Abstract
This study investigates the spectral shifting of UV-A radiation, using fluorescent material, as a tool for enhancing Chlorella sp. growth rate in simulated photobioreactors made of UV-stabilized polycarbonate (PC) and poly (methyl methacrylate) (PMMA). The feasibility of using a fluorescent coating as a wavelength shifter layer, to shift UV-A radiation to the PAR range, was explored. For this purpose, a variety of concentrations of fluorescent dye dissolved in thermoplastic acrylic resin were prepared and used to coat PMMA and PC sheets which then were placed between the radiation source and the culture flask. Compared with the uncoated sheets, the panels coated with the wavelength shifter layer exhibited 74% and 45% (for PC and PMMA substrates, respectively) increase in biomass productivity during the same culture period. It was also found that the elimination of UV-A radiation increased chlorophyll a content of the cells.
Introduction
Microalgae are unicellular photosynthetic microorganisms that use sunlight to synthesize organic materials. These materials can be a source of biofuels, foods, feeds and high-value bioactives. Indeed, these photosynthetic microorganisms are useful in bioremediation applications, such as nitrogen-fixing biofertilizers and carbon dioxide biofixation (Katsuda et al., Citation2006; Chisti, Citation2007; Ranjbar et al., Citation2008; Karatay & Dönmez, Citation2011; Borowitzka, Citation2013).
In photoautotrophic cultivation, photons are energy sources for growth. The properties of the light source, such as spectral power distribution and intensity, are critical for the growth of photoautotrophic microalgae. Thus, the specific growth rate, photosynthetic pigment content and biochemical composition (e.g. lipid content), could be influenced by the light source characteristics (Prokop et al., Citation1984; Chrismadha & Borowitzka, Citation1994; Matthijs et al., Citation1996; Hirata et al., Citation1998; Danesi et al., Citation2004). Many researchers have investigated the effect of wavelength on the growth of algae and cyanobacteria (Matthijs et al., Citation1996; Katsuda et al., Citation2006, Citation2008; Wang et al., Citation2010). Ideally, the wavelength of the incident light should be matched with the pigment spectral absorption curve of the individual microorganism, to maximize the amount of light utilized for photosynthetic processes. In the case of chlorophyll, absorption occurs in the blue and red regions of the visible spectra (Lee & Palsson, Citation1994; Kohen et al., Citation1995; Matthijs et al., Citation1996).
The energy of sunlight can be utilized as a source for carbon dioxide reduction by photosynthetic microorganisms. Although the spectrum of the sun at sea level includes wavelengths from 295 nm upwards, only the band between 400–700 (nm) represents photosynthetically active radiation (PAR) (Kohen et al., Citation1995); therefore, most of the radiation from the sun remains unused. In fact, considering the actual photochemical efficiency, all of the near-UV (290–400 nm) and IR, and part of the visible radiation spectrum, are not photosynthetically active (Kohen et al., Citation1995). The UV radiation spectrum ranges from 100 to 400 nm and is divided into UV-A (315–400 nm), UV-B (280–315 nm) and UV-C (<280 nm) (Sinha et al., Citation1998; Estevez et al., Citation2001; Holzinger & Lütz, Citation2006; Navntoft et al., Citation2009). Approximately 90–95% of the near-UV photons in sunlight are in the UV-A range while the remainder is composed of UV-B radiation. Both UV-A and UV-B have been shown to elicit physiological responses in a variety of organisms (Castenholz, Citation1997; Turcsányi & Vass, Citation2000; White & Jahnke, Citation2002). It has been demonstrated that exposing photosynthetic organisms to UV radiation may result in direct photosynthetic damage and cause photoinhibition (Helbling et al., Citation1992; Herrmann et al., Citation1997). Therefore, spectral conversion or spectral shifting of UV not only reduces damage to the cells but it may also lead to a higher number of photons being in the PAR region, which would increase the biomass productivity of microalgae.
Spectral conversion has been used to increase the efficiency of solar energy conversion processes in silicon solar cells since the early 1970s. Materials used for this purpose must exhibit: (i) a high quantum efficiency; (ii) a wide absorption band in the region where efficiency of the cell is low; (iii) a high absorption coefficient; (iv) a narrow emission band, coinciding with the peak of the maximum photovoltaic efficiency of the cell; (v) good separation between the absorption and emission bands in order to minimize losses due to re-absorption; and (vi) low cost and prolonged photo-stability (Hovel et al., Citation1979; Van Sark et al., Citation2005; Sloof et al., Citation2006; Strümpel et al., Citation2007; Van Sark, Citation2008; Klampaftis et al., Citation2009). These characteristics also seem to be of interest in wavelength-shifting strategies for photosynthetic applications. Wondraczek et al. (Citation2013) used a Sr0.4Ca0.59 Eu0.01S spectral converter in a flat panel reactor filled with Haematococcus pluvialis. The converter was placed with a mirror on the rear side of a reactor, to convert green light in the solar spectrum passing through the broth to red and reflect the red light back into the broth. An increase in cell generation of around 36% was observed compared with the flat plate reactor without modification. Xia et al. (Citation2013) used Ca0.4Sr0.6S:Eu2+ as the converter material on a backlight converter foil to increase photosynthetic activity in Spinacia oleracea and observed more than 25% increase in CO2 assimilation rate. Furthermore, Mohsenpour & Willoughby (Citation2013) used different Lumogen F dyes in the wall of a column photobioreactor to improve Chlorella vulgaris growth under Xenon arc lamp radiation. They observed up to ~20% increase in the biomass productivity and ~50% increase in maximum specific growth. Also, Delavari Amrei et al. (Citation2013) used the fluorescent dye Uvitex OB for converting UV-A to blue in 50 ml culture flasks filled with Synechococcus sp. They put coated polycarbonate sheets with different concentrations of the dye in front of each flask and experimentally observed up to 38% increase in the biomass productivity under UV-A lamp radiation.
The main materials used in photobioreactor walls are polycarbonate (PC), Poly (methyl methacrylate) (PMMA), PVC and glass; in this study the first two materials were chosen to investigate the effect of applying a wavelength-shifter layer containing a fluorescent dye on shifting the UV-A radiation to PAR. The effect on the specific growth rate and the biomass productivity of Chlorella sp., as a model microalga, was studied.
Methods
Microorganism
The microorganism Chlorella sp. (PTCC 6010, Persian Type Culture Collection, Tehran, Iran) was used to test the efficiency of the UV-A to PAR shifts. This microorganism was provided by the Iranian Research Organization for Science and Technology and pre-cultivated in rudic medium (pH ~8) (Imamoglu et al., Citation2010).
Culture media
After pre-cultivation of Chlorella sp. in 250 ml flasks (100 ml working volume) under cool white fluorescent lighting at approximately 25 μmol photons m−2 s−1, the broth (OD560≈2) was inoculated into a 2000 ml flask containing 900 ml standard inorganic medium. This medium contained per litre: NaNO3 (300 mg), KH2PO4 (20 mg), K2HPO4 (80 mg), NaCl (20 mg), CaCl2 (47 mg), MgSO47H2O (10 mg), ZnSO4.7H2O (0.1 mg), MnSO4.H2O (1.5 mg), CuSO4.5H2O (0.08 mg), H3BO3 (0.3 mg), (NH4)6Mo7O24.4H2O (0.3 mg), FeCl3.6H2O (17 mg), Co(NO3)2.H2O (0.2 mg) and EDTA (7.5 mg). Also, 33 g l−1 sea salt was added to the culture medium.
UV-A source
A black light blue lamp (Philips TL 8W/08, the Netherlands) was used as the UV-A source. This lamp showed a peak emission at ~365 nm with half bandwidth of 20 nm (see for full details).
Table 1. Properties of black light blue lamp.
Wavelength-shifter sheets
Solutions of different concentrations of the fluorescent dye Uvitex OB (BASF, Germany) in a thermoplastic acrylic resin (as a host material) were applied to PC and PMMA sheets with an air spray technique at 3.5 bars using an air spray gun (Iwata W101, Japan). The coatings were then dried after a short flash-off time at 60°C for 5 min.
Experimental set-up
After cultivation in 2000 ml flasks, 240 ml of the broth was distributed equally amongst six 40 ml tissue culture flasks (JET BIOFIL Vent Cap, China). The flasks were prepared by covering with aluminium foil, to reflect light, except for the side facing the UV-A source (). Coated and uncoated PC and PMMA sheets were placed between the open side of each flask and the UV-A source. Finally, the whole setup was placed in a black enclosure at 30°C for 16 days (see for details).
Fig. 1. Schematic diagram of experiment. 1, Controlled temperature black enclosure. 2, Culture flasks. 3, UV-A source. 4, Vent cap. 5, Wavelength-shifter sheet. 6, Visible light. 7, UV-A radiation. The bottom left panel is a cross section of the shifter layer and shows different light passes.
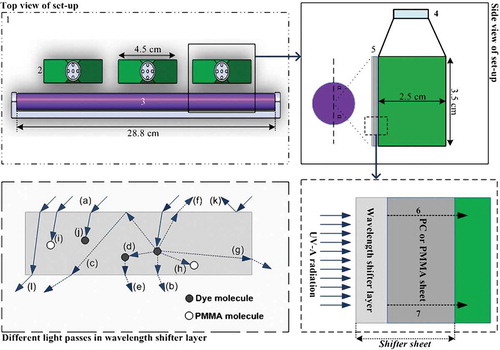
Table 2. Sample codes for test conditions of algal cultures and optical density of shifter layers.
The wavelength-shifter layer is assumed to be a homogeneous medium, consisting of PMMA and the fluorescent dye. In this medium a photon can experience different events. As can be seen in (bottom left panel), the incident photons (a) are absorbed and re-emitted at a wavelength longer than that of the absorption band of the wavelength-shifter layer. The majority of the shifted photons will leave the shifter layer, directly (b), or following internal reflection at air–shifter layer interface (c), or via re-absorption (d) and re-emission by dye molecules (e). A fraction of the shifted photons will leave through the top of the shifter layer (f) or through its sides (g); a small amount will be absorbed by the host material (h). Also, some of the incident photons could be absorbed completely by the host material (i), or dye molecules (j). A small amount of the incident photons can also be reflected (k) by the surface of the shifter layer, or unabsorbed (l) (Rowan et al., Citation2008; Klampaftis et al., Citation2009). In the case of air and PMMA (refractive index of 1.5), photons that escape from the top of the shifter layer have been quantified in the range of ~ 12.5% (Klampaftis et al., Citation2009). The losses from the sides of the large thin layer are usually not included in the calculation and estimation of overall losses (Klampaftis et al., Citation2009). Furthermore, based on the absorption curve of PMMA in , the number of emitted photons absorbed by the transparent host material is negligible. Thus, about 87% of emitted photons reach the broth.
Measurement of growth
The optical density (OD) of the broth was determined by measuring the absorbance of the broth at 560 nm in a double beam UV/Vis spectrophotometer (V-550JASCO, Japan) with cell path length of 1 cm. To measure biomass dry weight, a 10 ml sample of algal suspension was filtered through a pre-dried and pre-weighed 47 mm Whatman paper filter (GF/F, nominal pore size 0.7 μm), and washed twice with 20 ml of distilled water. The filter was dried at 105°C overnight then placed in a desiccator and weighed to the nearest 0.1 mg.
The relationship between the biomass concentration (X, mg l−1) or dry weight and optical density (OD560) is obtained as follows:
The biomass productivity (P, mg l−1 day−1) is estimated by Eq. (3):
Cell examination and measurement were carried out with an improved Neubauer haemocytometer (Brand, Wertheim, Germany) and an optical microscope (BIOVIDEO BEL, Italy) at 1000× magnification.
To determine the concentration and absorption spectra of pigments, cells collected in 2 ml centrifuge tubes were disrupted in an ultrasonic bath (LBS1, FALC, Italy) for 45 min. The pigments were extracted with 2 ml acetone overnight in an ice bath, then the mixture was centrifuged at 2500 × g for 10 min to sediment the cell debris. The supernatant was used for the determination of the absorption curve of the pigments using a UV/Vis spectrophotometer (Li et al., Citation2008). Finally, the content of chlorophyll a and b was calculated according to following equations:
Absorption and emission spectra
Absorption spectra of the shifter sheets were determined using the double beam UV-Vis spectrophotometer with air as reference. Also, absorption spectra of shifter layers for different samples were obtained by using uncoated PC sheet as reference. Using a high resolution spectrometer (HR4000 Ocean Optics, USA), the emission spectra of the shifter layer and output spectrum of the light source were obtained.
Visible light and UV-A intensity were measured with a light meter (TES-1330A, Taiwan) and UV light meter (UV-340, Lutron, Taiwan), respectively. TES-1330A was used at the pre-cultivation stage to measure light intensity. The bulb-sample distance was set so that the intensity of the light at the surface of the cultivation flask was 2000 lux. This corresponds to a photon flux rate of about 25 μmol photons m−2 s−1 for the cool white fluorescent bulb used in the pre-cultivation stage. A linear relationship was found between the intensity of the radiation, measured by the lux meter or the UV-A meter, and the area under the spectral distribution curve obtained by the spectrometer. This linear relationship was used to correlate the value of the area under the spectral distribution curve with the energy that reaches the surface of the cultivation flask for different wavelength shifter samples.
Results
Absorption, emission and transmittance spectra
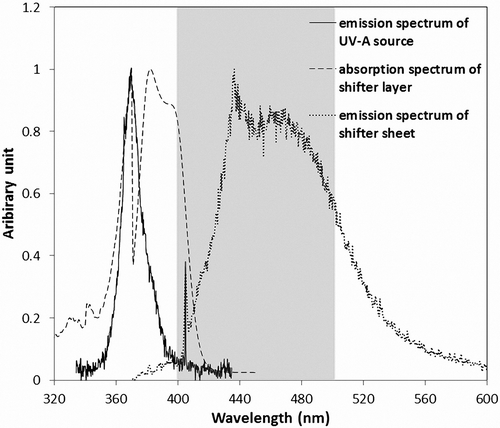
As presented in , the emission spectrum of the UV-A source reached its peak at 365 nm. The shifter layer shows two different absorption peaks, at 370 nm and about 380 nm. Emission spectra of the coated sheets exhibit two peaks at 435 nm and 465 nm. The former is close to the absorbance maximum of chlorophyll a at 430 nm (Kohen et al., Citation1995). It must be noted that chlorophyll a is the major light-harvesting pigment. On the other hand, both peaks of emission spectrum correspond to the absorption band of chlorophyll+carotenoids (Kohen et al., Citation1995; Solovchenko, Citation2010).
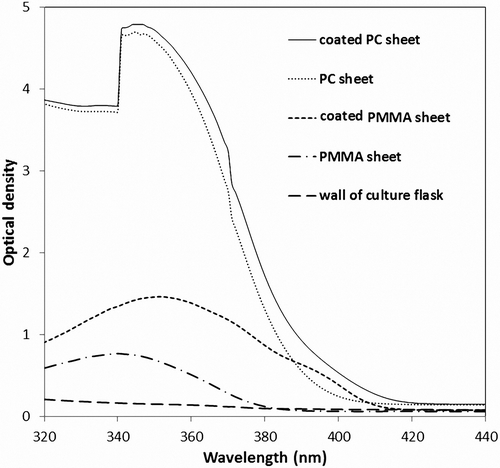
Photobioreactors are usually made of PC or PMMA plates or tubes. In order to obtain the highest photostability, PC is stabilized using UV absorbing additives. In PC plates, the light emerging at the broth contains a very low amount of UV-A radiation. However, for PMMA plates, most of the UV-A radiation is transmitted through the plate to the broth. After coating plates with fluorescent material, some of the UV-A radiation is converted to visible light and the transmittance of UV-A radiation through coated plates is less than that of uncoated plates. Therefore, it is rational to compare the effect of the coated and uncoated sheets to show how the wavelength-shifting strategy will influence specific growth rate and biomass productivity.
The uncoated PC sheet shows substantial absorption of UV-A wavelengths (), minimizing the transmission of the UV-A radiation through the sheet. In the coated PC sheet, due to the fluorescent characteristics of the wavelength-shifter layer, some of the UV-A radiation was converted to visible light (400–600 nm) when hitting this layer and most of the remaining UV-A radiation was absorbed by the polycarbonate itself. However in the uncoated PMMA sheets, most of the UV-A radiation was transmitted. In the case of the coated PMMA sheets, some UV-A radiation was absorbed by the shifter layer and converted to visible light whilst the remaining UV-A passed through the PMMA sheet and reached the broth. The absorption spectrum of the wall of the culture flask shows little absorption of light (), therefore its effect on extinction of UV-A radiation was ignored.
The spectral power distributions of the light reaching each sample are presented in and . Only samples PMMA-0 and PMMA-0.1 were exposed to UV-A radiation; in the other samples UV-A was absorbed by the shifter sheets (). Emission spectra of different sheets are presented in ; there are three peaks for each sample, two of which (435 and ~465 nm) are caused by emission spectra of Uvitex OB and the third corresponds to the UV-A source. As can be seen in , the energy of visible light that reaches samples PMMA-0.4 and PC-0.4 is greater than for the other samples.
Fig. 4. UV-A spectra after the light has passed through the shifter sheets before falling onto the broth.
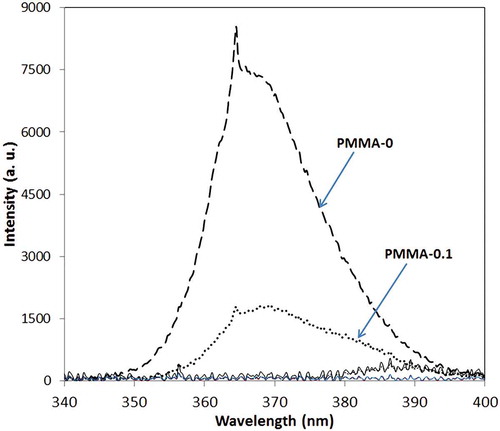
Fig. 5. Emission spectra of light after passing through shifter sheets and before falling onto the broth. a.u., arbitrary units.
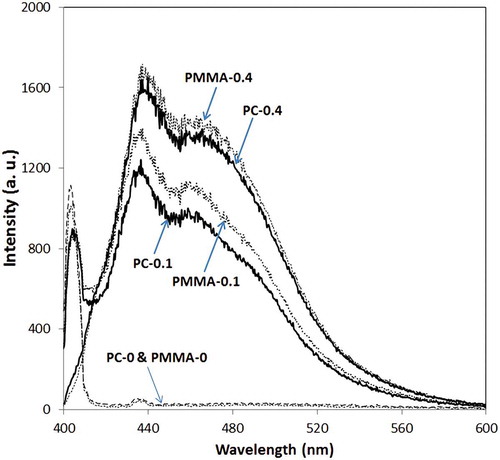
The energy reaching the broth of each culture flask, calculated from the surface area under the corresponding curve in and , is presented in ; surface area and energy have a linear relationship (R2 = 0.98). The calculations show that the amount of energy reaching the broth of sample PMMA-0 was higher than other samples and that the energy reaching sample PC-0 is very low. The percentage intensities of UV-A and visible light are also presented in . Considering OD at 380 nm for the shifter layer (), it is concluded that by increasing OD of the shifter layer or the concentration of optical brightener in the shifter layer, higher intensities of visible light and lower intensities of UV-A are achieved (). Therefore, a higher concentration of Uvitex OB in the shifter layer is able to convert more UV-A to visible light.
Table 3. Total energy and percentage of UV-A and visible light. a.u. = arbitrary units.
Biomass and pigment production
Because of the different wavelengths and energy intensities reaching the broth of each sample, different productivities and maximum specific growth rates were observed. The time course for cell growth of samples is illustrated in , and reports the growth parameter of Chlorella sp. in different conditions. Sample PMMA-0 had the highest maximum specific growth rate; however, because of the high UV-A intensity it did not have the highest biomass productivity. The overall growth pattern of the samples shows that in PMMA-0 and PMMA-0.1 the high dosage of UV-A radiation passing through the sheet resulted in photoinhibition (). As the amount of UV-A reaching the sample broth is increased, the speed at which photoinhibition occurs increases.
Fig. 6. Time course of cell density (measured as optical density at 560 nm) of cultures grown under different spectral conditions.
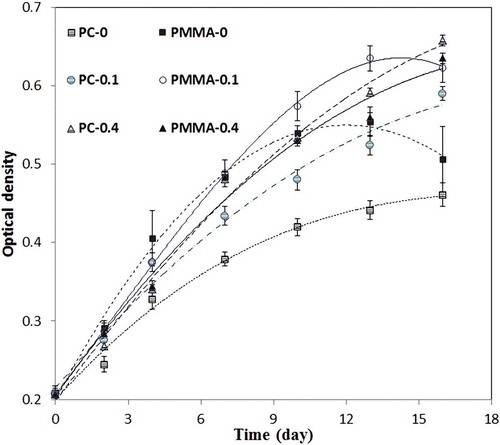
Table 4. Growth parameters of different samples. µmax = Maximum specific growth rate and P = Biomass productivity.
Samples PMMA-0.1 and PMMA-0.4 showed a high maximum specific growth rate of 0.16 d−1. The total energy reaching the broth of these samples was greater than for the others (), except for PMMA-0. For sample PC-0, because of the lower energy, the maximum specific growth rate was lower than for the others. Moreover, although the energy reaching the sample PC-0.4 broth is lower than for the PMMA samples, this sample had the highest biomass productivity. This is because very low UV-A intensity reached the broth of PC-0.4 and growth has been boosted by the total amount of visible light delivered to the broth. In other words, converting the UV-A radiation passing through the walls of a photobioreactor into visible light with a shifter layer increases biomass productivity. Compared with the uncoated sheets, the panels coated with the wavelength-shifter layer increased microalgal biomass productivity by 74% and 45% (for PC and PMMA substrates, respectively) during the same culture period.
Also, the chlorophyll a content of the cells in samples PC-0.1 and PC-0.4 was higher than the others () suggesting that reduction of UV-A radiation enhanced chlorophyll a content. Comparing cell sizes of samples grown under mixed UV-A and visible light, it was found that cell size remained around 2.2–2.4 µm, the same as on the first day of cultivation. However in samples receiving only 400–600 nm wavelengths, cell size increased up to 25% compared with the first day of cultivation, reaching a maximum size of 2.7–2.9 µm for the sample PC-0.4.
Discussion
A strategy to shift wavelengths from UV-A to the blue region was investigated for improving the growth rate of microalgae. Using Chlorella sp. as a model in flasks with coated PC and PMMA panels, increases in the biomass productivity of 74% and 45%, compared with flasks with uncoated PC and PMMA panels, respectively, were achieved. Despite the energy intensity reaching the broth of flasks being lower than that of uncoated flasks, biomass productivity in coated flasks was higher. In other words, converting the UV-A radiation reaching the walls of the photobioreactor into visible light with a shifter layer increased biomass productivity. Uvitex OB was used as the fluorescent dye to shift UV-A photons to PAR as the peaks of the emission spectrum of the dye correspond to the absorption band of chlorophyll+carotenoids (Kohen et al., Citation1995; Solovchenko, Citation2010). Furthermore, absorption and emission spectra of the dye overlapped within a very short range (~400–420 nm) () which can cause the re-absorption and energy loss to be minimized.
In our previous study (Delavari Amrei et al., Citation2013) it was demonstrated that using Uvitex OB on the wall of a 50 ml flask increased the productivity of Synechococcus sp. cyanobacteria by about 38%. This value was 50% lower than that of the Chlorella sp. in this work and shows that shifting UV-A to blue is more efficient for the growth of microalgae than cyanobacteria, due to differences in pigment composition.
Considering the polynomial interpolation of the data for a variety of crop plants in McCree (Citation1972), the average quantum efficiency in photosynthesis for blue and red light is ~0.67 and ~0.82, respectively. Therefore, it is possible that shifting of UV-A to the red region, in comparison to the blue region, would cause a greater increase in biomass production. Regardless of the shifting strategy, more work is needed, since fluorescent materials which are used for this purpose should be characterized for their quantum efficiency, photostability and filtration of PAR.
Since the fluorescent material used in this study is only excited by UV radiation and is transparent to PAR, the air spray technique and drying conditions of the shifter layer require care to prevent any translucency or opacity in the PAR range. Otherwise, the non-transparent sheet will filter PAR and reduce efficiency of the wavelength-shifting technique. Also, since both intensity and spectral distribution of the light have direct impacts on the biochemical composition of microalgal cells, including lipids and the metabolism of fatty acid synthesis (Sukenik, Citation1991; Chrismadha & Borowitzka, Citation1994), it is suggested that the biochemical composition of microorganisms should also be considered when choosing a suitable wavelength-shifting strategy.
In conclusion, converting the UV-A radiation reaching the surface of photobioreactor walls into visible light, using an integrated wavelength-shifting strategy, increases biomass productivity. This can be used to improve the quality and optimize the quantity of the light reaching the algal broth. Optical properties of the photobioreactor wall are important. Based on the growth parameters, eliminating UV-A wavelengths from the emerging light is a promising strategy in the process of producing chlorophyll a from microalgae.
Acknowledgements
We would like to thank Dr Madanipour and Mr Hosseini from the Optical Measurement Central Laboratory of the Optics, Laser & Photonics Institute of Amirkabir University of Technology for assisting us with light-measuring devices and methods. Also, the effort of Ms Nejadebrahim in making the wavelength-shifter sheets is gratefully acknowledged.
References
- Borowitzka, M. (2013). High-value products from microalgae – their development and commercialisation. Journal of Applied Phycology, 25: 743–756.
- Castenholz, R.W. (1997). Multiple strategies for UV tolerance in cyanobacteria. The Spectrum, 10: 10–16.
- Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25: 294–306.
- Chrismadha, T. & Borowitzka, M. (1994). Effect of cell density and irradiance on growth, proximate composition and eicosapentaenoic acid production of Phaeodactylum tricornutum grown in a tubular photobioreactor. Journal of Applied Phycology, 6: 67–74.
- Danesi, E.D.G., Rangel-Yagui, C.O., Carvalho, J.C.M. & Sato, S. (2004). Effect of reducing the light intensity on the growth and production of chlorophyll by Spirulina platensis. Biomass and Bioenergy, 26: 329–335.
- Delavari Amrei, H., Nasernejad, B., Ranjbar, R. and Rastegar, S. (2013). Spectral shifting of UV-A wavelengths to blue light for enhancing growth rate of cyanobacteria. Journal of Applied Phycology, doi:10.1007/s10811-013-0187-0.
- Estevez, M.S., Malanga, G. & Puntarulo, S. (2001). UV-B effects on Antarctic Chlorella sp cells. Journal of Photochemistry and Photobiology B, 62: 19–25.
- Helbling, E.W., Villafane, V., Ferrario, M. & Holm-Hansen, O. (1992). Impact of natural ultraviolet radiation on rates of photosynthesis and on specific marine phytoplankton species. Marine Ecology Progress Series, 80: 89–100.
- Herrmann, H., Häder, D.P. & Ghetti, F. (1997). Inhibition of photosynthesis by solar radiation in Dunaliella salina: relative efficiencies of UV-B, UV-A and PAR. Plant, Cell and Environment, 20: 359–365.
- Hirata, S., Taya, M. & Tone, S. (1998). Continuous cultures of Spirulina platensis under photoautotrophic conditions with change in light intensity. Journal of Chemical Engineering of Japan, 31: 636–639.
- Holzinger, A. & Lütz, C. (2006). Algae and UV irradiation: effects on ultrastructure and related metabolic functions. Micron, 37: 190–207.
- Hovel, H.J., Hodgson, R.T. & Woodall, J.M. (1979). The effect of fluorescent wavelength shifting on solar cell spectral response. Solar Energy Materials, 2: 19–29.
- Imamoglu, E., Dalay, M. & Sukan, F. (2010). Semi-continuous cultivation of Haematococcus pluvialis for commercial production. Applied Biochemistry and Biotechnology, 160: 764–772.
- Karatay, S.E. & Dönmez, G. (2011). Microbial oil production from thermophile cyanobacteria for biodiesel production. Applied Energy, 88: 3632–3635.
- Katsuda, T., Shimahara, K., Shiraishi, H., Yamagami, K., Ranjbar, R. & Katoh, S. (2006). Effect of flashing light from blue light emitting diodes on cell growth and astaxanthin production of Haematococcus pluvialis. Journal of Bioscience and Bioengineering, 102: 442–446.
- Katsuda, T., Shiraishi, H., Ishizu, N., Ranjbar, R. & Katoh, S. (2008). Effect of light intensity and frequency of flashing light from blue light emitting diodes on astaxanthin production by Haematococcus pluvialis. Journal of Bioscience and Bioengineering, 105: 216–220.
- Klampaftis, E., Ross, D., McIntosh, K.R. & Richards, B.S. (2009). Enhancing the performance of solar cells via luminescent down-shifting of the incident spectrum: a review. Solar Energy Materials and Solar Cell, 93: 1182–1194.
- Kohen, E., Santus, R. & Hirschberg, G.G. (1995). Photobiology. Academic Press, New York, NY.
- Lee, C.-G. & Palsson, B.Ø. (1994). High-density algal photobioreactors using light-emitting diodes. Biotechnology and Bioengineering, 44: 1161–1167.
- Li, Y., Horsman, M., Wang, B., Wu, N. & Lan, C. (2008). Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Applied Microbiology and Biotechnology, 81: 629–636.
- Matthijs, H.C.P., Balke, H., van Hes, U.M., Kroon, B.M.A., Mur, L.R. & Binot, R.A. (1996). Application of light-emitting diodes in bioreactors: flashing light effects and energy economy in algal culture (Chlorella pyrenoidosa). Biotechnology and Bioengineering, 50: 98–107.
- McCree, K.J. (1972). The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agricultural and Forest Meteorology, 9: 191–216.
- Mohsenpour, S.F. & Willoughby, N. (2013). Luminescent photobioreactor design for improved algal growth and photosynthetic pigment production through spectral conversion of light. Bioresource Technology, 142: 147–153.
- Navntoft, C., Dawidowski, L., Blesa, M.A., Fernandez-Ibanez, P., Wolfram, E.A. & Paladini, A. (2009). UV-A (315–400 nm) irradiance from measurements at 380 nm for solar water treatment and disinfection: comparison between model and measurements in Buenos Aires, Argentina and Almeria, Spain. Solar Energy, 83: 280–286.
- Prokop, A., Quinn, M.F., Fekri, M., Murad, M. & Ahmed, S.A. (1984). Spectral shifting by dyes to enhance algae growth. Biotechnology and Bioengineering, 26: 1313–1322.
- Ranjbar, R., Inoue, R., Katsuda, T., Yamaji, H. & Katoh, S. (2008). High efficiency production of astaxanthin in an airlift photobioreactor. Journal of Bioscience and Bioengineering, 106: 204–207.
- Rowan, B.C., Wilson, L.R. & Richards, B.S. (2008). Advanced material concepts for luminescent solar concentrators. IEEE Journal of Selected Topics in Quantum Electronics, 14: 1312–1322.
- Sinha, R.P., Klisch, M., Gröniger, A. & Häder, D.P. (1998). Ultraviolet-absorbing/screening substances in cyanobacteria, phytoplankton and macroalgae. Journal of Photochemistry and Photobiology B, 47: 83–94.
- Slooff, L.H., Kinderman, R., Burgers, A.R., Bakker, N.J., van Roosmalen, J.A.M. & Richards, B.S. (2006). Luminescent layers for enhanced silicon solar cell performance: down-conversion. Solar Energy Materials and Solar Cell, 90: 1189–1207.
- Solovchenko, A. (2010). Photoprotection in Plants; Optical Screening-based Mechanisms. Springer-Verlag, Berlin.
- Strümpel, C., McCann, M., Beaucarne, G., Arkhipov, V., Slaoui, A., Svrcek, V., del Cañizo, C. & Tobias, I. (2007). Modifying the solar spectrum to enhance silicon solar cell efficiency – an overview of available materials. Solar Energy Materials and Solar Cell, 91: 238–249.
- Sukenik, A. (1991). Ecophysiological considerations in the optimization of eicosapentaenoic acid production by Nannochloropsis sp. (Eustigmatophyceae). Bioresource Technology, 35: 263–269.
- Turcsányi, E. & Vass, I. (2000). Inhibition of photosynthetic electron transport by UV-A radiation targets the photosystem II complex. Photochemistry and Photobiology, 72: 513–520.
- Van Sark, W.G. (2008). Simulating performance of solar cells with spectral downshifting layers. Thin Solid Films, 516: 6808–6812.
- Van Sark, W.G., Meijerink, A., Schropp, R.E.I., van Roosmalen, J.A.M. & Lysen, E.H. (2005). Enhancing solar cell efficiency by using spectral converters. Solar Energy Materials and Solar Cell, 87: 395–409.
- Wang, W.J., Sun, X.T., Wang, G.C., Xu, P., Wang, X.Y., Lin, Z.L. & Wang, F.J. (2010). Effect of blue light on indoor seedling culture of Saccharina japonica (Phaeophyta). Journal of Applied Phycology, 22: 737–744.
- White, A.L. & Jahnke, L.S. (2002). Contrasting effects of UV-A and UV-B on photosynthesis and photoprotection of beta-carotene in two Dunaliella spp. Plant and Cell Physiology, 43: 877–884.
- Wondraczek, L., Batentschuk, M., Schmidt, M.A., Borchardt, R., Scheiner, S., Seemann, B., Schweizer, P. & Brabec, C.J. (2013). Solar spectral conversion for improving the photosynthetic activity in algae reactors. Nature Communication 4: 2047. doi:10.1038/ncomms3047.
- Xia, Q., Batentschuk, M., Osvet, A., Richter, P., P. Häder, D.P., Schneider,J., Brabec, C.J., Wondraczek, L. & Winnacker, A. (2013). Enhanced photosynthetic activity in Spinacia oleracea by spectral modification with a photoluminescent light converting material. Optics Express, 21: A909–A916.