Abstract
Stephanodiscus triporus was described in 1978, based on a study from the phytoplankton of Volgograd Reservoir that used transmission electron microscopy. This species is small, diameter 3.7–10.6 µm, with 14–30 striae and 30–60 areolae in 10 µm, and differs from other Stephanodiscus species by the presence of three satellite pores in the single central fultoportula. Later a new species similar in morphology to S. triporus, was described from Iowa, USA, namely S. vestibulis. A large population of S. vestibulis was found in Lake Balaton and the species also occurs in different Hungarian and French waters. Detailed comparison of S. triporus and S. vestibulis, based on our results and literature, showed they are very similar. Therefore we reinvestigated the type material of both and compared them with the Hungarian and French specimens. Conventional and geometric morphometric analyses were carried out, also including comparisons with the morphologically closest taxon, S. minutulus. There is a continuum of variation between S. triporus and S. vestibulis (in diameter, the number and morphology of the striae, the position of the valve face fultoportula with three satellite pores, the presence of a vestibulum having more or less the same shape). Hence we suggest that they are conspecific, with S. vestibulis a later synonym of S. triporus.
Introduction
Centric diatoms are one of the most important components of phytoplankton communities in large rivers and their reservoirs, in which the biomass of planktonic diatoms can reach 90% of the total phytoplankton (e.g. Kožova et al., 1982; Kiss & Nausch, Citation1988; Shcherbak et al., Citation1992; Ohapkin et al., Citation1997; further relevant references are given in the Supplementary information). Among freshwater centric diatoms, researchers have paid great attention in recent years to the genus Stephanodiscus and many small-celled new species have been described that are hardly determinable with light microscopy (LM), e.g. S. makarovae (Genkal, Citation1978), S. perforatus (Genkal & Kuzmin, Citation1978), S. parvus (Stoermer & Håkansson, Citation1984), S. delicatus (Genkal, Citation1985, Citation2004), S. binatus (Håkansson & Kling, Citation1990), S. inconspicuus (Makarova & Pomazkina, Citation1992), S. hankensis (Genkal & Ščur, Citation2000), and S. chantaicus (Genkal & Kuzmina, Citation1992). The systematic position of some of these species has been clarified in further studies and some have been reduced to synonymy (Kobayasi et al., Citation1985; Genkal & Korneva, Citation1990; Genkal, Citation2004, Citation2007, Citation2010).
Stephanodiscus triporus was described by Genkal & Kuzmin (Citation1978), based on a study of phytoplankton in the Volgograd Reservoir using transmission electron microscopy (TEM). It had small cells, 3.7–10.6 µm in diameter, 14–30 radial striae in 10 µm and 30–60 areolae in 10 µm. The areolae were arranged irregularly in the centre, but formed uniseriate striae on the valve face and biseriate striae close to the valve margin, and the interstriae ended in spines. There was a ring of marginal fultoportulae and a single rimoportula between them. Stephanodiscus triporus was thought to differ from other species of the genus by the presence of three satellite pores in the single central fultoportula. The species occurred not only in the Volgograd Reservoir but also in other Volga reservoirs, in the River Volga itself, in Lake Sevan (Armenia) and Lake Pertozero (Karelia).
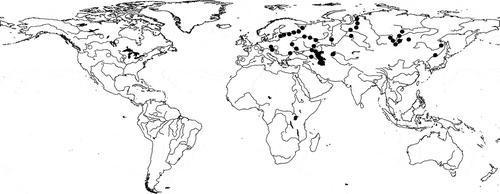
Later, a new species resembling S. triporus, Stephanodiscus vestibulis, was described from the ‘Lazy Lagoon’ (Iowa, USA) by Håkansson et al., Citation1986. According to the original description, S. vestibulis had valves 4–11 µm in diameter, with 12–14 striae in 10 µm. The central area was said to be convex or concave and the areolae had domed cribra. The puncta of the central area were disorderly or formed striae, and were arranged in fascicles of 2 to 4 striae towards the valve face–mantle junction, near which there was a ring of spines. As in S. triporus, there was one valve face fultoportula in the central area with three satellite pores. Several marginal fultoportulae were located on the mantle, one located beneath every third or fourth spine. The external opening of each marginal fultoportula was partly surrounded by an arch, the structure appearing porch-like, resembling a ‘vestibule’. One rimoportula was present, located a little above the ring of spines, with an external tube. Internally the rimoportula had the lip-like part inserted on an interfascicle near the junction of the valve face and mantle. Stephanodiscus vestibulis was later recorded in lakes and rivers in the USA, Canada, Japan, Korea, Nepal, Brazil, Switzerland, Spain and France (Håkansson & Kling, Citation1989; Yang et al., Citation1993; Genkal & Popovskaya, Citation1997; Risberg et al., Citation1999; Tuji & Houki, Citation2001; Laslandes, Citation2007; Naya et al., Citation2007; Hayashi, Citation2011) (; see also Supplementary information, references Sr 1–5).
Finally, a new variety of S. triporus, var. volgensis, was described by TEM and SEM from the phytoplankton of the Rybinsk Reservoir on the River Volga (Genkal & Korneva, Citation1990). This was said to differ from the type variety in its smaller size (diameter 3.7–8.7 µm, compared with 5.8–12.1 µm for var. triporus: Genkal & Korneva, Citation1990) and the presence of a large areola at the centre. Genkal & Korneva also clarified that var. triporus had 14–20 striae in 10 µm, one or sometimes two valve face fultoportulae, and usually three, but sometimes two or four satellite pores. Later, however, SEM studies clarified that the ‘large areola’ of S. triporus var. volgensis is the impression made by the valve face fultoportula of the sibling cell and that it exists on S. triporus var. triporus as well. Genkal (Citation2013) therefore returned S. triporus var. volgensis to synonymy with the type variety. Both varieties had frequently been found together (Genkal & Kuzmin, Citation1978; Genkal & Korneva, Citation1990; Genkal, Citation1992; Genkal et al., Citation2006; Pautova et al., Citation2009). Stephanodiscus triporus has now been recorded from many rivers, mainly in the territory of the former Soviet Union, including E Europe, Ukraine, the Baltic States, European Russia and Karelia, the Caucasus, Siberia and the Russian Far East (Genkal & Levadnaja, Citation1980; Genkal & Kuzmina, Citation1984; Genkal & Naumenko, Citation1985; Kiss & Genkal, Citation1993; Marvan et al., Citation2004; Genkal & Dmitriyeva, Citation2005; Genkal et al., Citation2009). Outside Eurasia, it has been recorded in Lake Erie in the USA (Genkal & Kuzmin, Citation1978) (for other references see Supplementary information, references Sr 7–10, 13).
However, having analysed the micrographs found in literature and our own samples taken from several water bodies in Europe, we discovered more and more similarities between S. triporus and S. vestibulis. The aim of this study was therefore to determine whether these two species can be separated morphologically, via conventional and geometric morphometric analyses.
Materials and methods
The type materials of S. triporus and S. vestibulis were re-examined, together with our own samples from various waters (Supplementary information, Table S1). The type material of S. triporus, from the Volgograd Reservoir, is held by the Institute of Biology of Inland Waters of the Russian Academy of Sciences, Borok. Isotype slides (No. 134 and 135) and an SEM stub (H. Håkansson #352) of S. vestibulis were obtained from the diatom collection of the Botanischer Garten und Botanisches Museum Dahlem, Freie Universität Berlin.
For detailed morphometric analyses, subsurface phytoplankton samples were taken from Lake Balaton in June 2010, at Siófok. Stephanodiscus vestibulis/triporus occurred in several other Hungarian water bodies as well (Table S1), but only a few cells were found. To study the Russian and Estonian populations of the diatom identified as S. triporus, many phytoplankton samples were taken from rivers, reservoirs and lakes in the territory of former Soviet Union (Table S1).
Samples from Russian and Estonian waters were treated according to Balonov (Citation1975) as follows: 0.5 to 1 ml of a solution of potassium dichromate in hot sulphuric acid (1 g K2Cr2O7 dissolved in100 ml H2SO4) was added to the concentrated sample and boiled for 2–3 min, then washed with distilled water and centrifuged for decantation. Washing was repeated three or four times. Hungarian and French samples were cleaned with hydrochloric acid and hydrogen peroxide, and subsequently washed three times with distilled water. For SEM, portions of samples were filtered through a 3-µm mesh polycarbonate membrane, which were then fixed on SEM stubs with carbon discs and coated with gold–palladium (105 s, 18 mA). Stubs were examined using a Hitachi S-2600N scanning electron microscope (Hitachi, Tokyo, Japan), for Hungarian populations and type materials, or a JSM-25S (JEOL, Tokyo, Japan) for Russian and Estonian populations. For SEM analysis of French populations, stubs were sputtered with gold (40 nm) using a Modular High Vacuum Coating System (BAL–TEC MED 020, Balzers, Liechtenstein) and studied with a Leica Stereoscan 430i (Leica, Cambridge, UK).
For LM analyses, diatoms were mounted in Naphrax® mounting medium (Brunel Microscopes, Chippenham, UK) and observed with an Olympus IX70 inverted light microscope (Olympus, Tokyo, Japan) equipped with differential interference contrast (DIC) optics at 1500× magnification. LM photos were obtained using an ARTCAM-500MI digital camera (ARTRAY, ESRI Hungary, Budapest). Micrographs of French populations were taken using a Leica DMRX light microscope with a Leica DC500 camera.
Structural elements of the valves were measured and analysed according to the methods of Genkal (Citation1977, Citation1984), taking into account Theriot (Citation1987). Terminology followed Anonymous (Citation1975), Ross et al. (Citation1979) and Theriot & Serieyssol (Citation1994).
Conventional morphometrics
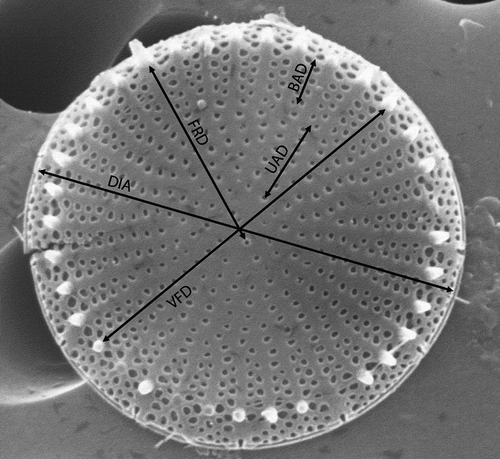
The following morphological characters (variables) of the frustule were measured on SEM micrographs (): valve diameter (DIA), valve face diameter (VFD), number of fasciculi (FAS), number of marginal fultoportulae (MFN), distance over which the striae are uniseriate (UAD), uniseriate areolae number (UAN), distance over which the striae are biseriate (BAD), biseriate areola number (BAN), number of areolae in a stria at the margin (NAS), distance between valve face fultoportula and rimoportula (FRD), width of the labia (lips) of the rimoportula (WLR). Uniseriate and biseriate areola distance and number were calculated as follows: we measured the length of the shortest and the longest uniseriate and biseriate areolae in every specimen, and also counted the number of areolae of each type, and their means were used as UAN, UAD, BAN and BAD respectively. Valve face diameter was measured between two opposite spines. Most of these morphological parameters selected for the conventional morphometric analysis were suggested by Tapia et al. (Citation2004).
Fig. 1. Conventional morphometric characters of Stephanodiscus triporus (shown on external valve face). See text for explanation.
Principal component analysis of the conventional morphometric characteristics was carried out using PAST, version 1.78 (Hammer et al., Citation2001). Data were standardized. To compare the characters of S. vestibulis and S. triporus with the morphologically closest taxon (S. minutulus), we measured 50 specimens of a Hungarian population of S. minutulus (collected in 2009 from Lake Széki, near Devecser, at 47° 7.843′ N, 17° 28.644′ E). We also tested populations of type material of S. vestibulis and S. triporus (t- or Welch-test) to look for statistical equality in each morphometric character (the Welch test was applied when the estimated variances were not equal).
For the above analysis we measured 50 specimens from Lake Balaton, 9 from French populations, 15 from type material of S. vestibulis, 50 from type material of S. triporus and 50 specimens of S. minutulus from Lake Széki (Table S1).
Geometric morphometrics
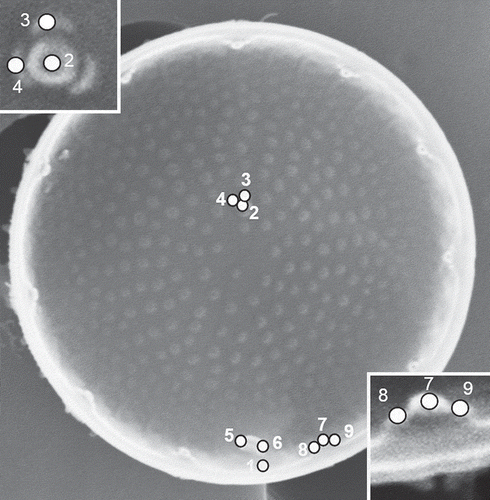
To analyse the characters of the valve interior, nine landmarks were placed as shown in and digitized using tpsDig2 software (Rohlf & Slice, Citation1990; Rohlf, Citation2004). The nine selected landmarks were as follows: (1) the edge of valve opposite the right-hand extremity of the rimoportula (RP); (2) the centre of the valve face fultoportula; (3) the satellite pore of the valve face fultoportula furthest from the rimoportula; (4) the satellite pore of the valve face fultoportula to the left of landmark 3; (5) the end of the slit of the RP closest to the valve centre; (6) the opposite (right-hand) end of the slit of the RP; (7) the top of the marginal fultoportula (MFP) lying immediately to the right of the RP; (8) the top of the left-hand satellite pore of the MFP lying immediately to the right of the RP; and (9) the top of the right satellite pore of the MFP lying immediately to the right of the RP. The Cartesian coordinates of the cells were aligned (translated, rotated and scaled) by the Procrustes generalized orthogonal least-squared superimposition procedure (Generalized Procrustes Analysis: Rohlf & Slice, Citation1990). To determine whether the amount of variation in shape of our geometric morphometric dataset was small enough to permit statistical analysis, the slope of the regression line of tangent space distances against Procrustes distances and their uncentred correlation coefficient were calculated using tpsSmall (Rohlf, Citation2003). The slope of the regression line was 0.98806, the correlation coefficient 0.99996, and inspection of the scatter of points revealed no large deviations of single points. These were accepted as indications of a good fit. Allometry was also investigated by a multivariate regression of shape variables onto diameter. These analyses were performed using TpsRegr 1.31 (Rohlf, Citation2005).
Fig. 2. Landmarks for geometric morphometrics, shown on the interior of the valve face of Stephanodiscus triporus. See text for explanation.
Canonical Variates Analysis (CVA) was carried out to distinguish individual groups using tangent space Procrustes coordinates. The Mann–Whitney U test was used to test whether the medians of S. vestibulis and S. triporus type materials are different. For this test we used the Euclidean distances of landmarks coordinates from the origin. The CVA and Mann–Whitney test were performed in PAST ver.1.74. To compare the selected characters of S. vestibulis and S. triporus to the most similar species (S. minutulus), we measured 50 specimens of S. vestibulis/triporus from Lake Balaton, 4 from French populations, 8 from type material of S. vestibulis, 40 from type material of S. triporus and 15 specimens of S. minutulus from Lake Széki. To analyse shape differences, the relative warp scores were used as an ordinary multivariate dataset.
The distribution of species in Hungary and the world was plotted using the ESRI ArcInfo 9.3 GIS program (ESRI Hungary, Budapest).
Results
Reinvestigation of type material of Stephanodiscus triporus
The type material of S. triporus was relatively rich in valves, so that it was possible to obtain 32 SEM micrographs of outside views and 16 inside views. In LM, valves usually appeared almost flat and radially striated; however, features such as slight elevations or depressions of the valve face, the valve face fultoportula, or the elongated tube of rimoportula could scarcely be distinguished (). The range of diameter was 6.8–9.9 µm ().
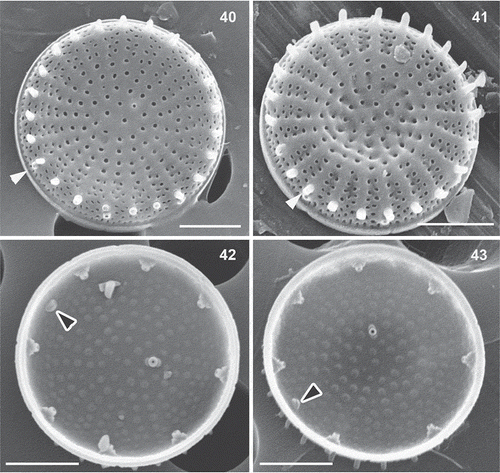
Figs 3–16. Type material of Stephanodiscus triporus () and S. vestibulis (), LM and SEM. Black arrowhead: external tube of rimoportula; white arrowhead: valve face fultoportula; black arrow: valve face fultoportula; white arrow: the inverse place of valve face fultoportula. Scale bars = 2 µm.
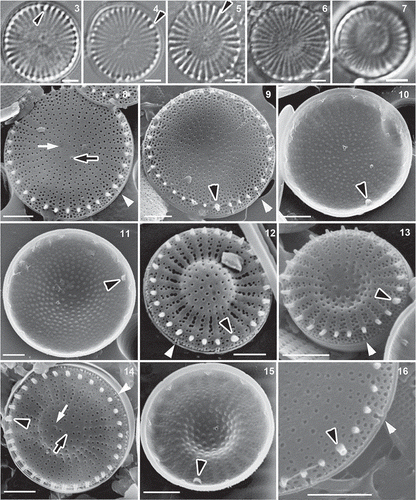
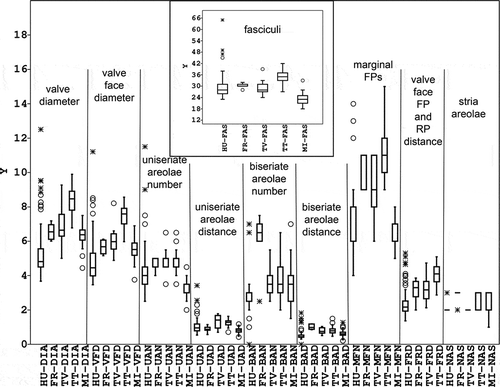
Fig. 17. Box-plots of measured conventional parameters. HU: Hungarian data, FR: French data, TV: type material of Stephanodiscus vestibulis, TT: type material of S. triporus, MI: S. minutulus. For other abbreviations, see the text. The minimal and maximal values, median, 25–75 percent quartiles, and outliers are shown.
In SEM studies, most valves were flat (), but some were slightly convex or concave (, ). The number of radial fasciculi was 27–42 (with 10–15 radial striae in 10 µm). The interstriae ended in short spines but sometimes these were very small and in other cases several were broken, so that they were easily overlooked. The areolae were usually round on valve face, but could be elongated or irregular close to the margin (). They were located irregularly in the valve centre and covered internally with domed cribra (). The single rows of areolae present towards the centre (containing 3.5–6.5 areolae: TT-UAN in ) became double (containing 2–6.5 areolae: TT-BAN in ) at the edge of valve face.
Table 1. The morphological variability of the valve face of Stephanodiscus triporus and S. vestibulis from SEM micrographs either from our study (where no source is given) or from literature.
In external views, the pore of a single valve face fultoportula could be seen near the centre (), although it was sometimes difficult to distinguish it from the areolae. The valve face fultoportula had three satellite pores internally () and there were marginal fultoportulae (internally with three satellite pores) on every second to fourth interstriae, below a spine (); the number of marginal fultoportulae was 9–15 (). Usually the external opening of marginal fultoportulae was partially surrounded by an arched structure (like a narrow vestibule: ). Sometimes the vestibule was not fully developed completely or was wide. A single rimoportula was present, situated above the ring of marginal fultoportulae (). It had a relatively long, large tube externally in the ring of spines () and small labia internally, which were oriented more or less perpendicular to nearby interstriae () or sometimes at other angles (supplementary SEM micrographs of all investigated materials are available from the first author on request).
Reinvestigation of type material of Stephanodiscus vestibulis
The type material of Stephanodiscus vestibulis contained few valves and only six SEM micrographs were taken of the exterior and five of the interior. Because of the sparseness of the type material, for further analysis the published micrographs by Håkansson were also measured.
In LM, the valves appeared radially striated, the elevation or depression of valve face was usually pronounced, but the elongated tube of rimoportula could rarely be seen. Valves were usually convex/concave (). Only one valve was found in which the elevation was not pronounced, only slight. Valve diameters were 5–9.3 µm ().
In the type material 10 strongly convex or concave valves () and one slightly convex valve were found with SEM (). The number of fasciculi was 24–39 (with 11–15 radial striae in 10 µm: ). The interstriae ended in short spines. The areolae were usually round on the valve face, but could be elongated or irregular in shape close to the margin. They were arranged irregularly in the valve centre and were covered internally with domed cribra (). The single rows of areolae towards the centre of the valve face (UAN: 3.5–6.5) became double (BAN: 2.5–5.5) at the edge of valve face (). As in S. triporus, the pore of a single valve face fultoportula was visible near the centre () but was sometimes difficult to distinguish from areolae (). It had three satellite pores internally (). There were marginal fultoportulae (internally with three satellite pores: ) on every second to third interstriae, below a spine (); the number of marginal fultoportulae was 6–11. The external opening of marginal fultoportulae was usually surrounded partially by an arched structure (like a narrow vestibule: ). The single rimoportula was situated in the ring of marginal fultoportulae and had a relatively long and large tube externally in the ring of spines () and small labia internally, oriented more or less perpendicular to the interstriae ().
Morphological characterization of Stephanodiscus triporus/vestibulis based on new investigations
Recent materials were investigated and morphologically analysed from Lake Balaton (8 LM and 107 SEM micrographs), six lakes or reservoirs and a river elsewhere in Hungary (27 SEM micrographs), and two rivers in France (7 LM and 24 SEM micrographs).
In LM, the valves were radially striated, with an elevated or depressed or flat valve face. The elongated tube of the rimoportula could be seen in some Hungarian (from Lake Balaton) () and French populations ().
Figs 18–33. Populations from Lake Balaton () and France (), LM and SEM. Black arrowheads: rimoportula, white arrowhead: vestibule; black arrows: valve face fultoportula; white arrows: the pit corresponding to the position occupied by the valve face fultoportula of the sibling valve. Scale bars = 2 µm () and1 µm ().
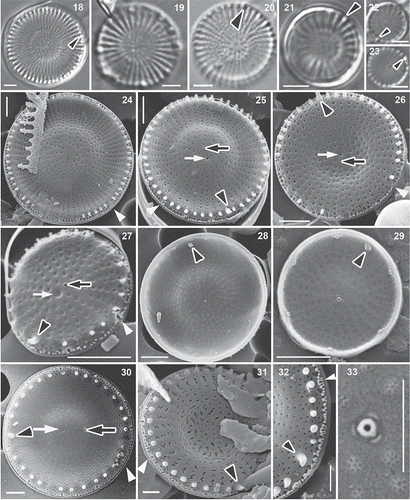
SEM confirmed that the valve face was usually convex or concave (), but also showed the presence of many valves with flat () or slightly convex or concave valve faces in Hungarian () and French populations. In the material from the Lake Balaton, 47% of specimens were flat, 18% were strongly convex or concave, and 35% were slightly convex/concave; in the French population 5% of specimens were flat, 80% were strongly convex or concave and 15% were slightly convex or concave (). In Russian material (including the type locality) valves were almost always strongly or slightly convex or concave, only a single flat valve being found ().
Valve diameters varied between 3.7 and 12.5 µm (). The number of fasciculi was 23–65 (with 11–21 radial striae in 10 µm). The interstriae ended in short spines. The areolae were usually round on the valve face (), but could be elongated or irregular close to the margin, or in some cases were zig-zag slits (). They were arranged irregularly in the valve centre and had domed () or flat () cribra internally. In heavily silicified valves the areolae were not or only partly discernible, or formed irregular striae (). The single rows of areolae (UAN: 2.5–11.5) became double (BAN: 0–7.5) at the edge of valve face. Externally, the pore of a single valve face fultoportula was visible near the centre (), but was sometimes difficult to distinguish among the areolae (). Internally, the valve face fultoportula had three satellite pores (), as did the marginal fultoportulae, which occurred on every second to third (rarely fourth or fifth) interstria below a spine; the number of marginal fultoportulae was 4–14. The external openings of the marginal fultoportulae were usually partially surrounded by an arched structure (like a narrow vestibule: ). The single rimoportula was situated above the ring of marginal fultoportulae and had a relatively long and large tube externally, lying in the ring of spines (); internally the rimoportula had small labia, oriented more or less parallel to the interstriae () or sometimes more or less perpendicularly in case of Lake Balaton specimens. In specimens from other Hungarian waters and in French specimens, the labia were oriented more or less perpendicularly.
Conventional and geometric morphometric analysis
To compare conventional characters in Stephanodiscus triporus and S. vestibulis type materials, we used t- or Welch tests. The diameters differed in the two populations and also those characters related to diameter, such as valve face diameter and the distance of the central fultoportula from the rimoportula, but other characters did not differ (). Multivariate regression of shape variables on diameter gave significant results (Wilks’ λ = 0.123; P = 5.591 × 10–34) indicating allometry.
Table 2. Results of t- or Welch and Mann–Whitney tests in comparisons of populations of Stephanodiscus triporus with those of S. vestibulis. The level of significance assumed for the ‘Differ?’ column was P ≤ 0.05.
The results of the Principal Component Analysis of conventional morphometric characters clearly demonstrated an overlap between the type materials of S. vestibulis and S. triporus along the first and second PC axes (). The French and Hungarian (Lake Balaton) populations partly overlapped with them. Stephanodiscus minutulus partly overlapped with the Hungarian population of S. vestibulis/triporus and segregated from the others. The variance explained by the first axis (λ1) was 54.4%, while the second (λ2) and third axes (λ3) explained 20.0% and 13.1%. PCA revealed that the first three factors explain almost all the variability (87.5 %) in the correlation matrix.
Fig. 34. Plot of principal component scores 1 and 2, based on the conventional morphometric dataset. Group outliers are connected by lines. Inverse triangles: population from Lake Balaton; triangles: French population; diamonds: type material of Stephanodiscus vestibulis; points: type material of S. triporus; squares: S. minutulus.
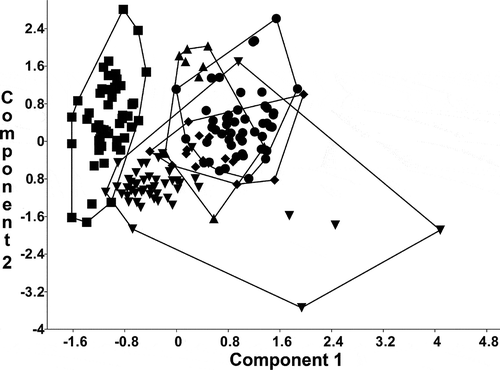
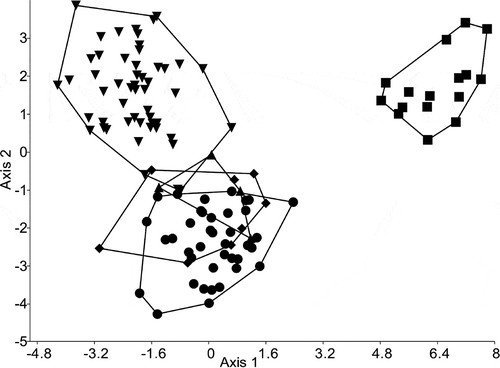
shows the results of a Canonical Variates Analysis, based on the geometric morphometric dataset. This clearly segregated S. minutulus from all other groups (Wilks’ λ = 0.01647, P << 0.0001). Stephanodiscus vestibulis and S. triporus type material and French population overlapped considerably, while only a small degree of overlap was observed with the Hungarian population. The first two canonical axes explained 64.7 % and 31.7 % of the variation, respectively. For all landmarks in the two type materials, Mann–Whitney tests were not significant, so the difference in their medians is small ().
Fig. 35. Plot of a Canonical Variates Analysis based on the geometric morphometric dataset. Group outliers are connected by lines. Inverse triangles: population from Lake Balaton; triangles: French population; diamonds: type material of Stephanodiscus vestibulis; points: type material of S. triporus; squares: S. minutulus.
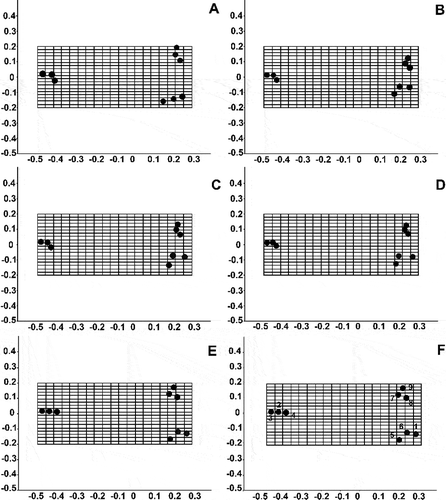
The specimens from the type materials of S. vestibulis and S. triporus and the French population presented similar relative warp grids (–D), while the population of Lake Balaton () and S. minutulus () differed from them. The Balaton population differed from the others in the angle of the interstria to the labia of the rimoportula. The population of S. minutulus differed in the shape of the marginal fultoportulae (S. minutulus has longer marginal fultoportulae) and the positions of the satellite pores of the valve face fultoportula (the angle is 180° in S. minutulus, but 120° in the others).
Fig. 36. Grids of relative warps and the average shape of populations from Lake Balaton (A) and France (B), the Stephanodiscus vestibulis type material (C), the S. triporus type material (D) and S. minutulus (E). F: landmarks 1 to 9 (see ).
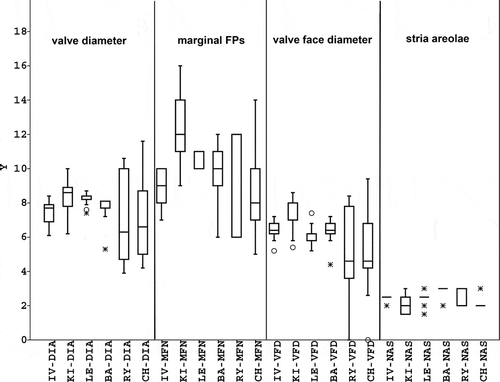
Because the type material of S. triporus comprises a population from only a single site and time, we investigated the real variability of this species by studying populations found in the area of former Soviet Union (Lake Leegu in Estonia; Kiev Reservoir, Ukraine; Ivankovo Reservoir, Lake Baikal, Rybinsk reservoir, and Cheboksary reservoir in Russia). Valves were 4–12 µm in diameter, the number of fasciculi was 25–48, the number of marginal fultoportulae was 5–16, and (1–) 2–3 (–4) areolae could be found at the ends of the striae ().
Fig. 37. Box-plots of measured conventional parameters in the area of former Soviet Union. IV: Ivankovo reservoir; KI: Kiev’s reservoir; LE: Lake Leegu; Ba: Lake Baikal; RY: Rybinsk reservoir; CH: Cheboksary reservoir. For other abbreviations, see the text. The minimal and maximal values, median, the 25–75 percent quartiles and outliers are shown.
Distribution of S. triporus and S. vestibulis
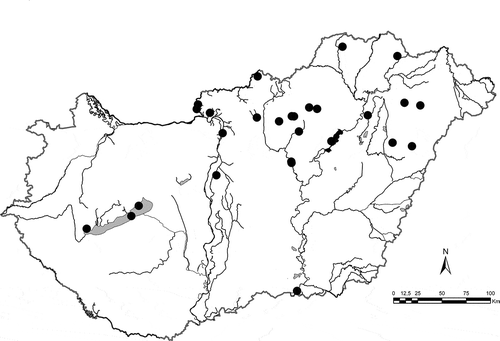
In a four-year long project (2008–2011) several Hungarian waters were studied in the eastern part of Hungary, and we also studied Lake Balaton. During these studies we frequently found S. vestibulis/triporus, in both lakes and rivers (). Previously there had been only one Hungarian record (in the River Danube), although the phytoplankton of Lake Balaton has been regularly studied since the 1960s (but only with LM). Our first thought was therefore that the species is a recent invader, but we reinvestigated (with SEM) a phytoplankton sample from Lake Balaton collected in 1997 and found the species in it. The literature shows that it is widespread in lakes, reservoirs, rivers and even the sea (close to a delta; ).
Discussion
Selection of morphological parameters
Most of the morphological parameters used here were chosen on the basis of suggestions by Tapia et al. (Citation2004). Some other characters have been considered (e.g. Theriot, Citation1987) as being important for delimiting particular Stephanodiscus species, like the widths of spines at their bases, the numbers of spines, or the horizontal width of the small rim encircling the valve. The number of spines usually has no taxonomical importance in Stephanodiscus (in S. triporus also) and spines sometimes break off leaving almost no trace of their presence, making it difficult to count them exactly (as noted also by Tapia et al., Citation2004). Furthermore, spines are usually situated at the end of each interstria, so that the number of spines is usually equal to that of the ‘costae’ and rows of striae. Spine width at the base varies rather little and it is practically impossible to measure it correctly. We did try measuring the basal widths of rimoprotulae, but the variability was smaller than the confidence of measurement, so we did not use it in the analysis. Measuring the horizontal width of the small rim encircling the valve would be important if we wished to investigate the degree of valve silicification, but this was not our goal and the degree of valve silicification is not a taxonomically useful character. Furthermore, it would have been practically impossible to measure it accurately in most of our valves.
Variability of morphological parameters
The diameter of the valves (6.8–9.9 µm) and the density of the striae (10–15 in 10 µm) in the type material of S. triporus were much less variable than in the original description (3.7–10.6 µm and 14–30 in 10 µm: Genkal & Kuzmin, Citation1978). This is because Genkal & Kuzmin constructed their description on the basis of samples taken from several different localities; the type material was less variable because it was taken from a single sampling site (Volvograd reservoir).
Notwithstanding, the typical characters – such as the well-developed external tube of the rimoportula, the vestibulum, the typically swollen wide labia, and the three satellite pores of the valve face fultoportula – could be found on almost all specimens in type material. Most cells were flat, a few were slightly convex/concave, but no strongly convex/concave valves were found. Stephanodiscus triporus was originally described as having a flat valve surface (Genkal & Kuzmin, Citation1978). This could be expected as the description was based on TEM observations, where the convex or concave nature of the valve surface can hardly be detected. We should note that two strongly convex, one slightly convex and one flat valve can be revealed by the detailed investigation of the original TEM pictures. In the following publications from Russia both strongly and slightly convex/concave valves are seen together with flat ones (Genkal & Makarova Citation1985; Genkal & Korneva, Citation1990; Genkal, Citation1992; Genkal & Kozyrenko, Citation1992; Genkal & Popovskaja, Citation1997, Citation2003; Genkal & Yarušina, Citation2002; Genkal & Trifonova, Citation2006; Majstrova et al., Citation2007; Genkal & Terenko, Citation2009; Genkal et al., Citation2011; Sr 11). Analysing in detail the ‘whole’ population from a lake or river and taking many SEM micrographs the ‘whole’ range of morphological variability of a given species can be found. The population from Lake Balaton showed the highest variability, where all types of valve surface was found. In our opinion, these populations demonstrate well that the valve face of this species can be characterized by continuity from strongly convex/concave to completely flat.
The diameter of cells in the presently investigated type material of Stephanodiscus vestibulis was smaller (5–9.3 µm), while the stria number was more variable (11–15 in 10 µm) than in the original description (4–11 µm and 12–14 in 10 µm: Håkansson et al., Citation1986). The specimens investigated by SEM were strongly convex/concave, albeit some slightly convex/concave cells were found during the LM observations; flat specimens were not found. The low number of S. vestibulis specimens on the stub of original material may be responsible for the low morphological variability. However, the characteristic features could be found on each specimen, like the well-developed tube of the rimoportula (this could be seen on a few specimens in LM as well), the vestibulum, the typically swollen, wide labium, and the three satellite pores of the valve face fultoportula. In the original description of S. vestibulis, and on the SEM figures only strongly convex/concave valves appear (Håkansson et al., Citation1986). The various morphological descriptions and SEM micrographs from the different parts of the world characterize S. vestibulis uniformly as a species with a strongly convex/concave valve (Håkansson & Kling, Citation1989; Gotoh et al., Citation1998; Tuji & Houki, Citation2001; Håkansson, Citation2002; Naya et al., Citation2007). This may be due to the prior expectations of observers searching for specimens resembling the original description and micrographs. In the publication of Gotoh et al., (Citation1998), specimens also have slightly or strongly convex/concave valves 6–13 µm in diameter, with stria densities of 12–15 in 10 µm and 3.4–6 marginal fultoportulae in 10 µm. Tuji & Houki (Citation2001) found the species in eutrophic Lake Biwa, with diameters of 4–11 µm, and stria densities of 11–14 in 10 µm. The specimens in their pictures are strongly convex/concave. Naya et al. (Citation2007) found the species in eutrophic Lake Kitaura and the specimens they illustrate have similarly strongly convex/concave valves, with diameters of 6–8.5 µm and 12–14 striae in 10 µm. The characteristics of the vestibula and the rimoportula are clearly visible on all published SEM micrographs, together with the three satellite pores of the valve face fultoportula. All the above-mentioned characteristics are in good accordance with our type material investigations and the population in the Lake Balaton.
The population from Lake Balaton shows the highest variability in conventional morphometric characters (). The stria density has a wider range than in the other populations investigated and overlaps with them and with stria densities reported for S. triporus and S. vestibulis in the literature. Furthermore, in the Hungarian populations all types of valve face occurred, from flat forms to slightly or strongly convex and concave ones. A continuum occurred too in the two French populations. In the Lake Balaton population, the areolae formed uniseriate striae even near the spines in some valves, while in others the uniseriate part was very short (consisting of one or two areolae). In about half of the specimens the labia were more or less parallel with the interstriae; in the other half, they were perpendicular to the interstriae. This is evident in the relative warp grids, where the mean position of the rimoportula relative to the mantle is illustrated by points 5 and 6. In some French specimens zig-zag slits were found instead of round areolae, because of the strong silicification.
Morphological disparities occur in some other centric diatoms. For example, the two forms of Stephanodiscus hantzschii (S. hantzschii f. hantzschii and S. hantzschii f. tenuis can frequently be found together in the same population, and even combined in the same frustule (Casper et al., Citation1987). Cyclotella hispanica is another polymorphic species and the same frustule may have a flat epivalve and a hypovalve with triangular depressions and elevations (Kiss et al., Citation2002). A very thorough study of Stephanodiscus minutulus type material and cultured material is available (Theriot & Jones, Citation2009) and shows that S. minutulus forms flat, weakly concentrically undulate and strongly concentrically undulate valves, like S. triporus.
Effects of environmental variables on cell morphology
Several studies have shown that some environmental variables can affect diatom morphology. Håkansson & Korhola (Citation1998) reported that a Cyclotella in their material showed morphological variation during a period of heavy pollution (highly nutrient-rich conditions or other ‘extreme’ environments), with changes in the number of valve face fultoportulae, the pattern of striae and costae, and the position and shape of the satellite pores of the mantle fultoportulae. They tentatively interpreted this diatom to be a form of C. meneghiniana, adapted to the changed nutrient conditions. Shirokawa et al. (Citation2012) studied the effect of salinity on the developmental plasticity of Cyclotella meneghiniana. They found that the numbers of valve face fultoportulae were significantly increased in all strains in seawater. Finally, Martín-Cereceda & Cox (Citation2011) found that the valve morphology of a Thalassiosira isolate changed with variation in the salinity and silicate concentration of the medium. They also gave a good review of literature about phenotypic plasticity of Thalassiosiraceae.
Outside diatoms, phenotypic plasticity has recently been demonstrated in the chrysophyte Synura echinulata, using geometric morphometrics. Changes in scale morphology were demonstrated in response to different combinations of light intensity and temperature, but taxonomically relevant characters remained unchanged (Němcová et al., Citation2010). Our materials from Lake Balaton were also influenced by changes in light conditions and temperature (see more chemical variables of Lake Balaton in Duleba et al., Citation2012).
These studies of Thalassiosiraceae and other algae are consistent with the idea that the extensive and overlapping ranges of variation in S. triporus and S. vestibulis reflect phenotypic plasticity in a single species, but that S. minutulus is a separate entity ().
Table 3. Differences between Stephanodiscus triporus (as redefined here to include S. vestibulis) and S. minutulus.
The taxonomic position of Stephanodiscus triporus and related taxa
On the basis of our study we conclude that S. vestibulis is conspecific with S. triporus. For example, populations of these two species have similar ranges of valve diameter, number of striae in 10 µm, number of rows of areolae, and number of satellite pores of the valve face and marginal fultoportulae. The main distinguishing feature of S. vestibulis was said to be the presence of small arches on the valve mantle, each similar to a vestibule (hence the name of the species: Håkansson et al., Citation1986) and covering the external aperture of one of the marginal fultoportulae. However, this feature is also present in S. triporus type material (), but was not noticed because the first studies of S. triporus (Genkal & Kuzmin, Citation1978) were made by TEM; it was also not mentioned during the description of S. triporus var. volgensis (Genkal & Korneva, Citation1990). The structure of the vestibules was later recognized from SEM investigations (Genkal et al., Citation2006, Citation2010; Genkal & Golokolenova, Citation2008; Genkal & Popovskaja, Citation2008a, b; Genkal & Trifonova, Citation2009).
As Håkansson et al. (Citation1986) noted, there is a small pit close to the valve face fultoportula in some valves of S. vestibulis, which is similar in size to a large areola. This has also been documented by other authors, including Gotoh et al. (Citation1998) and Tuji & Houki (Citation2001) and indicates continuity of variation between S. vestibulis and S. triporus var. volgensis. The first micrographs of S. vestibulis show the valve centre to be strongly convex or concave (Håkansson et al., Citation1986), but other researchers have published micrographs showing a less pronounced concentric convex or concave central area (Gotoh et al., Citation1998; Tuji & Houki, Citation2001). In our material the valve face was sometimes almost flat ().
Emended diagnosis of Stephanodiscus triporus
Stephanodiscus triporus Genkal & Kuzmin emend. Genkal, K.T. Kiss & Ács
Synonyms
S. vestibulis Håkansson, Theriot & Stoermer, S. triporus var. volgensis Genkal.
Description
Cells are solitary or in short chains. Valves usually concentrically convex–concave, sometimes almost flat, with a diameter of 3.7–12.5 µm. Number of fasciculi 23–65, radial striae 10–21(30) in 10 µm, composed of round or elongate areolae or sometimes zig-zag slits (26–61 in 10 µm). Interstriae end in spines. Areolae in the valve centre arranged irregularly. The single rows of areolae become double or triple, or rarely in fours, at the edge of valve face; very rarely they remain single. A single fultoportula with three satellite pores (rarely two or four) occurs near the centre of the valve face. Its round external opening is slightly elevated, like a tiny volcano. Opposite this elevated opening is a small depression (accommodating the valve face fultoportula of the sibling valve during valve formation). Marginal fultoportulae with three satellite pores occur on every second to fifth interstriae below a spine. External openings of marginal fultoportulae usually partially surrounded by arched structures (vestibules) but these are sometimes not fully developed. A single rimoportula is present, situated above the ring of marginal fultoportulae, with small labia oriented perpendicularly or at some other angle to the interstriae; it has a relatively long and large tube externally.
Distribution
The species has been recorded from eutrophic fresh waters of western North America, Japan and Korea (Gotoh et al., Citation1998), but we have also found it in waters with different trophic status (e.g. the oligo-mesotrophic Lake Balaton: Bolla et al., Citation2010), so we think it has a wide ecological range, occurring in oligotrophic to eutrophic waters. It occupies lakes, reservoirs and rivers in Asia, Europe, and North and South America. Previous to our investigations there was only a single Hungarian report of the species: Kiss & Genkal (Citation1993) observed it in the River Danube and identified it as S. triporus according to TEM pictures. It has not been reported previously from Lake Balaton, even though the phytoplankton of the lake has regularly been investigated (among others Tamás, Citation1961, Citation1975; Tóth & Padisák, Citation1978; Vörös, Citation1982; see also Sr 11, 12) . However, most previous studies used LM, by which it is almost impossible to differentiate S. triporus from S. minutulus. Kiss & Padisák (Citation1990) made a single EM study in Lake Balaton but the species was not found. Unfortunately the earliest sample that we have from Lake Balaton was collected in 1997 and so we cannot prove its earlier occurrence there. We suppose that S. triporus is not an invasive species but was hitherto overlooked, because it can be mistaken for other species only LM is available.
Supplementary information
The following supplementary material is available for this article, accessible via the Supplementary Content tab on the article’s online page at http://dx.doi.org/10.1080/09670262.2013.843204
Table S1. List of the Hungarian, French, Russian and Estonian waters from which samples were obtained.
Supplementary references: those references where data of occurrence were published from Stephanodiscus triporus or. S. vestibulis without micrographs.
Supplementary material
Download Zip (22.8 KB)Acknowledgements
The authors thank Dr Csilla Stenger-Kovács for the sample from Széki Lake; Dr Regine Jahn (Botanischer Garten und Botanisches Museum Dahlem, Freie Universität Berlin) for the original material of Stephanodiscus vestibulis; Áron Keve Kiss for final preparation of micrographs using Photoshop; Valerie Peeters for LM micrographs from the Vire River at Gourfaleur (France); and Christophe Bouillon for his technical assistance with SEM. This study was supported by Hungarian National Science Foundation (OTKA K 68327, KTIA-OTKA 80140).
References
- Anonymous (1975). Proposals for standardization on diatom terminology and diagnoses. Nova Hedwigia, Beiheft, 53: 323–354.
- Balonov, I.M. (1975). Podgotovka diatomovykh i zolotiskykh vodoroslej k elektronnoj mikroskopii [Preparation of diatoms and golden algae for electron microscopy]. In Metodika izucheniya biogeocenozov vnutrennikh vodoemov (Mordukhai-Boltovskoi, editor), 87–90. Nauka, Moscow. [In Russian.]
- Beszteri, B., Ács, É. & Medlin, L. (2005). Conventional and geometric morphometric studies of valve ultrastructural variation in two closely related Cyclotella species (Bacillariophyta). European Journal of Phycology, 40: 89–103.
- Blanco, S., Cejudo-Figueiras, C., Álvarez-Blanco, I., Bécares, E., Hoffmann, L. & Ector, L. (2010). Atlas de las Diatomeas de la cuenca del Duero/Diatom Atlas of the Duero Basin. Área de Publicaciones, Universidad de León, León.
- Bolla, B., Borics, G., Kiss, K.T., Reskóné, N.M., Várbíró, G. & Ács, É. (2010). Recommendations for ecological status assessment of Lake Balaton (largest shallow lake of central Europe), based on benthic diatom communities. Vie et Milieu, 60: 197–208.
- Casper, S.J., Scheffler, W., Augsten, K. & Peschke, T. (1987). Some observations on the Stephanodiscus hantzschii-group (Bacillariophyta) in waters of the GDR. I. Stephanodiscus hantzschii and S. “tenuis” in lakes Wentow, Tollense, Haussee, and Bautzen Reservoir. Archiv für Protistenkunde, 134: 17–34.
- Duleba, M., Bíró, P., Bolla, B., Borsodi, A.K., Kiss, K.T., Palatinszky, M., Pohner, Zs., Reskóné Nagy, M., Várbíró, G. & Ács, É. (2012). Comparison of benthic microbial community patterns and diversity in three basins with different trophic levels of Lake Balaton (Hungary). Vie et Milieu, 62: 143–157.
- Garrison, P. & LaLiberte, G. (2011a). Results of sediment core taken from White Ash and North White Ash lakes, Polk County, Wisconsin. In Aquatic Plant Management Plan: White Ash and North White Ash Lakes. White Ash Lakes APM Planning, Polk County, Wisconsin (Hendrickson S.E. & Blumer, D., editors), Appendix A, White Ash Lakes Paleocore Report, pp 1–9. WDNR No. AEPP-209-10, SEH No. WALPR 110599.
- Garrison, P. & LaLiberte, G. (2011b). Results of sediment core taken from Potato Lake, Washburn County, Wisconsin. In Aquatic Plant Management Plan: Potato Lake. Washburn County, Wisconsin (Hendrickson S.E. & Blumer, D., editors), Appendix B, Potato Lake Paleocore Report pp. 1–6. DNR No. LPL-1334-10 & LPL-1335-10, SEH No. POTAL 110812.
- Genkal, S.I. (1977). K metodike podscheta nekotorykh taksonomicheski znachimykh strukturnykh ehlementov stvorki u diatomovykh vodoroslej sem. Thalassiosiraceae Lebour emend. Hasle (Bacillariophyta). [On counting of some taxonomically significant structural elements of valves in the diatom algae of the family Thalassiosiraceae Lebour emend. Hasle (Bacillariophyta).] Botanicheskij Zhurnal, 62: 848–851. [In Russian.]
- Genkal, S.I. (1978). Novyj vid iz roda Stephanodiscus Ehr. (Bacillariophyta). [Species nova e genere Stephanodiscus Ehr. (Bacillariophyta).] Novosti Sistematiki Nizshikh Rastenij, 15: 11–14. [In Russian.]
- Genkal, S.I. (1984). O morfologicheskoj izmenchivosti osnovnykh elementov stvorki u vidov roda Stephanodiscus (Bacillariophyta). [On morphological variability of the main structural valve elements in the species of the genus Stephanodiscus (Bacillariophyta).] Botanicheskij Zhurnal, 69: 403–408. [In Russian.]
- Genkal, S.I. (1985). Novyj vid iz roda Stephanodiscus Ehr. (Bacillariophyta). [Species nova e genere Stephanodiscus Ehr. (Bacillariophyta).] Novosti Sistematiki Nizshikh Rastenij, 22: 30–32. [In Russian.]
- Genkal, S.I. (1992). Atlas diatomovykh vodoroslej planktona reki Volgi. [Atlas of planktonic diatoms of the river Volga.] Gidrometeoizdat, St. Petersburg. [In Russian.]
- Genkal, S.I. (2004). Taksonomiya melkokletochnykh vidov roda Stephanodiscus (Bacillariophyta). 1. Stephanodiscus delicatus. [Taxonomy of small-sized species of the genus Stephanodiscus (Bacillariophyta). 1. Stephanodiscus delicatus.] Botanicheskij Zhurnal, 89: 1814–1821. [In Russian.]
- Genkal, S.I. (2007). Morfologiya, taksonomiya, ehkologiya i rasprostranenie melkorazmernykh vidov Stephanodiscus (Bacillariophyta) 2. S. makarovae. [Morphology, taxonomy, ecology and distribution of small-sized species of the genus Stephanodiscus (Bacillariophyta). 2. S. makarovae.] Botanicheskij Zhurnal, 92: 241–248. [In Russian.]
- Genkal, S.I. (2010). Morfologiya, ehkologiya, rasprostranenie melkorazmernykh vidov Stephanodiscus (Bacillariophyta) i ikh taksonomiya. 3. S. minutulus. [Morphology, ecology, distribution of small-sized species of the Stephanodiscus (Bacillariophyta) and their taxonomy. 3. S. minutulus.] Botanicheskij Zhurnal, 95: 1247–1254. [In Russian.]
- Genkal, S.I. (2013). K morfologii, taksonomii, ehkologii i rasprostraneniyu Stephanodiscus triporus var. triporus i S. triporus var. volgensis (Bacillariophyta). [To morphology, taxonomy, ecology and distribution of Stephanodiscus triporus var. triporus and S. triporus var. volgensis (Bacillariophyta).] Novosti Sistematiki Nizshikh Rastenij, 47: in press [In Russian.]
- Genkal, S.I. & Dmitriyeva, O.A. (2005). New data on the flora of Centrophyceae (Bacillariophyta) of the Kursiu Maros Lagoon of the Baltic Sea. International Journal on Algae, 7: 181–193.
- Genkal, S.I. & Golokolenova, T.B. (2008). Tsentricheskie diatomovye vodorosli Tsimlyanskogo vodokhranilishcha. Povolzhskij ehkologicheskij Zhurnal, 3: 178–189. [In Russian.]
- Genkal, S.I. & Korneva, L.G. (1990). Morfologiya i sistematika nekotorykh vidov roda Stephanodiscus Ehr. [Morphology and taxonomy of some species of the genus Stephanodiscus Ehr.] In Flora i productivnost’ pelagicheskikh i litoralnykh fitotsenozov vodoemov bassejna Volgi (Ekzercev, editor), 219–236. Nauka, Leningrad. [In Russian.]
- Genkal, S.I. & Kozyrenko, T.F. (1992). [Materials to the algal flora (Bacillariophyta, Centrophyceae) of River Izhora]. Biologiya Vnutrennikh Vod, Informatsionnij Byulleten’, 95: 13–17. [In Russian.]
- Genkal, S.I. & Kuzmin, G.V. (1978). Novye taksony roda Stephanodiscus Ehr. (Bacillariophyta). [New taxa of the genus Stephanodiscus Ehr. (Bacillariophyta).] Botanicheskij Zhurnal, 63: 1309–1312. [In Russian.]
- Genkal, S. I. & Kuzmina, A.E. (1984). O novykh tsentricheskikh diatomejah dljy flory Yeniseya. [New centric diatoms on flora Yenisei.] Biologiya Vnutrennikh Vod, Informatsionnij Byulleten’, 62: 11-15. [In Russian.]
- Genkal, S.I. & Kuzmina, A.E. (1992). Novyj vid Stephanodiscus chantaicus Genkal et Kuzmina (Bacillariophyta). [New species of Stephanodiscus chantaicus Genkal et Kuzmina (Bacillariophyta).] Biologiya Vnutrennikh Vod, Informatsionnij Byulleten’, 92: 6–9. [In Russian.]
- Genkal, S.I. & Laugaste, R.A. (1985). Novye dannye k flore diatomovykh vodoroslej Ehstonii. [Novitates ad floram Bacillariophytorum in aquariis Estoniae.] Novosti Sistematiki Nizshikh Rastenij, 22: 32–35. [In Russian.]
- Genkal, S. I. & Levadnaja, G.D. (1980). Novye dannye k flore diatomovykh vodoroslej reki Obi. [Novitates ad floram Bacillariophytorum fluminis Obj.] Novosti Sistematiki Nizshikh Rastenij, 17: 3–7. [In Russian.]
- Genkal, S.I. & Makarova, I.V. (1985). Diatomovye vodorosli, novye dlya planktona Kaspijskogo i Azovskogo morej. [Bacillariophyta planctonica nova e maribus caspio et maeotico.] Novosti Sistematiki Nizshikh Rastenij, 22: 35–37. [In Russian.]
- Genkal, S.I. & Naumenko, Y.V. (1985). Novye dannye k flore diatomovykh vodoroslej Obi i Irtysha. [New data to the diatom flora of river Ob and Irtyš.] Biologiya Vnutrennikh Vod, Informatsionnij Byulleten’, 65: 16–19. [In Russian.]
- Genkal, S.I. & Nikulina, V.N. (1991). Novye i interesnye diatomovye vodorosli (Centrophyceae) ozera Sevan. [New and interesting diatom algae (Centrophyceae) of Lake Sevan.] Biologiya Vnutrennikh Vod, Informationnij Byulleten’, 90: 7–11. [In Russian.]
- Genkal, S.I. & Ohapkin, A.G. (2013) Tsentricheskie diatomovye vodorosli (Centrophyceae) nizhnevo techeniya r. Oki (Rossijskaya Federacia). [Centric diatoms of the river Oka (Russian Federation)]. Gidrobiologicheskij Zhurnal, 49: 41–57. [In Russian.]
- Genkal, S.I. & Popovskaya, G.I. (1997). [Materials to the diatom flora (Centrophyceae) of Lake Michigan]. Biologiya Vnutrennikh Vod, Informationnij Byulleten’, 2: 92–94. [In Russian.]
- Genkal, S.I. & Popovskaya, G.I. (2003). Tsentricheskie diatomovye vodorosli Selenginskogo melkovoda Ozera Baikal [Centric diatoms of Selenga shallows of the Baikal]. Biologiya Vnutrennikh Vod, Informationnij Byulleten’, 2: 9–14. [In Russian.]
- Genkal, S.I. & Popovskaja, G.I. (2008a). Tsentricheskie diatomovye vodorosli r. Selenga i eë deltovykh protok. [Centric diatom algae of the Selenga River and its delta tributaries]. Biologija Vnutrennikh Vod, Informationnij Bjulleten’, 2: 19–27. [In Russian.]
- Genkal, S.I. & Popovskaja, G.I. (2008b). Centric diatom algae of the Selenga River and its delta branches. Inland Water Biology, 1: 120–128.
- Genkal, S.I. & Ščur, L.A. (2000). New data on the flora of Bacillariophyta of Lake Hanka (Primorskiy region, Russia). Algologia, 10: 278–281.
- Genkal, S.I. & Terenko, L.M. (2009). Novye dannye po morfologii, ehkologii i rasprostraneniju Thalassiosira coronifera Pr.-Lavr. (Bacillariophyta) [New data on morphology, ecology and distribution Thalassiosira coronifera Pr.-Lavr. (Bacillariophyta)]. Ukrainian Botanical Journal, 66: 88–93. [In Russian.]
- Genkal, S.I. & Trifonova, I.S. (2006). Materialy k flore Bacillariophyta reki Narva I narvskogo vodokhranilishcha (severo-zapad Rossii). Centrophyceae. [Materials on the flora of Bacillariophyta of the Narva River and Narva Reservoir (North-Western Russia. 1. Centrophyceae.] Botanicheskii Zhurnal, 91: 693–697. [In Russian.]
- Genkal, S.I. & Trifonova, I.S. (2009). Diatomovye vodorosli planktona Ladozhskogo ozera i vodoemov ego bassejna. [Planktonic diatoms of Lake Ladoga and water bodies of its basin]. OAO Rybinsk Printing House, Rybinsk. [in Russian]
- Genkal, S.I. & Yarušhina, M.I. (2002). Materialy k flore tsentricheskikh (Centrophyceae) diatomovykh vodoroslej vodoemov Srednego Urala. [Materials to the flora of centric (Centrophyceae) diatoms in waterbodies of the Middle Urals]. Biologija Vnutrennikh Vod, Informationnij Bjulleten’, 2: 27–32. [In Russian.]
- Genkal, S.I., Pautova, V.N., Tarasova, N.G. & Nomokonova, V.I. (2006). Tsentricheskie diatomovye vodorosli Kujbyshevskogo vodokhranilishcha. [Centric diatoms in the Kujbyshev reservoir]. Izvestiya Samarskogo Nauchnogo Tsentra Rossijskoj Akademii Nauk, 8: 147–162. [In Russian.]
- Genkal, S.I., Teren’ko, L.M. & Nesterova, D.A. (2009). Novye dannye k flore tsentricheskikh diatomovykh vodoroslej (Centrophyceae) pridunajskogo rajona Chernogo morya. [New data to the flora of centric diatoms (Centrophyceae) of the Danube region of the Black Sea.] Gidrobiologicheskij Zhurnal, 45: 52–72. [In Russian.]
- Genkal, S.I., Shchur, L.A. & Yarushina, M.I. (2010). Diatom of some water bodies in northeastern West Siberia. Communication. 1. Centrophyceae. Contemporary Problems of Ecology, 3: 386–394.
- Genkal, S.I., Bondarenko, N.A. & Shchur, L.A. (2011). Diatomovye vodorosli ozer Juga i Severa Vostochnoj Sibiri [Diatom algae from lakes South and West-East Siberia]. Ribinskii Dom Petsati, Ribinsk. [In Russian.]
- Gotoh, T., Lee, J.H. & Tsugeki, N. (1998). Fine structure and ecology of a diatom Stephanodiscus vestibulis Håk., E.C. Ther. & Stoermer. Diatom, 14: 35–40 [In Japanese.]
- Håkansson, H. (2002). A compilation and evaluation of species in the genera Stephanodiscus, Cyclostephanos and Cyclotella with a new genus in the familyStephanodiscaceae. Diatom Research, 17: 1–139.
- Håkansson, H. & Kling, H. (1989). A light and electron microscope study of previously described and new Stephanodiscus species (Bacillariophyceae) from central and northern Canadian lakes, with ecological notes on the species. Diatom Research, 4: 269–288.
- Håkansson, H. & Kling, H. (1990). The current status of some very small freshwater diatoms of the genera Stephanodiscus and Cyclostephanos. Diatom Research, 5: 273–287.
- Håkansson, H. & Korhola, A. (1998). Phenotypic plasticity in the diatom Cyclotella meneghiniana or a new species? Nova Hedwigia, 66: 187–196.
- Håkansson, H., Theriot, E.C. & Stoermer, E.F. (1986). Morphology and taxonomy of Stephanodiscus vestibulis sp. nov. (Bacillariophyta). Nordic Journal of Botany, 6: 501–505.
- Hammer, Ø., Harper, D.A.T. & Ryan, P.D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4: 1–9.
- Hayashi, T. (2011). Monospecific planktonic diatom assemblages in the Paleo-Kathmandu Lake during the middle Brunhes Chron: implications for the paradox of the plankton. Palaeogeography, Palaeoclimatology, Palaeoecology, 300: 46–58.
- Kiss, K.T. & Genkal, S.I. (1993). Winter blooms of centric diatoms in the River Danube and in its side-arms near Budapest (Hungary). Hydrobiologia, 269/270: 317–325.
- Kiss, K.T. & Nausch, M. (1988). Comparative investigations of planktonic diatoms of section of the Danube near Vienna and Budapest. In Proceedings of the 9th International Diatom Symposium (Round, F.E., editor), 115–122. Biopress, Bristol.
- Kiss, K.T. & Padisák, J. (1990). Species succession of Thalassiosiraceae: Quantitative studies in a large, shallow lake (Lake Balaton, Hungary). In Proceedings of the 10th International Diatom Symposium (Simola, H., editor), 481–490. Koeltz, Koenigstein.
- Kiss, K. T., Hegewald, E. & Ács, É. (2002). Cyclotella hispanica a new dimorphic centric diatom species (Bacillariophyceae). Algological Studies, 106: 1–16.
- Kobayasi, H., Kobayashi, H. & Idei, M. (1985). Fine structure and taxonomy of the small and tiny Stephanodiscus (Bacillariophyceae) species in Japan. 3. Co-occurrence of Stephanodiscus minutulus (Kütz.) Round and S. parvus Stoerm. & Håk. Japanese Journal of Phycology, 33: 293–300.
- Kožhova, O.M., Basharova, N.I., Kobanova, G.I., Izmesteva, L.R. & Romanenko, T.I. (1982). Plankton Ust-Ilimskogo vodohranilishcha. [Plankton of the Ust-Ilimskij Reservoir]. Gidrometeoizdat, Leningrad. [In Russian.]
- Laslandes, B. (2007). Reconstitution de la variabilité climatique du littoral Fluminense (Rio de Janeiro, Brésil) au cours de l’Holocène par l’étude de bio-indicateurs (diatomées, coccolithophoridés). PhD thesis, University of Angers, France.
- Majstrova, N.V., Genkal, S.I., Ščerbak, V.I. & Semenjuk, N.E. (2007). Centrophyceae in the upper section of the Kanev water reservoir (Ukraine). Algologia, 17: 467–475.
- Makarova, I.V. & Pomazkina, G.V. (1992). Stephanodiscus inconspicuus sp. nov. (Bacillariophyta). Algologia, 2: 84–86.
- Martín-Cereceda, M. & Cox, E.J. (2011). Morphological variation in a small Thalassiosira species (Bacillariophyta) under different culture regimes. Botanica Marina, 54: 563–574.
- Marvan, P., Heteša, J., Hindák, F. & Hindáková, A. (2004). Phytoplankton of the Morava River in the Czech Republic and Slovakia: past and present. Oceanological and Hydrobiological Studies, 33: 41–60.
- Naya, T., Tanimura, Y., Nakazato, R. & Amano, K. (2007). Modem distribution of diatoms in the surface sediments of Lake Kitaura, central Japan. Diatom, 23: 55–70.
- Němcová, Y., Neustupa, J., Kvíderová, J. & Řezáčová-Škaloudová, M. (2010). Morphological plasticity of silica scales of Synura echinulata (Synurophyceae) in crossed gradients of light and temperature – a geometric morphometric approach. Nova Hedwigia, Beiheft 136: 21–32.
- Novais, M.H., Blanco, S., Hlúbiková, D., Falasco, E., Gomà, J., Delgado, C., Ivanov, P., Ács, É., Morais, M., Hoffmann, L. & Ector, L. (2009). Morphological examination and biogeography of the Gomphonema rosenstockianum and G. tergestinum species complex (Bacillariophyceae). Fottea, 9: 257–274.
- Ohapkin, A.G., Mikulchuk, I.A., Korneva, L.G. & Mineeva, N.M. (1997). [Phytoplankton of the Gorky Reservoir]. Institute of Ecology of Volga Basin of RAS, Toliatti. [In Russian.]
- Pautova, V.N., Genkal, S.I., Nomokonova, V.I. & Tarasova, N.G. (2009). The seasonal and interannual dynamics of centric diatoms in the Kuibyshev Reservoir. Inland Water Biology, 2: 213–222.
- Potapova, M. & Hamilton, P.B. (2007). Morphological and ecological variation within the Achnanthidium minutissimum (Bacillariophyceae) species complex. Journal of Phycology, 43: 561–575.
- Risberg, J., Sandgren, P., Teller, J.T. & Last, W.M. (1999). Siliceous microfossils and mineral magnetic characteristics in a sediment core from Lake Manitoba, Canada: a remnant of glacial Lake Agassiz. Canadian Journal of Earth Sciences, 36: 1299–1314.
- Rohlf, F.J. (2003). TpsSmall: thin plate spline small variation (Version 1.20). State University of New York at Stony Brook, Stony Brook, New York.
- Rohlf, F.J. (2004). TpsDig, digitize landmarks and outlines, version 2.0. Department of Ecology and Evolution, State University of New York at Stony Brook. http://life.bio.sunysb.edu/morph/
- Rohlf, F.J. (2005). TpsRegr. Department of Ecology and Evolution, State University of New York, Stony Brook, New York.
- Rohlf, F.J. & Slice, D. (1990). Extensions of the Procrustes method for the optimal superimposition of landmarks. Systematic Zoology, 39: 40–59.
- Ross, R., Cox, E.J., Karayeva, N.I., Mann, D.G., Paddock, T.B.B., Simonsen, R. & Sims, P.A. (1979). An amended terminology for the siliceous components of the diatom cell. Nova Hedwigia, Beiheft 64: 513–533.
- Shcherbak, V.I., Genkal, S.I. & Majstrova, N.V. (1992).Tsentricheskie diatomovye vodorosli v fitoplanktone Kievskogo i Kanevskogo vodokhranilishch. [Centric diatoms in the phytoplankton of the Kiev and Kanev reservoirs]. Biologii Vnutrennykh Vod, 93: 25–30. [In Russian.]
- Shirokawa,Y., Karino, K. & Mayama, S. (2012). Developmental plasticity and genotype–environment interactions influence valve morphology in the Cyclotella meneghiniana species complex (Bacillariophyceae). European Journal of Phycology, 47: 245–253.
- Stoermer, E.F. & Håkansson, H. (1984). Stephanodiscus parvus: validation of an enigmatic and widely misconstrued taxon. Nova Hedwigia, 39: 497–511.
- Tamás, G. (1961). Horizontale Plankton-Untersuchungen im Balaton. 2. Über das Phytoplankton in nordöstlichen Teil des Sees, auf Grund des Filtrate der in 1955, 1956 und 1958 entnommen Proben. Annales Instituti Biologici Tihany, 28: 143–149.
- Tamás, G. (1975). Horizontally occurring quantitative phytoplankton investigations in Lake Balaton, 1974. Annales Instituti Biologici Tihany, 42: 219–279.
- Tapia, P.M., Theriot, E.C., Fritz, S.C., Cruces, F. & Rivera, P. (2004). Distribution and morphometric analysis of Cyclostephanos andinus comb. nov., a planktonic diatom from Central Andes. Diatom Research, 19: 311–327.
- Theriot, E. (1987): Principal component analysis and taxonomic interpretation of environmentally related variation in silicification in Stephanodiscus (Bacillariophyceae). British Phycological Journal, 22: 359–373.
- Theriot, E.C. & Jones, B. (2009). The morphology, physiology and taxonomy of two small Stephanodiscus species in Yellowstone Lake and Jackson Lake, Wyoming, USA. Nova Hedwigia, Beiheft 135: 275–293.
- Theriot, E. & Serieyssol, K. (1994). Phylogenetic systematics as a guide to understanding features and potential characters of the centric diatom family Thalassiosiraceae. Diatom Research, 9: 429–450.
- Tóth, L.G. & Padisák, J. (1978). Short-term investigations on the phytoplankton of Lake Balaton at Tihany. Acta Botanica Hungarica, 24: 187–204.
- Tuji, A. & Houki, A. (2001). Centric diatoms in Lake Biwa. Lake Biwa Study Monographs, 7: 1–92.
- Vörös, L. (1982). Quantitative and structural changes of phytoplankton in Lake Balaton between 1965-1978. Aquacultura Hungarica (Szarvas), 3: 137–144.
- Yang, J.-R., Duthie, H.C. & Delorme, L.D. (1993). Reconstruction of the recent environmental history of Hamilton Harbour (Lake Ontario, Canada) from analysis of siliceous microfossils. Journal of Great Lakes Research, 19: 55–71.