Abstract
The Indo-Pacific Ocean is a biodiversity hotspot for marine organisms. In this area, most of the research has focused on marine animals, such as reef fish, molluscs and other associated coral fauna, but very little has been done on macroalgae. The Thai-Malay Peninsula is an important north–south barrier in this area, which faces two different oceans – the Indian Ocean and the Pacific Ocean. This study aims to investigate genetic distribution patterns of Padina boryana Thivy around the Thai-Malay Peninsula, where it is common. Three DNA marker regions, the mitochondrion-encoded cytochrome c oxidase subunit 3 gene (cox3); the plastid rbcL, and the nuclear internal transcribed spacer 2 (ITS2) were used to evaluate genetic diversity and the relationships within and between populations. Samples were collected from both the Andaman Sea and Gulf of Thailand sides of the peninsula. Parsimony networks and maximum likelihood and Bayesian analyses showed clearly that there are two separated P. boryana lineages, one restricted to the Gulf of Thailand and the other to the Andaman Sea and other areas of the Indo-Pacific. The effect of different ocean currents along the Andaman Sea and Gulf of Thailand may have shaped these populations of P. boryana. This phylogeographic separation, based on persistent currents in the area, may affect other marine organisms along the Thai peninsula.
Introduction
The Indo-Pacific Ocean is a biodiversity hotspot for marine organisms (Carpenter et al., Citation2011). The discontinuity produced by the many islands in the region is important, in that it provides both habitat complexity and potentially isolated populations, leading to a rich diversity of marine life (Briggs, Citation2000). In this area, research has mostly focused on marine animals (Briggs, Citation2005; Reaka et al., Citation2008; Carpenter et al., Citation2011; Mirams et al., Citation2011), rather than macroalgae. Benthic macroalgae are important primary producers in these coastal environments and structure ecological communities.
Thailand has a peninsula with coastlines on two different oceans. The west side faces the Andaman Sea, which is part of the Indian Ocean, and the east side faces the Gulf of Thailand, which is connected via the South China Sea to the Pacific Ocean. As a result, there are significant physical differences between the two sides of the Thai-Malay peninsula. The Gulf of Thailand has higher sedimentation, and therefore higher turbidity, than the Andaman Sea. A diurnal and mixed tidal cycle can be found in the Gulf of Thailand while the Andaman Sea has exclusively semidiurnal tides. Sea currents do not aid connectivity between the Gulf and Andaman Sea. In addition, the currents change seasonally in these areas. There are two monsoon seasons (Buranapratheprat et al., Citation2002; Buranapratheprat, Citation2008): the Southwest (May–October) and the Northeast (November–February) monsoons affect waters along the Thai-Malay Peninsula. These monsoons significantly influence the surface currents in the Gulf of Thailand (Buranapratheprat et al., Citation2002). During the Southwest monsoon, the surface current in the Gulf of Thailand moves in a clockwise direction. This current flows out from the Gulf in a northward direction and mixes with the South China Sea. During the Northeast monsoon, the water moves in a counter-clockwise direction in the Gulf of Thailand and remains mainly in the Gulf. The Thai-Malay coast of the Andaman Sea is connected with the open ocean, and is mainly influenced by the equatorial current in the monsoon season. The surface water current moves downward into the Malacca strait (Pornpinatepong, Citation2005), separately from currents in the Gulf of Thailand.
The different environmental factors and lack of current connectivity may influence species composition and population connectivity along the coasts. This lack of connectivity can be seen in the genetic differentiation of many organisms, for example, coral (Clifton, Citation1997; Knittweis et al., Citation2009), macroalgae (Maggs & Callow, Citation2002), crustaceans (de Bruyn et al., Citation2005; Obst et al., Citation2012) and plants (Liao et al., Citation2009). Moreover, molecular genetic analyses of anemone fish (Timm & Kochzius, Citation2008), seastars (Yasuda et al., Citation2009) and coral (Knittweis et al., Citation2009) have found a clear genetic break between Indian and Pacific Ocean populations, which has been attributed to the ocean current patterns in the region.
Padina boryana Thivy is distributed widely in the Indo-Pacific (Lüning, Citation1990) and in Thailand. It grows as an upright fan-shaped blade attaching to hard substrata in the intertidal and subtidal. Padina boryana exhibits an alternation of life history generations. However, sporophytes are always more common than gametophytes (Fagerberg & Dawes, Citation1973; Liddle, Citation1975; Lewis & Norris, Citation1987; Wichachucherd et al., Citation2010). Padina boryana reproduces throughout the year and has a high recruitment potential (Wichachucherd et al., Citation2010); thus P. boryana may be a good model for investigating the genetic diversity, population connectivity and barriers to gene flow of macroalgae in this area.
The present study aims to infer the phylogeographic patterns of P. boryana around the Thai-Malay Peninsula. We used genes from all three genomes (mitochondrion, plastid and nuclear) to evaluate genetic relationships within and between populations. This research will improve our understanding of the population genetics of P. boryana along the Thai-Malay peninsula.
Materials and methods
Sampling and collection
Samples were collected from several sites along the coast of the Andaman Sea and Gulf of Thailand (; ), both in the intertidal and subtidal (to c. 2–5 m depth), by snorkelling or sampling during low tide. Padina boryana grows as a patchy canopy of blades, so to avoid collecting samples from the same individual, they were collected from clearly distinct patches (this reduced the number of samples we could collect in some locations). After the specimens were collected, they were cleaned and vouchers made. An apical portion of each thallus was dried in silica gel for DNA extraction. The sample number of each specimen in silica gel could be linked with a voucher specimen for morphological investigation. Morphological characters of specimens, and identifications, were checked in the laboratory before DNA extraction. Selected voucher specimens were deposited in the Princess Maha Chakri Sirindhorn Natural History Museum at Prince of Songkla University (PSU).
Fig. 1. Collection sites along Thai coasts of the Thai-Malay Peninsula. For abbreviations of provinces see . Scale bar = 200 km.
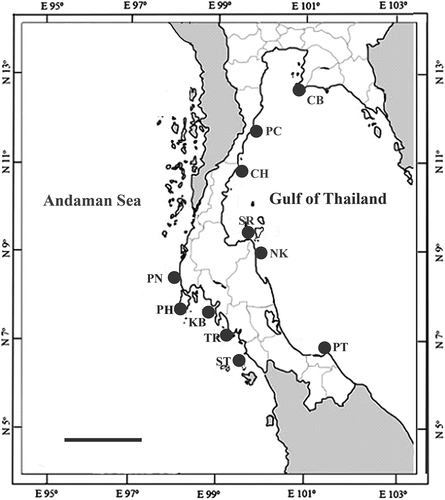
Table 1. Sample sites along the Thai-Malay Peninsula are shown, with number of samples sequenced.
DNA extraction
A modified CTAB protocol was used (Zuccarello & Lokhorst, Citation2005). Briefly, tissue samples (approximately 3 × 3 mm) were ground with a microfuge pestle in 500 µl CTAB buffer (0.1 M Tris-HCl pH 8, 1.4 M NaCl, 20 mM EDTA) plus 4 µg of RNase A and 0.1 µg of Proteinase K. After grinding, tissue was incubated at 60°C for 20 min. The aqueous phase was extracted twice with chloroform : isoamyl alcohol (24 : 1), mixed well and centrifuged at full speed for 10 min at room temperature. To the final aqueous phase an equal volume of 100% isopropanol was added. The tube was then centrifuged for 20 min at full speed. The DNA pellet was washed in 70% ethanol and then centrifuged for a further 5 min at full speed. The ethanol was decanted and the tube was air-dried at room temperature. To the final DNA pellet 50 µl of 0.1 × TE buffer (10 mM Tris (pH 8), 0.1 mM EDTA) was added and the sample was frozen at −20°C.
PCR amplification
PCR was used to amplify three target regions: the mitochondrion-encoded cytochrome c oxidase subunit 3 gene (cox3); the 3′ end of the plastid-encoded rbcL gene and the nuclear-encoded internal transcribed spacer 2 (ITS2). PCR reactions were in a 30 µl volume that contained: 1 µl of diluted DNA, 1× PCR buffer (New England Biolabs Inc.), 200 µM dNTP, 250 nM of each primer, 0.008% BSA, 1.0 U Taq polymerase (New England Biolabs). To obtain PCR products from some samples, 5% dimethyl sulfoxide or 0.8 M betaine was added.
Amplification of a fragment of cox3 was carried out using primers trnY-P1 (GAYCCWAGTCCMTGGCCWTTAG) and cox3-P6.3 (CCWACDATHGCRTGATGVGCCC; Ni-Ni-Win et al., Citation2008). Partial rbcL was amplified using primers rbcL-P1 (GKGTWATTTGTAARTGGATGCG; Ni-Ni-Win et al., Citation2008) and rbcS-P1 (GGATCATCTGYCCATTCTACAC; Ni-Ni-Win et al., Citation2008) or BB297F (YGGWGGTGGTACAATTGGTCA) and BB861 (TGTTCATCYGTTAARTCTGGT) for some difficult samples. ITS2 was amplified with the primers, ITS3 (GCATCGATGAAGAACGCAGC) and ITS4 (TCCTCCGCTTATTGATATGC) (White et al., Citation1990) or BB-ITS-F (CGCATCTTGCGCTTCCGGGATAC) and BB-ITS-R (AACATGTYGTGCGCGTGCARGAGAG). PCR conditions were as follows: an initial denaturation step at 94°C for 5 min, followed by 94°C 1 min, annealing at 47°C for 1 min, extension at 72°C for 1 min for 37 cycles, and a final extension at 72°C for 10 min. PCR products were checked for length and yield by electrophoresis. PCR products were cleaned using the ExoSAP-IT method (USB Affymetrix, Affymetrix, California, USA) and sequenced commercially in both directions using amplification primers (Macrogen Inc., Seoul, Korea).
Data analysis
Sequences were edited and aligned in Geneious (Biomatters, Auckland, New Zealand). Overlapping sequences, both forward and reverse directions, were assembled into a consensus. Haplotype networks were created using TCS 1.21 (Clement et al., Citation2000) to calculate the minimum number of mutational steps by which the sequences could be joined with 95% confidence.
Phylogenetic trees were inferred using Maximum likelihood (ML) and Bayesian inference (BI). The best-fit model of nucleotide substitution was calculated using the Akaike information criterion with jModelTest (Guindon & Gascuel, Citation2003; Posada, Citation2008). For cox3 and ITS2 datasets the best-fit model was K80, and for partial rbcL data it was GTR. ML analysis was carried out using PhyML 3.0 (Guindon et al., Citation2010). Bootstrap analysis (Felsenstein, Citation1985) was carried out to find support for individual internal branches in a heuristic search option with 100 replicates. Bayesian analyses were performed using MrBayes v3.2 (Ronquist et al., Citation2012). Two chains of Markov chain Monte Carlo iterations ran for 1 000 000 generations with sampling every 100 generations. A consensus topology and posterior probability values were calculated after a burn-in of 10 000 generations. The cox3 and partial rbcL datasets were combined with available sequences from previous studies (Ni-Ni-Win et al., Citation2011; Silberfeld et al., Citation2013) (supplementary information, Table S1) and other geographic regions in the Indian and Pacific Oceans.
Results
Final sample sets were 70 cox3 sequences, 42 partial rbcL sequences and 62 ITS2 sequences (). The alignments were 334 bp for cox3, 235 bp for partial rbcL and 439 bp for ITS2. Padina boryana could be found in large patches in most areas, but in only very small populations in other areas; for example stations CB and PT had small populations and correspondingly few samples. The number of sequences of each marker also varied as some samples were difficult to amplify.
Haplotype distribution
Mitochondrial and plastid markers showed little variability compared with the nuclear marker. Cox3 exhibited five haplotypes (C1–C5), partial rbcL had three haplotypes (R1–R3) and ITS2 had 10 ribotypes (I1–I10) (; details and GenBank Accession numbers in supplementary information, Table S2). All three marker networks were largely congruent, showing two main groups of haplotypes/ribotypes (–; supplementary information, Table S3). One group represented samples found in the Gulf of Thailand and the second group was mainly obtained from samples from the Andaman Sea and a few from the southern Gulf of Thailand.
Table 2. Distribution of haplotypes (partial cox3, partial rbcL) and ribotypes (ITS2) in the sampled populations. Population locations can be seen in and .
Fig. 2. Statistical parsimony network of Padina boryana partial cox3 gene sequences from the Thai-Malay Peninsula. n = number of samples. Lines indicate one base pair change. Filled circles indicate Gulf of Thailand lineage and open circles are the Andaman Sea lineage (the shaded segment is Gulf of Thailand samples assigned to the Andaman Sea lineage).
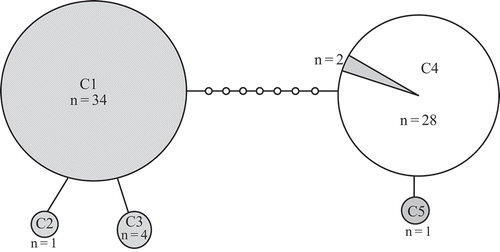
Fig. 3. Statistical parsimony network of Padina boryana partial rbcL gene sequences from the Thai-Malay Peninsula. n = number of samples. Lines indicate one base pair change. Filled circles indicate the Gulf of Thailand lineage and open circles are the Andaman Sea lineage (the shaded segment is Gulf of Thailand samples assigned to the Andaman Sea lineage).
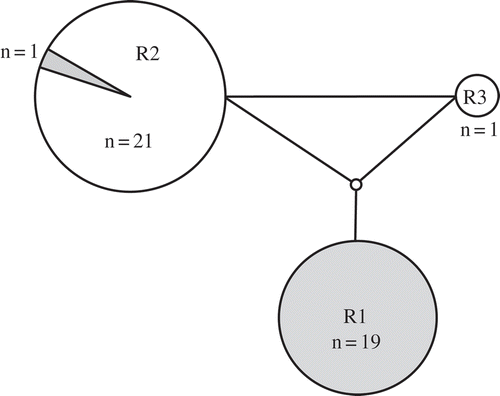
Fig. 4. Statistical parsimony network of Padina boryana ITS2 sequences from the Thai-Malay Peninsula. n = number of samples. Lines indicate one base pair change. Filled circles indicate the Gulf of Thailand lineage and open circles are the Andaman Sea lineage. The shaded segment is Gulf of Thailand samples assigned to the Andaman Sea lineage.
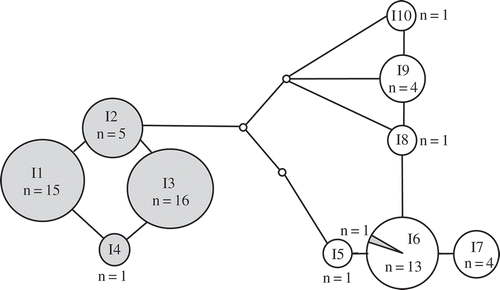
The cox3 dataset of 70 samples revealed five haplotypes (). Most of the samples belonged to two haplotypes (C1 and C4). Haplotypes C1 and C4 differed by 8 base pairs (bp). Haplotypes C1, C2 and C3 were from the Gulf of Thailand, whereas C4 was from the Andaman Sea. There were three Gulf of Thailand samples with haplotypes placed in the Andaman haplogroup. Haplotype C4 mostly came from the Andaman Sea except for two samples from Surat Thani province (SR91.4.F) and Pattani province (PT308.1) in the lower Gulf of Thailand. C5, one bp different from C4, was found in only one sample (NK336.4) which was from the Gulf of Thailand.
The partial rbcL dataset had 42 sequences and three haplotypes among samples obtained from Thai coasts. The majority of samples exhibited one of two haplotypes (R1 or R2; ) differing by 1–2 bp. Haplotype R1 was represented by samples from the Gulf of Thailand while haplotypes R2 and R3 were from the Andaman Sea except for one sample collected from Gulf of Thailand (NK336.4).
ITS2 showed more variation compared with the organellar markers. There were 10 ribotypes distributed in two groups (). Ribotype I1–I4 contained all sequences from the Gulf of Thailand. Another group of ribotypes, at least 3 bp different from the first group, was found mostly from the Andaman Sea (I5–I10). Three Gulf of Thailand sequences, SR91.4.F, NK336.4 and PT308.1, had ITS2 ribotypes similar to other Gulf of Thailand ribotypes (ribotype I6, ribotype I1 and ribotype I4, respectively). Two of these samples (NK336.4 and PT308.1) were grouped in cox3 and partial rbcL networks with Andaman Sea samples (haplotype C5 and haplotype C4 for cox3 respectively and haplotype R2 for partial rbcL; no rbcL sequence was available for PT308.1).
Phylogeographic relationships along the Thai coasts
Three molecular markers were used to produce phylogenetic trees to explore the relationship of all samples along the Thai-Malay Peninsula and from other Indo-Pacific regions (Ni-Ni-Win et al., Citation2011; Silberfeld et al., Citation2013, details in supplementary information, Table S1). Bayesian and Maximum Likelihood analyses gave the same tree topology (–). All phylogenetic trees showed patterns congruent with the networks. Two distinct groups of P. boryana were again evident.
Fig. 5. Bayesian tree of Padina boryana: the partial cox3 gene phylogeny (scale bar = 0.008 substitutions/site). Number above each node indicates Bayesian posterior probability followed by ML bootstrap percentages. GenBank sequences shown in bold text (Ni-Ni-Win et al., Citation2011; Silberfeld et al., Citation2013). The asterisk indicates the sample collected from the Gulf of Thailand grouped in the Andaman Sea clade. Padina minor was used as outgroup.
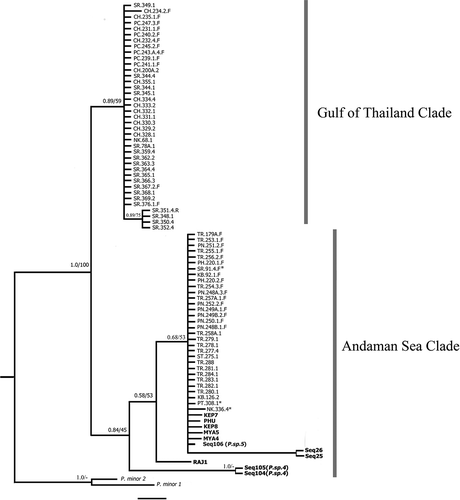
Fig. 6. Bayesian tree of Padina boryana: partial rbcL phylogeny (scale bar = 0.2 substitutions/site). Number above each node indicates Bayesian posterior probability followed by ML bootstrap percentages. GenBank sequences shown in bold text (Ni-Ni-Win et al., Citation2011; Silberfeld et al., Citation2013). The asterisk indicates the sample collected from the Gulf of Thailand grouped in the Andaman Sea clade. Padina minor was used as outgroup.
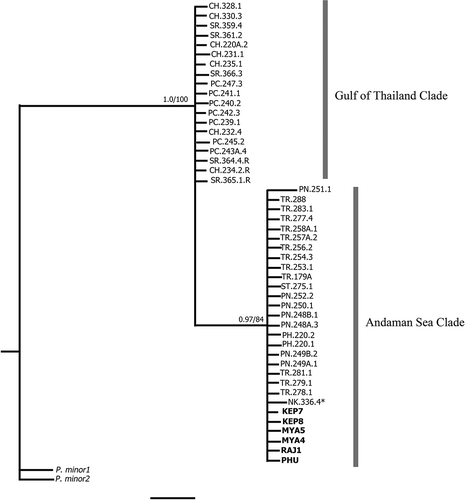
Fig. 7. Bayesian tree of Padina boryana: unrooted ITS2 phylogeny (scale bar = 0.002 substitutions/site). Number above each node indicates Bayesian posterior probability followed by ML bootstrap percentages. GenBank sequences shown in bold text (Ni-Ni-Win et al., Citation2011; Silberfeld et al., Citation2013). The asterisk indicates the sample collected from the Gulf of Thailand grouped in the Andaman Sea clade. Padina minor was used as outgroup.
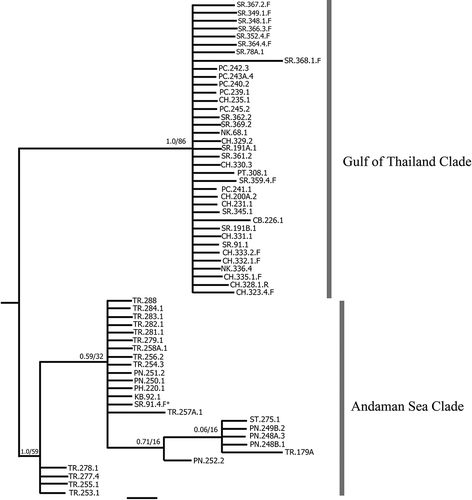
The cox3 phylogeny showed two diverse groups (). Most samples collected from the Gulf of Thailand formed a clade (with moderate support) with only three samples (SR91.4.F, NK336.4 and PT308.1) from the Gulf in the Andaman Sea clade (congruent with the haplotype network). All downloaded cox3 sequences from previous publications grouped with the Andaman Sea clade. Some sequences were divergent within this clade and these diverse clades showed low support. The samples from Tanzania and Kenya (Seq. 105 and Seq. 104) were a sister clade to the main group of Andaman Sea samples. This clade is called Padina sp. 4 in Silberfeld et al. (Citation2013). The next branch, sequence RAJ1, is from a sample collected in the Raja Ampat area close to West Papua in Indonesia. The main group of samples in the Andaman clade contained samples from Myanmar, Keplauan Seribu (Java Sea) in Indonesia, Phuket (Thai Andaman coast) and Sri Lanka plus our samples from the Andaman Sea. The sample from Sri Lanka was designated as Padina sp. 5 in Silberfeld et al. (Citation2013).
The phylogenetic tree of partial rbcL showed the same two groups (with strong to moderate support), which were from the Gulf of Thailand and the Andaman Sea (). Both clades are distinct from the closest sister species (P. minor Yamada). Available download sequences grouped in the Andaman Sea clade along with a sample from the Gulf of Thailand (NK.336.4). Even though the total number of sequences is lower, the relationships are congruent with the cox3 dataset.
An unrooted tree of the ITS2 dataset confirms the separation of P. boryana into two groups (). In the Gulf of Thailand clade, all sequences came from coastal Gulf of Thailand with no supported groupings within the clade. NK.336.4 and PT308.1 were also in this clade, whereas the mitochondrial and plastid markers placed it in the Andaman Sea clade. The Andaman Sea clade had more genetic variation.
Discussion
We used different markers to reveal genetic diversity within P. boryana along the Thai-Malay Peninsula, as different markers have different levels of variation (Hind & Saunders, Citation2013). For example, mitochondrial DNA and plastid DNA have been used to study populations, and for phylogenetic reconstruction, in the Fucales (Coyer et al., Citation2006; Engel et al., Citation2008; Dixon et al., Citation2012) and Dictyotales (De Clerck et al., Citation2006; Tronholm et al., Citation2010; Ni-Ni-Win et al., Citation2011; Silberfeld et al., Citation2013). The nuclear ITS region is also widely used in plants (Feliner & Rosselló, Citation2007) and marine organisms (Williams et al., Citation2002; Toews & Brelsford, Citation2012) for evolutionary studies and unravelling historical species dispersal, and is also useful in macroalgae (Lee et al., Citation1998; Vidal et al., Citation2003; Wang et al., Citation2008; Draisma et al., Citation2012) due to higher PCR amplification success rates and higher intraspecific variation (Schoch et al., Citation2012).
Our study using molecular markers from three genomes (mitochondrial, plastid and nuclear) found low, but significant, intraspecific variation for all markers (ITS2, up to 0.68%; cox3, up to 2.40%; partial rbcL, up to 0.85%) (Table S3). Padina boryana populations are maintained mostly by asexual tetraspore production (Wichachucherd et al., Citation2010). This could be a reason for the low variation found. Low levels of variation have been observed within species that have similar patterns of reproduction, for example, Sargassum polycystum (Chan et al., Citation2013) and Sargassum aquifolium (Chan et al., Citation2014). Most of the samples in this study fell into one of two groups (Gulf versus Andaman Sea) with all markers. However there were some samples that were in one group with cox3 and the other group with ITS2. This could be a case of ‘recent’ hybridization in a contact zone, as these samples were all found in the lower Gulf of Thailand populations in the area of Surat Thani (SR), Nakhon Si Thammarat (NK) and Pattani (PT). Similar incongruence between nuclear and organellar markers has been noted in several algal studies (Uwai et al., Citation2006; Andreakis et al., Citation2007; Neiva et al., Citation2010).
Our phylogenetic results using three genomic regions clearly indicated that P. boryana samples are found in two distinct lineages distributed in the Indo-Pacific Ocean. One lineage was restricted to the Gulf of Thailand. The second lineage or Andaman Sea clade showed a much wider distribution (from the western Indian Ocean to the southwest Pacific Ocean). Recently Silberfeld et al. (Citation2013) explored the biogeographic distribution of Padina species. They proposed two undescribed species sister to P. boryana (sp. 4 and sp. 5). Our results, based on more sampling, indicate that their samples are part of our Andaman Sea clade and do not support alternate species status. However, much more intensive sampling over a wider area (e.g. Malaysia and Vietnam), plus possibly other markers, is needed before conclusions on species status and distributional ranges (Provan & Bennett, Citation2008) can be made.
Physical oceanographic differences along the Thai coastlines could explain the pattern we see in the distribution of our two clades. Oceanic circulation has profound effects on population structure in addition to population connectivity. The circulation patterns in the Gulf of Thailand probably restrict connectivity between it and the Andaman Sea. The Gulf of Thailand is affected strongly by the South China Sea current during the Southwest monsoon season whereas the Andaman Sea faces the equatorial current coming from the west. Water in the Andaman Sea can flow through and mix with water masses north of Sumatra (Rizal et al., Citation2012; Yi-Neng et al., Citation2012) but not flow into the Gulf of Thailand because of the Malacca Strait barrier. Also, the occurrence of clockwise and counter-clockwise currents in the upper Gulf of Thailand (Buranapratheprat, Citation2008) will reduce connectivity to the open ocean. The different current directions restricting the water motion out of the Gulf may explain why samples from the Gulf of Thailand lineage are not found in the Andaman Sea and other areas. The effect of ocean currents on species and genotype distribution can be seen in other macroalgae, for example, Gelidium elegans (Kim et al., Citation2012), Carpophyllum maschalocarpum (Buchanan & Zuccarello, Citation2012), Sargassum polycystum (Chan et al., Citation2013) and Sargassum aquifolium (Chan et al., Citation2014). The currents in the Gulf of Thailand may affect genotype distribution of many organisms found on either side of the Thai-Malay Peninsula.
In conclusion, our results clearly show that along the east and west side of Thailand we have two divergent lineages identified as P. boryana. The ocean circulation patterns around the Thai-Malay peninsula may be responsible for the separation of these two lineages. This distinct phylogeographic pattern within the Gulf of Thailand will help in management of marine conservation in this area.
Supplementary information
The following supplemental material is available for this article, accessible via the Supplementary Content tab on the article’s online page at http://dx.doi.org/10.1080/09670262.2014.918658
Supplementary table S1. Sequences of Padina boryana and P. minor from previous studies cited here, showing GenBank Accession number and sampling locality.
Supplementary table S2. Haplotypes and ribotypes of Padina boryana deposited in GenBank.
Supplementary table S3. Distance matrices of p-distances between haplotypes with the three genetic markers (cox3, partial rbcL and ITS2).
Acknowledgements
We thank Eknarin Rodcharoen, Anuchit Darakrai, Pimonrat Thongroy, Prof. Dr Shigeo Kawaguchi, Dr Satoshi Shimada and Dr Olivier De Clerck who helped in providing samples or sequences. Thanks to Dr Warapond Wanna and her students for laboratory help. This work was supported by Faculty of Graduate School, Prince of Songkla University and Science Achievement Scholarship of Thailand, SAST.
References
- Andreakis, N., Procaccini, G., Maggs, C. & Kooistra, W.H.C.F. (2007). Phylogeography of the invasive seaweed Asparagopsis (Bonnemaisoniales, Rhodophyta) reveals cryptic diversity. Molecular Ecology, 16: 2285–2299.
- Briggs, J.C. (2000). Centrifugal speciation and centres of origin. Journal of Biogeography, 27: 1183–1188.
- Briggs, J.C. (2005). The marine East Indies: diversity and speciation. Journal of Biogeography, 32: 1517–1522.
- Buchanan, J. & Zuccarello, G.C. (2012). Decoupling of short- and long-distance dispersal pathways in the endemic New Zealand seaweed Carpophyllum maschalocarpum (Phaeophyceae, Fucales). Journal of Phycology, 48: 518–529.
- Buranapratheprat, A. (2008). Circulation in the upper Gulf of Thailand: a review. Burapha Science Journal, 13: 75–83.
- Buranapratheprat, A., Yanagi, T. & Sawangwong, P. (2002). Seasonal variations in circulation and salinity distributions in the upper Gulf of Thailand: modeling approach. La Mer, 40: 147–155.
- Carpenter, K.E., Barber, P.H., Crandall, E.D., Ablan-Lagman, M.C.A., Ambariyanto, Mahardika, G.N., Manjaji-Matsumoto, B.M., Juinio-Meñez, M.A., Santos, M.D., Starger, C.J., & Toha, A.H.A. (2011). Comparative phylogeography of the coral triangle and implications for marine management. Journal of Marine Biology, 2001. doi:10.1155/2011/396982
- Chan, S.W., Cheang, C.C., Chirapart, A., Gerun, G., Tharith, C. & Ang, P. (2013). Homogeneous population of the brown alga Sargassum polycystum in Southeast Asia: possible role of recent expansion and asexual propagation. PLoS One, 8 (10): e77662. doi:10.1371/journal.pone.0077662.
- Chan, S.W., Cheang, C.C., Yeung, C.W., Chirapart, A., Gerung, G. & Ang, P. (2014). Recent expansion led to the lack of genetic structure of Sargassum aquifolium populations in Southeast Asia. Marine Biology, 161: 785–795.
- Clement, M., Posada, D. & Crandall, K.A. (2000). TCS: a computer program to estimate gene genealogies. Molecular Ecology, 9: 1657–1659.
- Clifton, K.E. (1997). Mass spawning by green algae on coral reefs. Science, 275: 1116–1118.
- Coyer, J.A., Hoarau, G., Oudot-Le Secq, M.P., Stam, W.T. & Olsen, J.L. (2006). A mtDNA-based phylogeny of the brown algal genus Fucus (Heterokontophyta; Phaeophyta). Molecular Phylogenetics and Evolution, 39: 209–222.
- de Bruyn, M., Nugroho, E., Hossain, Md. M., Wilson, J.C. & Mather, P.B. (2005). Phylogeographic evidence for the existence of an ancient biogeographic barrier: the Isthmus of Kra Seaway. Heredity, 94: 370–378.
- De Clerck, O., Leliaert, F., Verbruggen, H., Lane, C.E., De Paula, J.C., Payo, D.A. & Coppejans, E. (2006). A revised classification of the Dictyoteae (Dictyotales, Phaeophyceae) based on rbcL and 26S ribosomal DNA sequence analysis. Journal of Phycology, 42: 1271–1288.
- Dixon, R.R.M., Huisman, J.M., Buchanan, J., Gurgel, C.F.D. & Spencer, P. (2012). A morphological and molecular study of austral Sargassum (Fucales, Phaeophyceae) supports the recognition of Phyllotricha at genus level, with further additions to the genus Sargassopsis. Journal of Phycology, 48: 1119–1129.
- Draisma, S.G.A., Eurlings, M.C.M. & Lim, P.E. (2012). High intra-individual sequence variation in the nuclear rDNA LSU-5s intergenic spacer in the Sargassaceae (Fucales, Phaeophyceae). Journal of Applied Phycology, 24: 1373–1379.
- Engel, C., Billard, E., Voisin, M. & Viard, F. (2008). Conservation and polymorphism of mitochondrial intergenic sequences in brown algae (Phaeophycae). European Journal of Phycology, 43: 195–205
- Fagerberg, W.R. & Dawes, C.J. (1973). An electron microscopic study of the sporophytic and gametophytic plants of Padina vickersiae Hoyt. Journal of Phycology, 9: 199–201.
- Feliner, G.N. & Rosselló, J.A. (2007). Better the devil you know? Guidelines for insightful utilization of nrDNA ITS in species-level evolutionary studies in plants. Molecular Phylogenetics and Evolution, 44: 911–919.
- Felsenstein, J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution, 39: 783–791.
- Guindon, S. & Gascuel, O. (2003). A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Systematic Biology, 52: 696–704.
- Guindon, S., Dufayard, J.F., Lefort, V., Anisimova, M., Hordijk, W. & Gascuel, O. (2010). New algorithms and methods to estimate Maximum-Likelihood phylogenies: assessing the performance of PhyML3.0. Systematic Biology, 59: 307–321.
- Hind, K.R. & Saunders, G.W. (2013). Molecular markers from three organellar genomes unravel complex taxonomic relationships within the coralline algal genus Chiharaea (Corallinales, Rhodophyta). Molecular Phylogenetics and Evolution, 67: 529–540.
- Kim, K.M., Guillaume, G.G. & Boo, S.M. (2012). Genetic structure and distribution of Gelidium elegans (Gelidiales, Rhodophyta) in Korea based on mitochondrial cox1 sequence data. Aquatic Botany, 98: 27–33.
- Knittweis, L., Kraemer, W.E., Timm, J. & Kochzius, M. (2009). Genetic structure of Heliofungia actiniformis (Scleractinia: Fungiidae) populations in the Indo-Malay Archipelago: implications for live coral trade management efforts. Conservation Genetics, 10: 241–224.
- Lee Jae, W., Yoon, H.S. & Boo, S.M. (1998). Phylogenetic relationships of Pelvetia and Pelvetiopsis (Phaeophyceae) based on small subunit ribosomal DNA sequences. Journal of Plant Biology, 41: 103–109.
- Lewis, J.E. & Norris, J.N. (1987). A History and Annotated Account of the Benthic Marine Algae of Taiwan. Smithsonian Contributions to the Marine Sciences 29, 11–12. Smithsonian Institution Press, Washington, DC.
- Liao, P.C., Chiang, Y.C., Huang, S. & Wang, J.C. (2009). Gene flow of Ceriops tagal (Rhizophoraceae) across the Kra Isthmus in the Thai Malay Peninsula. Botanical Studies, 50: 193–204.
- Liddle, L.B. (1975). The effect of intertidal stress on Padina sanctae-crucis (Phaeophyta). Journal of Phycology, 11: 327–330.
- Lüning, K. (1990). Seaweeds: Their Environment, Biogeography and Ecophysiology. Wiley-Interscience Publication, New York, NY.
- Maggs, C.A. & Callow, M.E. (2002). Algal spores. In Encyclopedia of Life Sciences, 1–6., Macmillan Nature Publishing Group, London.
- Mirams, A.G.K., Treml, E.A., Shields, J.L., Liggins, L. & Riginos, C. (2011). Vicariance and dispersal across an intermittent barrier: population genetic structure of marine animals across the Torres Strait land bridge. Coral Reefs, 30: 937–949.
- Neiva, J., Pearson, G.A., Valero, M. & Serrao, E.A. (2010). Surfing the wave on a borrowed board: range expansion and spread of introgressed organellar genomes in the seaweed Fucus ceranoides L. Molecular Ecology, 19: 4812–4822.
- Ni-Ni-Win Hanyuda, T., Arai, S., Uchimura, M., Abbott, I.A. & Kawai, H. (2008). Three new records of Padina in Japan based on morphological and molecular markers. Phycological Research, 56: 288–300.
- Ni-Ni-Win Hanyuda, T., Arai, S., Uchimura,M., Draisma, S.G.A., Phang, S.M., Abbott, I.A., Millar, A.J.K. & Kawai, H. (2011). A taxonomic study of the genus Padina (Dictyotales, Phaeophyceae) including the descriptions of four new species from Japan, Hawaii and the Andaman Sea. Journal of Phycology, 47: 1193–1209.
- Obst, M., Faurby, S., Bussarawit, S. & Funch, P. (2012). Molecular phylogeny of extant horseshoe crabs (Xiphosura, Limulidae) indicates Paleogene diversification of Asian species. Molecular Phylogenetics and Evolution, 62: 21–26.
- Pornpinatepong, S. (2005). Tidal circulation in Andaman Sea. Songklanakarin Journal of Science Technology, 27: 425–431.
- Posada, D. (2008). jModelTest: phylogenetic model averaging. Molecular Biology and Evolution, 25: 1253–1256.
- Provan, J. & Bennett, K.D. (2008). Phylogeographic insights into cryptic glacial refugia. Trends in Ecology and Evolution, 23: 564–571.
- Reaka, M.L., Rodgers, P.J. & Kudla, A.U. (2008). Speciation extinction dynamics and the topography of diversity on Indo-West Pacific coral reefs. Proceedings of the 11th International Coral Reef Symposium, Florida, 26: 1377–1381.
- Rizal, S., Damm, P., Wahid, M.A., Sündermann, J., Ilhamsyah, Y., Iskandar, T. & Muhammad (2012). General circulation in the Malacca Strait and Andaman Sea: a numerical model study. American Journal of Environmental Science, 8: 479–488.
- Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D.L., Darling, A., Hohnas, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model selection across a large model space. Systematic Biology, 61: 539–542.
- Schoch, C.L., Seifert, K.A., Huhndorf, S., Robert, V., Spouge, J.L., André Levesque, C., Chen, W. & Fungal Barcoding Consortium (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. PNAS, 109: 6241–6246.
- Silberfeld, T., Bittner, L., Fernandez-Garcıa, C., Cruaud, C., Rousseau, F., De Reviers, B., Leliaert, F., Payri, C.E. & De Clerck, O. (2013). Species diversity, phylogeny and large scale biogeographic patterns of the genus Padina (Phaeophyceae, Dictyotales). Journal of Phycology, 49: 130–142.
- Timm, J. & Kochzius, M. (2008). Geological history and oceanography of the Indo-Malay Archipelago shape the genetic population structure in the false clown anemone fish (Amphiprion ocellaris). Molecular Ecology, 17: 3999–4014.
- Toews, D.P.L. & Brelsford, A. (2012). The biogeography of mitochondrial and nuclear discordance in animals. Molecular Ecology, 21: 3907–3930.
- Tronholm, A., Steen, F., Tyberghein, L., Leliaert, F., Verbruggen, H., Siguan, M.A.R. & De Clerck, O. (2010). Species delimitation, taxonomy, and biogeography of Dictyota in Europe (Dictyotales, Phaeophyceae). Journal of Phycology, 46: 1301–1321.
- Uwai, S., Yotsukura, N., Serisawa, Y., Muraoka, D., Hiraoka, M. & Kogame, K. (2006). Intraspecific genetic diversity of Undaria pinnatifida in Japan, based on the mitochondrial cox3 gene and the ITS1 of nrDNA. Hydrobiologia, 553: 345–356.
- Vidal, R., Meneses, I. & Smith, M. (2003). Molecular genetic identification of crustose representatives of the order Corallinales (Rhodophyta) in Chile. Molecular Phylogenetics and Evolution, 28: 404–419.
- Wang, X., Zhao, F., Hu, Z., Critchley, A.T., Morrell, S.L. & Duan, D. (2008). Inter-simple sequence repeat (ISSR) analysis of genetic variation of Chondrus crispus populations from North Atlantic. Aquatic Botany, 88: 154–159.
- White, T.J., Bruns T., Lee, S. & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications (Innis, M.A., Gelfand, D.H., Sninsky, J.J. & White, T.J., editors), 315–322. Academic Press, New York, NY.
- Wichachucherd, B., Liddle, L.B. & Prathep, A. (2010). Population structure, recruitment, and succession of the brown alga, Padina boryana Thivy (Dictyotales, Heterokontophyta), at an exposed shore of Sirinart National Park and a sheltered area of Tang Khen Bay, Phuket Province, Thailand. Aquatic Botany, 92: 93–98.
- Williams, S.T., Gomez, J.J.E. & Knowlton, N. (2002). The marine Indo-West Pacific break: contrasting the resolving power of mitochondrial and nuclear genes. Integrative and Comparative Biology, 42: 941–952.
- Yasuda, N., Nagai, S., Hamaguchi, M., Okaji, K., Gérard, K. and Nadaoka, K. (2009). Gene flow of Acanthaster planci (L.) in relation to ocean currents revealed by microsatellite analysis. Molecular Ecology, 18: 1574–1590.
- Yi-Neng, L., Shi-Qiu, P. & Xue-Zhi, Z. (2012). Observations and simulations of the circulation and mixing around the Andaman-Nicobar submarine ridge. Atmospheric and Oceanic Science Letters, 5: 319–323.
- Zuccarello, G.C. & Lokhorst, G.M. (2005). Molecular phylogeny of the genus Tribonema (Xanthophyceae) using rbcL gene sequence data: monophyly of morphologically simple algal species. Phycologia, 44: 384–392.
