Abstract
The role of ecophysiological traits in the success and expansion of the toxic cyanobacterium Cylindrospermopsis raciborskii is still under debate. One key factor appears to be the high physiological flexibility of this organism when obtaining limiting resources. Recent studies have found that filamentous bloom-forming cyanobacteria are able to optimize their growth by adjusting phosphate uptake during fluctuating nutrient conditions. We investigated the growth response of two phosphate-deficient C. raciborskii isolates (MVCC19 from Uruguay and CCMP1973 from USA) to short-term fluctuations in phosphate supply. These isolates were exposed to five phosphate concentrations which were provided in two supply modes: a single pulse (SingleP) versus the same amount divided in 10 pulses (TenP), with one pulse applied every 6 min. Morphological traits and changes in chlorophyll a and phycocyanin fluorescence were also evaluated. Growth rates of CCMP1973 and MVCC19 almost doubled and tripled, respectively, when exposed to multiple rather than single phosphate additions. Different growth rates were observed with the same total added resource, thus contradicting the classical model of dependence of growth rate on absolute external concentration. Phosphate-deficient C. raciborskii showed a remarkable physiological flexibility in adapting to phosphate availability on a timescale from minutes to hours. The TenP mode provided an extension of phosphate exposure time that allowed the energetic optimization of uptake and growth. The morphological plasticity of the species in response to phosphate supply mode was also shown by differences in trichome length and individual size. Our results are the first evidence of growth optimization of phosphate-deficient C. raciborskii to short-term nutrient fluctuations, revealing its physiological flexibility. This adaptive behaviour may help to explain the invasive success of this diazotrophic cyanobacterium in a wide range of aquatic ecosystems where phosphorus is frequently the limiting resource.
Introduction
Phosphate availability controls phytoplankton growth in most fresh waters (Schindler, Citation1977). Nutrient depletion is a common feature of eutrophic lakes, implying that cyanobacteria and eukaryotic phytoplankton have to overcome suboptimal conditions for extended periods of time (Schwarz & Forchhammer, Citation2005; Catherine et al., Citation2008). Organisms are frequently exposed to fluctuating, rather than constant, phosphate concentrations, although this is a factor seldom considered in cyanobacterial growth studies (Wagner et al., Citation1995; Dignum et al., Citation2005; Pitt et al., Citation2010). The physiological capacity of cyanobacteria to deal with fluctuations in limiting environmental resources is critical to understanding their success (Falkner et al., Citation2006; Stomp et al., Citation2008; Wu et al., Citation2012) and recent studies show that filamentous cyanobacteria in phosphate-limiting conditions are capable of rapid regulation (in minutes) of phosphate uptake when exposed to consecutive pulses of nutrient concentration (Aubriot & Bonilla, Citation2012). This physiological response results in an energetic optimization of nutrient uptake and therefore positively affects further growth (Falkner et al., Citation2006; Aubriot et al., Citation2011). This adaptive uptake property has been studied in two cyanobacteria (Synechococcus leopoliensis and Anabaena variabilis: Wagner et al., Citation1995; Falkner et al., Citation2006) and in natural phytoplankton dominated by Planktothrix agardhii and Raphidiopsis mediterranea (Aubriot & Bonilla, Citation2012).
Blooms of the freshwater invasive cyanobacteria Cylindrospermopsis raciborskii (Order Nostocales) have expanded from the tropics to subtropical (Vidal & Kruk, Citation2008; Burford & Davis, Citation2011) and temperate regions (Hamilton et al., Citation2005; Stüken et al., Citation2006; Kokociński & Soininen, Citation2012) in recent decades. This species has gained scientific attention due to its capacity to produce potent toxins, such as cylindrospermopsin and saxitoxins (Chorus & Bartram, Citation1999). The factors that have promoted the geographical expansion of C. raciborskii are controversial and still under debate (Kokociński & Soininen, Citation2012). Some evidence suggests the existence of different ecotypes adapted to particular environmental conditions, and there are also data indicating that the species is morphologically and physiologically plastic (Saker & Neilan, Citation2001; Shafik et al., Citation2003; Briand et al., Citation2004; Chonudomkul et al., Citation2004; Piccini et al., Citation2011). Physiological traits are suggested to be advantageous for the spread of C. raciborskii (Padisák, Citation1997; Kokociński et al., Citation2010; Bonilla et al., Citation2012; Soares et al., Citation2013), particularly the high phosphate affinity of cellular uptake systems and the high nutrient storage capacity (Istvánovics et al., Citation2000).
There is only scant indirect evidence to suggest flexibility of C. raciborskii in relation to phosphate assimilation and growth under nutrient fluctuations; for example, the effect of daily phosphate additions promoted its dominance (Posselt et al., Citation2009) and its ability to acclimate physiologically to low ambient phosphate levels (Wu et al., Citation2012). Other supporting evidence includes rapid kinetic and energetic optimization of phosphate uptake (~ 20 minutes) during a sequence of phosphate pulses applied to phytoplankton dominated by Raphidiopsis mediterranea (Aubriot & Bonilla, Citation2012), a species phylogenetically related to C. raciborskii (Moustaka-Gouni et al., Citation2009). The sequence of phosphate pulses produced an increase in the exposure time of organisms to the nutrient and was shown to be the main regulatory factor in inducing phosphate uptake optimization.
In the present study, we investigated the physiological flexibility of two toxic American isolates of phosphate-deficient C. raciborskii (MVCC19, Uruguay and CCMP1973, USA) by examining the growth optimization achieved after short-term fluctuations in phosphate supply. According to previous findings, long exposure time to phosphate during phosphate fluctuations may be sufficient to induce adaptive responses in the uptake systems of filamentous cyanobacteria (Aubriot et al., Citation2011; Aubriot & Bonilla, Citation2012). We evaluated the dependence of growth rate on phosphate concentration with the classical Monod (Citation1950) equation and we discuss its utility in the light of cyanobacterial growth behaviour. The effect of pulse patterns on growth rates and trichome morphology are discussed in terms of the physiological flexibility of C. raciborskii and the implications for resilience and bloom formation under fluctuating limiting resources in lakes.
Materials and methods
Culture conditions
Isolate MVCC19 was obtained from Lago Javier, Uruguay (34° 51′S, 56° 02′W), and isolate CCMP1973 (collected at 27° 00′ N, 81° 00′ W, by Florida Department of Natural Resources, USA) was purchased from the National Center for Marine Algae and Microbiota (NCMA, Maine, USA). Both isolates are saxitoxin producers (Piccini et al., Citation2011, 2013). Cultures were kept in nitrogen-free modified BG11 culture medium (Stanier et al., Citation1971) at 25°C and under 80 µmol photons m−2 s−1 provided by daylight fluorescent tubes with 16 : 8 h light : dark photoperiod. Cultures were continuously bubbled with saturated humid air, which was previously filtered and bubbled in ultrapure sterilized water.
Phosphate deficiency conditions and growth experiments
Phosphate growth limitation was achieved by restricting the total phosphorus content of BG11-N to 10 µM K2HPO4 as follows: 17 ml of P-replete culture containing 10 µM of total phosphorus was diluted in 300 ml of BG11-N P-free medium. When growth reached the stationary phase (c. 5 days), the culture was diluted 1 : 1 (volume : volume) with fresh BG11-N containing 10 µM phosphate. This step was repeated twice a week. Phosphate deficiency was verified in two ways: firstly, by determining that orthophosphate concentrations in the culture medium were analytically undetectable by the molybdenum blue method (<0.03 µM; Murphy & Riley, Citation1962), and secondly, by the fact that the cultures attained stationary phase after each 1 : 1 renewal with fresh BG11-N with 10 µM phosphate and before the next renewal. Cultures were maintained under these phosphate limiting conditions for at least 1 month (about 9 doublings) before the beginning of the experiments.
Prior to growth experiments, the original P-deficient cultures were diluted with BG11-N P-free medium to an initial total P content of 3.3 µM. Phosphate-dependent growth rates were tested at five initial concentrations (0.5, 1, 2, 5 and 10 µM phosphate), each in 80-ml triplicates, that were maintained in the same growth conditions described above. The five concentrations selected are representative of natural phosphate availability in eutrophic lakes according to published information (e.g. Bonilla et al., Citation2012). The experiments had two phases. Firstly, to evaluate if a flexible physiological response took place, each tested phosphate concentration was applied in two different pulse patterns: single initial pulse (SingleP) versus the same amount divided into ten pulses (TenP) applied every 6 min (total phosphate exposure ≥ 54 min) () to mimic natural nutrient fluctuations. Secondly, growth was measured daily by the increase in optical density (OD, absorbance at 750 nm, Thermo Scientific™ Evolution 60 spectrophotometer; Thermo Fisher Scientific Inc., Waltham, USA) over 5 days. Since OD measurements can have interference (for example, from suspended solids and bacterial contamination), changes in major pigments, in vivo chlorophyll a and phycocyanin were also measured daily with an AquaFluor fluorometer (Turner Designs, Sunnyvale, USA), calibrated with a solid standard, and results expressed as fluorescence units (FU). The growth rate (µ, d–1) was calculated by integrating the biomass increase (as optical density, OD) in the exponential phase during the first 5 days and using the equation:
Fig. 1. Summary of the experimental design of phase 1 (minutes) and 2 (days). In phase 1, five initial phosphate concentrations (0.5, 1, 2, 5 and 10 µM) were tested. Each supplied concentration was added in two phosphate patterns: 10 pulse (TenP) and single pulse treatment (SingleP). The total added phosphate in TenP and SingleP at each concentration was the same by the end of the experiment. The dashed lines illustrate the theoretical representation of external phosphate ([Pe]) removal (phase 1) and the resulting growth curve (phase 2) by phosphate deficient C. raciborskii. Downward arrows indicate each phosphate pulse addition. In phase 2, biomass increase is evaluated daily by optical density (OD at 750 nm) on 5 subsequent days.
![Fig. 1. Summary of the experimental design of phase 1 (minutes) and 2 (days). In phase 1, five initial phosphate concentrations (0.5, 1, 2, 5 and 10 µM) were tested. Each supplied concentration was added in two phosphate patterns: 10 pulse (TenP) and single pulse treatment (SingleP). The total added phosphate in TenP and SingleP at each concentration was the same by the end of the experiment. The dashed lines illustrate the theoretical representation of external phosphate ([Pe]) removal (phase 1) and the resulting growth curve (phase 2) by phosphate deficient C. raciborskii. Downward arrows indicate each phosphate pulse addition. In phase 2, biomass increase is evaluated daily by optical density (OD at 750 nm) on 5 subsequent days.](/cms/asset/46b7761c-12eb-4ce5-8c21-c3aa19b9f457/tejp_a_897760_f0001_b.gif)
in which tf and ti are the final and initial time, respectively. The average growth rate of three replicates was calculated for each treatment.
The dependence of growth rates on phosphate concentrations was fitted with the empirical Monod equation (eq. 2; Monod, Citation1950). The relationship of growth rate (µ) and phosphate concentration (P, µM) is:
in which the maximum growth rate is µmax (d−1) and KP (µM) is the Monod constant.
In order to compare the µmax values derived from fitting eq. 2 to the OD data, the mean maximum growth rate (µB) was calculated with initial and final biovolume at the three highest tested phosphate concentrations (2, 5 and 10 µM). The µ data at 0.5 and 1 µM phosphate were excluded from the averaged higher values because they were expected to be below µmax, due to the dependency of µ on this range of low phosphate concentration.
Trichome concentrations and morphological features
Samples were taken at time zero of pulse treatments and at the end of incubation (day 5) in order to determine changes in trichome concentration, biovolume and morphological characteristics of both isolates. The lengths and widths of randomly selected trichomes (20 per replicate) were measured under a light microscope (Olympus BX 41, Olympus Corporation, Tokyo, Japan) at 100× magnification, and the volume (V, µm3) calculated according to Hillebrand et al. (Citation1999). One hundred trichomes were counted in random fields for each replicate according to Guillard (Citation1978) at 40× magnification using a 1 ml Sedgwick–Rafter chamber (Wildlife Supply Company, Yulee, USA). Biovolume was calculated as trichome volume × abundance (mm3 l−1).
Statistical analysis
Growth and morphological differences were analysed with two-way ANOVA (pulse supply treatment: fixed factor, with two levels (factor 1) and concentration: fixed factor, with five levels (factor 2)). When differences between isolates were analysed the fixed factors used were pulse treatment (factor 1) and isolate (factor 2), each factor with two levels. When data remained non-normal or had unequal variance, even after simple transformations, Mann–Whitney Rank Sum Tests (M–W) and Kruskal–Wallis tests were performed. Significant differences in the growth kinetic parameters of eq. 2 between pulse treatments (SingleP and TenP) were determined by a lack of overlap of the 95% confidence intervals of curve fitting. These analyses were performed using SigmaPlot 11 (Systat Software, Inc., Chicago, USA).
Results
The effect of phosphate concentration and pulse pattern on growth
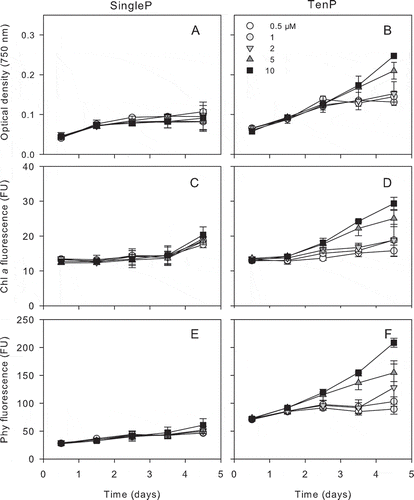
Both isolates reached higher final biomass (OD) when exposed to TenP in comparison to SingleP (P < 0.05 M–W) ( and ). The stationary phase was reached earlier by both isolates under SingleP treatment. Chlorophyll a and phycocyanin showed a similar temporal pattern ( and ) and no differences were found between treatments in the chlorophyll a : phycocyanin ratio (data not shown).The biomass estimates by pigment fluorescence confirmed the trend observed with OD data.
Fig. 2. Time course of optical density increase (750 nm, A and B), in vivo chlorophyll a (Chl a, C and D) and phycocyanin (phy, E and F) of MVCC19 isolate during 5-day incubation at five total phosphate concentrations for the single pulse treatment (SingleP, left plots) and the sequence of 10 pulses (TenP, right plots). Bars represent standard deviations.
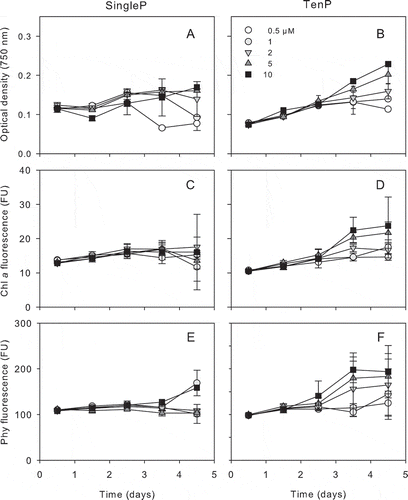
Fig. 3. Time course of optical density increase (750 nm, A and B), in vivo chlorophyll a (Chl a, C and D) and phycocyanin (phy, E and F) of CCMP1973 isolate during 5-day incubation at five total phosphate concentrations for the single pulse treatment (SingleP, left plots) and the sequence of 10 pulses (TenP, right plots). Bars represent standard deviations.
Notably, µ of both isolates was higher under the TenP rather than the SingleP addition (). The µ of MVCC19 was significantly higher when phosphate was added as TenP (; ANOVA, F = 186.8, P < 0.001). The same trend was observed for CCMP1973, however, differences were less marked than for MVCC19 (; ANOVA, F = 27.5; P < 0.001; mean µ: 0.282 ± 0.045 d−1 and 0.224 ± 0.018 d−1 for TenP and SingleP of CCMP1973, respectively). The maximum µ differences between pulse patterns were observed at 10 µM phosphate for CCMP1973 and 2 µM phosphate for MVCC19. The µ under TenP treatment was close to twice and three times as high as those obtained in CCMP1973 and MVCC19, respectively, compared with SingleP. Rates were not significantly dependent on total phosphate concentration in either isolate (ANOVA P > 0.05). highest µ in MVCC19 under TenP was reached at 2 µM phosphate (0.275 ± 0.023 d−1), while for SingleP the maximum µ was reached at 5 µM (0.143 ± 0.041 d–1). The maximum µ for CCMP1973 was reached at higher phosphate concentrations than for the South American isolate (0.355 ± 0.016 d−1 and 0.243 ± 0.034 d−1 at 10 µM phosphate TenP, and 5 µM phosphate SingleP, respectively). Despite the higher µ of both isolates in TenP mode, the response to this treatment was more pronounced in MVCC19 (ANOVA, F = 46.1, P < 0.001).
Fig. 4. Dependence of growth rates on phosphate concentrations (0.5, 1, 2, 5 y 10 µM) in two pulse patterns; single pulse (SingleP: closed circles) and sequence of 10 pulses (TenP: open circles). Solid lines represent the data fit obtained with eq. 2 and the dotted line corresponds to the 95% confidence interval. Bars represent standard deviation. Growth parameters are shown in .
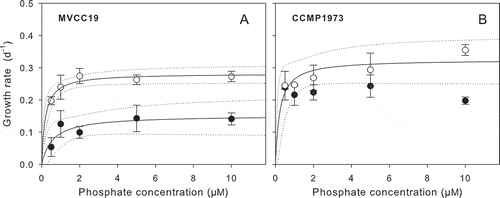
The two pulse patterns resulted in different kinetic growth parameters at the same added phosphate concentration (). The µmax of MVCC19 was twice as high as for TenP compared with SingleP, while KP in TenP was as low as a third of the value of SingleP (, ). For CCMP1973, only the µ from TenP could be fitted to eq. 2 (, ). No significant fit was obtained when eq. 2 was fitted to the combined µ data for SingleP and TenP for each isolate. The µB values calculated from biovolume at the three higher phosphate concentrations (2, 5 and 10 µM) are consistent with µmax values obtained from OD data ().
Table 1. Growth parameters derived from curve fitting of equation 2 (eq. 2) and morphological features comparing SingleP and TenP treatments for both isolates and for the applied phosphate concentrations (0.5–10 µM phosphate). The result of ANOVA curve fit parameters of eq. 2 (F, r2 and P) are indicated for each treatment and isolate. The µmax and KP are the maximum velocity and Monod constant derived from eq. 2, respectively. Trichome abundance and morphological features are averages for all phosphate levels in each treatment at initial and final incubation time. The µB values correspond to the mean growth rates calculated from biovolume (mm3 l–1) at 2, 5 and 10 µM phosphate concentrations.
Effect of phosphate concentration and pulse pattern on morphology
On the fifth day of incubation, the trichomes increased in length and volume, as well as concentration (). The morphological traits of the two isolates were differently affected by phosphate supply patterns. The trichomes of isolate CCMP1973 were significantly smaller when phosphate was added in TenP mode (; two-way ANOVA, F = 20.34, P < 0.001 and F = 13.26, P = 0.002, for trichome volume and length, respectively). No significant differences were observed between pulse patterns in MVCC19 trichome volume and length (; two-way ANOVA, P > 0.05). No correlation was found between µ and trichome length or volume in either isolate.
Discussion
Our study presents direct evidence of flexible behaviour in nutrient-deficient C. raciborskii, which permits it to optimize growth during fluctuations in the phosphate supply. The higher µ achieved after a sequence of phosphate pulses reveals the energy optimization enabled by short-term phosphate uptake regulation. The same phenomenon has been seen in natural phytoplankton dominated by Oscillatoriales (Aubriot et al., Citation2011; Aubriot & Bonilla, Citation2012). In the present study we applied a pulse sequence, which caused a phosphate exposure time equal to or greater than 54 minutes. The extended exposure time induced by the pulse sequence allowed C. raciborskii to significantly increase µ up to three times as much as in single pulse mode. The flexible response of organisms depends on the coupling between exposure time and environmental factors and the time required to adapt their physiology (reaction time) (Walters, Citation2005; Stomp et al., Citation2008). The longer exposure time in TenP treatment thus allowed the energetic optimization of resource acquisition and assimilation (Falkner et al., Citation2006; Aubriot et al., Citation2011). Recently, it has been shown that physiological adaptation to fluctuating phosphate concentrations in cyanobacteria depends on the exposure time to the nutrient prior to the onset of growth (Aubriot & Bonilla, Citation2012). The growth optimization observed in C. raciborskii under suboptimal conditions gives insight into the flexible ecophysiological traits of this invasive cyanobacterium.
Our µ data are lower than those reported for MVCC19 under sufficient P growth conditions (0.60 to 0.74 d−1 at 25°C: Piccini et al., Citation2011; Bonilla et al., Citation2012) and for other strains of C. raciborskii (Lürling et al., Citation2012). The lower µ values found in our study are consistent with values obtained under P-deficiency and semi-continuous culture (Wu et al., Citation2009, 2012), although these studies did not evaluate the growth optimization of C. raciborskii. However, the µ of our study are lower than values obtained with continuous chemostats (Istvánovics et al., Citation2000), presumably because this growth method produces a long exposure time to phosphate by continuous nutrient inflow.
Lower µ values imply severe constraints on cell function imposed by phosphate deficiency, which result in the modification of energetic metabolism and biochemical structure, particularly the photosynthetic apparatus (Schwarz & Forchhammer, Citation2005; Wu et al., Citation2012). Cylindrospermopsis raciborskii has been shown to overcome P-limitation by decreasing µ and activating extracellular phosphatases and antioxidant enzyme catalases (Wu et al., Citation2012). We determined that C. raciborskii is able to achieve µmax at phosphate concentrations an eighth of the value of those determined in other studies (e.g. Wu et al., Citation2012), when the timescale of phosphate fluctuations allows an adaptive response. In aquatic environments, including eutrophic lakes, phosphate availability is usually low or below detection limits (Aubriot et al., Citation2000) and thus, optimal growth conditions in nutrient-replete cultures are far from being representative of natural conditions. Although the determination of specific µ in natural phytoplankton is difficult, values are generally low (< 0.4 d−1) as a result of various constraints (Reynolds, Citation1997). As our experiments approximate the phosphate concentrations and patterns (pulses) that C. raciborskii may experience in the field, the determined µ may therefore reflect realistic responses under severe phosphate deficiency. In 5-day mesocosm experiments, Muhid et al. (Citation2013) found phytoplankton dominated by C. raciborskii to have a µ of 0.2 d−1, which is similar to our values. Therefore, the growth optimization found in our study has to be considered relative to nutrient deficiency state, and illustrates how C. raciborskii may rapidly overcome such functional constraints and reach a higher µ. This behaviour is in agreement with the beneficial acclimation hypothesis (Leroi et al., Citation1994) i.e. acclimating to a particular environmental condition provides an organism with advantages in fluctuating environments.
The flexible behaviour found in C. raciborskii may help to explain why this cyanobacterium dominates the phytoplankton in a wide range of lakes and climates, particularly at lower total P, relative to other bloom-forming cyanobacteria (Burford & O’Donohue, Citation2006; Bonilla et al., Citation2012: approx. 1.8 µM TP). Interestingly, we found the maximum µ of C. raciborskii to occur at similar phosphate levels (≥ 2 µM phosphate). Additionally, experimental evidence indicates that growth optimization occurs in C. raciborskii under fluctuating light and temperature due to its pigment acclimation capacity, which is greater than that of other bloom-forming species (Mehnert et al., Citation2010; Bonilla et al., Citation2012). Hence a number of factors may give C. raciborskii a higher capacity to bloom in lakes of lower trophic state than other common filamentous cyanobacteria (Bonilla et al., Citation2012): (1) the growth optimization response found in this study, together with (2) the high phosphate affinity of its uptake systems and its phosphate storage capacity (Istvánovics et al., Citation2000), (3) the capacity to dominate during low-frequency P pulses (Posselt et al., Citation2009), and (4) acclimation to low P environments (Wu et al., Citation2012). The phenotypic plasticity of multiple traits in C. raciborskii may therefore help to explain its resilience and ongoing expansion.
An additional complexity is the differential response found between isolates. Cylindrospermopsis raciborskii isolates differed in their growth and morphological response to phosphate availability, thus supporting the hypothesis of multiple ecotypes proposed for this species (Chonudomkul et al., Citation2004; Piccini et al., Citation2011). MVCC19 is more variable in terms of its physiology – specifically its µmax than CCMP1973 – whereas CCMP1973 is more changeable in terms of morphological features, including significant changes in trichome length and specific volume (27 and 47% higher for SingleP compared with TenP for length and volume, respectively). In this study, morphological plasticity may not necessarily reflect physiological flexibility.
The adaptive response described here, in which different µ occur with the same total added resource, contradicts the classical model of dependence of µ on absolute external concentration (Monod, Citation1950). The model does not adequately describe the growth behaviour of C. raciborskii because this organism adapts physiologically to varying resource availability. However, the growth kinetic parameters are currently used to identify species-specific traits (µmax and KP) in order to identify competitive advantages among phytoplankton and for constructing complex models to predict species dominance. Equation 2 could only be fitted to each individual pulse pattern, revealing the importance of growth history. Therefore, our results may contribute to the development of flexible growth models that consider the effect of rapid changes in external concentration on phosphate metabolism in order to help predict cyanobacterial growth in aquatic environments. Further studies are needed to elucidate the kinetic and energetic adjustments that occur in C. raciborskii during phosphate exposure, the effect on polyphosphate body formation and the subsequent membrane P-binding protein rearrangements.
Acknowledgements
We thank Amelia Fabre for technical collaboration and Dermot Antoniades for valuable linguistic corrections. This work was partially financed by Agencia Nacional de Investigación e Innovación (ANII), projects FCE2007_353 and PR_FCE_2009_1_2330.
References
- Aubriot, L. & Bonilla, S. (2012). Rapid regulation of phosphate uptake in freshwater cyanobacterial blooms. Aquatic Microbial Ecology, 67: 251–263.
- Aubriot, L., Bonilla, S. & Falkner, G. (2011). Adaptive phosphate uptake behaviour of phytoplankton to environmental phosphate fluctuations. FEMS Microbiology Ecology, 77: 1–16.
- Aubriot, L., Wagner, F. & Falkner, G. (2000). The phosphate uptake behaviour of phytoplankton communities in eutrophic lakes reflects alterations in the phosphate supply. European Journal of Phycology, 35: 255–262.
- Bonilla, S., Aubriot, L., Soares, M.C.S., González-PIana, M., Fabre, A., Huszar, V.L.M., Lürling, M., Antoniades, D., Padisák, J. & Kruk, C. (2012). What drives the distribution of the bloom-forming cyanobacteria Planktothrix agardhii and Cylindrospermopsis raciborskii? FEMS Microbiology Ecology, 79: 594–607.
- Briand, J., Leboulanger, C., Humbert, J., Bernard, C. & Dufour, P. (2004). Cylindrospermopsis raciborskii (Cyanobacteria) invasion at mid-latitudes: selection, wide physiological tolerance, or global warming? Journal of Phycology, 40: 231–238.
- Burford, M. & Davis, T. (2011). Physical and chemical processes promoting dominance of the toxic cyanobacterium Cylindrospermopsis raciborskii. Chinese Journal of Oceanology and Limnology, 29: 883–891.
- Burford, M.A. & O’Donohue, M.J. (2006). A comparison of phytoplankton community assemblages in artificially and naturally mixed subtropical water reservoirs. Freshwater Biology, 51: 973–982.
- Catherine, A., Quiblier, C., Yéprémian, C., Got, P., Groleau, A., Vinçon-Leite, B., Bernard, C. & Troussellier, M. (2008). Collapse of a Planktothrix agardhii perennial bloom and microcystin dynamics in response to reduced phosphate concentrations in a temperate lake. FEMS Microbiology Ecology, 65: 61–73.
- Chonudomkul, D., Yongmanitchai, W., Theeragool, G., Kawachi, M., Kasai, F., Kaya, K. & Watanabe, M.M. (2004). Morphology, genetic diversity, temperature tolerance and toxicity of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) strains from Thailand and Japan. FEMS Microbiology Ecology, 48: 345–355.
- Chorus, I. & Bartram, J. (1999). Toxic Cyanobacteria in Water. A Guide to their Public Health Consequences, Monitoring and Management. Chapman & Hall, London.
- Dignum, M., Matthijs, H.C.P., Pel, R., Laanbroek, H.J. & Mur, L.R. (2005). Nutrient limitation of freshwater Cyanobacteria. In Harmful Cyanobacteria (Huisman, J., Matthijs, H. & Visser, P., editors), 65–86. Springer, Dordrecht.
- Falkner, R., Priewasser, M. & Falkner, G. (2006). Information processing by cyanobacteria during adaptation to environmental phosphate fluctuations. Plant Signaling and Behavior, 1: 212–220.
- Guillard, R.R.L. (1978). Counting slides. In Phytoplankton Manual (Sournia, A., editor), 182–190. Unesco, Paris.
- Hamilton, P.B., Ley, L.M., Dean, S. & Pick, F.R. (2005). The occurrence of the cyanobacterium Cylindrospermopsis raciborskii in Constance Lake: an exotic cyanoprokaryote new to Canada. Phycologia, 44: 17–25.
- Hillebrand, H., Dürselen, C., Kirschtel, D., Zohary, T. & Pollingher, U. (1999). Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology, 35: 403–424.
- Istvánovics, V., Shafik, H.M., Présing, M. & Juhos, S. (2000). Growth and phosphate uptake kinetics of the cyanobacterium Cylindrospermopsis raciborskii (Cyanophyceae) in throughflow cultures. Freshwater Biology, 43: 257–275.
- Kokociński, M. & Soininen, J. (2012). Environmental factors related to the occurrence of Cylindrospermopsis raciborskii (Nostocales, Cyanophyta) at the north-eastern limit of its geographical range. European Journal of Phycology, 47: 12–21.
- Kokociński, M., Stefaniak, K., Mankiewicz-Boczek, J., Izydorczyk, K. & Soininen, J. (2010). The ecology of the invasive cyanobacterium Cylindrospermopsis raciborskii (Nostocales, Cyanophyta) in two hypereutrophic lakes dominated by Planktothrix agardhii (Oscillatoriales, Cyanophyta). European Journal of Phycology, 45: 365–374.
- Leroi, A.M., Bennett, A.F. & Lenski, R.E. (1994). Temperature acclimation and competitive fitness: an experimental test of the beneficial acclimation assumption. Proceedings of the National Academy of Sciences USA, 91: 1917–1921.
- Lürling, M., Eshetu, F., Faassen, E.J., Kosten, S. & Huszar, V.L.M. (2012). Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshwater Biology, 58: 552–559.
- Mehnert, G., Leunert, F., Cirés, S., Jöhnk, K.D., Rücker, J., Nixdorf, B. & Wiedner, C. (2010). Competitiveness of invasive and native cyanobacteria from temperate freshwaters under various light and temperature conditions. Journal of Plankton Research, 32: 1009–1021.
- Monod, J. (1950). La technique de culture continue: theorie et applications. Annales Institut Pasteur Lille, 79: 390–410.
- Moustaka-Gouni, M., Kormas, K.A., Vardaka, E., Katsiapi, M. & Gkelis, S. (2009). Raphidiopsis mediterranea Skuja represents non-heterocytous life-cycle stages of Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju in Lake Kastoria (Greece), its type locality: evidence by morphological and phylogenetic analysis. Harmful Algae, 8: 864–872.
- Muhid, P., Davis, T.W., Bunn, S.E. & Burford, M.A. (2013). Effects of inorganic nutrients in recycled water on freshwater phytoplankton biomass and composition. Water Research, 47: 384–394.
- Murphy, J. & Riley, J.P. (1962). A modified single solution method for the determination of phosphate in natural waters. Analytical Chemical Acta, 27: 31–36.
- Padisák, J. (1997). Cylindrospermopsis raciborskii (Woloszyska) Seenayya et Subba Raju, an expanding, highly adaptive cyanobacterium: worldwide distribution and review of its ecology. Archiv für Hydrobiologie, Supplement, 107: 563–593.
- Piccini, C., Aubriot, L., Fabre, A., Amaral, V., González-Piana, M., Giani, A., Figueredo, C.C., Vidal, L., Kruk, C. & Bonilla, S. (2011). Genetic and eco-physiological differences of South American Cylindrospermopsis raciborskii isolates support the hypothesis of multiple ecotypes. Harmful Algae, 10: 644–653.
- Piccini, C., Aubriot, L., D´aLessandro, B., Martigani, F. & Bonilla, S. (2013). Revealing toxin signatures in cyanobacteria: report of genes involved in cylindrospermopsin synthesis from saxitoxin-producing Cylindrospermopsis raciborskii. Advances in Microbiology, 3: 289–296.
- Pitt, F.D., Mazard, S., Humphreys, L. & Scanlan, D.J. (2010). Functional characterization of Synechocystis sp. strain PCC 6803 pst1 and pst2 gene clusters reveals a novel strategy for phosphate uptake in a freshwater cyanobacterium. Journal of Bacteriology, 192: 3512–3523.
- Posselt, A.J., Burford, M.A. & Shaw, G. (2009). Pulses of phosphate promote dominance of the toxic cyanophyte Cylindrospermopsis raciborskii in a subtropical water reservoir. Journal of Phycology, 45: 540–546.
- Reynolds, C.S. (1997). Vegetation Process in the Pelagic: A Model for Ecosystem Theory. Ecology Institute, Oldendorf, Germany.
- Saker, M.L. & Neilan, B.A. (2001). Varied diazotrophies, morphologies, and toxicities of genetically similar isolates of Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) from northern Australia. Applied and Environmental Microbiology, 67: 1839–1845.
- Schindler, D. (1977). Evolution of phosphorus limitation in lakes. Science, 195: 260–262.
- Schwarz, R. & Forchhammer, K. (2005). Acclimation of unicellular cyanobacteria to macronutrient deficiency: emergence of a complex network of cellular responses. Microbiology, 151: 2503–2514.
- Shafik, H.M., Vörös, L., Sprőber, P., Présing, M. & Kovács, A.W. (2003). Some special morphological features of Cylindrospermopsis raciborskii in batch and continuous cultures. Hydrobiologia, 506–509: 163–167.
- Soares, M.C.S., Lürling, M. & Huszar, V.L.M. (2013). Growth and temperature-related phenotypic plasticity in the cyanobacterium Cylindrospermopsis raciborskii. Phycological Research, 61: 61–67.
- Stanier, R., Kunisawa, R., Mandel, M. & Cohen-BAzire, G. (1971). Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriological Reviews, 35: 171–205.
- Stomp, M., Van Dijk, M.A., Van Overzee, H.M.J., Wortel, M.T., Sigon, C.A.M., Egas, M., Hoogveld, H., Gons, H.J. & Huisman, J. (2008). The timescale of phenotypic plasticity and its impact on competition in fluctuating environments. American Naturalist, 172: E169–E185.
- Stüken, A., Rücker, J., Endrulat, T., Preussel, K., Hemm, M., Nixdorf, B., Karsten, U. & Wiedner, C. (2006). Distribution of three alien cyanobacterial species (Nostocales) in northeast Germany: Cylindrospermopsis raciborskii, Anabaena bergii and Aphanizomenon aphanizomenoides. Phycologia, 45: 696–703.
- Vidal, L. & Kruk, C. (2008). Cylindrospermopsis raciborskii (Cyanobacteria) extends its distribution to latitude 34°53′S: taxonomical and ecological features in Uruguayan eutrophic lakes. Pan-American Journal of Aquatic Sciences, 3: 142–151.
- Wagner, F., Falkner, R. & Falkner, G. (1995). Information about previous phosphate fluctuations is stored via an adaptive response of the high-affinity phosphate uptake system of the cyanobacterium Anacystis nidulans. Planta, 197: 147–155.
- Walters, R.G. (2005). Towards an understanding of photosynthetic acclimation. Journal of Experimental Botany, 56: 435–447.
- Wu, Z., Shi, J. & Li, R. (2009). Comparative studies on photosynthesis and phosphate metabolism of Cylindrospermopsis raciborskii with Microcystis aeruginosa and Aphanizomenon flos-aquae. Harmful Algae, 8: 910–915.
- Wu, Z., Zeng, B., Li, R. & Song, L. (2012). Physiological regulation of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) in response to inorganic phosphorus limitation. Harmful Algae, 15: 53–58.
