Abstract
A new benthic phototrophic dinoflagellate is described from sediments of a tropical marine cove at Martinique Island and its micromorphology is studied by means of light and electron microscopy. The cell contains small golden-brown chloroplasts and the oval nucleus is posterior. It is laterally compressed, almost circular in shape when viewed laterally. It consists of a small epitheca tilted toward the right lateral side and a larger hypotheca. In the left view, the cingulum is more anterior and the epitheca is reduced. The cingulum is displaced and left-handed. This organism is peculiar in having no apical pore and its thecal plate arrangement is 2′ 1a 7′′ 5c 3s 5′′′ 1′′′′. The plates are smooth with small groups of pores scattered on their surface. An area with 60–80 densely arranged pores is found near the centre of the 2′′′ plate, on the left lateral side. Morphologically, these features are different from all other laterally compressed benthic genera. In addition, molecular genetic sequences of SSU and partial LSU form a distinct and well-supported clade among dinoflagellates and support the erection of a new genus. However, molecular phylogenies inferred from ribosomal genes failed to confirm any clear relationship with other benthic taxa and affinity with other laterally compressed dinoflagellates has not been demonstrated. Hence, the taxonomic affinity of Madanidinium loirii with a defined order and family is unclear at the moment.
Introduction
After being discovered and studied by Herdman (Citation1921, Citation1922, Citation1924a, Citationb), benthic dinoflagellates were then poorly studied for several decades (Balech, Citation1956; Dragesco, Citation1965). They gained new interest for scientists when the epiphytic species producing maitotoxin and ciguatoxin, Gambierdiscus toxicus, was associated with ciguatera disease in the tropical Pacific (Adachi & Fukuyo, Citation1979; Taylor, Citation1979). Subsequently, several other benthic taxa were found to be harmful and involved in the complex mechanism of ciguatera (Bomber & Aikman, Citation1989; Litaker et al., Citation2010). Because of this potential toxicity, several taxonomic studies were undertaken in tropical regions (Fukuyo, Citation1981; Besada et al., Citation1982; Berland et al., Citation1992; Grzebyk et al., Citation1994; Chinain et al., Citation1999) and also in temperate areas worldwide (e.g. Horiguchi & Chihara, Citation1983; Saunders & Dodge, Citation1984; Larsen, Citation1985; Hoppenrath, Citation2000b; Aligizaki & Nikolaidis, Citation2006; Murray, Citation2009; Chomérat et al., Citation2010b; Fraga et al., Citation2011).
In the Caribbean Sea, where ciguatera has been known since the late 18th century and has caused health problems for many years (Bagnis, Citation1981; Olsen et al., Citation1984; Tosteson, Citation2004; Tester et al., Citation2010), studies on benthic dinoflagellates have mostly focused on epiphytic species responsible for this disease, in order to better understand their distribution and assess the associated risk. Several investigations have been made in the northern and eastern parts of the basin (Ballantine et al., Citation1985, Citation1988; Taylor, Citation1985; Litaker et al., Citation2010). On the western side, studies were carried out on the Belize coast (e.g. Faust & Gulledge, Citation2002; Faust, Citation2009), and around Mexican and Cuban coasts (Hernández-Becerril & Almazán Becerril, Citation2004; Delgado et al., Citation2006). The southern Caribbean sea has only been investigated by Grzebyk et al. (Citation1998) who collected samples from a Panamanian island and Rodriguez et al. (Citation2010) who made a survey at San Andrés Island (Caribbean Colombia). In the French Antilles (French West Indies), where ciguatera poisoning is recurrent (Olsen et al., Citation1984; Vernoux, Citation1988; Pottier et al., Citation2001; Rosine et al., Citation2008; Tester et al., Citation2010), only a few surveys have been undertaken to check the presence and identity of toxigenic species (Besada et al., Citation1982; Taylor, Citation1985; Litaker et al., Citation2010).
Harmful species excepted, taxonomic studies on the diversity of benthic dinoflagellates are relatively scarce in the Caribbean. The first major contribution was made by Carlson (Citation1984) who collected samples in several places around the Virgin Islands and identified 38 benthic taxa. Then, M. A. Faust published a remarkable series of papers with descriptions or reinvestigations of taxa from coral-reef mangrove embayments in Belize (Faust, Citation1990, Citation1993a, Citationb, Citationc, Citation1994, Citation1996; Faust et al., Citation1996, Citation2008). In the course of her study, she described the very atypical and intriguing genus Plagiodinium (Faust & Balech, Citation1993), which has been found very infrequently in other areas (M. Saburova, pers. comm.) and still needs further investigation. Indeed, most of these taxa are known only from their morphology, and it would now be of great importance to complement this knowledge with DNA sequences to better understand their phylogenetic position within dinoflagellate lineages (Hoppenrath et al., Citation2013).
Since no taxonomic survey has been carried out to date in the French Antilles, we undertook to assess the diversity of benthic dinoflagellates in Martinique Island from occasional samples. During our study, we encountered a very atypical and interesting taxon, which is distinct from any armoured dinoflagellate genus hitherto described. Cells are strongly flattened laterally, and thecal plates are delicate and arranged in a unique pattern. In the present paper, we describe its morphology using light and scanning electron microscopy, and attempt to establish its phylogenetic position among other dinoflagellates using molecular data from environmental samples and clonal cultures.
Materials and methods
Sampling and cultivation
Martinique Island is a French volcanic island of the Lesser Antilles archipelago, located in the eastern part of the Caribbean Sea (). It is about 70 km long and 30 km wide. Samples of shallow sediments were collected by snorkelling (1–3 m depth) at Anse Dufour (14°31.538′N, 61°05.446′W), a cove located on the Caribbean side of the island (), 16 March 2010 and 22, 26, 30 March and 6 April 2013. All samples from March 2010 and March 2013 were immediately preserved with acidic Lugol’s solution (~5% final concentration) and stored in darkness at 4 °C before further examination. The 6 April 2013 aliquots were fixed and stored in the same conditions; some aliquots were kept fresh for algal isolation and cultivation. Immediately after collection, they were carefully packed to limit thermal variation and transferred to the Ifremer laboratory at Concarneau (France) by plane and train. Because of the travel time, isolation of living cells was carried out 2 days after sampling (8 April).
Fig. 1. Maps showing the location of Martinique Island in the Caribbean Sea (Atlantic Ocean), and the sampling area (Anse Dufour) on the western coast of the island.
For cultivation, single cells from the live sediment subsamples were identified and isolated with a micropipette under an IX41 (Olympus, Tokyo, Japan) inverted microscope. The cells were then rinsed in several drops of seawater and placed in 96-well culture plates containing seawater and medium. After several divisions, each clonal strain was transferred to culture plates with increasing well volume. Several cultures were established but only two were kept and grown in 50 ml culture flasks. The strain IFR-MLO-01M was grown in K medium (Keller et al., Citation1987) while the second strain IFR-MLO-02M was grown in f/2 medium (Guillard & Ryther, Citation1962; Andersen et al., Citation2005). Both strains were maintained in a growth chamber set up at 22 ± 1.0 °C and a 12 : 12 light: dark illumination cycle with ~ 50 µmol photons m−2 s−1 provided by white fluorescent tubes.
Observations
LM
Light microscopy observations were performed on isolated cells on a standard slide with a coverslip, using a BX41 (Olympus, Tokyo, Japan) upright microscope. It was equipped with brightfield, differential interference optics, epifluorescence filter sets U-MWU2 for DAPI stain (excitation: BP330-385; beamsplitter: DM400; emission: BA 420) and U-MWIB2 for chlorophyll autofluorescence (excitation: BP460-490; beamsplitter: DM505; emission: BA510IF), an Osram mercury short arc HBO 100W lamp as the light source for epifluorescence, and a DP72 (Olympus, Tokyo, Japan) colour digital camera.
To visualize the nuclei, some cells from the culture were isolated and fixed with 2% glutaraldehyde for 10–20 min at 4 °C, and then stained with 4′,6-diamidino-2-phenylindole (DAPI) according to Chomérat et al. (Citation2012). In addition, thecal plates were observed using Calcofluor White M2R (Sigma Aldrich) as fluorescent dye.
Scanning electron microscopy
Cells from the cultures were obtained after a vigorous shaking of the flask, and fixed with 2% formaldehyde. The specimens were then processed according to the methods described in Couté (Citation2002) and Chomérat & Couté (Citation2008). They were dehydrated and critical point dried, and observed with a Quanta 200 (FEI, Eindhoven, the Netherlands) scanning electron microscope with an acceleration voltage of 5 kV and a secondary electron detector.
Cells were measured from SEM digital micrographs using ImageJ software (Rasband, Citation1997–2006). SEM images were presented on a uniform background using Adobe Photoshop CS5 (v. 12.1, Adobe Systems).
DNA amplification and sequencing
Single cells from the fixed sediment sample from 16 March 2010 were isolated with a capillary pipette under the IX41 inverted microscope. They were rinsed in several drops of distilled water and placed in a 0.2 ml PCR tube containing 5 µl of distilled water. Living cells from the two cultures were isolated similarly and 1–5 cells were placed into PCR tubes. All tubes were stored at −20 °C prior to analysis. For PCR, tubes were thawed and processed as described previously in Chomérat et al. (Citation2010b).
Molecular analysis and phylogeny
The SSU and LSU sequences obtained were aligned with other dinoflagellate sequences and Perkinsus marinus (perkinsoid alveolate) as external group, using MAFFT software version 7 (Katoh & Standley, Citation2013) with selection of the Q-INS-i algorithm which considers the secondary structure for the alignment. This step was followed by refinement by eye with MEGA software version 5.2.1 (Tamura et al., Citation2011). For SSU a dataset of 77 sequences and 1691 aligned positions was used. For LSU, a matrix of 49 sequences and 860 positions was used. Ambiguous parts of the alignment (including the D2 domain) were excluded from the analysis using Gblocks software version 0.91b, with less stringent parameters. GenBank accession numbers of all sequences used are available in the supplementary material.
For each dataset, evolutionary models were examined using maximum likelihood (ML) and Bayesian Inference analysis (BI). The evolutionary model was selected using jModelTest version 0.1.1 (Posada, Citation2008). According to Akaike information criterion (AIC) and Bayesian information criterion (BIC), a general time reversible (GTR) model with a gamma correction (Γ) for among-site rate variation and invariant sites was chosen for the SSU dataset while a Tamura–Nei model with no invariant sites was chosen for the LSU dataset.
Maximum likelihood analyses were performed using PhyML version 3.0 (Guindon et al., Citation2010), and Bayesian analyses were run using MrBayes version 3.1.2 (Ronquist & Huelsenbeck, Citation2003). Bootstrap analysis (1000 pseudoreplicates) was used to assess the relative robustness of branches of the ML tree. Initial Bayesian analyses were run with a GTR model (nst = 6) with rates set to invgamma (gamma for LSU dataset). Each analysis was performed using four Markov chains (MCMC), with two million cycles for each chain. Trees were saved every 100 cycles and the first 2000 trees were discarded. Therefore, a majority-rule consensus tree was created from the remaining 18 000 trees in order to examine the posterior probabilities of each clade.
The consensus trees were edited using MEGA 5 software. The best ML phylograms are shown with robustness values for each node (ML/BI).
Results
Madanidinium loirii Chomérat, gen. et sp. nov. (Figs 2–28)
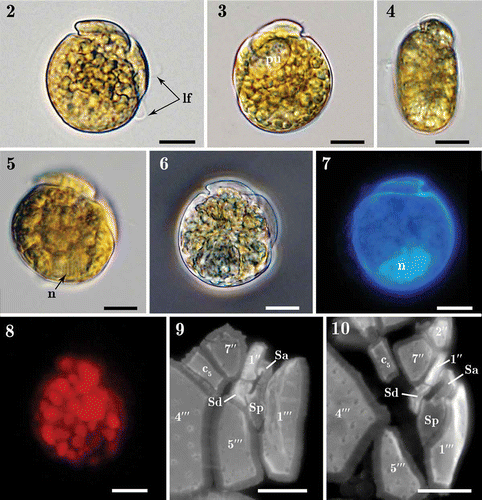
Figs 2–10. Light micrographs of Madanidinium loirii. Fig. 2. Right lateral view of a living cell with the longitudinal flagellum (lf) visible. Fig. 3. Left lateral view of a living cell with a pusule (pu) visible. Fig. 4. Dorsal view of a living cell showing the epitheca inclined toward the right side. Fig. 5. Right view of a cell with focus on the nucleus (n). Fig. 6. Right lateral view of a fixed environmental specimen used for single-cell molecular analysis (isolate IFR 12–200). Fig. 7. Left lateral view of a DAPI-stained specimen showing the posterior position of the nucleus (n). Fig. 8. Right lateral view of a living cell seen in epifluorescence (blue excitation) showing chlorophyll autofluorescence and the presence of small discoid chloroplasts. Figs 9–10. Detail of sulcal plates of two specimens stained with Calcofluor white. Except in Fig. 6, all specimens are from strain IFR–MLO–02M. Scale bars: 10 µm.
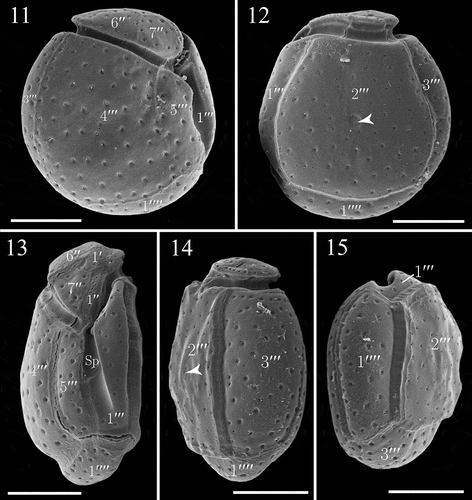
Figs. 11–15. SEM micrographs of Madanidinium loirii from strain IFR-MLO-02M. Fig. 11. Right lateral view (holotype specimen). Fig. 12. Left lateral view, note the reduced epitheca and area of densely arranged pores (arrowhead). Fig. 13. Ventral view showing the tilted epitheca. Fig. 14. Dorso-lateral view (arrowhead pointing to the area of densely arranged pores). Fig. 15. Antapical view. Scale bars: 10 µm.
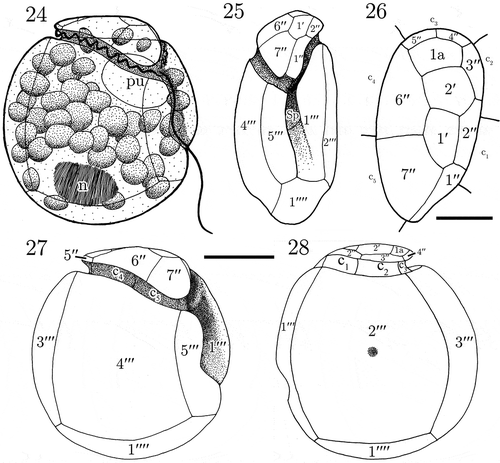
Figs. 16–23. Details of the theca of Madanidinium loirii in SEM. Fig. 16. Apical view. Fig. 17. Right lateral side of the epitheca. Fig. 18. Left lateral side of the epitheca. Fig. 19. Ventral view of the epitheca, note the flagellar pore (fp) visible partially. Fig. 20. Dorsal view. Fig. 21. Detail of thecal surface with groups of pores and some isolated pores. Fig. 22. Oval area of densely arranged pores on the 2′′′ plate. Fig. 23. Area of pores on the 2′′′ plate of another specimen, note that the shape is elongated. Scale bars: 5 µm in Figs 16–20; 1 µm in Figs 21–23.

Figs. 24–28. Line drawings of Madanidinium loirii. Fig. 24. Representation of a live cell in right lateral view (n: nucleus, pu: pusule). Fig. 25. Ventral view of the theca. Fig. 26. Apical view. Fig. 27. Right lateral view. Fig. 28. Left lateral view. Scale bars: 10 µm in Figs 24, 25, 27, 28 and 5 µm in Fig. 26.
DIAGNOSIS GENERICO-SPECIFICA (Art. 38.5, McNeill et al., Citation2012)
Genus repositum in Dinophyta; in incertum ordinem et incertam familiam; solitarium; marinum; cum theca et in arena vivens. Cellulae fere circulares in latere visu valdeque compressae a latere in ventrali visu. Longitudo: 25.2–31.0 µm; latitudo: 16.7–18.7 µm; dorsoventralis altitudo: 22.1–28.8 µm. Epitheca inclinata et deminuta; altior in dextero aspectu. Porus apicalis absens. Cingulum cellulam perfecte cingens et descendens; transversus in sinistro visu et obliquus in dextero visu. Hypotheca major. Thecae laminarum tabulatio: 2′ 1a 7′′ 5c 3s 5′′′ 1′′′′. Laminae thecae laeves cum poris in parvo numero agreggatis aequabiliterque dispersi in tota theca. Lamina 2′′′ cum parva regione praebenti poros dense compressos. Parvi chloroplasti numerosi. Nucleus ovalis in posteriore cellulae parte positus.
Etymology: The genus is named after Madanina, the ancient local name of Martinique Island (du Tertre, Citation1667–1671; Daney de Marcillac, Citation1846) and –dinium suffix for Dinophyceae. The specific epithet loirii commemorates Maurice Loir (French diatomist) who collected many samples from Martinique Island, and who kindly offered to the authors those used in the present study.
Type species: Madanidinium loirii
Holotype: (cell from the culture IFR–MLO–02M, SEM stub IFR-13H6 has been deposited in the Centre of Excellence for Dinophytes Taxonomy (CEDiT) with the accession reference CEDiT2013H22).
Isotypes: , fixed culture CEDIT2013I23.
Type locality: Anse Dufour (14°31.538′ N, 61°05.446′ W), Martinique Island, eastern Caribbean Sea.
DNA sequence information: Sequences have been deposited in GenBank under accession numbers KF751599, KF751600, KF751601, KF751602, KF751603 and KF751604.
The cells are laterally flattened, with their depth (i.e. dorso-ventral width) larger than lateral width. Hence, they are mostly observed in lateral view and their shape is almost circular (, , , ). They are 25.2–31.0 µm long (mean 28.9 µm, SD 1.4 µm, N = 16), 22.1–28.8 µm deep (mean 25.9 µm, SD 1.8 µm, N = 16) and 16.7–18.7 µm wide (N = 2). The length to depth ratio varies from 1.05 to 1.21 (mean 1.12, SD 0.05, N = 16). The cingulum is anterior and descending (left-handed) (, , ). Seen from the left side (, ), it is straight, anterior, and the epitheca is very small, emerging of 1.7–2.7 µm (N = 5) above the cingulum. In contrast, in the right lateral view (, ), the epitheca is higher (4.1–7.6 µm, N = 7), and the cingulum is conspicuously oblique, descending towards the ventral area (, , ).
Cells contain small yellow-brown chloroplasts. The oval nucleus is located posteriorly (, , ). Some cells have a large pusule located on the anterior ventral side, near the sulcal area (, ).
The thecal plate pattern is 2′ 1a 7′′ 5c 3s 5′′′ 1′′′′. The epitheca comprises 10 plates and does not have an apical pore (, ). Since the application of the Kofoid nomenclature of thecal plates was not straightforward, we decided that the apical plates were those in contact with the apex (geometrically speaking) of the cell and the unique plate actually not in contact with the apex and the cingulum is considered as an intercalary plate. In apical view, the epitheca is roughly pear-shaped, and tapers ventrally (). Plates are arranged asymmetrically and those on the right side are higher than those inserted on the left side (, ). The 1′ and 2′ plates are medium-sized, pentagonal and located at the apex of the slightly dome-shaped epitheca (, ). The 1′′ plate is elongated, five-sided and located ventrally (, ). The 2′′ and 3′′ plates are pentagonal and border the left side of the epitheca (, ). The 4′′ and 5′′ plates are very small, rectangular, four-sided, and located on the dorsal side of the epitheca (, ). The 6′′ plate, which is the largest of the epitheca, is six-sided (, ). The 7′′ plate is roughly trapezoidal and four-sided (, ), although it has a very short contact with the Sd plate ventrally (, , ). The unique intercalary plate 1a is pentagonal and in line with the two apical plates, but it is located more dorsally (, , ).
The cingulum completely encircles the cell and is composed of five plates unequal in size (). The c2 plate is large and runs along the left side of the theca, with its distal end facing the suture 2′′′/3′′′on the hypotheca (). The c3 plate is small and located dorsally, and runs along the width of the 3′′′ plate (). The sulcus is moderately long, and slightly oblique with respect to the longitudinal axis of the cell (). In SEM, we partially observed the flagellar pore, which is elongated oval in shape and located ventrally (). It is bordered by three major sulcal plates Sa, Sd and Sp (, ). Our observations of the sulcus using epifluorescence microscopy on several specimens confirm that the sulcus is composed of three plates (, ). The Sa plate is hook-shaped and in contact with the c1 plate. The Sd plate forms the end of the cingulum and connects the epitheca. The Sp plate is the largest of sulcal plates, and is posteriorly pointed (, ).
The hypotheca is formed of 6 major plates. The first postcingular plate 1′′′ is ventral and folds in order to form a flange covering the left side of the sulcus (, ). The 2′′′ plate, which is the largest of the hypotheca, is trapezoidal and four-sided, covering most of the left lateral side (). The 3′′′ plate is rectangular and is located on the dorsal side of the hypotheca (). The 4′′′ plate is large and four-sided (). The 5′′′ plate is the smallest of postcingular plates and contacts 6 plates, namely 1′′′, 4′′′, 1′′′′, c5, Sd and Sp (). The antapical plate 1′′′′ is pentagonal and elongated ().
Thecal plates are thin, delicate and smooth. They are covered by small groups of pores, and some isolated pores (0.1–0.2 µm in diameter) (). On the large lateral plate 2′′′, an area of closely arranged pores (68–86 in number; N = 4) of 0.08–0.1 µm in diameter is present nearly in the centre (, , ). This area is variable in shape, being circular to elongated (, ).
In culture, cells of M. loirii are almost always attached to the bottom of the container, and swimming cells are observed occasionally. The cells are strongly adherent to the substrate by their lateral sides and they appear almost always in lateral view. However, no particular structures such as stalks have been observed.
Molecular phylogeny
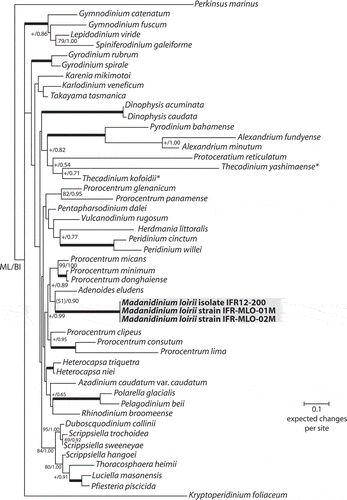
The results of the SSU and LSU phylogenetic analyses show that the sequences acquired from cultures and environmental specimens group together within a well-supported clade (, ). In the phylogeny inferred from SSU, the position of the Madanidinium clade is not supported and no clear relationships with other genera emerge (). In the LSU analysis, Madanidinium appears as a sister-clade to Adenoides eludens (), albeit without support (bootstrap value of 51 in ML and posterior probability of 0.90 in BI). In addition, the clade formed by Madanidinium and Adenoides forms a sister group with Prorocentrum species but without support.
Fig. 29. Maximum likelihood (ML) phylogenetic tree inferred from SSU rDNA (matrix of 77 sequences and 1691 aligned positions). The tree was rooted using Perkinsus marinus sequence as outgroup. Model selected GTR + I + Γ4. Log likelihood = −19792.7. Substitution rate matrix: A ↔ C = 1.52090, A ↔ G = 4.15185, A ↔ T = 1.43273, C ↔ G = 0.81766, C ↔ T = 9.38294, against G ↔ T = 1.00000. Assumed nucleotide frequencies: f(A) = 0.24690, f(C) = 0.19272, f(G) = 0.25795, f(T) = 0.30243. Among site rate variation: assumed proportion of invariable sites I = 0.317. Rates at variable site assumed to be gamma distributed with shape parameter α = 0.511. Bootstrap values (1000 pseudoreplicates) > 65 (in ML) and posterior probabilities > 0.5 (in BI) are shown at nodes, thick lines indicate full support of the branch (100/1.00). ‘+’ indicate nodes present but unsupported. Asterisks indicate benthic taxa with a lateral compression related to M. loirii by morphology.
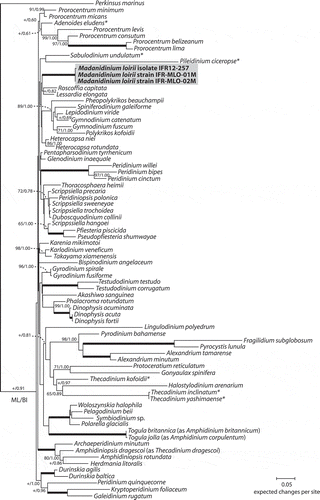
Fig. 30. Maximum likelihood (ML) phylogenetic tree inferred from partial LSU rDNA (matrix of 49 sequences and 860 aligned positions). The tree was rooted using Perkinsus marinus sequence as outgroup. Model selected TN93 + Γ4. Log likelihood = 14250.35343. Transition/transversion ratio for purines = 2.860; transition/transversion ratio for pyrimidines = 7.812. Nucleotides frequencies f(A) = 0.23690, f(C) = 0.18977, f(G) = 0.28854, f(T) = 0.28479. Rates at variable site assumed to be gamma distributed with shape parameter α = 0.528. Only bootstrap values (1000 pseudoreplicates) > 65 (in ML) and posterior probabilities > 0.5 (in BI) are shown at nodes; thick lines indicate full support of the branch (100/1.00); ‘+’ indicates a node present but unsupported. Benthic taxa with a lateral compression are highlighted with asterisks.
Discussion
Morphologically, Madanidinium has features closely related to other strongly laterally compressed sand-dwelling genera with a reduced epitheca like Plagiodinium, Planodinium, Sabulodinium, Cabra and Pileidinium () but also some Thecadinium species (Hoppenrath, Citation2000a; Yoshimatsu et al., Citation2004). In addition, a morphological resemblance can be found with the genus Sinophysis Nie et Wang (Dinophysales), that is also strongly laterally compressed and possesses a reduced epitheca (Hoppenrath, Citation2000b), but the thecal plate organization of Madanidinium is not of the dinophysoid type and no further comparison is possible. In Plagiodinium belizeanum, the epitheca is atypical, very small and slightly inclined to the ventral side (Faust & Balech, Citation1993), differing from M. loirii. The left-handed displacement of the cingulum in M. loirii is peculiar and reminiscent of that of Thecadinium yashimaense (Bolch & Campbell, Citation2004; Hoppenrath et al., Citation2004, Citation2005; Yoshimatsu et al., Citation2004), and also of the planktonic taxa Thecadiniopsis tasmanica and Pseudothecadinium campbellii (Croome et al., Citation1987; Hoppenrath & Selina, Citation2006). This is the reverse situation to the benthic genus Cabra where the epitheca is higher on the left side than on the right lateral side. When seen in the left lateral view, the outline of M. loirii is very similar to that of Sabulodinium, as the epitheca is almost invisible and the cingulum is short and very anterior. However, in Sabulodinium and Planodinium the cingulum is not displaced (Saunders & Dodge, Citation1984; Hoppenrath et al., Citation2007). In contrast with Pileidinium, the cingulum is complete in Madanidinium. Hence, owing to its peculiar overall morphology and position of the cingulum, M. loirii can be easily distinguished from most other benthic genera with the light microscope.
Table 1. Comparative features of Madanidinium loirii and other selected sand-dwelling dinoflagellate genera with a laterally compressed body (Thecadinium excluded), and the planktonic genera Thecadiniopsis and Pseudothecadinium.
Concerning the plate pattern, Madanidinium is also very atypical. It differs from other genera in the number and arrangement of epithecal plates (). The absence of an apical pore on the epitheca is a striking and uncommon feature which has been reported to date only in Planodinium striatum (Saunders & Dodge, Citation1984) and a few Thecadinium species, as shown by Hoppenrath (Citation2000a) and by Yoshimatsu et al. (Citation2004). Comparatively, in Plagiodinium belizeanum, the authors reported an unusual, minute plate provisionally named Po, which has been seen only at high magnification with the light microscope (Faust & Balech, Citation1993). Unfortunately, it has not been studied in SEM and no detailed information about this pore is available. In Pileidinium ciceropse, a simple circular pore has been found on the epitheca (Tamura & Horiguchi, Citation2005) and it is considered as a homologue of the apical pore present in other taxa. Interestingly, the asymmetric epitheca of Madanidinium, with precingular plates larger on the right side and smaller plates on the left side, is an unusual character not found in other genera with a displaced cingulum such as Cabra or Thecadiniopsis.
The presence of five cingular plates in Madanidinium is a feature found also in Plagiodinium, Sabulodinium, Thecadiniopsis and Thecadinium. Croome et al. (Citation1987) emphasized that this character is similar to freshwater peridinioids, while most gonyaulacoids have 6 plates. The reduced number and very simple arrangement of the sulcus of Madanidinium is remarkable and to date it is the smallest number of sulcal plates observed in a benthic genus. In other taxa, 4 or more sulcal plates have been described. Nevertheless, although we used epifluorescence microscopy and plate staining, it is possible that some very small platelets have been overlooked in our study, since the sulcus is difficult to study. In addition, since the 1′′ plate seems to have a short contact with the flagellar pore, it could be alternatively interpreted as a fourth sulcal (Sa) plate. However, as it is not part of the furrow and is actually completely in the epitheca, we considered that it fits better with the definition of a precingular plate. Moreover, the plate that we interpreted as Sa is hook-shaped, as in some gonyaulacoid genera like Alexandrium.
The arrangement of plates on the hypotheca of M. loirii is not distinctive and many benthic dinoflagellates like Cabra, Plagiodinium, Sabulodinium, Pileidinium (), Thecadinium pro parte and the planktonic genera Thecadiniopsis and Pseudothecadinium have a similar pattern of 5 postcingular and 1 antapical plate. However, the presence of an area of densely arranged pores near the centre of the 2′′′ plate on the left lateral side of the hypotheca is a very uncommon feature among the genera (). An area of grouped pores (or deep areolae) has been reported in Cabra and some other benthic genera such as Rhinodinium, Roscoffia and in some benthic Prorocentrum species. However this area is antapical and located on the 1′′′′ plate in Cabra, Rhinodinium and Roscoffia (Hoppenrath & Elbrächter, Citation1998; Murray et al., Citation2006; Chomérat et al., Citation2010a), which differs from Madanidinium where it is lateral as in Prorocentrum species. In Prorocentrum panamense and P. pseudopanamense, a roundish depression with a sieve-like bottom is present on the posterior dorsal side of the right lateral plate (Hoppenrath et al., Citation2013) while in P. glenanicum, a group of closely arranged pores, very similar to that observed in M. loirii, is found just above the centre of the right lateral plate (Chomérat et al., Citation2011). To date, the role of these structures has not been ascertained, but from observations of a live culture of P. panamense, it seems that cells can extrude mucus from the pores of this area, and attach to the substratum (M. Saburova, pers. comm.). Such fixation can be very efficient, and this may explain the strong adherence of cells of M. loirii in culture flasks. This is probably an adaptation to the benthic way of life, to resist water flow, but further ultrastructural studies are required to confirm this hypothesis.
Madanidinium is a phototrophic genus that can be maintained in culture, with plastids like Plagiodinium and Pileidinium (Faust & Balech, Citation1993; Tamura & Horiguchi, Citation2005). Interestingly, these two genera are from tropical areas, like Madanidinium. Among Thecadinium species, the type species T. kofoidii has chloroplasts (Hoppenrath, Citation2000a) and T. yashimaense and T. arenarium are phototrophic (or mixotrophic), as well as Pseudothecadinium (Hoppenrath & Selina, Citation2006). In contrast, the genera Cabra, Planodinium, Sabulodinium and most Thecadinium species are colourless and strictly heterotrophic (Saunders & Dodge, Citation1984; Chomérat et al., Citation2010a).
As a consequence, morphological features of Madanidinium are sufficiently different from all described genera to justify the establishment of a new genus.
Molecular phylogeny
Molecular data support the conclusion that Madanidinium loirii corresponds to a new dinoflagellate taxon, since its SSU and LSU sequences diverge from all other known genera. However, as previously shown by several authors, the resolution and support of deeper branches in the phylogenies inferred from ribosomal genes is non-existent or very low, and no clear relationship between Madanidinium and other taxa can be found in our analyses. With SSU, the position of this new genus is not stable in the trees, which indicates that this ribosomal gene lacks a good phylogenetic signal. This problem has already been pointed out with several other ‘unusual’ and monotypic genera of benthic dinoflagellates (Tamura & Horiguchi, Citation2005; Hoppenrath et al., Citation2007; Yamada et al., Citation2013). Moreover, no relationship was found to any of the morphologically related taxa with a lateral compression for which SSU rDNA sequences are available, such as Sabulodinium, Pileidinium and Thecadinium. Although the position of Sabulodinium and Pileidinium is uncertain in the SSU tree due to the lack of support, they are widely divergent from Madanidinium. From LSU, there is a weak indication that Madanidinium could be related to Adenoides eludens, another benthic and phototrophic genus, but there was no such support in the SSU phylogeny. Morphologically, Adenoides is also compressed laterally, but less than M. loirii, and no similarities in the thecal plate arrangement can be found between these two genera. Thus, this proposed phylogenetic relationship should be treated with caution. Moreover, there are almost no LSU sequences of morphologically related laterally compressed taxa available in GenBank, which can bias our analyses. The dataset should be improved with the addition of more taxa. As a consequence, the evolution of benthic and laterally compressed dinoflagellates is still unclear. It is not yet possible to infer whether these genera derived from a common benthic ancestor or if they resulted from a convergent evolution of similar traits well adapted to the benthic life. Hence, much more sequence acquisition remains to be carried out for benthic dinoflagellates, which is absolutely necessary in order to get a better understanding of the evolution within this very diverse and complex group of protists. This task is rendered difficult by the rarity of these organisms and the difficulty of keeping them in culture. In the case of phototrophic taxa, as with Madanidinium, the use of cultured strains can allow extensive ultrastructural, genetic and biochemical studies, which represents a great opportunity to increase our knowledge and understanding of the biology of benthic dinoflagellates.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at http://dx.doi.org/10.1080/09670262.2014.898797
Supplementary file S1. List of rDNA sequences used in the phylogenetic analyses (sequences acquired and deposited in this study are in bold type).
Supplementary material
Download MS Word (31.5 KB)Acknowledgements
The authors wish to thank Mrs E. Nézan for valuable advice and comments on the manuscript, Mr N. Gayet for preparing the samples and use of SEM, Mrs K. Chèze for DNA sequencing and Mrs M. Bouildé for providing useful literature. This work was supported by the Contrat de projet État-Région (PIDETOX) and is a contribution to the Brittany Culture Collection project (Souchothèque de Bretagne).
References
- Adachi, R. & Fukuyo, Y. (1979). The thecal structure of a marine toxic dinoflagellate Gambierdiscus toxicus gen. et sp. nov. collected in a ciguatera-endemic area. Bulletin of the Japanese Society of Scientific Fisheries, 45: 67–71.
- Aligizaki, K. & Nikolaidis, G. (2006). The presence of the potentially toxic genera Ostreopsis and Coolia (Dinophyceae) in the North Aegean Sea, Greece. Harmful Algae, 5: 717–730.
- Andersen, R.A., Berges, J.A., Harrison, P.J. & Watanabe, M.M. (2005). Appendix A – Recipes for freshwater and seawater media. In Algal culturing techniques (Andersen, R.A. editor), 429–538. Elsevier Academic Press, Burlington.
- Bagnis, R. (1981). L’ichtyosarcotoxisme de type ciguatera: phénomène complexe de biologie marine et humaine. Oceanologica Acta, 4: 375–387.
- Balech, E. (1956). Étude des dinoflagellés du sable de Roscoff. Revue Algologique, 2: 29–52.
- Ballantine, D.L., Bardales, A.T., Tosteson, T.R. & Dupont-Durst, H. (1985). Seasonal abundance of Gambierdiscus toxicus and Ostreopsis sp. in coastal waters of Southwest Puerto Rico. In Proceedings of the Fifth International Coral Reef Congress (Gabrié, C. & Salvat, B., editors), 417–422. MNHN-EPHE, Moorea, Tahiti.
- Ballantine, D.L., Tosteson, T.R. & Bardales, A.T. (1988). Population dynamics and toxicity of natural populations of benthic dinoflagellates in southwestern Puerto Rico. Journal of Experimental Marine Biology and Ecology, 119: 201–212.
- Berland, B., Grzebyk, D. & Thomassin, B.A. (1992). Benthic dinoflagellates from the coral reef lagoon of Mayotte Island (S-W Indian Ocean); identification, toxicity and preliminary ecophysiological study. Bulletin de la Société de Pathologie Exotique, 85: 453–456.
- Besada, E.G., Loeblich, L.A. & Loeblich, A.R., III (1982). Observations on tropical, benthic dinoflagellates from ciguatera-endemic areas: Coolia, Gambierdiscus and Ostreopsis. Bulletin of Marine Science , 32: 723–735.
- Bolch, C.J. & Campbell, C.N. (2004). Morphology and phylogenetic affinities of Thecadinium foveolatum sp. nov. (Dinophyceae: Thecadiniaceae), a new marine benthic dinoflagellate from the West of Scotland. European Journal of Phycology, 39: 351–360.
- Bomber, J.W. & Aikman, K.E. (1989). The ciguatera dinoflagellates. Biological Oceanography, 6: 291–311.
- Carlson, R.D. (1984). The distribution, periodicity, and culture of benthic/epiphytic dinoflagellates in a ciguatera endemic region of the Caribbean. PhD thesis. Southern Illinois University, Carbondale. 308 pp.
- Chinain, M., Faust, M.A. & Pauillac, S. (1999). Morphology and molecular analyses of three toxic species of Gambierdiscus (Dinophyceae): G. pacificus, sp. nov., G. australes, sp. nov., and G. polynesiensis, sp. nov. Journal of Phycology, 35: 1282–1296.
- Chomérat, N. & Couté, A. (2008). Protoperidinium bolmonense sp. nov. (Peridiniales, Dinophyceae), a small dinoflagellate from a brackish hypereutrophic lagoon (South of France). Phycologia, 47: 392–403.
- Chomérat, N., Couté, A. & Nézan, E. (2010a). Further investigations on the sand-dwelling genus Cabra (Dinophyceae, Peridiniales) in South Brittany (northwestern France), including the description of C. aremorica sp. nov. Marine Biodiversity, 40: 131–142.
- Chomérat, N., Sellos, D.Y., Zentz, F. & Nézan, E. (2010b). Morphology and molecular phylogeny of Prorocentrum consutum sp. nov. (Dinophyceae), a new benthic dinoflagellate from South Brittany (northwestern France). Journal of Phycology, 46: 183–194.
- Chomérat, N., Zentz, F., Boulben, S., Bilien, G., van Wormhoudt, A. & Nézan, E. (2011). Prorocentrum glenanicum sp. nov. and P. pseudopanamense sp. nov. (Prorocentrales, Dinophyceae), two new benthic dinoflagellate species from South Brittany (northwestern France). Phycologia, 50: 202–214.
- Chomérat, N., Saburova, M., Bilien, G. & al-Yamani, F.Y. (2012). Prorocentrum bimaculatum sp. nov. (Dinophyceae, Prorocentrales), a new benthic dinoflagellate species from Kuwait (Arabian Gulf). Journal of Phycology, 48: 211–221.
- Couté, A. (2002). Biologie et microscopie électronique à balayage. Mémoires de la Société Entomologique de France, 6: 31–44.
- Croome, R.L., Hallegraeff, G.M. & Tyler, P.A. (1987). Thecadiniopsis tasmanica gen. et sp. nov. (Dinophyta: Thecadiniaceae) from Tasmanian waters. British Phycological Journal, 22: 325–333.
- Daney de Marcillac, S. (1846). Histoire de la Martinique depuis la colonisation jusqu’en 1815. Tome 1er. E. Ruelle, Fort-Royal.
- Delgado, G., Lechuga-Devéze, C.H., Popowski, G., Troccoli, L. & Salinas, C.A. (2006). Epiphytic dinoflagellates associated with ciguatera in the northwestern coast of Cuba. Revista de Biologiá Tropical, 54: 299–310.
- Dragesco, J. (1965). Étude cytologique de quelques flagellés mésopsammiques. Cahiers de Biologie Marine, 6: 83–115.
- du Tertre, J.-B. (1667–1671). Histoire générale des Antilles habitées par les François. Tome II. T. Jolly, Paris.
- Faust, M.A. (1990). Morphologic details of six benthic species of Prorocentrum (Pyrrhophyta) from a mangrove island, Twin Cays, Belize, including two new species. Journal of Phycology, 26: 548–558.
- Faust, M.A. (1993a). Prorocentrum belizeanum, Prorocentrum elegans, and Prorocentrum caribbaeum, three new benthic species (Dinophyceae), from a mangrove island, Twin Cays, Belize. Journal of Phycology, 29: 100–107.
- Faust, M.A. (1993b). Surface morphology of the marine dinoflagellate Sinophysis microcephalus (Dinophyceae) from a mangrove island, Twin Cays, Belize. Journal of Phycology, 29: 355–363.
- Faust, M.A. (1993c). Three new benthic species of Prorocentrum (Dinophyceae) from Twin Cays, Belize: P. maculosum sp. nov., P. foraminosum sp. nov. and P. formosum sp. nov. Phycologia, 32: 410–418.
- Faust, M.A. (1994). Three new benthic species of Prorocentrum (Dinophyceae) from Carrie Bow Cay, Belize: P. sabulosum sp. nov., P. sculptile sp. nov. and P. arenarium sp. nov. Journal of Phycology, 30: 755–763.
- Faust, M.A. (1996). Dinoflagellates in a mangrove ecosystem, Twin Cays, Belize. Nova Hedwigia, 112: 447–460.
- Faust, M.A. (2009). Ciguatera-causing dinoflagellates in a coral-reef-mangrove ecosystem, Belize. Atoll Research Bulletin, 569: 1–30.
- Faust, M.A. & Balech, E. (1993). A further SEM study of marine benthic dinoflagellates from a mangrove island, Twin Cays, Belize, including Plagiodinium belizeanum gen. et sp. nov. Journal of Phycology, 29: 826–832.
- Faust, M.A. & Gulledge, R.A. (2002). Identifying harmful marine dinoflagellates. Smithsonian Institution. Contributions from the United States National Herbarium, 42: 1–144.
- Faust, M.A., Morton, S.L. & Quod, J.-P. (1996). Further SEM study of marine dinoflagellates: the genus Ostreopsis (Dinophyceae). Journal of Phycology, 32: 1053–1065.
- Faust, M.A., Vandersea, M.W., Kibler, S.R., Tester, P.A. & Litaker, R.W. (2008). Prorocentrum levis, a new benthic species (Dinophyceae) from a mangrove island, Twin Cays, Belize. Journal of Phycology, 44: 232–240.
- Fraga, S., Rodríguez, F., Caillaud, A., Diogène, J., Raho, N. & Zapata, M. (2011). Gambierdiscus excentricus sp. nov. (Dinophyceae), a benthic toxic dinoflagellate from the Canary Islands (NE Atlantic Ocean). Harmful Algae, 11: 10–22.
- Fukuyo, Y. (1981). Taxonomical study on benthic dinoflagellates collected in coral reefs. Bulletin of the Japanese Society of Scientific Fisheries, 47: 967–978.
- Grzebyk, D., Berland, B., Thomassin, B.A., Bosi, C. & Arnoux, A. (1994). Ecology of ciguateric dinoflagellates in the coral reef complex of Mayotte Island (S.W. Indian Ocean). Journal of Experimental Marine Biology and Ecology, 178: 51–66.
- Grzebyk, D., Sako, Y. & Berland, B. (1998). Phylogenetic analysis of nine species of Prorocentrum (Dinophyceae) inferred from 18S ribosomal DNA sequences, morphological comparisons, and description of Prorocentrum panamensis, sp. nov. Journal of Phycology, 34: 1055–1068.
- Guillard, R.R.L. & Ryther, J.H. (1962). Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Canadian Journal of Microbiology, 8: 229–239.
- Guindon, S., Dufayard, J.-F., Lefort, V., Anisimova, M., Hordijk, W. & Gascuel, O. (2010). New algorithms and methods to estimate Maximum-Likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology, 59: 307–321.
- Herdman, E.C. (1921). Notes on dinoflagellates and other organisms causing discolouration of the sand at Port Erin. Transactions of the Liverpool Biological Society 35: 59–63.
- Herdman, E.C. (1922). Notes on dinoflagellates and other organisms causing discolouration of the sand at Port Erin. II. Transactions of the Liverpool Biological Society, 36: 15–30.
- Herdman, E.C. (1924a). Notes on dinoflagellates and other organisms causing discolouration of the sand at Port Erin. III. Transactions of the Liverpool Biological Society, 38: 58–64.
- Herdman, E.C. (1924b). Notes on dinoflagellates and other organisms causing discolouration of the sand at Port Erin. IV. Transactions of the Liverpool Biological Society, 38: 75–84.
- Hernández-Becerril, D.U. & Almazán Becerril, A. (2004). Especies de dinoflagelados del género Gambierdiscus (Dinophyceae) del Mar Caribe mexicano. Revista de Biologiá Tropical, 52 (Suppl. 1): 77–87.
- Hoppenrath, M. (2000a). Morphology and taxonomy of the marine sand-dwelling genus Thecadinium (Dinophyceae), with the description of two new species from the North German Wadden Sea. Phycologia, 39: 96–108.
- Hoppenrath, M. (2000b). Taxonomische und ökologische Untersuchungen von Flagellaten mariner Sande. University of Hamburg, Hamburg. 311 pp.
- Hoppenrath, M. & Elbrächter, M. (1998). Roscoffia capitata (Dinophyceae) refound: notes on morphology and biology. Phycologia, 37: 450–457.
- Hoppenrath, M. & Selina, M. (2006). Pseudothecadinium campbellii gen. nov. et sp. nov. (Dinophyceae), a phototrophic, thecate, marine planktonic species found in the Sea of Okhotsk, Russia. Phycologia 45: 260-269.
- Hoppenrath, M., Saldarriaga, J.F., Schweikert, M., Elbrächter, M. & Taylor, F.J.R. (2004). Description of Thecadinium mucosum sp. nov. (Dinophyceae), a new sand-dwelling marine dinoflagellate, and an emended description of Thecadinium inclinatum Balech. Journal of Phycology, 40: 946–961.
- Hoppenrath, M., Bolch, C.J., Yoshimatsu, S.-A., Saldarriaga, J.F., Schweikert, M., Campbell, C.N., Toriumi, S., Dodge, J.D., Elbrächter, M. & Taylor, D.L. (2005). Nomenclatural note on a Thecadinium species (Dinophyceae, Gonyaulacales), which was described as new independently three times within two months. Journal of Phycology, 41: 1284–1286.
- Hoppenrath, M., Horiguchi, T., Miyoshi, Y., Selina, M., Taylor, F.J.R. & Leander, B.S. (2007). Taxonomy, phylogeny, biogeography, and ecology of Sabulodinium undulatum (Dinophyceae), including an emended description of the species. Phycological Research, 55: 159–175.
- Hoppenrath, M., Chomérat, N., Horiguchi, T., Schweikert, M., Nagahama, Y. & Murray, S. (2013). Taxonomy and phylogeny of the benthic Prorocentrum species (Dinophyceae) – a proposal and review. Harmful Algae, 27: 1–28.
- Horiguchi, T. & Chihara, M. (1983). Stylodinium littorale, a new marine dinococcalean alga (Pyrrhophyta). Phycologia, 22: 23–28.
- Katoh, K. & Standley, D.M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution, 30: 772–780.
- Keller, M.D., Selvin, R.C., Claus, W. & Guillard, R.R.L. (1987). Media for the culture of oceanic ultraphytoplankton. Journal of Phycology, 23: 633–638.
- Larsen, J. (1985). Algal studies of the Danish Wadden Sea II. A taxonomic study of psammobious dinoflagellates. Opera Botanica, 79: 14–37.
- Litaker, R.W., Vandersea, M.W., Faust, M.A., Kibler, S.R., Nau, A.W., Holland, W.C., Chinain, M., Holmes, M.J. & Tester, P.A. (2010). Global distribution of ciguatera causing dinoflagellates in the genus Gambierdiscus. Toxicon, 56: 711–730.
- McNeill, J., Barrie, F.R., Buck, W.R., Demoulin, V., Greuter, W., Hawksworth, D.L., Herendeen, P.S., Knapp, S., Marhold, K., Prado, J., Prud’homme van Reine, W.F., Smith, G.F., Wiersema, J.H. & Turland, N.J. (2012). International Code of Nomenclature for Algae, Fungi and Plants (Melbourne Code). Regnum Vegetabile Volume 154. Koeltz Scientific Books, Königstein.
- Murray, S. (2009). Diversity and Phylogenetics of Sand-dwelling Dinoflagellates. VDM Verlag Dr. Müller, Saarbrücken.
- Murray, S. & Patterson, D.J. (2009). Cabra matta, gen. nov., sp. nov., a new benthic, heterotrophic dinoflagellate. European Journal of Phycology, 39: 229–234.
- Murray, S., Hoppenrath, M., Preisfeld, A., Larsen, J., Yoshimatsu, S.-A., Toriumi, S. & Patterson, D.J. (2006). Phylogenetics of Rhinodinium broomeense gen. et sp. nov., a peridinioid, sand dwelling dinoflagellate (Dinophyceae). Journal of Phycology, 42: 934–942.
- Olsen, D.A., Nellis, D.W. & Wood, R.S. (1984). Ciguatera in the Eastern Caribbean. Marine Fisheries Reviews, 46: 13–18.
- Posada, D. (2008). jModelTest: phylogenetic model averaging. Molecular Biology and Evolution, 25: 1253–1256.
- Pottier, I., Vernoux, J.-P. & Lewis, R.J. (2001). Ciguatera fish poisoning in the Caribbean islands and Western Atlantic. Reviews of Environmental Contamination and Toxicology, 168: 99–141.
- Rasband, W.S. (1997–2006). ImageJ. National Institutes of Health, Bethesda, MD.
- Rodriguez, E.A., Mancera Pineda, J.E. & Gavio, B. (2010). Survey of benthic dinoflagellates associated to beds of Thalassia testudinum in San Andrés Island, Seaflower Biosphere Reserve, Caribbean Colombia. Acta Biologica Colombiana, 15: 229–246.
- Ronquist, F. & Huelsenbeck, J.P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19: 1572–1574.
- Rosine, J., Chappert, J.L., Cardoso, T. & Quénel, P. (2008). La ciguatéra dans les Antilles Françaises. In Premières journées interrégionales de Veille Sanitaire des Antilles Guyane. Schoelcher, Martinique.
- Saunders, R.D. & Dodge, J.D. (1984). An SEM study and taxonomic revision of some armoured sand-dwelling marine dinoflagellates. Protistologica, 20: 271–283.
- Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M. & Kumar, S. (2011). MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, evolutionary distance, and Maximum Parsimony methods. Molecular Biology and Evolution, 28: 2731–2739.
- Tamura, M. & Horiguchi, T. (2005). Pileidinium ciceropse gen. et sp. nov. (Dinophyceae), a sand-dwelling dinoflagellate from Palau. European Journal of Phycology, 40: 281–291.
- Taylor, F.J.R. (1979). A description of the benthic dinoflagellate associated with maitotoxin and ciguatoxin, including observations on Hawaiian material. In Toxic Dinoflagellate Blooms (Taylor, D. L. & Seliger, H. H., editors), 71–76. Elsevier North Holland, New York.
- Taylor, F.J.R. (1985). The distribution of the dinoflagellate Gambierdiscus toxicus in the eastern Caribbean. In Proceedings of the Fifth International Coral Reef Congress (Gabrié, C. & Salvat, B., editors), 423–428. MNHN-EPHE, Moorea, Tahiti.
- Tester, P.A., Feldman, R.L., Nau, A.W., Kibler, S.R. & Litaker, R.W. (2010). Ciguatera fish poisoning and sea surface temperatures in the Caribbean Sea and the West Indies. Toxicon, 56: 698–710.
- Tosteson, T.R. (2004). Caribbean ciguatera: a changing paradigm. Revista de Biologiá Tropical, 52: 109–113.
- Vernoux, J.-P. (1988). La ciguatera dans l’île de Saint-Barthélémy: aspects épidémiologiques, toxicologiques et préventifs. Oceanologica Acta, 11: 37–46.
- Yamada, N., Terada, R., Tanaka, A. & Horiguchi, T. (2013). Bispinodinium angelaceum gen. et sp. nov. (Dinophyceae), a new sand-dwelling dinoflagellate from the seafloor off Mageshima Island, Japan. Journal of Phycology, 49: 555–569.
- Yoshimatsu, S.-A., Toriumi, S. & Dodge, J.D. (2004). Morphology and taxonomy of five marine sand-dwelling Thecadinium species (Dinophyceae) from Japan, including four new species: Thecadinium arenarium sp. nov., Thecadinium ovatum sp. nov., Thecadinium striatum sp. nov. and Thecadinium yashimaense sp. nov. Phycological Research, 52: 211–223.
