Abstract
Glycerol production is modulated in some halo-tolerant organisms in response to extracellular osmotic stress. Glycerol-3-phosphate dehydrogenase (GPDH) is a rate-limiting enzyme of glycerol synthesis in both yeast and the green microalgae Dunaliella. In yeast, a High-Osmolarity-Glycerol (HOG) pathway, which is a mitogen-activated protein kinase (MAPK) cascade, has been suggested to activate the expression of GPDH and thus the accumulation of glycerol under osmotic stress. In Dunaliella tertiolecta, however, the osmo-regulatory mechanisms for glycerol synthesis are not well understood. In this study, the homologues of MAPK and GPDH in D. tertiolecta were cloned using Rapid Amplification of cDNA Ends (RACE) to investigate the molecular basis of the osmo-regulatory mechanisms. The isolated cDNA sequences were named DtMAPK (GenBank: KJ930518) and DtGPDH (GenBank: KJ930370), respectively. It was found that after osmotic shock DtMAPK and DtGPDH expression increased within 0.5 h and 1 h, respectively. In addition, glycerol production and DtGPDH expression level paralleled the expression of DtMAPK under various osmotic stress conditions. This suggests a close correlation between the expression of these two genes and glycerol production in D. tertiolecta. Moreover, suppressed transcription of DtGPDH and delayed accumulation of intracellular glycerol were observed in DtMAPK knock-down cells upon hyper-osmotic shock, providing further evidence that DtMAPK is involved in the regulation of DtGPDH expression and thus glycerol synthesis. Our findings demonstrate that a MAPK-mediated signalling pathway similar to the yeast HOG pathway may exist in D. tertiolecta in response to osmotic stress.
Introduction
The eukaryotic and photosynthetic microalga Dunaliella adapts to various osmotic stresses by varying its glycerol content (Ben-Amotz & Avron, Citation1990). In addition to accumulating intracellular glycerol, it has been found that Dunaliella tertiolecta also continuously releases large amounts of glycerol into the external medium (Chow et al., Citation2013). The massive production of glycerol in extremely high osmolarity environments makes Dunaliella a potential commercial source of glycerol (Ben-Amotz & Avron, Citation1981; Chisti, Citation2007; Chow et al., Citation2013). However, the osmo-regulatory mechanisms for glycerol production in Dunaliella under osmotic stress have not been completely understood.
Glycerol 3-phosphate dehydrogenase (GPDH) is an important rate-limiting enzyme for glycerol synthesis, and intracellular glycerol concentration functions as the counterbalancing osmolyte in both the unicellular green algae Dunaliella and other organisms such as the yeast Saccharomyces cerevisiae (Hohmann, Citation2002; Saito & Posas, Citation2012). It has been reported that a mitogen-activated-protein-kinase (MAPK) cascade, also known as the High-Osmolarity-Glycerol (HOG) signalling pathway, is activated to regulate glycerol synthesis during adaptation to osmotic stress in yeast (Saito & Tatebayashi, Citation2004; Hohmann, Citation2009). When yeast cells were stimulated by hyper-osmotic challenges, the accumulated and activated Hog1 triggers a cascade of signalling and transcription events which promote transcription of the GPDH enzyme and synthesis of glycerol (Saito & Posas, Citation2012). The core molecule Hog1 in the yeast HOG signalling pathway belongs to the MAPK family.
MAPK functions are highly conserved (Galcheva-Gargova et al., Citation1994; English et al., Citation2015); MAPK pathways are dynamic signalling modules present in all eukaryotic cells and mainly involved in the regulation of cellular responses to environmental stress inputs (Hohmann, Citation2002; English et al., Citation2015). MAP kinase proteins and genes have been studied in plants such as Arabidopsis, rice, maize and tobacco, as well as the green algae Dunaliella. A Dunaliella salina MAPK gene (DsMPK) was reported to be involved in D. salina response to hyper-osmotic shock (Lei et al., Citation2008) and a MAPK-like protein was suggested to be both up-regulated and phosphorylated in Dunaliella viridis in response to hypertonic shock (Jimenez et al., Citation2004). This protein could be detected by antibodies specific to yeast Hog1 and its mammalian homolog p38. Furthermore, an inhibitor of p38 MAP kinase (SB203580) was reported to markedly impair the adaptation of Dunaliella to osmotic stress (Jimenez et al., Citation2004). These investigations suggest that a MAPK signalling pathway may be operating in Dunaliella as the mechanism of osmosis regulation. A MAPK cascade similar to yeast HOG pathway may exist to regulate glycerol production in Dunaliella under osmotic stress.
The expression of GPDH enzyme as well as synthesis of glycerol is regulated by the MAPK protein Hog1 in yeast. To demonstrate that a MAPK cascade, which is similar to yeast HOG pathway, may exist in Dunaliella and regulate its glycerol synthesis in response to osmotic stress, the yeast Hog1 homologue MAPK encoding gene DtMAPK, as well as the GPDH encoding gene DtGPDH were isolated and characterized in D. tertiolecta. The sequence of DtMAPK is highly conserved. It was found that DtMAPK was involved in the regulation of DtGPDH expression and glycerol synthesis in D. tertiolecta. Based on the investigations, a MAPK meditated signalling pathway is suggested to control glycerol synthesis, which sheds light on the molecular basis of the osmo-regulatory mechanism in D. tertiolecta.
Materials and Methods
Strains and culture conditions
Dunaliella tertiolecta LB-999 was obtained from the Culture Collection of Algae at the University of Texas at Austin (UTEX). Dunaliella tertiolecta cells were grown in batch cultures, in sterile modified ATCC-1174 DA liquid medium (American Type Culture Collection at Manassas, Virginia) with various NaCl concentrations (0.5 M, 1 M, 2 M, 3 M and 4 M). The cultures were agitated by a rotary shaker at 100 rpm and incubated at 25°C, under a 14 h light:10 h dark photoperiod with approximately 50 µmol photons m−2 s−1 light intensity provided by cool white fluorescent lights. Irradiance was measured using a Model LI-189 Quantum Meter (LI-COR, USA).
Determination of cell density and glycerol concentration
Cell density was monitored by microscopy counting using a haemocytometer (Neubauer Improved Grid) loaded with 10 µl of cell culture. Before counting, cells were fixed with 2% (v/v) formaldehyde solution.
Glycerol content was measured using the Free Glycerol Determination kit (FG0100, Sigma-Aldrich, St Louis, Missouri, USA). Cells were collected by centrifugation at 5000 × g at 4°C for 10 min and then washed once with isotonic culture medium. The cell pellet was resuspended in distilled water and boiled for 10 min to release intracellular glycerol. The culture supernatant and intracellular glycerol contents were assayed. Both extracellular and intracellular glycerol production were calculated on a per cell basis.
RNA extraction and total cDNA synthesis
Total RNA was isolated from D. tertiolecta cells using the RNeasy® Plant Mini Kit (Qiagen) according to the manufacturer’s protocol. RNA samples were prepared from a minimum of 107 cells for each sample. The quality and concentration of RNA were measured using NanoDrop (NanoDrop 2000 UV-Vis Spectrophotometer, Thermo Scientific) immediately after extraction. The cDNA was prepared using 1µg RNA per sample by reverse transcription using the SuperScript™ III First-Strand Synthesis System (Invitrogen, USA) according to the manufacturer’s instructions.
Cloning of DtMAPK and DtGPDH in D. tertiolecta by Rapid Amplification of cDNA Ends (RACE)
RACE primers for the D. tertiolecta MAPK gene (DtMAPK) were designed from the conserved regions of MAPK from D. salina (NCBI GenBank: ABN03944.1), Chlamydomonas reinhardtii (NCBI GenBank: XP_001700291.1), Volvox carteri (NCBI GenBank: XP_002955338.1), Arabidopsis thaliana (NCBI GenBank: ABR46165.1) and the budding yeast Saccharomyces cerevisiae (NCBI GenBank: NP_013214.1). All primer sequences were minimized based on the D. tertiolecta codon bias from the website (http://www.kazusa.or.jp/codon/cgi-bin/showcodon.cgi?species=3047&aa=1&style=GCG) or the sequence of MAPK from D. salina. The forward primers DtMAPK-dgF1 (5’-AGGAGCATACGGTGTGGTTTG-3’) and DtMAPK-dgF2 (5’-AAAGTGGCCATCAAGAAAAT-3’) were designed from the conserved peptide sequences GAYGVVC and KVAIKK. The reverse primers DtMAPK-dgR1 (5’-TAGCTCAGGGGCTCTGTACCA-3’) andDtMAPK-dgR2 (5’-TAGTCCTTGCCGGGGAACA-3’) were designed from the conserved peptide sequence WYRAPEL and FPGKDY. DtMAPK related cDNA fragments were amplified by PCR and then sequenced.
The 5′- and 3′- ends of the D. tertiolecta MAPK cDNA (DtMAPK) were cloned using the SMARTer™ RACE cDNA Amplification Kit (Clontech, Mountain View, California, USA). DtMAPK 5′-RACE product was generated using 10×Universal Primer A Mix (UPM) or Nested Universal Primer A (NUP) provided with the kit as a forward primer and MAPK gene-specific primers DtMAPK-gsR1 (5’-ATCGCGGTGCAGGATGGCAG-3’) or DtMAPK-gsR2 (5’-CCACACCGTATGCTCCTTTGCCA-3’) as a reverse primer. DtMAPK 3′-RACE product was generated using a gene-specific forward primer DtMAPK-gsF1 (5’-TGCCAAACGCACACTGCGTG-3’) or DtMAPK-gsF2 (5’-CGCCTGATGTGGTGCGAGGC-3’) and UPM or NUP as a reverse primer.
RACE primers for amplifying the cDNA of D. tertiolecta GPDH (DtGPDH) were designed from the conserved regions of the GPDH from D. salina (NCBI GenBank: AY845323). The 5′- and 3′- ends of DtGPDH were cloned in the same way as DtMAPK. DtGPDH 5’-RACE product was generated using the UPM or NUP from the kit as a forward primer and GPDH gene-specific primers DtGPDH-gsR1 (5′-GTTGATGGTGGTGAACAAG-3′) or DtGPDH-gsR2 (5′-TGTACTCGATCAGGTTGC-3′) or DtGPDH-gsR3 (5′-GAAGGCATCTGCTCCATC-3′) as a reverse primer. DtGPDH 3′-RACE product was generated using a gene-specific forward primer DtGPDH-gsF1 (5′-AGTTCATCTCCCCCTCAGTGCGCGA-3′) or DtGPDH-gsF2 (5′-AGGCCTGGGCCCAGAAGAGGATCG-3′) (He et al., Citation2009) and UPM or NUP as a reverse primer.
Sequence analysis
The RACE-PCR products were cloned into pGEM®-T Easy Vector System (Promega, Madison, WI) for sequencing. RACE 5′- and 3′-overlapping sequences were assembled using the EMBOSS merger program to obtain the full-length sequences. The assembled sequences were named DtMAPK (NCBI GenBank: KJ930517) and DtGPDH (NCBI GenBank: KJ930370) respectively, and analysed for open reading frames using ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). The deduced amino acid sequences were compared with previously characterized protein sequences using the Blast algorithm (http://www.ncbi.nlm.nih.gov/BLAST/).
Construction of DtMAPK-RNAi plasmid
The pGreen-0229 binary vector HY104 (Yu et al., Citation2004) was modified and used as the D. tertiolecta transformation vector. To generate the DtMAPK knock-down construct DtMAPK-RNAi (), an 851-bp adenine methyltransferase (AMT) gene (NCBI GenBank: X06618.1) promoter from Chlorella virus was amplified using the PCR primer pair AMT-F-KpnI (5’-aaaGGTACCATCAGTAATGTGTTAATTGC-3’), which introduces a cutting site for the restriction enzyme KpnI, and AMT-R-XhoI (5’-ccgCTCGAGTTAAATAATATATAGTGTATTTTAG-3’), which introduces the XhoI site, and cloned into pGreen-0229 upstream of the gene fragments to drive its expression in D. tertiolecta (Mitra et al., Citation1994). A 526-bp bleomycin resistance gene (ble) derived from Streptoalloteichus hindustanus was amplified using the primers ble-F-XhoI (5′-AAACTCGAGATGGCCAAGCTGACCAGC-3′) introducing an XhoI site and ble-R2-ClaI (5′-CACATCGATTTAGTCCTGCTCCTCGGC-3′) introducing a ClaI site and inserted to the pGreen-0229 backbone as a dominant selectable marker to confer zeocin-resistance.
Fig. 1. Schematic diagram of DtMAPK-RNAi construct. Anti-sense and Sense: anti-sense sequence and sense sequence fragments of DtMAPK gene coding sequence.
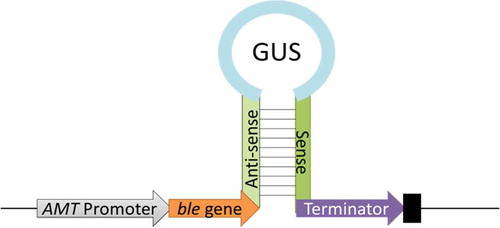
A 334-bp coding fragment of DtMAPK (bp 75–408) was used for the construction of DtMAPK-RNAi, which expressed self-complementary or hairpin RNA-containing sequences homologous to the target DtMAPK driven by the AMT promoter (). Sense and anti-sense sequences of this fragment were amplified by PCR from the total cDNA of D. tertiolecta. The primer pair for the sense fragment amplification were the forward primer DtMAPK-SF-PstI (5’-AACTGCAGAGTCCATCAACTATGAGGCCG-3’), which introduces a PstI site, and the reverse primer DtMAPK-SR-BamHI (5’-CGGGATCCTCGTACACCACATACAGGTCC-3’) introducing a BamHI site. Primers for the amplification of the anti-sense fragment were DtMAPK-AsF-ClaI (5’-CCATCGATTCGTACACCACATACAGGTCC-3’), which introduces a ClaI site, and DtMAPK-AsR-EcoRI (5’-CGGAATTCAGTCCATCAACTATGAGGCCG-3’), which introduces an EcoRI site. The amplified gene-specific anti-sense and sense fragments were then inserted into the modified pGreen-0229 vector before and after the β-glucuronidase encoding gene (GUS) fragment respectively.
Transformation of D. tertiolecta
Constructed plasmids were transformed into D. tertiolecta cells using the Agrobacterium-mediated Dunaliella transformation method as described previously (Kumar et al., Citation2004; Fang et al., Citation2012) with slight modifications. The DtMAPK-RNAi construct was transformed into Agrobacterium tumefaciens GV3101 via electroporation (2.5 kV). The transformed agrobacteria were subsequently co-cultured with logarithmic phase (5 d old) D. tertiolecta liquid culture in 0.05 M NaCl 1174 DA medium with 100 µM acetosyringone for 2 d. The transformed D. tertiolecta were plated onto 0.5 M NaCl 1174 DA medium agar plates containing 20 µg ml−1 zeocin (Zeocin™, Invitrogen) as the selection marker. Colonies that appeared within 2 weeks were subcultured in 96-well plates containing liquid selective medium (0.5 M NaCl ATCC-1174 DA medium containing 20 µg ml−1 zeocin). Individual D. tertiolecta colonies (transformants) were subjected to genotyping PCR using primers ble-gen-F (5’-GGAGCGGTCGAGTTCTGG-3’) and ble-gen-R (5’-CTCGCCGATCTCGGTCAT-3’) to confirm the presence of gene integration.
Osmotic stress treatments
Dunaliella tertiolecta wild-type (WT) cells or DtMAPK-RNAi transformants were subjected to osmotic stress treatments following the process described below. Cells were first grown in sterile 2 M NaCl ATCC-1174 DA liquid medium under photoperiod illumination for 5 d (logarithmic phase), and then harvested by centrifugation at 5000 × g for 10 min at 4°C. The microalgae pellets were suspended in iso-volumetric ATCC-1174 DA medium containing 0.5 M (hypo-osmotic shock), 1 M (hypo-osmotic shock), 2 M (iso-osmotic control), 3 M (hyper-osmotic shock) or 4 M (hyper-osmotic shock) NaCl.
Treated cells were harvested at 0.5 h, 1 h, 2 h, 4 h, 6 h and 8 h (short-term osmotic shock) or 5 d (long-term osmotic shock) by centrifugation at 5000 × g for 10 min at 4°C. The cell pellets were either frozen in liquid nitrogen followed by storage at −80 °C (for RNA extraction), or analysed immediately for glycerol determination. Each osmotic shock experiment was repeated at least three times.
Gene expression analysis by quantitative real-time PCR
Quantitative real-time PCR (QRT-PCR) was performed with the 2 × Maxima® SYBR Green/ROX qPCR Master Mix (Fermentas, Waltham, Massachusetts, USA) according to the manufacturer’s instructions, using an ABI PRISM® 7500 Real-Time PCR System (Applied Biosystems, Carlsbad, California). Specific primers for DtMAPK (forward: 5’-AGCCCCTGAGCTACTGCTCTCAT-3’; reverse: 5’-GTTCAGCTGGTGCACGTAGTCCT-3’), DtGPDH (forward: 5’-ATTAACCTGCTTGCGGATGT-3’; reverse: 5’-CATAGCTGCTGGCAATCAAA-3’) and D. tertiolecta β-tubulin gene (DtTUB) (forward: 5’-CAGATGTGGGATGCCAAGAACAT-3’; reverse: 5’-GTTCAGCATCTGCTCATCCACCT-3’) were designed to analyse gene expression. DtTUB was used as the endogenous control. Relative expression of DtMAPK or DtGPDH was calculated using the equation 2−ΔΔCt (Livak & Schmittgen, Citation2001). All reactions were carried out in parallel triplicates and the experiments were repeated three times.
Statistical analysis
All values are expressed as the means ± standard deviation of at least triplicate assays to measure technical variation and typically multiple experiments were performed with independent batches of cells to assess biological reproducibility. Statistical analyses were performed using SPSS Version 15 (SPSS Inc., Chicago, Illinois, USA). Student’s t-test was used for comparisons and Linear Regression was used for correlation analysis. A p-value ≤ 0.05 was considered as statistically significant.
Results
Identification and characterization of DtMAPK and DtGPDH in D. tertiolecta
Rapid Amplification of cDNA Ends (RACE) was performed in D. tertiolecta. A mitogen-activated protein kinase (MAPK) homologue was identified and named DtMAPK. The entire coding region of the isolated DtMAPK is 1471-bp in length, and encodes a putative protein containing 472 amino acids (GenBank: AIN35080.1). A BLASTX search against the National Center for Biotechnology Information (NCBI) protein database (http://www.ncbi.nlm.nih.gov/protein) revealed that the amino acid sequence is 98% and 81% similar to two MAPK proteins reported in D. salina (DsMPKs, NCBI GenBank: ABN03944.1 and AFJ54625.1, respectively) and 75% similar to a MAPK from Chlamydomonas reinhardtii (CrMAPK, NCBI GenBank: XP_001700291.1). A conserved MAPK family protein catalytic domain (including an active site, an ATP-binding site, a substrate-binding site, an activation loop and a KIM docking site) of the Serine/Threonine Kinases, TEY Mitogen-Activated Protein Kinases from plants was predicted to be located from position 43 to position 241. These features strongly suggest that the isolated DtMAPK cDNA encodes a putative mitogen-activated protein kinase of the MAPK family in D. tertiolecta.
The isolated DtGPDH is 2734-bp long with an ORF of 2106-bp. The predicted polypeptide codes for 701 amino acids with a molecular weight of 76.9 kDa and pI of 6.09. The putative amino acid sequence of DtGPDH shared a high homology with other Dunaliella GPDHs with the highest homology of 99% with the osmo-regulatory chloroplastic isoform DsGPDH2 in D. salina (NCBI GenBank: AY845323). The first 30 amino acids at the N terminal of the translated DtGPDH were predicted to be a chloroplast targeting peptide. Similar to other GPDHs cloned from Dunaliella, the DtGPDH enzyme contains phosphoserine phosphatase (position 106 to position 330) and GPDH (position 333 to position 682) catalytic domains.
Glycerol production and expression of DtMAPK and DtGPDH in D. tertiolecta are dependent on external osmolarity
In this investigation, glycerol content of D. tertiolecta as well as the expression levels of both DtMAPK and DtGPDH was determined at different external salinities (). & B show the intracellular and extracellular glycerol accumulated per cell over a 5 d period after the cells were transferred from 2 M NaCl to different salinities ranging from 0.5 M to 4 M NaCl. As can be seen, both intracellular and extracellular glycerol production by D. tertiolecta were proportional to the external salt concentrations (linear regression, P < 0.05). Intracellular glycerol content increased with increasing salinity (). This was expected as the intracellular glycerol functions as an osmolyte to maintain osmolality balance across the cell membrane. Extracellular glycerol was also positively correlated with external salinity () and D. tertiolecta has higher total glycerol production at high salt conditions. The results confirm that glycerol production of D. tertiolecta is regulated accordingly in the adaptation to various osmotic pressure conditions.
Fig. 2. Glycerol production and expression of DtMAPK and DtGPDH in D. tertiolecta under various osmotic stresses. Straight lines were generated by linear regression. R2 and P-value are shown for each regression, where P < 0.05 suggests a significant linear relationship. The values shown in the data were the average of triplicate experiments in parallel.
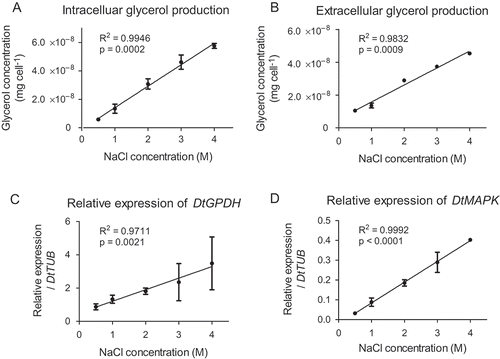
Moreover, & D show that the mRNA levels of both the DtGPDH and DtMAPK genes were positively correlated to the external salinity (linear regression, P < 0.05). Gene expression levels were higher in D. tertiolecta cells with higher salt stress condition. This observation is similar to the trend of glycerol production in D. tertiolecta at various salinities ( & B). Hence, glycerol production, as well as the expression of DtMAPK and DtGPDH, is dependent on external osmolarity, suggesting that correlations may exist between the gene expressions and glycerol synthesis in D. tertiolecta in response to osmotic stress.
Expression kinetics of DtMAPK in response to osmotic shocks
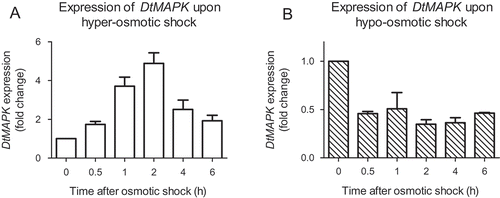
The expression kinetics of DtMAPK was further investigated on the response to changes in extracellular osmolarity. shows the relative fold change of DtMAPK mRNA level in response to a 2 M to 4 M hyper-osmotic shock as well as a 2 M to 0.5 M hypo-osmotic shock. As can be seen, transcriptional level of DtMAPK was regulated in response to osmotic shocks within the first 0.5 h after osmotic shock treatment. DtMAPK expression was up-regulated approximately fivefold within 2 h of hyper-osmotic shock treatment () and suppressed after hypo-osmotic shock treatment (). Hence the expression of DtMAPK rapidly responds to osmotic shocks in D. tertiolecta.
Fig. 3. Expression of DtMAPK in reponse to osmotic shocks. DtMAPK expression level at time 0 (before osmotic shock) was normalized as 1 in each set of experiment. Fold change was calculated and shown respectively based on the iso-osmotic control group (2 M NaCl to 2 M NaCl) taken at the same time points. DtTUB was used as the endogenous reference. A: hyper-osmotic shock (2 M NaCl to 4 M NaCl); B: hypo-osmotic shock (2 M NaCl to 0.5 M NaCl).
Expression kinetics of DtGPDH in response to hyper-osmotic shock
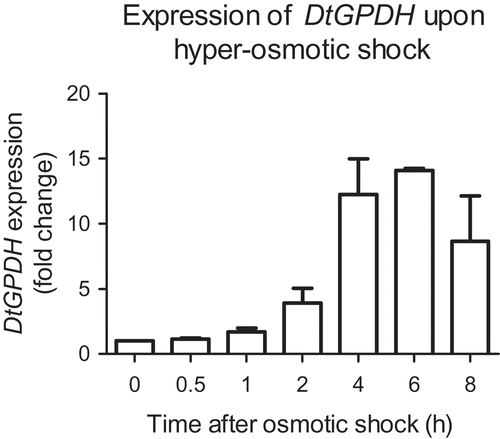
To explore the correlation between DtMAPK and DtGPDH in D. tertiolecta, kinetic expression of DtGPDH was determined under hyper-osmotic conditions where glycerol production of D. tertiolecta was increased. shows the fold change of DtGPDH in D. tertiolecta in response to a hyper-osmotic shock treatment from 2 M NaCl medium to 4 M NaCl medium. DtGPDH was up-regulated by hyper-osmotic stress within 1 h after the osmotic treatment. Transcriptional level of DtGPDH was increased by 10–15 times during the 4–8 h following hyper-osmotic shock. The prior up-regulation of DtMAPK expression within 0.5 h after hyper-osmotic treatment suggests that DtMAPK may be involved in the activation of DtGPDH transcription, which is required for the increase in glycerol synthesis of D. tertiolecta under hyper-osmotic stress.
Fig. 4. Expression of DtGPDH in response to a hyper-osmotic shock from 2 M NaCl to 4 M NaCl. DtGPDH expression level at time 0 (before osmotic shock) was normalized as 1 for each set of experiment. Fold change was calculated and shown respectively based on the iso-osmotic control group (2 M NaCl to 2 M NaCl) taken at the same time points. DtTUB was used as the endogenous reference.
Generation of the DtMAPK knock-down D. tertiolecta strain by RNA interference
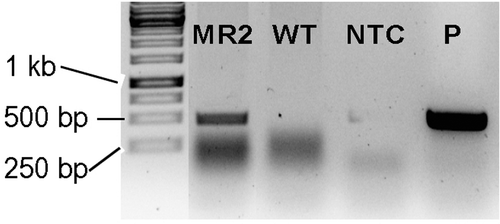
To further elucidate the biological function of DtMAPK in the osmo-regulation of D. tertiolecta, a DtMAPK knock-down D. tertiolecta strain was obtained by RNA interference (RNAi) technology. The DtMAPK-RNAi plasmid was constructed to express a hairpin RNA containing the endogenous DtMAPK coding fragment (), and then transformed into D. tertiolecta by the Agrobacterium-mediated gene transformation system. A 397-bp PCR product corresponding to the ble selection marker and anti-sense fragment targeted on DtMAPK was amplified by genotyping PCR, demonstrating the successful gene integration in D. tertiolecta MR2 strain ().
Fig. 5. Genotyping PCR of DtMAPK-RNAi-MR2 strain. MR2: DtMAPK knock-down D. tertiolecta MR2 strain; WT: D. tertiolecta wild-type; NTC: non-template control; P: positive control.
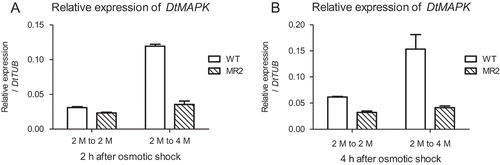
The transcriptional level in D. tertiolecta MR2 strain was measured during a hyper-osmotic shock (). The knock-down effect of DtMAPK gene was confirmed in the MR2 strain by three hyper-osmotic shock treatment experiments in parallel. As compared with DtMAPK expression in the wild-type which was up-regulated fourfold within 2 h of hyper-osmotic shock, DtMAPK expression in the MR2 strain was not significantly increased during the sudden increase in salinity (). DtMAPK expression of the MR2 strain was similar to the iso-osmotic control 2 h or 4 h after hyper-osmotic stress exposure. Moreover, when the MR2 and wild-type cells were subjected to the same treatment, DtMAPK expression in MR2 strain was always lower than that in D. tertiolecta wild-type (). These findings indicate that DtMAPK has been knocked down successfully in the MR2 strain even under hyper-osmotic stress.
Fig. 6. Expression of DtMAPK in the DtMAPK knock-down MR2 strain could not respond to hyper-osmotic shock. WT (blank): D. tertiolecta wild-type; MR2 (slash): DtMAPK knock-down MR2 strain. 2 M to 2 M, iso-osmotic control from 2 M NaCl to 2 M NaCl; 2 M to 4 M, hyper-osmotic shock treatment from 2 M NaCl medium to 4 M NaCl medium.
Expression of DtGPDH was suppressed in the DtMAPK knock-down MR2 strain
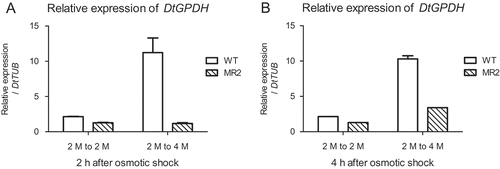
To evaluate whether DtGPDH was regulated by the expression of DtMAPK, transcriptional level of DtGPDH was determined in the DtMAPK knock-down D. tertiolecta MR2 strain. shows the relative expression of DtGPDH in the MR2 strain 2 h and 4 h after a hyper-osmotic shock from 2 M NaCl to 4 M NaCl. As can be seen, expression of DtGPDH was suppressed in the DtMAPK knock-down MR2 strain. Lower DtGPDH expression levels were observed in the MR2 strain compared with D. tertiolecta wild-type, in both the iso-osmotic control group and the 2 M to 4 M hyper-osmotic shock treatment group. The inhibition of DtGPDH expression in the MR2 strain suggests that DtGPDH transcription in D. tertiolecta may be regulated by DtMAPK, which is a possible explanation for the osmo-regulatory mechanism for glycerol production in D. tertiolecta in response to an osmotic shock.
Fig. 7. Expression of DtGPDH in response to hyper-osmotic shock in the DtMAPK knock-down strain MR2. WT (blank): D. tertiolecta wild-type; MR2 (slash): DtMAPK knock-down MR2 strain. 2 M to 2 M, iso-osmotic control from 2 M NaCl to 2 M NaCl; 2 M to 4 M, hyper-osmotic shock treatment from 2 M NaCl to 4 M NaCl.
Osmo-regulation of glycerol production was delayed in the DtMAPK knock-down MR2 strain
Glycerol content of D. tertiolecta upon hyper-osmotic shock was evaluated in the DtMAPK knock-down MR2 strain to demonstrate the effect of DtMAPK on the osmo-regulation of glycerol production. As the extracellular glycerol concentration in the media was negligible compared with the intracellular glycerol concentration after D. tertiolecta cells were transferred to fresh media for experimental treatments, only the intracellular glycerol concentration of the cells upon osmotic shock treatment was shown (). It was observed that the intracellular glycerol content of D. tertiolecta wild-type was generally increased 2 h and 4 h after a 2 M to 4 M hyper-osmotic shock treatment. However, the DtMAPK knock-down MR2 strain had significantly lower glycerol content than the wild-type 2 h after hyper-osmotic shock treatment (Student’s t-test, P < 0.05), with glycerol concentrations similar to that of iso-osmotic shock treated cells (). Therefore, unlike wild-type, the MR2 strain did not respond to a hyper-osmotic shock within the first 2 h of treatment. The osmo-regulation of glycerol production was delayed in the DtMAPK knock-down cells.
Fig. 8. Intracellular glycerol content of D. tertiolecta wild-type and MR2 strain in response to osmotic shock. WT (blank): D. tertiolecta wild-type; MR2 (slash): DtMAPK knock-down MR2 strain. 2 M to 2 M: iso-osmotic control from 2 M NaCl to 2 M NaCl; 2 M to 4 M: hyper-osmotic shock treatment from 2 M NaCl to 4 M NaCl.
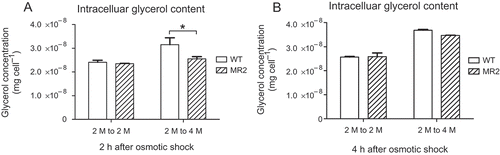
On the other hand, the intracellular glycerol content determined 4 h after treatment showed no significant difference between the wild-type and MR2 strain, under both hyper-osmotic shock and iso-osmotic conditions (). This correlates with an up-regulation of DtGPDH in MR2 strain 4 h after hyper-osmotic shock treatment, which was absent under iso-osmotic conditions (). The delayed increase in DtGPDH expression in MR2 compared with wild type under hyper-osmotic stress led to a slower osmotic response, as shown by the increase in glycerol production only 4 h after exposure to hyper-osmotic stress ().
Discussion
The isolated DtMAPK is highly conserved
A cDNA coding for MAPK has been previously reported in D. salina (DsMPK) (Lei et al., Citation2008). Alignment of DtMAPK with DsMPK showed a 98% similarly on their amino acid sequences. This suggests that DtMAPK is highly conserved and has a close relationship with DsMPK. The isolated DtMAPK was found to be up-regulated in D. tertiolecta upon hyper-osmotic shock and suppressed upon hypo-osmotic shock in this study (). The transient up-regulation of DtMAPK in response to hyper-osmotic stress could be due to a feedback from DtGPDH expression and glycerol synthesis 4–6 h after treatment, which is similar to the yeast HOG pathway (Jimenez et al., Citation2004). Moreover, a MAPK-like protein in D. viridis that could be detected by the yeast Hog1 antibody was reported to be up-regulated in response to hypertonic shock (Jimenez et al., Citation2004). The yeast MAPK Hog1 is activated and up-regulated by hyper-osmotic pressure as well (Saito & Tatebayashi, Citation2004). The conservation of MAPK function suggests that DtMAPK in Dunaliella has a similar function to yeast Hog1 in response to external osmotic stress.
Expression of DtGPDH is regulated by DtMAPK
Gene expression study on DtMAPK and DtGPDH showed that both genes were positively correlated to the external salinities ( & ). DtGPDH encodes the enzyme DtGPDH that catalyses the rate-limiting step in the glycerol synthetic pathway in D. tertiolecta. Glycerol production was also positively correlated to the external salinity ( & ). Thus, the increase in DtGPDH expression is expected with increased external salinity. The parallel increase in expression of DtMAPK with salinity can be explained by a potential relationship between DtMAPK and DtGPDH. GPDH expression is initiated by activation of the MAPK Hog1 in yeast (Hohmann, Citation2009). Therefore, it was proposed in this study that DtGPDH was mediated by DtMAPK and this has also been evidenced.
Kinetic studies on the expression of DtMAPK () and DtGPDH () in response to short-term hyper-osmotic shocks demonstrated that both genes were up-regulated upon hyper-osmotic shock treatment. However, the up-regulation of DtMAPK occurred at 0.5 h and peaked at 2 h after osmotic treatment (), while the expression of DtGPDH started to increase at 1 h after the transfer of cells to a higher salinity. The highest induction of DtGPDH occurred at 4–6 h after the hyper-osmotic shock treatment (). Therefore, it is suggested that DtGPDH is downstream of the expression of DtMAPK upon osmotic shock, resulting in a delay in its up-regulation.
DtMAPK is responsible for the immediate osmo-regulation of glycerol synthesis in D. tertiolecta
In this study, osmo-regulation in the DtMAPK knock-down MR2 strain was impaired as compared with D. tertiolecta wild-type upon hyper-osmotic shock ( & ). A delayed trigger of glycerol synthesis was observed in the DtMAPK knock-down MR2 cells treated with hyper-osmotic stress, while no difference in glycerol contents was observed in the cells subjected to an iso-osmotic treatment (). These findings suggest that DtMAPK is essential for the fast initial regulation of glycerol synthesis by activating the expression of DtGPDH in response to osmotic stress.
Similarly, it has been known that the transcription of GPDH in the budding yeast is activated in response to hyper-osmotic challenge by the HOG pathway, which is mediated by the MAPK protein Hog1 (Saito & Tatebayashi, Citation2004; Hohmann, Citation2009). Hence it can be suggested that a yeast HOG-like MAPK signalling pathway is involved in the osmo-regulation of D. tertiolecta under osmotic stress by activating the transcription of DtGPDH and thus, glycerol synthesis.
Other mechanisms might be involved in the osmo-regulation of D. tertiolecta
Glycerol production was delayed in DtMAPK knock-down MR2 cells in response to hyper-osmotic shock, as its intracellular glycerol content did not increase in response to a hyper-osmotic shock until 4 h after treatment (). In addition, DtGPDH expression in MR2 cells was slightly up-regulated 4 h after the hyper-osmotic shock treatment, though it was still inhibited as compared with wild-type (). It has been found that more than one MAPK-like protein was transiently phosphorylated under stress conditions in D. viridis (Jimenez et al., Citation2004). These different modules might function collaboratively or take part in the osmo-regulation of Dunaliella species. Therefore, other possible mechanisms and modules may be involved in the osmo-regulation in DtMAPK knock-down cells.
Conclusion
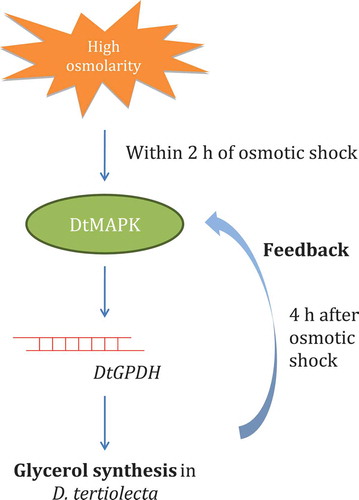
DtMAPK and DtGPDH coding for MAPK and GPDH respectively were isolated and characterized in D. tertiolecta for the first time in this study. Investigation on these genes suggests that a MAPK signalling pathway, similar to yeast HOG pathway, may be involved in the osmo-regulation of D. tertiolecta. A potential osmo-regulatory mechanism is proposed (), where the transiently up-regulated DtMAPK induces the expression of DtGPDH, thus activating glycerol synthesis in D. tertiolecta. These findings shed light on the molecular basis of osmo-regulatory mechanisms for glycerol synthesis in D. tertiolecta.
Fig. 9. Proposed information flow showing the response of D. tertiolecta to hyper-osmotic stress. We have shown that DtGPDH is up-regulated by the transient up-regulation of DtMAPK and causes activation of glycerol synthesis as an immediate response to high external osmolarity. Unknown mechanisms might be involved leading to up-regulation of DtGPDH mRNA levels 4 h after osmotic shock.
Acknowledgements
The authors would like to thank other members of the laboratory for helpful discussion and sharing of samples and reagents as needed.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Ran Zhao
Ran Zhao: planned and performed the experiments, analysed the data, wrote the paper; Daphne Ng: performed the experiments, analysed the data, wrote the paper; Lei Fang: contributed materials; Yvonne Chow: edited the paper; Yuan Kun Lee: planned the experiments, analysed the data, edited the paper.
References
- Ben-Amotz, A. & Avron, M. (1981). Glycerol and ß-carotene metabolism in the halotolerant alga Dunaliella: a model system for biosolar energy conversion. Trends in Biochemical Sciences, 6: 297–299.
- Ben-Amotz, A. & Avron, M. (1990). The biotechnology of cultivating the halotolerant alga Dunaliella. Trends in Biotechnology, 8: 121–126.
- Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25: 294–306.
- Chow, Y.Y., Goh, S.J., Su, Z., Ng, D.H., Lim, C.Y., Lim, N.Y., Lin, H., Fang, L., & Lee, Y.K. (2013). Continual production of glycerol from carbon dioxide by Dunaliella tertiolecta. Bioresource Technology, 136: 550–555.
- English, J.G., Shellhammer, J.P., Malahe, M., McCarter, P.C., Elston, T.C. & Dohlman, H.G. (2015). MAPK feedback encodes a switch and timer for tunable stress adaptation in yeast. Science Signaling, 8: ra5.
- Fang, L., Lin, H.X., Low, C.S., Wu, M.H., Chow, Y. & Lee, Y.K. (2012). Expression of the Chlamydomonas reinhardtii sedoheptulose-1,7-bisphosphatase in Dunaliella bardawil leads to enhanced photosynthesis and increased glycerol production. Plant Biotechnology Journal, 10: 1129–1135.
- Galcheva-Gargova, Z., Derijard, B., Wu, I.-H. & Davis, R.J. (1994). An osmosensing signal transduction pathway in mammalian cells. Science, 265: 806–808.
- He, Y., Meng, X., Fan, Q., Sun, X., Xu, Z. & Song, R. (2009). Cloning and characterization of two novel chloroplastic glycerol-3-phosphate dehydrogenases from Dunaliella viridis. Plant Molecular Biology, 71: 193–205.
- Hohmann, S. (2002). Osmotic stress signaling and osmoadaptation in yeasts. Microbiology and Molecular Biology Reviews, 66: 300–372.
- Hohmann, S. (2009). Control of high osmolarity signalling in the yeast Saccharomyces cerevisiae. FEBS Letters, 583: 4025–4029.
- Jimenez, C., Berl, T., Rivard, C.J., Edelstein, C.L. & Capasso, J.M. (2004). Phosphorylation of MAP kinase-like proteins mediate the response of the halotolerant alga Dunaliella viridis to hypertonic shock. Biochimica et Biophysica Acta (BBA)–Molecular Cell Research, 1644: 61–69.
- Kumar, S.V., Misquitta, R.W., Reddy, V.S., Rao, B.J. & Rajam, M.V. (2004). Genetic transformation of the green alga – Chlamydomonas reinhardtii by Agrobacterium tumefaciens. Plant Science, 166: 731–738.
- Lei, G., Qiao, D., Bai, L., Xu, H. & Cao, Y. (2008). Isolation and characterization of a mitogen-activated protein kinase gene in the halotolerant alga Dunaliella salina. Journal of Applied Phycology, 20: 13–16.
- Livak, K.J. & Schmittgen, T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods, 25: 402–408.
- Mitra, A., Higgins, D.W. & Rohe, N.J. (1994). A Chlorella virus gene promoter functions as a strong promoter both in plants and bacteria. Biochemical and Biophysical Research Communications, 204: 187–194.
- Saito, H. & Posas, F. (2012). Response to hyperosmotic stress. Genetics, 192: 289–318.
- Saito, H. & Tatebayashi, K. (2004). Regulation of the osmoregulatory HOG MAPK cascade in yeast. Journal of Biochemistry, 136: 267–272.
- Yu, H., Ito, T., Wellmer, F. & Meyerowitz, E.M. (2004). Repression of AGAMOUS-LIKE 24 is a crucial step in promoting flower development. Nature Genetics, 36: 157–161.
