is initiated from a row of transformed marginal cells that divide transversely to form a membranous margin and intercalary longitudinal lamellae. Cross-connecting strands mostly develop bilaterally and link bidirectionally to adjacent lamellae on the opposite side (Fig. 32). Expansion of the network is by intercalary cell divisions of primary longitudinal lamellae and the continued formation of cross-connecting strands (Figs 33–35). Numerous needle-like filaments are initiated from primary longitudinal lamellae and cross-connecting strands (Fig. 33). The cells at the base of the network divide longitudinally first, then anticlinally (Fig. 32, arrows) once or twice. Primary longitudinal lamellae are sheet-like, 20–30 cells (300–650 μm) wide and the basal membranous blades are composed of 2–3 cell layers, 20–50 μm thick. Numerous secondary pit-connections are formed between cells of the membranous parts of the blades.
Reproductive morphology. Tetrasporangial sori are round to oval in shape, 170–200 μm wide by 200–300 μm long, and are formed on primary longitudinal lamellae only. Mature tetrasporangia are tetrahedrally divided and measure 75–100 µm in diameter. Male plants were not found in this study. Female gametophytes are morphologically similar to the tetrasporophytes, but are relatively smaller. Cystocarps are 800–1100 µm in diameter, mostly scattered over the edges of the networks at the distal ends of blades (Fig. 36). Procarps and early post-fertilization stages were not observed, but the nuclei of the inner gonimoblast filaments enlarged and become darkly stained in mature cystocarps (Fig. 37). Carposporangia are pyriform, measuring 40–50 µm wide by 60–90 µm long.
Discussion
Based on rbcL sequence analysis, Lin et al. (2004a, 2009) showed that Martensia fragilis, a supposedly widespread species with multiple network bands, may encompass several cryptic species that have thus far gone unrecognized. They also suggested the need for a detailed investigation of critical stages of vegetative and reproductive development in M. ‘fragilis’ from different localities. Our analyses of additional rbcL sequences of M. fragilis-like specimens from the Indo-Pacific region, Korea and Japan in this study indicate that genuine M. fragilis may be restricted to Sri Lanka in the northern Indian Ocean. Unfortunately, Harvey (1854) did not provide an illustration when describing M. fragilis from Ceylon (=Sri Lanka), but the lectotype (TCD 0012254) is depicted at http://plants.jstor.org/specimen/tcd0012254, along with seven other plants from Harvey's collection from the type locality, Belligam (=Weligama). Our sequenced collection from Sri Lanka (the same taxon depicted in Coppejans et al., 2009. fig. 179a, b) agrees well with Harvey's description and the type material, and we provisionally recognize our material as authentic M. fragilis until a thorough taxonomic study of the genus around Sri Lanka can be undertaken. The genetic divergence distances among the six clades of M. ‘fragilis’ varied from 1.33% to 11.59%, which exceeded the intraspecific divergence (less than 1%) (see Lin et al. 2004a, 2012). As a result, we recognize these six clades as independent species separate from M. fragilis sensu stricto.
On the other hand, the collections of M. ‘fragilis’ from Japan and Korea turned out to be molecularly virtually identical to M. jejuensis (Lee, 2004, p. 256) and M. bibarii (Lee 2004, p. 258) from Jeju Island, Korea. Our analyses were based on two sets of seasonal collections of Martensia jejuensis/bibarii from the coasts of Jeju Island, including the type locality, Jongdal, that were made by the first author, S.-M. Lin, who also identified many forms intermediate between the type specimens of M. jejuensis and M. bibarii (see Figs 38–43). The pit-connections between linking cross-connecting strands and longitudinal lamellae in the networks can degenerate to varying degrees under differing environmental conditions or growth rates. Figs 38–40 show thalli with long, ribbon-like bladelets that were derived from the longitudinal lamellae of a broken network, whereas the networks of thalli shown in Fig. 43 remain intact. In some instances, the broadening longitudinal lamellae may bear marginal bladelets with hair-like networks (see Figs 41, 42, arrowheads). Thus it would appear environmental conditions can be a major factor affecting the morphology of Martensia in Jeju. In recent years, seashell culture has become popular around the coasts of Jeju Island and the ambient seawater is enriched. The changed environmental conditions might have considerable effects on the growth and thallus morphology of Martensia populations around Jeju Island. We obtained additional sequences from several collections of Martensia, displaying thallus morphologies similar to those of M. ‘fragilis’, M. jejuensis and M. bibarii (data not shown; their thallus morphologies shown in Figs 38–43). These additional rbcL sequences are all identical to those of typical M. jejuensis and M. bibarii. Based on these results, we conclude that these two species are conspecific. As the two names appeared in the same publication (Lee, 2004) they have equal priority. Herein we choose to adopt the name M. jejuensis (thereby establishing priority, International Code of Nomenclature for algae, fungi and plants, Art. 11.5: McNeill et al., 2012). Accordingly, the species bearing multiple bands of networks from Korea and Japan, i.e. M. ‘fragilis’ and M. bibarii, should all be treated as M. jejuensis, and this taxonomic conclusion is made formal below.
The three new Taiwanese species, M. leeii, M. kentingii and M. taiwanifretensis described in this study can be separated from similar Indo-Pacific species such as M. fragilis from Sri Lanka, M. denticulata from Western Australia and M. jejuensis from Korea, by a combination of their thallus habit, blade morphology and network structure (see for a morphological comparison). Among all the species with multiple bands of networks, M. jejuensis has the largest thallus (up to 30 cm) and shows a great variation of thallus habit (see Figs 38–43), whereas the thalli of genuine M. fragilis (see Coppejans et al., 2009. fig. 179a, b) and M. denticulata (Harvey 1855, pl. 127) are smaller, c. 5–10 cm in height. In contrast, the three new species from Taiwan are even smaller, ranging from 1–4 cm in height. M. taiwanifretensis can be separated from M. leeii and M. kentingii by its longer blades (up to 4 cm) with a conspicuous band of basal network (see Figs 40, 41). Martensia kentingii can be identified by its well-developed network, which almost covers the entire blade (see Figs 17, 18), whereas M. leeii can be characterized by the main blade having a wide band of network at the distal end and bearing numerous, fan-shaped, marginal lobes (see , ).
In this study, we have confirmed the observations by Lin et al. (2004a, 2009) that the morphology and the developmental patterns of networks are useful taxonomic features for delineating the species of Martensia. We anticipate that more new species of Martensia will be described when additional sequence analyses and careful, morphological comparison based on well-preserved specimens from warm water regions become available.
Taxonomic conclusion
Martensia jejuensis Y. Lee [(2004), Phycological Research 52: 256, figs 2–21] sensu emend. S.-M. Lin, W.-C. Yang, Huisman, De Clerk & W.J. Lee
Holotype: tetrasporic, LYP-1586 in the Herbarium of the Department of Biology, Cheju National University (CNU).
Type locality: Subtidal zone (6–8 m deep), Jongdal, Jeju Island, Korea, 7 August 2000.
Proposed synonym: Martensia bibarii Y. Lee (2004), Phycological Research 52: 258, figs 22–27. [Holotype: tetrasporic, LYP-1594 in the Herbarium of the Department of Biology, Cheju National University (CNU); Type locality: subtidal zone (6–8 m deep), Jongdal, Jeju Island, Korea, 3 August 2000.]
Distribution: Korea: Jeju Island, Korea (Lee, 2004; this study). Japan: Seto Inland Sea; Okinoshima, Chiba Prefecture (this study). Previous records of M. fragilis (e.g. Yoshida & Mikami, 1996) and M. denticulata (Okamura, 1936) from Japan should be treated as M. jejuensis.
Acknowledgements
This project was largely supported by a grant from National Science Council (Taiwan) (NSC 99-2621-B-019 -003 -MY3) and from NTOU's Center of Excellence for Marine Bioenvironment and Biotechnology to S.-M. Lin. J.M.H. acknowledges the support of the ‘Australian Biological Resources Study’. ODC is indebted to FWO – Vlaanderen. We thank Drs Eric Coppejans, S.-L. Liu and M. Suzuki for collecting Martensia used in this study.
References
- Coppejans , E. , Leliaert , F. , Dargent , O. , Gunasekara , R. and De Clerck , O. 2009 . Sri Lankan seaweeds – methodologies and field guide to the dominant species . Abc Taxa , 6 : 1 – 265 .
- Guiry, M.D. & Guiry, G.M. (2012). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 5 June 2012 (http://www.algaebase.org; searched on 5 June 2012)
- Harvey , W.H. 1854 . Short characters of three new algae from the shores of Ceylon . Hooker's Journal of Botany and Kew Garden Miscellany , 6 : 143 – 145 .
- Harvey , W.H. 1855 . Some account of the marine botany of the colony of western Australia . Transactions of the Royal Irish Academy , 22 : 525 – 566 .
- Hering , C. 1841 . Diagnoses Algarum novarum a cl. Dre. Ferdinand Krauss in Africa Australi lectarum . Annals and Magazine of Natural History , 8 : 90 – 92 . series 1,
- Lee , Y. 2004 . Two new species of Martensia (Delesseriaceae, Rhodophyta) from Jeju Island, Korea . Phycological Research , 52 : 255 – 265 .
- Lee , Y. 2005 . New red algae of Martensia (Delesseriaceae), M. palmata sp. nov. and M. projecta sp. nov. from Jeju Island, Korea . Algae , 20 : 279 – 294 .
- Lee , Y. 2006 . The genus Martensia Hering (Delesseriaceae, Rhodophyta) with M. albida sp. nov. and M. flammifolia sp. nov. on Jeju Island, Korea . Algae , 21 : 15 – 48 .
- Lin , S.-M. , Fredericq , S. and Liao , L.M. 2001a . Opephyllum martensii , 44 : 589 – 595 . ). Neotypification and taxonomic status of Schmitz in Schmitz et Hauptfleisch (Delesseriaceae, Rhodophyta) from Zamboanga, southern Philippines. Botanica Marina
- Lin , S.-M. , Fredericq , S. and Hommersand , M.H. 2001b . rbc , 37 : 881 – 899 . Systematics of the Delesseriaceae (Ceramiales, Rhodophyta) based on LSU rDNA and L sequences, including the Phycodryoideae subfam. nov. Journal of Phycology
- Lin , S.-M. , Hommersand , M.H. and Fredericq , S. 2004a . Martensia , 43 : 13 – 25 . Two new species of (Delesseriaceae, Rhodophyta) from Kenting National Park, southern Taiwan. Phycologia
- Lin , S.-M. , Fredericq , S. and Hommersand , M.H. 2004b . Augophyllum , 40 : 962 – 976 . a new genus of the Delesseriaceae (Rhodophyta) based on rbcL sequence analysis and cystocarp development. Journal of Phycology
- Lin , S.-M. , Hommersand , M.H. , Fredericq , S. and De Clerck , O. 2009 . Characterization of Martensia (Delesseriaceae, Rhodophyta) based on a morphological and molecular study of the type species, M. elegans Hering and M. natalensis sp. nov. from South Africa . Journal of Phycology , 45 : 678 – 691 .
- Lin , S.-M. , Yang , S.-Y. and Huisman , J.M. 2011 . Systematics of Liagora with diffuse gonimoblasts based on rbcL sequences and carposporophyte development, including the description of the new genera Neoizziella and Macrocarpus (Liagoraceae, Rhodophyta . European Journal of Phycology , 46 : 249 – 262 .
- Lin , S.-M. , Liu , L.-C. and Payri , C. 2012 . Characterization of Gracilaria vieillardii (Gracilariaceae, Rhodophyta) and molecular phylogeny of foliose species from the western Pacific Ocean, including a description of G. taiwanensis sp . nov. Phycologia , 51 : 421 – 431 .
- Littler , D.S. and Littler , M.M. 2000 . Caribbean reef plants. An identification guide to the reef plants of the Caribbean, Bahamas , Washington : Florida and Gulf of Mexico. Offshore Graphics .
- McNeill, J., Barrie, F.R., Buck, W.R., Demoulin, V., Greuter, W., Hawksworth, D.L., Herendeen, P.S., Knapp, S., Marhold, K., Prado, J., Prud'homme van Reine, W.F., Smith, G.F., Wiersema, J.H. & Turland, N.J. (2012). International Code of Nomenclature for algae, fungi and plants (Melbourne Code). Koeltz Scientific Books, Koenigstein.
- Millar , A.J.K. 1990 . Marine red algae of the Coffs Harbour region, northern New South Wales . Australian Systematic Botany , 3 : 293 – 593 .
- Okamura , K. 1936 . Nippon kaisô shi [Descriptions of Japanese algae] , Uchida Rokakuho : Tokyo .
- Ronquist , F. and Huelsenbeck , J.P. 2003 . MrBayes 3: Bayesian phylogenetic inference under mixed models . Bioinformatics , 19 : 1572 – 1574 .
- Swofford , D.L. 2003 . PAUP*: phylogenetic analysis using parsimony (*and other methods), Version 4 , MA : Sinauer Associates, Sunderland .
- Taylor , W.R. 1960 . Marine algae of the eastern tropical and subtropical coasts of the Americas , MI : University of Michigan Press, Ann Arbor .
- Taylor , W.R. 1969 . Notes on the distribution of West Indian marine algae particularly in the Lesser Antilles . Contributions of the University of Michigan Herbarium , 9 : 125 – 203 .
- Wittmann , W. 1965 . Aceto-iron-haematoxylin-chloral hydrate for chromosome staining . Stain Technology , 40 : 161 – 164 .
- Wynne , M.J. 2011 . A checklist of benthic marine algae of the tropical and subtropical western Atlantic: third revision . Nova Hedwigia, Beiheft , 140 : 7 – 166 .
- Yoshida , T. and Mikami , H. 1996 . Observations on Japanese species of the genus Martensia (Delesseriaceae, Rhodophyta), with the description of Neomartensia gen . nov. Phycological Research , 44 : 101 – 106 .
- Zwickl , D.J. 2006 . Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion , Austin : Ph.D. dissertation, University of Texas at Austin .