Abstract
Many species of macroalgae along the western Antarctic Peninsula have a high coverage of filamentous algal endophytes. A previous field study showed that endophyte presence negatively impacts growth and survival in some Antarctic algae, but can have no impact on others. We examined nine species of common macroalgal hosts from the area surrounding Palmer Station, Antarctica, to examine fine-scale impacts of endophyte presence on host physiology. Physiological parameters were selected based on their potential to influence fitness of host algae. These included photosynthetic parameters, thallus toughness and susceptibility to grazers in those species previously known to be chemically defended. We found that few macroalgal hosts are impacted by the presence of endophytes and that these impacts are not consistent across all physiological parameters. Iridaea cordata and Pachymenia sp. were the only species among the nine examined that demonstrated physiological stress in the presence of endophytes. Out of four species in a previous study, I. cordata was also the most heavily impacted by endophyte presence.
INTRODUCTION
The relationships formed between endophytes and their host macrophytes can be complex (Correa, Citation1994; Potin, Citation2012) and impacted by their environment (Russell et al., Citation2005; Eggert et al., Citation2010). Classification of these symbioses range from mutualistic to pathogenic depending on the species involved (Correa, Citation1994; Potin, Citation2012), therefore defining these relationships is important to understanding ecosystem processes. The high frequency of endophyte presence in many species of Antarctic macroalgae was first noted by Peters (Citation2003) and subsequently quantified by Amsler et al. (Citation2009). These endophytes are filamentous algae which are interstitial in the cortical cells of their host alga, rarely penetrating the medulla (personal observation). These algae are not host specific; they are present in seasonally adapted species, rhodophytes, phaeophytes, both calcified and fleshy algae (observation, Peters, Citation2003; Amsler et al., Citation2009) as well as invertebrates (Hommersand et al., Citation2009). In contrast, there are few incidences of free-living filamentous algae in the benthic subtidal environment along the western Antarctic Peninsula. These include the filamentous phaeophytes Geminocarpus geminatus on senescent Desmarestia antarctica R.L. Moe & P.C. Silva and of Elachista antarctica on Palmaria decipiens (Wiencke & Clayton, Citation2002; Peters, Citation2003).
Filamentous endophytes in Antarctic macroalgae have been shown to grow freely out of the host thallus when relieved of mesograzer presence (Amsler et al., Citation2009; Aumack et al., Citation2011b). Peters (Citation2003) hypothesized that the dearth of free-living filamentous algae and high incidence of endophytic filamentous algae in this region is the result of intense mesograzer pressure. Macroalgae in subtidal areas of the western Antarctic Peninsula support very high densities of amphipod mesograzers (Richardson, Citation1971, Citation1977; Huang et al., Citation2007; Aumack et al., Citation2011a) and a subsequent study confirmed that mesograzers associated with the chemically defended macroalgal species in the Antarctic subtidal do indeed ablate free-living filamentous algae, selecting for the endophytic habit (Amsler et al., Citation2012). Worldwide, research on endophyte–macroalgal symbioses has shown that endophytes have varied effects on their hosts (Potin, Citation2012; Schoenrock et al., Citation2013). In Antarctic macroalgae, high occurrence of endophyte presence throughout a host thallus has a negative impact on growth in three species and survival in one species of macroalgae, but no impact on another (Schoenrock et al., Citation2013). This implies that pathogenicity of endophyte presence may be host or endophyte-specific, but few studies have examined these symbioses.
Algal physiology can be measured in a variety of ways; the present study targeted parameters which directly affect the fitness of an alga, more specifically its ability to grow and survive in order to genetically contribute to future generations. The first goal of the present study was to determine the effect of endophyte presence on palatability of the host to sympatric herbivores, specifically common amphipod mesograzers. In previous studies endophytes have been shown to make the host more palatable to grazers (Correa & McLachlan, Citation1992; Craigie & Correa, Citation1996) and associated algae can make a host more desirable (Karez et al., Citation2000). Many Antarctic algae are known to elaborate chemical defences against herbivory (Amsler et al., Citation2005), but presence of palatable endophytes could make thalli more appetizing, compromising the advantage of chemical defences. Brown and green endophytes grown out of thallus material in culture are significantly more palatable to herbivores than their hosts (Amsler et al., Citation2009). Alternatively, hosts may elaborate defences in response to endophyte presence, thereby deterring herbivory as a side effect when infected with endophytes. For example fungal endophytes are responsible for production of a chemical anti-herbivore compound in terrestrial grasses (Clay, Citation1996).
The second goal of this study was to determine the effects of endophyte infection on host thallus toughness. Thallus toughness is a defence against grazer pressure and/or environmental stressors (Littler & Littler, Citation1980). Previous studies have shown that endophyte infection weakens the host thalli and decreases resistance to environmental stressors (Schaffelke et al., Citation1996; Ellertsdottir & Peters, Citation1997). Epiphytic algae associated with a host can increase drag in current or wave action and cause the thallus to break more frequently (D’Antonio, Citation1985; Hemmi et al., Citation2005; Anderson & Martone, Citation2014). Environmental stressors can act in two different ways on thalli: the force from wave action or current may damage a thallus by ripping it, tearing it from its substrate, or causing scour on crustose morphologies, while grazer pressure may puncture or scrape a thallus. Consequently we looked at both types of physical stress with the expectation that presence of endophytic algae would weaken the host thallus to both. Any weakening to grazer pressure may make that alga more accessible as a food source to local herbivores, especially if endophytes make the thallus material more palatable in that particular species.
The third goal of this study was to determine the effect of endophyte infection on photosynthetic parameters of the host. All known algal endophytes found along the western Antarctic Peninsula are pigmented and presumably provide their own energy resources through photosynthesis. By measuring chlorophyll a fluorescence one can define photosynthetic performance in algae and changes therein under the influence of stressors. If endophyte presence results in physiological stress to the algal host as it can in terrestrial plants (Pinto et al., Citation2000), the photocentres of the host adjacent to endophyte infection could be expected to be less efficient than those further away from infection (photosynthetic parameters would be negatively impacted). In previous studies pulse amplitude modulated (PAM) fluorometry has been used successfully with Antarctic algae to generate rapid light curves (RLCs) using lower irradiances and measure maximum quantum yield (MQY) (Runcie & Riddle, Citation2006, Citation2012). From these measurements one can get an accurate calculation of maximum electron transport rate (ETRmax), saturating irradiance (Ek) and the slope to saturation of photo centres (α).
The host macroalgae used in this study were chosen using information from a previous endophyte survey (Amsler et al., Citation2009) and field observations. Two conspicuous brown algae are included: Himantothallus grandifolius (A. Gepp & E.S. Gepp) Zanova and Desmarestia antarctica. Himantothallus grandifolius has an endophyte load that ranges from 0–10% cover of the thallus (Amsler et al., Citation2009), is kelp-like in morphology – growing to > 15 m in length – and is one of the more ecologically important macroalgae in Antarctica (Wiencke & Amsler, Citation2012). Desmarestia antarctica is a pseudo-perennial species that can grow in dense beds in the subtidal, but is more commonly found sporadically interspersed within the algal canopy. The age of individuals is distinguished by size and condition of thallus material and endophyte loads range from 0–60% cover (Amsler et al., Citation2009). High endophyte occurrence is more common in the second-year individuals which were used in this experiment. Both phaeophytes share three brown algal endophytes (genotypes) including Geminocarpus geminatus and genotypes C and D from Amsler et al. (Citation2009). Each has one distinct endophyte genotype: H. grandifolius has genotype B and D. antarctica has genotype H (Amsler et al., Citation2009).
Seven species of red macroalgae were included in this study: Iridaea cordata (Turner) Bory de Saint-Vincent, Myriogramme manginii (Gain) Skottsberg, Gymnogongrus turquetii Hariot, Curdiea racovitzae Hariot, Gigartina skottsbergii Setchell & N.L. Gardner, Pachymenia sp. and Trematocarpus antarcticus (Hariot) Fredericq & R.L. Moe. Iridaea cordata has endophyte loads ranging from 0–75% cover (Amsler et al., Citation2009) and inhabits the shallow subtidal. Myriogramme manginii has endophyte loads ranging from 0–40% cover (Amsler et al., Citation2009) and generally inhabits the shallow subtidal. Gymnogongrus turquetii has endophyte loads ranging from 0–35% (Amsler et al., Citation2009) and inhabits the shallow subtidal, sometimes in dense patches. Gigartina skottsbergii has endophyte loads ranging from 0–5% cover (observation, Amsler et al., Citation2009) and can be very large (~ 3 m in diameter) inhabiting a wide depth range. Curdiea racovitzae has endophyte loads ranging from 0–25% cover (Amsler et al., Citation2009) and is generally found in the shallow subtidal. Pachymenia sp. has endophyte loads ranging from 0–20% (observation) and is generally found ~ 5 m depth growing to 1 m in length alongside I. cordata. Lastly T. antarcticus has endophyte loads ranging from 0–40% cover (Amsler et al., Citation2009) and grows in large patches in the shallow subtidal, but can be found in deeper environments among the Desmarestia spp. canopy. Endophytes in all species include common brown genotypes as well as genotype G in I. cordata, genotype F in T. antarcticus, genotype E in C. racovitzae (unique to these species) and both green endophyte morphologies (Amsler et al. Citation2009). illustrates the range in size and morphology of the species used in this study. Not all species were used consistently throughout the experiments because of morphological or physiological differences (e.g. M. manginii is too brittle for tensometry) and frequency and location of endophyte presence in certain species/populations.
METHODS
All algae were collected using scuba diving from the archipelago surrounding Palmer Station, Antarctica (64°46′S, 64°03′W, see Amsler et al. Citation2009 for map), and placed in flow-through aquaria under ~22 μmol photons m−2 s−1 irradiance (~ 12h:12h day:night cycle) in ambient temperatures until used in experiments.
Palatability
Two different feeding studies were used to assess variance in palatability of the host thallus caused by endophyte infection. A common amphipod from the western Antarctica Peninsula, Gondogeneia antarctica, was used as the herbivore in these studies. To determine whether the endophyte itself makes the host more or less palatable a choice feeding assay was performed with thallus material with and without endophyte presence from the same individual (n = 30). To evaluate whether the chemistry of the host is altered by endophyte infection a choice feeding assay was performed with thallus material not containing endophyte material from two hosts: one with endophyte presence and one without endophyte presence (n = 10). Trematocarpus antarcticus, G. turquetii, I. cordata, H. grandifolius, M. manginii, D. antarctica and Pachymenia sp. were used in the first study, and only G. turquetii, C. racovitzae, M. manginii and I. cordata were used in the second study.
Feeding assays were run for ~96 h (or until a significant portion of the thallus material was missing) in 125 ml Nalgene bottles with filtered seawater (FSW), which was partially changed daily. Each assay used individual G. antarctica and included two thallus fragments as described above. A paired autogenic control in a separate bottle was used to correct for weight change in thallus material not due to herbivory, which was minimal. Thallus fragments were cut into different shapes in order to differentiate between material with and without endophyte presence. Each fragment was between 2–10 mg in weight and paired autogenic controls were within 0.1 mg of its wet weight. At the end of the experiment, all thallus fragments were reweighed with the amphipod and change in mass of fragments exposed to herbivory were corrected using % change in autogenic controls. Feeding rate was recorded as Δ µg thallus mg amphipod−1 time (h)−1. Mean feeding rates were log transformed and compared using a student’s t-test and a paired student’s t-test, accordingly (SPSS). One-sample t-tests were used to compare corrected feeding rates with an expected feeding rate of 0 μg thallus mg amphipod−1 h−1 to determine whether fragments were significantly consumed throughout the assay.
Thallus toughness
Thallus toughness was measured in two ways: penetrometry following the methods of Duffy & Hay (Citation1991) and tensometry following the methods of Carrington et al. (Citation2001). Pachymenia sp., I. cordata, H. grandifolius, M. manginii, D. antarctica, G. turquetii, C. racovitzae, T. antarcticus and Gigartina skottsbergii were used in penetrometry measurements. The force (N) required to puncture the thallus was measured in three locations across individual thalli (n = 10 per species) with and without endophyte presence (six locations per individual). Repeated measures were averaged for a thallus and all data were log transformed before analysis. Force to puncture a thallus was compared between areas of endophyte presence and absence using a paired t-test (SPSS).
Pachymenia sp., I. cordata, M. manginii, H. grandifolius, C. racovitzae and T. antarcticus were used in tensometry measurements. Locations with and without endophyte presence on the blade of an individual were cut from the thallus in a t-bone shape (Carrington et al., Citation2001). Each t-bone (n = 5 per species) was videotaped being broken by a Force Gauge Model M4-200 (Mark-10, Copiague NY) mounted on a palmer stand in ambient seawater (~2ºC). Dimensions of the t-bone (thickness, width and length between grips) were measured (cm) prior to testing, and length between grips was recorded at increasing force values. These values were used to create a stress-strain curve which calculates toughness (strain energy density, J m−3) in the thallus fragments (Denny, Citation1988). All data were log transformed and mean toughness was compared using a paired t-test (SPSS) for each species. All measurements were done within 48 h of collection to ensure that aquarium conditions did not change the properties of the thallus material, although such effects are probably miniscule (Harder et al., Citation2006).
Photosynthetic characteristics
A Diving-PAM fluorometer (WALZ, Germany) was used to measure photosynthetic parameters in thallus material adjacent to and furthest from endophyte presence in individuals. Iridaea cordata, M. manginii, Pachymenia sp., G. skottsbergii, C. racovitzae, T. antarcticus and G. turquetii were used in this experiment (n = 8, 6, 5, 4, 5, 5 and 3 respectively). Absorbance of the photosystems in each species was measured by placing a representative individual between the PAM light diode and a LI-COR sensor. Areas adjacent to endophyte infection and areas furthest away from infection were identified visually on each individual and were dark adapted for 30 min using dark adapting clips (WALZ, Germany). The area adjacent to endophyte presence was chosen so that the photocentres of the endophyte species did not contribute to the fluorescence signal of the host, but any negative impact to the host would presumably be the most extreme. MQY (Fv/Fm) was measured after dark adaptation followed shortly by RLCs (30 s increments, actinic light from 0–200 μmol photons m−2 s−1 irradiance). Ek, α and ETRmax were calculated from the RLCs which were fitted to a hyperbolic tangent function outlined in Jassby & Platt (Citation1976). All data were log transformed and paired t-tests (SPSS) were used to compare average MQY, Ek, α and ETRmax in areas adjacent to and furthest from endophyte presence in all species. Species were grouped and a paired t-test was used to compare areas adjacent to and far from endophyte presence to determine whether a larger sample size teases out significant differences in rhodophytes.
RESULTS
Palatability
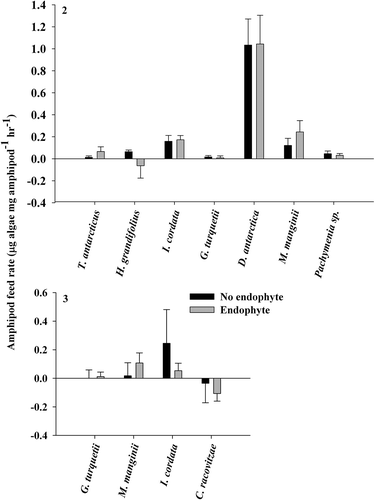
Palatability assays using thallus material from the same individual found no significant differences in amphipod feeding rate between material with and without endophyte presence (paired t-test, P > 0.05; ). Palatability assays using thallus material from hosts with and without endophyte presence (test for primed defences) also showed no significant differences in amphipod feeding rate (student’s t-test, P > 0.05; ). Thallus consumption was significantly greater than the expected 0 μg thallus mg amphipod−1 h−1 in the first assay in I. cordata, H. grandifolius (no endophyte), D. antarctica and M. manginii (endophyte) (one sample t-test, P < 0.05). In the second assay consumption was significantly greater than 0 μg thallus mg amphipod−1 h−1 in Pachymenia sp., but not in I. cordata, giving us differential results in that species.
Thallus toughness
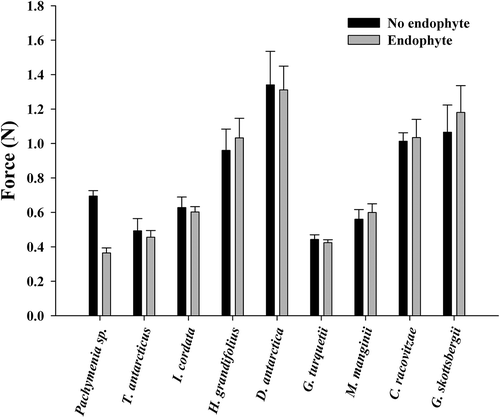
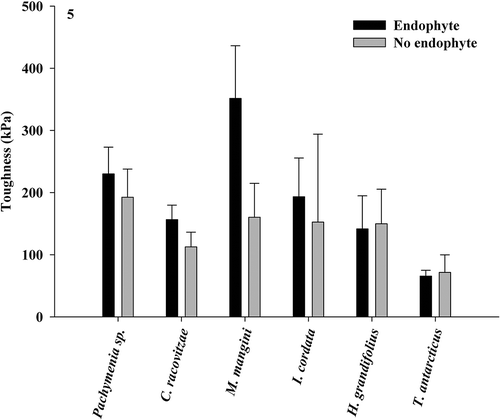
Penetrometry assays revealed a significant difference in the force required to puncture the thallus in one species, Pachymenia sp., where thallus material with endophyte presence was weaker than material without (paired t-test, P < 0.05; ). All other host species showed no significant difference in the force required to puncture the thallus with and without endophyte presence. Tensometry assays revealed no significant difference in toughness of thallus tissue with or without endophyte presence in any of the species tested (paired t-test, P > 0.05; ).
Photosynthetic characteristics
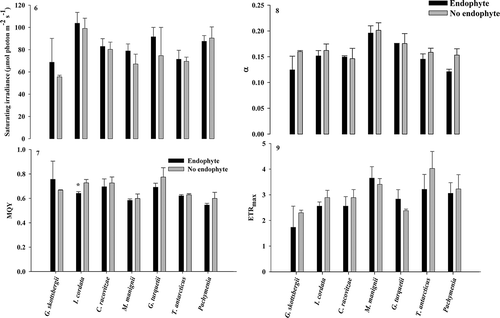
Analysis of photosynthetic parameters in most host species showed no significant differences between areas adjacent to and furthest away from endophyte presence (paired t-test, P > 0.05). The only exception was I. cordata, where MQY significantly decreased in areas adjacent to endophyte presence (paired t-test, P < 0.05; ). Paired t-tests showed no significant trends across species in all parameters (–).
DISCUSSION
Antarctica is a characteristically harsh environment: low light levels, extreme physical conditions (Wiencke et al., Citation2007) and large mesograzer populations (Amsler et al., Citation2008) make it so for the phycobenthos. Notably, the large mesograzer cohort is thought to be responsible for high levels of endophytic algae along the western Antarctic Peninsula (Peters, Citation2003; Amsler et al., Citation2014). However, from an evolutionary perspective the persistence of filamentous algal endophytes in Antarctic macroalgae may be credited to their benign impacts on many algal hosts, shown in most species in this study. Weakness in host physiology caused by endophyte presence, or pathogenicity, could reduce growth and survival of ‘infected’ individuals in a community, effectively reducing the fitness of that species. Loss of these individuals would include any symbionts, potentially reducing fitness of the filamentous endophytes as well.
A previous study showed that high endophyte presence within the hosts G. turquetii, T. antarcticus and I. cordata decreased growth late in the growing season (Schoenrock et al., Citation2013). Survival also decreased in individuals with high endophyte levels in the hosts G. turquetii and T. antarcticus (Schoenrock et al., Citation2013) showing that high endophyte presence probably hastens senescence. Still, evidence of whether endophyte presence directly impacts fitness of any of these host species is lacking. Early senescence could only be detrimental to fitness of a species if it occurs before spore/gamete dispersal or somehow reduces reproductive potential of an individual. The measurements used in this study targeted aspects of algal physiology that are fundamental to growth and survival in the subtidal and could impact reproductive potential (De Wreede & Klinger, Citation1988), however the impact of endophyte presence on fertility and spore dispersal were not directly measured.
In this study endophyte presence had no significant impact on palatability or consumption rate in any of the species assayed. Iridaea cordata, H. grandifolius, D. antarctica, M. manginii and Pachymenia sp. were consumed by G. antarctica in very low quantities (Figs 2 and 3). Consumption rates of D. antarctica were probably highest because individuals used in this study were second-year perennials that have the largest cover of endophytes and are potentially senescent. In a previous study (Amsler et al., Citation2009) D. antarctica was less palatable to herbivores, probably due to the fact that they were first-year individuals. Regardless, the absence of preferential consumption between thallus materials in assays shows that endophyte presence will not have any effect on grazer pressure in situ.
The mechanical measurements used in this study test two aspects of thallus toughness: resistance to puncture and breakage of the thalli. All species tested required less energy to puncture their thalli than previously recorded (Amsler et al., Citation2005), which may be related to the time of year in which the experiments took place (November–December versus March–April) or because endophytes often infect the older portions of thalli which may be deteriorating (specifically in D. antarctica and H. grandifolius). Strain energy density has never before been measured in algae from Antarctica. Breaking energy for these species was within the normal range for intertidal temperate species (Harder et al., Citation2006). Endophyte presence had no significant impact on toughness in any species with the exception of Pachymenia sp., specifically its resistance to puncture force (). Pachymenia sp. is often found at depths above 5 m (observation) which is a high-energy environment with fewer grazers. These results indicate that this species may be resilient only to the most aggressive stressors in its environment, however we did not evaluate materials properties to test this. In the future, evaluation of materials responsible for thallus toughness would be valuable in understanding the vulnerability of Pachymenia sp. to puncture force, as well as the mechanical properties of Antarctic algae in general. Overall, consumption and breakage of individuals should not be impacted by endophyte presence in situ, therefore would have no impact on fitness.
In pigmented organisms, one might expect the presence of a photosymbiont to be a stressor on the photophysiology of the host due to shading or another physical/chemical mechanism (Pinto et al. Citation2000; Enriquez & Borowitzka, Citation2010; Potin, Citation2012). Yield values are calculated as (Fm−Fo)/Fm and can be reduced by a reduction in Fm, indicating enhanced non-photochemical quenching (NPQ), or an increase in Fo that may reflect photodamage. Because all samples were dark adapted for 30 minutes prior to measurements NPQ should be relaxed initially (qE), and photodamage would cause reduction in yield values. Only one rhodophyte, I. cordata, showed a significant reduction in MQY in thallus material adjacent to endophyte presence (). No other photo-parameters were significantly impacted in this alga or any other species and there were no significant trends across species. Antarctic algae are well adapted to their environment and have very low optimal irradiance requirements (Wiencke et al., Citation2007) which may explain the low impact of a photosymbiont on most host species. Also, because of the experimental design we may not have captured a constitutive impact of the endophyte which we tested for in palatability assays. Iridaea cordata is the species where high levels of endophyte presence negatively impacted host growth the most (Schoenrock et al., Citation2013). Though palatability and thallus toughness of I. cordata were not impacted by endophytes, reduced MQY, or utilization of photon irradiance, indicates stress to host photosystems which could negatively affect fitness in this species. Measured photosynthetic parameters in this experiment differed slightly from previous studies on photophysiology of Antarctic macrophytes (Runcie & Riddle, Citation2006, Citation2012; Huovinen & Gomez, Citation2013), most likely due to individuals’ acclimation to aquarium conditions and a consistent source of light.
In conclusion, Antarctic endophytes should be classified as innocuous or harmful depending on their host alga. Thorough molecular identification has yet to be done on the green algal endophytes of these hosts, but most hosts do share brown genotypes and similar green endophyte morphologies (Amsler et al., Citation2009), so endophyte species is probably not a driver in mixed effects on examined hosts. Many of the experiments within this study had low sample sizes due to the natural history of these endophytes and their host algae and this increases the likelihood that there was not enough statistical power to reject our null hypotheses. The morphology of species, as well as the prevalence and distribution of endophytic algae within thallus material, made it hard to find individuals appropriate for these experiments; many individuals host at least one endophytic alga and some can have extreme coverage (Amsler et al., Citation2009). Future work should focus on nutritional aspects of the endophytes (carbon sequestration, etc.), high-resolution information on biomechanical properties of the algae, and microscale characterization of photosynthetic properties and palatability of the algae. Studies should also examine the effect of endophyte presence on reproductive potential and gamete dispersal in these algae, maybe the ultimate measure of fitness. Therefore this study provides a springboard for future investigations into the innocuous, sometimes pathogenic, relationships between many Antarctic macroalgae and their endophytes.
Disclosure statement
No potential conflict of interest was reported by the author(s).
ACKNOWLEDGEMENTS
This work was made possible by the Raytheon Polar Services/Antarctic Service Contract staff of Palmer Station and the University of Alabama at Birmingham/University of South Florida field teams including Maggie Amsler, Alan Maschek, Jason Cuce, Julie Schram, Bill Dent and Jackie Von Salm.
Additional information
Funding
Notes on contributors
Kathryn M. Schoenrock
K.M. Schoenrock: original concept, experiment, drafting and editing the manuscript; C.D. Amsler: funding, original concept and editing manuscript; J.B. McClintock: funding and editing manuscript; B.J. Baker: funding and editing manuscript.
Charles D. Amsler
K.M. Schoenrock: original concept, experiment, drafting and editing the manuscript; C.D. Amsler: funding, original concept and editing manuscript; J.B. McClintock: funding and editing manuscript; B.J. Baker: funding and editing manuscript.
James B. McClintock
K.M. Schoenrock: original concept, experiment, drafting and editing the manuscript; C.D. Amsler: funding, original concept and editing manuscript; J.B. McClintock: funding and editing manuscript; B.J. Baker: funding and editing manuscript.
Bill J. Baker
K.M. Schoenrock: original concept, experiment, drafting and editing the manuscript; C.D. Amsler: funding, original concept and editing manuscript; J.B. McClintock: funding and editing manuscript; B.J. Baker: funding and editing manuscript.
REFERENCES
- Amsler, C.D., Iken, K., McClintock, J.B., Amsler, M.O., Peters, K.J., Hubbard, J.M., Furrow, F.B. & Baker, B.J. (2005). Comprehensive evaluation of the palatability and chemical defenses of subtidal macroalgae from the Antarctic Peninsula. Marine Ecology Progress Series, 294: 141–159.
- Amsler, C.D., McClintock, J. & Baker, B.J. (2008). Macroalgal chemical defenses in polar marine communities. In Algal Chemical Ecology (Amsler, C.D., editors), 91–103. Springer-Verlag, Berlin.
- Amsler, C.D., Amsler, M.O., McClintock, J.B. & Baker, B.J. (2009). Filamentous algal endophytes in macrophytic Antarctic algae: prevalence in hosts and palatability to mesoherbivores. Phycologia, 48: 324–334.
- Amsler, C.D., McClintock, J.B. & Baker, B.J. (2012). Amphipods exclude filamentous algae from the western Antarctic Peninsula: experimental evidence. Polar Biology, 35: 171–177.
- Amsler, C.D., McClintock, J.B. & Baker, B.J. (2014). Chemical mediation of mutualistic interactions between macroalgae and mesograzers structure unique coastal communities along the western Antarctic Peninsula. Journal of Phycology, 50: 1–10.
- Anderson, L.M. & Martone, P.T. (2014). Biomechanical consequences of epiphytism in intertidal macroalgae. Journal of Experimental Biology, 217: 1167–1174.
- Aumack, C.F., Amsler, C.D., McClintock, J.B. & Baker, B.J. (2011a). Changes in amphipod densities among macroalgal habitats in day versus night collections along the western Antarctic Peninsula. Marine Biology, 158: 1879–1885.
- Aumack, C.F., Amsler, C.D., McClintock, J.B. & Baker, B.J. (2011b). Impacts of mesograzers on epiphyte and endophyte growth associated with chemically defended macroalgae from the western Antarctic Peninsula: a mesocosm experiment. Journal of Phycology, 47: 36–41.
- Carrington, E., Grace, S.P. & Chopin, T. (2001). Life history phases and the biomechanical properties of the red alga Chondrus crispus (Rhodophyta). Journal of Phycology, 37: 699–704.
- Clay, K. (1996). Interactions among fungal endophytes, grasses and herbivores. Researches on Population Ecology, 38: 191–201.
- Correa, J.A. (1994). Infections by pigmented algal endophytes: misuse of concepts and terminology. Revista Chilena De Historia Natural, 67: 4–8.
- Correa, J.A. & McLachlan, J.L. (1992). Endophytic algae of Chondrus crispus (Rhodophyta). IV. Effects on the host following infections by Acrochaete operculata and A. heteroclada (Chlorophyta). Marine Ecological Progress Series, 81: 73–78.
- Craigie, J.S. & Correa, J.A. (1996). Etiology of infectious diseases in cultivated Chondrus crispus (Gigartinales, Rhodophyta). Hydrobiologia, 327: 97–104.
- D’Antonio, C. (1985). Epiphytes on the rocky intertidal alga Rhodomela larix (Turner) C. Agardh.: negative effects on the host and food for herbivores. Journal of Experimental Marine Biology and Ecology, 86: 197–218.
- Denny, M.W. (1988). Biology and the Mechanics of the Wave-swept Environment. Princeton University Press, Princeton.
- De Wreede, R.E. & Klinger, T. (1988). Reproductive strategies in algae. In Plant Reproductive Ecology: Patterns and Strategies (Lovett-Doust, J. & Lovett-Doust, L., editors), 267–276. Oxford University Press,
- Duffy, J.E. & Hay, M.E. (1991). Food and shelter as determinants of food choice by an herbivorous marine amphipod. Ecology, 72: 1286–1298.
- Eggert, A., Peters, A. & Kupper, F. (2010). The potential impact of climate change on endophyte infections in kelp sporophytes. In Seaweeds and their Role in Globally Changing Environments (Israel, A., Elnar, R. & Seckbach, J., editors), 139–154. Springer, Berlin.
- Ellertsdottir, E. & Peters, A.F. (1997). High prevalence of infection by endophytic brown algae in populations of Laminaria spp. (Phaeophyceae). Marine Ecological Progress Series, 146: 135–143.
- Enriquez, S. & Borowitzka, M.A. (2010). The use of fluorescent signal in studies of seagrasses and macroalgae. In Chlorophyll a fluorescence in aquatic sciences. Methods and Applications (Sugett, D., Prasil, O. & Borowitzka, M.A., editors), 187–208. Springer, Dordrecht.
- Harder, D.L., Hurd, C.L. & Speck, T. (2006). Comparison of mechanical properties of four large, wave-exposed seaweeds. American Journal of Botany, 93: 1426–1432.
- Hemmi, A., Makinen, A., Jormalainen, V. & Honkanen, T. (2005). Responses of growth and phlorotannins in Fucus vesiculosus to nutrient enrichment and herbivory. Aquatic Ecology, 39: 201–211.
- Hommersand, M.H., Moe, R.L., Amsler, C.D. & Fredericq, S. (2009). Notes on the systematics and biogeographical relationships of Antarctic and sub-Antarctic Rhodophyta with descriptions of four new genera and five new species. Botanica Marina, 52: 509–534.
- Huang, Y.M., Amsler, M.O., McClintock, J.B., Amsler, C.D. & Baker, B.J. (2007). Patterns of gammaridean amphipod abundance and species composition associated with dominant subtidal macroalgae from the western Antarctic Peninsula. Polar Biology, 30: 1417–1430.
- Huovinen, P. & Gomez, I. (2013). Photosynthetic characteristics and UV stress tolerance of Antarctic seaweeds along the depth gradient. Polar Biology, 36: 1319–1332.
- Jassby, A.D. & Platt, T. (1976). Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnology and Oceanography, 21: 540–547.
- Karez, R., Engelbert, S. & Sommer, U. (2000). ‘Co-consumption’ and ‘protective coating’: two new proposed effects of epiphytes on their macroalgal hosts in mesograzer-epiphyte-host interactions. Marine Ecological Progress Series, 205: 85–93.
- Littler, M. M. & Littler, D. S. (1980). The evolution of thallus form and survival strategies in benthic marine macroalgae: field and laboratory tests of a functional form model. American Naturalist, 116: 25–44.
- Peter, A.F. (2003). Molecular identification, distribution and taxonomy of brown algal endophytes, with emphasis on species from Antarctica. Proceedings of the 17th International Seaweed Symposium, NY. 293–302.
- Pinto, L., Azevedo, J., Pereira, J., Vieira, M. & Labate, C. (2000). Symptomless infection of banana and maize by endophytic fungi impairs photosynthetic efficiency. New Phytologist, 147: 609–615.
- Potin, P. (2012). Intimate associations between epiphytes, endophytes, and parasites of seaweeds. In Seaweed Biology: Novel Insights into Ecophysiology, Ecology and Utilization (Wiencke, C. & Bischof, K., editors), 203–234. Springer, New York.
- Richardson, M.G. (1971). The ecology and physiological aspects of Antarctic weed dwelling amphipods (Preliminary report, II). British Antarctic Survey Report, N9/1971(-72) H: 1–16.
- Richardson, M.G. (1977). The ecology including physiological aspects of selected Antarctic marine invertebrates associated with inshore macrophytes. PhD dissertation, University of Durham. 165.
- Runcie, J.W. & Riddle, M.J. (2006). Photosynthesis of marine macroalgae in ice-covered and ice-free environments in East Antarctica. European Journal of Phycology, 41: 223–233.
- Runcie, J.W. & Riddle, M.J. (2012). Estimating primary productivity of marine macroalgae in East Antarctica using in situ fluorometry. European Journal of Phycology, 47: 449–460.
- Russell, B.D., Elsdon, T.S., Gillanders, B.M. & Connell, S.D. (2005). Nutrients increase epiphyte loads: broad-scale observations and an experimental assessment. Marine Biology, 147: 551–558.
- Schaffelke, B., Peters, A.F. & Reusch, T.B.H. (1996). Factors influencing depth distribution of soft bottom inhabiting Laminaria saccharina (L) Lamour in Kiel Bay, Western Baltic. Hydrobiologia, 327: 117–123.
- Schoenrock, K.M., Amsler, C.D., McClintock, J.B. & Baker, B.J. (2013). Endophyte presence as a potential stressor on growth and survival in Antarctic macroalgal hosts. Phycologia, 52: 595–599.
- Wiencke, C. & Clayton, M.N. (2002). Antarctic Seaweeds. A.R.G. Ganter Verlag KG, Koelz Scientific Books, Czech Republic.
- Wiencke, C. & Amsler, C.D. (2012). Seaweeds and their communities in polar regions. In Seaweed Biology: Novel Insights into Ecophysiology, Ecology and Utilization (Wiencke, C. & Bischof, K., editors), 265–294. Springer-Verlag, Berlin.
- Wiencke, C., Clayton, M.N., Gomez, I., Iken, K., Luder, U.H., Amsler, C.D., Karsten, U., Hanelt, D., Bischof, K. & Dunton, K. (2007). Life strategy, ecophysiology and ecology of seaweeds in polar waters. Review of Environmental Science and Biotechnology, 6: 95–126.