Abstract
The Chondrymeniaceae Rodríguez-Prieto, G. Sartoni, S.-M. Lin & Hommersand, fam. nov., is proposed for Chondrymenia lobata. Analyses of rbcL sequences place the new family in a large gigartinalean assemblage that comprises the Cystocloniaceae–Solieriaceae complex. Plants are decumbent and growth takes place by division of multiple apical cells at the margin of the blade. Thalli consist of an outer cortex of subspherical to elongate cortical cells arranged in anticlinal rows, a subcortex of cells cross-linked by lateral arms, and a large central medulla composed of primary medullary filaments intermixed with numerous rhizoidal filaments. Male stages are reported in monoecious individuals. Inactive carpogonial branches consist of a two-celled filament that is directed inwards from the supporting cell. Functional carpogonial branches are oriented outwardly, with the carpogonia and trichogynes pointed towards the thallus surface. After presumed fertilization, the carpogonium fuses with the hypogynous cell and transfers the zygote nucleus. The hypogynous cell, in turn, fuses with the supporting cell which contains many haploid nuclei. The resulting fusion cell functions as an auxiliary cell that cuts off a single gonimoblast initial, which produces the gonimoblast filaments. Gametophytic cells close to the auxiliary cell unite with it to form a placental fusion network of variable size and outline, and a placental fusion cell. Proximal gonimoblast cells fuse with the placental fusion cell, while the distal cells differentiate into branched chains of subspherical carposporangia. The superficial similarity of the outwardly developed osteolate cystocarp is responsible for Kylin's (1956) placement of Chondrymenia in his family Sarcodiaceae; however, the manner in which the placenta is formed is more like that seen in the Cystocloniaceae–Solieriaceae complex.
Introduction
Chondrymenia was established by Zanardini (Citation1860a , Citation1860b ) based on Halymenia lobata Meneghini (Citation1841) from Dalmatia in the eastern Adriatic Sea (). The original specimen in the G. Meneghini herbarium at the Università degli Studi di Firenze, Italy (FI) was said to be sterile (Zanardini, Citation1860a , Citation1860b ; Boudouresque & Huvé, Citation1969). Meneghini described it as being fleshy, planar, and extended into orbicular lobes from proliferous margins. Later collections from Dalmatia by Vidovich included female material, which led Zanardini to interpret Chondrymenia lobata as a member of the Gigartineae (sensu J. Agardh, Citation1851). A search did not locate the Vidovich material, thought to have been housed in the G. Zanardini herbarium at the Museo di Storia Naturale di Venezia (MCVE). Zanardini described the texture of Chondrymenia as fleshy-cartilaginous and somewhat rigid, the blades consisting of three distinct layers: a medulla composed of densely interwoven dichotomously branched segmented filaments, an intermediate layer of loosely reticulate anastomosing filaments, and a cortical layer of compact vertical moniliform filaments bound together within a dense mucilaginous matrix. Conspicuously ostiolate and protuberant cystocarps were present on both sides of the thallus, the carposporophytes consisting of moniliform multiseriate chains of compact carposporangia radiating from a basal placenta. Spermatangia and tetrasporangia were not seen. The specimens described by Meneghini and Zanardini grew on crustose corallines at a great depth. They were said to be dark purple and stipitate, up to 8 cm across, not encrusting the substratum, and divided into rounded, overlapping proliferous lobes.
Kützing (Citation1849) placed Halymenia lobata in his family Halymenieae, and Agardh (Citation1851) accordingly referred it with doubt to the Cryptonemieae. In a footnote to the genus Sarcodia, Agardh (Citation1876) stated that he had not seen Chondrymenia lobata but that, based on the appearance of the raised cystocarp and the manner in which the carposporangia radiated from a placenta, he concluded that it could not belong in the Gigartineae but should be referred instead to his ordo Spherococcoideae, tribus Gracilarieae. Ardissone (Citation1883) listed Chondrymenia as a genus of uncertain taxonomic position and extended its distribution to the region of Cagliari in Sardinia, based on collections in the Piccone herbarium. Hauck (1885) placed C. lobata in the family Spherococcaceae, providing a German translation of Zanardini's original Latin diagnosis along with engravings of three of his figures. Schmitz examined a collection of C. lobata from Dalmatia, Croatia, made by Vidovich and supplied by De Toni and Levi. Ten surviving slides from this material, including thick sections with cystocarps, are held in the Schmitz collection at the British Museum (BM000652410–19). Schmitz & Hauptfleisch (Citation1897) treated Chondrymenia as a member of the Spherococcaceae, section V, Melanthalieae, along with Sarcodia, Trematocarpus, Melanthalia and Curdiea. The habit figure in Schmitz & Hauptfleisch is a half-tone version of Zanardini's illustration. Schmitz & Hauptfleisch's description added details of the placenta, which they described as diffuse and thickly interwoven with rhizoidal filaments that were interconnected and basally fused to a central fusion cell. De Toni (Citation1903) adopted Schmitz & Hauptfleisch's classification and included the earlier references, and Preda (1908–1909) incorporated new illustrations of a cystocarpic plant. Kylin (Citation1932) transferred Melanthalia and Curdiea to the Gracilariaceae at the same time that he established the family Sarcodiaceae. Chondrymenia was not included in this treatment but was added to the Sarcodiaceae in Kylin's posthumous compendium of red algal genera (Kylin, Citation1956) in which he rephrased Schmitz & Hauptfleisch's description and incorporated their figure. Since then no further changes to the family placement of Chondrymenia have been suggested.
Chondrymenia lobata has a limited geographical distribution and has been reported only from the Adriatic Sea and from the Western Mediterranean. [The largest known collection came from Sardinia (Italy) cited by Ardissone (Citation1883).] Besides the holotype (Meneghini, Citation1841), this species has been recorded several times from the Adriatic Sea (Zanardini, Citation1860a , Citation1860b ; Ercegovic, Citation1957; Giaccone, Citation1978; Furnari et al., Citation1999). In the 20th century, Boudouresque & Huvé (Citation1969) extended the range of the species to the Mediterranean coast of France, at the Parc National de Port-Cross, where it was found growing on Lithothamnieae in deep water (60–90 m). Other Western Mediterranean records from the second half of the last century include those of Giaccone (Citation1969), Augier et al. (1971), Belsher et al. (Citation1976), Ballesteros (Citation1984), Barceló & Martí (1985), Rodríguez-Prieto et al. (1993) and Furnari et al. (Citation2003). The apparent rarity of this species undoubtedly stems from its virtual restriction to depths beyond most SCUBA access.
Details of tetrasporangial, spermatangial and gonimoblast development have been wholly or largely unreported up to now (Guiry & Guiry, Citation2013), and tetrasporophytes remain uncollected. We had the opportunity to examine recent French and Italian collections of Chondrymenia and discovered not only spermatangia but also a pattern of gonimoblast development that is unlike anything reported for the Sarcodiaceae or any of the other families with which Chondrymenia has been previously associated. We used molecular analyses to infer the placement of Chondrymenia within the broad group of gigartinalean families, including the Cystocloniaceae–Solieriaceae complex and its closest relatives.
Materials and methods
Morphological methods
Samples were obtained by Enric Ballesteros, Emma Cebrian and Conxi Rodríguez-Prieto in Corsica (France) and by Gianfranco Sartoni at Giglio Island (Italy), and supplemented by collections from Mediterranean France and Corsica by Eric Coppejans at the University of Ghent. Material was sectioned by hand or with a freezing microtome and the slide preparations stained either with 1% acidified aqueous aniline blue or with Wittmann's aceto-iron-haematoxylin-chloral hydrate (Wittmann, Citation1965) according to the method of Rodríguez-Prieto & Hommersand (Citation2009). Habit photos were taken with a Canon EOS 350D (Canon, Tokyo, Japan), and photomicrographs were made with an AxioCam MRc attached to an Axioskop 2 plus microscope (Zeiss, Oberkochen, Germany). Habit scans of historically important collections were provided by the curator of the G. Meneghini herbarium housed at the Università degli Studi di Firenze (FI). Voucher specimens have been deposited in the herbarium of the University of Girona, Spain (HGI). Herbarium abbreviations follow Thiers (2013).
Molecular methods
DNA from air-dried and silica gel-dried specimens was extracted using the DNeasy Plant Mini Kit (Qiagen, Valencia, California, USA) following the manufacturer's instructions, with DNA sequencing procedures as described in Lin et al. (Citation2001). New rbcL sequence data and those available from GenBank were compiled and aligned with Sequencher (Gene Codes, Ann Arbor, Michigan, USA). Twenty-one rbcL sequences were newly generated, and locality data for each sample analysed are provided in the Supplementary Table. Phylogenetic analyses were performed using maximum parsimony (MP) and maximum likelihood (ML) and calculations of bootstrap percentage values (BP) were conducted as described in Lin et al. (Citation2011). MP and ML analyses were performed using PAUP* v4.0 (Swofford, Citation2002) and MEGA version 5 (Tamura et al., Citation2011), with 1000 and 500 bootstrap replicates, respectively. The general time reversible model was selected for ML after 24 different nucleotide substitution models were tested in MEGA version 5. A Bayesian analysis was performed in MrBayes 3.1.2 (Ronquist & Huelsenbeck, Citation2003) using a GTR + I+ Γ model that allowed for rate variation among different codon positions. The analysis used four chains, one cold and three incrementally heated. Each run consisted of 106 generations and was sampled every 100 generations starting with a random tree. Burn-in was set at 2500 generations. A 50% consensus tree (majority rule as implemented by PAUP* v4.0) was computed from the 9975 + 1 trees saved after the burn-in point.
Results and discussion
Morphological observations
Chondrymenia lobata (Meneghini) Zanardini (Citation1860a, p. 65, pl. 6, )
(Figs 1–40)
Basionym: Halymenia lobata Meneghini (Citation1841, p. 427)
Lectotype (designated here): Specimen deposited in the Herbarium G. Meneghini at the Universita degli Studi di Firenze, Italy (FI), with a label specifying that it was collected by Hauptmann ().
Type locality: East coast, Dalmatia, Adriatic Sea.
Distribution: Western Mediterranean and Adriatic Seas.
Habitat: On encrusting Corallinales at depths of 20–70 m.
Seasonality: Sterile individuals are recorded from January to October; monoecious gametophytes with spermatangia and cystocarps were found from April to October (Ercegovic, Citation1957; this work).
Specimens examined: France: Port-Cros, Pta. du Joisseau, 13 September 1974, –22 m, legit. E. Coppejans, HEC 2254 female; La Gabinière, 15 September 1974, –43 m, legit. E. Coppejans, HEC 2272 female. Corsica: Cape La Rivellata, Calvi, 3 August 1977, –35 m, legit. E. Coppejans, HEC 3207 one female and six sterile, HEC 3206 sterile; La Girolata, Elmo, 11 August 1977, –35 m, legit. E. Coppejans, HEC 3267 female; Cape La Rivellata, Calvi, 23 August 1977, –35 m, legit. E. Coppejans, HEC 3174 female; Pta. de La Rivellata, Calvi, 03 April 1978, –43 m, legit. E. Coppejans, HEC 3692 female; La Bibliothèque, Calvi, 11 June 1979, legit. E. Coppejans, HEC 5431 sterile, HEC 3933 female; Messo Golfo, Calvi, 26 July 1979, –70 m, legit. E. Coppejans, HEC 4091 female; Bay of Elbu, 26 August 1992, –30 m, legit. C. Rodríguez-Prieto, HGI-A 2520 female, HGI-A 2521 female; Gargallu, 09 October 2006, –47 m, legit. E. Ballesteros, HGI-A 6905 female; Scandola, legit. E. Ballesteros, HGI-A 6960 sterile; Imbuttu, Scandola, 12 June 2006, –33 m, legit. E. Cebrian, HGI-A 8384 male and female; Jo Harmelin's Cave, Scandola, 12 June 2009, –20 m, 22 July 2011, legit. E. Cebrian, HGI-A 11104 sterile. Italy: Giglio I., 23 June 2006, –30 m, legit G. Sartoni, HGI-A 2617 female.
Habit
Thalli were dark red and cartilaginous, decumbent, flattened, up to 9 cm long and 16 cm wide, irregularly lobed, the lobes rounded and partially overlapping (, ). Blades were shortly stipitate, with a cylindrical and cartilaginous stipe up to 3.1 mm long by 2.1 mm in diameter and anchored by a discoid holdfast (). The thallus surface was smooth but spotted by protuberant cystocarps in mature fertile plants ().
Figs 1–9 Chondrymenia lobata. Habit and vegetative morphology (Figs 2, 4–7, 9: HGI-A 6905; Fig. 3: HGI-A 6960; Fig. 8: HGI-A 2617). Figs 5–7, 9, aniline-blue stained; Fig. 8, haematoxylin stained. 1. Lectotype of Halymenia lobata in FI. 2. Typical lobed habit of a female gametophyte. 3. Two stipes (arrows) arising from a common discoid holdfast. 4. Protuberant cystocarps scattered on both surfaces of blade. 5. Transverse section near thallus margin. 6. Longitudinal section of multiaxial margin showing elongated apical initials (arrows), primary cortical and medullary filaments, and lateral connections between files of subcortical cells (arrowheads). 7. Transverse section near apex showing lateral connections between files of subcortical cells by means of horizontal arms with pit connections (arrows). 8. An enucleate horizontal arm-bearing cell (arrow) that has linked to the cell on the left (arrowhead) by means of a conjunctor cell depositing its nucleus and leaving a pit connection (pc). Other arms and pit connections give rise to stellate-shaped cells. 9. Transverse section showing files of cortical and subcortical filaments linked by lateral arms and secondary pit connections in a stellate arrangement. Scale bars = 1 cm (Figs 2, 4), 2000 µm (Fig. 3), 100 µm (Fig. 5) and 20 µm (Figs 6–9).
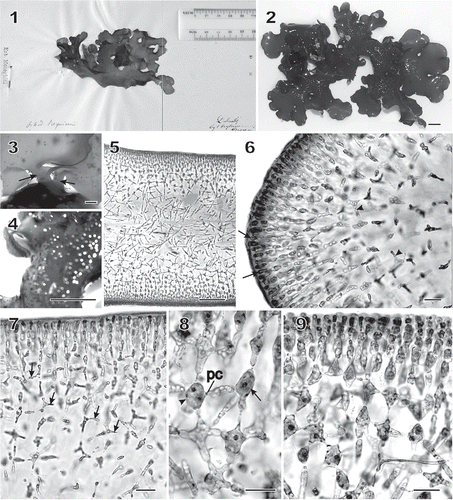
Vegetative development
The thalli were multiaxial and consisted of an outer cortex of anticlinal filaments composed of subspherical to elongate cortical cells, a subcortex of laterally linked cells, and a large central medulla composed of a few primary filaments interspersed by numerous rhizoidal filaments (). Growth took place at the margins of the lobes by anticlinal and periclinal divisions of the surface cells (). The broadly rounded apical regions quickly reached 450–550 µm in thickness (). The blade surfaces and cortex developed symmetrically on both sides. Subcortical cells cut off several lateral cells that fused apically to cells in adjacent cell rows, leaving behind numerous pit-connected horizontal arms (, ). These lateral cells behaved in the same way as the ‘conjunctor cells’ that form secondary pit connections in many Florideophyceae, although they were larger and longer than typical conjunctor cells (e.g. Coomans & Hommersand, Citation1990; Goff & Coleman, Citation1990). Such cells were readily seen in longitudinal section near the apex (, arrowheads) or throughout the breadth of the thallus in sections made further behind the apex (, arrows). Following fusion, the donor cell exhibited a short protrusion (, arrowhead) pit-connected to a long conjunctor cell that had fused with the recipient cell (, arrow). At maturity, the outermost cortical cells were 2–5 µm wide and 5–12 µm long, whereas those in the inner regions of the subcortex range were 4–6 µm wide and 8–12 µm long ().
The cortical cells were ovoid to elongate in cross section () and rounded to polygonal in surface view (), each containing a single nucleus 1–2 µm in diameter (). shows the multilayered network of arm-bearing cells that comprise the subcortex. Mature subcortical cells contained a reticulate plastid with many pyrenoid-like bodies () and an abundance of floridean starch grains (). Only one nucleus 3–4 µm in diameter remained in a subcortical cell after the nucleated process had fused with it. This nucleus was about twice the diameter of the original haploid nucleus, but we were not able to determine whether it represents the fusion of two nuclei or if one of the nuclei remains and enlarges as the other disappears.
Figs 10–20 Chondrymenia lobata. Vegetative and spermatangial morphology (Figs 10, 12, 13, 16, 17: HGI-A 6905; Figs 11, 14, 18, 19: HGI-A 2617; Fig. 15: HGI-A 8384; Fig. 20: HGI-A 3267). Figs 10, 11, 14–16, 20, haematoxylin stained; Figs 12, 13, 17–19, aniline-blue stained. 10. Transverse section of outer cortical layer showing uninucleate (n) cells. 11. Uninucleate (n) outer cortical cells in surface view. 12. Transverse section at boundary between subcortical and outer medullary layers. 13. Subcortical cell with a nucleus (n) and a reticulate plastid (pl) and containing pyrenoid-like structures (py). 14. A uninucleate (n) subcortical cell densely filled with floridean starch grains (fs). 15. Rhizoidal filament (rf) growing towards the medulla through a latticework of subcortical cells. 16. Single nuclei (n) in cells of medullary filaments. 17. A dense weft of rhizoidal filaments intermixed with primary medullary filaments. 18. Transverse section of a stipe. 19. Enlarged view of stipe cortex showing cellular arrangement. 20. Spermatangia differentiating into and releasing uninucleate (n), vacuolate (v) spermatia (s) from surface of spermatangial sorus. Scale bars = 20 µm (Figs 10–17, 20), 200 µm (Fig. 18) and 50 µm (Fig. 19).
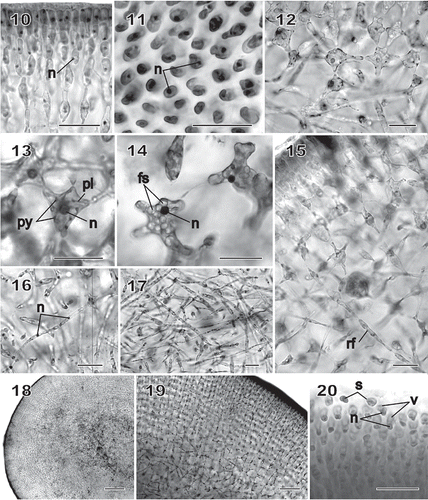
Cells of the subcortex, especially those bordering the medulla, gave rise to rhizoidal filaments near the thallus apex () composed of uninucleate cells 3–7 µm wide by 14–42 µm long (). Additional rhizoidal filaments issued progressively from inner subcortical cells as the medulla increased in thickness (), and a boundary formed between cells that make up the subcortex and the region composed mainly of entangled rhizoidal filaments that comprised the medulla (). In the stipe, the medulla () was more compact than in the blade, and the cortex, which was composed mainly of arm-bearing cells, was especially thick compared with the thickness of the blade ().
Reproductive development
Male gametes and their parent cells were found only on monoecious individuals. Spermatangia were produced from surface cortical cells in the vicinity of developing cystocarps, the surface cells dividing and maturing into spermatangia that differentiated and released their spermatia at the thallus surface. Mature, unreleased spermatia were unusual in that the nucleus sat at the base with the vacuole above it ().
Carpogonia and auxiliary cells formed procarps. The supporting cell of the carpogonial branch was a rounded subcortical cell produced at the boundary of the arm-bearing subcortical cells and the medulla (). The carpogonial branch was two-celled and in one instance it was seen directed towards the interior of the thallus. It consisted of a supporting cell filled with floridean starch bearing a small rectilinear hypogynous cell and an elongated carpogonium terminated by a short, apically inflated trichogyne (). This inwardly directed carpogonial branch appeared to be non-functional. Only post-fertilization stages were seen that showed functional carpogonial branches. In the youngest stage observed, the hypogynous cell appeared to have fused with the multinucleate supporting cell and to have borne a trichogyne laterally and directed towards the thallus surface (). shows a stage in which a haploid nucleus is present towards the tip of the trichogyne. This may be one of the two nuclei thought to enter the trichogyne after sperm attachment (Pickett-Heaps & West, Citation1998). The second nucleus had evidently effected fertilization by fusing with the nucleus of the carpogonium and had been deposited into the upper end of the fusion cell formed by fusion of the hypogynous cell with the supporting cell. A fine strand was often seen between the carpogonial remnant and the hypogynous cell, indicating that fusion had taken place between the two (, arrow). The diploid nucleus was located initially in the apical portion (hypogynous cell) of the fusion cell, while the original haploid nuclei remained in its lower portion (supporting cell) ().
Figs 21–29 Chondrymenia lobata. Female-gametangia and early post-fertilization stages (Figs 21, 22: HGI-A 2617; Fig. 23: HGI-A 8384; Figs 24, 29: HGI-A 3267; Fig. 25: HEC 3692; Figs 26–28: HGI-A 6905). Figs 21–27, 29, haematoxylin stained; Fig. 28 aniline-blue stained. 21. Early pre-fertilization configuration of the supporting cell (sc), hypogynous cell (hy) and carpogonium (cp) near boundary between subcortex and medulla. 22. Enlarged view of procarp in Fig. 21; the carpogonium (cp) is terminated by an inwardly directed trichogyne (t). 23. Early post-fertilization stage showing a trichogyne remnant (t) and fusion cell (fc) presumed to have been formed by the fusion of the diploidized hypogynous cell (hy) with the supporting cell (sc). 24. A presumed male nucleus (mn) inside the trichogyne (t) and zygote nucleus (dn) in upper part of fusion cell (fc). 25. Stage similar to that in Fig. 24 showing a remnant cytoplasmic strand (arrow) between the carpogonium (cp) and the fusion cell (fc). 26. Auxiliary cell (ac) containing a large anterior diploid nucleus (dn) and several small posterior haploid nuclei (hn). A lateral filament (lf) issues from the distal end of the auxiliary cell. 27. Initiation of lobes from an originally saccate auxiliary cell (ac). 28. Lobed auxiliary cell (ac) that appears to be linked to gametophytic filaments. 29. Early stage of gonimoblast development. The auxiliary cell (ac) has cut off a gonimoblast initial, seen as the primary gonimoblast cell (pg) that has formed a cluster of gonimoblast cells (g) and a gonimoblast filament initial (gfi). Lateral filaments (arrows) can be seen emerging from opposite sides of the auxiliary cell. Scale bars = 20 µm (Figs 22, 24–29) and 50 µm (Figs 21, 23).
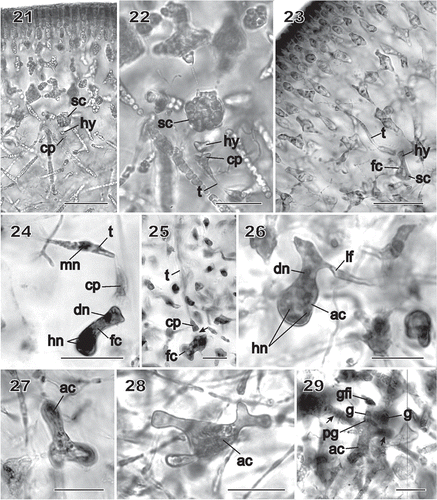
The diploidized fusion cell functioned as the auxiliary cell and was readily distinguishable because of its tendency to become lobed as it increased in size (), as well as by the large number of conspicuous haploid nuclei that were always present (). The auxiliary cell initiated a lateral filament filled with haploid nuclei that was attached by a pit connection (, lf). The upper part of the lobed auxiliary cell containing the diploid nucleus gave rise to the gonimoblast initial (seen as the primary gonimoblast cell) and the gonimoblast filaments ().
Large numbers of gametophytic subcortical and rhizoidal cells fused to each other and to the auxiliary cell in the vicinity of a fertilized procarp, leading to the formation of a large, irregularly shaped placental network below the developing pericarp (). Some of these cells broadened and became darkly staining (). Cells in the network continued fusing to produce a multinucleate placental fusion cell (). The innermost gonimoblast cells also fused at the tip of the auxiliary cell, forming a gonimoblast fusion cell (, gfc) that united with the placental fusion cell (). Gonimoblast filaments radiated and branched outwardly from the gonimoblast fusion cell in clusters (), terminated by uniseriate branched chains of uninucleate carposporangia 9–12 µm in diameter with nuclei 2–5 µm in diameter (). In addition, medullary cells belonging to the same branch lineage as the supporting cell could also fuse to form a long tubular ‘root’ that extended deep into the medulla (, arrow). Two opposite unsegmented filaments produced from the auxiliary cell grew horizontally through the inner cortex (, lf). The reticulate organization of the placental network was important in redirecting cystocarp growth towards the outside (). An enlarged view of a placental filament showed that it was filled with nuclei the size of the haploid gametophytic nuclei ().
Figs 30–35 Chondrymenia lobata. Stages of gonimoblast and associated placental gametophyte development (Figs 30, 31, 34, 35: HGI-A 6905; Fig. 32: HGI-A 6960; Fig. 33: HGI-A 8384). Figs 30, 31, aniline-blue stained; Figs 32–35, haematoxylin stained. 30. Placental network of interconnected gametophytic filaments presumed to be associated with nearby gonimoblast formation, surrounded by a thick pericarp (p). 31. Enlarged cells with dense contents within the placental network. 32. Fusion of placental filaments with the auxiliary cell to produce a placental fusion cell (pfc) from which the gonimoblast filaments (gf) arise. 33. Gonimoblast filaments (gf) radiating towards the thallus surface from a gonimoblast fusion cell (gfc) that has fused onto the placental fusion cell (pfc). 34. Elongation of the placental fusion cell (arrow) through the apparent incorporation of cell lineages that originally bore the supporting cell. See also the two opposite lateral filaments (lf) originally cut off from the auxiliary cell, and the fusion of medullary cells belonging to the same branch lineage to form a long tubular ‘root’ that extends deep into the medulla (arrow). 35. Enlarged view of chains of uninucleate (n) carposporangia (c). Scale bars = 100 µm (Fig. 30), 20 µm (Figs 31–33, 35) and 50 µm (Fig. 34).
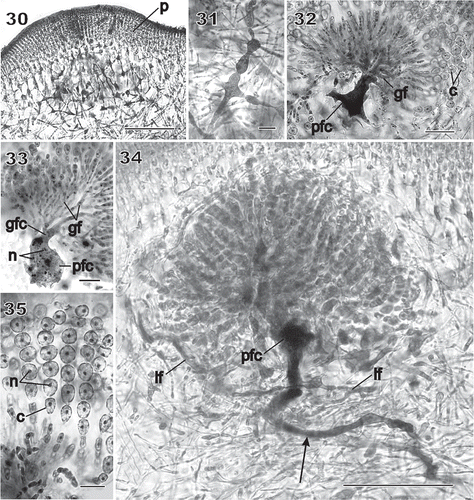
Figs 36–40 Chondrymenia lobata. Cystocarp (Fig. 36: HGI-A 2520; Figs 37–39: HGI-A 6905; Fig. 40: HGI-A 8384). Figs 36, 38–40, haematoxylin stained; Fig. 37, aniline-blue stained. 36. Immature cystocarp showing gonimoblast filaments (gf) radiating from a gonimoblast fusion cell (gfc) subtended by a placental fusion network (pfn) composed primarily of gametophytic filaments. 37. Cross-section of a mature cystocarp. The ostiolate (o) pericarp covers a chamber of carposporangial chains borne on a gonimoblast fusion cell (gfc) subtended by a placental fusion cell (pfc). 38. Apical region of a sinuous multinucleate lateral filament (lf) containing haploid nuclei (hn) spreading horizontally from the auxiliary cell through the inner cortex. 39. Anticlinal view of filaments forming the surface layers of the mature pericarp. 40. Detail of mature chains of carposporangia (c) internal to the pericarp (p). Scale bars = 100 µm (Figs 37, 38) and 20 µm (Figs 39–41).
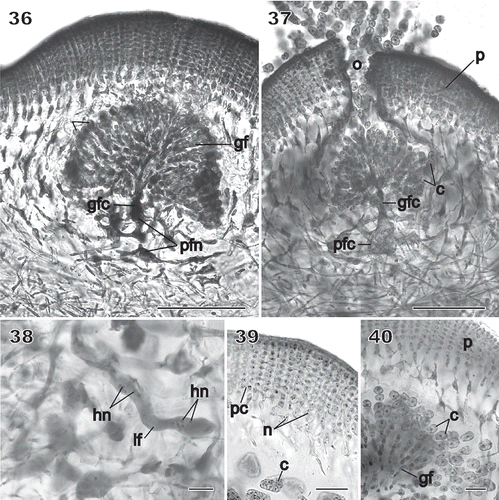
Cystocarps were sessile, slightly to prominently protuberant (), with a thick pericarp and a small central ostiole (). The pericarp consisted of a compact cortex of radiating files of 10–12 small cells, the outermost of which were ovoid, uninucleate and not connected by secondary pit connections. Inwardly, the cortex consisted of several layers of ovoid cells linked by secondary pit connections in which each cell contained two to three nuclei (), followed by a subcortex composed of several layers of multinucleate arm-bearing cells (). Mature carposporangia ranged from 60–200 µm in diameter.
Molecular studies
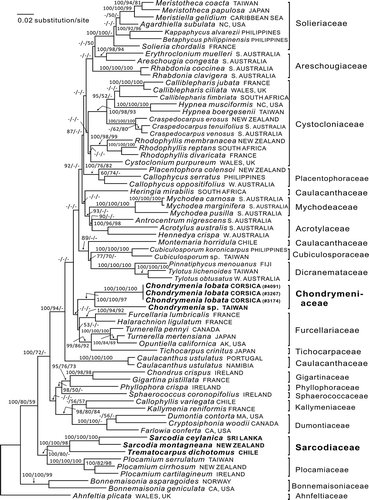
Twenty-one rbcL sequences were newly generated and analysed along with 48 sequences available from GenBank (Supplementary Table). Ahnfeltia plicata and two species of Bonnemaisonia were selected as the outgroup because the Ahnfeltiaceae is a member of the sister subclass Ahnfeltiophycidae (Saunders & Hommersand, Citation2004) and members of the Bonnemaisoniaceae sit near the base in the present limited analysis of the subfamily Rhodymeniophycidae. The rbcL data matrix included 1344 base pairs, of which 562 were parsimony-informative sites. The topologies of the ML, MP and BA trees are largely congruent and only the ML tree is shown (). The current members of the Sarcodiaceae split into two well-separated clades: one containing the genera Sarcodia and Trematocarpus that was distinct at the ordinal level from families regarded as belonging to the Gigartinales, the other containing representatives of four populations of Chondrymenia, that fell within a large gigartinalean assemblage that included the families Solieriaceae, Areschougiaceae, Cystocloniaceae, Placentophoraceae, Acrotylaceae, Mychodeaceae, Dicranemataceae, Cubiculosporaceae, Furcellariaceae, Tichocarpaceae and Caulacanthaceae (see ). Among the populations of Chondrymenia analysed was an undescribed species from Taiwan, which showed 9.6% interspecific genetic distance (129 pairwise base differences) from C. lobata in rbcL sequence analyses.
Fig. 41 RbcL phylogeny: ML tree (In L = −21877.5021) of the proposed new family Chondrymeniaceae from the Mediterranean Sea and north-western Pacific Ocean, compared with a selection of the related families. The numbers above the branches are the Bayesian posterior probabilities and the bootstrap values for the ML and the MP topology in that order. Dashes indicate less than 50% support values. The complex of families that include the Chondrymeniaceae are marked by an arrow on the left-hand side. Family names cited in the tree are from Guiry & Guiry (Citation2013), except that Callophycus is placed here in the Placentophoraceae.
Taxonomic conclusions
The traditional alliance of Chondrymenia with Sarcodia rests on the recognition by Agardh (Citation1876, footnotes on pp. 429, 430) that Chondrymenia appears to possess a protruding cystocarp with a prominent ostiole and outwardly radiating carposporangia, much like the features he had seen in Sarcodia. The association with Sarcodia was maintained by Schmitz & Hauptfleisch (Citation1897) who examined cystocarpic material collected by Vidovich, and was retained without comment by Kylin (Citation1956). None of this is surprising, in as much as the cystocarp was formed outwardly, much as in Sarcodia, and the developmental processes giving rise to the Chondrymenia cystocarp were unknown. Our initial discovery that an unfertilized carpogonial branch that was presumed to be non-functional grew inwardly was similar to that seen in many families among the Gigartinales. The evidence that functional carpogonial branches and the gonimoblasts grow outwardly, supported by a massive placenta composed of a fused network of secondary gametophytic filaments, and a placental fusion cell that has united with the gonimoblast fusion cell, is reminiscent of the similar placenta seen in some members of the Cystocloniaceae–Solieriaceae complex (as portrayed, for example, in Womersley, Citation1994). These facts, along with the unique features of carpogonial branch and cystocarp morphology, call for the establishment of a new family, Chondrymeniaceae, which is proposed formally herein.
The molecular data strongly support the removal of Chondrymenia from the Sarcodiaceae in agreement with the morphological observations. Its placement in a new family, Chondrymeniaceae, within the Cystocloniaceae–Solieriaceae complex of the order Gigartinales is less well supported, based as it is on evidence from a single gene, namely rbcL. In the limited tree presented here the Chondrymeniaceae is associated with 11 poorly understood families and two genera of uncertain placement. Additional genera and families are recognized within this group for which material was unavailable. A quick examination of the tree reveals that, whereas some individual families receive strong bootstrap support, there is no bootstrap support for the relationships among the families or for the topology of the tree. A similar assemblage is partly recognized within the Gigartinales in in Saunders et al. (Citation2004), based on a small-subunit rDNA analysis of the Gigartinales sensu stricto. Furthermore, a strongly supported terminal clade containing many of the same taxa seen in a terminal clade in our was reported within the Gigartinales by Verbruggen et al. (Citation2010, ). It is evident that, while the group as a whole that contains Chondrymenia is supported, the placing of any one family or genus within this assemblage will require new studies involving large numbers of taxa and several genes.
Chondrymeniaceae Rodríguez-Prieto, G. Sartoni, S.-M. Lin & Hommersand, fam. nov.
Description: Thallus multiaxial, growing primarily by a marginal meristem. Female reproductive development procarpic, the supporting cell cutting off a two-celled carpogonial branch from its inner or lateral face; the fertilized carpogonium evidently depositing its diploid nucleus into the hypogynous cell that fuses with the multinucleate supporting cell, thereby converting it into a generative auxiliary cell; the auxiliary cell enlarging and becoming lobed and filled with haploid nuclei at its posterior end, while cutting off opposite lateral filaments filled with haploid nuclei and a single gonimoblast initial terminally; inner gametophytic cortical and rhizoidal cells fusing with the auxiliary cell to produce a placental fusion network and central placental fusion cell; the primary gonimoblast cell producing the gonimoblast filaments; proximal cells of gonimoblast filaments fusing with the placental fusion cell and bearing branched chains of carposporangia; the carposporophyte enclosed by a protuberant, ostiolate pericarp.
Type: Chondrymenia Zanardini.
Supplementary material
Download PDF (29.8 KB)Acknowledgements
We thank Enric Ballesteros and Emma Cebrian for collecting some of the specimens examined in this study; Olivier De Clerck for the loan of the specimens of C. lobata housed at the herbarium of E. Coppejans at the University of Ghent; Egildo Luccioli and Raffaella Trabucco for providing photographs of G. Meneghini and G. Zanardini's specimens; J. Wilbraham for information and scans from the Schmitz slide collection at BM; and Paul C. Silva for comments on the nomenclature. This project was supported by two grants from the Spanish Ministry of Science and Technology (CGL2004-05556-C02-01 and CGL2008-00932); by grants from Taiwan's National Science Council (NSC 99-2621-B-019-003-MY3) and NTOU's Center of Excellence for Marine Bioenvironment and Biotechnology to S.-M. Lin; and by NSF grant DEB0937978 to J.M. Lopez Bautista, M.H. Hommersand and S. Fredericq. Special thanks go to Gerald T. Kraft, Heroen Verbruggen and two anonymous reviewers whose comments and suggestions greatly improved this paper.
References
- Agardh , J.G. 1851 . Species genera et ordines algarum. Volumen secundum: algas florideas complectens , Vol. Part 1 , Lund : C.W.K. Gleerup .
- Agardh , J.G. 1876 . Species genera et ordines algarum. Volumen tertium: de Florideis curae posteriores , Vol. Part 1 , Leipzig : C.W.K. Gleerup .
- Ardissone , F. 1883 . Phycologia mediterranea. Parte prima, Floridee . Memorie della Societa Crittogamologica Italiana , 1 : 1 – 516 .
- Augier , H. , Boudouresque , C.-F. and Laborel , J. 1971 . Végétation marine de l'Ile de Port Cros (Parc National) VII. -Les peuplements sciaphiles profonds sur substrat dur . Bulletin du Muséum d'Histoire Naturelle de Marseille , 31 : 153 – 183 .
- Ballesteros , E. 1984 . Contribució al coneixement algològic de la Mediterrània Espanyola, IV . Folia Botanica Miscelanea , 4 : 29 – 33 .
- Barceló & Martí, M.C . 1985 . Noves citacions de Chondrymenia lobata (Meneghini) Zanardini i Laminaria rodriguezii Bornet, per a la Península Ibèrica . Collectanea Botanica (Barcelona , 16 ( 1 ) : 229
- Belsher , T. , Augier , H. , Boudouresque , C.-F. and Coppejans , E. 1976 . Inventaire des algues benthiques marines de la rade et des îles d'Hyères (Méditerranée France . Travaux Scientifiques du Parc National de Port-Cros , 2 : 39 – 89 .
- Boudouresque , C.-F. and Huvé , H. 1969 . Végétation marine de l'île de Port Cros (Parc National) III. Sur la découverte de Chondrymenia lobata (Meneghini) Zanardini Rhodophycée nouvelle pour la flore Française . Bulletin du Muséum d'Histoire Naturelle de Marseille , 29 : 89 – 92 .
- Coomans , R.J. and Hommersand , M.H. 1990 . “ Vegetative growth and organization ” . In Biology of the red algae , Edited by: Cole , K.M. and Sheath , R.G. 275 – 304 . Cambridge : Cambridge University Press .
- De Toni , G.B. 1903 . Sylloge algarum omnium hucusque cognitarum. Vol. IV. Florideae. Sectio III , Padua : Sumptibus auctoris .
- Ercegovic , A. 1957 . La flore sous-marine de l'Ilot de Jabuka . Acta Adriatica , 8 ( 8 ) : 1 – 130 .
- Furnari , G. , Cormaci , M. and Serio , D. 1999 . Catalogue of the benthic marine macroalgae of the Italian coast of the Adriatic Sea . Bocconea , 12 : 1 – 214 .
- Furnari , G. , Giaccone , G. , Cormaci , M. , Alongi , G. and Serio , D. 2003 . Biodiversità marina delle coste italiane: catalogo del macrofitobenthos . Biologia Marina Mediterranea , 10 ( 1 ) : 1 – 482 .
- Giaccone , G. 1969 . Raccolte di fitobenthos sulla banchina continentale Italiana . Giornale Botanico Italiano , 103 : 485 – 514 .
- Giaccone , G. 1978 . Revisione della flora marina de Mare Adriatico . Annuario Parco Marino di Miramare , 6 ( 19 ) : 1 – 118 .
- Goff , L.J. and Coleman , A.W. 1990 . “ DNA: microspectrofluorometric studies ” . In Biology of the Red Algae , Edited by: Cole , K.M. and Sheath , R.G. 43 – 71 . Cambridge : Cambridge University Press .
- Guiry, M.D. & Guiry, G.M. (2013). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. searched on 29 October 2011. http://www.algaebase.org; (http://www.algaebase.org;)
- Hauck , F. Kryptogamen-Flora von Deutschland, Österreich und der Schweiz , Edited by: Rabenhorst , L. vol. 23 , Leipzig : Eduard Kummer . (1882–1885). Die Meeresalgen Deutschlands und Österreichs. In ed. 2 (editor), 1–575
- Kützing , F.T. 1849 . Species algarum , Leipzig : F.A. Brockhaus .
- Kylin , H. 1932 . Die Florideenordung Gigartinales . Acta Universitatis Lundensis , 28 ( 8 ) : 1 – 88 .
- Kylin , H. 1956 . Die Gattungen der Rhodophyceen , Lund : C.W.K. Gleerups .
- Lin , S.-M. , Fredericq , S. and Hommersand , M.H. 2001 . Systematics of the Delesseriaceae (Ceramiales, Rhodophyta) based on LSU rDNA and rbcL sequences, including the Phycodryoideae subfam . nov. Journal of Phycology , 37 : 881 – 899 .
- Lin , S.-M. , Yang , S.-Y. and Huisman , J.M. 2011 . Systematics of Liagora with diffuse gonimoblasts based on rbcL sequences and carposporophyte development, including the description of the new genera Neoizziella and Macrocarpus (Liagoraceae, Rhodophyta . European Journal of Phycology , 46 : 249 – 262 .
- Meneghini , G. 1841 . Memoria diretta a mostrare i rapporti di organizzazione fra le Alghe propriamente dette o Ficee, e le Alghe Terrestri o Licheni . Atti Della Terza Riunione Degli Scienziati Italiani tenuta in Firenze Nel Settembre del , 1841 : 417 – 431 .
- Pickett-Heaps , J.D. and West , J. 1998 . Time-lapse video observations on sexual plasmogamy in the red alga Bostrychia . European Journal of Phycology , 33 : 43 – 56 .
- Preda , A. Flora italica cryptogama Pars II: Algae Florideae. Vol. I. Fasc 2 , Rocca S. Casciano : Stabilimento typografico Capelli . (1908–1909).
- Rodríguez-Prieto , C. and Hommersand , M.H. 2009 . Behaviour of the nuclei in pre-and post-fertilization stages in Kallymenia (Kallymeniaceae, Rhodophyta . Phycologia , 48 : 138 – 155 .
- Rodríguez-Prieto , C. and Boudouresque , Ch. 1993 . Nouvelles observations sur les algues marines du Parc Naturel Regional de Corse . Travaux scientifiques du Parc Naturel Régional et des Réserves Naturelles de Corse , 41 : 53 – 61 . -F. & Marcot-Coqueugniot, J. (
- Ronquist , F. and Huelsenbeck , J.P. 2003 . MrBayeS 3: Bayesian phylogenetic inference under mixed models . Bioinformatics , 19 : 1572 – 1574 .
- Saunders , G.W. and Hommersand , M.H. 2004 . Assessing red algal supraordinal diversity and taxonomy in the context of contemporary systematic data . American Journal of Botany , 91 : 1494 – 1507 .
- Saunders , G.W. , Chiovitti , A. and Kraft , G.T. 2004 . Small-subunit rRNA gene sequences from representatives of selected families of the Gigartinales and Rhodymeniales (Rhodophyta). 3. Recognizing the Gigartinales sensu stricto . Canadian Journal of Botany , 82 : 43 – 74 .
- Schmitz , F. and Engler , A. 1892 . (6. Klasse Rhodophyceae). 2. Unterklasse Florideae . Syllabus der Vorlesungen über specielle und medicinisch-pharmaceutische Botanik. Grosse Ausgabe , : 16 – 23 . Borntraeger, Berlin.
- Schmitz , F. and Hauptfleisch , P. 1897 . Die natürlichen Pflanzenfamilien nebst ihren Gattungen und wichtigeren Arten insbesondere den Nutzpflanzen unter Mitwirkung zahlreicher hervorragender Fachgelehrte , Edited by: Engler , A. and Prantl , K. 382 – 396 . Leipzig : Wilhelm Engelmann . ). Sphaerococcaceae. vol. 1, part 2. 2. (editors)
- Swofford , D.L. 2002 . PAUP* Phylogenetic analyses using parsimony (*and other methods) Version 4b , MA : Sinauer Associates, Sunderland .
- Tamura , K. , Peterson , D. , Peterson , N. , Stecher , G. , Nei , M. and Kumar , S. 2011 . MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods . Molecular Biology and Evolution , 28 : 2731 – 2739 .
- Thiers, B. (2013, continuously updated). Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium. http://sweetgum.nybg.org/ih/ (http://sweetgum.nybg.org/ih/)
- Verbruggen , H. , Maggs , C.A. , Saunders , G.W. , Le Gall , L. , Yoon , H.S. and De Clerck , O. 2010 . Data mining approach identifies research priorities and data requirements for resolving the red algal tree of life . BMC Evolutionary Biology , 10 : 16
- Wittmann , W. 1965 . Aceto-iron-haematoxylin-chloral hydrate for chromosome staining . Stain Technology , 40 : 161 – 164 .
- Womersley, H.B.S. (1994). The marine benthic flora of southern Australia. Part IIIA. Bangiophyceae and Florideophyceae (Acrochaetiales, Nemaliales, Gelidiales, Hildenbrandiales and Gigartinales sensu lato). Australian Biological Resources Study, Canberra.
- Zanardini , G. 1860a . Scelta di ficee nuove o piu rare del mare Adriatico . Memorie dell' i reale Istituto Veneto di Scienze, Lettere ed Arti , 9 ( 1 ) : 41 – 78 .
- Zanardini , G. 1860b . Iconographia phycologica adriatica , Privately published : Venice . Vol. 1
Supplementary information
The following supplementary material is available for this article, accessible via the Supplementary Content tab on the article's online page at http://dx.doi.org/10.1080/09670262.2013.789931
Supplementary Table. List of species used in the rbcL analysis with GenBank numbers.