Abstract
Twenty-three freshwater springs of the Po plain (northern Italy) were surveyed and visited multiple times during 2011–2013 to assess water quality and algal taxa present. Despite poor water conditions of this human-altered floodplain, a relatively high number of taxa referable to the freshwater red algae, Batrachospermum and Sheathia, were observed. The identity of specimens collected was based on morphology and confirmed with sequence data. The specimens belonged to Sheathia arcuata, S. boryana, Batrachospermum atrum, B. gelatinosum and B. gelatinosum f. spermatoinvolucrum. This is the first report of B. gelatinosum f. spermatoinvolucrum outside North America. Niche analysis revealed a marked difference in the physical and chemical preferences of the freshwater red algae recorded. The occurrence of S. boryana was limited to relatively low nitrate and higher oxygen concentrations. Conversely, the spatial distribution of the other four taxa indicated that they were able to tolerate high concentrations of nitrate (up to 53.6 mg l–1) and low rates of dissolved oxygen saturation (as low as 3%). All the specimens of B. gelatinosum collected had few to many trichogynes deformed by a basal protuberance or knob, a structure previously unobserved in this species. No other taxa showed any deformities. The ecological analyses confirmed the existence of a relationship between the environmental conditions of the springs and the occurrence of knobs.
INTRODUCTION
Red algae (Rhodophyta) are an important component of the algal flora of inland aquatic ecosystems, and the Batrachospermales is the most complex and diverse order of these algae (Kumano, Citation2002; Eloranta et al., Citation2011). Several taxa within this order are considered to be sensitive to human impact and therefore to be reliable ecological bio-indicator species (Rott et al., Citation1999; Haury et al., Citation2006). Despite the conservation concern and their role as bio-indicators (Kwandrans & Eloranta, Citation2010), studies of the diversity and ecology of freshwater red algae are lacking, especially in Italy (Eloranta et al., Citation2011; Ceschin et al., Citation2013; Bolpagni et al., Citation2015).
Batrachospermales are reported to be less widespread in southern and central Europe than in northern Europe (Eloranta & Kwandrans, Citation2007; Kwandrans & Eloranta, Citation2010), possibly owing to the progressive worsening in water quality due to human activities and higher population density in the former regions, as suggested by Kwandrans & Eloranta (Citation2010). Nevertheless, an ecological survey of lowland freshwater springs, known as fontanili, located in northern Italy, revealed the presence of a relatively high number of taxa referable to the freshwater red algal genera Batrachospermum Roth and Sheathia Salomaki & M.L.Vis.
Sheathia is a new genus recently proposed for all taxa of Batrachospermum with heterocortication, formerly classified as Helminthoidea, and for B. arcuatum, a species with only cylindrical filaments covering axial cells (Salomaki et al., Citation2014). Heterocortication has been recognized as a synapomorphy of the clade, which is lost in B. arcuatum. In this paper, we adopt this taxonomic proposal and refer the specimens of B. arcuatum and B. boryanum recorded in this study to this new genus.
The fontanili are small, semi-artificial, aquatic ecosystems sensu Kløve et al. (Citation2011) that are typical of the Po River Basin, the largest Italian watershed (71 057 km2). This floodplain hosts one of the largest multilayer aquifers in Europe and is characterized by widespread NO3– contamination of both surface water and groundwater (Cinnirella et al., Citation2005; Bassanino et al., Citation2011). Accordingly, most of the fontanili show high nitrate levels (up to 90 mg l–1) due to widespread agricultural pollution (Rossetti et al., Citation2005; Laini et al., Citation2011; Sacchi et al., Citation2013). Being completely groundwater-dependent ecosystems, the fontanili exhibit low variation in temperatures, ranging from 10 to 17°C throughout the year, making them a good environment for cold stenotherm flora and fauna (Rossetti et al., Citation2005; Laini et al., Citation2011). Similarly, the dissolved oxygen saturation is also fairly constant, with seasonal fluctuations of the order of a few degrees (on average not more than 20%). In general, insignificant daily oxygen variations have been detected (Laini et al., Citation2011).
Although fontanili are all characterized by a low inter-annual variation in hydrological, hydro-chemical and thermal conditions (Rossetti et al., Citation2005; Laini et al., Citation2011), there is considerable variation between the fontanili, especially with regard to water nitrate and oxygen concentration (Laini et al., Citation2011). Therefore, the fontanili are ideal systems for investigating the autecology of freshwater red algae due to (1) the almost total lack of connection between them, which makes the springs isolated systems, (2) their high density within a relatively small area, and (3) their marked physical and chemical variability despite a homogeneous, shared, climatic zone.
The present study of 23 fontanili was undertaken to monitor these springs for a period of 3 years, collecting information on the physical and chemical characteristics of the water and on the presence/absence of Batrachospermum and Sheathia species. To our knowledge, the only study of the algal flora of these environments, if we exclude the citation of B. gelatinosum (Linnaeus) De Candolle by Guazzo Albergoni et al. (Citation1977), is a paper published by Zanfrognini (Citation1895).
The specific aims of the research were to: (i) document the diversity of Batrachospermum and Sheathia species in the fontanili; (ii) study the autecology of those species; and (iii) model their spatial pattern in relation to the physical and chemical characteristics of the springs. Furthermore, since the mechanism underlying the production of a basal protuberance (or knob) on trichogynes in Batrachospermales has not yet been elucidated, we also investigated the gradient of environmental stressors that may be correlated with the appearance of these structures.
MATERIALS AND METHODS
Study area
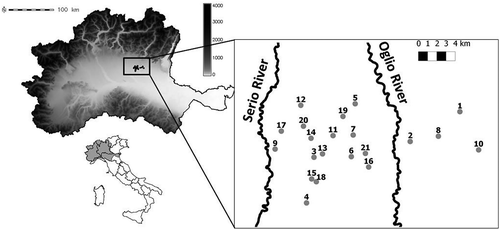
This study was conducted in 23 lowland springs in the Po River basin, selected from an area measuring 30 × 12 km located in the central-eastern part of the Lombardy Region that lies within the Oglio and Serio watersheds (). We focused our attention on the ‘head’ of each spring that is represented by small ponds created by the human-induced resurgence of groundwater. For each spring, the geographic coordinates, the hydrological domain (EO: east Oglio River; WO: west Oglio River), the substratum macro-typology (cobbles, pebbles, sand, mud), and the dominant primary producer (macroalgae, bryophytes, vascular) were recorded (Supplementary Table 1).
Fig. 1. Study area with the location of the lowland springs (fontanili) sampled (1: Averolda, 2: Becchilsù, 3: Belprato, 4: Boscovito, 5: Ca Nova, 6: Calcio, 7: Cavetti, 8: Chigaluzza, 9: Cna Volpe, 10: Crocetta, 11: Est Pompiano, 12: Fascina, 13: Isso Grande, 14: Isso Piccolo, 15: Lametta, 16: Mirandola, 17: Negroni, 18: Palazzina, 19: Pellegrina, 20: Pratizagni, 21: Saragozza, 22: Serafina, 23: Zemia). For more detailed location data see Supplementary . All fontanili were between 40° 23′−40° 30′ longitude and 9° 43′−10° 0′ latitude.
Collection of algae
Specimens of Batrachospermum and Sheathia were collected at the heads of the fontanili in 21 of the 23 springs visited in August 2011, March 2012 and January–February 2013 (Supplementary Table 1). They were kept cold (4°C) and stored in the laboratory (in a fridge) for no more than 24 h before analysis. After a preliminary morphological observation using a Leica DM750 microscope equipped with a Leica DFC 295 digital camera, each specimen was split into three samples. One sample was immediately fixed in 4% formaldehyde and was used for the detailed morphological analyses, the second sample was dried in silica gel and stored at −20°C for DNA analysis, and the third sample was dried and kept as a herbarium voucher specimen. Both the formaldehyde-fixed samples and the exsiccata are stored in the Laboratory of Ecology at the Department of Life Science of Parma University.
Water sampling and analyses
Water samples were collected at the same time as the red algal specimens (August 2011, March 2012 and January–February 2013) from all 23 springs. Water temperature, pH, specific conductivity (EC) and dissolved oxygen concentration (DO) were measured directly in situ using an YSI 560 multiprobe (Xylem Analytics, UK). In addition, a water sample was collected by hand just at the water surface using a 1 litre plastic bottle, filtered (~ 200 ml) and kept cold (4°C) in the dark until laboratory determination of NH4+ (Koroleff, Citation1970), NO2– (APHA, Citation1975), NO3– (Rodier, Citation1984), SiO2 (APHA, Citation1975) and PO43– (Valderrama, Citation1981) by spectrophotometry (Beckman DU 65). Filtered water subsamples were transferred to glass vials and analysed for dissolved inorganic carbon (DIC) using a six end-point titration method with 0.1N HCl (Anderson et al., Citation1986); CO2 concentrations were determined by back-calculation from DIC data (Talling, Citation1973).
Morphological analysis
Morphological observations were made using a Zeiss microscope equipped with a Leica DFC 42 digital camera. Qualitative morphological features were studied using 53 individuals collected from different springs, 43 of which belonged to Batrachospermum gelatinosum. To estimate the number of trichogynes with a knob and stalk per thallus of B. gelatinosum, we observed and photographed the first 15 consecutive trichogynes that appeared in the microscopic field of view after squashing the specimen on the slide. When fewer than five trichogynes with the knob and stalk were counted, the specimen was marked Ge.(sk) (as in –). When more than five trichogynes [from 6–7(10)] with the knob and stalk were counted, the specimen was marked Ge.sk (as in –). If the knob was absent or only hinted at and the stalk was long (as in –), the specimen was marked Ge.s(k).
Figs 2–14. Batrachospermum gelatinosum. Fig. 2. Whorls with carposporophytes. –. Lanceolate, sessile trichogynes with more or less elongated end and more or less swollen central part (marked Ge.(sk) in the text) from specimens collected at springs Pellegrina (), Isso Piccolo (), and Crocetta (sequenced specimen, ). , . Deformed trichogynes with long basal stalk or just a hinted knob (marked Ges(k) in the text) from a specimen collected at Cna Volpe, the spring with the best environmental conditions among the 23 investigated. –. Deformed trichogynes with more or less long stalk and knobs (marked Ge.sk in the text) from specimens collected in three springs with the poorest environmental conditions [Pratizagni (), Saragozza (), Fascina ()]. . Three carpogonia on the same fascicle branch from the sequenced specimen collected at the spring Averolda. Note the basal knob (arrow) on trichogyne of the central carpogonium (arrow). . Two carpogonia on the same fascicle branch from the sequenced specimen collected at the spring Est Pompiano, the one on the left with stalk and knob (arrow). , . B. gelatinosum f. spermatoinvolucrum. Specimen sequenced with spermatangia on the top of involucral filaments (arrowheads). , scale bar = 50 µm; –, scale bar = 20 µm.
![Figs 2–14. Batrachospermum gelatinosum. Fig. 2. Whorls with carposporophytes. Figs 3–5. Lanceolate, sessile trichogynes with more or less elongated end and more or less swollen central part (marked Ge.(sk) in the text) from specimens collected at springs Pellegrina (Fig. 3), Isso Piccolo (Fig. 4), and Crocetta (sequenced specimen, Fig. 5). Figs 6, 7. Deformed trichogynes with long basal stalk or just a hinted knob (marked Ges(k) in the text) from a specimen collected at Cna Volpe, the spring with the best environmental conditions among the 23 investigated. Figs 8–10. Deformed trichogynes with more or less long stalk and knobs (marked Ge.sk in the text) from specimens collected in three springs with the poorest environmental conditions [Pratizagni (Fig. 8), Saragozza (Fig. 9), Fascina (Fig. 10)]. Fig. 11. Three carpogonia on the same fascicle branch from the sequenced specimen collected at the spring Averolda. Note the basal knob (arrow) on trichogyne of the central carpogonium (arrow). Fig. 12. Two carpogonia on the same fascicle branch from the sequenced specimen collected at the spring Est Pompiano, the one on the left with stalk and knob (arrow). Figs 13, 14. B. gelatinosum f. spermatoinvolucrum. Specimen sequenced with spermatangia on the top of involucral filaments (arrowheads). Fig. 2, scale bar = 50 µm; Figs 3–14, scale bar = 20 µm.](/cms/asset/ad6ce946-5726-40c1-af41-44d172198979/tejp_a_1055592_f0002_b.gif)
Molecular data generation
For each taxon identified from morphology, at least one specimen was prepared for molecular analysis (Supplementary Table 2). The silica-desiccated specimens were homogenized using a mortar and pestle and DNA extraction performed using NucleoSpin Plant Genomic DNA kit (Macherey-Nagel, Bethlehem, Pennsylvania, USA) following the standard protocol with the cell lysis step increased to 30 min. A portion of the rbcL gene was amplified using the primer pair F160 and rbcLR or F650 and rbcLR, if the first pair did not yield a PCR product. PCR reactions were conducted using either 30 µl or 50 µl reaction. The 30 µl reaction consisted of 11 µl distilled water, 1.5 µl each of the primers, 15 µl AmpliTaq Gold master mix (Applied Biosystems, Carlsbad, California, USA) and 1 µl DNA. The 50 µl reaction consisted of 36.75 µl distilled water, 5 µl 10× Extaq® buffer (Clontech Laboratories Inc., Mountainview, California, USA), 4 µl dNTP, 1.5 µl each of the primers, 0.25 µl Extaq and 1 µl DNA. The PCR reaction was conducted in a 2720 Thermocycler (Applied Biosystems, Foster City, California, USA) using the following cycle: 95°C for 1 min, 35 cycles of 93°C for 30 s, 50°C for 30 s, 68°C for 1 min, and ending at 72°C for 10 min. The PCR products were purified using the UltraClean™ PCR Clean-up DNA Purification Kit (Mo Bio Laboratories, Carlsbad, California, USA). The sequence data were generated using an ABI 3100 Genetic Analyzer (Applied Biosystems, Foster City, California, USA) at the Ohio University Genomics Facility. Both amplification primers and in some cases internal primers were used for sequencing (Supplementary Table 2). The sequences were aligned in SequencherTM 4.10.1 (Gene Codes Corporation, Ann Arbor, Michigan, USA). All sequence data generated were submitted to GenBank.
Statistical analysis
To represent the ecological niche of the species of Batrachospermum and Sheathia from this study, we adopted the technique described by Dolédec et al. (Citation2000). This analysis places species along habitat conditions using a maximization of their mean Outlying Mean Index (OMI) and takes as inputs a set of abiotic variables and a matrix with species abundances as matrix entries. We used this technique simply to describe the Batrachospermum/Sheathia niche, without uncertainty evaluation of the results. The environmental variables selected for the statistical analysis were: pH, temperature, conductivity, dissolved oxygen (DO), dissolved inorganic carbon (DIC), soluble reactive silica (SiO2), ammonium (NH4+), nitrite (NO2–), nitrate (NO3–) and orthophosphate (SRP) ().
Table 1. Physical-chemical parameters for fontanili in which species of Batrachospermum and Sheathia occurred.
Poisson regression (Cameron & Trivedi, Citation1998) was applied to model count data. A basic assumption of the model is that data dispersion is equal to 1; we verified this assumption using the overdispersion test proposed by Cameron & Trivedi (Citation1990). In this study, Poisson regression was used to assess relationships between the specimens of B. gelatinosum with knobs and stalks counted during the observation time window and the mean of the physical and chemical parameters computed over the same time window. The regressions were computed using the total number of individuals in each fontanile observed during all sampling dates as a response variable and the mean of the physical and chemical parameters computed over the same time-window in each fontanile as independent variables.
RESULTS
Morphological description of the specimens sequenced
Sheathia arcuata (Kylin) Salomaki et M.L. Vis (Citation2014: 535)
Upright thalli with filaments covering axial cells composed only of cylindrical cells. Whorls spherical, separated or confluent, (362–)401–782(–932) µm in diameter, with flexuous, sometimes curved filaments. Thalli dioecious. Carpogonial branches undifferentiated from fascicle cells, numerous (up to six) on the same fascicle branch, surrounded by long involucral filaments composed of cylindrical cells. Carpogonia with sessile trichogynes, almost always clavate, rarely topped with spermatia (18.7)22–25(31) µm long. Spermatangia terminal or sub-terminal on fascicle branches. One or two pedicellate carposporophytes per whorl, exerted or near to axis, 75–204 µm in diameter. Molecular data were obtained from one male and two female specimens. GenBank numbers: KM077035, KM077036, KM077037.
Sheathia boryana (Sirodot) Salomaki et M.L. Vis (Citation2014: 535)
Upright thalli with filaments covering axial cells composed of both cylindrical and bulbous cells. Whorls separated or confluent, 411–942 µm in diameter, often bearing many unfertilized carpogonial branches. Thallus dioecious. Carpogonial branches undifferentiated from fascicle cells, sometimes two/three on the same fascicle branch, surrounded by short involucral filaments composed of 1–3(4) globose or barrel-shaped cells. Carpogonia 20.6–31.3 µm long (mean length of 15 measurements: 25.5 µm) with sessile trichogynes, mostly clavate, sometimes slightly constricted near the apex, almost always observed without spermatia attached. Spermatangia terminal or sub-terminal on fascicle branches. Carposporophytes 90–180 µm, briefly pedicellate, generally one per whorl. Molecular data were obtained from one female specimen. GenBank number: KM077043.
Batrachospermum gelatinosum (Linnaeus 1753) De Candolle (Citation1801: 21) emend. Vis et al (Citation1995: 52)
Upright thalli with filaments covering axial cells composed only of cylindrical cells. Whorls spherical, separated, 451–794(–1006) µm (). Thalli monoecious. Carpogonial branches undifferentiated from fascicle cells, sometimes more than one on the same fascicle branch (–), surrounded by long or short involucral filaments. Carpogonia 23.3–54.2 µm long and 7–12.2 µm in diameter (mean of 15 measurements: 34.6 µm × 9.1 µm) with sessile, lanceolate trichogynes having a more or less elongated end and a more or less swollen central part (–), frequently with 1–3 spermatia attached. Several trichogynes appeared deformed having basal stalks up to 11.5 µm long (, , ), and/or basal protuberance (knob) (–) that sometimes led the trichogyne to be curved (). Spermatangia terminal on fascicle branches. Three to six small carposporophytes per whorl, at various distances from axis, (56–)67–121 µm in diameter (). Molecular data were obtained from four specimens, all showing few or many deformed trichogynes. GenBank numbers: KM077030, KM077031, KM077032, KM077033.
Batrachospermum gelatinosum forma spermatoinvolucrum (M.L. Vis & Sheath, Citation1996) M.L. Vis & Sheath (Citation1998: 238)
Only one thallus of this form was collected in the Palazzina spring, in January 2013. The upright thallus displayed cylindrical cortication throughout. Whorls spherical and confluent, 416–750 µm in diameter. Thallus monoecious. Carpogonial branches undifferentiated from fascicle cells surrounded by short involucral filaments. Carpogonia 22.3–28.9 µm long and 8.3–10.5 µm wide (mean of 8 measurements: 26.1 µm × 9.1 µm) exhibiting obovoid or roughly fusiform trichogynes. Spermatangia terminal on fascicle branch or placed on involucral filaments of carpogonial branches (–). Carposporophytes small, 89–120 µm in diameter.
Molecular data were obtained from the single thallus. GenBank number: KM077034.
Batrachospermum atrum (Hudson, 1778) Harvey (Citation1841: 120)
Only one thallus of this species was collected in January 2013 in the Pellegrina spring. The upright thallus had reduced whorls, primary fascicles that did not adhere to the main axis and very short secondary fascicles. Thallus monoecious. Carpogonial branches arising mainly from periaxial cells. Carpogonia with obovoid trichogynes, 24.5–26 µm long. Spermatangia terminal on primary fascicle branches. Carposporophytes hemispherical, 155–170 µm in diameter, rare. Molecular data were obtained from the single thallus. GenBank number: KM077029.
Molecular data
Sequence data were generated for 10 specimens from the study sites and five specimens from a more distant stream (Supplementary Table 2). The sequence data for these specimens were compared with previous data in GenBank to determine taxonomic identity. One specimen from Pellegrina was identified as B. atrum having an identical sequence to a specimen of B. atrum (AF029139) collected from the UK. Five of the specimens were determined to be B. gelatinosum (Supplementary Table 2). These specimens differed from each other and from B. gelatinosum (GU810834) collected from France by 0−3 bp. The specimen from Palazzina had the morphology of B. gelatinosum f. spermatoinvolucrum, but differed from the only sequence of that taxon (AF029146) by 25 bp, whereas it was only 0−3 bp different from the B. gelatinosum specimens. Three specimens from two springs within the study area (Serafina and Belprato) as well as five specimens from a stream outside the study area were identical to each other and a specimen (DQ393131) of S. arcuata from Bulgaria. One of the two specimens from Averolda was identified to be S. boryana as the sequences were similar (6 bp different) to a specimen (JX669770) identified as this species from Lithuania.
Physical and chemical drivers and species spatial distribution
The graphs of the niche analysis (, ) highlighted a marked difference in physical and chemical features between the various springs. The first two axes explained 89% of the variability; the first axis showed a positive correlation with NH4+ followed by DO, and a negative correlation with NO3− and conductivity, while the second axis had a positive correlation with pH, and a negative correlation with SiO2.
Fig. 15. Biplot of the first two axes from the niche analysis. AR = Sheathia arcuata; BO = S. boryana; ATR = Batrachospermum atrum; SP = B. gelatinosum f. spermatoinvolucrum; Ge.sk = specimens of B. gelatinosum with more than 30% trichogynes deformed by knobs and stalks Ge.(sk) = specimens of B. gelatinosum with fewer than 30% trichogynes deformed by knobs and stalks; Ge.s(k) = specimens of B. gelatinosum with more than 30% trichogynes deformed almost exclusively by long stalks.
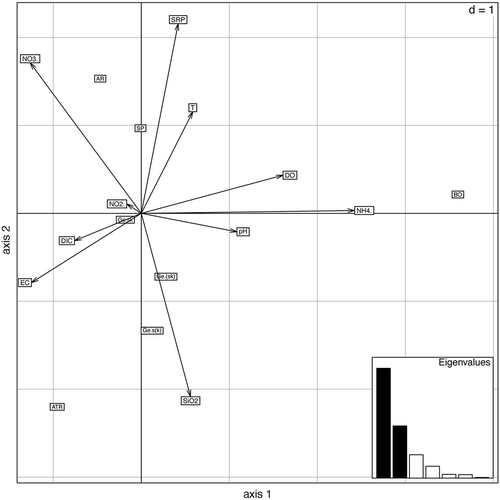
Fig. 16. Scatter plot on the first factorial plan from the niche analysis of species and springs. AR = Sheathia arcuata; BO = S. boryana; ATR = Batrachospermum atrum; SP = B. gelatinosum f. spermatoinvolucrum; Ge.sk = specimens of B. gelatinosum with more than 30% trichogynes deformed by knobs and stalks; Ge.(sk) = specimens of B. gelatinosum with fewer than 30% trichogynes deformed by knobs and stalks; Ge.s(k) = specimens of B. gelatinosum with more than 30% trichogynes deformed almost exclusively by long stalks.
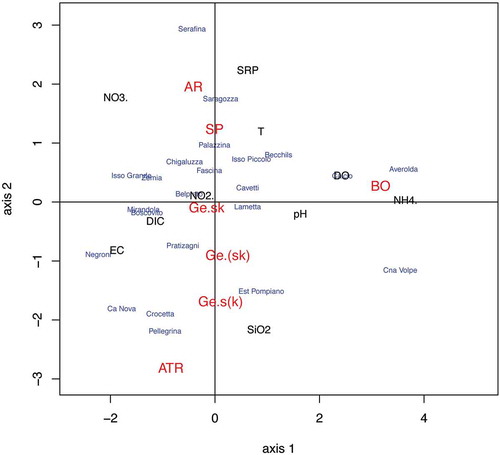
The niche analysis placed the four specimens of S. boryana studied in the sector corresponding to the springs with higher dissolved oxygen saturation (54.2–58.0%; Cna Volpe and Averolda) (; , ). However, this sector was also characterized by a positive correlation with NH4+. The Averolda spring displayed during 2011 and 2012 quantifiable concentrations of NH4+ (up to 60 μg l–1), close to the analytical detection limit in nearly all the other springs investigated (). Furthermore, the NO3− concentrations in these two springs were high (20.4–27.6 mg l–1) (), despite being among the lowest observed. Hence, the niche analysis suggested that S. boryana tolerates high nitrate and ammonium concentrations.
Sheathia arcuata occupied a sector characterized by high nitrate (37.0–43.4 mg l–1) and low dissolved oxygen concentrations in the niche analysis (e.g. Serafina exhibited a mean value of dissolved oxygen saturation equal to 18.3%, see ), although the data available for S. arcuata are somewhat limited as only the three dioecious specimens (one male and two female gametophytes) with sequence data collected at the Belprato and Serafina springs were utilized. However, these specimens indicate that this species seems to tolerate high concentrations of nitrate and low concentrations of dissolved oxygen.
Batrachospermum gelatinosum f. spermatoinvolucrum was collected only once. It was present in winter (February 2013) in a spring (Palazzina) with ecological conditions (mean nitrate value 37.3 mg l–1; oxygen saturation value 24.1%, see ) slightly better than those in which Sheathia arcuata was observed. Given the similar habitat, the niche analysis placed this specimen at the edge of the area occupied by S. arcuata (, ).
The position of Batrachospermum atrum was within the sector associated with environmental conditions characterized by low mean values of conductivity and SiO2, and somewhat moderate mean values of nitrate (, ). However, it should be noted that B. atrum was collected only once, in January 2013, in the Pellegrina spring, in conjunction with the highest nitrate values (37.7 mg l–1), and the lowest dissolved oxygen saturations (20.3%) detected in this spring.
For Batrachospermum gelatinosum, 43 specimens were investigated and three groups recognized on the basis of the number of trichogynes with stalks and/or knobs counted in each individual: (i) a group of 24 specimens (marked Ge.sk), in which more than 30% (up to 50%) of the trichogynes were deformed by stalks and knobs; (ii) a group of 13 specimens [marked Ge.(sk)], in which less than 30% of the trichogynes were deformed by stalks and knobs, and (iii) a group of six specimens [marked Ge.s(k)], in which more than 30% of the trichogynes were deformed almost exclusively by long stalks (as in ). In –, the group Ge.sk was at the intersection of the two axes, close to zero, thus indicating that the appearance of knobs and stalks on the trichogynes does not seem to be affected by changes in the average environmental conditions of the springs. The other two groups, Ge.(sk) and Ge.s(k), were in the space corresponding to springs with lower mean values of NO3– and higher mean values of SiO2 (, ).
More insight regarding the presence of knobs and stalks on trichogynes of Batrachospermum gelatinosum was gained from the Poisson regression analysis () which assessed the relationship between the different groups [Ge.sk, Ge.(sk), Ge.s(k)] of this species, and the mean physical and chemical conditions of the springs. The model obtained for Ge.(sk) revealed a clear correlation with pH, DIC and DO (inverse), and NH4+ and NO2– (direct). The model fitted the data with the per cent-explained variation of 57.8 %, and a correct prediction of 77.3 % of the cases. The model for Ge.s(k) indicated an inverse relationship with temperature and DIC and a direct relationship with NO2–. Although the fit of the model was not optimal, with a per cent-explained variation of 42%, the prediction was excellent (86%). With regard to Ge.sk, the model was not significant, as it revealed no statistically significant relationship with the mean environmental conditions of the springs, thereby confirming the results of the niche analysis.
Table 2. Results of the Poisson regression for Batrachospermum gelatinosum groups and fontanili environmental variables.
DISCUSSION
Morphological findings
Sheathia arcuata is difficult to distinguish from Batrachospermum gelatinosum as these two taxa share the same key characteristic of cylindrical cortication. Species of Sheathia are reported to produce abundant carpogonia and to contain numerous carpogonia within a single whorl (Entwisle et al., Citation2009). This feature was regularly observed in our specimens of S. arcuata, but was also found, albeit less frequently, in specimens of B. gelatinosum, either sequenced (see , ) or not. Therefore, this characteristic alone is not sufficient to distinguish the two species, though if the shape and the size of the trichogynes are also considered, the reliability appears to increase. The trichogynes in our specimens of S. arcuata were fusiform to clavate, as has also been described in the literature (Kumano, Citation2002; Sheath & Sherwood, Citation2011; Eloranta et al., Citation2011), and smaller than those of B. gelatinosum which were clavate to lanceolate when they were not deformed by knobs and stalks. Thus, the presence of numerous carpogonia within the whorls combined with smaller fusiform to clavate trichogynes appears to characterize S. arcuata well, and may differentiate this species from B. gelatinosum.
We observed a second morphological feature, besides the type of cortication, which clearly distinguished the specimens of S. arcuata from those of S. boryana, i.e. the type of involucral filaments around the carpogonial branch. In the non-heterocorticate specimens of S. arcuata, these filaments were long and composed of 8–10 cylindrical cells whereas in the heterocorticate specimens of S. boryana they were always short and composed of 1–3(4) globose or barrel-shaped cells. These different morphologies of the involucral filaments around the carpogonial branch had previously been observed and proposed as a diagnostic character by Eloranta et al. (Citation2011). Thus, this character can reliably be used to distinguish the dioecious thalli of both species.
All 43 specimens of B. gelatinosum displayed a few to many carpogonia with bumpy (knobs) and/or pedicellate (stalked) trichogynes. Such trichogynes have only been previously reported in Batrachospermum section Virescentia (Sheath et al., Citation1994; Kumano, Citation2002; Eloranta et al., Citation2011), though the presence of knobs on trichogynes has been considered to be an environmentally induced trait rather than a taxonomic character (Vis et al., Citation2001; Hanyuda et al., Citation2004). The molecular data from four specimens clearly showed them to be B. gelatinosum even though they displayed trichogynes with knobs and stalks.
A further specimen of B. gelatinosum was determined as B. gelatinosum f. spermatoinvolucrum as it exhibited spermatangia on the involucral filaments of its carpogonial branches. In the first specimen of this form collected in North America, in the cold boreal forest, this particular placement of spermatangia was interpreted as an adaptation to increase the reproductive success of the species in this biome (Vis & Sheath, Citation1996, Citation1998). Even in our case, this placement may represent a strategy of adaptation to the environment, related not so much to the low temperature as to the high levels of some nutrients and hypoxia of the water. Unfavourable environmental conditions, whether low temperatures or high levels of nutrients, may stimulate the production of large amounts of spermatia in a plant to increase the success of reproduction.
Batrachospermum atrum is considered to be rare, although widespread, in Europe (Kwandrans & Eloranta, Citation2010; Alcaraz et al., Citation2013) and also appears to be rare in Italy, having been reported only twice previously in the 19th century. The species was first collected in 1862 and voucher specimens distributed in Series I of the Erbario Crittogamico Italiano (as B. tenuissimum Bory, see Cuccuini, Citation1997). The second report comes from springs in the Emilia Romagna region (Zanfrognini, Citation1895). Similarly, previous reports in Italy of Sheathia boryana are few (Dell’Uomo, Citation1970 as B. ectocarpurm Sirodot; Abdelahad & Bazzichelli, Citation1995 as B. boryanum Sirodot). Sheathia arcuata has not previously been reported in Italy. However, its recent discovery (confirmed by molecular data, Supplementary Table 2) by the authors of this study, in a river in Lazio (River Rio, Central Italy) suggests that the species is fairly widespread in the territory and that it is likely to have been confused with B. gelatinosum in the past. Batrachospermum gelatinosum is the most frequently cited species in Italy since the mid-19th century (Massalongo, Citation1855).
Molecular findings
The rbcL sequence data corroborated the morphological identifications of the specimens. Although the specimens of B. gelatinosum had deformed trichogynes, these specimens were confirmed with the molecular data as being closely related to B. gelatinosum specimens without deformities from other European locations. For the remaining specimens, the sequences were either identical or very close to other European specimens with the same morphological identification. The sequence variation for the S. boryana specimen with other European specimens was slightly higher but still well within the range reported for intraspecific variation in freshwater red algae (Vis et al., Citation2001, Citation2010; Hanyuda et al., Citation2004). There was a single exception with the specimen morphologically identified as Batrachospermum gelatinosum f. spermatoinvolucrum as it differed considerably from the one sequenced specimen from Labrador, Canada, but was very similar to B. gelatinosum. Our finding lends further support for the hypothesis that spermatangia on the involucral filaments may be environmentally induced or a chance occurrence (Vis & Sheath, Citation1998).
Ecological findings
The result of Poisson regression analysis for the three groups of B. gelatinosum with up to 50% and less than 30% of the trichogynes deformed by knobs and stalks, was not surprising considering that the springs are characterized by variable nitrate concentrations and low oxygen rates. The Ge.sk group was collected in 14 of the 23 springs investigated and can be considered the ‘normal type’, i.e. the local ‘basal type’ of B. gelatinosum in the springs. The Ge.(sk) and Ge.s(k) groups diverged in relation to the baseline condition and were from springs characterized by a lower nitrate concentration. Thus, we surmise that a reduction in both the number and complexity of the deformations may result from the reduction in nitrate concentrations.
Members of Batrachospermum and Sheathia are generally observed in oligotrophic waters and are considered to be good indicators of the ecological status of aquatic systems (Haury et al., Citation2006; Carmona et al., Citation2011; Ceschin et al., Citation2012, Citation2013; García & Aboal, Citation2014). However, in the springs investigated in this paper, B. gelatinosum and S. arcuata were associated with high concentrations of nitrate (up to 56.3 mg l–1) and extremely low saturations of dissolved oxygen (as low as 3%). In addition, while the trichogynes in B. gelatinosum were significantly deformed by knobs and stalks, S. arcuata did not appear to be affected by the poor water quality. Therefore, B. gelatinosum may be more sensitive to pollution than S. arcuata in the investigated springs. However, the results of this study may not be applicable to all populations of S. arcuata, as recent molecular sequence data suggest that the binomial S. arcuata may hide more than one taxon (Salomaki et al., Citation2014). Comparable results were obtained for Batrachospermum atrum. There is also a recent report in Spain of this species being associated with high nitrate concentrations (11.1 mg l–1) (Alcaraz et al., Citation2013). However, at the spring where it was collected, B. atrum was able to withstand worse conditions, enduring nitrate concentrations of up to 37.7 mg l–1 and hypoxia (20.3%). In addition, this hard water species also displayed an ability to colonize neutral waters (7.0 pH units). With respect to S. boryana, although this taxon seems to require higher dissolved oxygen rates than the other taxa, our data indicate that it can tolerate some nitrate and ammonium pollution.
Our observations reveal that the species of Batrachospermum and Sheathia, which are widespread in Europe, recorded in the springs studied are distributed over a wider ecological range than previously reported in the literature. Moreover, the fontanili undeniably host, despite the poor conditions, a number of species and may consequently be considered an important refuge for these algae. On the basis of our observations, three factors may explain the presence of these taxa in conjunction with high nitrate levels: (1) the placement of specimens at the heads of the fontanili, thus exposing them to the direct influence of groundwater; (2) the very slight variations in temperature and pH that occur in the fontanili, and (3) the strong stoichiometric imbalance in the C:N:P ratio, which is due to the trapping of the dissolved reactive phosphorus in the soil-matrix groundwater system that results in the complete absence of this ion in the emergent waters.
The ecological and morphological data presented for Batrachospermum and Sheathia species make a significant contribution to our knowledge of the autecology of these species, highlighting their adaptability to hypoxia and/or high nitrate concentrations. It was possible to correlate, for the first time, the appearance of knobs on trichogynes with habitat quality. These results were obtained by studying only 23 fontanili distributed in an area measuring 30 × 12 km, a very small proportion of the estimated number of the 1000−2000 such springs in the central-western Po basin. Further investigations are warranted to adequately assess the diversity and the role that these systems play in the conservation of freshwater red algae at both the national and European scales.
Disclosure statement
No potential conflict of interest was reported by the author(s).
SUPPLEMENTARY INFORMATION
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at http://dx.doi.org/10.1080/09670262.2015.1055592.
Supplementary Table 1. Location and physical information for the fontanili, taxa found (with year of collection in parentheses), geographic coordinates (E = longitude, N = latitude), the hydrological domain (HD = EO: east Oglio River; WO: west Oglio River), the substratum macro-typology (SB = cobbles, pebbles, sand, mud), and the dominant primary producer (DPP = macroalgae, bryophytes, vascular). Spring number corresponds to those in .
Supplementary Table 2. Collection, molecular data and GenBank information for freshwater red algal specimens from which molecular data were obtained; * = primer used only for sequencing.
Supplementary Tables 1 and 2
Download MS Word (26.4 KB)ACKNOWLEDGEMENTS
We thank the editor and the two reviewers for their comments which have significantly improved the text.
Additional information
Notes on contributors
Nadia Abdelahad
N. Abdelahad, R. Bolpagni, G. Jona Lasinio, and M.L. Vis contributed equally to this study and are listed in alphabetical order.
Rossano Bolpagni
N. Abdelahad, R. Bolpagni, G. Jona Lasinio, and M.L. Vis contributed equally to this study and are listed in alphabetical order.
Giovanna Jona Lasinio
N. Abdelahad, R. Bolpagni, G. Jona Lasinio, and M.L. Vis contributed equally to this study and are listed in alphabetical order.
Morgan L. Vis
N. Abdelahad, R. Bolpagni, G. Jona Lasinio, and M.L. Vis contributed equally to this study and are listed in alphabetical order.
REFERENCES
- Abdelahad, N. & Bazzichelli, G. (1995). Occurrence of a Batrachospermum station in Latium. Giornale Botanico Italiano, 129: 38–45.
- Alcaraz, J.L.M., Canales, L.M. & Sanjurjo, M.A. (2013). Morphological description and ecology of some rare macroalgae in south-central Spanish rivers (Castilla-la Mancha Region). Anales del Jardín Botánico de Madrid, 70: 81–90.
- Anderson, L.G., Hall, P.O.J., Iverfeldt, A., van der Loeff, M.M.R., Sundby, B. & Westerlund, S.F.G. (1986). Benthic respiration measured by total carbonate production. Limnology and Oceanography, 31: 319–329.
- APHA, AWWA, WPCF (1975). Standard Methods for the Examination of Water and Wastewater. 14th ed. Washington.
- Bassanino, M., Sacco, D., Zavattaro, L. & Grignani, C. (2011). Nutrient balance as a sustainability indicator of different agro-environments in Italy. Ecological Indicators, 11: 715–723.
- Bolpagni, R., Amadio, C., Johnston, E.T. & Racchetti, E. (2015). New physical and chemical perspectives on the ecology of Thorea hispida (Thoreaceae). Journal of Limnology, 74: 294–301.
- Cameron, A. C. & Trivedi, P. K. (1990). Regression-based tests for overdispersion in the Poisson model. Journal of Econometrics, 46: 347–364.
- Cameron, A. C. & Trivedi, P. K. (1998). Regression Analysis of Count Data. Cambridge University Press, Cambridge.
- Candolle, A.P. de (1801). Extrait d’un rapport sur les conferves. Bulletin des Sciences par la Société Philomathique de Paris, 3: 17–21, pl. I.
- Carmona, J., Perona, E., Sánchez-Díaz, E. & Loza, V. (2011). Morphological and ecological characterization of Batrachospermales (Rhodophyta) in the Jarama Basin (Iberian Peninsula). Limnetica, 30: 117–128.
- Ceschin, S., Biscegli, S. & Ricci, S. (2012). Contribution to the knowledge of red algae (Rhodophyta) of some rivers in Central Italy. Crypogamie, Algologie, 33: 61–67.
- Ceschin, S., Ricci, S., Abati, S., Bisceglie, S., Minciardi, M.R. & Zuccarello, V. (2013). Distribution and ecology of red algae in Italian rivers. Fundamental and Applied Limnology, 183: 223–237.
- Cinnirella, S., Buttafuoco, G. & Pirrone, N. (2005). Stochastic analysis to assess the spatial distribution of groundwater nitrate concentrations in the Po catchment (Italy). Environmental Pollution, 133: 569–580.
- Cuccuini, P. (1997). L’Erbario Crittogamico Italiano. Storia e struttura di una collezione, con una introduzione storica di A. Graniti/ The Erbario Crittogamico Italiano. The history and structure of a collection with an historical note by A. Graniti. Museo Botanico dell’Università. Sezione del Museo di Storia Naturale, Firenze.
- Dell’Uomo, A. (1970). Contributo alla flora algale del fiume Potenza (Marche). Rivista di Idrobiologia, 9: 201–234.
- Dolédec, S., Chessel, D. & Gimaret-Carpentier, C. (2000). Niche separation in community analysis: a new method. Ecology, 81: 2914–2927.
- Eloranta, P. & Kwandrans, J. (2007). Freshwater red algae (Rhodophyta). Identification guide to European taxa, particularly to those in Finland. Norrlinia, 15, pp. 103.
- Eloranta, P., Kwandrans, J. & Kusel-Fetzmann, E. (2011). Rhodophyta and Phaeophyceae. Süßwasserflora von Mitteleuropa, Bd. 7.
- Entwisle, T.J., Vis, M.L., Chiasson, W.B., Necchi, Jr. O. & Sherwood, A.R. (2009). Systematics of the Batrachospermales (Rhodophyta) – a synthesis. Journal of Phycology, 45: 704–715.
- García, M.E. & Aboal, M. (2014). Environmental gradients and macroalgae in Mediterranean marshes: the case of Pego-Oliva marsh (East Iberian Peninsula). Science of the Total Environment 475: 216–224. doi:10.1016/j.scitotenv.2013.10.014.
- Guazzo Albergoni, F., Spreafico, E. & Toso, S. 1977. Profilo ecologico dei fontanili del Cremasco. Giornale Botanico Italiano, 111: 71–86.
- Hanyuda, T., Suzawa, Y., Suzawa, T., Arai, S., Sato, H., Ueda, K. & Kumano, S. (2004). Biogeography and taxonomy of Batrachospermum helminthosum (Batrachospermales, Rhodophyta) in Japan inferred from rbcL gene sequences. Journal of Phycology, 40: 581–588.
- Harvey, W.H. (1841). A Manual of the British Algae: Containing Generic and Specific Descriptions of the Known British Species of Sea-weeds and of Confervae both Marine and Fresh-water. John Van Voorst, London.
- Haury, J., Peltre, M.C., Trémolières, M., Barbe, J., Thiébaut, G., Bernez, I., Daniel, H., Chatenet, P., Haan-Archipof, G., Muller, S., Dutartre, A., Laplace-Treyture, C., Cazaubon, A. & Lambert-Servien, E. (2006). A new method to assess water trophy and organic pollution. The Macrophyte Biological Index for Rivers (IBMR): its application to different types of river and pollution. Hydrobiologia, 570: 153–158.
- Kløve, B., Ala-aho, P., Bertrand, G., Boukalova, Z., Ertürk, A., Goldscheider, N. Ilmonen, J., Karakaya, N., Kupfersberger, H., Kvœrner, J., Lundberg, A., Mileusnić, M., Moszczynska, A., Muotka, T., Preda, E., Rossi, P., Siergieiev, D., Šimek, J., Wachniew, P., Angheluta, V. & Widerlund, A. (2011). Groundwater dependent ecosystems. Part I: Hydroecological status and trends. Environmental Science & Policy, 14: 770–781.
- Koroleff, F. (1970). Direct determination of ammonia in natural waters as inophenol blue. Information on techniques and methods for seawater analisys. I.C.E.S. Interlaboratory Report, 3: 19–22.
- Kumano, S. (2002). Freshwater red algae of the World. Biopress Ltd, Bristol.
- Kwandrans, J. & Eloranta, P. (2010). Diversity of freshwater red algae in Europe. Oceanological and Hydrobiological Studies, 39: 161–169.
- Laini, A., Bartoli, M., Castaldi, S., Viaroli, P., Capri, E. & Trevisan, M. (2011). Greenhouse gases (CO2, CH4 and N2O) in lowland springs within an agricultural impacted watershed (Po River Plain, northern Italy). Chemistry and Ecology, 27: 177–187.
- Massalongo, A.B. (1855). De Cryptogamis nonnullis novis agri Veronensis. Flora (Regensburg), 38: 241–244.
- Rodier, J. (1984). L’analyse de l’eau. 7é éd. Dunod, Paris.
- Rossetti, G., Pieri, V. & Martens, K. (2005). Recent ostracods (Crustacea, Ostracoda) found in lowland springs of the provinces of Piacenza and Parma (Northern Italy). Hydrobiologia, 542: 287–296.
- Rott, E., Pfister, P., Van Dam, H., Pall, K., Pipp, E., Binder, N. & Ortler, K. (1999). Indikationslisten fur Aufwuchsalgen. Teil 2: Trophieindikation und autokologische Anmerkungen. Bundesministerium fur Land- und Forstwirtschaft, Wasser-wirtschaftkataster. Wien.
- Sacchi, E., Acutis, M., Batoli, M., Brenna, S, Delconte, C.A., Laini, A. & Pennisi, M. (2013). Origin and fate of nitrates in groundwater from the central Po plain: insights from isotopic investigations. Applied Geochemistry, 34: 164–180.
- Salomaki, E.D., Kwandrans, J., Eloranta, P. & Vis, M.L. (2014). Molecular and morphological evidence for Sheathia gen. nov. (Batrachospermales, Rhodophyta) and three new species. Journal of Phycology, 50: 526–542.
- Sheath, R.G., Vis, M.L. & Cole, K.M. (1994). Distribution and systematics of Batrachospermum (Batrachospermales, Rhodophyta) in North America. 4. Section Virescentia. Journal of Phycology, 30: 108–117.
- Sheath, R. G. & Sherwood, A.R. (2011). Phylum Rhodophyta (Red Algae). In The Freshwater Algal Flora of the British Isles. An Identification Guide to Freshwater and Terrestrial Algae. Second edition. (John, D.M., Whitton, B.A. & Brook, A.J., editors), 159–180. The Natural History Museum. Cambridge University Press, Cambridge.
- Talling, J.F. (1973). The application of some electrochemical methods to the measurement of photosynthesis and respiration in fresh water. Freshwater Biology, 3: 335–362.
- Valderrama, J.C. (1981). Methods used by the hydrographical department of the national board of fisheries. In Report of the Baltic Intercalibration Workshop (Grasshof, K., editor), 13–40. Interim Commission for the Protection of the Environment of Baltic Sea, Goteborg.
- Vis, M.L. & Sheath, R.G. (1996). Distribution and systematics of Batrachospermum (Batrachospermales, Rhodophyta) in North America. 9. Section Batrachospermum: description of five new species. Phycologia, 35: 124–134.
- Vis, M.L. & Sheath, R.G. (1998). A molecular and morphological investigation of the relationship between Batrachospermum spermatoinvolucrum and B. gelatinosum (Batrachospermales, Rhodophyta). European Journal of Phycology, 33: 231–239.
- Vis, M.L., Sheath, R.G. & Entwisle, T.J. (1995). Morphometric analysis of Batrachospermum section Batrachospermum (Batrachospermales, Rhodophyta) type specimens. European Journal of Phycology, 30: 35–55.
- Vis, M.L., Miller, E.J. & Hall, M.M. (2001). Biogeographical analyses of Batrachospermum helminthosum (Batrachospermales, Rhodophyta) in North America using molecular and morphological data. Phycologia, 40: 2–9.
- Vis, M.L., Feng, J., Chiasson, W.B., Xie, S.-L., Stancheva, R., Entwisle, T.J., Chou, J.-Y. & Wang, W.L. (2010). Investigation of the molecular and morphological variability in Batrachospermum arcuatum (Batrachospermales, Rhodophyta) from geographically distant locations. Phycologia, 49: 545–553.
- Zanfrognini, C. (1895). Contribuzione alla flora algologica del Modenese. Atti della Società dei Naturalisti di Modena, 13: 104–120.
