Abstract
In the present study we undertook an integrative approach, using both morphological and molecular data (COI-5P + rbcL), to assess the presence of Laurencia pyramidalis in Lusitanian Macaronesia. We studied type material of L. pyramidalis from the herbarium of the Naturalis Biodiversity Center, the Netherlands, and designated a lectotype and syntypes. Vegetative and reproductive features of L. pyramidalis were observed and we included a specimen from the type locality in our molecular analyses. We also investigated the geographical distribution of Laurenciella marilzae, a species recently described from the Canary Islands. Barcode sequences (COI-5P and rbcL) were generated for L. pyramidalis from the type locality (Normandy, France), the Azores, Madeira and the Canary Islands, and for L. marilzae from its type locality (Tenerife, Canary Islands), the Azores and Brazil.
Introduction
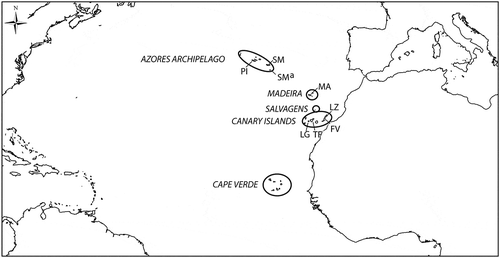
The Macaronesia region consists of five oceanic archipelagos in the north-eastern Atlantic Ocean between 39° N 31° W and 15° N 23° W. From north to south, these are the Azores, the Savage Islands (Ilhas Selvagens) and Madeira (Portugal), the Canary Islands (Spain) and the Cape Verde islands. The Macaronesian islands share many characteristics, although Cape Verde is quite distinct in terms of its climate and biota, having a more tropical climate; it is included within the West African transition province. In the present paper we will focus on the Azores, Madeira and Canary Islands, which belong to the same ecoregion within the Lusitanian province (Spalding et al., Citation2007). The volcanic islands of the Savage Islands, Madeira and the Canary Islands, along with related sea mounts, arose from several geological hotspots at various times during the last 60 Ma (Fernández-Palacios et al., Citation2010), whereas the Azores are much more recent, none emerging more than some 8 Ma ago. The Lusitanian Macaronesian islands have been colonized predominantly by the adjacent North African and European flora and fauna (Juan et al., Citation2000; Gillespie & Clague, Citation2009). Similarly, patterns of species richness and composition of algal assemblages across these islands result mainly from the proximity of continental African and European shores, combined with large and meso-scale oceanographic patterns (Tuya & Haroun, Citation2009).
The marine algal flora of Lusitanian Macaronesia has been investigated sporadically during the past centuries, that of the Canary Islands receiving particular attention (e.g. Bory de Saint-Vincent, Citation1803; Montagne, Citation1840; Børgesen, Citation1930). Since the 1980s, there has been a revival of systematics in this area (Gil-Rodríguez & Afonso-Carrillo, Citation1980a, b; Audiffred & Weisscher, Citation1984; Audiffred & Prud’homme van Reine, Citation1985; Prud’homme van Reine, Citation1988, Citation1990, Citation1998; Prud’homme van Reine et al., Citation1994, Citation2005; Afonso-Carrillo & Sansón, Citation1999).
The Laurencia complex currently encompasses six genera: Laurencia, Osmundea, Chondrophycus, Palisada, Yuzurua and the recently described Laurenciella (Cassano et al., Citation2012a). A list of records of the Laurencia complex for the Azores, Madeira, Savage Islands and Canary Islands is presented in . The Canary Islands, the southern most islands in Lusitanian Macaronesia but also the nearest to the continent, have the highest species richness with 26 species recorded (the number varies depending on the authors, see ), whereas only five species have been reported in the Azores, the northernmost, most isolated and most recently formed archipelago. All the species recorded in the Azores, Madeira and the Savage Islands are also encountered in the flora of the Canary Islands, with the exception of two species (Laurencia nidifica and Osmundea osmunda) recorded only from Madeira. Interestingly, all the members of the Laurencia complex found along the Atlantic European coasts have also been reported in Lusitanian Macaronesia, with the sole exception of Laurencia pyramidalis. One aim of the present study was therefore to investigate the presence of Laurencia pyramidalis in Lusitanian Macaronesia by conducting a floristic survey in the Azores, Madeira and Canary Islands.
Table 1. Laurencia complex species reported from Lusitanian Macaronesia, with sources of information.
Identification of species of the Laurencia complex based on anatomical and morphological characters is extremely difficult due to phenotypic plasticity and overlaps in many morphological characters. As a consequence, among the 28 species reported from Lusitanian Macaronesia, 14 species records have been regarded as doubtful (). DNA barcoding, based on a standardized sequence of the mitochondrial cytochrome c oxidase subunit I gene (COI-5P), has gained recognition as a tool for species delimitation and has proven useful for uncovering new and cryptic species of Rhodophyta, refining species distributions, and detecting invasive and alien species (Saunders, Citation2005, Citation2008, Citation2009; Robba et al., Citation2006; House et al., Citation2008; Walker et al., Citation2009; Clarkston & Saunders, Citation2010; Le Gall & Saunders, Citation2010; Manghisi et al., Citation2010). However, the molecular systematics of the Laurencia complex has been based mainly on sequences of the plastid-encoded large subunit of RuBisCO (rbcL), which has been used to infer interspecific relationships within the various genera (Nam et al., Citation2000; McIvor et al., Citation2002; Abe et al., Citation2006; Fujii et al., Citation2006; Díaz-Larrea et al., Citation2007; Cassano et al., Citation2009, Citation2012b; Gil-Rodríguez et al., Citation2009; Martín-Lescanne et al., Citation2010; Machín-Sánchez et al., Citation2012a, b).
In the present study we undertook an integrative approach, using both morphological and molecular data (COI-5P + rbcL), to assess whether L. pyramidalis is present in Lusitanian Macaronesia. This required us to locate and study the type of L. pyramidalis and include a specimen from the type locality in our molecular analyses. In addition, we investigated the distribution of Laurenciella marilzae, recently described from the Canary Islands as Laurencia marilzae by Gil-Rodríguez et al. (Citation2009) and transferred to Laurenciella by Cassano et al. (Citation2012a).
Materials and methods
Sampling sites
Collection sites in the Azores, Madeira, the Savage Islands and the Canary Islands are indicated in .
Fig. 1. Map of Atlantic Ocean showing the Macaronesian archipelagos. Sites where Macaronesian specimens were collected in the present study are in the Azores: Pico (PI: Pocinho-Monte Candelaria 38.49686466° N/ 28.53992863° W; Barca-Madalena 38.53988167° N/ 28.52058390° W; Prainha do Norte 38.47720139° N/ 28.20443794° W; Lajes do Pico-Poça de Baleia 38.38998195° N/ 28.25144459 W; Lajes do Pico-Fábrica de Baleia 38.38862491° N/ 29. 19430601° W; Santa Cruz Ribeiras 38.404° N/ 28.1872° W), São Miguel (SM: Cerco da Caloura-Baía 37.7570843° N/ 25.81716330° W; Ferraria 37.8579856° N/ 25.85265462° W; Mosteiros 37.8992112° N/ 25.82103420° W), Santa Maria (SMa: Boca de Ribeira Seca 36.94337327° N/ 25.16456403° W; Emissores 36.99720678° N/ 25.17678807° W; Anjos-Este 37.00542551° N/ 25.16444382° W; Anjos-Ponta dos Frades 37.00788999° N/ 25.15019092° W; Anjos-Piscinas 37.00458430° N/ 25.15727202° W); in Madeira (MA: Seixal-Praia da Laje 32.82554110° N/ 17.11529253° W; Porto Moniz-Piscinas 32.86802811° N/ 17.17135116° W; Ponta de Sao Jorge-Casi 32.8357° N/ 16.9053° W); in the Canary Islands: Fuerteventura (FV: Garcey 28.345492° N/ 14.178111° W; El Cotillo 28.7013° N/ 14.0182° W), Lanzarote (LZ: Arrecife 28.957972° N/ 13.544525° W; Pechigueras 28.855217° N/ 13.872631° W), La Gomera (LG: El Charco de las Condesas 28.0839772° N/ 17.33672469° W; El Charco del Conde 28.05150° N/ 17.20269° W; Punta de la Dama 28.031° N/ 17.183° W), Tenerife (TF: El Pris 28.50981317° N/ 16.42174616° W; Puerto de la Cruz 28.4175° N/ 165462° W; Punta del Hidalgo 28.5739° N/ 16.5462° W).
DNA analysis
Specimens for which new sequences were generated in the present study are listed in Supplementary Table S1. Samples for molecular analysis were cleaned, dried and preserved in silica gel. Total DNA was extracted, using a DNeasy Plant Mini Kit (QIAGEN, Hilden, Germany), according to the manufacturer’s instructions. A total of 64 COI-5P and 55 rbcL sequences were included in this study, 57 and 24 sequences being newly generated for COI-5P and rbcL respectively. For COI-5P, 670 bp were amplified using the forward primers GazF1 (Saunders, Citation2005) or GWSFn (Le Gall & Saunders, Citation2010) in combination with the reverse primers GazR1 (Saunders, Citation2005) or GWSRx (Saunders, Citation2009). A total of 1647 bp of the rbcL gene and rbcL–rbcS spacer was amplified in three fragments with the primer pairs F-rbcLstart and R-753 (Freshwater & Rueness, Citation1994) for the 5′ end, rbcLFC and 1011R (Nam et al., Citation2000) or F-577 and R1381 (Freshwater & Rueness, Citation1994) for the central fragment, and F-993 and R-rbcS start (Freshwater & Rueness, Citation1994) for the 3′ end. Sequencing reactions were performed by Genoscope (www.genoscope.fr, Evry, France) and Macrogen (dna.macrogen.com, Amsterdam, the Netherlands). Forward and reverse electropherograms were edited and assembled with the software Codoncode (Dedham, Massachusetts, USA) and multiple sequence alignments were constructed using ClustalX 2.1 (Larkin et al., Citation2007). The COI-5P alignment included 58 newly sequenced taxa (1 from the outgroup + 57 from the ingroup) and 10 taxa (3 outgroup + 7 ingroup) from GenBank. The rbcL dataset included 75 taxa from GenBank (3 outgroup + 72 ingroup), for which names were updated according to AlgaeBase (Guiry & Guiry, Citation2013), and 25 newly generated sequences (1 outgroup + 24 ingroup taxa).
Species assignment was performed (using MEGA version 5.1: Tamura et al., Citation2011) by cluster analysis of uncorrected p distances using the neighbour-joining (NJ) algorithm, with 10 000 replicates for bootstrapping. Phylogenetic analyses used Bayesian Inference (BI). jModelTest v. 0.1.1 (Posada, Citation2008) was used to select the most appropriate model of sequence evolution for BI analysis of the two datasets, under the Akaike Information Criterion (AIC). The General Time-Reversible model of nucleotide substitution with Gamma-distributed rates for the variable sites (GTR+G) was selected. BI analyses were performed with MrBayes v. 3.2 (Ronquist et al., Citation2012). The analyses were run with four heated Monte-Carlo Markov Chains for 1 × 106 generations, with sampling intervals of 100 generations, to produce 10 000 trees. After verifying that stationary stage had been reached by plotting –ln L against generation time, the first 2500 trees were discarded and majority rule consensus trees generated from the remaining (post-‘burn-in’) trees.
Morphological observations
Anatomical studies were performed on fresh specimens of Laurencia pyramidalis and Laurenciella marilzae fixed in 4% formalin seawater. Additionally, freshly collected specimens were examined to check for the presence of corps en cerise (Feldmann & Feldmann, Citation1950; Paradas et al., Citation2010). Transverse and longitudinal hand sections were made under a Leica MZ 12.5 stereoscopic dissection microscope (Leica, Wetzlar, Germany) using a stainless steel razor blade, and then stained with 0.5% aqueous aniline blue solution acidified with 1 N HCl (Tsuda & Abbott, Citation1985). Photomicrographs were taken with a Leica DFC290 digital camera coupled to a Leica DM 2000 microscope.
Voucher specimens were deposited in the herbarium of the University of La Laguna (TFC). Additionally, we examined specimens of the Laurencia complex deposited in the following herbaria: TFC, the herbarium of the Faculty of Biology of Marine Sciences, University of Las Palmas de Gran Canaria (BCM); the herbarium Ruy Telles Palhinga of the University of Azores (AZB); the herbarium of the Naturalis Biodiversity Center, the Netherlands (L); the herbarium of the Muséum National d’Histoire Naturelle, Paris, France (PC). Furthermore, we studied type material of L. marilzae in TFC. Herbarium abbreviations follow the on-line Index Herbariorum: http://sciweb.nybg.org/science2/IndexHerbariorum.asp (Thiers, Citation2013, continuously updated).
Results
Sampling and typification of Laurencia pyramidalis
In the course of our survey we collected specimens with a gross morphology similar to Laurencia pyramidalis in the Canary Islands, Madeira and the Azores, and we found Laurenciella marilzae in the Azores as well as in the Canary Islands (its type locality). Specimens were deposited in the TFC Herbarium (Supplementary material, Table S2).
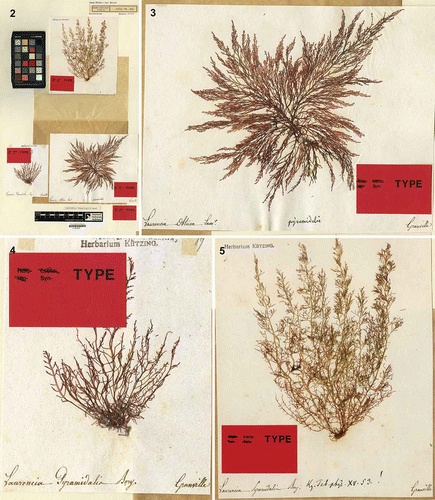
Laurencia pyramidalis was described by Kützing (1849, p. 854) based on a herbarium specimen from Lenormand collected in France (‘ad oras Galliae’), named as L. pyramidalis by Bory de Saint Vincent. Harvey (Citation1849), unaware of the description published earlier by Kützing (Citation1849), described a specimen from the Indian Ocean coast of South Africa as a variety of Laurencia obtusa (Hudson) J.V. Lamouroux, referring to it as L. obtusa var. pyramidalis Bory; however, since the name was never published by Bory, the correct attribution of the variety is to Bory ex Harvey. The name Laurencia obtusa var. pyramidalis had in fact been used previously by Zanardini (Citation1847) but without providing any description; therefore Zanardini’s name was not validly published. J. Agardh (1852, p. 752) claimed that L. obtusa var. pyramidalis Bory ex Harvey was a misapplied name for Laurencia virgata (C. Agardh) J. Agardh and he also listed L. obtusa var. pyramidata Bory ex J. Agardh with L. pyramidalis Kützing as a synonym. In contrast, Maggs & Hommersand (1993, p. 405) considered L. obtusa var. pyramidata Bory ex J. Agardh as a synonym of L. pyramidalis Bory ex Kützing and mentioned three probable syntypes from France (Cherbourg) in L, LD and BM. They claimed that ‘no specimens have been found in L that were obviously examined by Kützing’. We reviewed the material in L and found three samples from Granville (Normandy, France) in Kützing’s herbarium (). One of these () was clearly the one that Kützing used to prepare an illustration in his ‘Tabulae Phycologicae’ (1865, 15: p. 19, Tab. 53 a), in which he shows the habit of L. pyramidalis; we therefore here designate this specimen as the lectotype of Laurencia pyramidalis. This sample was stamped ‘Herbarium Kützing’, and on it was written ‘Laurencia pyramidalis Bory’ (in Lenormand’s handwriting), ‘Kg. Tab. Phyc. XV. 53. !’ (in Kützing’s handwriting) and ‘Granville’ (again in Lenormand’s handwriting). One of the remaining syntypes () was labelled ‘Laurencia obtusa Lamx.’ (handwriting of Lenormand), ‘pyramidalis’ (handwriting of Kützing) and ‘Granville’ (Lenormand’s handwriting), but had no ‘Herbarium Kützing’ stamp, while the second syntype () had a ‘Herbarium Kützing’ stamp and the inscriptions (in Lenormand’s handwriting) ‘Laurencia pyramidalis Bory’ and ‘Granville’, and the number 17. Thus Kützing’s comment that the species was ‘ad oras Galliae’ does not refer to Cherbourg but to Granville, which is a town along the western coast of Normandy. The three type samples are displayed in .
Molecular identification and phylogenetic analyses
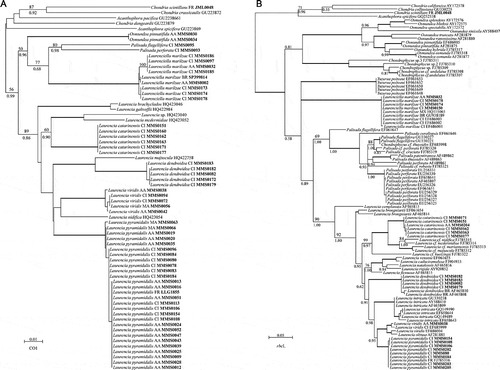
On the basis of the lectotypification of Laurencia pyramidalis on a specimen from Granville, we included in our molecular analysis a specimen recently collected at Chausey, a small archipelago which is under the jurisdiction of the town of Granville. The COI-5P sequence generated for this specimen (LLG1855) was identical to sequences obtained from 16 specimens collected in Macaronesia (). Moreover, these specimens belonged to a fully supported clade containing 11 additional specimens collected in Madeira, the Azores and the Canary Islands, whose sequences were very similar to LLG1855, though not identical. Altogether, three haplotypes were assigned to L. pyramidalis, displaying divergences of less than 0.52%. Eight rbcL sequences of L. pyramidalis were generated from Macaronesia and were clearly conspecific with a sequence from Brittany, France. Furthermore, two haplotypes were detected and were congruent with the CO1-5P data.
Fig. 6. Phylogram generated using neighbour-joining analyses from COI-5P sequences (A) and phylogenetic tree generated using Bayesian inference inferred from rbcL sequences (B). Voucher numbers (in bold) and the geographical origin of each specimen for which sequences were generated in the current study are indicated. FR: France, AA: Azores archipelago, MA: Madeira, CI: Canary Islands, MX: Mexico, BR: Brazil. Bootstrap values > 60% are indicated above nodes and Bayesian posterior probabilities are indicated under nodes. Bold branches indicate strongly supported nodes (bootstrap values > 95% and Bayesian probability > 0.99).
A specimen of Laurenciella marilzae collected from the type locality (Punta del Hidalgo Tenerife, Canary Islands) belonged to a fully supported clade also containing other specimens from the Canary Islands, and specimens from the Azores and Brazil (). The ten sequences belonged to three haplotypes with divergences ranging from 0.0% to 0.70%. Sequences of rbcL generated for three specimens from Canary Islands and one specimen from the Azores were identical to most (five out of six) sequences of L. marilzae available in GenBank. No sequence variation was observed in rbcL.
Other representatives of the Laurencia complex collected in Macaronesia and included in our analyses were resolved as distinct lineages (), confirming that they were distinct from L. pyramidalis and Laurenciella marilzae. The results include the first COI-5P for Palisada flagellifera (from the Canary Islands); Palisada perforata (a sample from the type locality – Tenerife, Canary Islands); Osmundea pinnatifida (from the Azores); Laurencia viridis (from the Azores, Madeira and the Canary Islands, including a sequence from the type locality, Punta del Hidalgo); and L. catarinensis and L. dendroidea (both from the Canary Islands).
All our phylogenetic analyses of rbcL sequences (NJ and BI) resolved the genus Laurencia sensu stricto as a monophyletic lineage (). However, relationships among Laurencia species were only moderately or poorly supported. Furthermore, although all the specimens of Laurenciella marilzae grouped together in a fully supported lineage, the phylogenetic affinities of this genus were not resolved. Interestingly, Laurencia catarinensis from Macaronesia was resolved with full support as sister species to a lineage encompassing specimens from the South Pacific.
Morphological observations
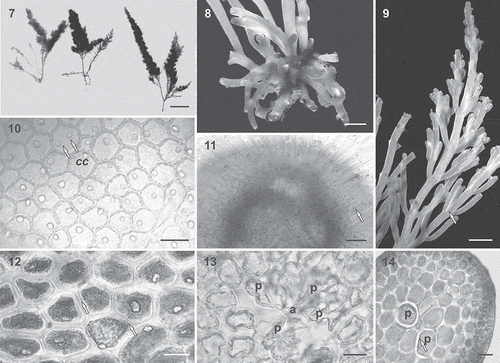
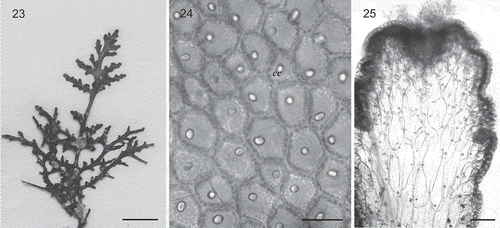
Supplementary Table S2 lists the specimens from TFC Herbarium for which we obtained morphological data. The type specimens and samples of Laurencia pyramidalis (from Normandy and Macaronesia) included in this study exhibited a similar habit, with spirally arranged branches around the main axes and densely ramified branchlets conferring a pyramidal shape to the plant. The Macaronesian specimens of L. pyramidalis (–) were in accordance with the descriptions given by Kützing (Citation1849), Maggs & Hommersand (Citation1993) and Serio et al. (Citation2004): the thalli were terete, 10–18 cm high, brownish red to purple in colour, soft in texture, attached to the substratum by a stoloniferous holdfast, and pyramidal in outline, with three to four orders of branches arranged in three whorls. In surface view, cortical cells usually contained one corps en cerise in living specimens and were connected to each other by secondary pit connections; the medullary cells lacked lenticular wall thickenings. The main difference between Macaronesian specimens and those from continental Europe was the presence in the former of two pericentral fertile cells in tetrasporangial segments (), instead of one as described by Serio et al. (Citation2004) for Mediterranean Sea samples. Furthermore, Macaronesian specimens were green to yellow-greenish and tended to be smaller than specimens from the continent. All specimens of Laurenciella marilzae observed in this study (e.g. ) fitted the descriptions given by Gil-Rodríguez et al. (Citation2009), Rocha-Jorge et al. (Citation2010) and Sentíes et al. (Citation2011). Corps en cerise structures were present in all cells of the thallus (, ), a unique and very distinctive feature of this species.
Figs 7–14. Laurencia pyramidalis from the Lusitanian Macaronesian region. 7. Habit. The main axes have sparse branching in the lower portions and first order laterals that decrease in length upwards and bear three further orders of branching in the same arrangement, resulting in a thallus that is pyramidal in outline. Scale bar = 2 cm. 8. Basal anchorage crust with stoloniferous branches. Scale bar = 1 mm. 9. Spiral branching with three or four orders of whorled branches. Branches issued from the first-order branches curved markedly towards the main axis, becoming almost parallel to it. Scale bar = 2 mm. 10. In surface view, cortical cells contain one or two corps en cerise (cc) in living specimens (arrows). Scale bar = 30 µm. 11. Branches with truncate tips showing dense hyaline trichoblasts with one corps en cerise per cell in living specimens (e.g. arrow). Scale bar = 50 µm. 12. Surface view showing polygonal cortical cells connected to each other by secondary pit connections (arrows). Scale bar = 30 µm. 13. Transverse section of the upper portion of a branch showing an axial cell (a) with four pericentral cells (p). Scale bar = 30 µm. 14. Transverse section of a thallus showing medullary cells with thickened walls. Note pericentral cells (p) with annular thickenings (arrow). Scale bar = 50 µm.
Figs 15–22. Laurencia pyramidalis from the Lusitanian Macaronesian region: reproductive structures. 15. Female gametophyte with cystocarps (arrows) located on the penultimate branches; they are subapical, sessile and prominent. Scale bar = 1 mm. 16. Longitudinal section showing a slightly pyriform cystocarp with a non-protuberant ostiole and clavate carposporangia. Scale bar = 200 µm. 17. Male gametophyte with spermatangial receptacles (arrows) located on the ultimate fertile branchlets. Scale bar = 500 µm. 18. Longitudinal section of a cup-shaped spermatangial receptacle. An axial cell (a) is discernible at the base bearing a fertile branch with many ovoid spermatangia. Scale bar = 50 µm. 19. Tetrasporangial plants with cylindrical branchlets. Scale bar = 2 mm. 20. Surface view of a tetrasporangium (te) with two presporangial cover cells (pr). Scale bar = 30 µm. 21. Transverse section near the apex of axial tetrasporangial segments with an axial cell (a), two vegetative pericentral cells (p1, p2) and two fertile pericentral cells (arrows). Scale bar = 30 µm. 22. Longitudinal section through a tetrasporangial branchlet showing the parallel arrangement of the tetrasporangia. Each fertile pericentral cell (arrows) cuts off presporangial cover cells (pr) distal to the initial tetrasporangium (te). Scale bar = 50 µm.

Figs 23–25. Laurenciella marilzae from the Azores. 23. Habit. Scale bar = 5 mm. 24. In surface view, cortical cells contain one corps en cerise (cc) in living specimens. Scale bar = 30 µm. 25. Longitudinal section through a branch showing corps en cerise in cortical and medullary cells. Scale bar = 10 µm.
Discussion
To the best of our knowledge, this study constitutes the first report of Laurencia pyramidalis for Macaronesia. This species has a broad distribution in the Atlantic, with records for France (Bouxin & Dizerbo, Citation1971, as L. obtusa var. pyramidata), Britain and Ireland (Maggs & Hommersand, Citation1993), Italy (Furnari et al., Citation1999; Serio et al., Citation2004), Portugal (Araújo et al., Citation2009), Spain (Conde et al., Citation1996; Gorostiaga et al., Citation2004; Bárbara et al., Citation2005; Cires Rodríguez & Cuesta Moliner, Citation2010) and Morocco (Dangeard, Citation1949). The presence of this species in the Azores, Madeira, the Savage Islands and the Canary Islands extends its distribution range westwards.
Individuals of L. pyramidalis from Lusitanian Macaronesia were found growing either in mid-to-lower intertidal rock pools or in turfs covering rocks, especially in the Azores, where the macroalgal turfs are one of the most conspicuous assemblages of the intertidal shores (Wallenstein et al., Citation2009). The species frequently occurred in non-calcareous turfs, sometimes together with L. viridis. The morphology of the specimens studied was in agreement with the type material of L. pyramidalis and with modern material from the type locality deposited in PC (PC0157557), which was included in our molecular analysis. It is noteworthy that L. pyramidalis specimens from Macaronesia were never taller than 7 cm, whereas mainland specimens are larger (13 cm in the lectotype, 17 cm for PC0157557). We observed the presence and absence of annular thickenings in medullary, pericentral and axial cells, and determined this to be a variable vegetative character. It would be interesting to test whether the size differences observed between L. pyramidalis specimens from Atlantic islands and the continent result from adaptive responses to environmental conditions.
Laurenciella marilzae was originally described as Laurencia marilzae, based on morphological and molecular studies from specimens collected in the Canary Islands (Gil-Rodríguez et al., Citation2009). However the distributional range of this species was not assessed in that study. Laurenciella marilzae has subsequently been reported from deep waters in south-eastern Brazil (Rocha-Jorge et al., Citation2010) and in the Mexican Caribbean (Sentíes et al., Citation2011). It occurs on both sides of the Atlantic and has probably a preference for tropical and warm temperate waters. It is noteworthy that, despite extensive sampling of members of the Laurencia complex in Brittany (Le Gall & Rousseau, unpublished data), Laurenciella marilzae has never been encountered there. It would be interesting to assess its presence in the warmer waters along the coasts of the Iberian Peninsula. The present report reveals that, within Macaronesia, L. marilzae is not restricted to the Canary Islands but also occurs in the Azores. The Azores specimens share similar morphological characters with specimens from the type locality in Tenerife, Canary Islands, i.e. yellow-orange thalli in the natural turf habitat, an irregularly pyramidal outline, discoid holdfast and a single corps en cerise in each cell of the thallus (Gil-Rodríguez et al., Citation2009). Moreover, in the Azores, specimens grow near the lower intertidal zone, generally forming turfs with other macroalgae, similar to L. marilzae in the Canary Islands.
The Laurencia complex provides an interesting model for understanding the biogeography of the Macaronesian algal flora; however, increased specimen sampling is required in both Macaronesia and along the continental coasts of Europe, Africa and America.
Supplementary information
The following supplementary material is available for this article, accessible via the Supplementary Content tab on the article’s online page at
Table S1. Specimens for which barcode (COI-5P) and rbcL sequences were generated in the present study, along with their valid names, vouchers, details of collection data, and GenBank accession numbers
Table S2. Collection details of specimens observed in this study along with their herbarium information, also indicating specimens newly collected for this study.
Supplementary material
Download Zip (38.3 KB)Acknowledgements
We are very grateful to our colleagues listed in Tables S1 and S2 for help with field collection. We thank Dr V. Garzón for his advice on cartography, Dr J.M. Utge and Dr A. Manghisi for help with molecular studies, and Dr M. Hernández-Ferrer for kindly hosting the first author in their laboratory in the University Institute of Tropical Diseases (University of La Laguna). We also thank the section NHN, Leiden, for providing the type specimens from and the curators of the BCM, AZB and PC herbarium for their support. Acquisition of molecular data was carried out at the CNRS-UMS 2700 in Service de Systématique Moléculaire, MNHN, Paris.
Funding
This project was supported by the network ‘Bibliothèque du Vivant’ funded by CNRS, Muséum National d’Histoire Naturelle, INRA and CEA (Centre National de Séquençage). M. Machín-Sánchez wishes to thank the Spanish Ministry of Education, Culture and Sport for the FPU grant. This work was supported by MEC [CGL 2010-14881] and partially by São Paulo Research Foundation [FAPESP, 2010/52244-2].
References
- Abe, T., Kurihara, A., Kawaguchi, S., Terada, R. & Masuda, M. (2006). Preliminary report on the molecular phylogeny of the Laurencia complex (Rhodomelaceae). Coastal Marine Science, 30: 209–213.
- Afonso-Carrillo, J. & Sansón. M. (1999). Algas, hongos y fanerógamas marinas de las islas Canarias. Clave analítica. Servicio de Publicaciones, Universidad de La Laguna.
- Agardh, J. (1852). Species genera et ordines Floridearum. 2. Floridearum classis constituitur. Part 3, fasc. 1: 701–786. C.W.K. Gleerup, Lund.
- Araújo, R., Bárbara, I., Tibaldo, M., Berecibar, E., Diaz-Tapia, P., Pereira, R., Santos, R. & Sousa-Pinto, I. (2009). Checklist of benthic marine algae and cyanobacteria of northern Portugal. Botanica Marina, 52: 24–46.
- Audiffred, P.A.J. & Prud’homme van Reine, W.F. (1985). Marine algae of Ilha do Porto Santo and Deserta Grande (Madeira Archipelago). Boletim do Museum Municipal do Funchal, 37: 20–51.
- Audiffred, P.A.J. & Weisscher, F.L.M. (1984). Marine algae of Selvagem Grande (Savage Islands, Macaronesia). Boletim do Museum Municipal do Funchal, 36: 5–37.
- Bárbara, I., Cremades, J., Calvo, S., López-Rodríguez, M.C. & Dosil, J. (2005). Checklist of the benthic marine and brackish Galician algae (NW Spain). Anales del Jardín Botánico de Madrid, 62: 69–100.
- Børgesen, F. (1930). Marine algae of the Canary Islands especially from Tenerife and Gran Canaria. III Rhodophyceae. Part III. Vol. IX, I. Det Kungelige Danske Videnskabernes Selskab. Biologiske Meddelelser. København, 1–159.
- Bory de Saint-Vincent, J.B.G.M. (1803). Essais sur les îles Fortunées et l’antique Atlantide, ou précis de l’histoire général de l’archipel des Canaries. Baudouin, Paris.
- Bouxin, H. & Dizerbo, A.H. (1971). Les algues de l’Archipel des Glénan (Finistère). Botanica Rhedonica, Sér. A, 10: 199–226.
- Cassano, V., Gil-Rodríguez, M.C., Sentíes, A. & Fujii, M.T. (2008). Laurencia caduciramulosa (Ceramiales, Rhodophyta) from the Canary Islands, Spain: a new record for the eastern Atlantic Ocean. Botanica Marina, 51: 156–158.
- Cassano, V., Díaz-Larrea, J., Sentíes, A., Oliveira, M.C., Gil-Rodríguez, M.C. & Fujii, M.T. (2009). Evidence for the conspecificity of Palisada papillosa with P. perforata (Ceramiales, Rhodophyta) from the western and eastern Atlantic Ocean on the basis of morphological and molecular analyses. Phycologia, 48: 86–100.
- Cassano, V., Oliveira, M.C., Gil-Rodríguez, M.C., Sentíes, A., Díaz-Larrea, J. & Fujii, M.T. (2012a). Molecular support for the establishment of the new genus Laurenciella within the Laurencia complex (Ceramiales, Rhodophyta). Botanica Marina, 59: 349–357.
- Cassano, V., Metti, Y., Millar, A.J.K., Gil-Rodriguez, M.C., Senties, A., Diaz-Larrea, J., Oliveira, M.C. & Fujii, M.T. (2012b). Redefining the taxonomic status of Laurencia dendroidea (Ceramiales, Rhodophyta) from Brazil and the Canary Islands. European Journal of Phycology, 47: 67–81.
- Cires Rodríguez, E. & Cuesta Moliner, C. (2010). Checklist of benthic algae from the Asturias coast (North of Spain). Boletín Ciencias Naturales R.I.D.E.A., 51: 135–212.
- Clarkston, B.E. & Saunders, G.W. (2010). A comparison of two DNA barcode markers for species discrimination in the red algal family Kallymeniaceae (Gigartinales, Florideophyceae), with a description of Euthora timburtonii sp. nov. Botany, 88: 119–131.
- Conde, F., Flores-Moya, A., Soto, J., Altamirano, M. & Sánchez, A. (1996). Check-list of Andalusia (S. Spain) seaweeds. III. Rhodophyceae. Acta Botanica Malacitana, 21: 7–33.
- Dangeard, P. (1949). Les algues marines de la côte occidentale du Maroc. Le Botaniste, 34: 89–189.
- Díaz-Larrea, J., Sentíes, A., Fujii, M.T., Pedroche, F.F. & Oliveira, M.C. (2007). Molecular evidence for Chondrophycus poiteaui var. gemmiferus comb. et stat. nov. (Ceramiales, Rhodophyta) from the Mexican Caribbean Sea: implications for the taxonomy of the Laurencia complex. Botanica Marina, 50: 250–256.
- Feldmann, J. & Feldmann, G. (1950). Les “corps en cerise” des Laurencia (Rhodomelacées, Ceramiales). Comptes Rendus de l’Académie des Sciences Paris, 266: 2393–2396.
- Fernández-Palacios, J.M., De Nascimento, L., Otto, R., Delgado, J.D., García-del-Rey, E., Arévalo, J.R. & Whittaker, R.J. (2010). A reconstruction of Palaeo-Macaronesia, with particular reference to the long-term biogeography of the Atlantic island laurel forest. Journal of Biogeography, 38: 226–246.
- Freshwater, D.W. & Rueness, J. (1994). Phylogenetic relationships of some European Gelidium (Gelidiales, Rhodophyta) species, based on rbcL nucleotide sequence analysis. Phycologia, 33: 187–194.
- Fujii, M.T., Guimarães, S.M.P.B., Gurgel, C.F. & Fredericq, S. (2006). Characterization and phylogenetic affinities of the red alga Chondrophycus flagelliferus (Rhodomelaceae, Ceramiales) from Brazil on the basis of morphological and molecular evidence. Phycologia, 45: 432–441.
- Furnari, G., Cormaci, M. & Serio, D. (1999). Catalogue of the benthic marine macroalgae of the Italian coast of the Adriatic Sea. Bocconea, 12: 1–214.
- Gillespie, R.G. & Clague, D.A. (2009). Encyclopedia of islands. University of California Press, CA.
- Gil-Rodríguez, M.C. & Afonso-Carrillo, J. (1980a). Catálogo de las algas marinas bentónicas (Cyanophyta, Chlorophyta, Phaeophyta y Rhodophyta) para el Archipiélago Canario. Aula de Cultura de Tenerife, Tenerife, Spain.
- Gil-Rodríguez, M.C. & Afonso-Carrillo, J. (1980b). Adiciones al la flora y catálogo ficológico para la isla de Lanzarote. Vieraea, 10: 59–70.
- Gil-Rodríguez, M.C. & Haroun, R. (1992). Laurencia viridis sp. nov. (Ceramiales, Rhodomelaceae) from the Macaronesian Archipelagos. Botanica Marina, 35: 227–237.
- Gil-Rodríguez, M.C. & Haroun, R. (1993). The genus Laurencia (Rhodomelaceae, Rhodophyta) in the Canary Islands. Courier Forschungs-Institut Senckenberg, 159: 113–117.
- Gil-Rodríguez, M.C., Sentíes, A., Díaz-Larrea, J., Cassano, V. & Fujii, M.T. (2009). Laurencia marilzae sp. nov. (Ceramiales, Rhodophyta) from the Canary Islands, Spain, based on morphological and molecular evidence. Journal of Phycology, 45: 264–271.
- Gil-Rodríguez, M.C., Cassano, V., Aylagas, E., Sentíes, A., Díaz-Larrea, J., Oliveira, M.C. & Fujii, M.T. (2010). Palisada flagellifera (Ceramiales, Rhodophyta) from the Canary Islands, Spain: a new record for the eastern Atlantic Ocean based on morphological and molecular evidence. Botanica Marina, 53: 31–40.
- Gil-Rodríguez, M.C., Fujii, M.T., Machín-Sánchez, M., Cassano, V., Aylagas, E. & Sentíes, A. (2012). Los géneros Laurencia, Laurenciella y Palisada (Rhodomelaceae, Rhodophyta) en las Islas Canarias. In Monografías ficológicas (Sentíes, A. & Dreckmann, K.M., editors), vol. 4, 43–110. UAM-Iztapalapa, México y Universidad de La Laguna, Tenerife, Spain.
- Gorostiaga, J.M., Santolaria, A., Secilla, A., Casares, C. & Díez, I. (2004). Check-list of the Basque coast benthic algae (North of Spain). Anales Jardín Botánico de Madrid, 61: 155–180.
- Guiry, M.D. & Guiry, G.M. (2013) AlgaeBase: world-wide electronic publication. National University of Ireland, Galway. Available from: http://www.algaebase.org (accessed 25 July 2013).
- Haroun, R.J., Gil-Rodríguez, M.C., Díaz de Castro, J. & Prud’homme van Reine, W.F. (2002). A checklist of the marine plants from the Canary Islands (central eastern Atlantic Ocean). Botanica Marina, 45: 139–169.
- Harvey, W.H. (1849). Nereis australis, or algae of the Southern Ocean: being figures and descriptions of marine plants, collected on the shores of the Cape of Good Hope, the extra-tropical Australian colonies, Tasmania, New Zealand, and the Antarctic regions; deposited in the Herbarium of the Dublin University. Reeve Brothers, London.
- House, D.L., Sherwood, A.R. & Vis, M.L. (2008). Comparison of three organelle markers for phylogeographic inference in Batrachospermum helminthosum (Batrachospermales, Rhodophyta) from North America. Phycological Research, 56: 69–75.
- John, D.M., Prud’homme van Reine, W.F., Lawson, G.W., Kostermans, T.B. & Price, J.H. (2004). A taxonomic and geographical catalogue of the seaweeds of the Western coast of Africa and adjacent islands. Nova Hedwigia, 127: 1–139.
- Juan, C., Emerson, B.C., Oromi, P. & Hewitt, G.M. (2000). Colonization and diversification: towards a phylogeographic synthesis for the Canary Islands. Trends in Ecology and Evolution, 15: 104–109.
- Kützing, F.T. (1849). Species algarum. F.A. Brockhaus, Lipsiae.
- Kützing, F.T. (1865). Tabulae phycologicae. Vol. 15. Gedruckt auf Kosten des Verfassers (in commission bei W. Köhne), Nordhausen, Germany.
- Larkin, M.A., Blackshields, G., Brown, N.P., Chenna, R., McGettigan, P.A., McWilliam, H., Valentin, F., Wallace, I.M., Wilm, A., Lopez, R., Thompson, J.D., Gibson, T. J. & Higgins, D.G. (2007). Clustal W and Clustal X version 2.0. Bioinformatics, 23: 2947–2948.
- Le Gall, L. & Saunders, G.W. (2010). DNA barcoding is a powerful tool to uncover algal diversity: a case study of the Phyllophoraceae (Gigartinales, Rhodophyta) in the Canadian flora. Journal of Phycology, 46: 374–389.
- Levring, T. (1974). The marine algae of the Archipelago of Madeira. Boletim do Museu Municipal do Funchal, 28: 5–111.
- Machín-Sánchez, M., Cassano, V., Díaz-Larrea, J., Sentíes A., Fujii, M.T. & Gil-Rodríguez, M. C. (2012a). Morphological and molecular evidence demonstrates the amphi-Atlantic distribution of Laurencia catarinensis (Ceramiales, Rhodophyta). Botanica Marina, 55: 241–252.
- Machín-Sánchez, M., Díaz-Larrea, J., Fujii, M.T., Sentíes, A., Cassano, V. & Gil-Rodríguez, M.C. (2012b). Morphological and molecular evidences within the species Osmundea (Ceramiales, Rhodophyta) from the Canary Islands, eastern Atlantic Ocean. African Journal of Marine Science, 34: 27–42.
- Maggs, C.A. & Hommersand, M.H. (1993). Seaweeds of the British Isles, vol. 1, Rhodophyta. Part 3 A Ceramiales. British Museum (Natural History), London.
- Manghisi, A., Morabito, M., Bertuccio, C., Le Gall, L., Cruaud, C. & Genovese, G. (2010). Is a routine DNA barcoding an efficient tool to reveal introductions of alien macroalgae? A case study of Agardhiella subulata (Solieriaceae, Rhodophyta) in Cape Peloro lagoon (Sicily, Italy). Cryptogamie, Algologie, 31: 423–433.
- Martín-Lescanne, J., Rousseau, F., de Reviers, B., Payri, C., Couloux, A., Cruaud, C. & Le Gall, L. (2010). Phylogenetic analyses of the Laurencia complex (Rhodomelaceae, Ceramiales) support recognition of five genera: Chondrophycus, Laurencia, Osmundea, Palisada and Yuzurua stat. nov. European Journal of Phycology, 45: 51–61.
- McIvor, L., Maggs, C.A., Guiry, M.D. & Hommersand, M.H. (2002). Phylogenetic analysis of the geographically disjunct genus Osmundea Stackhouse (Rhodomelaceae, Rhodophyta). Constancea, 83.9 [online publication of the Jepson Herbarium, UC, Berkeley: http://ucjeps.berkeley.edu/constancea/83/mcivor_etal/osmundea.html]
- Montagne, C. (1840). Plantae cellulares. Section 4. In Histoire naturelle des Iles Canaries. Phytographia canariensis (Barker-Webb, P. & Berthelot, S., editors), vol. 3 (2), sect. 4, 137–192. Mellier, Paris.
- Nam, K.W., Maggs, C.A., McIvor, L. & Stanhope, M.J. (2000). Taxonomy and phylogeny of Osmundea (Rhodomelaceae, Rhodophyta) in Atlantic Europe. Journal of Phycology, 36: 759–772.
- Neto, A.I. (1994). Checklist of the benthic marine macroalgae of the Azores Arquipelago. Ciências Biológicas e Marinhas, 12A: 15–34.
- Neto, A.I., Cravo, D.C. & Haroun, R.T. (2001). Checklist of the benthic marine plants of the Madeira Archipelago. Botanica Marina, 44: 391–414.
- Paradas, W.C., Salgado, L.T., Sudatti, D.B., Crapez, M.A., Fujii, M.T., Coutinho, R., Pereira, R.C. & Amado Filho, G.M. (2010). Introduction of halogenated vesicle transport in cells of red seaweed Laurencia obtusa. Biofouling, 26: 277–286.
- Parente, M.I., Gil-Rodríguez, M.C., Haroun, R.J., Neto, A.I., Smedt, G. de, Hernández, C.L. & Bericibar Zugasti, E. (2000). Flora marina de las Ilhas Selvagens: resultados preliminares de la expedición “Macaronesia 2000”. Revista de la Academia Canaria de las Ciencias, 12: 9–20.
- Posada, D. (2008). jModelTest: phylogenetic model averaging. Molecular Biology and Evolution, 25: 1253–1256.
- Prud’homme van Reine, W.F. (1988). Phytogeography of seaweeds of the Azores. Helgoländer Meeresuntersuchungen, 42: 165–185.
- Prud’ homme van Reine, W.F. (1990). Biogeography of Macaronesian seaweeds. Courier Forschungs-Institut Senckenberg, 129: 55–73.
- Prud’ homme van Reine, W.F. (1998). Seaweeds and biogeography in the Macaronesian Region. Boletim do Museu Municipal do Funchal, 5B: 307–331.
- Prud’ homme van Reine, W.F., Haroun, R. & Audiffred, P.A.J. (1994). A reinvestigation of Macaronesian seaweeds as studied by A. Piccone with remarks on those studied by A. Grunow. Nova Hedwigia, 58: 67–121.
- Prud’homme van Reine, W.F., Haroun, R.J. & Kostermans, L.B.T. (2005). Checklists on seaweeds in the Atlantic Ocean and in the Cape Verde Archipelago. In IV Simpósio Fauna e Flora das Ilhas Atlanticas, Praia 9–13 Setembro 2002, 13–26. Ministério do Ambiente, Agricultura e Pescas, Praia, Ilha de Santiago, República de Cabo Verde.
- Robba, L., Russell, S.J., Barker, G.L. & Brodie, J. (2006). Assessing the use of the mitochondrial cox1 marker for use in DNA barcoding of red algae (Rhodophyta). American Journal of Botany, 93: 1101–1108.
- Rocha-Jorge, R., Cassano, V., Oliveira, M.C. & Fujii, M.C. (2010). The occurrence of Laurencia marilzae (Ceramiales, Rhodophyta) in Brazil based on morphological and molecular data. Botanica Marina, 53: 143–162.
- Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D.L. Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61: 539–542.
- Saunders, G.W. (2005). Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications. Philosophical Transactions of the Royal Society, B: Biological Science, 360: 1879–1888.
- Saunders, G.W. (2008). A DNA barcode examination of the red algal family Dumontiaceae in Canadian waters reveals substantial cryptic species diversity. 1. The foliose Dilsea–Neodilsea complex and Weeksia. Botany, 86: 773–789.
- Saunders, G.W. (2009). Routine DNA barcoding of Canadian Gracilariales (Rhodophyta) reveals the invasive species Gracilaria vermiculophylla in British Columbia. Molecular Ecology Resources, 9 (Supplement 1): 140–150.
- Sentíes, A., Díaz-Larrea, J., Cassano, V., Gil-Rodriguez, M.C. & Fujii, M.T. (2011). Laurencia marilzae (Ceramiales, Rhodophyta) from the Mexican Caribbean: a new record for the tropical western Atlantic. Bulletin of Marine Science, 87: 681–686.
- Serio, D., Furnari, G. & Cormaci, M. (2004). On the occurrence of Laurencia pyramidalis Bory ex Kützing (Rhodophyta, Rhodomelaceae) in the Mediterranean Sea. Cryptogamie Algologie, 25: 329–336.
- Spalding, M.D., Fox, H.E., Allen, G.R., Davidson, N., Ferdana, Z.A., Finlayson, M., Halpern, B.S., Jorge, M.A., Lombana, A., Lourie, S.A., Martin, K.D., McManus, E., Molnar, J., Recchia, C.A. & Robertson, J. (2007). Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience, 57: 573–583.
- Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M. & Kumar, S. (2011). MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28: 2731–2739.
- Thiers, B. (2013). [continuously updated] Index herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/.
- Tittley, I., Neto, A.I. & Parente, M.I. (2009). The marine algal (seaweed) flora of the Azores: additions and amendments 3. Botanica Marina, 52: 7–14.
- Tsuda, R.T. & Abbott, I.A. (1985). Collecting, handling, preservation, and logistics. In: Handbook of phycological methods, Vol. 4. Ecological field methods: macroalgae. (Littler, M.M. & Littler, D.S., editors), 67–86. Cambridge University Press, Cambridge.
- Tuya, F. & Haroun, R.J. (2009). Phytogeography of Lusitanian Macaronesia: biogeographic affinities in species richness and assemblage composition. European Journal of Phycology, 44: 405–413.
- Walker, R.H., Brodie, J., Russell, L.S., Irvine, L.M. & Orfanidis, S. (2009). Biodiversity of coralline algae in the northeastern Atlantic including Corallina caespitosa sp. nov. (Corallinoideae, Rhodophyta). Journal of Phycology, 45: 287–297.
- Wallenstein, F.M., Terra, M.R., Pombo, J. & Neto, A.I. (2009). Macroalgal turfs in the Azores. Marine Ecology, 30 (Supplement 1): 113–117.
- Zanardini, G. (1847). Notizie intorno alle cellulari marine delle lagune e de’ litorali di Venezia. Atti del Reale Istituto Veneto di Scienze, Lettere ed Arti, 3: 205–300.