Abstract
Recent phylogenetic studies of Chlorella-like algae revealed that this group has diversified into free-living and endosymbiotic niches, the latter within protists and invertebrate organisms. Our previous studies using a long-term culture composed of an alga (Chlorella vulgaris, rDNA sequence unknown), a bacterium (Escherichia coli) and a ciliate (Tetrahymena thermophila), suggested that some clones in the algal population developed an endosymbiotic ability with T. thermophila while others developed an ectosymbiotic ability with E. coli from a non-associated stage. In this paper, an rDNA (18S, ITS1, 5.8S, ITS2)-based phylogeny of the ancestral strain and derived clones isolated from 5-year microcosm cultures was constructed and revealed that the alga belongs to the genus Micractinium. This result was supported by analysis of the secondary structure of the rRNA gene. No difference was observed in the sequence between the ancestral and derived clones. On the other hand, some morphological and physiological traits of the ancestral strain and the derived clones were analysed and revealed that several phenotypic changes had occurred among the algal clones. In particular, the derived clones obtained from long-term cultures had increased in cell size, changed in their ability to grow at pH 4.0, and developed a tendency for cell-aggregation or sedimentation.
Introduction
Chlorella-like green algae have diversified into free-living and endosymbiotic niches, and several species in this group are widely known as endosymbionts of heterotrophic organisms. For the past 100 years, these species have attracted much interest as a useful model for understanding general features of the evolution of endosymbiosis between autotrophic and heterotrophic organisms. However, the phylogenetic status of many symbiotic algae has long been unclear because the algae were classified by traditional methods based on their phenotypic properties, limiting the effective analysis of their evolution.
Recent rDNA-based phylogenetic analyses of Chlorella-like algae, including endosymbiotic and free-living (planktonic or edaphic) species, have revealed that endosymbiotic algae are polyphyletic, that a phylogenetic or macro-evolutionary pattern of these algae involves adaptive radiation into endosymbiotic and free-living niches, and that host–symbiont associations are not necessarily a one-to-one relationship but can involve one-to-many and many-to-one relationships (Hoshina et al., Citation2005; Pröschold et al., Citation2011). For example, it was believed that Paramecium bursaria harbours one algal lineage (e.g. Reisser, Citation1984), yet the North American ciliate strain contains different algal species from the European strain (Hoshina et al., Citation2005). Likewise, the same symbiotic species can associate with several different host species. Further examples of these contrasting possibilities include the demonstration by Summerer et al. (Citation2008), through phylogenetic analysis, that various ‘zoochlorellae’ (the traditional name for endosymbiotic Chlorella-like algae species) can be harboured by the same ciliate species, depending on their origin, whereas the same Chlorella-species can be found in various host species in the same lake. Pröschold et al. (Citation2011) reported a similar result: strains of P. bursaria have various endosymbionts, depending on their origin (as noted above), whereas the endosymbionts isolated from Climacostomum, Coleps and Euplotes, all growing in the same pond, were identical in their SSU and ITS sequences.
These studies suggest that endosymbiotic associations may evolve as a result of local co-evolutionary processes, which shape the phylogenetic pattern of the relationships between host and endosymbiont species. However, it is not known whether phylogeny can constrain the evolution of endosymbiosis by restricting the phenotypes generated in the lineage. For example, free-living Micractinium (of the Chlorella clade) forms bristles on its cell wall (Krienitz et al., Citation2004), which is considered to be an adaptation that inhibits predation by e.g. rotifers (Luo et al., Citation2006). The bristles may also prohibit cells from being ingested by prospective host organisms, reducing the opportunity for the potential establishment of endosymbiosis. Thus, if bristle-formation is a species-level phenotype, it would then affect the evolutionary fate of this lineage. However, things are not so straightforward because Micractinium reisseri isolated from P. bursaria does not form bristles even in the presence of rotifers (Pröschold et al. Citation2010).
Experimental microcosms can be a useful tool to directly observe and analyse the evolution of symbiosis. In previous studies, we developed an experimental microcosm called the CET microcosm, composed of a green alga (formerly known as Chlorella vulgaris), a bacterium (Escherichia coli) and a ciliate (Tetrahymena thermophila) (see Nakajima et al., Citation2009, for details). In static long-term cultures, it was observed that Chlorella-harbouring Tetrahymena cells (called ‘C-Tetrahymena’) increased in frequency to 80–90 % of the total population. Microscopical observation revealed that algal cells were ingested by the ciliate's phagocytotic activity, and egested or released by the host’s death, though the rates of ingestion and egestion are not known. The ingested algal cells were transmitted into daughter cells by host cell division. Sano et al. (Citation2009) revealed that a derived Tetrahymena population co-cultured with a derived algal population, both isolated from the microcosm after it had been cultured for about 1700 days, could survive longer in the absence of E. coli than the ancestral Tetrahymena population co-cultured with the ancestral algal clone under the same conditions. At present, we have no evidence for digestion of algal cells by the ciliate. These results suggest that an early stage of endosymbiotic association is emerging between the alga and the ciliate. Furthermore, it was observed that algal cell aggregates that incorporated E. coli cells within the aggregates were developed during the long-term culture, suggesting that a simple form of an ectosymbiotic association occurred between the alga and the bacterium (Nakajima et al., Citation2009). However, as far as we know, it has never been experimentally shown whether or not any species of Chlorella-like algae can evolve symbiotic traits when the selective environment favours such associations.
The ancestral algal strain used for the CET microcosm was obtained from a private collection and was previously identified as Chlorella vulgaris (see Materials and methods), based on traditional criteria of cell morphology and physiology rather than on rDNA sequencing and phylogenetic analyses. It is possible that this strain should be classified as another species or within a different genus, especially considering recent taxonomic revisions of Chlorella-like algae (e.g. Krienitz et al., Citation2004; Luo et al., Citation2009). In general – and our microcosm studies are no exception – analyses using experimental models focus on the interactions between component species, rather than their phylogenetic status and natural history. However, the phylogenetic positions of species used in experimental models can be significant, since the inherited characteristics of organism are the starting point for the evolutionary dynamics of the model systems and constrain them, leaving the possibility open that the evolutionary dynamics might have exhibited a different pattern if different species had been used, due to different lineage (species)-dependent genetic variability (i.e. evolvability).
Therefore, it was important to determine the phylogenetic position of the algal strain used in the microcosm, to determine its relationship to other Chlorella-like green algae, including endosymbiotic and free-living lifestyles. By doing so we can relate the micro-evolutionary tendency revealed by our previous investigations (Nakajima et al., Citation2009; Sano et al., Citation2009) to the macro-evolutionary patterns revealed by recent systematic studies (Luo et al., Citation2009; Summerer et al., Citation2007, Citation2008; Pröschold et al., Citation2011).
Our aim in this paper was therefore to determine the phylogenetic position of the ancestral alga used for the CET microcosm culture and describe some of its characteristics. As molecular markers we used the rDNA gene (SSU 18S, ITS1, 5.8S and ITS2) and the secondary structure of the rRNA ITS2 region, as proposed in recent studies (Luo et al., Citation2006, Citation2009; Hoshina et al., Citation2010; Pröschold et al., Citation2011). For comparison, we also sequenced some strains from the NIES culture collection (Micractinium pusillum NIES-151, Micractinium sp. NIES-455), and Chlorella sp. NIES-2171. We also sequenced the rDNA of derived algal clones isolated from the long-term microcosms after culture for approximately 5 years (1820–1847 days), to confirm that we are comparing the same algal strain during the culture of the CET microcosm. We also sought to determine whether phenotypic changes occurred within the algal lineage during the microcosm experiment; for this we investigated morphological and physiological characteristics of the microcosm’s ancestral strain and the derived clones, including cell size and the ability to metabolize various sugars.
Materials and methods
CET microcosm culture and isolation of algal clones from long-term cultures
The CET microcosm, a mixed culture of a green alga, a bacterium (E. coli), and a ciliate (T. thermophila) was cultured statically in MC medium (containing only mineral salts) with 11 replicate lines for five years on a 12 : 12 h light–dark cycle at 30°C. Organisms, MC medium and 3-year population dynamics of the microcosm have been described elsewhere (Nakajima et al., Citation2009). The algal strain, under the name ‘Chlorella vulgaris Beijerinck’, was supplied personally in 2001 by Dr Tomoaki Itayama at the National Institute for Environmental Studies (NIES), Japan. The strain was not at that time part of the Microbial Culture Collection at NIES, which was then under development; Dr Itayama received the strain from another researcher but unfortunately information on the origin of this strain was lost. The algal strain was purified on agar before use and stored at −85°C (in 15% glycerol). The name ‘Chlorella vulgaris Beijerinck’ was used for this strain in our previous studies (Nakajima et al., Citation2009; Sano et al., Citation2009).
Algal clones were isolated from both Tetrahymena cells and the liquid phase (i.e. outside of Tetrahymena cells) of CET microcosms that had been maintained for about 5 years. The clones coded ‘SC’ were isolated from within Tetrahymena cells and the clones coded ‘FC’ from the liquid phase (see Supplementary information 1). Three replicate lines of the microcosm were used for isolation: CET-3 (cultured for 1819–1826 days, comprising 1164 days of the original replicate culture, transfer by 10-fold dilution, and then a further 655–662 days’ culture), CET-9 (cultured for 1837 days without transfer), and CET-10 (cultured for 1820–1847 days without transfer). The algal clones used for this study are listed in . Samples of each of the isolated clones were stored at −85°C. Detailed information about the isolation procedure is available online in Supplementary Information 1.
Table 1. List of the strains and derived clones sequenced for this study; n/a = not applicable. NIES = Microbial culture collection at National Institute for Environmental Studies, Japan.
Molecular analyses and GenBank accession numbers
The strains and isolated clones used for the molecular phylogenetic analyses are presented in . They included some extra, newly sequenced, representative strains from the Microbial Culture Collection at NIES (Micractinium pusillum NIES-151; Micractinium sp. NIES-455 and Chlorella sp. NIES-2171). For each strain, 100 µl of algal cells from frozen samples were pre-cultured in MC medium for a week, under a 12 : 12 h light–dark regime at 30°C, which was the temperature used for the microcosm cultures (Nakajima et al., Citation2009). A small aliquot of cells was transferred into similar culture conditions for a week, after which the cells were collected for experiments.
Algal SSU 18S rDNA, ITS1, 5.8S and ITS2 regions were amplified with the AmpDirect®plus method (Shimadzu Biotech, Japan). The first PCR was carried out using the following two pairs of primers: SR-1 and Huss 2, and Huss 1 and CHspeHLR1R (see Supplementary Information 2 for a list of primers). Huss 1 and 2 are named after those used in Huss et al. (Citation2002). Samples were sequenced with the aforementioned primers and additional inner primers for the 18S region (see Supplementary Information 2). Chlo_1248R is a primer we designed and tested for this study using the free application AmplifX, version 1.5.4, developed by Nicolas Jullien (http://ifrjr.nord.univ-mrs.fr/AmplifX-Home-page). All samples were sequenced twice using ABI 3130.
For phylogenetic analyses, sequences of the ancestral alga and derived clones were added to available sequences of SSU and ITS regions from GenBank in 2012 to make a large dataset of 18S, ITS1, 5.8S, ITS2 sequences. The accession numbers of strains are listed in Supplementary Table 2. The sequences were aligned using ClustalW or Muscle (in MEGA5: Tamura et al., Citation2011) and adjusted by eye, taking into consideration the secondary structures of ITS found using Mfold 3.2 (Mathews et al., Citation1999; Zuker, Citation2003). Intron regions of the SSU were completely removed as suggested by previous studies (Hoshina et al., Citation2005, Citation2010; Pröschold et al., Citation2011). There were a total of 56 sequences and 2522 unambiguously aligned base positions in the final dataset. The final alignments are available in fasta format (see Supplementary Information 3). Species belonging to the Parachlorella clade were used as the outgroup (Krienitz et al., Citation2004). Evolutionary analyses were conducted in MEGA5 (Tamura et al., Citation2011).
The phylogenetic tree of the concatenated dataset () was inferred by maximum likelihood (ML) using the Tamura–Nei model (TN93+G+I model) (Tamura & Nei, Citation1993) which was chosen as the best model according to the Akaike Information Criterion of MEGA5 and Modeltest. Additional phylogenetic analyses using separate datasets were conducted to evaluate if the dataset used in should be analysed separately, as suggested in Luo et al. (Citation2009). For each dataset, the best evolutionary model was chosen accordingly. The results are summarized in Supplementary Table S1. To test the confidence of the tree topology (), a heuristic search was performed using the neighbour-joining tree as the starting tree and using nearest-neighbour-interchange algorithm. Bootstrap values were calculated by maximum likelihood (100 replicates), distance (neighbour-joining [NJ]; 1000 replicates), maximum parsimony (MP; 1000 replicates) and minimum evolution (ME; 1000 replicates).
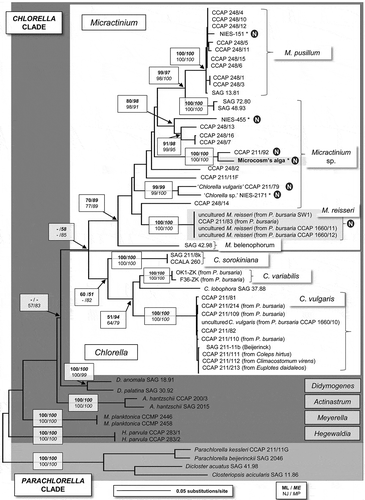
Fig. 1. Molecular phylogeny of the Chlorella clade based on SSU rDNA and ITS sequence comparisons. The phylogenetic tree shown was obtained by maximum likelihood method based on a 56-sequence dataset (alignment length 2522 bp). For the analysis, we used a Tamura–Nei evolutionary model, with the proportion of invariable sites = 0.67 and gamma distribution shape parameter = 0.55. Bootstrap values indicated in boxes were inferred from maximum likelihood (ML, bold), the minimum evolution of the maximum composite likelihood analyses (ME, bold italic), the neighbour-joining method of Jukes and Cantor (NJ, normal) and the maximum parsimony (normal italic), each with 1000 replicates. Micractinium symbiotic algae residing in Paramecium bursaria or Tetrahymena thermophila are indicated in boxes. Micractinium strains that were reported to have no bristles are indicated with a circled N. Strains sequenced de novo for this study are marked with an asterisk. Accession numbers of the strains are available in Supplementary Table 2.
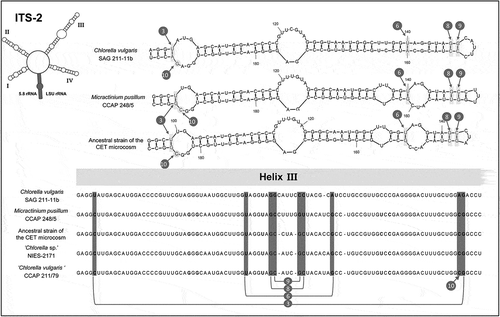
The secondary structure of ITS2 was investigated for the following strains, using the program Mfold (Mathews et al., Citation1999; Zuker, Citation2003): a representative species of Chlorella, Chlorella vulgaris SAG 211-11b (Beijerinck) and a representative species of Micractinium, Micractinium pusillum CCAP 248/5, together with Chlorella vulgaris CCAP 211/79, Chlorella sp. NIES-2171, Micractinium sp. NIES-455, and the ancestral algal strain of the CET microcosm. Sequences and structures were compared visually to identify the generic-level synapomorphic signatures of Chlorella and Micractinium proposed by Luo et al. (Citation2006).
Strain sequences were identified and renamed according to the results, then deposited in the NCBI database (GenBank) under the following accession numbers: Micractinium sp. Ehime (the ancestral strain of the alga used in CET microcosm, formerly described as C. vulgaris) (JX889639); Micractinium pusillum NIES-151 (JX889642); Micractinium sp. NIES-455 (JX889640) and Micractinium sp. NIES-2171 (formerly ‘Chlorella sp.’) (JX889641).
Morphology and phenotypic traits of the microcosm alga
General morphology of the microcosm ancestral algal strain and several isolated clones were observed under a microscope (Eclipse E600, ×1000; Nikon, Tokyo, Japan) using a digital camera (HDR-XR550V, Sony, Tokyo, Japan). For each strain or clone, a cell suspension of washed, pre-cultured algae were grown for 3 weeks in MC medium in similar growth conditions to the CET microcosm (30°C, 12 : 12 h light–dark cycle, 42 µmol m−2 s−1) prior to microscopical observations.
Cell size, aggregation ability, growth ability at pHs 4.0 and 7.2, sugar utilization ability, and susceptibility to antibiotics of algal clones, including the ancestral and derived clones isolated from the CET microcosm cultures, were examined and are recorded in . Except where specified, cells were grown in static culture in Erlenmeyer flasks plugged with an air-permeable plug without aeration in the same light and temperature regime as above.
Table 2. Phenotypic variations in cell size (mean ± SD; n = 50), growth ability at pHs 7.2 and 4.0, and cell state of derived algal clones isolated from the CET microcosm cultures after 1819–1847 days, together with the ancestral clone.
Measurement of cell size
Each algal strain or clone was pre-cultured by inoculating 0.3 ml of frozen stock into 30 ml of MC medium (pH 7.2), and incubating for 14 days. Then cells were washed three times with MC medium by centrifugation (800 × g, 20 min) and 0.5 ml of the washed algal suspension was inoculated into 50 ml fresh MC medium, and incubated for 9 days (early stationary phase). Photographs of cells extracted from the 9-day cultures were taken under a microscope (Eclipse E600, ×1000; Nikon, Tokyo, Japan) and the major and minor axes of 50 cells measured for each clone. Using JUMP 6.0 statistical software (SAS, Cary, North Carolina, U.S.A.), statistical comparisons of the cell sizes were performed with ANOVA, and statistical groups were determined by a post-hoc Tukey–Kramer test ().
Ability to aggregate
A washed pre-cultured algal suspension, grown from a frozen stock as described before, was inoculated into a MC medium (pH 7.2) with four replicates, and incubated for 10 days. A small aliquot of the 10-day culture was taken to observe whether cells formed aggregations. The tendency for cells to sediment in the flasks was also observed.
Growth ability at pHs 7.2 and 4.0
A washed, pre-cultured algal suspension was inoculated into a MC medium adjusted to pH 7.2 or pH 4.0 by adding drops of 1.2 m HCl. Four replicate cultures were incubated for 14 days and cells were counted as described in Sano et al. (Citation2009). Algal growth was recorded as ‘+’ if the cell population increased in density with time, ‘–’ if the cell density decreased over 14 days.
Sugar utilization and susceptibility to antibiotics
A small aliquot of a frozen stock was grown on MC medium agar plates for 15 days on a 12 : 12 h light–dark cycle (42 m−2 s−1). For each clone, a loopful of algal cells was then taken and streaked on agar plates containing MC medium supplemented with 0.1% sugars (either mannose, lactose, mannitol, xylose, galactose, glucose, maltose, sucrose or arabinose) which were added through a 0.2 µm cellulose acetate filter (ADVANTEC, California, U.S.A.). Each condition was tested separately and the cells were also streaked on MC medium agar without sugar as a control. After incubating in darkness for 21 days on these selective mediums, the colony formation was scored as ‘+’ or ‘–’. The same experiment was carried out using agar plates supplemented with 0.1% PYG (0.1% peptone, 0.1% yeast extract and 0.1% glucose) and 0.01% antibiotic (either streptomycin, kanamycin, or ampicillin, sterilized through a 0.2 µm filter) on a 12 : 12 h light–dark cycle to check susceptibility to the antibiotics, reported as resistant (‘r’), or sensitive (‘s’). Colony formation or absence was observed after 10 days’ incubation.
Results
Phylogenetic position of the ancestral and derived clones of the CET microcosm
The phylogenetic analyses of the SSU and ITS sequences shown in focus on the Chlorella clade (Krienitz et al., Citation2004) and especially on the known symbiotic algal species in Chlorella and Micractinium. The Parachlorella outgroup was clearly separated from the Chlorella clade. The genus Micractinium was composed of 27 operational taxonomic units (OTUs) and was distinct from the genus Chlorella (sensu stricto) (14 strains): separation of the genera was supported by high bootstrap values in all statistical analyses (ML, NJ, MP and ME). The Micractinium clade contained representatives of three named Micractinium species – Micractinium pusillum (for which Micractinium pusillum CCAP 248/5 is the authentic strain), Micractinium reisseri (Hoshina et al., Citation2010; Hoshina, Citation2011) and Micractinium belenophorum (a recently transferred species, Pröschold et al., Citation2011) – as well as several unnamed Micractinium species or lineages.
The phylogeny placed the microcosm’s ancestral algal strain (formerly described as C. vulgaris) within a group of Micractinium strains in the Chlorella clade (). This result was highly supported in all bootstrap analyses and by the presence in the ITS2 of molecular signatures typical of the genus Micractinium (see below). The CET microcosm ancestral strain and the clones derived from the long-term microcosm experiments comprised a single OTU in the phylogeny and no rDNA differences were found between them. The closest neighbour to the microcosm alga was Micractinium sp. CCAP 211/92, with 100% bootstrap support for a sister relationship in all analyses. The SSU of Micractinium sp. CCAP 211/92 was identical to that of the ancestral microcosm strain, except for a group I intron (349 bp) that was not present in the SSU of the microcosm alga. However, seven differences in the ITS nucleotide sequences were detected between the microcosm strain and its closest neighbour (see below). Also, 52 differences were found between the ITS2 sequences of the ancestral algal strain and Micractinium sp. NIES-455 (data not shown).
The phylogenetic analyses placed Chlorella sp. NIES-2171, Chlorella vulgaris CCAP 211/79 and Micractinium sp. NIES-455 strains within the Micractinium clade, among the lineages of Micractinium sp. strains. Two nucleotide variations in the newly sequenced SSU of NIES-2171, at positions 17 (A to T) and 35 (T insertion), were found when compared with the available sequence in GenBank (AB488574). The newly sequenced M. pusillum NIES-151 was placed within the M. pusillum group with 100% bootstrap support for all analyses.
Comparative analysis of the ITS2 secondary structure
represents an apomorphy analysis of the secondary structures of ITS 2, focused on helix 3 of ITS2, where the molecular signatures are located that separate the Chlorella and the genus Micractinium (Luo et al., Citation2006, Citation2009). Notably, the difference in paired nucleotides located at the top of the helix 3 (position 9 in ) is a synapomorphy for Micractinium (Luo et al., Citation2006, Citation2009). At this position, Chlorella sensu stricto species should exhibit a G–C pairing, whereas Micractinium species should exhibit a C–G pairing (Luo et al., Citation2006, Citation2009). The ancestral microcosm strain, along with Chlorella vulgaris CCAP 211/79 and Chlorella sp. NIES-2171, possessed the compensatory base change (CBC), along with three other synapomorphies, of the genus Micractinium, which support their classification within this genus. Moreover, the helix 3 sequence of the ancestral microcosm alga was identical to that of its closest neighbour, CCAP 211/92 (). A detailed comparison of the whole ITS2 structures of those strains, revealing three nucleotide substitutions between the two strains (in helix 2, helix 4 and the internal loop), is provided in the Supplementary Fig. S1.
Fig. 2. Comparison of algal rRNA secondary structures of the upper parts of Helix III of ITS 2. The synapomorphic signatures for Micractinium and Chlorella are indicated by a number, as presented in Luo et al. (Citation2006). The strains ‘Chlorella sp.’ NIES-2171, ‘Chlorella vulgaris’ 211/79, and the ancestral strain used for the CET microcosm (formerly described as C. vulgaris), possess four signatures identical to the representative species Micractinium pusillum CCAP 248/5, indicating they belong to the genus Micractinium. The G-C nucleotide pairing at the signature number 8 differs with one of the representative species M. pusillum CCAP 248/5, as observed in the secondary structure proposed for M. reisseri (Hoshina et al., Citation2010).
General morphology and cytology of ancestral and derived clones
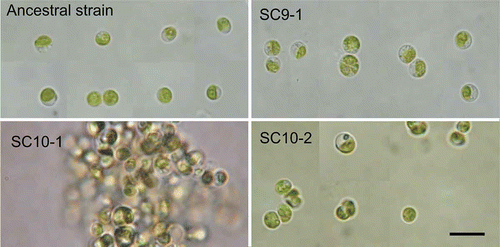
The ancestral and three derived algal clones were cultured in the same conditions and their cell morphologies were compared (). The vegetative algal cells were similar in shape, being spherical or ovoid, and contained a single chloroplast; however, they differed in size (see below). The cell morphologies of the Micractinium or Chlorella sp. strains obtained from the NIES culture collection are available for comparison in Supplementary Information 3.
Fig. 3. Cell morphology of the ancestral strain of the CET microcosm and the derived clones SC9-1, SC10-1 and SC10-2 isolated from 5-year long-term cultures. Algal cells were cultivated for 3 weeks in MC medium prior to light microscopical observation. The derived clone SC10-1 formed large cell-aggregations in bacteria-free static cultures while the other clones did not. Scale bar = 10 µm.
Variations in the phenotypic traits of derived algal clones of the microcosm
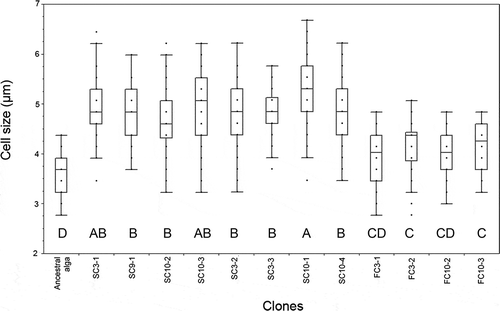
Measurement of cell size of the algal clones isolated from the microcosm cultures () revealed significant differences between the ancestral and derived clones (ANOVA, P < 0.001). The vegetative cells of the ancestral strain were smallest, with the major axis measuring between 2.7–4.37 µm in diameter. The FC clones (FC3-1 and FC3-2: see Supplementary information 1) had similar shape and average sizes of 3.97 and 4.16 µm, respectively, for the major axis. Multiple comparisons using a Tukey–Kramer test were used to define statistical groups, which placed the ancestral strain in group D (smallest cells), followed by the FC clones in groups C and CD (). Additional post hoc comparisons between the FC clones and the ancestral strain showed that despite the overlap in the ranges of dimensions, the ancestral cells were smaller (Tukey’s HSD, P < 0.05). Clones SC3-2, SC3-3, SC9-1, SC10-1, SC10-2, SC10-3 and SC10-4, isolated from 5-year cultures, presented a spherical or ovoid shape. Results from ANOVA followed by post hoc comparisons between values showed that the SC isolates were larger (in average or maximal size) than either the ancestral or FC clones (Tukey’s HSD, P < 0.05), with the largest cell sizes observed for SC10-1 (, ). The general increase in cell size of all isolates was proportional for both cells’ axes, following a linear regression (y = 0.9461x – 0.2505; R² = 0.8325) indicating the cells tended to maintain their spherical shape.
Fig. 4. Average cell size (major axis) of the ancestral alga and derived clones isolated from 5-year microcosm cultures (N = 50). Box-plots represent the median values and the 25th and 75th percentiles (quartiles). Points located at the outside of the boxplot represent possible outliers. Capital letters (A to D) indicate the statistical groups determined with a multiple comparison test (Tukey–Kramer, P < 0.05).
Cell-aggregation was not observed in the ancestral strain but did occur in many of the derived clones (). Microscopical observations revealed that the SC10-1 cell-aggregations were the largest (data not shown). Also, a tendency for cell sedimentation was observed for the ancestral strain and for most of the derived clones, but not for SC9-1, SC10-2, SC10-3 and SC10-4.
All algal clones could grow in pH 7.2 MC medium, whereas at pH 4.0 the growth was inhibited, except for the ancestral strain and isolates SC3-1 and SC3-2 (). The population density of FC10-2 declined over the first 7 days of culture then increased slowly (data not shown). The algal growth on sugar-supplemented MC medium in darkness exhibited a similar pattern throughout (). Ancestral and derived clones grew on glucose or galactose, but not on mannose, lactose, mannitol, xylose, maltose, sucrose or arabinose. The algal resistance to antibiotics was tested in a similar experiment and showed that all clones were resistant to kanamycin and ampicillin (). Most clones, including the ancestral one, were resistant to streptomycin, whereas SC9-1 and SC10-2 were sensitive.
Table 3. Ability to use sugars and susceptibility towards antibiotics of the ancestral and derived algal clones isolated from the CET microcosm cultures. Abbreviations: man (mannose), lac (lactose), mtl (mannitol), xyl (xylose), gal (galactose), glc (glucose), mal (maltose), suc (sucrose), ara (arabinose), str (streptomycin), kan (kanamycin), amp (ampicillin), + (growth), – (no growth), r (resistant), s (sensitive).
Discussion
Recent developments in reconstructing phylogenetic relationships between strains or species in Chlorella-like algae (Hoshina et al., Citation2005, Citation2010; Luo et al., Citation2006; Pröschold et al., Citation2011) not only provide an important foundation for species identification, but also pave the way for analysis of their evolutionary history, including adaptive radiation by coevolution with heterotrophic species and adaptations to free-living niches.
rDNA-based identification
The origin of the ancestral strain (formerly known as ‘Chlorella vulgaris’) including its history prior to its arrival in our laboratory, is unknown; it was obtained from a private culture collection and records were lost before we acquired it, due to difficulties in culture management. This makes it impossible to know the strain’s natural habitat and its interactions with other species prior to isolation. However, its identity is now clear: bootstrapped phylogenetic analyses of molecular genetic data (comprising the whole of the 18S and ITS regions) confidently place the microcosm alga (both the ancestral strain and derived clones) in the genus Micractinium, the near neighbour of the Chlorella group sensu stricto (). The two genera are clearly separated which is in accordance with recent studies (e.g. Luo et al. Citation2006). Moreover, the ancestral strain, along with the strains Chlorella sp. NIES-2171 and C. vulgaris 211/79, showed the characteristic molecular synapomorphies of the genus Micractinium () that were proposed by Luo et al. (Citation2006, Citation2009). Therefore, these strains should be placed within the genus Micractinium and renamed accordingly. For the moment, we propose referring to the microcosm ancestral strain as ‘Micractinium sp. Ehime; Chlorella sp. NIES-2171 as ‘Micractinium sp. NIES-2171; and Chlorella vulgaris CCAP 211/79 as ‘Micractinium sp. CCAP 211/79. We think it is premature to describe a new Micractinium species for the microcosm alga or for the other strains identified as Micractinium sp., because the species concept is not well developed within this genus (Luo et al., Citation2006).
As for the algal clones isolated from 5-year cultures, the results indicated no 18S rDNA or ITS differences between them and the ancestral strain. However, the present results may not necessarily indicate that the rDNA sequences, which are present in multiple copies in the genome, are all exactly the same in the derived clones and identical to those of the ancestral strain. The use of AFLP methods has been shown to reveal considerable genomic variation in Chlorella-like algae when their rDNA sequences are similar (Müller et al. Citation2005). Hence, in a future study of the microcosm strains, it may be worthwhile to use an AFLP approach to give supplementary information on the microcosm strain and its derived algal clones and possible variation at an intraspecific level.
Phenotypic changes of the alga in the microcosm cultured over 5 years
Both the ancestral and the derived algal clones could grow on galactose and glucose in darkness; this ability is shared with algae formerly known as ‘Chlorella pyrenoidosa’ (Samejima & Myers, Citation1958), though the use of this name is now outdated as a result of recent phylogenetic revisions (Krienitz et al., Citation2004). The ancestral strain and the algal isolates tested in this study all exhibited the same sugar utilization pattern. However, our results indicate that variations occurred in other phenotypic traits in several algal clones derived from a single ancestral cell, after microcosm culture (, ). These variations concern (1) cell size, (2) cell aggregation or sedimentation, (3) growth rate at pHs 7.2 and 4.0, and (4) sensitivity to antibiotic drugs.
All algal clones isolated from the 5-year cultures showed an increased average size compared to the ancestral clone (). Remarkably, the cell sizes of ‘FC’ clones – originally isolated from the outside of Tetrahymena cells – were smaller than the ‘SC’ cells isolated from within host cells (P < 0.001). At present, the mechanism for the increased cell size of the derived clones, and particularly the SC clones, is not known. The measurement of algal cell size, as well as the assessment of other traits, was conducted in a standard single culture using fresh MC medium: the algal clones were grown in two consecutive batch cultures (i.e. a culture was transferred from a frozen sample into fresh MC medium and grown, then transferred into further fresh MC medium and grown for a second time before measurement).The algal populations grew 100-fold in each batch culture, which implies that, during the course of two consecutive periods of batch culture, about 13 generations or divisions occurred (~2 × log2100), in addition to the generations that occurred during the process of isolation from within Tetrahymena cells and purification on agar before making frozen stocks (Supplementary Information 1). Therefore, any phenotypic differences between clones likely reflect genetic differentiation rather than phenotypic plasticity induced by environmental conditions, though we have not examined the clones using molecular methods designed to detect differences at the DNA level. If so, our results indicate that selection favoured a relatively large cell size in Micractinium sp. Ehime through the intimate interaction with the ciliate. Further investigation is required to determine the selection mechanism for this change in cell size.
Also, our data indicate that many algal clones have reduced or repressed growth at pH 4.0. It is known that a Tetrahymena pyriformis cell vacuole’s pH is approximately 3.5–4 after ingestion of food particles (Nilsson, Citation1977). The non-growth at pH 4.0 could be related to the repeated ingestion or a hypothetic regulation of the endosymbiont population within the cell vacuoles of T. thermophila. However, most of the aggregate-forming cells also exhibited similar repressed growth at pH 4.0 (), which makes explanation difficult.
The tendency of algal cells to be dispersed or to aggregate or sink to the bottom is likely to affect the interaction between the algae and the other species (E. coli and T. thermophila) in the microcosm. Cell aggregation will strongly affect the ingestion rate of algal cells by the ciliate due to differences in the effective size of the alga relative to the ciliate’s ‘mouth’. The outer opening diameter (major axis) of the oral groove of the T. thermophila ancestral strains, CU427 and CU438, were 10.0 µm ± 1.1 and 10.2 µm ± 1.2, respectively (n = 32) (Nakajima et al., Citation2009); this means that although it is physically possible for single algal cells to be ingested, it is less likely if they are aggregated (see for the algal cell sizes), though a small percentage of the aggregate-forming algae may occasionally be ingested by hosts, as some aggregation-forming clones were found in the host cells (). On the other hand, it may be expected that algal cells with suspended and non-aggregating forms (e.g. SC9-1, SC10-2, SC10-3) would tend to be more easily ingested by the ciliate than the other types of cells. It was demonstrated that these non-aggregating algal isolates clones enhanced the longevity of a Tetrahymena clone when co-cultured in the absence of bacteria, suggesting that these algal clones developed an endosymbiotic phenotype (Germond et al., Citation2013; Nakajima et al., Citation2013). The aggregated form of the algal cells is likely to facilitate interactions with the bacterium by harbouring the bacterial cells within the aggregates.
Protist hosts may ingest algae and thus the size and shape of the algae is clearly one of the first barriers to the evolution of endosymbiosis. In fact, many autotrophic organisms have developed behavioural or morphological features that appear to be countermeasures to avoid grazing. Phytoplankters can reduce grazing by zooplankton through various features of their morphology, life-history and behaviour, including changes in size and cell wall shape (Lass & Spaak, Citation2003; Van Donk, Citation2005). Bristle formation in Micractinium strains is of particular interest in relation to adaptive change of algae in response to grazing and the impact on the establishment of algal symbioses. Some algae among the Chlorellaceae develop bristles or spines, and most Micractinium strains can develop bristles on their cell walls (bristles differ from spines in that they contain no cellulose fibres; Schnepf et al., Citation1980). Pröschold et al. (Citation2010) showed a polyphyletic distribution of bristle-formation in the three genera Micractinium, Didymogenes and Hegewaldia. Variations exist within a genus in terms of presence or absence or numbers of bristles (Schnepf et al., Citation1980). In the genus Micractinium, the formation of bristles depends on environmental conditions and biotic interactions, and is suggested to be a protection against grazing pressure from rotifer predators such as Brachionus (Schlüter et al., Citation1987; Luo et al., Citation2006).
Bristle formation can be induced in most non-bristled strains; for example, bristles were easily induced in several strains of M. pusillum within 24 hours using the culture filtrate of Brachionus calyciflorus (Luo et al., Citation2006). However, several Micractinium strains have been reported to be unable to produce bristles in Brachionus cultures, such as the strains CCAP 211/11F and CCAP 211/92 (Luo et al., Citation2009) and endosymbiotic strains CCAP 211/83 and SAG 241.80 of P. bursaria (Pröschold et al., Citation2011). For the first two strains, the authors explained the absence of bristles by the absence of grazing pressure in the edaphic environment. As for the endosymbiotic strains, it is impossible to know if the bristle-forming mechanism was not present, present but not expressed, or lost through the adaptation to endosymbiosis. During the long-term culture of the CET microcosm, Micractinium sp. Ehime has never developed bristles, despite the various conditions of culture, including temperature, pH, light intensity/cycle and medium composition (data not shown). The algal cells have also been grown in the presence of T. thermophila and T. pyriformis, but bristles were not observed. Luo et al. (Citation2009) proposed a ‘standard test’ of the ‘Brachionus factor’, in which algal strains would be exposed to grazing using Brachionus calyciflorus. In our study, systematic testing of this kind was not carried out for the ancestral strain. However, the fact that the ancestral alga and all the isolates of Micractinium used in the microcosm study formed no bristles in the presence of T. thermophila implies that the strain does not respond to grazing by producing bristles and hence instead has the potential to evolve an endosymbiotic association with the ciliate under the culture conditions. Further investigations are needed to determine if the ancestral strain, along with its neighbouring CCAP 211/92, the strains Micractinium sp. NIES-2171, NIES-455 and Micractinium sp. 211/79, are representative members of a non-bristle species of Micractinium.
More generally, particular phenotypic traits of unicellular algae, such as a cell size smaller than the mouth of prospective hosts, the absence of defence mechanisms against grazing, and the capacity to resist host digestion, must be important factors allowing repeated algal ingestion and the possibility of intimate intracellular interactions with prospective hosts. If these conditions are met, endosymbiosis between algae and heterotrophic organisms can evolve under a particular selective force (Nakajima et al. Citation2009).
In conclusion, the results from the present study demonstrate that a Chlorella-like alga, now identified as Micractinium sp. Ehime, has diversified phenotypically from a single ancestral clone during the 5-year culture of the microcosm. These results suggest that local selection pressures operating on Chlorella-like algae in natural habitats may diversify a variety of phenotypes to adapt to various niches, including symbiotic association with other species, which will consequently shape their macro-evolutionary phylogenetic patterns.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at dx.doi.org/10.1080/09670262.2013.860482
Supplementary Information 1. Experimental procedure for the isolation of algal clones obtained from the long-term cultures of the CET microcosm.
Supplementary Information 2. List of primers used in this study. The site number 1 corresponded to the position 1 of SSU rDNA sequence of Micractinium pusillum 248/5, as referred in GenBank (accession number FM205836). The list includes primer information from Nakayama et al. (1996), not otherwise referred to in the text.
Supplementary Information 3. Concatenated SSU–ITS alignment (fasta file)
Supplementary Table S1. Comparison of bootstrap support for the six genera using different datasets. Each dataset was analysed using Modeltest to test which evolutionary model fits best (according to the Akaike Information Criterion, corrected) for the phylogenetic analyses (see Materials and methods). The bootstrap values were calculated by MEGA5 using maximum likelihood (ML, using the best model, 100 replicates), distance neighbour-joining (NJ, using the best model; 1000 replicates), maximum parsimony (MP, 1000 replicates) and maximum evolution (ME, using best model, 1000 replicates).
Supplementary Table S2: List of strains used for the phylogenetic analysis (). The GenBank entries or references give the origin, year of isolation and the name of the isolator.
Supplementary Figure S1: Comparison of the ITS2 secondary structures of the ancestral alga of the CET microcosm and its closest neighbour CCAP 211/92. The data were obtained from mfold analysis (see Materials and methods). Four nucleotide differences can be found between the two strains (black arrow).
Supplementary Figure S2: Cell morphology of the algal strains obtained from the NIES culture collection (Japan) and sequenced de novo in this study: Micractinium pusillum NIES-151, Micractinium sp. NIES-455 and Micractinium sp. NIES-2171 (formerly named ‘Chlorella sp.’). Scale bar is 10 µm.
Supplementary material
Download Zip (2.9 MB)Acknowledgements
We thank Todd Miller for English revisions. The isolation of algal clones from 5-year microcosm cultures was assisted by Mimi Karita. We also thank the editors and the two anonymous reviewers for their insightful and valuable comments that helped to improve the quality of the manuscript.
References
- Germond, A., Kunihiro, T., Inouhe, M. & Nakajima, T. (2013). Physiological changes of a green alga (Micractinium sp.) involved in an early-stage of association with Tetrahymena thermophila during 5-year microcosm culture. BioSystems, 7: 164–171.
- Hoshina, R. (2011). Comments on the taxonomic treatment of Micractinium reisseri (Chlorellaceae, Trebouxiophyceae), a common endosymbiont in Paramecium. Phycological Research, 59: 269–272.
- Hoshina, R., Kato, Y., Kamako, S.I. & Imamura, N. (2005). Genetic evidence of ‘American’ and ‘European’ type symbiotic algae of Paramecium bursaria Ehrenberg. Plant Biology, 7: 526–532.
- Hoshina, R., Iwataki, M. & Imamura, N. (2010). Chlorella variabilis and Micractinium reisseri sp. nov. (Chlorellaceae, Trebouxiophyceae): redescription of the endosymbiotic green algae of Paramecium bursaria (Peniculia, Oligohymenophorea) in the 120th year. Phycological Research, 58: 188–201.
- Huss, V.A.R., Ciniglia, C., Cennamo, P., Cozzolino, S., Pinto, G. & Pollio, A. (2002). Phylogenetic relationships and taxonomic position of Chlorella-like isolates from low pH environments (pH < 3.0). BMC Evolutionary Biology, 2:1–3.
- Krienitz, L., Hegewald, E.H., Hepperle, D., Huss, V.A.R., Rohr, T. & Wolf, M. (2004). Phylogenetic relationship of Chlorella and Parachlorella gen. nov. (Chlorophyta, Trebouxiophyceae). Phycologia, 43: 529–542.
- Lass, S. & Spaak, P. (2003). Chemically induced antipredator defences in plankton: a review. Hydrobiologia, 491: 221–239.
- Luo, W., Pflugmacher, S., Pröschold, T., Walz, N. & Krienitz, L. (2006). Genotype versus phenotype variability in Chlorella and Micractinium (Chlorophyta, Trebouxiophyceae). Protist, 157: 315–333.
- Luo, W., Pröschold, T., Bock, C. & Krienitz, L. (2009). Generic concept in Chlorella-related coccoid green algae (Chlorophyta, Trebouxiophyceae). Plant Biology, 12: 545–553.
- Mathews, D.H., Sabina, J., Zuker, M. & Turner, D.H. (1999). Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. Journal of Molecular Biology, 288: 911–940.
- Müller, J., Friedl, T., Hepperle, D. & Lorenz, M. (2005). Distinction between multiple isolates of Chlorella vulgaris (Chlorophyta, Trebouxiophyceae) and testing for conspecificity using amplified fragment length polymorphism and ITS rDNA sequences. Journal of Phycology, 41: 1236–1247.
- Nakajima, T., Sano, A. & Matsuoka, H. (2009). Auto-/heterotrophic endosymbiosis evolves in a mature stage of ecosystem development in a microcosm composed of an alga, a bacterium and a ciliate. BioSystems, 96: 127–135.
- Nakajima, T., Matsubara, T, Ohta, Y. & Miyake, D. (2013). Exploitation or cooperation? Evolution of a host (ciliate)-benefiting alga in a long-term experimental microcosm culture. BioSystems, 113: 127–139.
- Nakayama, T., Watanabe, S., Mitsui, K., Uchida, H. & Inouye, I. (1996). The phylogenetic relationship between the Chlamydomonadales and Chlorococcales inferred from 18S rDNA sequence data. Phycological Research, 44: 47–55.
- Nilsson, J.R. (1977). On food vacuoles in Tetrahymena pyriformis GL. Journal of Protozoology, 24: 502–507.
- Pröschold, T., Bock, C., Wei, L. & Krienitz, L. (2010). Polyphyletic distribution of bristle formation in Chlorellaceae: Micractinium, Diacanthos, Didymogenes and Hegewaldia gen. nov. (Trebouxiophyceae, Chlorophyta). Phycological Research, 58: 1–8.
- Pröschold, T., Darienko, T., Silva, P.C., Reisser, W. & Krienitz, L. (2011). The systematics of Zoochlorella revisited employing an integrative approach. Environmental Microbiology, 13: 350–364.
- Reisser, W. (1984).The taxonomy of a green algae endosymbiotic in ciliates and a sponge. British Phycological Journal, 19: 309–318.
- Samejima, H. & Myers, J. (1958). On the heterotrophic growth of Chlorella pyrenoidosa. Journal of General Microbiology, 18: 107–117.
- Sano, A., Watanabe, M. & Nakajima, T. (2009). Adaptive characteristics of a ciliate Tetrahymena thermophila in endosymbiotic association with a green alga Chlorella vulgaris derived in a long-term microcosm culture. Symbiosis, 47: 151–160.
- Schlüter, M., Groeneweg, J. & Soeder, C.J. (1987). Impact on rotifer grazing on population dynamics of green microalgae in high-rate ponds. Water Research, 21: 1293–1297.
- Schnepf, E., Deichgräber, G., Glaab, M. & Hegewald, E. (1980). Bristles and spikes in Chlorococcales: ultrastructural studies in Acanthosphaera, Micractinium, Pediastrum, Polyedriopsis, Scenedesmus, and Siderocystopsis. Journal of Ultrastructural Research, 72: 367–379.
- Summerer, M., Sonntag, B. & Sommaruga, R. (2007). An experimental test of the symbiosis specificity between the ciliate Paramecium bursaria and strains of the unicellular green alga Chlorella. Environmental Microbiology, 9: 2117–2222.
- Summerer, M., Sonntag, B. & Sommaruga, R. (2008). Ciliate-symbiont specificity of freshwater endosymbiotic Chlorella (Trebouxiophyceae, Chlorophyta). Journal of Phycology, 44: 77–84.
- Tamura, K. & Nei, M. (1993). Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution, 10: 512–526.
- Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M. & Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 10: 2731–2739.
- Van Donk, E. (2005). Planktonic interactions: developments and perspectives. Verhandlungen. Internationale Vereinigung für Theoretische und Angewandte Limnologie, 29: 61–72.
- Zuker, M. (2003). Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Research, 31: 3406–3416.