Abstract
Pellicle or temporary cysts of Pyrodinium bahamense var. compressum (Böhm) Steidinger, Tester & F.J.R. Taylor and their role in bloom dynamics have not yet been adequately characterized and understood. We investigated the role of temperature- and nutrient-mediated stress as factors that could induce pellicle formation in batch cultures. Cellular features and their implications for temporary cyst viability were examined using confocal laser scanning microscopy (CLSM). Our data suggest that temperature change is one of the key factors influencing pellicle formation, preserving viability at low temperature (i.e. 13°C). Hypnocysts (resting cysts) were not observed. During pellicle formation, motile cells generally undergo ecdysis, extrusion of cytoplasmic materials and bacteria, compaction of the nucleus and non-motility. The outermost covering of the temporary cysts shows red autofluorescence and it contains lower concentrations of chlorophyll (chl) a and no detectable chl c. The nuclear region is surrounded by transitional red bodies and other unidentified cellular structures. Temporary cysts can immediately revert back to the motile state upon exposure to optimum conditions. This is accompanied by the expansion of the nuclear region, regeneration of the chloroplasts and enlargement of the cell. Developmental changes during reversal of temporary cysts to motile forms were also observed to cause breaks in the cell covering that could serve as sites for bacterial entry. Though observed in vitro, such behaviour may also be occurring in nature especially as a response to drastic short-lived environmental changes. This is the first detailed report on the characteristics of temporary cysts of P. bahamense var. compressum.
Introduction
Dinoflagellates exhibit intricate life cycles in response to varying environmental conditions and other unknown internal regulating factors that induce them to exist in different forms. These stages are necessary for the survival and propagation of the organism as well as in the succession of the population. Complete knowledge of the life cycle of each species is important in understanding the effects of environmental variation and in interpreting monitoring data (Figueroa et al., Citation2008). However, many of these life stages are difficult to recognize using light microscopy, hence many life forms, such as those in Pyrodinium bahamense var. compressum (Böhm) Steidinger, Tester & F.J.R. Taylor, have not yet been identified and characterized (Khan et al., Citation1995; Kai et al., Citation2006). Azanza (Citation1997) suggested a life cycle for P. bahamense var. compressum which included the formation of haploid resting cysts (hypnocysts), their regeneration to motile cells and development of pellicle forms (temporary cysts). Many studies have been made to understand the resting cysts and the motile forms, but only a few have focused on the pellicle cyst and other possible unknown stages.
Pellicle formation was first observed in Pyrodinium cells collected from a bloom that were left to stand in the dark for a night. The cells were able to revert back to the vegetative state immediately and underwent cell division when conditions became favourable (Azanza, Citation1997). The formation of pellicle cysts in dinoflagellates has been suggested as a means of escape from harsh or unfavourable environments (Garcés et al., Citation2002; Olli, Citation2004; Bravo et al., Citation2010). Pellicle cyst formation has also been observed in algal species that were digested by filter feeders and were able to survive and revert back to the vegetative state (Persson & Rosenberg, Citation2003). Observations in the field indicate that pellicle formation is a response to conditions such as turbulence (Azanza, Citation1997), temperature change (Anderson et al., Citation1984; Jensen & Moestrup, Citation1997), bacterial/viral attack (Nagasaki et al., Citation2000; Tarutani et al., Citation2001), light attenuation and high albedo (Rintala et al., Citation2007) and nutrient starvation (Azanza & Larsen, Citation1997). However, despite several studies including field observations, the critical and specific factors influencing pellicle formation and excystment are not yet fully understood.
In this study we report in detail on developmental processes in Pyrodinium which have not been characterized previously, particularly pellicle cyst formation. No detailed investigations have been made of this stage and its presence in the field has been overlooked because of this. We investigated potential stresses that may induce formation of temporary cysts. Laboratory induction is required to directly associate environmental parameters causing vegetative or motile cells to give rise to the pellicle stage, which will then be useful for field investigations. In this study, cyst refers to temporary cysts unless otherwise specified.
Materials and methods
Morphological characterization
Pyrodinium cultures (PbcMZRVA042595) were obtained from the RVA Microalgal Culture Collection of the Harmful Algal Blooms Laboratory at The Marine Science Institute, University of the Philippines Diliman. The monoculture used was isolated on April 1995 in Masinloc Bay, Zambales, Northwestern Philippines and routinely maintained in Guillard’s f/2 medium (Guillard & Ryther, Citation1962) without silica at 24°C (Azanza, Citation1997).
15–20 Pyrodinium cells were inoculated in 24-well plates with 1.5 ml f/2 enriched seawater and exposed to temperature stress by incubating at 30°C and 13°C. Cells were also maintained at 24°C as positive controls. The 13°C regime cultures were grown in a refrigerated incubator (Kolin, USA) while others were incubated at 30°C in a temperature-gradient chamber (Anderson et al., Citation1984). Cultures were monitored using an inverted microscope (PhotoZoom, Bausch & Lomb) and morphological changes were noted every 30 min for the first 4 h, every hour after that for 24 h, then once a day for the next 10 days.
To further understand morphology and composition, immotile temporary cysts obtained in the stressful conditions in upscaled cultures (200 ml) were fixed (1% glutaraldehyde, final concentration) for at least 30 min at 4ºC, centrifuged (2300 × g for 13 min) and further characterized by confocal laser scanning microscopy (CLSM) as described by Onda et al. (Citation2013) with few modifications. In brief, the pelleted, preserved cells were resuspended in 500 µl sterile phosphate buffer saline (filter-sterilized and adjusted to pH 8.0); 50 µl of resuspended cells was mounted on a polycarbonate filter with a hole at the centre and embedded in a glass slide. Cells were stained with Calcofluor white (0.15%, final concentration; Sigma Aldrich) and Sybr Green (100× final concentration; Invitrogen, USA). A confocal laser scanning microscope (LSM 710 Carl Zeiss, Germany) was used for imaging and Z-stack images were reconstructed to 3D-images. The stained thecal plates were imaged by exciting the fluorescent dye at 355 nm and capturing emissions at 360–443 nm (Sigma-Aldrich). Associated bacteria/bacteria-like forms (BLF – intracellular and extracellular) were also visualized by staining with Sybr Green (excitation 497 nm; Invitrogen, USA) and detection under a 520 nm filter. A pure culture of known bacteria and the non-axenic algal medium with bacterial co-cultures were used as controls. Autofluorescent materials were detected at 650 nm (excitation 580 nm). Motile cells were also subjected to the same process and bacteria-like particles characterized by their coccoid shape. Brownian motion, green fluorescence and localization were imaged, for both motile cells and temporary cysts.
To investigate the formation of temporary cysts in situ, field samples were collected in Matarinao Bay, Samar, Eastern Philippines on May 2010. A 10 l Niskin type bottle was used for the collection and samples were transferred to sterile 1 l Nalgene bottles. Some of the samples were fixed with glutaraldehyde (1% final concentration) immediately after collection but others were transported without fixing. Cells were examined as discussed previously.
Effects of temperature and nutrient stress
After characterization and identification of cell differentiation during pellicle formation, another experiment with additional treatments was conducted to investigate effects of changing conditions, such as temperature and nutrient stresses. Cultures of P. bahamense var. compressum were pre-grown in routine laboratory conditions (i.e. in f/2 medium at 24°C) for 10 days. When the appropriate cell density was reached (~2000 cells ml−1), 100 ml aliquots were taken and then grown with additional 100 ml media or sterile seawater under nine experimental conditions (i.e. treatments), representing the different levels of temperature and nutrient conditions investigated in this study.
Three nutrient regimes were tested together with the three temperature regimes (in a 3 × 3 factorial design), namely, routine nutrient condition (f/2), low nitrogen (f/2 without NaNO3) and low total nutrients (surface seawater without nutrient supplements). Salinity at 34 ± 1 ppt was maintained throughout the experiment in all treatments. Negative controls (sterile seawater + f/2 without inoculum) were used to detect possible bacterial and fungal contamination. The temperature regimes tested were low (13°C), routine (24°C), and high (30°C) using the incubators previously described. Irradiance in all set-ups was provided by cool-white fluorescent bulbs at approximately 200 µmol photons m−2 s−1 with a 12 : 12 h light: dark (LD) cycle. Due to limitations in space in the incubators and chambers, treatments were limited to duplicates (N = 2). One ml of cells from each set-up was sampled after the first 4 hours and then after every 24 hours from the start of sub-culturing. Cells were immediately fixed in 1% formaldehyde (final concentration; Sigma-Aldrich) after every sampling to arrest cell differentiation (Garcés et al., Citation2002). The flasks were lightly swirled before pipetting, to sample cysts that had settled at the bottom. Then, 100 µl from the cell aliquot were resuspended in 1 ml sterile seawater in a Sedgwick-Rafter counting chamber (motile cells and cysts) under a light microscope (Axioscope, Carl Zeiss, Germany). Samples were observed for changes in morphology.
During counting, cells were classified as either vegetative/motile cell or temporary cyst based on established parameters and criteria: vegetative cells are generally larger, have darker cell bodies and intact appendages (i.e. horns and cingulum; Azanza-Corrales & Hall, Citation1993; Azanza & Larsen, Citation1997), while the pellicle or temporary cysts have smaller nuclei, a smoother surface and appear to have already undergone excretion of intracellular components and primary and/or secondary ecdysis, based on previous observations (see Results and Supplementary –). Total cell count was the summation of vegetative/motile cells and temporary cysts. Encystment efficiency (EE [cyst cell−1]) was calculated by taking the ratio of cysts and the total cell count every sampling (Anderson et al., Citation1984).
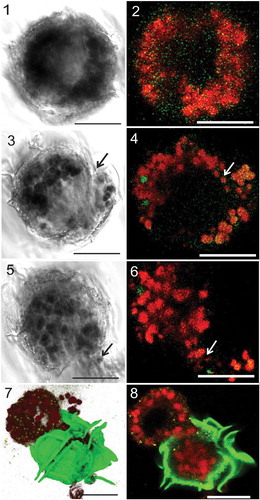
Figs 1–8. Light and confocal laser scanning microscopy (CLSM) of Pyrodinium bahamense var. compressum showing intracellular changes during pellicle formation. , Cells were first observed to exhibit aggregation of the red autofluorescent (RAF) organelles (plastids) during pellicle formation, followed by formation of the opening in the membrane (arrows) and in between the thecal plates () and extrusion of the RAF materials via the opening in the wall as indicated by the arrows (). . Extruded materials might possibly include bacteria-like forms (BLFs), plastids and other cytoplasmic materials (BLFs pseudocoloured yellow stained with Sybr Green, theca pseudocoloured green stained with Calcofluor White, RAF-bodies/plastids pseudocoloured red). Scale bars are 20 µm.
A randomized complete block design was used in the experiments. Statistical analysis by Repeated Measure ANOVA was done using PAST (Paleontological Statistics; Hammer et al., Citation2001) to test for significant differences in the means and interactions of variables.
Reversal to motile state
We also investigated the viability of the observed pellicle forms. Twenty-eight days after sub-culturing, 100 ml of ~100% temporary cysts from the low and high-temperature treatments were transferred to new flasks with 100 ml of freshly prepared f/2 medium without silica and incubated at the routine laboratory temperature condition. One ml of cells was taken immediately after transfer, every 30 min for the first 3 hours, and every hour after that, until mature forms of vegetative cells were observed. Samples were immediately fixed with ~1% glutaraldehyde (final concentration) and prepared for microscopy and imaged using CLSM as described previously.
Chlorophyll extraction and analysis
Cells for chlorophyll (chl) analysis were prepared as described above with some modifications. To investigate the effect of nutrient depletion on chl concentration, one set of cultures was cultivated in 100 ml sterile-filtered seawater (low total nutrient) and another in routine laboratory conditions in f/2 medium. Negative controls were also maintained using the same nutrient conditions but without any algal cultures. Three replicate cultures grown in 250 ml Erlenmeyer flasks were used for each treatment. All treatments were first grown at 24°C for 7 days, and then three flasks for each nutrient treatment (f/2 and low total nutrient) were transferred to 13°C to induce formation of temporary cysts and incubated for another 6 days until they reached ~100% cysts, while positive controls (with an algal inoculum) were retained at 24°C. After incubation, the cultures were mixed thoroughly and 1 ml of cells were withdrawn and fixed (in 1% glutaraldehyde). Cells (cysts and motile) were counted as described in the preceding section.
Chlorophyll extraction followed the acetone method described by Aminot & Rey (Citation2001) with some adjustments. 100 ml of the well-mixed culture were filtered using GF/C filter paper (Whatman) by a vacuum pump in subdued light. The filtrates were immediately folded and transferred to a 15 ml conical tube wrapped with foil, stored in an ice box to prevent possible pigment degradation, and soaked in 5 ml 90% acetone. To lyse the cells and liberate the chlorophyll, filtrates were sonicated for 1 min in an ultrasonicator (Cole-Parmer, USA) whilst in an ice-bath and under subdued light. 90% acetone was added to the sonicated filtrate to make it up to 10 ml and it was then centrifuged at 500 × g for 10 min at 15°C to separate the debris from the extracted pigments.
Absorbance of three chlorophyll pigments was measured, namely chl a, b and c using a spectrophotometer (UV1800 Shimadzu, Japan), following the spectrophotometric-trichromatic method. Immediately after centrifugation, 1 ml of the supernatant was transferred to a 1 ml cuvette. Absorbance at 630, 647, 664 and 750 nm were measured against 90% acetone as a blank. Chlorophyll concentrations (µg l−1) of each replicate were then calculated using equations suggested by Jeffrey & Humphrey (Citation1975). To get the chl content per cell (µg cell−1), the total chlorophyll concentration (µg l−1) was divided by the total number of cells (cells l−1) and the average for the three replicates was calculated.
DNA extraction and gene amplification
Total genomic DNA extraction was performed using the DNeasy Plant Mini-kit (Qiagen, USA). 50 ml of ~100% temporary cysts, induced at low temperature in f/2, were collected and pelleted at 3000 × g for 12 min to concentrate cells and remove the free DNA in the medium. The supernatant was removed and extracted in duplicate as described by Onda et al. (Citation2013). The pelleted cysts were resuspended in 400 µl AP1 buffer (Qiagen, USA) and 4–6 sterile silica beads were added. Cysts were bead-beaten in a vortex (Quick Geenie) for 3–4 min. 4 µl of RNAse (Qiagen, USA) was added and incubated at 65ºC for 15 min with frequent inversion, then at 90ºC for an additional 10 min to stop the reaction. Extraction was completed as prescribed by the manufacturer. Extracted DNA was stored at −20ºC until use.
To test the integrity of the genome, 3 markers were amplified. For the nuclear genes, the universal primers 4616F (5′-AACCTGGTTGATCCTGCCAG-3′) and 4618R (5′-GATCCTTCTGCAGGTTCACCTAC-3′), DinFi (5′-GCATATAAGTAMGYGGWGG-3′) and DinRi (5′-CCGTGTTTCAAGACGGGTC-3′) were used to amplify the 18S and 28S rRNA markers respectively. PCR was conducted as reported by Logares et al. (Citation2008), but using 60°C as the annealing temperature. The mitochondrial cytochrome oxidase b (cob) gene was amplified using the barcoding primers (Dinocob4F 5′-AGCATTTATGGGTTATGTNTTACCTTT-3′ and Dinocob3R 5′-AGCTTCTANDGMATTATCTGGATG-3′) at conditions suggested by Zhang et al. (Citation2008). PCR reactions were carried out in a 25 µl reaction mixture with 0.26 U Titanium Taq DNA polymerase, 1× Taq buffer, 0.13 µM of each dNTP (Clontech), and at least 50 ng of template DNA, and were purified using QIAquick Gel Purification kit (Qiagen, USA).
Amplicons were sent to 1st Base (Malaysia) for sequencing, where both forward and reverse fragments were sequenced. Sequences were then viewed, edited and aligned in BioEdit v.7.0 (see Supplementary Materials for alignments). The most similar matches were found using BLASTn against the NCBI GenBank nuclear database. The TN93+G (Tamura-Nei 93 for 18S and 28S) and HKY+G (Hasegawa-Kishino-Yano for cob) models were used to calculate the distance matrix as determined by the substitution model test in MEGA 5.0 (Tamura et al., Citation2011) for maximum likelihood (ML), utilizing neighbour joining (NJ). Phylogenetic trees were generated separately for each gene in MEGA 5.0 with bootstrap support (resampled 100 times) using apicomplexans as the outgroup sequences.
Results
Induction of pellicle formation
A potential process of pellicle formation was determined (, Supplementary –). At the start, red autofluorescent (RAF) organelles accumulated and aggregated in the periphery of the cell, while the theca started to peel off (, [primary ecdysis]). The cytoplasm decreased in size while creating a small opening at one side where RAF-organelles, cytoplasm and bacteria-like forms (BLFs) were extruded (–). After extrusion, the inner cell covering closed and the cells further decreased in size, leaving only nuclear materials (NR) and a few RAF-materials (i.e. transitional red bodies), while BLFs were undetectable inside the cell (). The remnant cytoplasmic body continued to shrink, until it separated and/or was dislodged from the surrounding thecal wall (secondary ecdysis). ‘Ecdysis’ or the shedding of the theca may or may not lead to total liberation of the pellicle body. However, it was clear that due to cell shrinkage, the pellicle body detached from the surrounding theca. Most of the inactive forms observed, however, were not freed from the old theca (). At this stage, the cysts were smaller, more compact, irregular in shape and enclosed within a thin autofluorescent outermost layer that acted as protective covering (–) similar to the outermost covering above the theca observed in the motile cells (Supplementary –).
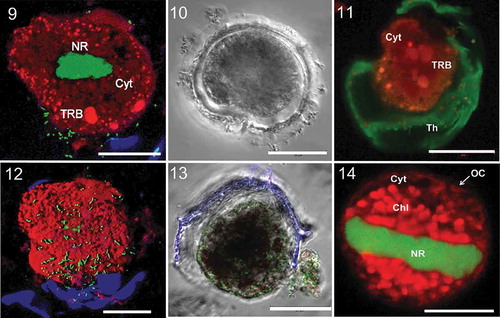
Figs 9–14. Light and pseudocoloured CLSM images of Pyrodinium temporary cysts. Midsection showing the more compact nuclear region (NR), the RAF-cytoplasm (Cyt) and three visible transitional red bodies (TRB). Extracellular bacteria (green dot-like/cocci particles) are also seen in the periphery of the cell. A DIC image showing the space that forms between the pellicular body and the old theca as a result of reduction in size. The cyst still encapsulated in its old theca (Th) with some visible structures such as the autofluorescent cytoplasmic matrix (Cyt) and TRBs. A 3D reconstructed image of the RAF-pellicle body showing the outer surface with attached bacteria (green cocci particles) and the old theca (blue). A DIC image showing the clear outline of the outermost covering that is not stainable by Calcofluor White as compared with the theca (blue). BLFs (green dots, stained with Sybr Green) are also seen attached to outside wall of the cyst. The pellicle body is also about to be dislodged off from the theca. Regeneration of the chloroplast (Chl) and the expansion of the nuclear region (NR) during reversal from temporary cyst to motile form. The other visible components in the image are the cytoplasm (Cyt) and the outermost covering (OC). Scale bars are 20 µm.
Extracellular BLF, or those attached to the outside surface of the cells, were observed in both motile cells and temporary cysts. They were stained with Sybr Green and some were mobile (). Some of these BLFs were oriented perpendicular to the surface of the temporary cysts.
Structure and composition
The temporary cysts of Pyrodinium were 25–45 μm (some reached 50 μm), and immotile. Temporary cysts did not possess defined rigid walls; instead they appeared to be covered by a thin outermost covering. The outermost covering was non-stainable with Calcofluor White, as indicated by the absence of its signal in the micrographs (–).
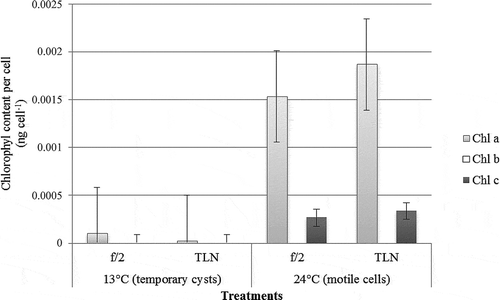
The majority of the cytoplasmic matrix exhibited strong autofluorescence, observed in red and far-red spectra. Other prominent RAF-structures were the round red bodies (; Supplementary –), similar to the red bodies described in resting cysts, with the same fluorescence exhibited by chloroplasts (). In addition, there were also distinct smaller granular RAF-emitting bodies scattered throughout the cell, possibly the retained reduced plastids. Temporary cysts had no detectable chl c and only 2.6 × 10-5 ng cell−1 and 1.03 × 10−4 ng cell−1 chl a for those obtained in f/2 and seawater respectively. On the other hand, the concentration of chl c was significantly lower than chl a in vegetative cells with only 2.67 × 10-4 ng cell−1 in f/2 and 3.33 × 10−4 ng cell−1 in the low total nutrient treatment. Vegetative cells had 1.53 × 10−3 ng cell−1 and 1.87 × 10−3 ng cell−1 chl a for cultures grown in medium with f/2 and seawater respectively. Neither form contained any chl b. Nutrient depletion had no significant effect on pigment concentration ().
Fig. 15. Chlorophyll content analyses showing the concentration of different pigments (ng cell−1), namely chl a, b and c, in temporary cysts and motile cells of Pyrodinium bahamense var. compressum grown in f/2 and total low nutrient conditions.
Genomic extraction yielded a high amount of DNA (~102 ng µl−1) with1.8 OD purity and a single bright band in agarose gel, suggesting that the material was of high molecular weight. Sequencing also showed that the amplified genes were still intact and useful for molecular identification. Phylogenetic analyses of the three genes verified the identity of the cysts as Pyrodinium bahamense var. compressum with relatively high bootstrap support (Supplementary –).
Effects of temperature and nutrient stress
The proportion of temporary cysts in low and high temperature conditions (13°C and 30°C) was generally higher (83–100%) at the end of incubation (10 days), regardless of nutrient conditions, than in routine laboratory conditions [24°C, f/2] (). After subcultures were transferred to experimental conditions, within the first 4 hours loss of motility and development of cyst-like structures (e.g. thecal ecdysis) was observed, except in the positive controls. Some cells in low-temperature cultures (13°C) and those in low total nutrient conditions (under both normal and high temperature) transformed into temporary cysts within 24 hours. The relative proportion of temporary cysts was also high under high-temperature treatments (regardless of nutrient conditions) but the absolute number of cells generally decreased over time and cell lysis was observed. Exposure to high temperature (30°C) had a significant effect on the viability of both vegetative/motile-like cells and cysts. The number of vegetative/motile-like cells decreased drastically apparently owing to a high rate of temporary cyst formation and/or lysis. It took only 6 days before all cells had become cysts. This is corroborated by the results of the regeneration experiment in which inocula from treatments exposed to 30°C did not produce any motile cells after return to normal culture conditions. There is a linear relationship between observed cell debris and incubation period, suggesting that further exposure to high temperature rendered the cysts non-viable.
Fig. 16. Matrix of figures showing the trends for motile cell, temporary cysts and total cell counts (◊ motile cells, □ temporary cysts, ∆ total).
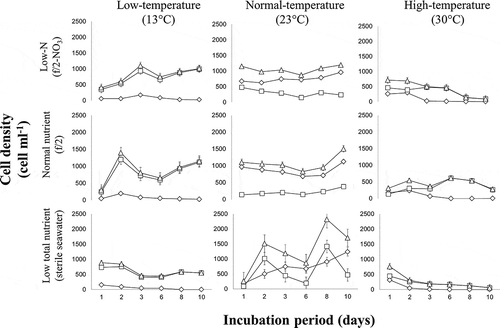
Also, the low total nutrient treatments generally produced more temporary cysts over a shorter period of time, followed by the low-N treatments both in 13ºC and 30ºC conditions (). Results also indicate that the relative effect of nutrient stress was apparently less pronounced compared with that of temperature stress. Within a temperature treatment, the proportion of cysts in cultures grown under low N or low total nutrients was, generally, only slightly higher (although significantly so, based on ANOVA results) compared with those under normal nutrient conditions ().
Reversal to motile state
Reversal to motile state was observed just minutes after re-exposure to standard laboratory culture conditions, though mature motile cells were seen after almost a day and some were only short-lived (1–4 days). Expansion of the nuclear region was first observed, occupying most of the cellular space. This was followed by the regeneration and enlargement of chloroplasts which emitted strong red autofluorescence (). The transitional red bodies also became undetectable when organelles regenerated. The redevelopment and expansion of the organelles inside the cell could have caused the expansion and breakage of the outermost covering. These breaks/tears could provide entry for the bacteria present in the outside environment to the regenerating cyst. This is supported by CLSM z-stack images showing what seem to be greater numbers of bacteria in the outermost part of the cell compared with the innermost, implying that the bacteria are coming from the outer part of the cell (Supplementary ).
Simultaneously, a new wall was created which maintained the integrity of the cell by an unknown mechanism. As the breaks/tears were continuously patched up and closed, bacterial cells originally from outside the cell were observed to become trapped inside and in the periphery of the regenerating wall (Supplementary ). Though such changes were observed immediately after re-exposure, mature motile cells were only seen after a few hours to one day but their longevity is unknown. Also, not all cells regenerated to motile forms.
Discussion
Our data indicate that temperature stress could be one of the key factors in the formation of pellicle or temporary cysts in vitro. When cells pre-grown under routine laboratory conditions were transferred to a lower temperature regime (13°C), the cells exhibited inactivity/inhibited cell division almost immediately after exposure; the cultures attained almost ~100% temporary cyst population in 5–6 days. Interestingly, increase in cyst population without any change in vegetative activity (e.g. motility, morphology and cell division) was also observed in cultures in 13ºC by day 10. Previous studies have shown that in some species, temporary cysts are capable of division forming so-called division cysts (Bravo & Figueroa, Citation2014) but these were not actually observed in this study and possible explanations are still unclear. We also observed that a temperature regime of 30°C induced pellicle formation, indicating that temperature stress (both low and high) can cause transformation but with different effects on cell viability and integrity. For example, cells at 30°C exhibited a drastic reduction in total cell counts. Pyrodinium bahamense var. compressum is capable of growth at higher temperatures (37°C) as reported by Blackburn & Oshima (Citation1989). Gedaria et al. (Citation2007) also reported that an isolate of the same species of Pyrodinium from Bamban Bay, Zambales, Philippines was able to grow at temperatures as high as 36°C (after gradual acclimatization) but with decreased cell division rate. Sea surface temperatures in some areas where blooms have been observed in the Philippines ranged from 18–31ºC, which varied with monsoons (Villanoy et al., Citation2006).
Nutrient stress (i.e. low N or low total nutrient) also induced pellicle formation although patterns were inconsistent in some conditions (i.e. low N-normal temperature). The effect of nutrient stress was also less immediate and remarkable than that of temperature. The increase in cell count in low N and low total nutrient media could be attributed to the carry-over effect of the previous culture inoculum. Nutrient stress has previously been suggested to significantly elevate temporary cyst production (Olli, Citation2004) and could influence sexuality or fusion of gametes, triggering hypnocyst/resting cyst formation in many species of dinoflagellates (Sako et al., Citation1984, Citation1985; Anderson et al., Citation1984; Olli, Citation2004; Smith & Persson, Citation2005; Shikata et al., Citation2008). Though several investigations report that temperature- and nutrient-stress can induce sexuality and resting cyst formation (e.g. Anderson et al., Citation1984, Citation1985; Binder, Citation1986; McQuiod et al., 2002; Kremp & Parrow, Citation2006), hypnocysts were not observed in this study.
The temporary cysts induced in this study possessed characteristics unreported for Pyrodinium. As shown by the microscopic analysis and imaging, it seems that the temporary cyst was actually enclosed by some form of thin covering that encapsulates the periphery of the cell and acts as cell wall. Pyrodinium motile cells have an outer membranous covering found above the theca (Onda, Citation2011) and the inner membrane beneath the theca found before the cytoplasm (Vicente, Citation2005). This layering is similar to the complex described by Morrill & Loeblich (Citation1983) as the amphiesma. During pellicle formation, the motile cell sheds its theca in a process called ecdysis, which has also been observed during pellicle formation in other species such as Alexandrium minutum, A. catenella and Lingulodinium polyedrum (Matsuoka et al., Citation1989; Bolli et al., Citation2007; Mayali et al., Citation2007; Bravo et al., Citation2010). Pellicle shrinkage, accompanied by its separation from the theca, necessitated the formation of a protective layer that would become the interface between the internal and external environments of the cell. It is possible that ecdysis only caused shedding of the outermost membrane and the theca; the inner membrane is retained to serve as the protective coating, similar to what has been described in Scrippsiella hexapraecingula (Sekida et al., Citation2001). The outermost covering of Pyrodinium temporary cysts, however, seems not to be thickened and rigid like those observed in the temporary cysts of Scrippsiella hangoei (Rintala et al., Citation2007) and S. hexapraecingula (Sekida et al., Citation2001). In addition, it appears that the shape of the Pyrodinium temporary cysts was defined by the cellular contents resulting in an irregular shape. In contrast, resting cysts of dinoflagellates have sturdy double-layered walls with protruding processes which allow them to survive in harsh environments for longer periods of time (Wall, Citation1971; Dale, Citation1977; Anderson, Citation1980; Binder, Citation1986).
The tough thecal plates of the vegetative cells are composed of structural polysaccharide fibrils such as cellulose, hemicelluloses and pectin that can be specifically stained with calcofluor white (Herth & Schnepf, Citation1980). However, our results suggest that the walls of the temporary cysts differ in composition from the theca, such that these are no longer stainable with Calcofluor White (Seo & Fritz, Citation2000; Pozdnyakov & Skarlato Citation2012). Sekida et al. (Citation2004) also reported variation and changes in the composition of the membrane sugars with life stages and physiological state. For example, in Heterocapsa niei, the resistant layer of the pellicle was suggested to consist of plant sporopollenin or dinosporin (Morrill & Loeblich, Citation1981). The cysts of Pyrocystis noctiluca produced after ecdysis were also coated with dinosporin, which has been found widely in resistant cysts of dinoflagellates and is usually related to cyst formation (Seo & Fritz, Citation2000). No distinct boundary between the outermost covering which we propose to be the ‘pellicular wall’ and the cytoplasm was observed in Pyrodinium temporary cysts. This is different to what has been seen in other species such as Alexandrium and Scrippsiella, where the pellicular wall forms a rigid and defined outline (Rintala et al., Citation2007; Bravo et al., Citation2010). The CSLM images also revealed that the fluorescence intensity of the nuclear material in the cyst remained the same when compared with motile cells; the presence of this region distinguishes cysts from dead cells. PCR amplification of a few marker genes also verified their integrity and usability for phylogenetics, with strong bootstrap support. Interestingly, the observed autofluorescence of the pellicular cytoplasm was also detected in the lipid membrane of the motile cells. Thus, it would be logical to suggest, based on autofluorescence, that temporary cysts were mostly made up of lipid globules and fatty materials; this has been found in a number of dinoflagellate cysts and generally characterizes the reduction in metabolic activity (Binder, Citation1986). On the other hand, it could also be possible that some of the organelles were reverse-synthesized into storable reserves (Rintala et al., Citation2007). Another prominent structure within the pellicle body that exhibited strong autofluorescence was the round transitional red body (TRB). In light microscopy, the red bodies appeared as reddish or orange coloured material; they are seen in many resting cysts and are thought to be food stored during inactivity (McQuiod et al., 2002; Bravo et al., Citation2012). Lirdwitayaprasit et al. (Citation1990) reported that red bodies are made up of sugars such as fructose, xylose, mannose and glucose and are easily degraded/consumed when the cell reverts back to its vegetative state. However, detailed characterization of the red body of S. trochoidea revealed that it is mainly composed of carotene and xanthophyll, proteins made up mainly of glutamic acid and glycine, but no sugars (Meksumpun & Montani, undated). In Pyrodinium, the red bodies became undetectable during the process of regeneration, as in other species, strongly suggesting that their role is transitional and confined to the cyst phase. Although the shape, size and fluorescence of the TRBs are similar to chloroplasts, other criteria such as changes during cyst formation, intensity of fluorescence in UV excitation and localization allowed us to differentiate the two structures.
Remarkably, temporary cysts contained reduced amounts of chl a and no detectable chl c. These two pigments are the dominant pigments in the thylakoids of most dinoflagellates (Ogata et al., Citation1994) and some macroalgae (Strain et al., Citation1943), and are closely associated with one another via a pigment protein complex. Chl c, naturally occurring in small amounts, is important in the production of organic matter especially by marine plants due to the large amount of light that it absorbs (Strain et al., Citation1943). Dinoflagellates contain a single type of chl c which serves as the accessory pigment to the chl a-protein complex, common in dinoflagellates as the peridinin (Boczar & Prézelin, Citation1987) but has not yet been investigated in Pyrodinium. In Gonyaulax and Glenodinium, chls a and c occur in a molar ratio of 4 : 1 (Prézelin & Alberte, Citation1978) while in Pyrodinium, it occurs in a ratio of ~5 : 1 (ng cell−1) for both vegetative cells grown in f/2 medium and low total nutrient. The absence of chl b in either stage of the dinoflagellate corroborates the findings of Strain et al. (Citation1943) who claimed that it was not observed in majority of marine plants with chl c.
The characteristics mentioned earlier (except for the chl profiles) were also observed in the temporary cysts formed from the field samples collected in Matarinao Bay, Samar, Eastern Philippines. The same temporary cysts were also seen in culture isolates of Pyrodinium from Honda Bay, Palawan, Southwestern Philippines (Arielle Sulit, pers. comm.) and in field samples from other bays fixed with Lugol’s solution (Garry Benico, pers. comm.), suggesting that it is a universal response of the species and possibly occurs naturally in situ.
Our data also showed the viability of the low-temperature induced temporary cysts and their capability for regeneration (i.e. reversal to motile state). In this study, temporary cysts from all treatments in the low temperature regime were able to produce live motile cells. Low nutrient concentration during pellicle formation seemed to have no effect on pellicle viability, but is characterized by the enlargement of the pellicle body as a consequence of regeneration of the chloroplasts. It was also observed that temporary ‘breaks’ or ‘tears’ formed in the surrounding wall, perhaps as a result of such enlargement; at this stage the cyst remains immotile. It is possible that free-living bacteria in the medium or in the field could enter the intracellular environment through these ‘breaks’ and become trapped as the cell developed a new amphiesmal layer and thecal wall. This mechanism has not yet been reported in any species of dinoflagellates or microalgae, the majority of which have been shown to harbour bacterial endosymbionts including Pyrodinium (Azanza et al., Citation2006; Onda, Citation2011; Santos & Azanza, Citation2012). More studies, perhaps using labelling and time-lapse microscopy, should be done to elucidate this hypothesis and to determine any specificity in the establishment of the endosymbionts in the host cell.
Pellicle formation has previously been regarded to be just a laboratory or culturing and sampling artefact, but a number of studies have demonstrated their occurrence and role in field blooms (e.g. Garcés et al., Citation2002, Citation2004; Olli, Citation2004; Bravo et al., Citation2010). Several internal morphological changes were also observed that differed from what has been previously reported (Matsuoka et al., Citation1989). Although this study only focused on samples in vitro, the processes we observed may also occur in nature.
Supplementary information
The following supplemental material is available for this article, accessible via the Supplementary Content tab on the article’s online page at http://dx.doi.org/10.1080/09670262.2014.905710
Supplementary figs 1–8. Photomicrographs showing the apparent morphological changes in the process of pellicle formation. Cells were first observed to shed their membrane and theca accompanied by aggregation of intracellular materials (), followed by extrusion of cytoplasmic materials in the apical region of the cell in between the thecal plates (), then closing of the covering wall (), formation of red bodies (), secondary ecdysis (shedding and dislodging of the remaining thecal wall) ().
Supplementary figs 9–10. CLSM images of the () cross section of a whole motile cell and the () enlarged periphery of the cell showing the distinct layers of outer membrane (OM), theca (Th) and the cytoplasm (Cyt). Scale bar is 20 µm.
Supplementary figs 11–13. Phylogenetic trees constructed from the partial () 18s rDNA, () 28s rDNA and () cytochrome oxidase b, extracted from temporary cysts. The bootstrap supports are indicated by the numbers near the node and the GenBank accession number of reference sequences are found after the scientific names. Scale refers to the substitution rate.
Supplementary figs 14–17. CLSM images of the regenerating cyst taken at different ocular depths and showing the entry and attachment of the bacteria (green dots/cocci particles) to the intracellular region of the cell.
Supplementary file 1. 18s rRNA gene of Pyrodinium temporary cyst.
Supplementary file 2. 28s rRNA gene of Pyrodinium temporary cyst.
Supplementary file 3. cob gene of Pyrodinium temporary cyst.
Supplementary material
Download Zip (47.2 MB)Acknowledgements
This study is part of the research programme ‘Ecology and Oceanography of Harmful Algal Blooms in the Philippines (PhilHABs), Project 1: Biodiversity/Genetic Diversity of selected HAB-forming species in the Philippines and their associated bacterial communities’, funded and supported by the Department of Science and Technology (DOST) through the Philippine Council for Aquatic and Marine Research and Development (PCAMRD now PCAARRD) and a national project of the UNESCO IOC-SCOR programme entitled ‘Global Ecology and Oceanography of Harmful Algal Blooms’ (GEOHAB). We would also like to thank the Marine Toxinology Laboratory of the MSI headed by Dr Lourdes J. Cruz for the use of the sonicator and the spectrophotometer, Emelita Eugenio for her help in maintaining the algal cultures, and Garry Benico, Johanna Munar and Powell Marquez for some technical assistance. Also, we are grateful to the comments of some anonymous reviewers and to Dr Mary Anne Gonzales-Santos for her insights into Pyrodinium biology and ecology.
References
- Aminot, A. & Rey, F. (2001). Chlorophyll a: determination by spectroscopic methods. ICES Techniques in Marine Environmental Science, 30: 1–18.
- Anderson, D.M. (1980). Effects of temperature conditioning on development and germination of Gonyaulax tamarensis (Dinophyceae) hypnozygotes. Journal of Phycology, 16: 166–172.
- Anderson, D.M., Kulis, D.M. & Binder, B.J. (1984). Sexuality and cyst formation in the dinoflagellate Gonyaulax tamarensis: cyst yield in batch cultures. Journal of Phycology, 20: 418–425.
- Anderson, D.M., Coats, D.W. & Taylor, M.A. (1985). Encystment of dinoflagellate Gyrodinium uncatenum: temperature and nutrient effects. Journal of Phycology, 21: 200–206.
- Azanza, M.P., Azanza, R.V., Vargas, V.M.D. & Hedreyda, C.T. (2006). Bacterial endosymbionts of Pyrodinium bahamense var. compressum. Microbial Ecology, 52: 756–764.
- Azanza, R.V. (1997). Contributions to the understanding of the bloom dynamics of Pyrodinium bahamense var. compressum: a toxic red tide causative organism. Science Diliman, 9: 1–6.
- Azanza, R.V. &Larsen, J. (1997). Variation in nutrient concentration: effects on Pyrodinium cells in culture. (Abstract). 8th International Conference on Harmful Algae. Vigo, Spain.
- Azanza-Corrales, R.V. & Hall, S. (1993). Isolation and culture of Pyrodinium bahamense var. compressum from the Philippines. In Toxic Phytoplankton Blooms in the Sea (Smayda, T.J. & Shimizu, Y., editors), 725–730. Elsevier Science Publishing Inc., Amsterdam.
- Binder, J. F. (1986). The physiology or dormancy and germination in cysts of the marine dinoflagellate Scrippsiella trochoidea. PhD Dissertation, Massachusetts Institute of Technology/Woodshole Oceanographic Institution.
- Blackburn, S.I. & Oshima, Y. (1989). Review of culture methods for Pyrodinium bahamense. In Biology, Epidemiology and Management of Pyrodinium Red Tides (Hallegraeff, G.M. & Maclean, J.L., editors), 257–266. ICLARM Conference Proceedings 21, Fisheries Department, Ministry of Development, Brunei Darussalam, and International Centre for Living Aquatic Resources Management, Manila.
- Boczar, B.A. & Prezélin, B.B. (1987). Chlorophyll-protein complexes from the red-tide dinoflagellate, Gonyaulax polyedra, Stein. Plant Physiology, 83: 805-812.
- Bolli, L., Llaveria, G., Garcés, E., Guaodayol, O., van Lenning, K., Peters, F. & Berdalet, E. (2007). Modulation of ecdysal cyst and toxin dynamics of two Alexandrium (Dinophyceae) species under small-scale turbulence. Biogeosciences Discussion, 4: 893–908.
- Bravo, I. & Figueroa, R.I. (2014). Towards an ecological understanding of dinoflagellate cysts. Microoorganisms, 2: 11–32.
- Bravo, I., Figueroa, R.I., Garcés, E., Fraga, S. & Massanet, A. (2010). The intricacies of dinoflagellate pellicle cysts: the example of Alexandrium minutum cysts from a bloom-recurrent area (Bay of Baiona, NW Spain). Deep-Sea Research Part II: Topical Studies in Oceanography, 57: 166–174.
- Bravo, I., Vila, M., Casabianca, S., Rodriguez, F., Rial, P., Riobo, P. & Penna, A. (2012). Life cycle stages of the benthic palytoxin-producing dinoflagellate Ostreopsis cf. ovata (Dinophyceae). Harmful Algae, 18: 24–34.
- Dale, B. (1977). Cysts of the toxic red-tide dinoflagellate Gonyaulax excavata (Braarud) Balech from Oslofjörden, Norway. Sarsia, 63: 29–34.
- Figueroa, R.I., Bravo, I. & Garcés, E. (2008). The significance of sexual versus asexual cyst formation in the life cycle of the noxious dinoflagellate Alexandrium peruvianum. Harmful Algae, 7: 653–663.
- Garcés, E., Masoand, M. & Camp, J. (2002). Role of temporary cysts in the population dynamics of Alexandrium taylori (Dinophyceae). Journal of Plankton Research, 24: 681–686.
- Garcés, E., Bravo, I., Vila, M., Figueroa, R.I., Maso, M. & Sampedro, N. (2004). Relationship between vegetative cells and cyst production during Alexandrium minutum bloom in Arenysde Mar harbor (NW Mediterranean). Journal of Plankton Research, 26: 637–645.
- Gedaria, A.I., Luckas, B., Reinhardt, K. & Azanza, R.V. (2007). Growth response and toxin concentration if cultured Pyrodinium bahamense var. compressum to varying salinity and temperature conditions. Toxicon, 50: 518–529.
- Guillard, R.R.L. & Ryther, J.H. (1962). Studies of marine planktonic diatoms I. Cyclotella nana Hustedt and Detonula confervaceae (Cleve) Gran. Canadian Journal of Microbiology, 8: 229–239.
- Hammer, Ø., Harper, D.A.T. & Ryan, P.D. (2001). PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica, 4: 1–9.
- Herth, W. & Schnepf, E. (1980). The fluorochrome, calcofluor white, binds oriented to structural polysaccharide fibrils. Protoplasma, 105: 129–133.
- Jeffrey, S.W. & Humphrey, G.F. (1975). New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochemie und Physiologie der Pflanzen, 167: 191–194.
- Jensen, M.O. & Moestrup, Ø. (1997). Autecology of the toxic dinoflagellate Alexandrium ostenfeldii: life history and growth at different temperatures and salinities. European Journal of Phycology, 32: 9–18.
- Kai, A.K.L., Cheung, Y.K., Yeung, P.K.K. & Wong, J.T.Y. (2006). Development of single-cell PCR methods for the Raphidophyceae. Harmful Algae, 5: 649–657.
- Khan, S., Arakawa, O. & Onoue, Y. (1995). Effects of physiological factors on morphology and motility of Chattonella antiqua (Raphidophyceae). Botanica Marina, 38: 347–353.
- Kremp, A. & Parrow, M.W. (2006). Evidence for asexual resting cysts in the life cycle of the marine peridinoid dinoflagellate, Scrippsiella hangoei. Journal of Phycology, 42: 400–409.
- Lirdwitayaprasit, T., Okaichi, T., Montani, T. & Anderson, D.M. (1990). Changes in cell chemical composition during the life cycle of Scrippsiella trochoidea (Dinophyceae). Journal of Phycology, 26: 99–106.
- Logares, R., Shalchian-Tabrizi, K., Boltovskoy, A. & Rengefors, K. (2008). Extensive dinoflagellate phylogenies indicate infrequent marine–freshwater transitions. Molecular Phylogenetics and Evolution, 45: 887–903.
- Matsuoka, K., Fukuyo, Y. & Anderson, D.M. (1989). Methods for modern dinoflagellate cyst studies. In Red Tides: Biology, Environmental Science and Toxicology (Okaichi, T., Anderson, D.M. & Nemoto, T., editors), 461–479. Elsevier, Amsterdam.
- Mayali, X., Franks, P.J.S. & Azam, F. (2007). Bacterial induction of temporary cyst formation by the dinoflagellate Lingulodinium polyedrum. Aquatic Microbial Ecology, 50: 51–62.
- McQuoid, M.R., Godhe, A. & Nordberg, K. (2002). Viability of phytoplankton resting stages in the sediments of a coastal Swedish fjord. European Journal of Phycology, 37: 191–201.
- Meksumpun, S. & Montani, S. (undated). Chemical components of red-brown materials on cysts of Scrippsiella trochoidea (Dinophyceae). Retrieved from <http://pikul.lib.ku.ac.th/Fulltext_FISHB/TAB000125520885c.pdf>
- Morrill, L.C. & Loeblich, A.R. III. (1981). The dinoflagellate pellicular wall layer and its occurrence in the division Pyrrhophyta. Journal of Phycology, 17: 315–323.
- Morrill, L.C. & Loeblich, A.R. III. (1983). Ultrastructure of the dinoflagellate amphiesma. International Review of Cytology, 82:151–180.
- Nagasaki, K., Yamaguchi, M. & Imai, I. (2000). Algicidal activity of a killer bacterium against the harmful red tide dinoflagellate Heterocapsa circularisquama isolated from Ago Bay, Japan. Nippon-Suisan-Gokkaish, 66: 666–673.
- Ogata, T., Kodama, M., Nomura, S., Kobayashi, M., Nozawa, T., Katoh, T. & Mimuro, M. (1994). A novel peridinin-chlorophyll a protein (PCP) from the marine dinoflagellate Alexandrium cohorticula: a high pigment content and plural spectral form of peridinin and chlorophyll a. FEBS Letters, 356: 367–371.
- Olli, K. (2004). Temporary cyst formation of Heterocapsa triquetra (Dinophyceae) in natural populations. Marine Biology, 145: 1–8.
- Onda, D.F.L. (2011). Bacterial genetic diversity and association in Pyrodinium bahamense var. compressum (Böhm) Steidinger, Tester and Taylor. MSc Thesis. University of the Philippines Diliman, Quezon City.
- Onda, D.F.L., Benico, G., Sulit, A.L., Gaite, P.L., Azanza, R.V. & Lluisma, A.O. (2013). Morphological and molecular characterization of some HAB-forming dinoflagellates from Philippine waters. Philippine Science Letters, 6: 1–10.
- Persson, A. & Rosenberg, R. (2003). Impact of grazing and bioturbation of marine benthic deposit feeders on dinoflagellate cysts. Harmful Algae, 2: 43–50.
- Pozdnyakov, I. & Skarlato, S. (2012). Dinoflagellate amphiesma at different stages of the life cycle. Protistology, 2:108–115.
- Prézelin, B.B. & Alberte, R.S. (1978). Photosynthetic characteristics and organization of chlorophyll in marine dinoflagellates. Proceedings of the National Academy of Sciences USA, 75: 1801–1804.
- Rintala, J.M., Spilling, K. & Blomster, J. (2007). Temporary cyst enables long-term dark survival of Scrippsiella hangoei (Dinophyceae). Marine Biology, 152: 57–62.
- Sako, Y., Ishida, Y., Kadota, H. & Hata, Y. (1984). Sexual reproduction and cyst formation in the freshwater dinoflagellate Peridinium cunningtonii. Bulletin of the Japanese Society of Scientific Fisheries, 50: 743–750.
- Sako, Y., Ishida, Y., Kadota, H. & Hata, Y. (1985). Excystment in the freshwater dinoflagellate Peridinium cunningtonii. Bulletin of the Japanese Society of Scientific Fisheries, 51: 267–272.
- Santos, M.A.G. & Azanza, R.V.A. (2012). Responses of Pyrodinium bahamense var. compressum and associated cultivable bacteria to antibiotics. Journal of Applied Phycology, 24: 825–835.
- Sekida, S., Horiguchi, T. & Okuda, K. (2001). Development of the cell covering in the dinoflagellate Scrippsiella hexapraecingula (Peridiniales, Dinophyceae). Phycological Research, 49:163–176.
- Sekida, S., Horiguchi, T. & Okuda, K. (2004). Development of the thecal plates and pellicle in the dinoflagellate Scrippsiella hexapraecingula (Peridiniales, Dinophyceae) elucidated by changes in stainability of the associated membranes. European Journal of Phycology, 39: 105–114.
- Seo, K.S. & Fritz, L. (2000). Cell-wall morphology correlated with vertical migration in the non-motile marine dinoflagellate Pyrocystis noctiluca. Marine Biology, 137: 589–594.
- Shikata, T., Nagasoe, S., Matsubara, T., Yamasaki, Y., Shimasaki, Y., Oshima, Y., Uchida, T., Jenkinson, I.A. & Honjo, T. (2008). Encystment and excystment of Gyrodinium instriatum Freudenthal et Lee. Journal of Oceanography, 64: 355–365.
- Smith, B.C. & Persson, A. (2005). Synchronization of encystment of Scrippsiella lachrymose (Dinophyta). Journal of Applied Phycology, 17: 317–321.
- Strain, H.H., Manning, W.M. & Hardin, G. (1943). Chlorophyll c (chlorofucine) of diatoms and dinoflagellates. Journal of Biological Chemistry, 148: 655–661.
- Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M. & Kumar, S. (2011). MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28: 2731–2739.
- Tarutani, K., Nagasaki, K., Itakura, S. & Yamaguchi, M. (2001). Isolation of a virus infecting the novel shellfish-killing dinoflagellate Heterocapsa circularisquama. Aquatic Microbial Ecology, 23: 103–111.
- Vicente, H.J. (2005). In situ and in vitro developmental stages and ultrastructure of Pyrodinium bahamense plate var. compressum (Böhm) in Taguines Lagoon, Binoni, Mahinog, Camiguin Island, Philippines. PhD Dissertation. University of the Philippines Diliman.
- Villanoy, C., Azanza, R.V., Altemerano, A. & Casil, A.L. (2006). Attempts to model the blooms dynamics of Pyrodinium, a tropical toxic dinoflagellate. Harmful Algae, 5: 156–183.
- Wall, D. (1971). Biological problems concerning fossilizable dinoflagellates. Geoscience and Man, 3: 1–15.
- Zhang, H., Bhattacharya, D., Miranda, L. & Lin, S. (2008). Mitochondrial cob and cox1 genes and editing of the corresponding mRNAs in Dinophysis acuminata from Narragansett Bay, with special reference to the phylogenetic position of the genus Dinophysis. Applied and Environmental Microbiology, 74: 1546–1557.
