Abstract
Fucoid macroalgae are important primary producers and habitat modifiers on North Atlantic intertidal rocky shores. With decreasing latitude, western European fucoid populations display reduced levels of abundance, biomass and recruitment, while experiencing higher levels of physical environmental stress during summer months. We hypothesized that such reduction in the south is accompanied by a detectable decline in fucoid reproductive capacity. To test this hypothesis, morphological and reproductive traits of core (Welsh) and marginal (Portuguese) populations of two common fucoid species, Fucus vesiculosus and F. spiralis (Ochrophyta, Fucales), were examined. Morphological measurements showed that for a given thallus length, both fucoid species had smaller thallus volume and lower biomass in the southerly marginal part of the range. Significantly lower biomass of reproductive tissue of F. vesiculosus and a smaller number of receptacles per individual on specimens of both species indicate that levels of reproductive output are probably lower in southern populations. Despite the differences in reproductive traits observed between regions, reproductive effort (measured as the percentage of total dry biomass represented by reproductive tissue) of both species remained similar, as algae from both regions made similar investments in reproduction. The results indicate that stressful conditions reduced growth and number of receptacles of both species and amount of reproductive biomass of F. vesiculosus in the south but do not seem to change the way these algal species invest their energy. The decline in mass and reproductive biomass of specimens from southern shores found in this study, when combined with the lower abundance of adults and lower recruitment levels previously observed, is a strong indication of fucoid populations with lower levels of propagule output. This is an important factor when considering responses of these populations to a changing environment.
INTRODUCTION
Large brown fucoid macroalgae dominate rocky intertidal areas at wave-protected sites across the North Atlantic (Chapman, Citation1995; Jenkins et al., Citation2008). They play an important structuring role, with both positive and negative influences on the rest of the community (Chapman & Johnson, Citation1990; Eriksson et al., Citation2007; Jenkins et al., Citation2008). Their three-dimensional structure provides habitat space and reduces environmental extremes during emersion, thus enhancing the survival of many organisms (Thompson et al., Citation1996; Irving & Connell, Citation2006; Moore et al., Citation2007; Viejo et al., Citation2008). They also have a negative effect on others, through competition for resources (e.g. Jenkins et al., Citation1999; Hancock & Petraitis, Citation2001) or physical damage to organisms from the sweeping effects of their fronds (e.g. Hawkins, Citation1983; Jenkins & Hawkins, Citation2003).
Fucoid macroalgae are important intertidal primary producers throughout Europe, but their biomass and percentage cover decline from northern to southern European regions (Ballantine, Citation1961; Hawkins et al., Citation1992; Ferreira, Citation2012), with rapid decay at the southern range limits (Ardré, Citation1970; Boaventura et al., Citation2002; Lima et al., Citation2007). The southern continental distributional limit of important fucoid species, such as Fucus vesiculosus Linnaeus, Fucus serratus Linnaeus and Ascophyllum nodosum (Linnaeus) Le Jolis, occurs in Portugal (Araujo et al., Citation2009a, Citation2009b; Nicastro et al., Citation2013), while Fucus spiralis Linnaeus extends its distribution to Morocco (Ribera et al., Citation1992; Southward et al., Citation1995). The processes determining the decrease are not fully understood, yet factors such as grazing pressure, emersion stress and recruitment failure have been shown to affect fucoid abundance over this gradient (Jenkins et al., Citation2005; Davies et al., Citation2007; Viejo et al., Citation2011; Ferreira et al., Citation2014). Towards their range limits in southern Europe, the physiological performance of fucoid macroalgae is impacted by increased solar radiation and rising seawater and air temperatures (Pearson et al., Citation2009; Viejo et al., Citation2011; Martínez et al., Citation2012; Ferreira et al., Citation2014). The intensities of these physical parameters have risen over recent decades as a consequence of climate warming (IPCC, Citation2007; Lima et al., Citation2007; Sanchez-Lorenzo et al., Citation2009). The extent to which environmental change has a negative effect on fucoid physiology has been demonstrated (Martinez et al., Citation2012; Ferreira et al., Citation2014), but its impact on growth and reproductive capacity and consequently fitness of southern fucoid populations is still largely undetermined (Viejo et al., Citation2011; Wahl et al., Citation2011; Martínez et al., Citation2012).
The role of macroalgal density in maintaining viable populations is complex (Hixon et al., Citation2002), with effects on reproductive processes, dispersal and settlement (Schiel & Foster, Citation2006). However, in general, populations with greater density and biomass are expected to have increased fitness and reproductive output, at least up to the point when resources become scarce (Leimu et al., Citation2006). In such cases, high density can lead to limitations on settlement and recruitment (self-thinning) with consequent negative effects on population abundance over time (Reed, Citation1990; Creed et al., Citation1998; Scrosati, Citation2005). The major negative consequences for population maintenance occur when densities are low and mortality rates exceed reproductive replacement over a long enough period (Sodhi et al., Citation2009). The observed decline of fucoid biomass and percentage cover in populations from the southern limits of distribution (Ballantine, Citation1961; Ferreira, Citation2012) raises the possibility of reduced reproductive capacity in such populations, which in time could contribute to and further aggravate species decline in the area.
Different survival strategies to cope with variable environmental conditions are adopted by different species through the application of divergent patterns of reproductive investment (e.g. McCourt, Citation1985). Fucoid algae show variation in their investment in reproduction over different environmental gradients (Mathieson & Guo, Citation1992; Back et al., Citation1993; Araujo et al., Citation2011; Viejo et al., Citation2011). For example, populations of Fucus spiralis over a salinity gradient showed a reduction in reproductive biomass allocation in estuarine waters (Mathieson & Guo, Citation1992). A similar effect can also occur in populations exposed to greater wave action. In this case wave action, which shapes the horizontal distribution patterns of fucoid species, also reduces the reproductive biomass allocation of F. spiralis populations subject to high wave stress (Mathieson & Guo, Citation1992).
In the North East Atlantic, the overall level of environmental stress experienced by fucoid algae during summer increases with decreasing latitude, with evidence of negative effects on the physiological performance of these organisms (Martínez et al., Citation2012; Ferreira et al., Citation2014). Increasing stress combined with the decline of fucoid biomass (Ballantine, Citation1961; Ferreira, Citation2012) and poor levels of recruitment, even in the absence of dominant grazers (Coleman et al., Citation2006; Ferreira et al., Citation2015) in southern regions, led us to hypothesize that southern fucoid populations may exhibit diminished reproductive capacity when compared with populations from core areas of their distribution. To examine this hypothesis we sampled populations of F. vesiculosus and F. spiralis from near the edge of their range in Portugal and in the centre of their distribution in North Wales. Specimens were collected from within a fixed and restricted range of frond lengths (20 to 30 cm), from fully saline shores exposed to moderate wave action. This stratified approach allowed us to formally test if specimens from range centre and southern populations, exposed to different environmental conditions influenced by latitude, possess distinct morphological and reproductive traits. Vegetative and reproductive biomass were quantified for each population and also used to determine whether reproductive strategies differed between populations. Documenting the reproductive capacity of range edge populations in comparison to those in the range centre will enable an understanding of the dynamics of populations. In turn, this will allow insight into the role of climate change in the observed poleward contraction of southern European populations of canopy-forming macroalgae (Lamela-Silvarrey et al., Citation2012; Nicastro et al., Citation2013).
MATERIALS AND METHODS
Collection and preservation of specimens
The work focused exclusively on specimens of Fucus vesiculosus and F. spiralis (Ochrophyta, Fucales), as these species had previously been shown to be susceptible to insolation stress under summer conditions along the Portuguese coast (Pearson et al., Citation2009; Ferreira et al., Citation2014) and show a decline in abundance from central to southern areas of their distribution (Ballantine, Citation1961; Jenkins et al., Citation2008; Ferreira, Citation2012).
To test our hypothesis, collection of fertile reproductive F. vesiculosus and F. spiralis individuals was made on three shores, which were at least 2 km apart, in northern Portugal and Wales. These geographic regions are separated by 11º of latitude and correspond to areas of high (Wales) and low (Portugal) biomass and percentage of cover for these species (Ferreira, Citation2012). Portuguese populations of both species are near their southern limit of distribution. In Wales, the collection was made at Porth Cwyfan (53°10’58.2”N, 4°29’23.4”W), Cemlyn Bay (53°24’24.8”N, 4°32’03.6”W) and Trearddur Bay (53°16’15.9”N, 4°37’27.9”W), while in Portugal algae were collected from Viana do Castelo (41°41’33.6”N, 8°51’02.0”W), Areosa (41°42’45.7”N, 8°51’49.2”W) and Carreço (41°43’46.5”N, 8°52’23.5”W) populations. All the locations were from fully saline environments and moderately exposed to wave action (verified using the wave fetch model of Burrows et al., Citation2008) with maximal tidal range varying from 6.1 m in Wales to 3.6 m in Portugal.
The reproductive period of F. vesiculosus, more precisely the period of gamete release, takes place in the UK normally between April and July (Knight & Parke, Citation1950), while in Portugal the release of gametes occurs at least as early as April extending until June (Ladah et al., Citation2003, Citation2008). The period of gamete release of F. spiralis in the UK normally occurs between May to August (Vernet & Harper, Citation1980), while in Portugal it has been shown to start as early as April and finish in June (Ladah et al., Citation2003, Citation2008). Samples were collected during the last 10 days of May; a period that incorporates the peak of the reproductive season of F. vesiculosus and is within the reproductive season of F. spiralis. Adult specimens, between 20 and 30 cm, were collected from quadrats placed randomly across a 100 m stretch of shore. From each quadrat (starting from the top left corner to the bottom right corner) up to a maximum of four fucoid individuals within the specified size range were collected. This approach was taken to ensure random selection of appropriately sized individuals across a stretch of shoreline. Post hoc analyses confirmed, as expected, that significant differences in total length were not detected across distinct geographic regions (, ; , ). In total, due to their local abundance, 40 specimens of F. vesiculosus and 30 specimens of F. spiralis were collected from each shore. To avoid areas where the presence of F. guiryi and fucoid hybrids was more probable, F. spiralis fronds were collected high on the shore (see Zardi et al. (Citation2011) for description of fucoid vertical distribution on European shores), while the presence of vesicles made F. vesiculosus easily identifiable. After collection, the algae were transported in cold boxes to the laboratory where they were frozen to avoid tissue deterioration until later assessment.
Table 1. Two-way mix model ANOVA of Fucus vesiculosus length, volume, dry total biomass, dry vegetative biomass, dry reproductive biomass, dry single receptacle biomass, number of receptacles and reproductive effort.
Table 2. Two-way mix model ANOVA of Fucus spiralis length, volume, dry total biomass, dry vegetative biomass, dry reproductive biomass, dry single receptacle biomass, number of receptacles and reproductive effort.
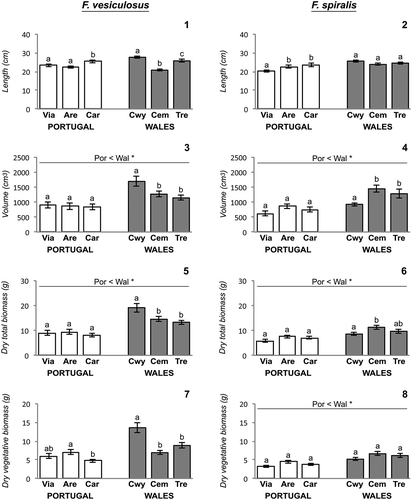
Figs 1–8. Fucus morphology across different shores at Portugal and Wales. Fig. 1. Fucus vesiculosus length. Fig. 2. Fucus spiralis length. Fig. 3. Fucus vesiculosus volume. Fig. 4. Fucus spiralis volume. Fig. 5. Fucus vesiculosus dry total biomass. Fig. 6. Fucus spiralis dry total biomass. Fig. 7. Fucus vesiculosus dry vegetative biomass. Fig. 8. Fucus spiralis dry vegetative biomass. Error bars = ±1SE; Only post hoc SNK tests of significant differences are presented; different lower case letters indicate significant differences of fucoid responses variables on populations from different shores within the same geographic regions, while line over bars indicate significant differences of fucoid responses variables between geographic regions; symbols *(P < 0.05) and **(P < 0.01) indicate levels of significant difference. Abbreviations used – Por: Portugal; Wal: Wales; Via: Viana do Castelo; Are: Areosa; Car: Carreço; Cwy: Porth Cwyfan; Cem: Cemlyn Bay; Tre: Trearddur Bay.
Measurement of response variables
Seven different response variables were analysed for each of the species. In addition, fucoid length, which was not expected to vary across geographic regions due to sampling stratification, was also re-measured in laboratory conditions. Morphological variables included volume (bushiness) and total and vegetative dry tissue biomass. Reproductive variables measured included quantification of the number of receptacles, mean reproductive dry tissue biomass and mean dry biomass of a single receptacle, while differences in reproductive investment, perceived as the amount of available energy channelled to reproduction, were informed by data on reproductive effort of specimens.
The number of receptacles per specimen and a crude estimate of thallus volume (bushiness) were determined using fresh algal tissue. The measurement of the maximum circumference, as described by Åberg (Citation1990), consisted of placing all fronds from one individual parallel to each other and measuring the circumference of the assembled fronds in several places until the maximum value was determined. Using the determined value it was possible to achieve a crude estimate of volume that reflects ‘bushiness’ of each individual by applying the formula for the volume of a cylinder (V = π r2 h). After this, algal tissue types (reproductive and somatic) were separated and dried, over a period of 72 h at 60ºC, to determine the total, vegetative and reproductive dry tissue biomass of specimens. In this study reproductive tissues were defined as just the receptacles (tips of vegetative branches). Finally, the mean dry single receptacle biomass per individual and an index of reproductive effort (as defined in Ang, Citation1992), comparable between specimens, were calculated using the following formulae:
Statistical analysis
In order to analyse the effects of geographic region and shore identity on the morphology and reproductive biomass allocation of F. vesiculosus and F. spiralis, two-way nested ANOVAs were used separately for each species. Geographic origin was treated as a fixed factor, while shore was considered random and nested in geographic origin. Significant results from the two-way ANOVA were further investigated with SNK (Student–Newman–Keuls) multiple comparisons. The assumption of homogeneity of variances was analysed with Cochran’s test (Cochran, Citation1951) and when necessary data were transformed (Supplementary Table). Despite multiple testing, we have not applied Bonferroni correction, following the arguments presented by Moran (Citation2003). The statistical package WinGMAV5 (EICC, University of Sydney) was chosen to run all the ANOVA analyses.
Linear regressions were used to analyse the relationship between total number of receptacles and total plant dry weight for populations from Portugal and Wales of each species. Data were transformed (√3(x+1)) to improve the normality of distribution of residuals and assure homoscedasticity. Differences between regions were analysed through t-tests on slopes of regression lines. These statistical analyses were undertaken using SPSS Statistics Version 20.
RESULTS
Fucoid morphology
Volume (Bushiness)
The volume of F. vesiculosus and F. spiralis individuals differed among geographic regions (, ). For both fucoid species, individuals from Portugal had a significantly smaller volume than individuals from Wales (, ), being 37% and 39% less than those of core populations of F. vesiculosus and F. spiralis, respectively. Significant differences among shores were only detected among Welsh shores (, ; , ).
Total dry tissue biomass
Amounts of total dry tissue biomass in both species reflected the results for volume. Heavier specimens were from core populations, with differences of total mean mass between geographic regions of 44% and 31% in F. vesiculosus and F. spiralis, respectively (, ; , ). Variability of total dry biomass of F. vesiculosus and F. spiralis populations among shores was exclusively observed among Welsh shores (, ).
Vegetative dry tissue biomass
Differences in the amounts of vegetative dry tissue biomass occurring at a local scale were observed for the two fucoid species, but differences at larger spatial scales were only detected in F. spiralis populations (, ; , ). Welsh F. spiralis specimens exhibited greater quantity of mean vegetative dry tissue biomass with mean values (mean ± SE; n = 90) reaching 6.0 ± 0.3 g, which were significantly greater than the mean of 3.8 ± 0.2 g shown by Portuguese populations. On a local scale, amounts of vegetative dry tissue biomass from both fucoid species varied among shores at both geographic regions (, ; , ).
Reproductive traits
Reproductive dry tissue biomass
Fucus vesiculosus populations from Welsh and Portuguese regions showed different levels of reproductive dry tissue biomass (). Specimens of F. vesiculosus from Welsh populations produced more than twice the amount of reproductive tissue biomass compared with the populations from Portuguese shores, with mean values (mean ± SE; n = 120) of 5.9 ± 0.3 g and 2.8 ± 0.2 g, respectively (). In F. spiralis, however, differences between geographic regions were not detected ().
Figs 9–16. Fucus reproductive traits across different shores at Portugal and Wales. Fig. 9. Fucus vesiculosus dry reproductive biomass. Fig. 10. Fucus spiralis dry reproductive biomass. Fig. 11. Fucus vesiculosus dry single receptacle biomass. Fig. 12. Fucus spiralis dry single receptacle biomass. Fig. 13. Fucus vesiculosus number of receptacles. Fig. 14. Fucus spiralis number of receptacles. Fig. 15. Fucus vesiculosus reproductive effort. Fig. 16. Fucus spiralis reproductive effort. Error bars = ±1SE; Only post hoc SNK tests of significant differences are presented; different lowercase letters indicate significant differences of fucoid responses variables on populations from different shores within the same geographic regions, while lines over bars indicate significant differences of fucoid responses variables between geographic regions; *(P < 0.05) and **(P < 0.01) indicate levels of significant difference. Abbreviations used – Por: Portugal; Wal: Wales; Via: Viana do Castelo; Are: Areosa; Car: Carreço; Cwy: Porth Cwyfan; Cem: Cemlyn Bay; Tre: Trearddur Bay.
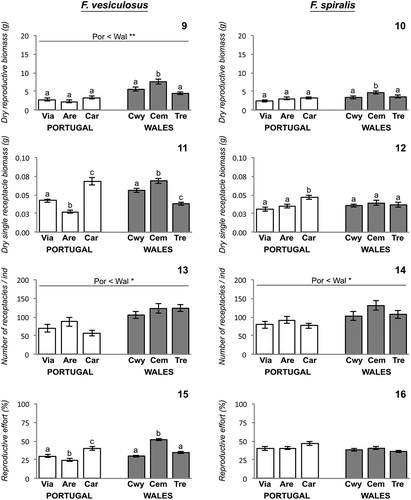
In both F. vesiculosus and F. spiralis reproductive dry tissue biomass varied between shores exclusively at Welsh shores (, ; , ).
Single receptacle dry tissue biomass
The mean dry biomass of a receptacle from either species did not significantly differ between geographic regions (, ). Results from both fucoid species receptacles were still variable between shores (, ). Variability in mean dry biomass of receptacles between shores occurred at both geographic regions for F. vesiculosus, while for F. spiralis it was only detected at Portuguese shores (, ).
Number of receptacles per individual
The mean number of receptacles (mean ± SE; n = 120) present in individuals of F. vesiculosus was less on Portuguese 71.1 ± 5.8 than on Welsh shores 117.0 ± 6.0 (). Portuguese populations of F. spiralis also had lower mean numbers of reproductive receptacles per individual (mean ± SE; n = 90), 82.6 ± 5.0 compared with 113.2 ± 7.0 in Wales (). These differences between geographic regions were significantly different (, ), indicating that Portuguese populations were developing fewer receptacles per individual alga in both species. The mean values among shores from each of the geographic regions were similar for both species (, ).
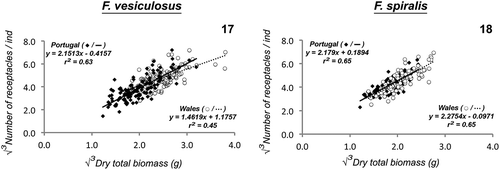
The number of receptacles also showed a strong positive relationship with total dry tissue biomass in populations from either geographic regions of F. vesiculosus and F. spiralis (F. vesiculosus (Portugal): r2 = 0.629, P < 0.01, n = 120; F. vesiculosus (Wales): r2 = 0.445, P < 0.01, n = 120; F. spiralis (Portugal): r2 = 0.655, P < 0.01, n = 90; F. spiralis (Wales): r2 = 0.651, P < 0.01, n = 90; , ). The regression coefficients (slope) were not significantly different (P = 0.69, t-test) between F. spiralis populations from distinct geographic regions but differed between F. vesiculosus populations, with a regression coefficient significantly lower in populations from Wales (P < 0.01, t-test). This indicates that larger F. vesiculosus specimens in Portugal have more receptacles per unit of biomass, which means a greater percentage of the energy available is diverted into reproduction rather than growth in larger specimens in Portugal (, ).
Reproductive effort
Significant differences in reproductive effort of F. vesiculosus populations from distinct geographic regions were not detected (), despite some variability observed in populations from different shores within Portuguese and Welsh regions (; ). Overall, the mean reproductive effort (mean ± SE; n = 40) of F. vesiculosus populations ranged between 24.4 ± 2.0% and 39.9 ± 2.2% in Portuguese shores and 30 ± 1.4% and 51.8 ± 1.8% in Welsh shores ().
Fucus spiralis reproductive effort was not significantly different across both geographic regions and within each geographic region (). The mean reproductive effort (mean ± SE; n = 30) for populations of this species was between 39.7 ± 2.6% and 46.5 ± 2.3% in Portuguese shores and 36.1 ± 1.7% and 40.3 ± 2.1% in Welsh shores ().
DISCUSSION
We tested the hypothesis that the decline in fucoid abundance and overall biomass from core (Wales) to marginal regions (Portugal), described by Ballantine (Citation1961) and quantified by Ferreira (Citation2012), is accompanied by detectable changes in reproductive capacity.
Our results showed that morphological traits of F. vesiculosus and F. spiralis varied between populations from Wales and Portugal. For plants of equivalent length, the volume of northern plants was over 37% higher for both fucoid species. These ‘bushier’ plants were also heavier, showing regional differences in total dry biomass for both species and in vegetative dry biomass in F. spiralis. These results are in agreement with previous studies that demonstrated the presence of smaller and lighter fucoid specimens in marginal populations (Araujo et al., Citation2011, Citation2014; Viejo et al., Citation2011) and with physiological experiments that demonstrated that specimens subjected to higher physiological stress show reduced growth rates (Martínez et al., Citation2012).
In addition to the decline in total biomass, reproduction-related variables of F. vesiculosus and F. spiralis were also shown to vary between populations from distinct regions. As has been shown for fucoid populations from other regions (e.g. Robertson, Citation1987), the number of receptacles per alga is significantly influenced by the specimen own total dry mass. For both species, the number of receptacles per specimen in our study was significantly lower in populations from the Portuguese region. The influence of their lower mean total dry weight meant that even F. vesiculosus populations from Portugal, where a greater rate of number of receptacles per unit of mass in larger individuals was observed, produced a lower number of receptacles. The mean mass per receptacle in contrast was similar across populations for both species. This combination of factors led to a significant decline in reproductive dry tissue biomass from core to southern regions in F. vesiculosus, with a similar, although not significant, trend also observed in F. spiralis. These observations led to the conclusion that there is a reduction in reproductive output in range edge populations. However, this conclusion makes an important assumption, namely that the density of viable gametes released per receptacle is equivalent across populations. It could be argued that receptacles from different areas could produce different number of gametes (Kalvas & Kautsky, Citation1993; Berger et al., Citation2001). However, the possibility of reduced reproductive output in southern populations is supported by previous studies, which have shown that, although southern fucoid populations present high fertilization rates during spawning events (Ladah et al., Citation2003), they still possess low fucoid recruitment at Portuguese shores even under reduced grazing pressure and ameliorated physical conditions (Ferreira et al., Citation2015). Further support is given by the observed reduction in reproductive capacity of the closely related F. serratus in nearby populations from the northern coast of the Iberian Peninsula (Viejo et al., Citation2011).
Latitudinal scale changes in the reproductive output of both species could be caused by a reduction in total biomass in southern populations or additionally by a decline in proportional investment in reproduction. Populations of F. vesiculosus exposed to different salinity levels have been shown to invest differently in reproduction, leading to differences in the reproductive output between populations (Back et al., Citation1993; Kalvas & Kautsky, Citation1993). In contrast, our results on reproductive effort of F. vesiculosus and F. spiralis, measured as the proportion of dry reproductive biomass to total dry biomass, showed no significant differences between populations from different geographic regions. Similarly, Creed et al. (Citation1996) demonstrated no change in reproductive effort in F. vesiculosus growing under different levels of intraspecific competition in the Isle of Man, UK. Our observations showed that reproductive effort was approximately 35% and 40% for F. vesiculosus and F. spiralis, respectively, independent of geographic region, values which are within the range previously reported for these species (Mathieson & Guo, Citation1992; Brenchley et al., Citation1996). The absence of differences in reproductive effort between populations indicates that the increase in stressful conditions in southern regions does not seem to change the way these fucoid species invest their energy. Therefore, the observed decline in reproductive biomass and number of receptacles available is not due to a decline in proportional investment in reproduction, but is only a consequence of the overall reduction in growth and total biomass presumably caused by an increase in stressful conditions experienced by southern populations.
The morphological and reproductive variability shown by populations over this large spatial scale could be due to long periods of adaptation to regional climate or be the result of disruptions imposed by harsh climatic conditions, as severe stress conditions have consistently been shown to negatively impact the amount of reproductive biomass of fucoid species (Dethier et al., Citation2005; Dethier & Williams, Citation2009). In either case, differences are likely to impact on the dynamics of populations. For example, regional differences, such as those shown between populations in our study, will affect how populations respond to a reduction in grazing pressure. It seems likely that as a consequence of the lower abundance, smaller plant size and reduced reproductive capacity of fucoid populations in Portugal, local or general reductions in grazing pressure only lead to limited fucoid recruitment (as shown by Coleman et al., Citation2006; Ferreira et al., Citation2015), a result that contrasts with the strong response to reduced grazing pressure shown in northern regions (Coleman et al., Citation2006; Ferreira et al., Citation2015).
In addition to the regional differences observed, local differentiation of populations was also apparent. Variability among shores within each of the geographic regions, measuring variation on the scale of tens of kilometres, influenced total dry tissue biomass, volume (bushiness), dry vegetative and reproductive tissue biomass and mean receptacle dry biomass of both fucoid species. The influence of these local scale effects was also shown on the total reproductive effort of F. vesiculosus. Variability at this spatial scale was expected and has previously been reported at several locations. Some examples include the variability in receptacle dry biomass of F. vesiculosus across different shores on Merseyside, UK (Russell, Citation1979) or the variability of Ascophyllum nodosum total reproductive effort observed across shores in New England, USA (Mathieson & Guo, Citation1992). The observation of fucoid populations with distinct reproductive traits is not uncommon and has normally been associated with local changes in salinity or wave exposure levels (Mathieson & Guo, Citation1992; Blanchette, Citation1997). The results of our study highlight the fact that apart from those factors, others not controlled by our experiment also cause local variability in morphological and reproductive characteristics of F. vesiculosus and F. spiralis populations.
In summary, our work shows the effects of local environment and latitudinal environmental changes on morphology and reproductive traits of fucoids. We demonstrate that levels of stress occurring on the Portuguese coast, which have already been shown to affect physiological performance of fucoid species (Martinez et al., Citation2012; Ferreira et al., Citation2014), also reduced growth and reproductive capacity of local F. vesiculosus and F. spiralis populations. Although reproductive effort remains similar, the number of receptacles in both species and reproductive biomass of F. vesiculosus in southern populations is reduced due to the lower biomass of specimens. Such reductions are likely to affect reproductive output and consequently reduce recruitment success, which has already been shown to be low (Coleman et al., Citation2006; Ferreira et al., Citation2015), and could consequently explain the observed decline of fucoid abundance at this southern edge of their distribution (Ballantine, Citation1961; Ferreira, Citation2012). Our results also suggest that increased levels of the stresses limiting reproductive capacity of these fucoid species may lead to a northward contraction of their southern range edge, with consequent impacts on the associated community and on local levels of intertidal primary productivity. This scenario is supported by the recently described contraction observed in the distribution of F. vesiculosus in Morocco and in the Bay of Biscay (Nicastro et al., Citation2013). Studies on the closely related F. serratus have also shown that southern populations of this species present lower stochastic population growth when compared with populations from central areas of distribution and are therefore more susceptible to environmental variation (Araujo et al., Citation2014). Therefore, future work should not only address the effects of individual stresses acting on Fucus spp., but also try to understand the consequences of higher order interactions between stresses on fucoid spatial distribution and survival across marginal and core populations. Such information is needed for the development and testing of models that can predict the consequences of future climatic changes to the survival and population dynamics of these important intertidal primary producers across their distribution range.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
SUPPLEMENTARY INFORMATION
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at http://do.org.doi/10.1080/09670262.2015.1066036
Supplementary Table 1. Information on data transformation.
Supplementary Table
Download MS Word (34.5 KB)ACKNOWLEDGEMENTS
We are grateful to Dr Fernando Lima and Dr Francisco Arenas for their helpful advice on the distribution of local Portuguese fucoid populations. We dedicate this paper to the memory of George Russell who got SJH interested in fucoids.
Additional information
Funding
Notes on contributors
João G. Ferreira
João G. Ferreira: original concept, field sampling, laboratory work, statistical analysis, drafting and editing manuscript; Stephen J. Hawkins: original concept, statistical analysis, drafting and editing manuscript; Stuart R. Jenkins: original concept, statistical analysis, drafting and editing manuscript.
Stephen J. Hawkins
João G. Ferreira: original concept, field sampling, laboratory work, statistical analysis, drafting and editing manuscript; Stephen J. Hawkins: original concept, statistical analysis, drafting and editing manuscript; Stuart R. Jenkins: original concept, statistical analysis, drafting and editing manuscript.
Stuart R. Jenkins
João G. Ferreira: original concept, field sampling, laboratory work, statistical analysis, drafting and editing manuscript; Stephen J. Hawkins: original concept, statistical analysis, drafting and editing manuscript; Stuart R. Jenkins: original concept, statistical analysis, drafting and editing manuscript.
REFERENCES
- Åberg, P. (1990). Measuring size and choosing category size for a transition matrix study of the seaweed Ascophyllum nodosum. Marine Ecology Progress Series, 63: 281–287.
- Ang, P.O. (1992). Cost of reproduction in Fucus distichus. Marine Ecology Progress Series, 89: 25–35.
- Araújo, R., Barbara, I., Tibaldo, M., Berecibar, E., Tapia, P.D., Pereira, R., Santos, R. & Pinto, I.S. (2009a). Checklist of benthic marine algae and cyanobacteria of northern Portugal. Botanica Marina, 52: 24–46.
- Araújo, R., Vaselli, S., Almeida, M., Serrão, E. & Sousa-Pinto, I. (2009b). Effects of disturbance on marginal populations: human trampling on Ascophyllum nodosum assemblages at its southern distribution limit. Marine Ecology Progress Series, 378: 81–92.
- Araújo, R., Serrão, E.A., Sousa-Pinto, I. & Åberg, P. (2011). Phenotypic differentiation at southern limit borders: the case study of two fucoid macroalgal species with different life-history traits. Journal of Phycology, 47: 451–462.
- Araújo, R., Serrão, E.A., Sousa-Pinto, I., & Åberg, P. (2014). Spatial and temporal dynamics of fucoid populations (Ascophyllum nodosum and Fucus serratus): a comparison between central and range edge populations. PloS ONE, 9: e92177.
- Ardré, F. (1970). Contribution à l’étude des algues marines du Portugal I. La Flore. Portugaliae Acta Biologica (B), 10: 1–423.
- Back, S., Collins, J.C. & Russell, G. (1993). Comparative reproductive biology of the Gulf of Finland and the Irish Sea Fucus vesiculosus L. Sarsia, 78: 265–272.
- Ballantine, W.J. (1961). A biologically defined exposure scale for the comparative description of rocky shores. Field Studies, 1: 1–19.
- Berger, R., Malm, T., & Kautsky, L. (2001). Two reproductive strategies in Baltic Fucus vesiculosus (Phaeophyceae). European Journal of Phycology, 36: 265–273.
- Blanchette, C.A. (1997). Size and survival of intertidal plants in response to wave action: a case study with Fucus gardneri. Ecology, 78: 1563–1578.
- Boaventura, D., Ré, P., Da Fonseca, L.C. & Hawkins, S.J. (2002). Intertidal rocky shore communities of the continental Portuguese coast: analysis of distribution patterns. Marine Ecology–Pubblicazioni Della Stazione Zoologica Di Napoli I, 23: 69–90.
- Brenchley, J.L., Raven, J.A. & Johnston, A.M. (1996). A comparison of reproductive allocation and reproductive effort between semelparous and iteroparous fucoids (Fucales, Phaeophyta). Hydrobiologia, 327: 185–190.
- Burrows, M.T., Harvey, R. & Robb, L. (2008). Wave exposure indices from digital coastlines and the prediction of rocky shore community structure. Marine Ecology Progress Series, 353: 1–12.
- Chapman, A.R.O. (1995). Functional ecology of fucoid algae: twenty-three years of progress. Phycologia, 34: 1–32.
- Chapman, A.R.O. & Johnson, C.R. (1990). Disturbance and organization of macroalgal assemblages in the northwest Atlantic. Hydrobiologia, 192: 77–121.
- Cochran, W.G. (1951). Testing a linear relation among variances. Biometrics, 7: 17–32.
- Coleman, R.A., Underwood, A.J., Benedetti-Cecchi, L., Åberg, P., Arenas, F., Arrontes, J., Castro, J., Hartnoll, R.G., Jenkins, S.R., Paula, J., Della Santina, P. & Hawkins, S.J. (2006). A continental scale evaluation of the role of limpet grazing on rocky shores. Oecologia, 147: 556–564.
- Creed, J.C., Norton, T.A. & Kain, J.M. (1996). Are neighbours harmful or helpful in Fucus vesiculosus populations? Marine Ecology Progress Series, 133: 191–201.
- Creed, J.C., Kain, J.M. & Norton, T.A. (1998). An experimental evaluation of density and plant size in two large brown seaweeds. Journal of Phycology, 34: 39–52.
- Davies, A.J., Johnson, M.P. & Maggs, C.A. (2007). Limpet grazing and loss of Ascophyllum nodosum canopies on decadal time scales. Marine Ecology Progress Series, 339: 131–141.
- Dethier, M.N. & Williams, S.L. (2009). Seasonal stresses shift optimal intertidal algal habitats. Marine Biology, 156: 555–567.
- Dethier, M.N., Williams, S.L. & Freeman, A. (2005). Seaweeds under stress: manipulated stress and herbivory affect critical life-history functions. Ecological Monographs, 75: 403–418.
- Eriksson, B.K., Rubach, A. & Hillebrand, H. (2007). Dominance by a canopy forming seaweed modifies resource and consumer control of bloom-forming macroalgae. Oikos, 116: 1211–1219.
- Ferreira, J.G. (2012). Latitudinal and climatic driven changes in local patterns of intertidal macroalgae: implications for biodiversity and ecosystem functioning. PhD thesis, School of Ocean Sciences, Bangor University, Bangor.
- Ferreira, J.G., Arenas, F., Martínez, B., Hawkins, S.J. & Jenkins, S.R. (2014). Physiological response of fucoid algae to environmental stress: comparing range centre and southern populations. New Phytologist, 202: 1157–1172.
- Ferreira, J.G., Hawkins, S.J., & Jenkins, S.R. (2015). Physical and biological control of fucoid recruitment in range edge and range centre populations. Marine Ecology Progress Series, 518: 85–94.
- Hancock, K.M. & Petraitis, P.S. (2001). Effects of herbivorous snails and macroalgal canopy on recruitment and early survivorship of the barnacle Semibalanus balanoides (L.). Journal of Experimental Marine Biology and Ecology, 257: 205–218.
- Hawkins, S.J. (1983). Interactions of Patella and macroalgae with settling Semibalanus balanoides (L). Journal of Experimental Marine Biology and Ecology, 71: 55–72.
- Hawkins, S.J., Hartnoll, R.G., Kain, J.M. & Norton, T.A. (1992). Plant–animal interactions on hard substrata in the north-east Atlantic. In Plant–Animal Interactions in the Marine Benthos (John, D.M., Hawkins, S.J. & Price, J.H., editors), 1–32. Oxford University Press, Oxford.
- Hixon, M.A., Pacala, S.W., & Sandin, S.A. (2002). Population regulation: historical context and contemporary challenges of open vs. closed systems. Ecology, 83: 1490–1508.
- IPCC (2007). Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge.
- Irving, A.D. & Connell, S.D. (2006). Predicting understorey structure from the presence and composition of canopies: an assembly rule for marine algae. Oecologia, 148: 491–502.
- Jenkins, S.R. & Hawkins, S.J. (2003). Barnacle larval supply to sheltered rocky shores: a limiting factor? Hydrobiologia, 503: 143–151.
- Jenkins, S.R., Hawkins, S.J. & Norton, T.A. (1999). Interaction between a fucoid canopy and limpet grazing in structuring a low shore intertidal community. Journal of Experimental Marine Biology and Ecology, 233: 41–63.
- Jenkins, S.R., Coleman, R.A., Della Santina, P., Hawkins, S.J., Burrows, M.T. & Hartnoll, R.G. (2005). Regional scale differences in the determinism of grazing effects in the rocky intertidal. Marine Ecology Progress Series, 287: 77–86.
- Jenkins, S.R., Moore, P., Burrows, M.T., Garbary, D.J., Hawkins, S.J., Ingolfsson, A., Sebens, K.P., Snelgrove, P.V.R., Wethey, D.S. & Woodin, S.A. (2008). Comparative ecology of North Atlantic shores: do differences in players matter for process? Ecology, 89: S3–S23.
- Kalvas, A. & Kautsky, L. (1993). Geographical variation in Fucus vesiculosus morphology in the Baltic and North Seas. European Journal of Phycology, 28: 85–91.
- Knight, M. & Parke, M. (1950). A biological study of Fucus vesiculosus L and F serratus L. Journal of the Marine Biological Association of the United Kingdom, 29: 439–514.
- Ladah, L., Bermudez, R., Pearson, G. & Serrão, E. (2003). Fertilization success and recruitment of dioecious and hermaphroditic fucoid seaweeds with contrasting distributions near their southern limit. Marine Ecology Progress Series, 262: 173–183.
- Ladah, L.B., Feddersen, F., Pearson, G.A. & Serrão, E.A. (2008). Egg release and settlement patterns of dioecious and hermaphroditic fucoid algae during the tidal cycle. Marine Biology, 155: 583–591.
- Lamela-Silvarrey, C., Fernandez, C., Anadon, R. & Arrontes, J. (2012). Fucoid assemblages on the north coast of Spain: past and present (1977–2007). Botanica Marina, 55: 199–207.
- Leimu, R., Mutikainen, P., Koricheva, J. & Fischer, M. (2006). How general are positive relationships between plant population size, fitness and genetic variation? Journal of Ecology, 94: 942–952.
- Lima, F.P., Ribeiro, P.A., Queiroz, N., Hawkins, S.J., & Santos, A.M. (2007). Do distributional shifts of northern and southern species of algae match the warming pattern? Global Change Biology, 13: 2592–2604.
- Martínez, B., Arenas, F., Rubal, M., Burgues, S., Esteban, R., Garcia-Plazaola, I., Figueroa, F.L., Pereira, R., Saldana, L., Sousa-Pinto, I., Trilla, A. & Viejo, R.M. (2012). Physical factors driving intertidal macroalgae distribution: physiological stress of a dominant fucoid at its southern limit. Oecologia, 170: 341–353.
- Mathieson, A.C. & Guo, Z.Y. (1992). Patterns of fucoid reproductive biomass allocation. British Phycological Journal, 27: 271–292.
- McCourt, R.M. (1985). Reproductive biomass allocation in three Sargassum species. Oecologia, 67: 113–117.
- Moore, P., Hawkins, S.J. & Thompson, R.C. (2007). Role of biological habitat amelioration in altering the relative responses of congeneric species to climate change. Marine Ecology Progress Series, 334: 11–19.
- Moran, M.D. (2003). Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos, 100: 403–405.
- Nicastro, K.R., Zardi, G.I., Teixeira, S., Neiva, J., Serrão, E.A. & Pearson, G.A. (2013). Shift happens: trailing edge contraction associated with recent warming trends threatens a distinct genetic lineage in the marine macroalga Fucus vesiculosus. BMC Biology, 11: 6.
- Pearson, G.A., Lago-Leston, A. & Mota, C. (2009). Frayed at the edges: selective pressure and adaptive response to abiotic stressors are mismatched in low diversity edge populations. Journal of Ecology, 97: 450–462.
- Reed, D.C. (1990). An experimental evaluation of density dependence in a subtidal algal population. Ecology, 71: 2286–2296.
- Ribera, M.A., Garreta, A.G., Gallardo, T., Cormaci, M., Furnari, G. & Giaccone, G. (1992). Checklist of Mediterranean seaweeds. 1. Fucophyceae (Warming, 1884). Botanica Marina, 35: 109–130.
- Robertson, B.L. (1987). Reproductive ecology and canopy structure of Fucus spiralis L. Botanica Marina, 30: 475–482.
- Russell, G. (1979). Heavy receptacles in estuarine Fucus vesiculosus L. Estuarine and Coastal Marine Science, 9: 659–661.
- Sanchez-Lorenzo, A., Calbo, J., Brunetti, M. & Deser, C. (2009). Dimming/brightening over the Iberian Peninsula: trends in sunshine duration and cloud cover and their relations with atmospheric circulation. Journal of Geophysical Research – Atmospheres, 114: D00D09.
- Schiel, D.R. & Foster, M.S. (2006). The population biology of large brown seaweeds: ecological consequences of multiphase life histories in dynamic coastal environments. Annual Review of Ecology, Evolution, and Systematics, 37: 343–372.
- Scrosati, R. (2005). Review of studies on biomass-density relationships (including self-thinning lines) in seaweeds: main contributions and persisting misconceptions. Phycological Research, 53: 224–233.
- Sodhi, N.S., Brook, B.W. & Bradshaw, C.A.J. (2009). Causes and consequences of species extinctions. In Princeton Guide to Ecology (Levin, S.A., editor), 514–520. Princeton University Press, Princeton, NJ.
- Southward, A.J., Hawkins, S.J., & Burrows, M.T. (1995). Seventy years observations of changes in distribution and abundance of zooplankton and intertidal organisms in the Western English channel in relation to rising sea temperature. Journal of Thermal Biology, 20: 127–155.
- Thompson, R.C., Wilson, B.J., Tobin, M.L., Hill, A.S., & Hawkins, S.J. (1996). Biologically generated habitat provision and diversity of rocky shore organisms at a hierarchy of spatial scales. Journal of Experimental Marine Biology and Ecology, 202: 73–84.
- Vernet, P. & Harper, J.L. (1980). The costs of sex in seaweeds. Biological Journal of the Linnean Society, 13: 129–138.
- Viejo, R.M., Arenas, F., Fernandez, C., & Gomez, M. (2008). Mechanisms of succession along the emersion gradient in intertidal rocky shore assemblages. Oikos, 117: 376–389.
- Viejo, R.M., Martínez, B., Arrontes, J., Astudillo, C. & Hernandez, L. (2011). Reproductive patterns in central and marginal populations of a large brown seaweed: drastic changes at the southern range limit. Ecography, 34: 75–84.
- Wahl, M., Jormalainen, V., Eriksson, B.K., Coyer, J.A., Molis, M., Schubert, H., Dethier, M., Karez, R., Kruse, I., Lenz, M., Pearson, G., Rohde, S., Wikstrom, S.A. & Olsen, J.L. (2011). Stress ecology in Fucus: abiotic, biotic and genetic interactions. Advances in Marine Biology, 59: 37–105.
- Zardi, G.I., Nicastro, K.R., Canovas, F., Costa, J.F., Serrão, E.A. & Pearson, G.A. (2011). Adaptive traits are maintained on steep selective gradients despite gene flow and hybridization in the intertidal zone. PloS ONE, 6: e19402.