Abstract
Hormosira banksii, the most successful intertidal fucoid in the southern hemisphere, usually inhabits only the lower shore. On Kainga Reef, at 36°S on the New South Wales coast, it is one of only two macroalgae in most of the pools on a platform above xthe lowest high water; 29 pools in the middle of the platform were monitored photographically for 12 years. Recruitment was not seasonal. It took about 5–8 months for germlings to become visible, when they consisted of about 3 vesicles. The growth rate of germlings was between linear and exponential. Individuals could become fertile when they were 11–14 months old. Adult frond growth rate in the mid-platform pools, measured in situ, was highest in autumn but similar in the other seasons. In summer, however, in pools at the edge of the reef, often flushed by waves, the growth rate was as fast in summer as in autumn, either because of better provision of scarce nutrients or because of less inhibition by high temperature. Mortality, both of individuals and of fronds, was highest in the summer when pool isolation for one or more tidal cycles, caused by the coincidence of low high tide levels and a calm sea, was more common. Hormosira could tolerate full summer sunlight, water temperatures of up to 40°C, 140 psu (four times the strength of seawater) but not dehydration. Dehydration caused inter-annual variation in cover and in the total number of plants present, but with no overall trend over 12 years. Sand cover and rotting cast weed could also cause mortality. Survivorship was Slobodkin’s Type IV, with few plants reaching 4 years but high longevity after that, due to the establishment of a holdfast which could regenerate new fronds. Compared with other fucoids, growth was slow. The lifestyle resembles that of Ascophyllum nodosum.
INTRODUCTION
Hormosira banksii (Turner) Decaisne is the most successful intertidal fucoid in the southern hemisphere. Hormosira is monospecific (Clarke & Womersley, Citation1981), although there is considerable variation in morphology in different habitats (Bergquist, Citation1959a; Ralph et al., Citation1998; Macinnis-Ng et al., Citation2005), in contrast to Fucus in the northeast Atlantic which has diversified into specialist species found at particular levels of the shore or in estuaries. It appears relatively versatile because it inhabits a considerable proportion of the vertical range of intertidal rock and withstands estuarine salinities. It is temperate, with northern limits near Ballina, New South Wales and Norfolk Island, both at 29°S (Millar, Citation1999) and a southern limit on Stewart Island at 47°S (Adams, Citation1994). While this is a narrow latitudinal range compared with Fucus spp., sea surface isotherms are closer together in the Tasman Sea than in the East Atlantic. Hormosira can endure sea surface temperatures down to 9°C (Stewart Island winter) and up to 26°C (Ballina summer) (NODC, Citation2009).
Hormosira, being a fucoid, grows apically, producing a chain of vesicles, branching at irregular intervals (Clayton et al., Citation1985; Womersley, Citation1987). Plants are dioecious, with conceptacles under the surface of mature vesicles at all times of year (Clayton, Citation1990). Multiple fronds arise from a single holdfast. Each vesicle has a parenchymatous cortex and loose medulla around water and gas, regarded as a mechanism for retaining water and increasing drought resistance (Bergquist, Citation1959b; Dromgoole, Citation1980; Chapman, Citation1995). Hormosira recovers poorly from desiccation (Brown, Citation1987) and on open rock is usually confined to around mid-shore or lower, where it is never emersed for more than one tidal cycle (Underwood, Citation1981; Schiel & Taylor, Citation1999; Dunmore, Citation2006; Schiel, Citation2006). Often the dominant alga on fairly sheltered shores (Osborn, Citation1948; Underwood, Citation1998; Dunmore, Citation2006; McKenzie & Bellgrove, Citation2009), on Kainga Reef this alga occupied many pools above neap tides (Kain, Citation2008). Pools in this high shore position, together with supratidal pools, can be dependent on waves to flush them, so in calm weather they can be isolated for several tidal cycles and become hyposaline in rainy conditions or hypersaline through evaporation, in addition to temperature fluctuations with the possibility of drying out.
In rockpools in the Baltic Sea, temperature, rather than salinity, was thought to be the most important factor determining the community present (Ganning, Citation1971). In high rockpools in the North Atlantic (Pyefinch, Citation1943; Goss-Custard et al., Citation1979; Femino & Mathieson, Citation1980; Sze, Citation1980; Wolfe & Harlin, Citation1988; Björk et al., Citation2004), North Pacific (Dethier, Citation1984) and New Zealand (Ambler & Chapman, Citation1950), Chlorophyta, in particular the euryhaline Ulva intestinalis, are dominant though in Massachusetts, USA, this depended on grazer density (Lubchenco, Citation1978) and in Maine, USA, Fucus distichus occurred in high pools (Pearson & Brawley, Citation1996). On the coast of Portugal, Lithophyllum incrustans was also important (Araújo et al., Citation2006) in high-shore pools
In contrast, on Kainga Reef only brown algae occupied this niche and Hormosira was one of only two macroalgae occupying most of the pools, the other being the crust of Colpomenia bullosa (Saunders) Yamada (Kain et al., Citation2010). Even the obligate epiphyte Notheia anomala (Hallam et al., Citation1980) was absent from the pools in the middle of the platform. The following investigation was designed to determine the attributes that allow Hormosira to be successful where other macrophytic species fail.
MATERIALS AND METHODS
Study site
Kainga Reef (35°49′52′′S, 150°13′21′′E) is a rocky, partly intertidal, platform with numerous pools. Most of the pools are situated around four much larger pools at slightly different heights (). Isolation of pools could occur when the higher of the two daily high waters (HHW) was below the height of a pool and the swell was negligible. The median swell height on the platform was 0.5 m and most of the pools were rarely isolated for two tidal cycles (Kain, Citation2008; , ). Maximum pool temperatures in summer could reach 10°C above that of the sea, the mean reaching 35°C in summer (Kain, Citation2008; ).
Fig. 1. Photograph of Kainga Reef from the cliff, facing southwest. Black bar, 2 m; red dots, mid pools; blue dots, edge pools.

Fig. 2. A diagrammatic explanation of how growth in vesicle number (Rv) was measured in situ in mature fronds of Hormosira. Letters to the left name the vesicles, lower case for Time 1, upper case for Time 2. What was the third vesicle at Time 1 was designated the scale vesicle and its diameter assumed to be unchanged by Time 2 (see text). The formulae for determining the proportional size of the two apical vesicles relative to the appropriate mature vesicles are shown to the right. The equation expresses the calculated final (at Time 2) number of vesicles apical to the original third vesicle minus the same at Time 1, divided by the time interval in days.
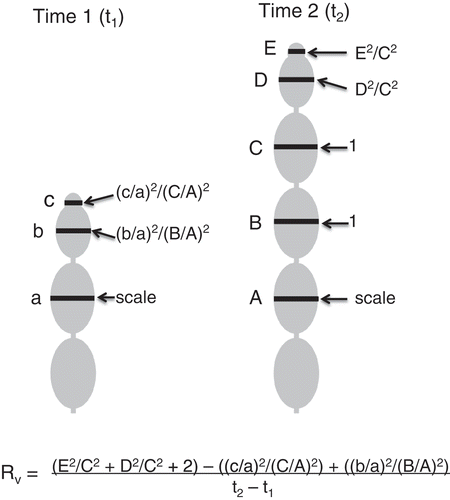
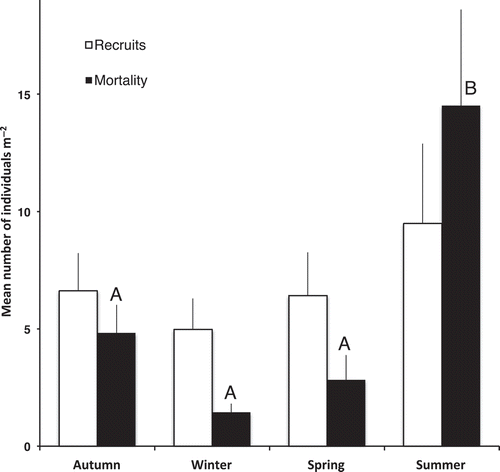
Fig. 3. Mean densities of recruits and deaths during the four seasons from 1 March 2002 to 28 February 2012 in 29 mid pools on Kainga Reef with a total area of 3.46 m2. Mean recruitment over 10 years was 27.5 germlings m−2 year−1. Different letters indicate significant difference (P < 0.05, Tukey HTD test and P = 0.0018, Kruskal–Wallis). Bars, standard error.
The site was occupied by considerable numbers of three species of grazing gastropods (Nerita atramentosa, Austrocochlea porcata and Austrolittorina unifasciata), in the pools and on surrounding rock (Kain, Citation2013).
Nitrate levels in the sea and two pools were determined from samples collected at four times of year, in different seasons, by Testing and Analytical Services: CSIRO Land and Water.
Hormosira monitoring in situ
The site was visited at approximately monthly intervals when low water was near the middle of the day from early 2002 to 2011 and less frequently until 2013. A digital photograph was taken of each pool, or part of it, from directly above the pool. A designated area (235–2828 cm2) was monitored, the whole of small pools and part of larger pools, delimited by rock structures. The total monitored area in 29 ‘mid’ pools was 3.46 m2 and the pool heights varied from 1.3 to 2.7 m above lowest astronomical tide (LAT). Individual Hormosira plants within the designated areas were marked on the photographs. Recruits could be recognized initially when they had 2–3 vesicles. The vesicles of some recruits were counted for about a year during 2003–7 for growth rate calculation. Recruitment and mortality of individuals were recorded and their lifespan was calculated during 2002–11 (9 years) when observations were sufficiently frequent. Seasonal recruitment and mortality was calculated for 2002–12 and annual cohorts and totals were calculated for 2002–13.
The area covered by some of the mature plants of Hormosira was measured in photographs viewed in Photoshop Elements, using the ellipse tool providing vertical and horizontal pixels. These were scaled by similarly measuring a white washer with a diameter of 24 mm that had been placed in the pool. Mortality records for pools were searched for temporal clusters, which would indicate a common cause. In some cases this could be attributed to prolonged sand incursion. When sand had not been observed it was assumed that the pools had been isolated by the combination of low HHW and a calm sea, causing desiccation and hypersalinity. A few pools were observed to be vulnerable to cast weed being trapped for long periods, resulting in the death of all resident Hormosira. At the beginning of 2004, eight pools dried out completely and all the plants in them died. Monthly counts of recruits in these pools before and after this event were used to estimate the time it took for zygotes to develop to 3-vesicle germlings. Salinity measurements were made in other pools on the second day of the 4-day dry-out (Kain, Citation2013). The fate of Hormosira plants was assessed 2 weeks later.
In September 2008, 78 Hormosira germlings were removed from unmonitored pools at the same levels as some of the monitored pools. Their lengths were measured, fresh weight determined and vesicles counted. They were then sectioned to determine fertility and sex.
From early 2009 to early 2012 the growth rate of Hormosira fronds, as increase in vesicle number, was determined in situ. Any form of tagging of fronds could lead to damage and/or loss, so a non-invasive technique was devised. Pools with suitable mature plants were chosen. In 2009–10, 26 pools near the middle of the reef were used (‘mid pools’, ), with heights of 1.3–1.7 m (mean 1.5 m) above LAT but from September 2010 the number was reduced to 10 pools at the same mean height. For the purpose of growth rate comparison an additional group of five pools was adopted on the edge of the reef (‘edge pools’, ) at heights of 1.0–1.4 m (mean 1.2 m). The accessibility of these pools was limited by frequent exposure to waves. Each pool was photographed and the plants numbered. From each plant a single frond was chosen and photographed. Paper prints were made of the pool and frond photographs and after a time interval used to find each frond for further photographing. If a frond could not be found another was chosen from the same plant. On no occasion was more than one frond knowingly used concurrently from the same plant. Individual fronds of Hormosira are very different in their branching pattern and therefore recognizable.
The growth rate, as vesicles per unit time (Rv), was calculated from the change in number of vesicles on the fastest-growing branch of the frond. Because this was a low value it was necessary to measure fractions of mature vesicles. It was assumed that the third vesicle from the apex was mature. (In 16 cases where the third vesicle size was measured with a reliable scale the mean growth was only 3% of the total.) This was designated the scale vesicle. On each photograph the maximum diameter of the apical three vesicles was measured in pixels. As vesicles are circular in section this value would be the same even if the camera had not been quite perpendicular to the frond; also the 3 measured vesicles, being next to each other, would be a similar distance from the camera. There were two possible ways of calculating what proportion of their mature size the 2 apical vesicles were (, Time 1 and Time 2). The first used the mature sizes of Time 2 apical vesicles but clearly could not be used if the frond was not found again. The second (as shown in ) used a scale vesicle, further from the apex. Each diameter in pixels was squared to represent the cross-section and divided by the square of the diameter of the chosen mature vesicle. At each time the total number of vesicles distal to the last branch was counted and the increase (2 in the example in ) added to the apical proportions at T2. The difference between the vesicle numbers on the two occasions was divided by the time change (). For a comparison between seasons, the longest time period within the 3-month season was selected and the growth of the fronds that spanned it was calculated, ignoring intervening observations. For comparison between groups of pools, the same time period (identical if possible) for both was selected.
Total vesicle number was counted on each photograph in the 8 longest surviving fronds with minimal loss of frond branches.
Mature vesicle length was determined by selecting a series of them lying in a straight line in a photograph and measuring the total length in pixels. This was scaled using a 25 mm circle drawn on the white plastic disc and measuring the diameter parallel to the vesicle line.
Salinity experiments
The hypersalinity tolerance of Hormosira was tested by exposing single fronds, in shallow dishes of seawater, to evaporation in summer sunlight. On 26 January 2011 single fronds were collected (each from a separate plant) from a large mid pool at 1.51 m LAT on Kainga Reef, transported in seawater 130 m to an outdoor site and each placed in a white glass dish containing 500 ml seawater at 34–35 practical salinity units (psu) 25 mm deep. The 31 dishes were on a stone table exposed to full sun until about 15.00 h each day. Depths were measured daily and sufficient distilled water added to three control dishes to return the depth to 25 mm. Daytime temperatures averaged 24.2°C with a maximum of 35°C. After 6–12 days the remaining water in each of most of the dishes was sampled for salinity measurement, transferred to conical flasks together with the frond and an equal volume of seawater and removed from sunlight. In some dishes the fronds were transferred to conical flasks without dilution of the hypersaline water until 2 or 5 days had elapsed when seawater of the same volume was added. Twenty-four hours after dilution each frond was placed in normal seawater (34–35 psu) and changed every 2 days. Plant stress was tested by measuring quantum yield: after 30 minutes in darkness at ~23°C the Fv/Fm of each frond was determined damp with a Hansatech Plant Efficiency Analyser. Salinities were measured with a refractometer after measured dilution when necessary. In a further experiment, two fronds were placed in each dish, one from the same mid pool and the other from open rock at the side of the reef at 1.20 m LAT. The experiment was disrupted by rain but a comparison of the pairs of fronds was possible.
Statistical tests were made using VassarStats: Website for Statistical Computation. For differences in location the Mann–Whitney U test was used for unpaired and the Wilcoxon signed-ranks test for paired data. For correlation Spearman’s rank correlation coefficient was used.
RESULTS
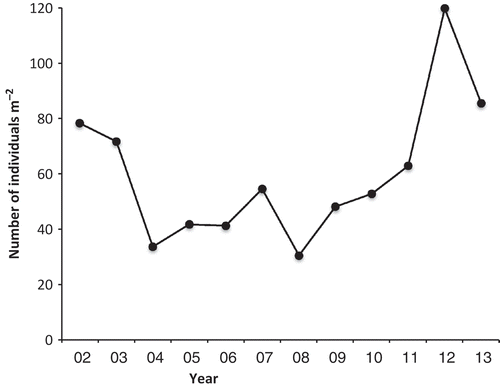
The number of recruits appearing in the 29 pools during the different quarters designated as the seasons, starting with February 2002 and ending with January 2012, varied from 3 to 111. The recruitment means for each season did not vary significantly from each other (). The total number of recruits present at the end of each summer showed no trend over 10 years ().
Fig. 4. The density of individuals of Hormosira in 29 mid pools on Kainga Reef (total number of plants in the monitored areas divided by the total monitored area of 3.46 m2) on 1 March each year. Error bars would be inappropriate because pools had different areas.
In the eight pools that dried out at the beginning of January 2004 there was a gap of 8 months in recruiting whereas there were recruits in all but 2 of the 11 preceding and 12 ensuing months. From this it would seem that it took about 8 months for zygotes to reach a size of about 3 vesicles when they could be recognized in photographs. However, it is possible that recruitment was initially prevented on the previously dried surface. In 12 germlings observed in mid pool photographs the mean increase in number of vesicles on the longest frond 6 months after it was 3 vesicles in length was 3.35 (SE 0.44), or 0.56 vesicles/month. It was therefore likely to have taken over 5 months to produce the first 3 vesicles.
Most germlings soon had more than one upright branch (frond), often arising in a circle. It was not possible to see a holdfast at that stage when the substratum was often the crust of Colpomenia bullosa.
Two-thirds of the growth rates in vesicle number of 21 germlings had a higher correlation coefficient with time when expressed exponentially () than when expressed linearly and the mean relative growth rate R was 0.00886 per day. Allowing 5–8 months before it had 3 vesicles, after 17–20 months an average germling would have had 76 vesicles in total (all fronds). Its length would then be about 60 mm. In both vesicle increase in mature fronds and the rate of increase in radius of mature plants the rate was equally close to linear and exponential ().
Table 1. A comparison of the growth of Hormosira expressed linearly or as natural logarithms (exponential growth) correlated with time.
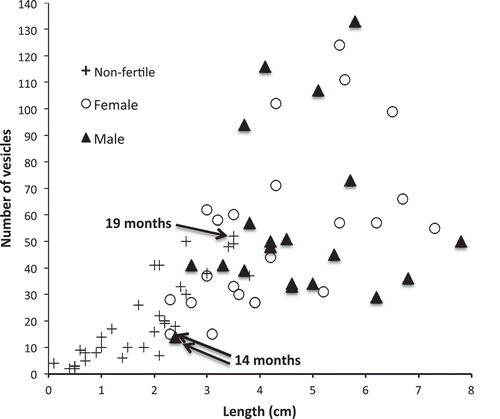
Male and female germlings became fertile at about the same size: the smallest sampled in September 2008 were probably about 11–14 months and the largest non-fertile about 16–19 months old (). In photographs of in situ germlings taken during 2003–7 the smallest with visible conceptacles was 10–13 months and the largest apparently sterile 16–19 months old (n = 24).
Fig. 5. The number of vesicles and lengths of non-fertile, fertile female and fertile male germlings (total of 78 plants) sampled in September 2008. The age of the plants shown was calculated from the vesicle number of each, assuming it took 5–8 months to reach 3 vesicles, and using the mean relative growth rate in vesicle number of 0.00886 vesicles day-1 observed in situ.
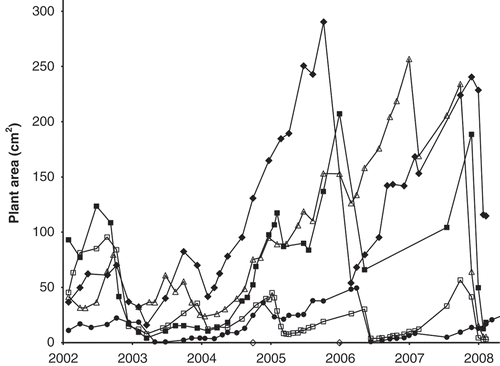
The area covered by five mature plants fluctuated on a mainly seasonal basis, usually peaking at the end of winter, with marked reductions in some summers and inter-annual differences ().
Fig. 6. The area covered by each of five plants of Hormosira in five different mid pools on Kainga Reef (each with different symbol) during 6 years from the start of 2002.
The growth rate, in terms of vesicles per apex of fronds, in mid pools was significantly faster in autumn than in the other seasons in all 3 years. In edge pools the growth rate was significantly faster than mid pools in summer but similar to mid pools in the other seasons, also in all 3 years (, Table S1). The mean number of vesicles produced per year was 6.59 in mid and 7.81 in edge pools. With a mean vesicle length of 9.05 mm this would be 60 and 71 mm per year respectively. The mid pool annual rate would be a mean of 0.55 vesicles month−1, remarkably similar to the rate in the observed germlings, from their 3-vesicle size, of 0.56 vesicles month−1. There was no correlation of growth rate with pool height on the shore in summer, when edge plants grew significantly faster than mid ().
Fig. 7. The mean growth rates of single apices of fronds over 3 years in pools on Kainga Reef. Mid, pools >6 m from edge of the reef (mean n (fronds) = 30); edge, pools <3 m from edge (mean n (fronds) = 17). Bars, standard error. Overall mean for mid pools was 6.59 and edge pools 7.81 vesicles year-1.
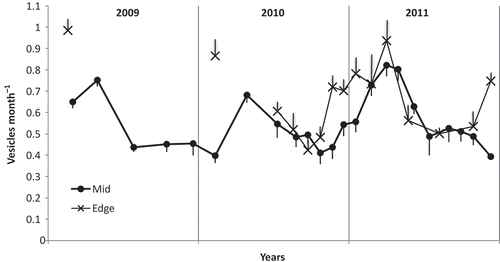
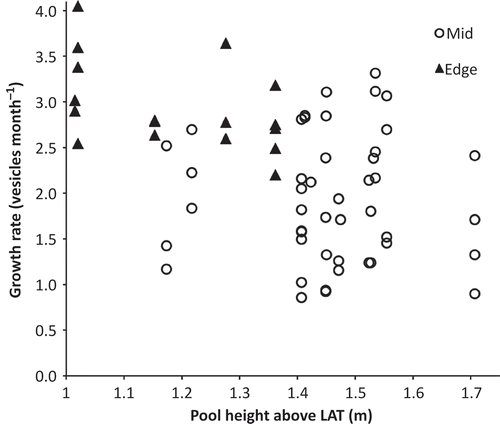
Fig. 8. The growth rate of fronds of Hormosira in pools at various heights on the Kainga Reef in late summer 2009. Mid, pools >6 m from edge of the reef; edge, pools <3 m from edge. The difference between the mid and edge pools between 1.1 and 1.4 m LAT (n = 6 and 11 respectively) was significant at P = 0.0105, Mann–Whitney.
Nitrate was undetectable (< 0.30 µmol l−1) in mid pools on all occasions and in the sea in April and November but it was just detectable in the sea in July (0.56 ±0.05 µmol l−1, when there was 0.33 ±0.1 µmol l−1 in the edge pools) and in September (0.66±0.13 µmol l−1) (Table S2).
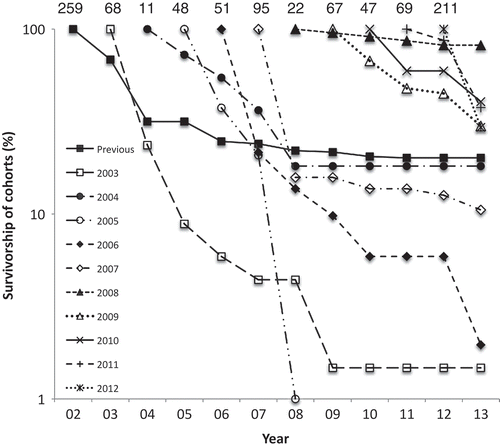
Mortality was significantly highest in the summer (). Most germlings died within a year from first observation and only three juvenile plants survived more than 4 years (). Fifty original plants, in 11 pools, were still present after 11 years. The survivorship of annual cohorts was variable as some numbers were low, particularly in 2004 just after a dry-out (). Plants originally present in 2002 (‘previous’) fared well but were not in an annual cohort: they had already been partly naturally selected. The calculated annual mortality as a proportion of the number at the start of each year decreased significantly (P = 0.00033, Spearman’s rank correlation) during the first 6 years of the first five cohorts (Fig. S1).
Fig. 9. The lifespan from first observation, in 2-month intervals, of plants of Hormosira in 29 mid pools on Kainga Reef monitored from 2002 to 2011. The last column represents the number of originally observed plants that were still present in March 2013.
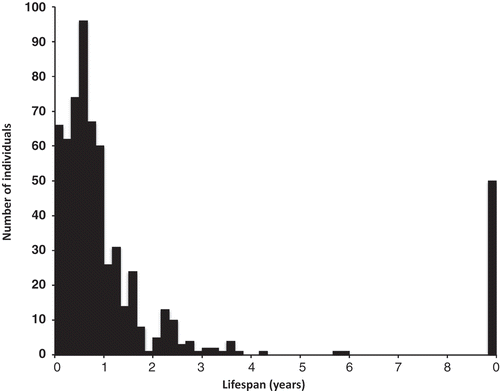
Fig. 10. Per cent survivorship of Hormosira, in 29 mid pools on Kainga Reef, in 10 annual cohorts plus those present previously (n shown above), each being the number at the beginning of March that had arisen during the previous 12 months.
Nearly half of the mortality was attributable to pool evaporation, being in clusters affecting four or more pools simultaneously and associated with times of low HHW (). Only 11 of the 29 pools were vulnerable to sand incursion but in those prolonged residence caused mortality. Cast weed was the only other identifiable cause of mortality ().
Table 2. Numbers of plants of Hormosira which died in clusters with a known or unknown cause each calendar year (number of pools in brackets). Eleven of the 29 mid pools studied were vulnerable to sand incursion.
In the drying event at the beginning of 2004 the salinity of all the pools was raised on the second day. Of the 29 pools monitored, four were devoid of Hormosira before the incident; five dried out completely and all plants died. In 12 pools there was some mortality (partly due to drying at the edge) but some plants survived a measured salinity of 127 psu and an estimated 160 psu, though an estimated 170 psu was lethal (Table S3). In eight pools there were no deaths, the highest salinity being 104 psu. During this event the mean temperature in pools that had not dried out reached 35.0°C and one pool reached 40.4°C, while the same 22 pools had reached a mean of 34.1°C on a hot day without being isolated, with no mortality.
The mortality of fronds of mature plants was significantly higher during the summer quarter and higher in spring than winter (Fig. S2).
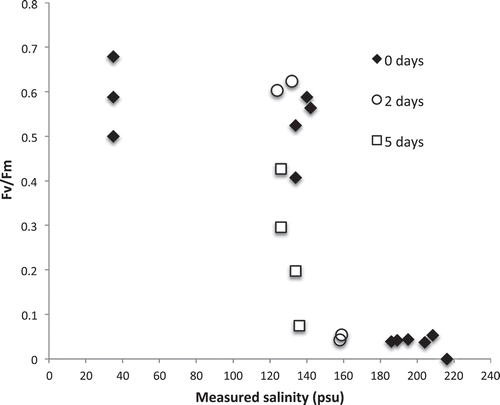
When individual fronds of Hormosira were subjected to evaporating seawater in individual dishes, they were apparently unstressed up to a salinity of 140 psu for a short time but 5 days at 126–136 psu reduced fv/fm (). In a further experiment, fronds from open rock at a lower tidal level were directly compared with pool fronds at salinities up to 170 psu and there was no significant difference between their Fv/Fm (Wilcoxon signed-ranks test).
Fig. 11. Fluorescence of Hormosira vesicles after exposure in evaporating seawater and either gradual return to normal seawater or being held for 2 or 5 days in hypersalinity. Between 124 and 142 psu 5-day exposure was significantly more stressful than 0- and 2-day pooled (P = 0.05, Mann–Whitney).
There was no browning of Hormosira vesicles in 21 of the pools after exposure to the highest possible radiation in 2008; the only browning was of vesicles which could have been exposed to the air (, ). An increase in occasional browning in 2011 was possibly due to higher biomass, pushing fronds above the surface, after two benign summers when no evaporation-linked mortalities were recorded (). Vesicles with some browning of the surface in response to stress were shown to be capable of complete recovery in at least 10 instances (e.g. , blue arrows). Prolonged emersion of fronds in shallow water caused severe browning where almost all fronds less than 35 mm deep browned (). A well-developed plant was found to have browned completely under a large frond of detached, fetid, Phyllospora comosa which had been in the pool (). The Hormosira died back, possibly to the holdfast, but later produced new fronds (). Such resurrection was observed in 12 other cases, but because the browned fronds remained for some time they hid the base so it was not apparent whether basal vesicles had survived the stress, except in three cases where groups of new fronds appeared on what had been flat brown surfaces at a site previously occupied by a mature plant (as in ). On no occasion, however, was resurrection observed after complete drying out including the holdfast. Prolonged (3 months) sand cover also killed holdfasts.
Figs 12–17. Browning of Hormosira. Fig. 12. A shallow pool in January showing browning of the upper sides of vesicles after exposure to air (blue arrows). Fig. 13. Partially brown vesicles (blue arrow) in February. Fig. 14. Complete recovery 45 days later. Fig. 15. Pool with a deeper central basin (water depths in blue) in March after partially drying out. Fig. 16. Browning of plant, seen healthy 26 days previously, after removal of a decomposing Phyllospora comosa frond from the pool in December. Fig. 17. Regrowth 8 months later.
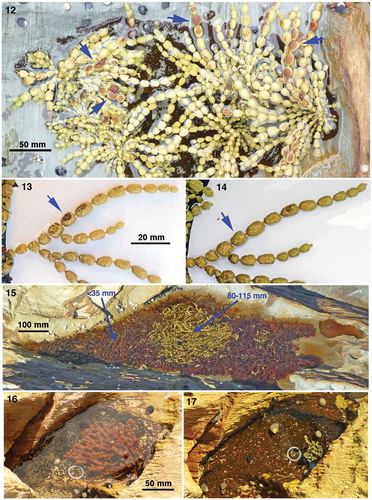
Table 3. The assessed state of Hormosira, in pools where it was present (out of 29 monitored), after a series of sunny days in near midsummer, when the pools were isolated at mid-day.
Notheia, the obligate epiphyte on Hormosira, was never seen in mid pools but in edge pools it was observed on 7% of the frond photographs (n = 241).
DISCUSSION
Recruitment of new plants clearly depends on the availability of propagules. Even over beds of Hormosira, propagules have been hard to find in seawater (Bellgrove et al., Citation1997, Citation2004) but detached fronds can provide a source of gametes (McKenzie & Bellgrove, Citation2008). In some Fucus spp., gamete release is timed to be favourable for attachment of the zygote (Brawley, Citation1992; Pearson & Brawley, Citation1996; Serrao et al., Citation1996). A single wave dislodged zygotes of both Hormosira (Taylor & Schiel, Citation2003) and Ascophyllum nodosum (Vadas et al., Citation1990) which had been settled on artificial substrata in the laboratory. An existing canopy favours fucoid early survival, particularly lower on the shore (Brawley & Johnson, Citation1991; Dunmore, Citation2006). On the other hand the density of natural recruits of Hormosira reached about 500 cm−2 week−1 on sheltered experimental panels in New Zealand (Dunmore, Citation2006), although Hormosira, together with Ascophyllum nodosum, has a lower egg output than species of Fucus (Vernet & Harper, Citation1980; Clayton, Citation1990). By the time fucoid recruits can be detected in the field without the deployment of artificial panels, their numbers are likely to have dropped. The mean density of Hormosira recruits found in the current study, 27 m−2 y−1 (), was similar to the 40 m−2 y−1 found for Ascophyllum in Sweden (Åberg & Pavia, Citation1997). Fucus spp., however, can recruit in several thousands m−2 in the northeast Pacific (Ang, Citation1991a), the northwest Atlantic (Menge, Citation1991) and in the northeast Atlantic (Cervin et al., Citation2005).
The 5–8 months that it apparently took for Hormosira germlings to reach 3 vesicles and be identifiable was similar to field observations of the timing of reappearance of Hormosira after complete clearance in two instances (Underwood, Citation1998; Lilley & Schiel, Citation2006) but not a third observation where it was detected 3 months after clearance by exposing virgin rock surface with a chisel (Airoldi, Citation2003).
As Hormosira is fertile throughout the year, it was no surprise that there was no significant difference in recruitment between the seasons in the present study, although in New Zealand recruitment was higher in spring and summer (Dunmore, Citation2006). Kainga Reef was rich in parental source, the large deep (unmonitored) pools on the platform housing many mature plants, and as shown in this study, new plants could be fertile in 11–14 months from settlement ().
The ring of fronds formed after the appearance of the initial axis at an early stage of germling growth could have indicated either a holdfast or connecting rhizoidal filaments shown to form embryos in several species of Fucus (McLachlan & Chen, Citation1972). Most fucoids can regenerate fronds from holdfasts and this is sometimes an important contribution to succession (McCook & Chapman, Citation1992). In Sargassum spp. more than half the annual recruitment was from this source (Kendrick & Walker, Citation1994), while in Ascophyllum nodosum recruitment from zygotes was unimportant so replacement fronds must have arisen from holdfasts (Keser et al., Citation1981; Vadas & Wright, Citation1986). In the present study there were only 13 observed cases of reappearance of plants from blackened holdfasts while 970 new recruits were observed during 11 years. On the other hand there were many observations of parts of plants dying back to stumps on well-developed holdfasts. On open rock Hormosira holdfasts have been shown to be important for regeneration after damage by storms or trampling (Povey & Keough, Citation1991; Underwood, Citation1998; Schiel & Taylor, Citation1999). During autumn, winter and most of spring there was no significant difference between the growth rate in mid and edge pools – both were higher in autumn than winter or spring (), indicating a factor not associated with water movement being less favourable in winter and spring. The mean daylength is higher in spring than autumn (), making light an unlikely factor. The mean sea surface temperature off Batemans Bay (10 km north of Kainga Reef) was similar in winter and spring but higher in autumn () so perhaps this was the factor responsible.
Fig. 18. The means during the seasonal quarters of daylength, 10-year mean of the sea surface temperature (SST) off Batemans Bay, ~20 km north of Kainga Reef (Manly Hydraulics Laboratory) and the mean maximum temperature of the mid pools; bars, standard error.
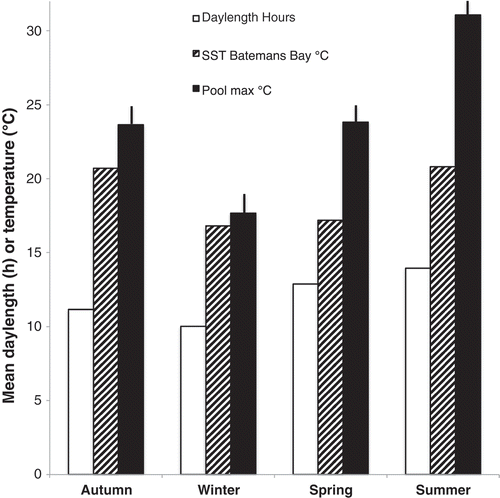
The difference between mid and edge pool growth rates, from late spring to the end of summer () was presumably due to the fact that edge pools were flushed more often. This could have had several consequences important for Hormosira photosynthesis and growth. Low oxygen concentration at night and low carbon concentration during the day have been reported for tidepools isolated from the sea (Truchot & Duhamel-Jouve, Citation1980; Morris & Taylor, Citation1983). However, nights are shorter in summer than autumn when Hormosira growth was maximal and carbon extraction depends on biomass, which would have been lower in summer because of high mortality. On the other hand mid pools were exposed to temperatures considerably higher than the sea; in summer the maximum pool temperature was much greater than in autumn (). Quantum yield by Hormosira embryos was reduced at 28°C (Clark et al., Citation2013) and, while adults can tolerate higher temperatures (Clark, personal communication) mean mid pool temperature could reach 35°C (Kain, Citation2008), likely to reduce photosynthesis and growth. Another likely factor was the provision of nutrients, particularly nitrate, low in the summer (Scott, Citation1981; Rochford, Citation1984) and undetectable in this study (Table S2). Not only would the edge pools be replenished with nutrients, albeit at a low level, but uptake by algae is aided by water movement (Hurd et al., Citation1996). There is no evidence to favour temperature, lack of nutrients or both being responsible for the low summer growth rate in mid pools.
The growth rate of Hormosira in this study was slow compared with other fucoids. Annual growth in length was about a third of that of four species of Fucus and about half that of Ascophyllum from the northern hemisphere (Table S4). Slow growth could be due to the paucity of nitrate in the sea off Kainga Reef (Table S2) rather than an innate characteristic of Hormosira. In the absence of data on growth rates of this alga at other sites, a comparison can only be made between early growth in culture where nutrient supply was plentiful: with one exception (Fucus gardneri), Hormosira germlings were smaller than all the other fucoids at any given time (Fig. S3). So it seems that the species is probably just a slow grower but is successful in spite of that, perhaps because it is stress tolerant. In terrestrial plants slow growth can be associated with stress tolerance (Grime, Citation1977).
Germling mortality could have been increased by the fairly abundant gastropods whose faeces occasionally contained fucoid zygotes (Kain, Citation2013) and the survivorship of Fucus spp. in intertidal pools was improved when gastropods were excluded (Chapman, Citation1990; Chapman & Johnson, Citation1990; Masterson et al., Citation2008). The lethal temperature for embryos was reported to be 30°C (Clark et al., Citation2013), well below some summer temperatures of mid pools. Some of the pools sometimes contained stones which, when moved by wave action, cleared recruits from the bottom of the pool. Finally, rotting seaweed could create a toxic environment, presumably through production of H2S (Andrews, Citation1991).
Some of the overall mortality could be attributed to sand incursion, if it was prolonged (). A variety of algae can withstand the stresses of scouring and burial and hence gain advantage over species that cannot on sand-influenced rocky shores (Markham & Newroth, Citation1972; Markham, Citation1973; Daly & Mathieson, Citation1977; Mathieson, Citation1982; Trowbridge, Citation1996). On emersed rock, however, some water would be expected to drain out of superimposed sand at low water and would therefore be replaced with the rising tide. In a pool the water could be static for extended periods. The only plant able to survive sand-filled pools in California was a seagrass with a rhizomatous root system (Littler et al., Citation1983). So it is hardly surprising that Hormosira succumbed to prolonged cover by sand in Kainga Reef pools.
Hormosira’s stress tolerance has been further demonstrated by its ability to survive, albeit short-term, a salinity of 140 psu, four times that of seawater. A frequent occupant of supratidal pools, Ulva intestinalis, while tolerant of hyposalinity, was found intolerant of 102 psu (Reed & Russell, Citation1979). However, a few zygotes of Fucus spiralis, an upper shore alga, survived a 6 hour treatment of 200 psu (Li et al., Citation1998). On the other hand there was no development in the same species continuously exposed to 65 psu (Wright & Reed, Citation1990): the time of exposure is clearly important. Turning to hyposalinity, F. spiralis embryos were unaffected by 9 psu (Wright & Reed, Citation1990) whereas Hormosira embryos were adversely affected by 21 psu (Doblin & Clayton, Citation1995; Kevekordes, Citation2000). Tolerance of low salinity is essential to intertidal algae but embryos in pools would be likely to avoid rain, which would remain near the surface (Naylor & Slinn, Citation1958; Pearson & Brawley, Citation1996).
Both photosynthetically active and ultraviolet radiation (PAR and UVR) are high in summer at the latitude of Kainga Reef (Wood, Citation1987; Alados-Arboledas et al., Citation2000). In Victoria sun-induced browning of Hormosira was attributed to ‘sunburning’ and was shown to involve the release and oxidation of phenolic compounds from physodes packing the peripheral cells (Schoenwaelder, Citation2002). On Kainga Reef there was no evidence that browning was due to radiation since maximal or near-maximal levels over several days had no effect on vesicle colour on three occasions (). Nor was immersion likely to have been protective: UVB radiation would have been reduced by 2% due to reflection and less than 1% at 50 mm depth through attenuation (Jerlov, Citation1968; Smith & Baker, Citation1979; Kirk Citation2010). There was, however, evidence that emersion caused browning because this occurred whenever evaporation left the vesicles dry (e.g. and ). It would be difficult to separate the two factors in an open rock population.
The survivorship of cohorts of Hormosira in the present study was inter-annually variable but appeared ( and ) to approximate to Type IV (Slobodkin, Citation1966). It was unlikely to be Type II which Slobodkin defined as ‘a constant number of deaths per unit time’ and there is evidence against Type III, ‘found when the risk of death is constant with age’ because proportional mortality decreased with time (Fig. S1). In Type IV mortality is greatest early in life but survivors can be long-lived. High early mortality was observed for germlings of Hormosira (Dunmore, Citation2006) and zygotes of Ascophyllum nodosum (Vadas et al., Citation1990) and high longevity demonstrated in Hormosira (present study) and in Ascophyllum (Peckol et al., Citation1988; Åberg, Citation1992). Thus these genera seem to share Type IV, in contrast to Fucus distichus and Undaria pinnatifida which show Type I where mortality increases as plants get older, resulting in a shorter lifespan (Ang, Citation1991a; Schiel & Thompson, Citation2012). The difference in the fucoids may lie in the fact that Hormosira and Ascophyllum regenerate from their holdfasts, prolonging life, but F. distichus relies on zygote recruits (Ang, Citation1991b). In Carpophyllum maschalocarpum the survivorship type depended on the plant density (Schiel, Citation1985). On Kainga Reef, 50 plants were known to be more than 11 years old. In this environment it was the young plants which were vulnerable: once they were more than 4 years old they were likely to survive a long time. By then there would be a substantial holdfast capable of producing fronds apparently indefinitely and able to survive stresses that killed the vesicles of fronds. Although growth was slow, such regrowth would be almost a year ahead of a zygote.
The fronds used for growth rate determinations were all on plants with established holdfasts. It was clear again that mortality was highest in summer. The level of 77% for the summer quarter (13 weeks) was similar to the summer frond mortality of 58% over 10 weeks at Crofts Bay, Victoria, Australia (McKenzie & Bellgrove, Citation2009). There was clearly a turnover of fronds all through the year on Kainga Reef and fluctuations in plant size according to conditions, as shown by area measurements (), must have been due to frond breakage. On the other hand, in Victoria the holdfast usually came away from the rock, rather than fronds breaking, when plants were pulled off the shore (McKenzie & Bellgrove, Citation2009) but it is unlikely that on Kainga Reef mechanical stress would be the major one.
The absence of Notheia from mid but presence in edge pools accentuates the harshness of the mid pool environment. In Victoria Notheia was more common in pools than on emerged Hormosira (Hallam et al., Citation1980). It seems the epiphyte is less tolerant than the host.
So how did Hormosira occupy these pools when almost all other species failed? It is possible that ephemerals with small propagules were excluded through grazing by the numerous gastropods on the reef (Kain, Citation2013). Zygotes and germlings of Hormosira might escape grazing enough of the time for the establishment of the holdfasts which allowed lengthy lives. The species must be tolerant of rapidly fluctuating temperature: some pools reached 10°C above the sea which could then flush them suddenly. There must also be a tolerance of high PAR and UVR: fucoids in rockpools showed photoinhibition by both (Huppertz et al., Citation1990; Häder et al., Citation2001) and radiation is high on the southern Australian coast (Wood, Citation1987; Karsten & West, Citation2000) but in the edge pools Hormosira grew at maximal rate in summer. It could withstand the hypersalinity if a pool dried to a quarter of its original volume. It was vulnerable to desiccation but its key defence was in its holdfast, which, although not able to withstand complete drying out, was less vulnerable than the vesicles and contained cells capable of regrowth.
That there was no significant change in numbers of plants of Hormosira over more than a decade might come as no surprise. Ironically, two predicted consequences of climate change could work in opposite directions as stresses in the upper intertidal pools: higher sea level, by reducing periods of pool isolation, could mitigate the effect of raised temperature.
It can be concluded that in a number of ways Hormosira resembles Ascophyllum nodosum of the northern hemisphere. In both, the reproductive effort is low compared with other measured fucoids and regeneration from holdfasts can be more important than recruitment from zygotes. Both grow slowly, though Hormosira is slower than Ascophyllum. Both show Type IV survivorship which allows a long lifespan. In the North Atlantic Ascophyllum is harvested and recovers in spite of its slow growth rate, providing the holdfasts remain (Sharp, Citation1987). If harvesting of Hormosira were proposed, its slow growth rate and the protection of its holdfasts would need consideration.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
SUPPLEMENTARY INFORMATION
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at http://dx.doi.org/10.1080/09670262.2015.1075594
Supplementary table S1. Significance of differences (Mann–Whitney) between growth rates in vesicle number in mid pools during autumn and the following seasons followed by the significance of difference between mid pools and edge pools at during identical periods in four summers.
Supplementary table S2. Concentrations of nitrate in seawater found by Rochford (1984) and in the present study at different times of year.
Supplementary table S3. Pools grouped according to the fate of Hormosira in them, arranged in order of height above LAT and showing salinities measured on the second day of the 2004 dry-out with estimated salinities attained before flushing on subsequent days (Kain, 2013).
Supplementary table S4. The growth in length in the field in mm year−1 of Hormosira compared with intertidal fucoids from the northern hemisphere.
Supplementary Fig. S1. The number of plants in each of five cohorts that died in each year expressed as a proportion of the number at the start of the year.
Supplementary Fig. S2. The apparent mortality, including failure to recognize them, of fronds of Hormosira being observed for growth rate determination in mid pools on Kainga Reef from March 2009 to June 2010.
Supplementary Fig. S3. Length (without rhizoid) in mm of germlings of fucoids in culture at different ages after fertilization (Knight & Parke 1950; McLachlan et al. 1971; McLachlan, 1974; Sheader & Moss, 1975; Norton, 1977; Forbes & Hallam, 1979; Clayton et al. 1985; Kevekordes & Clayton, 2000; Altamirano et al., 2003; Choi & Norton, 2005; Dunmore 2006).
Supplementary Figures
Download PDF (116.4 KB)Supplementary References
Download PDF (85 KB)Supplementary Tables
Download MS Word (23 KB)Acknowledgements
I am very grateful to Veronica Briceno who ably helped with fluorescence measurements and to Jennifer Clark and Diana Walker for helpful comments on the manuscript. I am extremely grateful to Margaret Clayton who gave great support throughout the project and discussed the manuscript at length.
REFERENCES
- Åberg, P. (1992). A demographic study of two populations of the seaweed Ascophyllum nodosum. Ecology, 73: 1473–1487.
- Åberg, P. & Pavia, H. (1997). Temporal and multiple scale spatial variation in juvenile and adult abundance of the brown alga Ascophyllum nodosum. Marine Ecology Progress Series, 158: 111–119.
- Adams, M.N. (1994). Seaweeds of New Zealand. University of Canterbury, Christchurch.
- Airoldi, L. (2003). Effects of patch shape in intertidal algal mosaics: roles of area, perimeter and distance from edge. Marine Biology, 143: 639–650.
- Alados-Arboledas, L., Olmo, F.J., Alados, I. & Pérez, M. (2000). Parametric models to estimate photosynthetically active radiation in Spain. Agricultural and Forest Meteorology, 101: 187–201.
- Ambler, M.P. & Chapman, V.J. (1950). A quantitative study of some factors affecting tide pools. Transactions of the Royal Society of New Zealand, 78: 394–409.
- Andrews, J.W. (1991). The ecology of the Manx strandline. PhD thesis. University of Liverpool.
- Ang, P. (1991a). Age- and size-dependent growth and mortality in a population of Fucus distichus. Marine Ecology Progress Series, 78: 173–187.
- Ang, P. (1991b). Natural dynamics of a Fucus distichus (Phaeophyceae, Fucales) population: reproduction and recruitment. Marine Ecology Progress Series, 78: 71–85.
- Araújo, R., Sousa-Pinto, I., Bárbara, I. & Quintino, V. (2006). Macroalgal communities of intertidal rock pools in the northwest coast of Portugal. Acta Oecologica, 30: 192–202.
- Bellgrove, A., Clayton, M.N. & Quinn, G.P. (1997). Effects of secondarily treated sewage effluent on intertidal macroalgal recruitment processes. Marine and Freshwater Research, 48: 137–146.
- Bellgrove, A., Clayton, M.N. & Quinn, G.P. (2004). An integrated study of the temporal and spacial variation in the supply of propagules, recruitment and assemblages of intertidal macroalgae on a wave-exposed rocky coast, Victoria, Australia. Journal of Experimental Marine Biology and Ecology, 310: 207–225.
- Bergquist, P.L. (1959a). A statistical approach to the ecology of Hormosira banksii. Botanica Marina, 1: 22–53.
- Bergquist, P.L. (1959b). The effect of cations and anions on the respiration rate of the brown alga Hormosira banksii. Physiologia Plantarum, 12: 30–36.
- Björk, M., Axelsson, L. & Beer, S. (2004). Why is Ulva intestinalis the only macroalga inhabiting isolated rockpools along the Swedish Atlantic coast? Marine Ecology Progress Series, 284: 109–116.
- Brawley, S.H. (1992). Fertilization in natural populations of the dioecious brown alga Fucus ceranoides and the importance of the polyspermy block. Marine Biology, 113: 145–157.
- Brawley, S.H. & Johnson, L.E. (1991). Survival of fucoid embryos in the intertidal zone depends upon developmental stage and microhabitat. Journal of Phycology, 27: 179–186.
- Brown, M.T. (1987). Effects of desiccation on photosynthesis of intertidal algae from a southern New Zealand shore. Botanica Marina, 30: 121–127.
- Cervin, G., Åberg, P. & Jenkins, S.R. (2005). Small-scale disturbance in a stable canopy dominated community: implications for macroalgal recruitment and growth. Marine Ecology Progress Series, 305: 31–40.
- Chapman, A.R.O. (1990). Effects of grazing, canopy cover and substratum type on the abundance of common species of seaweeds inhabiting littoral fringe tidepools. Botanica Marina, 33: 319–326.
- Chapman, A.R.O. (1995). Functional ecology of fucoid algae: twenty-three years of progress. Phycologia, 34: 1–32.
- Chapman, A.R.O. & Johnson, C.R. (1990). Disturbance and organization of macroalgal assemblages in the Northwest Atlantic. Hydrobiologia, 192: 77–121.
- Clark, J.S., Poore, A.G.B., Ralph, P.J. & Doblin, M.A. (2013). Potential for adaptation in response to thermal stress in an intertidal macroalga. Journal of Phycology, 49: 630–639.
- Clarke, S.M. & Womersley, H.B.S. (1981). Cross-fertilization and hybrid development of forms of the brown alga Hormosira banksii (Turner) Decaisne. Australian Journal of Botany, 29: 497–505.
- Clayton, M.N. (1990). The adaptive significance of life history characters in selected orders of marine brown macroalgae. Australian Journal of Ecology, 15: 439–452.
- Clayton, M.N., Hallam, N.D., Luff, S.E. & Diggins, T. (1985). Cytology of the apex, thallus development and reproductive structures of Hormosira banksii (Fucales, Phaeophyta). Phycologia, 24: 181–190.
- Daly, M.A. & Mathieson, A.C. (1977). The effects of sand movement on intertidal seaweeds and selected invertebrates at Bound Rock, New Hampshire, USA. Marine Biology, 43: 45–55.
- Dethier, M.N. (1984). Disturbance and recovery in intertidal pools: maintenance of mosaic patterns. Ecological Monographs, 54: 99–118.
- Doblin, M.A. & Clayton, M.N. (1995). Effects of secondarily-treated sewage effluent on the early life-history stages of two species of brown macroalgae: Hormosira banksii and Duvillaea potatorum. Marine Biology, 122: 689–698.
- Dromgoole, F.I. (1980). Desiccation resistance of intertidal and subtidal algae. Botanica Marina, 23: 149–159.
- Dunmore, R.A. (2006). Demography of early life stages of habitat-forming intertidal fucoid algae. PhD thesis. University of Canterbury.
- Femino, R.J. & Mathieson, A.C. (1980). Investigations of New England marine algae IV. The ecology and seasonal succession of tide pool algae at Bald Head Cliff, York, Maine, USA. Botanica Marina, 23: 319–332.
- Ganning, B. (1971). Studies on chemical, physical and biological conditions in Swedish rockpool ecosystems. Ophelia, 9: 51–105.
- Goss-Custard, S., Jones, J., Kitching, J.A. & Norton, T.A. (1979). Tide pools of Carrigathorna and Barloge Creek. Philosophical Transactions of the Royal Society of London Series B, 287: 1–44.
- Grime, J.P. (1977). Evidence fot the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist, 111: 1169–1194.
- Häder, D.-P., Porst, M. & Lebert, M. (2001). Photosynthetic performance of the Atlantic brown macroalgae Cystoseira abies-marina, Dictyota dichotoma and Sargassum vulgare, measured in Gran Canaria on site. Journal of Photochemistry and Photobiology B: Biology, 45: 21–32.
- Hallam, N.D., Clayton, M.N. & Parish, D. (1980). Studies on the association between Notheia anomala and Hormosira banksii (Phaeophyta). Australian Journal of Botany, 28: 239–248.
- Huppertz, K., Hanelt, D. & Nultsch, W. (1990). Photoinhibition of photosynthesis in the marine brown alga Fucus serratus as studied in field experiments. Marine Ecology Progress Series, 66: 175–182.
- Hurd, C.L., Harrison, P.J. & Druehl, L.D. (1996). Effest of seawater velocity on inorganic uptake by morphologically distinct forms of Macrocystis pyrifera from wave-sheltered and exposed sites. Marine Biology, 126: 205–214.
- Jerlov, N.G. (1968). Optical Oceanography. Elsevier, New York.
- Kain, J.M. (2008). Winter favours growth and survival of Ralfsia verrucosa (Phaeophyceae) in high intertidal rockpools in southeast Australia. Phycologia, 47: 498–509.
- Kain, J.M. (2013). Colpomenia bullosa crust (Phaeophyceae) stressed less by grazing than by abiotic factors in high intertidal rockpools in southeast Australia. Botanica Marina, 56: 441–450.
- Kain, J.M., Buchanan, J., Boo, S.M. & Lee, K.M. (2010). Colpomenia bullosa crust masquerading as Ralfsia verrucosa (Phaeophyceae) in southeast Australia. Phycologia, 49: 617–627.
- Karsten, U. & West, J.A. (2000). Living in the intertidal zone – seasonal effects on heterocides and sun-screen compounds in the red alga Bangia atropurpurea (Bangiales). Journal of Experimental Marine Biology and Ecology, 254: 221–234.
- Kendrick, G.A. & Walker, D.I. (1994). Role of recruitment in structuring beds of Sargassum spp. (Phaeophyta) at Rottnest Island, Western Australia. Journal of Phycology, 30: 200–208.
- Keser, M., Vadas, R.L. & Larson, B.R. (1981). Regrowth of Ascophyllum nodosum and Fucus vesiculosus under various harvesting regimes in Maine, U.S.A. Botanica Marina, 24: 29–38.
- Kevekordes, K. (2000). The effects of secondarily-treated sewage effluent and reduced salinity on the early life stages of Hormosira banksii (Phaeophyceae). European Journal of Phycology, 35: 365–371.
- Kirk, J.T.O. 2010. Light and Photosynthesis in Aquatic Ecosystems. 3rd ed. Cambridge University Press, Cambridge.
- Li, R., Brawley, S.H. & Close, T.J. (1998). Proteins immunologically related to dehydrins in fucoid algae. Journal of Phycology, 34: 642–650.
- Lilley, S.A. & Schiel, D.R. (2006). Community effects following the deletion of a habitat-forming alga from rocky shores. Oecologia, 148: 672–681.
- Littler, M.M., Martz, D.R. & Littler, D.S. (1983). Effects of recurrent sand deposition on rocky intertidal organisms: importance of substrate heterogeneity in a fluctuating environment. Marine Ecology Progress Series, 11: 129–139.
- Lubchenko, J. (1978). Plant species diversity in a marine intertidal community: importance of herbivore food preference and algal competitive abilities. American Naturalist, 112: 23–39.
- Macinnis-Ng, C.M.O., Morrison, D.A. & Ralph, P.J. (2005). Temporal and spatial variation in the morphology of the brown macroalga Hormosira banksii (Fucales, Phaeophyta). Botanica Marina, 48: 198–207.
- Markham, J.W. (1973). Observations on the ecology of Laminaria sinclairii on three northern Oregon beaches. Journal of Phycology, 9: 336–341.
- Markham, J.W. & Newroth, P.R. (1972). Observations on the ecology of Gymnogongrus linearis and related species. In Proceedings of the Seventh International Seaweed Symposium (Nisizawa, K. editor), 127–130. Tokyo University Press, Tokyo.
- Masterson, P., Arenas, F.A., Thompson, R.C. & Jenkins, S.R. (2008). Interaction of top down and bottom up factors in intertidal rockpools: effects on early successional macroalgal community composition, abundance and productivity. Journal of Experimental Marine Biology and Ecology, 363: 12–20.
- Mathieson, A.C. (1982). Field ecology of the brown alga Phaeostrophon irregulare Setchell et Gardner. Botanica Marina, 25: 67–85.
- McCook, L.J. & Chapman, A.R.O. (1992). Vegetative regeneration of Fucus rockweed canopy as a mechanism of secondary succession on an exposed rocky shore. Botanica Marina, 35: 35–46.
- McKenzie, P.F. & Bellgrove, A. (2008). Dispersal of Hormosira banksii (Phaeophyceae) via detached fragments: reproduction viability and longevity. Journal of Phycology, 44: 1108–1115.
- McKenzie, P.F. & Bellgrove, A. (2009). Dislodgement and attachment strength of the intertidal macroalga Hormosira banksii (Fucales, Phaeophyceae). Phycologia, 48: 335–343.
- McLachlan, J. & Chen, L.C.-M (1972). Formation of adventive embryos from rhizoidal filaments in sporelings of four species of Fucus (Phaeophyceae). Canadian Journal of Botany, 50: 1841–1844.
- Menge, B. (1991). Relative importance of recruitment and other causes of variation in rocky intertidal community structure. Journal of Experimental Marine Biology and Ecology, 146: 69–100.
- Millar, A.J.K. (1999). Marine benthic algae of Norfolk Island, South Pacific. Australian Systematic Botany, 12: 479–547.
- Morris, S. & Taylor, A.C. (1983). Diurnal and seasonal variation in physico-chemical conditions within intertidal rockpools. Estuarine, Coastal and Shelf Science, 17: 339–355.
- Naylor, E. & Slinn, D.J. (1958). Observations on the ecology of some brackish water organisms in pools at Scarlett Point, Isle of Man. Journal of Animal Ecology, 27: 15–25.
- NODC (2009). World Ocean Atlas. U.S. Government Printing Office, Washington DC.
- Osborn, J.E.M. (1948). The structure and life history of Hormosira banksii (Turner) Decaisne. Transactions of the Royal Society of New Zealand, 77: 47–71.
- Pearson, G.A. & Brawley, S.H. (1996). Reproductive ecology of Fucus distichus (Phaeophyceae): an intertidal alga with successful external fertilization. Marine Ecology Progress Series, 143: 211–223.
- Peckol, P., Harlin, M.M. & Krumscheid, P. (1988). Physiological and population ecology of intertidal and subtidal Ascophyllum nodosum (Phaeophyta). Journal of Phycology, 24: 192–198.
- Povey, A. & Keough, M.J. (1991). Effects of trampling on plant and animal populations on rocky shores. Oikos, 61: 355–368.
- Pyefinch, K.A. (1943). The intertidal ecology of Bardsey Island, North Wales, with special reference to the recolonization of rock surfaces, and the rock-pool environment. Journal of Animal Ecology, 12: 82–108.
- Ralph, P.J., Morrison, D.A. & Addison, A. (1998). A quantitative study of the patterns of morphological variation within Hormosira banksii (Turner) Decaisne (Fucales: Phaeophyta) in south-eastern Australia. Journal of Experimental Marine Biology and Ecology, 225: 285–300.
- Reed, R.H. & Russell, G. (1979). Adaptation to salinity stress in populations of Enteromorpha intestinalis (L.) Link. Estuarine and Coastal Marine Science, 8: 251–258.
- Rochford, D.J. (1984). Nitrates in eastern Australian coastal waters. Australian Journal of Marine and Freshwater Research, 35: 385–397.
- Schiel, D.R. (1985). Growth, survival and reproduction of two species of marine algae at different densities in natural stands. Journal of Ecology, 73: 199–217.
- Schiel, D.R. (2006). Rivets or bolts? When single species count in the function of temperate rocky reef communities. Journal of Experimental Marine Biology and Ecology, 338: 233–252.
- Schiel, D.R. & Taylor, D.I. (1999). Effects of trampling on a rocky intertidal assemblage in southern New Zealand. Journal of Experimental Biology and Ecology, 235: 213–235.
- Schiel, D.R. & Thompson, G.A. (2012). Demography and population biology of the invasive kelp Undaria pinnatifida on shallow reefs in southern New Zealand. Journal of Experimental Marine Biology and Ecology, 434–435: 25–33.
- Schoenwaelder, M.E.A. (2002). Physode distribution and the effect of ‘thallus sunburn’ in Hormosira banksii (Fucales, Phaeophyceae). Botanica Marina, 45: 262–266.
- Scott, B.D. (1981). Hydrological structure and phytoplankton distribution in the region of a warm-core eddy in the Tasman Sea. Australian Journal of Marine and Freshwater Research, 32: 479–492.
- Serrao, E.A., Pearson, G., Kautsky, L. & Brawley, S.H. (1996). Successful external fertilization in turbulent environments. Proceedings of the National Academy of Sciences USA, 93: 5286–5290.
- Sharp, G. 1987. Ascophyllum nodosum and its harvesting in Eastern Canada. In Case Studies of Seven Commercial Seaweed Resources (Doty, M.S., Caddy, J.F. & Santelices, B., editors), 3–48. Food and Agriculture Organization of the United Nations, Rome.
- Slobodkin, L.B. (1966). Growth and Regulation of Animal Populations. Holt, Rinehart and Winston, New York.
- Smith, R.C. & Baker, K.S. (1979). Penetration of UV-B and biologically effective dose-rates in natural waters. Photochemistry and Photobiology, 29: 311–323.
- Sze, P. (1980). Aspects of the ecology of macrophyte algae in high rockpools at the Isles of Shoals. Botanica Marina, 23: 313–318.
- Taylor, D.I. & Schiel, D.R. (2003). Wave-related mortality in zygotes of habitat-forming algae from different exposures in southern New Zealand: the importance of ‘stickability’. Journal of Experimental Marine Biology and Ecology, 290: 229–245.
- Trowbridge, C.D. (1996). Demography and phenology of the intertidal green alga Codium setchellii: the enigma of local scarcity on sand-influenced rocky shores. Marine Biology, 127: 341–351.
- Truchot, J.-P. & Duhamel-Jouve, A. (1980). Oxygen and carbon dioxide in the marine intertidal environment: diurnal and tidal changes in rockpools. Respiration and Physiology, 39: 241–254.
- Underwood, A.J. (1981). Structure of a rocky intertidal community in New South Wales: patterns of vertical distribution and seasonal changes. Journal of Experimental Marine Biology and Ecology, 51: 57–85.
- Underwood, A.J. (1998). Grazing and disturbance: an experimental analysis of patchiness in recovery from a severe storm by the intertidal alga Hormosira banksii on rocky shores in New South Wales. Journal of Experimental Marine Biology and Ecology, 231: 291–306.
- Vadas, R.L. & Wright, W.A. 1986. Recruitment, growth and management of Ascophyllum nodosum. In Actas Segundo Nacional Sobre Algas Marinas Chilenas (Westermeier, R., editor), 101–113. Universidad Austral de Chile, Valdivia.
- Vadas, R.L., Wright, W.A. & Miller, S.L. (1990). Recruitment of Ascophyllum nodosum: wave action as a source of mortality. Marine Ecology Progress Series, 61: 263–272.
- Vernet, P. & Harper, J.L. (1980). The cost of sex in seaweeds. Biological Journal of the Linnean Society, 13: 129–138.
- Wolfe, J.M. & Harlin, M.M. (1988). Tidepools in southern Rhode Island, U.S.A. II. Species diversity and similarity analysis of macroalgal communities. Botanica Marina, 31: 537–546.
- Womersley, H.B.S. (1987). The marine benthic flora of southern Australia. Part II. South Australian Government Printing Division, Adelaide.
- Wood, W.F. (1987). Effect of solar ultra-violet radiation on the kelp Ecklonia radiata. Marine Biology, 96: 143–150.
- Wright, P.J. & Reed, R.H. (1990). Effects of osmotic stress on gamete size, rhizoid initiation and germling growth in fucoid algae. British Phycological Journal, 25: 149–155.
