Abstract
The large diatom genus Nitzschia and the morphologically similar Hantzschia are currently distinguished on the basis of cell symmetry and division. In Hantzschia, the eccentrically placed raphe systems of the two valves are always on the same side of the frustule (‘hantzschioid’ symmetry), never diagonally opposite (‘nitzschioid’ symmetry). Although some Nitzschia species produce hantzschioid cells, it has previously been thought that these cells never breed true but divide to give one nitzschioid daughter and one hantzschioid daughter. Sublittoral marine epipelon from Loch Goil, W Scotland, contained three undescribed species of Bacillariaceae whose cells were always hantzschioid, but which possessed a valve structure (silica flaps on either side of the raphe, no central raphe endings, a slit-like entrance to the raphe canal that narrows towards the poles, and discrete bar-like fibulae) linking them to N. spathulata and the type species of Nitzschia, N. sigmoidea. They also agreed with these species in chloroplast morphology and auxosporulation and are therefore assigned to Nitzschia as N. dicrogramma, N. brachygramma and N. parkii, spp. nov. Consequently the only type of cell division found in all Hantzschia species (in which a hantzschioid parent cell divides to give two hantzschioid daughters) is not unique to Hantzschia but also occurs in at least one lineage of Nitzschia. ‘Core’ Hantzschia species may nevertheless comprise a monophyletic group, characterized by an unusual kind of internal central raphe endings. Four symmetry categories are now known in Hantzschia and Nitzschia: (1) all cells hantzschioid; (2) hantzschioid and nitzschioid cells in a 2 : 1 ratio; (3) hantzschioid and nitzschioid cells in a 1 : 1 ratio; and (4) all cells nitzschioid. The division pattern seems to be constant within species and may therefore be useful as a taxonomic character above the species level. Hantzschia segmentalis is given its first written description.
Introduction
Two long-established genera of raphid diatoms, Nitzschia and Hantzschia, were originally separated on the basis of cell symmetry (e.g. Hustedt, Citation1930; Ricard, Citation1987). In both genera, as in most other genera within the Bacillariaceae (e.g. Cymbellonitzschia, Denticula, Fragilariopsis, Psammodictyon, Pseudo-nitzschia and Tryblionella: Round et al., Citation1990), the raphe system is generally positioned eccentrically on the valve and thus frustules can potentially exist in two configurations. In the first, the raphe systems of the two valves are on different sides of the cell, so that the raphes are diagonally opposite each other in cross sections; this configuration is referred to as ‘nitzschioid’ symmetry (). In the second, the raphes are on the same side of the cell, referred to as ‘hantzschioid’ symmetry (); hantzschioid cells therefore exhibit mirror symmetry about the median valvar plane. Hantzschia cells are always hantzschioid and are also usually asymmetrical, the side bearing the raphes (the ‘ventral’ side) being concave or straight, whereas the opposite (‘dorsal’) side is convex. In contrast, the vast majority of nitzschioid cells have a symmetrical outline and exhibit 180° rotational symmetry about the median valvar plane. Because of the diagonal positioning of the raphes, any nitzschioid frustule with an asymmetrical outline will have dissimilar valves. For example, Cymbellonitzschia minima, which has cells shaped like orange segments (http://rbg-web2.rbge.org.uk/algae/research/types/Cymbellonitzschia_minima_type.html), produces both hantzschioid and nitzschioid cells (Cocquyt & Jewson, Citation1994; Trobajo et al., Citation2012) and in the nitzschioid cells one valve has a straight raphe running along the ventral margin, while the other has a curved raphe running along the convex dorsal margin. This kind of dimorphism seems to be extremely rare and indeed, the only example known to us is C. minima.
Fig. 1. Diagrammatic cross sections through nitzschioid (N) and hantzschioid (H) cells, showing the positions of the raphe systems (r), which are subtended by bridges of silica (the fibulae); and the five types of division (I–V) known in Bacillariaceae. In all the diagrams, the epitheca is to the left; thus, although types III and IV have the same outcome (H → N + H), they are not exactly equivalent.
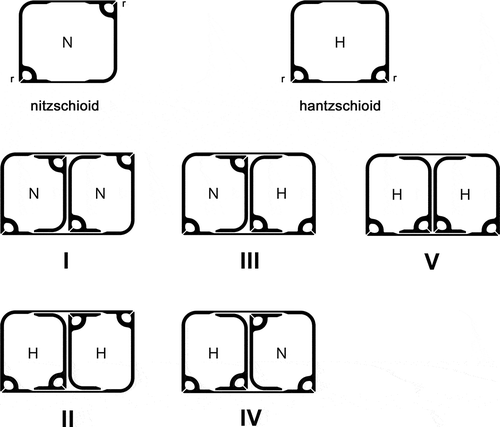
Early 20th century taxonomic accounts of the Bacillariaceae (e.g. Hustedt, Citation1930) generally equated the presence of nitzschioid symmetry with Nitzschia and hantzschioid symmetry with Hantzschia, but two papers published almost 50 years ago (Lauritis et al., Citation1967; Geitler, Citation1968a) showed that some Nitzschia species, including the heterotrophic N. alba and even the type species of Nitzschia, N. sigmoidea, produce both nitzschioid and hantzschioid cells. This led to the suggestion by Lauritis et al. (Citation1967), who studied N. alba, that Hantzschia ‘as it is now defined’ cannot be distinguished from Nitzschia. However, following comparative studies of cell division in Hantzschia and Nitzschia, Mann (Citation1980a) and Pickett-Heaps (Citation1983) proposed a new method of separating the genera, not on the basis of whether the frustules are hantzschioid or nitzschioid, but according to the relative positions of the raphe systems in the sibling valves formed during cell division (see also Round, Citation1970); they suggested that, in Nitzschia, the raphe systems of sibling valves are always formed on opposite sides of the parent cell (, division types I–IV), whereas in Hantzschia they are formed on the same side (, division type V). Consequently, hantzschioid cells of Nitzschia species will never breed true but will always divide to give one nitzschioid and one hantzschioid cell (, division types III and IV), and the proportions of nitzschioid and hantzschioid cells in a population will depend on how the pair of new valves are orientated in relation to the parent cell (Mann, Citation1980a). In contrast, the hantzschioid cells of Hantzschia always breed true (, division type V), producing two hantzschioid daughters (Mann, Citation1980a; Pickett-Heaps, Citation1983; see also summaries by Kociolek & Williams, Citation1987, p. 277; Round et al., Citation1990, pp. 23, 610).
In some marine sublittoral samples from Loch Goil, western Scotland (Park, Citation1992), we found some dorsiventral diatoms that clearly belonged to the Bacillariaceae, since they possessed an eccentric raphe system with rib-like fibulae (Round et al., Citation1990). The raphe systems were always on the straight ventral sides of the cells (–) and the frustules were consistently hantzschioid (H), so that the only pattern of cell division present must have been H → H + H (, ). According to the Mann (Citation1980a) and Pickett-Heaps (Citation1983) hypothesis, therefore, the unidentified diatoms should belong to Hantzschia. In the light microscope, however, the valves appeared to be very similar in structure to Nitzschia sensu stricto (i.e. the group of species allied to the type species, N. sigmoidea), which possesses a suite of characters – notably the presence of siliceous flaps (conopea) on either side of the raphe system – that are not found in other Nitzschia species that have been studied, nor in Hantzschia (Mann, Citation1986). Within Nitzschia sensu stricto, the species most similar to the dorsiventral species, in overall appearance, dimensions and fibula density, was N. distans and Park (Citation1992) accordingly identified the hantzschioid species as ‘Nitzschia aff. distans’ in his ecological studies of Loch Goil. However, N. distans has a symmetrical outline and the raphe is close to the midline of the valve (Gregory, Citation1857), not eccentric as in the Loch Goil forms. An irregular texturing of the dorsal part of the valve in some of the Goil forms reminded us of H. segmentalis (Brun, Citation1895), but apart from this no similar diatoms were found illustrated in floras (e.g. Peragallo & Peragallo, Citation1897–1908; Desikachary, Citation1986–1989; Hartley et al., Citation1996; Witkowski et al., Citation2000) or detailed studies of benthic marine material (e.g. Brockmann, Citation1950; Montgomery, Citation1978; Kuylenstierna, Citation1990; Poulin et al., Citation1990), apart from three illustrations of a diatom from North Carolina, USA, identified as N. insignis var. adriatica (Hustedt, Citation1955, pl. 15, –), and a photograph of ‘N. distans’ by Foged (Citation1978, pl. 45, ).
The aims of the present study were therefore to (1) examine the hantzschioid Loch Goil diatoms by light and scanning electron microscopy (LM and SEM) to establish their likely relationship to other Bacillariaceae, especially Nitzschia sect. Nitzschia and Hantzschia species; (2) determine whether any of them have been described before and specifically whether any are synonymous with Hantzschia segmentalis, Nitzschia insignis var. adriatica or N. distans; (3) document chloroplast morphology and sexual reproduction for comparison with other Bacillariaceae; and (4) review the use of symmetry in differentiating Hantzschia from Nitzschia. Unfortunately, all of the material from Loch Goil was cleaned with acids long ago and re-collecting these diatoms (obtained from c. 6–10 m depth) has not been possible. Hence molecular methods could not be applied.
Materials and methods
Study site and sampling
All samples of the new Nitzschia species came from Loch Goil, a sea-loch in Argyll, W Scotland. The site sampled was located c. 5 km from Lochgoilhead, along the western shore of the loch, at 56° 7.7′ N, 4° 54.2′ W. The site was distant from urban centres, though subject to the discharges from the greater Glasgow area. Although located only 75 m from a stream (the Cormonachan Burn), there was only ever a shallow superficial layer (of a few cm) of slightly brackish water (at a salinity of 29); beneath this was a fully marine water column with salinity ≥ 33. In 1988–90, the levels of dissolved oxygen recorded at the sediment–water interface at 9 m depth varied from 5.5 ppm in September to 11 ppm in January. Other site data are given by Park (Citation1992) and an introduction to the Clyde Sea is given by Connor & Little (Citation1998). Extensive monitoring of Loch Goil was carried out before and during the sampling period by the Clyde River Purification Board (e.g. Citation1985, Citation1986).
In depth transects by Park (Citation1992), the dorsiventral species (recorded as an undifferentiated ‘Nitzschia aff. distans’) were found at 6, 12 and 15 m in June 1989, and at 6 and 12 m in July 1990, but not at 20, 30 and 40 m on these dates. Park therefore classified ‘Nitzschia aff. distans’ as part of the ‘shallow-water’ community. The salinities at the depths where ‘Nitzschia aff. distans’ was recorded were c. 36 (all three depths in 1989), 35 (at 6 m in 1990), and 35.5 (at 12 m in 1990). In a two-year study (June 1988 to June 1990) of the seasonal cycle of epipelon at 9 m, ‘Nitzschia aff. distans’ was present on 35 occasions out of 40, but never reached more than 0.4% of the epipelic assemblage. During this time the Secchi depth of the water column only reached 9 m four times and was generally c. 5 m or less (Park, Citation1992, ).
The sediments at the study site comprised muddy sand, which formed a gently sloping plain 9–12 m below mean low water, spring tides. Sediment samples were collected by hand by scuba divers and were used for the ecological and biodiversity studies of Park (Citation1992). Extra material was provided to us by Dr Park, allowing the cytological and auxospore studies described here. Epipelon was obtained the day after sampling, using lens tissue, cover slips or thin polythene to harvest the algae that had moved up towards the light from the sediment (from which excess water had been removed by suction after the samples had stood for several hours). Some cover-slips with trapped epipelon were then incubated in enriched seawater medium, prepared either by adding N, P and Si to seawater at the concentrations specified for WC medium (Guillard & Lorenzen, Citation1972), or by adding the following to 10 litres of filtered (Whatman No. 3) Loch Goil seawater: 50 ml of a N–P stock solution (containing 22.5 g NaNO3, 0.9 g Na2HPO4 and 0.75 g K2HPO4 per litre); 5 ml of a Se stock (5 mg Na2SeO3 per litre); and 5 ml and 10 ml, respectively, of the trace element and silicate stocks specified for WC medium. The rough cultures were incubated for up to 3 weeks in the laboratory or in an incubator, at temperatures of c. 15–18°C, with low light (not measured, but c. 5 µmol photons m–2 s–1) from cool-white fluorescent tubes and long days (14–16 h light). For incubations of more than a week, the medium was changed every 3–5 days.
The principal samples used to generate the data on living cells and sexual reproduction reported here were collected in February 1988, April 1989, January 1990 and March 1990 (for the hantzschioid Nitzschia spp.), and January 1984, November 1989 and April 1990 (for N. spathulata). All samples of the dorsiventral species and N. spathulata were from the Loch Goil sites described above, except some material of N. spathulata obtained from Portobello Beach, Edinburgh (55° 57.16′ N, 3° 06.21′ W) in 1984.
Specimen preparation and microscopy
For studies of frustules, harvested epipelon was cleaned by boiling in 70% nitric acid. Living material was studied by mounting large coverslips (24 × 50 mm), incubated as described above, on slides with drops of seawater medium and ringing them with vaseline to prevent evaporation. Cell contents were preserved by fixing and staining cells that had colonized cover-slips; the fixative was Flemming’s weak solution (Johansen, Citation1940), applied for 15 min, followed by staining with Harris’s haematoxylin and mounting in Canada Balsam. The permanent preparations used are listed in .
Table 1. Permanent voucher slides used for studies of hantzschioid Nitzschia species (including types A and B) and Hantzschia segmentalis. All preparations are held in the diatom herbarium of the Royal Botanic Garden Edinburgh (E), except the Brun preparation G00295660, which is in the Conservatoire et Jardin Botaniques, Geneva (G).
For LM, preparations were mounted in Naphrax (Brunel Microscopes: http://www.brunelmicroscopes.co.uk/) and examined using either a Reichert Polyvar photomicroscope, with Plan-Apochromat 100× objective (N.A. 1.32), or a Zeiss Axio Imager M2 (Zeiss, Oberkochen, Germany) with a Plan-Apochromat 100× objective (N.A. 1.4); most observations were made by differential interference contrast optics. Where it was important to obtain maximum resolution to confirm (via stria density measurements) that valves found in SEM were conspecific with particular populations distinguished in LM, the condenser was oiled to the slide, allowing measurement of stria densities of up to 48 in 10 µm. In the case of the Axio Imager, photographs were taken using an Axiocam HRc digital camera and Axiovision software (Zeiss). Film photographs from the Polyvar (taken using the built-in camera) were digitized using a Nikon 5000 Coolscan. Digital images were processed with Adobe Photoshop (CS5 Extended, version 12.0.4), by general application of the Levels, Curves and Unsharp Mask tools without localized enhancements, and, for images captured from the Axio-Imager, the use of different digital filters to transform the original colour images to greyscale, depending on the colour stained by the haematoxylin. Drawings were made using the drawing attachment of the Polyvar.
The Axio Imager stage was motorized, allowing capture of stacks of images at defined vertical intervals of 0.1–0.2 µm, which were stored as .zvi files (Axiovision, Zeiss). These were then used to produce the videos available in the Supplementary information by processing in Axiovision to resample to smaller image dimensions, crop, and enhance contrast and brightness (global application). The stacks were then exported as .avi files with 10–15% compression.
For SEM, cleaned frustules were dried onto 13-mm diameter glass coverslips, which were attached to aluminium stubs by carbon discs, coated with platinum, and studied in a LEO Supra 55VP at 5 or 6 kV.
Terminology follows Ross et al. (Citation1979) and Kaczmarska et al. (Citation2013).
Results
Living cells and LM observations
Within the Loch Goil samples, hantzschioid Bacillariaceae were uncommon and usually comprised much less than 0.1% of the epipelic diatom population. Nevertheless, they were quite easily spotted when alive because, unlike most other diatoms in the Loch Goil epipelon apart from Amphora sensu lato, cells almost always lay and moved in girdle view (, –; Supplementary videos 1–4), with their raphes appressed to the substratum. However, even though they rarely presented themselves in valve view, their dorsiventral shape was detectable by careful focusing, because the ventral side was clearly planar whereas the dorsal side was domed (compare with , with , and with ; see also Supplementary videos 1–4).
Figs 2–17. Three hantzschioid species from Loch Goil, LM, differential interference contrast optics: fixed and haematoxylin-stained cells (, , –) and acid-cleaned thecae and valves (–). –. Type A1 (Nitzschia dicrogramma): cell, frustule and theca in girdle view (–, respectively); note the line parallel to the raphe (arrow), which curves in towards the raphe at the pole (arrowhead; cf. ), reflecting the presence of a conopeum. , . Type A1 valve, two focuses (holotype of N. dicrogramma); note the line parallel to the raphe (, arrow). . Type A1 valve. . Type A2 valve (holotype of N. brachygramma); note the line parallel to the raphe (arrow). . Type B valve (holotype of N. parkii); note the line parallel to the raphe (arrow). , . Type A1 cell (N. dicrogramma) in ventral and dorsal focus: note the two ventral chloroplasts (and large dorsal nucleus with two nucleoli). –. Type A2 cell (N. brachygramma) in ventral, mid and dorsal focus: the four chloroplasts are appressed to the valves, leaving a strip clear along the midline of the girdle on either side. –. Type B cell (N. parkii) in ventral, mid and dorsal focus: note the lobes of the chloroplast reaching the ventral side (), the small central nucleus, and the central part of each chloroplast lying against the dorsal side (). Scale bars = 10 µm (for –, see ; for –, see ).
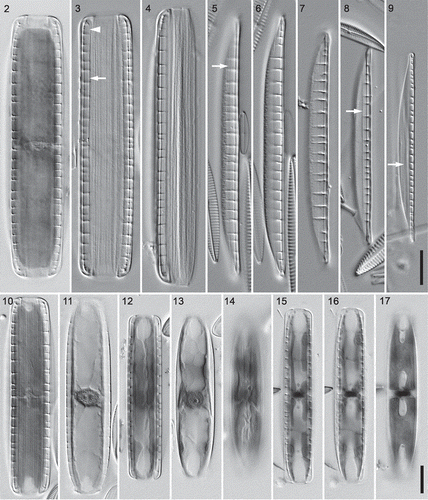
Different cell sizes and pattern metrics were observed among the hantzschioid forms. A combination of generally greater girdle width and lower fibula density suggested that some specimens (‘type A’: e.g. –, –) might not belong to the same species as the remainder (‘type B’: , –). Moreover, types A and B differed in chloroplast structure and arrangement. The smaller-celled type B had two plates appressed to the dorsal side, which were constricted longitudinally making an ‘H’ shape (). Lobes (usually two on each side) extended out from the plates onto the valves and reached the ventral side via a narrow isthmus beneath the raphe (Supplementary videos 3 and 4); in ventral focuses of cells the ends of the lobes appeared as narrow longitudinal strips running more or less parallel to the raphe slits (). In contrast, type A cells never had chloroplasts appressed to the dorsal side. Instead, most had two simple plates appressed to the ventral side (, ; Supplementary video 1), which therefore appeared in focus with the fibulae. The plates were sometimes slightly constricted longitudinally, but never as deeply as in type B cells (, ; contrast ). One type A specimen was found with a different chloroplast arrangement (–). Here the chloroplasts (there appeared to be four) were appressed to the valves and were separated by a clear space running along the midline of the girdle on both the ventral () and dorsal () sides (see also Supplementary video 2). This specimen – hereafter referred to as belonging to type A2 – had more closely spaced fibulae and a narrower girdle than the cells with two simple ventral chloroplasts (type A1).
The nuclei lay on the opposite side to the chloroplasts (i.e. dorsally) in type A1 cells (, Supplementary video 1), whereas in types A2 and B they appeared to be more or less equidistant from each side of the girdle (Supplementary videos 2–4). Type A cells (both A1 and A2) had much larger nuclei than type B cells (, , contrast ) and contained prominent nuclei and granular nucleoplasm.
Cleaned material contained isolated valves, which lay in valve or girdle view. In valve view the valves could be seen to have a straight or very slightly convex ventral margin and a strongly convex dorsal margin (–), though in the central part of the valve the sides were parallel (linear). The valve apices were acute in type A valves (–) and rostrate in type B (). Frustules and valves usually appeared almost structureless (‘hyaline’) in LM, apart from the prominent, bar-like fibulae (–), some longitudinal lines on the girdle (apparently the edges of individual girdle bands: , ), and two longitudinal lines on the valve (, , arrows), one on each side of the raphe. Careful focusing on specimens in girdle view indicated that the longitudinal lines were the edges of flap-like structures extending out from the raphe system (, arrowhead; cf. , ). In addition to the longitudinal lines and fibulae, type A1 valves had a zone of irregular texture towards the dorsal side of the valve (–, ; Supplementary video 5), visible even without oiling the condenser. No such texture was visible in type A2 valves (, Supplementary video 6), which were also generally smaller and narrower (Supplementary ).
Striae were always undetectable in LM in type B cells (e.g. in the valve shown in ), whereas in type A cells striae could sometimes be seen when the condenser was oiled (hence maximizing the numerical aperture). Type A1 valves had c. 43 striae in 10 µm on the valve (, ) and on the ventral side of the girdle (), and perhaps slightly fewer (c. 38 in 10 µm: only one measurement!) on the dorsal side of the girdle (). Each stria on the girdle bands of type A1 cells could be seen to contain several (3–5) pores, which formed irregular longitudinal lines (, ). With extreme difficulty, it was possible to resolve the striae in a single type A valve that lacked the zone of irregular texture and hence was assigned to type A2. In this the stria density was c. 48 in 10 µm ().
Figs 18–22. Details of type A frustules: high resolution light microscopy (condenser oiled, NA = 1.4). , . Type A1 (Nitzschia dicrogramma): ventral and dorsal focuses of a frustule, girdle view, showing porous girdle bands. , . Type A1 (N. dicrogramma): two focuses of a valve in valve view with c. 43 striae in 10 µm, irregular texture towards the dorsal side (to left), and transapically elongate fibulae. . Type A2 valve (N. brachygramma) in valve view with c. 48 striae in 10 µm. Scale bars = 5 µm (for , , see ; for –, see ).
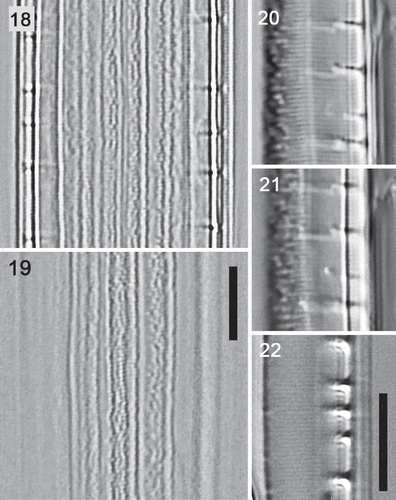
In type A2 and B valves, the fibulae appeared as short bars in both girdle and valve view (, , , ). In type A1 valves, while the fibulae again appeared as short bars in girdle view (, ), valve views (–) showed that they extended much further across the valves than in types A2 and B, extending into the zone of irregular patterning (see also Supplementary videos 5 and 6). There were 2.9–4.2 fibulae in 10 µm in type A1 cells (mean ± SD = 3.45 ± 0.34, n = 14), 2.9–4.5 in type A2 cells (3.88 ± 0.57, n = 6) and 3.4–6.3 in 10 µm in type B (4.13 ± 0.50, n = 40). Although the ranges of fibula densities overlapped, so that this metric cannot be used for identifying individual valves, the mean fibula densities of type A1 and B cells were clearly different (t test, P < 0.001). Type A2 cells did not differ significantly from either A1 or B (P > 0.05).
Nitzschia spathulata had chloroplasts like those of type B cells, both lying on one side of the girdle and extending beneath the valves onto the far side of the girdle (Supplementary video 7) and, again as in type B, the nucleus was small and central.
SEM observations
All of the hantzschioid forms possessed essentially the same valve ultrastructure. Internal views showed a narrow slit-like opening into the raphe canal, which became even narrower away from the centre () but opened out again at each pole (e.g. ); the fibulae were discrete bars, whose bases were not interconnected by thickened ridges running longitudinally along the valve (). Externally, all had siliceous flaps (conopea) extending out from the raphe canal on either side, covering the proximal parts of the valve face and mantle (, , , ). The conopea were always plain and were not attached to the raphe sternum but to the wall of the raphe canal, the base of the conopeum being separated from the raphe sternum by striae like those of the valve face (, , ). At the poles, there were elliptical openings into the space beneath each conopeum (, , , ). The striae were simple and uniseriate, containing tiny round poroids (, , ). The valves had a curved profile, with no clear separation of valve face and mantle on the side opposite the raphe (, , ). The raphe lacked central raphe endings (e.g. ).
Figs 23–31. Interior views of type A and B valves and Nitzschia spathulata, SEM. , . Type A1 (N. dicrogramma), valve and detail: note the greatly extended fibulae, which fork distally, and the sparsely and irregularly porous dorsal striae. , . Type A2 (N. brachygramma), valve and detail, showing simple, orderly, uninterrupted striae. , . Type B (N. parkii), valve and detail: note the plain strips bordering the fibula bases and interrupting the striae. , . Type B: narrower variant. . Nitzschia spathulata. Scale bars = 10 µm (, bar in ) and 5 µm (; , bar in ).
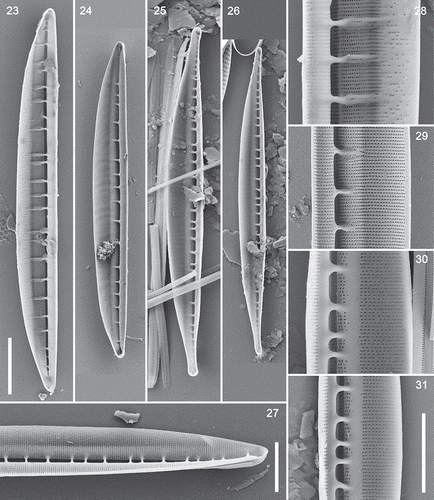
Figs 32–42. Exterior views (except ) of type A and B valves and Nitzschia spathulata, SEM. . Type A1 (N. dicrogramma): valve in girdle view (), valve pole showing the conopeum () and the sparsely and irregularly porous dorsal side (). . Type A2 (N. brachygramma) valve in valve view: note the acute apices. . Type A valve: note the flap (conopeum) running parallel to the raphe, corresponding to the line visible in LM (cf. ). . Details of type A2 (N. brachygramma) valves: note the narrow entrance to the raphe canal and porous girdle bands (), continuity of the raphe across the centre of the valve () and hooked terminal fissure (turned towards the ventral side: ). . Type B (N. parkii): detail of valve pole with conopea and dorsally hooked terminal fissure () and porous girdle bands (: the ridged sides are exterior). . Nitzschia spathulata, whole valve. Note the symmetrical outline of the valve and slightly eccentric raphe. Scale bars = 10 µm (, , ), 5 µm () and µm (, ).
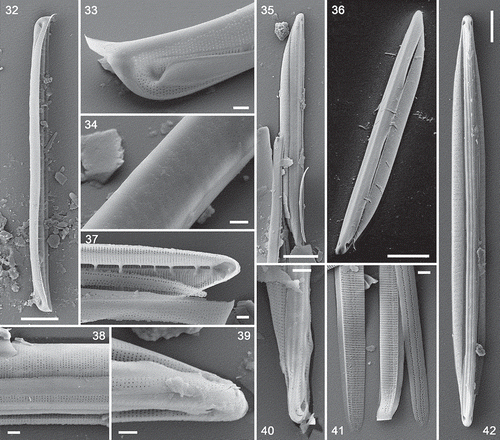
However, the stria pattern and fibula structure showed some variation among the hantzschioid forms. In valves identifiable as the LM type A1 (recognizable from the greater widths of the valves and striation densities of c. 43–44 in 10 µm), the poroids became irregularly and widely spaced in the dorsal part of the valve (, ). This corresponded to the zone of irregular texture visible in LM (compare and with ). SEM also showed that type A1 valves had fibulae that extended approximately halfway across the valve face and were usually forked distally (), extending along two or three transapical ribs (interstriae). In A2 valves (identified by having a valve shape identical to A1 but a striation density of c. 46–48 in 10 µm), the striae continued uninterrupted from the raphe to the distal margin (); the fibulae here were shorter and simpler than in A1 valves ().
In valves corresponding to the LM type B cells (which were more clearly rostrate in SEM than in LM: see , and compare ), the striae were interrupted by a strip of plain, unthickened silica running along the valve on either side of the entrance to the raphe canal (, ). There seemed to be two variants of type B valves in SEM, differing in valve width (6.6 vs 4.8 µm), but in their ultrastructure they appeared identical, both had the same stria density (c. 46 in 10 µm), and their fibula densities were both within the range determined for type B in LM (3.4–6.3 in 10 µm). The girdles of type A2 () and type B cells () contained bands bearing closely spaced striae, as shown by LM in type A1 cells (, ). Those of type B were ridged externally ().
Nitzschia spathulata had the same frustule characteristics as the hantzschioid forms, possessing conopea externally, closely spaced uniseriate striae of small round poroids, discrete bar-like fibulae, and a slit-like entrance into the raphe canal that tapered towards the poles (, ). It differed, however, in its symmetrical outline and only slightly eccentric raphe (), and the special elevations of the raphe near the poles that give the species its name (Supplementary video 7; Mann, Citation1978, figs 247, 816, 829).
Sexual reproduction
Auxosporulation was observed in type A1 cells () and N. spathulata (), and was similar in both. Cells paired via their girdles (e.g. ) and almost always lay in girdle view. Type A1 cells paired via the flatter ‘ventral’ sides (). The pairing cells (gametangia) were orientated with their apical axes roughly parallel (; observations of N. spathulata not shown) and meiotic prophase took place without any rearrangement of the chloroplasts, which therefore lay one towards each pole as in vegetative cells (compare and ; ). The gametangium nucleus enlarged during meiotic prophase and became ± spherical with a clearly defined outline (e.g. ).
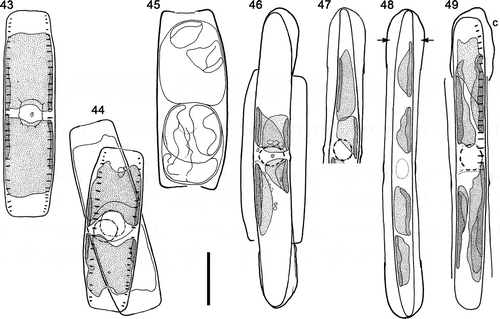
Figs 43–49. Sexual reproduction in type A1 (N. dicrogramma): camera lucida drawings of living cells. . Vegetative cell, girdle view (cf. , ). . Paired cells in meiotic prophase, with their ventral girdles adjacent. . Gametangium with rounded-up, rearranged gametes. . Expanded auxospore: protoplast beginning to contract prior to formation of the initial epivalve. Note the caps over the ends of the auxospore, the four chloroplasts, and the restored diploid nucleus. . The same auxospore after 23.5 h: the chloroplasts have begun to become redistributed along the auxospore and the contraction of the protoplast has created a narrower asymmetrical cell contracted away from the perizonium, except at its tip, which remains linked to the end of the auxospore. . Fully formed dorsiventral initial cell in valve view still enclosed within the parallel-sided perizonium; note the spaces separating the initial cell from the auxospore wall (arrows) and the four chloroplasts in a line along the initial cell. . Later stage: initial cell (in girdle view) escaping from the perizonium, from which the incunabular caps (c) have become detached and in one case lost. The four chloroplasts now lie in two pairs, associated one pair with each valve, so that at the first mitotic division of the cell two chloroplasts will be segregated to each daughter. Scale bar = 20 µm.
Figs 50–60. Sexual reproduction in type A1 (Nitzschia dicrogramma: , ) and N. spathulata (), LM, interference contrast optics. , . Rearranged gametes () and plasmogamy (): note that the gametangia are appressed via their straight ventral sides (). , . Gametangia in girdle view during meiotic prophase, with expanded, strongly demarcated spherical nuclei. In both cases a second gametangium was present immediately beneath the one shown. Note the characteristic flared ends of the N. spathulata valves (also visible in ). . Part of copulation tube (arrow), through which gametes will move to effect plasmogamy. , . Young linear-ellipsoidal zygotes in girdle view: note the two unfused gametic nuclei (, arrows). . Early stage of expansion: the auxospore has begun to push the gametangial thecae apart (cf. ). One of the two unfused haploid gametic nuclei is visible at the centre. . Early stage of expansion: note the four chloroplasts (their pointed tips nearly meet at the centre); the sister auxospore is slightly oblique and mostly out of focus. . Almost fully expanded auxospore (note the different scale relative to other N. spathulata shown) still containing two unfused haploid nuclei at the centre (arrows). Four chloroplasts are present, two near each pole. The gametangial thecae have been pushed far apart. . Initial cell escaping from the auxospore (cf. ). The elongate incunabular caps (c) have become detached and (note that each is approximately half the size of the zygotes shown in , ) between them the perizonium can be seen, consisting of a series of transverse bands (seen in section as regularly spaced thickenings of the discarded auxospore wall): the primary transverse band (black arrow) is slightly wider than the secondary bands (e.g. white arrows). The size of the initial cell relative to the perizonium shows that a strong contraction occurs during initial cell formation in N. spathulata, like that in type A1 () but symmetrical. Scale bars = 10 µm (for , , see ; for and , see ).
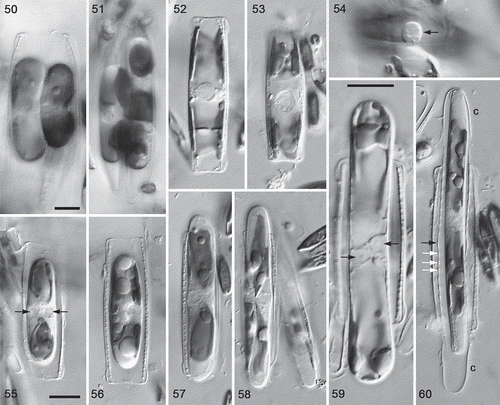
In both diatoms, two morphologically similar gametes (isogametes) were produced per gametangium (, ; not illustrated for N. spathulata), as a result of a cell division at meiosis I, and in type A1 cells the gametes rounded up and rearranged themselves to lie one towards each end of the gametangium. Each gamete contained two chloroplasts (e.g. , top gamete). One gamete in each gametangium was active, the other passive; plasmogamy involved the movement of the active gamete out of each gametangium to fuse with the passive gamete in the other gametangium (), i.e. these species exhibit ‘trans’ physiological anisogamy. Consequently, each of the two zygotes lay within one of the gametangia. In N. spathulata, plasmogamy took place via a copulation tube formed between the gametangia (), but in type A1 no tubes were evident, although the movements of the gametes seemed to be restricted, the exchange of gametes taking place via a relatively small area in the central parts of the cells ().
Following plasmogamy, the zygotes became linear-ellipsoidal, still lying within the gametangial frustules (, ), which by now had split apart. Subsequently, each zygote (auxospore) expanded parallel to the apical axes of the gametangia (, ) to form a roughly isodiametric cylinder (, ). The auxospores bore incunabular caps at each end (, ), representing the halves of the zygote wall, and contained four chloroplasts (, , , ) derived from the gametes. In N. spathulata the gametic nuclei were closely associated but unfused during auxospore expansion (); no comparable observations of type A1 were made. Fully expanded auxospores contained a single nucleus (e.g. ). During the formation of the initial cells, the cell contracted away from the auxospore wall, revealing the presence of a perizonium composed of transverse bands (, , ); the contraction was asymmetrical in type A1 so that, although the auxospore was parallel-sided, the initial cell was dorsiventral, like the vegetative cells and gametangia (, ). Finally, the initial cell escaped from the auxospore casing via one end (, ), following the detachment of the incunabular caps from the perizonium.
Hantzschia segmentalis
The only examples of H. segmentalis authenticated by Brun seem to be the two specimens marked on slide G00295660 (Conservatoire et Jardin botaniques de la Ville de Genève), from material from Morris Cove (Brun’s ‘Mories Cove’), near New Haven, Connecticut, USA. The two specimens agree well with the two illustrations Brun provided for the original description (Brun, Citation1895, pl. 17) and are an isolated valve in valve view (Brun’s fig. 109) and a frustule in girdle view (his fig. 110). The valve is illustrated here in and (see also Supplementary video 8) and is asymmetrical about the apical plane, with a convex dorsal margin and a biarcuate ventral margin; it measures 92.5 µm long and c. 10 µm wide. The specimen in girdle view, corresponding to Brun’s (Citation1895) fig. 110, measures 97 µm. Although this specimen is partly disassembled and is lying obliquely, careful focusing shows that it has hantzschioid symmetry (Supplementary video 9). As Brun indicated in his fig. 109, H. segmentalis has highly elongate fibulae () and a dorsal zone in which the striae contain widely and somewhat irregularly spaced poroids (), as in type A1 valves from Loch Goil. However, there is no evidence of conopea in the two specimens of H. segmentalis on G00295660.
Figs 61–69. Hantzschia segmentalis, LM photographs and camera lucida drawings. , . Two focuses of the lectotype; Morris Cove, Connecticut. . Valves () and girdle details (); Barnstable, Massachusetts. and show the same valve. Scale bars = 10 µm (for , see , for , see bar below ).
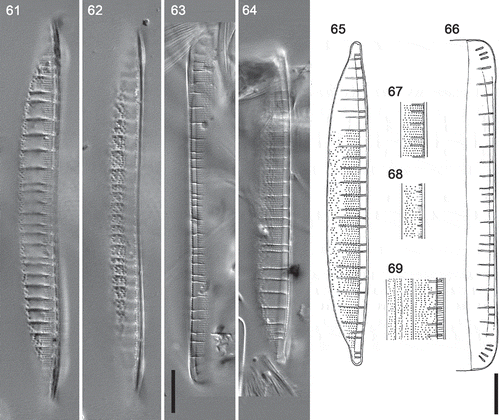
We also found a few specimens of what appears to be H. segmentalis on a slide made by F.E. Round from material he collected in the 1960s from Barnstable, Massachusetts, USA (), some 220 km east from Morris Cove. The Barnstable specimens had the same striation density (19 in 10 µm) and elongate fibulae () as Brun’s Morris Cove material, and had widely and irregularly spaced poroids towards the dorsal margin (, , ). As in the Morris Cove valves, there were no lines parallel to the raphe that might indicate the presence of conopea (, ). The fibula density was 3.5–3.8 in 10 µm (three measurements). Some specimens still had girdle bands attached, which bore short striae containing two or three poroids ().
Discussion
The Loch Goil forms are new to science
Our examination of H. segmentalis shows that it is not conspecific with any of the Loch Goil forms. Although H. segmentalis resembles Loch Goil type A1 in its dorsiventral organization, elongate fibulae, a change in stria structure across the valve (with closely and evenly spaced poroids near the raphe but very widely and rather irregularly spaced poroids towards the dorsal margin), two or more rows of poroids on the most advalvar girdle bands, and the absence of central raphe endings, the two differ in (1) the absence of conopea in H. segmentalis, and (2) the much finer structure of type A1 valves, with 43 striae in 10 µm and poroids that are unresolvable in LM – H. segmentalis having 16–20 striae in 10 µm and easily resolvable poroids (the fibula densities are similar: 2.5–4 in 10 µm in H. segmentalis and 2.9–4.2 in type A1).
No other described species of Hantzschia agree with any of the Loch Goil forms. However, seen from some angles, Nitzschia species can appear to have asymmetrical outlines, so we also searched in this genus for previous reports of the Loch Goil species, focusing on the sect. Spathulatae because this group is exclusively marine and was founded for species possessing conopea (visible in LM as longitudinal lines parallel to the raphe) and because it comprises species without central raphe endings: Grunow’s description of the sect. Spathulatae (in Cleve & Grunow, Citation1880, p. 87) stated that they have ‘meist sehr zart gestreiften Schaalen. Kiel in der Schaalenansicht meist von 2 parallelen Begleitlinien eingefasst … Andeutung eines Mittelknotens nie bemerkbar?’ [mostly very faintly striated valves. Keel in valve view mostly bordered by two parallel accompanying lines … suggestion of a central nodule never noticeable?]. However, none of the species listed by Grunow (in Cleve & Grunow, Citation1880, which is still the most complete account of the Spathulatae) are dorsiventral. It is not impossible that some of the examples of N. distans drawn by Peragallo & Peragallo (Citation1897–Citation1908, pl. 73, and the left-hand drawing in ) could in fact be Loch Goil type A1 or A2, but if so, these were misidentifications since the original description of N. distans indicates clearly that N. distans has a symmetrical outline (Gregory, Citation1857, p. 58, fig. 103). Likewise, a single figure of an east Australian specimen identified by Foged (Citation1978, pl. 45, ) as N. distans appears to show an asymmetrical diatom and could conceivably be the same as our type A2 (since the fibulae are not extended across the valve face).
In Nitzschia outside sect. Spathulatae we again found no similar species. The possibility that the Loch Goil A1 forms could be identified as N. insignis var. adriatica (because of the irregular spacing of the poroids towards the distal side of the valves shown in the illustrations by Hustedt, Citation1955) can be dismissed because of the much coarser striae in var. adriatica (11.5 in 10 µm, as opposed to 43 in A1: see Cleve & Grunow, Citation1880) and the absence of conopea in sect. Insignes (Mann, Citation1978, and unpublished observations). In fact, Hustedt’s specimens correspond to Brun’s H. segmentalis (discussed below).
The Loch Goil forms must therefore be described as new species. The differences among them in valve and fibula ultrastructure, chloroplast morphology and stria density (a conservative character within Nitzschia that seems to remain more or less constant within clones, except sometimes in the smallest cells: Trobajo et al., Citation2011, Citation2012) supports the view that each of types A1, A2 and B are separate species. Whether they should be assigned to Nitzschia or Hantzschia is discussed below.
The absence of previous reports of similar hantzschioid diatoms is strange. Although they were never abundant in the Loch Goil sediments, the hantzschioid forms were common enough to be detected in Park’s (Citation1992) routine seasonal counts, and their size makes them quite easy to find when scanning slides at low magnification (e.g. using a ×25 or ×40 objective). Furthermore, our unpublished observations of shallow sublittoral samples from the Isles of Scilly reveal further hantzschioid forms (Supplementary fig. 2), similar to type A1 but with lower stria densities (39–40 striae in 10 µm, as opposed to 43 in 10 µm) and no irregular dorsal patterning. These hantzschioid species have probably been overlooked because of (1) their apparent restriction to sublittoral sediments (we have never found them in hundreds of samples from the marine intertidal in the UK), which have been studied relatively little by diatomists, relative to intertidal or freshwater benthos; and (2) the ease of overlooking dorsiventrality when, as often occurs, thecae or frustules of these species are seen in girdle view.
The hantzschioid Loch Goil forms agree with Nitzschia subgenus Nitzschia in frustule structure, chloroplasts and auxosporulation
The type A and B forms have the same valve and raphe structure as a group of Nitzschia species that includes not only N. spathulata (illustrated here and by Sims & Paddock, Citation1979, ) and its allies (e.g. Lobban & Mann, Citation1987), but also the type species of Nitzschia, N. sigmoidea and freshwater species of the sections Nitzschia (‘Sigmoideae’) and Dissipatae (Mann, Citation1978, Citation1986; Lange-Bertalot & Metzeltin, Citation1996). In SEM, the most obvious characteristic of this group – referred to by Mann (Citation1986) as Nitzschia subgenus Nitzschia – is the presence of siliceous flaps (conopea), which are attached to the valve on either side of the raphe and create a longitudinal canal that opens to the exterior via an expanded aperture at each pole (cf. ). Throughout subgenus Nitzschia the conopeum is attached, not to the raphe-sternum, but to the wall of the raphe canal, and is separated from the raphe-sternum by a few rows of poroids (, ; see also Mann, Citation1986, ). Furthermore, in all of the conopeum-bearing species, including the Loch Goil forms, the raphe lacks central raphe endings and the internal entrance to the raphe canal tapers towards the poles before suddenly opening out again at the pole itself (e.g. ; Mann, Citation1978). The stria density is generally high (rarely as low as 25 in 10 µm and often more than 40 in 10 µm, making the striae invisible during routine LM work) and the fibulae are usually discrete rib- or bar-like structures, which are sometimes extended transapically across the valve but are not usually connected by longitudinal internal ridges, in contrast to most other Bacillariaceae (e.g. Mann, Citation1978; Round et al., Citation1990; Lange-Bertalot & Metzeltin, Citation1996, pls 68, 122; Trobajo et al., Citation2013); however, exceptions occur in the conopeum-bearing N. wuellerstorfii and N. sigmoidea, which do have linked fibula bases (Lange-Bertalot & Krammer, Citation1987; Knattrup et al., Citation2007). Finally, as far as is known, all conopeum-bearing species have cingula containing several bands bearing multiple longitudinal rows of poroids (Mann, Citation1978; Lange-Bertalot & Krammer, Citation1987, pl. 1, –, pl. 21, ; Knattrup et al., Citation2007). Individually, several of these features are not unique to the conopeum group. For example, the absence of central raphe endings is seen also in a wide variety of other Bacillariaceae, including some species in other sections of Nitzschia and some species of Denticula, Hantzschia and Cylindrotheca (Mann, Citation1982a); discrete, longitudinally ± unconnected fibulae are present in e.g. Nitzschia linearis and N. tenuis (Kobayasi & Kobori, Citation1990); and species with multiple rows of poroids per girdle band are seen also in e.g. N. heufleriana (Lange-Bertalot & Krammer, Citation1987, pl. 2, –) and N. monachorum (Lange-Bertalot & Krammer, Citation1987, pl. 6, ), though not in many other Nitzschia species, where there are only one or no rows of poroids per band (e.g. Mann, Citation1978; Trobajo et al., Citation2013). However, as a whole, the syndrome of raphe and girdle characters seems to be characteristic of the conopeum-bearing Bacillariaceae, including the Loch Goil forms.
Although sexual reproduction has been reported in fewer than 20 species of Bacillariaceae so far, it is noteworthy that all the conopeum-bearing species investigated show a particular kind of physiological anisogamy (the ‘trans’ type) in which each gametangium produces one active and one passive gamete, which become rearranged within the gametangium before plasmogamy; in addition, the zygotes are highly elongate and expand parallel to the apical axes of the gametangia. These characteristics occur in N. sigmoidea (Geitler, Citation1949), N. dissipata (Geitler, Citation1958), N. recta (Mann, Citation1986) and N. spathulata (this paper), and also in Loch Goil type A1 (this paper). They have also been reported in N. flexoides (Geitler, Citation1968b), a sigmoid species lacking central raphe endings that appears to be related to N. sigmoidea. In contrast, Bacillariaceae that lack conopea generally exhibit other types of auxosporulation. Nitzschia fonticola (in a population from Lunz, Austria), N. angustata and N. amphibia are isogamous (Geitler, Citation1932, Citation1954, Citation1969). Pseudo-nitzschia species, which nest within Nitzschia in molecular phylogenies (Lundholm et al., Citation2002; Mann et al., Citation2013a) are physiological anisogamous and produce two gametes per gametangium like the conopeum-bearing species, but they exhibit the cis variant of physiological anisogamy, in which both active gametes are produced by one gametangium and both passive gametes by the other (e.g. Davidovich & Bates, Citation1998; Amato et al., Citation2005; Chepurnov et al., Citation2005); furthermore, in Pseudo-nitzschia auxospore expansion takes place at right angles to the axes of the gametangia, rather than parallel as in the conopeum-bearing species. Fragilariopsis and Cylindrotheca are also nested within Nitzschia in molecular phylogenies (Lundholm et al., Citation2002, Trobajo et al., Citation2009, Citation2010) and their sexual reproduction has recently been reported by Fuchs et al. (Citation2013) and Vanormelingen et al. (Citation2013). In Fragilariopsis kerguelensis fertilization was not observed and it is unclear whether this species is physiologically anisogamous, but auxosporulation is generally like that of the related Pseudo-nitzschia (Fuchs et al., Citation2013). Cylindrotheca also produces spherical zygotes and is isogamous (Vanormelingen et al., Citation2013), like the similarly shaped and possibly related Nitzschia species studied by Mann (Citation1993). Finally, it may be noted that the conopeum-bearing Nitzschia species, including the Goil forms, have thin smooth incunabula (the coverings secreted by the zygote before auxospore expansion), which persist during expansion as caps over the ends of the auxospores (Geitler, Citation1958; Mann, Citation1986; this paper); there is no hint in these species of the presence of silica strips like those secreted by N. fonticola (Geitler, Citation1932; Trobajo et al., Citation2006). As with frustule morphology, therefore, auxosporulation characteristics indicate agreement between the hantzschioid species from Loch Goil and conopeum-bearing Nitzschia species allied to the type species, N. sigmoidea, suggesting that they form a group to the exclusion of some other Nitzschia species and of other currently recognized genera of Bacillariaceae.
The Loch Goil forms must also be compared with Hantzschia species, given that they consistently exhibit hantzschioid symmetry and therefore must always divide by division type V (). Unfortunately, there have been no reports of auxosporulation in Hantzschia species and there are few molecular data for the genus, since the only species brought into culture are members of the H. amphioxys group (e.g. Souffreau et al., Citation2013); all of our own attempts to culture the common marine littoral species (H. virgata sensu lato and H. marina sensu lato) have failed. The type species of Hantzschia, H. amphioxys, which is nested within Nitzschia sensu lato in molecular phylogenies (Lundholm et al., Citation2002; Mann et al., Citation2013a), differs from the hantzschioid Loch Goil diatoms in its (1) lack of conopea, (2) possession of central raphe endings and (3) H-shaped chloroplasts (with two plates lying against the sides of the girdle, linked by a narrow bridge containing the pyrenoid: see e.g. Heinzerling, Citation1908, pl. 3, , ; Mann, Citation1978, figs 103, 104). None of the H. virgata and H. marina groups of species possess the same chloroplast morphologies as type A and B cells, and none lack central raphe endings or have conopea (Mann, Citation1981 and unpublished). Members of the H. amphioxys group of species have unusual internal central raphe endings (deflected in opposite directions: Mann, Citation1977, Citation1978; Lange-Bertalot, Citation1993) that link them to marine species such as H. virgata var. wittii (Mann, Citation1981), H. marina (Mann, Citation1978) and H. distinctepunctata (Garcia-Baptista, Citation1993), despite some striking differences in valve structure and fibula morphology. This type of central ending is otherwise unknown, except in the enigmatic eunotioid genus Eunophora (Vyverman et al., Citation1998) and in Cocconeis, Achnanthidium and their allies (Round et al., Citation1990), which are not closely related to Bacillariaceae (Ruck & Theriot, Citation2011). Hence it is possible that oppositely deflected central endings may be a synapomorphy for ‘core’ Hantzschia. Other species currently classified in Hantzschia (including H. spectabilis, H. vivax and H. segmentalis) differ from the type species and resemble the Goil forms in lacking central raphe endings, but none of these Hantzschia species possess conopea (Mann, Citation1978; Lange-Bertalot, Citation1993; this paper). Thus, Hantzschia species agree with the Loch Goil diatoms in the consistent hantzschioid symmetry and dorsiventrality of the frustules, but possess nothing else in common with them that would argue for classifying the Goil diatoms in Hantzschia. In contrast, all characters argue for classification in Nitzschia subgenus Nitzschia, except symmetry. We therefore assign the Goil forms to Nitzschia and suggest that type B may be closely related to N. spathulata because of the similarity of their chloroplasts; the chloroplasts of type A1 are more akin to those of N. sigmoidea or N. recta (Mann, Citation1986) and raise the question of whether constant hantzschioid symmetry (i.e. division type V) evolved once or several times in subgenus Nitzschia.
Consistent hantzschioid symmetry (division type V) does not define a single monophyletic group in the Bacillariaceae
Our direct assessments of frustule symmetry (nitzschioid or hantzschioid) in the Loch Goil species were based on the rather few complete cells found in the haematoxylin-stained slides made in February 1988: c. 10 cells of type A1, only one of type A2, and c. 25 of type B. In addition, tens of cells of type A1 were seen alive during observations of material containing auxosporulating cells in January 1990. All these complete cells were hantzschioid, with the raphe systems on the straight ventral side. These few observations of frustules are complemented, however, by observations of many tens of single cleaned valves of types A1 and A2, fewer of type B, all of which had their raphes on the ventral side; and by Park’s observations of hundreds of type A cells and valves during a two-year period. Taken together, these observations indicate that most or all type A and B frustules are hantzschioid and that the sole or predominant method of cell division is type V ().
Round et al. (Citation1990) suggested that ‘few characters are common to all Hantzschia spp. but absent from all Nitzschia spp. All Hantzschia spp., however, seem to have a type of division in which cells of hantzschioid symmetry always give rise to two daughter cells both of which have hantzschioid symmetry: this contrasts with the situation in Nitzschia.’ The implication of our discovery of consistently hantzschioid, dorsiventral species within Nitzschia subgenus Nitzschia is that Round et al.’s attempt to define Hantzschia is inadequate: cell division patterns are not a foolproof criterion for separating Nitzschia and Hantzschia. It seems instead that there are at least three groups in which the only method of cell division is type V (): (1) ‘core’ Hantzschia, including the H. amphioxys, H. virgata, H. marina and H. distinctepunctata species groups (Mann, Citation1977, Citation1978, Citation1980b, Citation1981); (2) the type A and B species from Loch Goil and any others with similar characteristics (e.g. the hantzschioid conopeum-bearing species in littoral samples from the Isles of Scilly; Supplementary fig. 2); and (3) Cymbellonitzschia diluviana, which unlike the type species of Cymbellonitzschia (C. minima, for which see Cocquyt & Jewson, Citation1994; Trobajo et al., Citation2012), almost always has its raphe on the straight ventral side of the valve (Hustedt, Citation1950; Round et al., Citation1990; Jewson & Lowry, Citation1993). Apart from symmetry, there are no obvious characteristics that could be suggested as synapomorphies linking these three groups together.
In fact, contrary to the traditional view (e.g. Hustedt, Citation1930), in which Nitzschia is characterized by nitzschioid symmetry and Hantzschia by hantzschioid symmetry, symmetry seems to be quite labile within the Bacillariaceae. summarizes published and new data on symmetry in 37 taxa of Bacillariaceae. Four categories of Bacillariaceae can be distinguished. In category 1, the only kind of division is type V, so that all frustules are hantzschioid. Conversely in category 4, the only kind of division is type I since all of the frustules are nitzschioid (N. sinuata var. tabellaria and N. solgensis make occasional ‘mistakes’, creating a tiny minority of hantzschioid cells). Category 3 taxa have all of types I–IV, producing equal ratios of nitzschioid and hantzschioid cells. Category 2 is perhaps the most curious. Here, although both types of frustule symmetry are produced, nitzschioid cells never breed true, i.e. type I division is absent, the consequence being a 2 : 1 ratio of hantzschioid: nitzschioid cells, first detected in N. hierosolymitana (Mann, Citation1980a) and now found also in N. barrowiana and N. reversa. Most likely, this ratio is also produced in the heterotrophic N. alba, since Lauritis et al. (Citation1967) found only H → N + H (i.e. division types III or IV) and N → H + H (Type II).
Table 2. Published data on frustule symmetry and new counts for Bacillariaceae. H = number of hantzschioid cells counted; N = number of nitzschioid cells counted; P {1:1}= P value from hypothesis {H:N = 1:1} (χ2 test); P {2:1} = P value from hypothesis {H:N = 2:1} (χ2 test); N/NN, N/HH, H/NH, H/HH = numbers of each of division types I, II, III+IV, and V counted; {H:N} = interpreted ratio of H : N suggested by the proportions of H and N cells and the types of division observed; ‘all’ restates a claim made in the reference cited (in which no counts were given); ‘0’ is the converse of ‘all’; nd = no data. *, ** and *** indicate P < 0.05 > 0.01, P < 0.01 > 0.001, and P < 0.001, respectively. The slides used for new counts were from the Academy of Natural Sciences of Philadelphia (prefixed by ‘ANSP’) or the collections of the Royal Botanic Garden Edinburgh (all other slides).
Individual species seem to behave consistently. For example, different populations of Tryblionella hungarica, N. sinuata var. tabellaria and N. sigmoidea have the same symmetry characteristics (), as do all populations of the three most commonly recorded Hantzschia species we have studied over the past 40 years. Hence, it is appropriate to add frustule symmetry to the characters that are routinely recorded in taxonomic descriptions of Bacillariaceae species. For the moment it is difficult to draw phylogenetic conclusions about the distribution of the different symmetry categories, because there are so few data. However, the genera Tryblionella and Psammodictyon seem to be consistently nitzschioid, belonging to symmetry category 4 ( and our unpublished observations), whereas ‘core’ Nitzschia – the conopeum-bearing species of subgenus Nitzschia – are variable, belonging to symmetry categories 1 (the Loch Goil species), 3 (N. sigmoidea, N. vermicularis, N. dissipata) and 4 (N. recta). Our impression, without having made counts for more than a few species (), is that most species in the large Lanceolatae section of Nitzschia are consistently nitzschioid, but that a few, such as N. alba and two species whose true identities are unknown (recorded by Geitler, Citation1968a, as ‘N. palea’ and ‘N. kützingiana’) produce both hantzschioid and nitzschioid frustules (). However, the sect. Lanceolatae is not monophyletic according to published molecular phylogenies (e.g. Trobajo et al., Citation2009; Rimet et al., Citation2011).
The creation of shape during auxosporulation is not always a function of perizonium development
Shape is created de novo in diatoms during auxosporulation. The zygotes of pennate diatoms are frequently spherical or ellipsoidal, whereas the expanded auxospores can be linear, lanceolate or rhombic, centrally constricted or centrally expanded, curved or straight (e.g. see Mann, Citation1994a). The transformation of shape during auxospore expansion is usually mediated by a system of siliceous bands, the perizonium, whose structure was first revealed in detail by von Stosch (Citation1962, Citation1982; see also Mann, Citation1982b; Idei et al., Citation2013; Kaczmarska et al., Citation2013; Mann et al., Citation2013a) and which is built up sequentially, hardening the auxospore wall so that further expansion can occur only at the tips, where the perizonium is absent. In this ‘canonical’ type of development, the balance between hardening and tip growth determines the shape an auxospore will acquire (Mann et al., Citation2013b). Thus, curved shapes, such as those that characterize Cymbella and Amphora, are produced through faster hardening on the dorsal side of the auxospore, through the perizonial bands being wider on this side (Geitler, Citation1927; Mann, Citation1994b; Nagumo, Citation2003), cf. the abdomen of shrimps. It might have been expected that type A1 auxospores would likewise exhibit differential expansion of the perizonium to create an asymmetrical initial cell. Instead, however, the auxospores of type A1 are straight, as in N. spathulata, and the dorsiventral shape is produced through an unequal contraction of the cell away from the perizonium during formation of the initial cell (). This ‘non-canonical’ generation of shape, via post-expansion modification within the perizonium, is also seen in Pleurosigma and Entomoneis, where sigmoid or twisted shapes are produced by contraction within a simple linear auxospore (Karsten, Citation1899). It will be interesting to see whether Hantzschia species produce asymmetry by post-expansion modification, as in N. dicrogramma, or by the canonical method. In Cymbellonitzschia diluviana it appears that development is canonical, since the initial valves are moulded by the interior of the perizonium (Jewson & Lowry, Citation1993), implying no contraction of the auxospore protoplast.
Taxonomic conclusions
The three hantzschioid Nitzschia species from Loch Goil, types A1, A2 and B are described here as new. Brun’s publication of 1895 did not contain a description of H. segmentalis, only the comment ‘Belle et très distincte espèce, qui n’a guère d’analogie qu’avec la H. virgata Roper. Mories Cove, Connecticut U.S. (Tempère). Sondage. Rare.’ accompanying two illustrations. The publication of the two illustrations constitutes valid publication under Articles 38.8–38.10 of the International Code of Nomenclature (Melbourne Code: McNeill et al., Citation2012) but it is not helpful for identification. We therefore supply the first written description of H. segmentalis.
Nitzschia dicrogramma D.G. Mann & Trobajo, sp. nov
, , , , , ,
[Loch Goil ‘Type A1’]
Description: Frustules always hantzschioid. Valves dorsiventral, with a straight ventral margin and a convex dorsal margin (though this is straight in the centre of the valve), 61–117 × 6.5–8 µm (N = 28, including three initial cells and eight gametangia). Poles acute. Striae uniseriate, c. 43–44 in 10 µm, containing regularly spaced round poroids near the raphe but widely and irregularly spaced poroids dorsally, creating an irregular texture in LM. Conopea present on either side of the raphe. Raphe lacking central raphe endings; terminal fissures hooked towards the ventral side. Fibulae 2.9–4.2 in 10 µm, extended transapically and often forked, the extensions continuing along two or three adjacent valve ribs. Epicingulum containing several porous bands with striae of 2–4 pores. Chloroplasts two, plate-like, ventral; nucleus dorsal.
Distinguished from N. parkii and N. brachygramma by its coarser striation, irregular pore spacing on the dorsal side of the valve, more elongate and forked fibulae, and simple plate-like chloroplasts lying on the ventral side. In cleaned material in LM, only the irregular dorsal spacing (visible as texture) and elongate fibulae are useful for identification.
Holotype: Slide M3/10 (at E): valve at England Finder L38, centre circle, close to sector 1. Context photograph and image stack available at http://rbg-web2.rbge.org.uk/algae/research/types/Nitzschia_dicrogramma_type.html.
Type locality: Loch Goil, Argyll, W Scotland (56° 7.7′ N, 4° 54.2′ W): sublittoral silty sediments, fully marine. Not yet known elsewhere.
Etymology: From the Greek ‘dicros’ = forked, bifurcate, and ‘gramma’ = writing (strokes of a pen), referring to the extended, forked fibulae.
Nitzschia brachygramma D.G. Mann & Trobajo, sp. nov
, , , , ,
[Loch Goil ‘Type A2’]
Description: Frustules always hantzschioid. Valves dorsiventral, with a straight ventral margin and a convex dorsal margin (though this is straight in the centre of the valve), 51–67 × 5.6–6.7 µm (N = 8, initial cells and gametangia unknown). Poles acute. Striae uniseriate, c. 46–48 in 10 µm, containing regularly spaced round poroids throughout. Conopea present on either side of the raphe. Raphe lacking central raphe endings; terminal fissures hooked towards the ventral side. Fibulae 2.9–4.5 in 10 µm, rib-like, short, not extending beyond the conopea. Epicingulum containing several porous bands with striae of 2–4 pores. Chloroplasts four, lying against the valves and extending from there onto the side of the girdle, leaving a clear space along the midline of the girdle, nucleus large with granular nucleoplasm, ± equidistant from each side of the girdle.
Distinguished from N. dicrogramma by its shorter fibulae and higher stria density (only visible in LM when the condenser is oiled); from N. parkii by its acute poles and uninterrupted striae; from both by having four chloroplasts instead of two. In LM, N. brachygramma resembles N. dicrogramma except for the shorter fibulae.
Holotype: Slide M3/10 (at E): valve at England Finder M44, sector 2, close to the junction of L44, L45, M44 and M45. Context photograph and image stack available at http://rbg-web2.rbge.org.uk/algae/research/types/Nitzschia_brachygramma_type.html.
Type locality: Loch Goil, Argyll, W Scotland (56° 7.7′ N, 4° 54.2′ W): sublittoral silty sediments, fully marine. Not yet known elsewhere.
Etymology: From the Greek ‘brachys’ = short, and ‘gramma’ = writing (strokes of a pen), referring to its short fibulae.
Nitzschia parkii D.G. Mann & Trobajo, sp. nov
, , , , ,
[Loch Goil ‘Type B’]
Description: Frustules always hantzschioid. Valves dorsiventral, with a straight ventral margin and a convex dorsal margin (though this is straight in the centre of the valve), 53–65 × 5.0–6.8 µm (N = 23, initial cells and gametangia unknown). Poles rostrate. Striae uniseriate, c. 46–48 in 10 µm, containing regularly spaced round poroids but interrupted by a plain band running along the valve beneath the conopea, distal to the fibula bases. Conopea present on either side of the raphe. Raphe lacking central raphe endings; terminal fissures hooked towards the dorsal side. Fibulae 3.4–6.3 in 10 µm, rib-like, short, not extending onto the plain area beneath the conopea. Epicingulum containing several porous bands with striae of 2–4 pores and transverse ridges externally. Chloroplasts two, lying against the dorsal side of the girdle, each with two lobes on either side that extend beneath the valves onto the ventral side of the girdle; the dorsal part of the chloroplast constricted along the midline of the girdle; nucleus small without obviously granular nucleoplasm, ± equidistant from each side of the girdle.
Distinguished from N. dicrogramma by its shorter fibulae, higher stria density (apparently invisible in LM, even when the condenser is oiled) and lobed dorsal chloroplasts; from N. brachygramma by its rostrate poles and interrupted striae, and by having two chloroplasts instead of four. Identifying N. parkii from N. brachygramma in LM is extremely difficult unless chloroplast detail is available.
Holotype: Slide E5648, Loch Goil ‘Large box’, coll. March 1989: valve at England Finder M37, centre circle, near the border with sector 2. Context photograph and image stack available at http://rbg-web2.rbge.org.uk/algae/research/types/Nitzschia_parkii_type.html.
Type locality: Loch Goil, Argyll, W Scotland (56° 7.7′ N, 4° 54.2′ W): sublittoral silty sediments, fully marine. Not known elsewhere.
Etymology: This species is named in honour of Dr Richard Park, whose studies of Loch Goil epipelon made this paper possible.
Hantzschia segmentalis Brun, emend. D.G. Mann & Trobajo
Original publication: Brun (Citation1895, figs 109, 110), by provision of an illustration with analysis.
Description: Frustules apparently always hantzschioid. Valves dorsiventral, with a slightly convex (in smaller valves), straight or concave (in larger valves) ventral margin and a convex dorsal margin, 76–131 µm long (but the initial cells and gametangia are unknown), c. 10–11 µm wide at the centre. Poles rostrate. Striae uniseriate, 16–20 in 10 µm, containing obvious (LM!) pores that are closely spaced (c. 20–25 in 10 µm) near the raphe, but much more widely and somewhat irregularly spaced (c. 10 in 10 µm, even as low as 5 in 10 µm in places) towards the dorsal margin. Raphe system eccentric, close to the ventral margin; central raphe endings apparently absent. Fibulae narrow and greatly extended across the valve, sometimes almost to the dorsal margin, appearing rib-like in LM in both valve and girdle view (but therefore in fact shaped like flat partitions), rather irregularly spaced, 2.5–4 in 10 µm. Longitudinal lines parallel to the raphe absent and hence conopea almost certainly absent. Girdle bands porous, at least three per theca, each bearing short striae of 2–4 pores, the striae being equally or slightly more dense than those of the valve. Marine epipelic, on intertidal and subtidal sediments.
Distinguished from the similarly coarsely structured H. virgata and H. vivax by the irregular, wide spacing of the stria pores towards the dorsal margin, and from H. virgata also by the lack of central raphe endings; from Nitzschia dicrogramma, which is also dorsiventral and has widely spaced stria pores on the dorsal side, by its much coarser structure (< 20 as opposed to >40 striae in 10 µm) and lack of conopea.
Lectotype: We hereby designate the marked specimen in valve view (an isolated valve) on slide G00295660 in G as the lectotype of H. segmentalis (our , and Supplementary video 8).
Distribution: Known from the east coast of the USA, from Massachusetts to North Carolina. F.E. Round’s specimens from Barnstable, Massachusetts, are documented here (). As mentioned previously, Hustedt (Citation1955, pl. 15, ) provided drawings of a hantzschioid diatom that he identified as ‘N. insignis var. adriatica’, which was collected by Harold Humm in 1949 at Beaufort, North Carolina, from beach mud and harbour piles. Though resembling N. dicrogramma in the irregular arrangement of the pores on the dorsal sides of the valves, Hustedt’s drawings resemble much more the type material of H. segmentalis from Morris Cove, Connecticut. Hustedt gave no dimensions or striation densities for ‘N. insignis var. adriatica’, but measurements from his three drawings on pl. 15, , gave lengths of 127, 78 and 76 µm, the single specimen lying more or less in valve view (pl. 15, ) having a width of 9 µm. The fibula densities are 3.7, 3.1 and 3.7 in 10 µm (measured over 50 µm), respectively, and the stria densities apparently 16, 16.5 and 19 in 10 µm (although it is difficult to be sure because of the fibulae). These measurements agree well with H. segmentalis. In the online database of the Hustedt collection (https://web-apps.awi.de/Hustedt-Diatoms/Curator/), there are 18 images of ‘Nitzschia insignis var. adriatica’ from the Beaufort material, from Hustedt collection slides ZT1/81, ZT/85 and W1/34; these specimens have lengths of 77–131 µm, fibula densities of 3.0–4.6 in 10 µm, and stria densities of c. 16–20 in 10 µm. The database images clearly show the asymmetrical outline of the valve that H. segmentalis also has (e.g. images H65486-1a, H65068-1 [the numbers given here are the file names, all in .jpg format]) and the irregular and wide spacing of the poroids towards the dorsal side (H65293-1, H65036-1a and H65260-1a; compare our , and ). The shape of the valve in girdle view is identical to H. segmentalis from Barnstable (H65404-1a, H65404-1a; compare our ). In all respects, then, Hustedt’s specimens correspond to Brun’s H. segmentalis and disagree with Grunow’s original description of Nitzschia insignis var. adriatica (in Cleve & Grunow, Citation1880, p. 84), which states that var. adriatica is an even coarser diatom than H. segmentalis, with a striation density of 11.5 in 10 µm. Furthermore, at least as shown by Peragallo & Peragallo (Citation1897–Citation1908, pl. 75, ), var. adriatica has a symmetrical outline in valve view.
The only places where H. segmentalis has been found are from the eastern seaboard of the USA; possibly it is endemic to this region. As well as the confirmed reports analysed here, the species has apparently also been found at Wareham in Massachusetts (Smithsonian Institution record for USNM slide D14839, Paul S. Conger collection: Wareham oyster beds, Plymouth County, Massachusetts). We have found no other online or literature records. The species is rare in the Morris Cove and Barnstable samples, but at Beaufort Hustedt (Citation1955) commented that it was ‘rather frequent’.
Supplementary information
The following supplemental material is available for this article, accessible via the Supplementary Content tab on the article’s online page at http://dx.doi.org/10.1080/09670262.2014.915063
Supplementary fig. 1. Valves of N. dicrogramma (A–E) and N. brachygramma (F–J) from Loch Goil. Scale bar = 10 µm.
Supplementary fig. 2. A hantzschioid, conopeum-bearing Nitzschia species from the Isles of Scilly, Cornwall, SW England: three valves (A–C), and details of the striae (D, E). Scale bars = 10 µm.
Supplementary videos 1–4. Through-focus image stacks, presented as .avi files (Cinepak codec), of fixed and haematoxylin-stained cells of Nitzschia dicrogramma (type A1: video 1), N. brachygramma (type A2: video 2) and N. parkii (type B: videos 3 and 4). The cells shown have lengths of 76, 60, 60 and 63 µm, respectively.
Supplementary videos 5, 6. Image stacks of cleaned valves of N. dicrogramma (type A1) and N. brachygramma (type A2) in valve view. Valve lengths 73.5 and 57 µm, respectively.
Supplementary video 7. Image stack of a fixed and haematoxylin-stained cell of N. spathulata. Cell length 54.5 µm.
Supplementary videos 8, 9. Image stacks of a cleaned valve (the lectotype) and a poorly preserved frustule of Hantzschia segmentalis, apparently corresponding to the two specimens illustrated by Brun (1895, figs 109, 110); their lengths are 92.5 and c. 97 µm, respectively.
Supplementary material
Download Zip (61.9 MB)Acknowledgements
We thank Dr Richard Park for donating his collections of diatoms to the Royal Botanic Garden Edinburgh, making it possible to check the identity of ‘Nitzschia aff. distans’; Frieda Christie for advice on using the FE-SEM; the Royal Society for a grant allowing the purchase of the Polyvar photomicroscope; Dr Philippe Clerc, Cryptogam curator at the Conservatoire et Jardin botaniques de la Ville de Genève for locating and sending the H. segmentalis type; and the late Dr Ruth Patrick for kindly making it possible (via a Jessup–McHenry award) for D.G.M. to work at the Academy of Natural Sciences of Philadelphia in summer 1981, during which some of the ‘new’ symmetry counts reported here were made.
References
- Amato, A., Orsini, L., D’Alelio, D. & Montresor, M. (2005). Life cycle, size reduction patterns, and ultrastructure of the pennate planktonic diatom Pseudo-nitzschia delicatissima (Bacillariophyceae). Journal of Phycology, 41: 542–556.
- Brockmann, C. (1950). Die Watt-Diatomeen der schleswig-holsteinischen Westküste. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft, 478: 1–26.
- Brun, J. (1895). Diatomées lacustres, marines ou fossiles. Espèces nouvelles ou insuffisamment connues. Le Diatomiste, 2: pls 14–17.
- Chepurnov, V.A., Mann, D.G., Sabbe, K., Vannerum, K., Casteleyn, G., Verleyen, E., Peperzak, L. & Vyverman, W. (2005). Sexual reproduction, mating system, chloroplast dynamics and abrupt cell size reduction in Pseudo-nitzschia pungens from the North Sea (Bacillariophyta). European Journal of Phycology, 40: 379–395.
- Cleve, P.T. & Grunow, A. (1880). Beiträge zur Kenntniss der arctischen Diatomeen. Kongliga Svenska Vetenskaps-Akademiens Handlingar, 17: 1–121.
- Clyde River Purification Board (1985). Pollution in Scottish sealochs. Unpublished report, Clyde River Purification Board, Technical Report 81.
- Clyde River Purification Board (1986). Water quality. A ten year review, 1976–1985. Unpublished report, Clyde River Purification Board.
- Cocquyt, C. & Jewson, D.H. (1994). Cymbellonitzschia minima Hustedt (Bacillariophyceae), a light and electron microscopic study. Diatom Research, 9: 239–247.
- Connor, D.W. & Little, M. (1998). Clyde Sea (MNCR Sector 12). In Marine Nature Conservation Review. Benthic marine ecosystems of Great Britain and the north-east Atlantic (Hiscock, K., editor), 339–353. Joint Nature Conservation Committee, Peterborough.
- Davidovich, N.A. & Bates, S.S. (1998). Sexual reproduction in the pennate diatoms Pseudo-nitzschia multiseries and P. pseudodelicatissima (Bacillariophyceae). Journal of Phycology, 34: 126–137.
- Desikachary, T.V. (1986–1989). Atlas of Diatoms. Madras Science Foundation, Madras. 6 vols.
- Foged, N. (1978). Diatoms in Eastern Australia. Bibliotheca Phycologica, 41: 1–243.
- Fuchs, N., Scalco, E., Kooistra, W.H.C.F., Assmy, P. & Montresor, M. (2013). Genetic characterization and life cycle of the diatom Fragilariopsis kerguelensis. European Journal of Phycology, 48: 411–426.
- Garcia-Baptista, M. (1993). Observations on the genus Hantzschia Grunow at a sandy beach in Rio Grande do Sul, Brazil. Diatom Research, 8: 31–43.
- Geitler, L. (1927). Die Reduktionsteilung und Copulation von Cymbella lanceolata. Archiv für Protistenkunde, 58: 465–507.
- Geitler, L. (1932). Der Formwechsel der pennaten Diatomeen (Kieselalgen). Archive für Protistenkunde, 78: 1–226.
- Geitler, L. (1949). Die Auxosporenbildung von Nitzschia sigmoidea und die Geschlechtsbestimmung bei den Diatomeen. Portugaliae Acta Biologica, ser. A, R.B. Goldschmidt volume: 80–86.
- Geitler, L. (1954). Paarbildung und Gametenfusion bei Anomoeoneis exilis und einigen anderen pennaten Diatomeen. Österreichische Botanische Zeitschrift, 101: 441–452.
- Geitler, L. (1958). Notizen über Rassenbildung, Fortpflanzung, Formwechsel und morphologische Eigentümlichkeiten bei pennaten Diatomeen. Österreichische Botanische Zeitschrift, 105: 408–442.
- Geitler, L. (1968a). Die Lage der Raphen in den Zellen von Nitzschia-Arten. Berichte der Deutschen Botanischen Gesellschaft, 81: 411–413.
- Geitler, L. (1968b). Auxosporenbildungen bei einigen pennaten Diatomeen und Nitzschia flexoides n. sp. in der Gallerte von Ophrydium versatile. Österreichische Botanische Zeitschrift, 115: 482–490.
- Geitler, L. (1969). Die Auxosporenbildung von Nitzschia amphibia. Österreichische Botanische Zeitschrift, 117: 404–410.
- Gregory, W. (1857). On new forms of marine Diatomaceae, found in the Firth of Clyde and in Loch Fine. Transactions of the Royal Society of Edinburgh, 21: 473–542.
- Guillard, R.R.L. & Lorenzen, C.L. (1972). Yellow-green algae with chlorophyllide c. Journal of Phycology, 8: 10–14.
- Hartley, B., Barber, H.G., Carter, J.R. & Sims, P.A. (1996). An Atlas of British Diatoms. Biopress, Bristol.
- Heinzerling, O. (1908). Der Bau der Diatomeenzelle, mit besonderer Berücksichtigung der ergastischen Gebilde und der Beziehung des Baues zur Systematik. Bibliotheca Botanica, 69: 1–88.
- Hustedt, F. (1930). Bacillariophyta (Diatomeae). In Die Süsswasser-Flora Mitteleuropas (Pascher, A., editor), vol. 10, 2nd ed. G. Fischer, Jena.
- Hustedt, F. (1950). Die Diatomeenflora norddeutscher Seen mit besonderer Berücksichtigung des holsteinischen Seengebiets. V.–VII. Seen in Mecklenburg, Lauenburg und Nordostdeutschland. Archiv für Hydrobiologie, 43: 329–458.
- Hustedt, F. (1955). Marine littoral diatoms of Beaufort, North Carolina. Duke University Marine Station, Bulletin, 6: 1–67.
- Idei, M., Sato, S., Watanabe, T., Nagumo, T. & Mann, D.G. (2013). Sexual reproduction and auxospore structure in Diploneis papula (Bacillariophyta). Phycologia, 52: 295–308.
- Jewson, D.H. & Lowry, S. (1993). Cymbellonitzschia diluviana Hustedt (Bacillariophyceae): habitat and auxosporulation. Hydrobiologia, 269/270: 87–96.
- Johansen, D.A. (1940). Plant Microtechnique. McGraw-Hill, New York, NY.
- Kaczmarska, I., Poulíčková, A., Sato, S., Edlund, M.B., Idei, M., Watanabe, T. & Mann, D.G. (2013). Proposals for a terminology for diatom sexual reproduction, auxospores and resting stages. Diatom Research, 28: 263–294.
- Karsten, G. (1899). Die Diatomeen der Kieler Bucht. Wissenschaftliche Meeresuntersuchungen, N.F. (Kiel), 4: 1–205.
- Knattrup, A., Yde, M., Lundholm, N. & Ellegaard, M. (2007). A detailed description of a Danish strain of Nitzschia sigmoidea, the type species of Nitzschia, providing a reference for future morphological and phylogenetic studies of the genus. Diatom Research, 22: 105–116.
- Kobayasi, H. & Kobori, S. (1990). Nitzschia linearis and two related diatom species. In Proceedings of the Tenth International Diatom Symposium (Simola, H., editor), 183–193. Koeltz Scientific Books, Königstein.
- Kociolek, J.P. & Williams, D.M. (1987). Unicell ontogeny and phylogeny: examples from the diatoms. Cladistics, 3: 274–284.
- Kuylenstierna, M. (1990). Benthic algal vegetation in the Nordre Älv estuary (Swedish west coast). Department of Marine Botany, University of Göteborg, Sweden. 2 vols, 244 pp, 76 plates.
- Lange-Bertalot, H. (1993). 85 neue Taxa und über 100 weitere neu definierte Taxa ergänzend zur Süßwasserflora von Mitteleuropa Vol. 2/1–4. Bibliotheca Diatomologica, 27: 1–454.
- Lange-Bertalot, H. & Krammer, K. (1987). Bacillariaceae Epithemiaceae Surirellaceae. Neue und wenig bekannte Taxa, neue Kombinationen und Synonyme sowie Bemerkungen und Ergänzungen zu den Naviculaceae. Bibliotheca Diatomologica, 15: 1–289.
- Lange-Bertalot, H. & Metzeltin, D. (1996). Oligotrophie-Indikatoren. 800 Taxa repräsentativ für drei diverse Seen-Typen. Kalkreich – Oligodystroph – Schwach gepuffertes Weichwasser. In Iconographia Diatomologica, vol. 2 (Lange-Bertalot, H., editor), 3–390. Koeltz Scientific Books, Königstein.
- Lauritis, J.A., Hemmingsen, B.B. & Volcani, B.E. (1967). Propagation of Hantzschia sp. Grunow daughter cells by Nitzschia alba Lewin and Lewin. Journal of Phycology, 3: 236–237.
- Lobban, C.S. & Mann, D.G. (1987). The systematics of the tube-dwelling diatom Nitzschia martiana and Nitzschia section Spathulatae. Canadian Journal of Botany, 65: 2396–2402.
- Lundholm, N., Daugbjerg, N. & Moestrup, Ø. (2002). Phylogeny of the Bacillariaceae with emphasis on the genus Pseudo-nitzschia (Bacillariophyceae) based on partial LSU rDNA. European Journal of Phycology, 37: 115–134.
- Mann, D.G. (1977). The diatom genus Hantzschia Grunow – an appraisal. Nova Hedwigia, Beiheft, 54: 323–354.
- Mann, D.G. (1978). Studies in the family Nitzschiaceae (Bacillariophyta). 2 vols. Ph.D. thesis, University of Bristol, Bristol, UK. Available at http://rbg-web2.rbge.org.uk/algae/publications_mann_thesis.html.
- Mann, D.G. (1980a). Hantzschia fenestrata Hust. (Bacillariophyta) – Hantzschia or Nitzschia? British Phycological Journal, 15: 249–260.
- Mann, D.G. (1980b). Studies in the diatom genus Hantzschia. II. H. distinctepunctata. Nova Hedwigia, 33: 341–352.
- Mann, D.G. (1981). Studies in the diatom genus Hantzschia 3. Infraspecific variation in H. virgata. Annals of Botany, 47: 377–395.
- Mann, D.G. (1982a). The use of the central raphe endings as a taxonomic character. Plant Systematics and Evolution, 141: 143–152.
- Mann, D.G. (1982b). Structure, life history and systematics of Rhoicosphenia (Bacillariophyta). II. Auxospore formation and perizonium structure of Rh. curvata. Journal of Phycology, 18: 264–274.
- Mann, D.G. (1986). Nitzschia subgenus Nitzschia (Notes for a monograph of the Bacillariaceae 2). In Proceedings of the 8th International Diatom Symposium (Ricard, M., editor), 215–226. O. Koeltz, Königstein.
- Mann, D.G. (1993). Sexual reproduction in a marine member of the Bacillariaceae. Diatom Research, 8: 109–116.
- Mann, D.G. (1994a). The origins of shape and form in diatoms: the interplay between morphogenetic studies and systematics. In Shape and Form in Plants and Fungi (Ingram, D.S. & Hudson, A.J., editors), 17–38. Academic Press, London.
- Mann, D.G. (1994b). The systematics of amphoroid diatoms: the life history of Amphora arcus. Nova Hedwigia, 58: 335–352.
- Mann, D.G., Sato, S., Rovira, L. & Trobajo, R. (2013a). Paedogamy and auxosporulation in Nitzschia sect. Lanceolatae (Bacillariophyta). Phycologia, 52: 204–220.
- Mann, D.G., Idei, M. & Sato, S. (2013b). How the diatom got its shape. Phycologia, 52 (4 Supplement): 67.
- McNeill, J., Barrie, F.R., Buck, W.R., Demoulin, V., Greuter, W., Hawksworth, D.L., Herendeen, P.S., Knapp, S., Marhold, K., Prado, J., Prud’homme van Reine, W.F., Smith, G.F., Wiersema, J.H. & Turland, N.J. (2012). International Code of Nomenclature for Algae, Fungi, and Plants (Melbourne Code). Koeltz Scientific Books, Königstein.
- Montgomery, R.T. (1978). Environmental and ecological studies of the diatom communities associated with the coral reefs of the Florida Keys. PhD dissertation, Florida State University. 2 vols.
- Nagumo, T. (2003). Taxonomic studies of the subgenus Amphora Cleve of the genus Amphora (Bacillariophyceae) in Japan. Bibliotheca Diatomologica, 49: 1–265.
- Palmer, J.D. & Round, F.E. (1967). Persistent, vertical-migration rhythms in benthic microflora. VI. The tidal and diurnal nature of the rhythm in the diatom Hantzschia virgata. Biological Bulletin, 132: 44–55.
- Park, R.A. (1992). The ecology of epipelic diatoms in Loch Goil. Ph.D. thesis, University of Edinburgh, Edinburgh, UK. Available at http://rbg-web2.rbge.org.uk/algae/RPark.html.
- Peragallo, H. & Peragallo, M. (1897–1908). Diatomées marines de France et des districts maritimes voisins. Micrographe-Éditeur, Grez-sur-Loing. 2 vols.
- Pickett-Heaps, J. (1983). Valve morphogenesis and the microtubule center in three species of the diatom Nitzschia. Journal of Phycology, 19: 269–281.
- Poulin, M., Bérard-Therriault, L., Cardinal, A. & Hamilton, P.B. (1990). Les diatomées (Bacillariophyta) benthiques de substrats durs des eaux marines et saumâtres du Québec. 9 Bacillariaceae. Le Naturaliste Canadien, 117: 73–101.
- Ricard, M. (1987). Diatomophycées. In Atlas du phytoplancton marin (Sournia, A., editor), vol. 2, 1–297. Éditions du Centre National de la Recherche Scientifique, Paris.
- Rimet, F., Kermarrec, L., Bouchez, A., Hoffmann, L., Ector, L. & Medlin, L.K. (2011). Molecular phylogeny of the family Bacillariaceae based on 18S rDNA sequences: focus on freshwater Nitzschia of the section Lanceolatae. Diatom Research, 26: 273–291.
- Ross, R., Cox, E.J., Karayeva, N.I., Mann, D.G., Paddock, T.B.B., Simonsen, R. & Sims, P.A. (1979). An amended terminology for the siliceous components of the diatom cell. Nova Hedwigia, Beiheft, 64: 513–533.
- Round, F.E. (1970). The genus Hantzschia with particular reference to H. virgata v. intermedia (Grun.) comb. nov. Annals of Botany, 34: 75–91.
- Round, F.E., Crawford, R.M. & Mann, D.G. (1990). The Diatoms. Biology and Morphology of the Genera. Cambridge University Press, Cambridge.
- Ruck, E.C. & Theriot, E.C. (2011). Origin and evolution of the canal raphe system in diatoms. Protist, 162: 723–737.
- Sims, P.A. & Paddock, T.B.B. (1979). Observations and comments on some prominent morphological features of naviculoid genera. Nova Hedwigia, Beiheft, 64: 169–191.
- Souffreau, C., Vanormelingen, P., Van de Vijver, B., Isheva, T., Verleyen, E., Sabbe, K. & Vyverman, W. (2013). Molecular evidence for distinct Antarctic lineages in the cosmopolitan terrestrial diatoms Pinnularia borealis and Hantzschia amphioxys. Protist, 164: 101–115.
- Stosch, H.A. von (1962). Über das Perizonium der Diatomeen. Vorträge aus dem Gesamtgebiet der Botanik, 1: 43–52.
- Stosch, H.A. von (1982). On auxospore envelopes in diatoms. Bacillaria, 5: 127–156.
- Trobajo, R., Mann, D.G., Chepurnov, V.A., Clavero, E. & Cox, E.J. (2006). Taxonomy, life cycle, and auxosporulation of Nitzschia fonticola (Bacillariophyta). Journal of Phycology, 42: 1353–1372.
- Trobajo, R., Clavero, E., Chepurnov, V.A., Sabbe, K., Mann, D.G., Ishihara, S. & Cox, E.J. (2009). Morphological, genetic and mating diversity within the widespread bioindicator Nitzschia palea (Bacillariophyceae). Phycologia, 48: 443–459.
- Trobajo, R., Mann, D.G., Clavero, E., Evans, K.M., Vanormelingen, P. & McGregor, R.C. (2010). The use of partial cox1, rbcL and LSU rDNA sequences for phylogenetics and species identification within the Nitzschia palea species complex (Bacillariophyceae). European Journal of Phycology, 45: 413–425.
- Trobajo, R., Rovira, L., Mann, D.G. & Cox, E.J. (2011). Effects of salinity on growth and on valve morphology of five estuarine diatoms. Phycological Research, 59: 83–90.
- Trobajo, R., Mann, D.G. & Cox, E.J. (2012). Studies on the type material of Nitzschia abbreviata (Bacillariophyta). Nova Hedwigia, Beiheft, 141: 185–199.
- Trobajo, R., Rovira, L., Ector, L., Wetzel, C.E., Kelly, M. & Mann, D.G. (2013). Morphology and identity of some ecologically important small Nitzschia species. Diatom Research, 28: 37–59.
- Vanormelingen, P., Vanelslander, B., Sato, S., Gillard, J., Trobajo, R., Sabbe, K. & Vyverman, W. (2013). Heterothallic sexual reproduction in the model diatom Cylindrotheca. European Journal of Phycology, 48: 93–105.
- Vyverman, W., Sabbe, K., Mann, D.G., Kilroy, C., Vyverman, R., Vanhoutte, K. & Hodgson, D. (1998). Eunophora gen. nov. (Bacillariophyta) from Tasmania and New Zealand: description and comparison with Eunotia and amphoroid diatoms. European Journal of Phycology, 33: 95–111.
- Witkowski, A., Lange-Bertalot, H. & Metzeltin, D. (2000). Diatom flora of marine coasts I. In Iconographia Diatomologica. Annotated Diatom Micrographs, Vol. 7 Diversity–Taxonomy–Identification. A.R.G. Gantner Verlag K.G., Ruggell.
