Abstract
Alexandrium tamarense is a cyst-forming dinoflagellate that can cause toxicity in shellfish. Belfast Lough, in northeast Ireland, has experienced toxicity events due to the presence of A. tamarense, which are monitored because of shellfish farms in the Lough. Since 1992 anthropogenic influences on the Lough have changed with the introduction of a ‘fast cat’ ferry service and an increase in mussel farming. In 2002 Belfast Lough was surveyed for A. tamarense cyst distribution in the sediments and the results are compared to a previous cyst survey carried out in the Lough in 1992. Cyst numbers were generally lower in 2002 than in 1992 (t-test, P < 0.01). The highest concentration found in 2002 was 1058 cysts g−1 dry sediment compared with 3330 in 1992. Although sediment disturbance increased in the period between the surveys and plays a role in cyst distribution, A. tamarense cysts are still present in the seabed of the Lough. These cysts remain an important repository of inoculating cells for A. tamarense blooms that have not been removed by the recent anthropogenic activity in the Lough and therefore the requirement for monitoring remains. Comparisons between cyst counts per volume of wet sediment and per weight of dry sediment were carried out, and although the first is needed for ecological studies allowing a deeper analysis, it is also recommended that cyst counts per dry weight of sediment are always reported for wider comparative purposes.
Introduction
Dinoflagellates are a group of protists of which approximately half of the species are photosynthetic (Taylor, Citation1987). These protists include many harmful species, particularly in the genus Alexandrium (Halim), which produce paralytic shellfish toxins (PSTs) (Taylor et al., Citation2003). PSTs accumulate in shellfish and are the cause of a serious form of food poisoning. Symptoms appear 30 minutes after ingestion and range from diarrhoea, nausea and vomiting to respiratory paralysis and death (Backer et al., Citation2003). Shellfish fishery closures and related negative publicity can have a major economic impact on coastal communities, affecting not only fishermen and aquaculturists, but also the tourist industry. Monitoring is necessary to prevent algal toxins reaching humans by the consumption of shellfish, direct contact or drinking water, and to minimize economic loss to the affected industries (Andersen et al., Citation2003). At the same time aquaculture deployments may in themselves influence bloom events. Filter-feeding shellfish may stimulate a bloom as they selectively ingest the natural phytoplankton community and supplement phytoplankton biomass by nutrient excretion (Smayda, Citation2003).
Some dinoflagellates produce a cyst following sexual reproduction (Pfiester & Anderson, Citation1987). Newly formed cysts will act as silt particles, being transported by currents until deposited onto the seabed (Dale, Citation1983). Dinoflagellate cysts in sediments provide an integrated record of water column activity of cyst-producing species, determining their potential distribution in the water column (Dale, Citation1983). Thus cyst assemblages in surface sediments can indicate changes in water quality of enclosed seas (Matsuoka, Citation1999) and they can also prove useful for tracing pollution and changes in pollution (Sætre et al., Citation1997). Cyst records can be used as indicators of different types of eutrophication, as well as of climate variations (Dale, Citation2009). Alexandrium species are of especial interest in the coastal zone because of the role they play in PST events (Hallegraeff, Citation1991; Anderson, Citation1998). Changes in cyst numbers in sediments represent the net result of increases to and losses from the benthic environment. Such changes might be mediated through variation in production of cysts in the water column, cyst sedimentation and redistribution patterns and the fate of cysts once they are in the sediment. In shallow coastal areas sediment disturbance might affect the fate and distribution of dinoflagellate cysts in several different ways. Cysts need oxygen to germinate (Anderson et al., Citation1987; Rengefors & Anderson, Citation1998; Kremp & Anderson, Citation2000; Blanco, Citation2005), therefore cysts buried in anoxic layers of the sediment will not excyst unless removed from these anoxic layers. Sediment disturbance will be likely to re-expose buried cysts to oxygen, increasing the number of cysts able to germinate (viable cysts) and thus, over time potentially decreasing the number of viable cysts in the sediment through excystment. Kirn et al. (Citation2005) showed that resuspended cysts contribute to the seasonal vegetative cell population. However, this phenomenon might be offset by the seeding of a higher population of the motile stages in the water column, thus providing more cysts to the sediments. Shull et al. (in press), developed a model that predicted these events, showing how high germination rates were followed by high cyst deposition. In addition, resuspended cysts that do not germinate while in the water column could be redistributed when redeposited. For instance, A. fundyense cysts in the nepheloid layers, which are an important source of germinating cysts, are transported southwards in the gulf of Maine (Pilskaln et al., in press). This redistribution might be to areas where they were not originally present (Anderson et al., Citation1982b), thus it is important to revisit cyst distributions through time to review the effect of such ecological changes.
Belfast Lough is a shallow bay with maximal depths of about 10 m in the inner Lough and 20 m at the mouth (Tylor et al., Citation1995), covering about 130 km2. It is situated on the east coast of Northern Ireland and has a history of PSP (paralytic shellfish poisoning) toxicity in shellfish (Ayres et al., Citation1982; McCaughey & Campbell, Citation1992), due to the presence of the A. tamarense species complex. Although we are unable to distinguish between A. tamarense and A. catenella cysts morphologically (Fukuyo, Citation1985), A. catenella has not been reported in Belfast Lough. Dinoflagellate cyst distribution was previously studied in the Lough in 1992 (Tylor et al., Citation1995; Tylor, Citation1997). Since that time there have been two changes in anthropogenic activity in the Lough: the introduction of the high speed catamaran ferry service in 1996 and the expansion of shellfish farming in the inner Lough, increasing bottom mussel cultivation and establishing at least one new scallop farm by 2000. Of particular interest to this study was the increased sediment disturbance in the shallow Lough caused by the fast cat service that operates several times a day, year round. Any changes in A. tamarense occurrence might have consequences for the increased shellfishery activity in the area.
There are few studies reporting long-term cyst distributions in surface sediments (Anderson et al., Citation2005; Martin et al., in press). This study reports on a comparative study of A. tamarense cyst distribution in Belfast Lough with a 10-year gap (1992–2002) to examine two questions: has there been any change in sediment cyst concentration over this period? If there has been a change in cyst concentration, what impact might this have on monitoring strategies for the Lough? In carrying out this study we were also keen to explore a methodological question: does reporting cysts per dry weight of sediment and cysts per volume of sediment produce the same comparative results?
Materials and methods
Sampling
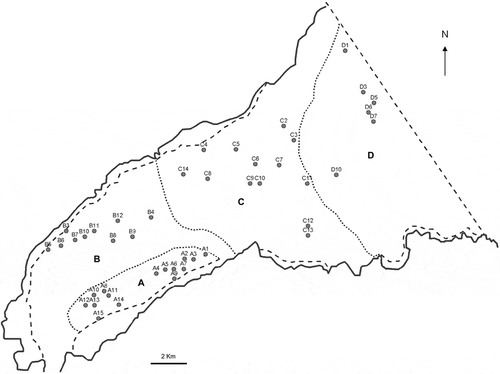
Sediment samples were collected in November 2002 and in April 2003 with a Craib corer (Craib, Citation1965). The top 5 cm of each core was extruded on site with the overlying water and stored in jars in the dark at 4ºC. The Lough was divided into four regions (A, B, C and D) as in Tylor et al. (Citation1995) (). Fifteen sites were chosen randomly in each region from a square grid with potential points separated by c. 550 m (). Only 44 of the 60 identified sites were sampled due to failure of the corer or because of bad weather conditions.
Cyst counts
Sediment samples were used for cyst identification and counts. Samples were processed and counted as in Blanco et al. (Citation2009). Briefly, sediment aliquots were sonicated for 4 minutes and sieved to retain the 20–80 µm particle fraction. These were concentrated using a density separation method using sodium polytungstate (Bolch, Citation1997), with a density gradient of 1.4 g ml−1 based on the lower densities of various cysts (Anderson et al., Citation1985), the work carried out by Tylor (Citation1997), and because it removed most particles that could interfere with the counts. Samples were then counted in a Sedgewick-Rafter chamber under a light microscope. Cyst numbers were determined both by sediment volume (cysts ml−1) and sediment dry weight (cysts g−1) for comparison purposes. For the latter, known volumes of each sediment sample were dried at 80°C for 4 days and weighed. Salinity corrections were applied to remove the weight of the salt content of the samples, assuming a bottom salinity of 33, as in Tylor et al. (Citation1995).
Triplicate samples were available for some of the sites, however, for other sites only two replicates were available due to corer failure or loss during sample transportation. From the replicates, at least one count was carried out under the light microscope, two counts when all three replicates were available. The third replicate was then analysed using the primuline staining method as described by Yamaguchi et al. (Citation1995), to make Alexandrium counting faster. Alexandrium tamarense cysts in primuline-stained samples were counted in a Sedgewick–Rafter chamber under a fluorescence microscope with a filter of 400–410 nm. Random checks were made under the light microscope to ensure that only full A. tamarense cysts were counted (empty cysts could be distinguished because they were not as bright as full cysts). Both counting methodologies were compared to validate the primuline staining method and no significant differences were found between light microscopy counts and fluorescence microscopy counts (t-test, P < 0.05), therefore, the results from both were averaged. Alexandrium tamarense cyst counts from Tylor (Citation1997) were available for comparison ().
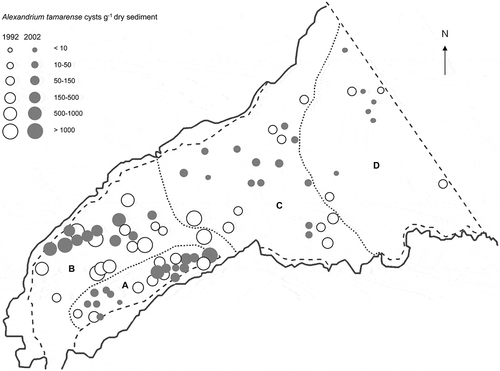
Fig. 2. Mean concentration of Alexandrium tamarense (cysts g−1 dry weight) in the surface sediments (0–5 cm depth) from 44 stations sampled in Belfast Lough in November 2002 and April 2003 (filled) and those obtained by Tylor (Citation1997) (open).
Sediment analysis
The sediment particle size data from 1992 were available from Tylor (Citation1997). Subsamples from 2002 were analysed at CEFAS (Burnham Laboratory) with sieving and laser diffraction techniques (Mason, Citation2011). Dry weights ml−1 sediment were calculated in triplicate for each sediment sample and the two datasets compared to evaluate the units in which cysts should be reported.
Statistical analysis
Alexandrium tamarense mean cyst concentrations in the four regions were compared using the Welch t-test and one-way analysis of means not assuming equal variances with the computer program R (R Core Team, Citation2013). Alexandrium tamarense cyst concentrations in the Lough were also compared with those from 1992 found by Tylor (Citation1997) by running a Welch t-test with the same program. Differences between light microscope and fluorescence microscope counts were analysed with the same test. Correlations of the cyst data expressed using two different units were carried out with GraphPad Prism v. 6.0a (Graphpad Software Inc., California, USA). The silt/clay fraction in each region of the Lough in 2002 was compared with those of 1992 with unpaired t-tests using Prism. Assumptions were checked for the choice of the appropriate analysis, and in all cases P < 0.05 unless otherwise stated.
Results
Results for cyst concentration in terms of volume and dry weight are shown in . Cyst concentrations in a sample varied depending on the unit of measurement; however there was a positive and significant correlation between the concentrations expressed as cysts g−1 dry sediment and cysts ml−1 wet sediment, with the exception of A. tamarense in region B ().
Table 1. Concentration of cysts (all species) in surface sediments from Belfast Lough collected in November 2002 and April 2003. Percentage of cysts from each station was in relation to the whole Lough, and percentage of A. tamarense cysts also was in relation to the whole Lough. All are expressed as both cysts ml−1 wet sediment and as cysts g−1 dry sediment. The stations have been sorted by descending values of cysts ml−1 wet sediment (second column).
Table 2. Correlation coefficient, P value and regression equation of the cyst concentration expressed as cysts ml−1 and cysts g−1 for the cyst concentration (all species) and for A. tamarense cyst concentration in the four regions () of Belfast Lough.
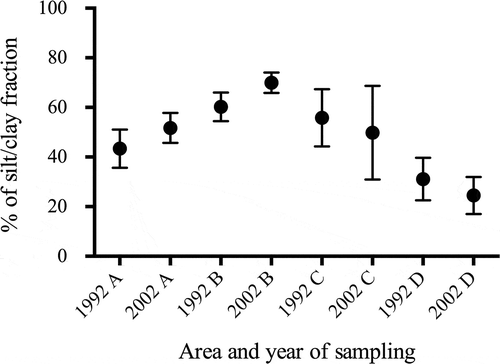
The silt/clay fraction in 2002 was lower in the outer Lough and higher in the inner Lough than in 1992, but no statistical difference was found between regions ().
Fig. 3. Silt/clay fraction (%) in the sediments of Belfast Lough divided into four regions () from the 1992 sampling (Tylor, Citation1997) and during this study (2002).
The dry weight of sediment in 1 ml of wet sediment in the Lough was 0.77± 0.28 g (mean ± SD). These results by region were: 0.74 ± 0.26 g for region A, 0.64 ± 0.16 g for region B, 0.71 ± 0.20 g for region C and 1.21 ± 0.18 g for region D. The minimum and maximum recorded dry weights of 1 ml of wet sediment were 0.35 and 1.42 g respectively.
Dinoflagellate cyst species found in Belfast Lough were Alexandrium tamarense (Lebour), A. minutum (Halim), Spiniferites spp., Scrippsiella trochoidea (Stein), Polykrikos sp. (Bütschli), Brigantedinium sp. and several Protoperidinium spp. (P. conicum (Gran), P. claudicans (Paulsen), P. conicoides (Paulsen) and P. minutum (Kofoid)).
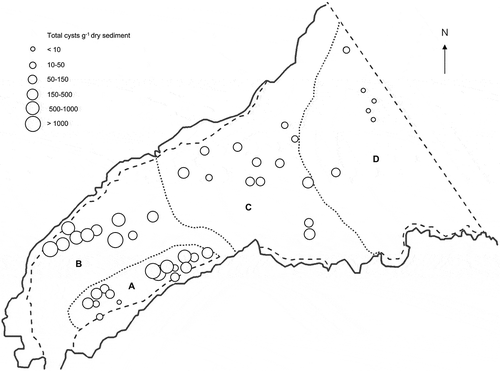
Total dinoflagellate cyst concentrations in Belfast Lough () were higher in the inner Lough (t-test, P < 0.01) with a mean concentration of 315 ± 390 cysts g−1 dry weight in region A (mean ± SD) and of 662 ± 330 cysts g−1 dry weight in region B, which correspond to the areas of finer sediments. Mean dinoflagellate cyst concentrations in the outer Lough were of 97 ± 81 cysts g−1 dry weight and 20 ± 31 cysts g−1 dry weight in regions C and D respectively. When comparing regions, region B, with the highest cyst concentration, differed significantly from all the others (t-test, P < 0.05) and regions A and C differed from region D, which showed the lowest concentration of cysts (t-test, P < 0.05). These results were equally valid using both cysts g−1 dry weight and cysts ml−1 wet sediment.
Fig. 4. Mean concentration of dinoflagellate cysts (cysts g−1 dry weight) in the surface sediments (0–5 cm depth) from 44 stations sampled in Belfast Lough in November 2002 and April 2003.
Alexandrium tamarense cyst concentrations were higher in the inner Lough, up to 611 ± 184 cysts g−1 dry weight and 335 ± 10 cysts ml−1 wet sediment in zone A; and 1058 ± 75 cysts g−1 dry weight and 640 ± 85 cysts ml−1 wet sediment in zone B (), following a similar pattern to the total dinoflagellate cyst concentration. The highest cyst concentrations were found in region B, where the mean concentration was 442 ± 294 cysts g−1 dry sediment and 239 ± 166 cysts ml−1 wet sediment. The lowest values, of 0.2 cysts (both g−1 dry sediment ± 0.4 and ml−1 wet sediment ± 0.5), were found in zone D. Differences in A. tamarense cyst concentration, as cysts g−1 dry sediment, between the four areas were statistically significant (one-way, P < 0.05), with the exception of regions A and C which did not differ statistically. Alexandrium tamarense cyst concentrations were generally lower (t-test, P < 0.01) than those found by the 1992 survey (Tylor, Citation1997). However, when the inner and the outer Lough were compared between surveys, a significant difference was only found for the inner Lough (t-test, P < 0.01). This change seemed marked in region B (t-test, P < 0.01), which was the only one to show a significant difference when comparing the two surveys region by region. When comparing cysts ml−1 wet sediment, there were stronger differences between surveys. In this case both the outer and inner Lough showed significant differences (t-test, P < 0.01), and all regions differed from the same region in the previous survey (t-test, P < 0.01 for regions B, C and D).
Discussion
Methodology
As discussed by Cho & Matsuoka (Citation2001), sediments have a variable water and salt content depending on where and how they have been collected. Dale (Citation2001) advised measurement of cyst concentration as cysts g−1 dry weight to remove the water factor, which can significantly affect cyst concentrations. Also Dale (Citation2001) discussed the unsuitability of measurements as cysts ml−1 wet sediment for estimating changes in cyst productivity. The results presented in this paper showed differences in peak numbers of cysts depending on the method of expression (for example sites B8 and B3 had respectively 471 and 482 cysts g−1 dry weight of sediment, but 1019 and 555 cysts ml−1 wet sediment). Otherwise, a good overall correlation was found between both units of measurement, likewise within each region, with the exception of A. tamarense in region B. However, although correlations were good, the slopes varied. In the Gulf of Maine in 2005, cyst abundance measured in both units showed a good correlation and had similar slopes (Anderson et al., in press), but these were very different from the slopes in this study. We obtained up to 35% variation in the weight of sediment per ml in one of the study regions where the sediments have similar characteristics. We consider that this variability must be mostly due to differences in water content which should be normalized for interpretation of cyst data. Even if sediments had similar water content, sampling in itself might introduce variation in this content, and also evaporation may occur to some extent: samples that have been stored for longer may have less water than when collected. Thus, sediments from different sites and areas in the world will be more likely to have different properties and might have larger variations in water content, and also in salt content, as well as a different relation with the volumetric unit.
However, we recognize that cyst numbers per volume of sediment are very important for ecological purposes, where information is required per unit area, to drive models for example. The general correlation of both units within one geographical area validates the use of these units. Perhaps in this case the results expressed per unit volume should be preferred as both datasets come from the same geographical area, but still, we found differences in the sediment dry weight and in particle size distribution over time. However, it is important to note that the weight of the sediment was well correlated with the silt/clay fraction: the higher the silt/clay fraction the lower the weight of the sediments. This information might be helpful when deciding how to compare cyst concentrations, if we know how similar or different the seabed is in a study area. Still, we recommend that cysts per dry weight of sediment are always reported for wider comparative purposes even where the study in question uses cysts per volume for other purposes.
Cyst distribution
PSP toxins occur in shellfish in the inner part of Belfast Lough, especially along the north shore (McCaughey & Campbell, Citation1992). Cyst distribution in the Lough seems to be influenced by both particle deposition and by cyst production in the water column (Tylor et al., Citation1995). Total cyst concentrations followed broadly the same distribution pattern as those for A. tamarense cysts. Alexandrium tamarense cyst concentrations in the Lough were generally lower in 2002 than in 1992 when the highest concentration was 3330 cysts g−1 dry sediment or 2696 cysts ml−1 wet sediment (Tylor et al., Citation1995) against 1058 cysts g−1 dry sediment or 216 cysts ml−1 wet sediment in the 2002 survey. Alexandrium spp. cyst concentrations as cysts g−1 dry sediment equalling the 1992 survey have also been found in other areas in the world, as well as others well below the values from the 2002 survey (). Other concentration values around the world are given as cysts ml−1 wet sediment. The highest value of cysts g−1 dry weight in the Belfast 2002 survey corresponded to 216 cysts ml−1 wet sediment, although the highest concentration, of 640 cysts ml−1 wet sediment, was found in a different site within the Lough and both were lower than the 2696 cysts ml−1 wet sediment found in 1992. In other regions of the world the concentration of A. tamarense cysts varied from few hundreds to thousands of cysts ml−1 wet sediment (). The concentration of A. tamarense cysts in Belfast Lough in the 2002 survey might seem low compared with other parts of the world when expressed as cysts ml−1 wet sediment, but this difference is not so marked when numbers are expressed in cyst g−1 dry weight (). The mean concentration of 442 cysts g−1 dry weight found in 2002 in region B is still a relatively high figure for the UK. In comparison, the highest concentration found in the Firth of Forth, where cysts in the sediment seem to be responsible for the PSP episodes in the area by direct toxicity and by inoculating the water column, was 404 cysts g−1 dry sediment (Higman, Citation1997; Joint et al., Citation1997).
Table 3. Alexandrium spp. cyst concentration in different areas worldwide expressed as cyst g−1 dry weight or cyst ml−1 wet sediment.
The lower cyst concentrations found in the 2002 Belfast survey relative to 1992 might be due to various factors. It is possible that environmental conditions from 1992 to 2002 contributed to increased excystment rates or lower encystment rates, thus decreasing cyst concentrations over time. A more probable explanation for the decrease in cyst concentrations is the increase in sediment disturbance in the Lough since 1992 due to the newly introduced ferry routes and mussel dredging. The main shipping line is dredged for maintenance every five years, while dredging in the mussel farms can occur throughout the year (Service & Dunbar, Citation2008). The effect of sediment disturbance in the Lough can also explain the better sorting of particles, with an increase of the silt/clay fraction in the inner Lough and a decrease in the outer Lough, in comparison with 1992, that was also reflected in the distribution of A. tamarense cysts. Boat-generated disturbance has been shown to affect macrobenthic fauna (Bishop & Chapman, Citation2004) and macroscopic vegetation (Eriksson et al., Citation2004) by increased wave action, turbidity and removal of sediment. Besides increasing germination potential of cysts, disturbance increases turbidity in the water column potentially affecting the growth and survival of A. tamarense motile cells and reducing cyst production. An increase in turbidity in the Lough was confirmed in 2004 by the Agri-Food and Biosciences Institute (AFBI), which has monitored the Lough historically through regular surveys at coastal sites and recently by collecting data remotely. Alexandrium tamarense motile cells have been documented in the inner Lough (A. McKinney, AFBI, personal communication) from 1995 to 2002 as part of the AFBI monitoring programme. Highest cell abundances were near the north shore of the inner Lough, with concentrations ranging from 0 to 8.89 × 105 cells l−1. The highest abundances of A. tamarense cells in the water column appeared between May and July every year, and the species disappeared from October to February. This higher abundance on the north shore corresponds with the higher cyst concentrations in the sediments of the inner north shore (region B). Over the duration of monitoring there was no reduction in motile cell numbers in the water column reported, despite a possible increase in boat-generated turbidity.
Shellfish farming (mussels) also increased in the Lough between the two surveys, especially on the north shore of the Lough (Brittain, Citation2001), with 12 new sites in 2002, covering most of the inner Lough (H. Moore, AFBI, personal communication). These activities might affect dinoflagellate populations by moving cysts out of the Lough through mussel dredging, therefore decreasing their numbers, but at the same time spreading them into other areas. This could explain why major differences were found between surveys in region B and also when comparing the inner with the outer Lough, although particle sorting has affected all areas.
In other areas of the world where cyst concentrations in the sediments have been tracked from year to year, considerable variations can be found (Anderson et al., Citation2005). Although we cannot rule out such inter-annual variation for Belfast Lough with these data (only two datasets considered, 10 years apart), in the 2 years surveyed there was a reduction of cysts in the sediments. The annual presence of A. tamarense motile cells, and the fact that motile cells were not detected in early spring or autumn suggests that A. tamarense populations in Belfast Lough are seeded and maintained by excystment of cysts in the sediments of the Lough. Furthermore, taking into account the cyst deposits in the Lough, which are still extensive after 10 years, it would seem that A. tamarense will remain a feature of the plankton in the Lough. Considering also the shellfish activity in the area, it will therefore remain important to maintain monitoring programmes to protect shellfish consumers from paralytic shellfish poisoning.
Acknowledgements
The authors would like to thank Dr Tim Tylor for providing his data from 1992 for comparison purposes, and to Drs April McKinney, Matt Service and Heather Moore for their help in sample collection and the data and information provided, as well as Drs Adam Mellor, M. E. Charlesworth and all the crew who helped during sample collection from the Aquatic Sciences Research Division (ASRD) of the Department of Agriculture in Northern Ireland. This work was funded by the Centre for the Environment, Fisheries and Aquaculture Science (CEFAS).
References
- Agri-Food and Biosciences Institute. Available from: www.afbini.gov.uk/index/services/services-specialist-advice/coastal-science/coastal-monitoring/monitored-sites.htm [Last accessed 4 June 2011].
- Andersen, P., Enevoldsen, H. & Anderson, D. (2003). Harmful algal monitoring programme and action plan design. In Manual on Harmful Marine Microalgae (Hallegraeff, G.M., Anderson, D.M. & Cembella, A.D., editors), 627–647. UNESCO Publishing, Paris.
- Anderson, D.M. (1998). Physiology and bloom dynamics of toxic Alexandrium species, with emphasis on life cycle transitions. In Physiological Ecology of Harmful Algal Blooms (Anderson, D.M., Cembella, A.D. & Hallegraeff, G.M., editors), Vol. G 41, 29–48. Springer-Verlag, Berlin Heidelberg.
- Anderson, D.M., Aubrey, D.G., Tyler, M.A. & Coats, D.W. (1982a). Vertical and horizontal distributions of dinoflagellate cysts in sediments. Limnology and Oceanography 27: 757–765.
- Anderson, D.M., Kulis, D.M., Orphanos, J.A. & Ceurvels, A.R. (1982b). Distribution of the toxic dinoflagellate Gonyaulax tamarensis in the Southern New England region. Estuarine, Coastal and Shelf Science, 14: 447–458.
- Anderson, D.M., Lively, J.J., Reardon, E.M. & Price, C.A. (1985). Sinking characteristics of dinoflagellate cysts. Limnology and Oceanography, 5: 1000–1009.
- Anderson, D.M., Taylor, C.D. & Armbrust, E.V. (1987). The effects of darkness and anaerobiosis on dinoflagellate cyst germination. Limnology and Oceanography, 32: 340–351.
- Anderson, D.M., Stock, C.A., Keafer, B.A., Nelson, A.B., Thompson, B., McGillicuddy, D.J., Keller, M., Matrai, P.A. & Martin, J. (2005). Alexandrium fundyense cyst dynamics in the Gulf of Maine. Deep-Sea Research Part II, 52: 2522–2542.
- Anderson, D.M., Keafer, B.A., Kleindinst, J.L., McGillicuddy Jr., D.J., Martin, J.L., Norton, K., Pilskaln, C.H., Smith, J.L., Sherwood, C.R. & Butman, B. (in press). Alexandrium fundyense cysts in the Gulf of Maine: long-term time series of abundance and distribution, and linkages of past and future blooms. Deep-Sea Research Part II.
- Ayres, P.A., Seaton, D.D. & Tett, P.B. (1982). Plankton blooms of economic importance to fisheries in UK waters 1968–1982. 21 pp. CM1982/L:38, Biological Oceanography Committee, ref. Marine Environmental Quality Committee.
- Backer, L.C., Fleming, L.E., Rowan, A.D. & Baden, D.G. (2003). Epidemiology, public health and human diseases associated with harmful marine algae. In Manual on Harmful Marine Microalgae (Hallegraeff, G.M., Anderson, D.M. & Cembella, A.D., editors), 723–749. UNESCO Publishing, Paris.
- Bishop, M.J. & Chapman, M.G. (2004). Managerial decisions as experiments: an opportunity to determine the ecological impact of boat-generated waves on macrobenthic infauna. Estuarine, Coastal and Shelf Science, 61: 613–622.
- Blanco, E.P. (2005). A study of the distribution and germination dynamics of Alexandrium cysts in UK sediments. PhD thesis. University of Westminster. London, United Kingdom.
- Blanco, E.P., Lewis, J. & Aldridge, J. (2009). The germination characteristics of Alexandrium minutum (Dinophyceae), a toxic dinoflagellate from the Fal estuary (UK). Harmful Algae, 8: 518–522.
- Bolch, J. (1997). The use of sodium polytungstate for the separation and concentration of living dinoflagellate cysts from marine sediments. Phycologia, 36: 472–478.
- Brittain, K. (2001). Minister welcomes development of farmed mussel industry in Belfast Lough. DARD Media Services, Northern Ireland. Available from: www.dardni.gov.uk/pr2001/pr010010.htm [Accessed 29 June 2005].
- Cho, H.-J. & Matsuoka, K. (2001). Distribution of dinoflagellate cysts in surface sediments from the Yellow Sea and East China Sea. Marine Micropaleontology, 42: 103–123.
- Cox, A.M., Shull, D.H. & Horner, R.A. (2008). Profiles of Alexandrium catenella cysts in Puget Sound sediments and the relationship to paralytic shellfish poisoning events. Harmful Algae, 7: 379–388.
- Craib, J.S. (1965). A sampler for taking short undisturbed marine cores. Journal du Conseil, 30: 34–39.
- Dale, B. (1983). Dinoflagellate resting cysts: “benthic plankton”. In Survival Strategies of the Algae (Fryxwell, G.A., editor), 69–137. Cambridge University Press, Cambridge.
- Dale, B. (2001). Marine dinoflagellate cysts as indicators of eutrophication and industrial pollution: a discussion. Science of the Total Environment, 264: 235–245.
- Dale, B. (2009). Eutrophication signals in the sedimentary record of dinoflagellate cysts in coastal waters. Journal of Sea Research, 61: 103–113.
- Eriksson, B.K., Sandström, A., Isæus, M., Schreiber, H. & Karås, P. (2004). Effects of boating activities on aquatic vegetation in the Stockholm archipelago, Baltic Sea. Estuarine, Coastal and Shelf Science, 61: 339–349.
- Fukuyo, Y. (1985). Morphology of Protogonyaulax tamarensis (Lebour) and Protogonyaulax catenella (Whedon and Kofoid) Taylor from Japanese coastal waters. Bulletin of Marine Science, 37: 533–534.
- Hallegraeff, G.M. (1991). Aquaculturists’ Guide to Harmful Australian Microalgae. CSIRO, Division of Fisheries, Tasmania.
- Higman, W. (1997). The distribution and toxicity of Alexandrium of the North East coast of Britain. PhD thesis. University of Westminster, London.
- Horner, R.A., Greengrove, C.L., Davies-Vollum, K.S., Gawel, J.E., Postel, J.R. & Cox, A.M. (2011). Spatial distribution of benthic cysts of Alexandrium catenella in surface sediments of Puget Sound, Washington, USA. Harmful Algae, 11: 96–105.
- Joint, I., Lewis, J., Aiken, J., Proctor, R., Moore, G., Higman, W. & Donald, M. (1997). Interannual variability of PSP outbreaks on the north east UK coast. Journal of Plankton Research, 19: 937–956.
- Kirn, S.L., Townsend, D.W. & Pettigrew, N.R. (2005). Suspended Alexandrium spp. hypnozygotes cysts in the Gulf of Maine. Deep-Sea Research II, 52: 2543–2559.
- Kremp, A. & Anderson, D.M. (2000). Factors regulating germination of resting cysts of the spring bloom dinoflagellate Scrippsiella hangoei from the northern Baltic Sea. Journal of Plankton Research, 22: 1311–1327.
- Martin, J.L., LeGresley, M.M. & Hanke, R. (in press). Thirty years: Alexandrium fundyense cysts, bloom dynamics and shellfish toxicity in the Bay of Fundy, eastern Canada. Deep-Sea Research II.
- Mason, C. (2011). NMBAQC’s Best Practice Guidance: Particle Size Analysis (PSA) for Supporting Biological Analysis. 72 pp. National Marine Biological AQC Coordinating Committee.
- Matsuoka, K. (1999). Eutrophication process recorded in dinoflagellate cyst assemblages – a case of Yokohama Port, Tokyo Bay, Japan. Science of the Total Environment, 231: 17–35.
- McCaughey, W.J. & Campbell J.N. (1992). Monitoring in Belfast Lough. Harmful Algal News, 3: 3.
- Orozco, F.E. & Carreto, J.I. (1989). Distribution of Alexandrium excavatum resting cysts in a patagonic shelf area (Argentina). In Red Tides: Biology, Environmental Sciences and Toxicology (Okumura, Y., Anderson, D.M. & Nemoto, T., editors), 309–312. Elsevier, London.
- Pfiester, L.A. & Anderson, D.M. (1987). Dinoflagellate reproduction. In The Biology of Dinoflagellates (Taylor, F.J.R., editor), 611–648. Blackwell Scientific Publications, Oxford.
- Pilskaln, C.H., Hayashi, K., Keafer, B.A., Anderson, D.M. & McGillicuddy Jr. D.J. (in press). Benthic nepheloid layers in the Gulf of Maine and Alexandrium cyst inventories. Deep-Sea Research II.
- R Core Team (2013). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. www.R-project.org.
- Rengefors, K. & Anderson, D.M. (1998). Environmental and endogenous regulation of cyst germination in two freshwater dinoflagellates. Journal of Phycology, 34: 568–577.
- Sætre, M.M.L., Dale, B., Abdullah, M.I. & Sætre, G.-P. (1997). Dinoflagellate cysts as potential indicators of industrial pollution in a Norwegian Fjord. Marine Environmental Research, 44: 167–189.
- Service, M. & Dunbar, K. (2008). Sanitary Survey Report Belfast Lough. Available from: www.food.gov.uk/multimedia/pdfs/belfastlough.pdf [Accessed 1 November 2013].
- Shull, D.H., Kremp, A. & Mayer, L.M. (in press). Bioturbation, germination and deposition of Alexandrium fundyense cysts in the Gulf of Maine. Deep-Sea Research II.
- Smayda, T.J. (2003). Environmental monitoring, with examples from Narrangansett Bay. In Manual on Harmful Marine Microalgae (Hallegraeff, G.M., Anderson, D.M. & Cembella, A.D., editors), 595–625. UNESCO Publishing, Paris.
- Taylor, F.J.R. (1987). General group characteristics; special features of interest; short history of dinoflagellate study. In The Biology of Dinoflagellates (Taylor, F.J.R., editor), 1–23. Blackwell Scientific Publications, Oxford.
- Taylor, F.J.R., Fukuyo, Y., Larsen, J. & Hallegraeff, G.M. (2003). Taxonomy of harmful dinoflagellates. In Manual on Harmful Marine Microalgae (Hallegraeff, G.M., Anderson, D.M. & Cembella, A.D., editors), 389–432. UNESCO Publishing, Paris.
- Turgeon, J., Cembella, A.D., Therriault, J.-C. & Beland, P. (1990). Spatial distribution of resting cysts of Alexandrium spp. in sediments of the lower St. Lawrence Estuary and the Gaspe coast (eastern Canada). In Toxic Marine Phytoplankton (Granéli, E., Sundström, B., Edler, L. & Anderson, D.M., editors), 238–243. Elsevier, London.
- Tylor, T.J.M. (1997). Studies of Alexandrium sp. hypnocysts in Belfast Lough. PhD Thesis, University of Westminster, London.
- Tylor, T.J.M., Lewis, J. & Heaney, S.I. (1995). A survey of Alexandrium sp. cysts in Belfast Lough, 1992. In Harmful Marine Algal Blooms (Lassus, P., Arzul, G., Erard, E., Gentien, P. & Marcaillou, C., editors), 835–840. Lavoisier Science Publishers, Paris.
- Wang, Z., Matsuoka, K., Qi, Y., Chen, J. & Lu, S. (2004a). Dinoflagellate cyst records in recent sediments from Daya Bay, South China Sea. Phycological Research, 52: 396–407.
- Wang, Z., Qi, Y., Lu, S., Wang, Y. & Matsuoka, K. (2004b). Seasonal distribution of dinoflagellate resting cysts in surface sediments from Changjiang River Estuary. Phycological Research, 52: 387–395.
- Yamaguchi, M., Itakura, S., Imai, I. & Ishida, Y. (1995). A rapid and precise technique for enumeration of resting cysts of Alexandrium spp. (Dinophyceae) in natural sediments. Phycologia, 34: 207–214.