Abstract
The unconsolidated sediment of intertidal mudflats constitutes a highly unstable environment, due to continuously changing water levels and currents as well as temporary exposure to the air. Therefore, diatoms inhabiting marine intertidal areas are subjected to strongly changing surface light and UV intensities due to exposure at low tide. Five marine intertidal diatoms (Achnanthes exigua, Cocconeis peltoides, Diploneis littoralis, Navicula digitoradiata and Amphora exigua) were isolated from the Solthörn tidal flat (Lower Saxony, southern North Sea). Semi-continuous cultures were used to determine the effect of UV radiation (photosynthetically active radiation only [PAR], PAR+UV-B, PAR+UV-A, PAR+UV-B+UV-A) during short- and long-term exposure (6 h or 30 days). Growth rates, chlorophyll a (chl a), antioxidant capacities, accumulation of phenolic compounds (e.g. flavonoids) and DMSP, and activities of antioxidant enzymes (superoxide dismutase, ascorbate peroxidase, monodehydroascorbate reductase and glutathione reductase) were assessed. UV-A had only minor effects on cells, while growth rate, chl a content and protein content were significantly reduced after long-term UV-B exposure. Achnanthes exigua extracts showed the highest antioxidant capacity. The highest activity of SOD, APX and MDHAR was found under long-term combined UV exposure (PAR+UV-B+UV-A). Overall, the antioxidative defence of the five isolates was stimulated during exposure to UV radiation, as may be found during emersion. Emersion induces oxidative stress and, as a result, growth of the five diatom taxa was inhibited to suit changing environmental conditions. All five taxa tested in the present study showed species-specific acclimatization potentials, providing possible explanations for variability in population, species composition and ecosystem structures in the face of climatic variations.
Introduction
Depletion of the ozone layer is leading to increasing levels of ultraviolet radiation (UV; 280–400 nm) reaching the earth’s surface (Madronich et al., Citation1995). The effects of this increased UV on primary producers in aquatic systems have been a topic of considerable interest but, although microphytobenthos are important primary producers in many shallow-water and intertidal habitats (Admiraal, Citation1984; Pinckney & Zingmark, Citation1993; Heip et al., Citation1995), the effect on them of UV has not been widely investigated (exceptions include Garcia-Pichel & Castenholz, Citation1994; Peletier et al., Citation1996; Sundbäck et al., Citation1997; Odmark et al., Citation1998; Rijstenbil, Citation2003).
Benthic diatoms, which are usually the major group of microalgae on tidal flats, may contribute up to ~50% of total areal primary production in coastal waters (Rijstenbil, Citation2003); the highest production rates occur at low tide (Underwood & Kromkamp, Citation1999). Intertidal benthic diatom communities are mainly composed of multi-layered communities of epipelic species, which often perform vertical migrations. These may limit exposure to ultraviolet (UV) and offer photoprotection by self-shading (Underwood & Kromkamp, Citation1999) within the thin layer of intertidal sediments in which photosynthesis is possible (2–4 mm: Kühl et al., Citation1996; Garcia-Pichel et al., Citation1999). However, exposure of intertidal communities of benthic diatoms to air poses a photo-damage threat, making it necessary for them to protect themselves against bright sunlight and UV. UV radiation has been shown to negatively affect benthic microalgae in various ways, with a potential cascade effect on the whole ecosystem (Karsten et al., Citation2009). For example, UV reduces the photosynthetic performance of microphytobenthos (reviewed in Franklin & Forster, Citation1997). Primary targets are the D1/D2 protein complex in photosystem II (PS II), and the water-splitting complex (e.g. Zsiros et al., Citation2006). UV-induced production of active oxygen species via photochemical reactions in the aqueous environment suggests that such photoreactions may also be a result of oxidative stress in benthic diatoms during tidal emersion (Zhou & Mopper, Citation1990; Scully et al., Citation1996). In addition, UV also stimulates intracellular active oxygen production in plants and algae (e.g. Mallick & Mohn, Citation2000; Rijstenbil, Citation2003; Küpper et al., Citation2008). The photosynthetic electron transport system is the major source of reactive oxygen species (ROS) in plant tissues, having the potential to generate singlet oxygen (1O2) and superoxide (O2–) (Asada, Citation1994a, b). Furthermore, during reduction of dioxygen (O2), hydrogen peroxide (H2O2) and hydroxyl radicals (OHS) are formed. All these ROS, except hydrogen peroxide, are characterized by a short lifetime, as they interact rapidly with either water or cellular components (Asada, Citation1994a). Hydrogen peroxide itself is not particularly reactive with most biologically important molecules, but it is probably an intracellular precursor for more reactive oxidants as it passes quickly through membranes by diffusion (Apostol et al., Citation1989). If accumulation of ROS exceeds the capacity of enzymatic and non-enzymatic antioxidant systems, the photosynthetic apparatus is damaged due to destruction of lipids, proteins and nucleic acids, leading finally to cell death (e.g. Karpinski et al., Citation1999). Both UV-A (e.g. Vega & Pizarro, 2000; Rijstenbil, Citation2001) and UV-B (e.g. Hideg & Vass, Citation1996; Rijstenbil, Citation2005) can induce oxidative stress. Antioxidative defence enzymes may be induced, thereby providing protection against cell damage (in particular lipid peroxidation) by UV-induced active oxygen species (Mackerness, Citation2000; Mallick & Mohn, Citation2000; Vincent & Neale, Citation2000). In this context, Rijstenbil (Citation2002) reported that the activities of superoxide dismutase (scavenging active oxygen) and glutathione reductase (reducing GSSG to GSH; glutathione exists in reduced GSH and oxidized GSSG states) increase in response to UV-B exposure in the planktonic diatom Thalassiosira pseudonana, whereas ascorbate peroxidase activities did not change. Le Clainche et al. (Citation2004) found that dimethylsulfonio-propionate (DMSP) is also involved in coping with oxidative stress.
Organisms have evolved a wide range of adaptive strategies that may reduce the impact of solar UV (Riegger & Robinson, Citation1997; for further overviews, see Roy, Citation2000 and Karsten et al., Citation2009). These include repair of UV-induced damage to DNA (e.g. Laurion & Roy, Citation2009) and the production of UV-absorbing substances, such as mycosporine-like amino acids (MAAs; e.g. Hernando et al., Citation2002; Callone et al., Citation2006; Ingalls et al., Citation2010), which act as UV ‘sunscreens’. For instance, gene regulation of the key enzyme of the flavonoid biosynthetic pathway, chalcone synthase, has been demonstrated to be directly regulated by UV-A (320–400 nm) and blue light (Strid et al., Citation1994). Flavonoids are the substances providing sunscreen protection in higher plants (e.g. Ryan et al., Citation2002). In general, phenolic compounds and their derivatives, including simple phenols, flavonoids, phenylproponoids, tannins, lignins and many other substances, contain aromatic rings and hydroxyl groups that determine the radical scavenging power of the compound (Rozema et al., Citation2002). Many phenolic compounds have been described as antioxidants, e.g. phaeophytin from green microalgae (e.g. Goh et al., Citation2010) and phlorotannins from brown microalgae (Nagayama et al., Citation2003). However, they might not be major contributors to the antioxidant capacities of microalgae (Li et al., Citation2007).
During a one-year investigation of the microphytobenthic communities in the Solthörn tidal flat (southern North Sea) we observed significant differences in community composition, particularly in the benthic diatom community in relation to seasonal succession patterns (Scholz & Liebezeit, Citation2012a, b). None of the abiotic parameters determined during the annual cycle provided sufficient explanation for species occurrences. Physiological acclimatization to fluctuating temperatures and salinities was found in several diatom taxa isolated from the Solthörn tidal flat, indicating the ability of single taxa to tolerate such environmental conditions by changes in their biochemical compositions (Scholz & Liebezeit, Citation2012c, d, 2013). The present study is the first part of an extensive description of the physiological acclimatization potential of five intertidal microphytobenthic diatoms to photosynthetically active radiation (PAR) supplemented with UV-A and/or UV-B radiation; the influence of PAR on benthic diatom growth and antioxidative defence strategies is also discussed. The second part describes effects on carbohydrate, amino- and fatty acid compositions (Scholz et al., Citation2014). The taxa were isolated from the Solthörn tidal flat in summer 2008, when relatively high UV was recorded on the sediment surface (up to 118 W m−2 in June 2008; Scholz & Liebezeit, Citation2012b). The main objectives of the present investigation were to (1) determine growth rates, chl a and protein content under different UV-radiations during short- (6 h) and long-term (30 days, with a daily dose of 4 h) experiments; (2) evaluate the antioxidant capacities of the five diatom taxa using different scavenging activity assays; (3) test for the presence of phenolic compounds; (4) quantify activities of the antioxidative key enzymes, viz. superoxide dismutase (SOD), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR) and glutathione reductase (GR); and (5) examine possible additional defence strategies, e.g. the accumulation of DMSP.
Materials and methods
Organisms
The diatoms Achnanthes exigua, Cocconeis peltoides, Diploneis littoralis, Navicula digitoradiata and Amphora exigua were present on the Solthörn tidal flat (southern North Sea, Germany) in summer 2008 (Scholz & Liebezeit, Citation2012a) and isolated as described by Scholz & Liebezeit (Citation2012d). The diatoms were purified from bacterial contaminants by spreading cells on 1.5% f/2 (Guillard, Citation1975) agar plates containing 5 μg ml−1 tetracycline and 5 μg ml−1 kanamycin for 3 weeks (if not otherwise mentioned, all of the chemicals used in this study were of the highest purity from Sigma–Aldrich). The absence of bacterial contaminants was verified by epifluorescence microscopy using 4′,6-diamidino-2-phenylindol (DAPI). summarizes sampling stations and dates.
Table 1. Characteristics of the Solthörn tidal flat isolates. all grown in f/2 medium (Guillard, Citation1975). Sampling locations in the Solthörn tidal flat, sampling dates, cell volumes (*1) and growth medium.
Cultivation of monocultures prior to experiments
Axenic cultures of individual species were grown in 2-l Erlenmeyer flasks (approximately 600 ml culture volume), using f/2 Medium as a base medium (pH 8.04, salinity = 30 psu). To reflect Solthörn tidal flat conditions (Scholz & Liebezeit, Citation2012b), the medium was modified (1) by changing the N:P ratio to 17:1, and (2) adding NaHCO3 after autoclaving to a final concentration of 2 mM. The photon flux rate was 600 µmol photons m−2 s−1 (Master TL-D 18W/840, Philips, Germany) in a light : dark regime of 16 : 8 h; cultures were maintained at 18 ± 0.5°C. A layer of glass beads (diameter: 425‒600 µm; Sartorius AG, Göttingen, Germany) approximately 3 mm thick, was placed on the bottom of culture vessels, used as substratum. Salinity, pH and conductivity were measured using handheld probes (YK-31SA, YK-2001PH SI Model 33, Engineered Systems and Designs-Model 600, Philips W9424). Cultures were supplied with new medium every 7 days to maintain optimal growth. After biofilm development (2 weeks after inoculation) the culture broths were removed with a sterile tube until only a thin liquid film remained over the biofilms. Subsequently, the experiments were started by addition of fresh culture medium.
Experimental design
Axenic isolates were cultivated in 2 l semi-continuous cultures under sterile conditions as described above, using sterilized UV-transparent quartz bottles. Once per day 60 ml of culture suspension were replaced with fresh medium, which gave a dilution rate of 0.6 d−1. UV-A radiation was supplied from four Philips TLK09N 40 W fluorescent tubes (maximum emission at 354 nm) and UV-B from two Spectroline EB160C fluorescent tubes (Laboratory Products Sales, Rochester, New York, USA; maximum emission at 312 nm) pre-burned for c. 110 h to stabilize the lamp output. Lamp radiation of less than 290 nm was eliminated by covering the UV-B tubes with 0.08 mm cellulose acetate, replaced daily. PAR, UV-A and UV-B irradiances were adjusted with neutral density screens positioned in front of the fluorescent tubes. Irradiances were determined with a Biospherical QSL100 quantum scalar irradiance meter for PAR, and with an International Light IL1700 radiometer (International Light Technologies, Massachusetts, USA) for UV-A and UV-B (the photodetectors were SUD033 and SUD240, respectively), resulting in 350 W m−2 PAR, 42 W m−2 UV-A, and 0.47 W m−2 UV-B. The UV values correspond to average values in the environment, as measured in 2008 with a MACAM SR9910 double monochromator scanning spectroradiometer (Macam Photometrics, Livingston, UK) and a cosine sensor in air. PAR corresponded to 1580 µmol photons m−2 s−1 when measured with a LI-COR LI-250 light meter (LI-COR, Lincoln, Nebraska, USA) and a flat sensor. Due to lateral heterogeneities in the light field, PAR0 (photosynthetic active radiation at the point 0) was measured inside culture flasks containing medium but no algae. The first test series was the short-term experiment, running for 6 h. The second test series, the long-term experiment, was started concurrently and ran for 30 days. Six replicate flasks per treatment and species were deployed in the experiment. Taxa were grown in biofilms on a glass bead layer in order to simulate environmental conditions. The position of replicate flasks was randomly changed every other day to eliminate any location effects due to minor changes in external conditions.
Cell counting and monitoring
Cultures were counted microscopically, using an Improved-Neubauer counting chamber with 0.1 mm depth (LO-Laboroptik, Friedrichsdorf, Germany). Only cells exhibiting red fluorescence under UV light were counted. At least 1000 cells per sample were counted at 400× magnification. The specific growth rate (µ) for the long-term experiment was calculated according to the equation
where c1 and c0 are the cell densities at times t1, and t0, respectively. The maximum specific growth rate (µmax) for the short-term experiment was calculated according to the method of Bolch (Citation2004), using a Lineweaver–Burk plot, where values for μmax can be derived by calculating the y-intercepts of the line (y-intercept = 1/μmax).
Harvesting and sample treatment
Biofilms grew without physical disturbance on the glass beads. Diatoms were sampled after dislodging the biofilm from the substrate by rotary shaking (350 rpm). Microscopical observation of the beads after homogenization confirmed release of cells. Biomass from the short-term (6 h) and long-term experiments (30 days) was harvested by centrifugation at 680 × g for 10 min. The six replicates with the diatom biomass were each subdivided into ten aliquots. Six of them were used for different extraction procedures as described below.
Biochemical analysis
Protein content
Protein content was estimated by the Bradford assay (Bio-Rad Laboratories, Hercules, California, USA), using bovine serum albumin as standard (Bradford, Citation1976) and expressed in µg cell−1.
Chlorophyll a content
A spectrophotometric method adapted from Parsons et al. (Citation1984) using Lorenzen’s equations (Lorenzen, Citation1967) was used to ascertain chlorophyll a (chl a) and phaeopigment concentrations in the samples. Measurements were conducted following the method described in Brito et al. (Citation2009). Chl a and phaeopigment contents were calculated in µg cell−1.
Scavenging activity assays
The extraction procedure included three steps, each with different solvents (hexane, dichloromethane and methanol). In the first step, two grams of freeze-dried samples was soaked in 200 ml of solvent and shaken for 24 h, prior to filtration. The filtrate was suspended in 200 ml of the next solvent, which was treated as described above. The process was repeated for the last solvent. After extraction, all solvents were pooled and rotary evaporated (Heidolph VV2000, Heidolph, Schwabach, Germany) at 35°C, and kept at −18°C until further analysis.
2,2-Diphenyl-1-picrylhydrazyl-free-radical-scavenging activity (DPPH)
Stable DPPH radicals were subject to scavenging by the components of extracts, using a modified method proposed by Brand-Williams et al. (Citation1995). A 2 ml fraction of extract was mixed thoroughly with 2 ml freshly prepared 3 × 10–5 M DPPH solution in dimethyl-sulfoxide (DMSO). The reaction mixture was incubated for 1 h and the absorbance of the supernatant measured at 517 nm using an UV-VIS spectrophotometer (PEQLAB Biotechnology, Erlangen, Germany).
Superoxide-anion-scavenging assay (SAS)
The superoxide-anion-scavenging effect was tested following Nagai et al. (Citation2003). A reaction mixture consisting of 0.48 ml of 0.05 M sodium carbonate buffer (pH 10.5), 0.02 ml xanthine (3 mM), 0.02 ml EDTA (3 mM), 0.02 ml bovine serum albumin (= BSA, 0.15%), 0.02 ml NBT (nitro blue tetrazolium salt; 0.75 mM), and 0.02 ml extract was incubated at 25°C for 10 min. Then the reaction was started by adding 6 mU xanthine oxidase while maintaining the mixture at 25°C. The reaction was stopped by adding 0.02 ml CuCl2 (6 mM) after 20 min. The absorbance was measured at 560 nm.
Hydrogen-peroxide-scavenging activity (HPS)
Hydrogen-peroxide-scavenging activity was determined according to Rice-Evans et al. (Citation1995). A sample comprising 80 µl extract, 100 µl phosphate buffer saline (= PBS, 0.1 M, pH 5.0), and 20 µl H2O2 (10 mM) prepared in a Eppendorf cap and incubated at 37°C for 5 min. Thereafter, 30 µl ABTS (2,2-azinobis (3-ethylbenthiazoline)-6-sulfonic acid; 1.25 mM) and 30 µl peroxidase (1 unit ml−1) were added. The absorbance at 405 nm was recorded after incubating the mixture at 37°C for 10 min.
Hydroxyl-radical-scavenging activity (HRS)
The hydroxyl-radical-scavenging effect was assessed according to Chung et al. (Citation1997). A Fenton reaction mixture of 200 µl FeSO4.7H2O (10 mM), 200 µL EDTA (10 mM) and 200 µl 2-deoxyribose (10 mM) was added to 1.2 ml PBS (0.1 M, pH 7.4) and 200 µl extract solution. Then 200 µl H2O2 (10 mM) were added and the mixture incubated at 37°C for 4 h. Afterwards 1 ml trichloroacetic acid (TCA; 2.8%) and 1 ml 2-thiobarbituric acid (TBA; 1%) were added and the mixture kept in a boiling water bath for 10 min. After cooling, the mixture was centrifuged for 5 min at 395 × g, and the absorbance measured at 532 nm.
Ferric-reducing antioxidant power assay (FRAP)
The FRAP assay was carried out as suggested by Omidreza et al. (Citation2005), with a modified concentration. FRAP reagents were freshly prepared for each measurement by mixing 2,4,6-tripyridyl triazine (TPTZ; 10 mM) with HCl (40 mM), acetate buffer (0.3 M, pH 3.6), and ferric chloride (20 mM) in double-distilled water in a ratio 1 : 10 : 1. A 180 μl volume of pre-warmed FRAP reagent (37°C) was added to 20 μl of the sample solution and incubated at 37°C for 30 min, before measurement of absorbance at 593 nm. All the results were expressed as μmol Trolox Equivalent (TE) per gram dry weight of the diatom.
Ferrous-ion chelating assay (FIC)
The ferrous-ion chelating assay was carried out according to Decker & Welch (Citation1990). First, 100 μl of the sample solution were mixed with 100 μl of double-distilled water, followed by addition of 25 μl of ferrous chloride (0.5 mM). Absorbance was measured at 550 nm before as well as 20 min (at room temperature) after 2.5 mM of ferrozine (final concentration) was added. All results were expressed as μmol EDTA equivalent per gram dry weight of the diatom.
Phenolic compounds
Two grams of freeze-dried sample was soaked in 500 ml of methanol, shaken for 24 h, and filtered through a membrane filter. The solvent was then rotary evaporated to dryness at 35°C, and kept at −18°C until further analysis. Phenolic compounds, flavonoids (Shinoda test) and tannins were determined according to the methods described by Scholz & Liebezeit (Citation2006).
Total phenolics assessment was carried out following LeBlanc et al. (Citation2009). In brief, the initial solution was prepared by mixing 300 μl HCl (3%) with 200 μl of the sample extract. The resulting mixture was vigorously vortexed and left still for 3 min. Then, 100 μl of the initial solution was added to 1000 μl sodium bicarbonate (3%). The new mixture was likewise thoroughly vortexed and left still for 2 min before 20 μl of the Folin–Ciocalteu reagent was added. The new solution was vortexed and left undisturbed for 30 min at room temperature. After that, 200 μl of the final solution was loaded into each well of a 96-well plate. All experiments were run in triplicate and the results were reported as μmol of gallic acid equivalents per gram of dried extract.
The total flavonoid content determination was run using the colorimetric method described by Woisky & Salatino (Citation1998). Before measuring the absorbance at 420 nm, 0.5 ml of 2% AlCl3 ethanol solution was added to 0.5 ml of extract, and kept at room temperature for 1 h. Total flavonoid content was calculated as kaempferol equivalents from the calibration curve.
Antioxidant defence enzymes
Samples were freeze-dried and subsequently transferred into an extraction buffer, and sonicated on ice. Homogenates were centrifuged (20 min; 7000 × g; 4°C) and the supernatants (extracts) were kept on ice, prior to activity measurements. A subsample of extract (50 μl) was stored (at −18°C) for protein analysis.
Superoxide dismutases (SOD, EC 1.15.1.1)
Measurements of SOD activity were based upon the principle that superoxide anions (O2–) generated by the xanthine–xanthine oxidase system reduce cytochrome c (cyt c), and that SOD inhibits cyt c reduction (McCord & Fridovich, Citation1969). Specific SOD activities were measured in enzyme units (U mg protein−1).
Ascorbate peroxidase (APX)
Assessment of activity relied on the decrease in the absorption of ascorbate at 290 nm (Weckx & Clijsters, Citation1996).
Glutathione reductase (GR)
GR activity measurements were based on the spectrophotometrically measured determination of NADPH oxidation rate (Weckx & Clijsters, Citation1996).
Monodehydroascorbate reductase (MDHAR, EC 1·6·5·4)
Activity measurements were based on the conversion of ascorbate with ascorbate oxidase to MDHA radicals, and the reduction of MDHA to ascorbate catalysed by MDHAR, whereby the NADP+ yield from NADPH is monitored spectrophotometrically (Jahnke et al., 1991). The NADPH absorption decreasing rates were read during 3 min, at 25°C and λ = 340 nm (specific NADPH absorbance, ε = 6.22 mM−1 cm−1) in a 1 cm quartz cuvette (μmol NADPH [ml extract]−1 min−1). Specific activities of APX, MDHAR and GR, normalized to protein (Rijstenbil, Citation2002), were presented as enzyme units, U mg protein−1 defined, respectively, as μmol ascorbate mg protein−1 min−1 (APX) or as μmol NADPH mg protein−1 min−1 (MDHAR, GR).
Dimethylsulfoniopropionate (DMSP)
DMSP was separated on 0.25 mm silica gel G plates (Machery–Nagel, Düren, Germany) developed with methanol: acetone: concentrated HCl (90 : 10 : 4, v/v/v) (Summers et al., 1998), and visualized using Dragendorff reagent according to Awwad & Adelstein (Citation1966). DMSP was estimated by area measurement of Dragendorff-positive TLC zones (Aronoff, Citation1967). The plots of zone area versus the logarithm of the standard quantity (Fisher Scientific, Loughborough, UK) were linear (r2 ≥ 0.92, P < 0.01).
Statistical analysis
For all measured parameters, the effects of the experimental light treatments were assessed with XLSTAT 2009, version 2009.4.03 (Addinsoft, New York, USA). Results were expressed as mean value ± standard deviation (SD) (N = 3). One way ANOVA and Duncan post hoc tests were applied to test for significant differences at P < 0.05. In addition, the correlation between antioxidant assay, total phenolic and DMSP contents, as well as the activities of antioxidative defence enzymes, were analysed using the Pearson correlation test.
Results
Growth responses, protein and chlorophyll a contents
Cell sizes did not vary significantly during UV exposure (P > 0.05, ). Growth was recorded as the variation in biomass (protein, chl a) and cell numbers (). The maximum growth rates (μmax, 6 h) calculated from the Lineweaver–Burke plot ranged from 0.025 h−1 (UV-B assay, Navicula digitoradiata) to 0.69 h−1 (PAR, Achnanthes exigua, ). The specific growth rate for the short-term exposure in the assays did not show significant differences when compared with the PAR control treatment (P > 0.05), but 4 h of daily exposure to UV-B and UV-A + UV-B during 30 days led to decreasing cell numbers in all tested species (ANOVA F1,10 [PAR:UV-B:UV-A+UV-B] = 20.3, P < 0.0001, ). Significant differences were found for Amphora exigua in the combined PAR+UV-A+UV-B treatment compared with the PAR control (ANOVA F1,6 [PAR: PAR+UV-A+UV-B] =36.1, P < 0.0001).
Fig. 1. Maximum specific growth rate (µmax, A), specific growth rate (µ, B), chl a content (C, D) and protein content (E, F) of Achnanthes exigua, Amphora exigua, Cocconeis peltoides, Diploneis littoralis and Navicula digitoradiata obtained from short- (6 h) and long-term UV (30 days) experiments. All values are means ± SD.
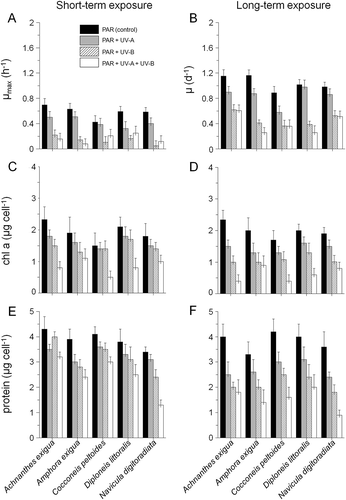
The chl a content of most tested species changed with UV treatment (, D); taxa grown at UV-B and UV-A+UV-B yielded lower chl a than in the PAR-control treatment after short- as well as long-term exposure (average differences = 8–13%, ANOVA F1,12 [PAR:PAR+UV-A+UV-B long/short-term] = 32.3, P < 0.0001, ). The effect of the combined PAR+UV-B+UV-A-treatment was particularly noticeable in Achnanthes exigua and Cocconeis peltoides from the sixth hour onwards (ANOVA F1,18 [PAR+UV-A+UV-B] = 27.7, P < 0.0001).
The protein content was highly variable during short- and long-term experiments and showed significant decreases in the UV-A and UV-B treatments after 30 days’ exposure (ANOVA F1,12 [PAR:PAR+UV-A/PAR+UV-B] = 34.9, P < 0.0001, , F). This effect was most noticeable in N. digitoradiata (difference between PAR and PAR+UV-A+UV-B-treatment: 27%, post hoc P < 0.05, ).
Scavenging activities
With the exception of the FRAP assay in A. exigua during short-term UV exposure, solvent extracts of the five isolates showed the highest antioxidant activities during the long-term treatments (). Here, A. exigua showed the highest antioxidant capacity on average compared with the control and among all tested species (P < 0.05). Furthermore, extraordinarily high activities were recorded for the SAS assay, being significant for Diploneis littoralis in the combined PAR+UV-B+UV-A long-term treatment compared with the PAR control (ANOVA F1,6 [PAR :PAR+UV-A+UV-B] = 30.5, P < 0.0001; assay A4, ). In contrast, the highest FRAP activities were found in Amphora exigua in the long-term UV-B treatment (assay A3). In the remaining antioxidant assays, most species showed activities in the order DPPH > FIC > HPS > HRS, but in Achnanthes exigua, the order was DPPH > HPS > HRS > FIC. The UV-A exposure resulted in lower activities than those of the UV-B treatments in the antioxidant assays (only 13–25%). Finally, the PAR control did not show any significant activities at all (ANOVA F1,16 = 0.64, P = 0.72).
Table 2. Antioxidant effects of methanol extracts on different radical screening tests, expressed as µmol g–1 DW (mean ± SD. Assays: A1 = PAR, A2 = PAR+UV-A, A3 = PAR+UV-B, A4 = PAR+UV-A+UV-B). Abbreviations: DPPH = 2,2-diphenyl-1-picrylhydrazyl-free-radical-scavenging activity, SAS = superoxide-anion-scavenging assay, HPS = hydrogen-peroxide-scavenging activity, HRS = hydroxyl-radical-scavenging activity, FRAP = ferric-reducing antioxidant power assay, FIC = ferrous-ion chelating assay, n.d. = not determined, – = not detected.
Presence and quantity of phenolic compounds
Phytochemical screening results varied significantly between species, irradiance composition and duration of exposure (). The strongest positive colorimetric reactions with the ferric chloride reagent were observed after 30 days’ UV-exposure (), being significant for D. littoralis and N. digitoradiata in the PAR+UV-B+UV-A-treatment compared with the PAR control (ANOVA: F1,24 [PAR:PAR+UV-A+UV-B] = 24.9, P < 0.0001; post hoc: P < 0.05). In this context, the absence of phenolic compounds in the long-term UV-A treatments of C. peltoides and D. littoralis is noteworthy.
Fig. 2. Results from phytochemical screening. Presence of phenolic compounds (A), flavonoids (B: dark grey) and tannins (B: light grey) in biomass extracts of the five isolates. Data were obtained from short- and long-term exposure to PAR, PAR+UV-A, PAR+UV-B and PAR+UV-A+UV-B. The dimensions of circles indicate slight (small circles), medium (small-medium circles), medium-strong (large-medium circles) and strong (large circles) colorimetric reactions.
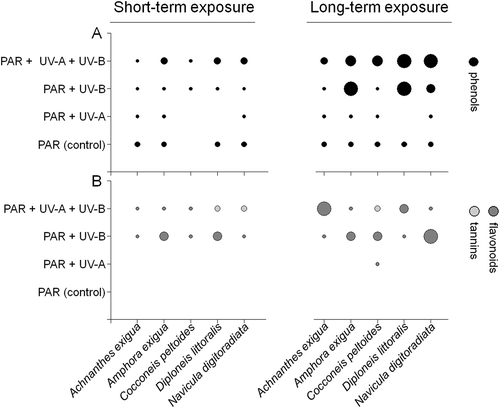
Flavonoid accumulation was stimulated in 18 out of the 40 assays, whereas tannins were detected in only three cases. A significant positive reaction in the Shinoda test was observed for N. digitoradiata in the UV-B assay (ANOVA: F1,12 = 28.2, P < 0.0001, ). Total polyphenolic (TPPC) and flavonoid contents (TFC) varied similarly as a function of irradiance composition and time (). The highest TPPC contents were recorded for long-term UV-A+UV-B exposure, being significant for N. digitoradiata (ANOVA: F1,24 = 25.3, P < 0.0001, ). In contrast, TFC was up to 93% lower than TPPC values after long-term exposure ().
Table 3. Total polyphenolic and flavonoid content obtained from the short- and long-term UVR experiments. Data are expressed as mg 100 g-1, including mean values ± SD. Assays: A1 = PAR, A2 = PAR+UV-A, A3 = PAR+UV-B, A4 = PAR+UV-A+UV-B. Abbreviations: TPPC = total polyphenolics, TFC = total flavonoid content, – = not detected.
Enzyme activities
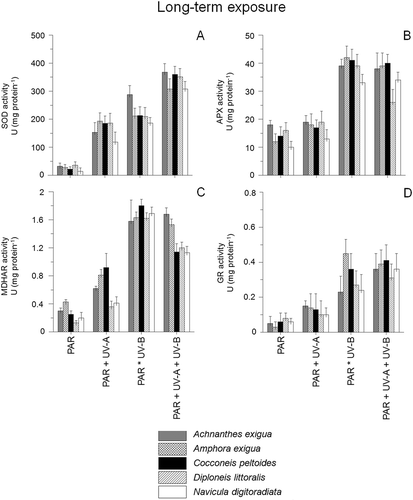
The activity of superoxide dismutase (SOD), ascorbate peroxidase (APX), mono-dehydroascorbate reductase (MDHAR) and glutathione reductase (GR) varied considerably as a function of species, time and UV exposure (, ). The highest activities were recorded in the combined PAR+UV-B+UV-A-treatment after both short- and long-term exposure. Differences occurred mainly in response to the short-term UV-exposure, while long-term experiments led to increasing activities in all tested species (P < 0.05). The highest enzyme activities were observed for SOD in the PAR+UV-B+UV-A-assay after long-term exposure, ranging from 306.4 mg protein−1 in N. digitoradiata to 357.3 in A. exigua (), whereas short-term exposure led to significantly lower activities (). In the short-term experiments, C. peltoides showed the highest SOD activity in the UV-B- and UV-A+UV-B-assays (68.3–74.2 mg protein−1, ), whereas activities of APX, MDHAR and GR were much lower (only 3–18% of SOD activity, –D, 4B–D). Significant APX activities were detected for A. exigua (ANOVA: F1,21 [PAR:PAR+UV-A+UV-B] = 23.1, P < 0.0001, ), whereas GR activities of Achnanthes exigua were the highest in all assays during short-term exposure (ANOVA: F1,20 [PAR:PAR+UV-A+UV-B] = 29.1, P < 0.0001, ).
Fig. 3. Enzyme activities of Achnanthes exigua, Amphora exigua, Cocconeis peltoides, Diploneis littoralis and Navicula digitoradiata after 6 h exposure to PAR, PAR+UV-A, PAR+UV-B and PAR+UV-A+UV-B. (A) Superoxide dismutases (= SOD), (B) Ascorbate peroxidase (= APX), (C) Monodehydroascorbate reductase (= MDHAR) and (D) Glutathione reductase (= GR).
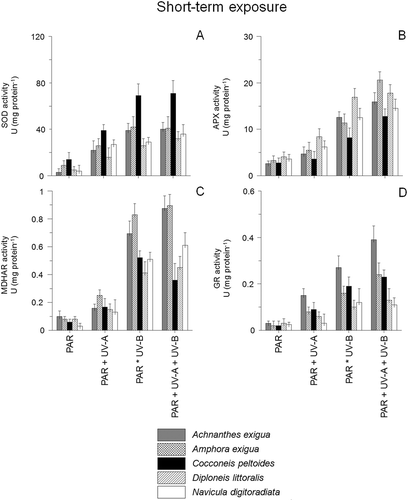
Fig. 4. Enzyme activities of Achnanthes exigua, Amphora exigua, Cocconeis peltoides, Diploneis littoralis and Navicula digitoradiata after 30 days exposure to PAR, PAR+UV-A, PAR+UV-B and PAR+UV-A+UV-B. (A) Superoxide dismutases (= SOD), (B) Ascorbate peroxidase (= APX), (C) Monodehydroascorbate reductase (= MDHAR) and (D) Glutathione reductase (= GR).
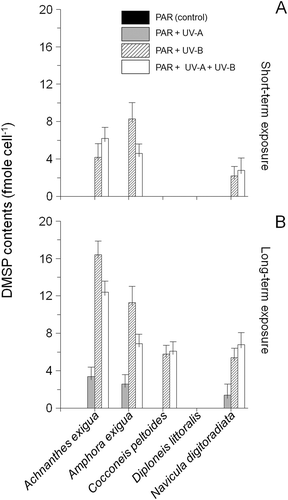
Presence of DMSP
DMSP was detected neither in the PAR controls nor in the short-term UV-A assays (). In contrast, significant accumulations of DMSP were detected in the combined PAR+UV-B+UV-A assay after long-term exposure (ANOVA F1,15 = 29.4, P < 0.0001, ); DMSP was not found at all in extracts of D. littoralis.
Discussion
Effects on growth, chl a and protein content
The relative growth rates of Achnanthes exigua, Amphora exigua, C. peltoides, N. digitoradiata and D. littoralis treated with UV-A and UV-B decreased compared with that of the controls after long-term exposure, while negative effects were not recorded for the short-term treatments. Furthermore, the chl a and protein content concomitantly decreased with exposure to UVR (). Differences in all growth responses measured during the exposures occurred mainly after long-term exposure to PAR+UV-A+UV-B. Nielsen & Ekelund (1995) and Zhang et al. (Citation2005) have reported similar results for some phytoplankton diatoms, but due to differences in the experimental set up, their results are difficult to compare with ours. In plants, UV-B damage has been observed at low radiation doses, causing a general decrease in protein synthesis and mRNA transcripts for proteins involved in chloroplasts and photosynthesis (Strid et al., Citation1994). Hence the reduction in growth rates we observed may be a result of UV-induced oxidative damage to proteins, DNA and membranes, as well as an extra investment in energy (ATP) and reductive capacity needed for enzymatic repair and antioxidative defence (Buma et al., Citation1995; Dawar et al., Citation1998; Mallick & Mohn, Citation2000).
Antioxidant capacities
Recently, there has been increased interest in antioxidants from natural sources, such as plants, because they produce polyphenolics, nitrogen-containing compounds, phytosterols, carotenoids and chlorophyll derivatives, which are known to be able to reduce reactive oxygen species (e.g. Singh et al., Citation2012). In the present study, antioxidant potential was determined by using different kinds of antioxidant assays of the pooled solvent extracts of each of the five Solthörn isolates. Although the results obtained showed differences among the five isolates, there are difficulties in comparing the data with existing literature. In most cases, other authors have compared antioxidant capacity to commercial available antioxidants such as α-tocopherol and butylated hydroxytoluene (BHT), without presenting hard data that could be used for comparison. For example, using the commercial antioxidant α-tocopherol as equivalent in the present study for the long-term UV-B exposure, the antioxidant activities of the pooled extract of Achnanthes exigua would be 83.5%, 36.4%, 41.5% and 67.1% on DPPH radical, superoxide anion scavenging, hydrogen peroxide scavenging and hydroxyl radical, respectively. In contrast, Affan et al. (Citation2006) found up to 36.8% lower values in the scavenging activities for 80% methanolic extracts of the benthic diatom Grammatophora marina. Such differences might have arisen from using only one solvent extract but can also be species specific. Furthermore, carotenoids and naturally occurring tocopherol probably contribute to the high scavenging power exhibited by microalgae such as Grammatophora marina or Chaetoceros sp. (e.g. Affan et al., Citation2006; Goh et al., Citation2010). According to Hanaa et al. (Citation2008) these compounds are extracted by dichloromethane and might contribute to the high scavenging activities found in the present study. However, we can only speculate about this since we did not identify the active substances present in the diatom extracts.
Relationship between phenolic compounds and antioxidant capacity
Due to the differences observed between the 80% methanolic extracts of C. peltoides and D. littoralis in the test for phenolic compounds and the pooled extracts used in the tests for antioxidant capacities, the antioxidant capacity of these two species has been suggested as not being related to phenolic compounds. Overall, there was no significant correlation between the DPPH assay and phenolic content after long-term PAR+UV-A exposure (R2 = 0.192 and 0.102). Furthermore, the results of NBT and the FRAP assays for D. littoralis were moderately inversely correlated with the total phenolic content, explaining at least 23 to 29% of the variation (R2 = −0.231 and −0.298, respectively). Only the FIC assay results for C. peltoides were correlated significantly (P < 0.05) with total phenolic content (R2 = 0.633). Similar observations have been reported by different authors (e.g. Nagayama et al., Citation2003; Affan et al., Citation2006; Goh et al., Citation2010) and are consistent with the fact that microalgae, and in particular diatoms, bear a number of antioxidant compounds, including carotenoids, polyunsaturated fatty acids and polysaccharides (Mohamed, Citation2008; Holtin et al., Citation2009; Scholz et al., Citation2014). In contrast, both the long-term UV-B- and combined UV-treatments of the five diatom taxa showed significant correlations between the DPPH and FRAP assays and the total phenolic contents (P < 0.05), suggesting that phenolic compounds could be involved in acclimatization to at least UV-B long-term exposure.
Activities of antioxidant defence enzymes
Antioxidative defence enzymes may be induced when cells are exposed to UV radiation, offering protection against cell damage (in particularly lipid peroxidation) posed by UV-induced active oxygen species (e.g. Jaleel et al., Citation2009). One of the important features of antioxidant enzymes is their inducibility under conditions of oxidative stress, and such induction could be an important adaptation to UV-B radiation stress (Rijstenbil, Citation2002; Zhang et al., Citation2005). Furthermore, microalgae are damaged when the UV-B dose is relatively high and the production of protective enzymes is reduced, so the antioxidant systems cannot scavenge the excess active oxygen (Rijstenbil, Citation2002). In the present study, the dose of UV-B radiation was moderate and comparable to those conditions found in the Solthörn tidal flat (Scholz & Liebezeit, Citation2012b). The SOD activities of the five Solthörn isolates were within the activity range of Cylindrotheca closterium (Rijstenbil, Citation2002), and higher than those values for UV-B-exposed dinoflagellates Symbiodinium bermudense and Prorocentrum micans Ehrenberg (Lesser, Citation1996). APX activities were much higher in our diatoms than those reported for the two dinoflagellate species (Lesser, Citation1996; Butow et al., Citation1997: 0.2–11 U mg protein−1) but a significant increase in the activities of APX and MDHAR was found only in long-term UV-B-exposed cells. MDHAR activities were far greater than in Peridinium gatunense (Butow et al., Citation1997: 0 to 0.05 U mg protein−1), but similar to those in C. closterium (Rijstenbil, Citation2002). GR activities were similar to those in P. gatunense and C. closterium (Butow et al., Citation1997; Rijstenbil, Citation2002).
Since APX transforms H2O2 produced by SOD to H2O, APX activity was expected to increase with SOD activity, but this was not found in the present study (P > 0.05). Butow et al. (Citation1997) have also shown that a coupling between SOD and APX activity under oxidative stress does not always exist in phytoplankton. GR participates in the ascorbate–glutathione cycle, in which GSH reduces dehydroascorbate to ascorbate (Mallick & Mohn, Citation2000). In the process of active oxygen elimination, GR activity generally follows the pattern of SOD and APX (Halliwell–Asada cycle; Foyer et al., Citation1994) and thus, the GR activity increase under UV-B conditions may result from enhanced H2O2 production.
DMSP accumulation
The so-called ‘compatible solutes’ are small molecules with low molar weight that have little adverse effect on cellular functions (Brown, Citation1976). These organic solutes are accumulated by many microorganisms through synthesis or through uptake from the environment to counteract the outflow of water under hypertonic growth conditions (e.g. Karsten et al., Citation1996). DMSP is such a compatible solute and is produced as an osmolyte in relation to salinity stress (e.g. Krell, Citation2006; Scholz & Liebezeit, Citation2012c). Besides this, it is also found in temperature-stressed marine diatoms, especially at low temperatures (Karsten et al., Citation1996; van Rijssel & Gieskes, Citation2002; Scholz & Liebezeit, Citation2013). The ratio of DMSP to chl a in phytoplankton is normally highest near the surface and tends to decrease with depth (Belviso et al., Citation1993; Dacey et al., Citation1998). These observations suggest that the production of DMSP and related compounds is probably associated with high irradiation or nutrient limitation events. Such conditions can elevate oxidative stress in marine organisms and Sunda et al. (Citation2002) suggested that DMSP and its enzymatic breakdown products (DMS and acrylate) may protect phytoplankton from ROS. Although in the present study DMSP content increased with the duration of UV-exposure, tests showed only weak correlations with the antioxidant capacity assays (R2 = 0.089–0.099), suggesting that DMSP was not actively involved in acclimatization to UV and seemed to be rather an indicator of elevated stress in the tested diatom taxa. Similar results have been obtained for different phytoplankton species (e.g. van Rijessel & Buma, 2002; Harada et al., Citation2009), showing no alteration of intracellular DMSP concentration during and after UV stress.
UV defence strategies in laboratory experiments and tidal environments – a short comparison
In the environment and in laboratory trials, microalgae may respond to UV in two ways: adaptation or avoidance. Moreover, UV effects on microalgae are often species-specific (e.g. Karentz et al., 1991). Previous laboratory studies on the effects of UV-B on cohesive diatom mats (dominated by Gyrosigma balticum) found that, whereas elevated UV-B reduced the rate of primary production (Sundbäck et al., Citation1997), removal of ambient levels of UV-B had little effect (Sundbäck et al., 1996). As in the present study, Underwood et al. (Citation1999) showed that elevated UV-B can have significant effects on a range of variables over a time scale ranging from hours to days. Importantly, they showed, using non-invasive fluorescence techniques, that UV-B can affect the vertical positioning of cells within diatom mats during the first few days of exposure, when other techniques do not detect such effects. Thus, although microphytobenthos may be exposed to high levels of UV, sediments may act as a refuge. Both PAR (Kühl et al., Citation1994) and UV (Garcia-Pichel & Bebout, Citation1996) are rapidly attenuated by the fine sediments inhabited by microphytobenthos, although backscattering can result in elevated irradiance in the topmost layers (Garcia-Pichel & Bebout, Citation1996). Additionally, Wulff et al. (Citation1999) have shown that UV-B radiation is able to penetrate down to 0.6 mm sediment depth and thus species may escape UV by vertical movement as long as the oxygen-depleted zone allows this. In the present study, all five diatom taxa were grown on glass bead layers to simulate the effect found in the environment and to allow them to develop biofilms, in which the species were able to migrate. But measurements of primary production or oxygen levels were not made in our study, nor were migration movements investigated. Due to the multi-species nature of microphytobenthic biofilms and the complex relationships of abiotic and biotic parameter in natural environments, the determination of antioxidative defence mechanisms in individual taxa is more or less restricted to laboratory trials, although these may be compromised by difficulties in the simulation of environmental conditions. Thus, while laboratory experiments should never be uncritically extrapolated to determine community responses, they nevertheless give valuable information on underlying mechanistic processes.
Concluding remarks
Our results demonstrate that UV-B radiation induced a significant decline in the relative growth rate, protein and chl a contents of five microphytobenthic diatom taxa. Furthermore, all tested species displayed antioxidant reactions, accumulating phenolic compounds in response to UV-exposure. The changes induced by UV were diverse. The main factors controlling the different responses within species were the wavelength and duration of irradiances. Here, shorter UV wavelengths and longer exposure times caused the most significant effects. In particular, Achnanthes exigua extracts showed an UV-induced increase in their antioxidant capacity over the control, being the highest for all species studied. In addition, the highest activities of SOD, APX and MDHAR were observed under UV-B- and combined UV-treatments. Finally, DMSP was not significantly involved in the acclimatization process.
Acknowledgements
We are grateful to Dr Daniel Ziehe and Professor Dr Ken Ryan for their really helpful comments on the manuscript.
References
- Admiraal, W. (1984). The ecology of estuarine sediment inhabiting diatoms. Progress in Phycological Research, 3: 269–322.
- Affan, A., Karawita, R., Jeon, Y.-J., Kim, B.-Y. & Kim, J.-B. (2006). Growth characteristics, biochemical composition and antioxidant activities of benthic diatom Grammatophora marina from Jeju Coast, Korea. Algae 21: 141–148.
- Apostol, I., Heinstein, P.F. & Low, P.S. (1989). Rapid stimulation of an oxidative burst during elicitation of cultured plant cells. Plant Physiology, 90: 109–116.
- Aronoff, S. (1967). Techniques of radiobiochemistry. Hafner, New York, NY.
- Asada, K. (1994a). Mechanisms for scavenging reactive molecules generated in chloroplasts under light stress. In Photoinhibition of photosynthesis: from molecular mechanisms to the field (Post, A., Baker, N.R. & Bowyer, J.R., editors), 128–140. BIOS Scientific Publishers, Oxford.
- Asada, K. (1994b). Production and action of active oxygen species in photosynthetic tissues. In Causes of photooxidative stress and amelioration of defence systems in plants (Foyer, C.H. & Mullineaux, P.M., editors), 77–104. CRC Press, Boca Raton, FL.
- Awwad, H.K. & Adelstein, S.J. (1966). A quantitative method for the determination of the specific radioactivity of sulfur-containing amino acids separated by paper chromatography. Analytical Biochemistry, 16: 433‒437.
- Belviso, S., Buat-Menard, P., Putaud, J.-P., Nguyen, B., Claustre, H. & Neveux, J. (1993). Size distribution of dimethylsulfoniopropionate (DMSP) in areas of the tropical northeastern Atlantic Ocean and the Mediterranean Sea. Marine Chemistry, 44: 55–71.
- Bolch, C. (2004). KQA 201 - Intensive algal culture practical manual. School of Aquaculture, University of Tasmania, Launceston, Australia.
- Bradford, M.M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 180: 136–139.
- Brand-Williams, W., Cuvelier, M.E. & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. Lebensmittel-Wissenschaft und Technologie, 28: 25–30.
- Brito, A., Newton, A., Tett, P. & Fernandes, T.F. (2009). Temporal and spatial variability of microphytobenthos in a shallow lagoon: Ria Formosa (Portugal). Estuarine Coastal and Shelf Science, 83: 67–76.
- Brown, A.D. (1976). Microbial water stress. Bacteriological Reviews, 40: 803–846.
- Buma, A.G.J., van Hannen, E.J., Veldhuis, M.J.W., Roza, L. & Gieskes, W.W.C. (1995). Monitoring UV-B induced DNA damage in individual diatom cells by immunofluorescent thymine dimer detection. Journal of Phycology, 31: 314–321.
- Butow, B.J., Wynne, D. & Tel-Or, E. (1997). Antioxidative protection of Peridinium gatunense in Lake Kinneret: seasonal and daily variation. Journal of Phycology, 33: 780–786.
- Callone, A.I., Carignan, M., Montoya, N.G. & Carreto, J.I. (2006). Biotransformation of mycosporine like amino acids (MAAs) in the toxic dinoflagellate Alexandrium tamarense. Journal of Photochemistry and Photobiology B Biology, 84: 204–212.
- Chung, S.K., Osawa, T. & Kawakishi, S. (1997). Hydroxyl radical scavenging effects of spices and scavengers from black mustard (Brassica nigra). Bioscience, Biotechnology, and Biochemistry, 61: 118–123.
- Dacey, J.W.H., Howse, F.A., Michaels, A.F. & Wakeham, S.G. (1998). Temporal variability of dimethylsulfide and dimethylsulfoniopropionate in the Sargasso Sea. Deep-Sea Research, 45: 2085–2104.
- Dawar, S., Vani, T. & Singhal, G.S. (1998). Stimulation of antioxidant enzymes and lipid peroxidation by UV-B irradiation in thylakoid membranes of wheat. Plant Biology, 41: 65–73.
- Decker, E.A. & Welch, B. (1990). Role of ferritin as a lipid oxidation catalyst in muscle food. Journal of Agricultural and Food Chemistry, 38: 674–677.
- Foyer, C.H., Lelandais, M. & Kunert, K. J. (1994). Photooxidative stress in plants. Physiologia Plantarum, 92: 696–717.
- Franklin, L.A. & Forster, R.M. (1997). The changing irradiance environment: consequences for marine macrophyte physiology, productivity and ecology. European Journal of Phycology, 32: 207–232.
- Garcia-Pichel, F. & Bebout, B.M. (1996). Penetration of ultraviolet radiation into shallow water sediments: high exposure for photosynthetic communities. Marine Ecology Progress Series, 131: 257–262.
- Garcia-Pichel, F. & Castenholz, R.W. (1994). On the significance of solar ultraviolet radiation for the ecology of microbial mats. In Microbial mats. NATO ASI Series G: Ecological Sciences, Vol. 35 (Stal, L.J. & Caumette, P., editors), 77–84. Springer, Berlin.
- Garcia-Pichel, F., Kühl, M., Nübel, U. & Muyzer, G. (1999). Salinity dependent limitation of photosynthesis and oxygen exchange in microbial mats. Journal of Phycology, 35: 227–238.
- Goh, S.H., Yusoff, F.M. & Loh, S.P. (2010). A comparison of the antioxidant properties and total phenolic content in a diatom, Chaetoceros sp. and a green microalga, Nannochloropsis sp. Journal of Agricultural Science, 2: 123–130.
- Guillard, R.R. (1975). Culture of phytoplankton for feeding marine invertebrates. In Culture of marine invertebrate animals (Smith, W.L. & Chanley, M.H., editors), 26‒60. Plenum Press, New York, NY.
- Hanaa, H.A.E., Hussein, M.M. & Gamal, S.E. (2008). Algal extracts improve antioxidant defense abilities and salt tolerance of wheat plant irrigated with sea water. Electronic Journal of Environmental, Agricultural and Food Chemistry, 7: 2812–2832.
- Harada, H., Vila-Costa, M., Cebrian, J. & Kiene, R.P. (2009). Effects of UV radiation and nitrate limitation on the production of biogenic sulfur compounds by marine phytoplankton. Aquatic Botany, 90: 37–42.
- Heip, C.H.R., Goosen, N.K., Herman, P.M.J., Kromkamp, J., Middelburg, J.J. & Soetaert, K. (1995). Production and consumption of biological particles in temperate tidal estuaries. Oceanography and Marine Biology Annual Review, 33: 1–149.
- Hernando, M., Carreto, J.I., Carignan, M.O., Ferreyra, G.A. & Gross, C. (2002). Effects of solar radiation on growth and mycosporine-like amino acids content in Thalassiosira sp., an Antarctic diatom. Polar Biology, 25: 12–20.
- Hideg, E. & Vass, I. (1996). UV-B induced free radical production in plant leaves and isolated thylakoid membranes. Plant Science, 115: 251–260.
- Holtin, K., Kuehnle, M., Rehbein, J., Schuler, P., Nicholson, G. & Albert, K. (2009). Determination of astaxanthin and astaxanthin esters in the microalgae Haematococcus pluvialis by LC-(APCI)MS and characterization of predominant carotenoid isomers by NMR spectroscopy. Analytical and Bioanalytical Chemistry, 395: 1613–1622.
- Ingalls, A.E., Whitehead, K. & Bridoux, M.C. (2010). Tinted windows: the presence of the UV absorbing compounds called mycosporine-like amino acids embedded in the frustules of marine diatoms. Geochimica et Cosmochimica Acta, 74: 104–115.
- Jahnke, L.S., Hull, M.R. & Long, S.P. (1991). Chilling stress and oxygen metabolizing enzymes in Zea mays and Zea diploperennis. Plant Cell Environment, 14: 97–104.
- Jaleel, C.A., Riadh, K., Gopi, R., Manivannan, P., Inès, J., Al-Juburi, H.J., Zhao, C.X., Hong-Bo, S. & Panneerselvam, R. (2009). Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiologiae Plantarum, 31: 427–436.
- Karentz, D., McEuen, F.S., Land, M.C. & Dunlap, W.C. (1991). Survey of mycosporinelike amino acid compounds in Antarctic marine organisms: potential protection from ultraviolet exposure. Marine Biology, 129: 157–66.
- Karpinski, S., Reynolds, H., Karpinska, B., Wingsle, G., Creissen, G. & Mullineaux, P. (1999). The role of hydrogen peroxide and antioxidants in systemic acclimation to photo-oxidative stress in Arabidopsis. In Plant responses to environmental stress (Smallwood, M.F., Calver, C.M. & Bowes, D.J., editors), 25–32. BIOS Scientific Publishers, Oxford.
- Karsten, U., Kück, K., Vogt, C. & Kirst, G.O. (1996). Dimethylsulfoniopropionate production in phototrophic organisms and its physiological function as a cryoprotectant. In Biological and environmental chemistry of DMSP and related sulfonium compounds (Kiene, R.P., Visscher, P.T., Keller, M.D. & Kirst, G.O., editors), 143–153. Plenum Press, New York, NY.
- Karsten, U., Wulff, A., Roleda, M., Müller, R., Steinhoff, F.S., Fredersdorf, J. & Wiencke, C. (2009). Physiological responses of polar benthic algae to ultraviolet radiation. Botanica Marina, 52: 639–654.
- Krell, A. (2006). Salt stress tolerance in the psychrophilic diatom Fragilariopsis cylindrus. Ph.D. Thesis, University of Bremen, Germany.
- Kühl, M., Lassen, C. & Jørgensen, B.B. (1994). Light penetration and light intensity in sandy marine sediments measured with irradiance and scalar irradiance fibre optic microprobes. Marine Ecology Progress Series, 105: 139–148.
- Kühl, M., Glud, R.N., Ploug, H. & Ramsing, N.B. (1996). Microenvironmental control of photosynthesis and photosynthesis coupled respiration in an epilithic cyanobacterial biofilm. Journal of Phycology, 32: 799–812.
- Küpper, F.C., Carpenter, L.J., McFiggans, G.B., Palmer, C.J., Waite, T.J., Boneberg, E.-M., Woitsch, S., Weiller, M., Abela, R., Grolimund, D., Potin, P., Butler, A., Luther, G.W., Kroneck, P.M.H., Meyer-Klaucke, W. & Feiters, M.C. (2008). Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proceedings of the National Academy of Sciences of the United States of America, 105: 6954–6958.
- Laurion, I. & Roy, S. (2009). Growth and photoprotection in three dinoflagellates (including two strains of Alexandrium tamarense) and one diatom exposed to four weeks of natural and enhanced ultraviolet-B radiation. Journal of Phycology, 45: 16–33.
- LeBlanc, B.W., Davis, O.K., Boue, S., DeLucca, A. & Deeby, T. (2009). Antioxidant activity of Sonoran Desert bee pollen. Food Chemistry, 115: 1299–1305.
- Le Clainche, Y., Levasseur, M., Vézina, A., Dacey, J.W.H. & Saucier, F.J. (2004). Behaviour of the ocean DMS(P) pools in the Sargasso Sea viewed in a coupled physical‐biogeochemical ocean model. Canadian Journal of Fisheries and Aquatic Sciences, 61: 788–803.
- Lesser, M.P. (1996). Acclimation of phytoplankton to UV-B radiation: oxidative stress and photoinhibition of photosynthesis are not prevented by UV-absorbing compounds in the dinoflagellate Prorocentrum micans. Marine Ecology Progress Series, 132: 287–297.
- Li, H.B., Cheng, K.W., Wong, C.C., Fan, K.W., Chen, F. & Jiang, Y. (2007). Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chemistry, 102: 771–776.
- Lorenzen, C.J. (1967). Determination of chlorophyll and pheopigments: spectrophotometric equations. Limnology and Oceanography, 12: 343–346.
- Mackerness, S.A.-H. (2000). Plant responses to ultraviolet-B (UV-B: 280–320 nm) stress: what are the key regulators? Plant Growth Regulation, 32: 27–39.
- McCord, J.M. & Fridovich, I. (1969). Superoxide dismutase an enzymatic function for erythrocuprein (hemocuprein). Journal of Biological Chemistry, 244: 6049–6055.
- Madronich. S., McKenzie, R.L., Caldwell, M.M. & Bjorn, L.O. (1995). Changes in ultraviolet radiation reaching the earth’s surface. Ambio, 24: 143–152.
- Mallick, N. & Mohn, F.H. (2000). Reactive oxygen species: response of algal cells. Journal of Plant Physiology, 157: 183–193.
- Mohamed, Z.A. (2008). Polysaccharides as a protective response against microcystin induced oxidative stress in Chlorella vulgaris and Scenedesmus quadricauda and their possible significance in the aquatic ecosystem. Ecotoxicology, 17: 504–516.
- Nagai, T., Inoue, I., Inoue, H. & Suzuki, N. (2003). Preparation and antioxidant properties of water extract of propolis. Food Chemistry, 80: 29–33.
- Nagayama, K., Shibata, T., Fujimoto, K., Honjo, T. & Nakamura, T. (2003). Algicidal effect of phlorotannin from brown alga Ecklonia kurome on red tide microalgae. Aquaculture, 218: 601–611.
- Nielsen, T. & Ekelund, N. (1995). Influence of solar ultraviolet radiation on photosynthesis and motility of marine phytoplankton. FEMS Microbiology Ecology, 18: 281–288.
- Odmark, S., Wulff, A., Wängberg, S.-A., Nilsson, C. & Sundbäck, K. (1998). Effects of UVBR radiation in a microbenthic community of a marine shallow-water sandy sediment. Marine Biology, 132: 335–345.
- Omidreza, F., Antonio, L., Rita, P., Giancarlo, M. & Luciano, S. (2005). Evaluation of the antioxidant activity of flavonoids by ferric reducing antioxidant power Q assay and cyclic voltammetry. Biochimica et Biophysica Acta, 1721: 174–184.
- Parsons, T., Maita, Y. & Lalli, C. (1984). A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford.
- Peletier, H., Gieskes, W.W.C. & Buma, A.G.J. (1996). Ultraviolet-B radiation resistance of benthic diatoms isolated from tidal flats in the Dutch Wadden Sea. Marine Ecology Progress Series, 135: 163–168.
- Pinckney, J. & Zingmark, R.G. 1993. Photophysiological response of intertidal benthic microalgal communities to in situ light environments: methodological considerations. Limnology and Oceanography, 38: 1373–1383.
- Rice-Evans, C.-A., Miller, N.J., Bolwell, P.G., Bramley, P.M. & Pridham, J.B. (1995). The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radical Research, 22: 375–383.
- Riegger, L. & Robinson, D. (1997). Photoinduction of UV-absorbing compounds in Antarctic diatoms and Phaeocystis antarctica. Marine Ecology Progress Series, 160: 13–25.
- Rijstenbil, J.W. (2001). Effects of periodic, low UVA radiation on cell characteristics and oxidative stress in the marine planktonic diatom Ditylum brightwellii. European Journal of Phycology, 36: 1–8.
- Rijstenbil, J.W. (2002). Assessment of oxidative stress in the planktonic diatom Thalassiosira pseudonana in response to UVA and UVB radiation. Journal of Plankton Research, 24: 1277–1288.
- Rijstenbil, J.W. (2003). Effects of UVB radiation and salt stress on growth, pigments and antioxidative defence of the marine diatom Cylindrotheca closterium. Marine Ecology Progress Series, 254: 37–48.
- Rijstenbil, J.W. (2005). UV- and salinity-induced oxidative effects in the marine diatom Cylindrotheca closterium during simulated emersion. Marine Biology, 147: 1063–1073.
- Roy, S. (2000). Strategies for the minimisation of UV-induced damage. In The effects of UV radiation in the marine environment (de Mora, S.J., Demers, S. & Vernet, M., editors), 177–205. Cambridge University Press, Cambridge.
- Rozema, J., Björn, L.O., Bornman, J.F., Gaberščik, A., Häder, D.-P., Trošt, T., Germ, M., Klisch, M., Gröniger, A., Sinha, R.P., Lebert, M., He, Y.-Y., Buffoni-Hall, R., de Bakker, N.V.J., van de Staaij, J. & Meijkamp, B.B. (2002). The role of UV-B radiation in aquatic and terrestrial ecosystems – an experimental and functional analysis of the evolution of UV-absorbing compounds. Journal of Photochemistry and Photobiology B: Biology, 66: 2–12.
- Ryan, K.G., Swinny, E.E., Markham, K.R. & Winefield, C. (2002). Flavonoid gene expression and UV photoprotection in transgenic and mutant Petunia leaves. Phytochemistry, 59: 23–32.
- Scholz, B. & Liebezeit, G. (2006). Chemical screening for bioactive substances in culture media of microalgae and cyanobacteria from marine and brackish water habitats: first results. Pharmaceutical Biology, 44: 544–549.
- Scholz, B. & Liebezeit, G. (2012a). Microphytobenthic dynamics in a Wadden Sea intertidal flat – Part I: Seasonal and spatial variations of diatom communities in relation to macronutrient availability. European Journal of Phycology, 47: 105–119.
- Scholz, B. & Liebezeit, G. (2012b). Microphytobenthic dynamics in a Wadden Sea intertidal flat – Part II: Seasonal and spatial variation of non-diatom community components in relation to abiotic parameters. European Journal of Phycology, 47: 120–137.
- Scholz, B. & Liebezeit, G. (2012c). Compatible solutes in three marine intertidal microphytobenthic Wadden Sea diatoms exposed to different salinities. European Journal of Phycology, 47: 393–407.
- Scholz, B. & Liebezeit, G. (2012d). Growth responses of 25 benthic marine Wadden Sea diatoms isolated from the Solthörn tidal flat (southern North Sea) in relation to varying culture conditions. Diatom Research, 27: 65–73.
- Scholz, B. & Liebezeit, G. (2013). Compatible solutes and fatty acid compositions of five marine intertidal microphytobenthic Wadden sea diatoms exposed to different temperatures (southern North Sea). Diatom Research, 28: 337–358.
- Scholz, B., Rúa, A. & Liebezeit, G. (2014). Effects of UV radiation on five marine microphytobenthic Wadden sea diatoms, isolated from the Solthörn tidal flat (Lower Saxony, southern North Sea) – Part II: carbohydrate, amino- and fatty acid compositions. European Journal of Phycology, 49: 97–114.
- Scully, N.M., McQueen, D.J., Lean, D.R.S. & Cooper, W.J. (1996). Hydrogen peroxide formation: the interaction of ultraviolet radiation and dissolved organic carbon in lake waters along a 43–75°N gradient. Limnology and Oceanography, 41: 540–548.
- Singh, A., Jain, A., Sarma, B.K., Jha, A. & Singh, H.B. (2012). Natural antioxidants and their role in cancer prevention. In Nutrition, diet and cancer (Shankar, S. & Srivastava, R.K., editors), 563–583. Springer, the Netherlands.
- Strid, Å., Chow, W.S. & Anderson, J.M. (1994). UV-B damage and protection at the molecular level in plants. Photosynthesis Research, 39: 475–489.
- Summers, P.S., Nolte, K.D., Cooper, A.J.L., Borgeas, H., Leustek, T., Rhodes, D. & Hanson, A.D. (1998). Identification and stereospecificity of the first three enzymes of 3-dimethylsulfoniopropionate biosynthesis in a chlorophyte alga. Plant Physiology, 116: 369–378.
- Sunda, W., Kieber, D.J., Kiene, R.P. & Huntsman, S. (2002). An antioxidant function for DMSP and DMS in marine algae. Nature, 418: 317–320.
- Sundbäck, K., Nilsson, P., Nilsson, C. & Jonsson, B. (1996). Balance between autotrophic and heterotrophic components and processes of sandy sediments: a field study. Estuarine, Coastal and Shelf Science, 43: 689–706.
- Sundbäck, K., Odmark, S., Wulff, A., Nilsson, C. & Wängberg, S.A. (1997). Effects of enhanced UVB radiation on a marine benthic diatom mat. Marine Biology, 128: 171–179.
- Underwood, G.J.C. & Kromkamp, J. (1999). Primary production by phytoplankton and microphytobenthos in estuaries. Advances in Ecological Research, 29: 93–153.
- Underwood, G.J.C., Nilsson, C., Sundbäck, K. & Wulff, A. (1999). Short-term effects of UVB radiation on chlorophyll fluorescence, biomass, pigments, and carbohydrate fractions in a benthic diatom mat. Journal of Phycology, 35: 656–666.
- Van Rijssel, M. & Buma, A.G.J. (2002). UV radiation induced stress does not affect DMSP synthesis in the marine prymnesiophyte Emiliania huxleyi. Aquatic Microbial Ecology, 28: 167–174.
- Van Rijssel, M. & Gieskes, W.W.C. (2002). Temperature, light, and the dimethylsulfonio-propionate (DMSP) content of Emiliania huxleyi (Prymnesiophyceae). Journal of Sea Research, 48: 17–27.
- Vega, M.P. & Pizzaro, R.A. (2000). Oxidative stress and defence mechanisms of the freshwater cladoceran Daphnia longispina exposed to UV radiation. Journal of Photochemistry and Photobiology B: Biology, 54: 121–125
- Vincent, W.F. & Neale, P.J. (2000). Mechanisms of UV damage to aquatic organisms. In The effects of UV radiation in the marine environment (de Mora, S.J., Demers, S. & Vernet, M., editors), 149–176. Cambridge University Press, Cambridge.
- Weckx, J.E.J. & Clijsters, H.M.M. (1996). Oxidative damage and defense mechanisms in primary leaves of Phaseolus vulgaris as a result of root assimilation of toxic amounts of copper. Physiologia Plantarum, 96: 506–512.
- Woisky, R.G. & Salatino, A. (1998). Analysis of propolis: some parameters and procedures for chemical quality control. Journal of Apicultural Research, 37: 99–105.
- Wulff, A., Nilsson, C., Sundbäck, K., Wängberg, S.-A. & Odmark, S. (1999). UV radiation effects on microbenthos – a four month field experiment. Aquatic Microbial Ecology, 19: 269–278.
- Zhang, P.-Y., Yu, J. & Tang, X.-X. (2005). UV-B radiation suppresses the growth and antioxidant systems of two marine microalgae, Platymonas subcordiformis (Wille) Hazen and Nitzschia closterium (Ehrenb.) W. Sm. Journal of Integrative Plant Biology, 47: 683–691.
- Zhou, X. & Mopper, K. (1990). Determination of photochemically produced hydroxyl radicals in seawater and freshwater. Marine Chemistry, 30: 71–78.
- Zsiros, O., Allakhverdiev, S.I., Higashi, S., Watanabe, M., Nishiyama, Y. & Murata, N. (2006). Very strong UV-A light temporally separates the photoinhibition of photosystem II into light-induced inactivation and repair. Biochimica et Biophysica Acta, 1757: 123–129.